Translate this page into:
Synthesis, structure and anticancer studies of Cu (II), Ni (II) and Co (II) complexes based on 2,3-dihydroxybenzaldehyde-2-(2-aminophenyl)benzimidazole Schiff base
⁎Corresponding authors at: School of Basic Courses, Anhui Province Key Laboratory of Translational Cancer Research, Bengbu Medical College, Bengbu 233030, China. lwg1010@163.com (Wen Ge Li), 0200017@bbmc.edu.cn (Jing Tong)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Three metal complexes [Cu(HL)Cl](C2H5OH)2] (complex 1), [Ni4L2(CH3COO)4(C2H5OH)4] (complex 2) and [CoHL2] (complex 3) based on 2,3-dihydroxybenzaldehyde-2-(2-aminophenyl)benzimidazole Schiff base (H2L) were synthesized by solvothermal reaction. The three complexes were characterized by FT-IR spectrometer, elemental analysis, thermogravimetric analysis, and X-ray single-crystal diffraction. X-ray single-crystal diffraction analysis confirmed that complex 1 belongs to monoclinic crystal system with P21/c space group, and complex 3 crystallizes in trigonal crystal system with R-3 space group. However, complex 2 crystallized in triclinic crystal system with P-1 space group and exhibits centrosymmetric tetranuclear crystallographic structure. Besides, MTT assay was studied antitumor activities against different tumor cells. The results show that the three complexes all have fine anticancer activities. Among them, complex 2 has significantly better anticancer properties against CNE-2Z cells than that of cisplatin. Finally, apoptosis and cell migration were studied to prove that complex 2 could inhibit tumor cells from proliferating in the manner of apoptosis and migration.
Keywords
Schiff base
Benzimidazole derivative
Crystal structure
Metal complex
Anticancer activity
1 Introduction
Currently, cancer is the major cause of human mortality, and a variety of treatments are used to fight the battle against cancer, in which chemotherapy is the most effective treatment to destroy cancer cells (Bray et al., 2018; Torre et al., 2016). Cisplatin is one of the earliest and most effective anticancer drugs. However, its drug resistance and nonspecific toxicity restrict its clinical application (Ghosh et al., 2019; Sun et al., 2019; Köberle et al., 2010). Consequently, many non-platinum metal complexes were designed to develop anticancer drugs with high efficiency and few side effects. So far, the benzimidazole moiety is a key pharmacophore in medicinal industry due to its biological activities, such as anticancer, antibacterial, anti-inflammatory and analgesic effects (Yadav et al., 2015; Ates-Alagoz et al., 2016; Keri et al., 2015; Keri et al., 2016; Akhtar et al., 2017; Sirim et al., 2020). For instance, Hranjec M et al synthesized a series of novel benzimidazole Schiff bases. In vitro anticancer results showed that some of compounds exerted excellent inhibitory effect on HeLa and MCF-7 cell lines (Hranjec et al., 2011).
Schiff bases are a class of organic compounds formed by the condensation of amines with carbonyl groups. Owing to Schiff bases' lively imine groups, they are easily coordinated with metal ions to form various complexes. (Reshma et al., 2022; Kostova and Saso, 2013; Khan et al., 2022; Xu et al., 2020; Fontana et al., 2022). In addition, structural modifications of ligands are key aspects to the relations of their biological activities. Thus, a variety of benzimidazole Schiff base metal complexes are designed to improve their diverse biological activities as potential anticancer drugs (Casanova et al., 2018; Skrodzki et al., 2021; Magd-El-Din et al., 2018; Galal et al., 2009). Anup Paul et al prepared three benzimidazole-based Schiff base copper(II) complexes. Anticancer results showed one of Cu (II) complexes exhibits significant cytotoxic effect on A549 cancer cells. (Paul et al., 2015). Lotfi M et al synthesized benzimidazole schiff base ligand and its Cr (III), Mn (II) and Zn (II) metal complexes. The cytotoxicity results showed that Mn (II) complexes had good inhibitory effects on HCT 116 cells, HepG2 cells and MCF-7 cell (Aroua et al., 2023).
Meanwhile, the current studies show that most of the benzimidazole Schiff base complexes do not have precise molecular structure and their anticancer activities need to be improved (Kumaravel et al., 2018; Tahlan et al., 2019; Aragón-Muriel et al., 2021). Non-platinum metal elements, such as copper, nickel, and cobalt are essential nutrients in the human body. In this paper, three new 2,3-dihydroxybenzaldehyde-2-(2-aminophenyl)benzimidazole Schiff base metal complexes [Cu(HL)Cl](C2H5OH)2, [Ni4L2(CH3COO)4(C2H5OH)4] and [Co(HL)2] were synthesized by the solvothermal method. Their crystal structures were resolved by X-ray single crystal diffraction and their anticancer activities were also studied.
2 Experimental part
2.1 Materials and instruments
Chemical reagents and solvents were provided by Shanghai Aladdin Biochemical Technology Co. MTT reagents (Shanghai, China) were provided by BTS Biotech. Apoptosis assay kit (Nanjing, China) was provided by KeyGEN Biotech.
IR spectra were measured by Nicolet Model Nexus 470 FT-IR spectrometer, using KBr pellets. Elemental analyses for C, H, and N were carried out with Elementar Vario EL cube analyzer. X-ray crystallographic data were obtained from Bruker Apex APEX II X-ray single crystal diffractometer. Powder X-ray diffraction (PXRD) patterns were obtained on the Bruker D8 diffractometer. TG was performed with STA 449-F3 instrument.
2.2 Synthesis of the Schiff base ligand
2-(2-aminophenyl)benzimidazole (2.0 g, 9.56 mmol) was added to 80 mL ethanol, then slowly dripped 2,3-dihydroxybenzaldehyde (1.32 g, 9.56 mmol) (Fig. 1). The mixture was refluxed for 4–5 h at 60 ℃ and obtained white precipitate. The white precipitate was filtered and then washed with cold ethanol. (Yield: 74.78%). 1H NMR: δ 9.69 (s, 1H), 9.16 (s, 1H), 7.97 (d, J = 7.6 Hz, 1H), 7.65 (d, J = 7.96 Hz 1H), 7.28––7.06 (m, 6H), 6.88 (d, J = 8.1 Hz, 1H), 6.80 (t, J = 7.48 Hz, 1H), 6.74 (d, J = 7.8 Hz, 1H), 6.45 (t, J = 7.9 Hz, 1H), 6.19 (t, J = 7.8 Hz, 1H) (Fig. S1). 13C NMR: δ 147.7, 145.9, 144.3, 144.0, 143.0, 133.4, 132.0, 127.5, 125.1, 122.6, 122.4, 119.7, 119.0, 118.3, 116.8, 116.0, 115.0, 112.0, 110.7, 63.2 (Fig. S2).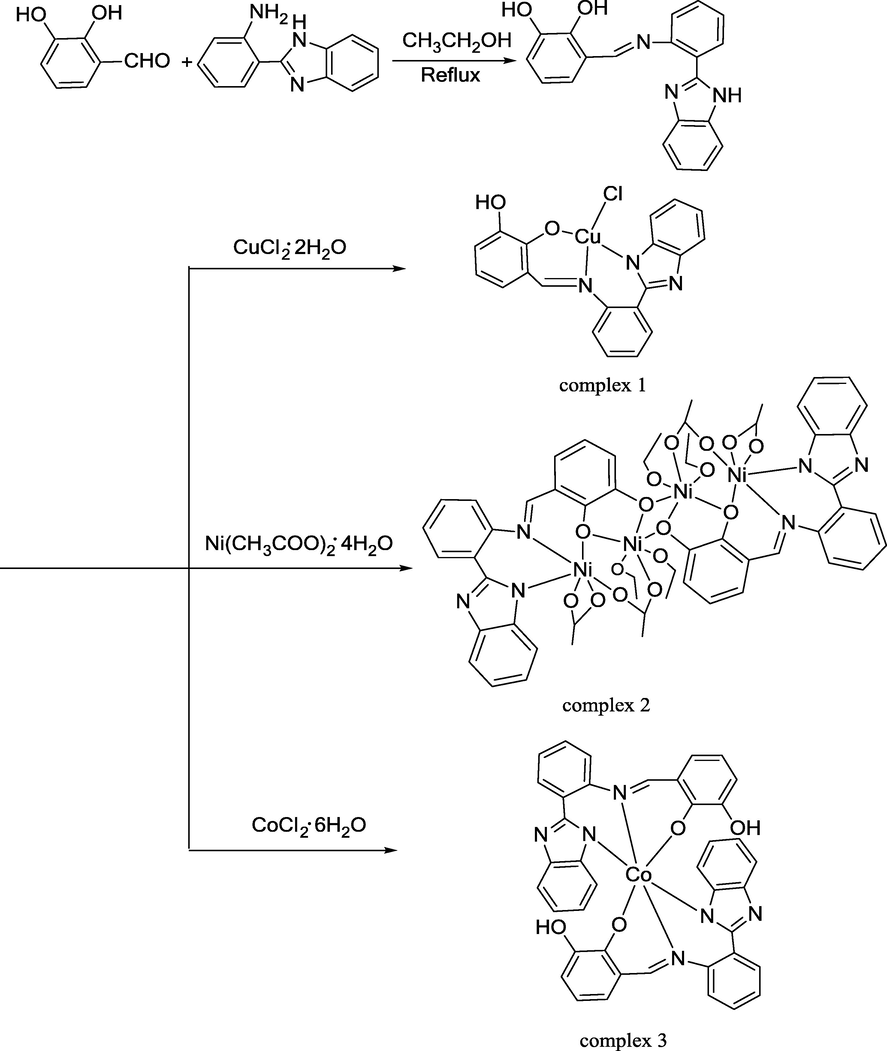
Synthetic pathway of ligand and complexes 1, 2 and 3.
2.3 Synthesis of the Cu (II) complex
CuCl2·2H2O (0.005 g, 0.032 mmol), H2L (0.02 g, 0.061 mmol), 0.01 mL triethylamine and 8 mL ethanol were mixed (Fig. 1). The reactants were transformed into a 20 mL teflon-sealed autoclave and subsequently heated to 75 ℃ for 3 d. Cooling down to room temperature, the dark green crystals were got. (Yield: 53.65%). C24H26ClCuN3O4(%): C: 55.49, H: 5.04, N: 8.09, Found: C: 55.57, H: 4.92, N:8.17. FT-IR (KBr, cm−1): ν (O–H) 3615, ν (C = N) 1652, ν (C-O) 1176, ν (Cu–O) 517 (Fig. S3).
2.4 Synthesis of the Ni (II) complex
Ni(CH3COO)2·4H2O (0.02 g, 0.080 mmol), H2L (0.01 g, 0.030 mmol) and 0.03 mL triethylamine were added to 8 mL ethanol and heated at 70 ℃ for 4 d (Fig. 1). The yellow-green crystals were obtained by similar method to that of Cu (II) complex (Yield: 55.32%). C56H62N6Ni4O16: C: 51.35, H: 4.77, N: 6.42, Found: C: 51.42, H: 4.70, N: 6.51. FT-IR (KBr, cm−1): ν (O–H)3748, ν (C = N) 1634, ν (C-O)1079, ν (Ni-O) 504 (Fig. S4).
2.5 Synthesis of the Co (II) complex
CoCl2·6H2O (0.004 g, 0.017 mmol), H2L (0.015 g, 0.046 mmol), 0.04 mL triethylamine, 5 mL ethanol, and 3 mL ethyl acetate were mixed fully, heated at 65 ℃ for 3 d (Fig. 1). Afterwards, the dark purple crystals were obtained by the same method as Cu (II) complex (Yield: 62.43%). C40H27CoN6O4: C: 67.23, H: 3.81, N: 11.76. Found: C: 67.16, H: 3.73, N: 11.89. FT-IR (KBr, cm−1): ν (O–H) 3626, ν (C = N) 1617, ν (C-O) 1076, ν (Co-O) 502 (Fig. S5).
2.6 Structure determination
The single crystals were examined with a Bruker APEX II diffractometer. Software SAINT and SADABS were used to calibrate the data (BrukerSAINT, 2012). For the empirical adsorption correction, SADAB was employed. SHELX was employed to solve the structures (Krause. et al., 2015; Hübschle et al., 2011). Anisotropic displacement parameters were utilized to refine all atoms with the exception of H. Crystallographic data are shown in Table 1. The selected bond lengths and angles are shown in Table 2.
Complex
complex 1
complex 2
complex 3
Empirical formula
C24H26ClCuN3O4
C56H62N6Ni4O16
C40H27CoN6O4
Molecular weight
519.47
1309.88
714.60
Crystal system
monoclinic
triclinic
trigonal
Crystal size(mm3)
0.37 × 0.23 × 0.14
0.07 × 0.04 × 0.03
0.25 × 0.23 × 0.18
Space group
P21/c
P-1
R-3
λ/Å
0.71073
0.71073
0.71073
a (Å)
12.7552(5)
10.974(14)
33.6912(8)
b (Å)
13.5677(5)
12.6947(13)
33.6912(8)
c/(Å)
14.3188(4)
12.7848(19)
17.9473(9)
α(°)
90
119.453(3)
90
β(°)
112.033(2)
99.642(5)
90
γ(°)
90
101.753(4)
120
V (Å3)
2297.02(14)
1441.6(3)
17642.6(12)
Z
4
1
18
ρcalc.(g·m−3)
1.502
1.509
1.211
angles of data collected
0.65–0.75
0.60–0.75
0.66 – 0.75
μ(mm−1)
1.104
1.360
0.483
F(0 0 0)
1076
680
6624
Data/restraint/parameters
4156/ 75/ 344
4929/ 3/ 383
7106 / 0 / 460
Goodness − of − fit on F2
1.053
1.033
1.050
R indexes [all data]
R1 = 0.0417
wR2 = 0.0804R1 = 0.1427
wR2 = 0.0924R1 = 0.1090,
wR2 = 0.1597
Final R1, wR2[I>=2σ (I)]
0.0307, 0.0732
0.0658, 0.0792
0.0510, 0.1263
Largest diff. peak/hole / eÅ−3
0.749 and −0.306
0.632 and −0.625
0.460 and −0.440
CCDC No
2,244,067
2,244,085
2,244,086
complex 1
Cu1 − Cl1
2.297(6)
O2 − Cu1 − Cl1
157.8(8)
N1 − Cu1 − Cl1
93.8(6)
Cu1 − O2
1.889(1)
O2 − Cu1 − N3
94.5(7)
N3 − Cu1 − Cl1
155.2(6)
Cu1 − N1
1.947(1)
N1 − Cu1 − N3
91.0(8)
Cu1 − N3
1.985(1)
O2 − Cu1 − Cl1
90.1(5)
complex 2
Ni1 − O2
1.997(4)
O2 − Ni1 − O3
98.4(1)
O1 − Ni2 − O1
84.4(1)
Ni1 − O3
2.026(4)
O2 − Ni1 − O5
94.0(1)
O2 − Ni2 − O4
104.8(1)
Ni1 − O5
2.139(5)
O2 − Ni1 − O6
154.6(1)
O2 − Ni2 − O7
160.4(1)
Ni1 − O6
2.241(5)
O2 − Ni1 − N1
90.9(1)
O2 − Ni2 − O8
87.7(1)
Ni1 − N1
1.997(6)
O2 − Ni1 − N2
103.5(1)
O1 − Ni2 − O2
79.8(1)
Ni1 − N2
2.015(5)
O3 − Ni1 − O5
91.2(1)
O4 − Ni2 − O7
93.3(1)
Ni2 − O1
2.009(4)
O3 − Ni1 − O6
84.1(2)
O4 − Ni2 − O8
87.8(1)
Ni2 − O2
2.134(5)
O3 − Ni1-N1
169.4(1)
O4 − Ni2 − O1
90.5(1)
Ni2 − O4
2.026(4)
O3 − Ni1 − N2
89.9(2)
O7 − Ni2 − O8
85.6(1)
O5 − Ni1 − O6
60.5(1)
O7 − Ni2 − O1
94.0(1)
O5 − Ni1 − N1
93.2(1)
O8 − Ni2 − O1
178.4(1)
O5 − Ni1 − N2
162.0(1)
O1-Ni2-O4
173.3(1)
O6 − Ni1 − N1
89.6(1)
O1 − Ni2 − O7
82.7(1)
O6 − Ni1 − N2
101.7(1)
O1 − Ni2 − O8
97.0(1)
N1 − Ni1 − N2
83.0(1)
O1 − Ni2 − O2
79.8(1)
complex 3
Co1 − O4
1.893(2)
O4 − Co1 − O2
87.5(1)
O2 − Co1 − N1
90.0(1)
Co1 − O2
1.903(3)
O4 − Co1 − N6
92.1(1)
N6 − Co1 − N1
90.7(1)
Co1 − N6
1.921(3)
O2 − Co1 − N6
174.9(1)
N4 − Co1 − N1
93.9(1)
Co1 − N4
1.921(3)
O4 − Co1 − N4
90.9(1)
N3 − Co1 − N1
85.8(1)
Co1 − N3
1.931(3)
O2 − Co1 − N4
90.3(1)
N6 − Co1 − N3
94.5(1)
Co1 − N1
1.944(3)
N6 − Co1 − N4
84.6(1)
N4 − Co1 − N3
179.2(1)
O4 − Co1 − N3
89.2(1)
O4 − Co1 − N1
174.5(1)
O2 − Co1 − N3
90.4(1)
2.7 Cytotoxicity assay (MTT)
The antitumor activity of the three complexes on human MAD-MB-231, A549, CNE-2Z, and SMMC-7721 cells was determined by the MTT method. The cells were inoculated when the cell density reached 80% in every well. Then the compounds were diluted to different concentrations in the medium and added to 96-well plates. Meanwhile, cisplatin and DMSO were used as positive and negative controls, respectively. Cultivated (37 ℃, 5% CO2) for 72 h, 10 mL of MTT was added and incubated in a cell incubator for 4 h. After removing the culture medium, the formazan crystals were dissolved with 150 uL DMSO. The absorbance value at 490 nm was measured with a microplate reader, and then the IC50 was calculated (Kumar et al., 2018).
2.8 Cell migration
The migratory ability of the complexes was studied against CNE-2Z cells. Cells in the logarithmic growth phase were collected, centrifuged, and resuspended in medium containing 10% serum. Subsequently, cells were seeded in 6-well plates and incubated at 37 ℃, 5% CO2. When the cells density grew to 90%, a straight line was drawn in the central area, and the suspended cells were gently washed away twice with PBS. Different concentrations of the complexes were prepared with 4% serum in the medium and then added to a 6-well plates. Pictures of the migration were taken at 0, 12, and 48 h with an inverted microscope (Peng et al., 2022).
2.9 Exploration of apoptosis
Cell apoptosis of CNE-2Z cells was tested by Annexin-VFITC/PI method. The labeled Annexin V can be used as a probe to detect the changes of cell membrane caused by apoptosis, which can be used for examining apoptotic cells. Cells were seeded at a density of 1 × 106 cells/well in a 6-well plate and cultured (37 ℃, 5% CO2) for 24 h. CNE-2Z cells were treated with different concentrations of Ni (II) complexes for 24 h. After trypsinization without EDTA, the cells were colllected by centrifugation. Then washed twice with cold PBS and centrifuged for 5 min at 2000 r. 400 uL of Binding Buffer solution was added to resuspend cells, and then 5 uL of FITC and 10 uL of PI were added to the cell suspension in turn, mixing them gently. Next, cells were incubated for 5–10 min at room temperature in a dark environment. The cells were detected by flow cytometry (FACS) within 1 h (Wang et al., 2022).
2.10 Statistical analysis
The experimental data were analyzed and processed using SPSS 19.0 statistical software and expressed as mean ± SD. The differences between groups were compared by one-way ANOVA (One-way ANOVA) and t-test, and P < 0.05 was considered statistically significant between the two groups.
3 Results and discussion
3.1 Crystal structure analysis
Complex 1 crystallizes in tetragonal structure with space group P21/c (Table 1). As shown in Fig. 2a, the tetra-coordinate center Cu (II) ion is coordinated to N1, N3 O2 and Cl1 forming planar quadrilateral structure. Among which N1 and N3 atoms are from the C = N bond and imidazole of one 2,3-dihydroxybenzaldehyde-2-(2-aminophenyl)benzimidazole Schiff base ligand, O2 is from the hydroxyl group of the same Schiff base ligand and Cl1 is from copper chloride. As a result of the bond angles of tetra-coordinate center Cu (II) ion are ∠N1 − Cu1 − Cl1 = 93.8(2)°, ∠N3 − Cu1 − O2 = 94.5(7)°, ∠N3 − Cu1 − N1 = 91.0(8)° and ∠O2 − Cu1 − Cl1 = 90.1(5)° respectively, constructing a slightly distorted planar square geometry. The stacking diagram of complex 1 reveals that imidazole (benzene) rings of Schiff base ligands are parallel to the other adjacent imidazole (benzene) rings of Schiff base ligands, forming face-to-face (3.6575 Å, 3.9051 Å) intermolecular stacking (Fig. 2b). There are intramolecular hydrogen bonds O—H⋅⋅⋅O (D H1A⋅⋅⋅O2 = 2.2604 Å) in complex 1. Finally, the complex 1 forms three-dimensional structure through intermolecular π⋅⋅⋅π stacking interactions and hydrogen bonding.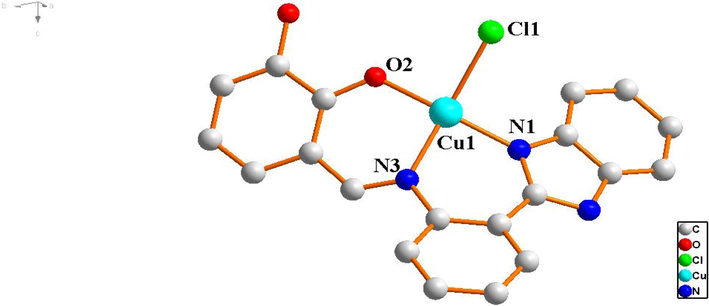
The molecular structure of the Cu (II) complex 1.
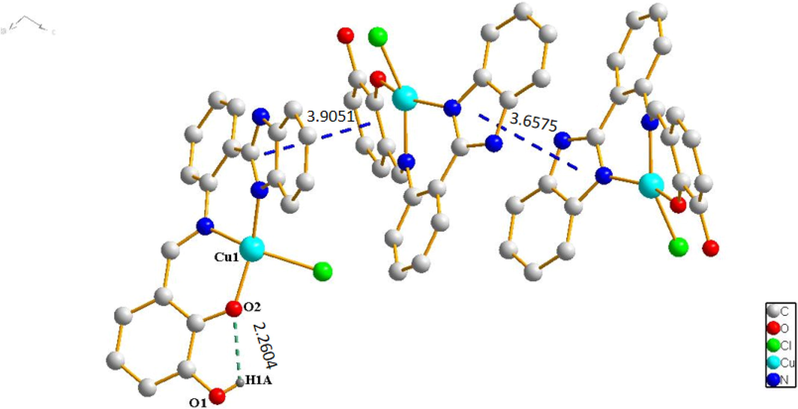
Hydrogen bonds (green dashed lines) and π⋅⋅⋅π interactions (blue dashed lines) in Cu (II) complex 1.
Complex 2 belongs to trigonal system with P-1 space group (Table 1). Its independent molecular unit is made up of four Ni (II) ions, two Schiff base ligands (L2-), four CH3COO– ions, and four C2H5OH molecules. As illustrated in Fig. 3a, its asymmetric unit contains two Ni (II) ions, one Schiff base ligand (L2-), two CH3COO– ions, and two C2H5OH molecules. The two asymmetric units exhibit the same coordination structural environment. Ni1 ion is coordinated by O3, O5 and O6 (from the carboxyl groups of two different acetate ions), O2 (from one hydroxyl of Schiff base ligand), N1 (from C = N bond of Schiff base ligand) and N2 (from imidazole of Schiff base ligand) to form six-coordinated octahedral structure. The bond angles of central Ni1(II) ion are ∠N1-Ni1-O6 = 89.6(1)°, ∠O6-Ni1-O3 = 84.1(2)°, ∠O3-Ni1-O2 = 98.4(2)°, and ∠O2-Ni1-N1 = 90.9(1)° respectively, indicating N1, O2, O3, and O6 atoms are located in an approximate plane. Therefore, the six-coordinated [Ni1O4N2] moiety forms a distorted octahedron. In Fig. 3b, the Ni2 ion is coordinated by O1 and O2 (from two hydroxys of one Schiff base ligand), O4 (from carboxyl groups of the acetate ion), O7 and O8 (from two ethanol molecules) and O1′ (from one hydroxys of the other Schiff base ligand) to form the similar octahedral structure. Owing to the approximate plane, the [Ni2O6] moiety also forms a slightly distorted octahedron geometry. Ni1 and Ni2 atoms are bridged by O3, O4 (from one acetate ion) and O2 (from one hydroxy of Schiff base ligand) to form a distorted hexagon structure geometry. Finally, Ni2 and Ni2′ atoms are bridged by two O1 and O1′ atoms of hydroxy from two different Schiff base ligands to form centrosymmetric independent unit.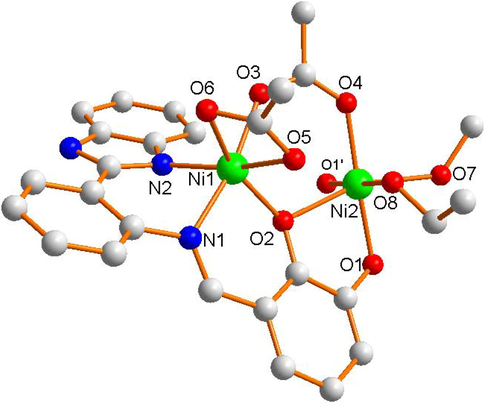
The asymmetric unit of the Ni (II) complex 1.
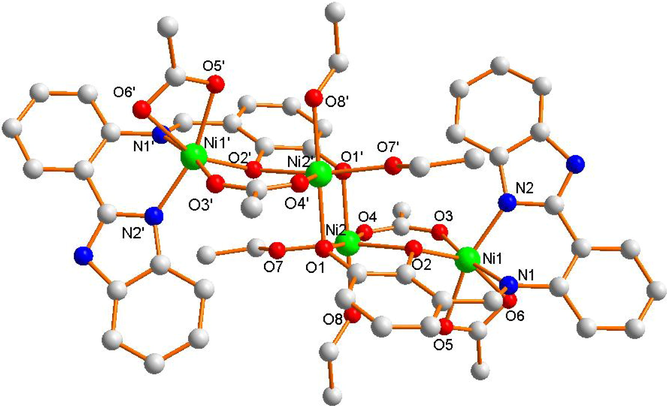
Molecular structure of ni (ii) complex 2.
As depicted in Fig. 3c, the imidazole ring of the molecule is almost parallel to the benzene ring of the adjacent complex molecule, forming face-to-face (3.7905 Å) intermolecular stacking. There are intramolecular hydrogen bonds (O-H⋅⋅⋅O, D H7A⋅⋅⋅O2 = 2.2232 Å, DH8A⋅⋅⋅O5 = 1.8969 Å) and intermolecular hydrogen bonds (N-H⋅⋅⋅O, D H3A⋅⋅⋅O6 = 1.9546 Å) in complex 2. Ultimately, complex 2 forms three-dimensional structure through π⋅⋅⋅π stacking and intramolecular hydrogen bonding interaction.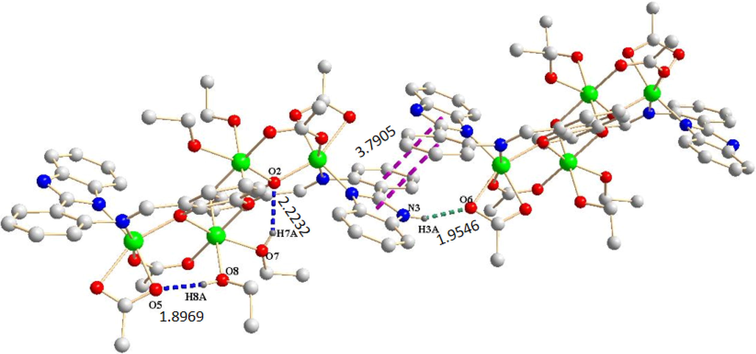
Hydrogen bonds (blue,green dashed lines) and π⋅⋅⋅π interactions(purple dashed lines) in Ni(II) complex 2.
Complex 3 crystallizes in tripartite system with R-3 space group (Table 1). Complex 3 is six-coordinated octahedral configuration, in which central Co (II) ion is coordinated by N1, N4 from two benzimidazole ring of two Schiff base ligands, N3, N6 from two imine group of two Schiff base ligands and two O2, O4 from hydroxyl of two different Schiff base ligands, respectively (Fig. 4a). The bond angles of central Co (II) ion are ∠N6 − Co1 − N4 = 84.6(1)°, ∠N6 − Co1 − O4 = 92.1(1)°, ∠N4 − Co1 − O2 = 90.3(1)°, and ∠O2 − Co1 − O4 = 87.5(1)° respectively. As a result, the [ Co N1 N6 O2 O4] moiety is located in an approximate plane geometry. Eventually, the Co (II) forms a slightly distorted octahedral geometry. Their bond lengths Co1-N1 = 1.944(3) Å, and Co1-N6 = 1.921(3) Å, and Co1-O2 = 1.903(3) Å and Co1-O4 = 1.893(2) Å are in normal range.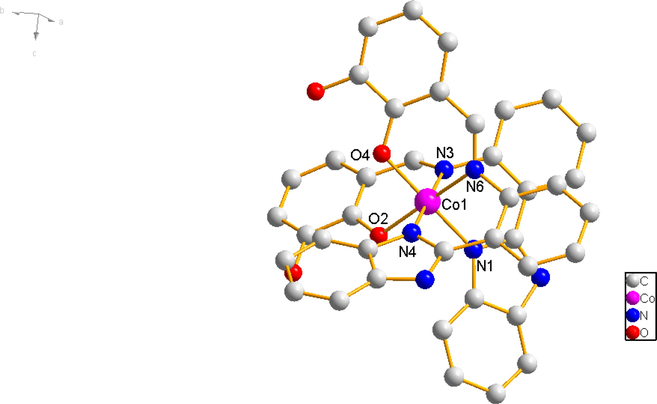
Molecular structure of co (ii) complex 3.
As shown in Fig. 4b, there are intramolecular hydrogen bonds (O-H⋅⋅⋅O, D H1A⋅⋅⋅O2 = 2.3025 Å, D H3A⋅⋅⋅O4 = 2.2932 Å) and intermolecular hydrogen bonds (N-H⋅⋅⋅N, D H5A⋅⋅⋅N2 = 1.9408 Å) within the complex 3. The complex 3 produce C-H⋅⋅⋅π stacking interactions, which the phenyl ring of 2,3-dihydroxysalicylaldehyde from one ligand is approximately perpendicular to the imidazole ring of 2-(2-aminophenyl)benzimidazole from the other, with an edge-to-face distance of 2.9196 Å. At last, the complex 3 monomer molecules are connected through C-H⋅⋅⋅π interactions and intermolecular hydrogen bonds.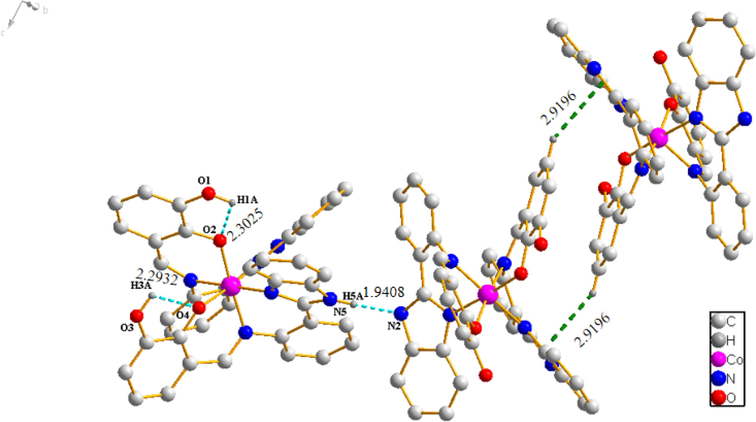
Hydrogen bonds (cyan dashed lines) and C-H⋅⋅⋅π interactions (green dashed lines) in Co (II) complex 3.
3.2 Thermal stability of complexes 1, 2 and 3
As shown in Fig. 5a, the TG-DTG curves of the three complexes were studied. The weight loss process consists of four steps in complex 1. Firstly, the two weight losses are 8.92% and 8.95%, corresponding to loss of two coordinated ethanol molecules (98 ℃, 254 ℃), respectively. Secondly, the three weight loss is 26.5%, assigning to lose 2,3-dihydroxybenzaldehyde in 293 to 437 ℃ range. Finally, the skeleton of the complex 1 collapses and decomposes in 437 to 800 ℃ range. As shown in Fig. 5b, there are three weight loss steps in complex 2, in which weight losses are 9.03% (149 ℃), 24.84% (149 to 387 ℃) and 48.05% (387 to 685 ℃) respectively. The three weight losses correspond to the departure of two coordinated acetate ions, one 2,3-dihydroxybenzaldehyde-2-(2-aminophenyl)benzimidazole Schiff base ligand molecule, and the other 2,3-dihydroxybenzaldehyde-2-(2-aminophenyl)benzimidazole Schiff base ligand molecule, two coordinated ethanol molecules, and four coordinated acetate ions, respectively. Complex 2 produces stable residues above 685℃. Complex 3 also takes place in three stages, corresponding to losses of mass of 29.31% (285 ℃) and 18.79% (356 to 528 ℃), respectively (Fig. 5c), attributed to eliminating 2,3-dihydroxybenzaldehyde-2-(2-aminophenyl)benzimidazole Schiff base ligand, and 2,3-dihydroxybenzaldehyde. Complex 3 is inconstant in the third stage above 528 ℃. According to TG-DTG curves of the three complexes, complex 3 has higher thermal stability than complex 1 and complex 2.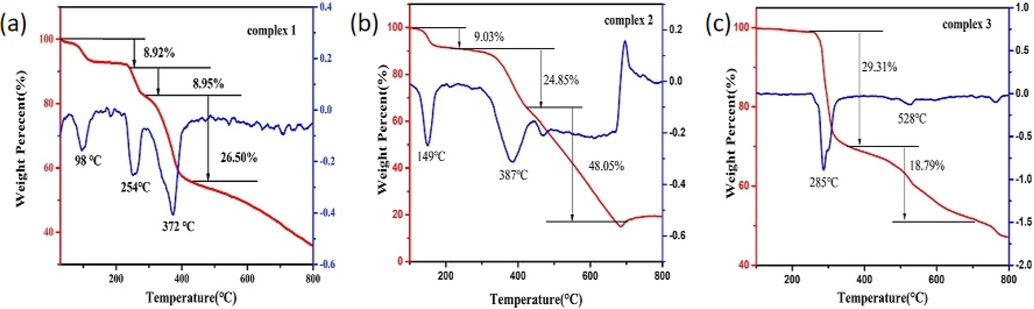
TG and DTG curves of complexes 1, 2 and 3.
3.3 Powder XRD patterns of complexes 1,2 and 3
The powder XRD patterns of complex 1, 2 and 3 was further investigated by powder X-ray diffraction (PXRD) (Fig. 6). The intensities of diffraction peaks of complex 1, 2 and 3 are in accordance with their theoretical diffraction peaks generated from single crystal X-ray analysis. This indicates that the crystal samples of the three complexes conform to the crystal structures determined by single crystal analysis.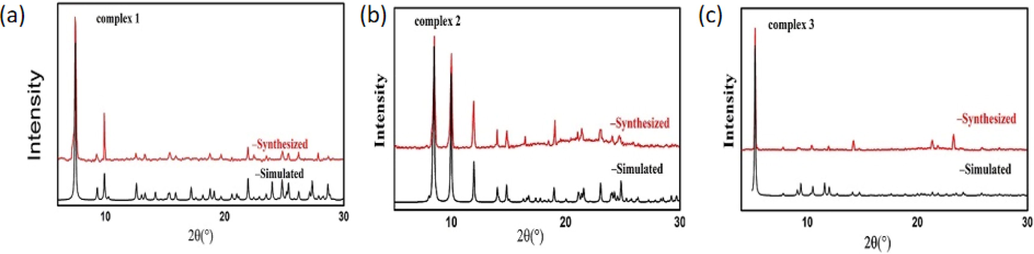
XRD patterns of complexes 1, 2 and 3 (Black- simulated from CIFs, Red-experimentally synthesized).
3.4 Cytotoxicity assay (MTT)
The anticancer effect of the compounds was evaluated against the human cancer cell lines and the IC50 values are in Table 3. The results indicate that complex1-3 exhibit greater anticancer activity than ligand. Among them, complex 2 shows stronger anticancer activity than the other two complexes against four human cancer cell lines. Meanwhile, complex 2 exhibits lower cytotoxicity than cisplatin on CNE-2Z cells. It may be related to the tetranuclear complex of complex 2.
Compounds
IC50 (µM ± SD)
CNE − 2Z
MDA − MB − 231
A549
SMMC − 7721
complex 1
20.05 ± 0.72
15.43 ± 0.48
37.72 ± 0.65
54.79 ± 1.12
complex 2
1.99 ± 1.60
8.84 ± 0.30
23.14 ± 2.73
21.50 ± 1.07
complex 3
17.18 ± 0.14
15.98 ± 0.78
43.34 ± 0.60
73.10 ± 2.01
H2L
24.36 ± 2.02
38.79 ± 0.79
100.69 ± 1.99
41.03 ± 1.50
Cisplatin
4.54 ± 0.04
4.02 ± 0.08
15.74 ± 0.54
4.42 ± 0.11
3.5 CNE-2Z cell migration were inhibited by complexes
The effect of complexes 1, 2 and 3 against the migration of CNE-2Z cells was observed using a wound-healing assay. According to Fig. 7, complex 1 was applied to CNE-2Z cells at 0 µM, 8 µM and 16 µM. After 24 h, the cell migration rates were 75.64%, 53.68%, and 46.31%, respectively. Complex 2 was applied to CNE-2Z cells at concentrations of 8 µM and 16 µM. After 24 h, the cell migration rates were 37.90% and 26.30%, respectively. Similarly, for complex 3, at 0 µM, 8 µM and 16 µM, the cell migration rates were 44.88%, 38.63% and 31.64% respectively after 12 h. The cell migration rate was more than 65% at 24 h. According to the results of the study, complex 2 inhibits the lateral migration ability in a concentration-dependent manner, complex 1 can inhibit the migration of CNE-2Z cells at high concentrations, and complex 3 has little effect on the migration ability of CNE-2Z cells at low or high concentrations.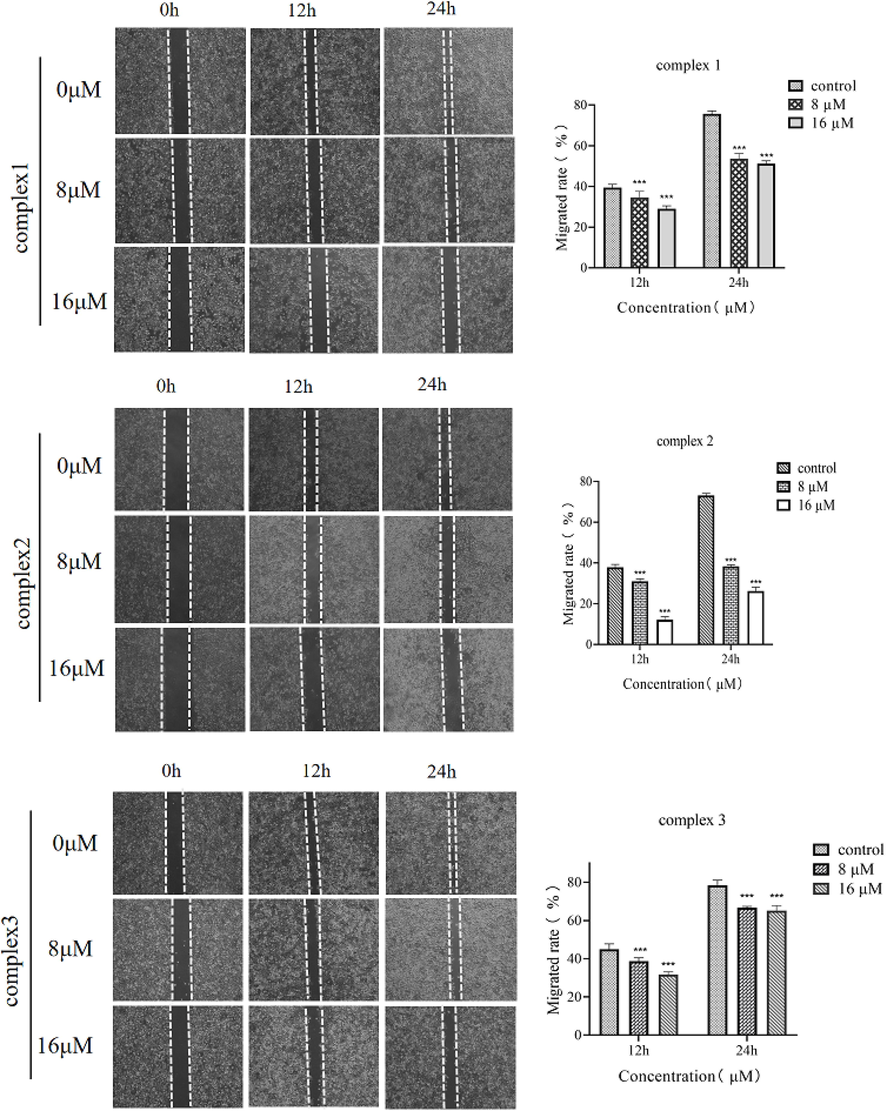
Effect of complex 1, complex 2 and complex 3 on the migration ability of CNE-2Z cells. Data represent mean ± SD (n = 3) and use one-way ANOVA with Tukey’s multiple comparison test, ***p < 0.005 vs. Control.
3.6 Cells apoptosis
To further investigate the inhibitory effect of complex 2 on CNE-2Z cells, the FITC-PI double staining method was used in this experiment to study. The effect of different concentrations of complex 2 on the apoptosis of CNE-2Z cells for 24 h (Fig. 8). Approximately 10.06%, 15.14% and 26.10% of apoptotic cells were observed when CNE-2Z cells were treated with 4 µM, 8 µM and 16 µM of complex 2, respectively. This shows that complex 2 can significantly stimulate apoptosis of CNE-2Z cells with increasing concentration, and the effect is proportional to the concentration of the Ni (II) complex.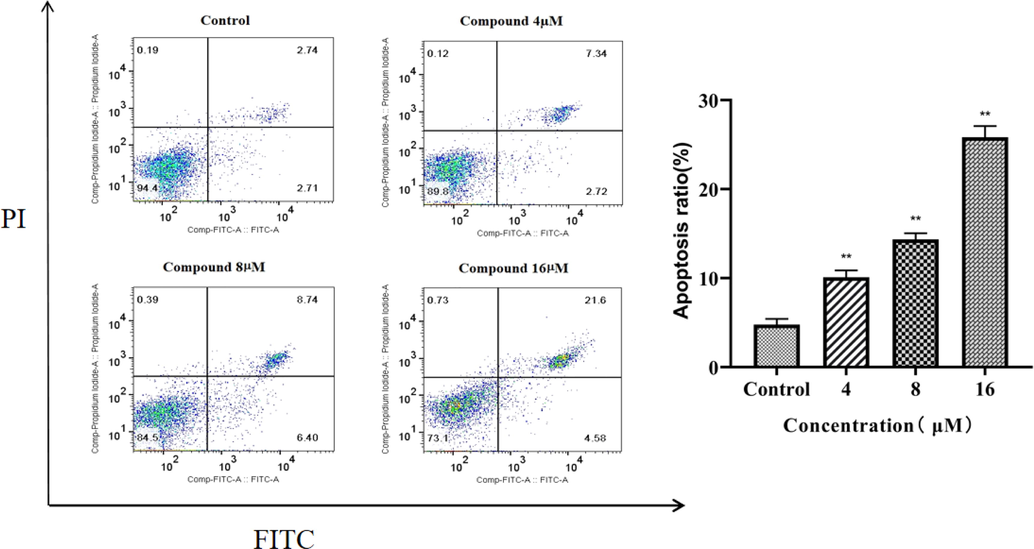
The effect of complex 2 on CNE − 2Z cell apoptosis rate: a Effect of different concentrations of complex 2 on apoptosis of CNE-2Z cells for 24 h. Apoptosis rate of complex 2 at 0 µM, 4 µM, 8 µM, and 16 µM (**P < 0.05).
4 Conclusion
In our study, 2-(2-aminophenyl)benzimidazole and 2,3-dihydroxybenzaldehyde were used to synthesize schiff base ligand, and three metal complexes [Cu(HL)Cl](C2H5OH)2, [Ni4L2(CH3COO)4(C2H5OH)4] and [Co(HL)2] were obtained by solvothermal method. X-ray single crystal diffraction analysis confirmed that Cu (II) complex is tetra-coordinated mononuclear, Ni (II) complex is six-coordinated tetranuclear molecule, and Co is tetra-coordinated mononuclear complex. MTT assay was studied for anticancer activities against different cancer cells. The anticancer activities of the three complexes are significantly better than that of the Schiff base ligand. It is worth mentioning that the anti-proliferative activity of complex 2 against CNE-2Z cells is better than that of cisplatin. Cell scratch assay shows that migration ability against CNE-2Z cells for complex 2 is high relative to increasing concentration. The results of apoptosis experiments also showed that complex 2 can significantly promote the apoptosis of CNE-2Z cells, and the apoptosis rate is proportional to the drug concentration.
Acknowledgments
This work was supported by the Key Natural Science Research Foundation for the University of Anhui (KJ2021A0770), the Major project of the Natural Science Research Foundation for the University of Anhui (KJ2021ZD0079), the Key Special Project of Transforming Medicine of Bengbu Medical College (BYTM2019001), and the Graduate student scientific research innovation projects of Bengbu Medical College (Byycx22064).
Author contributions
Min Hou: Performed the research, wrote the paper and financial support. Hou Cong Li: Synthesized the complexes. Ning An: performed the PXRD and TGA tests.Wen Ge Li*: Designed the research, performed the crystal structure study, wrote the paper and financial support. Jing Tong*: Designed the research, performed the crystal structure study, wrote the paper and financial support.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Therapeutic evolution of benzimidazole derivatives in the last quinquennial period. Eur. J. Med. Chem.. 2017;27(126):705-753.
- [CrossRef] [Google Scholar]
- In vitro evaluation of the potential pharmacological activity and molecular targets of new benzimidazole-based schiff base metal complexes. Antibiotics (Basel).. 2021;10(6):728.
- [CrossRef] [Google Scholar]
- Synthesis and characterization of different complexes derived from Schiff base and evaluation as a potential anticancer, antimicrobial, and insecticide agent. Saudi J Biol Sci.. 2023;30(3):103598
- [CrossRef] [Google Scholar]
- Antimicrobial Activities of 1-H-Benzimidazole-based Molecules. Curr. Top. Med. Chem.. 2016;16(26):2953-2962.
- [CrossRef] [Google Scholar]
- Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin.. 2018;68(6):394-424.
- [CrossRef] [Google Scholar]
- BrukerSAINT, Bruker AXS Inc. (Madison, Wisconsin, USA, 2012).
- Metal complexes of a novel heterocyclic benzimidazole ligand formed by rearrangement-cyclization of the corresponding Schiff base. Electrosynthesis, structural characterization and antimicrobial activity. Dalton Trans.. 2018;47(12):4325-4340.
- [CrossRef] [Google Scholar]
- Novel chitosan-based Schiff Base compounds: chemical characterization and antimicrobial activity. Molecules. 2022;27(9):2740.
- [CrossRef] [Google Scholar]
- New transition metal ion complexes with benzimidazole-5-carboxylic acid hydrazides with antitumor activity. Eur J Med Chem. 2009 Apr;44(4):1500-1508.
- [CrossRef] [Google Scholar]
- Cisplatin: The first metal based anticancer drug. Bioorg. Chem.. 2019;88:102925
- [CrossRef] [Google Scholar]
- Synthesis, spectroscopic characterization and antiproliferative evaluation in vitro of novel Schiff bases related to benzimidazoles. Eur. J. Med. Chem.. 2011;46(6):2274-2279.
- [CrossRef] [Google Scholar]
- ShelXle: a Qt graphical user interface for SHELXL. J. Appl. Cryst.. 2011;44(Pt 6):1281-1284.
- [CrossRef] [Google Scholar]
- Comprehensive review in current developments of benzimidazole-based medicinal chemistry. Chem. Biol. Drug Des.. 2015;86(1):19-65.
- [CrossRef] [Google Scholar]
- Benzimidazole-core as an antimycobacterial agent. Pharmacol. Rep.. 2016;68(6):1254-1265.
- [CrossRef] [Google Scholar]
- Recent advances and therapeutic journey of schiff base complexes with selected metals (Pt, Pd, Ag, Au) as potent anticancer agents: a review. Anticancer Agents Med Chem.. 2022;22(18):3086-3096.
- [CrossRef] [Google Scholar]
- Cisplatin resistance: preclinical findings and clinical implications. BBA. 2010;1806(2):172-182.
- [CrossRef] [Google Scholar]
- Advances in research of Schiff-base metal complexes as potent antioxidants. Curr. Med. Chem.. 2013;20(36):4609-4632.
- [CrossRef] [Google Scholar]
- Comparison of silver and molybdenum microfocus X-ray sources for single-crystal structure determination. J. Appl. Crystallogr.. 2015;48(Pt 1):3-10.
- [CrossRef] [Google Scholar]
- Analysis of Cell Viability by the MTT Assay. Cold Spring Harb Protoc.. 2018;2018(6)
- [CrossRef] [Google Scholar]
- Exploiting the biological efficacy of benzimidazole based Schiff base complexes with l-Histidine as a co-ligand: combined molecular docking, DNA interaction, antimicrobial and cytotoxic studies. Bioorg. Chem.. 2018;77:269-279.
- [CrossRef] [Google Scholar]
- Benzimidazole - Schiff bases and their complexes: synthesis, anticancer activity and molecular modeling as Aurora kinase inhibitor. Z. Naturforsch., C: J. Biosci.. 2018;73(11–12):465-478.
- [CrossRef] [Google Scholar]
- Synthesis, DNA binding, cellular DNA lesion and cytotoxicity of a series of new benzimidazole-based Schiff base copper(II) complexes. Dalton Trans.. 2015;44(46):19983-19996.
- [CrossRef] [Google Scholar]
- Layered double hydroxides-loaded sorafenib inhibit hepatic stellate cells proliferation and activation in vitro and reduce fibrosis in vivo. Front. Bioeng. Biotechnol.. 2022;27(10):873971
- [CrossRef] [Google Scholar]
- Tridentate imidazole-based Schiff base metal complexes: molecular docking, structural and biological studies. J. Biomol. Struct. Dyn.. 2022;40(18):8602-8614.
- [CrossRef] [Google Scholar]
- Novel benzimidazole-acrylonitrile hybrids and their derivatives: Design, synthesis and antimycobacterial activity. Eur. J. Med. Chem.. 2020;15(188):112010
- [CrossRef] [Google Scholar]
- Schiff Base Cobalt(II) complex-catalyzed highly markovnikov-selective hydrosilylation of alkynes. Org. Lett.. 2021;23(3):663-667.
- [CrossRef] [Google Scholar]
- Phytochemicals: current strategy to sensitize cancer cells to cisplatin. Biomed. Pharmacother.. 2019;110:518-527.
- [CrossRef] [Google Scholar]
- 2-Mercaptobenzimidazole Schiff Bases: Design, Synthesis, Antimicrobial Studies and Anticancer Activity on HCT-116 Cell Line. Mini Rev. Med. Chem.. 2019;19(13):1080-1092.
- [CrossRef] [Google Scholar]
- Global cancer incidence and mortality rates and trends–an update. Cancer Epidemiol. Biomark. Prev.. 2016;25(1):16-27.
- [CrossRef] [Google Scholar]
- Paradol induces cell cycle arrest and apoptosis in glioblastoma cells. Nutr. Cancer. 2022;74(8):3007-3014.
- [CrossRef] [Google Scholar]
- A novel and green cellulose-based Schiff base-Cu (II) complex and its excellent antibacterial activity. Carbohydr. Polym.. 2020;15(230):115671
- [CrossRef] [Google Scholar]
- Structure activity relationship (SAR) study of benzimidazole scaffold for different biological activities: a mini-review. Eur. J. Med. Chem.. 2015;5(97):419-443.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2023.105144.
Appendix A
Supplementary data
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







