Translate this page into:
The removal of Cu, Ni, and Zn in industrial soil by washing with EDTA-organic acids
⁎Corresponding author. qintlin@163.com (Qintie Lin)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
In order to improve the heavy metal removal ability of traditional single washing agents and explore the removal mechanism of heavy metals. Then, the washing reagents that mixed by low-molecular weight organic acids (citric acid, oxalic acid, and tartaric acid) and artificial chelating compound ethylenediaminetetraacetic acid disodium (EDTA) were selected. Furthermore, the effect of soil washing parameters, the variation of leaching toxicity, mobility, stability and speciation of heavy metals were also considered. The results of soil washing experiments showed that mixing an equal volume of 0.05 M EDTA and 0.2 M organic acids (citric acid, oxalic acid, and tartaric acid) could remove more than about 80% heavy metals from soil under the optimal conditions. In addition, the soil leaching toxicity was decreased and the stability of remaining heavy metals was increased, indicating that EDTA-organic acid washing reagents could effectively reduce the ecological risk of contaminated soil. EDTA had a stronger chelating ability with heavy metals than the organic acids, and the organic acids could not only chelate heavy metals but also decrease the pH of the mixture for promoting the desorption of heavy metals. Thus, mixing EDTA and organic acids was advisable method to improve soil washing technology.
Keywords
Soil washing
EDTA-organic acids
Heavy metals
Chelating
Desorption
1 Introduction
Soil is an important part of terrestrial ecosystems and maintains basic ecological functions (i.e., primary production and decomposition) (Elouear et al., 2016). Heavy metals with strong migration ability exist in abandoned industrial soils at high concentrations, posing a threat to human health in recent years (Bolan et al., 2014; Li et al., 2017; Maas et al., 2010; Zhao et al., 2017). Therefore, the restoration of those polluted soils is crucial for the reuse of land resources. Unfortunately, heavy metals, characterized by their inaccessibility, persistence and undegradability, have caused substantial challenges in the remediation of soil around the world (Arwidsson et al., 2010; Moutsatsou et al., 2006).
Some soil remediation technologies have been used widely in heavy metal-contaminated sites, including solidification, stabilization, electrokinetic extraction, soil washing and bioremediation (Bolan et al., 2014; Cameselle and Gouveia, 2019; Rosestolato et al., 2015; Yan and Lo, 2012; Yoo et al., 2018; Zhai et al., 2018). Among these approaches, soil washing has attracted considerable attention for its ability to permanently remove heavy metals, short duration, and remarkable cost effectiveness compared to other methods (Beiyuan et al., 2018; Dermont et al., 2008; Khalid et al., 2017; Xu et al., 2014; Zhao et al., 2017). As far as soil washing is concerned, the selection of effective and harmless washing reagents should be carefully considered. Currently, organic acids (citric acid and oxalic acid), chelating agents (EDTA), inorganic acids (hydrochloric acid), surfactants (sodium dodecyl sulfate and Triton X-100) and some inorganic salt (calcium chloride) have been widely used as washing reagents (Jiang et al., 2017; Li et al., 2017; Li et al., 2019; Mao et al., 2015; Nagai et al., 2012; Tang et al., 2017; Yoo et al., 2017). Surfactants have good performance in the removal of organic pollutants due to their ability to form micelles in solution but are not ideal for removing heavy metals from soil (Ishiguro and Koopal, 2016; Muñoz-Morales et al., 2017). Although inorganic acids can achieve high efficiencies in the removal of heavy metals, such reagents have irreversible effects on the physical and chemical properties of soil, such as the loss of soil organic matter and essential nutrients, increasing environmental risks. Many researchers have shifted their attention to artificial chelating agents such as EDTA, which can bind multiple heavy metals to form soluble and stable complexes. However, the use of high concentrations of EDTA may impose a burden on environmental capacity owing to the unfavorable biodegradability of EDTA (Guo et al., 2016; Jelusic et al., 2014; Jez and Lestan, 2016). Organic acids with certain functionalities could promote the desorption of heavy metals from soil particles. Nevertheless, a mediocre removal efficiency of heavy metals has often been observed because organic acids have short half-lives in soil (1.5–5.7 d) (Cao et al., 2018; Chen et al., 2016; Meers et al., 2005; Wen et al., 2009). For the past few years, the combined utilization of multiple soil washing reagents has become a research focus. Some studies have shown that a higher removal efficiency of heavy metals in contaminated soil always has been obtained though sequential extraction with different washing reagents (Guo et al., 2018; Wang et al., 2019). But the increase of frequency of sequential extraction leaded directly to higher costs.
Previous studies had focused on the remediation of low concentrations of pollutants in farmland soil using mixed washing reagents (Li et al., 2015). Actually, the soil washing is greatly appropriate for remediation of high-concentration contaminated industrial soils considering the above characteristics of this technology (Qiu et al., 2010). However, few studies were focused on the innovation of washing reagents and migration and transformation of pollutants in industrial soil remediation by soil washing technology. A new mixed washing reagent that mixed by low concentration of EDTA and three organic acids (citric acid, oxalic acid, and tartaric acid) was curial for treating heavy metal-contaminated industrial soil, aiming to make up for the shortcomings of the above soil washing technology. Furthermore, the changes of leaching toxicity, mobility, stability and speciation of heavy metals and leaching toxicity were investigated. Finally, a further treatment of soil washing wastewater was conducted.
2 Materials and methods
2.1 Soil sampling
According to the previous detailed survey report of the site Soil samples were collected with a depth of 0–50 cm from the abandoned sewage treatment station located in the relocated electroplating factory Dongguan City, Guangdong Province, Southern, China. These soil samples were air dried for 7 d, mixed fully, ground and passed through a 2 mm soil sieve for further use.
2.2 Soil analysis
The total content of heavy metals in soil was determined by Atomic Absorption Spectrometer Z2000 (Hitachi, Japan) after the soil sample was digested completely by MARS6 (CEM, America) with HF-HCl-HNO3-HClO4 (Guo et al., 2018). The leaching toxicity was measured by the Sulfuric acid and Nitric acid method (HJ/T299-2007, China) and Horizontal Vibration method (HJ 557-2010, China). Soil particle size was measured by Mastersizer 3000 (Marlvern, UK). The pH, soil organic matter (SOM) and cation exchange capacity (CEC) of soil were measured according to Soil-Determination of pH-Potentiometry (HJ 962-2018, China), Potassium Dichromate Oxidation Spectrophotometric method (HJ 615-2011, China) and NH4Cl-NH4OAc method (GB 7863-87, China), respectively. EDTA (C10H14N2Na2O8), citric acid (C6H8O7), oxalic acid (C2H2O4) and tartaric acid (C4H6O6) were purchased from Aladdin Reagents Co., Ltd. (Shanghai).
2.3 Soil washing experiment
A batch washing experiment was carried out by using a horizontal constant-temperature washing method (Jiang et al., 2017). Soil samples with a mass of approximately 2 g were placed in a series of 50 mL centrifuge tubes. The reagents for the experimental groups were made by mixing equal volumes of citric acid, oxalic acid, and tartaric acid along a concentration gradient (0.01, 0.05, 0.1, 0.2 and 0.4 M) and EDTA (0.005, 0.01, 0.05, 0.1 and 0.2 M). The pH of the mixed reagent solutions was adjusted to 3.0. The washing reagent used in the control group was deionized water. Both the experimental and control groups were added 20 mL their respective washing reagents and placed in a water bath constant-temperature oscillator at 180 r/min for 6 h at room temperature and then centrifuged at 4000 r/min for 10 min. The upper layer of liquid was filtered through a 0.45-µm microporous membrane for further analysis. We also investigated the effect of different solid-liquid ratios (g/mL) with 1:5, 1:7.5, 1:10, 1:15 and 1:20 by adding different qualities of soil samples and equal volume (20 mL) of washing reagents with optimum concentration. Finally, the different soil washing time (0.5, 1, 2, 6 and 12 h) the initial pH values (3.0, 4.0, 5.0, 6.0 and 7.0) of the mixed washing reagents was considered. All experiments were repeated three times. The removal efficiencies of heavy metals were calculated as follows:
2.4 Heavy metal speciation analysis
To analyze the redistribution of heavy metals in treated soils and to assess environmental risks, the soil sample were extracted by BCR fractionation from. We measured the heavy metals contents of acid-soluble fraction (F1), reducible fraction (F2), oxidizable fraction (F3) and residual fraction (F4), respectively. The specific extraction steps are shown in Table 1 (Liu et al., 2019; Ure et al., 1993).
Procedure
Speciation
Reagents and conditions
1
Acid-soluble fraction (F1)
0.11 M acetic acid (CH3COOH), 40 mL
2
Reducible fraction (F2)
0.5 M HONH3Cl (pH = 2.00, HNO3), 40 mL
3
Oxidizable fraction (F3)
10 mL, 30% hydrogen peroxide (H2O2) at 85 °C and 50 mL, 1.0 M ammonium acetate (CH3COONH4)
4
Residual fraction (F4)
6 mL HCl, 2 mL NHO3, 1 mL HF, 160 °C
2.5 Heavy metal mobility and stability analysis
MF is an important indicator of heavy metal mobility and bioavailability in soil, which are defined as follows (Gusiatin and Klimiuk, 2012):
The stability was accessed by the reduced partition index of an element, IR, which was defined as follows (Gusiatin and Klimiuk, 2012; Han et al., 2003):
3 Results and discussion
3.1 Analysis of soil components
The total heavy metal contents and several fundamental parameters of the original soil samples are presented in Table 2. The soil showed weak acidity with a pH value of 5.50. Both SOM and CEC were measured in a low value. In addition, the soil sample was classified as loamy fine sand because it was composed of sand (81.6%), silt (17.5%) and clay (0.93%). The total Cu and Ni contents were about 2 and 4 times higher than the regulatory limits issued in the Risk Control Standard for Soil Contamination of Development Land (GB36600-2018, China), respectively. Therefore, the contents of Cu, Ni and Zn were selected as the test indices in our experiments. Moreover, these above characteristics of soil sample showed that soil washing could effectively remove heavy metals from the soil because these pollutants in soil with an unstable state (Delil and Köleli, 2019).
Parameters
pH
CEC (cmol/kg)
Organic matte (%)
Sand (%)
Silt (%)
Clay (%)
Values
5.50
8.00
0.96
81.6
17.5
0.93
Parameters
Total contents (mg/kg)
Leaching toxicity (HJ/T299-2007) (mg/L)
Leaching toxicity (HJ557-2010) (mg/L)
Cu
Ni
Zn
Cu
Ni
Zn
Cu
Ni
Zn
Values
3884.8
624.48
280.25
27.96
23.51
8.67
24.96
20.89
3.56
3.2 Effect of different concentrations of washing reagents
3.2.1 Single washing reagents
The determination of the optimum concentration of a single washing reagent is beneficial to the process of mixing in next step. The removal efficiencies of Cu, Ni, and Zn using different concentrations of citric acid, oxalic acid, tartaric acid and EDTA are summarized in Fig. S1a-d. When the concentration of EDTA increased from 0 to 0.05 M, the removal of Cu, Ni and Zn increased continuously to 66.1%, 62.5% and 67.3%, respectively. Further increases in the concentrations of washing reagents did not improve the heavy metal removal efficiencies. The removal tendencies of Cu, Ni, and Zn using other washing reagents with the selected concentration gradient were similar. The highest removal efficiencies of heavy metals in soil samples were achieved with the addition of 0.2 M citric acid, oxalic acid and tartaric acid and 0.05 M EDTA. In the summary, the above four kinds of washing reagents could apparently enhance the removal rates of Cu, Ni, and Zn above with in their optimal concentrations. There were two possible reasons: (1) these washing reagents could release more H+, which is conducive to the dissolution of heavy metals; (2) more oxygen-containing groups provided by the washing reagents would combine with heavy metals (Begum et al., 2012; Chen et al., 2003; Qin et al., 2004).
It is worth noting that oxalic acid had the highest removal of Ni (70.4%) among these washing reagents, but the worst Cu (51.1%) removal. In fact, the difference in removal efficiency always occurred in soils contaminated by multiple heavy metals (Guo et al., 2018; Jiang et al., 2017). Therefore, it was difficult to effectively remove Cu, Ni and Zn at the same time when using only one washing reagent. A possible reason is that the diverse molecular structure of organic acids leads to varying formation and stability of reaction products. Moreover, there was competition among the heavy metals to combine with the active sites on washing reagents (Di Palma and Mecozzi, 2007; Kim et al., 2003).
3.2.2 The mixed washing reagents
An artificial chelating reagent (EDTA) was mixed with natural chelating reagents (organic acids) to make up for the limitations of single washing reagents in complex heavy metal removal in soil. The washing reagent mixed by an equal volume of 0.05 M EDTA and 0.2 M oxalic acid could remove Cu (85.3%), Ni (86.9%), and Zn (84.0%) (Fig. 1d-f), achieving a higher removal efficiency of heavy metals than that obtained with single oxalic acid. Moreover, compared with the single organic acid systems, significant improvement was also observed in the EDTA-citric acid (Fig. 1a-c) and EDTA-tartaric acid systems (Fig. 1g-i). Meanwhile, the fact that the removal efficiency of Cu, Zn, and Ni by EDTA-organic acids was higher than that by EDTA alone was testified. Hydrogen ions provided by organic acids can promote the dissolution of carbonate minerals, oxides and hydroxides. And then the released heavy metal ions combined with the organic acids to generate monodentate complexes (Jiang et al., 2017). On this basis, the addition of EDTA would enhance the chelating ability of mixed reagents to heavy metals. In addition, mixed washing reagents could achieve high removal efficiencies of heavy metals at a lower concentration, reducing the cost and secondary pollution to the environment.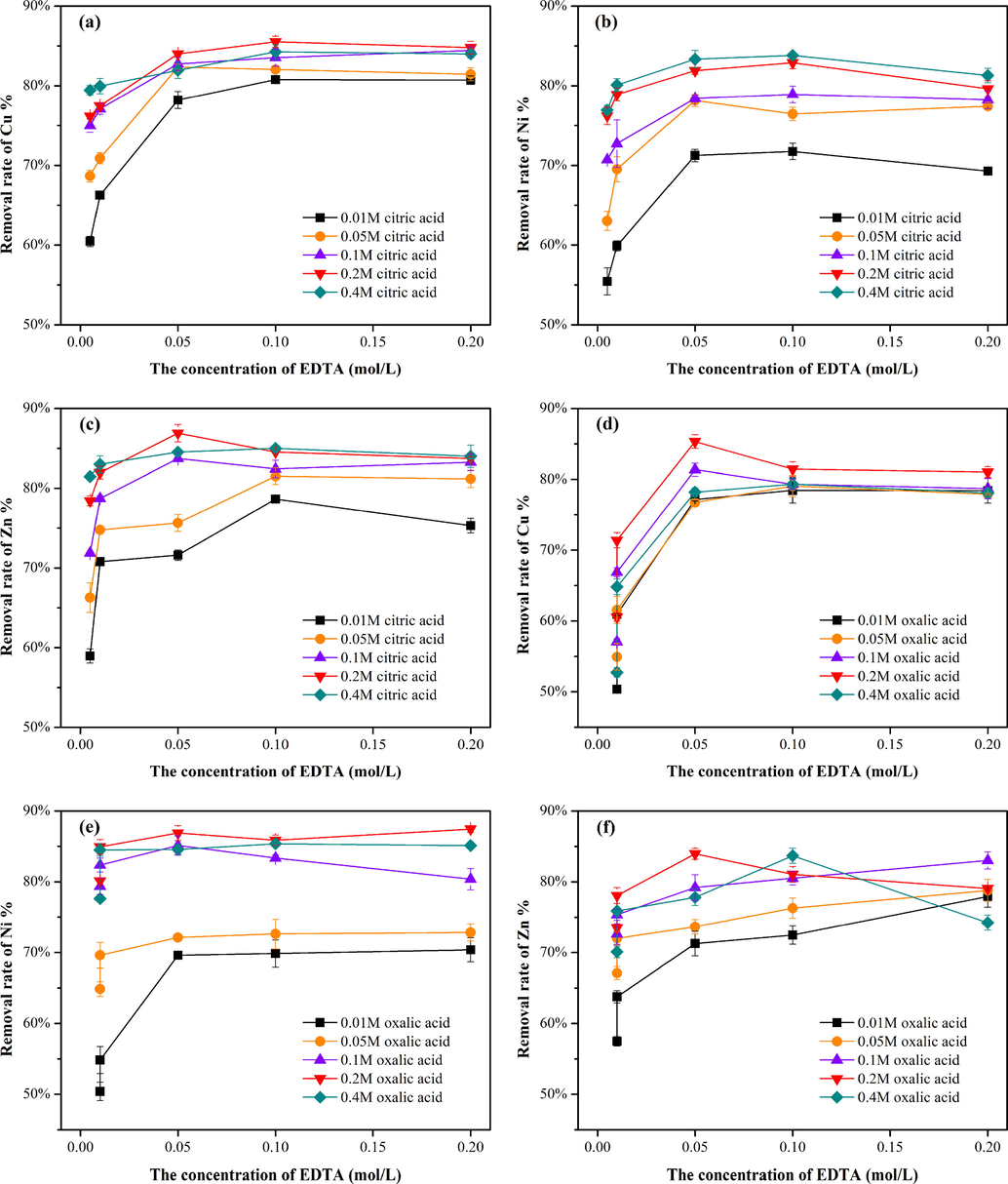
Removal rates of Cu, Ni and Zn using mixed reagents: equal volumes of EDTA (0.005/0.01/0.05/0.1/0.2 M) and citric acid (a-c), oxalic acid (d-f), and tartaric acid (g-i) (0.01/0.05/0.1/0.2/0.4 M) under an initial pH of 3.0, solid-liquid ratio of 1:10 and washing time of 6 h.
Removal rates of Cu, Ni and Zn using mixed reagents: equal volumes of EDTA (0.005/0.01/0.05/0.1/0.2 M) and citric acid (a-c), oxalic acid (d-f), and tartaric acid (g-i) (0.01/0.05/0.1/0.2/0.4 M) under an initial pH of 3.0, solid-liquid ratio of 1:10 and washing time of 6 h.
3.3 The effect of other washing parameters
3.3.1 Solid-liquid ratio
The solid-liquid ratio is a crucial factor in soil washing technology as this parameter is directly related to the difficulty and cost of subsequent wastewater treatment. Therefore, it was necessary to minimize the amount of mixed washing reagents while maximizing the heavy metal removal efficiency. As presented in Fig. S2a, the removal rates of Cu, Ni and Zn by EDTA-citric acid increased from 69.1% to 84.5%, 66.1% to 82.1%, and 68.0% to 86.1% as the solid-liquid ratio changed from 1:5 to 1:10, respectively. Nevertheless, the heavy metal removal efficiencies remained stable as the solid-liquid ratio decreased from 1:10 to 1:20. The other mixed washing reagents, EDTA-oxalic acid (Fig. S2b) and EDTA-tartaric acid (Fig. S2c), showed similar trends at these solid-liquid ratios. A possible reason is that the removal efficiency of heavy metals was determined by the molar ratio of reagents to heavy metals. Therefore, increasing the amount of washing reagent initially benefited the removal of heavy metals. Nevertheless, some heavy metals were fixed in the interior of soil particles and were hard to release. Thus, the removal of heavy metals was minimally improved when using an overdose of mixed washing reagent.
3.3.2 Washing time
The washing time determined the degree of heavy metal desorption and affected the washing effectiveness of heavy metals (Yip et al., 2009). As shown in Fig. S3a-c, in the three mixed washing reagent systems, the removal efficiencies of Cu, Zn and Ni increased significantly with reaction time (0–6 h) and tended towards stability after 6 h. For example, the removal rates of Cu, Ni, and Zn by EDTA-tartaric acid (Fig. S3c) increased from 57.9% to 83.5%, from 65.9% to 80.5%, and from 55.1% to 82.9% from 0.5 to 6 h but ranged from 83.9% to 84.1%, from 82.5% to 82.1%, and from 82.0% to 85.3% from 12 to 24 h, respectively. There were two stages of heavy metal desorption: the first stage was the fast desorption of weakly bound heavy metals on the soil surface; the second stage was the slow release of strongly bound heavy metals in the soil particles (Zhang et al., 2013). In our study, the process of heavy metal desorption was consistent with the above research, therefore the variation tendency of removal efficiencies of Cu, Zn and Ni could be approximate predictable. Considering the balance between efficiency and cost, the optimal washing time was approximately 6 h.
3.3.3 Initial pH of mixed washing reagents
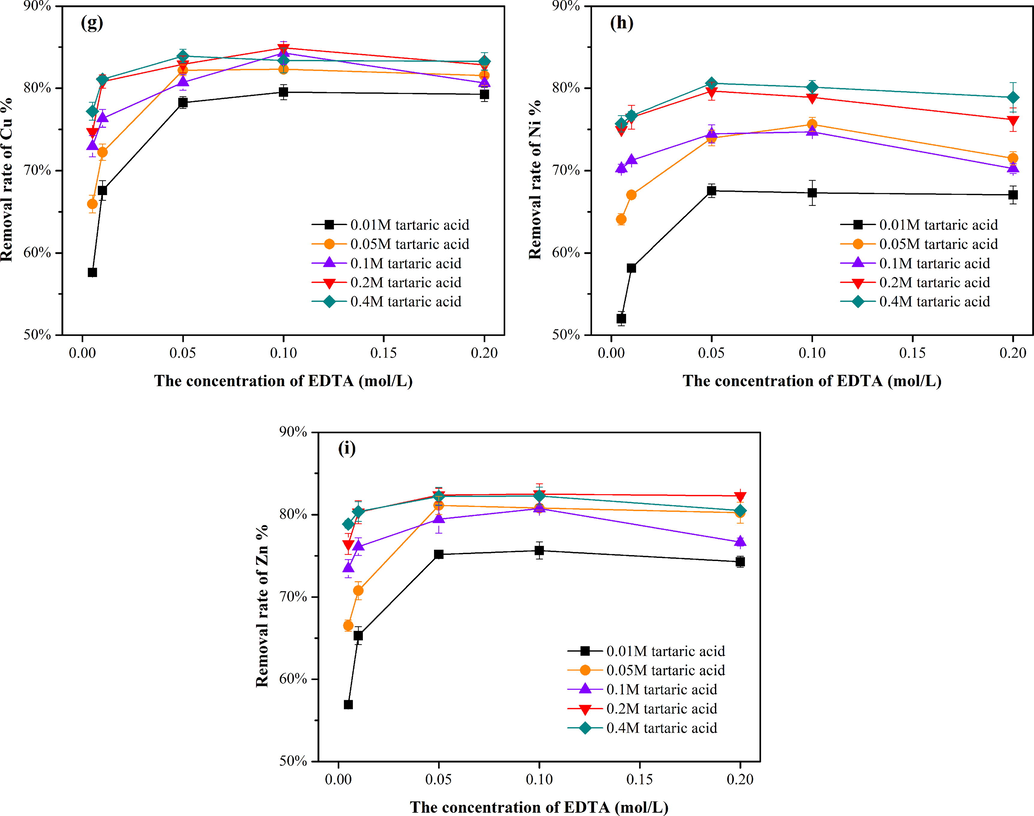
As seen from Fig. 2a-d, the removal efficiencies of Cu, Ni, and Zn using the three kinds of mixed washing reagents showed similar trends with varying pH values. When the pH increased from 3.0 to 7.0 in the EDTA-tartaric acid system, the Cu, Ni, and Zn removal efficiencies decreased from 83.1% to 69.9%, from 80.5% to 63.1%, and from 82.1% to 69.5%, respectively (Fig. 2c). An appropriate pH is conducive to the dissolution and further complexation removal of heavy metals, especially at low pH values. Fig. 2d shows the removal efficiency of heavy metals by EDTA solution with different pH values. The EDTA-organic mixtures could always remove more heavy metals (7.0–8.9% of Cu, 17.4–23.5% of Ni and 11.5–15.4% of Zn) than EDTA at pH 3.0. A probable reason is that in addition to providing hydrogen ions, organic acids can provide certain functional groups, such as —COOH and —OH, which can facilely bind heavy metals (Qin et al., 2004).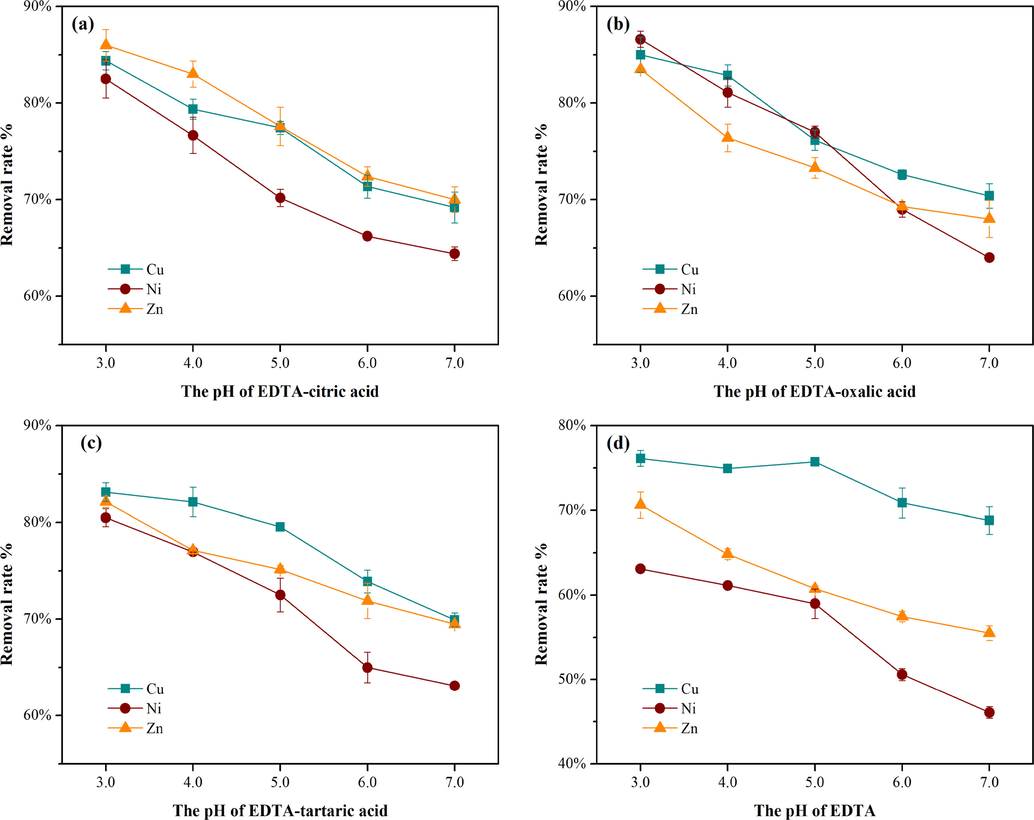
Removal rates of Cu, Ni and Zn by EDTA-citric acid (a), EDTA-oxalic acid (b), EDTA-tartaric (c) acid and EDTA (d) under pH of 3.0, 4.0, 5.0, 6.0 and 7.0.
3.4 Changes of soil leaching toxicity
In the investigation and remediation of decommissioned industrial lands, the leaching toxicity is considered highly important as an index to judge the risk of contaminated soil. When measuring leaching toxicity, one method alone may not reflect the real situation due to the interference of organic matter, inorganic colloids and pH in the soil. Currently, there are two acknowledged methods for determining leaching toxicity in China: Solid Waste-Extraction Procedure for Leaching Toxicity-Sulphuric Acid & Nitric Acid Method (HJ/T299-2007) and Solid Waste-Extraction Procedure for Leaching Utilization-Horizontal Vibration Method (HJ557-2010). The above two standards were adopted in this paper to comprehensively analyze the leaching toxicity of heavy metals. According to method 2, the leaching toxicity of Cu, Zn and Ni in the sample leached by EDTA-oxalic acid was reduced to 5.85 mg/L, 2.82 mg/L and 1.27 mg/L, respectively, corresponding to the most effective group (Fig. 3a). In the other groups, the leaching toxicity of heavy metals treated by washing was also effectively decreased under the two determination methods (Fig. 3b), further indicating that the risk in later construction and utilization of the site was successfully controlled.
Changes in leaching toxicity measured by method 1 (a; sulfuric acid solution, pH = 3.20 ± 0.01, turned constantly for 16 h) and method 2 (b; deionized water, oscillated horizontally for 8 h).
3.5 Speciation analysis
To explore the migration and transformation of Cu, Ni and Zn in soils, an extraction experiment (BCR) was conducted. As shown in Fig. 4a, Cu in the original soil mainly existed in F1 (68.4%), followed by F2 (7.7%), F3 (16.5%) and F4 (7.4%). After washing treatment with EDTA-citric acid, EDTA-oxalic acid and EDTA-tartaric acid, the F1 fraction of Cu was removed at rates of 94.2%, 93.5%, and 93.3%, respectively, exceeding the removal efficiency of other fractions of Cu. Similar tendencies were also observed for the removal of Ni (Fig. 4b) and Zn (Fig. 4c). The F1 and F2 fractions of Cu, Ni, and Zn were more significantly removed by EDTA-oxalic acid than EDTA-citric acid and EDTA-tartaric acid. This difference in removal was because oxalic acid could release more anions than the other organic acids used in this experiment, accelerating heavy metal desorption more due to the pKa values of oxalic acid (pKa1 = 1.23, pKa2 = 4.19) (Jiang et al., 2017). Meanwhile, redox reactions between a portion of the heavy metals and oxalic acid occurred because of the reducibility of oxalic acid (Jiang et al., 2017).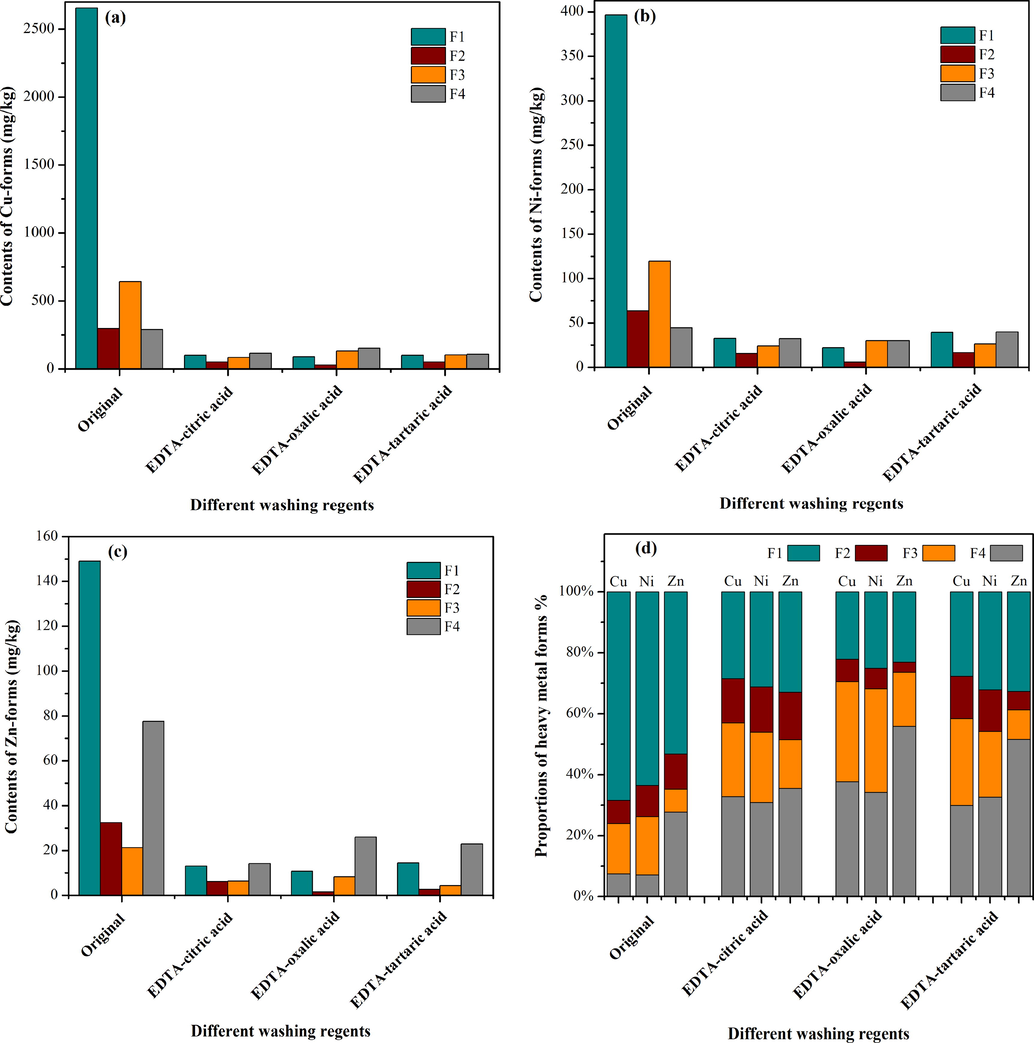
Changes in the content (a-c) and proportion (d) of Cu, Ni and Zn in different speciations.
In addition, the speciation distribution of heavy metals in the soil changed significantly through soil washing (Fig. 4d). Compared with the original soil, the proportions of the F1 of Cu, Ni and Zn were obviously decreased, and F4 accounted for a greater percentage, while F2 and F3 changed slightly after washing with the three mixed washing reagents. Since the degree of soil risk is mainly determined by the content of acid-extractable heavy metals with strong migration ability, the speciation distribution of soil samples treated by washing indicated preliminarily that the stability of the remaining heavy metals was enhanced.
3.6 Changes of heavy metal mobility and stability in soils
The mobility and stability of heavy metals in soil were accessed by MF and IR values, respectively. In fact, the heavy metal contained soils with high MF values always possess higher toxicity and bioavailability (Ma and Rao, 1997). In addition, the IR index which could reflect the heavy metal binding intensity in soils was also analyzed (Gusiatin and Klimiuk, 2012; Han et al., 2003). As shown in Fig. 5a, the MF values of Cu (0.68), Ni (0.64) and Zn (0.53) in original soil dropped evidently after washing with EDTA-organic acids, especially oxalic acid, which could decrease most effectively the MF of Cu (0.22), Ni (0.25) and Zn (0.23). The tendency of IR was presented in Fig. 5b. After washing by EDTA-organic acids, the IR of Cu, Ni and Zn in soil almost all were more than 0.5, while the index in original soil was only 0.23 of Cu, 0.24 of Ni and 0.38 of Zn. It was indicated that the residual heavy metals in the soil existed in a more stable state.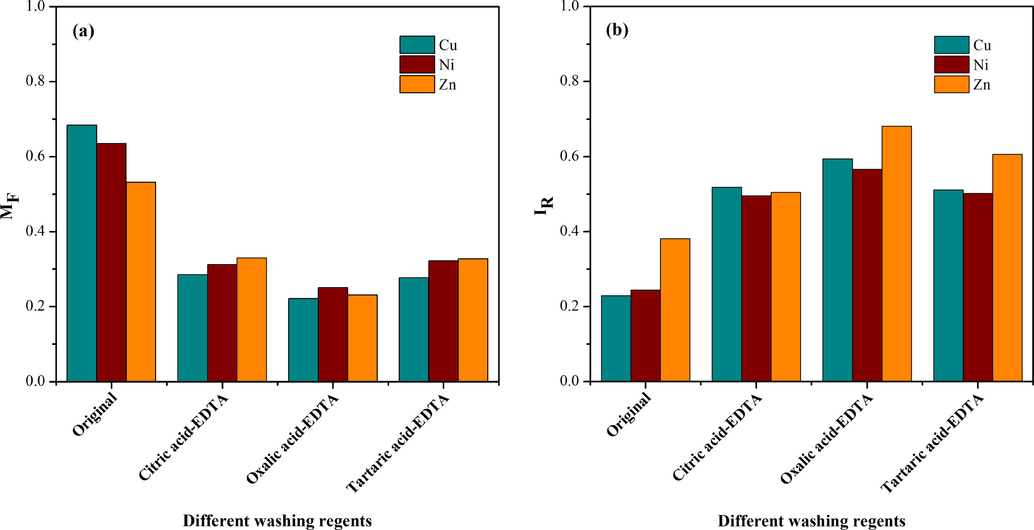
The changes of MF (a) and IR (b) of Cu, Ni and Zn in the soil before and after soil washing.
Considering these changes of MF and IR, the one reason could be that heavy metals in F1 was the main part to be removed. The other possible reason was that it could enable more heavy metals to be absorbed onto the soil due to the increase of organic components in soil by washing with EDTA-organic acids, and the movement of heavy metals was restricted (Udom et al., 2004). The above results further confirmed the positive function of EDTA-organic acids in reducing soil toxicity and ecological risks.
3.7 The treatment of soil washing wastewater
Finally, due to the strong stability of the EDTA-heavy metals, the Advanced Oxidation Process (AOP) was used to generate the decomplexation of the complexes in soil washing wastewater. Subsequently, the released heavy metal irons were removed by alkaline precipitation (the specific produce was shown in SI Text 1). The preliminary experiment results showed that the combination method was effective to treat soil washing wastewater (Fig. S4). Of course, the treatment of wastewater is briefly mentioned in this paper, and further research will be elaborated in future papers.
The costs of the washing reagents were calculated to further verify the feasibility of removing heavy metal by EDTA-organic acid washing reagents. Most of the organic acids used in this paper, such as citric acid and oxalic acid cost less than EDTA, therefore the addition of organic acids reduced the application and subsequent treatment costs while promoting the removal efficiency of heavy metals.
4 Conclusions
In this study, heavy metals-contaminated soil collected from a relocated industrial site in Guangdong Province in China was washed and analyzed. The concentration and initial pH of the washing reagents, washing time and solid-liquid ratio were critical parameters influencing the removal of heavy metals. The washing reagents produced by mixing 0.05 M EDTA with 0.2 M citric acid, oxalic acid and tartaric acid could remove 81.5%, 85.5% and 85.0% of Cu, 85.9%, 82.9% and 78.9% of Ni, 81.1%, 84.6% and 82.5% of Zn under an initial pH of 3.0, solid-liquid ratio of 1:10 and washing time of 6 h. Compared with single organic acid or EDTA solutions, the mixed washing reagents had obvious improvements in the total heavy metal removal effect. In addition, the leaching toxicity and F1 of Cu, Ni and Zn could be removed more than 60% and 90% by washing with EDTA-organic acids, respectively, indicating that the washing reagents had good performance in the reduction of toxicity and the restriction of migration of Cu, Zn, and Ni. Moreover, low concentrations of EDTA were used in the mixed washing agents, which reduced the burden on the environment and the cost of subsequent treatment processes. In summary, the effectiveness and necessity of mixing citric acid/oxalic acid/ tartaric acid and EDTA were proven, demonstrating the feasibility and applicability of mixed reagents in the remediation engineering of polluted industrial soils.
Acknowledgements
This work was financially supported by the National Key Research and Development Program of China (2018YFC1802803), the National Natural Science Foundation of China (21677041, 41371317), and the Science and Technology Project of Guangdong Province (2017B020216002).
References
- Remediation of heavy metal contaminated soil washing residues with amino polycarboxylic acids. J. Hazard. Mater.. 2010;173:697-704.
- [Google Scholar]
- Remediation of toxic metal contaminated soil by washing with biodegradable aminopolycarboxylate chelants. Chemosphere. 2012;87:1161-1170.
- [Google Scholar]
- Chelant-enhanced washing of CCA-contaminated soil: Coupled with selective dissolution or soil stabilization. Sci. Total. Environ.. 2018;612:1463-1472.
- [Google Scholar]
- Remediation of heavy metal(loid)s contaminated soils – To mobilize or to immobilize. J. Hazard. Mater.. 2014;266:141-166.
- [Google Scholar]
- Phytoremediation of mixed contaminated soil enhanced with electric current. J. Hazard. Mater.. 2019;361:95-102.
- [Google Scholar]
- Feasibility of nanoscale zero-valent iron to enhance the removal efficiencies of heavy metals from polluted soils by organic acids. Ecotox. Environ. Safe. 2018;162:464-473.
- [Google Scholar]
- Effects of surfactants on low-molecular-weight organic acids to wash soil zinc. Environ. Sci. Pollut. R. 2016;23:4629-4638.
- [Google Scholar]
- The role of citric acid on the phytoremediation of heavy metal contaminated soil. Chemosphere. 2003;50:807-811.
- [Google Scholar]
- Investigation of a combined continuous flow system for the removal of Pb and Cd from heavily contaminated soil. Chemosphere. 2019;229:181-187.
- [Google Scholar]
- Soil washing for metal removal: A review of physical/chemical technologies and field applications. J. Hazard. Mater.. 2008;152:1-31.
- [Google Scholar]
- Heavy metals mobilization from harbour sediments using EDTA and citric acid as chelating agents. J. Hazard. Mater.. 2007;147:768-775.
- [Google Scholar]
- Application of sheep manure and potassium fertilizer to contaminated soil and its effect on zinc, cadmium and lead accumulation by alfalfa plants. Sustainable Environ. Res.. 2016;26:131-135.
- [Google Scholar]
- Effect of soil washing with only chelators or combining with ferric chloride on soil heavy metal removal and phytoavailability: Field experiments. Chemosphere. 2016;147:412-419.
- [Google Scholar]
- Effect of mixed chelators of EDTA, GLDA, and citric acid on bioavailability of residual heavy metals in soils and soil properties. Chemosphere. 2018;209:776-782.
- [Google Scholar]
- Metal (Cu, Cd and Zn) removal and stabilization during multiple soil washing by saponin. Chemosphere. 2012;86:383-391.
- [Google Scholar]
- New approach to studies of heavy metal redistribution in soil. Adv. Environ. Res.. 2003;8:113-120.
- [Google Scholar]
- Surfactant adsorption to soil components and soils. Adv. Colloid Interfac.. 2016;231:59-102.
- [Google Scholar]
- Effect of EDTA washing of metal polluted garden soils. Part II: Can remediated soil be used as a plant substrate. Sci. Total. Environ.. 2014;475:142-152.
- [Google Scholar]
- Removal of toxic metals from vanadium-contaminated soils using a washing method: Reagent selection and parameter optimization. Chemosphere. 2017;180:295-301.
- [Google Scholar]
- A comparison of technologies for remediation of heavy metal contaminated soils. J. Geochem. Explor.. 2017;182:247-268.
- [Google Scholar]
- Factors affecting EDTA extraction of lead from lead-contaminated soils. Chemosphere. 2003;51:845-853.
- [Google Scholar]
- Evaluation of the potential redistribution of chromium fractionation in contaminated soil by citric acid/sodium citrate washing. Arabian J. Chem.. 2017;10:S539-S545.
- [Google Scholar]
- Remediation of cadmium- and lead-contaminated agricultural soil by composite washing with chlorides and citric acid. Environ. Sci. Pollut. R. 2015;22:5563-5571.
- [Google Scholar]
- The combined effects of surfactant solubilization and chemical oxidation on the removal of polycyclic aromatic hydrocarbon from soil. Sci. Total. Environ.. 2019;647:1106-1112.
- [Google Scholar]
- Potassium lignosulfonate as a washing agent for remediating lead and copper co-contaminated soils. Sci. Total. Environ.. 2019;658:836-842.
- [Google Scholar]
- Chemical fractionation of cadmium, copper, nickel, and zinc in contaminated soils. J. Environ Qual. 1997:26.
- [Google Scholar]
- Spatial distribution of heavy metal concentrations in urban, suburban and agricultural soils in a Mediterranean city of Algeria. Environ. Pollut.. 2010;158:2294-2301.
- [Google Scholar]
- Use of surfactants for the remediation of contaminated soils: A review. J. Hazard. Mater.. 2015;285:419-435.
- [Google Scholar]
- Enhanced phytoextraction: I. effect of EDTA and citric acid on heavy metal mobility in a calcareous soil. Int. J. Phytorem.. 2005;7:129-142.
- [Google Scholar]
- Washing as a remediation technology applicable in soils heavily polluted by mining–metallurgical activities. Chemosphere. 2006;63:1632-1640.
- [Google Scholar]
- Remediation of soils polluted with lindane using surfactant-aided soil washing and electrochemical oxidation. J. Hazard. Mater.. 2017;339:232-238.
- [Google Scholar]
- Ecological risk assessment of on-site soil washing with iron(III) chloride in cadmium-contaminated paddy field. Ecotox. Environ. Safe.. 2012;80:84-90.
- [Google Scholar]
- Effects of low-molecular-weight organic acids and residence time on desorption of Cu, Cd, and Pb from soils. Chemosphere. 2004;57:253-263.
- [Google Scholar]
- Removal of trace and major metals by soil washing with Na2EDTA and oxalate. J. Soils Sediments. 2010;10:45-53.
- [Google Scholar]
- Electrokinetic remediation of soils polluted by heavy metals (mercury in particular) Chem. Eng. J.. 2015;264:16-23.
- [Google Scholar]
- Extraction of rare earth elements from a contaminated cropland soil using nitric acid, citric acid, and EDTA. Environ. Technol.. 2017;38:1980-1986.
- [Google Scholar]
- Distributions of zinc, copper, cadmium and lead in a tropical ultisol after long-term disposal of sewage sludge. Environ. Int.. 2004;30:467-470.
- [Google Scholar]
- Speciation of heavy metals in soils and sediments. An account of the improvement and harmonization of extraction techniques undertaken under the auspices of the BCR of the commission of the European communities. Int. J. Environ. An. Ch.. 1993;51:135-151.
- [Google Scholar]
- Effects of biodegradable chelator combination on potentially toxic metals leaching efficiency in agricultural soils. Ecotox. Environ. Safe.. 2019;182 109399
- [Google Scholar]
- Biodegradation of rhamnolipid, EDTA and citric acid in cadmium and zinc contaminated soils. Soil Biol. Biochem.. 2009;41:2214-2221.
- [Google Scholar]
- Influence of particle size distribution, organic carbon, pH and chlorides on washing of mercury contaminated soil. Chemosphere. 2014;109:99-105.
- [Google Scholar]
- Pyrophosphate coupling with chelant-enhanced soil flushing of field contaminated soils for heavy metal extraction. J. Hazard. Mater.. 2012;199–200:51-57.
- [Google Scholar]
- Kinetic interactions of EDDS with soils. 1. Metal resorption and competition under EDDS deficiency. Environ. Sci. Technol.. 2009;43:831-836.
- [Google Scholar]
- A combination of ferric nitrate/EDDS-enhanced washing and sludge-derived biochar stabilization of metal-contaminated soils. Sci. Total. Environ.. 2018;616–617:572-582.
- [Google Scholar]
- Simultaneous application of chemical oxidation and extraction processes is effective at remediating soil Co-contaminated with petroleum and heavy metals. J. Environ. Manage.. 2017;186:314-319.
- [Google Scholar]
- Remediation of multiple heavy metal-contaminated soil through the combination of soil washing and in situ immobilization. Sci. Total. Environ.. 2018;635:92-99.
- [Google Scholar]
- Remediation of an electroplating contaminated soil by EDTA flushing: chromium release and soil dissolution. J. Soils Sediments. 2013;13:354-363.
- [Google Scholar]
- Development of phosphate rock integrated with iron amendment for simultaneous immobilization of Zn and Cr(VI) in an electroplating contaminated soil. Chemosphere. 2017;182:15-21.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2020.02.015.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







