Translate this page into:
Synthesis, structure elucidation and plants growth promoting effects of novel quinolinyl chalcones
⁎Corresponding author. mmhassan121@yahoo.com (Mohamed M. Hassan)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The readily synthesized 3-(4-Hydroxy-1-methyl-1,2-dihydro-2-oxoquinolin-3-yl)-1-phenyl-1H-pyrazole-4-carbaldehyd (5) and 3-(2-Oxo-2H-chromen-3-yl)-1-phenyl-1H-pyrazole-4-carbaldehyde (6) were utilized as a convenient starting precursor materials for synthesis of novel enone system 4-hydroxy-1-methyl-3-(4-(2H-2-oxo-chromen-3-yl)prop-2-enoyl)-1-phenyl-1H-pyrazol-4-yl)quinolin-2(1H)-one (7) and4-hydroxy-1-methyl-3-(2E)-3-(3-(2-oxo-2H-chromen-3-yl)-1-phenyl-1H-pyrazol-4-yl)acryloyl)quinolin-2(1H)-one (8). Simple homonuclear NOE experiment (NOESY 1D) method was performed for structure elucidation of the novel quinolinyl chalcones. The synthesized compounds have been estimated for their effect of growth on some selective crop of plants (Hibiscus, Mint and Basil).
Keywords
Pyrazolocarbaldehydes
Chalcones
Homonuclear
Hibiscus
Basil
1 Introduction
Synthetically, 2-quinolinones are considered as active classes of heterocyclic organic compounds. They received much interest in the latter years because of their wide variety of applications in organic chemistry (Huang and Chang, 2008). Also, coumarine (2H-1-benzopyran-2-one) derivatives had recently a considerable interest due to their significant usage as starting material for synthesis of many active organic compounds (Špirtović-Halilović et al., 2014). A growing interest in synthesis of compounds with the backbone of chalcone as a reactive keto-vinyl chain (—CO—CH⚌CH—). Chalcones had confirmed their efficiency in considerable biological and pharmacological effectiveness like, antimicrobial (Solankee et al., 2010), anti-inflammatory (Bandgar et al., 2010; Vogel et al., 2010; Kim et al., 2007), antimalarial (Hans et al., 2010), antifungal (Bag et al., 2009) antitumor (Echeverria et al., 2009; Modzelewska et al., 2006), and antioxidant activity (Venkatachalam et al., 2012; Doan and Tran, 2011; Vogel et al., 2008). In the light of this information, we expected that the conjunction between both 2-quinolinone and coumarine moieties via chalcone linkage may promote their activities. We planned to prepare a new crossbred chalcones type of quinolinone and coumarine nucleus together in the same framework. Homonuclear NOE (NOESY 1D) spectral tool was used for structure elucidation of the novel chalcones in addition to their spectral data (IR, 1H NMR, and ESI-MS).
It is important to remind that chalcone derivatives attracted researchers interest not only for their synthetic uses but also for their biological implementations. Recently, Kalambe et al., (Kalambe et al., 2015) reported the effects of the substituted chalcones on different crop plant growth as the medicinal plants cultivation needs more new fertilizers for the enhancement of their growth (Bhattacharjee et al., 2020). In view of these facts, our newly synthesized chalcons were examined for the growth effects on some selective agriculture crop plants namely; Hibiscus, Mint and Basil because of its various medical and pharmaceutical applications.
Hibiscus (Malvaceae) have gained researchers attention. The Hibiscus genus contains more than 300 species, but kenaf (Hibiscus cannabinus L.) and roselle (Hibiscus sabdariffa L.) are the most two important species within the genus (Wang et al., 2012). Hibiscus anthocyanins (HAs), as a set of naturalistic dyes present in the plant calyx, showed antioxidant action. Many researchers indicated that anthocyanins have the ability to slow down the development of cancer cells and depresses the lipoprotein (LDL) oxidation (Mohamed et al., 2012). Roselle seeds are perfect lipid source which dissolve antioxidants, in particular copherol. Diversity of plant kinds are widely utilized due to its hypoglycemic or anti-diabetic character (Puro et al., 2014).
Mint is one of the most famous flavors coming after vanilla and citrus aromas. Fresh or dried leaves of menthe species are used as a condiment and also their essential oils are produced (Arslan et al., 2010). Mint is considered as one of the most public and cultivated aromatic plants. Mint showed a wide range of uses of carminative and antiemetic in addition to providing a major source of dietary phenolic compounds, which are considered the most abundant natural antioxidants (Figueroa Pérez et al., 2014; Dorman et al., 2003). Mint oil is widely spread as an effective ingredient in trade medicines, for example in cough drops and syrups. It can also be applied for local analgesic, cramps, arthritis, sprains, and muscle aches. Mint oil has antibacterial activities because it was contained menthol (Schuhmacher et al., 2003). It is also examined as environmental secure pesticides (Akbari et al., 2015).
Basil or sweet basil (Ocimumbasilicum L.) is considered as an perennial shrub which follows Lamiaceae (Labiatae) family. Traditionally, the plant has been employed in folk medicine for its carminative, stimulant, and antispasmodic properties (Marotti et al., 1996). Basil is used as a medicinal herb in medical treatments such as for headaches, coughs, diarrhea, worms, and kidney malfunctions. Basil considered as a source of aroma compounds, and it possesses a range of biological activities such as insect repellent, nematocidal, antibacterial, antifungal agents and antioxidants activities (Telci et al., 2006).
2 Results and discussion
2.1 Synthesis and structure elucidation of the novel quinolinyl chalcones
Treatment of 3-acetyl-4-hydroxyquinolin-2(1H)-one (1) (Tomita, 1951) and/or 3-acetyl-2H-chromen-2-one (2) (Siddiqui et al., 2009; Sahu et al., 1996) with phenylhydrazine afforded the corresponding hydrazones 3 and 4 according to the literature methods (Kappe et al., 1995; Stadlbauer and Hojas, 2004; Chodankar et al., 1986). The synthesized hydrazones were reacted with Vilsmeier-Haack reagent (DMF-POCl3) (Singh et al., 2005) furnishing the pyrazolo-4-carbaldehydes 5 and 6, respectively (Abdel-Megid et al., 2007; Laxmi et al., 2013).
The desired chalcones yielded efficiently under the well established conditions of the base-catalyzed Claisen– Schmidt reaction (Mogilaiah et al., 2010). Hence, condensation of equivalent quantities of pyrazole-4-carbaldehydes 5 and 6 with the proper 3-acetyl derivatives in the presence of piperidine as catalyst furnished the desirable chalcones 7 and 8 (Scheme 1). The assuming novel E-form enones 7 and 8 were identified from their spectral analysis. 1H NMR spectrum displayed the vanishing of both pyrazolo-4-carbaldehydes 5 and 6 (—CHO) protons (Abdel-Megid et al., 2007; Laxmi et al., 2013) and instead the outcrop of ortho-coupled two doublets at δ range between 7.00 ppm and 8.00 ppm characteristic for both Hα and Hβ. Coupling constant in the range among 15–16 Hz confirm the trans geometry at the vynilic protons of both 7 and 8 products. The structure of the suggested chalcones 7 and 8 were also supported on the basis of their mass (ESI) spectra which showed the promising peaks at m/z 538 [(M + Na)]+ and 518 [(M + H)]+, respectively.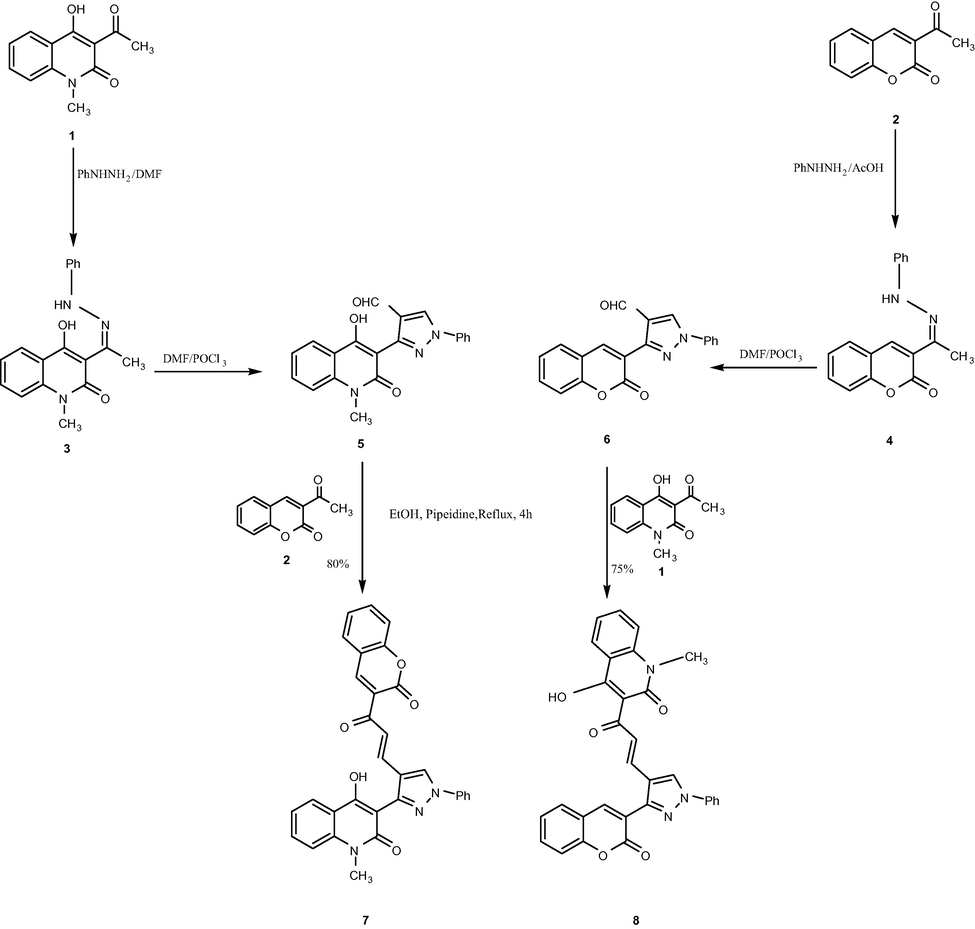
Synthesis of the new chalcones 7 and 8.
Common analytical methods were not able to distinguish between both 7 and 8 products due to their similar spectrum data. In a very elegant way, the structures elucidation was performed using homonuclear NOE experiments (NOESY 1D) as an excellent distinguishable tool. Using this approach has never been used according to our knowledge for structural determination of such chalcones. NOE experiment was carried out on the distinct OH groups at the quinolinone moiety of both products 7 and 8 (Fig. 1). Thus, irradiation of the OH singlet of one of the resulting chalcones that exhibits a high proton frequency (δ11.50 ppm), gives an adverse impact on one of the olefinic chalcone protons beside H-5 of the quinolinone ring indicating the proposed structure of chalcone 7 (Fig. 2), whilst running the same experiment on the other chalcone product gave a positive effect on only H-5 of the quinolinone ring as a strong support for the other suggested chalcone structure 8 (Fig. 3).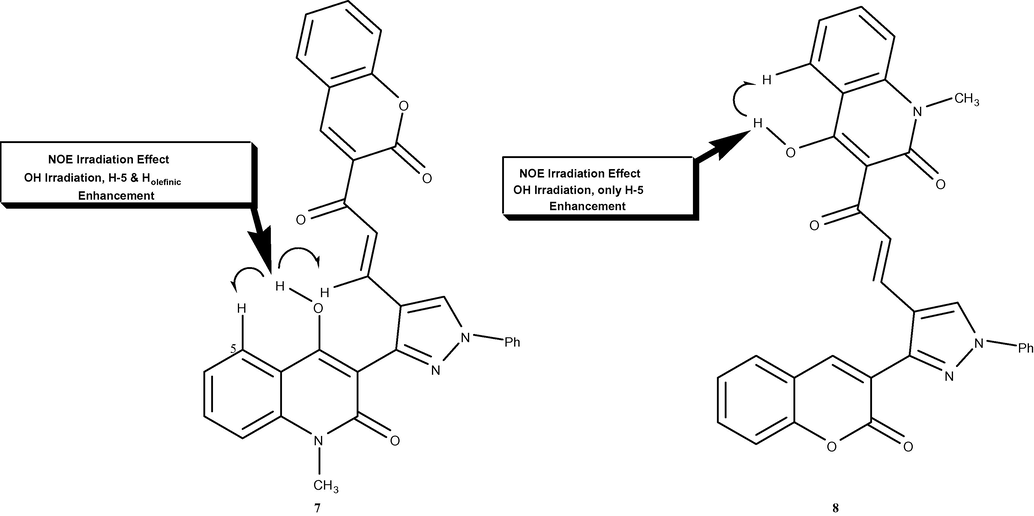
Confirmation of compound 7 and 8 structures utilizing NOE effect.
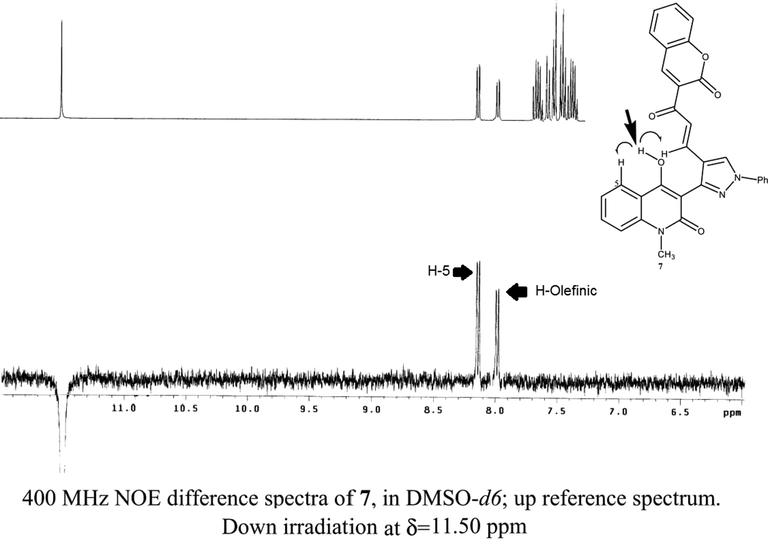
Confirmation of compound 7 structures utilizing NOE effect.
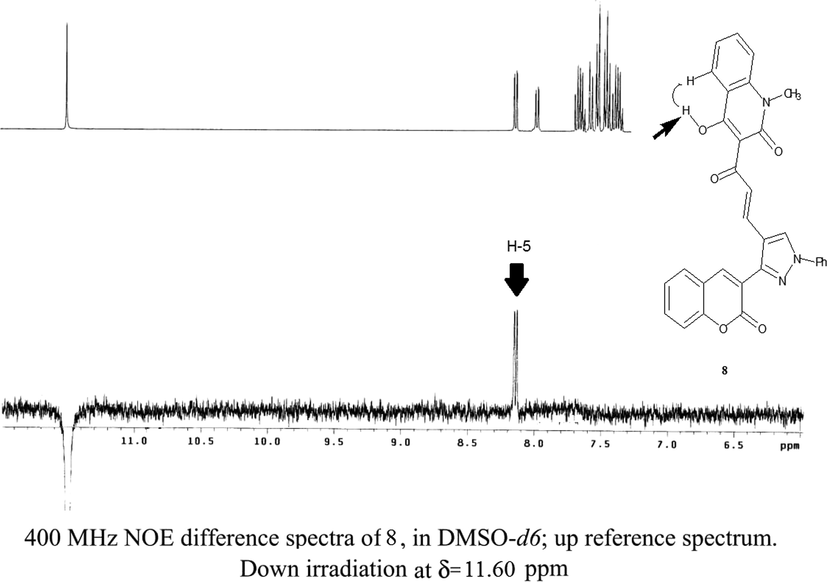
Confirmation of compound 8 structures utilizing NOE effect.
2.2 Growth promoting effect of the novel quinolinyl chalcones
On an open field a beds of black cotton ground were arranged. Selective seeds of Hibiscus, Mint and Basil were selected accurately. The three types of seeds were separately planted and irrigated in the ready beds using the conventional way. From every bed crops were divided to two separate categories, category A (control category) and category B (treated category). Category (A) group were preserved without spraying, while that from category (B) were treated with the examined compounds. Category (B) were addressed with examined compounds before sowing to test promoting growth effects. Novel chalcones solutions were prepared individually in 1,4-dioxane (0.01 M) and were used for bed spraying thrice at fortnightly intervals (15, 30, 45, 60, 75 and 90 days).
Tests were performed to match the category (B) plants with that plants from category (A). Samples were taken at 15, 30, 45, 60, 75 and 90 days after sowing. Plants were accurately checked, and the number of functional leaves and heights of shoots were registered accurately. The percent of change in the shoot height and the No. of leaves was calculated according to the following equation:
Results in Tables 1 and 2 show that all treated plants possess noteworthy growth in shoot in addition to a remarkable enhancement in the leaves numbers against the untreated samples. This enhancement of the growth in the treated plants is similar to (Kalambe et al., 2015) who reported the positive effect of substituted chalcones on some crop plants. The positive effect of substituted chalcones can be attributed to the fact that chalcones as flavonoids in plants have several roles such as suppression the inhibitors of auxin (the key growth hormones), pigments production, phytoalexins production, UV protectants, signal molecules in plantmicrobe interactions, antioxidants, and pollinator attractants or feeding deterrents (Rozmer and Perjési, 2016). These compounds have a critical role in the interaction of plants with their environment (Dao et al., 2011).
Periodicity of the observation (days)
Cultivated Crops
Hibiscus
Mint
Basil
Shoot height
No. of leaves
Shoot height
No. of leaves
Shoot height
No. of leaves
C
T
% change
C
T
% change
C
T
% change
C
T
% change
C
T
% change
C
T
% change
15
8
13
62.5
5
8
60.0
8
10
25.0
8
10
25.0
10
13
30.0
14
18
28.6
30
16
22
37.5
8
11
37.5
11
13
18. 2
14
18
28.6
14
16
14.3
24
32
33.3
45
36
44
22.2
9
14
55. 6
13
15
15.4
17
20
17.6
16
22
37.5
38
46
21.1
60
54
62
14.8
11
16
45. 5
16
18
12.5
30
39
30.0
20
25
25.0
44
51
15.9
75
72
78
8.3
14
18
28.6
19
22
15.8
32
39
21.9
24
32
33.3
53
62
17.0
90
89
95
6.7
16
22
37.5
20
24
20.0
38
42
10.5
26
33
26.9
61
74
21.3
Periodicity of the observation (days)
Cultivated Crops
Hibiscus
Mint
Basil
Shoot height
No. of leaves
Shoot height
No. of leaves
Shoot height
No. of leaves
C
T
% change
C
T
% change
C
T
% change
C
T
% change
C
T
% change
C
T
% change
15
7
20
185.7
4
10
150.0
9
12
33.3
7
19
171.4
11
17
54.5
16
30
87.5
30
17
30
76.5
9
14
55.5
12
14
16. 7
14
26
85.7
13
20
53.8
22
38
72.7
45
32
50
56.3
11
18
63.6
14
17
21.4
18
35
94.4
25
30
20.0
36
54
50.0
60
55
74
34.5
13
20
53.8
17
20
17.6
28
44
57.1
26
35
34.6
42
66
57.1
75
73
85
16.5
16
22
37.5
20
23
15.0
31
59
90.3
30
38
26. 7
50
78
56.0
90
90
100
11.1
18
25
38. 9
21
26
23.8
36
66
83.3
31
40
29.0
63
89
41.3
Interestingly chalcone 8 displayed more strong vegetative growth than chalcon7, as this appeared in the percent of the change in the chalcone 8 treated plants were obvious higher than that in chalcon 7 treated plants (Tables 1 and 2). This may be attributed to the far distance between the olefinic chalcone proton and the (—OH) group at the quinolinone moiety in chalcone 8 which permitted more stability in aqueous soil and more effective due to its free-soil mobility so be easy to be absorbed with plants (Salem et al., 2019).
3 Materials and methods
3.1 General
Optimal automated melting point system was applied for melting points measurements and are not corrected. Purification of products was performed using thin layer chromatography with 0.2-mm silica gel F-254 (Merck) plates, visualization with ultra violet source (254 and 366 nm) exposure. (JASCO,V-670 UV–VIS–IR) double-beam spectrophotometer were applied for recording of IR spectra. Micro mass LC-ZMD spectrometric apparatus was used for detecting electrospray ionization (ESI) mass spectra. Varian Mercury VX-400apparatus was used for proton 1H NMR spectral detection using(CD3)2SO as solvent and TMS as a reference. Perkin-Elmer 2400I was used for elemental microanalyses. 3-(4-Hydroxy-1-methyl-1,2-dihydro-2-oxoquinolin-3-yl)-1-phenyl-1H-pyrazole-4-carbaldehyd (5) and 3-(2-Oxo-2H-chromen-3-yl)-1-phenyl-1H-pyrazole-4-carbaldehyde (6) have been synthesized according to the reported methods (Abdel-Megid et al., 2007; Laxmi et al., 2013).
3.2 Procedure for preparation of 3-(4-Hydroxy-1-methyl-1,2-dihydro-2-oxoquinolin-3-yl)-1-phenyl-1H-pyrazole-4-carbaldehyde (5)
Phosphoryl chloride POCl3 (30 mmol) was added up to DMF (30 mmol) with continuous stirring. The reaction content was left to cool down before adding the hydrazone3 (10 mmol) solution in DMF (10 ml). Stirring condition was maintained for 1 h at room temperature and then gradually raised to 70–80 °C for 4 h. The reaction content was decanted onto mashed ice and then cold potassium carbonate solution was used for neutralization. The precipitated product was separated by filtration before subjected to purification using flash-chromatography with ethyl acetate/petroleum ether as eluent (1:1, v/v). Finally crystallization was performed from ethanol to yield the product as yellow crystals; (40% yield); m.p 230–232 °C (Lit. 228–230 °C, Abdel-Megid et al., 2007).
3.3 Procedure for preparation of 3-(2-Oxo-2H-chromen-3-yl)-1-phenyl-1H-pyrazole-4- carbaldehyde (6)
POCl3 (14 mmol) was added to cold DMF solution (14 mmol) at 0–5 °C. 3-[1-(phenyl-hydrazono)-ethyl]-chromen-2-one (4) (3.5 mmol) was added and the content was subjected to contentious stirring for 4 h at room temperature. Accomplishment of the reaction was adjusted by TLC. The reaction content was poured to ice before neutralization with 10% NaOH solution. The precipitate was filtered off, dried, and recrystallized from ethanol as yellow crystals; (60% yield); m.p 179–182 °C (Lit.180–185 °C, Laxmi et al., 2013).
3.4 Procedure for preparation of 4-hydroxy-1-methyl-3-(4-((2H-2-oxo-chromen-3-yl)prop-2-enoyl)-1-phenyl-1H-pyrazol-4-yl)quinolin-2(1H)-one (7)
Magnetically stirred solution of 5 (1 mmol) and 2 (1 mmol) in absolute ethanol (15 ml) was heated at 80 °C for 8 h with addition of catalytic piperidine drops. The resulted crystalline product that produced while hot was separated by filtration and purified by crystallized from ethanol to yield 7 as pale-yellow crystals, yield (0.412 g) 80%, m.p. > 300 °C. IR (KBr), (νmax, cm−1): 3430–3200 (br, OH), 3040 (C—Harom.), 2930 (C—Haliph.),1660–1630 (3C⚌O), 1590 (C⚌N), 1530 (C⚌C) cm−1. 1H NMR (400 MHz, DMSO): δ 3.65 (s, 3H, NCH3),7.50 (d,1H,J 15.6 Hz, Hα), 7.26–8.15(m, 15H, Ar–H), 8.00(d, 1H, J 15.8 Hz, Hβ),11.50 (s, 1H, OH). MS(ESI) m/z:MS (ESI) m/z 538 [(M + Na)+, 45%], 516 [(M + H)+, 20%], 515(1 0 0). Anal.calc. for C31H21N3O5 (515.52): C, 72.23; H, 4.11; N, 8.15. Found: C, 72.10; H, 3.95; N, 8.00%.
3.5 Procedure for preparation of 4-hydroxy-1-methyl-3-((2E)-3-(3-(2-oxo-2H-chromen-3-yl)-1-phenyl-1H-pyrazol-4-yl)acryloyl)quinolin-2(1H)-one (8)
Solution of 6 (1 mmol) and 1 (1 mmol) in absolute ethanol (15 ml) was heated under reflux for 8 h with addition of drops of catalytic piperidine. The resulted crystalline product that produced while hot was filtered and recrystallized using ethanol to yield 7 as pale yellow crystals, yield (0.386 g) 75%, m.p. > 300 °C. IR (KBr), (νmax, cm−1): 3410–3250 (br, OH), 3030 (C—Harom.), 2910 (C—Haliph.),1665–1635 (3C⚌O), 1580 (C⚌N), 1510 (C⚌C) cm−1.1H NMR (400 MHz, DMSO): δ 3.60 (s, 3H, NCH3),7.40 (d,1H,J 15.6 Hz, Hα), 7.26–8.15(m, 15H, Ar–H), 7.95(d, 1H, J 15.8 Hz, Hβ),11.60 (s, 1H, OH).MS(ESI) m/z :MS (ESI) m/z 538 [(M + Na)+, 25%], 516 [(M + H)+, 40%], 515(1 0 0). Anal.calc. for C31H21N3O5 (515.52): C, 72.23; H, 4.11; N, 8.15.Found: C, 71.95; H, 3.50; N, 7.90%.
4 Conclusion and prospective
In our research work, we reported simple synthesis of new hybrid chalcones 7 and 8 via base-catalyzed claisen– schmidt condensation reaction. It allows some advantages including moderate conditions, high products yield and simple work-up procedure. The novel synthesized chalcones were characterized using homonuclear (NOE) experiments in addition to their IR, NMR, mass spectral data and elemental analyses. Finally, growth promoting effect was done in the soil field and the results shows an important feature about the ability of the synthesized chalcons to enhance the growth of selective agriculture crop plants. The toxicity of the new synthesized chalcones should be studied in subsequent studies to ensured their use as a crop fertilizer.
Acknowledgements
We acknowledge Chemistry Department, Faculty of Education, Ain Shams University, Roxy, 11711, Cairo-Egypt, Biology Department, Faculty of Sciences & Arts (Almikhwah), Al Baha University, KSA and MSA University, Faculty of pharmacy, 26 July Mehwer Road intersection with Wahat Road, 6th October City, Cairo, Egypt. for supporting and following up our research work.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Substituted quinolinones. Part 13 a convenient route to heterocyclization reactions with 3-substituted 4-hydroxyquinolin-2(1H)-one. J. Heterocyclic Chem.. 2007;44:315-322.
- [Google Scholar]
- Physiological and pharmaceutical properties of peppermint as a multipurpose and valuable medicinal plant: A Systematic Review. Bangladesh J. Med. Sci.. 2015;4:413-420.
- [Google Scholar]
- Evaluation of drying methods with respect to drying parameters, some nutritional and colour characteristics of peppermint (Mentha × piperita L.) Energy Conver Mgmt.. 2010;51:2769-2775.
- [Google Scholar]
- Synthesis and biological evaluation of α, β-unsaturated ketone as potential antifungal agents. Med. Chem. Res.. 2009;18:309-316.
- [Google Scholar]
- Synthesis and biological evaluation of nitrogen-containing chalcones as possible anti-inflammatory and antioxidant agents. Bioorg. Med. Chem. Lett.. 2010;20:730-733.
- [Google Scholar]
- Cultivation of Medicinal Plants: Special Reference to Important Medicinal Plants of India. In: Sen S., Chakraborty R., eds. Herbal Medicine in India. Singapore: Springer; 2020.
- [Google Scholar]
- Synthesis of 3-hetarylcoumarins from 3-acetylcoumarinst. Dyes Pigments. 1986;7:231-236.
- [Google Scholar]
- Chalcone synthase and its functions in plant resistance. Phytochem. Rev.. 2011;10:397-412.
- [Google Scholar]
- Synthesis, antioxidant and antimicrobial activities of a novel series of chalcones, pyrazolicchalcones, and allylic chalcones. J. Pharm. Pharmacol.. 2011;2:282-288.
- [Google Scholar]
- Antioxidant properties and composition of aqueous extracts from Mentha species, hybrids, varieties, and cultivars. J. Agr. Food. Chem.. 2003;51:4563-4569.
- [Google Scholar]
- Structural antitumoral activity relationships of synthetic chalcones. Int. J. Mol. Sci.. 2009;10:221-231.
- [Google Scholar]
- Effect of chemical elicitors on peppermint (Menthapiperita) plants and their impact on the metabolite profile and antioxidant capacity of resulting infusions. Food Chem.. 2014;156:273-278.
- [Google Scholar]
- Synthesis, antimalarial and antitubercular activity of acetylenic chalcones. Bioorg. Med. Chem. Lett.. 2010;20:942-944.
- [Google Scholar]
- Kalambe, N., Maldhure, A., Raghuvanshi, P., 2015. Synthesis and study of 2-hydroxy substituted Chalcone dibromide effects on different crop plant growth. Der Pharma Chemica. 7, 279–283.
- Reactions of 3-acyl-4-hydroxy-2(1H)-quinolones with nitrogen bases. Heterocycl. Commun.. 1995;1:341-352.
- [Google Scholar]
- Anti-inflammatory activity of the synthetic chalcone derivatives: inhibition of inducible nitric oxide synthase-catalyzed nitric oxide production from lipopolysaccharide-treated RAW 264.7 cells. Biol. Pharm. Bull.. 2007;30:1450-1455.
- [Google Scholar]
- Synthesis and antimicrobial activity of coumarin pyrazole pyrimidine 2,4,6(1H,3H,5H)triones and thioxopyrimidine4,6(1H,5H)diones. Med. Chem Res.. 2013;22:768-774.
- [Google Scholar]
- Differences in essential oil composition of basil (Ocimumbasilicum, L.) Italian cultivars related to morphological characteristics. J. Agric. Food Chem.. 1996;44:3926-3929.
- [Google Scholar]
- Anticancer activities of novel chalcone and bis-chalcone derivatives. Bioorg. Med. Chem.. 2006;14:3491-3495.
- [Google Scholar]
- Claisen-Schmidt condensation under solvent free conditions. Indian J. Chem.. 2010;49:382-385.
- [Google Scholar]
- Roselle (Hibiscus sabdariffa L.) in Sudan, cultivation and their uses. Bull. Environ. Pharmacol. Life Sci.. 2012;1:48-54.
- [Google Scholar]
- Medicinal uses of Roselle plant (Hibiscus sabdariffa L.): A Mini Review. Indian J. Hill Fmg.. 2014;27:81-90.
- [Google Scholar]
- Naturally occurring chalcones and their biological activities. Phytochem. Rev.. 2014;15:87-120.
- [Google Scholar]
- Synthesis of thiazole, benzothiazole, oxadiazole, thiadiazole, triazole and thiazolidinone incorporated coumarins. Indian J. Heterocyclic Chem.. 1996;6:91-94.
- [Google Scholar]
- Substitution at phenyl rings of chalcone and schiff base moieties accounts for their antiproliferative activity. Anticancer Agents Med. Chem.. 2019;19:620-626.
- [Google Scholar]
- Virucidal effect of peppermint oil on the enveloped herpes simplex virus type 1 and type 2 in vitro. Phyto. Med.. 2003;10:504-510.
- [Google Scholar]
- Synthesis of some new coumarin incorporated thiazolyl semicarbazones as anticonvulsants. Acta Pol. Pharm.. 2009;66:161-167.
- [Google Scholar]
- Vilsmeier-Haack reaction on hydrazones: a convenient synthesis of 4-formylpyrazoles. J. Chem. Res.. 2005;5 316–315
- [Google Scholar]
- Synthesis of some new S-triazine based chalcones and their derivatives as potent antimicrobial agents. Eur. J. Med. Chem.. 2010;45:510-518.
- [Google Scholar]
- DFT study and microbiology of some coumarin-based compounds containing a chalcone moiety. J. Serb. Chem. Soc.. 2014;79:435-443.
- [Google Scholar]
- Ring closure reactions of 3-arylhydrazonoalkyl-quinolin-2-ones to 1-aryl-pyrazolo[4,3-c]quinolin-2-ones. J. Heterocycl. Chem.. 2004;41:681-690.
- [Google Scholar]
- Variability in essential oil composition of Turkish basils (Ocimumbasilicum) Biochem. Syst. Ecol.. 2006;34:489-497.
- [Google Scholar]
- The antibacterial properties of compounds containing the tricarbonyl-methane Group.V. the synthesis of 3-acyl-2,4-dijhydroxyquinolines and of the related compounds. With respect to their Antibacterial Properties. Pharm. Soc.. 1951;Jpn:71,1100.
- [Google Scholar]
- Evaluation of the antioxidant activity of novel synthetic chalcones and flavonols. Int. J. Chem. Eng. Appl.. 2012;3:216-219.
- [Google Scholar]
- Synthesis, cytotoxicity, anti-oxidative and anti-inflammatory activity of chalcones and influence of A-ring modifications on the pharmacological effect. Eur. J. Med. Chem.. 2010;45:2206-2213.
- [Google Scholar]
- Natural and non-natural prenylated chalcones: Synthesis, cytotoxicity and anti-oxidative activity. Bioorg. Med. Chem.. 2008;16:4286-4293.
- [Google Scholar]
- Assessment of oil content and fatty acid composition variability in two economically important hibiscus species. J. Agric. Food Chem.. 2012;60:6620-6626.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2020.05.024.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







