Translate this page into:
Synthesis, physicochemical property, and antibacterial activity of novel nonionic 1-alkylaminoglycerol Gemini surfactants
⁎Corresponding author. junb112he@whpu.edu.cn (Junbo He)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Four novel nonionic 1-alkylaminoglycerol Gemini surfactants, represented by m-s-m (m = C8 and C12, s = 3 and 4), were synthesized through a two-step reaction in high yields, and confirmed by 1H NMR, 13C NMR, and HRMS. The synthesized Gemini surfactants exhibited low CMC (1.41–5.25 mmol/L), excellent surface-tension-reducing ability (25.45–26.83 mN/m), and wetting ability on hydrophobic interface. Gemini surfactants in aqueous solutions were in stable nanoscale dispersion, and exhibited strong and long-lasting foaming ability and emulsifying abilities for benzene/water and triacylglyceride/water systems. Especially, 12–4-12 solution displayed excellent tolerance to wide pH range and high salinity. Biological evaluation demonstrated that these Gemini surfactants, except for 8–4-8, showed significant antibacterial activities against Staphylococcus aureus and Escherichia coli at 25 and 50 µg/mL. Hence, these Gemini surfactants have the potential to be used in a varieties of fields as highly efficient agents.
Keywords
1-alkylaminoglycerol
nonionic Gemini surfactant
Physicochemical property
Antibacterial activity
1 Introduction
Surfactants, a group of amphiphilic substances with hydrophilic and lipophilic parts, can dramatically lower the surface or interfacial tension. They serve as emulsifiers, dispersants, foaming agents, wetting agents, solubilizers, disinfectants, and other functions in a variety of industrial products (Sharma et al., 2017). Gemini surfactant, a type of diversified surfactants, has a unique structure that consists of two hydrophilic headgroups and two hydrophobic chains that are covalently linked by a spacer (Fig. 1). This structure is typically represented by m-s-m, where m and s are the carbon numbers of the chain and spacer, respectively (Menger and Littau 1993). In comparison to the related single-chain surfactant, it has improved physicochemical features due to their structure, including lower critical micelle concentration (CMC), stronger solubilization power, wetting, and foaming properties (Menger and Keiper 2000). Due to these special features, Gemini surfactant is regarded as the ‘next generation’ of high-quality surfactants and has attracted a great deal of interest from the scientific community for various applications (Sharma et al., 2017, Ahmady et al., 2022).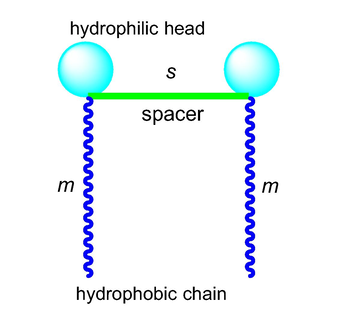
Structure diagram of Gemini surfactant represented by m-s-m.
Gemini surfactant can be classified into three types, cationic, anionic, and nonionic, according to the characteristics of the hydrophilic headgroups (Sharma et al., 2017). Anionic Gemini surfactant carries the sulphonate, carboxylate, and phosphate headgroups, while cationic Gemini surfactant is virtually exclusively composed of quaternary ammonium headgroups. There are relatively fewer reports concerning the synthesis and physicochemical characterization of nonionic Gemini surfactants. The hydrophilic heads of nonionic Gemini surfactants are usually derived from sugars in terms of sustainability and environmental safety. Liu et al. (Liu et al., 2013) reported the facile synthesis of nonionic Gemini alkyl O-glucoside surfactants through the glycosylation of Gemini alkyl glycerol ether with ethylene glycol as the spacer, showing excellent surface activity. Similarly, the reduced glucose-based Gemini surfactants linked through their tertiary amino headgroups were investigated to consider the effects of alkyl chain length and spacer on their properties (Bergsma et al., 2001, Fielden et al., 2001, Johnsson et al., 2003). Due to the protonation of nitrogen caused by the presence of two tertiary amines, this form of surfactants was sensitive to the pH of solution during the vesicle-to-micelle transition. Additionally, nonionic Gemini surfactants of the gluconamide and polyoxyethylene types were reported (Nieh et al., 2004, FitzGerald et al., 2005, Sakai et al., 2008). These nonionic Gemini surfactants all have relatively complex synthetic routes and low yields, hance it is necessary and useful to produce Gemini surfactants with comparable structure and properties but simple synthesis.
Glycerol, which makes up around 10% of the weight of oil during transesterification, is the major and large amount by-product of the biodiesel industry. The use of glycerol as a raw material to increase value-added products is therefore highly explored due to its affordability and renewability in nature (Singh et al., 2009). The single-chain glycerol-based surfactants were reported (Bigot et al., 2011, Kandeel 2012, Moity et al., 2013), but they are uncommon for the Gemini type. Zhang et al. reported the efficient emulsifying properties of two glycerol-based nonionic Gemini surfactants, 1,3-bis(octylamino)propan-2-ol and 1,3-bis(dodecylamino)propan-2-ol (Zhang et al., 2018). Due to the water-soluble and renewable properties of glycerol, structure-diversified glycerol-based surfactants, particularly Gemini surfactants, require additional exploration from both the theoretical and practical viewpoints.
In this work, we synthesized and characterized a class of novel m-s-m nonionic Gemini surfactants (m = 8 and 12, s = 3 and 4) using 1-aminoglycerol as two headgroups (Fig. 2). Their surface property, wettability, foaming property, emulsifying property, water dispersion, pH and salt tolerance, water sorption, and antibacterial activity against Staphylococcus aureus (S. aureus) and Escherichia coli (E. coli) were studied. These Gemini surfactants were discovered to have excellent antibacterial and emulsifying properties.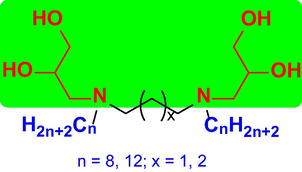
Structural representation of the 1-aminoglycerol-based m-s-m nonionic Gemini surfactants.
2 Materials and methods
2.1 Materials
Glycidol (96%), octylamine (99%), dodecylamine (98%), 1,3-dibromopropane (98%), and 1,4-dibromobutane (98%) were purchased from Shanghai Aladdin Biochemical Technology Co., Ltd. Ethanol, ethyl acetate, and K2CO3 were purchased from Tianjin Cameo Chemical Reagent Co., Ltd. (Tianjin, China). The bacterial strains of S. aureus (BNCC 186335) and E. coli (BNCC 336902) were purchased form Bena Culture Collection (Beijing, China). Milli-Q water was used in the preparation of samples.
2.2 Synthesis of Gemini surfactant
Compounds 1a and 1b were synthesized according to the report (Lamanna et al., 2004). Octylamine or dodecylamine (30 mmol) dissolved in ethanol (10 mL) were added with glycidol (30 mmol) droplet and magnetically stirred at 25℃ and 600 rpm for 3 h. Then, ethanol was removed by Buchi R-100 rotary evaporator (Fisher Scientific, Loughborough, UK) under reduced pressure to give the compounds 1a and 1b as yellow oil and white solid, respectively.
1a or 1b (20 mmol) were further mixed with 1,3-dibromopropane or 1,4-dibromobutane (10 mmol) and reacted at 80℃ and 600 rpm for 4 h, using dry K2CO3 (40 mmol) as base to neutralize the formed HBr. After which, ethyl acetate (50 mL) was added and stirred for 0.5 h to dissolve the obtained surfactants. The final nonionic 1-alkylaminoglycerol Gemini surfactants were obtained by filtration and concentration under reduced pressure to remove ethyl acetate.
2.3 Structural characterization
1H NMR and 13C NMR spectra were recorded on at a 400 MHz in DMSO‑d6 solution on a Varian Mercury Plus 400 spectrometer (Varian, Inc., Palo Alto, CA, USA) and chemical shifts were recorded in parts per million (ppm). High resolution mass spectrometry (HRMS) was performed on a Thermo Scientific Q Exactive mass spectrometer (Thermo Fisher Scientific Inc., Waltham, MA, USA). Fourier transform infrared spectra (FTIR) were performed on a Thermo Nicolet NEXUS 670 FTIR spectrometer (Thermo Nicolet Corporation, Madison, WI).
2.4 Hydrophilic-lipophilic balance (HLB) calculation
HLB numbers of 1-alkylaminoglycerol Gemini surfactants were calculated from the following equation by the Griffin method (Hait and Moulik 2001, Fu et al., 2020).
Where X and T are the molecular mass of the hydrophilic portion and the total molecular mass, respectively.
2.5 Surface property measurement
The surface tensions of Gemini surfactant solutions were measured by the drop volume method using a DSA30 automatic tensiometer (Kruss, Germany). Critical micelle concentration (CMC) was determined by the cross-point of the two lines before and after CMC on the γ-lgC curve. The measurement temperature was controlled at 25℃.
The maximum surface excess concentration (Гmax) is calculated by the following Gibbs equation,
The minimum area occupied per surfactant molecule (Amin) at air/water interface is related to the surface excess Гmax as follows:
2.6 Wettability
According to the literature (Nascimento et al., 2015), the wettability was measured using an optical contact angle meter (DSA30, Kruss, Germany) at 25℃ by determining the contact angles of Gemini surfactant solutions on the paraffin film. Gemini surfactant concentrations were set as 0.1, 0.2, 0.4, 0.6, 0.8, 1.0, 2.0, 4.0, and 6.0 mg/mL. Each measurement was repeated 5 times.
2.7 Foaming property
Foaming property was characterized as the report with little modification (Fu et al., 2020). A 100 mL graduated cylinder with a plug was filled with 20 mL of Gemini surfactant solutions at the concentration of 2 mg/mL while the temperature was kept at 25℃. The initial foam volume (V0) was recorded after 1 min of intense shake manually. After 3 min and 5 min of standing, the foam’s volumes (V3 and V5) were recorded.
2.8 Emulsifying property
According to the report (Fu et al., 2020), the emulsifying property was defined. Initially, aqueous solutions of Gemini surfactants were produced at a concentration of 2 mg/mL. In a 100 mL graduated cylinder with a plug, equal volumes (20 mL) of aqueous solution and oil phase was combined, and the solution was rapidly vibrated manually for 1 min before remaining static for 1 h. Following that, the volumes of the water phase, oil phase, and emulsified phase were measured. The volume of emulsified phase was used to assess the emulsifying ability of Gemini surfactants.
2.9 Particle size, polydispersity index (PDI), and Zeta-potential determination
Particle size, PDI, and Zeta-potential measurements of Gemini surfactants in aqueous solution (2 mg/mL) were performed using a Malvern Zetasizer Nano ZS90 Particle Analyzer (Malvern Instruments Ltd., Worcestershire, UK). All samples were determined directly without dilution.
2.10 pH and salt tolerance
The pH and salt tolerance of Gemini surfactant aqueous solutions (2 mg/mL) was evaluated by the turbidity method. The pH of Gemini surfactant solution (10 mL) in glass bottle was adjusted to 2, 4, 6, 8, 10, and 12 using 1 M HCl solution. The salt concentration of Gemini surfactant solution (10 mL) was adjusted to 5, 10, 20, 30, 40, and 50 mg/mL by adding an aqueous 3 M NaCl stock solution. Then all the solutions were incubated at 25℃ for 24 h. Turbidity was calculated by measuring the transmittance at 600 nm using a visible spectrophotometry (METASH UV-6100, Shanghai, China).
2.11 Water sorption at constant humidity
Water sorption isotherms were determined similarly with the report (Durkin et al., 2022). Dry Gemini surfactants (500 mg) accurately weighted in glass plates were transferred into the chamber with constant relative humidity (RH) of 81% and 43%, respectively, controlled by saturated (NH4)2SO4 and Na2CO3 solutions, respectively. The water sorption rate (WSR) was calculated using equation 4 by weighting samples at predetermined time interval.
Where W1 represents the mass of sample after water sorption. Each sample were evaluated in triplicate.
2.12 Antibacterial activity
Antibacterial activity of Gemini surfactants against S. aureus and E. coli was determined in vitro by the 96-well microtiter plate technique (He et al., 2019). Briefly, Gemini surfactants were dissolved in Milli-Q water to get the solutions with concentrations of 25 and 50 μg/mL. To wells of microtiter plates were added 100 μL of bacterial culture (ca. 106 CFU/mL bacteria) and 100 μL of Gemini surfactant solution. Absorbance of wells at 590 nm was measured on a SpectraMax iD5 microplate reader (Molecular Devices, San Jose, CA) before and after 24 h incubation at 37℃. Milli-Q water was used as control. All experiments were performed in triplicate.
3 Results and discussion
3.1 Synthesis and characterization
The ring-opening of glycidol with octylamine and dedecylamine followed by substitution reaction with 1,3-dibromopropane or 1,4-dibromobutane, as shown in Fig. 3A, made it simple to create the Gemini surfactants. Considering the accessibility, cost-effectiveness, and virtually atomic economic, this synthesis approach is extremely favorable. The surfactant structures were confirmed by characterization with 1H NMR, 13C NMR, FTIR, and HRMS, and the appearances were shown in Fig. 3B.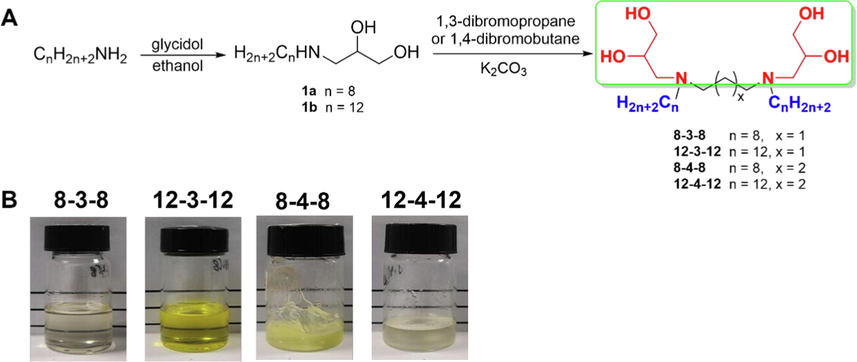
Synthetic route for the nonionic 1-alkylaminoglycerol Gemini surfactants (A) and appearance of Gemini surfactants (B).
8–3-8 (3,3′-(propane-1,3-diylbis(octylazanediyl))bis(propane-1,2-diol)). Light yellow liquid. Yield: 90%. 1H NMR (400 MHz, DMSO‑d6) δ 0.80 (t, J = 4.0 Hz, 6H, CH3), 1.19 (s, 22H, CH2), 1.35 (s, 4H, CH2), 2.34–2.52 (m, 10, CH2), 3.13–3.24 (m, 2H, CH2), 3.27–3.34 (m, 4H, CH2), 3.51–3.57 (m, 2H, CH); 13C NMR (100 MHz, DMSO‑d6) δ 14.02, 14.05, 22.33, 26.99, 27.03, 29.01, 29.22, 31.54, 52.40, 55.39, 58.10, 64.52, 64.72, 68.97. FTIR (KBr): 3353, 2918, 2849, 1456, 1375, 1107, 1048 cm−1. HRMS (ESI): calcd. For C25H54O4N2 [M + 1]+ 447.41563, found 447.41553. Calculated HLB = 7.97.
12–3-12 (3,3′-(propane-1,3-diylbis(dodecylazanediyl))bis(propane-1,2-diol)). Yellow liquid. Yield: 88%. 1H NMR (400 MHz, DMSO‑d6) δ 0.83 (t, J = 4.0 Hz, 6H, CH3), 1.21 (s, 38H, CH2), 1.38 (s, 3H, CH2), 1.45–1.51 (m, 1H, CH2), 2.31–2.49 (m, 10, CH2), 3.14–3.24 (m, 2H, CH2), 3.29–3.33 (m, 4H, CH2), 3.51–3.52 (m, 2H, CH); 13C NMR (100 MHz, DMSO‑d6) δ 13.93, 13.96, 22.15, 26.17, 26.30, 26.84, 26.90, 28.79, 29.10, 29.14, 31.36, 52.29, 55.28, 58.16, 64.45, 69.05. FTIR (KBr): 3353, 2918, 2849, 1456, 1375, 1107, 1048 cm−1. HRMS (ESI): calcd. For C33H70O4N2 [M + 1]+ 559.54084, found 559.54077. Calculated HLB = 6.37.
8–4-8 (3,3′-(butane-1,4-diylbis(octylazanediyl))bis(propane-1,2-diol)). Yellow thick liquid. Yield: 92%. 1H NMR (400 MHz, DMSO‑d6) δ 0.81 (t, J = 4.0 Hz, 6H, CH3), 1.19 (s, 22H, CH2), 1.33–1.70 (m, 6H, CH2), 2.26–2.50 (m, 6H, CH2), 2.60–2.87 (m, 2H, CH2), 3.16–3.55 (m, 10H, CH2); 13C NMR (100 MHz, DMSO‑d6) δ 14.02, 22.20, 22.22, 26.96, 28.63, 28.69, 28.80, 28.89, 29.15, 31.32, 31.42, 53.53, 55.42, 55.50, 58.26, 62.73, 63.27, 63.59, 63.95, 64.53, 69.26. FTIR (KBr): 3353, 2918, 2849, 1456, 1375, 1107, 1048 cm−1. HRMS (ESI): calcd. For C26H56O4N2 [M + 1]+ 461.43128, found 461.43076. Calculated HLB = 7.73.
12–4-12 (3,3′-(butane-1,4-diylbis(dodecylazanediyl))bis(propane-1,2-diol)). Light yellow thick liquid. Yield: 89%. 1H NMR (400 MHz, DMSO‑d6) δ 0.84 (t, J = 8.0 Hz, 6H, CH3), 1.22 (s, 38H, CH2), 1.34–1.64 (m, 6H, CH2), 2.27–2.50 (m, 7H, CH2), 3.18–3.56 (m, 11H, CH2); 13C NMR (100 MHz, DMSO‑d6) δ 13.92, 22.16, 25.93, 26.52, 26.71, 26.92, 28.81, 29.11, 29.15, 31.38, 53.38, 53.58, 55.40, 58.25, 62.61, 63.16, 63.57, 64.44, 64.49, 69.25. FTIR (KBr): 3353, 2918, 2849, 1456, 1375, 1107, 1048 cm−1. HRMS (ESI): calcd. For C34H72O4N2 [M + 1]+ 573.55649, found 573.55566. Calculated HLB = 6.22.
3.2 Surface property
Fig. 4 shows the surface tension curves for compounds 1a and Gemini surfactants as a function of the surfactant concentrations at 25℃. The CMC values were obtained from the γ-lgC curves. Surface tension (γ) reduced noticeably for all surfactants as surfactant concentration increase and nearly remained constant above the critical concentration. The observed CMC and γCMC values for the water-soluble single-chain surfactant 1a (C8) were 11.48 mmol/L and 25.26 mN/m, respectively (Table 1). Due to compound 1b’s weak solubility, the CMC and γCMC were not measured. These Gemini surfactants significantly reduced the CMC compared to 1b (Table 1), particularly 12–3-12 and 8–4-8 with about 8-times lower values (1.58 and 1.41 mmol/L, respectively), demonstrating their superiority over single-chain surfactant in this regard. Additionally, CMC values of Gemini surfactants in Table 1 show that the alkyl-chain length m and spacer carbon number s worked together to determine the CMC. Because the longer hydrophobic chain makes the molecule more hydrophobic and increases the potential to form micelles (Verma and Ghosh 2011), the CMC for 8–3-8 (C8) and 12–3-12 (C12) dropped when s = 3. However, the CMC rose from 8 to 4-8 (C8) (1.41 mmol/L) to 12–4-12 (C12) (4.57 mmol/L) at s = 4. According to Grosmaire et al. (Grosmaire et al., 2002), the carbon numbers of spacer have an impact on the CMC. The CMC gradually rises as the spacer lengthens from 2 to 4 carbon atoms, but further spacer extension causes the CMC to fall once more. The spacer effect is due to its hydrophobicity, that is, short spacers are fully extended on the air–water interface, but long spacers start to fold into air due to their stronger hydrophobicity and flexibility (Wettig and Verrall 2001). The finding shows that by adjusting the spacer chain length, the Gemini surfactants can be structurally improved even more.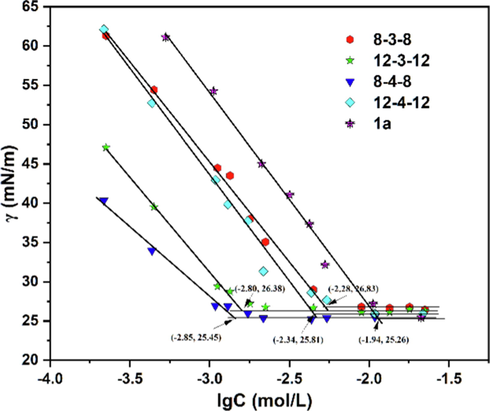
Surface tension as function of the lgC of aqueous 1a and Gemini surfactant solutions at 25℃.
Sample
CMC (mmol/L)
CMC (mg/mL)
γCMC (mN/m)
Гmax (10-6, mol/m2)
Amin (Å2/moleclue)
8–3-8
5.25
2.35
26.83
2.65
62.66
12–3-12
1.58
0.88
26.38
1.33
124.85
8–4-8
1.41
0.65
25.81
1.20
138.38
12–4-12
4.57
2.62
25.45
2.03
81.80
1a
11.48
2.16
25.26
4.76
34.89
Single-chain surfactant 1a at CMC had a surface tension of 25.26 mN/m, which is very close to the surface tension of monooctyl ether of glycerol (24 mN/m) (Bigot et al., 2011), indicating that the interchange of –NH- and -O- groups in the headgroup had no impact on the surface tension. In comparison to their monomeric surfactants, dimerization of 1a and 1b to Gemini surfactants has little effect in lowering the surface tension.
The Гmax values, defined as the amount of surfactant adsorbed at the air/water interface, are also listed in Table 1, and followed the same trends as CMC depending on the Gemini surfactant structures. The greater Гmax values of 8–3-8 and 12–4-12 suggested that denser arrangement at interface. Correspondingly, the greater Amin values of 12–3-12 and 8–4-8 (Table 1) indicated that the loose packing at the air/water interface leads to a larger average area per molecules.
3.3 Wetting property
The surface tension relationship between a liquid–solid system and how thoroughly the liquid wets the solid is known as the wetting property. Fig. 5A depicts the contact angles of Gemini surfactants at various concentrations with the hydrophobic surface (Paraffin film). Deionized water was reported to have a contact angle of roughly 110° with the Paraffin film (Caskey and Barlage 1971), while Gemini surfactants dramatically lowered contact angles, and the observations were associated with the surfactant concentrations. Gemini surfactant structure and contact angle are also highly correlated. With a minimum value of 34.5° at 4 mg/mL (Fig. 5B), 8–3-8 had the best capacity to reduce the contact angles, suggesting its favorable interaction with the hydrophobic surface of Paraffin film, which can lower the surface tension. However, the 8–4-8 solution’s contact angle at 4 mg/mL concentration is still over 70° (Fig. 5B), indicating that it has a limited capacity to adsorb on hydrophobic surface and displace water molecules. The finding is consistent with the report that surfactant solutions have varied contact angles on the same substrate even when they have the same surface angles, and that the surface tension data alone are insufficient to predict contact angle (Kovalchuk and Simmons 2021).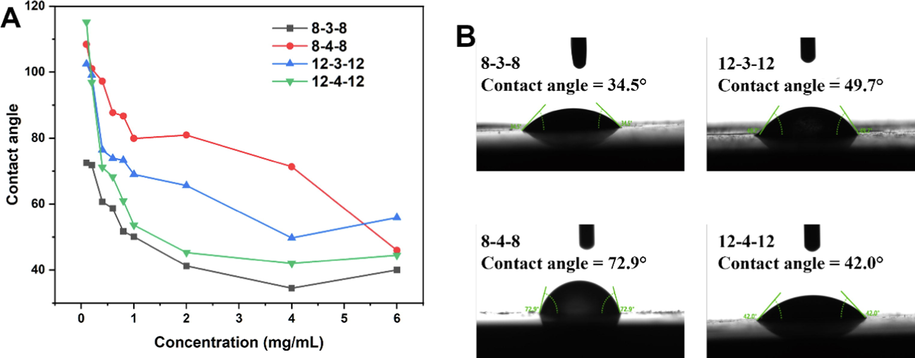
Contact angles of aqueous Gemini surfactant solutions with different concentrations (A) and concentration at 4 mg/mL (B) on the Paraffin film at 25℃.
3.4 Particle size, PDI, and Zeta-potential
Fig. 6A displays the particle size, PDI, and Zeta-potential of Gemini surfactant solutions. According to particle size, the Gemini surfactant aggregates were dispersed in nanoscale profile, with sizes ranging from 186.5 ± 3.2 nm (8–3-8), to 353.3 ± 8.5 nm (12–3-12), to 227.3 ± 8.0 nm (8–4-8), and to 27.3 ± 0.2 nm (12–4-12). Particle size distribution in Fig. 6B shows that the particle sizes of 8–3-8 and 8–4-8 are concentrated in a narrow region with just one main peak, however, 12–3-12 and 12–4-12 have two main peaks. More specifically, 12–4-12 predominantly formed micelles in the range of 10 nm, although by adjusting the surfactant concentration, bigger aggregates can be eliminated. When administrated intravenously, the extremely small size aggregates of 12–4-12 can be exploited as effective nanocarriers that can extravasate from blood vessels (Qin et al., 2013). The uniformity of the particle size distribution is described by the PDI parameter (Danaei et al., 2018). Fig. 6A demonstrates that the polydisperse indexes for the 12–3-12 and 12–4-12 were relatively higher, being 0.575 ± 0.016 and 0.540 ± 0.042, respectively, indicating a wide size distribution as opposed to a tight distribution, which is implied by a PDI below 0.5 (Yang et al., 2017). All the Gemini surfactant solutions had the Zeta-potential values greater than + 50 mV. The high values indicated the excellent physical stability since there is adequate drive force from electrostatic repulsion to prevent accumulation (He et al., 2022). To put it simply, the Gemini surfactants produced can create stable nanoscale micelles with positive Zeta-potentials.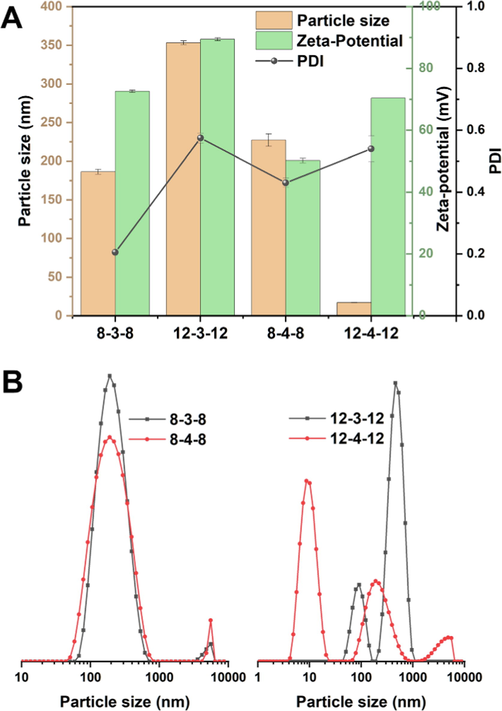
Particle size, PDI, and Zeta-potential of Gemini surfactants (A), and particle size distribution of Gemini surfactants (B).
3.5 Foaming property
Gemini surfactants' foaming properties, such as foaming capacity and foam stability, were assessed. Initial foam volume (V0) was used to explore the foaming capacity, while V3 and V5 were used to measure the change in foam volume over the course of 3 and 5 min to determine foam stability. According to the Table 2., the initial foaming volume (V0) rose for s = 3 as the alkyl chain grew from C8 (8–3-8, 20.5 mL, HLB = 7.97) to C12 (12–3-12, 38.0 mL, HLB = 6.37), showing that an increase in hydrophobicity is beneficial for the formation of foaming. 8–4-8 has the extremely lowest foaming capacity for s = 4 (V0 = 3.0 mL, HLB = 7.73). This might be because the greater flexibility of spacer prevents it from dispersing at the gas/liquid interface and lowering the interfacial tension. The best foaming capacity, however, belonged to 12–4-12 (V0 = 54.5 mL, HLB = 6.22). Comparing the foaming capacity of Gemini surfactants expect for 8–4-8, they follow the patterns that longer spacer, but less than 6 (Kuliszewska and Brecker 2014), and hydrophobic chains favor the foam capacity. Additionally, good foam stability was seen, with a slight reduction after 3 min and a nearly constant change from 3 to 5 min. An innovative low foaming surfactant is 8–4-8.
Sample
V0 (mL)
V3 (mL)
V5 (mL)
8–3-8
20.5
18.0
17.5
12–3-12
38.0
36.5
36.0
8–4-8
3.0
3.0
3.0
12–4-12
54.5
51.0
51.0
3.6 Emulsifying property
Emulsions can serve as the foundation for a variety of industries, including the pharmaceutical, cosmetic, agrochemicals, and petrochemicals (Shahin et al., 2011). A crucial indicator for the Gemini surfactants is their emulsifying property. Fig. 7 depicts the results of an investigation on the emulsifying ability of Gemini surfactants in four different two-phase systems, including the benzene/water system, the rapeseed oil/water system, the cyclohexane/water system, and the medium carbon chain triglyceride/water system. For benzene/water and cyclohexane/water systems, Gemini surfactants, 8–3-8, 12–3-12, and 12–4-12, showed much greater emulsification to benzene/water system, and formed Winsor I microemulsion (i.e., excess oil layer and emulsified layer). However, all Gemini surfactants formed Winsor III microemulsion (i.e., excess oil and water layers, emulsified layer) in cyclohexane/water system, where the emulsified layers were thin. The selectivity of emulsifying ability indicates that these Gemini surfactants are good emulsifier for the aromatic substances, such as aromatic essential oils. In the rapeseed oil/water and medium carbon chain triglyceride/water systems, 12–3-12 and 12–4-12 both have good emulsifying ability with Winsor II microemulsion (i.e., emulsified layer, excess water) formation. The difference between 8 and 3-8 and 8–4-8 and how successfully 12–3-12 and 12–4-12 emulsified the oil phase in should be attributed to the superior interactions between the twelve-alkyl chain of Gemini surfactants and oils.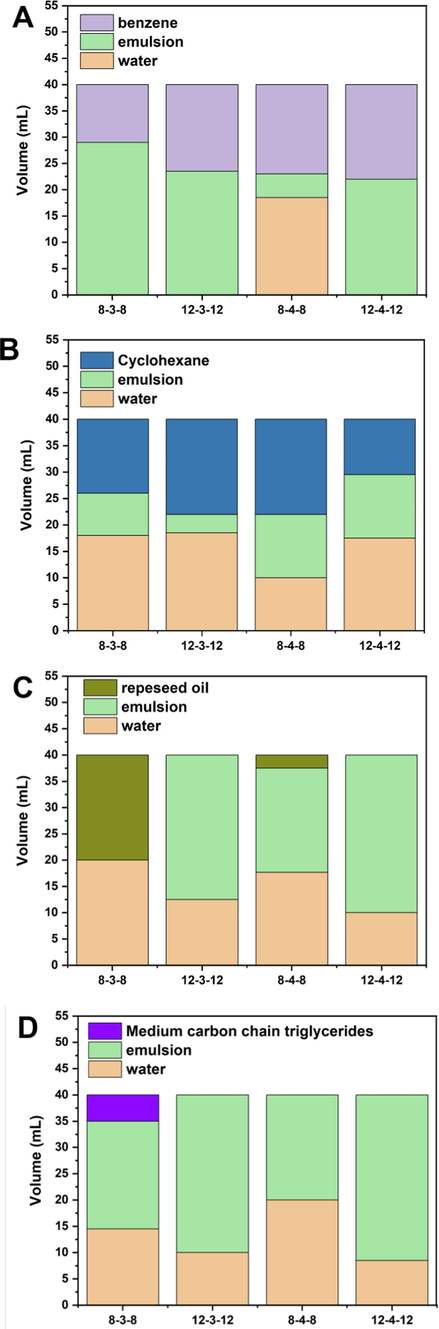
Emulsifying property of Gemini surfactants in the (A) benzene/water system, (B) cyclohexane/water system, (C) rapeseed oil/water system, (D) medium carbon chain triglyceride/water system.
The benzene/water system was classified as O/W emulsion (Winsor I). The low viscosity of benzene can be adsorbed in the Gemini surfactant micelle in water, which is how O/W emulsion was explicated. However, in the case of high viscosity oil (rapeseed oil and medium carbon chain triglyceride), they formed W/O emulsions (Winsor II), since the severe vibration caused water droplets to scatter in surfactant-loaded oil phase. In addition, the calculated HLB values of 12–3-12 and 12–4-12 (6.37 and 6.22, respectively), which are typically used as W/O emulsifiers, also supported the existence of W/O emulsions.
3.7 pH and salt tolerance
Gemini surfactant utilization frequently involves conditions with wide pH ranges and high salt concentrations, as a results, the pH and salt tolerance were assessed using the turbidity method by measuring the transmittance at 600 nm with a visible spectrophotometer. Except for 12–3-12, which showed nearly 90% transmittance at pH 4.0, all Gemini surfactants shown very good water solubility at pH 2.0 and 4.0, according to the Fig. 8A and 8B. This suggests complete micellization and can be explained by the protonation of tertiary amine to produce ammonium salt with improved water solubility (Bergsma et al., 2001). When the pH raised from 6.0 to 12.0, 8–3-8, 12–3-12, and 8–4-8 all showed signs of turbidity increase, which suggested the breaking up of vesicles (Fig. 8B). Nevertheless, the transmittance of 12–4-12 remained consistent from acidic to basic conditions, demonstrating its great pH tolerance. At basic conditions, the low water solubility of 8–3-8, 12–3-12, and 8–4-8 should be the result of the molecular electrostatic repulsion interaction between the hydroxy groups and electronegativity-rich amine groups. The phenomenon indicates these three Gemini surfactants have potential as pH sensitive nanocarriers in basic conditions.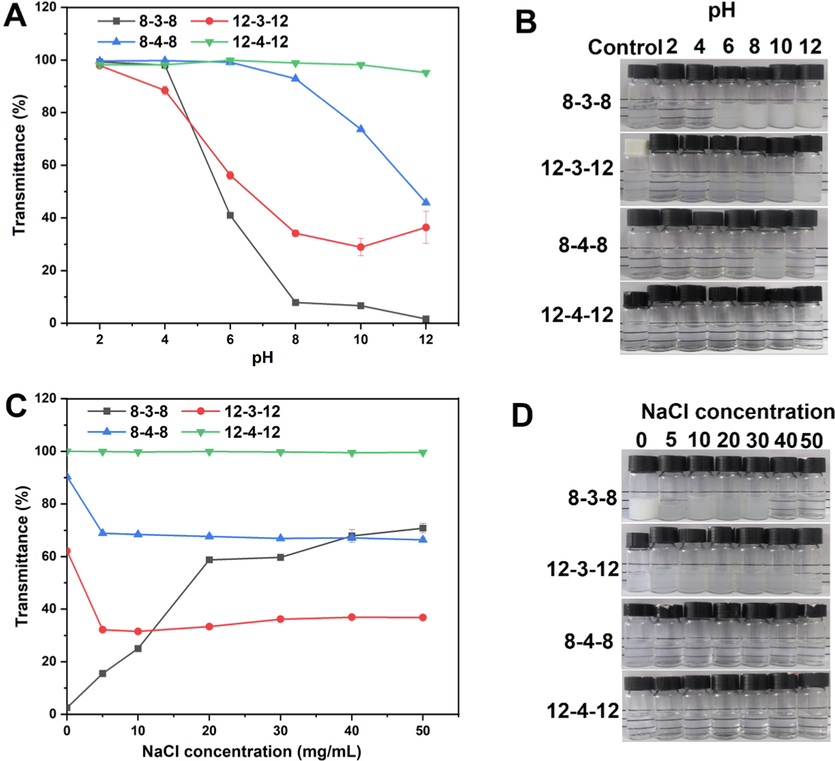
Transmittance of aqueous Gemini surfactant solution at different pH (A), salt concentration (C), and appearances at pH (B) and salt concentration (D).
The assessment of salt tolerance to NaCl is depicted in Fig. 8C and 8D. It showed perfect transmittance at salt concentrations ranging from 0 to 50 mg/mL, demonstrating the same strong tolerance to ionic strength as the pH tolerance of 12–4-12. The water solubility of 12–3-12 and 8–4-8 is affected by the salt addition at a concentration of 5 mg/mL and afterwards changes little even when the salt concentration is increased to 50 mg/mL. It is interesting to see that the water solubility of 8–3-8 grew to match 8–4-8′ performance as the salt concentration rose from 40 to 50 mg/mL.
The hydrophobic chain length and the spacer usually influence the physicochemical properties of Gemini surfactants (Hussain et al., 2019). The aforementioned results clearly showed the Gemini surfactant 12–4-12 had excellent pH and salt tolerance, which had wide application.
3.8 Water sorption property
By weighting the amount of water sorption in a chamber with controlled relative humidity (RH) using saturated salt solutions (RH 43% of Na2CO3 solution and RH 81% of (NH4)2SO4 solution), the water sorption capacities of Gemini surfactants, which can reveal the hydration forces (Kocherbitov 2023), was studied. Fig. 9 shows the water sorption profiles of Gemini surfactants at 43% and 81% RH. The 8–4-8 had the highest water sorption property at 43% RH, demonstrating around a 3.0% mass increase after 72 h of storage, followed by the 12–4-12 (2.75%), 8–3-8 (2.25%), and 12–3-12 (1.85%), according to the results in Fig. 9A. When the RH increased 81%, the mass increase amplitude significantly increased, reaching 14% for 8–4-8, 9% for 12–4-12 and 8–3-8, 7% for 12–3-12 (Fig. 9B), with an increase of at least three times that at 43% RH. However, these Gemini surfactants followed the same tendencies, with 8–4-8 and 12–3-12 exhibiting the strongest and weakest water sorption capacities, respectively, whether the RH was 43% or 81%. The findings supported the hypothesis that the synergetic effect of hydrophobic chain and spacer influences the water sorption capacity. With regard to 8–3-8 and 8–4-8, the longer spacer may increase the flexibility of 8–4-8, giving it more room to interact with water molecules and higher water sorption capacities. The same pattern applied to 12–3-12 and 12–4-12, with s = 3 bringing the distribution of 12–3-12 closer and reducing the interaction between the hydrophilic head and the water molecules.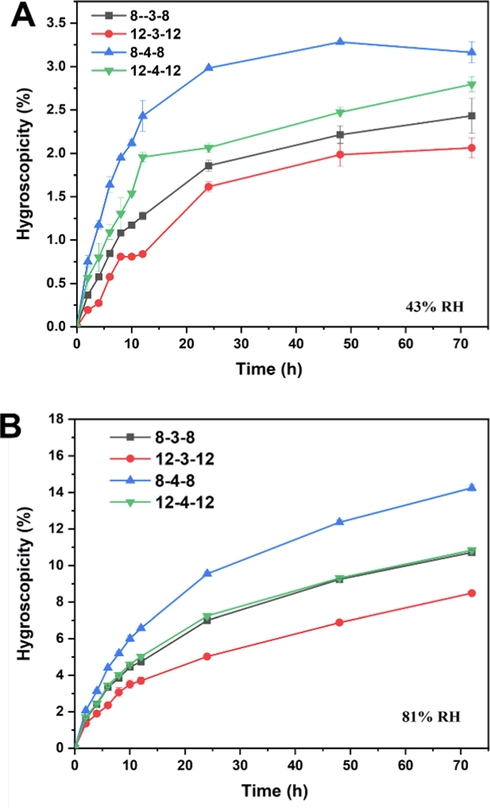
Water sorption profiles for the Gemini surfactant at different time and different relative humidity (RH). (A) 43% RH, (B) 81% RH.
3.9 Antibacterial activity
Gemini surfactants usually display potent antibacterial activity; therefore, the synthesized Gemini surfactants were tested for their antibacterial activities against S. aureus and E. coli at 25 and 50 μg/mL, and the results were shown in Table 3. At 25 and 50 μg/mL, 8–3-8 and 12–3-12 both exhibited strong inhibitory activity against S. aureus and E. coli. However, 8–4-8 and 12–4-12 showed selective inhibitory activity for S. aureus and E. coli when s = 4, that 8–4-8 nearly has no antibacterial activity. Additionally, 12–4-12 displayed strong inhibition against S. aureus but extremely low against E. coli at the dosage of 25 μg/mL, demonstrating its selectivity between Gram-positive and Gram-negative bacteria at low dosage.
Sample
S. aureus (μg/mL)
E. coli (μg/mL)
25
50
25
50
8–3-8
92.8 ± 0.1%
95.5 ± 0.2%
91.4 ± 0.3%
98.4 ± 0.2%
12–3-12
90.0 ± 0.3%
98.2 ± 0.1%
90.3 ± 0.3%
99.0 ± 0.1%
8–4-8
0.7 ± 1.0%
9.9 ± 0.2%
6.3 ± 0.7%
9.00 ± 1.2%
12–4-12
93.3 ± 0.5%
94.0 ± 0.3%
3.0 ± 0.9%
91.6 ± 0.1%
Surfactants usually displayed antimicrobial activity by interacting with cell membranes and interfering various cellular processes (Brycki et al., 2017). The contact angle assessment showed that 8–4-8 had a limited capacity to adsorb on hydrophobic surfaces, which may decrease its ability to the interface with hydrophobic cell membranes and reduced its antibacterial activity. According to reports, cationic Gemini surfactants with longer spacer had stronger antimicrobial activity as they adapt to cell surface better (Bogumil et al., 2017). The present nonionic Gemini surfactants, however, exhibited the opposite trend, with s = 3 being preferable to s = 4. This may be due to the large increase in lipophilicity, which makes it more difficult to cross the cell membrane and causes activity to decline.
4 Conclusion
In the present study, we synthesized four nonionic 1-aminoglycerol-based Gemini surfactants with high isolated yields (88–92%) and evaluated their physicochemical properties and antibacterial activity. Surface properties revealed that while all of them had similar γcmc, 12–3-12 and 8–4-8 had lower CMC than 8–3-8 and 12–4-12. Gemini surfactants in aqueous solutions were in stable nanoscale dispersion, and exhibited strong and long-lasting foaming ability, emulsifying abilities for benzene/water and triacylglyceride/water systems, and low water sorption property. Especially, 12–4-12 solution displayed excellent tolerance to wide pH range and high salinity. Antibacterial activity showed that Gemini surfactants, with the exception of 8–4-8, have excellent inhibition of S. aureus and E. coli at 25 and 50 µg/mL. Thus, these novel Gemini surfactants can be employed as good emulsifier and nanocarrier system with good antibacterial activity.
Funding
This work was supported by the National Natural Science Foundation of China (No. 21807084), Open Project Program of Key Laboratory for Deep Processing of Major Grain and Oil (Wuhan Polytechnic University), and Ministry of Education (2020JYBQGDKFB16).
CRediT authorship contribution statement
Ruifeng Liao: Investigation, Formal analysis, Writing – original draft. Liangliang Shi.: Investigation, Formal analysis, Writing – original draft. Yi Zhou: Investigation, Validation. Chenyue Jia: . Jiangtao Feng: Data curation, Writing – review & editing. Weinong Zhang: Supervision, Writing – review & editing. Junbo He: Conceptualization, Funding acquisition, Supervision, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Cationic gemini surfactant properties, its potential as a promising bioapplication candidate, and strategies for improving its biocompatibility: a review. Adv. Colloid Interface Sci.. 2022;299:102581
- [CrossRef] [Google Scholar]
- Alkanediyl-.alpha.,omega.-bis(dimethylalkylammonium bromide) surfactants. 3. Behavior at the air-water interface. Langmuir. 1993;9:1465-1467.
- [CrossRef] [Google Scholar]
- pH-Dependent Aggregation Behavior of a Sugar-Amine Gemini Surfactant in Water: Vesicles, Micelles, and Monolayers of Hexane-1,6-bis(hexadecyl-1′-deoxyglucitylamine) J. Colloid Interface Sci.. 2001;243:491-495.
- [CrossRef] [Google Scholar]
- Synthesis and surface properties of glycerol based C8 chain monoethers. Ind. Eng. Chem. Res.. 2011;50:9870-9875.
- [CrossRef] [Google Scholar]
- Brycki, B. E., Kowalczyk, I. H., Szulc, A., et al., 2017. Multifunctional Gemini surfactants: structure, synthesis, properties and applications. Application and Characterization of Surfactants. N. Reza. Rijeka, IntechOpen.
- An improved experimental technique for determining dynamic surface tension of water and surfactant solutions. J. Colloid Interf. Sci.. 1971;35:46-52.
- [CrossRef] [Google Scholar]
- Impact of particle size and polydispersity index on the clinical applications of lipidic nanocarrier systems. Pharmaceutics. 2018;10:57.
- [CrossRef] [Google Scholar]
- Fiber-welded polyionic biocomposites using 1-alkyl-3-vinylimidazolium alkylphosphonate ionic liquids. J. Ionic Liq.. 2022;2:100024
- [CrossRef] [Google Scholar]
- Sugar-based tertiary amino gemini surfactants with a vesicle-to-micelle transition in the endosomal pH range mediate efficient transfection in vitro. Eur. J. Biochem.. 2001;268:1269-1279.
- [CrossRef] [Google Scholar]
- Micellar structure in Gemini nonionic surfactants from small-angle neutron scattering. Langmuir. 2005;21:7121-7128.
- [CrossRef] [Google Scholar]
- Water solubility and surface activity of alkoxyethyl beta-d-maltosides. J. Agric. Food Chem.. 2020;68:8330-8340.
- [CrossRef] [Google Scholar]
- Alkanediyl-α, ω-bis(dimethylalkylammonium bromide) surfactants: 9. Effect of the spacer carbon number and temperature on the enthalpy of micellization. J. Colloid Interf. Sci.. 2002;246:175-181.
- [CrossRef] [Google Scholar]
- Determination of critical micelle concentration (CMC) of nonionic surfactants by donor-acceptor interaction with lodine and correlation of CMC with hydrophile-lipophile balance and other parameters of the surfactants. J. Surfactants Deterg.. 2001;4:303-309.
- [CrossRef] [Google Scholar]
- Carvacrol loaded solid lipid nanoparticles of propylene glycol monopalmitate and glyceryl monostearate: preparation, characterization, and synergistic antimicrobial activity. Nanomaterials. 2019;9:1162.
- [CrossRef] [Google Scholar]
- Novel polyglycerol-10 dialdehyde mediated cross-linking of sodium caseinate: preparation, characterization, and improved emulsifying properties. Colloids Surf. A. 2022;647:128957
- [CrossRef] [Google Scholar]
- Surface and thermal properties of synthesized cationic poly(ethylene oxide) Gemini surfactants: the role of the spacer. RSC Adv.. 2019;9:30154-30163.
- [CrossRef] [Google Scholar]
- Sugar-based Gemini surfactant with a vesicle-to-micelle transition at acidic pH and a reversible vesicle flocculation near neutral pH. J. Am. Chem. Soc.. 2003;125:757-760.
- [CrossRef] [Google Scholar]
- Synthesis and performance of glycerol ester-based nonionic surfactants. J. Disper. Sci. Technol.. 2012;33:949-954.
- [CrossRef] [Google Scholar]
- A model for water sorption isotherms and hydration forces in sugar surfactants. J. Colloid Interf. Sci.. 2023;633:343-351.
- [CrossRef] [Google Scholar]
- Surfactant-mediated wetting and spreading: recent advances and applications. Curr. Opin. Colloid In.. 2021;51:101375
- [CrossRef] [Google Scholar]
- Gemini surfactants foam formation ability and foam stability depends on spacer length. J. Surfactants Deterg.. 2014;17:951-957.
- [CrossRef] [Google Scholar]
- Synthesis and biological evaluation of 3-alkyloxazolidin-2-ones as reversible MAO inhibitors. ARKIVOC. 2004;2004:118-130.
- [CrossRef] [Google Scholar]
- A novel type of highly effective nonionic gemini alkyl O-glucoside surfactants: a versatile strategy of design. Langmuir. 2013;29:8511-8516.
- [CrossRef] [Google Scholar]
- Surface tension, interfacial tension and emulsification of sodium dodecyl sulfate extended surfactant. Colloids Surf. A. 2016;494:201-208.
- [CrossRef] [Google Scholar]
- Gemini surfactants: a new class of self-assembling molecules. J. Am. Chem. Soc.. 1993;115:10083-10090.
- [CrossRef] [Google Scholar]
- Hydrotropic properties of alkyl and aryl glycerol monoethers. J. Phys. Chem. B. 2013;117:9262-9272.
- [CrossRef] [Google Scholar]
- Wettability of paraffin surfaces by nonionic surfactants: Evaluation of surface roughness and nonylphenol ethoxylation degree. Colloids Surf. A. 2015;480:376-383.
- [CrossRef] [Google Scholar]
- Effect of the hydrophilic size on the structural phases of aqueous nonionic Gemini surfactant solutions. Langmuir. 2004;20:9061-9068.
- [CrossRef] [Google Scholar]
- Polymeric micelles for enhanced lymphatic drug delivery to treat metastatic tumors. J. Control. Release.. 2013;171:133-142.
- [CrossRef] [Google Scholar]
- Adsorption and micellization behavior of novel gluconamide-type Gemini surfactants. J. Colloid Interf. Sci.. 2008;318:440-448.
- [CrossRef] [Google Scholar]
- Development of stable O/W emulsions of three different oils. Int. J. Pharm. Stud. Res.. 2011;2:45-51.
- [Google Scholar]
- Advances in the synthesis, molecular architectures and potential applications of gemini surfactants. Adv. Colloid Interf. Sci.. 2017;248:35-68.
- [CrossRef] [Google Scholar]
- Synthesis of glycerol-based pyridinium surfactants and appraisal of their properties. Ind. Eng. Chem. Res.. 2009;48:1673-1677.
- [CrossRef] [Google Scholar]
- Micellar and surface properties of some monomeric surfactants and a Gemini cationic surfactant. J. Surfactants Deterg.. 2011;14:347-352.
- [CrossRef] [Google Scholar]
- Thermodynamic studies of aqueous m–s–m Gemini surfactant systems. J. Colloid Interf. Sci.. 2001;235:310-316.
- [CrossRef] [Google Scholar]
- Preparation and characterization of emamectin benzoate solid nanodispersion. J. Nanomater. 2017:6560780.
- [CrossRef] [Google Scholar]
- Efficient emulsifying properties of glycerol-based surfactant. Colloids Surf. A. 2018;553:225-229.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2023.105111.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







