Translate this page into:
Synthesis of N, N-Bis([1,1′-Biphenyl]-4-ylmethyl)-4-morpholinoaniline derivatives via SMC reaction: Assessing their anti-seizure potential through electroencephalogram evaluation and molecular docking studies
⁎Corresponding author. nasirrasool@gcuf.edu.pk (Nasir Rasool)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
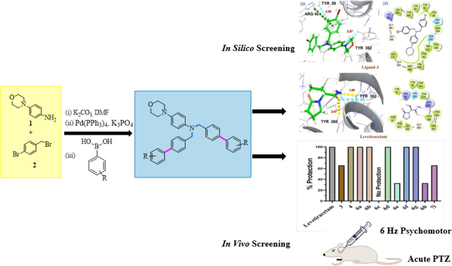
A new series of N, N-bis([1,1′-biphenyl]-4-ylmethyl)-4-morpholinoaniline derivatives (6a − h, 7i) were synthesized and evaluated their anticonvulsant potential through in vivo and in silico studies. 6 Hz Psychomotor and acute pentylenetetrazol (PTZ) seizure models were used for in vivo anticonvulsant activity. A glide program in a standard precision (SP) model, and the targeted protein 8G5G, 3O7P, and 1PW4 were used for in silico studies. Through in silico and in vivo experiments, it was determined that compounds 6f and 6 h, which exhibit no neurotoxicity when compared to standard drugs (levetiracetam and diazepam), were the most potent among all the synthesized compounds (6a-h, 7i). Multiple binding affinities of synthetic compounds (6a-h, 7i) to localize binding receptors were also observed by in silico study. Based on the docking studies of active molecules with different targets, the aforementioned drugs most likely work via binding to the SV2A transporter and GABA-A receptor.
Keywords
N, N-bis(4-bromobenzyl)-4-morpholinyl amine
Suzuki Miyaura reactions
Anticonvulsant potential
Acute PTZ and 6 Hz psychomotor seizure model
Electroencephalogram
Molecular docking studies
1 Introduction
Epilepsy is a critical neurological disorder pertaining to high economic costs, stigma, and psychological multimorbidity affecting nearly 65 million people worldwide (Begley and Beghi, 2002; Löscher et al., 2020; Dash et al., 2024; Leonardi et al., 2024). Neural activity may alter as a consequence of damage to brain tissue resulting from a stroke, which may act as a trigger for seizures. Furthermore, multiple sclerosis and neurodegenerative illnesses are among the neurological disorders that are frequently linked to an increased risk of epilepsy (Tellez-Zenteno et al., 2007; Fiest et al., 2014). These varied aspects highlight the etiology of epilepsy's complexity and its correlation with a range of underlying brain-related abnormalities. The estimated lifetime chronicity of active epilepsy is 7.60 per 1000 individuals (Begley et al., 2022). Pharmacoresistant epilepsy is intimately linked to uncontrolled seizures, or the inability to achieve effective seizure control using standard anticonvulsant drugs. It is one of the foremost triggers for mortality in both developing and developed nations, with a staggering prevalence (Sharma et al., 2015). Despite triggering uncontrolled seizures, drug-resistant epilepsy (DRE) disrupts brain chemistry, causing neuropsychiatric dysfunctions, lifetime dependency, and a socioeconomic burden (Kwan and Brodie 2002, Sheng et al., 2018). The entire incidence and prevalence of DRE increased from 0.11 to 0.58 % and 0.06 to 0.51 %, respectively, as reported in a recent systematic study (Fattorusso et al., 2021). Moreover, the frequent use of anticonvulsant drugs is associated with undesirable effects that potentially reveal incurable (Zaccara et al., 2007; Obniska et al., 2012; Gaitatzis and Sander 2013, Perucca 2021). Due to the above-stated reasons, scientists are consistently endeavoring to synthesize new molecules with better safety profiles of antiepileptic potential.
Various types of amines are stated to be crucially significant pharmacophores of anticonvulsant drugs as brivaracetam, levetiracetam, diazepam, and carbamazepine (Banerjee and Sharma 2012, Mittapalli and Roberts 2014). Tertiary amines rationalize 60 % of entire medicinal amines (Roughley and Jordan 2011). According to the World Drug Index, currently, morpholine-bearing tertiary amine scaffold has been used in more than 100 drugs (i.e., Gefitinib, Linezolid, and Aprepitant) containing a morpholine ring system such as scaffold, side-chain, or part of integrated ring systems (Tereshchenko et al., 2017).
Morpholine and amines are key functionalities in many FDA approved drugs. Generally, morpholine enhanced the medicinal effect of a drug (Delost et al., 2018; Jarzyński et al., 2023). Some of the FDA approved drugs has been depicted in the (Fig. 1a). Saravanan and coauthors reported the synthesis of isoxazole-bearing morpholinyl derivatives exhibits significant antiepileptic activities on the MEZ and scPTZ model found (a) as a potent compound (Saravanan et al., 2014). Similarly, Rybka and coworkers found 1,3-substituted pyrrolidine-2,5-dione as potential anticonvulsant agents, compound (b) found as pilot compound on MES and scPTZ model (Rybka et al., 2017). Abdelli et al. synthesized morpholine amine analogues; compound (c) found to be effective (Abdelli et al., 2024). Moreover, Parkash et al. evaluated the 1-(substituted benzylidene)-3-(1-(morpholino/piperidino methyl)-2,3-dioxoindolin-5-yl) urea derivatives and found (d) as potent compound bearing morpholine moiety against antiepileptic activities on the scPTZ model (Fig. 1b) (Prakash and Raja 2011). Also, Maharramova and Martynenko coauthors reported N-substituted tertiary amines demonstrating significant antiseizure potential in in-vivo animal models (Maharramova et al., 2018, Martynenko et al., 2020). Zareba and coauthors reported tricyclic N-benzyl-4-hydroxybutanamide analogs bearing tertiary amines as CNS active scaffolds as blockers of mGAT1-4 GABA transporters possessing significant anticonvulsant potential (Zaręba et al., 2021).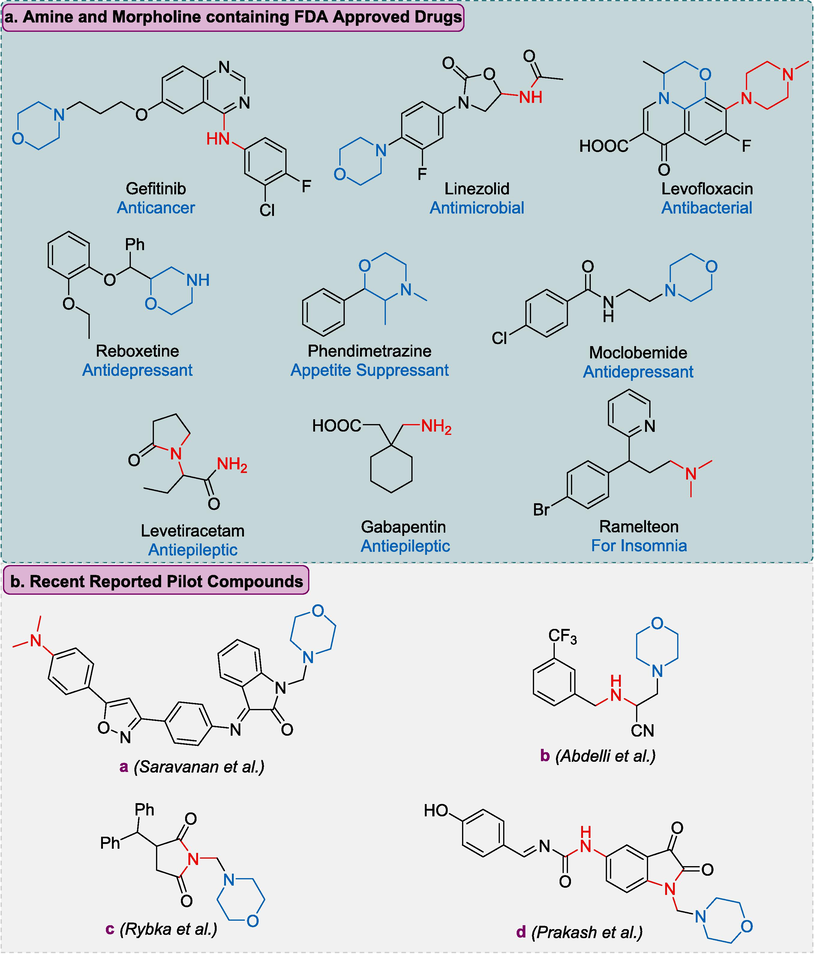
Summary of Morpholine and amine containing FDA approved drugs and some recently reported morpholine amine based anti-epileptic drugs.
Our current study focuses on the design and synthesis of neural active morpholinyl amine derivatives, especially tertiary amines as anticonvulsant agents (Prakash et al. 2012). We selected morpholine anilines core for rationalization. Our designed molecular structure comprises hydrophobic aryl rings adjacent to C-N alkylated amines, providing active pharmacophores together in one molecule to avoid the succession of epilepsy (Simonetti et al. 2017). Here, we followed Pd(0) catalyzed Suzuki-Miyaura cross-coupling (SMC), which is a highly versatile and efficient method for preparing new compounds (Miyaura and Suzuki 1995).
2 Materials and method
2.1 General information
Melting points of the newly synthesized compounds (6a-h, 7i) were determined using a Buchi B-540 instrument. Macklin China provided all of the chemicals that were employed in this research. In the spectroscopic analysis, the Avance III Bruker spectrometer was used to analyze the 1H/13C NMR spectra of synthesized compounds at 400 MHz in deuterated solvents CDCl3. The coupling constant was measured in Hz while the chemical shift measurements were ascertained in ppm. To purify the chemicals, Column chromatography with silica gel was used. For elemental analysis, the LECO 630–200-200 TruSpec CHNS microanalyzer was applied.
2.1.1 Synthesis of N, N-bis(4-bromobenzyl)-4-morpholinyl amine (3)
To a solution of 4-morpholinyl aniline (1.0 equiv, 0.5 g, 2.81 mmol) and K2CO3 (3.0 equiv., 1.24 g, 8.43 mmol) in DMF (40 mL). The reaction mixture was agitated at room temperature for 30 min. Then added 4-bromobenzyl bromide (2.2 equiv., 1.55 g, 6.2 mmol) into it and taken into isotherm for 5–6 h. The reaction mixture was stirred continuously at room temperature until completion, which occurred within 24 h. The reaction progress was observed using TLC, EtOAc, and n-hexane (3:7) as solvent systems. An off-white solid crystal was extracted using DMF in water, after completion of the reaction. Pure crystals crystallized out in DMF, while, other side impurities dissolve in water and we obtain the pure product in excellent yield. The synthesized compounds (6a-h, 7i) were investigated through elemental analysis and NMR spectroscopy.
2.1.2 General procedure for synthesis of N, N-bis([1,1′-biphenyl]-4-ylmethyl)-4-morpholinoaniline derivatives (6a − h, 7i)
N, N-bis(4-bromobenzyl)-4-morpholinyl amine (1.0 equiv, 0.3 g, 0.58 mmol), Pd(PPh3)4 catalyst (0.046 g, 4.07 mmol, 7 mol%), and 1,4-dioxane (7 mL) were put in a Schlenk flask. The reaction mixture underwent degassing using argon. After stirring for 30 min, boronic acid (0.22 g, 1.45 mmol, 2.5 equiv) and K3PO4 (0.49 g, 2.32 mmol, 4.0 equiv) were introduced into the reaction mixture. The mixture was stirred for an additional 10 min. Later, distilled water (0.5 mL) was added and refluxed for 24 h at 90 °C. Completion of the reaction was observed through TLC via using n-hexane and EtOAc (3:7) as a solvent system. The product underwent filtration and purification using column chromatography, after reaction completion (Maqbool et al. 2023). The synthesized compounds were investigated through elemental analysis and NMR spectroscopy.
2.2 Characterization*
2.2.1 N, N-bis(4-bromobenzyl)-4-morpholinyl amine (3)
Yield 95 %, Dark brown crystals; M.P. 194 °C. 1H NMR (400 MHz, CDCl3) δ 7.43 (d, J = 8.3 Hz, 4H), 7.10 (d, J = 8.3 Hz, 4H), 6.89 (s, 2H), 6.67 (d, J = 7.0 Hz, 2H), 4.48 (s, 4H), 3.89 (s, 4H), 3.07 (s, 4H). 13C NMR (101 MHz, CDCl3) δ 137.50, 131.69, 128.62, 120.71, 114.47, 66.71, 54.43, 51.19. Elemental Anal. Calcd. for C24H24Br2N2O (516.28): C, 55.84; H, 4.69; N, 5.43 % Observed: C, 55.79; H, 4.64; N, 5.44 % (supplementary data, S1-S2).
2.2.2 N-(4-bromobenzyl)-4-morpholinoaniline (4)
Yield 5 % (minor product), brown crystals; M.P. 160 °C. 1H NMR (400 MHz, CDCl3) δ 7.45 (d, J = 8.3 Hz, 2H), 7.24 (d, J = 8.2 Hz, 2H), 7.04 – 6.78 (m, 3H), 6.64 (dd, J = 20.6, 8.3 Hz, 2H), 4.26 (s, 2H), 3.93 (s, 4H), 3.09 (s, 4H). 13C NMR (101 MHz, CDCl3) δ 156.13, 131.94, 131.68, 129.84, 129.19, 122.12, 121.04, 118.88, 116.06, 114.29, 66.46, 51.79, 48.45. Elemental Anal. Calcd. for C17H19BrN2O (347.26): C, 58.80; H, 5.52; N, 8.07 % Observed: C, 58.78; H, 5.49; N, 8.04 % (supplementary data, S3-S4).
2.2.3 N, N-bis((3′,5′-dimethyl-[1,1′-biphenyl]-4-yl) methyl)-4-morpholinoaniline (6a)
Yield 84 %, pale yellow solid; M.P. 168 °C. 1H NMR (400 MHz, CDCl3) δ 7.55 (d, J = 7.9 Hz, 4H), 7.33 (d, J = 7.9 Hz, 4H), 7.22 (s, 4H), 7.01 (s, 3H), 6.83 (d, J = 9.1 Hz, 3H), 4.66 (s, 4H), 3.91 (s, 4H), 3.13 (d, J = 33.5 Hz, 4H), 2.40 (s, 12H). 13C NMR (101 MHz, CDCl3) δ 140.91, 140.13, 138.25, 128.85, 127.37, 127.19, 124.99, 114.00, 66.75, 54.57, 51.47, 21.42. Elemental Anal. Calcd. for C40H42N2O (566.79): C, 84.77; H, 7.47; N, 4.94 % Observed: C, 84.73; H, 7.44; N, 4.89 % (supplementary data, S5-S6).
2.2.4 Dimethyl-4′,4′''-(((4-morpholinophenyl) azanediyl) bis(methylene)) bis([1,1′-biphenyl]-4 carboxylate) (6b)
Yield 81 %, colorless solid; M.P. 141–142 °C. 1H NMR (400 MHz, CDCl3) δ 8.10 (d, J = 8.4 Hz, 4H), 7.65 (d, J = 8.4 Hz, 4H), 7.59 (d, J = 7.9 Hz, 4H), 7.35 (d, J = 7.8 Hz, 4H), 7.03 (ddd, J = 30.3, 21.5, 8.3 Hz, 2H), 6.82 – 6.70 (m, 2H), 4.69 (s, 4H), 3.94 (s, 6H), 3.26 – 3.05 (m, 4H). 13C NMR (101 MHz, CDCl3) δ 166.95, 145.14, 130.12, 128.86, 127.90, 127.58, 127.34, 126.87, 54.60, 52.13. Elemental Anal. Calcd. for: C40H38N2O5 (626.75): C, 76.66; H, 6.11; N, 4.47 % Observed: C, 76.66; H, 6.11; N, 4.47 % (supplementary data, S7-S8).
2.2.5 N, N-bis((3′,4′-dichloro-[1,1′-biphenyl]-4-yl) methyl)-4-morpholinoaniline (6c)
Yield 79 %, white solid; M.P. 139–140 °C. 1H NMR (400 MHz, CDCl3) δ 7.65 (d, J = 1.9 Hz, 2H), 7.49 (d, J = 8.2 Hz, 6H), 7.39 (dd, J = 8.3, 1.9 Hz, 2H), 7.34 (d, J = 8.0 Hz, 4H), 6.98 – 6.64 (m, 4H), 4.64 (s, 4H), 3.88 (s, 4H), 3.07 (s, 4H). 13C NMR (101 MHz, CDCl3) δ 140.83, 138.86, 137.45, 132.82, 131.34, 130.69, 130.40, 130.01, 128.79, 127.54, 127.17, 126.20, 114.25, 66.79, 54.70, 51.16. Elemental Anal. Calcd. for: C36H30Cl4N2O (648.45): C, 66.68; H, 4.66; N, 4.32 % Observed: C, 66.69; H, 4.61; N, 4.28 % (supplementary data, S9-S10).
2.2.6 N, N-bis((4′-chloro-[1,1′-biphenyl]-4-yl) methyl)-4-morpholinoaniline (6d)
Yield 78 %, dark brown solid; M.P. 137–138 °C. 1H NMR (400 MHz, CDCl3) δ 7.55 – 7.47 (m, 8H), 7.40 (d, J = 8.4 Hz, 4H), 7.33 (d, J = 7.7 Hz, 4H), 7.03 (ddd, J = 22.5, 15.9, 8.9 Hz, 2H), 6.79 (d, J = 18.7 Hz, 2H), 4.67 (s, 4H), 3.93 (ddd, J = 26.9, 12.5, 3.6 Hz, 4H), 3.18 (d, J = 9.9 Hz, 4H). 13C NMR (101 MHz, CDCl3) δ 139.20, 133.33, 130.34, 128.92, 128.22, 127.24, 119.02, 60.39, 54.60. Elemental Anal. Calcd. for: C36H32Cl2N2O (579.57): C, 74.61; H, 5.57; N, 4.83 % Observed: C, 74.57; H, 5.59; N, 4.86 % (supplementary data, S11-S12).
2.2.7 N, N-bis((4′-methyl-[1,1′-biphenyl]-4-yl) methyl)-4-morpholinoaniline (6e)
Yield 82 %, light yellow solid; M.P. 177–178 °C. 1H NMR (400 MHz, CDCl3) δ 7.53 (d, J = 8.0 Hz, 4H), 7.47 (d, J = 8.1 Hz, 4H), 7.31 (d, J = 8.0 Hz, 4H), 7.24 (d, J = 7.8 Hz, 4H), 7.07 – 6.84 (m, 2H), 6.77 (s, 2H), 4.65 (s, 4H), 3.88 (dd, J = 33.5, 8.9 Hz, 4H), 3.12 (d, J = 25.0 Hz, 4H), 2.39 (s, 6H). 13C NMR (101 MHz, CDCl3) δ 139.85, 137.95, 136.98, 129.48, 127.17, 126.85, 113.88, 66.74, 54.48, 21.10. Elemental Anal. Calcd. for: C38H38N2O (538.74): C, 84.72; H, 7.11; N, 5.20 % Observed: C, 84.68; H, 7.13; N, 5.22 % (supplementary data, S13-S14).
2.2.8 4-morpholino-N, N-bis(4-(thiophen-3-yl) benzyl) aniline (6f)
Yield 81 %, purple solid; M.P. 149–150 °C. 1H NMR (400 MHz, CDCl3) δ 7.56 (d, J = 8.1 Hz, 4H), 7.45 – 7.42 (m, 2H), 7.40 – 7.36 (m, 4H), 7.28 (d, J = 7.9 Hz, 4H), 6.95 (dd, J = 36.4, 6.3 Hz, 2H), 6.76 (d, J = 7.0 Hz, 2H), 4.64 (s, 4H), 3.87 (dd, J = 25.6, 20.9 Hz, 4H), 3.13 (s, 4H). 13C NMR (101 MHz, CDCl3) δ 141.95, 134.67, 132.13, 132.03, 131.91, 128.55, 128.43, 127.26, 126.69, 126.24, 120.12, 77.33, 54.55. Elemental Anal. Calcd. for: C32H30N2OS2 (522.73): C, 73.53; H, 5.79; N, 5.36; S, 12.27 % Observed: C, 73.49; H, 5.73; N, 5.39; S, 12.18 % (supplementary data, S15-S16).
2.2.9 N, N-bis((4′-methoxy-[1,1′-biphenyl]-4-yl)methyl)-4-morpholinoaniline (6 g)
Yield 83 %, brown solid; M.P. 175–176 °C. 1H NMR (400 MHz, CDCl3) δ 7.70 – 7.64 (m, 4H), 7.46 (ddd, J = 7.2, 5.3, 2.3 Hz, 6H), 7.30 (d, J = 7.9 Hz, 4H), 6.97 (d, J = 8.7 Hz, 4H), 6.78 (d, J = 6.5 Hz, 2H), 4.66 (s, 4H), 3.94 (s, 4H), 3.85 (s, 6H), 3.12 (s, 4H). 13C NMR (101 MHz, CDCl3) δ 167.76, 159.09, 139.55, 133.35, 133.03, 132.42, 132.13, 132.03, 131.99, 131.94, 131.91, 130.88, 128.79, 128.55, 128.43, 128.01, 127.20, 126.93, 114.19, 68.13, 55.32, 54.47. Elemental Anal. Calcd. for: C38H38N2O3 (570.73): C, 79.97; H, 6.71; N, 4.91 % Observed: C, 79.94; H, 6.66; N, 4.94 % (supplementary data, S17-S18).
2.2.10 N, N-bis((3′-chloro-4′-fluoro-[1,1′-biphenyl]-4-yl) methyl)-4-morpholinoaniline (6 h)
Yield 78 %, yellow solid; M.P. 141–142 °C. 1H NMR (400 MHz, CDCl3) δ 7.60 (dd, J = 7.0, 2.2 Hz, 2H), 7.48 (d, J = 8.1 Hz, 4H), 7.42 (ddd, J = 8.5, 4.5, 2.3 Hz, 2H), 7.35 (d, J = 8.1 Hz, 4H), 7.19 (t, J = 8.7 Hz, 2H), 6.84 (d, J = 49.1 Hz, 4H), 4.65 (s, 4H), 3.88 (s, 4H), 3.06 (s, 4H). 13C NMR (101 MHz, CDCl3) δ 158.81, 156.33, 138.54, 138.13, 138.09, 137.67, 129.07, 127.52, 127.18, 126.64, 126.57, 121.32, 121.15, 118.25, 116.93, 116.72, 114.28, 66.87, 54.70, 51.12. Elemental Anal. Calcd. for: C36H30Cl2F2N2O (615.55): C, 70.25; H, 4.91; N, 4.55 % Observed: C, 70.26; H, 4.98; N, 4.53 % (supplementary data, S19-S20).
2.2.11 1-(4′-(((4-bromobenzyl) (4-morpholinophenyl) amino) methyl)-[1,1′-biphenyl]-3-yl) ethan-1-one (7i)
Yield 77 %, colorless solid; M.P. 157–158 °C. 1H NMR (400 MHz, CDCl3) δ 8.17 (s, 1H), 7.92 (d, J = 7.7 Hz, 1H), 7.77 (d, J = 7.4 Hz, 1H), 7.56 (dt, J = 15.6, 6.9 Hz, 4H), 7.38 – 7.30 (m, 4H), 7.28 (s, 1H), 6.99 (d, J = 9.6 Hz, 2H), 6.74 (s, 2H), 4.64 (s, 4H), 4.01 – 3.75 (m, 4H), 3.12 (s, 4H), 2.65 (s, 3H). 13C NMR (101 MHz, CDCl3) δ 198.10, 141.34, 138.89, 137.60, 131.60, 130.36, 130.00, 129.05, 128.65, 127.80, 127.43, 127.16, 126.99, 126.80, 54.86, 26.76. Elemental Anal. Calcd. for: C32H31BrN2O2 (555.52): C, 69.19; H, 5.63; N, 5.04 % Observed: C, 69.19; H, 5.63; N, 5.04 % (supplementary data, S21-S22).
2.3 Screening for anticonvulsant potential
2.3.1 Animals
C57BL/6 male mice weighing 18–25 g were used in the current study. The mice were brought up and kept in the Faculty of Pharmacy's animal facility at Bahauddin Zakariya University in Multan. Every mouse was kept at a constant temperature of 25 °C with a 12-hour day and night cycle. They were also given unbounded access to normal rodent water and food. To reduce handling-induced stress, the mice were acclimated to the testing surroundings and the experimenter's control prior to the experiment. All the animal experiments were endorsed by the Department of Pharmacology's Ethics Committee at BZU, Multan (05-PHL-S22), and the “Institute of Laboratory Animal Resources” (ILAR) provided guidelines that were followed. Commission on Bioscience, National Research Council (NRC, 1996).
2.3.2 Drugs and chemicals
Animals were divided into different groups. Both standard and experimental medications (6a-h, 7i) for antiseizure potential were evaluated. Group 1: named as a seizure control group, treated with PTZ 80 mg/kg (Nieoczym et al., 2013). Group 2: The reference group received 5 mg/kg diazepam (Rehman et al., 2022). Whereas the remaining groups (6a-h, 7i) were primarily treated with synthesized compounds (100 mg/kg) followed by administration of PTZ. The PTZ and diazepam were dissolved and diluted in normal saline while test compounds were suspended in a 1 % Tween 80. All dilutions were prepared fresh and administered via intraperitoneal (i.p.) route. In acute PTZ testing diazepam acted as the standard drug while in 6 Hz levetiracetam(LEV) (50 mg/kg) was used as the standard therapy, and the results were compared with the mice that were given 1 % Tween 80 (1 ml/kg) injections (Góra et al., 2020).
2.3.3 6 Hz psychomotor seizure test
Before stimulation, a drop of 1 % lidocaine HCl solution was utilized in the corneas of the mouse to induce local anesthesia and enhance current conductivity, ensuring optimal conditions for the stimulation intended to cause psychomotor seizures at 6 Hz, 32 mA, with a rectangular pulse width of 0.2 ms for 3 s duration (Barton et al., 2001). The animal exhibited a shocked posture during the 60–120 sec 6 Hz convulsions, which included rearing, twitching of the vibrissae, and clonus of the forelimb. Animals were regarded as protected if they returned to their regular behavior within 10 s of stimulation. Approximately, 45–60 min prior to corneal stimulation of 6 Hz, all synthesized compounds (100 mg/kg) via intraperitoneal route were delivered in the current study.
2.3.4 PTZ seizure test
The animals divided group-wise were first treated with designated compounds and then PTZ. After 40–45 min pretreatment with synthesized compound, PTZ (80 mg/kg) was injected, and animals were observed for the next 30 min to detect clonic episodes occurrence. Clonic episodes were rendered as jerking of the whole body remains for exceeding 3 sec, accompanied by the absence of the righting reflex (Calderon 2018). Different parameters like frequency of seizure occurrence, the onset to the first clonus, tonic clonic convulsions, tonic hind limb stretching, and the latency to death were noted and analyzed among the reference and drug-treated groups. The absence of clonic seizures analyzed during the research duration was referred to as the compound's capability to prevent in contrast to the PTZ-induced seizures.
2.3.5 Stereotaxic surgery and EEG acquisition
Each animal was given an intraperitoneal injection of a combination of ketamine (87.5 mg/kg) and xylazine (12.5 mg/kg) to induce unconsciousness before the insertion of an EEG electrode. The animals' level of consciousness was tracked using toe reflexive pinching withdrawal (Rasool et al., 2023). To perform sterile surgery the skull was cleansed and shaved with 70 % alcohol after the animals were set in a thermostatic frame (Stoelting, USA) equipped for thermoregulation with a heating pad.
A 2-centimeter incision was performed, and the exposed skull surface's soft tissue was cleansed an electrically powered driller was used to carefully drill the holes in the skull, taking care not to puncture the meningeal membrane with the drill bit. A screw was inserted at AP − 2 mm and L − 1.5 mm as a reference electrode, and cortical tripolar electrodes were implanted at AP + 2 mm and LL ± 1.5 mm from the bregma (Anjum et al., 2018). Dental cement was used to secure the mounted implants and to prevent dehydration, a subcutaneous bolus of 0.9 % normal saline was administered. Every mouse implanted with an EEG electrode was monitored until it recovered from anesthesia, moved freely, and restarted its typical dietary routine. Before being used in the experiment, every animal was given a 5–7-day post-operative healing span.
Following a period of 5–7 days for healing, the mice in individual cages were linked to an analog–digital converter (PowerLab 8/30 ML870, ADInstruments) and 8-channel bioamplifiers (ADInstruments Ltd., Sydney, Australia) for EEG acquisition. Laptop was used and connected with a Logitech camera for video EEG recording. The animals were placed into EEG-designated cages and given 30 min to acclimate. The baseline was recorded for 15–20 min, and then injections of the synthesized compounds 6b and 6f (100 mg/kg; i.p.) were given to three different groups of animals. An hour later, PTZ (80 mg/kg; i.p.) was given, and vEEG was recorded for 30 min with a signal sampling rate of 200 Hz and bandpass filtered between 0.1 and 60 Hz (Anjum et al., 2018). The EEGs were examined for electrographic modifications and spikes, which were electroencephalogram changes of at least double the amplitude of the EEG baseline. Muneeb et al.'s previously published spike analysis method was used to assess the spiking intensity. The results were reported as the latency (s) to the first post-PTZ spike in the 10 s following the initial spike (Anjum et al., 2018).
2.3.6 Statistical analysis
Statistical analysis of all data was conducted utilizing Graph Pad Prism version 8.0. A one-way ANOVA following a post-hoc Dunnett test for multiple comparisons was employed to evaluate the statistical differences between groups across all behavioral parameters. Every data was presented as mean ± SD and P < 0.05 indicated that the results were statistically significant.
2.3.7 Molecular docking methodology
To investigate the anticonvulsant activity, the X-ray crystal structures of GABA-A receptor from mice (PDB ID: 8G5G) (Sun et al., 2023) and SV2A protein in outward open state (PDB ID: 3O7P) (Dang et al., 2010) and inward open state (PDBID: 1PW4) (Huang et al., 2003) were retrieved from RCSB, Protein Data Bank ( https://www.rcsb.org). The Protein structures were prepared by deleting water molecules, adding polar hydrogens, correcting geometry for het-atoms, and repairing missing atoms/loops with prime module in Protein Preparation Wizard. The hydrogen bond optimization was performed by the hydrogen bond assignment tool and protein structures were then subjected to energy minimization with an RMSD constraint set at 0.3 Å, utilizing the OPLS-2005 forcefield. The GLIDE grid generation wizard was utilized to define the docking space, centered on the crystallized ligand of each respective protein.
The 2D structure of investigated ligands and reference drugs (Levetiracetam and Diazepam) were sketched using 2D ChemDraw and subsequently saved in sdf format. Ligand preparation was conducted using the LigPrep wizard application within Maestro 12.0 (Schrodinger 2016). The structures were converted to 3D with adjustment of bond lengths and angles. The OPLS 2005 forcefield was utilized for energy minimization and optimization of ring conformations. The ionization and tautomeric states were generated with the Epick tool and specified chiralities were maintained with default parameters employed in Maestro 12.0.
To evaluate the effectiveness of the investigated ligand’s interactions with the receptor, the docking process was carried out using the Glide program in standard precision SP (standard precision) mode (Friesner et al., 2004). The glide docking scores and glide E-model scores were computed for best conformational pose of each ligand and results were compared with scores of reference drugs utilized in this study.
3 Results and discussions
3.1 Chemistry
The current research is a synthetic strategy for the synthesis of N, N-bis(4-bromobenzyl)-4-morpholinyl amine derivatives (6a-h, 7i) which is based upon chemical derivatization of morpholine aniline for improving their safety profile. In 2017, Castillo and coworkers reported direct C-N alkylated tertiary amines through Cs2CO3 with primary benzylamines and anilines with a wide range of alkyl halides (Castillo et al., 2016). Barraza and Denmark developed the C-N Alkylation of 2-bromoaniline with benzyl bromide under K2CO3 basic N-alkylation conditions (Barraza and Denmark 2017). Despite all these methodologies, there is a big barrier to directly synthesizing tertiary amines from primary amines with minimum side reactions. We here, synthesized direct synthesis of C-N alkylated tertiary amines through well-suited base K2CO3. In this study, N, N-bis(4-bromobenzyl)-4-morpholinyl amine (3) that corresponds to tertiary amines as a major product, and N-(4-bromobenzyl)-4-morpholino aniline (4) as a minor trace were obtained by the reaction of 4-morpholinyl aniline (1) with K2CO3 in the presence of dimethylformamide solvent, following after 30 min addition of 4-bromobenzyl bromide (2) at ambient temperature, then allowed it 3 h for isotherm and finally obtained good yield (95 % and 5 %) (Scheme 1). In the aforementioned reaction, C-N alkylated tertiary amines were achieved in an excellent yield. Here, surprisingly, we focus on tertiary amines but some minor traces of secondary amines were also observed during spectroscopic identification, which were further evaluated for screening of antiepileptic potential. In Table 1. Different bases like Cs2CO3 and CaH2, were also used to synthesize intermediate molecules, but due to their high cost and relatively less yield comparable with K2CO3, they were exploited further to synthesize new molecules. Herein, the foremost benefit of using K2CO3 was due to its low cost and highly selective product.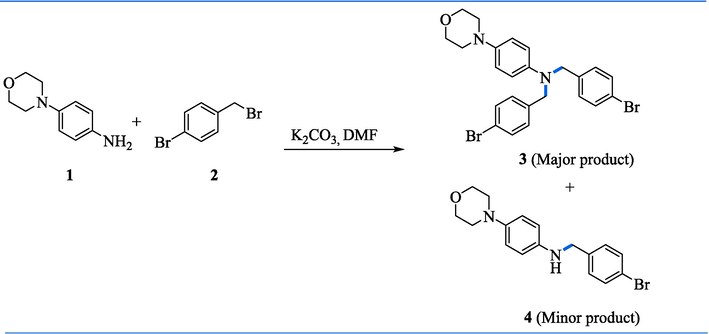
Synthesis of N, N-bis(4-bromobenzyl)-4-morpholinyl amine (3)a. aConditions: (i) 1 (1 equiv, 0.5 g, 2.81 mmol), 2 (2.2 equiv., 1.55 g, 6.2 mmol), K2CO3 (3 equiv., 1.24 g, 8.43 mmol), and DCM (40 mL), stirring 24 h, rt.
Entry
Base
Equivalent
3 % yield
1.
Cs2CO3,
3
90 %
2.
CaH2,
3
88 %
3.
K2CO3,
3
95 %
Here, the plausible mechanism is shown in scheme 2. The potassium cation and primary amines (1) lead to the formation of complex A, which increases the primary amine acidity. Then the acidic hydrogen linked with nitrogen is removed by carbonate ion resulting in the formation of potassium amide B. The resulting amide strong nucleophilicity promotes substitution reactions with 4-brmo benzyl bromide more likely, which synthesize secondary amine (4). Tertiary amines (3) were then obtained when secondary amine (4) reacted with benzyl bromide (Scheme 2).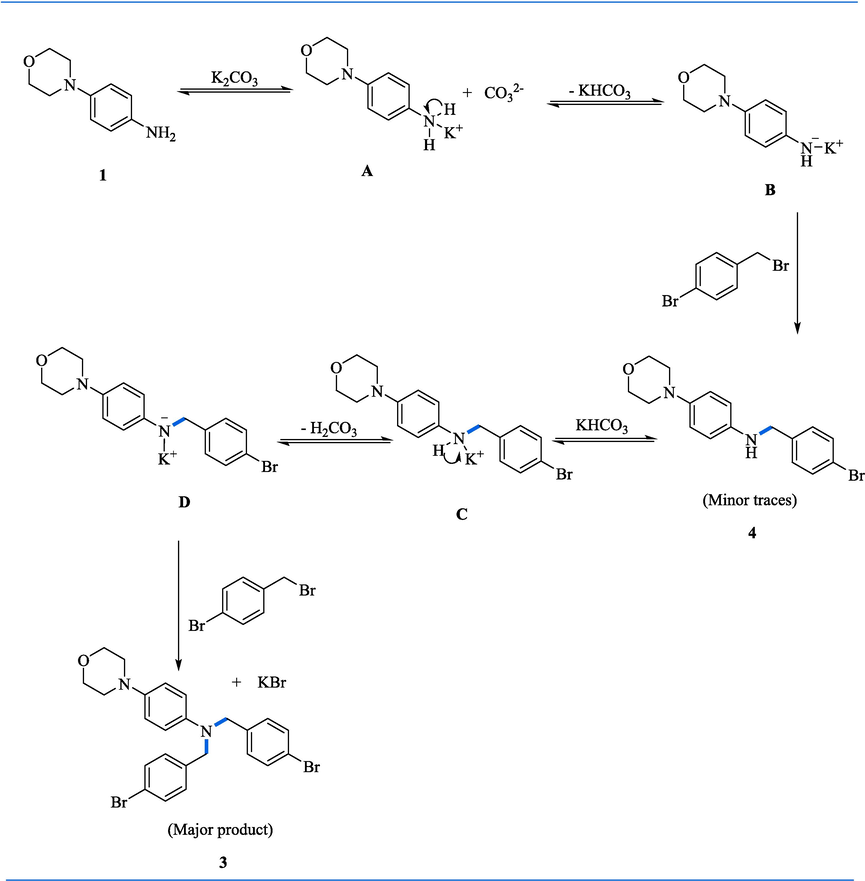
Proposed mechanism of C-N alkylation of morpholine anilines.
To broaden the scope of our study, we conducted the Pd(0) catalyzed Suzuki reaction involving N, N-bis(4-bromobenzyl)-4-morpholinyl amine (3) with various phenyl boronic acids (5), resulting in the formation of the corresponding N, N-bis(4-bromobenzyl)-4-morpholinyl (6a − h, 7i) derivatives ranging from moderate to good yields (77–84 %) (Scheme 3). Surprisingly, all the compounds are synthesized by double coupling but exceptionally, only one undergoes mono coupling, during evaluating the spectroscopic identification of newly synthesized molecules. To the best of our understanding, the synthesized derivatives represent new compounds. The presence of the bulky ligand (PPh3)4 on the commercially available Pd-metal catalyst promoted heterocoupling among the reacting species instead of undergoing homocoupling, thereby enabling the reaction to proceed effectively. The solvent employed in this coupling combines water and dioxane, as polar solvents in the transition phase with the palladium complex can be coordinated and give favorable results (Proutiere and Schoenebeck 2011, Chen et al., 2018). Due to the reduced nucleophilicity of aryl boronic acids comprising electron deficient groups, transmetalation proceeds at a slower rate compared to neutral species, making them more prone to side reactions. Similarly, boronic acids with steric hindrance tend to yield lower product yields.![Synthesis of N, N-bis([1,1′-biphenyl]-4-ylmethyl)-4-morpholino aniline derivatives (6a-h)a and 1-(4′-(((4-bromobenzyl) (4-morpholinophenyl) amino) methyl)-[1,1′-biphenyl]-3-yl) ethan-1-one (7i)a. aConditions: 3 (0.3 g, 0.58 mmol, 1 equiv), 5 (0.22 g, 1.45 mmol, 2.5 equiv), Pd(PPh3)4 (0.046 g, 4.07 mmol, 7 mol %), K3PO4 (0.49 g, 2.32 mmol, 4 equiv), 1,4- dioxane (8 mL), water (0.5 mL), 120 °C, 24 h under argon.](/content/184/2024/17/9/img/10.1016_j.arabjc.2024.105889-fig5.png)
Synthesis of N, N-bis([1,1′-biphenyl]-4-ylmethyl)-4-morpholino aniline derivatives (6a-h)a and 1-(4′-(((4-bromobenzyl) (4-morpholinophenyl) amino) methyl)-[1,1′-biphenyl]-3-yl) ethan-1-one (7i)a. aConditions: 3 (0.3 g, 0.58 mmol, 1 equiv), 5 (0.22 g, 1.45 mmol, 2.5 equiv), Pd(PPh3)4 (0.046 g, 4.07 mmol, 7 mol %), K3PO4 (0.49 g, 2.32 mmol, 4 equiv), 1,4- dioxane (8 mL), water (0.5 mL), 120 °C, 24 h under argon.
This study applied to PTZ (80 mg/kg) and 6 Hz (32 mA) mice models to preliminary analyze the antiseizure efficacy of the synthesized morpholinyl amine derivatives (6a-h, 7i). To confirm the antiseizure potential of potent drugs, this study assessed how they affected the PTZ model's electroencephalogram (EEG) activity.
3.2 6 Hz seizure model
Out of eleven, in only six synthesized compounds (4, 6a, 6b, 6d, 6f, 6 g), 100 % protection was noticed as shown in Fig. 2. The animals were 66 % protected against psychomotor seizures by receiving treatment with 3 and 7i compounds. Whereas the compounds namely 6e and 6 h showed 33 % protection. However, the mice administered with the remaining compounds showed complete (100 %) seizure protection, with all three tested animals returning to their normal exploratory actions within 10 s of the shock application. Despite using the same dosage for testing the 6c, none of the animals were retrieved from seizures. Levetiracetam (LEV) (50 mg/kg) was taken as standard, and results were compared with it and protected 3/3 of the animals as shown in Fig. 2.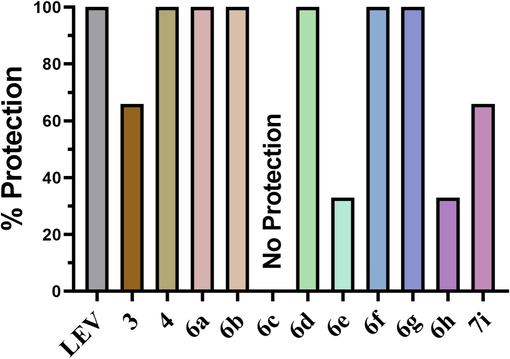
The % protection noted in animals pre-treated with test compounds followed by testing in 6 HZ seizure model.
3.3 Acute PTZ model
In animals treated with test compounds, latency to first myoclonic seizures was noted in all groups and it was clearly indicated that PTZ control animals developed first myoclonus twitch within 34 ± 10.78 sec as compared to others. Whereas the standard drug diazepam significantly delayed the seizure potential 240 ± 35.00 sec (P < 0.0001). The 6d compound produced the delayed development of the first myoclonus jerk with latency of 122 ± 41.72 sec. Further, the animals treated with 6b and 6f exhibited prominent delay of 155.66 ± 9.01 sec (P < 0.01) and 152.66 ± 65.74 sec (P < 0.01) in appearance of initial myoclonic twitch when compared to the PTZ control drug.
Similarly, latency to tonic-clonic seizures was also observed in animals pretreated with synthesized compounds. Animals just received chemo convulsive agent show tonic-clonic jerking within 59.33 ± 3.51 sec. Only two compounds 6b and 6f out of all exhibited significant protection from tonic-clonic seizure after 368.6 ± 188.28 sec (P < 0.05) and 400.23 ± 191.79 sec (P < 0.01), respectively.
Moreover, 30 min after PTZ injection, a prominent latency to tonic hind limb extension was monitored. The generalized tonic-clonic seizures progressed to hind limb extension within 94.66 ± 10.50 sec in PTZ control mice. However, mice pre-treated with 6b and 6f remained completely protected from tonic hind limb extension for 30 min with P < 0.001. Whereas 6a and 6 g treatment caused a significant delay of 1470.6 ± 39 sec (P < 0.01) and 1585 ± 372.39 sec (P < 0.01) in the development of seizures with hind limb extension.
Additionally, mice were also observed for post-PTZ latency to death and outcomes showed complete protection from death in mice pre-treated with 6b and 6f. While compounds 6a, 6 h and 6 g showed significant delay of 1507.33 ± 360.95 sec, 1536 ± 457.26 sec and 91594 ± 356.82 sec in death, in comparison to PTZ control mice (Fig. 3).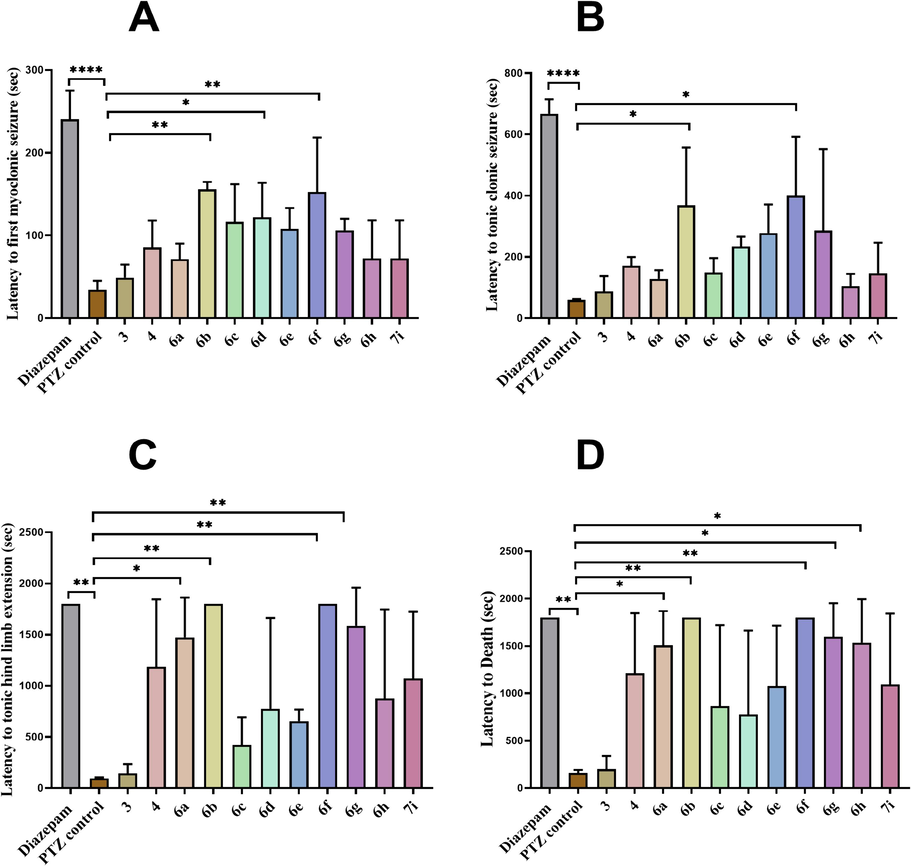
Represents anti-seizure potential of synthesized compounds in acute PTZ model. The data is represented as (mean ± SD). Statistical analysis was conducted by employing one-way ANOVA pursued by Dunnett's test. (A) latency to first myoclonic jerking (B) latency to tonic clonic seizures (C) latency to tonic hind limb extension (D) latency to death. Significant difference is shown when analyzed to the control group: *p = 0.05, ** p < 0.01, *** p < 0.001 and ****p < 0.0001.
3.4 Validation of anti-seizure potential of 6b and 6f through EEG
EEG was noted in electrode-implanted mice pre-treated with 6b and 6f to further validate the anti-seizure potential of both compounds. The electrographic changes were consistent with behavioral development of seizures as PTZ administration caused a continuous train of epileptic spikes that continued to increase in amplitude and became the double of normal pre-PTZ baseline as shown in Fig. 4.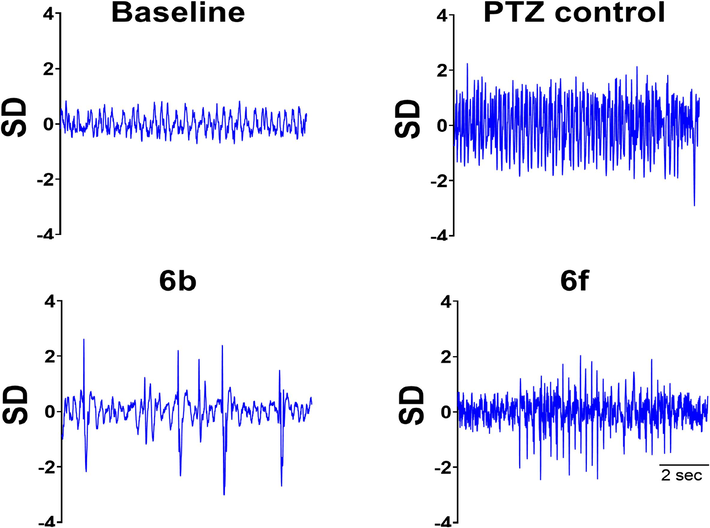
Demonstrates the EEG traces observed throughout the experiment. Baseline EEG of sample obtained before PTZ administration. PTZ control group receiving only chemo convulsive agent. While groups receiving treatment (Compound 6b + PTZ) and (Compound 6f + PTZ) showed less epileptic spikes when compared to control group.
In mice pre-treated with 6b and 6f mice were protected from hind limb extension and death and EEG studies validated these observations as these mice had reduced frequency of epileptic spikes while inter-spike interval was notable greater in comparison to PTZ control mice as depicted in real-time EEG tracing presented in Fig. 4. An electrical activity of 10 sec were observed after appearance of first epileptic spike post-partum injection, represents EEG tracing of a high timed resolution of spike.
3.5 Structure-activity relationship (SAR)
SAR of the synthesized compounds (6a-h, 7i) demonstrates that the substitution of methoxy carbonyl, methyl, chloro, fluoro, and thiophene group on the distal aryl rings showed the most significant activity as shown in Fig. 5.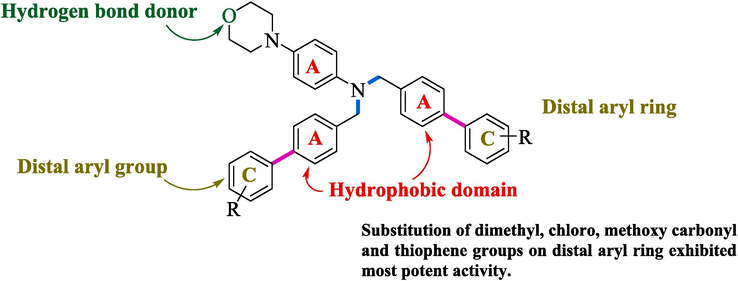
SAR of synthesized compounds (6a-h, 7i).
In Fig. 2 6b and 6f showed 100 % survival and exhibited most potent activity due to thiophene and methoxy carbonyl substitution at the para position. However, 6a, 6d, 6 g, and 6 h exhibited 66 % survival, and showed significant activity than 3, 6c, 6e, and 7i molecules exhibited 33 % survival against acute seizure PTZ (80 mg/kg) kindling model. Compound 6c exhibits less activity due to the substitution of the dichloro group at the 3 and 4th position making it relatively less susceptible for seizure control. Compounds 6e and 7i exhibit relatively less activity due to methyl and acetyl moiety at the para position, which significantly lose its efficacy against antiepileptic potential (Fig. 6).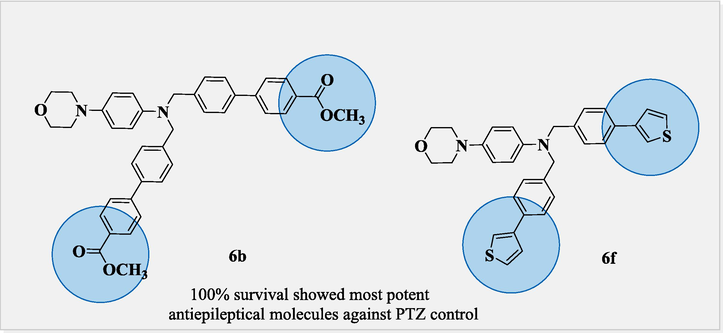
100% survival of most potent anticonvulsant molecules 6b and 6f.
In Fig. 8 Compounds 6a, 6b, 6d, and 6f, also exhibit 100 % protection against the 6 Hz psychomotor seizure model (Levetiracetam as standard drug) due to dimethyl substitution at meta position on both sides in 6a, similarly 6b was also due to the substitution of methoxy carbonyl at para position increases its retention stability. Likewise, 6d and 6f activity was also due to the chloro, and thiophene moiety at the para position, which enhances the lipophilicity to cross the BBB (blood–brain barrier) and exerts its efficacy towards seizure control. Compound 4 which is accidently obtained as a minor product significantly exhibits 100 % protection than compound 3 (66 % protection) (Fig. 7).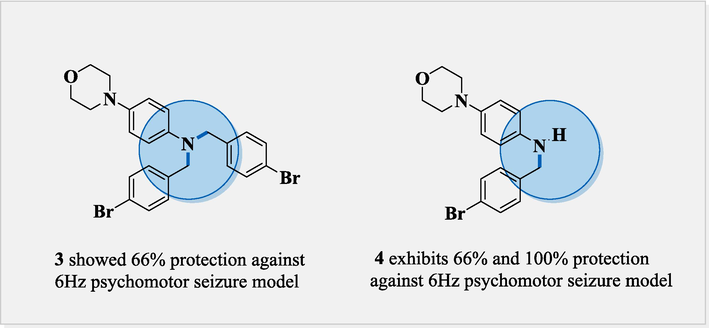
Comparison between anticonvulsant molecules 3 and 4.
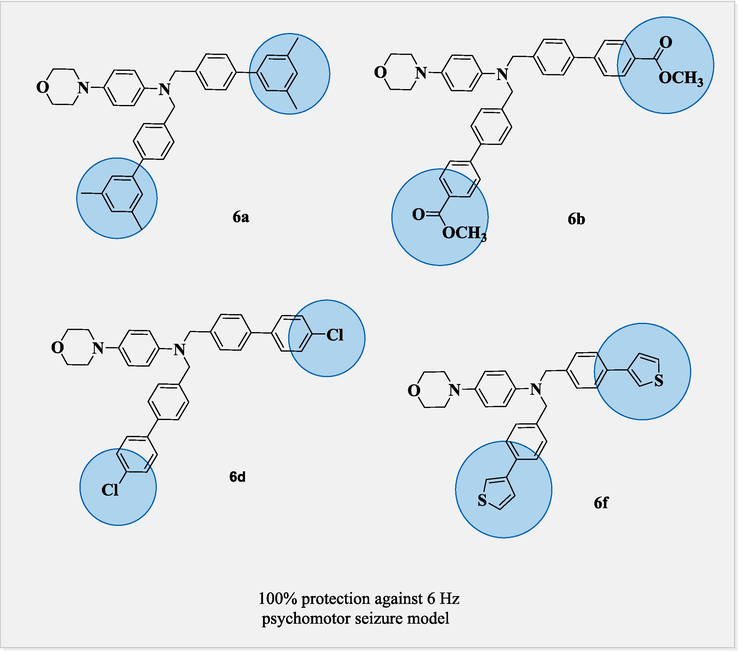
Comparison between anticonvulsant molecules of 6a, 6b, 6d and 6f.
3.6 Molecular docking analysis
Molecular docking studies revealed a consistent position and alignment of investigated ligands within the potential binding site of GABA-A receptor (PDB code: 8G5G), in comparison to the co-crystallized ligand, zolpidem. Table 2 provides comprehensive information on the binding energies and interactions between the investigated ligands and the target protein 8G5G. The H-bonding interaction with SER229, PHE77, and pi-cation interaction with TYR184 is found to be crucial for the anticonvulsant activity of zolpidem (Sancar et al., 2007; Vijayan et al., 2012). The levetiracetam and diazepam, used as a reference in this study, exhibited H-bonding and π-π interaction with aforementioned amino acid residues and exhibited high binding affinity with G-scores of −7.1 kcal/mol and −7.4 kcal/mol, respectively. The proposed binding mode of ligand 4 in GABA-A receptor binding site revealed strong binding affinity (G-score = -6.6 kcal/mol) through H-bonding interaction with key amino acid TYR184 and hydrophobic interaction with aminoacid residue SER229 along with PHE124, ILE227, ARG228, SER230, THR231, TYR234, PHE77, MET130, LEU140 and THR142 within binding pocket. In the case of ligands 6c and 6d, para positioned chloro group on the terminal phenyl ring is engaged in halogen interactions with amino acid residues THR142 and TYR231, maintaining a G-score higher than −5.0 kcal/mol. Fig. 9 illustrates the 2D and 3D binding interactions of ligands 4, 6c and 6d with GABA-A receptor (PDB ID: 8G5G). The replacement of the chloro group with other halogens or change in position from para to meta as in the case of ligand 6 h, resulted in the loss of halogen bonding interactions with these amino acid residues. However, π –hydrogen bonding interactions with residues ASN60, ASP192 and ARG228 may contribute towards a significant binding affinity with G-score −6.1 kcal/mol. The ligand 7i established H-bonding interactions with THR142, and THR231 via acetyl substituent on the terminal phenyl ring, along with aromatic hydrogen bonding interactions involving ASN60, ASP192, ARG228 and GLU225. Additionally, it exhibited π- π interactions with TYR58, resulting in a substantial binding affinity indicated by a G-score of −6.2 kcal/mol. On the other hand, ligand 6f demonstrated significant π-π interactions with residues TYR58, PHE77, HIS126, π-ionic interaction with LYS180, as well as aromatic H-bonding interactions with ASN60 and ARG228 resulted in significant binding affinity with G-score of −6.7 kcal/mol, as illustrated in Fig. 10. The binding interactions of investigated ligands were compared with those of reference compounds, including levetiracetam, diazepam and crystallized ligand (zolpidem) as shown in Fig. 11. CL: Crystallized Ligand (Zolpidem) H. B. I: Hydrogen Bonding Interactions.
Ligands
G-Score
(kcal/mol)
E-model
Score
H.B.I
Residue (Distance Å)
Hydrophobic & other interacting residues
4
−6.6
−50.51
TYR184 (2.43)
C: PHE124, HIS126, SER183, ILE227, ARG228, SER229, SER230, THR231, TYR234
D: ASP56, TYR58, PHE77, ALA79, MET130, LEU140, THR142, GLU189
6c
−5.8
−71.64
ASN60 (2.71)
THR142 (2.52)
GLU189 (2.64)
ARG228 (2.30)
THR231 (2.41)
C: TYR184, GLU225, ILE226, ILE227, SER229, SER230, TYR234
D: TYR58, PHE77, MET130, GLY191, ASP192,
6d
−5.1
−63.54
ASN60 (2.74)
THR142 (2.57)
ARG228(2.45) THR231 (2.37)
C: LYS180, GLU225, ILE226, ILE227, ARG228, SER229, SER230, THR231, TYR234
D: TYR58, PHE77, MET130, GLU189, GLY191, ASP192
6f
−6.7
−78.44
ASN60 (2.38)
ARG228 (2.18)
C: PHE124, HIS126, ASN127, PRO164, PRO178, LYS180, ILE227, ARG228, SER229, SER230, THR231, TYR234
D: TYR58, PHE77, ALA79, THR142, GLU189, ASP192
6 h
−6.1
−69.77
ASN60 (2.21)
ASP192 (2.42)
ASP192 (2.43)
C: HIS126, PRO164, PRO178, LYS180, TYR184, ILE227, ARG228, SER229, THR231, TYR234
D: TYR58, PHE77, MET130, THR142, SER195
7i
−6.2
−75.09
ASN60 (2.54)
THR142 (2.35)
ASP192 (2.49)
ASP192 (2.73)
GLU225 (2.37)
ARG228 (2.45)
THR231 (1.98)
C: PHE124, HIS126, LYS180, SER183, TYR184, ILE227, SER229, SER230, TYR234
D: TYR58, PHE77, MET130, GLU189, VAL190, GLY191,
Levetiracetam
−7.1
−43.32
HIS126 (2.59)
TYR184 (2.38)
SER183 (2.15)
SER229 (2.33)
C: PHE124, SER183, TYR184, ILE227, SER229, THR231, TYR234
D: TYR58, ASN60, PHE77, MET130, THR142
Diazepam
−7.4
−54.41
ASN60 (2.53)
TYR184 (2.51)
C: PHE124, SER183, ILE227, ARG228, SER229, SER230, THR231, TYR234
D: TYR58, PHE77, ALA79, MET130, THR142
CL
−9.7
−88.75
PHE77 (2.79)
SER229 (1.81)
SER230 (2.56)
C: PHE124, HIS126, SER183, TYR184, ALA185, ILE227, ARG228, SER229, SER230, THR231, TYR234
D: MET57, TYR58, ASN60, PHE77, PHE78, ALA79, MET130, THR142, GLU189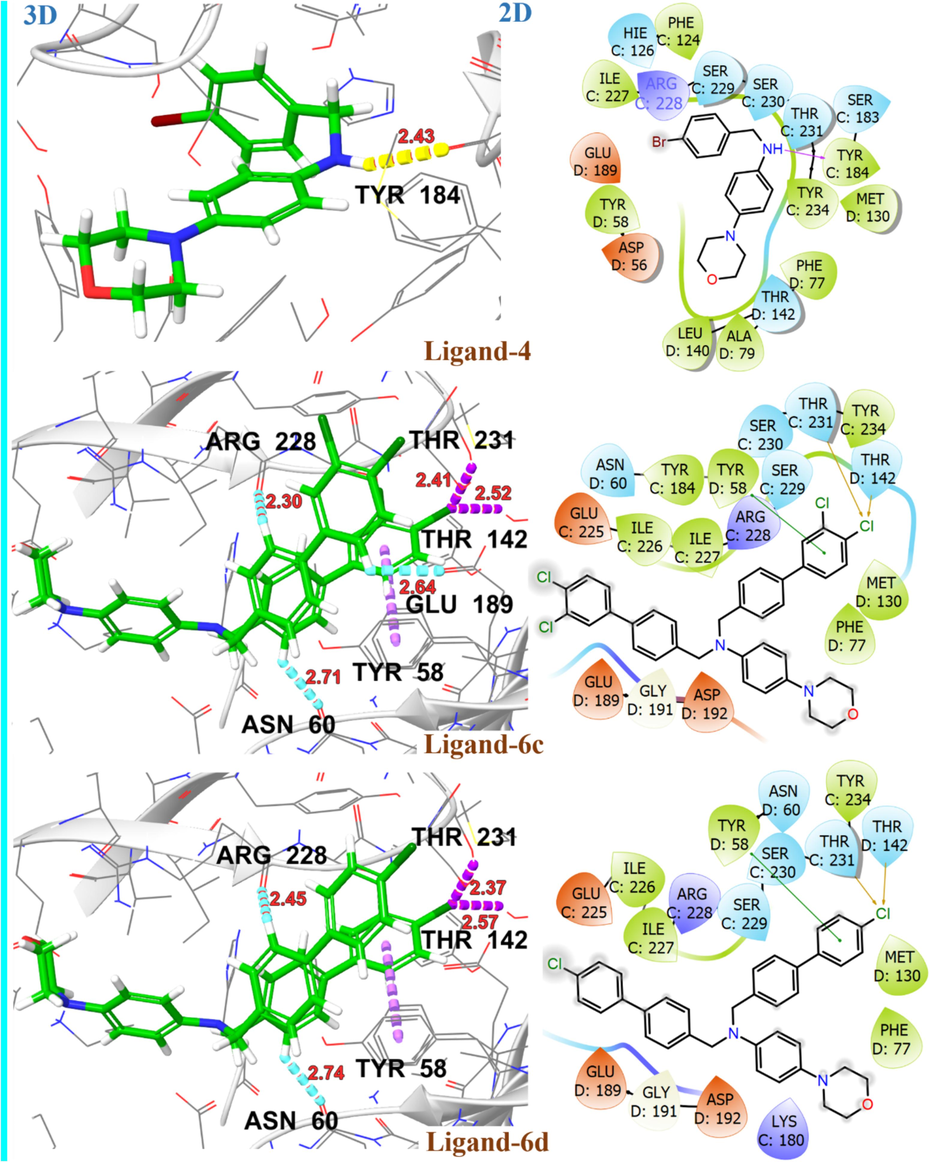
2D and 3D representation of ligands 4, 6c and 6d with GABA-A receptor (PDB ID: 8G5G).
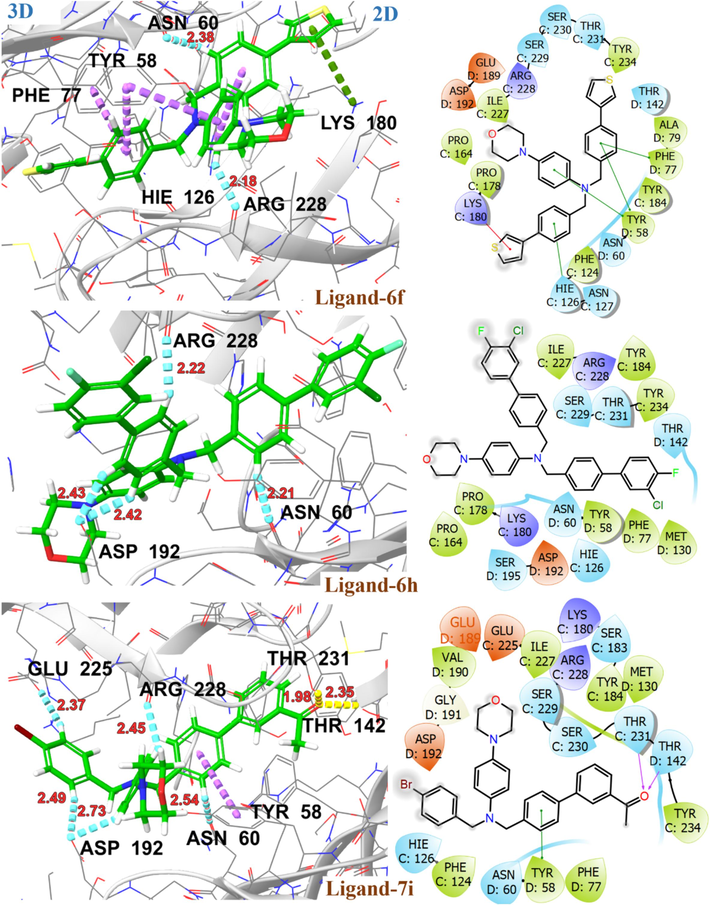
2D and 3D representation of ligands 6f, 6 h and 7i with GABA-A receptor (PDB ID: 8G5G).

2D and 3D representation of reference and crystallized ligands with GABA-A receptor (PDB ID: 8G5G).
The upregulation of SV2A resulted in increased neuronal activity and is considered to be a potential target for the treatment of epilepsy (Lynch et al., 2004). The investigated ligands have been evaluated for their binding affinity with the SV2A target protein in its outward (PDB ID: 3OP7) and inward (PDB ID: 1PW4) open state. Table 3 presented the glide docking score, H-bonding, and hydrophobic interactions for investigated ligands with target protein 3O7P and 1PW4. Most of these ligands exhibited a high binding affinity for 3O7P in an outward open state with G-score ranging from −8.4 kcal/mol to −9.6 kcal/mol. The amino acid residues ASP46, GLU135 and ALA162 are involved in substrate recognition as well as protonation and deprotonation steps in the transportation process. After substrate binding and protonation of GLU135, there is a conformational change resulting in an outward open state of 3O7P with substrate release (Dang, Sun et al. 2010). The ligand 6d exhibited substantial binding affinity with G-score −9.6 kcal/mol, possibly attributed to two aromatic H-bonding interactions with GLU135, a key amino acid involved in substrate recognition. The ligands 6c, 6f and 6 h demonstrated H-bonding and hydrophobic interactions with key amino acid residues ASP46, GLU135 and ALA162 resulting in significant binding affinity with G-scores > -8.0 kcal/mol. The 2D and 3D interactions of investigated ligands and crystallized ligands with target protein 3O7P are shown in Fig. 12 and Fig. 13. The levetiracetam showed significant binding affinity for both outward (3O7P) and inward (1PW4) open states of the target protein with G-scores of −6.7 kcal/mol, and −6.2 kcal/mol, respectively. Among the investigated ligands, only ligand 3 demonstrated high affinity for 1PW4 with a G-score of −7.0 kcal/mol, most probably due to its π-ionic interaction with ARG45. Fig. 14 represents the 2D and 3D interactions of ligand 3 and levetiracetam with target protein 1PW4. CL: Crystallized Ligand H. B. I: Hydrogen Bonding Interactions.
Ligands
G-Score
(kcal/mol)
E-model
Score
H.B.I
Residue (Distance Å)
Hydrophobic & other interacting residues
3O7P
6c
−8.7
−49.94
GLU135 (2.48)
LEU31, LEU34, PHE35, TRP38, ALA39, ASN42, ASN43, PHE70, TYR74, ILE94, LEU98, LEU131, LEU134, ALA138, GLN159, ALA162, SER163, ILE167, VAL222, ILE226, VAL271, GLN274, THR275, TRP278, MET305, PHE308, ARG312, ILE391, GLY394
6d
−9.6
−95.57
LEU34 (2.51)
GLU135 (2.39)
GLU135 (2.43)LEU31, PHE35, TRP38, ALA39, ASN42, ASN43, PHE70, TYR74, ILE94, LEU98, LEU131, LEU134, ALA138, GLN159, ALA162, SER163, ALA166, ILE167, ILE226, VAL271, GLN274, THR275, TRP278, MET305, PHE308, PHE309, ARG312, ILE391, GLY394
6f
−8.8
−93.19
SER163 (2.43)
LEU34, PHE35, TRP38, ALA39, ASN42, ASN43, ILE94, LEU98, LEU134, GLU135, ALA138, ASN139, GLN159, ALA162, SER163, ALA166, VAL222, TYR270, VAL271, GLN274, TRP278, TYR365, ARG312, ILE391
6 h
−8.4
−88.15
GLU135 (2.32)
LEU31, TRP38, ALA39, ASN42, ASN43, ASP46, ILE94, LEU98, LEU134, GLU135, GLN159, ALA162, SER163, ALA166, VAL170, VAL222, ILE226, TYR270, VAL271, GLN274, THR275, TRP278, MET305, ARG312, TYR365, ILE391
Levetiracetam
−6.7
−37.81
TRP38 (2.02)
GLU135 (1.64)PHE35, ALA39, ASN42, TRP74, LEU131, LEU134, GLU135, ALA138, ASN139, ALA162, GLN159, ARG312
CL
−6.7
−56.52
PHE35 (2.09)
PHE35 (2.77)
ARG312 (1.92)LEU31, LEU34, TRP38, ALA39, ASN42, ASN43, ILE94, LEU98, LEU134, GLU135, ALA138, ASN139, GLN159, ALA162, SER163, ALA166, VAL222, ILE226, PHE308
1PW4
3
−7.0
−67.21
TYR38 (2.65)
TYR362 (2.57)TYR42, ARG45, TYR76, LYS80, TRP161, ASN162, ALA164, HIS165, TYR266, VAL365, MET366, GLY369, LEU370, GLY386, GLY389, LEU390, TYR393
Levetiracetam
−6.2
−31.33
TYR266 (2.07)
TYR362 (1.96)TYR38, TYR42, ARG45, TYR76, TRP161, HIS165, TYR270, ARG269, VAL365, GLY389, LEU390, TYR393
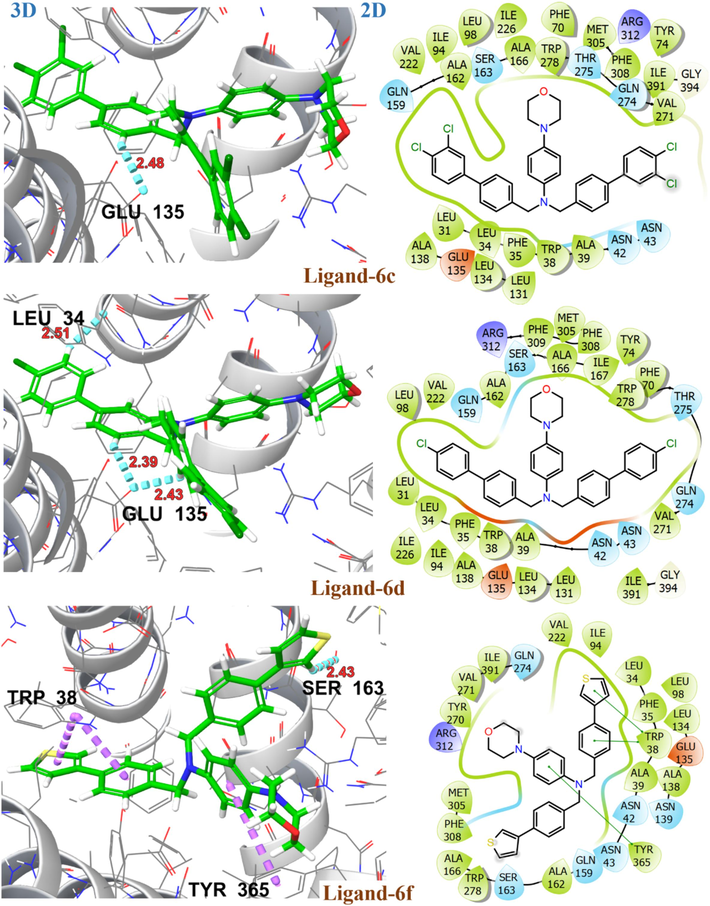
2D and 3D representation of ligands 6c, 6d and 6f with target protein S2VA in outward open state (PDB ID: 3O7P).
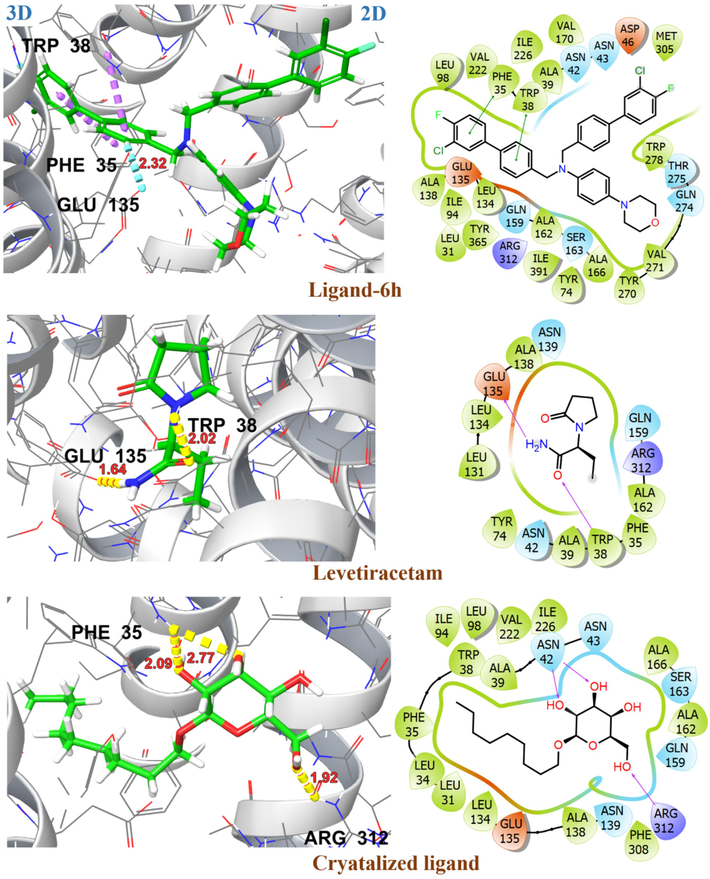
2D and 3D representation of ligands 6 h, levetiracetam and crystallized ligand with target protein S2VA in outward open state (PDB ID: 3O7P).
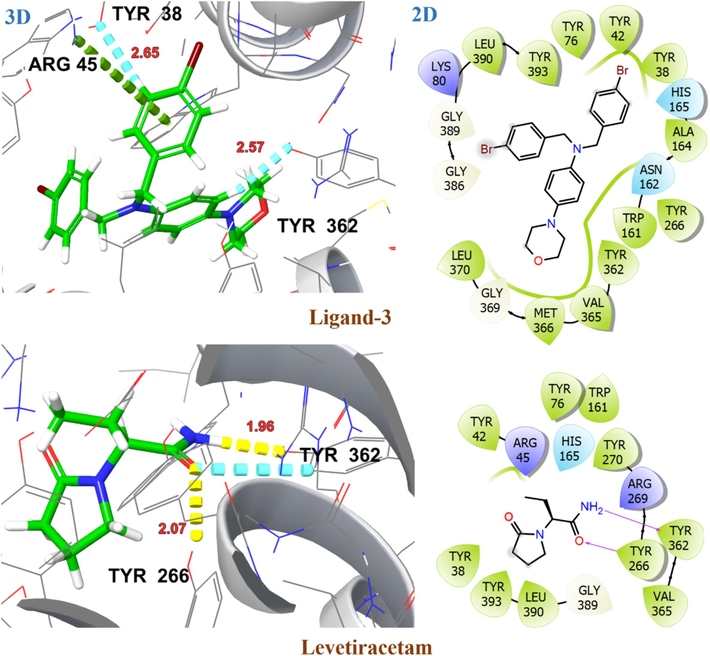
2D and 3D representation of ligand 3 and levetiracetam with target protein SV2A in the inward open state (PDB ID: 1PW4).
3.7 Lipophilicity profile analysis (Method)
PkCSM (predicting small-molecule pharmacokinetic properties using graph-based signatures) is a freely available online software to predict pharmacokinetic properties and toxicity, including ADMET (Absorption, Distribution, Metabolism, Excretion, Toxicity) for drug development. The performance of the pkCSM software can also determine the partition coefficient value (Log P) of a drug with the web on the site (https://biosig.unimelb.edu.au/pkcsm/prediction). SMILES string of the ligand structure must be input to the sites to get the Log P value and logBB values.
4 Results
The log P value describes the lipophilicity of a drug, the greater the log P value, the higher the solubility in lipids, the more non-polar the compound will be. Hence, the more non-polar nature of a compound is a clear indication of greater lipophilicity of a drug, enabling them to cross the BBB easily. LogP > 0, states the drug is lipophilic and LogP < 0 states the drug is hydrophilic in nature (Kharwar et al., 2024). So, the investigated compounds are the promising agents to cross the BBB due to their lipophilic nature as is indicated by their high LogP (except the reference drug L) which is slightly less in value than zero as shown in Table 4, with especial inherent ability of 6b and 6f compounds to exert anti-epileptic therapeutic effect.
Smiles Strings
Code of ligand
LogP
Numeric (log BB)
>0.3 good
<-1 poorBBB permeability
CC1 = CC(C(C = C2) = CC = C2CN(CC3 = CC = C(C4 = CC(C) = CC(C) = C4)C = C3)C(C = C5) = CC = C5N6CCOCC6) = CC(C) = C1
6a
9.30
1.40
Yes
O = C(OC)C(C = C1) = CC = C1C(C = C2) = CC = C2CN(CC3 = CC = C(C4 = CC = C(C(OC) = O)C = C4)C = C3)C(C = C5) = CC = C5N6CCOCC6
6b
7.64
−0.94
Yes
ClC1 = C(Cl)C = CC(C(C = C2) = CC = C2CN(CC3 = CC = C(C4 = CC = C(Cl)C(Cl) = C4)C = C3)C(C = C5) = CC = C5N6CCOCC6) = C1
6c
10.68
1.25
Yes
ClC(C = C1) = CC = C1C(C = C2) = CC = C2CN(CC3 = CC = C(C4 = CC = C(Cl)C = C4)C = C3)C(C = C5) = CC = C5N6CCOCC6
6d
9.37
1.25
Yes
CC(C = C1) = CC = C1C(C = C2) = CC = C2CN(CC3 = CC = C(C4 = CC = C(C)C = C4)C = C3)C(C = C5) = CC = C5N6CCOCC6
6e
8.68
1.32
Yes
N1(C2 = CC = C(N(CC3 = CC = C(C4 = CSC = C4)C = C3)CC5 = CC = C(C6 = CSC = C6)C = C5)C = C2)CCOCC1
6f
8.19
1.16
Yes
COC(C = C1) = CC = C1C(C = C2) = CC = C2CN(CC3 = CC = C(C4 = CC = C(OC)C = C4)C = C3)C(C = C5) = CC = C5N6CCOCC6
6 g
8.08
−0.02
Yes
FC(C(Cl) = C1) = CC = C1C(C = C2) = CC = C2CN(CC3 = CC = C(C4 = CC(Cl) = C(F)C = C4)C = C3)C(C = C5) = CC = C5N6CCOCC6
6 h
9.65
1.29
Yes
BrC(C = C1) = CC = C1CN(CC2 = CC = C(C3 = CC = CC(C(C) = O) = C3)C = C2)C(C = C4) = CC = C4N5CCOCC5
7i
7.36
0.06
Yes
CCC(C(=O)N)N1CCCC1 = O
L
−0.13
−0.28
Yes
CN1C(=O)CN = C(C2 = C1C = CC(=C2)Cl)C3 = CC = CC = C3
D
3.15
0.33
Yes
CC1 = CC = C(C = C1)C2 = C(N3C = C(C = CC3 = N2)C)CC(=O)N(C)C
Z
3.25
0.53
Yes
The prediction of the ability of a substance to cross the BBB into pkCSM is based on the model by A. Yan’s et al., (Yan et al., 2013), which contains 320 data points. The result of the prediction is the LogBB value, defined as the logarithm of the ratio of the drug concentration in the brain to the concentration in blood measured at a steady state. According to the prediction results, substances with a value of LogBB > 0.3 were intended to cross the BBB, and substances with LogBB < -1.0 face difficulty in crossing the BBB (Vilar et al., 2010). The hit compounds 6b and 6f can cross BBB with LogBB of −0.94 (although < 0.3 but > -1.0) and 1.16 (which > 0.3) respectively, thus favoring their tendency to cross the BBB. However, the reference drug (L) has the LogBB value of −0.28 (although < 0.3 but > -1.0) its BBB permeability too which could be of less extent.
5 Conclusion
In search of better anticonvulsive agents, the new derivatives of N, N-bis([1,1′-biphenyl]-4-ylmethyl)-4-morpholinoaniline derivatives (6a − h, 7i) were synthesized through Palladium catalyzed Suzuki reactions. The data collected following characterization, in silico, and in vivo analyses affirmed the anticonvulsant potential of the synthesized derivatives (6a-h, 7i). Among all, compounds 6a, 6d, 6 h, and 6 g were found to exhibit significant anticonvulsive potential against PTZ control with standard drug diazepam with no neurotoxicity. However, 6b and 6f exhibit most significant antiepileptic potential in both seizure control models compared with reference drugs diazepam and levetiracetam. Molecular docking analysis revealed substantial binding affinity of investigated ligands with target proteins, GABA-A receptor (PDB ID: 8G5G) and SV2A transporter (PDB ID: 3O7P and 1PW4) with G-score −5.1 kcal/mol to −9.6 kcal/mol. The ligands 4 and 7i displayed aromatic H-bonding interactions with TYR184 and hydrophobic interactions with PHE77 representing strong interactions with target protein 8G5G. The ligands 6d and 6c showed significant interactions for target protein 3O7P with G-score −9.6 and −8.7 kcal/mol, respectively. The ligands 6f and 6 h exhibited strong interactions with both target proteins, contributing to their significant anti-epileptic potential. The molecular docking results are perfectly aligned with experimental in vivo studies, emphasizing the potential therapeutic significance of the investigated ligands, particularly 6f and 6 h, in anti-epileptic applications.
Funding
This work was funded by Distinguished Scientist Fellowship program at King Saud University, Riyadh, Saudi Arabia through research supporting project Number (RSP2024R131).
Acknowledgments
The present data are the part of Master’s thesis of Jawaria Hafeez. The authors gratefully acknowledge the Pakistan Council of Scientific and Industrial Research (PCSIR) (Ministry of Science and Technology), which provided financial support to access the sophisticated equipment through the data repository of the scientific instrumentation development program initiated in 2021. Furthermore, the authors are thankful to Mr. Muhammad Imran, an animal house attendant for taking care of animals. Furthermore, the authors extended their appreciation to Distinguished Scientist Fellowship program at King Saud University, Riyadh, Saudi Arabia for funding this work through Research Supporting Project Number (RSP2024R131).
Declaration of competing interest
The authors indicated no potential conflicts of interest.
References
- Conception, Synthesis and In silico Assessment of New Morpholine-Containing Compounds Against Human Legumain and Cholinesterase Enzymes. Chemistry Africa. 2024;7(2):605-619.
- [Google Scholar]
- Automated quantification of EEG spikes and spike clusters as a new read out in Theiler's virus mouse model of encephalitis-induced epilepsy. Epilepsy Behav.. 2018;88:189-204.
- [Google Scholar]
- New antiepileptic agents: structure–activity relationships. Med. Chem. Res.. 2012;21:1491-1508.
- [Google Scholar]
- Unexpected Rearrangement of 2-Bromoaniline under Biphasic Alkylation Conditions. Synlett: Accounts Rapid Communications in Synthetic Organic Chemistry. 2017;28(20):28-91.
- [Google Scholar]
- Pharmacological characterization of the 6 Hz psychomotor seizure model of partial epilepsy. Epilepsy Res.. 2001;47(3):217-227.
- [Google Scholar]
- The global cost of epilepsy: A systematic review and extrapolation. Epilepsia. 2022;63(4):892-903.
- [Google Scholar]
- Calderon, O. (2018). “Santiva∼ nez-Acosta R, Pari-Olarte B, Enciso-Roca E, Montes VMC, Acevedo JLA.” J Anticonvulsant effect of ethanolic extract of Cyperus articulatus L. leaves on pentylenetetrazol induced seizure in mice. J Tradit Complement Med 8: 95-99.
- Castillo, J. C., J. Orrego‐Hernández and J. J. E. J. o. O. C. Portilla (2016). “Cs2CO3‐Promoted Direct N‐Alkylation: Highly Chemoselective Synthesis of N‐Alkylated Benzylamines and Anilines.” 2016(22): 3824-3835.
- Synthesis of new 2-arylbenzo [b] furan derivatives via palladium-catalyzed Suzuki cross-coupling reactions in aqueous media. Molecules. 2018;23(10):24-50.
- [Google Scholar]
- Structure of a fucose transporter in an outward-open conformation. Nature. 2010;467(7316):734-738.
- [Google Scholar]
- Review of machine and deep learning techniques in epileptic seizure detection using physiological signals and sentiment analysis. ACM Transactions on Asian Low-Resource Language Information Processing. 2024;23(1):1-29.
- [Google Scholar]
- From oxiranes to oligomers: Architectures of US FDA approved pharmaceuticals containing oxygen heterocycles. J. Med. Chem.. 2018;61(24):10996-11020.
- [Google Scholar]
- The pharmacoresistant epilepsy: an overview on existant and new emerging therapies. Front. Neurol.. 2021;12:674.
- [Google Scholar]
- Glide: a new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J. Med. Chem.. 2004;47(7):1739-1749.
- [Google Scholar]
- Synthesis, anticonvulsant and antinociceptive activity of new hybrid compounds: derivatives of 3-(3-methylthiophen-2-yl)-pyrrolidine-2, 5-dione. Int. J. Mol. Sci.. 2020;21(16):50-57.
- [Google Scholar]
- Structure and mechanism of the glycerol-3-phosphate transporter from Escherichia coli. Science. 2003;301(5633):616-620.
- [Google Scholar]
- Mechanochemical synthesis and anticonvulsant activity of 3-aminopyrrolidine-2, 5-dione derivatives. Biomed. Pharmacother.. 2023;168:115749
- [Google Scholar]
- A computational approach to identify natural putative inhibitors to combat monkeypox. Nanotechnology and In Silico Tools: Elsevier; 2024. p. :285-308.
- Refractory epilepsy: a progressive, intractable but preventable condition? Seizure. 2002;11(2):77-84.
- [Google Scholar]
- The World Health Organization Intersectoral Global Action Plan on Epilepsy and Other Neurological Disorders and the headache revolution: from headache burden to a global action plan for headache disorders. J. Headache Pain. 2024;25(1):1-16.
- [Google Scholar]
- Drug resistance in epilepsy: clinical impact, potential mechanisms, and new innovative treatment options. Pharmacol. Rev.. 2020;72(3):606-638.
- [Google Scholar]
- Lynch, B. A., N. Lambeng, K. Nocka, P. Kensel-Hammes, S. M. Bajjalieh, A. Matagne and B. Fuks (2004). “The synaptic vesicle protein SV2A is the binding site for the antiepileptic drug levetiracetam.” Proceedings of the National Academy of Sciences 101(26): 9861-9866.
- Synthesis, characterization, antioxidant, antidiabetic, anticholinergic, and antiepileptic properties of novel N-substituted tetrahydropyrimidines based on phenylthiourea. J. Biochem. Mol. Toxicol.. 2018;32(12):e22221.
- [Google Scholar]
- Synthesis of 1-(4-Bromobenzoyl)-1, 3-dicyclohexylurea and Its Arylation via Readily Available Palladium Catalyst─ Their Electronic, Spectroscopic, and Nonlinear Optical Studies via a Computational Approach. ACS Omega. 2023;8(33):30306-30314.
- [Google Scholar]
- 2-[(3-Aminoalkyl-(alkaryl-, aryl-))-1 H-1, 2, 4-triazol-5-yl] anilines: synthesis and anticonvulsant activity. Turk. J. Chem.. 2020;44(3):746-755.
- [Google Scholar]
- Structure activity relationships of novel antiepileptic drugs. Curr. Med. Chem.. 2014;21(6):722-754.
- [Google Scholar]
- Palladium-catalyzed cross-coupling reactions of organoboron compounds. Chem. Rev.. 1995;95(7):2457-2483.
- [Google Scholar]
- Effect of sildenafil, a selective phosphodiesterase 5 inhibitor, on the anticonvulsant action of some antiepileptic drugs in the mouse 6-Hz psychomotor seizure model. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2013;47:104-110.
- [Google Scholar]
- Synthesis, anticonvulsant activity and 5-HT1A/5-HT7 receptors affinity of 1-[(4-arylpiperazin-1-yl)-propyl]-succinimides. Pharmacol. Rep.. 2012;64(2):326-335.
- [Google Scholar]
- The pharmacological treatment of epilepsy: recent advances and future perspectives. Acta Epileptologica. 2021;3(1):1-22.
- [Google Scholar]
- Design, synthesis and antiepileptic properties of novel 1-(substituted benzylidene)-3-(1-(morpholino/piperidino methyl)-2, 3-dioxoindolin-5-yl) urea derivatives. Eur. J. Med. Chem.. 2011;46(12):6057-6065.
- [Google Scholar]
- Anticonvulsant Activity of Novel 1-(Substituted Benzylidene)-4-(1-(Morpholino/Piperidino Methyl)-2, 3-Dioxoindolin-5-yl) Semicarbazide Derivatives in Mice and Rats Acute Seizure Models. Chem. Biol. Drug Des.. 2012;80(4):524-532.
- [Google Scholar]
- Solvent effect on palladium-catalyzed cross-coupling reactions and implications on the active catalytic species. Angew. Chem. Int. Ed.. 2011;50(35):8192-8195.
- [Google Scholar]
- A facile synthesis of 1, 3, 4-oxadiazole-based carbamothioate molecules: Antiseizure potential, EEG evaluation and in-silico docking studies. Arab. J. Chem.. 2023;16(4):1-14.
- [Google Scholar]
- Combination of levetiracetam with sodium selenite prevents pentylenetetrazole-induced kindling and behavioral comorbidities in rats. Saudi Pharmaceutical Journal. 2022;30(5):494-507.
- [Google Scholar]
- The medicinal chemist’s toolbox: an analysis of reactions used in the pursuit of drug candidates. J. Med. Chem.. 2011;54(10):3451-3479.
- [Google Scholar]
- Synthesis and evaluation of anticonvulsant properties of new N-Mannich bases derived from pyrrolidine-2, 5-dione and its 3-methyl-, 3-isopropyl, and 3-benzhydryl analogs. Bioorg. Med. Chem. Lett.. 2017;27(6):1412-1415.
- [Google Scholar]
- Structural determinants for high-affinity zolpidem binding to GABA-A receptors. Mol. Pharmacol.. 2007;71(1):38-46.
- [Google Scholar]
- Anticonvulsant activity of novel 1-(morpholinomethyl)-3-substituted isatin derivatives. Bulletin of Faculty of Pharmacy, Cairo University. 2014;52(1):115-124.
- [Google Scholar]
- Pharmacoresistant epilepsy: a current update on non-conventional pharmacological and non-pharmacological interventions. Journal of Epilepsy Research. 2015;5(1):1-8.
- [Google Scholar]
- Biaryl synthesis via C-H bond activation: strategies and methods. Adv. Organomet. Chem., Elsevier.. 2017;67:299-399.
- [Google Scholar]
- Cryo-EM structures reveal native GABAA receptor assemblies and pharmacology. Nature. 2023;622(7981):195-201.
- [Google Scholar]
- Psychiatric comorbidity in epilepsy: a population-based analysis. Epilepsia. 2007;48(12):2336-2344.
- [Google Scholar]
- Synthesis of 3-oxadiazolyl/triazolyl morpholines: Novel scaffolds for drug discovery. Tetrahedron. 2017;73(6):750-757.
- [Google Scholar]
- Deciphering the binding mode of Zolpidem to GABA A α 1 receptor–insights from molecular dynamics simulation. J. Mol. Model.. 2012;18:1345-1354.
- [Google Scholar]
- Prediction of passive blood–brain partitioning: straightforward and effective classification models based on in silico derived physicochemical descriptors. J. Mol. Graph. Model.. 2010;28(8):899-903.
- [Google Scholar]
- In-silico prediction of blood–brain barrier permeability. SAR QSAR Environ. Res.. 2013;24(1):61-74.
- [Google Scholar]
- Idiosyncratic adverse reactions to antiepileptic drugs. Epilepsia. 2007;48(7):1223-1244.
- [Google Scholar]
- Development of tricyclic N-benzyl-4-hydroxybutanamide derivatives as inhibitors of GABA transporters mGAT1-4 with anticonvulsant, antinociceptive, and antidepressant activity. Eur. J. Med. Chem.. 2021;221:113512
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2024.105889.
Appendix A
Supplementary data
The following are the Supplementary data to this article:Supplementary Data 1
Supplementary Data 1







