Translate this page into:
Tetraethylenepentamine-containing adsorbent with optimized amination efficiency based on grafted polyolefin microfibrous substrate for CO2 adsorption
⁎Corresponding author at: Malaysia-Japan International Institute of Technology, Universiti Teknologi Malaysia, Jalan Sultan Yahya Petra, 54100 Kuala Lumpur, Malaysia. mahmoudeithar@cheme.utm.my (Mohamed Mahmoud Nasef)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Preparation and optimization of tetraethylenepentamine (TEPA) content in polyethylene/polypropylene (PE/PP) grafted with poly(glycidyl methacrylate) (PGMA) adsorbent for CO2 capture were investigated. The amination reaction parameters such as solvent type, amine to solvent ratio and reaction time were optimized using the Taguchi method to maximize the level of amination in the adsorbent. The obtained data were statistically analysed using the analysis of variance (ANOVA) and signal to noise (S/N) ratio. The chemical, morphological, structural, and thermal properties of the aminated fibrous adsorbent were investigated using Fourier transform infrared (FTIR), scanning electron microscope (SEM), X-ray diffraction (XRD) and thermal gravimetric analysis (TGA). The performance of the obtained TEPA-containing fibrous adsorbent was evaluated for adsorption of pure and mixed CO2/N2 gases under varying pressure up to 30 bar. The optimization of the amination parameters led to an adsorbent with improved properties in forms of not only 23% higher amination level and 27% greater TEPA utilisation efficiency but also 31.6% bigger CO2 adsorption capacity than its corresponding adsorbent obtained without optimization. The adsorbent also demonstrated high CO2/N2 selectivity and regeneration stability. The results suggest that the obtained TEPA-containing fibrous adsorbent is a promising candidate for CO2 capture from CO2/N2 gaseous mixture under various pressures.
Keywords
Solid aminated adsorbent
Polyolefin substrate
Tuning amination level
Taguchi method
CO2 capture
1 Introduction
Development of highly efficient materials and processes for CO2 capture has been widely investigated for mitigation of CO2 emission, which is continuously rising by the soaring demand on fossil (Andreoli, 2017). Majority of CO2 emissions come from energy production (55%) which is driven by fossil fuel (natural gas, petroleum, and coal) combustion and its capturing in industrial power plants follows 3 main approaches pre-combustion, post-combustion and oxyfuel combustion (Leung et al., 2014). Various technologies using chemical and physical solvents, solid adsorbents and membrane separators have been employed to capture CO2 from various effluents (Luis Míguez et al., 2018). Chemical absorption with aqueous alkanolamine solutions is the most matured technology due to high CO2 capturing efficiency and selectivity at low partial pressure (Abu-Zahra et al., 2013). However, this process is challenged by high-energy demand, equipment corrosion and high operation cost (Petrovic et al., 2020). Thus, adsorption by solid support materials such as activated carbon, zeolites, metal organic frameworks (MOFs), porous and mesoporous silica and functionalized polymer supports, has been proposed as reliable alternative adsorbents materials for CO2 capture (Jafari et al., 2018; Zhang et al., 2019). Adsorbents are commonly hosted in columns which operated based on pressure swing adsorption, temperature swing adsorption, vacuum swing adsorption or electric swing adsorption, which varies depending on the adsorbent regeneration strategy (Songolzadeh et al., 2014).
Porous supports fall into micro- and nano-scaled categories and possess advantages in terms of large surface area, high chemical stability and high CO2 selectivity when functionalized with chemical groups such as amines (Luo et al., 2016). Particularly, solid fibrous supports are suitable for hosting amines using techniques like physical (impregnation) and chemical (grafting) interactions. The former method has the disadvantage of not only slow adsorption rate caused by diffusion limitation accompanied pore-filling with amines but also amine leaching and evaporation as a result of weak physical interactions with porous support (Jahandar Lashaki et al., 2019; Liu et al., 2018). On the other hand, chemical grafting leads to stable adsorbents with better kinetics compared to physically impregnated ones but with lower CO2 adsorption capacity (Varghese and Karanikolos, 2020). Thus, chemical impregnation of amine is highly advantageous for adsorbent development and the level of amination must be carefully optimized. Free radical grafting is an appealing method for covalent incorporation of amine-containing grafts to various polymeric substrates and can be carried out using chemical, photochemical, plasma or radiochemical initiators. This method allows desired control to the type of the grafting monomer and amine groups providing tailored adsorbents with desired properties. Radiation induced graft copolymerization (RIGC) is a versatile method to impart desired chemical moiety to inert fibrous polymer substrates by grafting with vinylic (e.g. vinyl benzyl chloride) or acrylate monomers such as glycidyl methacrylate (GMA) (Nasef and Güven, 2012).
Grafting of GMA introduces epoxy groups to various fibrous polymer substrates such as polyethylene/polypropylene (PE/PP) nonwoven sheets and PP nanofibrous sheets, which undergo a mild ring-opening reaction such as amination converting the grafted PGMA precursor into CO2 adsorbent (Abbasi et al., 2019b, 2018; Nasef et al., 2014b, 2014a). The selection of polyolefins such as PE/PP substrate is motivated by its low cost, abundance, stability, great process ability and high radiation resistance (Graziano et al., 2018). The performance of the obtained adsorbents is mainly controlled by the type of amine and its loading level. Particularly, the amine loading level is a function of amination reaction parameters such as concentration of amine agent, type of diluting solvent, temperature and reaction time. Hence, it is essential to optimize the amination reaction parameters to incorporate the desired level of amine (amine density). Tetraethylene pentamine (TEPA) is a branched polyamine that has abundant amino functional groups and potential to enhance CO2 adsorption of TEPA impregnated solid porous inorganic substrates such as nanosepiolite (Irani et al., 2015) and mesocellular silica sponge (Dao et al., 2020). However, amination of the PGMA grafted fibrous substrates with TEPA and optimization of its density have not been reported.
Design of experiment (DOE) is a statistical tool than was used in a number of studies as a tool for optimization of the treatment parameters to maximize CO2 adsorption capacity in amine containing adsorbents through applying response surface methodology (RSM) (Selin and Emik, 2018; Thouchprasitchai et al., 2017). However, studies employing DOE through Taguchi method for maximizing the amine level to enhance CO2 adsorption performance have not been reported. Taguchi method, is a unique and powerful technique that allows economical optimization of process parameters with minimum number of experiments to determine the best factor combination by studying the effects of individual factors on the response (Davis and John, 2018).
The objective of this study is to systematically investigate the effect of reaction parameters on amination efficiency of PGMA grafted PE/PP fibrous substrate obtained by RIGC of GMA using Taguchi method. Particularly, the parameters of amination with TEPA such as solvent types, amine to solvent ratio and reaction time are optimized to achieve a maximum level of amination. The various physico-chemical properties of the obtained adsorbent are investigated. The theoretical CO2 adsorption, TEPA utilisation efficiency and adsorbent selectivity are studied. The performance of the obtained adsorbent with optimum amine content was evaluated for CO2 adsorption.
2 Experimental section
2.1 Chemicals and reagents
The PE/PP nonwoven sheet grafted with PGMA with a degree of grafting of 166%, was prepared by RIGC as described in a previous communication (Nasef et al., 2014a). The PE/PP sheets were irradiated by an electron beam (EB) accelerator (NHV-Nissin High Voltage, EPS3000) operated at an acceleration energy of 2 MeV and a beam current of 10 mA with a total dose of 50 kGy at 25 kGy/pass to create the free radicals. The irradiated PE/PP sheets were placed in a grafting system having an evacuated glass ampoule containing N2 bubbled emulsion grafting mixture containing 10% vol% of GMA in deionized water (DI) and 0.5% Tween 20 (surfactant). The reaction was carried out at a temperature of 50 °C for 35 min and the obtained grafted samples are denoted as PE/PP-g-PGMA. The degree of grafting in the samples was determined as the weight increase after grafting compared to initial sample weight. TEPA (purity ≥ 95%) was purchased from Acros Organics (California, United States). Solvents such as methanol, ethanol and isopropanol were analytical grade and purchased from Merck (Darmstadt, Germany). DI was obtained from Barnstead Nanopure Diamond Lab Water Purification System (ThermoFisher, Waltham, USA) and used for washing of samples and dilution of TEPA. Pure CO2 gas (99.8%) and pure N2 gas (99.999%) were supplied by Linde Malaysia (Sdn Bhd).
2.2 Functionalization of adsorbent precursors
A sample of known weight of PE/PP-g-PGMA sheet (adsorbent precursors) in a form small pieces of known weight was added to a 100 ml round bottom flask containing 30 ml of TEPA solutions having different solvents (water, methanol, ethanol, and isopropanol) and TEPA/solvent ratios as shown in Table 1. The reaction was carried out under reflux taking the boiling point of each solvent (100 °C, 65 °C, 78 °C and 82 °C) into account and at continuous stirring for a time range of 1–4 h. After reaction completion, the aminated sample was removed and repeatedly washed with DI and ethanol followed by drying in a vacuum oven at 60 °C for 5 h. All experiments were repeated 3 times and the reported data for the percent of amination is an average of three readings. The weight change in the sample after amination reaction, which is denoted as PE/PP-g-PGMA/TEPA was determined and used to calculate the amination efficiency (percent of amination) as per the following equation:
Variables
Levels
1
2
3
4
Solvent type
Water
Methanol
Ethanol
Isopropanol
TEPA/solvent ratio
1:3
1:1
2:1
3:1
Reaction time (h)
1
2
3
4
2.3 Characterization of adsorbent
The chemical, morphological, textural, structural, and thermal properties of the aminated fibrous adsorbent were evaluated in comparison of the pristine PE/PP and the grafted fibrous substrates before functionalization. The changes in the chemical composition were analysed using a Nicolet iS50 FT-IR spectrometer. The spectra were obtained in a frequency range of 500–4000 cm−1 with 32 scans and 4 cm−1 resolution. The morphological properties of the samples were investigated using a GEMINISEM 500 microscope. The textural properties were obtained from nitrogen adsorption/desorption isotherms recorded at −196 °C on Quantachrome NOVAtouch™ instrument. The surface area, total pore volume and pore size were measured by using the multipoint Brunauer–Emmett–Teller (BET) method. The crystalline structure of the samples was investigated by X-ray diffraction (XRD) using a PANalytical Empyrean analyser at Bragg’s angle in the range of 5–80°. The thermal stability of the samples was carried out using a Q50 thermogravimetric analyser (TA Instruments) in a temperature range of 30–700 °C at a heating rate of 10 °C /min.
2.4 Design of experiment (DOE)
Before designing the experiment, a few initial experiments were performed to screen the significant independent parameters and find their suitable levels. Based on the literature analysis and preliminary experimental results, the significant parameters were identified, and their levels were determined as presented in Table 1 (Abbasi et al., 2019b; Othman et al., 2019). The experimental design was set to maximize the amination percentage and thus a quality characteristic was employed for the analysis. Three experimental parameters such as solvent type, TEPA/solvent ratio and reaction time were investigated and optimized through Taguchi design. The analysis of variance (ANOVA) and signal-to-noise (S/N) (available in Minitab 17.0 software) were used to monitor the effect of the parameters on amination efficiency and optimize the process. Experimental runs were designed through Taguchi orthogonal array method of L16.
2.5 CO2 adsorption tests
The CO2 adsorption capacity measurement studies was carried out using the magnetic suspension balance (MSB) from isoSORP® Gravimetric Analyzer that was manufactured by RUBOTHERM (Bochum, Germany). Details of the basic principles, components and operational procedure of MSB was described elsewhere (Fujii et al., 2010; Schell et al., 2012). Completion of one cycle of CO2 adsorption capacity measurement consists of three steps comprising pre-treatment, buoyancy, and adsorption measurement. All the data were recorded by RUBOTHERM system control software (RSCS-2016) and each measurement was configured step by step. A pre-treatment was conducted for both fresh and regenerated adsorbent samples by heating 80 °C for 4.5 h under vacuum until the weight loss of adsorbent became constant indicating a complete removal of any trapped moisture in the former and adsorbed CO2 in the latter. Buoyancy measurement was also carried out for fresh and regenerated samples to precisely determine the adsorbents weight and volume using purified N2 gas at 30 °C in a varying pressure from vacuum condition to 30 bar. Finally, the adsorption measurements of both pure CO2 and N2 gases and their mixture containing 40% CO2 was carried out by the sample evacuation for 30 to 60 min followed by gas introduction variation of the pressure from 5 to 30 bar with a gas flowrate of 500 ml/min at 30 °C. The equilibrium sorption was achieved in about 50 min for every pressure reading. Desorption is carried after each adsorption measurement was completed at 30 bars. The desorption process was performed by depressurizing sample holding vessel until reaching vacuum before heating to 80 °C for 4.5 h until no weight loss was detected in the adsorbents. This was followed by buoyancy and adsorption measurements to achieve another adsorption cycle. All adsorption/desorption cycles experiments were repeated 3 time and the average values were reported.
2.6 Calculation of theoretical CO2 adsorption, TEPA utilisation efficiency and selectivity
The theoretical CO2 adsorption capacity (νT) in mg/g of the adsorbent (PP/PP-g-PGA/TEPA) was calculated taking the reaction of 2RNH2 + CO2 → R2NH2+ + R2NCOO– (in the absence of water) into account using equation (2) (Guo et al., 2020):
The TEPA utilization efficiency or adsorption capacity per unit TEPA (Q) in mg/g, which refers to the amount of adsorbed CO2 per unit of loaded TEPA, can be calculated as follows (Guo et al., 2020):
The adsorption selectivity was calculated using the following equation:
3 Results and discussion
Fig. 1 shows reaction scheme for preparation of CO2 fibrous adsorbents by RIGC of GMA onto PE/PP fibrous sheet and subsequent functionalization of the obtained PE/PP-g-PGMA with TEPA. The incorporation of TEPA conferred the basicity if forms of polyamines which are essential for CO2 capture (chemisorption) which is categorized as Lewis acid (Varghese and Karanikolos, 2020).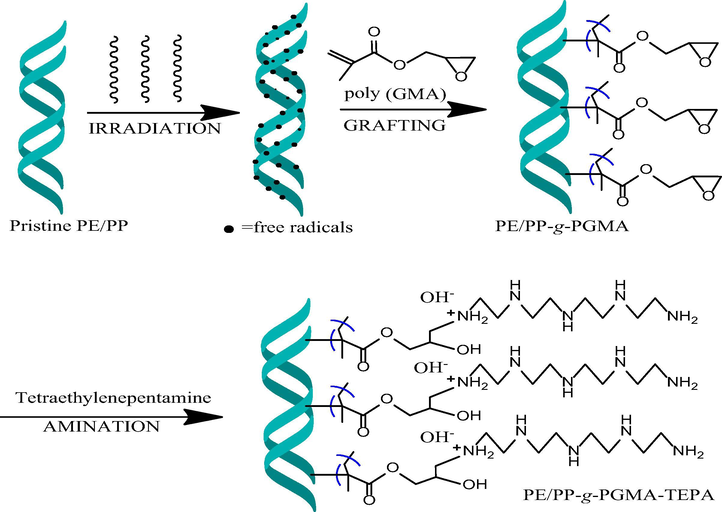
Reaction scheme for preparation of fibrous adsorbent by RIGC of GMA onto PE/PP nonwoven sheet and subsequent amination with TEPA.
3.1 Changes in chemical structure
Fig. 2a shows the spectra of pristine PE/PP and PGMA grafted PE/PP nonwoven sheets together with the corresponding aminated adsorbent. The pristine PE/PP polymer was marked by the presence of 2 peaks representing the antisymmetric and asymmetric stretching vibrations of C-H at 2917 and 2847 cm−1, respectively. This was coupled with bending vibration peaks at 1457 cm−1 for CH2 and 1376 cm−1 for CH3 of PP (Kavakli et al., 2014). The incorporation of PGMA led to the emergence of 2 new bands at 840 and 905 cm−1, which are assigned for epoxy group confirming the presence of successful grafting (Nasef et al., 2014a). Besides, the peak at 1740 cm−1 was assigned for the carbonyl groups from the ester in PGMA present in both PE/PP-g-PGMA and PE/PP-g-PGMA/TEPA samples (Kavaklı et al., 2016). The disappearance of epoxy group representative bands at 840 and 905 cm−1 after amination provides a strong evidence for the successful epoxy ring-opening by TEPA. The peaks at 1527 cm−1 and 1658 cm−1 were assigned for the secondary and primary amine from TEPA. From these results, it can be concluded that a fibrous adsorbent was successfully prepared by PGMA grafting and subsequent amination. Furthermore, an investigation of the changes took place in the adsorbent chemical structure after CO2 adsorption compared to pristine adsorbent was made and the obtained spectra are shown in Fig. 2b. The adsorbent spectrum showed 2 new peaks at 1590 cm−1 and 1490 cm−1, which are assigned for COO− stretching vibrations after capturing of CO2 molecules by the adsorbent. The appearance of a small peak at 1320 cm−1 originated from the formation of carbamate (NCOO−) skeletal vibration confirms the interaction of CO2 with the TEPA amine group (Dao et al., 2020; Wang et al., 2020).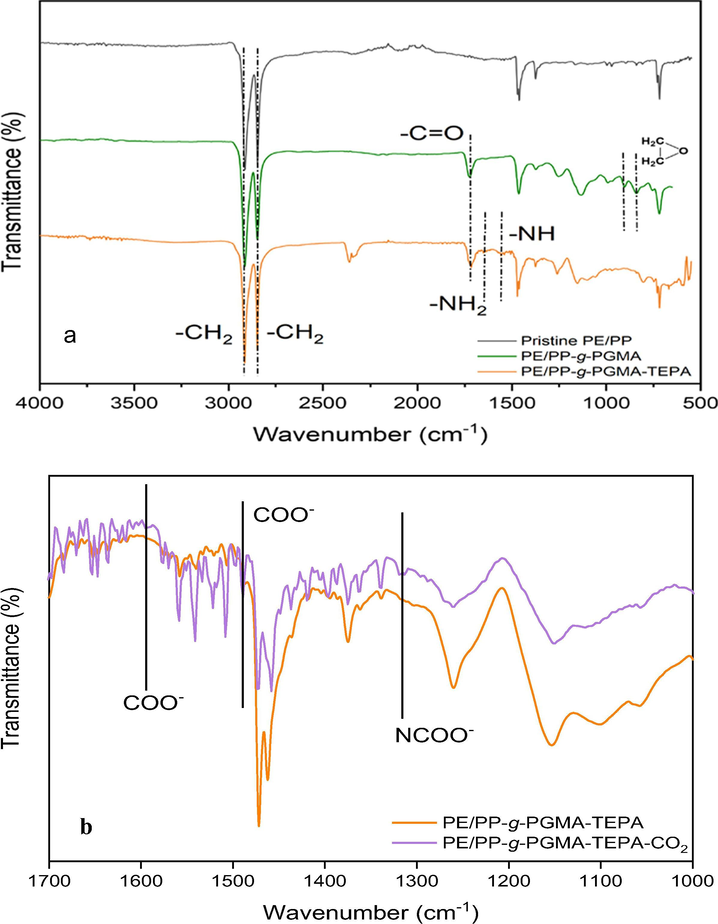
FTIR spectra of: (a) pristine PE/PP, PE/PP-g-PGMA and PE/PP-g-PGMA/TEPA samples and (b) adsorbent of before and after CO2 adsorption.
3.2 Changes in surface morphology
Fig. 3 shows the changes in the surface morphology of PE/PP-g-PGMA/TEPA compared to pristine PE/PP substrate and that of the corresponding grafted PE/PP-g-PGMA precursor. The average of fibre diameter of pristine PE/PP substrate was increased significantly after grafting of PGMA and covalent impregnation of TEPA. For instance, the average of fiber diameter increased from 12.89 µm in pristine PE/PP to 20.92 µm in PE/PP-g-PGMA and to 32.54 µm in PE/PP-g-PGMA/TEPA. This was accompanied by a reduction in the free volume (pores) between the fibers forming denser and lesser porous structure in the adsorbent sheet. These observations further confirm the success of PGMA grafting and TEPA loading.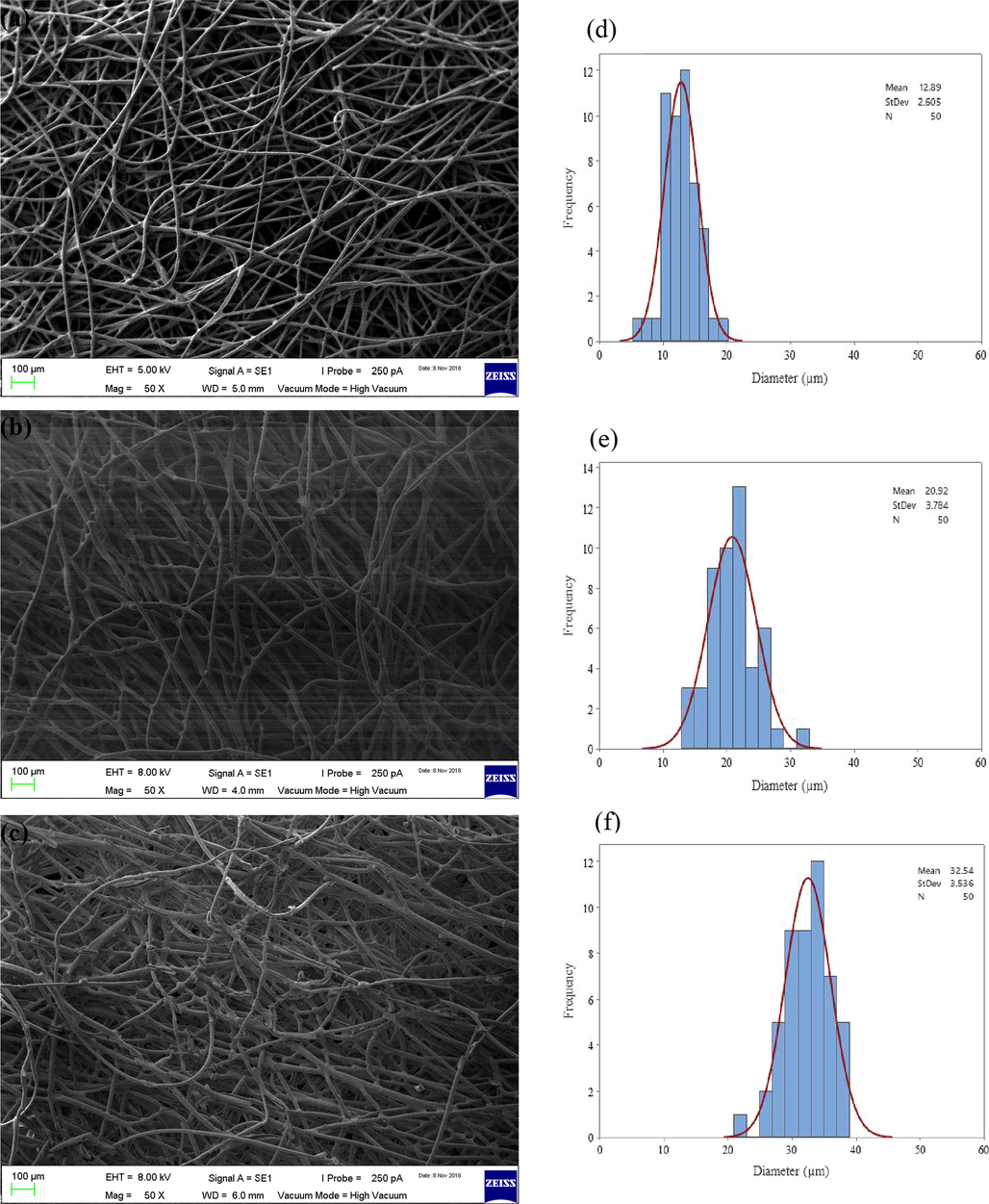
SEM images of pristine PE/PP (a), PE/PP-g-PGMA (b) and PE/PP-g-PGMA/TEPA samples (c) together with their related histograms of diameter size distribution (d,e and f).
From the previous sample analysis, the chemical and textural properties of the best PE/PP-g-PGMA/TEPA adsorbent can be summarized as presented in Table 2. As can be seen, the adsorbent has a small surface area compared to inorganic porous aminated adsorbents reported in the literature. However, the adsorption in this material is rather dependent upon the large number of amine groups present on its fibrous surfaces. This suggests that CO2 adsorption is not only predominantly controlled by the number of the polyamine ligands and their distribution on the surface of the adsorbent but also by the amine sites accessibility.
Properties
Description/value
Substrate
Physical formPE/PP
Non-woven sheet
Ionic moiety
TEPA
Chemical structure
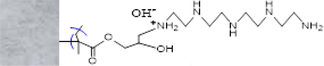
Average fiber diameter (μm)
32.54
Amine density (mmol/g)
Surface area (m2/g)
Pore volume (cm3/g)
Pore radius (nm)3.23
1.8
2.3 × 10−3
2.55
3.3 Changes in crystalline structure
Fig. 4 shows the XRD diffractograms of the pristine PE/PP, PE/PP-g-PGMA and PE/PP-g-PGMA/TEPA samples. All the diffractograms showed almost similar crystalline peak patterns without significant changes in the intensities of major peaks indicating that the crystalline structure of pristine PE/PP was well-preserved even after the two-step procedure, i.e. grafting and amination. Moreover, there is no new peak emerged after the amination of PE/PP-g-PGMA precursor confirming that amines has no contribution to diffraction pattern (Hashim, 1998; Sharif et al., 2013). However, the diffraction angles showed minor shifts to higher Bragg’s angle after incorporation of PGMA and subsequent amination with TEPA. These observations suggest that the grafting of GMA took place in areas close to the surface of the crystallites of PE/PP substrate causing a partial disorder in the crystalline structure which slightly increased with loading of TEPA. It can be concluded that the incorporation of PGMA/TEPA ligand took place mainly on the surfaces of PE/PP fibrous support in a way affecting the porous structure of the sheet without invading the core structure of the fibers.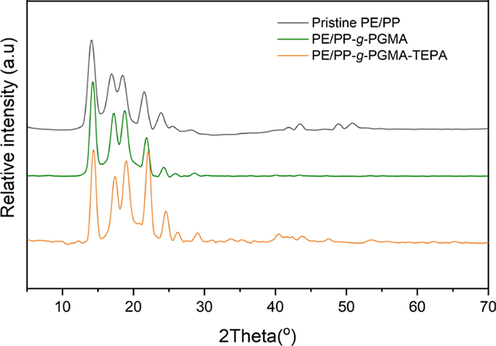
XRD diffractograms of pristine PE/PP, PE/PP-g-PGMA and PE/PP-g-PGMA/TEPA samples.
3.4 Changes in thermal stability
Fig. 5 shows the TGA thermograms of pristine PE/PP, PE/PP-g-PGMA and PE/PP-g-PGMA/TEPA samples. Compared to the single-step weight loss observed in the pristine PE/PP substrate starting from >300 °C, the depolymerization of PE/PP-g-PGMA started <300 °C following a single step decomposition pattern suggesting the presence of high compatibility between PGMA and PE/PP substrate. In the aminated sample, three-step decomposition pattern was observed with the first degradation stage started at >100 °C and continued to 150 °C is due to the dehydration of water molecules and the desorption of pre-adsorbed CO2 (Liu et al., 2013). The continuous weight loss up to ∼200 °C is mostly due to the decomposition of amines. The third weight loss, which was in the range of 250–350 °C is due to the decomposition of grafted region (Xu et al., 2015). The third stage of the weight loss in PE/PP-g-PGMA/TEPA sample at 350 °C and beyond is attributed to the decomposition of the main chains of PE/PP polymer backbone.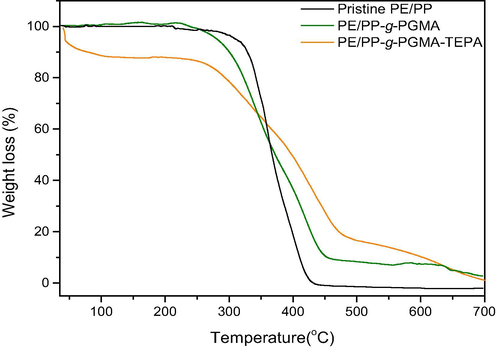
TGA thermograms of pristine PE/PP, PE/PP-g-PGMA and PE/PP-g-PGMA/TEPA samples.
3.5 Effect of reactions conditions on percent of amination
The percent of amination was calculated using equation (1) and the obtained values are presented in Table 3. Three variables including type of solvent, ratio of TEPA to solvent and the reaction time were considered. A total number of 16 experiments were suggested from Taguchi experimental design, each of the reaction took place at the boiling point of each solvent. As can be seen, water show the lowest percent of amination (0.08%) at 1:3 TEPA/water ratio and 1 h reaction time whereas isopropanol yielded the highest percent of amination (57.15) at 1:1 TEPA/isopropanol ratio and 3 h reaction time. Low amination in presence of TEPA dilution by water was also reported elsewhere (Choi, 1999). Furthermore, the ethanol also showed no significant increase in the percent of amination where amination percentage as high as 14.05% was obtained at 3:1 TEPA/ethanol ratio and 2 h reaction time. Unlike ethanol, methanol increased the percent of amination leading to an amination percentage of 43.66% at 2:1 TEPA/methanol ratio and 4 h reaction time. It can be concluded that the sequence of solvent efficiency in amination is water < ethanol < methanol < isopropanol. Hence, isopropanol was chosen as the best solvent for the amination reaction.
Experiment no.
Solvent
TEPA: solvent ratio
Reaction time (h)
Amination (%)
1
Water
1:3
1
0.08
2
Water
1:1
2
1.06
3
Water
2:1
3
1.18
4
Water
3:1
4
3.53
5
Methanol
1:3
2
19.05
6
Methanol
1:1
1
37.24
7
Methanol
2:1
4
43.66
8
Methanol
3:1
3
32.95
9
Ethanol
1:3
3
1.11
10
Ethanol
1:1
4
4.31
11
Ethanol
2:1
1
6.22
12
Ethanol
3:1
2
14.05
13
Isopropanol
1:3
4
26.35
14
Isopropanol
1:1
3
57.15
15
Isopropanol
2:1
2
49.03
16
Isopropanol
3:1
1
55.84
Considering solvent ratio, higher amine content led to an increase in the percent of amination with ethanol as a solvent. This is likely due to the increase in the access of TEPA to epoxy groups in the PE/PP-g-PGMA substrate. However, a slightly different trend was observed when methanol and isopropanol were used as solvents. For instance, the increase in TEPA content in methanol led to an increase in the percent of amination until it achieved 43.66% yield at a ratio of 2:1 TEPA/methanol beyond which it decreased to 32.95% with a higher content of TEPA (3:1 ratio) in the mixture. However, isopropanol showed inconsistent results as the highest percent of amination was 57.15% when the TEPA/isopropanol ratio was 1:1, but slightly decreased when the ratio was 2:1 with 49.03% of amination and again increased when the ratio was 3:1 with the percent of amination increased to 55.84%, which is almost similar to the level obtained with the ratio of 1:1. Such inconsistency in the results could be due to the mutual changes in TEPA/isopropanol ratio and reaction time parameters at the same time. However, the longer reaction time should have led to higher percent of amination since it allows higher chances for reaction of TEPA with epoxy rings.
3.6 Optimization of percent of amination using Taguchi method
The main effects plots for signal-to-noise (S/N) ratio and means are shown in Fig. 6. The significance of every parameter was measured from the slope of the main effect plot. The line of high slope angle representing such parameter is more significant, which means, there is no main effect present when the line is horizontal (parallel to the x-axis). The greater the difference in the vertical position of the plot points, the greater is the magnitude of the main effect (Antony et al., 2006; Thakker et al., 2018). From the results in Fig. 6 a, it can be inferred that solvent type is more significant than the other factors. As can be seen, the highest percent of amination was obtained when isopropanol was used as a solvent whereas water showed the worst performance for amination. The reaction time showed a small effect on the S/N ratio compared to the amine to solvent ratio. The experimental runs at 2 and 3 h also had a small effect on the S/N ratio. However, longer reaction time showed higher impact on the percent of amination. It is also obvious that increasing the amount of TEPA in the reaction solution as in the case of 3:1 amine to solvent ratio led to a higher effect on the amination reaction. It can be also observed that the highest value of S/N ratio calculated at each level for each factor was selected to have the best parameters combinations to achieve the maximum percent of amination. So, to achieve the optimum percent of amination (amination efficiency), the applied parameters should be isopropanol as a solvent, 3:1 amine to solvent ratio and 4 h of reaction time.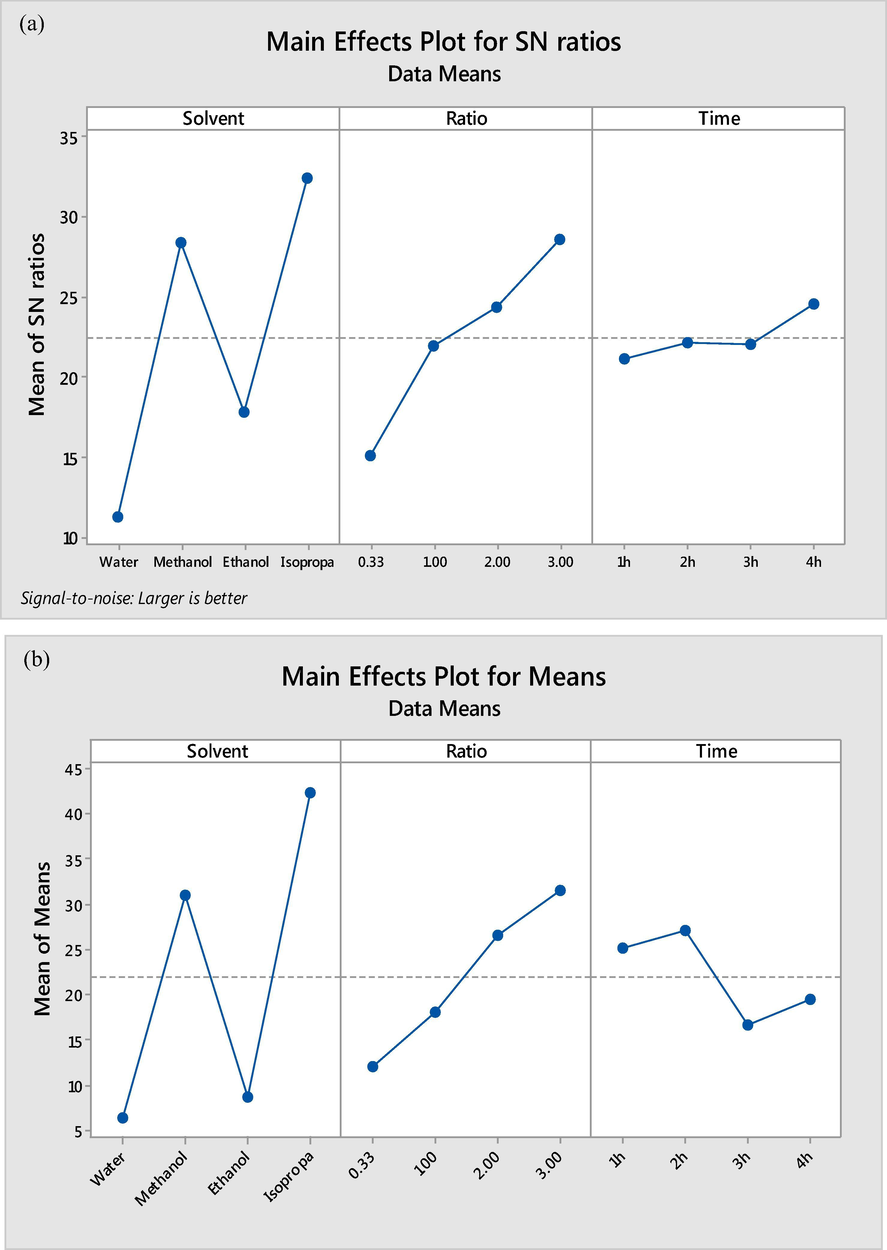
Main effects plots for SN ratios (a) and main effects plots for means (b) for reaction parameters.
ANOVA was conducted to identify significant parameters affecting the percent of amination and the results are presented in Table 4. A parameter with higher F-value and lower P-value has the most significant effect on the response (Liao et al., 2008; Nik et al., 2012). The solvent type appears to be more significant factor than TEPA to solvent ratio as indicated by the higher F-value of the former (25.22) compared to 18.27 for the latter. Furthermore, the P-value for the solvent is smaller (0.000) than that of the ratio value (0.001). Thus, the solvent type is the most important factor and this model can be regarded as significant since F-value for both solvent and amine/solvent ratio is >1 and P-value is <0.01.
Source
DF
Adj SS
Adj MS
F-value
P-value
Regression
4
4536.2
1134.04
23.48
0.000
Ratio
1
882.4
882.39
18.27
0.001
Solvent
3
3653.8
1217.92
25.22
0.000
Error
11
531.2
48.29
Total
15
5067.3
Fig. 7 shows the residual plots, which depicts a comparison between the experimental and predicted data. From the normal probability plot in Fig. 7 a, it seems that the plot is scattered along a straight line, which means that the experimental and predicted data have a linear relationship. Furthermore, the data are normally distributed, and the variables are affecting the response. Accordingly, the residual vs. fitted plot in Fig. 7 b shows a structure loss of the plot pattern around the residual points, which is representing the validity of the assumptions and a constant variance. Moreover, the histogram from Fig. 7 c shows that the data are not skewed and no outliers presented and the residuals vs. order from Fig. 7 d shows the time or data collection order indicating that there are systematic effects (Panda and Singh, 2013).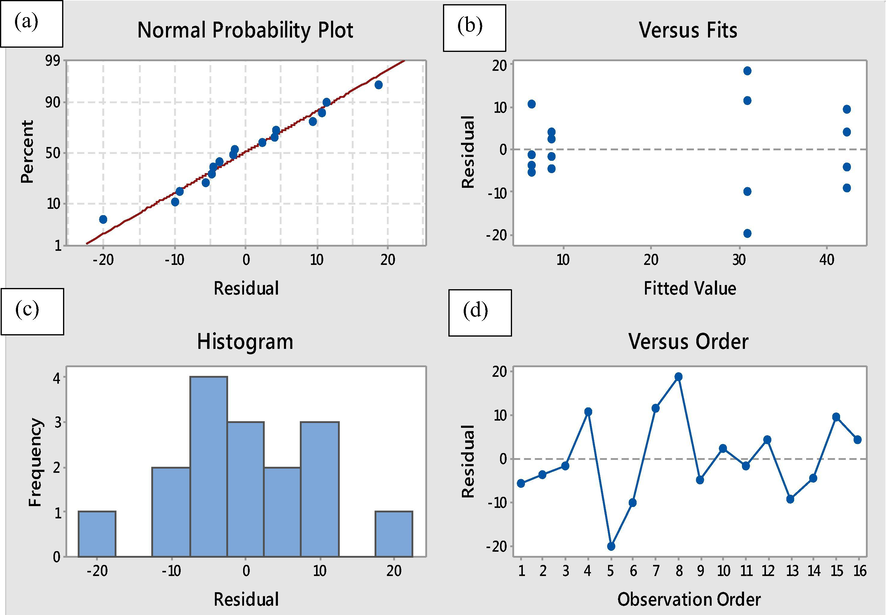
Plots of normal probability, variation of the standardized residuals with fitted values, histogram of vairation frequency with residuals and variation of the standardized residuals with observed order values of the data.
The Taguchi method predicts the optimum parameters required to validate the model and it suggests that higher percent of amination is desired. This experiment was performed using isopropanol as a solvent, 3:1 amine to solvent ratio and reaction time is 4 h. Thus, from the reaction, the obtained percent of amination is 70.42%, which is not only as high as predicted but also is the highest among all the results obtained from the 16 runs of experiments presented and discussed earlier. Considering the experimental run number 16, one can observe that the only different parameter was the prolonged reaction that was increased from 1 to 4 h leading to 26% increase in the percent of amination. This allowed more interactions between the aminating agent and the epoxy groups available in the PE/PP-g-PGMA adsorbent precursor leading to higher amine immobilisation (Zhuang et al., 2013). The PE/PP-g-PGMA/TEPA-V adsorbent sample obtained under optimised conditions is likely to have more CO2 adsorption capacity compared counterparts with lower TEPA content. It can be concluded that the model developed from Taguchi method is proven to be capable of prediction of the percent of amination and optimisation of the reaction parameters to achieve a maximum amination efficiency in PE/PP-g-PGMA fibrous substrates.
3.7 CO2 adsorption performance
The CO2 adsorption capacity of PE/PP-g-PGMA/TEPA with the highest and lowest amine content was investigated not only to observe the impact of amine content on the CO2 adsorption ability but also to validate the design of experiment created by Taguchi method at different operating pressures in various ranges up to 30 bar. The adsorbent performance was tested with pure gases (CO2 and N2) followed by adsorption measurements with a binary gaseous mixture of CO2/N2 containing 40% CO2. Fig. 8A shows the pure CO2 adsorption isotherms for pristine PE/PP, PE/PP-g-PGMA, PE/PP-g-PGMA/TEPA3 and PE/PP-g-PGMA/TEPA14 (prepared in run 3 and 14 of Table 3), and PE/PP-g-PGMA/TEPA-V, which are the samples to validate the model from Taguchi method. Both pristine PE/PP and PE/PP-g-PGMA samples were used as adsorbent references even though they do not have functional groups, and this is to evaluate the presence of any significant physisorption associated with strong hydrophobic nature of these samples.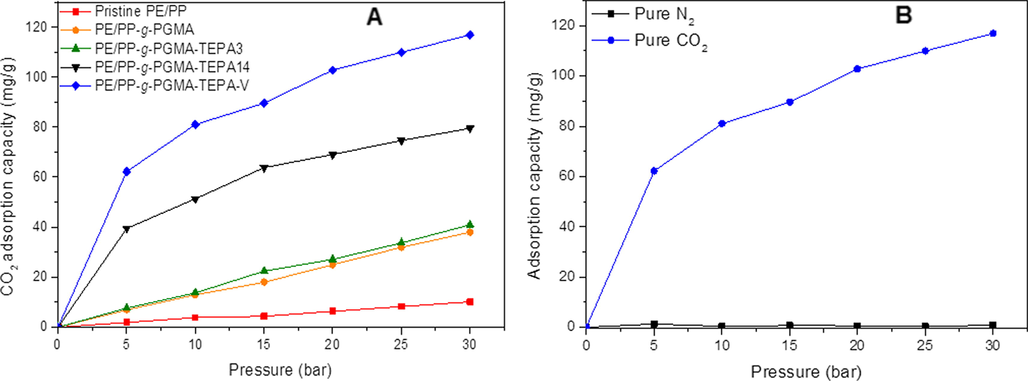
Variation of adsorption with pressure for: A) pure CO2 on pristine, grafted substrates and various adsorbents and for B) pure CO2 and N2 adsorption on PE/PP-g-PGMA/TEPA-V at 30 °C.
As can be seen, the pristine PE/PP sample showed very low CO2 adsorption yet pressure dependent isotherm with maximum adsorption capacity of 10 mg/g compared to 38 mg/g for PE/PP-g-PGMA sample at 30 bar indicating the presence of a level of affinity towards CO2. Since, such adsorbent samples do not have any functionality, thus, the observed CO2 adsorption is likely to be due to physical sorption in forms of the hydrophobic associations (Van der Waals force) in fibrous substrates that was increased by the incorporation of strongly hydrophobic PGMA imparting more hydrophobicity to PE/PP fibrous sheet (Younas et al., 2016). It can be also noticed that the CO2 adsorption capacity of PE/PP-g-PGMA/TEPA3 with an amination efficiency of 1.18% (after H2O/TEPA mixture treatment) was as low as 41 mg/g at 30 bar, which is slightly higher than that of PE/PP-g-PGMA. This suggests that the hydrophobic fibrous structure have a more important role than the TEPA functionalities in this sample. In other words, physisorption is predominantly controlling the adsorption behavior of this slightly aminated sample having a curve shape matching those of pristine and grafted samples and resembling multi-layer adsorption.
At higher amination levels of 57.15% and 70.42%, the shape of the pressure-dependent curves (isotherms) started to shift towards Type-I suggesting a monolayer adsorption dominated by chemisorption. The CO2 adsorption capacity of PE/PP-g-PGMA/TEPA14 (obtained from isopropanol/TEPA mixture treatment without optimization) almost doubled to 80 mg/g at 30 bar, which was considerably increased to 117 mg/g for PE/PP-g-PGMA/TEPA-V (obtained from isopropanol/TEPA mixture treatment optimization) due to the increase in basic TEPA sites causing a stronger affinity to CO2 which has an acidic nature. When this adsorbent was tested for pure N2 adsorption under similar conditions, it showed a negligible affinity to CO2 as indicated by the tiny adsorption capacity (≥1.29 mg/g) at various pressures up to 30 bar as shown in Fig. 8B. This indicates that the adsorbent is highly selective to CO2.
The adsorption behavior of binary CO2/N2 gaseous mixture on 5 adsorbent samples at various pressures were measured and is represented by adsorption isotherms depicted in Fig. 9. As in pure gases, the adsorption has a pressure-dependant behavior and the performance followed pure CO2 gas increasing trend. However, the performance of all samples is approximately reduced by 44% upon using a gas mixture of 40% CO2 with 60% N2. The reduction in CO2 sorption in gas mixture is due to the competition between CO2 and N2 gases which led to a reduction in the accessibility of amine groups for CO2 molecules (Rodríguez et al., 2019). Moreover, PE/PP-g-PGMA/TEPA-V showed the highest performance at all pressures.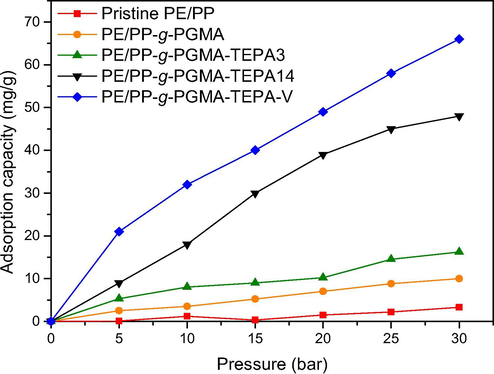
Variation of CO2 adsorption with pressure on pristine and grafted substrates and adsorbents for a mixture of CO2 and N2 containing 40% CO2.
Table 5 shows the variation of the adsorption selectivity of best adsorbent, which was estimated at various pressures at 30 °C. The PE/PP-g-PGMA/TEPA-V adsorbent exhibited high adsorption selectivity of CO2 over N2 at all pressures with the highest value of 1194 obtained at 5 bar which decreased to 136 at 30 bar. These observations indicate that a large amount of CO2 can be adsorbed at low pressures compared to N2, which is scarcely adsorbed. This indicated that the interaction between polar surface of the adsorbent and quadrupolar molecule of CO2 is favourably occurred at low pressure which is explained by the high selectivity at 5 bar operating pressure. Similar observation was reported for selective adsorption of CO2 from a mixture with CH4 (Tamnanloo et al., 2014). The high selectivity of this adsorbent is caused by the strong affinity of CO2 towards TEPA groups unlike N2. Moreover, the variation in the selectivity depends on level of accessibility to TEPA groups. It is noteworthy mentioning that the adsorption selectivity of such fibrous aminated adsorbent is not only reported for first time but also the obtained selectivity values are interestingly high and can match those of established CO2 adsorbents (Varghese and Karanikolos, 2020; Yang et al., 2017). It can be concluded PE/PP-g-PGMA/TEPA-V is a potential adsorbent for separation of CO2 from its mixtures with N2.
Pressure (bar)
Adsorption selectivity
5
1194
10
281
15
160
20
181
25
189
30
136
3.8 TEPA utilisation efficiency and selectivity of adsorbents
The experimental adsorption capacity is compared with the theoretical adsorption capacity for 3 adsorbents with different TEPA content and the data is present in Fig. 10A. The experimental adsorption capacities of the two adsorbents with high TEPA content were smaller than their corresponding theoretical adsorption capacities but maintained equal differences. For instance, PE/PP-g-PGMA/TEPA-V (70.42 amine %) and PE/PP-g-PGMA/TEPA14 (57.15 amine %) showed about 47% lower adsorption capacities than their corresponding theoretical values that were 221 and 149 mg/g, respectively. Such difference seems to be reasonable taking the low surface area of the present fibrous adsorbent into consideration. The unusual behavior of PE/PP-g-PGMA/TEPA3 in which the theoretical adsorption capacity was found to be inferior to that of experimental value by 85.3% is caused the extremely low amination efficiency of 1.18% leaving the physical adsorption observed in the hydrophobic grafted sample to play a bigger role in CO2 adsorption than the tiny TEPA content. The increase in the amination level to 57.15% and above imparted strong hydrophilic moieties to the adsorbent surface leading to the dominance of chemical adsorption and the suppression of the physical adsorption.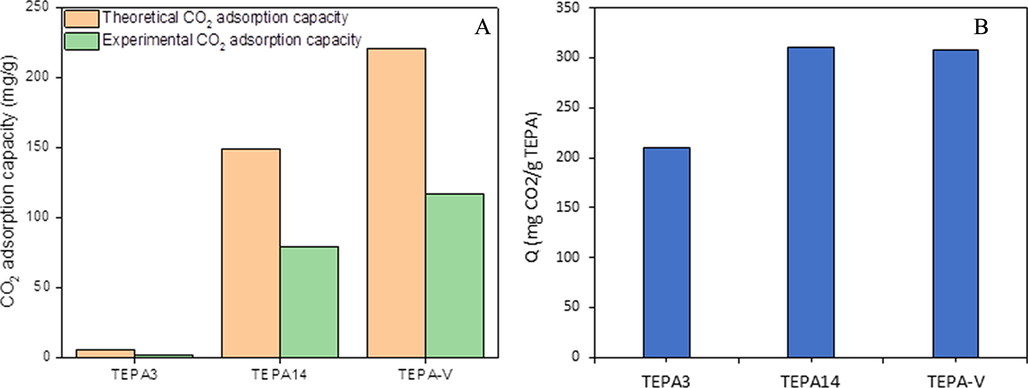
Experimental and theoretical CO2 adsorption capacities (A) and TEPA utilisation efficiency (B) on 3 adsorbents with different amination levels.
The TEPA utilization efficiency or CO2 adsorption capacity per unit TEPA (Q) of 3 functionalised adsorbents with different amination percentages is presented in Fig. 10B. As can be seen, Q increased from 210 to 310 mg/g with the rise in the TEPA content from 1.18% in PE/PP-g-PGMA/TEPA3 to 57.15% in PE/PP-g-PGMA/TEPA14. The physisorption of CO2 was excluded from the former sample. The increase in TEPA content to 70.42% in PE/PP-g-PGMA/TEPA-V sample showed almost no change in Q and stands at 308 mg/g. This trend is most likely attributed to the presence of incomplete amination level (i.e., not achieving100%) in the two samples despite the variation of amination percentage. This allowed more access to amine sites with the pressure increase by enhancing the CO2 diffusion that overcome the mass transfer limitation. This observation suggests that TEPA utilization efficiency is mainly dependent on the content and the access to amine sites which plays a crucial role in determining the overall adsorption capacity of the present adsorbent. Similar observations were reported for nanofibrous polypropylene-based adsorbents containing PGMA grafts modified with various amines (Abbasi et al., 2019a; Lin et al., 2013).
3.9 Regeneration of PE/PP-g-PGMA/TEPA-V adsorbent
The stability of PE/PP-g-PGMA/TEPA-V sample was evaluated for 11 adsorption/desorption cycles and the results are present in Fig. 11. The adsorption was conducted at a temperature of 30 °C with a pure CO2 and a flowrate of 500 ml/min and the adsorbent was regenerated by placing it inside the sample container of MSB and heating to 80 °C for 4.5 h under vacuum conditions until a constant weight was obtained in the adsorbent. The adsorbent showed a reasonable stability with an average adsorption capacity of 114 mg/g (∼3% loss) after 11 cycles at a pressure of 30 bar. Such negligible loss in the adsorption capacity is possibly due to the leaching of few unbound chains that were likely occluded among PGMA/TEPA grafts covalently bonded to the surface of the adsorbent. Furthermore, the applied desorption method involving a combination of vacuum and heating treatments was effective in reversing the chemical interaction between CO2 and PE/PP-g-PGMA/TEPA-V and the adsorbent can be used for prolonged cyclic operation. It can be concluded that the performance of PE/PP-g-PGMA/TEPA-V adsorbent is almost retained after 11 adsorption/desorption cycles suggesting that it is stable enough with an excellent regeneration for column and scaled application of CO2 capture.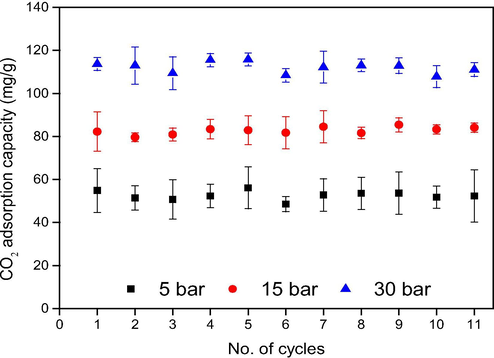
CO2 adsorption/desperation cycles for PE/PP-g-PGMA/TEPA-V at different pressures.
Table 6 shows a comparison between the adsorbent developed in this study and various researched polymeric adsorbents. As can be seen, the present adsorbent has very good adsorption capacity compared to all aminated adsorbents of same category. Considering the nature of present adsorbent and the method used of its preparation, its properties including stability, regeneration, and its chemisorption adsorption mechanism, it can be suggested that PE/PP-g-PGMA/TEPA-V is a promising adsorbent for CO2 capture. Moreover, the adopted RIGC preparation method is not only environmentally friendly but also cost effective and versatile in allowing the use of various waste fibers and fabrics as substrates for developing CO2 adsorbents. Finally, the fibrous nature of the present adsorbent provides advantages in its handling in industrial application including reduction of pressure loss, where competing adsorbents in particulate forms such as COP, zeolite, COF and glucose-based carbon having higher CO2 adsorption performance are likely to undergo serious challenges such as dustiness, clogging, channelling and pressure drop when applied in columns (Pu et al., 2018).
Materials
Functional group
Adsorption method
CO2 adsorption capacity (mg/g)
Temperature (°C)
CO2 composition (%)
Refs.
PMMA
PEI
Column
155
45
100
(Gray et al., 2009)
Viscose-PGMA
TETA
Column
103
25
20
(Lin et al., 2013)
PP-g-PGMA (microfibers)
TEPA
Column
99
30
15
(Zhuang et al., 2013)
Porous polystyrene resin
TEPA
Column
53
30
10
(Liu et al., 2017)
PP-g-PGMA (nanofibers)
EA
Column
126
30
15
(Abbasi et al., 2019b)
PP-g-PGMA (nanofibers)
DEA
Column
91
30
15
(Abbasi et al., 2019a)
PP-g-PGMA (nanofibers)
TEA
Column
41
30
15
(Abbasi et al., 2019b)
Ion exchange resin
Benzyl amine
Column
79
30
10
(Alesi and Kitchin, 2012)
Polyamide-6/CNT nanofibers
PEI
Column
71
25
100
(Zainab et al., 2017)
PE/PP-g-PVAm
–
MSB
60
30
100
(Zubair et al., 2020)
PE/PP-g-PGMA
TEPA
MSB
117
30
100
This study
4 Conclusion
An interesting fibrous adsorbent made of PGMA/TEPA grafted PE/PP fibrous sheet and containing a maximum amount of TEPA was successfully prepared and tested for CO2 adsorption. The amination efficiency of PE/PP-g-PGMA precursor substrates was optimized by controlling the reaction parameters such as type of solvent, amine to solvent ratio and reaction time using Taguchi method. The parameters’ optimization increased the amination efficiency from 57.15% (obtained with 1:1 isopropanol/TEPA ratio at 3 h) to 70.42% (obtained with 3:1 isopropanol/TEPA ratio at 4 h) leading to a remarkable increase in the CO2 adsorption capacity from 80 to 117 mg/g in the adsorbent. Thus, Taguchi method is proven to be highly effective in optimization of reaction parameters and endowing the maximum amination efficiency. The PE/PP-g-PGMA/TEPA-V adsorbent was found to be highly selective for CO2 over N2 and has a high amine utilization efficiency coupled with a competitive adsorption capacity. Furthermore, the adsorbent was found to have very good stability and can be easily regenerated at 80 °C rendering it suitable for column and scaled CO2 capture applications.
Acknowledgement
This work was supported by Malaysia Thailand Joint Authority (MTJA) under vote no. 4C116. MMN is debt for the partial support for this work from International Atomic Energy Agency (IAEA) under from coordinated research projects (CRP # F22072).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Carbon dioxide adsorption on grafted nanofibrous adsorbents functionalized using different amines. Front. Energy Res.. 2019;7:1-14.
- [CrossRef] [Google Scholar]
- Highly flexible method for fabrication of poly (Glycidyl Methacrylate) grafted polyolefin nanofiber. Radiat. Phys. Chem.. 2018;151:283-291.
- [CrossRef] [Google Scholar]
- Amine functionalized radiation induced grafted polyole fi n nano fi bers for CO2 adsorption. Radiat. Phys. Chem.. 2019;156:58-66.
- [CrossRef] [Google Scholar]
- Carbon dioxide post-combustion capture: solvent technologies overview, status and future directions. Mater. Process. Energy Commun. Curr. Res. Technol. Dev. 2013:923-934.
- [Google Scholar]
- Evaluation of a primary amine-functionalized ion-exchange resin for CO 2 capture. Ind. Eng. Chem. Res.. 2012;51:6907-6915.
- [CrossRef] [Google Scholar]
- Materials and processes for carbon dioxide capture and utilisation. J. Carbon Res.. 2017;3:16.
- [CrossRef] [Google Scholar]
- An application of Taguchi method of experimental design for new product design and development process. Assem. Autom.. 2006;26:18-24.
- [CrossRef] [Google Scholar]
- Choi, S., 1999. Adsorption of Pb 2+ , Cu 2+ and Co 2+ by polypropylene fabric and polyethylene hollow fiber modified by radiation-induced 16, 241–247.
- Enhancement of CO2 adsorption/desorption properties of solid sorbents using tetraethylenepentamine/diethanolamine blends. ACS Omega. 2020;5:23533-23541.
- [CrossRef] [Google Scholar]
- Davis, R., John, P., 2018. Application of Taguchi-based design of experiments for industrial chemical processes. Stat. Approaches With Emphas. Des. Exp. Appl. to Chem. Process. https://doi.org/10.5772/intechopen.69501.
- Sorption characteristics of CO2 on rocks and minerals in storing CO2 processes. Nat. Resour.. 2010;01:1-10.
- [CrossRef] [Google Scholar]
- Parametric study of solid amine sorbents for the capture of carbon dioxide. Energy Fuels. 2009;23:4840-4844.
- [CrossRef] [Google Scholar]
- Review on modification strategies of polyethylene/polypropylene immiscible thermoplastic polymer blends for enhancing their mechanical behavior. J. Elastomers Plast. 2018
- [CrossRef] [Google Scholar]
- Optimization of CO2 adsorption on solid-supported amines and thermal regeneration mode comparison. ACS Omega. 2020;5:9641-9648.
- [CrossRef] [Google Scholar]
- Cation exchange membranes by radiation-induced graft copolymerization of styrene onto PFA copolymer films. I. Preparation and characterization of the graft copolymer. J. Appl. Polym. Sci.. 1998;73:2095-2102.
- [Google Scholar]
- Modified nanosepiolite as an inexpensive support of tetraethylenepentamine for CO2 sorption. Nano Energy. 2015;11:235-246.
- [CrossRef] [Google Scholar]
- CO2capture by amine-based aqueous solution containing atorvastatin functionalized mesocellular silica foam in a counter-current rotating packed bed: Central composite design modeling. Chem. Eng. Res. Des.. 2018;129:64-74.
- [CrossRef] [Google Scholar]
- Stability of amine-functionalized CO2 adsorbents: A multifaceted puzzle. Chem. Soc. Rev.. 2019;48:3320-3405.
- [CrossRef] [Google Scholar]
- Preparation and characterization of glycidyl methacrylate grafted 4-amino-1,2,4-triazole modified nonwoven fiber adsorbent for environmental application. Radiat. Phys. Chem.. 2014;94:111-114.
- [CrossRef] [Google Scholar]
- Activation of polyethylene/polypropylene nonwoven fabric by radiation-induced grafting for the removal of Cr(VI) from aqueous solutions. Water. Air. Soil Pollut.. 2016;227
- [CrossRef] [Google Scholar]
- An overview of current status of carbon dioxide capture and storage technologies. Renew. Sustain. Energy Rev.. 2014;39:426-443.
- [CrossRef] [Google Scholar]
- Applications of Taguchi and design of experiments methods in optimization of chemical mechanical polishing process parameters. Int. J. Adv. Manuf. Technol.. 2008;38:674-682.
- [CrossRef] [Google Scholar]
- Design of a viscose based solid amine fiber: Effect of its chemical structure on adsorption properties for carbon dioxide. J. Colloid Interface Sci.. 2013;407:425-431.
- [CrossRef] [Google Scholar]
- Synthesis and CO2 adsorption behavior of amine-functionalized porous polystyrene adsorbent. J. Appl. Polym. Sci.. 2017;134:1-7.
- [CrossRef] [Google Scholar]
- Liu, L., Chen, H., Shiko, E., Fan, X., Zhou, Y., Zhang, G., Luo, X., Hu, X. (Eric), 2018. Low-cost DETA impregnation of acid-activated sepiolite for CO 2 capture. Chem. Eng. J. 353, 940–948. https://doi.org/10.1016/j.cej.2018.07.086.
- CO2 adsorption properties and thermal stability of different amine-impregnated MCM-41 materials. J. Fuel Chem. Technol.. 2013;41:469-475.
- [CrossRef] [Google Scholar]
- Evolution of CO2capture technology between 2007 and 2017 through the study of patent activity. Appl. Energy. 2018;211:1282-1296.
- [CrossRef] [Google Scholar]
- Sisal fiber-based solid amine adsorbent and its kinetic adsorption behaviors for CO 2. RSC Adv.. 2016;6:72022-72029.
- [CrossRef] [Google Scholar]
- New CO2 adsorbent containing aminated poly(glycidyl methacrylate) grafted onto irradiated PE-PP nonwoven sheet. Radiat. Phys. Chem.. 2014;103:72-74.
- [CrossRef] [Google Scholar]
- Radiation-grafted copolymers for separation and purification purposes: Status, challenges and future directions. Prog. Polym. Sci.. 2012;37:1597-1656.
- [CrossRef] [Google Scholar]
- Radiation grafted adsorbents for newly emerging environmental applications. Radiat. Phys. Chem.. 2014;118:55-60.
- [CrossRef] [Google Scholar]
- Surface grafting of FAU/EMT zeolite with (3-aminopropyl)methyldiethoxysilane optimized using Taguchi experimental design. Chem. Eng. Res. Des.. 2012;90:1313-1321.
- [CrossRef] [Google Scholar]
- Selectivity of copper by amine-based ion recognition polymer adsorbent with different aliphatic amines. Polymers (Basel).. 2019;11
- [CrossRef] [Google Scholar]
- Panda, A.K., Singh, R.K., 2013. Optimization of Process Parameters by Taguchi Method : Catalytic degradation of polypropylene to liquid fuel 50–54.
- Influence of surface modification on selective CO2 adsorption: A technical review on mechanisms and methods. Microporous Mesoporous Mater.. 2020;110751
- [CrossRef] [Google Scholar]
- Performance comparison of metal-organic framework extrudates and commercial zeolite for ethylene/ethane separation. Ind. Eng. Chem. Res.. 2018;57:1645-1654.
- [CrossRef] [Google Scholar]
- Experimental study of adsorption on activated carbon for CO2 capture. Intech. 2019;32:137-144.
- [Google Scholar]
- Pure and binary adsorption of CO 2, H 2, and N 2 on activated carbon. Adsorption. 2012;18:49-65.
- [CrossRef] [Google Scholar]
- Fast and highly ef fi cient removal of 2, 4-D using amino-functionalized poly (glycidyl methacrylate) adsorbent: optimization, equilibrium, kinetic and thermodynamic studies. J. Mol. Liq.. 2018;260:195-202.
- [CrossRef] [Google Scholar]
- Graft copolymerization of glycidyl methacrylate onto delignified kenaf fibers through pre-irradiation technique. Radiat. Phys. Chem.. 2013;91:125-131.
- [CrossRef] [Google Scholar]
- Carbon dioxide separation from flue gases: A technological review emphasizing reduction in greenhouse gas emissions. Sci. World J.. 2014;2014
- [CrossRef] [Google Scholar]
- Binary equilibrium adsorption data and comparison of zeolites with activated carbon for selective adsorption of CO2 from CH4. Adsorpt. Sci. Technol.. 2014;32:707-716.
- [CrossRef] [Google Scholar]
- Synergism between ionic liquid and ultrasound for greener extraction of geraniol: Optimization using different statistical tools, comparison and prediction. Chem. Eng. Res. Des.. 2018;134:162-171.
- [CrossRef] [Google Scholar]
- Optimization of CO2 adsorption capacity and cyclical adsorption/desorption on tetraethylenepentamine-supported surface-modified hydrotalcite. J. Environ. Sci.. 2017;65:293-305.
- [CrossRef] [Google Scholar]
- CO2 capture adsorbents functionalized by amine – bearing polymers: A review. Int. J. Greenh. Gas Control. 2020;96:103005
- [CrossRef] [Google Scholar]
- Mechanism and kinetics of CO2 adsorption for TEPA-impregnated hierarchical mesoporous carbon in the presence of water vapor. Powder Technol.. 2020;368:227-236.
- [CrossRef] [Google Scholar]
- Preparation of polypropylene based hyperbranched absorbent fibers and the study of their adsorption of CO2. RSC Adv.. 2015;5:32902-32908.
- [CrossRef] [Google Scholar]
- Cation-exchanged zeolitic chalcogenides for CO2 adsorption. Inorg. Chem.. 2017;56:14999-15005.
- [CrossRef] [Google Scholar]
- Feasibility of CO2 adsorption by solid adsorbents: a review on low-temperature systems. Int. J. Environ. Sci. Technol.. 2016;13:1839-1860.
- [CrossRef] [Google Scholar]
- Free-standing, spider-web-like polyamide/carbon nanotube composite nanofibrous membrane impregnated with polyethyleneimine for CO2 capture. Compos. Commun.. 2017;6:41-47.
- [CrossRef] [Google Scholar]
- Separation and Purification Technology A novel amine double functionalized adsorbent for carbon dioxide capture using original mesoporous silica molecular sieves as support. Sep. Purif. Technol.. 2019;209:516-527.
- [CrossRef] [Google Scholar]
- Preparation of a solid amine adsorbent based on polypropylene fiber and its performance for CO2 capture. J. Mater. Res.. 2013;28:2881-2889.
- [CrossRef] [Google Scholar]
- Kinetic studies of radiation induced grafting of N-vinylformamide onto polyethylene/polypropylene fibrous sheets and testing its hydrolysed copolymer for CO2 adsorption. Radiat. Phys. Chem.. 2020;171
- [CrossRef] [Google Scholar]







