Translate this page into:
Thermodynamics, a suitable reporter in the design of mercury (II) ion selective electrodes
⁎Corresponding author:. danil-de-namor@surrey.ac.uk (Angela F. Danil de Namor)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
A fully functionalised calix[4]pyrrole derivative, namely, meso-tetramethyl-tetrakis[(diethylthiocarbamoyl)phenoxy] calix[4]-pyrrole, 1 has been synthesised and structurally characterised. Its complexing properties with metal cations in acetonitrile were investigated with particular emphasis on the thermodynamics of these systems. These data and those previously reported for a partially functionalised calix[4]arene based receptor, 5,11,17,23-tetra-tert-butyl[25,27 bis(diethylthiocarbamoyl)oxy]calix[4]arene, 2 were used to, i) further corroborate their use to predict the selectivity coefficients of carrier mediated mercury (II) ion selective electrodes (ISEs) and ii) to obtain approximate stability constant data from selectivity coefficients. The optimum working conditions of both electrodes were determined. It is shown that while the ISE based on 1 is characterised by a wider linear range and a lower mercury (II) detection than the one based on 2, the latter has the advantage of lower interference of other metal cations due to the much lower stability constants of bivalent cations relative to Hg(II). The response characteristics of these electrodes are compared with those found in the literature. SEM micrographs along with EDX spectra of the PVC membranes containing ionophore 1 or 2 unloaded and loaded with Hg(II) are reported.
Keywords
Calix based Ionophores
Ion selective electrodes
Thermodynamics
Mercury (II)
1 Introduction
The negative impact produced by the presence of mercury species in the environment and their detrimental effect on human health is a topic extensively discussed in the literature (Park and Zheng, 2012; Rice et al., 2014; World Health Organisation, 2017; European Environment Agency, 2018a,b). The removal of mercury speciation from water and the development of monitoring systems for its quantitative detection are areas of considerable interest. The relatively low cost of ion selective electrodes (ISEs) and the possibility of their use ‘in situ’ make them attractive tools for assessing the presence of mercury in water.
Receptors based on Supramolecular Chemistry (Ganjali et al., 2006) have been the subject of considerable interest due to their selective properties for the target species and therefore these have been used in the design of ISEs. Among these receptors, calixarenes (Gutsche, 1978, 1990; Danil de Namor et al., 1998) and calixpyrroles (Gale, 2000; Sessler et al., 2000; Gale et al., 2001; Danil de Namor and Shehab, 2003; Danil de Namor et al., 2007a,b) offer advantages in that these can be easily synthesised and functionalised. Within this context, being mercury (II) a soft metal cation, receptors containing sulphur donor atoms offer a potential choice for the development of carrier-mediated ISEs. A number of sulphur containing calix[4] receptors have been reported (Lee and Lindsey, 1994; Jang et al., 2000; Danil de Namor and Abbas, 2007; Danil de Namor et al., 2007a,b). Among them a narrow-rim partially substituted p-tert-butylcalix[4]arene with thioacetamide functionalities namely 5,11,17,23-tetra-tert-butyl[25,27 bis(diethylthiocarbamoyl)oxy]calix[4]arene, 2 was reported by Danil de Namor and Pawlowski (2011) (Fig. 1). Detailed thermodynamic studies on the complexation of 2 with different cations in dipolar aprotic media were conducted. Investigations have been carried out on calix based receptors containing sulphur donor atoms for the development of carrier mediated Hg(II)ISEs (Lu et al., 2003; Danil de Namor, 2007; Tyagi et al., 2010; Abbas, 2012; Gupta et al., 2013). In all cases, the design of ISEs was based on qualitative rather than quantitative information regarding ion-receptor interactions. In a previous paper (Danil de Namor et al., 2018) we have demonstrated using two calix[4] based receptors that provided their conformation in solution and in the membrane are the same, from thermodynamic parameters of complexation of metal cations in a model solvent, the selectivity coefficient of cations relative to Hg(II) can be predicted without the need of constructing the electrode. When data using different receptors are available, the thermodynamics of cation complexation processes provide quantitative information regarding the most selective receptor for the target cation to be introduced in the membrane for the production of a carrier mediated ISEs. The aim of this paper is i) to further explore our previous findings by using a new calix[4]pyrrole derivative, namely, meso-tetramethyl-tetrakis[(diethylthiocarbamoyl)phenoxy] calix[4]pyrrole, 1 and its interaction with metal cations ii) to predict qualitatively the stability constant of 2 with metal cations in acetonitrile from selectivity coefficients of cations relative to Hg(II) using 2 as a carrier mediated ISE and the stability constant of Hg(II) and 2 in the model solvent. Therefore we report here the synthesis, structural and thermodynamic characterisation of 1 with metal cations as well as the design of two carrier mediated electrodes based on 1 and 2 ionophores.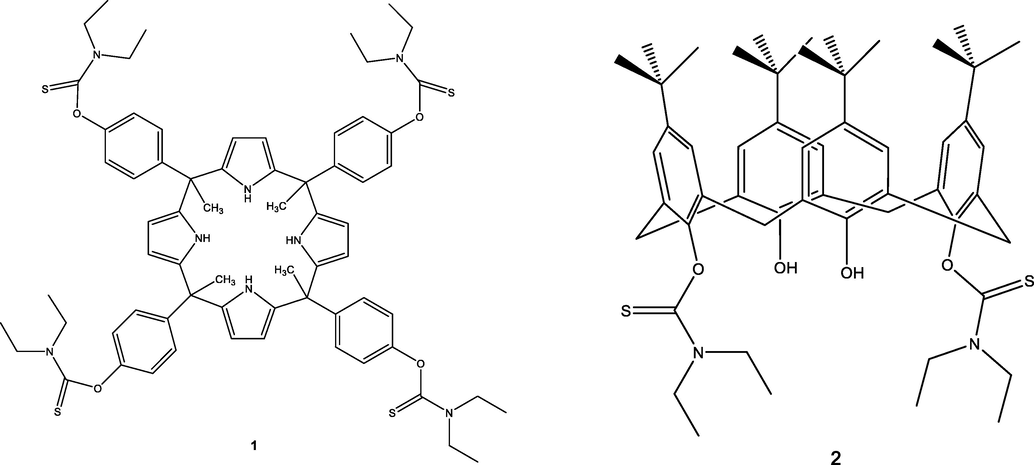
2D structures of 1 and 2.
2 Materials and methods
2.1 Chemicals
Pyrrole (C4H4NH, 99%), p-tert-Butyl calix[4]arene (C44H56O4, ≥97%) diethylthiocarbamoyl chloride ((C2H5)2NCSCl, 95%), 18-crown-6 (C12H24O6, 99%) [mercury (II) nitrate monohydrate (Hg(NO3)2·H2O, ≥99.99%), silver nitrate (AgNO3, ≥99%), 4′-hydroxyacetophenone (C8H8O2, 99%), methanesulfonic acid (CH3SO3H, ≥ 99.5%), sulfuric acid (H2SO4, 95–98%), acetone (C3H6O, 99%), dioctyl sebacate (CH2)8(COOC8H17)2, 2-nitrophenyl octyl-ether (C14H21ONO2, 99%), oleic acid (C18H34O2, ≥99%), polyvinyl chloride (CH2CHCl)n, methanol (CH3OH, HPLC grade, 99.7%) were purchased from Sigma-Aldrich. Hydrochloric acid (HCl, 35–38%), sodium hydroxide (NaOH, ≥97%), acetonitrile (CH3CN, HPLC grade, 99.99%), ethanol (C2H6O, reagent grade, 99.98% v/v), ethanoic acid (CH3COOH, glacial analytical reagent grade 99.7+ %) and potassium carbonate (K2CO3, 99+ %) were obtained from Fisher Scientific. Salts used throughout the study were placed in a vacuum oven and then stored in vacuum desiccators over phosphorus pentoxide, P4O10 for several days to remove water, before being used for experimental purposes. Deuterated solvents used in NMR experiments, acetonitrile‑d3 (CD3CN, 99.8%), acetone‑d6 (C2D6O, 99.9%), dimethyl sulfoxide-d6 (C2D6OS, 99.9%), methanol‑d4 (CD3OD, 99.8%), tetrahydrofuran‑d8 (C4D8O, 99.5%) and chloroform-d (CDCl3, 99.8% + 0.05% v/v TMS) were purchased from Cambridge Isotope Laboratories. For acetonitrile purification, the solvent was collected from PureSolv Micro solvent purification system (Inert Technology Inc. MA, USA) in a single-necked round-bottom flask containing activated 4 Å molecular sieves.
2.2 Synthesis of meso-tetramethyl-tetrakis[(diethylthiocarbamoyl)phenoxy] calix[4]-pyrrole, 1
The synthetic procedure used for the preparation of the ligand is described in Scheme 1 was prepared from meso-tetramethyl-tetrakis-(4-hydroxyphenyl)calix[4]pyrrole (CPI), (1.7 g, 2.3 mmol), 18-crown-6 (0.5 g, 1.9 mmol) and potassium carbonate (7.5 g, 54.3 mmol) using dry acetonitrile, MeCN (150 cm3) as solvent. The reaction mixture was stirred under a nitrogen atmosphere and refluxed for 1 h. Then, the content was cooled down after which diethylthiocarbamoyl chloride dissolved in MeCN (7.58 g, 50 mmol) was added gradually. The mixture was then refluxed at 70 °C and left overnight. TLC was used to monitor the progress of the reaction using a dichloromethane/methanol (9:1) mixture as the developing solvent system. After cooling, MeCN was removed under reduced pressure. The solid afforded was dissolved in dichloromethane, extracted several times with water saturated with sodium bicarbonate. The organic phase was separated and dried with magnesium sulphate, then filtered. The solvent was evaporated in vacuo and the obtained crude oil product dissolved in methanol. The solid material was filtered and re-crystallized from methanol. The obtained solid was filtered, washed with cold methanol and dried under vacuum at 60 °C. (0.68 g, 40% yield). The ligand was characterised by 1H NMR (500 MHz) at 298 K. The following signals (ppm) were observed: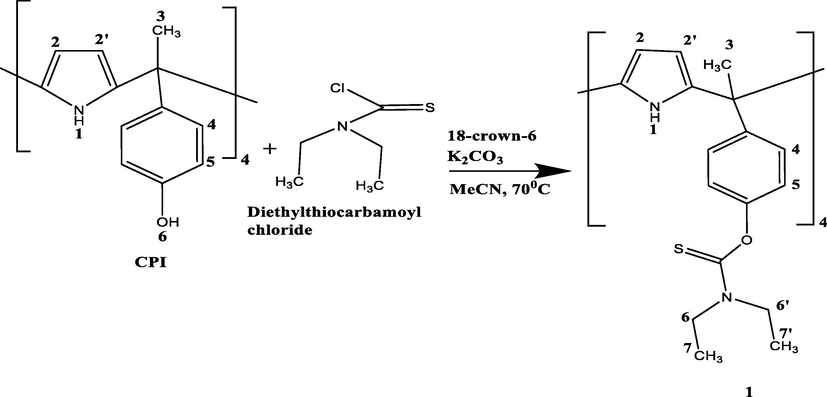
Synthetic procedure used for the preparation of 1.
1H NMR (500 MHz, DMSO‑d6, δ in ppm); 6.96 (s broad, NH, 4H (1)); 6.67 (d, J = 8.9, ArH, 8H (5)); 6.63 (d, J = 8.9, ArH, 8H (4)); 5.93 (s, pyrrole-H, 4H (2)); 5.92 (s, pyrrole-H, 4H (2′) 3.93 (q, J = 7.0, CSNCH2CH3, 8H (6)); 3.70 (q, J = 7.1, CSNCH2′CH3′, 8H (6′));1.71 (s, CH3, 12H (3)); 1.21 (t, J = 7.2, N-CH2-CH3, 12H (7)); 1.09 (t, J = 7.2, N-CH2′-CH3′, 12H (7′)). 1H NMR spectrum (S1) is given under Supplementary Material.
Elemental analysis was carried out in duplicate at the University of Surrey; (C68H80N8O4S4) MW. (1201.68); C68H80N8O4S4·H2O; Calculated %; C, 66.96; H, 6.80; N, 9.20. Found %; C, 66.60; H, 6.85; N, 9.38.
2.3 Synthesis of 5,11,17,23-tetra-tert-butyl[25,27 bis(diethylthiocarbamoyl)oxy]calix[4]arene, 2
The receptor was prepared as reported by Danil de Namor and Pawlowski (2011).
1H NMR (500 MHz, CD3CN, δ in ppm); 7.14 (s, ArH’, 4H (2)); 4.85 (s, ArH, 4H (2′)); 5.06 & 5.04 (s,s ArOH, 2H (4)); 3.41 (q, CSNCH2CH3, 4H (5)); 3.35(CSNCH2′CH3′, 4H (5′)); 3.29 (d, H-axial, 4H (3)); 3.41 (d, H-equatorial, 4H (3)); 1.14(t, CSNCH2CH3, (6)); 1.09 (t, N-CH2′-CH3′, (6′)); 1.23 (s, C(CH3)3), (1′)); 0.88 (s, C(CH′3)3, (1)). 1H NMR spectrum (S2) is given under Supplementary Material.
2.4 1H NMR complexation studies and calorimetric titration experiments have been carried out as previously reported (Chaaban et al., 2020)
Bruker DRX-500 pulse Fourier Transform NMR Spectrometer was used for 1H NMR complexation experiments. Runs were made at 298 K by dissolving the ionophore (1) (5 × 10−4 mol.dm−3) in CD3CN (deuterated acetonitrile), then adding the metal cation salt (2 × 10−3 mol·dm−3) into an NMR tube using TMS as an internal standard. Chemical shift changes (Δδ, ppm) were determined by subtracting the chemical shift of the free ionophore (reference spectrum) from the chemical shift of the complex.
For calorimetric titration experiments, samples (ionophore 1 and salts) were prepared in anhydrous acetonitrile. The titration runs were carried out at 298.15 K in Nano ITC2G calorimeter instrument, model 5300, TA Co.
2.5 Mercury (II) ion selective electrodes based on 1 and 2
The design of Hg(II)-ISEs was reported by Danil de Namor and co-workers (Danil de Namor et al., 2015). However, the construction of the Hg(II)-ISE setup employed in this study involves different ionophores (1 or 2). The preparation of the Hg(II)-ISE membranes and their behaviour under different conditions are described.
2.5.1 Hg (II)-ISEs cell assembly
The potential measurements (EMF, mV) of the fabricated Hg (II)-ISEs were recorded in a Fluke 73 Series III Digital Multimeter. The apparatus reads over a wide measurement range (1000 V) with a good precision. The multimeter was conjugated to an external reference electrode (silver/silver chloride that is equipped with a Flexible Connector (MF-2052, RE-5B) filled with sodium chloride solution (3 mol dm−3) and the designed internal reference electrode was a silver wire (6 cm × 0.15 mm) coated with a thin layer of silver chloride by an electroplating. The electrochemical cell assembly was as follows, Ag/AgCl/internal solution (1.0 × 10−2 mol dm−3 Hg(II)(nitrate as counter-ion) in 0.1 M KCl)/PVC membrane/test solution/KCl (std.)/Ag/AgCl.
The procedure used in the fabrication of mercury (II) selective membrane electrodes was previously described in the literature (Meier, 1982; Gale, 2000). Composition of PVC based membranes used in this work are summarised in Table 1. - o-NPOE: 2-Nitrophenyl octyl ether. - DOS: Dioctyl sebacate. -OA: Oleic acid. -PVC: Polyvinyl chloride. -Number of trials (N) = 4.
Electrode No.
Membrane mass composition, wt %
Ionophore
PVC %
Plasticiser
Additive
Name
%
Name
%
Name
%
E-1&E-6
–
–
30
o-NPOE
68
OA
1
E-2
1
1
30
o-NPOE
68
–
–
E-7
2
1
30
o-NPOE
68
–
–
E-3
1
1
30
DOS
68
OA
1
E-8
2
1
30
DOS
68
OA
1
E-4
1
2
30
o-NPOE
68
OA
1
E-9
2
2
30
o-NPOE
68
OA
1
E-5
1
1
30
o-NPOE
68
OA
1
E-10
2
1
30
o-NPOE
68
OA
1
2.5.2 Potential measurements of the Hg (II)-ISEs based on 1 and 2
In order to obtain the optimal potential response, the performance of the developed Hg (II)-ISEs was determined by the potential recorded across the prepared membrane during the addition of mercury nitrate solutions to a container with de-ionised water (75 cm3) at room temperature under a constant stirring rate (180 rpm). The analyte solutions were prepared in de-ionised water in the concentration range of 6.67 × 10−8 to 4.32 × 10−2 mol dm−3. Addition of the prepared standard solutions was made every three minutes from low to high concentrations, where emf values (mV) were recorded following each addition. Then the calibration curve was constructed by plotting the variation of the recorded potential vs the logarithm of the Hg(II) activity (log aHgII).
2.5.3 Effects of soaking, pH, dynamic response time and life-span on the Hg (II)-ISEs potential
The developed electrodes were soaked in aqueous solution of Hg(NO3)2 (1 × 10−2 mol dm−3) at room temperature for different periods of time (6, 12, 24, 30, 36 and 48 h). Calibration curves were established following each period and slope values were determined from each plot. These values (slope/mV pHg−1) were plotted as a function of time (hour).
The response of the Hg (II)-ISEs based on 1 and 2 over a wide range of pH (2–12) was examined at two different concentrations of Hg(NO3)2 (1.0 × 10−2 and 1.0 × 10−3 mol dm−3). The pH was adjusted by the addition of small drops of nitric acid (0.1 mol dm−3) and sodium hydroxide (0.1 mol dm−3). EMF values (mV) were recorded and plotted against the pH of the solution.
This experiment was achieved in the same electrochemical cell but at different concentrations of the Hg (II) cation salt (1 × 10−7, 1 × 10−6, 1 × 10−5, 1 × 10−4, 1 × 10−3 and 1 × 10−2 mol dm−3). EMF values (mV) were recorded after the addition of each concentration of the Hg (II) cation salt and the membrane potential was plotted against the dynamic response time to Hg (II). On the other hand, the electrode was immersed at two different concentrations of aqueous solution of Hg(NO3)2 (1 × 10−4 and 1 × 10−3 mol dm−3) respectively, and potential values were plotted as a function of response time to demonstrate the reversibility of the Hg (II) selective electrodes.
The life time of the Hg (II)-ISEs was examined over a period of four months. This was achieved my running a calibration experiment that involved the addition of the Hg(II) salt (6.67 × 10−8 to 4.32 × 10−2 mol dm−3 concentration range) over a period of time (1, 7, 20, 40, 60, 90 & 120 days) where the potential values were recorded. Slope values were calculated from the calibration curves after which they were plotted as a function of time (days).
2.5.4 Selectivity measurements: effect of interfering ions
The matched potential method (MPM) recommended by IUPAC (International union of pure and applied chemistry, 2000), was used to assess the potentiometric selectivity coefficients of the membrane electrode. Thus, the concentration of the reference solution (Hg(NO3)2) was 1 × 10−4 mol dm−3, while that of the primary ion was 1 × 10−2 mol dm−3 whereas the foreign ions concentration was 1 × 10−1 mol dm−3. The potential change (ΔE) was recorded for the primary ion ( ) as the addition of the interfering ion ( ) was made until the same potential difference (ΔE) was obtained.
2.5.5 Analytical application of 1 and 2 based Hg(II) ISEs
The use of the fabricated Hg(II)-ISEs based on the ionophores 1 and 2 as indicator electrodes for the potentiometric determination of Hg(II) ion in the dental amalgam filling samples were carried out as previously reported (Danil de Namor et al., 2015).
3 Results and discussion
3.1 1H NMR investigations on the complexation of 1 with metal cations in CD3CN at 298 K
In the present study, recognition sites were introduced through the substituted pendant arms which include phenolic oxygens in addition to sulphur, nitrogen and oxygen donor atoms from the thioacetamide functionalities.
Therefore, 1H NMR measurements were conducted to assign the sites of interaction of 1 with metal cations in a protophobic dipolar aprotic solvent (CD3CN) at 298 K. Chemical shift changes (Δδ, ppm) in the 1H NMR spectrum of 1 after the addition of metal cations relative to the free ligand in CD3CN at 298 K are listed in Table 2. No chemical shift changes were observed by the addition of alkali-metal cations. Thus 1 displays significant downfield shifts in H-4, H-6, H-6′, H-7 and H-7′ protons by the addition of the silver salt (nitrate as counter-ion) suggesting an interaction of Ag(I) with the sulphur donor atoms of the thioacetamide moiety. However for the alkaline-earth metal cations, small downfield Δδ values upon the addition of Mg(II) (H-7 & H-7′), Ca(II) (H-7 & H-7′), Sr(II) (H-7) and Ba(II) (H-7) are observed which are indicative of a relatively weak interaction occurring between these cations (hard cations) and the amide functionality of the receptor which seems to decrease down the group. [Mn+] = 1.10 × 10−3 mol·dm−3; V = 0.5 cm3. [1] = 2.17 × 10−4 mol·dm−3; V = 0.5 cm3.
δ (ppm)
H-1
H-2
H-2′
H-3
H-4
H-5
H-6
H-6′
H-7
H-7′
δRef
8.06
6.079
6.074
1.88
6.75
6.94
3.82
3.62
1.13
1.06
Mg(II)
−0.03
−0.01
−0.05
−0.01
−0.02
−0.01
0.01
0.01
0.1
0.1
Ca(II)
−0.07
−0.02
−0.03
−0.03
−0.01
−0.02
0.01
0.04
0.1
0.11
Sr(II)
−0.04
−0.02
−0.06
−0.01
−0.02
−0.02
0.00
−0.01
0.1
0.08
Ba(II)
−0.1
−0.02
−0.06
−0.02
−0.03
−0.03
0.00
−0.02
0.1
0.07
Ag(I)
−0.08
−0.02
−0.03
0.08
0.2
−0.08
0.13
0.1
0.2
0.2
Zn(II)
−0.04
−0.01
−0.02
−0.01
0.17
0.01
−0.02
0.15
0.08
0.05
Pb(II)
0
0.03
0.03
0.2
0.17
0.02
0.03
0.04
0.1
0.18
Ni(II)
0.11
0.18
0.14
0.05
0.14
0.21
0.2
0.18
0.3
0.27
Cd(II)
0.28
0.01
0.00
−0.52
0.2
0.16
0.14
0.24
0.1
0.00
Hg(II)
−0.1
0.75
0.66
−0.63
0.43
0.3
0.27
0.21
0.04
0.04
Deshielding of protons (H-5) and (H-6) were observed upon the addition of Zn(II) revealing an interaction with the ligand. However, a precipitate was observed in the NMR tube that is attributed to the formation of a complex of lower solubility than that of the free receptor in this solvent. Significant downfield chemical shift changes were also seen in most of the ligand protons particularly H-4, H-5, H-6 and H-6′ upon the addition of Ni(II), Cd(II) and Hg(II). Although the shielding effect observed for the latter two cations in H-3 is not observed for Ni(II). These changes in chemical shifts suggest that phenolic oxygen, nitrogen and particularly sulphur donor atoms are likely to be the sites of ligand interaction with the mentioned metal cations. However, the largest changes in the chemical shifts of 1 are found by the addition of the Hg(II) salt.
Based on this information we proceede with thermodynamics studies described below.
3.2 Thermodynamic parameters of complexation of 1 with metal cations in acetonitrile at 298.15 K
1H NMR measurements showed the interaction of 1 with metal cations in acetonitrile. Table 3 lists stability constant (log Ks) and derived standard Gibbs energies (ΔcG0), enthalpies (ΔcH0) and entropies (ΔcS0) of complexation for alkaline-earth metal cations (Mg(II), Ca(II), Sr(II) and Ba(II)), transition (Ni(II), Co(II) and Ag(I)) and heavy metal cations (Cd(II), Pb(II) and Hg(II)) and 1 in acetonitrile at 298.15 K. Standard deviations of the data are also listed in the Table. The thermodynamic data for 1 with cations except for Hg(II) fit into a 1:1 (ligand: metal cation) model (Eq. (1)) whereas data for 1 with Hg(II) fit into a 1:2 model (Eq. (2)). As pointed out in previous publications (Danil de Namor et al., 2018) it may be argued that thermodynamics does not give structural information but it is indisputable that any model proposed must fit the experimental thermodynamic data.
1
Cation
(
)
log KS
(kJ mol−1)
(kJ mol−1)
(J mol−1 K−1)
Ag(I)
1:1
3.60 ± 0.01
−20.6 ± 0.2
−45.7 ± 0.1
−84
Mg(II)
1:1
4.56 ± 0.05
−26.0 ± 0.1
−30.0 ± 0.3
−13
Ca(II)
1:1
4.06 ± 0.07
−23.2 ± 0.2
−8.1 ± 0.3
50
Sr(II)
1:1
3.8 ± 0.2
−21.7 ± 0.5
−14.8 ± 0.5
25
Ba(II)
1:1
3.69 ± 0.02
−21.1 ± 0.1
−13.66 ± 0.04
25
Co(II)
1:1
5.20 ± 0.06
−29.7 ± 0.4
−65.8 ± 0.3
−121
Ni (II)
1:1
4.88 ± 0.05
−27.9 ± 0.3
−68.7 ± 0.5
−136
Zn(II)
Precipitation of the Complex
Cd(II)
1:1
5.9 ± 0.1
−33.5 ± 0.2
−79.7 ± 0.5
−155
Pb(II)
1:1
5.21 ± 0.03
−29.7 ± 0.1
−79.5 ± 0.4
−2
Hg(II)
1:1
6.98 ± 0.04
−39.4 ± 0.1
−43.4 ± 0.1
−13
1:2
5.00 ± 0.04
−28.4 ± 0.1
−14.7 ± 0.1
46
(1 + 2)
11.98 ± 0.04
−67.8 ± 0.1
−58.1 ± 0.1
33
In these equations MII+ denotes the bivalent metal cation.
Stability constant values show that the relatively small chemical shift changes observed in the 1H NMR spectrum of 1 with alkaline-earth metal cations with H-7 and H-7′ were indicative of complex formation possibly with oxygen and nitrogen donor atoms of the pendant arms. It is observed that the trend followed is in accord with the charge density of these cations with the highest selectivity for Mg(II) but very poor selectivity for the remaining alkaline-earth metal cations. The small difference observed in stability constants of the latter cations in this solvent led to the conclusion that there is a high degree of enthalpy-entropy compensation effect (Ryde, 2014). Again the receptor hardly distinguishes between Co(II), Ni(II) and Pb(II) in MeCN since the differences in the log Ks values are relatively small. It seems that the significant chemical shift changes observed in the1H NMR spectra for addition of these cations to 1 are reflected in the enthalpy which is the parameter contributing to their stability. Moreover, the highest stability constant value for 1:1 complexes are shown for Hg(II) followed by Cd(II). Therefore, the stability constant findings show that 1 is not only selective for Hg(II) but in addition has a higher hosting capacity for this cation. It should be noted that the higher capacity of 1 relative to 2 must be attributed to the fact that the former is fully functionalised while the latter is partially functionalised and consequently the stability constant for the 1:1 complex of 1 for Hg(II) is expected be higher than corresponding data for 2. This statement is based on the fact that for 1 to host two Hg(II) cations, each one will interact with neighbouring arms while for 2 the cation will interact with alternate functional groups. This is corroborated by the higher stability constant of the Hg(II)1 complex in MeCN shown in Table 3 relative to that for the Hg(II)2 complex in the same solvent (log Ks = 4.5) (Danil de Namor and Pawlowski, 2011). As far as Ag(I) and this receptor is concerned, a high stability is not found which was expected given the higher solvation of this cation in this solvent as assessed from the transfer Gibbs energy of this cation from H2O to MeCN = -21.8 kJ mol−1 (data based on the Ph4As Ph4B convention) (Cox et al., 1982). This is reflected in the lower enthalpic stability and lower entropy loss relative to other cations such as Ni(II), Co(II), Pb(II) and Cd(II).
Inspection of Table 3 reveals that the complexation process of 1 with metal cations in MeCN is enthalpically controlled ( except for Ca(II) cation where it is entropically favoured. For Sr(II) and Ba(II), although the processes are enthalpically controlled, the entropy contributes favourably to the stability of the complex in acetonitrile. It is interesting to assess the complexation enthalpies and entropies for the Hg(II) and this receptor in acetonitrile in that the formation of the 1:1 complex follows the expected trend in that there is a loss of entropy as two species, host and guest interact to form one species, namely the complex while the enthalpy is responsible for the high stability observed for the complex. However the formation of the 1:2 complex is favourable in terms of enthalpy and entropy to the point that their contribution to the stability of the complex is about the same. It is quite clear that the entrance of a second cation will produce a significant disorder in the system.
The role played by the solvation of the cation (free and complex) to the binding process and to a lesser extent, the receptor (neutral molecule) has been emphasised in a number of papers by Danil de Namor and co-workers (Danil de Namor and Abbas, 2007; Danil de Namor et al., 2007; Danil de Namor and Khalife, 2008; Danil de Namor and Pawlowski, 2011). In an attempt to find if the free cation plays a significant role in the complexation of these cations with this receptor as previously demonstrated for some systems by this Group, the standard Gibbs energies of complexation shown in Table 3 are plotted against corresponding data for the hydration of these cations, Δhyd.G◦. Strictly speaking the standard Gibbs energies of solvation, ΔsolvG◦ should be considered. This parameter can be calculated from Eq. (3)
In Eq. (3), ΔtG◦ (M (II) (H2O → MeCN) denotes the standard transfer Gibbs energy of the cation from water to acetonitrile (Data based on the Parker convention). Gibbs energies in Eq. (3) correspond to the following processes, respectively
The limitation is that there are very few data on the ΔtG◦ for bivalent cations from H2O to MeCN. However the trend of solvation and hydration would be the same, although the individual values will change slightly due to the medium effect. This statement is based on the fact that the contribution of the transfer parameter is very small as compared to that of hydration. Fig. 2 is a plot of ΔcG◦ (data from Table 3) against Δhyd.G◦. As can be seen there is a selectivity peak for Hg(II). There are two predominant processes in the binding of macrocycles with guest species, binding and cation de-solvation upon complexation. Interpretation of the data in this Figure demonstrates that from Ba(II) to Hg(II) the binding process predominates and as result the stability of the complex increases significantly up to Hg(II). However as the solvation of the cation increases from Hg(II) to Mg (II), the stability of the complex decreases. It should be emphasised that as cation solvation increases, the competition of the receptor and the solvent for the cation increases. Essentially if the cation sits comfortably in the solvent it becomes more reluctant to interact with the receptor. It is quite clear that the selectivity peak observed in terms of Gibbs energies is not controlled only by the enthalpy given that the highest enthalpic stability is found for Cd(II) and Pb(II). It is therefore concluded that the selectivity peak observed in terms of Gibbs energies (hence complex stability) results from both, the enthalpy and the entropy of these system but there is no doubt that the solvation of the cation plays a significant role in the complexation process. To a lesser extent; the complex and the ligand solvation are likely to have an effect on the complexation process.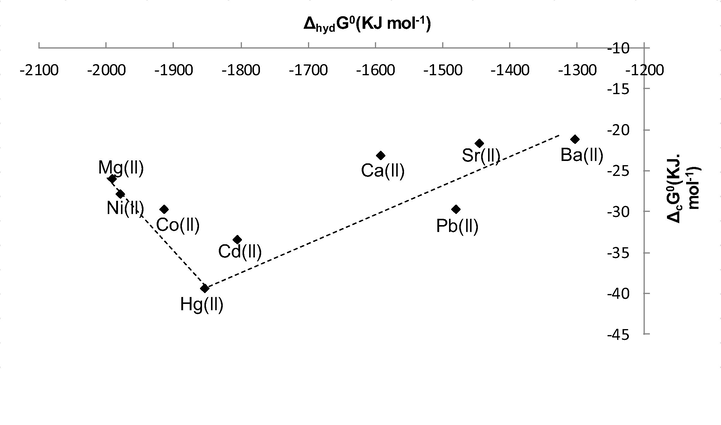
Plot of Standard Gibbs Energies of complexation (ΔcG0) of 1 and bivalent metal cations in acetonitrile at 298.15 K against Gibbs Energies of hydration (ΔhydG0).
Following fundamental studies on the interaction of 1 and metal cations and previously reported data on receptor 2 (Danil de Namor and Pawlowski, 2011) attempts were made to use these receptors for the design of ISEs.
3.3 Hg(II)ISEs based on 1 and 2 receptors
Based on the above discussion the following steps were undertaken to investigate whether or not fundamental studies in MeCN allow to predict the behaviour shown by the receptor based membrane towards Hg(II) relative to other cations.
3.3.1 Potentiometric response of carrier mediated ISEs towards Hg(II) as function of the membrane composition
The potentiometric characteristics (slope, linear range, detection limit and response time) of the electrodes towards Hg(II) were investigated for five membranes with different compositions and mixing ratios of 1 and 2, plasticizer and additive while a fixed amount of PVC was used.
Data on the response characteristics for the investigated Hg(II)-ISEs are listed in Table 4 and calibration plots for 1 (E1- E5) and 2 (E6-E10) are presented in Fig. 3a and b respectively.
Slope
(mV/pHg)Linear Range
LR (mol dm−3)Detection Limit
DL (mol dm−3)Response Time
RT (sec.)
E-1
No ionophore, 68% o-NPOE, 30% PVC, 1% OA
6.7 ± 0.5
7.10 × 10−4 −1.34 × 10−3
3.48 × 10−4
25 ± 2
E-2
1% 1, 68% o-NPOE, 30% PVC, no additive
15.9 ± 0.3
3.48 × 10−4-1.34 × 10−3
1.67 × 10−4
20 ± 2
E-3
1% 1, 68% DOS, 30% PVC, 1% OA
21.8 ± 0.7
1.13 × 10−6- 1.51 × 10−2
6.78 × 10−7
15 ± 2
E-4
2% 1, 68% o-NPOE, 30% PVC, 1% OA
27.8 ± 0.6
6.78x 10−7- 3.48 × 10−4
1.36 × 10−7
13 ± 1
E-5
1% 1, 68% o-NPOE, 30% PVC, 1% OA
29.4 ± 0.3
6.78 × 10−7- 1.34 × 10−3
3.16 × 10−7
11 ± 1
E-6
No ionophore, 68% o-NPOE, 30% PVC, 1% OA
6.1 ± 0.1
4.75 × 10−6-3.48 × 10−4
2.03 × 10−6
30 ± 2
E-7
1% 2, 68% o-NPOE, 30% PVC, no additive
16.7 ± 0.3
1.38 × 10−5-1.67 × 10−4
4.75 × 10−6
20 ± 2
E-8
1% 2, 68% DOS, 30% PVC, 1% OA
18.2 ± 0.1
1.13 × 10−6-7.71 × 10−5
6.78 × 10−7
15 ± 1
E-9
2% 2, 68% o-NPOE, 30% PVC, 1% OA
20.7 ± 0.6
1.13 × 10−6-3.48 × 10−4
6.78 × 10−7
13 ± 1
E-10
1% 2, 68% o-NPOE, 30% PVC, 1% OA
29.7 ± 0.3
1.13 × 10−6-1.34 × 10−3
6.78 × 10−7
10 ± 1
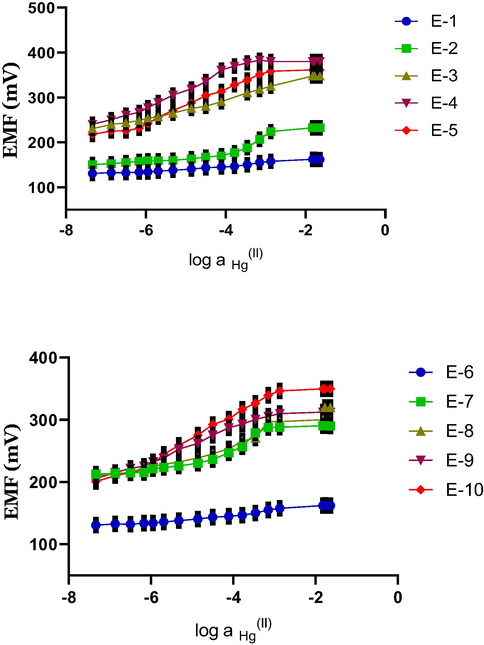
Effect of different membrane compositions on the potential responses of ISEs based on t ionophores 1 and 2. Number of trial (N) = 4.
In the absence of the ionophore (E1 and E6, blank experiments) the response characteristics displayed no selectivity towards the Hg(II) cation. In the absence of additive (E 2 and E 7) the response characteristics (slope, linear range, detection limit and response time) of the Hg(II)-ISE along with the selectivity towards Hg(II) were negatively affected. As previously shown (Tavakkoli and Shamsipur, 1996; Fakhari et al., 1997) the presence of the lipophilic additive can reduce the resistance of the membrane, reduce ionic interference and thereby improve the sensitivity towards Hg(II) cation. However, this additive (oleic acid) was selected for this study since unlike sodium tetraphenylborate (Danil de Namor et al., 2015). It showed no interaction with Hg(II) cation.
The nature of the plasticiser was examined using membrane electrodes either with dioctyl sebacate, DOS (E-3 and E8) or o-nitrophenyl octyl ether, o-NPOE (E-5 and E10). These plasticisers showed no interactions with Hg(II) (Danil de Namor et al., 2015). This is a very important point to emphasise because if there is an interaction of the plasticizer with Hg(II) this is most likely to have an impact on the selectivity coefficient and would reflect not only the selectivity of the receptor for Hg(II) but also that of the plasticiser for this cation. Results showed the best response behaviour of the electrode was that involving the o-NPOE plasticiser (E-5 and E10) showing for 1 a slope value of 29.4 ± 0.3 mV decade−1 (near Nernstian behaviour of a slope value of 29.5 mV decade−1), a linear range of 6.78 × 10−7- 1.34 × 10−3 mol dm−3, a detection limit of 3.16 × 10−7 mol dm−3 and response time of 11 ± 1 sec. For 2 the best performance in the ISE was seen in E-10 with membrane composition of 2 (1%), o-NPOE (68%), PVC(30%) and OA (1%). The slope value for this membrane was 29.7 ± 0.3 mV decade−1, close to the value expected from Nernst equation, detection limit of 6.78 × 10−7 mol dm−3, linear range of 1.13 × 10−6-1.34 × 10−3 mol dm−3 Hg(II) and a response time of 10 ± 1 sec. So far advantages of the E-5 membrane containing 1 relative to E-10 containing 2 are the wider linear range and the lower activities that can be detected for the former relative to the latter. However the electrode response time is approximately the same for both membranes. E5 and E 10 were selected for further investigations. It is generally found that the use of o-NPOE as plasticizer leads to a longer linear section in the calibration curve and a lower detection limit than the use of DOS as plasticizer (Bedlechowicz and Hulanicki, 2002).
3.3.2 Effect of solution pH at different internal solution concentration, soaking, response time and reversibility of the Hg(II)ISEs
See Supplementary Material for interpretation.
3.3.3 Selectivity of 1 and 2 based Hg(II)ISEs
Selectivity coefficients (
) of the Hg(II)-ISEs based on 1 and 2 determined by the Matched Potential Method recommended by IUPAC are shown in Table 5. Conditions: reference solution: 1.0 × 10−4 mol dm−3 Hg(NO3)2. Primary ion (A): 1 × 10−2 mol dm−3 Hg(II). Interfering ions (B): 1 × 10−1 mol dm−3.
Matched Potential Method (MPM)
(Ionophore 1)
(Ionophore 2)
Mg(II)
1.2 × 10−3
−2.88
1.01 × 10−3
−2.99
Ca(II)
1.18 × 10−3
−2.92
2.17 × 10−3
−2.66
Sr(II)
5.92 × 10−4
−3.23
5.26 × 10−4
−3.28
Ba(II)
4.38 × 10−4
−3.36
3.94 × 10−4
−3.40
Cd(II)
7 × 10−2
−1.15
2.96 × 10−4
−3.53
Cu(II)
5.16 × 10−4
−3.29
7.2 × 10−4
−3.14
Zn(II)
3.8 × 10−4
−3.42
2.07 × 10−4
−3.68
Pb(II)
2 × 10−2
−1.70
1.13 × 10−3
−2.95
Ni(II)
1 × 10−2
−2.0
1.18 × 10−3
−2.93
Co(II)
1 × 10−2
−2.0
2.37 × 10−4
−3.63
Mn(II)
2.22 × 10−4
−3.65
1.98 × 10−4
−3.7
Al(III)
4.79 × 10−4
−3.32
2.15 × 10−4
−3.67
Fe(III)
3.25 × 10−4
−3.49
6.63 × 10−4
−3.18
It can be observed from these values that the electrode showed a high selectivity to Hg(II) with no significant interference from other cations particularly for the ISE containing receptor 2. In fact for the ISE containing 1, the selectivity coefficients for Ni(II), Pb(II) and Cd(II) follow the same pattern as that found from 1HNMR in that the significant chemical shifts indicating ion-receptor interactions leads to a decrease (less negative) in the selectivity coefficients for these cations relative to Hg(II). This also applies to the ISE containing 2 in that among the bivalent cations investigated in solution, this receptor interacts only with Hg(II).
The following correlation (Eq. (7)) involving the difference between the stability constant of a given cation and the selectivity coefficient of the same cation, both relative to mercury (II) was previously demonstrated for two calix[4] based receptors and now this correlation is shown for 1 (Table 6). As pointed out before (Danil de Namor et al., 2018), validity of Eq. (7) requires that the conformation of the receptor is not altered from that in solution by its incorporation in the IS membrane. We have also explained previously that i) the transfer of the ion from water to the membrane phase must be related to the response time of the electrode rather than to the equilibrium potential and ii) ion pair formation in the membrane phase will lead to a non- linear response of the electrode. Therefore these two issues do not seem to present any problem regarding the selectivity of the electrode in acetonitrile and in the membrane phase (Danil de Namor et al., 2018).
1
Δ (log Ks)
Mg(II)
−2.88
−2.42
Ca(II)
−2.92
−2.92
Sr(II)
−3.23
−3.18
Ba(II)
−3.36
−3.29
Zn(II)
Precipitation of the complex
Cd(II)
−1.15
−1.08
Hg(II)
0
0
Pb(II)
−1.70
−1.77
Ni(II)
−2
−2.1
Co(II)
−2
−1.78
A direct implication of Eq. (7) is that selectivity coefficients of carrier mediated ISEs involving calix[4] receptors can be obtained from thermodynamic investigations without the necessity of constructing the electrode which as stated previously is a time consuming process. In order to explore further the validity of this equation with other calix[4] based carrier ISEs, Eq. (7) is applied using stability constant data for 1 and Hg(II) (Table 3) and selective coefficients for the same receptor from Table 5. Stability constants for other metal (II) complexes can be calculated.
As far as the Hg(II) ISE based on receptor 2 is concerned, application of Eq. (7) using the stability constant data previously reported in the literature (log Ks = 4.5 in MeCN at 298.15 K, Danil de Namor and Pawlowski, 2011) and the selectivity coefficients for this receptor and metal cations relative to Hg(II) given in Table 5, approximate values for stability constants of other cations and 2 are calculated and these are shown in Table 7. These data show that very weak complexes are formed to an extent that no chemical shift changes were observed in 1H NMR studies previously reported in CD3CN. Again log Ks values lower than 2 are below the lower limit of detection of calorimetry.
2
log Ks
Mg(II)
1.51
Ca(II)
1.84
Sr(II)
1.22
Ba(II)
1.10
Zn(II)
0.82
Cd(II)
0.99
Pb(II)
1.55
Ni(II)
1.57
Co(II)
0.87
3.3.4 SEM-EDX studies of ISE membranes based on 1 and 2
SEM micrographs along with EDX spectra of the PVC membranes containing ionophore 1 or 2 unloaded (4a & 4c) and loaded with Hg(II) (4b & 4d). The SEM micrograph (4a) validates a uniform microporous membrane with honeycomb structure before being superimposed in the ISE with no particles or dark patches. EDX spectrum shows the bulk elemental composition of the membrane grafted with the receptor. The relative peak heights of these elements in the membrane are correlated with their corresponding concentrations. The scanning electron micrograph (4b) shows distorted micropores indicating ageing of the membrane. The EDX spectrum shows a remarkable reduction in the heights of O Kα, N Kα, and Cl Kα with the appearance of new characteristic peaks of Hg, which provides clear evidence of the cation assembly to the hydrophilic binding sites of the ionophore. The reduction in the Cl peak is justified by the leaching of anionic impurities in the membrane support (Park et al., 2001).
As far as SEM images of PVC membranes with ionophore 2 is concerned, significant morphological changes in both micrographs (4c & 4d) are clearly seen with uniform micropores and no dark areas in the control membrane (c). On the other hand, white patches spreading over the membrane surface was observed in Fig. 4d indicating the presence of Hg(II) cation. The EDX analysis of the scanning electron micrograph (Fig. 4d) displayed mercury characteristic peaks in the membrane components confirming the presence of the analyte in the IS membrane.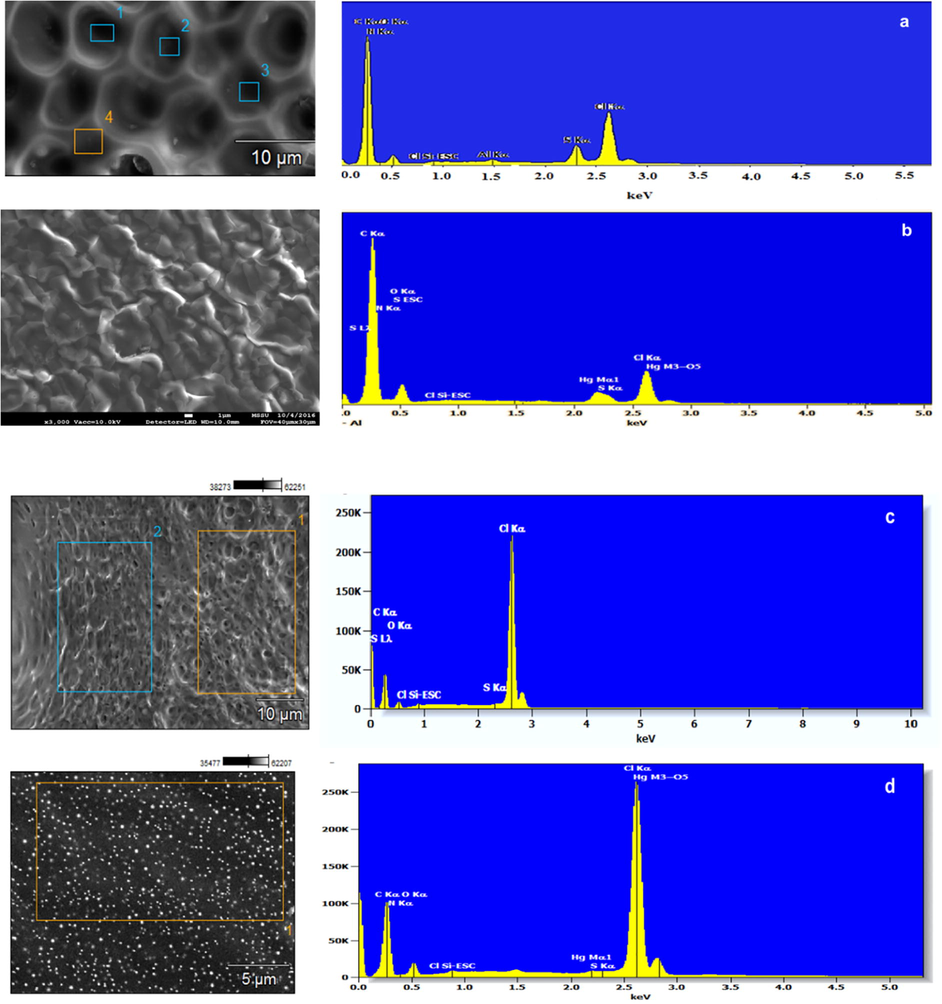
SEM micrographs coupled with EDX spectra of control membranes (a) & (d) and membranes used for sensing Hg(II) as nitrate salts in water (b). Aluminium peak (Al) in the spectra referred to the aluminium stub used for samples’ mounting.
3.3.5 Testing the electrodes
A number of analytical techniques are available for the quantitative determination of Hg (II). Among them are Cold-Vapour Atomic Absorption Spectrometry, CV-AAS, Ion Coupled Plasma Spectroscopy, ICP-OES and others (Bagheri and Gholami, 2001; Ferrúa et al., 2007; Martinis et al., 2009; Jiang et al., 2010; Zavvar Mousavi et al., 2010). These techniques are not always available in Developing Countries due to their high cost and maintenance. In addition samples need to be transported from the site to the Laboratory. This is not the case with ISEs which also have the advantage of analysing samples ‘in situ’. The Ion Selective electrodes described in this paper were tested by measuring the amount of Hg(II) in a filling dental amalgam provided by Nordiska Dental AB Industries (Sweden) and the results compared with those obtained by Cold Vapour-Atomic Absorption Spectrometry (CV-AAS) are shown in Table 8. The findings obtained by both electrodes were found to be in good agreement with those obtained by the CV-AA. Accordingly, the electrodes are good alternative for Hg(II) quantitative determination in real dental amalgam samples.
Ionophore
Mercury(II) concentration (mol.dm−3)
CV-AAS
mol. dm−3
Hg-ISE
mol.dm−3
Recovery (%)
1
1.00 × 10−5
9.81 × 10−6
98.1
2
1.00 × 10−5
9.62 × 10−6
96.2
3.3.6 Response characteristics comparison of 1 and 2 and with other reported calix[4]pyrroles, calix[4]arenes based Hg(II) ISEs and other macrocycles
As mentioned earlier, 1 and 2 have the same pendant arms (thioacetamide) but the former is fully functionalised while the latter is partially functionalised and their fundamental studies revealed high selectivity for Hg(II) particularly for 1 in the formation of the 1:1 complex. In terms of response characteristics of Hg(II)-ISE based on these two ionophores, both electrodes (E-5 & E-10) displayed a Nernstian behaviour. However, E-5 based on 1 showed a slightly lower detection limit (3.16 × 10−7 mol dm−3) and a wider linear range (6.78 × 10−7 to 1.34 × 10−3 mol dm−3) than E-10 based on 2. The response time of both electrodes is practically the same (11 ± 1 sec for E-5 and 10 ± 1 sec for E-10). Having stated it the ISE based on 2 has the advantage of interacting weakly with other bivalent cations relative to Hg(II) and therefore interference with other cations are unlikely to occur.
Calix[4]pyrrole based Hg(II) ISEs are rarely reported in the literature. Calix[2]thieno[2]pyrrole and meso-tetramethyl-tetrakis-(4 N,N-diethylacetamide)phenoxymethylcalix[4]pyrrole were used as ionophores for Hg(II)-ISEs (Danil de Namor, 2007; Abbas, 2012; Danil de Namor et al., 2015). However, the former was excluded from the comparative study due to the use of NaBPh4 additive in the PVC membrane, which as reported before (Danil de Namor et al., 2015), interacts with Hg(II). Therefore it is not possible to assess whether or not the electrode response to Hg(II) is due entirely to the ionophore or is the result of the contribution (positive or negative) of the additive and the cation. On the other hand, the calix[4]pyrrole amide ionophore based Hg(II)-ISE showed similar response characteristics for the slope value (30 ± 1 mV decade−1) and response time (10 sec.) with 1 based Hg(II)-ISE (E-5) but a slightly lower detection limit and a slightly wider working concentration range than 2 (see Table 4).
The Hg(II)ISEs based on a thia-bridge modified calix[4]arene developed by Mahajan and co-workers (Mahajan et al., 2008) had a detection limit of 10−6 mol dm−3 and a response time of 20 sec. Moreover, Tyagi and co-workers (Tyagi et al., 2010) constructed a calix[4]arene thioether derivative based Hg(II) ISE using NaBPh4 additive in the preparation of PVC membrane. Though the authors reported a wide concentration range (7.2 × 10−8-1.0 × 10−1 mol dm−3) and a fast time response (14 sec.) for it, this cannot be included in the comparative study for the reasons stated above. Another Hg(II)-ISE based on calix[4]arene derivative with thiazole moieties was reported by Lu and co-workers (Lu et al., 2003) with a slope of 28.79 ± 0.3 mV decade−1 and concentration range of 5 × 10−6 to 1x 10−2 mol dm−3. As far as other macrocycles are concerned a number of Hg(II) ISEs have been reported using crown ether derivatives as carriers. Among these 1,4 -dithia-12-crown −4 and 15-crown-5 have been reported, linear range of 10−6-10−2 mol dm−3 which works at a pH ≥ 2 (Lai and Shih, 1986), hexa-thia-18-crown-6 tetraone with a linear range of 4. 10−6-1.10−2 mol dm−3, life-time, 3 months and a pH range 0.5–2 (Fakhari et al., 1997), dibenzo-diazathia 18-crown-6- dione, linear range 8. 10−6-1.10−2 mol dm−3, response time, 10 s, life-time, 3 months, pH range, 0.5–2.5 (Javanbakht et al.,1999), pentathia15-crown-5, linear range, 2.51. 10−5-1.10−1 mol dm−3 (Gupta et al., 1997). To a much lesser extent ISEs based on a cryptand (as defined by the authors) have also been reported, linear range 1.10−6-1.10−1 mol dm−3, detection limit, 5.10−6 mol dm−3.slope, 30.2, stable in 3–6 pH range (Patel et al., 2009) as well as that based on polyaniline-cryptand 222, linear range, 10−12-1.10−8 mol dm−3 (Muthukumar et al.,2007). The above calix[4] based ISEs offer advantages relative to crown ethers and cryptands in the design of carrier mediated ISEs.
4 Conclusions
Receptor 1 based on a calix[4]pyrrole derivative is selective for Hg(II) as assessed from 1H NMR and thermodynamic studies. In addition it has a greater ability to host for this cation relative to others although the electrode response is so fast that the uptake of a second Hg(II) cation to form a 1:2 complex is unlikely to occur in the membrane electrode but it is likely to serve as an efficient extracting agent for removing Hg(II) from water. Based on fundamental studies two ISEs were constructed. For 1 the linear correlation found between the difference in stability constant of mercury (II) relative to corresponding data for other cations and the selectivity coefficient of the primary cation with respect to others is held. Agreement was found between experimental values of stability constant of metal cations (obtained from calorimetry) and calculated values (obtained from selectivity coefficients and stability constant for Hg(II)). As far as 2 is concerned the response of the electrode is in agreement with the outcome of NMR studies in that this receptor only interacts with Hg(II) and to a lesser extent with Ag(I) while interaction with other bivalent cations is weak.
Acknowledgements
The authors thank the Leverhulme Trust (Emeritus Fellowship awarded to AFDdeN), Taif University Researchers Supporting Project number (TURSP-2020/90), Taif University, Taif, Saudi Arabia for the financial support and Prof. John F Watts and staff for invaluable assistance in the use of equipment available in the Micro Structural Unit and Surface Analysis Laboratory (University of Surrey, UK).
References
- Mercury(II) selective membrane electrode based on calix[2]thieno[2]pyrrole. Int. J. Chem.. 2012;4:23-29.
- [Google Scholar]
- Determination of very low levels of dissolved mercury(II) and methylmercury in river waters by continuous flow with on-line UV decomposition and cold-vapor atomic fluorescence spectrometry after pre-concentration on a silica gel-2-mercaptobenzimidazol sorbent. Talanta. 2001;55:1141-1150.
- [Google Scholar]
- Effect of a plasticizer on the detection limit of calcium selective electrodes. J. Electroanalyt. Chem.. 2002;517:111-118.
- [Google Scholar]
- An asymmetric N-rim partially substituted calix[4]pyrrole: Its affinity for Ag(I) and its destruction by Hg(II) Arab. J Chem.. 2020;13:4824-4834.
- [Google Scholar]
- Gibbs free energies of transfer of cryptand (2,2,2) and silver (2,2,2) perchlorate from water to acetonitrile-water mixtures. J. Am. Chem. Soc.. 1982;104:3789-3792.
- [Google Scholar]
- Selective recognition of halide anions by calix[4]pyrrole: a detailed thermodynamic study. J. Phys. Chem. B. 2003;107:6462-6468.
- [Google Scholar]
- Plenary lecture. Neuquen, Argentina: XV Argentinian Congress of Toxicology; 2007.
- Sulfur-containing hetero-calix[4]pyrroles as mercury(II) cation-selective receptors: thermodynamic aspects. J. Phys. Chem.. 2007;B111:5803-5810.
- [Google Scholar]
- A new calix[4]pyrrole derivative and its anion (fluoride)/cation (mercury and silver) recognition. J. Phys. Chem. B.. 2007;111:3098-3105.
- [Google Scholar]
- Modified calix[4]pyrrole receptor: solution thermodynamics of anion complexation and a prelimenary account on the phosphate extraction ability of its oligomer. J. Phys. Chem. B. 2007;111:12177-12184.
- [Google Scholar]
- Calix [4] Pyrrole derivative: recognition of fluoride and mercury ions and extracting properties of the receptor-based new material. J. Phys. Chem. B. 2008;112:15766-15774.
- [Google Scholar]
- A new calix[4]arene derivative and its ionic recognition for silver (I) and mercury (II) in dipolar aprotic media. New J. Chem.. 2011;35:375-384.
- [Google Scholar]
- A ditopic calix[4]pyrrole amide derivative: highlighting the importance of fundamental studies and the use of NaPh4B as additive in the design and applications of mercury(II) ion selective electrodes. J. Mater. Chem. A. 2015;3:13016-13030.
- [Google Scholar]
- Calix[4]based Hg(II) ion selective electrodes: A thermodynamic protocol to address the selectivity versus the hosting capacity paradigm in the selection of the carrier. Electrochimica Acta. 2018;290:686-694.
- [Google Scholar]
- European Environment Agency, 2018a. Mercury: a persistent threat to the environment and people's health, 17/9/2018.
- European Environment Agency, 2018b. Mercury pollution remains a problem in Europe and globally, 19/9/2018.
- M PVC-based hexathia-18crown-6-tetraone sensor for mercury(ll) ions. Anal. Chem.. 1997;69:3693-3696.
- [Google Scholar]
- On-line preconcentration and determination of mercury in biological and environmental samples by cold vapor-atomic absorption spectrometry. J. Hazard. Mater.. 2007;141:693-699.
- [Google Scholar]
- Anion coordination and anion-directed assembly: highlights from 1997 and 1998. Coord. Chem. Rev.. 2000;199:181-233.
- [Google Scholar]
- A PVC based penta-thia-15-crown-5 membrane potentiometric sensor for mercury (II) Electroanalysis. 1997;9:478-480.
- [Google Scholar]
- A. Mercury selective potentiometric sensor based on low rim functionalized thiacalix [4]-arene as a cationic receptor. J. Molec. LIquids. 2013;177:114-118.
- [Google Scholar]
- Calixarenes, A Versatile Class of Macrocyclic Compounds. Dordrecht, Netherlands: Kluwer Academic Publishers; 1990. p. :3. (Chapter 1)
- Calixarenes. 1. Analysis of the product mixtures produced by the base-catalyzed condensation of formaldehyde with para-substituted phenols. J. Org. Chem.. 1978;43:4905-4906.
- [Google Scholar]
- International Union of Pure and Applied Chemistry, IUPAC, 2000. Potentiometric selectivity coefficients of ion-selective electrodes Technical Report 72, 1851-2082.
- Synthesis of calix [n] furano [n] pyrroles and calix [n] thieno [n] pyrroles (n= 2, 3, 4) by '3+ 1'approach. Tetahedron Lett.. 2000;41:2919-2923.
- [Google Scholar]
- Mercury(II) Ion Selective Electrode based on dibenzo-diaza thia 18-crown-6-dione. Electroanalysis. 1999;11:81-84.
- [Google Scholar]
- Determination of mercury by electrochemical cold vapor generation atomic fluorescence spectrometry using polyaniline modified graphite electrode as cathode. Spectrochim. Acta. Part B At. Spectrosc.. 2010;65:171-175.
- [Google Scholar]
- Mercury (II) and silver(I) ion selective electrodes based on dithia crown ethers. Analyst. 1986;111:891-895.
- [Google Scholar]
- One-flask synthesis of meso-substituted dipyrromethanes and their application in the synthesis of trans-substituted porphyrin building blocks. Tetrahedron Lett.. 1994;50:11427-11440.
- [Google Scholar]
- X.W. A mercury ion-selective electrode based on a calixarene derivative containing the thiazole azo group. Electroanal. Chem.. 2003;540:111-117.
- [Google Scholar]
- Mercury(II) sensors based on calix[4]arene derivatives as receptor molecules. Sens. Actuators B. 2008;130:290-294.
- [Google Scholar]
- Trace mercury determination in drinking and natural water samples by room temperature ionic liquid based-preconcentration and flow injection-cold vapor atomic absorption spectrometry. J. Hazard. Mater.. 2009;167:475-481.
- [Google Scholar]
- Two-parameter Debye-Hückel approximation for the evaluation of mean activity coefficients of 109 electrolytes. Anal. Chim. Acta.. 1982;136:363-368.
- [Google Scholar]
- Conductimetric mercury (II) sensor based on polyaniline cryptand 222 hybrid. J. Electroanalyt. Chem.. 2007;602:172-180.
- [Google Scholar]
- Human exposure and health effects of inorganic and elemental mercury. J. Prev. Med. Public Health. 2012;45:344-352.
- [Google Scholar]
- Structural selectivity of calix[m]pyrroles[n]furans (m + n = 4) as ionophores in Ag(I) ion-selective electrodes. J. Incl. Phenom. Macrocycl. Chem.. 2001;39:295-301.
- [Google Scholar]
- Mercury selective membrane electrode based on dithio derivatised macrotricyclic compound. J. Incl. Phenom. Macrocyclic Chem.. 2009;64:101-108.
- [Google Scholar]
- Environmental mercury and its toxic effects. J. Prev. Med. Public Health. 2014;47:74-83.
- [Google Scholar]
- Fundamental view of enthalpy-entropy compensation. Med. Chem. Commun. 2014;5:1324-1336.
- [Google Scholar]
- Lead selective electrode membrane based on dibenzopyridino-18-crown-6. Anal. Lett.. 1996;29:2269-2273.
- [Google Scholar]
- Potentiometric polymeric membrane electrodes for mercury detection using calixarene ionophores. Water. Sci. Techn.. 2010;61:693-704.
- [Google Scholar]
- World Health Organisation, 2017. Mercury and health, 31, 3, 2017.
- Determination of Hg in water and wastewater samples by cv-aas following online preconcentration with silver trap. J. Anal. Chem.. 2010;65:935-939.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2020.09.059.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary Data 1
Supplementary Data 1







