Translate this page into:
Syzygium samarangense leaf extract exhibits distinct antidiabetic activities: Evidences from in silico and in vivo studies
⁎Corresponding authors. rasha.rashied@gmail.com (Rasha M.H. Rashied), r.rashied@herts.ac.uk (Rasha M.H. Rashied), mansour.sobeh@um6p.ma (Mansour Sobeh)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The wax apple, Syzygium samarangense, is widely used in traditional medicine. We have previously described a plethora of biological activities from its leaf extract. These include antioxidant, anti-inflammatory, antiulcer, antitrypanosomal and hepatoprotective effects. Here, we explored the antidiabetic activities from the bioactive leaf extract in silico on two crucial receptors involved in the management of diabetes disease namely peroxisome proliferator activated receptor gamma (PPARɣ) and glucagon like peptide-1 (GLP-1) and in vivo against streptozotocin-induced diabetic rats. Altogether, 457 secondary metabolites belonging to 10 classes (phenolic acids (86 compounds), flavonoids (139 compounds), anthocyanins (61 compounds), alkylphenols (17 compounds), chalcone (15 compounds), stilbenes (9 compounds), lignans (29 compounds), tannins (29 compounds), tyrosols (13 compounds), and terpenes and others (59 compounds), were docked into the active site of PPARɣ and GLP-1 receptors. From the PDB codes used for each receptor, the co-crystallized ligand was extracted and docked together with a known reference ligand. This was done simultaneously with docking the extract’s compounds to serve as references for comparative purposes. Out of the docked candidates, the top 30 compounds affording the best docking scores were compiled for further inspection and they appeared to exhibit better scores than the respective co-crystallized and reference ligands, highlighting the antidiabetic potential of the tested extract. Nine compounds exhibited highly negative scores on both receptors, demonstrating their high probability of being potent antidiabetic agents through forming stable ligand-receptor complexes. These activities were also confirmed in STZ diabetic rats where the extract reduced the elevated levels of serum glucose and lipid peroxides and increased the declined serum insulin hormone level. Taking all together, S. samarangense can be a potential candidate for further investigations for the treatment of numerous health disorders including diabetes.
Keywords
Syzygium samarangense
Diabetes mellitus
PPARɣ
GLP-1
Molecular docking
1 Introduction
Diabetes mellitus is a chronic disease happening due to limitations related to either insulin release or/and insulin action. This defect is accompanied with hyperglycemia which is the gate to further complications of the disease (American Diabetes Association, 2013). It is estimated that 642 million adults aged 20–79 years would experience diabetes globally by 2040. In addition, diabetes was responsible for 6.8% of global deaths in 2010 for people from the same age category (Zheng et al., 2018). These alarming statistics would assuredly view diabetes as one of the leading diseases contributing to high morbidity and mortality rates worldwide.
Various types of diabetes exist; type I diabetes mellitus (T1DM), type II diabetes mellitus (T2DM) and gestational diabetes (GDM), however, most of the cases fall into the first two categories. T1DM is characterized by decreased insulin secretion from the pancreas, particularly due to autoimmune destruction of pancreatic β-cells (Tan et al., 2019). It accounts for only 5–10% of diabetes cases. On the other hand, T2DM is a more prevalent form of diabetes (90% −95%) which is usually due to a combination of insulin resistance and decreased insulin secretion (American Diabetes Association, 2013, Tan et al., 2019). The risk of developing T2DM increases with age, obesity, lack of physical activity and it is also associated with genetic factors. In this form of diabetes, hyperglycemia resulting from insulin resistance is accompanied with a relatively insufficient compensatory release of insulin. This hyperglycemic status usually goes undetected initially, and then ends up with complications to various organs. T2DM complications are serious and costly and hence negatively impact the patient’s quality of life (Tan et al., 2019). They are mainly sorted into macrovascular, and microvascular complications. Macrovascular complications refer to the large blood vessels of the circulatory system, particularly those supplying heart, brain, and legs, the commonest of which is atherosclerosis of coronary arteries being the major death cause among diabetic patients. Microvascular complications of diabetes affect smaller blood capillaries distributed all over the human body, causing complications such as diabetic retinopathy and nephropathy. Moreover, nerve damage (neuropathy) is also manifested as a very common complication of diabetes. It can involve pain, numbness, ulcers, or atrophy of the proximal organ (Zheng et al., 2018; Guthrie and Guthrie, 2004).
Management of T2DM involves lifestyle interventions and weight control. This is usually paired with pharmacological drug approaches which aim at lowering blood glucose levels by different mechanisms. Some drugs affect insulin secretion either directly by stimulating the pancreas to release insulin (sulfonylureas) or indirectly by providing exogenous insulin analogues. Other drugs work on increasing insulin sensitizations (thiazolidinediones) or decreasing gluconeogenesis in the liver (biguanides) (Kaul et al., 2013).
Peroxisome proliferator activated receptor gamma (PPARɣ) is an isoform of PPARs, which belong to the nuclear receptors superfamily and are key transcription factors that regulate lipid and glucose metabolism. PPARɣ is expressed in adipose tissue and in skeletal muscles, it regulates glucose homeostasis, lipid uptake and adipocyte differentiation (Ammazzalorso and Amoroso, 2019; Loza-Rodríguez et al., 2020). Full and partial PPARγ agonists can activate the receptor causing improvements in glycemic control. However, numerous side effects were experienced with full agonists use; such as weight gain, renal fluid retention, loss of bone density, and congestive heart failure. This made research interests now more focused on developing candidates possessing partial agonistic effect on PPARγ, with reduced undesired adverse effects (Frkic et al., 2021; Guasch et al., 2011; Cuthbertson et al., 2012). Glucagon like peptide-1 (GLP-1) receptor is a G-protein coupled receptor (GPCR) that belongs to class B family. It is expressed in pancreatic islets, brain, kidneys, stomach, heart, and adipocytes, and its activation by indigenous GLP-1 enhances insulin secretion. This makes GLP-1 receptor an established target for the development of drugs as antidiabetic agents (Cuthbertson et al., 2012).
The use of pharmaceuticals to treat T2DM is hindered by their high cost and their possible side effects. Medical expenditure for diabetes patients is almost 3 times more than that of non-diabetic individuals (Zheng et al., 2018). Furthermore, various shortcomings were reported for diabetes medications, including hypoglycemia, weight gain, water retention and risks of developing congestive heart failure (Tan et al., 2019; Kaul et al., 2013). Consequently, the aim of this work is to find safer and more cost-effective alternatives for T2DM management, with potentially less adverse effects and accordingly a better patient compliance. These advantages could be the highlights of encouraging the use of natural phytochemicals for the treatment of type 2 diabetes (Sobeh et al., 2019b).
Syzygium samarangense (commonly known as wax apple, Myrtaceae family), is a fruiting plant that is well-known for its wide array of biological activities (Mahmoud et al., 2021; Sobeh et al., 2016, 2018, 2019a). Previous analysis of the plant’s constituents has revealed plenty of polyphenols which are linked to the treatment of different diseases (Khandaker et al., 2015). Leaf extracts of S. samarangense were reported to have antiulcer, anti-inflammatory, analgesic, antioxidant, antifungal, spasmolytic, cytotoxic, antihyperglycemic, antidepressant and hepatoprotective effects. In addition, they were used for the treatment of fever, eczema and diarrhea in southern China (Raj et al., 2021; Yang et al., 2018).
The phytoconstituents in the leaf extract were previously described using LC-MS, and NMR (Sobeh et al., 2018, 2019a). Herein, we surveyed all the identified metabolites from the species and we found 457 compounds (Mahmoud et al., 2021). The possible mechanisms of the antidiabetic effects of the leaf extract were explored in silico using a computational approach on two different receptors that are recognized for their roles in T2DM management; peroxisome proliferator activated receptor gamma (PPARɣ) and glucagon like peptide-1 receptor (GLP-1). Furthermore, the antidiabetic effects were investigated in vivo in STZ diabetic animals.
2 Materials and methods
2.1 Plant material
S. samarangense (Blume) Merr. & L.M.Perry leaves were collected from full trees grown in private garden, 30 km away from Cairo on Cairo-Alexandria desert road during the springtime. The leaves were air dried, ground and extracted with methanol (100 g, 3 × 0.5 L). The combined extracts were filtered off and evaporated at 40 °C under reduced pressure till dryness. The obtained residue was kept at − 80 °C then lyophilized to yield a fine dry powder (Sobeh et al., 2018).
2.2 Molecular docking
Molecular modeling was performed through docking the major metabolites identified in the extract into the active sites of PPARɣ and GLP-1 receptors. The proteins’ structures were downloaded from the Protein Data Bank ( www.rcsb.org, PDB IDs: 2Q5S and 6ORV). The downloaded proteins were prepared before the docking process, where the unnecessary protein chains or ligands were removed, and the crystal structures were protonated. The docked compounds were either downloaded directly as sdf files from PubChem or drawn using the builder tool of Molecular Operating Environment (MOE) software and saved as mol files. The compounds were classified into 10 databases grouped according to their phytochemical classes. Compounds’ ionization, partial charges, as well as tautomeric states at pH 7.0 were checked and adjusted, if necessary. Energy minimization was done using MMFF94x force field. Structural water molecules at the protein ligand interface, whenever available, were considered as an integral part of the binding site since they are crucial in mediating and improving hydrogen bonds. Docking was done using the default MOE settings of placement (Triangle Matcher) and scoring (London dG), and refinement was set to force field. The scoring function was tested by a self-docking study which indicated its ability to retain the docked poses with root mean squared deviations below 2A°. The docked compounds were evaluated based on the interactions of each pose to the surrounding residues and the poses’ scores as an indication of their binding affinity to the target protein. The poses’ scores were compared to both the docked co-crystallized ligand present in the PDB code as well as a docked reference ligand that was previously reported to activate the receptor.
2.3 Antidiabetic activity
2.3.1 Animals
Male Wister rats (170–260 g) were provided by the animal facility unit of King Fahd Medical Research Center, King Abdulaziz University, Jeddah, Saudi Arabia. The local ethical committee of Taibah University, Jeddah, Saudi Arabia had approved the applied experimental protocol. According to standard conditions, rats were housed under a temperature of 24 ± 5 °C, a relative humidity of 55 ± 5%, and normal 12 h light:12 h dark cycle. Rats had free access to food and water. Animals were treated in accordance to care of animals guidelines specified by WHO (Sobeh et al., 2017a).
2.3.2 Experimental protocol
Diabetes was induced as previously described (Singab et al., 2005). Rats were randomly divided into 4 groups of 10 animals each. The first group received vehicle for 10 days and was considered as the control group. The second group had rats with the induced diabetes and named STZ-diabetic control (STZ dose was 60 mg/Kg B.W, IP). Group 3 and 4 consisted of STZ-diabetic rats which received daily (for 10 days) an oral dose of 600 µg of glibenclamide (GLB) and 100 mg/kg of the leaf extract, respectively. On day 11, the rats were anaesthetized and sacrificed. Trunk blood was then collected, the serum was prepared, and the biochemical parameters were determined as previously described (Omar et al., 2011; Sobeh et al., 2017a). In brief, blood samples were centrifuged at 4 °C for 15 min at 3000 rpm and the sera obtained were used for biochemical analyses. Serum glucose was estimated by the glucose oxidase method. Serum immunoreactive insulin was determined by the radioimmunoassay method using Amarsham insulin RIA kit using rat insulin as the standard. Lipid peroxides expressed as thiobarbituric acid reactive substance, (TBARS) production were determined according to the previous method (Sobeh et al., 2017b).
2.4 Statistical analysis
Values were expressed as mean ± SEM. To analyze the differences between groups, statistical analysis was performed by one-way ANOVA followed by Tukey's post hoc test using the software (GraphPad Prism, version 5, USA). Significance was considered at a p value < 0.01.
3 Results and discussions
3.1 Molecular docking
Structure-based molecular docking has become a common tool in early drug discovery process. It allows a rapid exploration of vast chemical candidates and categorize them as potential hits able to interact with a given target protein for the management and treatment of a particular disorder. In the current work, the major compounds that were identified and reported from the previous literature in S. samarangense were docked into PPARγ and GLP-1 receptors, which are substantially involved in the prognosis of diabetes disease.
PPARγ consists of an N-terminal ligand independent transactivation domain (AF1), a DNA-binding domain and a C-terminal ligand-binding domain (LBD). The LBD contains a ligand dependent transactivation function (AF2), it is a Y-shaped structure formed of three arms: a hydrophobic entrance (arm III) that branches into a polar arm (arm I) and a hydrophobic arm (arm II) (Guasch et al., 2011; Zoete et al., 2007). When ligands bind to PPARγ, they specifically attach to the ligand-binding domain of the receptor. The receptor in turn forms a heterodimer with Retinoid X Receptor α (RXRα) and binds to peroxisome proliferator response element (PPRE). Coactivators or corepressors are then recruited to the PPARγ/RXRα complex promoting or repressing genes transcription and consequently controlling cellular metabolic homeostasis (Frkic et al., 2021). Full and partial PPARγ agonists promote coactivator binding and genes transcription to a different extent, where partial agonists have a weaker transactivation activity on PPARγ than full agonists. Nevertheless, the antidiabetic activity of partial agonists is still maintained via a different mechanism, which is the inhibition of PPARγ phosphorylation at Ser273.
Full agonists demonstrated hydrogen bond interactions with Tyr473 (H12), Ser289 (H3), His323 (H6) and His449 (H11), they occupy arm I and arm II in the LBD and eventually contribute to H12 stabilization. The degree of transactivation of the full agonist is directly correlated to the degree of H12 stabilization. Compared to the full agonist rosiglitazone, partial agonists occupy arms II and III only in the LBD, and they show statistically insignificant stabilization of H12 (residues 470 – 477) of the LBD (Guasch et al., 2011; Bruning et al., 2007). Since partial agonists exhibit transactivation responses without significantly stabilizing H12, they control coactivator recruitment through a different approach, independent of H12. X-ray crystallography as well as proton deuterium exchange (HDX) studies were used to explain the transactivation response of partial agonists that appeared to happen by stabilizing regions in the LBD other than H12, specifically the β-sheet (residues 341–351) and H3 (residues 277–302). Since partial agonists are unable to contact H12, the degree of transactivation of the partial agonist is directly correlated to the degree of H3 stabilization (Bruning et al., 2007). In addition to changes observed in H12 stabilization, partial agonists demonstrated protection to exchange in the β-sheet of the receptor, this can be explained by the hydrogen bond network afforded between partial agonists and the β-sheet (Bruning et al., 2007).
The crystal structure of PPARγ bound to the partial agonist nTZDpa is deposited in the protein data bank (PDB ID: 2Q5S). The partial agonist nTZDpa forms a hydrogen bond with Ser342 through its’ 2-carboxy group of the indole ring and another hydrogen bond with Glu343 that is water-mediated. It also makes several hydrophobic interactions with the β-sheet, particularly observed with Ile341. Other reported partial agonists adopted hydrophobic interactions with Cys285, Gly284, and Ile281 and a hydrogen bonding with Arg288 in H3 (Bruning et al., 2007; Nolte et al., 1998).
As for GLP-1 receptor, it consists of 7 transmembrane (TM) helices, a C-terminal intracellular domain, and an N-terminal extracellular domain. Similar to other GPCRs, binding of a ligand to the extracellular domain of GLP-1 is transduced to the inner of the cell, initiating a cascade of events. For instance, when activated by GLP-1, the receptor stimulates the adenylate cyclase pathway leading to increased cAMP as well as increased intracellular calcium concentrations and consequently boosting exocytosis of insulin-containing granules, thus enhancing insulin release.
The crystal structure of GLP-1 receptor in complex with a non-peptide agonist, TT-OAD2, was resolved (PDB ID: 6ORV). Compared to the peptide GLP-1, TT-OAD2 adopts a different binding orientation within GLP-1 receptor, with only 10 out of 29 common amino acid residues in their interactions, indicating a limited overlap. The non-peptide agonist experienced an orientation within the binding site that allowed its protrusion through transmembrane helices 2 and 3, interacting with residues located at the top of these helices, particularly Trp203. This is distinct from the interactions adopted by GLP-1, which was more into TM5, TM6 and TM7 of the receptor as well as side chains located deep in the bundle (particularly interacting with residues Tyr69, Arg121, Leu123 and Glu128) (Underwood et al., 2010). On the other hand, most of the reported interactions for TT-OAD2 were hydrophobic contacts with Trp297, Phe230, Tyr145, Leu201, Ile196, Ala200, Leu217, Val229 and Met204. Additionally, the compound forms an ionic interaction with Lys197 and interacts through a hydrogen bond with Tyr220.
The docked compounds included in our study, with their polyphenolic nature, are expected to maintain a polar network and hydrophobic contacts within the binding sites of the two receptors (Zhao et al., 2020). Docking of the databases on each target was simultaneous with docking two compounds, the co-crystallized ligand that is bound to the protein in the crystal structure used, as well as a reference ligand that represents another compound known to activate the receptor. For PPARɣ receptor, the co-crystallized ligand in the crystal structure used was nTZDpa (PDB ID:2Q5S), while the reference ligand was the partial agonist AL26-29. As for GLP-1 receptor, the co-crystallized ligand is TT-OAD2 (PDB ID: 6ORV) and the reference ligand is the non-peptide CHU-128. The docking results of the co-crystallized and reference ligands in the binding site of their respective receptor are shown in Table 1.
Compound
Docking scores (kcal/mol)
PPARɣ
GLP-1
Co-crystallized ligand*
−14.71
−11.58
Reference ligand**
−11.47
−11.89
Phenolic acids are classified into hydroxybenzoic acids, hydroxycinnamic acids, hydroxyphenylacetic acids and hydroxyphenylpropanoic acids. They act as antioxidant, anti-inflammatory, immunoregulatory, anti-microbial, anti-thrombotic, cardioprotective, and they are also reported to have anti-cancer and antidiabetic properties (Rashmi and Negi, 2020). The top phenolic acid derivative on PPARɣ receptor was 1-Sinapoyl-2-feruloylgentiobiose, scoring −28.79 kcal/mol. The compound forms a hydrogen bonding network with Arg288, Ser342, Glu343 and forms hydrophobic interactions with amino acids Leu330, Ile281, Cys285, Table 1S. Concerning results on GLP-1 receptor, the compound 1,2-Disinapoylgentiobiose was the top phenolic acid derivative with a score of −12.63 kcal/mol. It forms a hydrogen bond network with Tyr220 and Arg299 in addition to hydrophobic interactions with Trp297 and Arg299.
Flavonoids constitute a broad characteristic class of natural polyphenols that can modulate enzyme functions and hence possess numerous biological activities. They are known for their antioxidant, antimutagenic and antitumor effects (Panche et al., 2016). The prepared database of flavonoids consists of 139 compounds, 113 of which showed better docking score than both the co-crystallized compound (nTZDpa) and the reference compound (AL26-29) on PPARɣ, Table 2S. Interestingly, only three compounds in this large database exhibited worse docking scores than the reference compound (AL26-29). The interactions of the top scoring compound in this class, Naringin 6′-malonate, included hydrogen bonds to the residues Ser342, Glu259, Glu343 and Ser289 along with hydrophobic interactions with Ile281, Ile341 and Arg288. Docked naringin 6′-malonate scored 2 folds better than the co-crystallized ligand, with an exact score of −30.90 kcal/mol. When flavonoids were docked in the binding pocket of GLP-1, 27 compounds exhibited more negative scores than both the co-crystallized ligand TT-OAD2 and the reference ligand CHU-128. Myricetin 3-O-glucoside was the best flavonoid in terms of its docking score to GLP-1 receptor with a score of −16.24 kcal/mol. It interacted with amino acids in the binding site by a hydrogen bonding to Cys226 and pi-pi stacking interactions to Trp297. The hydrophobic interactions were extended also to the amino acid residue Trp203.
Compound name
PPARɣ docking score (kcal/mol)
Anthocyanins (9 compounds)
Glycoside
Respective aglycone
Petunidin 3,5-O-diglucoside
−30.74*
−19.50
Petunidin 3-O-glucoside
−27.75
−19.50
Pinotin A
−27.96
–22.14
Petunidin 3-O-galactoside
−26.99
−19.50
Cyanidin 3-O-sophoroside
−27.87
−18.37
Cyanidin 3-O-(6′'-succinyl-glucoside)
−27.43
−18.37
Pelargonidin 3-O-glucosyl-rutinoside
−27.62
−16.92
Pelargonidin 3-O-sophoroside
−26.76
−16.92
Petunidin 3-O-(6′'-p-coumaroyl-glucoside)
−26.94
−19.50
Flavonoids (11 compounds)
Naringin 6′-malonate
−30.73*
−15.72
Rhoifolin 4′-O-glucoside
−30.70*
−24.99
Kaempferol 3-O-xylosyl-rutinoside
−29.51
−16.22
Kaempferol 3-O-rhamnosyl-rhamnosyl-glucoside
−29.24
−16.22
Chrysoeriol 7-O-(6′'-malonyl-apiosyl-glucoside)
−29.34
−14.00
Isorhamnetin 3-O-rutinoside
−28.77
−17.33
Quercetin 3-O-xylosyl-rutinoside
−28.15
−16.15
Myricetin 3-O-rutinoside
−28.17
−17.95
Quercetin 3-O-(6′'-acetyl-galactoside) 7-O-rhamnoside
−28.08
−16.15
Neodiosmin
−26.54
−15.97
Kaempferol 3-O-(2′'-rhamnosyl-galactoside) 7-O-rhamnoside
−27.30
−16.22
Phenolic acids (2 compounds)
1-Sinapoyl-2-feruloylgentiobiose
−28.72
1,2-Disinapoylgentiobiose
−27.69
Tannins (8 compounds)
Theaflavin 3′-O-gallate
−31.64*
Procyanidin C1
−31.08*
Theaflavin 3-O-gallate
−30.17
Prodelphinidin dimer B3
−29.26
Epiafzelechin-(4b->8)-epicatechin 3,3′-digallate
−29.05
Procyanidin dimer B2 3′-gallate
−28.48
Procyanidin C2
−27.21
Procyanidin dimer B5
−26.81
Lignans are natural compounds having a core of two or more phenylpropanoid units. They mostly exist as dimers and are widely distributed across the plant kingdom. They are reported to have a wide spectrum of activities, such as antioxidant, antibacterial, antiviral, and antitumor effects due to their structural diversity (Cui et al., 2020). Among the 29 lignans docked, 21 possessed better scores on PPARɣ receptor than the co-crystallized and reference ligands, Table 3S. Lariciresinol was the lignan with the best score of −18.71 kcal/mol, it interacts with the binding site through water mediated hydrogen bonding with Gly284 and a hydrophobic interaction with Ile281. However, isolariciresinol was the best scoring lignan on GLP-1 receptor with a docking score of −12.62 kcal/mol. Interactions to the binding site were afforded via a pi-pi stacking interaction with Trp297, a hydrophobic interaction with Lys197 and a hydrogen bond with Tyr220.
Compound name
GLP-1 docking score (kcal/mol)
Anthocyanins (9 compounds)
Glycoside
Respective aglycone
Pinotin A
−13.51
−10.07
Cyanidin 3-O-sophoroside
−12.84
−7.76
Cyanidin 3-O-glucosyl-rutinoside
−13.73
−7.76
Pelargonidin 3-O-sambubioside
−13.31
−7.97
Malvidin 3,5-O-diglucoside
−15.81ǂ
−8.84
Peonidin 3-O-rutinoside
−13.14
−8.53
Pelargonidin 3-O-glucoside
−13.86
−7.97
Cyanidin 3-O-xyloside
−14.41
−7.76
Cyanidin 3-O-galactoside
−14.74ǂ
−7.76
Flavonoids (12 compounds)
Kaempferol 3-O-xylosyl-rutinoside
−14.53
−7.85
Myricetin 3-O-rutinoside
−13.31
−10.43
Quercetin 3-O-sophoroside
−12.98
−9.36
Luteolin 7-O-rutinoside
−14.54
−8.13
Diosmin
−15.91ǂ
−7.53
Hesperidin
−14.19
−9.32
Kaempferol 3-O-xylosyl-glucoside
−13.82
−7.85
Myricetin 3-O-glucoside
−16.35ǂ
−10.43
Apigenin 6,8-C-arabinoside-C-glucoside
−14.69
−7.65
Quercetin 3-O-rutinoside
−14.08
−9.36
Narirutin
−13.60
−7.80
Mearnsitrin
−13.34
−13.41
Tannins (8 compounds)
Theaflavin 3′-O-gallate
−13.09
Procyanidin C1
−15.33ǂ
Theaflavin 3-O-gallate
−13.72
Prodelphinidin dimer B3
−14.15
Epiafzelechin-(4b->8)-epicatechin 3,3′-digallate
−13.86
Theaflavin
−14.50
Castalagin
−13.55
Catechin 3′-glucoside
−13.21
−8.83
Tyrosols (1 compound)
Demethyloleuropein
−13.63
Tannins are phenolic compounds, where the phenolic groups act as electron scavengers allowing for the powerful antioxidant properties of these compounds. They are also used as anti-inflammatory agents, astringents for diarrhea and diuretics (Khanbabaee and Van Ree, 2001; de Hoyos-Martínez et al., 2019). Docking of the tannins database, which consists of 30 metabolites revealed that 26 compounds scored better than the co-crystallized and reference ligands on PPARɣ receptor, Table 4S. Theaflavin 3′-O-gallate possessed a docking score of −31.77 kcal/mol, which is the best docking score achieved across all classes. Examining the binding mode of this compound, it was shown to directly make a hydrogen bond to the essential residue Ser342 as well as Ile326, and it forms a water mediated hydrogen bond network with Arg288 and Glu343. Moreover, it interacts also with Ile326 via a hydrophobic interaction. Docking results of this class of compounds on GLP-1 receptor also seems promising, with 15 compounds achieving more negative scores than the co-crystallized and the reference ligands. The best scoring tannin on GLP-1 receptor was procyanidin C1 with a score of −15.15 kcal/mol, it forms a hydrogen bond with both Lys197, Cys296 and hydrophobic interactions with Trp203 and Ala200.
Compound name
Docking scores (kcal/mol)
Anthocyanins (9 compounds)
Petunidin 3,5-O-diglucoside
−17.76
Petunidin 3-O-glucoside
−21.14
Pinotin A
−18.16
Petunidin 3-O-galactoside
−21.06
Cyanidin 3-O-sophoroside
−20.21
Cyanidin 3-O-(6′'-succinyl-glucoside)
−20.57
Pelargonidin 3-O-glucosyl-rutinoside
−18.37
Pelargonidin 3-O-sophoroside
−17.62
Petunidin 3-O-(6′'-p-coumaroyl-glucoside)
−21.00
Flavonoids (11 compounds)
Naringin 6′-malonate
−27.86
Rhoifolin 4′-O-glucoside
−19.71
Kaempferol 3-O-xylosyl-rutinoside
−24.32
Kaempferol 3-O-rhamnosyl-rhamnosyl-glucoside
−24.81
Chrysoeriol 7-O-(6′'-malonyl-apiosyl-glucoside)
–23.69
Isorhamnetin 3-O-rutinoside
–23.82
Quercetin 3-O-xylosyl-rutinoside
–23.88
Myricetin 3-O-rutinoside
–23.11
Quercetin 3-O-(6′'-acetyl-galactoside) 7-O-rhamnoside
−21.73
Neodiosmin
–22.23
Kaempferol 3-O-(2′'-rhamnosyl-galactoside) 7-O-rhamnoside
−25.76
Phenolic acids (2 compounds)
1-Sinapoyl-2-feruloylgentiobiose
−18.68
1,2-Disinapoylgentiobiose
−18.28
Tannins (8 compounds)
Theaflavin 3′-O-gallate
−25.75
Procyanidin C1
–22.92
Theaflavin 3-O-gallate
−21.57
Prodelphinidin dimer B3
−26.14
Epiafzelechin-(4b->8)-epicatechin 3,3′-digallate
−20.55
Procyanidin dimer B2 3′-gallate
–23.69
Procyanidin C2
−26.63
Procyanidin dimer B5
–23.29
Anthocyanins are glycosylated anthocyanidins, they are colored, water-soluble phenolic compounds. Their intake is associated with protection from neurogenerative and cardiovascular diseases, in addition to their known antioxidant properties (Mattioli et al., 2020). Docking of anthocyanins revealed very promising results on PPARɣ receptor, where 60 compounds had more negative scores than nTZDpa and AL26-29 out of 61 compounds in this database, Table 5S. The best anthocyanin was petunidin 3,5-O-diglucoside whose best binding mode achieved a score of −30.61 kcal/mol. Examining this pose, the compound forms direct hydrogen bonding with Ser342, hydrogen bonding to Arg288, Gly284 and Glu343 that are all mediated via structural water molecules. It also forms Vander Waal interactions with Ile281. Thirty-three compounds belonging to anthocyanins showed promising results on GLP-1 receptor with either equal docking scores or more negative scores than both TT-OAD2 and CHU-128. Malvidin 3,5-O-diglucoside had a top score of −15.73 kcal/mol on GLP-1 receptor among anthocyanins. It binds to Trp297 through a pi-pi stacking interaction, and one of its glucosidic moieties interacts with Ile196 through a hydrogen bond.
Chalcones are naturally occurring aromatic ketones. Their structures include a reactive α, β-unsaturated carbonyl function which is highly electrophilic contributing to the biological activities of this class. Chalcones are known for their anti-inflammatory and their antitumor effect (Goyal et al., 2021). The compound with the best score on PPARɣ receptor was 3-hydroxyphloretin 2′-O-xylosyl-glucoside, with a docking score of −25.44 kcal/mol, Table 6S. The compound made hydrogen bonds with the backbone of Ser342, and with the sidechains of the amino acids Arg288, Glu259 and Met329. It formed hydrophobic interactions with Arg288 and Ile341. On the other hand, docking of the database compounds in the active site of GLP-1 receptor revealed that the best binder to GLP-1 was floridzin, scoring −12.27 kcal/mol. Interactions to the binding site of GLP-1 protein included hydrogen bonds to Met204 and Cys296 as well as pi-pi stacking with Trp297.
Stilbenes (1,2-diphenylethylenes) are naturally occurring phenolic compounds having antioxidant, neuroprotective and antitumor effects. In addition, they are prescribed as anti-obesity agents for their roles in regulating fat metabolism (Benbouguerra et al., 2021). E-viniferin was predicted to have the best binding affinity on PPARɣ with a score of –23.09 kcal/mol, Table 7S. The binding of e-viniferin was supported by hydrogen bonds to Glu343 and Glu259 as well as hydrophobic interactions with Arg288, Ile281 and Ile341. For binding to GLP-1 receptor, pallidol was the top scoring stilbene with −11.85 kcal/mol. The compound’s phenol groups interacted with Trp203 and Leu217 through hydrogen bonds and with Met233 through hydrophobic interactions.
Alkylphenols are organic compounds that can be found naturally in plants extracts, in essential oils or from thermal cracking of natural products such as brown coal and wood. They are produced industrially mainly by catalytic alkylation of phenols, cresols, or xylenols. Their main industrial use is the manufacture of surfactants (Fiege et al., 2000). Docking of alkylphenols into the active site of PPARɣ receptor revealed that the top scoring compounds were resorcinol derivatives, with 5-tricosenylresorcinol having the most negative score −17.53 kcal/mol, Table 8S. The best binding mode for this compound showed a hydrogen bond network with Arg288 and Glu343 mediated through structural water molecules as well as a hydrophobic interaction with Ile341. However, docking results on GLP-1 receptor afforded scores that were less negative than both the co-crystallized ligand as well as the reference ligand.
Tyrosol is a secoiridoid derivative present in olive oil. Tyrosol, and its derivatives, have a wide spectrum of biological effects against hypertension, atherosclerosis, coronary heart disease and insulin resistance (Karković Marković et al., 2001). Docking in the binding site of PPARɣ receptor retrieved 10 out of 13 compounds scoring better than the docked co-crystallized and reference ligands, Table 9S. The best scoring tyrosol was oleuropein which made hydrogen bonds with Ser342, Arg288 and Glu343. It also formed a hydrophobic interaction with Ile281, all of which contributed to a docking score of −21.41 kcal/mol. Its demethylated analog, demethyloleuropein, was the best docked tyrosol on GLP-1 receptor with a docking score of −13.81 kcal/mol. The glucosidic part of the compound afforded 2 hydrogen bonds with Tyr220. The rest of the interactions are hydrophobic ones, such as interactions with Trp203 and Trp297.
Terpenes, also called isoprenoids, are a large group of natural compounds that are known for their antiplasmodial, antiviral, anticancer and antidiabetic activities. They are subdivided according to the number of isoprene units in their structures (Cox-Georgian et al., 2019). The top scoring terpene on PPARɣ receptor was carnosic acid, with a score of −20.07 kcal/mol, Table 10S. The compound forms a salt bridge with Arg288. Phlorin possessed the best score on GLP-1 receptor among this class of compounds, with a score of −12.01 kcal/mol. Phlorin afforded a hydrogen bond with Tyr220 and a hydrophobic interaction with Trp203.
Comparing the results obtained across all docked classes of compounds, it was found that the best results were obtained for anthocyanins, tannins, and flavonoids on both receptors, PPARɣ and GLP-1. >80% of the compounds in each of the three databases scored better than nTZDpa and AL26-29 on PPARɣ. As for GLP-1, 54% of anthocyanins database and 51.7% of tannins database scored better than TT-OAD2 and CHU-128 while flavonoids followed afterwards with 19.4%. Anthocyanins was the class of compounds with the best results on both receptors; where 98.3% and 54% of the compounds scored better than the relevant co-crystallized as well as reference ligands on PPARɣ and GLP-1, respectively. The top 30 compounds across all classes with the most negative scores on PPARɣ and GLP-1 are compiled in Table 2 and Table 3, respectively. The listed scores are average scores of three different simulations. The detailed results of the three individual simulations on each of the two receptors were compiled in Table 11S and Table 12S, and the standard deviations were calculated. Noteworthy, the compounds docked to PPARɣ receptors showed a very comparable binding fashion to the partial agonists as they afforded interactions with the amino acids in H3 and β-sheet (particularly Ser342) but not with amino acids in H12 (particularly Tyr473). In addition, the docked poses of the compounds on GLP-1 receptor mimic the binding modes of the reference non peptides, TT-OAD2 and CHU-128. This was illustrated through preserving polar and hydrophobic interactions with the reported amino acids in TM2 and TM3. Nine compounds were among the top 30 on both receptors. These compounds are: cyanidin 3-O-sophoroside, epiafzelechin-(4b->8)-epicatechin 3,3′-digallate, kaempferol 3-O-xylosyl-rutinoside, myricetin 3-O-rutinoside, procyanidin C1, prodelphinidin dimer B3, theaflavin 3′-O-gallate, pinotin A, and theaflavin 3-O-gallate.
Among this compiled list of top compounds, the structures of the best scoring 5 compounds on each of PPARɣ and GLP-1 receptors were grouped in Fig. 1. The top 5 compounds on PPARɣ receptor were two tannins: theaflavin-3′-O-gallate (a, CID: 71307578), procyanidin C1 (b, CID: 169853), two flavonoids: naringin-6′-malonate (c, CID: 74819370), rhoifolin-4′-O-glucoside (d, CID: 5282150) and one anthocyanin: petunidin 3,5-O-diglucoside (e, CID: 71587075). Their best poses demonstrated average scores of −31.64, −31.08, −30.73, −30.70 and −30.74 kcal/mol, respectively. Moreover, the best scoring 5 compounds on GLP-1 were two flavonoids: myricetin-3-O-glucoside (f, CID: 22841567), diosmin (g, CID: 5281613), one tannin: procyanidin C1 (b, CID: 169853) and two anthocyanins: malvidin 3,5-O-diglucoside (h, CID: 159287), cyanidin 3-O-galactoside (i, CID: 441699). Their best poses demonstrated average scores of −16.35, −15.91, −15.33, −15.81 and −14.74 kcal/mol, respectively. It was recognized that compound (b) was among the top 5 scorers on both PPARɣ and GLP-1 receptors. This latter finding suggests a possible multi-target antidiabetic activity for this compound, and hence it can serve as a lead compound for further modifications to obtain candidates with potentially high antidiabetic activities on either or both receptors.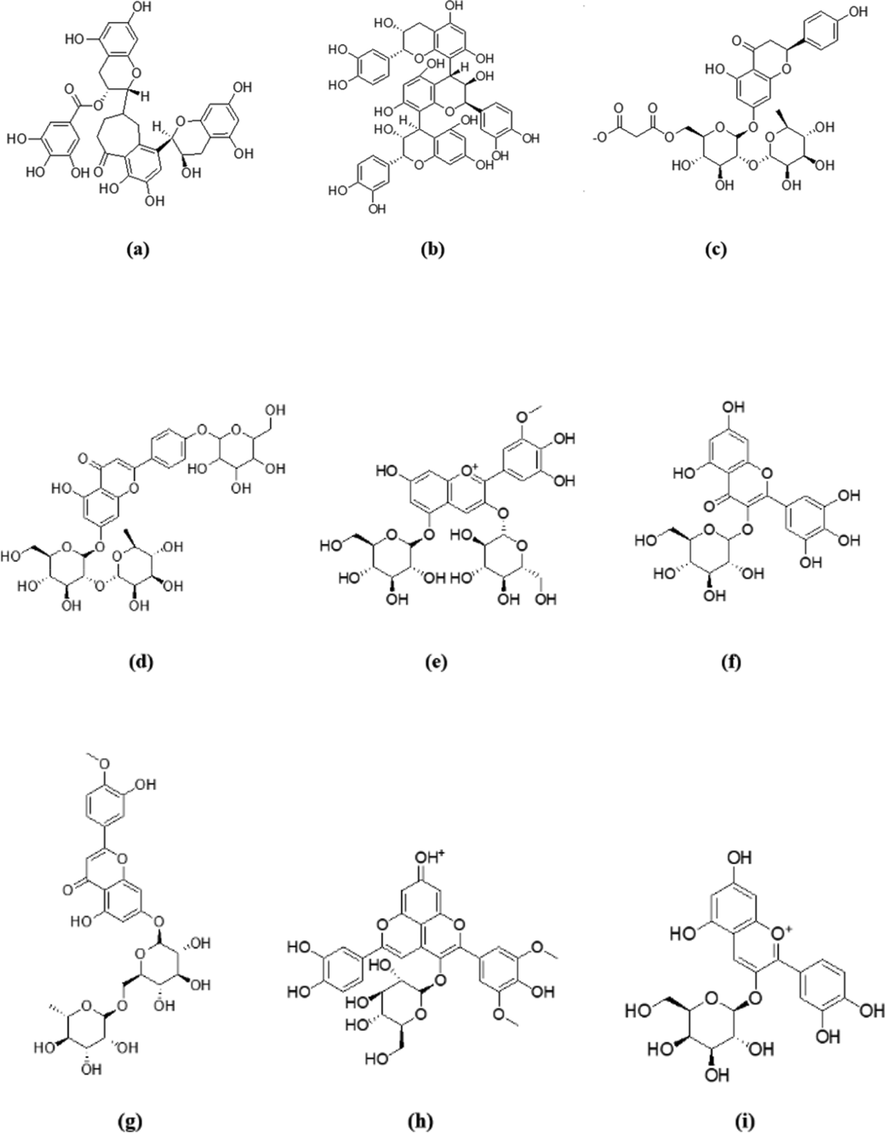
Chemical structures of the top 5 compounds on each of PPARɣ and GLP-1 receptor. (a) theaflavin-3′-O-gallate, (b) procyanidin C1, (c) naringin-6′-malonate, (d) rhoifolin-4′-O-glucoside, (e) petunidin 3,5-O-diglucoside, (f) myricetin-3-O-glucoside, (g) diosmin, (h) malvidin 3,5-O-diglucoside, (i) cyanidin 3-O-galactoside.
From the top 5 scoring candidates on each target, the best compound from each phytochemical class was picked for visualization of its 2D interactions with the amino acids of the binding site. Fig. 2 highlights the interactions of compounds (a), (c) and (e) with PPARɣ receptor, while Fig. 3 highlights those of compounds (f), (b) and (h) with GLP-1 receptor. Moreover, the binding mode of the top scoring compound was examined on each receptor (Fig. 4). In Fig. 4I, compound (a) afforded hydrogen bonds and hydrophobic interactions with the amino acid residues in PPARγ binding site as discussed previously. It is clear from the figure that the residues involved in the full agonist action (represented by the red ribbon on the top right of the figure) were spatially away from the binding site and thus are not involved in the interactions with the docked ligand, suggesting the ligand’s activity on the receptor in a partial agonistic fashion. In Fig. 4II, compound (f) preserved the hydrophobic and hydrogen bonding interactions with the residues of GLP-1 receptor. This was particularly evident through the pi-pi stacking between compound (f) and Trp297.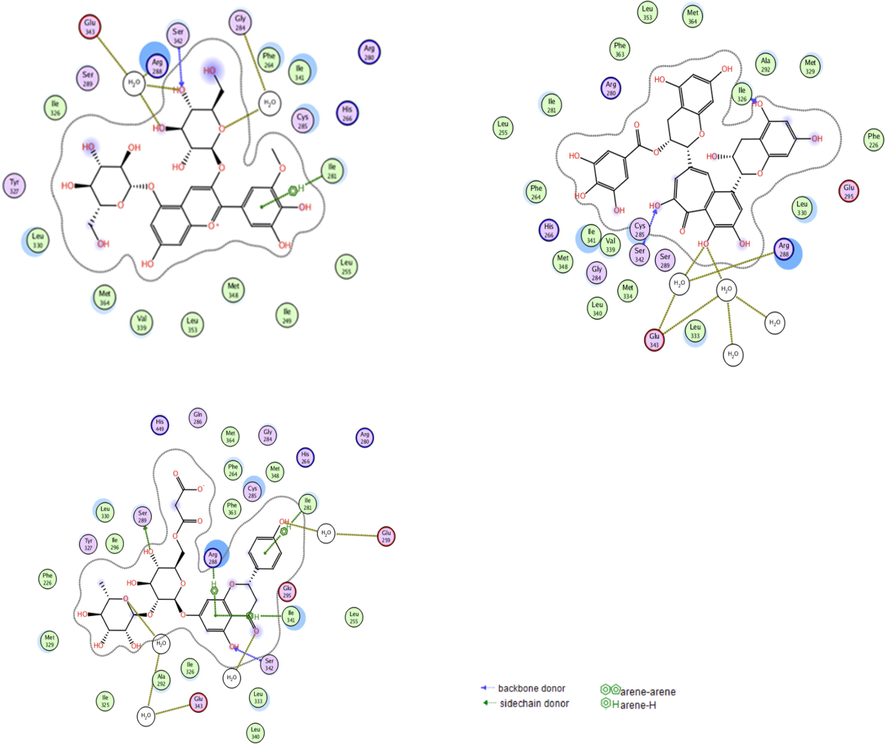
The interactions of the best scoring anthocyanin (e; top left), tannin (a; top right), and flavonoid (c; bottom) with the residues in the binding site of PPARɣ receptor. Blue dotted lines represent direct hydrogen bonding with residues’ backbones, green dotted lines represent direct hydrogen bonding with residues’ sidechains, gold dotted lines represent interactions through solvent molecules, and green arene-hydrogen lines indicate hydrophobic interactions.
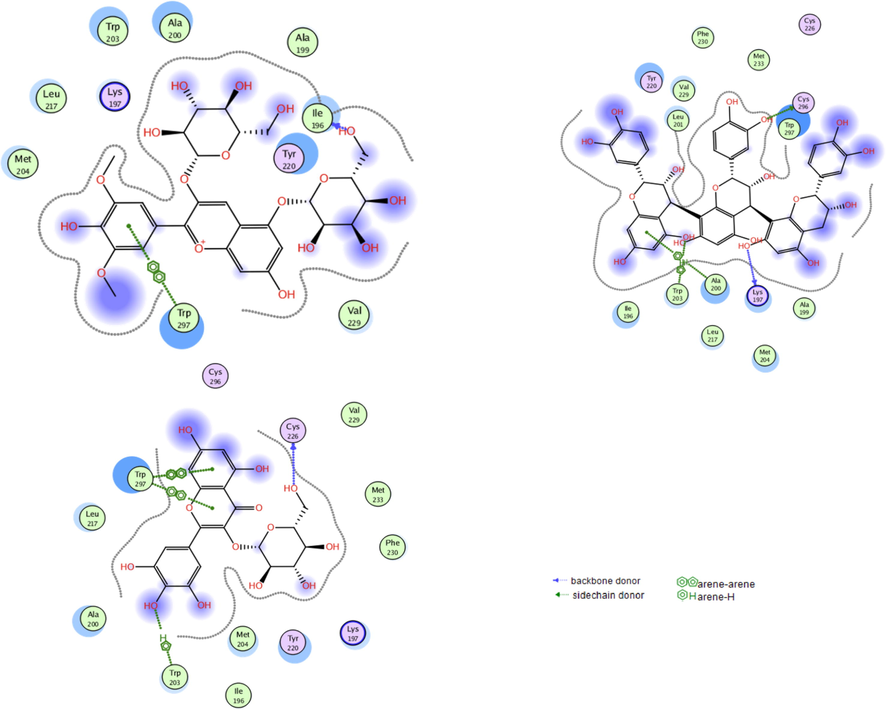
The interactions of the best scoring anthocyanin (h; top left), tannin (b; top right), and flavonoid (f; bottom) with the residues in the binding site of GLP-1 receptor. Blue dotted lines represent direct hydrogen bonding with residues’ backbones, green dotted lines represent direct hydrogen bonding with residues’ sidechains, green arene-hydrogen lines as well as green arene-arene lines indicate hydrophobic interactions.
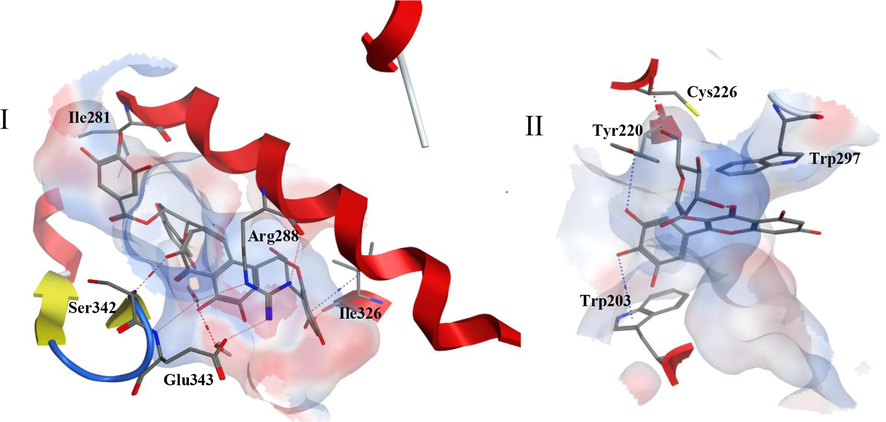
I. Three dimensional representation of compound (a) in the active site of PPARɣ receptor. II. Three-dimensional representation of compound (f) in the active site of GLP-1 receptor. An electrostatic molecular surface was created surrounding the amino acids constituting the binding site. Side chains of amino acids were omitted for clarity. Red dotted lines indicate hydrogen bonding while blue dotted lines indicate hydrophobic interactions. Secondary structures of receptors including red alpha helices, yellow beta sheets and blue turns are displayed.
To exclude the potential full agonistic activity of the compounds, the best 30 compounds in terms of their docking scores on PPARɣ were compiled into a database and were subjected to a secondary virtual screening step. The compounds were docked into a different crystal structure of PPARɣ which is complexed with the full agonist rosiglitazone (PDB ID: 4EMA). The docking results are shown in Table 4. All the 30 compounds exhibited more negative docking scores in the partial agonists’ binding site than their scores in the full agonists’ binding site. This revealed that the compounds docked better into the partial agonists’ site. Through the inspection of the binding modes as well as the interactions of the 30 compounds with the amino acids lining the binding site of full agonists, it was found that most of the compounds preserved their binding modes like partial agonists and they maintained the crucial interactions needed for partial agonistic effect without interacting with amino acids reported for full agonistic activity. Out of the 30 compounds, only 4 compounds showed several poses displaying interactions with amino acids reported for full agonistic activity; petunidin 3-O-glucoside, cyanidin 3-O-sophoroside, pelargonidin 3-O-sophoroside and isorhamnetin 3-O-rutinoside. This requires additional inspection of these compounds for possible presence of full-agonists’ side effects.
In an attempt to compare the binding modes of our ligands to reported partial as well as full agonists, the best candidate, theaflavin-3′-O-gallate (a), was superimposed separately on each of the co-crystallized ligands in the two crystal structures 2Q5S and 4EMA. The compound appears to be well overlayed with the partial agonist ligand in 2Q5S than with the full agonist ligand of 4EMA. In Fig. 5I, compound (a) displays interactions with crucial amino acids for partial agonistic activity in agreement with the co-crystallized partial agonist. On the other hand, it appears in Fig. 5II that, unlike the co-crystallized full agonist rosiglitazone, compound (a) didn’t display interactions with the crucial residues for full agonistic activity, such as Ser289 and Tyr473.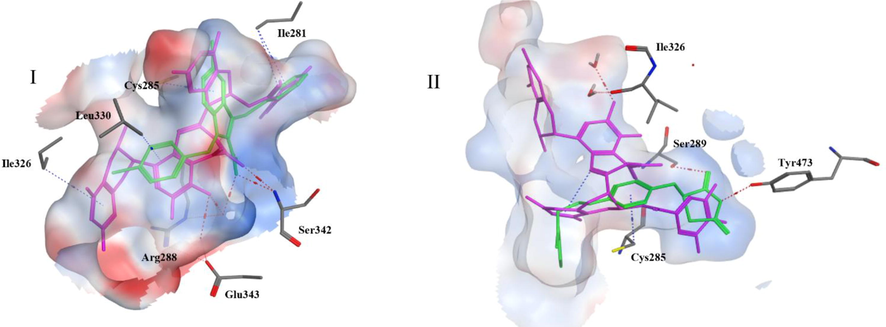
I: Superimposition of the top compound theaflavin 3′-O-gallate (magenta) on the partial agonist nTZDpa (green) in the binding site of 2Q5S, II: Superimposition of the top compound theaflavin 3′-O-gallate (magenta) on the full agonist rosiglitazone (green) in the binding site of 4EMA. Red dotted lines indicate hydrogen bonding while blue ones indicate hydrophobic interactions.
Herein, we present polyphenolic compounds that can be further modified for possible optimum antidiabetic hits either through their action on PPARɣ or GLP-1 receptors. Some of the compounds are glycosides, and it's hard to control the bioavailability of glycosylated compounds as their bioavailability depends on the type of sugar moiety involved. Consequently, the extent of absorption and hydrolysis of such compounds can’t be predicted unless pharmacokinetic studies were performed. To address this issue, the respective aglycones of all glycosylated compounds present among the top candidates were docked to both PPARɣ and GLP-1. The docking results of aglycones were worse than that of the glycosylated derivatives on the two targets. However, the aglycones still display docking scores that are better than (in PPARɣ) or comparable to (in GLP-1) the co-crystallized/reference ligands. The docking scores of the aglycones on PPARɣ and GLP-1 are shown in Tables 2 and 3, respectively.
3.2 In vivo antidiabetic activities
Motivated by the promising data obtained from the in silico studies, we decided to evaluate the potential of the oral administration of S. samarangense extract to mitigate the diabetic status in rats with STZ-induced diabetes. The effects of the studied extract on the serum levels of glucose, insulin, and lipid peroxides is shown in Fig. 6. Blood glucose level is a perfect biomarker to follow up and monitor the patients’ diabetic status as it meets the necessary criteria needed for this purpose. It enables diagnosis, evaluating the management and predicting the complications of the disease (Krentz and Hompesch, 2016). It was shown that STZ injection lead to 243% increase in the serum glucose level, Fig. 6I. Oral administration of S. samarangense extract resulted in 53.2% reduction in the elevated serum glucose level (compared to STZ diabetic animals), which was very comparable to the effect of the reference drug glibenclamide. Noteworthy, this effect obtained by the extract showed to be significantly different from the diabetic control (p < 0.01) and did not differ significantly from the normal control group.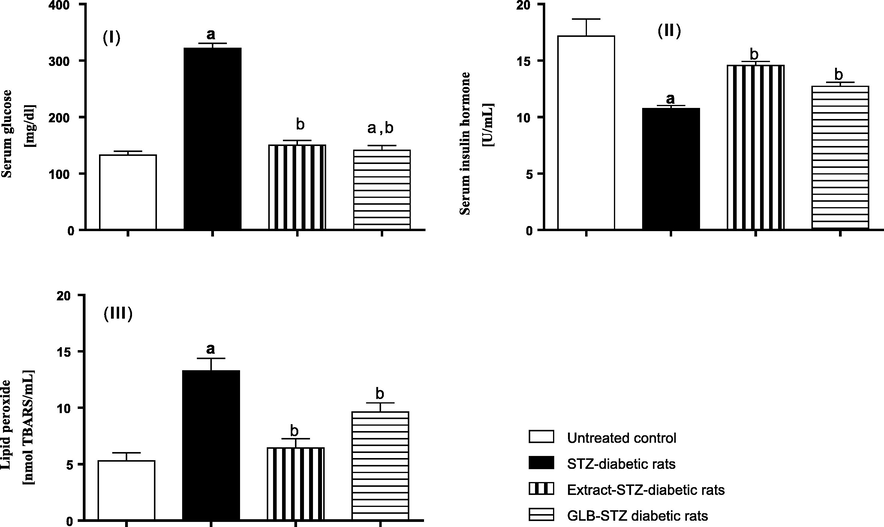
In vivo antidiabetic results of S. samarangense extract (100 mg/kg) on STZ-induced diabetic rats. (I) serum glucose, (II) serum insulin hormone, and (III) lipid peroxide. Results are shown as mean ± S.E.M. (n = 10). a,bSignificantly different from normal control, and diabetic group at p < 0.01.
Oral hypoglycemic therapies used to manage diabetes disease usually exert their effect by stimulating the pancreatic beta cells to secrete insulin hormone, hence lead to rise in the serum insulin level following administration (Ganesan et al., 2018). Fig. 6II reveals that diabetic rats had their serum insulin hormone reduced by 37.4% compared to the normal control group. Rats orally treated with the studied extract showed 35.6% elevation in their serum insulin level compared to the diabetic group, an effect that showed to be significantly different (p < 0.01) from STZ-diabetic rats and not significantly different from that of the reference drug used in the study.
Lipid peroxides are the oxidation products of different fat materials such polyunsaturated fatty acids and phospholipids. Lipid peroxides are mainly associated with the vascular complications of diabetes mellitus as they can degenerate cellular proteins, inactivate vital enzymes, destroy phospholipid-based structures like the cell membrane and some subcellular structures such as lysosomes, microsomes, and mitochondria leading to their malfunction (Vedasree et al., 2022) . There is, indeed, considerable evidence that hyperglycemia associated with diabetes disease leads to an increased production of reactive oxygen species (ROS) due to an altered erythrocytes’ membrane function, which in turn inhibits the activity of superoxide dismutase enzyme. Accumulation of ROS results in lipids peroxidation and hence, elevated lipid peroxides level. As shown in Fig. 6III, induction of diabetes by STZ in rats resulted in 250% increase in the serum level of peroxides relative to normal animals. Oral administration of S. samarangense extract showed 48.5% reduction of the lipid peroxides serum level, an effect that surpassed that of the reference drug, glibenclamide, and showed to be significantly different from the diabetic control group (p < 0.01). This enhanced effect of the extract could be mainly attributed to its rich content of different classes of polyphenols including flavonoids, tannins, glycosides, and phenolic acids that are well renowned with their powerful antioxidant potential.
Taking together the results of both in silico and in vivo studies conducted in this work, it is obvious that the considerable antidiabetic potential of the extract is attributed to its high content of polyphenols. Both PPARɣ and GLP-1 receptors are possible molecular targets for the extract’s components (mentioned above). Our results came in agreement with the previously related literature that reported the antidiabetic activity of the polyphenols identified in the S. samarangense extract. For instance, anthocyanins, which are among the major extract’s components, have been recently reported to exert hypoglycaemic effect through the inhibition of alpha glucosidase and alpha amylase enzymes, thus interfere with the starch digestion leading to a consequent hypoglycaemia (Oliveira et al., 2020). Kaempferol and myricetin along with their derivatives are as well among the compounds prevailing in S. samarangense extract. Different mechanisms of action were reported to explain the antidiabetic effect of kaempferol derivatives including enhancing the cellular uptake of glucose, stimulating the pancreatic beta cells function and leptin hormone regulation (Zang et al., 2015). On the other hand, myricetin derivatives, in accordance with our in silico results, were reported to have an agonistic effects on GLP-1 receptors, which in turn lead to insulinotropic action (Li et al., 2017). In view of our results, we propose, here in, that the components of S. samarangense extract could as well activate the PPARɣ receptors and hence improve the glucose homeostasis via increasing the expression of glucose transporters and enhancing the insulin sensitivity. Moreover, they can increase insulin secretion via activating GLP-1 receptors.
The condensed tannin, procyanidin C1, was found to be a potential insulin sensitizer that works through improving the differentiation of 3 T3-L1 adipocytes, which in turn enhances insulin-induced glucose uptake (Sun et al., 2019). Our in silico data revealed the potential of this compound to activate PPARɣ receptors, an action that leads to enhancing insulin sensitivity. A recent study has reported the regulatory effect of theaflavin-rich fraction from green tea on adipogenesis, a pathway that involves the PPARɣ receptors (Park et al., 2019). In our study, two theaflavin derivatives were among the top compounds that docked successfully to PPARɣ receptors. Moreover, different hydrolysable and condensed tannins isolated from several plant extracts such as Stachytarpheta indica, amaranth leaves, finger millet, and sunflower seeds were reported to exert an antidiabetic effects (Kasali et al., 2016; Kunyanga et al., 2011). It is also crucial to consider the substantial antioxidant potential of the polyphenol-rich extracts that alleviates the cellular oxidative stress and guard against the vascular complications of diabetes disease. Similar antidiabetic effects were reported from tannins rich extracts (Ximenia americana var. caffra and Albizi harveyi) and polyphenols rich extracts (Eugenia uniflora and Eremophila maculata) (Sobeh et al., 2017a,b; 2019b; Youssef et al., 2017).
4 Conclusions
The leaf extract of S. samarangense contains plenty of previously characterized polyphenols. These compounds were compiled into various chemical classes and docked into the active sites of two receptors that are recognized for their roles in T2DM management, PPARɣ and GLP-1. Our molecular modelling results revealed that anthocyanins, tannins and flavonoids were the best scoring classes on the two receptors. The top candidates on each receptor were highlighted and it was found that, out of the best scoring candidates, nine compounds demonstrated good binding affinities on both receptors suggesting their possible roles as antidiabetic agents with dual mechanisms. Moreover, the studied extract at 100 mg/kg was able to improve the glycemic parameters, including serum glucose, insulin, and lipid peroxide levels in STZ-diabetic rats. Further studies are recommended to isolate and investigate, on a molecular level, the binding potential of the nine compounds identified in our study towards PPARɣ and GLP-1 to present them as lead compounds for treatment of diabetes disease.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- American Diabetes Association Diagnosis and Classification of Diabetes Mellitus. Diabetes Care 2013, 36, S67–S74, doi:10.2337/dc13-S067.
- Global Aetiology and Epidemiology of Type 2 Diabetes Mellitus and Its Complications. Nat Rev Endocrinol. 2018;14:88-98.
- [CrossRef] [Google Scholar]
- Type 1 and 2 Diabetes Mellitus: A Review on Current Treatment Approach and Gene Therapy as Potential Intervention. Diabetes Metab. Syndrome Clin. Res. Rev.. 2019;13:364-372.
- [CrossRef] [Google Scholar]
- Pathophysiology of Diabetes Mellitus. Critical Care Nursing Quarterly. 2004;27:113-125.
- [Google Scholar]
- Kaul, K.; Tarr, J.M.; Ahmad, S.I.; Kohner, E.M.; Chibber, R. Introduction to Diabetes Mellitus. In Diabetes: An Old Disease, a New Insight; Ahmad, S.I., Ed.; Advances in Experimental Medicine and Biology; Springer: New York, NY, 2013; pp. 1–11 ISBN 978-1-4614-5441-0.
- Inhibition of Pparγ by Natural Compounds as a Promising Strategy in Obesity and Diabetes. Open Med. Chem. J.. 2019;13
- [Google Scholar]
- Oleanolic Acid Induces a Dual Agonist Action on PPARγ/α and GLUT4 Translocation: A Pentacyclic Triterpene for Dyslipidemia and Type 2 Diabetes. Eur. J. Pharmacol.. 2020;883:173252
- [Google Scholar]
- The Therapeutic Potential of Inhibiting PPARγ Phosphorylation to Treat Type 2 Diabetes. J. Biol. Chem.. 2021;297
- [Google Scholar]
- Structural Insights for the Design of New PPARgamma Partial Agonists with High Binding Affinity and Low Transactivation Activity. J. Comput. Aided Mol. Des.. 2011;25:717-728.
- [Google Scholar]
- Improved Glycaemia Correlates with Liver Fat Reduction in Obese, Type 2 Diabetes, Patients given Glucagon-like Peptide-1 (GLP-1) Receptor Agonists. PLoS ONE. 2012;7:e50117
- [Google Scholar]
- Syzygium Samarangense Leaf Extract Mitigates Indomethacin-Induced Gastropathy via the NF-ΚB Signaling Pathway in Rats. Biomed. Pharmacother.. 2021;139:111675
- [CrossRef] [Google Scholar]
- Chemical Profiling of the Essential Oils of Syzygium Aqueum, Syzygium Samarangense and Eugenia Uniflora and Their Discrimination Using Chemometric Analysis. Chem. Biodivers.. 2016;13:1537-1550.
- [Google Scholar]
- Isolation of Myricitrin and 3, 5-Di-O-Methyl Gossypetin from Syzygium Samarangense and Evaluation of Their Involvement in Protecting Keratinocytes against Oxidative Stress via Activation of the Nrf-2 Pathway. Molecules 2019:24.
- [Google Scholar]
- Chemical Profiling of Secondary Metabolites of Eugenia Uniflora and Their Antioxidant, Anti-Inflammatory, Pain Killing and Anti-Diabetic Activities: A Comprehensive Approach. J. Ethnopharmacol.. 2019;240:111939
- [Google Scholar]
- High Resolution UPLC-MS/MS Profiling of Polyphenolics in the Methanol Extract of Syzygium Samarangense Leaves and Its Hepatoprotective Activity in Rats with CCl4-Induced Hepatic Damage. Food Chem. Toxicol.. 2018;113:145-153.
- [CrossRef] [Google Scholar]
- Bioactive Constituents, Antioxidant and Antimicrobial Activities of Three Cultivars of Wax Apple (Syzygium Samarangense. Res. J. Biotechnol.. 2015;10:1.
- [Google Scholar]
- Acylphloroglucinol Derivatives from the Leaves of Syzygium Samarangense and Their Cytotoxic Activities. Fitoterapia. 2018;129:1-6.
- [CrossRef] [Google Scholar]
- Hypoglycemic Effect of Egyptian Morus Alba Root Bark Extract: Effect on Diabetes and Lipid Peroxidation of Streptozotocin-Induced Diabetic Rats. J. Ethnopharmacol.. 2005;100:333-338.
- [Google Scholar]
- Antioxidant Activity of Artocarpus Heterophyllus Lam. (Jack Fruit) Leaf Extracts: Remarkable Attenuations of Hyperglycemia and Hyperlipidemia in Streptozotocin-Diabetic Rats. TheScientificWorldJOURNAL. 2011;11:788-800.
- [Google Scholar]
- Hepatoprotective and Hypoglycemic Effects of a Tannin Rich Extract from Ximenia Americana Var. Caffra Root. Phytomedicine. 2017;33:36-42.
- [CrossRef] [Google Scholar]
- Albizia Harveyi: Phytochemical Profiling, Antioxidant, Antidiabetic and Hepatoprotective Activities of the Bark Extract. Med Chem Res. 2017;26:3091-3105.
- [CrossRef] [Google Scholar]
- Zoete, V.; Grosdidier, A.; Michielin, O. Peroxisome Proliferator-Activated Receptor Structures: Ligand Specificity, Molecular Switch and Interactions with Regulators. Biochimica et Biophysica Acta (BBA)-Molecular and Cell Biology of Lipids 2007, 1771, 915–925.
- Partial Agonists Activate PPARγ Using a Helix 12 Independent Mechanism. Structure. 2007;15:1258-1271.
- [Google Scholar]
- Ligand Binding and Co-Activator Assembly of the Peroxisome Proliferator-Activated Receptor-γ. Nature. 1998;395:137-143.
- [Google Scholar]
- Crystal Structure of Glucagon-like Peptide-1 in Complex with the Extracellular Domain of the Glucagon-like Peptide-1 Receptor. J. Biol. Chem.. 2010;285:723-730.
- [CrossRef] [Google Scholar]
- Activation of the GLP-1 Receptor by a Non-Peptidic Agonist. Nature. 2020;577:432-436.
- [Google Scholar]
- Antifungal activity of Syzygium samarangense leaf extracts against Candida. Letters in Applied. 2021;73:31-38.
- [Google Scholar]
- Phenolic Acids from Vegetables: A Review on Processing Stability and Health Benefits. Food Res. Int.. 2020;136:109298
- [Google Scholar]
- Tannins Extraction: A Key Point for Their Valorization and Cleaner Production. J. Cleaner Prod.. 2019;206:1138-1155.
- [Google Scholar]
- Anthocyanins: A Comprehensive Review of Their Chemical Properties and Health Effects on Cardiovascular and Neurodegenerative Diseases. Molecules. 2020;25:3809.
- [Google Scholar]
- Chalcones: A Review on Synthesis and Pharmacological Activities. J. Appl. Pharmaceut. Sci.. 2021;11:1-14.
- [Google Scholar]
- Stilbenes in Grape Berries and Wine and Their Potential Role as Anti-Obesity Agents: A Review. Trends Food Sci. Technol. 2021
- [Google Scholar]
- Fiege, H.; Voges, H.-W.; Hamamoto, T.; Umemura, S.; Iwata, T.; Miki, H.; Fujita, Y.; Buysch, H.-J.; Garbe, D.; Paulus, W. Phenol Derivatives. Ullmann’s Encyclopedia of Industrial Chemistry 2000.
- Hydroxytyrosol, Tyrosol and Derivatives and Their Potential Effects on Human Health. Molecules. 2001;2019:24.
- [Google Scholar]
- Cox-Georgian, D.; Ramadoss, N.; Dona, C.; Basu, C. Therapeutic and Medicinal Uses of Terpenes. In Medicinal Plants: From Farm to Pharmacy; Joshee, N., Dhekney, S.A., Parajuli, P., Eds.; Springer International Publishing: Cham, 2019; pp. 333–359 ISBN 978-3-030-31269-5.
- Glucose: Archetypal Biomarker in Diabetes Diagnosis. Clin. Managem. Res. Biomarkers Med.. 2016;10:1153-1166.
- [CrossRef] [Google Scholar]
- Ganesan, K.; Rana, M.B.M.; Sultan, S. Oral Hypoglycemic Medications. 2018.
- Oliveira, H.; Fernandes, A.; F Brás, N.; Mateus, N.; de Freitas, V.; Fernandes, I. Anthocyanins as Antidiabetic Agents—in Vitro and in Silico Approaches of Preventive and Therapeutic Effects. Molecules 2020, 25, 3813.
- The Anti-Obesity and Anti-Diabetic Effects of Kaempferol Glycosides from Unripe Soybean Leaves in High-Fat-Diet Mice. Food Funct.. 2015;6:834-841.
- [Google Scholar]
- Myricetin: A Potent Approach for the Treatment of Type 2 Diabetes as a Natural Class B GPCR Agonist. FASEB J.. 2017;31:2603-2611.
- [Google Scholar]
- Procyanidin C1, a Component of Cinnamon Extracts, Is a Potential Insulin Sensitizer That Targets Adipocytes. J. Agric. Food. Chem.. 2019;67:8839-8846.
- [Google Scholar]
- Theaflavin-Enriched Fraction Stimulates Adipogenesis in Human Subcutaneous Fat Cells. Int. J. Mol. Sci.. 2019;20:2034.
- [Google Scholar]
- Comparative Hypoglycemic Activity of Flavonoids and Tannins Fractions of Stachytarpheta Indica (L.) Vahl Leaves Extracts in Guinea-Pigs and Rabbits. Int. J. Pharm. Pharm. Res. Hum.. 2016;5:48-57.
- [Google Scholar]
- Antioxidant and Antidiabetic Properties of Condensed Tannins in Acetonic Extract of Selected Raw and Processed Indigenous Food Ingredients from Kenya. J. Food Sci.. 2011;76:C560-C567.
- [Google Scholar]
- Efficacy of Cyanotis tuberosa (Roxb.) Schult. Schult. f. root tubers' active fraction as anti-diabetic, antihyperlipidemic and antioxidant in Streptozotocin-induced diabetic rats. J. Ethnopharm.. 2022;285:114856.
- [Google Scholar]
- Antihyperglycaemic Activity of the Methanol Extract from Leaves of Eremophila Maculata (Scrophulariaceae) in Streptozotocin-Induced Diabetic Rats. J. Pharm. Pharmacol.. 2017;69:733-742.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2022.103822.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







