Translate this page into:
The effect of organic additives in electrodeposition of Co from deep eutectic solvents
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
A range of organic/ inorganic additives have traditionally been added to electroplating solutions to improve brightness and encourage levelling. A limited number of studies have shown that some brighteners function in ionic liquids. In this study the effect of four additives; nicotinic acid (NA), methyl nicotinate (MN), 5,5-dimethyl hydantoin (DMH) and boric acid (BH), have been measured on the electrodeposition of cobalt in the 1choline chloride (ChCl): 2 ethylene glycol (EG) based deep eutectic solvent (DES). In general the addition of these additives causes the deposition potential of Co to be shifted to more negative over-potentials. No apparent change in speciation and coordination environment around the CoII centre was observed. The surface morphology was significantly changed by the addition of each of the additives, suggesting that they function by modifying the double layer. The nucleation and growth mechanism of Co deposition was found to change in the presence of these additives. Flat, shiny and high uniform cobalt layers were obtained with the additives whereas in their absence the deposit was black and dull. The additives significantly increased the hardness of the cobalt deposit and this was shown to be related to the crystal structure of the deposit which was determined using XRD.
Keywords
Electroplating
Cobalt
Deep eutectic solvents
Additives
1 Introduction
Both electrodeposition and electropolishing processes possess important aspect industrially (Abbott et al., 2013; Abbott et al., 2015a,b; Karim et al., 2018; Karim, 2021). The electrodeposition of cobalt as film on a substrate enables surface resistant and has plausible magnetic property (Al-Esary, 2017). The electrodeposition can be carried out from aqueous solutions but the narrow electrochemical potential window of water complicates the process. However, ionic liquids (ILs) and DESs have recently been extensively used in metal finishing processes (Karim, 2016). The influence of temperature on electrodeposition of cobalt was carried out in urea-choline chloride mixture (Li et al., 2014).
Recently, the effect of additives on metal electrodeposition has been conducted using deep eutectic solvents (Al-Esary et al. 2019; Abbott, et al., 2017).
Deep eutectic solvent (DES) are mixture of quaternary ammonium salts with hydrogen bond donors such as ethylene glycol (Abbott et al., 2015a,b,). They have been used for a wide variety of applications including metal finishing and some of these have even been used at > 1 tonne scale (Endres et al., 2008). The electrodeposition of many metals and alloys have been studied and the topic of brighteners has been investigated for zinc, aluminium and copper (Abbott et al., 2011; Gu et al., 2012; Abbott et al., 2010). In all cases it is thought that the brighteners function by changing adsorption at the electrode-solution interface.
In the present study, four common additives have been examined as brighteners with the electrodeposition of cobalt in a eutectic mixture of EG and choline chloride which is commonly known by its trade name, Ethaline. Two important electrochemical techniques have been implemented; cyclic voltammetry and chronoamperometry, in an attempt to investigate the reduction mechanism of cobalt with different additives. The effect of the additives on speciation and the physical properties of the DES has also investigated. The influence of the additives on the crystal structure of the deposit have been studied and related the coating hardness.
2 Experimental
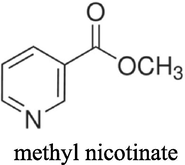
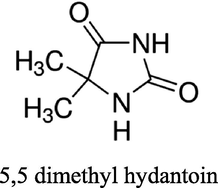
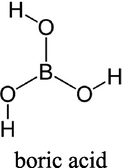
Choline chloride, [HOC2H4N(CH3)3Cl] (ChCl) (Aldrich 99%) was recrystallised from absolute ethanol, filtered and dried under vacuum. Ethylene glycol (EG) (Aldrich + 99%), was used as received. The two components have been mixed together by stirring (in a 1: 2 M ratio of ChCl: hydrogen bond donor) at 60 °C until a homogeneous, colourless liquid formed. The cobalt salt; CoCl2·6H2O (Aldrich ≥ 98%) was used as received. The concentration of cobalt salts in all liquids was 0.4 mol dm−3. The additives nicotinic acid (NA) (Sigma ≥ 99.5%), methyl nicotinate (MN) (Aldrich 97%), 5,5 dimethyl hydantoin (DMH) (Sigma ≥ 99.5%), hydantoin (HD) (Aldrich 98%) and boric acid (BH) (Analar 99.8%) were all used as received. The structures of the additives can be seen in Table 1.
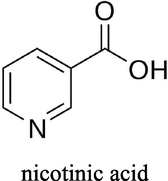
nicotinic acid
methyl nicotinate
5,5 dimethyl hydantoin
boric acid
The conductivity of the liquids were measured as a function of temperature using a Jenway 4510 conductivity meter fitted with an inherent temperature probe (cell constant = 1.01 cm−1). Cyclic voltammetry investigations were carried out using an Autolab/PGSTAT12 potentiostat controlled with GPES2 software. A three-electrode system was used, consisting of a platinum working-electrode (0.12 cm2 area), a platinum flag counter-electrode and a silver wire pseudo-reference electrode. The working electrode was polished with 0.05 μm γ-alumina paste and cleaned by rinsing with deionised water followed by acetone prior to each experiment. All cyclic voltammograms were recorded at 90 °C and at a scan rate of 10–70 mV s−1.
Bulk electrolysis was carried out using cathodic plates (copper, 50 mm × 42 mm × 1 mm) which were mechanically polished and cleaned with acetone and rinsed with water and dried. An iridium oxide-coated titanium mesh electrode, 40 mm × 50 mm, was used as an anode and prepared in the same manner. In all of the experiments the solution temperature was 90 °C and deposition was carried out using a constant current for 3 h, after which the substrates were removed from solution and washed with water and acetone.
Surface microstructure analysis: The surface morphology was characterised using scanning electron microscopy (SEM) and elemental analysis of the deposit compositions was carried out by energy dispersive X-ray spectroscopy (EDX), using a Phillips XL30 ESEM instrument with an accelerating voltage between 15 and 20 keV, giving an average beam current of ca. 120 μA.
Cross-section microstructure: The samples were mounted in a resin using a Struers Labo Press 3. The samples were then polished first with 240 grit silicon carbide paper to make them flat, then with diamond abrasives of successively 9 μm and 3 μm size and finally with 0.5 μm colloidal silicon carbide paste. UV visible spectrophotometer: A Shimadzu model UV-1601 spectrophotometer was used with the cell path length equal to 10 mm. Values for λmax were determined using the spectrophotometer’s built-in peak-pick feature, using uv- probe software.
3 Results and discussion
Cyclic voltammetry using a platinum working electrode was carried out in Ethaline at 90 °C containing 0.4 mol dm−3 CoCl2·6H2O using a Pt electrode in the potential range of 0.5 V to −1.0 V as presented in Fig. 1. The cyclic voltammogram shows a maximum current at approximately −0.84 V during the cathodic sweep and a maximum current at about −0.04 V during the anodic sweep. The difference between the onset potentials for deposition and reduction is relatively large c.a. 0.3 V and this quasi-reversible response is similar to Ni, Pd and Pt (Van der Linden and Dix, 1979; Abbott et al., 2008a,b).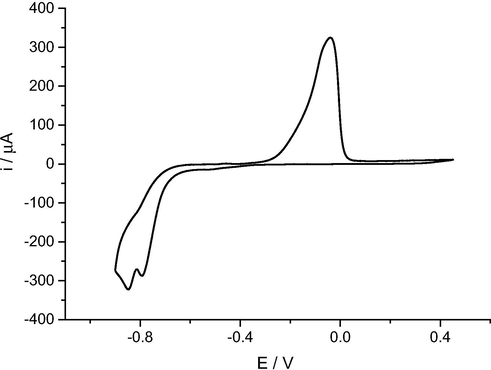
Cyclic voltammogram of 0.4 mol dm−3 CoCl2·6H2O in Ethaline at 90 °C on a Pt electrode, at a sweep rate of 10 mV s−1.
Redox couples such as Cu (II) and Zn (II) show only a small difference in the onset potentials for deposition and reduction (c.a. 20 mV) which is unusual as they possess similar speciation ([MCl4]2- complexes) and this anomaly is as yet unexplained (Hartley et al., 2014).
Bulk electrolysis was carried out on a copper electrode using a current density of 0.66 A dm−3 for 3 h. The deposit obtained was black with an average film thickness of approximately 8.2 µm as can be seen from Fig. 2. EDX analysis confirmed the deposit consisted only of cobalt.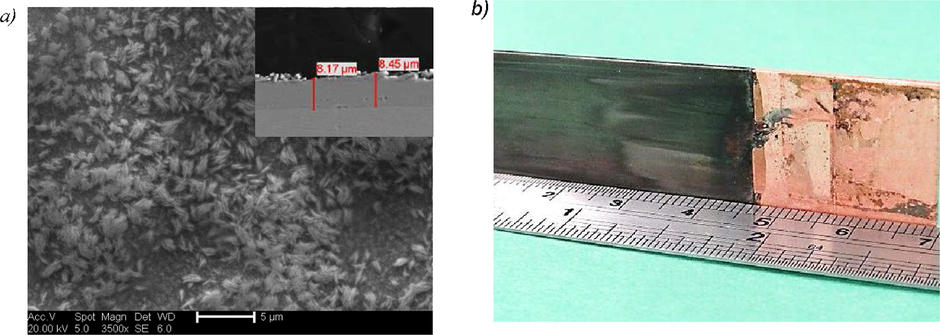
Scanning electron micrograph shows the bulk electrodeposition with photo images of the 0.4 mol dm−3 CoCl2·6H2O in Ethaline system at 90 °C for 3 h on a nickel electrode at an applied current density of 0.66 A dm−3).
Fig. 3a also displays the voltammetry of the Ethaline system as a function of cobalt chloride concentration. The deposition charge is also proportional the concentration of cobalt chloride. In all solutions the metal is present at very high concentrations as usual, it is seen that the deposition is under diffusion control. Moreover, as the concentration of cobalt chloride increases from 0.2 to 0.8 mol dm−3 the onset potential of the reduction metal shift to more positive by 0.23 V. This is could be due to availability of relatively high Co (II) ion at the interfacial range and this is more likely be concentration dependent. Fig. 3b shows that the anodic and cathodic charges for Fig. 3a are, the same at each concentration showing that the deposition of cobalt is fully reversible.![a) Cyclic voltammogram for a Pt disc (1.0 mm diameter) electrode immersed in [CoCl2·6H2O] in Ethaline200 as a function of different concentration at sweep rate 10 mV / s (all potentials versus Ag wire b) relationship between stripping and deposition charge.](/content/184/2021/14/4/img/10.1016_j.arabjc.2021.103036-fig3.png)
a) Cyclic voltammogram for a Pt disc (1.0 mm diameter) electrode immersed in [CoCl2·6H2O] in Ethaline200 as a function of different concentration at sweep rate 10 mV / s (all potentials versus Ag wire b) relationship between stripping and deposition charge.
Additives can function by changing speciation which changes the reactivity of the metal complex and accordingly this changes the redox potential. They can also change the mass transport of species to and from the electrode by changing viscosity which alters the kinetics of growth. Finally the additives could adsorb at the electrode-solution interface and change the kinetics of nucleation and growth.
3.1 Speciation
The coordination environment of a metal species in an ionic liquid will have an effect on the electrochemical behaviour and to test this Uv–vis spectroscopy was performed on the solutions. Fig. 4a shows the absorption spectra of Ethaline containing 0.015 mol dm−3 CoCl2·6H2O and 0.01 mol dm−3 with different additives (NA, MN, DMH and BH) at room temperature. There was no significant difference between the speciation before and after addition of those additives, indicating that these additives do not change the coordination environment of CoII.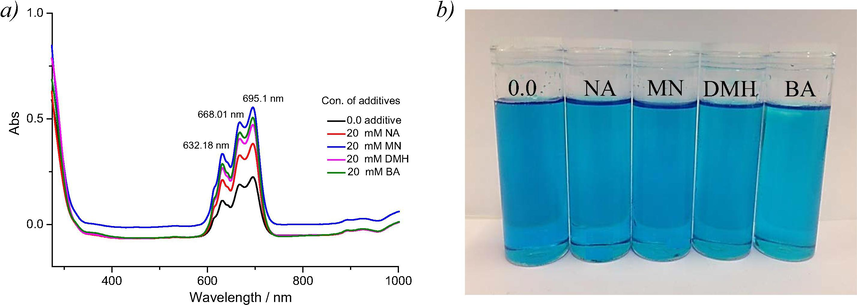
(a) UV–visible spectra measured at 25C° from Ethaline using 0.015 mM CoCl2·6H2O with the additives shown in Table 1. (b) photograph of solutions showing the lack of a colour change.
Many octahedral Co complexes are typically pale red or purple, while many common tetrahedral ones are intensely blue. It can be seen from Fig. 4b that in Ethaline, CoCl2 forms a blue coloured solution. EXAFS analysis has recently shown that the main species is [CoCl4]2-. A recent study on the analogous nickel chloride system showed that there was a marked thermochromic effect where the solution changes colour from green at 25 °C to blue at 90 °C. This is thought to occur due to a ligand exchange from ethylene glycol at lower temperatures to the [NiCl4]2- at higher temperatures. The same thermochromic effect is not observed for CoCl2 in Ethaline (Abbott et al., 2008a,b).
3.2 Effect of additives on physical properties
To test whether the additives were changing the physical properties of the DESs, the viscosity and conductivity of the Ethaline with the 4 additives listed above were measured. Fig. 5 shows that the viscosity of the liquid increases slightly and the conductivity decreases slightly at ambient temperatures when additives are put into the solution. At the operating conditions of the plating bath, however the changes in viscosity are negligible and hence any changes in morphology of deposition kinetics are unlikely to be due to mass transport issues.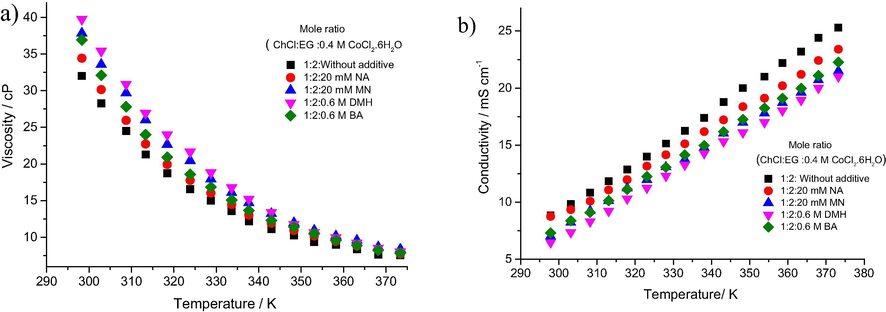
a) The viscosity and b) conductivity of 0.4 moldm−3 CoCl2·6H2O in Ethaline as a function of temperature and different additives, as a function of temperature.
3.3 Cyclic voltammetry with additives
Fig. 6a and 6b show the cyclic voltammetry for 0.4 mol dm−3 CoCl2·6H2O in Ethaline at 90 °C on a Pt electrode as a function of different concentrations of nicotinic acid (NA), methyl nicotinate (MN), 5,5 dimethylhydantoin (DMH) and boric acid (BH). The concentrations for the first two are different from the last two and this is due to solubility differences. These concentrations were chosen to be comparable with those used in aqueous solutions when they are applied as brighteners. It is noted from UV–Vis (Fig. 4) that these additives cannot affect speciation. Fig. 6 shows lowering in current at cathodic an anodic sides generally in the presence of NA and MN which is expectable (viscosity was increased). Despite the CoCl2 being present in a 20 to 80 fold excess, the additive still has a marked effect upon the voltammetric responses, indicating surface modification by the additives. From Fig. 6, it is seen that the reversibility of the electrodeposition process is quite different qualitatively for each additive. The presence of two anodic peaks has previously been shown to be due to the formation of different morphologies of deposits. The AFM images in situ was used to study the electrodeposition of Zn from the same liquid and it was shown that the currents at smaller over-potentials was due to the electrodeposition of nano-particles whereas that for larger over-potentials was due to macroscopic deposits (Abbott et al., 2011).![Cyclic voltammograms for a Pt electrode immersed in 0.4 mol dm−3 [CoCl2·6H2O] in Ethaline as a function of various concentration of the organic additive (a)NA (b)MN (c)DMH and (d)BH at sweep rate 10 mV s−1.](/content/184/2021/14/4/img/10.1016_j.arabjc.2021.103036-fig6.png)
Cyclic voltammograms for a Pt electrode immersed in 0.4 mol dm−3 [CoCl2·6H2O] in Ethaline as a function of various concentration of the organic additive (a)NA (b)MN (c)DMH and (d)BH at sweep rate 10 mV s−1.
Fig. 6c and 6d exhibit the impact of DMH and BH to the Co (II) ion electrodeposition. It can be seen that DMH substantially decreases both the cathodic and anodic current as a consequence of increasing viscosity. Conversely, the addition of the BH to the Co solution increases both the anodic and cathodic currents. This is might be due to that boric acid acts as a homogeneous catalyst which drops the overvoltage metal deposition which is adsorbed on the cathode surface and therefore, decreases the active site for H2 evolution. This causes lower current efficiency (Santos et al., 2007; Hoare, 1986).
3.4 Chronoamperometry
Fig. 6 suggests that the additives significantly change the cobalt electrochemical response potentially function by adsorbing at the electrode-solution interface which should result in a difference in the nucleation mechanism compared to the additive free system. Using chronoamperometry with these additives it should be possible to detect changes in the nucleation mechanisms with these additives. The chronoamperometric responses were compared to the theoretical model developed by Scharifker and Hills for the nucleation of metals (Gunawardena et al., 1982). Fig. 7 shows the analysis of the chronoamperometric resulting from a potential step to −0.8 V. The data are represented as dimensionless current - time plots for 3D instantaneous, and progressive as calculated by Equations (1) and (2) respectively.
![Chronoamperometric responses with/without additives for the deposition of 0.4 M [CoCl2·2H2O] in Ethaline at −0.8 V. Plots of t/tmax vs I/Imax (Eqs (1) and (2)). (a) no additive (b) 20 mM NA, (c) 20 mM MN, (d) 0.6 M DMH, and (e) 0.6 M BH.](/content/184/2021/14/4/img/10.1016_j.arabjc.2021.103036-fig7.png)
Chronoamperometric responses with/without additives for the deposition of 0.4 M [CoCl2·2H2O] in Ethaline at −0.8 V. Plots of t/tmax vs I/Imax (Eqs (1) and (2)). (a) no additive (b) 20 mM NA, (c) 20 mM MN, (d) 0.6 M DMH, and (e) 0.6 M BH.
In the absence of additives, cobalt shows an instantaneous nucleation and growth mechanism. The addition of 0.02 moldm−3 nicotinic acid to the plating solution shows the same initial instantaneous nucleation mechanism which changes as the nuclei grow and deviates significantly beyond tmax. In the case of MN the initial nucleation follows more closely a 3 dimensional progressive mechanism which tends to dominate for slower nucleation and growth mechanisms. The fits for 5, 5 dimethylhydantoin and boric acid, do not fit well either of the 3D nucleation models. It can at least be qualitatively concluded that the 4 additives each change the nucleation mechanism which most probably results from different adsorption mechanisms.
3.5 Deposit morphology
Fig. 8 shows SEM images, cross sectional views and optical images of Co electroplated on copper from Ethaline with different additives at 90 °C for 3 hrs with a current density of 0.66 A dm−2. The SEM images of the surfaces show very different morphologies to that shown in Fig. 2 without additives. The samples are metallic looking, rather than black, with a mirror finish in all cases. It should also be noted that despite the deposition currents being smaller when additives were included in the solution (Fig. 6) the Co films obtained with the additives are 3 to 4 times thicker than those without additives (Fig. 2).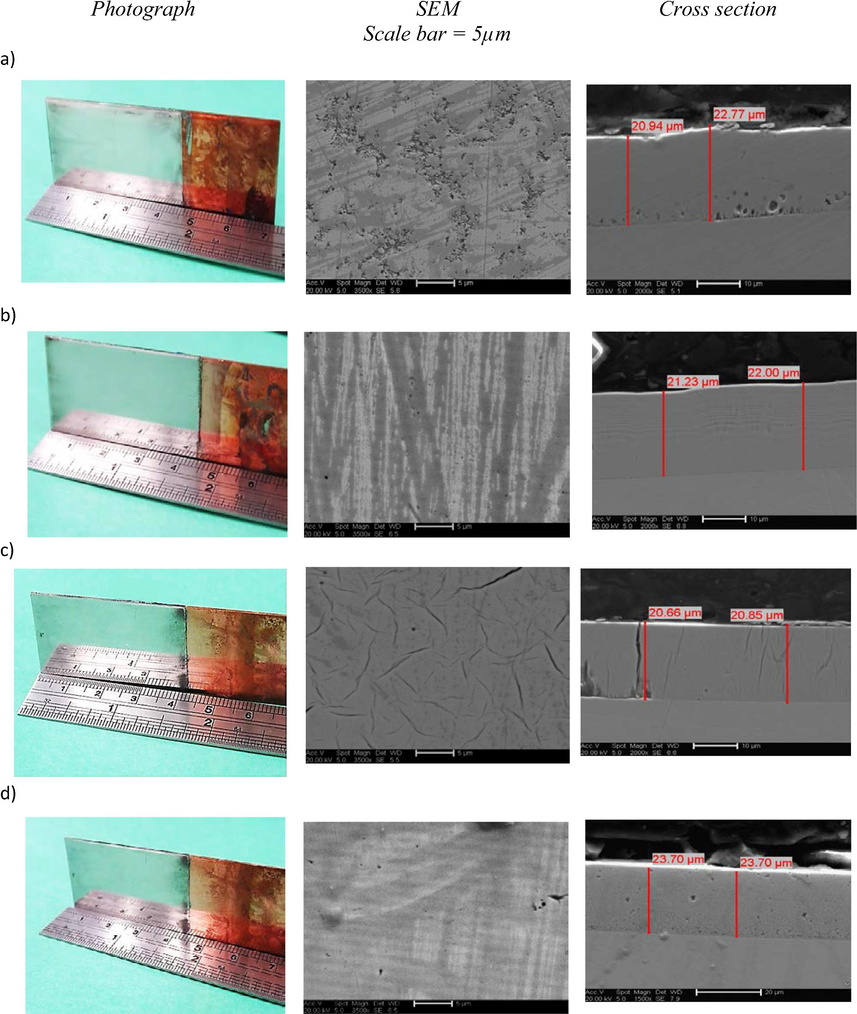
Scanning electron micrographs with cross section showing samples after bulk electrodeposition from Ethaline systems, 0.4 mol dm−3 CoCl2·6H2O (all at 90 °C for 3 hr. on a copper electrode at an applied current density of 0.66 A dm−3) ,(a) with 0.02 mol dm-3NA, b) 0.02 mol dm−3 MN, c) 0.6 mol dm−3 DMH and d) 0.6 mol dm−3 BH.
This shows that some aqueous brighteners can function in the same method in ionic liquids when used at comparable concentrations. The cross section in Fig. 8a shows the effect of adding 0.020 moldm−3 nicotinic acid which results in a tightly packed cobalt layer without any discernible structure or any cracking suggesting that the coatings are not stressed. Close to the copper surface there are some small voids which may result from hydrogen evolution with the acidic additive.
Fig. 8b shows the effect of adding 0.02 mol dm−3 methyl nicotinate to the plating solution. While it appears that a structure-less layer is produced close inspection shows tightly packed layers parallel to the substrate. The additive also shows a good levelling effect with an even film thickness. Fig. 8c shows the effect on the deposit morphology of adding 0.6 mol dm−3 5,5 dimethyl hydantoin to the cobalt containing solution. It can be seen that the cobalt contains a significant number of micro and macro-cracks some of which penetrate right the way through the cobalt layer. This is characteristic of the morphology obtained for chromium from an aqueous chromic acid solution. This is often due to inbuilt stress in the deposited layer but often results in a high deposit hardness.
Fig. 8d shows the deposit morphology for the cobalt film formed by adding 0.6 mol dm−3 boric acid to the plating solution. This is very flat and contains a larger number of small voids evenly distributed throughout the cobalt layer. This is presumably due to the acidity of the additive resulting in hydrogen evolution.
Fig. 9 shows the hardness of the whole samples. All of the additives result significantly in hardening the cobalt film than that of absent additives in DES-containing Co(II) ion. The hardest sample deposited was obtained using DMH as an additive. Fig. 9c shows that the layer was highly cracked suggesting a stressed film.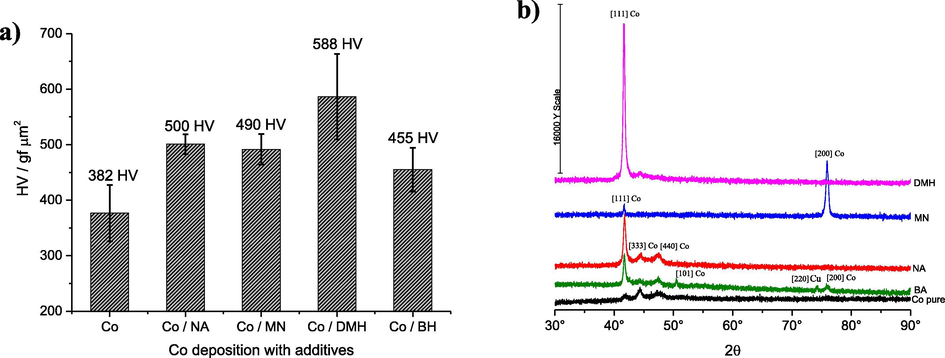
Vickers hardness (a) and XRD spectra (b).
Fig. 9b shows that the [1 1 1] crystal structure dominates in the presence of additives while without additives the [3 3 3] and [4 4 0] dominate. The coating obtained using MN shows that the [2 0 0] face is significant and this could be why the stratified morphology is observed in Fig. 9b. The markedly different XRD spectra obtained with each additive support the idea that the additives adsorb differently at the electrode-solution interface which changes the deposit morphology, nucleation mechanism and deposit hardness.
4 Conclusions
It is concluded that cobalt film can be obtained from the Ethaline 200. The additives; nicotinic acid (NA), Methyl nicotinate (MN), 5,5-dimethyl hydantoin (DHM) and boric acid (BA), cause altering the chemistry of Co (II) ion electrodeposition in the electrolytic bath at 90 °C. The mass transport can be slowed which in turn impacts the mechanism of electrodeposition of Co (II) ion. The adsorption of additives on Cu-sheet as a substrate is likely and the additive molecules have not been involved in the Co (II) ion complex. The composition of interfacial region has been changed via adsorption on substrate surface, changing the crystal faces of the deposit. The deposit hardness can be enhanced via appearance of [1 1 1] crystal faces when these additives have been involved. It is shown that the additives change the nucleation mechanism and they significantly increase the thickness of Co film.
Acknowledgements
The author gratefully acknowledge the financial support for this study from Koya University , Kurdistan –Iraq and Ministry of Higher Education and Scientific Research , Kurdistan Regional Government /Iraq.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Electroplating using Ionic Liquids. Annu. Rev. Mater. Res.. 2013;43:335-358.
- [CrossRef] [Google Scholar]
- A comparative study of nickel electrodeposition using deep eutectic solvents and aqueous solutions. Electrochimica Acta.. 2015;176:718-728.
- [CrossRef] [Google Scholar]
- Electropolishing of nickel and cobalt in deep eutectic solvents. Transactions of the IMF.. 2018;96:200-205.
- [CrossRef] [Google Scholar]
- Electropolishing of pure metallic titanium in a deep eutectic solvent. Arab. J. Chem.. 2021;2021(14):102906-102910.
- [CrossRef] [Google Scholar]
- Influence of additives on Electrodeposition of metals from deep eutectic solvents. University of Leicester; 2017. PhD Thesis
- Anodic dissolution of metals in ionic liquids. University of Leicester; 2016. PhD Thesis
- Cobalt electrodeposition using urea and choline chloride. Electrochim. Acta.. 2014;123:325-331.
- [CrossRef] [Google Scholar]
- Influence of additives on the electrodeposition of zinc from a deep eutectic solvent. Electrochim. Acta.. 2019;304:118-130.
- [CrossRef] [Google Scholar]
- Bright metal coatings from sustainable electrolytes: the effect of molecular additives on electrodeposition of nickel from a deep eutectic solvent. Phys. Chem. Chem. Phys.. 2017;19:3219-3231.
- [CrossRef] [Google Scholar]
- Anodic dissolution of metals in ionic liquids. Prog. Nat. Sci.. 2015;25:595-602.
- [CrossRef] [Google Scholar]
- Electrodeposition from Ionic Liquids. Wiley; 2008.
- The effect of additives on zinc electrodeposition from deep eutectic solvents. Electrochim. Act.. 2011;56:5272-5279.
- [CrossRef] [Google Scholar]
- Electrodepsoition, structural and corrosion properties of Cu films from a stable deep eutectic system with additive of ethylene diamine. Surf. Coat. Technol.. 2012;209:117-123.
- [CrossRef] [Google Scholar]
- Double layer, diluent and anode effects upon the electrodeposition of aluminium from chloroaluminate based ionic liquids. Phys. Chem. Chem. phys.. 2010;12:1862-1872.
- [CrossRef] [Google Scholar]
- Electrochemical redox behavior of dithiocarbamates and diseenocarbamates of nickel, palladium, and platinium. Inorganica Chim. Acta.. 1979;35:65-71.
- [CrossRef] [Google Scholar]
- Metal complexation in ionic liquids. Annu. Rep. Sec. “A” (Inorg. Chem.). 2008;104:21-45.
- [CrossRef] [Google Scholar]
- EXAFS study into the speciation of metal salt dissolved in ionic liquids and deep eutectic solvents. Inorg. Chem.. 2014;53:6280-6288.
- [CrossRef] [Google Scholar]
- Electrodeposition of nickel using deep eutectic based ionic liquids. Trans. Inst. Met. Finish.. 2008;86:234-240.
- [CrossRef] [Google Scholar]
- Effect of temperature on Co electrodeposition in the presnce of boric acid. Electrochim. Acta. 2007;53:644-649.
- [CrossRef] [Google Scholar]
- On the role of boric acid in the Watts bath. J. Electrochem. Soc.. 1986;133:2491-2494.
- [CrossRef] [Google Scholar]
- Electrochemical nucleation : Part I. General consideration. J. Electroanal. Chem.. 1982;138:225-239.
- [CrossRef] [Google Scholar]







