Translate this page into:
Synthesis of eco-friendly porous g-C3N4/SiO2/SnO2 composite with excellent visible-light responsive photocatalysis
⁎Corresponding authors. custkjy@163.com (Da-wei Feng), yh2001101@163.com (Hui Yu)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Forming eco-friendly heterojunction photocatalysts is excellent method to accelerate the separation rate of photogenerated charge carriers, which is attracting more and more attention. In this study, a novel and stable disordered porous g-C3N4/SiO2/SnO2 (DOP-CSiSn) heterojunction composites was fabricated by a sol-gel hard template method, and the optimal g-C3N4 doped ratio was adjusted in DOP-CSiSn. The DOP-CSiSn photocatalyst had the much larger specific surface area and disordered porous structure, which exhibited strong photocatalytic effect to degrade Rhodamine B (RhB), Methylene blue (MB) and Methyl orange (MO) under visible light. When the g-C3N4 doping content was 30 wt%, the highest photocatalytic activities were obtained, and the degradation rate of MB and MO were 99.73% and 95.58% after 50 min, respectively. Degradation rate of RhB was 95.10% after 90 min. Photocatalytic degradation rate of organic pollutants were still more than 90% after six time consecutive cycles, the composite had wonderful stability and potential value in environmental purification.
Keywords
Disorder mesoporous SnO2 aerogel
G-C3N4
Hard template method
Photocatalytic
Visible-light
1 Introduction
The global population growth led to sustained growth in energy demand, which induced a series of environmental issues. The environmental problems solving needs to be started from advanced technologies of green fuel production and effective elimination of harmful pesticides and organic pollutants (Wang and Wu, 2013). Since Fujishima and Honda reported that TiO2 electrode could produce H2 and O2 in 1972, the semiconductor-based photocatalysis seemed to be a versatile concept of green technology because of their capacities for water decontamination and water splitting (Zheng and Yang, 2018; Yan et al., 2018). The wolframate and titanate heterostructure materials have been used to degrade organic pollutants (Zhao et al., 2018; Mohnamed et al., 2012; Kumar et al., 2014). The most of oxide semiconductors have been used as photocatalysis, such as TiO2 (Jallouli et al., 2019; Nadarajan et al., 2018; Zheng et al., 2017; Wu et al., 2017; Hao et al., 2017), ZnO (liu et al., 2017; Rashid et al., 2014), SnO2 (You et al., 2013; Wu et al., 2011), Fe2O3 (Tian et al., 2014; Xu et al., 2018), CdSe (Shi et al., 2017) etc.
Due to widespread use, the tin oxide (SnO2) has aroused people’s attention. SnO2 is a wide band gap (3.6 eV) n-type semiconductor (Rakibuddin et al., 2017; Zhang et al., 2013), which has been widely used in photo-catalytic degradation of organic dyes (Li et al., 2013), rechargeable lithium ion batteries (Reddy et al., 2016; Chen et al., 2017), gas sensors (Lin et al., 2015; Du et al., 2015; Qi et al., 2014; Liu et al., 2010), fuel sensitized solar cells (Son et al., 2015) and supercapacitor (Liu et al., 2014). Davis et al. (Davis et al., 2012) successfully prepared ZnO-SnO2 nanocomposites photocatalysts. Huang et al. (2013) prepared ZnO nanorod-SnO2 nanoparticle (ZnO NR-SnO2 NPs) heterostructures through a simple way, the RhB degradation efficiency was 78% after 60 min. Kar et al. (2012) prepared SnO2/CdS composite, and reported its photocatalytic properties, the photocatalytic degradation rate of Congo red dye was found to be 97% under UV light irradiation. Park et al. (2014) synthesized hierarchical mesoporous SnO2 spheres which showed high photocatalytic properties under UV light. Due to the broad energy bandgap, SnO2 can only be used as a active photocatalyst under ultraviolet light irradiation. It is all known that the UV region occupies only about 4% of all solar spectrum, while the visible light accounts for 45% of all solar spectrum. In order to broaden the applications of SnO2 photocatalyst, visible light response must be achieved. Therefore, the energy band of SnO2 needs to be modified. In order to reduce the band gap, there are three conventional strategies, which includes modifying of valence band, adjusting of conduction band and continuous modulation of valence and conduction bands. Many attempts have been made to improve visible-light photocatalysis of SnO2 by doping with metal and non-metal. The purpose of these methods are achieve efficient separation of electron-holes by designing to construct a heterostructure and change the direction of electron transfer.
The non-metal doping has achieved remarkable results in recent years. For example, graphene and other carbon materials were used as absorbing material, electrochemical, which were also used to enhance the conductivity of semiconductor to evolve its response. But graphene requires complex processing procedures (Yang et al., 2018; Xia et al., 2018). The graphitic carbon nitride (g-C3N4) is an ideal non-metal. The g-C3N4 can be applied to SnO2 band modification. As a perfect polymer n-type semiconductor, g-C3N4 attracted a lot of attention in degradation of organic pollutants (Jiang et al., 2015). The band gap of g-C3N4 is 2.73 eV (Thomas et al., 2008; Naseri et al., 2017), which indicates that the response wavelength of g-C3N4 is up to 450 nm. g-C3N4 has appropriate band gap to absorb the visible-light (He et al., 2017; Wen et al., 2017; Wei et al., 2017; Wang et al., 2017; Yu et al., 2017; Zhao et al., 2017; Zhou et al., 2018), it can be easily prepared by calcining of melamine, urea and cyanamide (Wang et al., 2014; Kuang et al., 2015) which has been used in the field of photocatalysts. Kong et al. (2016) prepared low cost Ni/g-C3N4 photocatalyst, and 4318 μmolg−1 h−1 H2 production rate was obtained when Ni was loaded to 7.40 wt%. However, the photocatalytic efficiency of g-C3N4 is still limited because of relatively fast recombination rate of photogenerated electron-hole pairs. In addition, the less surface area reduces absorption efficiency of g-C3N4 to visible-light. Therefore, g-C3N4 can be doped in porous SnO2 matrix as visible light driven photocatalysts. On the other hand, the more active sites are provided by porous SnO2.
In the current work, we successfully prepared porous g-C3N4/SiO2/SnO2 composites by sol-gel method. The prepared g-C3N4/SiO2/SnO2 was characterized through powder X-ray diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscope (TEM), ultraviolet-visible spectrum (UV–vis) and N2 adsorption-desorption measurements. The prepared disordered porous g-C3N4/SiO2/SnO2 composite material had the higher photocatalytic activities to the decomposition of RhB, MB and MO under visible light (>420 nm) irradiation, which had potential applications in the decomposition of the organic pollutants.
2 Experimental
2.1 Materials
Tin (II) chloride dihydrate (SnCl2·2H2O, A.R.) was produced by Xilong Chemical Research Institute in China. Tetraethyl silicate (TEOS, A.R.) was produced by Beijing Chemical Works Research Institute in China, melamine (C3H6N6, A.R.) was produced by Tianjin Guangfu Fine Chemical Research Institute. Isopropy alcohol (C3H2O, A.R.) and p-Benzoquinone (C6H4O2, A.R.) were produced by Tianjin Fuyu Fine Chemical Co., Lt. Sodium oxalate (Na2C2O4, A.R.) was produced by East China Reagent Factory. All reactants were analytical grade, and used without further purification. Deionized water and absolute alcohol were used throughout dissolution and filtration process.
2.2 Preparation of porous g-C3N4/SiO2/SnO2 composites
G-C3N4 was prepared according to the literature reported procedure (Sun et al., 2014). The detailed preparation process as follow: melamine was calcined at 550 °C with 10 °C·min−1 heat rate for 2 h.
The g-C3N4/SiO2/SnO2 composite was synthesized by a sol-gel method. The detailed preparation process as follow:11.283 g of SnCl2·2H2O was dissolved in 6.2 mL ethyl alcohol with stirring for 30 min, 3.8 mL of distilled water and different qualities g-C3N4 were added into the above solution with continue stirring for 2 h, and 4 mL of TEOS was added into the solution to form the gel. The gel was aged in room temperature for 24 h. The aged gel was soaked in deionized water to continue age at 70 °C for 2–4 d. The solvent of the gel was replaced by n-hexane solution at 50 °C for 2 d. The gel was dried, and calcined at 550 °C for 4 h with 1 °C·min−1 heat rate. S0, S1, S2, S3 and S4 were used for signing samples whose g-C3N4 doped quantity were 0 wt%, 10 wt%, 20 wt%, 30 wt% and 40 wt%, respectively.
2.3 Photodegradation character measurement
Under visible light, photocatalytic activities of the prepared g-C3N4/SiO2/SnO2 composites were evaluated through the photodegradation efficiency to organic dye solution (including RhB, MB and MO) at room temperature. 5 mg prepared composite was soaked in 95 mL deionized water, and 5 mL organic dye solution (0.0100 g/L) was added into the above solution. The mixture was continuously stirred for 1 h to ensure adsorption-desorption equilibrium of organic dye on the sample surfaces in the dark. Then, the mixture was radiated under a 500 W xenon lamp with continuously stirring. Current was set to 12.5 A, and light intensity was 150 Mw/cm2. Every 10 min, 4 mL suspension liquid was taken out, and centrifuged to remove the particles of catalyst. The concentration of organic dye solution was determined by the maximum absorbance at fixed wavelength. Photocatalytic activities of the g-C3N4/SiO2/SnO2 was compared with SiO2/SnO2 and mechanical mixture (SiO2/SnO2 and g-C3N4) under the same experimental conditions.
2.4 Characterization
The morphologies of the samples were obtained by a scanning electron microscopy (SEM, JEOL JSM-5600L). Elemental composition of the g-C3N4/SiO2/SnO2 was obtained from energy dispersive x-ray spectroscopy (EDS) which was assembled on the SEM instrument. The microstructures were characterized using transmission electron microscope (TEM, JEOL 2010 TEM instrument). The X-ray diffraction (XRD) patterns of the prepared composites were recorded by a Siemens D5005 diffractometer using Cu-Kα radiation (λ = 1.5418 Å and operating at 30 kV and 20 mA). The ultraviolet-visible spectrum of sample was determined by an UV-1240 ultraviolet-visible spectrophotometer. Bet surface area (BET) and pores parameters were identified by physical adsorption of nitrogen on a Micromeritics ASAP2010M volumetric adsorption analyzer. Sample was degassed in vacuum at 573 K for 3 h before measurement. The pore size was computed using the BJH method.
3 Results and discussion
3.1 Structure characterization of porous g-C3N4/SiO2/SnO2 composites
The crystal phases of S3 sample was confirmed by X-ray diffraction (XRD) analysis (Fig. 1). In the XRD pattern, the peaks at 2θ = 26.6°, 33.9°, 37.9°, 51.7°,57.8°,64.7°,78.7° and 84.2° could be assigned to (1 1 0), (1 0 1), (2 0 0), (2 1 1), (0 0 2), (1 1 2), (3 2 1) and (4 1 0) planes of tetragonal rutile phase SnO2 (JCPDS: 41-1445), respectively. The peaks at 2θ = 26.6°could also be assigned to (0 0 2) planes of g-C3N4 (JCPDS: 87-1526). There was no characteristic diffraction peak of SiO2 in S3 sample, it could be attributed to automatic remediation of instrument because quartz sample trough was used in XRD measurement process. From Fig. 1, the g-C3N4 content has been assembled into the tetragonal rutile phase SnO2 matrix.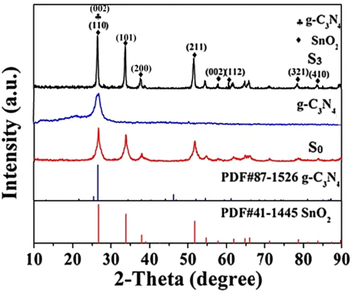
XRD patterns of S3, SnO2 and g-C3N4 samples.
Morphologies of the S3 sample were characterized using SEM and TEM. From SEM image (Fig. 2a), the S3 sample showed gel blocky structure with slice layer which was assembled with gel particles. Fig. 2b and Fig. 2c showed the TEM image and HRTEM image of S3 sample, respectively. It could be clearly seen that surfaces of the gel particles were distributed with disordered loosened pores. The 0.336 nm and 0.347 nm interplanar crystal spacing could be corresponded to the (0 0 2) plane of g-C3N4 and (1 1 0) plane of SnO2, which was consistent with the results of the XRD test. Elementary composition of S3 sample was detected using EDS which was showed in Fig. 2d. Sn, O, C, N and Si elementary signals peaks were clearly seen in the EDS spectrum of S3 sample which revealed the presence of Sn, O, C, N and Si in the prepared materials.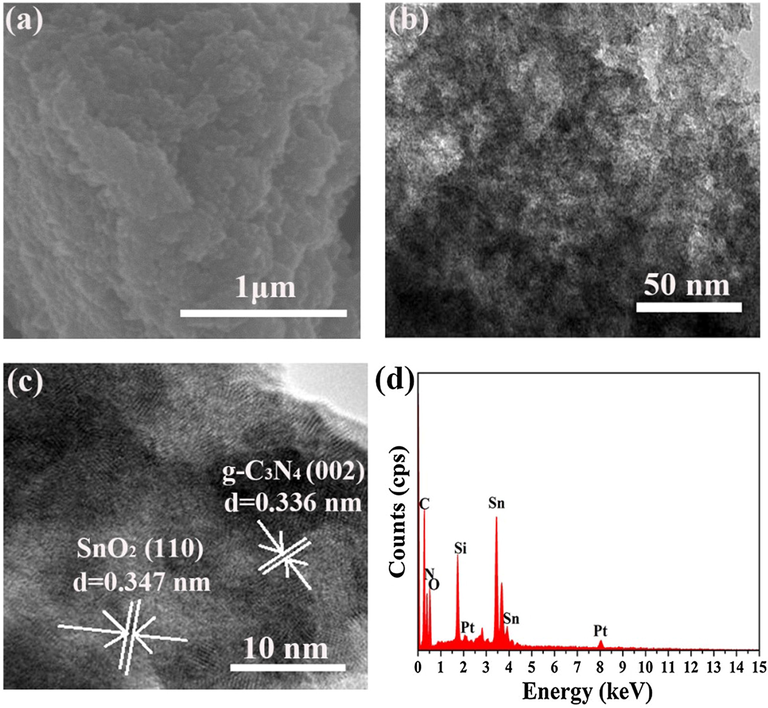
(a) SEM image, (b) TEM image, (c) HRTEM image and (d) EDS spectrum of the prepared S3 sample.
Fig. 3(a–e) showed SEM images of S0, S1, S2, S3 and S4 composites, respectively. With the increase of content of g-C3N4, the particles in gel bulk structure became smaller. When the doped amount of g-C3N4 was 40 wt%, the morphology of the composite material changed significantly, and the gel bulk structure became an aggregate granular structure. It was attributed to which the superfluous g-C3N4 lead to gel bulk broke, and g-C3N4 reunite.
SEM images of (a) S0, (b) S1, (c) S2, (d) S3 and (e) S4 samples.
The nitrogen adsorption-desorption isotherms of S3 sample was displayed in Fig. 4. It showed typical IV type isotherms with H1 hysteresis loop, which was the character of porous materials with cylindrical pores. From pore size distribution curves (Fig. 4 illustration), pore size distribution of S3 sample was much narrow, which indicated that the prepared material had much homogeneous pores. Specific surface area of the S3 sample was obtained using the BET method which was about 99.716 m2/g. Pore diameter was calculated by the BJH method which was about 3.56 nm.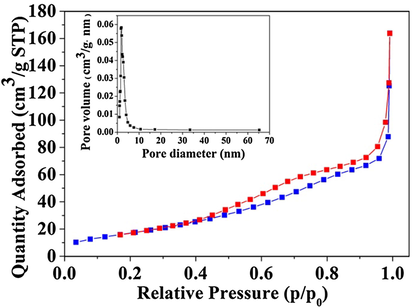
Nitrogen adsorption-desorption isotherms and pore size distribution curves (inset) of the S3 sample.
3.2 Photocatalytic experiment
Photocatalytic activities of the prepared materials were evaluated through decomposition of MO, MB and RhB. Fig. 5(a–c) showed the degradation rates of S0, S1, S2, S3 and S4 catalysts to MO, MB and RhB. For MO, the degradation rates of the five samples were 16.31%, 94.48%, 95.24%, 95.58% and 92.09% under visible light irradiation for 50 min, respectively. For MB, the degradation rates were 21.53%, 97.60%, 97.78%, 99.73% and 98.79% under visible light irradiation for 50 min, respectively. For RhB, the degradation rates respectively were 7.22%, 52.63%, 64.19%, 77.79% and 55.73% under visible light irradiation for 50 min, respectively. The prepared sample had the much better degradation effect to all three organic pollutants, and the degradation effects to MB and MO were much better than RhB. It could be seen that the S3 sample had the best catalytic degradation effect to MB. From Fig. 5, the degradation rates of three organic pollutants increased with the increase of the g-C3N4 content, it reached the biggest when the g-C3N4 content was 30%, and it reduced when the g-C3N4 content was 40%. SnO2 is a wide band gap (3.6 eV) n-type semiconductor which shows the photocatalytic activity under the UV-light, the band gap of g-C3N4 is 2.73 eV which has appropriate band gap to absorb the visible-light, however, the photocatalytic efficiency of g-C3N4 is limited because of relatively fast recombination rate of photogenerated electron-hole pairs. In g-C3N4/SiO2/SnO2 composite, the g-C3N4 can enhance the absorption capacity of visible light, and the porous SnO2 matrix can weaken the recombination rate of photogenerated electron-hole and the agglomeration of g-C3N4, their interaction improves the photocatalytic performance. Such, when g-C3N4 doped amount was increased, the absorption capacity of visible light was enhanced, and photocatalytic activities was enhanced. But, the agglomeration was happened (as Fig. 3-e) when the g-C3N4 doped amount was higher than 30%, it lead to the photocatalytic activities of the materials reduce.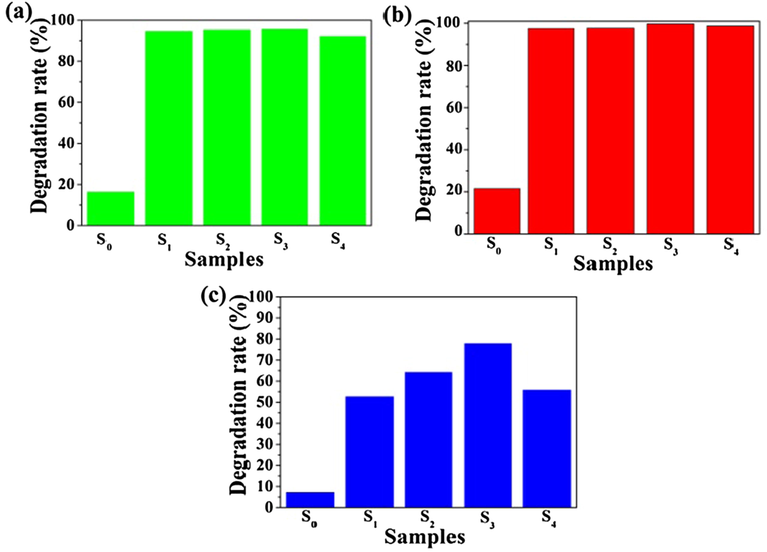
Degradation rate histogram degradation of S0, S1, S2, S3 and S4 samples to MO (a), MB (b), and RhB (c) under visible light irradiation for 50 min.
From Fig. 6(a–c), S represented a mechanical mixture of g-C3N4 and SiO2/SnO2 matrix according to proportion of S3, which was tested under the same experimental conditions. Photocatalytic performance of the g-C3N4/SiO2/SnO2 samples were observably higher than that of SiO2/SnO2 and S samples. The degradation rates of S, S0, S1, S2, S3 and S4 samples to RhB were 76.19%, 21.65%, 86.20%, 90.93%, 95.10% and 77.23% under visible light irradiation after 90 min. With increasing of g-C3N4 doped amount, the photocatalytic activity was increased firstly, and then weakened. Photocatalytic activity reached the biggest when g-C3N4 doped amount was 30 wt%. When the doped amount of g-C3N4 was increased from 0 to 30 wt%, g-C3N4 was well dispersed in the matrix, which increased the contact area of g-C3N4 with the matrix, and also increased the reactive site, thereby improved photocatalytic performance of composite material. When g-C3N4 doped amount was exorbitant, dispersibility of g-C3N4 was decreased, and an aggregation would be occurred. It increased recombination probability of electron-hole, and photocatalytic effect was weakened. The characteristic absorption of MO, MB and RhB at λ = 460 nm, λ = 660 nm and λ = 554 nm were respectively monitored during photocatalytic degration process. Fig. 6(d–f) were UV–visible absorption spectrum of MO, MB and RhB by catalysis of S3 under the different visible light radiation time. MO and MB were almost completely degraded after 50 min, and RhB was completely degraded after 90 min. The photocatalytic activities of the prepared materials to RhB in 50 min was shown in supporting information Fig. S1.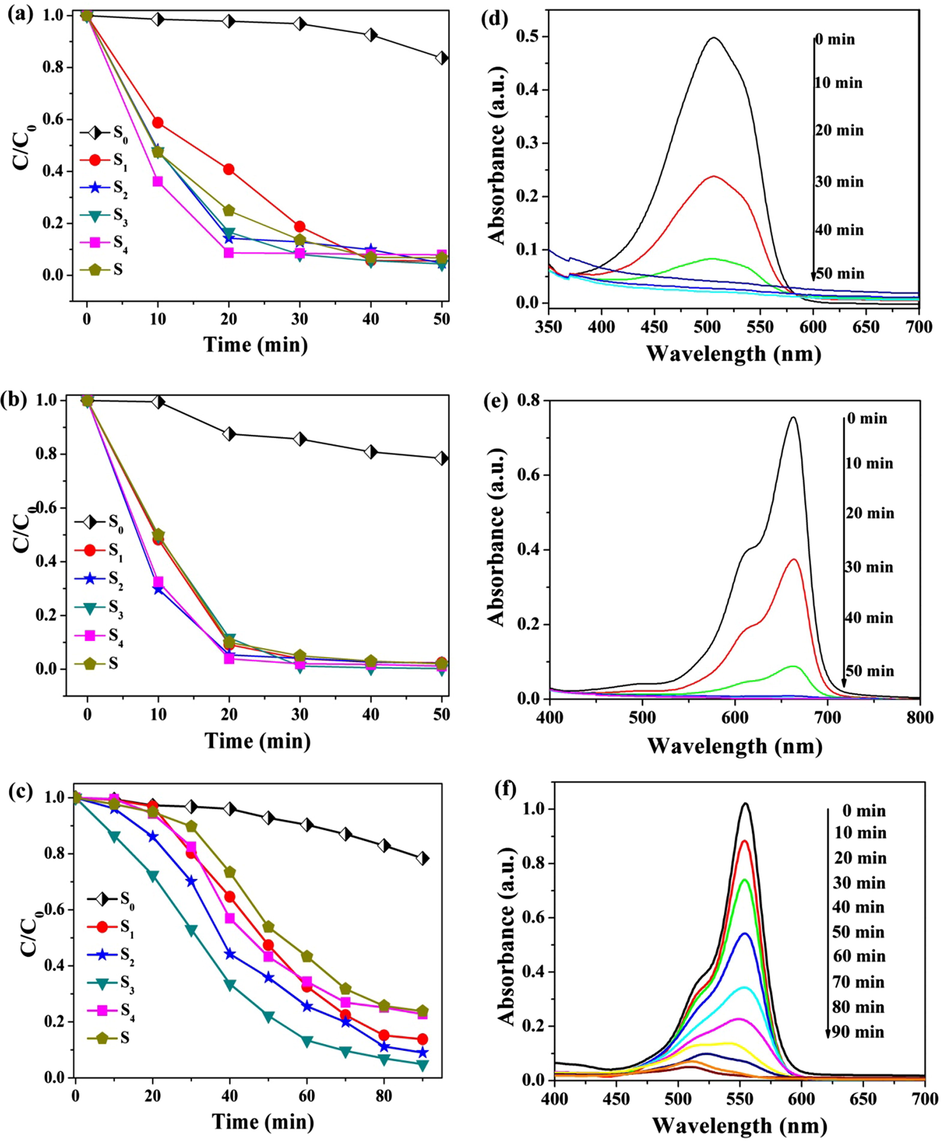
Photocatalytic activity of samples to MO (a), MB (b) and RhB (c), and UV–Visible absorption spectrum of MO (d), MB (e) and RhB (f) under catalysis of S3 in different visible light radiation time.
The pseudo-first-order kinetic model (Formula (1)) was used to study the photocatalytic activity.
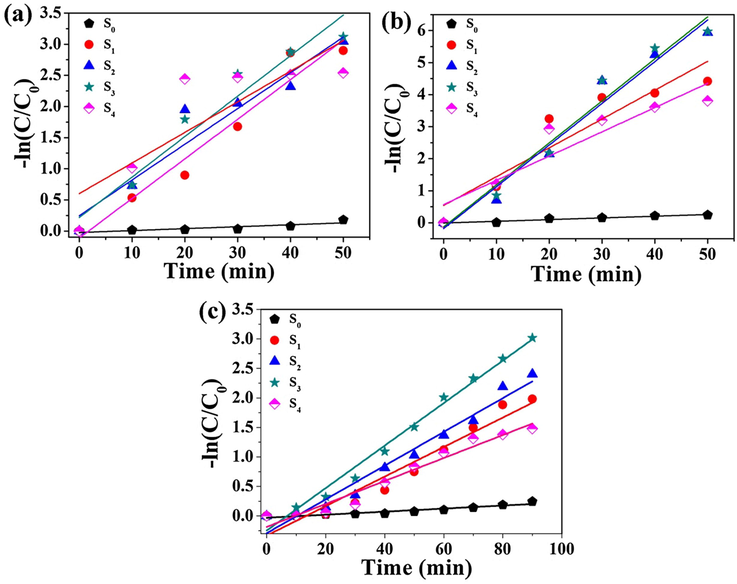
Plots of –Ln(C/C0) versus irradiation time of S0, S1, S2, S3 and S4 samples to (a) MO, (b) MB and (c) RhB photocatalytic degradation.
Under visible light, MO, MB and RhB degradation cycling stability tests of the S3 sample were researched, and the results were presented in Fig. 8. During the recycling experiment, the catalyst was collected by centrifugation, washed with deionized water, dried at 60 °C, and reused in the next cycles. For MO and MB degradation, the photocatalytic degradation rates of the S3 sample were respectively maintained up 88.91% and 92.58% in 50 min after 10 time cycles. For RhB, the photocatalytic degradation rate was remained up 87.63% in 90 min after ten time cycles. The detailed test results were showed in supporting information Table S1. It indicated that the prepared material had the better stability, and had the potential application value in environmental purification.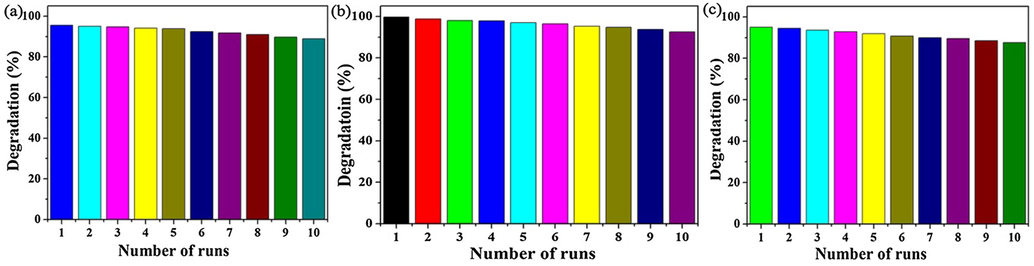
Cycling stability test results of S3 sample for MO (a), MB (b) and RhB (c).
3.3 Photocatalytic mechanism
It is well known that the rapid photogenerated electrons-hole pair transfer rate and the slower photogenerated electron-hole recombination rates are benefit to improve the photocatalytic performance of the materials. The transfer efficiency of photogenerated electrons-hole pairs can be evaluated by PL spectrum. The materials have the lower PL luminescence intensity which will have the better photocatalyst performance. Fig. 9 presents the PL spectra of all catalysts which are excited by 371 nm light. A strong emission peak shows at around 437 nm which was attributed to the band-band PL phenomenon. It is found that the luminescent intensities are gradually decreased with the increase of g-C3N4 content, and the PL intensities are enhanced when the content of g-C3N4 is 40 wt%, which are inverse with the photocatalytic performances of samples. It is consistent with above theory. When the content of g-C3N4 is increased, the heterojunction interface may be reduced, the electron-hole recombination rate is increased, and the recombination center is increased.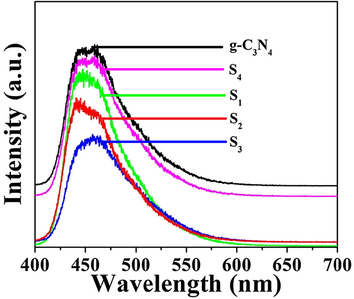
Room temperature PL spectra of g-C3N4, S1, S2, S3 and S4 samples.
Fig. 10(a) are the UV–Vis absorption spectra of all samples. The estimated band gaps of samples are showed in Fig. 10(b) which are obtained from UV–vis absorption spectra of the prepared samples. The band gap energy (Eg) values of all samples are calculated by the Kubelka-Munk theorem. The band gaps of S0, S1, S2, S3 and S4 are 3.10, 2.51, 2.50, 2.47 and 2.48 eV, respectively. G-C3N4 exhibits light absorption over nearly the visible light range, while SnO2 merely responds to ultraviolet light. Doped g-C3N4, a visible light absorbance is observably enhanced for g-C3N4/SiO2/SnO2 composite.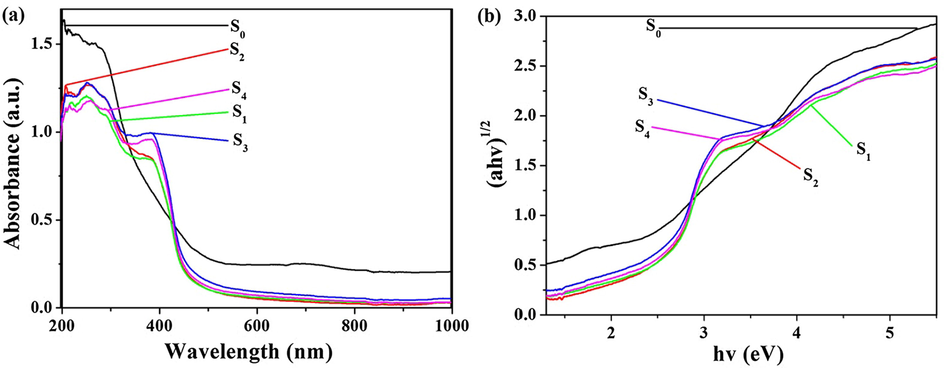
(a) UV–Vis diffuse reflectance spectra and (b) (α hv)1/2-hv curves of S0, S1, S2, S3 and S4 sample.
Many reactive groups including •OH, •O2− and h+ are possible drawn into photocatalytic reaction, which are much significant to photocatalytic reaction. In order to verify their effect, isopropyl alcohol (IPA), 1,4-benzoquinone (BQ) and ethanedioic acid (K2C2O4) are respectively used as scavenger of •OH, •O2− and h+, and the results are showed in Fig. 11(a). Photocatalytic effects of S3 sample are significantly decreased when BQ is added to eliminate •O2−, which verifies that •O2− is a much important active specie in the photocatalytic reaction. Degradation rate is reduced when IPA and K2C2O4 are respectively added in photocatalytic system of S3 sample, which suggests that h+ and •OH play an equally significant role in the catalysis degradation. In order to prove the reactive species in photocatalytic degradation process, the electron spin resonance (ESR) is an ideal test method, which is used to detect reactive species under dark and visible light irradiation. No signal of •O2− is detected in dark, and the signals of •O2− are detected when it is radiated by the visible light which is showed in Fig. 11(b). As showing in Fig. 11(c), the signal of •OH has never appeared either in dark or in visible light conditions. The test results show that •O2− can be generated, and •OH almost is not produced during photocatalytic degradation.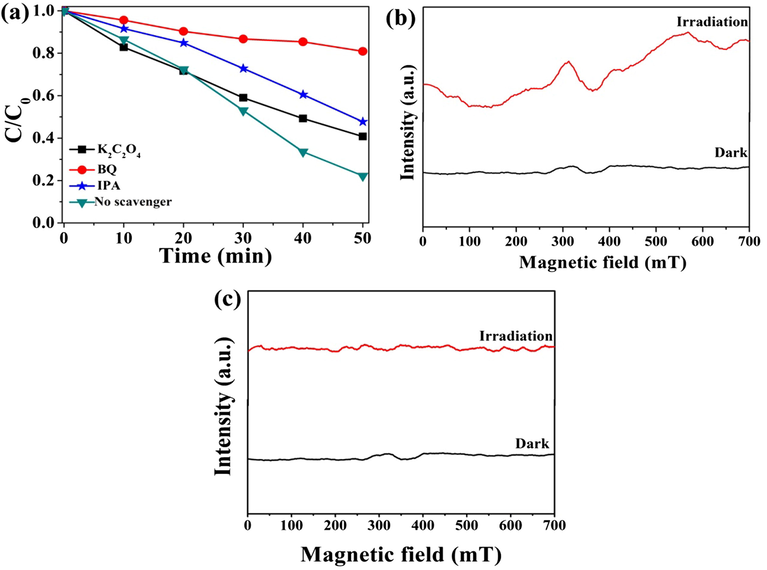
(a) Photodegradation of RhB over S3 sample with different quenchers; ESR spectra of radical actives trapped by DMPO for S3 sample: (b) DMPO- •O2−: (c) DMPO- •OH.
When g-C3N4/SiO2/SnO2 nanocomposites are irradiated by visible light, the photoexcited electrons are generated in conduction band (CB) of g-C3N4, and finally transfer to the conduction band of SnO2 in order to reduce potential energy. At same time, h+ are injected in the opposite direction for appropriate VB offsets, and the same amounts of h+ are enriched in the VB of g-C3N4, and h+ on VB of SnO2 are transferred to the VB of g-C3N4. Due to the presence of S3 catalyst, the recombination rate of photo-generated electron-hole pairs in system is sharply reduced. Under the experimental condition, the electrons in CB of SnO2 contact with adsorbed O2 to form •O2− on the surface. h+ combines with water, and •OH is formed. By this way, electron-hole pair recombination is reduced. Because the •OH and •O2− are power oxidants, the organic pollutes can be decomposed effectively (Li et al., 2015). Schematic illustration of photocatalytic reaction and charge transfer of the S3 sample under visible light irradiation is showed in Fig. 12.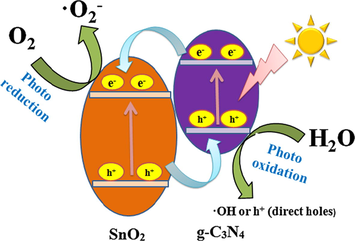
Schematic illustration of photocatalytic reaction and charge transfer of the S3 sample under visible-light irradiation.
In order to discuss SiO2 effect, NaOH (2 mol/L) solution is used to remove SiO2 from the sample, photocatalytic tests are carried out under the same conditions, the result is showed in Fig. 13. It is found that the photocatalytic effect is significantly reduced after the removal of SiO2, only 29.31% RhB has been degraded after 90 min. Zhou et al. (Zhou et al., 2013) indicated that SiO2 can forbid the electron transfer effectively in SiO2-Ag-SiO2-TiO2 composite. It is speculated that SiO2 may forbid the electron effectively transfer during photocatalysis process in this study, which reduces the recombination rate of photogenerated electron-hole pairs in system. In this study, TEOS has catalysis for SnO2 gelation, which make the structure of the prepared material become more loosen, defect concentration increase, active site increase, and photocatalytic activity is enhanced. According to aerogel theory, the SiO2 is porous structure in this system, which can not only increase sample's adsorb ability to organic contaminants, but also the porous SiO2 provides a good high-speed channel for the migration of electrons, which effectively promotes the electron-hole separation ability in sample, thereby improves the photocatalytic performance of sample. Yu et al. has even studied the relevant research on this issue. (Yu et al., 2019) The loose porous structure of the prepared material was destroyed when the SiO2 was removed, it lead to the structure collapse, and the photocatalytic performance of sample was dropped sharply.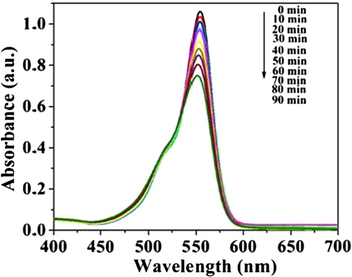
UV–Visible absorption spectrum of Rh Bunder catalysis of S3 removed SiO2 sample with in different visible light radiation time.
4 Conclusions
In this study, g-C3N4/SiO2/SnO2 composite was synthesized by sol-gel hard-template method. The MO, MB and RhB photodegradation catalytic effects of g-C3N4/SiO2/SnO2 composite were inspected under visible light, which realized effective separation of photogenerated electron-hole pairs. When loaded g-C3N4 was 30 wt%, the highest photocatalytic activity was obtained. This work provided a facile method for synthesis organic-inorganic catalytic material, and g-C3N4/SiO2/SnO2 heterojunction structure materials could serve as a promising candidate as the catalytic material in pollution treatment applications.
Acknowledgements
We gratefully acknowledge the support of the work by the National Natural Science Foundation of China (Grant No. 51572034).
Declaration of Competing Interest
All authors declare that they have no conflict of interest.
References
- Synergistic effect induced ultrafine SnO2/graphene nanocomposite as an advanced lithium/sodium-ion batteries anode. J. Mater. Chem. A. 2017;5:10027-10038.
- [Google Scholar]
- Aerogel nanocomposites of ZnO–SnO2 as efficient photocatalysts for the degradation of rhodamine B. Catal. Sci. Technol.. 2012;2:922-924.
- [Google Scholar]
- Sens. Investigation of gas sensing properties of SnO2/In2O3 hetero-nanofibers treated by oxygen plasma. Actuat. B: Chem.. 2015;206:753-763.
- [Google Scholar]
- In situ hydrothermal synthesis of g-C3N4/TiO2 heterojunction photocatalysts with high specific surface area for Rhodamine B degradation. Appl. Surf. Sci.. 2017;411:400-410.
- [Google Scholar]
- Enhanced visible light photocatalytic H2 production over Z-scheme g-C3N4 nansheets/WO3 nanorods nanocomposites loaded with Ni(OH)x cocatalysts. Chinese J. Catal.. 2017;38:240-252.
- [Google Scholar]
- Type-II ZnO nanorod–SnO2 nanoparticle heterostructures: characterization of structural, optical and photocatalytic properties. Nanoscale. 2013;5:3828-3833.
- [Google Scholar]
- Photocatalytic degradation of paracetamol on TiO2 nanoparticles and TiO2/cellulosic fiber under UV and sunlight irradiation. Arab. J. Chem.. 2019;10:S3640-S3645.
- [Google Scholar]
- In situ construction of α-Bi2O3/g-C3N4/β-Bi2O composites and their highly efficient photocatalytic performances. RSC Adv.. 2015;5:92963-92969.
- [Google Scholar]
- Photocatalytic properties of semiconductor SnO2/CdS heterostructure. RSC Adv.. 2012;2:10222-10230.
- [Google Scholar]
- Light-assisted rapid preparation of Ni/g-C3N4 magnetic compositefor robust photocatalytic H2 evolution from water. J. Mater. Chem. A. 2016;4:9998-10007.
- [Google Scholar]
- g-C3N4 decorated ZnO nanorod arrays for enhanced photoelectrocatalytic performance. Appl. Surf. Sci.. 2015;358:296-303.
- [Google Scholar]
- Cost-effective and eco-friendly synthesis of novel and stable N-doped ZnO/g-C3N4 core-shell nanoplates with excellent visible-light responsive photocatalysis. Nanoscale. 2014;6:4830-4842.
- [Google Scholar]
- Preparation and enhanced visible light photocatalytic activity of novel g-C3N4 nanosheets loaded with Ag2CO3 nanoparticles. Nanoscale. 2015;7:758-764.
- [Google Scholar]
- ersatile nanobead- scaffolded N-SnO2 mesoporous microspheres: one - step synthesis and superb performance in dye - sensitized solar cell, gas sensor, and photocatalytic degradation of dye. J. Mater. Chem.. 2013;1:524-531.
- [Google Scholar]
- A new type of acetylene gas sensor based on a hollow heterostructure. RSC Adv.. 2015;5:61521-61527.
- [Google Scholar]
- Fabrication of In2O3/ZnO@Ag nanowire ternary composites with enhanced visible light photocatalytic activity. RSC Adv.. 2017;7:37220-37229.
- [Google Scholar]
- Hierarchical SnO2 nanostructures made of intermingled ultrathin nanosheets for environmental remediation. ACS Appl. Mater. Interfaces. 2014;6:2174-2184.
- [Google Scholar]
- Novel sea urchin-like hollow core–shell SnO2 superstructures: Facile synthesis and excellent ethanol sensing performance. Sens. Actuat. B: Chem.. 2010;151:229-235.
- [Google Scholar]
- Photocatalytic degradation of 1,2-dichlorobenzene using immobilized TiO2/SnO2/WO3 photocatalyst under visible light: Application of response surface methodology. Arab. J. Chem.. 2018;11:34-47.
- [Google Scholar]
- Graphitic carbon nitride (g-C3N4) - based photocatalysts for solar hydrogen generation: recent advances and future development directions. J. Mater. Chem. A. 2017;5:23406-23433.
- [Google Scholar]
- One-pot synthesis of hierarchical mesoporous SnO2 spheres using a graft copolymer: enhanced photovoltaic and photocatalytic performance. RSC Adv.. 2014;4:31452-31461.
- [Google Scholar]
- SnO2 nanoparticle-coated In2O3 nanofibers with improved NH3 sensing properties. Sens. Actuat. B: Chem.. 2014;194:440-446.
- [Google Scholar]
- Fabrication of graphene aerosol hybridized coordination polymer derived CdO/SnO2 heteronanostructure with improved visible light pgotocatalytic performance. Sol. Energ. Mat. Sol. C.. 2017;162:62-71.
- [Google Scholar]
- Ag/ZnO nanoparticles thin films as visible light photocatalysts. RSC Adv.. 2014;4:56892-56899.
- [Google Scholar]
- Synergistic effect induced ultrafine SnO2/graphene nanocomposite as an advanced lithium/sodium-ion batteries anode. Nanoscale. 2016;8:471-482.
- [Google Scholar]
- Self-assembled Au/CdSe nanocrystal clusters for plasmon mediated photocatalytic hydrogen evolution. Adv. Mater.. 2017;29:1700803.
- [Google Scholar]
- Understanding the role of the dye/oxide interface via SnO2-based MK-2 dye - sensitized solar cells. Phys. Chem. Chem. Phys.. 2015;17:15193-15200.
- [Google Scholar]
- Enhanced visible–light photocatalytic activity of g-C3N4/Zn2GeO4 heterojunctions with effective interfaces based on band match. Nanoscale. 2014;6:2649-2659.
- [Google Scholar]
- Chemlnform abstract: graphitic carbon nitride materials: variation of structure and morphology and their use metal-free catalysts. J. Mater. Chem.. 2008;18:4893-4908.
- [Google Scholar]
- Tube-like ternary α-Fe2O3@SnO2@Cu2O sandwich heterostructures: synthesis and enhanced photocatalytic properties. ACS Appl. Mater. Interfaces. 2014;6:13088-13097.
- [Google Scholar]
- Controllable synthesis of nanotube-type graphitic C3N4 and their visible-light photocatalytic and fluorescent properties. J. Mater. Chem. A. 2014;2:2885-2890.
- [Google Scholar]
- Fabrication of WO3@g-C3N4 with core@shell nanostructure for enhanced photocatalytic degradation activity under visible light. Appl. Surf. Sci.. 2017;423:197-204.
- [Google Scholar]
- Nanotechnology-enabled energy harvesting for self-powered micro – nanosystems. Angew. Chem. Int. Ed.. 2013;51:1170-11721.
- [Google Scholar]
- Facile fabrication of mesoporous g-C3N4/TiO2 photocatalyst for efficient degradation of DNBP under visible light irradiation. Appl. Surf. Sci.. 2017;426:1271-1280.
- [Google Scholar]
- Highly enhanced photocatalytic degradation of methylene blue over the indirect all-solid-state Z-scheme g-C3N4-RGO-TiO2 nanoheterojunctions. Appl. Surf. Sci.. 2017;405:60-70.
- [Google Scholar]
- Controlled synthesis of magnetic iron oxides@SnO2 quasi-hollow core-shell heterostructures: formation mechanism, and enhanced photocatalytic activity. Nanoscale. 2011;3:4676-4684.
- [Google Scholar]
- Enhanced electromagnetic wave absorption properties of laminated SiCNW-Cf/thium-aluminum-silicate (LAS) composites. J. Alloy. Compd.. 2018;748:154-162.
- [Google Scholar]
- Constructing 2D/2D Fe2O3/g-C3N4 direct Z-scheme photocatalysts with enhanced H2 generation performance. Sol. RRL. 2018;2:1800006.
- [Google Scholar]
- Enhanced photocatalytic activity of surface disorder-engineered CaTiO3. Mater. Res. Bull.. 2018;105:286-290.
- [Google Scholar]
- Fe3O4@LAS/RGO composites with a multiple transmission-absorption mechanism and enhanced electromagnetic wave absorption performance. Chem. Eng. Jour.. 2018;352:510-518.
- [Google Scholar]
- Gold nanoparticle doped hollow SnO2 supersymmetric nanostructures for improved photocatalysis. J. Mater. Chem. A. 2013;1:4097-4104.
- [Google Scholar]
- Novel SiO2 nanoparticle-decorated BiOCl nanosheets exhibiting hight photocatalytic performances for the removal of organic pollutants. Chinese J. Catal.. 2019;40:1212-1221.
- [Google Scholar]
- Alkali-assisted synthesis of nitrogen deficient graphitic carbon nitride with tunable band structures for efficient visible-light-driven hydrogen evolution. Adv. Mater.. 2017;29:1605148.
- [Google Scholar]
- Controllable synthesis of recyclable core–shell γ-Fe2O3@SnO2 hollow nanoparticles with enhanced photocatalytic and gas sensing properties. Phys. Chem. Chem. Phys.. 2013;15:8228-8236.
- [Google Scholar]
- Enhanced photocatalytic hydrogen evolution along with byproducts suppressing over Z-scheme CdxZn1-xS/Au/g-C3N4 photocatalysts under visible light. Sci. Bull.. 2017;62:602-609.
- [Google Scholar]
- Synthesis and theoretical study of large-sized Bi4Ti3O12 square nanosheets with high photocatalytic activity. Mater. Res. Bull.. 2018;107:180-188.
- [Google Scholar]
- Assembly of Ag3PO4 nanoparticles on rose flower-like Bi2WO6 hierarchical architectures for achieving high photocatalytic performance. J. Mater. Sci.: Mater. Electron.. 2018;29:29291-29300.
- [Google Scholar]
- Humic acid-mediated visible-light degradation of phenol on phosphate-modified and nafion-modified TiO2 surface. Chinese J. Catal.. 2017;38:2076-2084.
- [Google Scholar]
- SiO2-Ag-SiO2-TiO2 multi-shell structures: plasmon enhanced photocatalysts with wide-spectral-response. J. Mater. Chem. A. 2013;1:13128-13138.
- [Google Scholar]
- Template-free large-scale synthesis of g-C3N4 microtubes for enhanced visible light-driven photocatalytic H2 production. Nano Res.. 2018;11:3462-3468.
- [Google Scholar]
Appendix A
Supplementary material
Supporting information contains the degradation profile of RhB over S3 sample in visible light exposed to 50 min in Fig. S1, and the photocatalytic degradation rate of S3 sample for MO, MB and RhB in cycling experiments in Table S1. Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2019.07.009.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary Fig. S1 and Table S1
Supplementary Fig. S1 and Table S1







