Translate this page into:
The therapeutic effects of tumor treating fields on cancer and noncancerous cells
⁎Corresponding author. ahasan@qu.edu.qa (Anwarul Hasan)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Tumor treating fields (TTFields) are among clinically active anticancer modalities that utilize low‐intensity, intermediate frequency (IF), and alternating electric fields (AEFs) to selectively disrupt mitosis in cancerous cells. Application of TTFields in the range of 100–900 kHz in cancer therapy and its effect on normal and cancer cells have attracted a great deal of interest in recent years. TTFields affect solid tumors by introducing increased chromatid aberrations that reduce the capacity to repair DNA damage and chromosome segregation, resulting in autophagy and subsequent cell death. In this review, we present an overview of the applications of TTFields in the treatment of cancer. We discuss several practical applications of TTField frequencies combined with metallic nanoparticles (NPs) (magnetic or nonmagnetic NPs) for internalization into cancer cells. In addition, TTFields can be combined effectively with chemotherapy and radiotherapy.
Keywords
Therapeutic effects
Tumor treatment field
Cancer cells
1 Introduction
The effects of electric fields on cancerous and noncancerous cells have been studied extensively using both high and low-frequency electric fields. Alternating Electric Fields or Tumor Treating fields (TTFields) are low intensity (1–3 V/cm) fields with an intermediate frequency range of 100–300 kHz. The application of these frequencies to cancer cells has been approved by the FDA (Carrieri et al., 2020) and has been shown to produce significant positive results. The TTFields frequencies work on cell physiology and molecular alteration on cells positioned around the tumor region's area, with side effects mostly limited to the skin. Additionally, TTFields do not interrupt the viability of normal cells, nerve, or muscle cells because of their low power current, occurrence specificity, and locoregional system usage. These dielectrophoretic forces of polar molecules move toward higher field intensity and prevent polymerization (See Table 1).
Cell line
Tissue
Disease
Karyotype
(kHz)
Duration (h)
Ref.
A2780
Ovary
Carcinoma
Model chromosome number = 46
200 kHz
18.7 hr
(Giladi, 2015; Chu et al., 2009)
A549
Lung
Adenocarcinoma
Hypertriploid modal chromosome number = 66 in 24% of cells
150 kHz
23.8 hr
(Giladi, 2015) (Pless et al., 2013)
APC-1
Pancreas
Adenocarcinoma
Not specified
150 kHz
54.0 hr
(Giladi, 2015; Giladi, 2014; Pless et al., 2013)
HeLa
Cervix
Adenocarcinoma
Model number = 82
150 kHz
24 hr
(Giladi, 2015; Giladi, 2014)
MCF-7
Mammary gland Breast
Adenocarcinoma
Hypertriploid to hypo-tetraploid Model chromosome number=
150 kHz
29.3 hr
(Giladi, 2015; Acuna, 1999; Noguchi, 2006; Zasadil, 2014)
MDA-MB-231
Mammary gland Breast
Adenocarcinoma
Near triploid Model chromosome number = 64
150 kHz
29.1 hr
(Giladi, 2015; Acuna, 1999; Noguchi, 2006; Zasadil, 2014)
MSTO-211H
Lung
Stage 4, mestothelioma
Model chromosome number = 72
150 kHz
26.4 hr
(Giladi, 2015; Pless et al., 2013)
NCI-H1299
Lung
NSCLC carcinoma
Not specified
150 kHz
23.1 hr
(Giladi, 2015; Pless et al., 2013)
NCI-2052
Lung
Biphasic mesothelioma
Not specified
200 kHz
18.9 hr
(Giladi, 2015; Pless et al., 2013)
U-87 MG
Brain
Grade-IV Glioblastoma astrocytoma
Hypo-diploid modal chromosome number = 44 in 48%
200 kHz
34.0 hr
(Giladi, 2015; Turner et al., 2014; Kirson, 2004)
U-118 MG
Brain
Grade-IV Glioblastoma astrocytoma
Hypo-diploid modal chromosome number = 44 in 48%
200 kHz
18.5 hr
(Giladi, 2015; Turner et al., 2014; Kirson, 2004)
TTFields affect solid tumors by introducing increased chromatid aberrations that reduce the capacity to repair DNA damage and chromosome segregation, resulting in autophagy and subsequent cell death. The alteration in cancer cells as a result of exposure to TTField has been investigated using gene expressions. Alterations were observed in cell proliferation pathways and cell cycles, particularly in BRCA1, the DNA-damage response that decreases their expression (p < 0.05), and DNA double-strand break (DSB) repair was noticeable. The combined treatment of TFField with radiotherapy shows even more substantial effects on molecular bases of the cells, such as induced chromatid aberrations and inhibition of capacity to repair DNA DSBs, which were adequately identified to be responsible for a significant part of the enhanced cell death seen in combination therapy. Furthermore, the death of cancer cells increases when TTField is added to radiotherapy because of enhancing BRCAness that enhances the sensitivity of the cancer cells to the radiations accordingly (Karanam et al., 2018; Cao and Scarfi, 2014).
Moreover, the same mutant of TP53 was discovered in four glioblastoma cell lines using gene expression and profiling. Primarily, TP53 mutant is associated with the cell cycle, cell death, and immune response. The investigators observed altered TP53 status of the genes after exposure to TTFields, which appeared to exert enhanced anticancer effects by altering the immune system in the inflammatory environment and regulating cell cycle- and cell death-related genes. However, the specific gene influence varied according to TP53 status (Lee et al., 2020; Giladi et al., 2014; Schneiderman et al., 2010). Furthermore, a new study demonstrated that a combination of particular types of nanoparticles (NPs) with the influence of TTField is more effective than the use of TTField alone (Fröhlich, 2012; Sun et al., 2017). TTFields activated the accumulation of NPs which caused alterations in cell proliferation pathways and cell cycles. Therefore, using nanomaterials in combination with TTFields- can result in better responses in cancer cells.
This review presents an overview of the application of TTFields in cancer therapy and the combination of TTField with radiotherapy and chemotherapy (Carrieri et al., 2020). It summarizes the progress and results from recent in-vitro studies investigating the disruptive effects of TTFields in different cancer cells and recent and ongoing clinical trials on the application of TTFields in treating different types of solid tumors.
2 The effect of high-electric MF (1 MHz – 900 MHz) on human noncancerous and cancer cells
High-frequency fields have been used in some studies to induce genotoxic responses in different types of cells. This frequency range commonly causes heat, primarily if it is introduced into the tissue in combination with 1.5 Gy gamma radiation (GR). In those investigations, bone marrow stromal cell (BMSC) cancer cells were exposed to 900 MHz magnetic frequencies at a power flux density of 120 μW/cm2 for three h per day. The authors used the dose for genotoxicity at this point in the study as 1.5 Gy GR. However, exposure of cancer and normal cells high-frequency fields in the 1.5 Gy GR has the disadvantage of increasing body temperature, disrupting cellular membranes, and increasing the effects of electroporation effects, which lead to cell death (Sannino, 2011; Giladi, 2014).
High frequencies (300 kHz-1 MHz) of intermediate magnetic frequency (IMF) were used on noncancerous human cells as a negative control and then on human cancer cells (solid tumors) after short-term exposure. There was no apparent effect on noncancerous human cells. As mentioned earlier, the WHO (Shi, 2018) describes the hazard range of IF to public health as ranging from 300 Hz to 10 MHz. However, in this study, the investigator applied ionizing radiation (IR) as an adaptive dose (AD) and then observed the effect on noncancerous and cancer cells. The results demonstrated no effect of exposure to IF on public health ranging from 100 kHz −300 kHz, but human or animal health relevance was inconclusive. However, other studies reported an increase in inflammatory mediators and oxidative stress markers in the young mouse brain after IF and IR exposure (Win-Shwe et al., 2015), as described further in the review. These studies indicate the disadvantage of the significant heterogeneity in study design, in addition to variations in study design and conclusions.
3 The effect of a low frequency MFs on human noncancerous and cancer cells
Researchers have declared that frequencies within the IF spectrum emitted from induction cooktop heaters are approximately 23 kHz in recent studies. Different reports (Sannino, 2011; Giladi, 2014) described that CHO-K1 cells have been exposed to 23 kHz for 2 h with 532 µT and 6.05 µT magnetic flux densities. Cell growth and genotoxicity were assessed using the comet assay.
The comet assay detects micronucleus formation and gene mutations in hypoxanthine–guanine phosphoribosyltransferase (HPRT) (Cao and Scarfi, 2014). Combined exposure to different agents; such as non-IR and chemicals/pharmaceuticals or IR [challenge dose (CD)] (Dimova et al., 2008), induces the Adaptive response (AR). For example, a previous study (Sannino, 2011) examined AR in human lymphocytes in vitro after exposure to RF at 1950 MHz for AD and later to 1.0 or 1.5 Gy as a CD (Dimova et al., 2008). In recent work, researchers described a significant decrease in IR that induced a regular rate of a micronuclei formation when lymphocytes were pre-exposed to RF-EMF. In earlier studies (Sannino, 2011); AR was also detected in human lymphocytes using 900 MHz RF-EMF, and the chemical mutagen mitomycin C yielded the same AR from the same cell types. However, human lymphocytes exhibited some side effects on the patient's skin, such as itching, redness, and a burning sensation due to RF exposure. Giladi (Giladi, 2015) explains that low frequencies (under 1 kHz) of AEFs stimulate nerves and muscles by depolarizing the cell membrane
4 The effect of TTField on different cell type
TTField is an advanced noninvasive technique used to treat cancer cells. The fields used for treatment are low currency fields in the range of 100 kHz to 300 kHz called alternating MFs (AMFs). These fields are created using ceramic transducers placed on the opposite side of organ regions with cancer in the patient's body (Munster, 2015; Soni and Yanagihara, 2019), which generates an AMF. The mechanism of TTFields is used in many cancer therapy applications, including immunology, migration, and autophagy of the cancer cells (Bomzon, 2016); (Bomzon and Wenger, 2017). Gene toxicity is the result of long-term exposure to the AMF's IFs, which comprise the TTField. Sun et al. (Cao and Scarfi, 2014) exposed retinal pigment epithelial cells to 100 kHz for 24 h and did not observe any difference in the comet assay DNA bands. Meanwhile, additional studies conducted (Brech et al., 2019) evaluated the possible gene toxicity of exposure to 123.90 kHz and 250.80 kHz IF-MF in canine and human blood (Table 2). The blood of different types of vertebrates was exposed to IF-MF at 630 A/m (0.79 mT) and 80 A/m (0.10 mT). The time of exposure was between 1 and 5 h (hly), and gene toxicity was assayed using the comet assay after 20 and 24 h of exposure.
Type of normal cells
Type of MF
Time duration
Genotoxic Effect
References
Retinal pigment epithelial cells
100 kHz
24 h
After 24 h, there is no apparent effect
(Németh et al., 2019)(Sun et al., 2017)
Human blood cells
123.90 kHz and 250.80 kHz IF-MF
1 to 5 h for 20 to 24 h
Only after 20 h exposure, there is slide DNA damage recorded in both frequencies
(Németh et al., 2019)
Canine blood cells
123.90 kHz and 250.80 kHz IF-MF
1 to 5 h for 20 to 24 h
Only after 20 h exposure, there is slide DNA damage recorded in both frequencies
(Brech et al., 2019)
Leukocytes in human blood cells
250.80 kHz IF-MF with IR (1.5 Gy X-ray)
20 h and 24 h
Before 20 h, there is no apparent effect in 20 h.
(Németh et al., 2019)
5 The effect of TTField on cell membrane permeability
The electric power frequency of TTField and total power addition within the membrane can cause significant changes in the chemical and physical parameters of the cell membrane. These changes can be observed at higher frequencies. Most treatments do not include a complete picture of the effects. In recent years, Chang et al. (Chang, et al., 2018) studied the effects of TTFields on the cellular membrane structure, observing that cells are more permeant to chemotherapeutics when exposed to TTField. The study revealed that membrane permeability was stimulated by introducing a TTField, as shown by several approaches in the experiment. 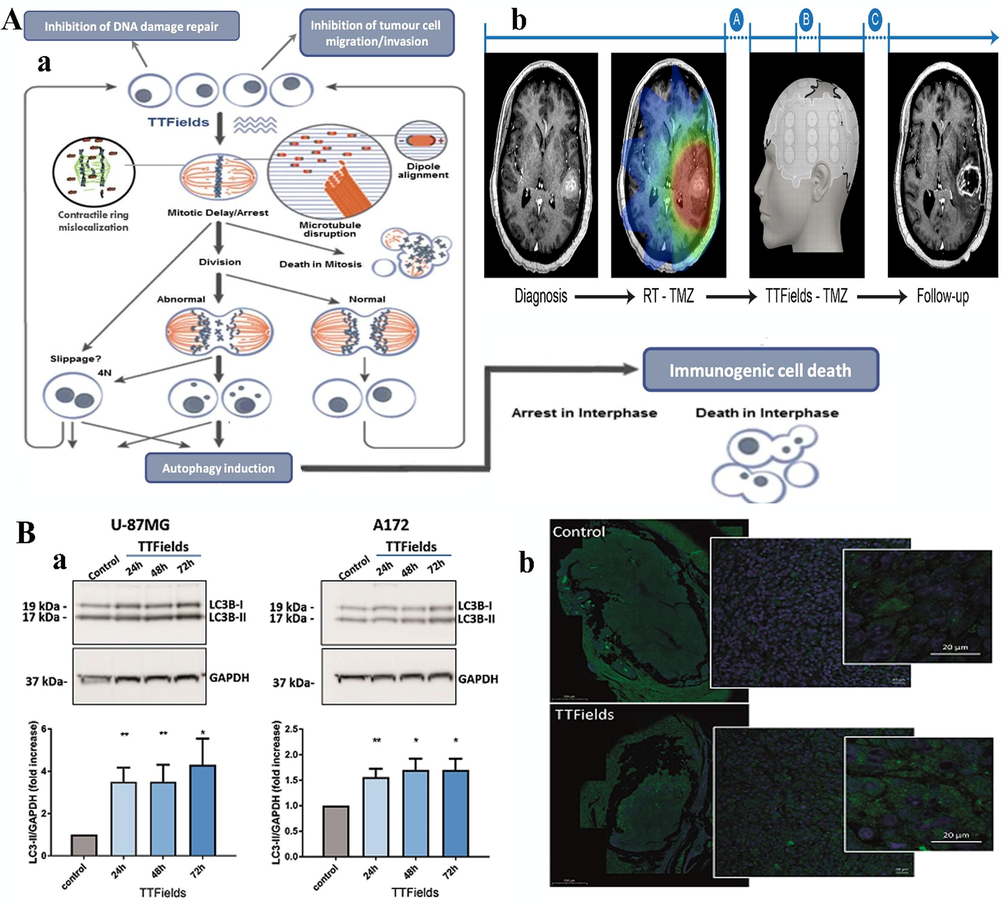
A: In this study, the investigator anticipated cellular mechanisms to endure the treatment resulting from TTFields. In vitro and in vivo experiments confirmed that TTFields at a frequency between 100 kHz and 300 kHz leads to delayed cell division, resulting from cell proliferation and encouraging cell proliferation membrane breaking. (b) The descriptive therapy time period in a patient with glioblastoma. The treatment protocol was as follows: for approximately 2–6 weeks, patients diagnosed early with glioblastoma were exposed to combined radiation with concurrent TMZ. This exposure period should have a break of approximately one month, followed by TTFields with concurrent TMZ for at least six months or until treatment failure. In Point A, imaging biomarkers were used as a predictive tool to determine whether to continue the therapy. Evaluation of the effect of the treatment is explained in Point B or ceasing the treatment due to the success of therapy evaluation is explained in Point C (Soni and Yanagihara, 2019). B: (a) TTFields induced autophagy in a U-87 MG GBM cell line. A172 cells either escaped the first treatment or were treated with a short exposure to TTFields at 24 h, 48 h, or 72 h in vitro. All cell lines were cultured simultaneously, incubated for 18 h to allow cell attachment, and collected 72 h thereafter. Cells were pelleted and lysed, and examined using western blotting for LC3 and GAPDH. Upper panel: WB bands. Lower panel: densitometric quantification of WB bands. The Figure shows an average of three independently performed experiments. (b) Paraffin-embedded cells from sham- or TTFields-in vivo treated animals were stained with anti-LC3 Ab (green) and DAPI substrate (Shteingauz, 2018). Figures reprinted with Creative Commons Attribution 2.0 (CC-BY-0.2) License from Refs. (Soni and Yanagihara, 2019) and (Shteingauz, 2018).
On the other hand, the permeability of the cell membrane was observed by the increased binding of some reagents, such as dextran-FITC or ethidium D. However, upon exposure to TTField for 24 hr, the holes on the cellular membrane increased in size, the bioluminescence of the cell membrane increased and membrane hole production was upgraded(Voloshin, 2016; Cosette et al., 2016). Thus; the study demonstrated that these effects are temporary. Preliminary investigations revealed that TTFields did not induce membrane holes in normal human fibroblasts using scanning electron microscopy (SEM), suggesting that the phenomenon was specific to cancer cells. This study demonstrated that membrane permeability significantly increased due to underexposure to the AMF. Chemotherapeutic agents and therapeutic compounds find their way into cancer cells due to their capability to differentiate cancerous cells from normal cells. This process also explains the preservative and synergistic effects between TTField and chemotherapy in the past year. These findings have directed studies toward combining combination therapies in most solid tumors, which would pointedly change the standard of care strategies for these diseases (Voloshin, 2017; Ram et al., 2017).
6 The effect of TTField on cell division
TTFields cause dividing cells to orient in the applied area. Such details have been described in cultured human corneal epithelial cells exposed to a constant TTField or AMFs, leading to a similar effect. Recent studies have found evidence indicating that TTFields disrupt typical microtubule polymerization during mitotic cell cycles (Giladi, 2014). Giladi et al. (Giladi, 2014) described abnormal mitotic configurations observed after exposure to an AMF or TTField. TTFields decreased the ratio between polymerized and total tubulin in their study and prevented proper mitotic spindle assembly in cancer cells (Giladi, 2015). The aberrant mitotic events induced by TTFields lead to abnormal chromosome segregation, cellular multinucleation, and caspase-dependent apoptosis of daughter cells. The TTField effect on cell viability and clonogenic survival substantially depends upon the cell division rate. Research by Giladiet al. (Giladi, 2015) revealed that extending exposure to AMF affected dividing cells. MF frequencies significantly affected cancer cell division and increased cell membrane permeability. In addition, the optimum MF frequency exposure duration usually leads to the highest reduction in cell numbers.
A previous study (Soni and Yanagihara, 2019) showed that the NovoTTF™-100A system is a portable machine that generates TTFields settled transducer arrays when set on the patient's head near glioblastoma cells in the scalp (Fig. 1A). This experiment measured the TTFields' effect on newly diagnosed glioblastoma (GBM) using imaging tests (Voloshin, 2017). TTField has been FDA-approved for recurrent GBM. The frequency of TTFields affects cancer cell resistance, leading to mitosis. The mitotic effects of TTFields include abnormal chromosome segregation and ER stress, which induce different types of cell death, specifically in cancer cells.
7 The effect of TTField on inducing autophagy
Autophagy is the survival-promoting pathway that recycles proteins produced by cells and organelles inside lysosomes (White, 2015; Shteingauz, 2018). Autophagy restores organelle function, prevents the toxic accumulation of unused cellular products, and delivers substrates to prevent starvation. However, autophagy suppresses tumorigenesis in some contexts (Shteingauz, 2018). In most studies, it has been found that autophagy promotes tumorigenesis, where cancer cells can control autophagic metabolism to sustain the microenvironment. In addition, cancer cells exhibit increased growth and aggressiveness. Autophagy mechanisms that promote cancer include inhibiting p53 tumor suppressor protein induction and maintaining mitochondrial metabolic function. Applying AMFs or TTFields to constrain autophagy, which improves cancer therapy, has attracted great interest. Time-lapse immunofluorescence microscopy revealed a significant upregulation in LC3 marker proteins specific to cells dividing during TTField application. Calculation of particular cell stress factors revealed an increase in the expression of the endoplasmic reticulum (ER) stress marker GRP78. Additionally, TTField application decreases intracellular ATP levels, indicative of increased pro-toxic pressure, AMP-activated-protein-kinase (AMPK) upregulation, and autophagy-related processes, including autophagic cell death(Shteingauz, 2018; Lam et al., 2010; Ullén, 2010).
Furthermore, AMF-induced upregulation of autophagy prepares cells for treatment. The Findings in previous studies suggested sorafenib treatment of cells exposed to an AMF. This finding was promising for successful treatment in different chemotherapy and immunotherapy stages for different types of solid tumors (Wilhelm et al., 2008; Coventon, 2017). In an additional study, the authors observed that the TTFields prevent the cells from dividing due to aneuploidy and ER stress. This procedure stimulates autophagy, inducing aneuploidy and ER stress, and AMPK serves as a critical regulator of this process (Fig. 1B) (Shteingauz, 2018). However, autophagy-related protein 7 (ATG7) suppresses autophagy stimulation in response to AMF, indicating that cancer cells employ autophagy as a defense mechanism against TTField (Shteingauz, 2018). In addition, lysosomes accumulated in response to TTField in all cell lines tested. TTField exposure resulted in variations in cellular size. Finally, the cell physiology changes led to lysosome buildup as shown by fluorescence microscopy in a study by Yunhui et al. (Jo, 2018).
8 The application of TTField on cell initialization
The effect of NPs as imaging and drug delivery tools, with NP sizes between 20 and 200 nm, has been evaluated. NPs are made from easy blood clearance metals, such as silver, barium, and copper. NPs are also used for treating and diagnosing many diseases established in previous studies (Fröhlich, 2012; Yoon et al., 2020; Forest and Pourchez, 2017). The use of NPs also typically increases a drug's ability to reach the cytoplasm at a safe dose. This treatment can induce genotoxicity and also NP-induced cell death can dysregulate mitochondrial metabolism (Al-Rawi et al., 2011).
Therefore, the authors observed that electric field-responsive NPs such as barium titanate (BTNPs) were useful as TTFields-active sensitizers to increase the therapeutic efficacy of TTFields in cancer cells. Lysosomal induction of NP metal generation leads to intracellular reactive oxygen species (ROS) secretion (Albers et al., 2013; Fröhlich, 2012). As shown in those studies, lysosomal interruption leads to cellular self-destruction (Fröhlich et al., 2012; Fröhlich, 2012). In addition, metal NPs generate intracellular connections to cytoskeletal components that initiate chromosome segregation and protein mutations, cell cycle apoptosis, and cell death (Albers et al., 2013; Forest and Pourchez, 2017). However, the addition of TTField ensures that more NPs internalize the target cell's cytoplasm in nanosafe amounts and accurate sizes, increasing cancer cell death. The TTField that promotes internalization of NPs of different sizes is superficially that larger NPs are more easily internalized with TTFields (Forest and Pourchez, 2017). NP internalization is needed to allow NPs to selectively enter cancer cells. The use of NPs for drug delivery is usually controlled by size, charge, and surface shape. The addition of TTField improves the efficacy of NP characterization. Gene insertion of the drug transfer can increase the genotoxicity of such constructs, as previously reported (Fröhlich, 2012; Naderi-Meshkin et al., 2015; Yoon et al., 2020).
Many studies have recently investigated the effectiveness of TTFields using a field strength of 1–3 v/cm and frequency of 100–300 kHz, which increases the permeability of the cells to allow NPs to enter through the cells' open holes to reach the anatomical region of the tumor (Yoon et al., 2020; Kirson, 2004; Giladi, 2015). In this study, the investigator observed that TTField and NP combinations yielded far better results in the cell cycle. Programmed cell death (apoptosis) was examined to compare the effect of combining the two types of therapy on cancer cells, and western blot, cell cycle and apoptosis assays showed the same results (Fröhlich, 2012; Yoon et al., 2020). Yoon et al. (Yoon et al., 2020) studied whether NPs combined with TTFields encourages the internalization flow of human carcinoma of the breast. In addition, using TTFields made cellular uptake for immunofluorescent imaging of drug delivery more practical (Fröhlich, 2012).
The combination of NPs and TTFields provided a better indication of the antitumor effect on breast cancer cells. Indeed, the study demonstrated that barium titanate (BT) NPs in combination with TTFields exhibited a potential antitumor impact on breast cancer cells by triggering cell cycle arrest and apoptosis (Forest and Pourchez, 2017; Yoon et al., 2020). However, the size of NPs and the type of cancer cell may significantly influence how significantly TTField exerts its antitumor activity. In a previous study, y (Yoon et al., 2020), researchers observed that TTField alone and BTNP treatment increased the resistance of the MCF-7 cell line. However, as reported from X-ray diffraction data in (Jo, 2018) and (Yoon et al., 2020), they used more significant NPs to treat breast cancer cells with BTNPs that were 200 nm larger than 100 nm BTNPs alone. Therefore, the combination of BTNPs of 200 nm with TTField was much more effective as an antitumor treatment, explaining why 200 nm BTNPs induce a higher dielectric and average grain size value than 100 nm BTNPs.
9 The combination of TTField with ionizing radiation (IR) for cancer therapy
TTFields exposure in non-small cell lung cancer disease combined with IR resulted in increased chromatid irregularities, as shown by western blot analysis. It significantly decreased the expression of the BRCA1 DNA damage repair protein (P < 0.05) (Karanam et al., 2018). These intense reactions to the use of TTFielids as a combined modality therapy with radiation or other DNA-damaging agent combinations can be a strong basis for using TTFielids followingIR for patient therapy (Karanam et al., 2017; Karanam et al., 2018). Therefore, it is the most effective method to prepare these cells for radioionization and different drug delivery types.
An advanced study by Giladi et al. (Fröhlich, 2012) investigated the effect of TTField and IR on cell genotoxicity. The study used a combination treatment of exposure to TTFields and IR to leukocytes and the adrenocortical carcinoma cell line H295R. In brief, the researcher exposed leukocytes and the adrenocortical carcinoma cell line H295R in vitro 250.8 kHz TTField strength at 80 A/m (equivalent to 100 μT magnetic flux density) for 20 h. Additionally, both cell lines were exposed to 123.90 kHz −250.80 kHz for 20 h and 24 h after treatment with IR. These experimental data suggested that IR exposure induces PARP mRNA protein expression (Sannino, 2011). The PARP-1-mRNA protein has a role in suppressing the cell growth and migration of protein in cancer cells. This DNA damage was detected using the alkaline comet assay to determine the DNA damage due to the treatment. The images showed a significant genotoxic effect of exposure to 123.90 kHz-250.80 kHz TTField combined with IR in cancer cells compared to controls.
10 The combination of TTField and chemotherapy for cancer therapy
It has been well documented that chemotherapy drugs, such as paclitaxel, cisplatin, and pemetrexed, in combination with TTFields potentially inhibit the proliferation of cancer cells (Bomzon and Wenger, 2017; Pless et al., 2013; Schneiderman et al., 2010). These data are in agreement with many investigations in different cancer cells, such as non-small cell lung carcinoma (NSCLC) cells in vitro and in vivo (Bomzon and Wenger, 2017; Pless et al., 2013). In a recent study, TTFields and chemotherapy used to treat NSCLC cell lines improved therapeutic efficacy across all cell lines. The researcher observed a potential response of Lewis lung carcinoma and KLN205 squamous cell carcinoma in mice to the same doses of treatment with TTFields combined with pemetrexed, cisplatin, or paclitaxel. Indeed, TTFields in combination with these therapeutic agents significantly increased therapeutic efficacy compared to the respective single agents and control groups in all animal models (Giladi et al., 2014; Schneiderman et al., 2010).
Suppressing cell division is the primary effect of combining TTFields and withaferin A (chemotherapy drug) in glioblastoma cancer therapy, as reported in detail in this study. This shows the dramatic inhibition of human glioblastoma cell growth, monitored through a bioluminescence test that progressed effectively by reducing cell number (Cosette et al., 2016) in agreement with other studies. The investigator believed that TTFields interfere with forming the mitotic spindle and affect polar molecules during telophase, preventing cell division (Zhu, 2017).
Furthermore, a combination of TTFields and chemotherapy in a different study was used to treat a newly diagnosed glioblastoma patient with high efficiency compared to temozolomide chemotherapy alone. Cognitive screening was improved in response to temozolomide and TTField at 150 kHz. Therefore, the combination of TTFields with paclitaxel represents a new hope for promising therapy in ovarian cancer patients. In vitro application of TTFields on humans has been described (Voloshin, 2016). Voloshin et al. (Voloshin, 2016) described the effect on ovarian cancer cells when cells were treated with TTFields 175 kHz and paclitaxel for 72 h. The cells exhibited a significant reduction in cell viability of more than 44.6% of the total cell count and a reduction in clonogenic potential (23.8%) in the whole-cell count. These cell numbers were significantly reduced in comparison to the negative control and blank cells (p < 0.001), as shown in Fig. 2, which adds more value to chemotherapy's efficiency.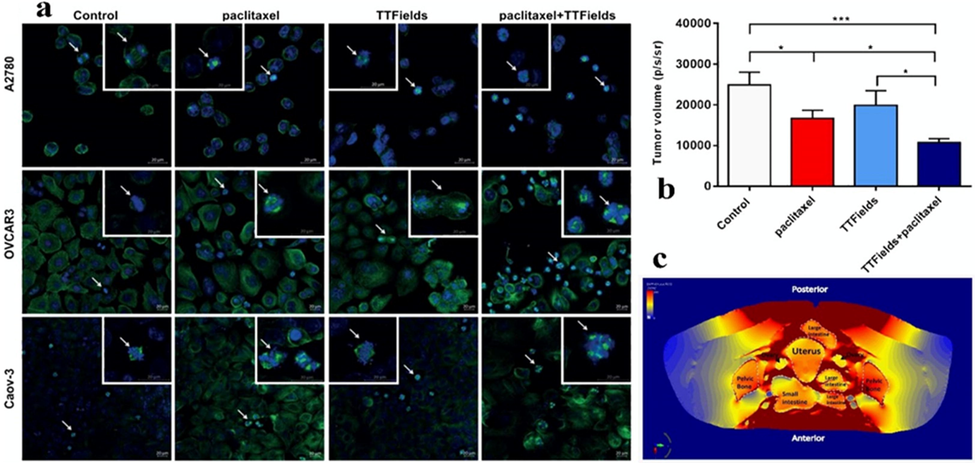
(a) Mitotic Figures shown using confocal fluorescence microscopy in A2780 cells for 8 hr, OVCAR-3 cells for 16 hr, and Caov-3 cells for 72 hr). The small micrographs represent the cell phases of metaphase and late anaphase. Green, tubulin, blue, DAPI-stained DNA. (b) The bioluminescent signal from tumors was used to predict tumor growth. (c) TTField intensity focused on the organ directly affected by the treatment (Voloshin, 2016). Figures reprinted with Creative Commons Attribution 2.0 (CC-BY-0.2) License from Ref. (Voloshin, 2016).
For the in vivo efficacy of C57Bl/6, the investigator injected MOSE-L FFL luciferase-positive cells that cause breast tumors in mice. Finite element mesh (FEM) simulations were performed using the Sim4life software package (ZMT, Zurich, Switzerland) to calculate the electric field intensities around the ovaries in addition to the injection of paclitaxel treatment.
In their results, the researcher demonstrated that the treatment of 200 kHz combined with paclitaxel resulted in a further reduction in the number of viable cells. The cell number reduction was calculated using the tumor luminescence test (40%, p < 0.01). These results confirmed a significant effect of TTField combined with paclitaxel in treating breast cancer cells in vivo . Furthermore, the tumor weight decreased compared to untreated tumor-bearing mice in the range of 55%, p < 0.05. Finite element mesh (FEM) simulations revealed that electric field strengths inside and in the area of the ovaries of an accurate human anatomy model are approximately 1 and 2 V/cm RMS above the minimum threshold required for the TTFields response(Munster, 2015; Vergote et al., 2017). These findings demonstrated that the effective combination of TTField and chemotherapy doses could play a vital role in inhibiting cancer cells.
11 Future potential and challenges of TTFields
Cancer therapy generally considers a standard protocol with measurable results and realistic data for chemotherapy and immunotherapy. In contrast, TTFields and the combination of TTField and chemotherapy, immunotherapy, or NPs are all advances with poorly traceable results, even though many studies are already done on the preclinical level with massive data. Therefore, an optimum method to assume or measure the efficacy of TTFields leads to a blind investigation period while receiving the treatment. The challenge is to optimize accurate processes that determine the efficiency of TTFields.
The limitation of TTFields could be observed in animal model research because there is no practical device that can reach the rodent brain. Delivering TTFields to rodent brains is not yet possible. Although. The FDA has approved many ranges for specific tumor cells, such as glioblastoma cells, using 200 kHz TTFields frequency, researchers can optimize higher frequencies or longer durations of exposure, but that cannot be permitted and causes limitations in the treatment (Chang, et al., 2018).
Different doses of effective drugs described in this review are combined with TTField. This combination works based on their pharmacodynamics and pharmacokinetics (Voloshin, 2016; Ballo et al., 2019; Bomzon et al., 2020). Such a general result will need to be further investigated in preclinical and clinical investigations. Ongoing research on phase III studies in TTFields has to add secondary endpoints that measure the effect of TTField on cancer organs compared to the impact on the whole human body in patients receiving TTFields compared to control patients.
On the other hand, the combination of TTField with immunotherapy was used in an early investigation and had a significant effect. TTFields improved treatment by adding to individual cytotoxic doses and specific immunogenic cell death. At the same time, a patient received immune checkpoint inhibitors (Mun et al., 2018). However, such combination therapy involving TTFields requires further evaluation (Mun et al., 2018).
Indeed, delivering an AMF or TTField directly to affected tissues using a specifically designed machine needs a more optimized and newer model of devices. In addition, the small amount of noncancerous tissue exposure in these different anatomic regions will continue to be an area of investigation.
Furthermore, performing such a changeable method in real-life drug treatment requires a restricted and scientifically established structure to apply TTFields doses representing clear data on how dose distribution impacts disease development in different malignancies (TTFields dosimetry). Bomzon et al. (Bomzon and Wenger, 2017) described several studies that discussed critical components related to TTFields dosimetry and treatment planning. These studies build the foundation for developing TTFields dosimetry and treatment planning (Bomzon et al., 2020). From that point, TTField has great potential in cancer therapy and will facilitate significant success in improving the effects of chemotherapy, radiotherapy, and immunotherapy.
The researcher's adaptive plan for each patient can be described in a few steps, starting with tumor imaging data determining the tumor position and building a specific model. Then, the treatment plan can be optimized using numerical stimulations followed by a physician's evaluation. After all these steps, the treatment can be initiated, followed by a subsequent imaging process to monitor the quality of treatment. Finally, the patient would continue the approved optimized treatment.
12 Conclusions
TTFields is noninvasive and advances therapy of solid tumors. The FDA has approved TTField alone and in combination with different types of chemotherapy and radiotherapy against glioblastoma. Meanwhile, continuing studies are investigating TTFields for various solid tumors, such as ovarian, NSCLC, pancreatic metastases from NSCLC, brain, and malignant mesothelioma. TTFields interrupts mitosis and rapidly kills cancer cells through continuous exposure (at least 18 h per day) in the range of 100–900 kHz. Optimizing the maximum IF for treating each type of cancer cell in several studies proved to be adjustable to reach the maximum anticancer therapy. In addition, the use of TTField reduces the induction of cytotoxicity to noncancerous human cells due to chemicals or radiation with a dose duration of fewer h of exposure.
Acknowledgments
This research was made possible by grant NPRP10-120-170-211 from the Qatar National Research Fund (QNRF). The statements made herein are the sole responsibility of the authors.
Declaration of Competing Interest
None.
References
- Carrieri, F.A., Smack, C., Siddiqui, I., Kleinberg, L.R., Tran, P.T., 2020. Tumor treating fields: at the crossroads between physics and biology for cancer treatment. Front Oncol 2020, 10.
- Tumor treating fields elicit a conditional vulnerability in non-small cell lung cancer cell lines through the down-regulation of key DNA repair and replication stress pathways that when targeted with chemoradiation results in synergistic cell killing. Int. J. Radiat. Oncol. Biol. Phys.. 2018;102(3):e184
- [Google Scholar]
- Adaptive response in mammalian cells exposed to non-ionizing radiofrequency fields: A review and gaps in knowledge. Mutat. Res. Mutat. Res.. 2014;760:36-45.
- [Google Scholar]
- Gene expression profiling of glioblastoma cell lines depending on TP53 status after tumor-treating fields (TTFields) treatment. Sci. Rep.. 2020;10(1):1-14.
- [Google Scholar]
- Alternating electric fields (tumor-treating fields therapy) can improve chemotherapy treatment efficacy in non-small cell lung cancer both in vitro and in vivo. Semin. Oncol.. 2014;41:S35-S41.
- [CrossRef] [Google Scholar]
- TTFields alone and in combination with chemotherapeutic agents effectively reduce the viability of MDR cell sub-lines that over-express ABC transporters. BMC Cancer. 2010;10(1):229.
- [Google Scholar]
- The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int. J. Nanomedicine. 2012;7:5577.
- [Google Scholar]
- Impact of magnetic field generated by wireless power transfer system of electric vehicle on retinal pigment epithelium cell in vitro. Asia-Pacific International Symposium on Electromagnetic Compatibility (APEMC). 2017;2017:385-387.
- [Google Scholar]
- Induction of adaptive response in human blood lymphocytes exposed to 900 MHz radiofrequency fields: Influence of cell cycle. Int. J. Radiat. Biol.. 2011;87(9):993-999.
- [Google Scholar]
- Alternating electric fields (tumor-treating fields therapy) can improve chemotherapy treatment efficacy in non-small cell lung cancer both in vitro and in vivo. Semin. Oncol.. 2014;41:S35-S41.
- [Google Scholar]
- Reliability of whole-exome sequencing for assessing intratumor genetic heterogeneity. Cell Rep.. 2018;25(6):1446-1457.
- [Google Scholar]
- Early exposure to intermediate-frequency magnetic fields alters brain biomarkers without histopathological changes in adult mice. Int. J. Environ. Res. Public Health. 2015;12(4):4406-4421.
- [Google Scholar]
- Adaptive response: some underlying mechanisms and open questions. Genet. Mol. Biol.. 2008;31(2):396-408.
- [Google Scholar]
- Mitotic spindle disruption by alternating electric fields leads to improper chromosome segregation and mitotic catastrophe in cancer cells. Sci. Rep.. 2015;5:18046.
- [Google Scholar]
- Alternating electric fields (TTFields) in combination with paclitaxel are therapeutically effective against ovarian cancer cells in vitro and in vivo. AACR 2015
- [Google Scholar]
- Tumor treating fields in the management of Glioblastoma: opportunities for advanced imaging. Can. Imag.. 2019;19(1):76.
- [Google Scholar]
- Using computational phantoms to improve delivery of Tumor Treating Fields (TTFields) to patients. In: 2016 38th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC). 2016. p. :6461-6464.
- [Google Scholar]
- Of fields and phantoms: the importance of virtual humans in optimizing cancer treatment with tumor treating fields. IEEE Pulse. 2017;8(4):46-49.
- [Google Scholar]
- Potent inhibition of tubulin polymerisation and proliferation of paclitaxel-resistant 1A9PTX22 human ovarian cancer cells by albendazole. Anticancer Res.. 2009;29(10):3791-3796.
- [Google Scholar]
- A phase I/II trial of Tumor Treating Fields (TTFields) therapy in combination with pemetrexed for advanced non-small cell lung cancer. Lung Can.. 2013;81(3):445-450.
- [Google Scholar]
- Vinorelbine and paclitaxel as first-line chemotherapy in metastatic breast cancer. J. Clin. Oncol.. 1999;17(1):74.
- [Google Scholar]
- Predictive factors for response to docetaxel in human breast cancers. Can. Sci.. 2006;97(9):813-820.
- [Google Scholar]
- Cytotoxicity of paclitaxel in breast cancer is due to chromosome missegregation on multipolar spindles. Sci. Transl. Med.. 2014;6(229)
- [Google Scholar]
- The effect of field strength on glioblastoma multiforme response in patients treated with the NovoTTFTM-100A system. World J. Surg. Oncol.. 2014;12(1):162.
- [Google Scholar]
- Disruption of cancer cell replication by alternating electric fields. Can. Res.. 2004;64(9):3288-3295.
- [Google Scholar]
- Intermediate frequency magnetic field at 250.8 kHz does not induce DNA damage or ‘Adaptive Response’ in vitro. Genet. Appl.. 2019;3(1):42-50.
- [Google Scholar]
- Genotoxic effects of intermediate frequency magnetic fields on blood leukocytes in vitro. Mutat. Res. Toxicol. Environ. Mutagen. 2019
- [Google Scholar]
- E. Chang et al., 2018. Tumor treating fields increases membrane permeability in glioblastoma cells. Cell Death Discov, 4.
- Alternating electric fields (TTFields) in combination with paclitaxel are therapeutically effective against ovarian cancer cells in vitro and in vivo. Int. J. Can.. 2016;139(12):2850-2858.
- [Google Scholar]
- Bioluminescence-based tumor quantification method for monitoring tumor progression and treatment effects in mouse lymphoma models. JoVE Journal Vis. Exp.. 2016;113:e53609
- [Google Scholar]
- Tumor Treating Fields (TTFields) plus anti-PD-1 therapy induce immunogenic cell death resulting in enhanced antitumor efficacy. AACR 2017
- [Google Scholar]
- ACTR-27. Compliance and treatment duration predict survival in a phase 3 EF-14 trial of tumor treating fields with temozolomide in patients with newly diagnosed glioblastoma. Neuro. Oncol.. 2017;19(Suppl)
- [Google Scholar]
- AMPK-dependent autophagy upregulation serves as a survival mechanism in response to Tumor Treating Fields (TTFields) Cell Death Dis.. 2018;9(11):1074.
- [Google Scholar]
- The many roles of NOX2 NADPH oxidase-derived ROS in immunity. Sem. Immunopathol.. 2010;32(4):415-430.
- [Google Scholar]
- Sorafenib induces apoptosis and autophagy in prostate cancer cells in vitro. Int. J. Oncol.. 2010;37(1):15-20.
- [Google Scholar]
- Preclinical overview of sorafenib, a multikinase inhibitor that targets both Raf and VEGF and PDGF receptor tyrosine kinase signaling. Mol. Cancer Ther.. 2008;7(10):3129-3140.
- [Google Scholar]
- A review of the mechanism of action and clinical applications of sorafenib in advanced osteosarcoma. J. Bone Oncol.. 2017;8:4-7.
- [Google Scholar]
- Functional biological activity of sorafenib as a tumor-treating field sensitizer for glioblastoma therapy. Int. J. Mol. Sci.. 2018;19(11):3684.
- [Google Scholar]
- Barium titanate nanoparticles Sensitise treatment-Resistant Breast cancer cells to the Antitumor Action of tumour-treating fields. Sci. Rep.. 2020;10(1):1-9.
- [Google Scholar]
- Preferential binding of positive nanoparticles on cell membranes is due to electrostatic interactions: a too simplistic explanation that does not take into account the nanoparticle protein corona. Mater. Sci. Eng. C. 2017;70:889-896.
- [Google Scholar]
- Uptake and intracellular localization of submicron and nano-sized SiO 2 particles in HeLa cells. Arch. Toxicol.. 2011;85(7):813-826.
- [Google Scholar]
- In vitro cytotoxicity of silver nanoparticles on osteoblasts and osteoclasts at antibacterial concentrations. Nanotoxicology. 2013;7(1):30-36.
- [Google Scholar]
- Cytotoxity of nanoparticles is influenced by size, proliferation and embryonic origin of the cells used for testing. Nanotoxicology. 2012;6(4):424-439.
- [Google Scholar]
- Strategies to improve homing of mesenchymal stem cells for greater efficacy in stem cell therapy. Cell Biol. Int.. 2015;39(1):23-34.
- [Google Scholar]
- Tumor-treating fields elicit a conditional vulnerability to ionizing radiation via the downregulation of BRCA1 signaling and reduced DNA double-strand break repair capacity in non-small cell lung cancer cell lines. Cell Death Dis.. 2017;8(3)
- [CrossRef] [Google Scholar]
- Health-related quality of life, cognitive screening, and functional status in a randomized phase III trial (EF-14) of tumor treating fields with temozolomide compared to temozolomide alone in newly diagnosed glioblastoma. J. Neurooncol.. 2017;135(3):545-552.
- [Google Scholar]
- INNOVATE: A phase II study of TTFields (200 kHz) concomitant with weekly paclitaxel for recurrent ovarian cancer—Updated safety and efficacy results. Am. Soc. Clin. Oncol. 2017
- [Google Scholar]
- Correlation of tumor treating fields dosimetry to survival outcomes in newly diagnosed glioblastoma: A large-scale numerical simulation-based analysis of data from the phase 3 EF-14 randomized trial. Int. J. Radiat. Oncol. Biol. Phys.. 2019;104(5):1106-1113.
- [Google Scholar]
- Tumor-treating fields at EMBC 2019: A roadmap to developing a framework for TTFields dosimetry and treatment planning. Brain Hum. Body Model.. 2020;2020:3-17.
- [Google Scholar]
- Tumor-treating fields: a fourth modality in cancer treatment. Clin. Can. Res.. 2018;24(2):266-275.
- [Google Scholar]







