Translate this page into:
Synthesis of new cadmium(II) complexes of Schiff bases as alkaline phosphatase inhibitors and their antimicrobial activity
⁎Corresponding authors. ashraf.shaheen@uos.edu.pk (Muhammad Ashraf Shaheen), mmojzych@yahoo.com (Mariusz Mojzych)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The present investigation deals with the synthesis, characterization and pharmacological evaluation of four cadmium complexes (1–4) with Schiff bases (1L-4L) derived from the substituted amines and aryl aldehydes. Amantadine is reacted with different aryl aldehydes (salicylaldehyde, 3-ethoxysalicylaldehyde and 4-(diethylamino) salicylaldehyde) to prepare Schiff base ligands 1L-3L, while 4L was prepared using 4-methylaniline. The complexes were characterized by elemental analysis, spectroscopic techniques and thermal analysis, and the structure of one of the ligands, 3L was determined by X-ray crystallography. The spectroscopic data indicate that the ligands coordinate with Cd(II) in a bidentate fashion to give octahedral geometry. Single crystal XRD analysis shows that 3L is orthorhombic with the space group P212121. The ligands and their Cd(II) complexes were evaluated for antimicrobial activities, while the complexes were also investigated for inhibition effects towards an enzyme, alkaline phosphatase. The anti-microbial screening results showed that cadmium complex 1 exhibited significant antibacterial potential, particularly against Staphylococcus aureus with respect to the corresponding ligand suggesting that the complexes are more effective than their ligands for antimicrobial activity. In case of alkaline phosphatase inhibition screening, the synthesized cadmium complex 1 was found to maximally inhibit the activity of alkaline phosphatase in comparison to rest of three complexes.
Keywords
Schiff bases
Cadmium complexes
Alkaline phosphatase inhibition
Antimicrobial studies
1 Introduction
The Since the discovery of Schiff bases by Hugo Schiff 1864 (Schiff, 1864; Tidwell, 2008); these compounds have been exploited extensively due to their easy preparation, versatile ligation modes, stereogenicity, chirality, thermal stability, medicinal utility, solubility in common solvents and ability to stabilize different oxidation states of metals (Collinson and Thielemans, 2010; Nath et al., 1997; Siddiqui et al., 2006). Schiff bases have been employed as models for biological systems (Corden and Wallbridge, 1999) and for tuning the stereochemistry of hexa-coordinated transition metal complexes, which are expected to be potential catalysts for alkene polymerization (Nozaki et al., 1968). For example, Ryoji Noyori has been awarded a share of the 2001 Nobel Prize in chemistry for developing a copper-II Schiff base complexes for the metal-carbenoid cyclopropanation of styrene (Vigato and Tamburini, 2004). Metal complexes of Schiff bases have been found to be potential antimicrobial, antifungal, antitumor, antimalarial, antiviral, and anti-Alzheimer agents (Jones et al., 1979; Kenche and Barnham, 2011). A large number of Schiff bases and their complexes have been studied for their interesting and important properties such as their ability to reversibly bind oxygen, catalytic activity in hydrogenation of olefins, transfer of an amino group and photo-chromic properties (Henrici-Olivé and Olivé, 2012; Dugas and Penney, 2013; Margerum and Miller, 1971; Zuo et al., 2013; Uddin et al., 2020; Abu-Dief and Mohamed, 2015; Miroslaw, 2020; Alagarraj and Natarajan, 2020; Shazia, 2020).
Schiff bases are versatile ligands and ligate via azomethine nitrogen atom with a variety of metals such as Ni, Cu, Co, Pb, Bi, Zn, Cd and many other forming metal complexes (Cozzi, 2004; Gupta et al., 2008). Amantadine is a clinically safe compound for humans and has been reported for the treatment of several diseases i.e., Influenza, fitness impairment and Parkinson’s disease (Deyde et al., 2007; Vollum et al., 1971). Several Schiff bases of amantadine and their complexes have been reported for biological applications like anti-microbial and inhibitors for carbonic anhydrases II and Schiff bases of amantadine with salicylaldehyde, 5-chlorosalicylaldehyde and O-vanillin exhibited anti-viral potential. Furthermore, water soluble synthesized polymers of amantadine have also been reported having antiviral activity (Holt, 1987).
Although cadmium(II) is an environmental pollutant and human carcinogen, which inhibits RNA polymerase activity in vivo and reacts readily with proteins and other biological molecules but several stable cadmium complexes with different ligands have been reported (Anacona and Rodriguez, 2005; Drew et al., 1979; Guha et al., 2011; Hakimi, 2013; Naik et al., 2002; Nelson et al., 1977; Saghatforoush et al., 2008; Sultana et al., 2005; Jimmy et al., 2008). It has been revealed that organo-Cd complexes are potent inhibitor of proteasomal chymotrypsin-like (CT-like) activity and are capable of inducing apoptosis in a cancer cell-specific manner. Cd(II) complexes with tridentate Schiff base having HNNS donor sequence have been found to exhibit cytotoxicity with CD50 value of 2.30 μg mL−1 against the T-lymphoblastic leukemic cells (Tarafder et al., 2001, 2000). The Cd(II) complexes were also effective against colon cancer cells with CD50 values of 3.10 μg mL−1. These compounds demonstrated a higher antioxidant activity than the α-tocopherol (vitamin E) and was comparable with butylated hydroxytoluene (BHT), a commercially used synthetic antioxidant (Tarafder et al., 2001). Furthermore, Cd(II) ions showed stronger inhibitory effect for alkaline phosphatase (ALP) than other heavy metals, such as Hg(II), Cu(II), Ni(II), Zn(II), Mn(II) and Pb(II) (Suzuki et al., 1989). Several studies have been reported, which describe the inhibition of ALP using Cd ions in the presence of other metals and additives (Renella et al., 2003; Tan et al., 2018).
Inspired by the interesting biological activities of cadmium complexes, herein we report the synthesis, characterization, antimicrobial properties and ALP inhibition study of four cadmium(II) complexes (1–4) of Schiff bases derived from amantadine or 4-methylaniline and salicylaldehyde or its derivatives. The structure of one of the ligands, 3L is also presented, while those of other ligands are known in the literature (Fernández-G et al., 2001; Shaheen et al., 2012a, 2012b).
2 Experimental
2.1 Materials and measurements
All the solvents and reagents used i.e., Ethanol, Methanol, n-Hexane, Ethyl acetate, Magnesium sulfate, amantadine, salicylaldehyde, 3-ethoxysalicylaldehyde, 4-(diethylamino)salicylaldehyde, 4-methylaniline and cadmium chloride were of analytical grade and purchased from Sigma-Aldrich, Merck and Alpha Aesar Companies. These were used without further purification. The solid-state IR spectra of the ligands and their complexes were measured on Prestige-21, Shimadzu IR Spectrophotometer. NMR spectra were recorded on Bruker 300 MHz UltraShield NMR spectrometer.
2.2 Preparation of ligands
2.2.1 N-adamantylsalicylimine (1L)
The ligand 1L was prepared according to a published procedure with little modification (Fernández-G et al., 2001). To 15 mL of ethanolic solution of amantadine (2 mmol, 0.302 g) was added to equimolar solution of salicylaldehyde (2 mmol, 0.194 mL) drop wise and the reaction mixture was refluxed for 3 h. The resulting solution was concentrated in rotary evaporator and allowed to stand overnight and filtered. Fine fluorescent yellow crystals of 1L were washed with ice cold ethanol and dried. Yield: 75%. M.P.: 91 °C. 1H NMR (300 MHz, CD3OH): δ = 12.74 (s, 1H), 8.54 (s, 1H), 7.34 (t, 2H), 6.75 (m, 2H), 2.16 (s, 6H), 1.96 (d, 3H), 1.79 (d, 6H) ppm. Elemental analysis (%): Calculated: C: 79.96, H: 8.29, N: 5.49; Found: C: 79.48, H: 8.03, N: 5.31.
2.2.2 N-adamantyl(3-ethoxy)salicylimine (2L)
The equimolar ethanolic (15 mL) solutions of 3-ethoxysalicylaldehyde (2 mmol, 0.332 g) and amantadine (2 mmol, 0.302 g) were added to round bottom flask and refluxed for 3 h. The resultant solution was evaporated to one-third of original. The yellow crystals of the Schiff base were obtained after 36 h. The crystals were washed with cold ethanol and dried in desiccator. Yield: 80%. M.P.: 82 °C. 1H NMR (300 MHz, CD3OH): δ = 13.12 (s, 1H), 8.54 (s, 1H), 6.96 (d, 2H), 6.71 (t, 1H), 4.46 (dd, 2H), 2.25 (s, 3H), 1.96 (s, 6H), 1.79 (m, 6H), 1.77 (t, 3H) ppm. Elemental analysis (%): Calculated: C: 76.22, H: 8.42, N: 4.68; Found: C: 75.98, H: 8.35, N: 4.51.
2.2.3 N-adamantyl(4-diethylamino)salicylimine (3L)
To 15 mL hot ethanolic solution of 4-(diethylamino)salicylaldehyde (2 mmol, 0.386 g,) was added ethanolic (15 mL) solution of amantadine (2 mmol, 0.302 g) and the reaction mixture was refluxed for 4 h. The resultant solution was concentrated and left for crystallization. Rod like red crystals of N-adamantyl(4-diethylamino)salicylimine were obtained after 48 h. Crystals were washed with cold ethanol and dried. Yield: 75%. M.P.: 147 °C. 1H NMR (300 MHz, CD3OH): δ = 12.96 (s, 1H), 7.94 (s, 1H), 7.11 (d, 1H), 6.15 (d, 1H), 5.92 (s, 1H), 3.51 (m, 4H), 2.21 (s, 3H), 1.85 (d, 6H), 1.66 (m, 6H), 1.23 (t, 6H) ppm. Elemental analysis (%): Calculated: C: 77.26, H: 9.26, N: 8.58; Found: C: 77.56, H: 9.13, N 8.47. CCDC NUMBER 989105.
2.2.4 3-ethoxy-6-{(E)-[(4-methylphenyl)imino]-methyl}phenol (4L)
To 15 mL ethanolic solution of 4-methylaniline (2 mmol, 0.214 g) was added drop wise to ethanolic solution (15 mL) of 3-ethoxysalicylaldehyde (2 mmol, 0.332 g). Resultant solution was refluxed for 1 h. The needle like crystals was obtained on cooling (Shaheen et al., 2012). The crystals were washed with cold ethanol and dried in air. Yield: 85%. M.P.: 80 °C. 1H NMR (300 MHz, CD3OH): δ = 12.54 (s, 1H), 8.69 (s, 1H), 7.78 (s, 1H), 7.35 (s, 4H), 7.12 (dd, 1H), 6.75 (t, 1H), 4.21 (m, 2H), 3.34 (s, 3H), 1.51 (t, 3H) ppm. Elemental analysis (%): Calculated: C: 75.27, H: 6.71, N: 5.49; Found: C: 75.43, H: 6.45, N: 5.31.
2.3 Synthesis of Cd(II) complexes
For preparation of complexes, 15 mL ethanolic solution of cadmium chloride (1 mmol, 0.183 g) was added drop wise to 2 mmoles of the ligands (1L-4L) in 15 mL ethanol. The reaction mixture was refluxed for 2 h. The resulting yellow precipitates of the complexes were filtered, washed with ice cold ethanol and dried under vacuum (Mriganka et al., 2017).
2.4 Analytical data of complexes
Complex 1:
Yield: 65%. M.P.: 285 °C; 1H NMR (300 MHz, CD3OD): δ = 8.44 (s, 2H), 7.32 (t, 4H), 6.77 (m, 4H), 2.1 (s, 12H), 1.94 (d, 6H), 1.85 (d, 12H) ppm. Elemental analysis (%): Calculated: C: 65.75, H: 6.49, N: 4.51; Found: C: 64.98, H: 6.07, N: 5.11. Conductivity: 2.02 µS.
Complex 2:
Yield: 70%. M.P.: >300 °C; 1H NMR (300 MHz, CD3OD): δ = 8.44 (s, 2H), 6.93 (d, 4H), 6.65 (t, 2H), 4.41 (dd, 4H), 2.20 (s, 6H), 1.98 (s, 12H), 1.74 (m, 12H), 1.74 (t, 6H) ppm. Elemental analysis (%): Calculated: C: 64.35, H: 6.82, N: 3.95; Found: C: 64.48, H: 6.35, N: 4.21. Conductivity: 4.35 µS.
Complex 3:
Yield: 67%. M.P.: 270 °C. 1H NMR (300 MHz, CD3OD): δ = 7.96 (s, 2H), 7.07 (d, 2H), 6.19 (d, 2H), 5.96 (s, 2H), 3.44 (m, 8H), 2.17 (s, 6H), 1.95 (d, 12H), 1.76 (m, 12H), 1.20 (t, 12H) ppm. Elemental analysis (%): Calculated: C: 66.08, H: 7.66, N: 7.34; Found: C: 65.84, H: 7.25, N: 7.67. Conductivity: 3.73 µS.
Complex 4:
Yield: 75%. M.P.: >300 °C; 1H NMR (300 MHz, CD3OD): δ = 8.79 (s, 2H), 7.88 (s, 2H), 7.25 (s, 8H), 7.07 (dd, 2H), 6.85 (t, 2H), 4.16 (m, 4H), 3.30 (s, 6H), 1.45 (t, 6H) ppm. Elemental analysis (%): Calculated: C: 61.89, H: 5.19, N: 4.51; Found: C: 61.43, H: 4.95, N: 5.01. Conductivity: 2.76 µS.
The structures of ligands and the complexes are shown in Scheme 1.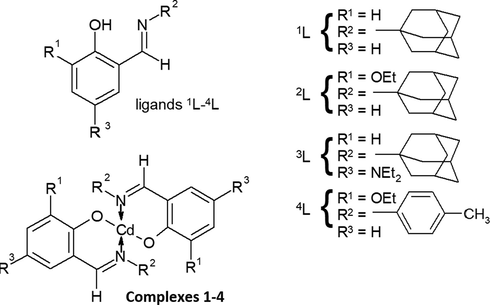
Structures of ligands 1L to 4L and complexes 1–4.
2.5 Thermal studies
Thermogravimetric studies of compounds were carried out in dynamic air atmosphere using SDT Q600 Thermo gravimetric analyzer. Approximately 2 mg of samples were heated with the heating rate of 50 °C per minute using platinum crucibles in temperature from ambient to 800 °C.
2.6 Conductivity measurements
Conductance values of complexes were noted at room temperature in DMSO using Conductivity meter SDT-600. Conductivity meter was calibrated with the solutions of standard potassium chloride electrolytes.
2.7 X-ray structure determination of 3L
The absolute ethanol was used for recrystallization of Schiff bases. Fine crystals of Schiff base 3L were selected for single crystal studies. The single crystal X-ray diffraction data of 3L was measured on Bruker Kappa Apex-II diffractometer using SAINT (Bruker) for cell refinement and data reduction. The SHELXS97 program (Sheldrick, 2008) was used to solve the structure.
2.8 Antimicrobial studies
Antibacterial activities of the ligands and their complexes were determined by reported methods (Carron et al., 1987). The bacteria strains used include: Escherichia coli, Bacillus subtilis, Shigella flexeneri, Staphylococcus aureus, Pseudomonas aeruginosa and Salmonella typhi. The 0.1 mg/mL of sample in DMSO was used for well of 6 mm diameter. 10 µg amikacin per disc was used as a standard drug.
2.9 Alkaline phosphatase (ALP) inhibition assay protocol
For the preparation of assay same method was used as reported earlier with slight modifications (Chaudhry et al., 1983; Hu et al., 2017). Working substrate was made by mixing the four parts of reagent A (diethanolamine pH 10.2, 1.4 mol/L and magnesium chloride 0.5 mmol/L) and one part of reagent B (p-nitrophenyl phosphate 50 mmol/L). Substrate was incubated for five minutes at 25 °C. In a cell cuvette 500 µL of the substrate and 10 µL of human serum were taken. After incubation of 1-minute absorbance was measured to check the activity of enzyme (ΔA/min) × 2750, which was found to be 172 U/I. ALP hydrolysed the p-NPP and yellow colored p-nitrophenol was produced that absorbs at 405 nm. where Pi is inorganic phosphate.
Then various amounts of cadmium(II) complexes were added periodically from the 12.5 mmolar stock solution and again incubated for 3 min. Change in absorbance was recorded again after 1, 2, 3, 4 and 5 min thereafter. At the end their average was taken and % age inhibition was calculated.
3 Results and discussions
3.1 Synthesis of Schiff base ligands and complexes
The Schiff base ligands, 1L, 2L and 3L were prepared by condensation of amantadine with aryl aldehydes i.e., salicylaldehyde, 3-ethoxysalicylaldehyde and 4-(diethylamino)salicylaldehyde. The ligand 4L was prepared by reacting 4-methylaniline with 3-ethoxysalicylaldehyde as represented in Scheme 2. The physical properties such as colors, melting points are given in Table S1 (Supplementary Information).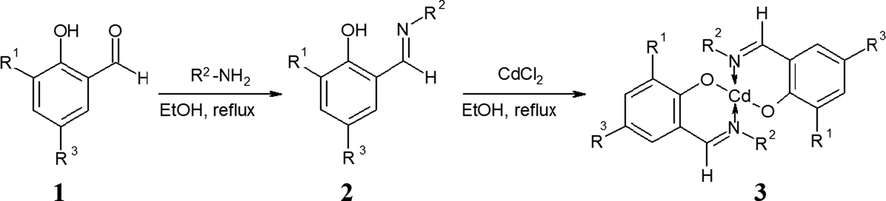
Synthesis of ligands 2 and their complexes 3 from precursor aryl aldehydes 1 upon treatment with amantadine. R1, R2 and R3 have been represented in Scheme 1.
3.2 Spectroscopic studies
3.2.1 FT-IR
The Infra-red spectra of compounds were recorded in the range of 4000–400 cm−1. In these spectra the absorptions for important functional groups like N—C, C⚌C, C—O, —OH and HC⚌N were observed and are listed in Table S2 (Supplementary Information). The absorption peaks were attributed to different functional groups with the help of the literature on IR spectroscopy. The peak characteristic of azomethine group (HC⚌N) of Schiff bases was observed in region of 1588–1691 cm−1. In complexes, this absorption shifted towards lower frequencies due to coordination through nitrogen atom. Stretching vibrations in the region of 1446–1463 cm−1 were attributed to the unsaturation of aromatic ring, C⚌C. Sharp bands were observed in the region of 2904–2910 cm-1 due to C—H stretching. Phenolic C—O functional groups absorb at 1205–1255 cm−1, while in complexes it shifted to 1190–1238 cm−1, which indicates the binding of phenolic oxygen to the metal atom. Coordination of ligands to metal is further evidenced by absence of OH peaks in the spectra of complexes, which appear in the range of 3380–3465 cm−1 for ligands.
3.2.2 NMR
The detailed 1H NMR data of the ligands and the complexes (1–4) is given in experimental section. The spectra of complexes show that the signal of phenolic proton is absent (present in ligand- spectra) confirming the binding of ligands to the metal atom. Moreover, small shifts of 0.2–0.3 ppm in peak positions of protons, as compared to ligands further suggest the formation of complexes. In case of N-adamantylsalicylimine-Cd(II), the single resonance of azomethine proton was observed at 8.44 ppm. Multiple peaks were observed in the region of 6.67–7.32 ppm, which are attributed to the protons of aromatic ring. Three protons of amantadine gave signals at 2.20 ppm and the remaining protons in the range of 1.74–1.99 ppm. For complex 1, a triplet was observed at 1.39–1.44 ppm, which is assigned to CH3 of ethoxy substituent of salicylaldehyde. The CH2 protons give quartet at 4.07–4.14 ppm. The peak at 8.44 ppm was attributed to the HC⚌N proton. The protons on phenolic ring give peaks in the range of 6.51–6.93 ppm. Complex 2 contains a Schiff base of amantadine and 4-diethylaminosalicylaldehyde. A doublet of single proton was observed at 7.04–7.07 ppm and attributed to the proton of benzene ring. A quartet of four protons of diethyl group was observed at 3.37–3.44 ppm and a quintet of the same substituent was observed at 1.15–1.20 ppm. Three protons of amantadine give singlet at 2.17 ppm. A singlet at 7.96 ppm was attributed to the azomethine proton. In case of 3, the singlet of azomethine proton was observed at 8.79 ppm. The CH3 protons of 4-methylaniline of 4L show a triplet at 1.06–1.45 ppm. A quartet is observed at 4.09–4.16 ppm and attributed to the two protons of aniline. A doublet of doublets is observed for the protons of benzene ring of 3-ethoxysalicylaldehyde.
3.3 Thermal analysis
Thermal properties of the compounds were studied by TGA. All complexes show two stages of weight loss for ligands 1L, 2L, 3L and 4L. Table 1 shows the details of different stages of decomposition of the complexes. The weight loss was due to loss of metal oxide (CdO) residue upon heating.
Complexes
Temp. range °C
% Weight loss
Residue
1
284–342
25.34
CdO
(22.21%)
579–746
40.70
2
268–336
28.82
CdO
(18.70%)
336–541
18.28
541–679
27.84
3
275–328
17.89
CdO
(18.01%)
328–591
15.14
591–835
45.20
4
235–271
34.66
CdO
(22.15%)
271–357
17.27
357–576
8.62
576–775
10.23
3.4 Crystal structure of 3L
The crystallographic data of compound 3L is given in Table 2. The molecular structure of 3L including the atom-numbering scheme is depicted in Fig. 1. The Compound crystallized in the solid state into the phenol-imine tautomeric form. In this compound the N1⚌C7 bond distance of 1.2791 Å is in agreement with the imine group. Furthermore, the ranges of this bond length and that between the C6 atom of the aromatic ring and the C7 atom of the imine group correspond to conjugated C⚌N group. The range of C-O bond length corresponds to the value described for the C-O bond in phenols (∼1.36 Å). The group A (O1/C1/C2…/C7/N1) is planar with r. m. s. deviation of 0.006 Å. The dihedral angle between B (N2/C8/C9) and C (N2/C10/C11) is 63.24 (18)°. There exist an intramolecular H-bond between O1----H1…N1 forming an S(6) loop. The molecules are essentially stabilized due to van der Waals forces (Table 2).
Compound
3L
Chemical Formula
C21H30N2O
Formula weight
326.47
T (K)
296(2)
Wavelength (Å) Mo Kα
0.71073
Crystal system
Orthorhombic
Space group
P212121
a (Å)
6.8045(4)
b (Å)
7.6589(5)
c (Å)
34.546(2)
V (Å3)
1800.36(19)
Z
4
Absorption coefficient (mm−1)
1.204
F(000)
712
Crystal size (mm)
0.35 × 0.28 × 0.25
θ-range for data calculation (°)
2.687 to 26.00
Reflections collected
8068
Independent reflections
3241
Reflections ≥ 2σ(I)
2974
Goodness-of-fit on F2
1.050
Final R indices
[I > 2σ(I)]R1 = 0.0328
wR2 = 0.0784
R indices (all data)
R1 = 0.0364
wR2 = 0.0877
Data/restrains/parameters
3241/0/222
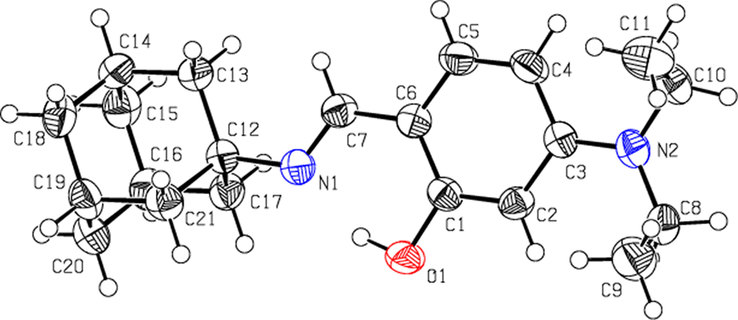
Molecular structure of N-adamantyl(4-diethylamino)salicylimine (3L).
3.5 Antimicrobial study
All the ligands were found to be mild active against the various strains of bacteria. The most significant activity was exhibited by complex 1 against Staphylococcus aureus, while other complexes did not show any significant activity. The positive control was carried out using amikacin as standard drug against antimicrobial analysis (Patil and Pawar, 2019; Rakshani et al., 2019). The results are summarized in Table 3. Our reported metal complexes exhibited better anti-bacterial activity against tested strain when amikacin was used as standard drug in comparison to the recent reported antibacterial organic smart molecular metal complexes (Mahmoud et al., 2015; Mosmann, 1983).
Samples
E. coli
B. subtilis
S. flexeneri
S. aureus
P. aeruginosa
S. typhi
Standard
25
50
28
48
23
28
1L
18 ± 0.31
32 ± 0.25
21 ± 0.25
30 ± 0.03
17 ± 0.03
20 ± 0.17
2L
17 ± 0.31
30 ± 0.12
18 ± 0.25
27 ± 0.03
14 ± 0.03
17 ± 0.15
3L
20 ± 0.31
34 ± 0.25
22 ± 0.20
31 ± 0.04
17 ± 0.03
21 ± 0.11
4L
14 ± 0.31
31 ± 0.12
17 ± 0.20
27 ± 0.03
13 ± 0.05
16 ± 0.12
1
30 ± 0.31
56 ± 0.12
30 ± 0.20
52 ± 0.02
28 ± 0.02
31 ± 0.10
2
23 ± 0.31
42 ± 0.12
25 ± 0.25
42 ± 0.03
18 ± 0.03
22 ± 0.17
3
27 ± 0.31
46 ± 0.25
27 ± 0.25
48 ± 0.04
21 ± 0.03
26 ± 0.11
4
24 ± 0.31
45 ± 0.12
26 ± 0.25
46 ± 0.03
20 ± 0.03
25 ± 0.10
3.6 Alkaline phosphatase inhibition activity
Cadmium metal was found to be an inhibitor of ALP (Suzuki et al., 1989). The inhibition might be due to the blockage of the active sites of the enzyme by it. Enzyme was inhibited in the concentration-dependent manner.
Fig. 2 shows the ALP inhibition data of Cd(II) complexes 1–4. The ligands itself was inactive against ALP and percentage inhibition for it is zero at all concentrations. Among the complexes 1 showed maximum inhibition of ALP. The results revealed that inhibition of ALP by the synthesized complexes is concentration dependent. An increase in concentration of complexes decreased enzymes activity and there was total loss of activity at 0.247 mM concentration.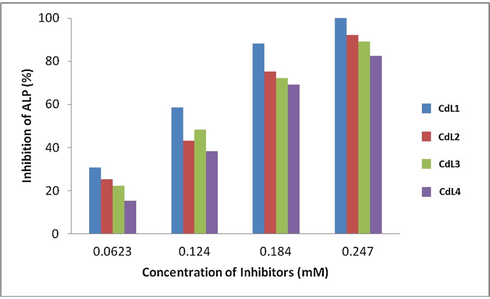
Concentration dependent inhibition of alkaline phosphatase (ALP) by cadmium complexes.
4 Conclusions
In summary, herein we have synthesized Schiff base ligands and their cadmium(II) complexes. Characterization of the synthesized materials were performed by FT-IR analysis, NMR spectroscopic analysis, elemental analysis, thermal analysis and conductivity measurements. For better insight toward the ligand structure, the fine crystal of ligand was grown and analyzed for Single crystal XRD analysis. In order to investigate the bioprofile of the synthesized material, the antimicrobial activities of ligands and their complexes were performed using various bacterial strains and the results showed better anti-microbial potential of the cadmium complexes in comparison of the ligands. In case of enzymatic inhibition analysis, the synthesized compounds were observed to exhibit appreciable response toward alkaline phosphatase inhibition.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- A review on versatile applications of transition metal complexes incorporating Schiff bases. Beni Suef Univ. J. Basic Appl. Sci.. 2015;4:119.
- [Google Scholar]
- Biological response of Schiff base metal complexes incorporating amino acids - a short review. J. Coord. Chem.. 2020;73:2095.
- [Google Scholar]
- Synthesis and antibacterial activity of ceftriaxone metal complexes. Transit. Met. Chem.. 2005;30:897-901.
- [Google Scholar]
- Antimicrobial properties of some extracts obtained from some Mediterranean plants of medicinal value. Plantes Med. Phyther.. 1987;21:195-202.
- [Google Scholar]
- A kinetic study of rat salivary gland alkaline phosphatase and its inhibition by cadmium. Arch. Oral Biol.. 1983;28:741-744.
- [Google Scholar]
- The catalytic oxidation of biomass to new materials focusing on starch, cellulose and lignin. Coord. Chem. Rev.. 2010;254:1854-1870.
- [Google Scholar]
- Stereochemical control of cis-and trans-TiCl 2 groups in six-coordinate complexes [(L) TiCl 2](L 2–= N 2 O 2-donor Schiff base) and reactions with trimethylaluminium to form cationic aluminium species. Chem. Commun. 1999:323-324.
- [Google Scholar]
- Metal-Salen Schiff base complexes in catalysis: practical aspects. Chem. Soc. Rev.. 2004;33:410-421.
- [Google Scholar]
- Surveillance of resistance to adamantanes among influenza A (H3N2) and A (H1N1) viruses isolated worldwide. J. Infect. Dis.. 2007;196:249-257.
- [Google Scholar]
- Pentagonal-pyramidal cadmium (II) and mercury (II) complexes of the quinquedentate macrocyclic ligand 2, 15-dimethyl-3, 7, 10, 14, 20-penta-azabicyclo [1431] eicosa-1 (20), 2, 14, 16, 18-pentaene. J. Chem. Soc. Dalt. Trans. 1979:575-581.
- [Google Scholar]
- Bioorganic Chemistry: A Chemical Approach to Enzyme Action. Springer Science & Business Media; 2013.
- The structures of some ortho-hydroxy Schiff base ligands. J. Mol. Struct.. 2001;561:197-207.
- [Google Scholar]
- Fluorescent Au@ Ag core–shell nanoparticles with controlled shell thickness and HgII sensing. Langmuir. 2011;27:13198-13205.
- [Google Scholar]
- Coord. Chem. Rev. 2008
- Structural and spectral characterization of a chromium (III) picolinate complex: introducing a new redox reaction. J. Korean Chem. Soc.. 2013;57:721-725.
- [Google Scholar]
- The Chemistry of the Catalyzed Hydrogenation of Carbon Monoxide. Springer Science & Business Media; 2012.
- Sensitive and selective colorimetric assay of alkaline phosphatase activity with Cu (II)-phenanthroline complex. Talanta. 2017;163:146-152.
- [Google Scholar]
- Bis[1-(1-adamantyliminomethyl)-2-naphtholato-κ2N, O]cobalt(II) Acta Cryst. E. 2008;64:m1223
- [CrossRef] [Google Scholar]
- Synthetic oxygen carriers related to biological systems. Chem. Rev.. 1979;79:139-179.
- [Google Scholar]
- Alzheimer’s disease & metals: therapeutic opportunities. Br. J. Pharmacol.. 2011;163:211-219.
- [Google Scholar]
- Synthesis, spectral characterization, thermal, anticancer and antimicrobial studies of bidentate azo dye metal complexes. J. Therm. Anal. Calorim. 2015
- [CrossRef] [Google Scholar]
- Margerum, J.D., Miller, L.J., 1971. Photochromism, Interscience.
- Homo- and hetero-oligonuclear complexes of platinum group metals (PGM) coordinated by imine Schiff base ligands. Int. J. Mol. Sci.. 2020;21:3493.
- [Google Scholar]
- Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55.
- [Google Scholar]
- Targeted synthesis of cadmium(ii) Schiff base complexes towards corrosion inhibition on mild steel. RSC Adv.. 2017;7:48569.
- [Google Scholar]
- Synthesis, spectral and thermal degradation kinetics of divalent cadmium complexes of dothiepine and diphenhydramine. Turkish J. Chem.. 2002;26:565-572.
- [Google Scholar]
- Synthesis, characteristic spectral studies and in vitro antimicrobial and antitumour activities of organotin (IV) complexes of Schiff bases derived from amino-acids. Appl. Organomet. Chem.. 1997;11:727-736.
- [Google Scholar]
- Crystal structures of four complexes of quinquedentate macrocyclic ligands with novel co-ordination geometries containing five-co-ordinate silver (I), six-co-ordinate cadmium (II), six-co-ordinate mercury (II), and seven-co-ordinate cadmium (II) J. Chem. Soc., Chem. Commun. 1977:167-168.
- [Google Scholar]
- Homogeneous catalysis in the decomposition of diazo compounds by copper chelates: asymmetric carbenoid reactions. Tetrahedron. 1968;24:3655-3669.
- [Google Scholar]
- Patil, R.V., Pawar, K.D., 2019. In planta synthesis of nanomaterials for environmental remediation. In: Plant-Metal Interactions. Springer, pp. 283–307.
- Design and fabrication of novel thiourea coordination compounds as potent inhibitors of bacterial growth. J. Antibiotics. 2019;72:260.
- [Google Scholar]
- Additive effects of copper and zinc on cadmium toxicity on phosphatase activities and ATP content of soil as estimated by the ecological dose (ED50) Soil Biol. Biochem.. 2003;35:1203-1210.
- [Google Scholar]
- Preparation of zinc (II) and cadmium (II) complexes of the tetradentate schiff base ligand 2-((E)-(2-(2-(pyridine-2-yl)-ethylthio) ethylimino) methyl)-4-bromophenol (PytBrsalH) Molecules. 2008;13:804-811.
- [Google Scholar]
- Mittheilungen aus dem Universitätslaboratorium in Pisa: eine neue Reihe organischer Basen. Justus Liebigs Ann. Chem.. 1864;131:118-119.
- [Google Scholar]
- 5-Hydroxy-2-{(E)-[(3-nitrophenyl) iminio] methyl} phenolate. Acta Crystallogr. Sect. E Struct. Reports Online. 2012;68 o2622–o2622
- [Google Scholar]
- 2-[(E)-N-(Adamantan-1-yl) carboximidoyl]-6-ethoxyphenol. Acta Crystallogr. Sect. E Struct. Reports Online. 2012;68 o2588–o2588
- [Google Scholar]
- Recent advances in anticancer ruthenium Schiff base complexes. Appl. Organometallic Chem.. 2020;34:e5687
- [Google Scholar]
- Synthesis and spectroscopic studies of new Schiff bases. Molecules. 2006;11:206-211.
- [Google Scholar]
- Synthesis and antibacterial activity of cephradine metal complexes: part II complexes with cobalt, copper, zinc and cadmium. Pak. J. Pharm. Sci.. 2005;18:36-42.
- [Google Scholar]
- Preventive effects of zinc on cadmium-induced inhibition of alkaline phosphatase activity and mineralization activity in osteoblast-like cells, MC3T3-E1. J. Pharmacobiodyn.. 1989;12:94-99.
- [Google Scholar]
- Soil mineral alters the effect of Cd on the alkaline phosphatase activity. Ecotoxicol. Environ. Saf.. 2018;161:78-84.
- [Google Scholar]
- Coordination chemistry and biological activity of two tridentate ONC and NNS Schiff bases derived from S-benzyldithiocarbazate. Transition Met. Chem.. 2000;25:295.
- [Google Scholar]
- Synthesis and characterization of Zn(II) and Cd(II) complexes of S-benzyl-β-N-(2-pyridyl)methylenedithiocarbazate (HNNS): bioactivity of the HNNS Schiff base and its Zn(II), Cu(II) and Cd(II) complexes and the X-ray structure of the [Zn(NNS)2] complex. Polyhedrone. 2001;20:2363.
- [Google Scholar]
- Synthesis and characterization of Zn (II) and Cd (II) complexes of S-benzyl-β-N-(2-pyridyl) methylenedithiocarbazate (HNNS): bioactivity of the HNNS Schiff base and its Zn (II), Cu (II) and Cd (II) complexes and the X-ray structure of the [Zn (NNS) 2] com. Polyhedron. 2001;20:2363-2370.
- [Google Scholar]
- Hugo (Ugo) Schiff, Schiff bases, and a century of β-lactam synthesis. Angew. Chemie Int. Ed.. 2008;47:1016-1020.
- [Google Scholar]
- Uddin, Mohammad N., Sayeda, S.A., Rahatul Alam, S.M., 2020. REVIEW: Biomedical applications of Schiff base metal complexes. J. Coord. Chem. 73(23), 3109–3149. https://doi.org/10.1080/00958972.2020.1854745.
- The challenge of cyclic and acyclic Schiff bases and related derivatives. Coord. Chem. Rev.. 2004;248:1717-2128.
- [Google Scholar]
- Cellular and computational studies of proteasome inhibition and apoptosis induction in human cancer cells by amino acid Schiff base–copper complexes. J. Inorg. Biochem.. 2013;118:83-93.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2021.103308.
Appendix A
Supplementary material
The following are the Supplementary data to this article:







