Translate this page into:
Synthesis, in vitro biological analysis and molecular docking studies of new thiadiazole-based thiourea derivatives as dual inhibitors of a-amylase and a-glucosidase
⁎Corresponding author. sono_waj@yahoo.com (Wajid Rehman)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University. Production and hosting by Elsevier.
Abstract
Diabetes mellitus is a syndrome that is caused due to the imbalance of insulin production in the body. In the present study we have synthesized a class of fifteen compounds (1–15) based on thiadiazole-bearing thiourea that were assessed for in vitro alpha-amylase and alpha-glucosidase inhibitory potentials against standard drug acarbose. In this series, all the synthesized scaffolds were recognized as potentials inhibitors of both targeted enzymes, α-amylase having varied range from IC50 values = 35.70 ± 0.70 µM to 1.30 ± 0.05 µM against standard drug acarbose (10.30 ± 0.20 µM) while for α-glucosidase IC50 values = 37.60 ± 0.80 µM to 2.20 ± 0.10 µM against standard drug acarbose (9.80 ± 0.20 µM). Among the series, nine scaffolds such as 4, 6, 7, 9, 8, 11, 12, 14 and 15 showed excellent activity against a-amylase and a-glucosidase and were found many folds more potent than standard acarbose drugs due to the change in nature and number/s of substituent along the entire skeleton. A molecular docking study was conducted against active compounds to understand the binding modes of the synthetic analogs and how they show interaction with the active part of the enzymes. To confirm the structure of synthetic analogs different spectroscopic techniques will be used like NMR and HREI-MS.
Keywords
Thiadiazole
Thiourea
α-amylase
α-glucosidase
SAR and Docking study
1 Introduction
Diabetes, a serious metabolic disease caused by a high plasma glucose concentration can occur (Rasheed et al., 2023; Fall, Holen, Davis, Krieg, & Koster, 2006).Type-II diabetes also known as cure able disorder found to be the most common than Type-I. The change between insulin release and glucose intake is a common source of type-II diabetes. Controlling blood sugar is the primary source to cure type-II diabetes (Baron, 1998).It is also gain this purpose with active insulin release through recommended diet guidance (Goldberg, 1998; Porte Jr & Kahn, 2001; Rabasa‐Lhoret & Chiasson, 2003). Group for Research on Diabetes in the UK (UKPDS) 1998, advice to controlling one's diet is one of the primary diabetes treatments. One of the most frequently recommended health remedies right now is eating diet based foods that are low in sugar. It was determined that using nutritional therapy along with traditional clinical medications would have a greater impact(Albright et al., 2000; Jenkins et al., 1981; Wolever et al., 1994). It is also maintained through the limiting diet plan and amounts of food consumed.Another possible solution is to slow down the nutritional source, the main nutritional source is glucose, to slow down the rate of glucose consumption in the small intestine. Nutritional therapy is also known as more effective than insulin regulation (Porte Jr & Kahn, 2001). The process of controlling the level of glucose has been focused on by blocking proteins such as alpha-amylase/glucosidase, which convert the nutritional source to glucose (Ahrén et al., 2004; Ali, Stone, Peters, Davies, & Khunti, 2006; Geng, Qiu, Zhu, & Bai, 2008). Alpha-amylase, catalyze the hydrolysis of glycosidic linkages, releasing maltose as well as glucose into starch (Svensson & Søgaard, 1993; Takkinen et al., 1991), while maltose and other possible sucrose are released down the glucose (Anderson et al., 2003; Mohan, Sim, Rose, & Pinto, 2007). While only a-amylase is present in salivation, the two of them are expelled in the small digestive tract. Through the controlling of glucose in the circulatory system to maintain diabetes –II. Researchers continuously try to make these enzymes a part of food materials or food additives (Seo, Suh, Bae, & Jung, 2005; Wakita et al., 2005). However, the fact demonstrated that some phenolic compounds with sugar-like properties may have a-glucosidase inhibitory potential. The majority of the research suggested the use of phenolic compounds to reduce these two enzymes(Bhandari, Jong-Anurakkun, Hong, & Kawabata, 2008; Hermeking, 2007).
It was reported that heteroarene serves as the primary moiety in the structures of many commercially available drugs(Enguehard-Gueiffier & Gueiffier, 2007; Leeson & Springthorpe, 2007; Makhova et al., 2020; Nimesh et al., 2014; Thiel, 2013).Due to its prominence in many biologically active analogs for drug discovery, fused heteroarene is one of the major heteroarene-bearing scaffolds that has drawn the attention of medicinal chemists. It is also found in many biologically active drugs. Additionally, heterocyclic compounds showed interesting biological applications like benzothiazole combined with imidazole, which is reported as an antitumor precursor which is used for the treatment of cancer(Amino et al., 2006).
Five-membered heterocyclic analogues such as thiadiazole shown a wide range of biological applications such as anti-bacterial as well as neuroprotective (Fang, Zhu, Xu, Wang, & Ji, 2017; Perlovich, Proshin, Volkova, Petrova, & Bachurin, 2012). Due to their biological profile condensed heterocyclic compounds thiadiazole(Gummidi et al., 2021; Javid et al., 2018; Palamarchuk, Shulgau, Dautov, Sergazy, & Kulakov, 2022) and thiourea (Ahmed et al., 2022; Naz et al., 2019) got attention in the field of drug discovery, hence we were introduced a new class of heterocyclic compounds such as such as 1,2,4-thiadiazole, which incorporates a thiourea moiety and is used to treat diabetic patients. We purposefully omitted the synthesis of 1-(2-nitrophenyl)-3-(5-((3-(trifluoromethyl)phenyl)amino)-1,2,4-thiadiazol-3-yl)thiourea and examined their alpha-amylase and alpha-glucosidase profile to keep in mind the biological applications of fused heterocyclic compounds. To find out the findings SAR and molecular docking study was performed (see Fig. 1).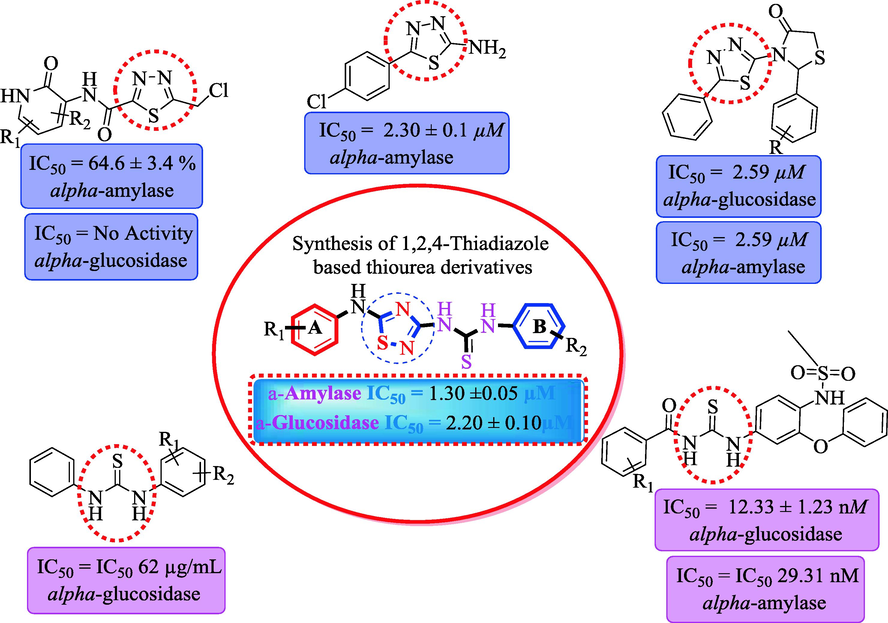
Rational of current study.
2 Materials and methods
2.1 Materials
All of the chemicals were used in this research work were purchased from Merck (Germany) and Sigma Aldrich (USA). For the analysis of 1H NMR and 13C NMR Bruker Avance (600 MHz) instrument were used. HREI-MS data were recorded on the mass spectrometer JMS-600H JEOL. For the identification of spots in TLC plate UV 366 and 254 nm lamp was use. DMSO‑d6 used as reference, chemical shift was measured in ppm and coupling constant was calculated in Hz.
2.2 Chemistry
In the first step, solution of guanidine salt (a I equivalent) was added to the solution of isothiocyanate (b I equivalent) in THF(10 mL)in the presence of ET3N (2–3 drops) and remaining mixture was reflux and stirred for 8 hrs to afford the formation of substrate (c I equivalent) was re-dissolved in 1,4-Dioxane(10 mL), followed by the addition of molecular iodine and potassium carbonate in sequence. The remaining residue was stirred and refluxed until the oxidative cyclization N-S bond formation was done and conversion will be monitored through TLC reflux for 14 hrs. After being cooled to 25 °C, the residue was reacted with 5% sodium thiosulphate solution and extracted with ethyl acetate to afford the synthesis of 3-amino-1,2,4-thiadiazole as an intermediate (d I equivalent). In the next step, the 3-amino-1,2,4-thiadiazole intermediate (d I equivalent) was further reacted with different substituted isothiocyanate in tetrahydrofuran(10 mL)and Et3N (2–3 drops) and the resulting reaction residue was refluxed and stirred until the reactants were disappeared to yield 1,2,4-thiadiazole based urea analogs (1–15) (Scheme 1). Further, these derivatives (1–15) could be further cyclized with chloroacetic acid in glacial acetic acid and sodium acetate to afford thiadiazole-based thiazolidinone analogues in future.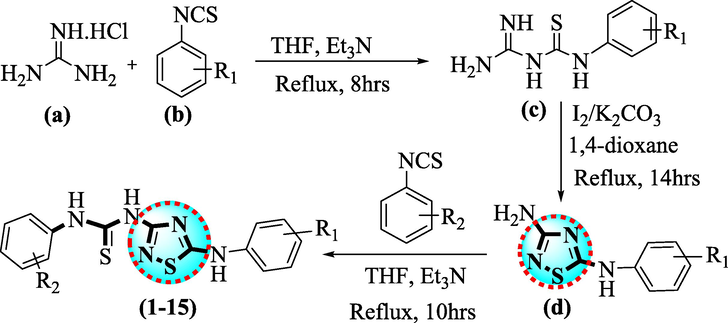
Synthesis of thiadiazole-based thiourea scaffolds.
2.3 Spectral analysis
Spectral analysis was provided in the supplementary information.
2.4 Assay protocol for a-amylase
It has been carried out according to our previously reported work (Salar et al., 2017).
2.5 Assay protocol for a-glucosidase
It has been carried out according to our previously reported work (Ramírez-Escudero et al., 2016).
2.6 Assay protocol for molecular docking investigation
It has also been performed according to our previously published work (Khan et al., 2022).
3 Results and discussion
3.1 Biological analysis (1–15)
3.1.1 In vitro α-amylase and α-glucosidase inhibitory activities
All synthetic scaffolds based on 1,2,4-thiadiazole (Gummidi et al., 2021; Javid et al., 2018; Palamarchuk, Shulgau, Dautov, Sergazy, & Kulakov, 2022) (Ahmed et al., 2022; Naz et al., 2019; Mitrakou et al., 1992) bearing thiourea (1–15) were assessed for in vitro alpha-glucosidase and alpha-amylase inhibition profiles. It was revealed by structure–activity relationship studies that all the newly afforded thiadiazole-based thiourea scaffolds illustrated moderate to good inhibition profiles against both these targeted enzymes when compared to acarbose as a reference drug. Moreover, it was suggested by limited SAR studies that entire structural features such as 1, 2, 4-thiadiazole, thiourea, aryl part ‘A’ and aryl part ‘B’ actively contributed to inhibiting the action of both targeted enzymes and any variation in the potency is attributed to diverse substitutions pattern on both aryl parts ‘A’ and ‘B’ respectively.
3.1.1.1 SAR study of alpha-amylase and alpha-glucosidase inhibitory potentials
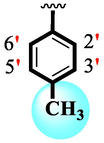
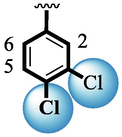
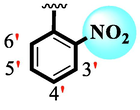
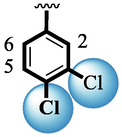
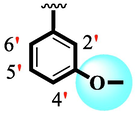
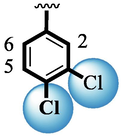
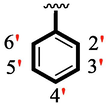
Among scaffolds (4 and 6–8), scaffold (7) bearing –F moiety at the 2nd position of “B” anddi-Cl moieties at 3,4-position of the ring ‘A’ was found as the potent inhibitor of both these targeted enzymes. This elevation in the potency of these scaffolds is due to better interaction of these di-Cl as well as –F moieties with the active residue of amino acid and hence, enhanced the inhibition profile. Scaffold (6) that holds di-Cl moieties on 3rd and 4thposition of both rings ‘A’ and ‘B’ displayed 8-fold more activity than standard acarbose drug. These di-Cl moieties on both rings reduce the electronic density on both rings ‘A’ and ‘B’, making them e-deficient species which in turn attain stability by establishing key interactions like pi-cationic interaction with an active pocket of the amino residue of both targeted α-amylase and α-glucosidase enzymes. The inhibitory potentials of compound (6) was drops down too many folds by de-attachment of di–Cl moieties of ring ‘B’ followed by consequent addition of –OCH3 moiety at 2-position of ring ‘B’ as in scaffold (8). This discrepancy in potency found in both these scaffolds was due to different number/s as well as the diverse nature of attached substituents showing that number/s as well as natures of substituents is actively participating in the potency. However, scaffold (7) that holds –F moiety at the 2-position of ring ‘B’ along with di-Cl moieties at 3,4-position of the ring ‘A’ displayed somewhat better potency than its counterpart (6). Moreover, the decline in the activity of the compound (75) was observed by replacing –F moiety from its 2-position of ring ‘B’ with –NO2 moiety as in scaffold (4). This was due to the stronger e-withdrawing nature of the fluorine atom present at the 2-position of ring ‘B’ of the scaffold (7) shown in (Fig. 2).
SAR study of analogues 4, 6, 7 and 8.
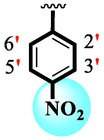
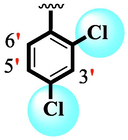
Analog (9) bearing -F moiety attached to the 4-position of the ring ‘A’ and –NO2 moiety present at the 3-position of another ring ‘B’ displayed excellent potency for both targeted alpha-amylase and alpha-glucosidase enzymes. These -F and –NO2 moieties established interesting interactions such as conventional hydrogen and halogen-bond interactions and hence, improved the enzymatic inhibition of both targeted α-amylase and α-glucosidase. The analog (10) that holds –NO2 moieties on the 3-position of both rings ‘A’ and ‘B’ respectively, exhibited 3-fold lower potency against α-amylase and α-glucosidase enzymes than its counterpart (9) owing to the diverse nature of substituent around ring ‘A’ although the ring ‘B’ holds same –NO2 moiety at 3-position. The decline in the potency of the scaffold (10) was observed by shifting the positions of –NO2 moiety from its 3-position of the ring ‘B’ to its 4-position of the ring ‘B’ as in compound (11). This difference in activity found in these scaffolds (10) and (11)was due to different position of –NO2 moiety around ring ‘B’ showing that the inhibition profile against both these targeted enzymes was greatly affected by changing the position of a substituent to either side of rings ‘A’ and ‘B’. The enhanced inhibitory potential of either compound (10)or analog (11)was observed by the introduction of di-Cl moietiesat the 2,4-position of ring ‘B’ instead of –NO2moiety as in the case of compound (12). The superior potency of scaffold (12) was due to a greater number of –Cl moietiesalong with –NO2 moiety on the 3-position of the ring ‘A’. These di-Cl and –NO2 moieties interact well with active residues of both targeted a-amylase and a-glucosidase enzymes and hence, enhanced the enzymatic potentials shown in Fig. 3.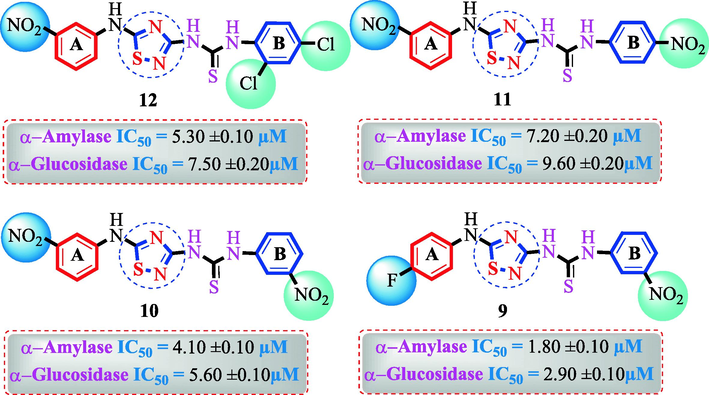
SAR study of analogues 9, 10, 11 and 12.
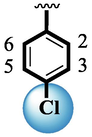
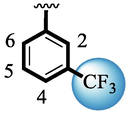
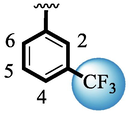
Compound (15) bearing tri-fluoro methyl moiety attached to the 3-position of the ring ‘A’ and –NO2 moiety present at the 2-position of another ring ‘B’ showed remarkable potency for both targeted alpha-amylase and alpha-glucosidase enzymes. These tri-fluoro methyl and –NO2 moieties form better interactions such as halogen-bond and conventional hydrogen bond interactions and hence, enhanced the enzymatic potentials of α-amylase and α-glucosidase. The scaffold (14) that holds tri-fluoro methyl moiety on 3-position of ring ‘A’ and –Cl moiety linked to 4-position of other ring ‘B’ displayed 7-folds less inhibition profile against α-amylase and α-glucosidase enzymes than its counterpart (15) owing to different nature of substituent around ring ‘B’ although the ring ‘A’ holds same tri-flouro methyl moiety at 3-position. The decrease in the potency of either scaffold (15) or compound (14) was observed by changing the nature of substituent through de-attachment of 3-trifluoro methyl moiety followed by subsequent attachment of –Cl moiety to 4-position of the ring ‘A’ as in scaffold (2) that holds –NO2 moiety to 4-position of ring ‘B’ along with –Cl moiety at 4-position of ring ‘A’. This discrepancy in potency found in these scaffolds (15) and (2) was due to better interactions of tri-fluoro methyl moiety with the active residue of amino acid than –Cl moiety which is unable to interact like tri-fluoro methyl moiety. The inhibitory potential of compound (2)was further diminished by the introduction of a substituent of bulky nature like –Br in place of –NO2 moiety as in the case of compound (1). Compound (1) displayed lower potency when compared to either scaffold (15) or compound (2). The lower potency of scaffold (1) was due to the bulky nature of –Br which is unable to interact well with both targeted α-amylase and α-glucosidase enzymes and hence, lower the enzymatic activity shown in (Fig. 4).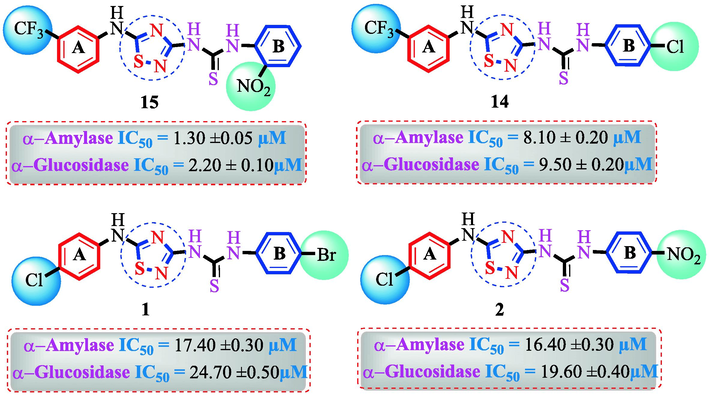
SAR study of analogues 1, 2, 14 and 15.
Overall it was summarized based on the aforementioned observation, that inhibitory potentials for each category against alpha-amylase and alpha-glucosidase enzymes were greatly affected by bringing varied substitutions in different number/s around aromatic parts on both sides ‘A’ and ‘B’. Hence, it was also noted down by changes in the nature and position around the aromatic parts on both sides ‘A’ and ‘B’ were found to be helpful for enzymatic potentials against targeted enzymes.
3.2 Molecular Docking:
The synthesized analogs and their measured inhibition against alpha-amylase and alpha-glucosidase enzymes are shown in Table 1. It was prominent from IC50 values of thiadiazole-based thiourea analogs that alpha-amylase and alpha-glucosidase inhibitors showed strong effect due to the position, numbers/s and nature of the attached substituent of both aryl parts “A” and “B” at the basic skeleton. However, Molecular docking analyses were performed in order to note down the position, numbers and natures of the attached substituents and enzymatic profile furthermore, to develop the binding modes of the newly afforded analogs with the active part of the targeted enzymes. 0.20 µM
S.No.
Ring A
Ring B
α-amylase inhibition (µM ± SEM)
α-glucosidase inhibition (µM ± SEM)
Solubility
1
17.40 ± 0.30
24.70 ± 0.50
DMSO
2
16.40 ± 0.30
19.60 ± 0.40
DMSO
3
23.50 ± 0.30
24.80 ± 0.40
DMSO
4
2.50 ± 0.10
4.10 ± 0.20
DMSO
5
20.60 ± 0.30
25.80 ± 0.40
DMSO
6
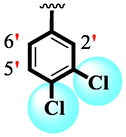
2.10 ± 0.10
3.20 ± 0.10
DMSO
7
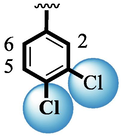
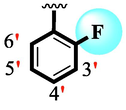
1.90 ± 0.10
2.70 ± 0.10
DMSO
8
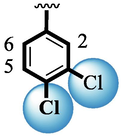
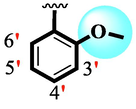
25.30 ± 0.50
28.40 ± 0.50
DMSO
9
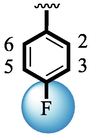
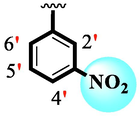
1.80 ± 0.10
2.90 ± 0.10
DMSO
10
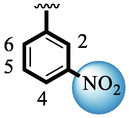
4.10 ± 0.10
5.60 ± 0.10
DMSO
11
7.20 ± 0.20
9.60 ± 0.20
DMSO
12
5.30 ± 0.10
7.50 ± 0.20
DMSO
13
35.70 ± 0.70
37.60 ± 0.80
DMSO
14
8.10 ± 0.20
9.50 ± 0.20
DMSO
15
1.30 ± 0.05
2.20 ± 0.10
DMSO
Standard Acarbose
10.30 ± 0.20 µM
---
It was shown that from the PLI study details of both active analogs 9 and 15 against the targeted enzymes that they are developed interactions of the active part of both enzymes, which also help in the enhancement of the inhibition profile of these potent analogues against the targeted enzymes. It was noted that analog 9 adopted various interactions with the active residue of amino acid of alpha-glucosidase enzymes including various interaction such as Ph3476 (conventional hydrogen bond and pi-pi stacking), ILE233 (pi-sigma), Lys506 (pi-cation and pi-alkylation), Ala231 (pi-alkylation), Asn496 (conventional hydrogen bond and halogen (fluorine)) and Ser505 (halogen (fluorine)) (Fig. 5A). On the other hand, protein–ligand interaction (PLI) of same scaffold 9 against α-amylase enzyme revealed several important interactions such as Arg343 (halogen (fluorine)), Gly359 (halogen (fluorine)), Pro57 (carbon hydrogen bond), Val358 (carbon hydrogen bond and pi-alkylation) and Asn362 (conventional hydrogen bonding) (Fig. 5C).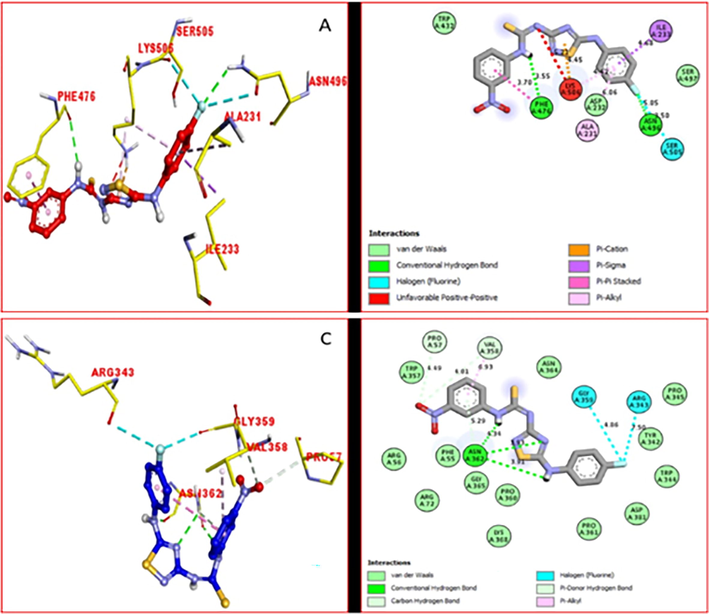
PLI profile of the active compounds 9 against the alpha-amylase and alpha-glucosidase (A) for 9 against alpha-glucosidase, while (C) for same compound 9 against alpha-amylase enzymes.
Similarly, the PLI profile of potent analog 15 against alpha-glucosidase enzyme showed various interactions with the active site of alpha-glucosidase including residues ILE233 (pi-sigma), Ala231 (pi-alkylation), Ser505 (conventional hydrogen bond) Lys506 (pi-cation and pi-alkylation), Phe476 (pi-sulfur and pi-alkylation) and Trp432 (pi-alkylation) (Fig. 6B), while this scaffold against α-amylase enzyme adopted key interactions with Asp381 (attractive charge) and Asn362 (conventional hydrogen bond) (Fig. 6D). The high enzymatic inhibition of this scaffold may be due to the –NO2and fluoro moieties on both ends of scaffolds, where they rescue the electronic density from pi-system making the partial positive charge over pi-system, and further via adopting several key interactions with the active residues this ph-rings regain stability, and hence enhanced the inhibition profile.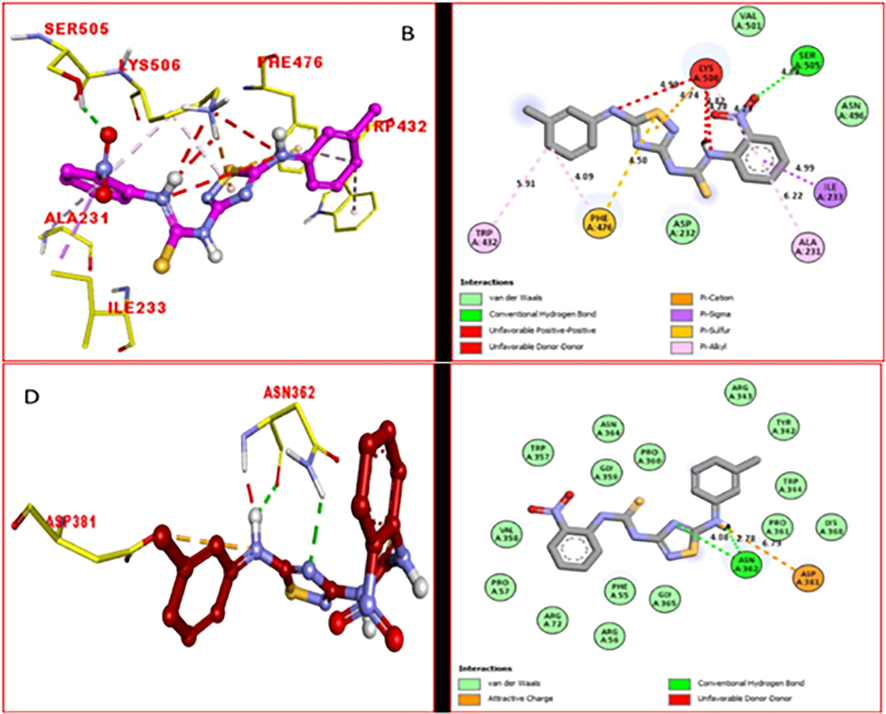
The PLI profile of the potent compound 15 against alpha-glucosidase and alpha-amylase (B) for analogue 15 against alpha-glucosidase, while (D) for analogue 15 against alpha-amylase enzyme.
4 Conclusion
In this work, we have synthesized a class of fifteen compounds (1–15) based on thiadiazole-bearing thiourea that were synthesized and assessed for in vitro a-amylase and a-glucosidase inhibitory potentials and an SAR study was conducted to understand in a better way. All the synthesized scaffolds were recognized as potential inhibitors of both targeted α-amylase and α glucosidase enzymes. Among the seriesnine scaffolds such as 4, 6, 7, 9, 8, 11, 12, 14 and 15 showed considerable activity against alpha-glucosidase and alpha-amylase and found many folds more potent than standard acarbose drug. While remaining analogs such as 1, 2, 3, 5, 10 and 13also displayed considerable inhibitory potentials both for α-amylase and α glucosidase enzymes, but found as less active than standard.Additionally, to find out the binding interactions molecular docking study was conducted against both enzymes several key interactions were found such as pi-alkylation, pi-cation, pi-sulfur, conventional hydrogen bond, pi-fluorine etc.The results shown that selected compounds shown interesting interactions with the selected enzymes, also enhance the enzymes, enzymatic activity. Additionally, several analytical tools were used such as 1H NMR, 13CNMR and HREI-MS were employed to confirm the structure of the synthesized analogs. Future studies could involve additional optimization of the analogs, in vivo assessments, and clinical trials to fully understand their efficacy and potential for therapeutic applications.
Acknowledgement
The authors extend their appreciation to Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2023R342), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia for funding this research. The authors also extend their appreciation to the Researchers Supporting Project number (RSPD2023R754), King Saud University, Riyadh, Saudi Arabia for funding this research.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Nimesulide linked acyl thioureas potent carbonic anhydrase I, II and α-glucosidase inhibitors: Design, synthesis and molecular docking studies. European Journal of Medicinal Chemistry Reports. 2022;6:100082
- [Google Scholar]
- Inhibition of dipeptidyl peptidase-4 reduces glycemia, sustains insulin levels, and reduces glucagon levels in type 2 diabetes. J. Clin. Endocrinol. Metab.. 2004;89(5):2078-2084.
- [Google Scholar]
- American College of Sports Medicine position stand. Exercise and type 2 diabetes. Med. Sci. Sports Exerc.. 2000;32(7):1345-1360.
- [Google Scholar]
- The prevalence of co-morbid depression in adults with Type 2 diabetes: a systematic review and meta-analysis. Diabet. Med.. 2006;23(11):1165-1173.
- [Google Scholar]
- YM-201627: An orally active antitumor agent with selective inhibition of vascular endothelial cell proliferation. Cancer Lett.. 2006;238(1):119-127.
- [Google Scholar]
- Intracellular bacterial biofilm-like pods in urinary tract infections. Science. 2003;301(5629):105-107.
- [Google Scholar]
- Postprandial hyperglycaemia and α-glucosidase inhibitors. Diabetes Res. Clin. Pract.. 1998;40:S51-S55.
- [Google Scholar]
- α-Glucosidase and α-amylase inhibitory activities of Nepalese medicinal herb Pakhanbhed (Bergenia ciliata, Haw.) Food Chem.. 2008;106(1):247-252.
- [Google Scholar]
- Recent Progress in the Pharmacology of Imidazo [1, 2-a] pyridines. Mini Rev. Med. Chem.. 2007;7(9):888-899.
- [Google Scholar]
- Fall, J. A., Holen, D. L., Davis, B., Krieg, T., & Koster, D. (2006). Subsistence harvests and uses of wild resources in Iliamna, Newhalen, Nondalton, Pedro Bay, and Port Alsworth, Alaska, 2004. Alaska Department of Fish and Game Division of Subsistence, Technical Paper(302).
- Aerobic radical-cascade cycloaddition of isocyanides, selenium and imidamides: facile access to 1, 2, 4-selenadiazoles under metal-free conditions. Green Chem.. 2017;19(7):1613-1618.
- [Google Scholar]
- Four acarviosin-containing oligosaccharides identified from Streptomyces coelicoflavus ZG0656 are potent inhibitors of α-amylase. Carbohydr. Res.. 2008;343(5):882-892.
- [Google Scholar]
- Proteasome inhibitors: valuable new tools for cell biologists. Trends Cell Biol.. 1998;8(10):397-403.
- [Google Scholar]
- Multicomponent reaction for the synthesis of new 1, 3, 4-thiadiazole-thiazolidine-4-one molecular hybrids as promising antidiabetic agents through α-glucosidase and α-amylase inhibition. Bioorg. Chem.. 2021;115:105210
- [Google Scholar]
- Synthesis, in vitro α-glucosidase inhibitory potential and molecular docking study of thiadiazole analogs. Bioorg. Chem.. 2018;78:201-209.
- [Google Scholar]
- Glycemic index of foods: a physiological basis for carbohydrate exchange. Am. J. Clin. Nutr.. 1981;34(3):362-366.
- [Google Scholar]
- Synthesis, in vitro α-amylase, α-glucosidase activities and molecular docking study of new benzimidazole bearing thiazolidinone derivatives. J. Mol. Struct. 2022
- [Google Scholar]
- The influence of drug-like concepts on decision-making in medicinal chemistry. Nat. Rev. Drug Discov.. 2007;6(11):881-890.
- [Google Scholar]
- Progress in the chemistry of nitrogen-, oxygen-and sulfur-containing heterocyclic systems. Russ. Chem. Rev.. 2020;89(1):55.
- [Google Scholar]
- Role of reduced suppression of glucose production and diminished early insulin release in impaired glucose tolerance. N. Engl. J. Med.. 1992;326(1):22-29.
- [Google Scholar]
- Synthesis of S-alkylated sulfonium-ions and their glucosidase inhibitory activities against recombinant human maltase glucoamylase. Carbohydr. Res.. 2007;342(7):901-912.
- [Google Scholar]
- Enzyme inhibitory, antioxidant and antibacterial potentials of synthetic symmetrical and unsymmetrical thioureas. Drug Des. Devel. Ther.. 2019;13:3485.
- [Google Scholar]
- Synthesis and biological evaluation of novel bisbenzimidazoles as Escherichia coli topoisomerase IA inhibitors and potential antibacterial agents. J. Med. Chem.. 2014;57(12):5238-5257.
- [Google Scholar]
- Design, synthesis, spectroscopic characterization, computational analysis, and in vitro α-amylase and α-glucosidase evaluation of 3-aminopyridin-2 (1 H)-one based novel monothiooxamides and 1, 3, 4-thiadiazoles. Org. Biomol. Chem.. 2022;20(45):8962-8976.
- [Google Scholar]
- Novel 1, 2, 4-thiadiazole derivatives as potent neuroprotectors: approach to creation of bioavailable drugs. Mol. Pharm.. 2012;9(8):2156-2167.
- [Google Scholar]
- beta-cell dysfunction and failure in type 2 diabetes: potential mechanisms. Diabetes. 2001;50(suppl_1):S160.
- [Google Scholar]
- Structural analysis of β-fructofuranosidase from Xanthophyllomyces dendrorhous reveals unique features and the crucial role of N-glycosylation in oligomerization and activity. J. Biol. Chem.. 2016;291(13):6843-6857.
- [Google Scholar]
- Rasheed, L., Rehman, W., Rahim, F., Ali, Z., Alanazi, A.S., Hussain, R., Khan, I., Alanazi, M.M., Naseer, M., Abdellattif, M.H., 2023. Molecular modeling and synthesis of indoline-2, 3-dione-based benzene sulfonamide derivatives and their inhibitory activity against α-glucosidase and α-amylase enzymes. ACS omega.
- New hybrid hydrazinyl thiazole substituted chromones: As potential α-amylase inhibitors and radical (DPPH & ABTS) scavengers. Sci. Rep.. 2017;7(1):1-17.
- [Google Scholar]
- Differentiation of human adipose stromal cells into hepatic lineage in vitro and in vivo. Biochem. Biophys. Res. Commun.. 2005;328(1):258-264.
- [Google Scholar]
- An active single-chain antibody containing a cellulase linker domain is secreted by Escherichia coli. Protein Eng. Des. Sel.. 1991;4(7):837-841.
- [Google Scholar]
- Heterocyclic Chemistry in Drug Discovery. Wiley Online Library; 2013.
- Production of infectious hepatitis C virus in tissue culture from a cloned viral genome. Nat. Med.. 2005;11(7):791-796.
- [Google Scholar]
- Glycaemic index of 102 complex carbohydrate foods in patients with diabetes. Nutr. Res.. 1994;14(5):651-669.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2023.105078.
Appendix A
Supplementary data
The following are the Supplementary data to this article:Supplementary Data 1
Supplementary Data 1







