Translate this page into:
Thymol bioactivity: A review focusing on practical applications
⁎Corresponding author at: Centro de Investigación y Desarrollo en Tecnología de Pinturas (CIDEPINT), CICPBA-CONICET-UNLP, Calle 52 e/ 121 y 122, 1900 La Plata, Argentina. g.blustein@cidepint.ing.unlp.edu.ar (Guillermo Blustein)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Thymol is a natural volatile monoterpenoid phenol that is the main active ingredient of oil extracted from species Thymus vulgaris L., commonly known as thyme, and other plants such as Ocimum gratissimum L., Origanum L., Carum copticum L., different species of the genus Satureja L., Oliveria decumbens Vent, and many others. It is a versatile molecule with a wide variety of practical applications such as medical, dentistry, veterinary, food, and agrochemicals, among others. Its pharmacological applications have been the most investigated and reported, focusing on its prominent antimicrobial, antioxidant, anti-inflammatory, cicatrizing activities. Furthermore, it is noteworthy that the research on its agricultural applications has increased, highlighting its uses as a natural agrochemical and preservative to safeguard foods from pathogenic microorganisms both in sowing and storage, which could have a beneficial effect on human health and the environment. Research has also been reported on its activity as an insecticide, acaricide, and animal repellent. This review summarizes important aspects of thymol such as its bioavailability, synthesis, and biological activities, with special interest in practical applications.
Keywords
Thymol
Synthesis
Mechanism of action
Bioactivity
1 Introduction
Thymol, also known by the chemical names 2-isopropyl-5-methylphenol and 5-methyl-2-isopropylphenol, is a natural monoterpenoid phenol, crystalline and colorless, with a characteristic odor. It is also an isomer with carvacrol and is the principal active ingredient of oil extracted from the species Thymus vulgaris, commonly known as thyme. It is a perennial aromatic plant, woody, very branched and of small height, of the mint family Lamiaceae, with a useful life of approximately 10–15 years, native to the Mediterranean and neighboring countries, Northern Africa, and parts of Asia, and is cultivated all over the world (Fig. 1) (Kuete, 2017). T. vulgaris presents bilabiate flowers, white, yellow or purple in color, which bloom in spring. When its leaves are touched, it gives off a pleasant and sweet smell. Thyme is a relative of the oregano genus Origanum, and people have used it for many centuries as a food seasoning and medicinal herb, but its antioxidant and antimicrobial properties have also been studied (Mandal and DebMandal, 2016).
Thyme vulgaris plant and flowers.
The reputation of thyme goes back thousands of years. In Ancient Rome, it was believed to be an antidote. Eating thyme before or during meals protected from poisons. This fame made it one of the favorite herbs of emperors. Time later, during the Black Death around 1340, it was used as the main ingredient in medicinal concoctions and ointments applied directly to the blistered skin (Dunn, 2013). Although at that time it was unknown what the pharmacological principle was, it was not until 1719 that thymol was first isolated by the scientist Caspar Neumann. Later, between 1879 and 1880, Italian doctors discovered its anti-hookworm activity, when fighting the hookworm epidemic, a disease caused by a type of intestinal parasite (Sharma and Anand, 1997). Today it is known that thymol is a powerful antiseptic that is currently widely used in mouthwash, hand sanitizer, and acne medications, etc. (Nagoor Meeran et al., 2017).
Thymol has been isolated from other plants such as Ocimum gratissimum L., Origanum L., Trachyspermum ammi (L.), different species of the genus Satureja L. and Monarda L. (Lamiacaeae), Carum copticum L. and Oliveria decumbens Vent (Apiaceae), Anemopsis californica (Saururaceae) and species of Verbenaceae, Scrophulariaceae and Ranunculaceae (Bassole, 2003; Boskabady et al., 2014; dos Santos et al., 2019; Marchese et al., 2016; Medina et al., 2005; Moein et al., 2015; Mohagheghzadeh et al., 2007; Öztürk, 2012). These extracts have been used as medicinal herbs by different cultures in many countries.
Among the medicinal uses that have been reported for T. vulgaris essentials oils and other oils that contain thymol, the following can be listed: treatment of disorders that affect the respiratory and digestive systems (antitussive, expectorant, stomachic, digestive, carminative, and antispasmodic) (Gavliakova et al., 2013; Kissels et al., 2017; Negahban et al., 2007; Salehi et al., 2018; Wan et al., 2018), activity against oral diseases such as caries (Preston et al., 2007; Rezaeian et al., 2019), cicatrizing (Mollarafie et al., 2015), antioxidant (Nikolić et al., 2014), anti-inflammatory (Fachini-Queiroz et al., 2012; Oliveira et al., 2017), antifungal (Borugă et al., 2014; Jafri and Ahmad, 2019), and antimicrobial activities (Costa et al., 2018; Reyes-jurado et al., 2019), among others. This variety of proven pharmacological applications, the identification of their mechanisms of action, and their pharmacokinetic study postulate thymol as a possible medicinal treatment of natural origin (Milovanovic et al., 2019; Nagoor Meeran et al., 2017). There are commercial pharmaceuticals whose main component is Shirazi thyme (Zataria multiflora) essential oil, commonly used in medicine for the treatment of respiratory diseases (Ebrahimzadeh et al., 2003). Another world-famous product that contains thymol is Listerine, which gets its name thanks to Joseph Lister, who discovered the properties of thymol to treat mouth problems such as mouth, throat and gingivitis infections caused by bacteria (Buckle, 2014). This is because the oils that contain thymol have shown antibacterial activity against Gram-positive (Staphylococcus aureus, Streptococcus faecalis, Bacillus subtilis, and Bacillus cereus) and Gram-negative (Proteus mirabilis, Escherichia coli, Salmonella Typhimuium Ty2, and Pseudomonas aeruginosa) bacteria (De Feo et al., 2003; Nabavi et al., 2015; Tohidpour et al., 2010).
But the applications of thymol are not only at the pharmacological level, since from the moment it was included in the list of ‘Generally Recognized As Safe’ (GRAS) for use as a food additive with negligible toxicity by the United States Food and Drug Administration (FDA) (U.S. Food and Drug Administration (FDA), 2020), research on its activity as a natural food preservative has increased significantly, highlighting its antimicrobial and antioxidant activity. Due to these studies, it is now known that its antioxidant activity is because the compounds containing phenolic groups both by absorbing or neutralizing free radicals and by augmenting endogenous antioxidants (Heydari et al., 2019; Marchese et al., 2016). In addition, due to its antimicrobial properties, it prevents or slows the growth of fungi and bacteria in food. Finally, among its applications, thymol also stands out in commercial formulations for use as insecticide (Benelli et al., 2017), acaricide (Tabari et al., 2017), insect and animal repellent (Park et al., 2005), fungicide, and medical disinfectant, presenting itself as an alternative to reduce the employment of synthetic fungicides (Dingfei Hu, 2008; Robledo et al., 2019).
All the above-mentioned uses have led to study the composition and properties of a wide variety of essential oils containing thymol. These studies show that thymol and the oils that contain it have potential applications in pharmaceutical, cosmetic, food, agronomic, and veterinary industries, among others, and that their biological activity varies depending on the chemical composition of the oil, which is determined by genotype of the plant and influenced by agronomic and environmental conditions, cultivation, and plant growth stage (Rota et al., 2008).
Some critical reviews of pharmacological properties of thymol in different areas of knowledge have been published in the last years (Alagawany et al., 2020; Islam et al., 2019; Marchese et al., 2016; Nagoor Meeran et al., 2017). The multiple therapeutic action of thymol against various cardiovascular, neurological, rheumatological, gastrointestinal, metabolic and malignant diseases have been reviewed by Nagoor Meeran et al. (2017) Furthermore, Islam et al. (2019) have documented a critical review on the anticancer activity of thymol, emphasizing its mechanism of pharmacological action. Another article regarding the antibacterial and antifungal effects of thymol, focused on human pathogens, was summarized by Marchese et al. (2016). On the other hand, the use of thymol in aquaculture was also reported (Alagawany et al., 2020). These authors have recently reviewed the beneficial effect of thymol on health and production of fish.
The innovativeness and originality of this review is to critically analyze the available literature on thymol bioactivities with emphasis on specific practical applications (medical materials, dentistry, veterinary, food preservatives, feed additives for animal production, agrochemical and antifouling, among others). The present review work aims to complement the information so far described in the literature. Important information on the chemistry, synthesis, and bioavailability of thymol was also gathered, providing a complete review of this versatile molecule.
2 Natural sources of thymol
The natural sources of thymol, their content and properties are summarized in Table 1.
Plant species
Plant families
Part of the plant
Amount thymol (%)
Activity
References
Thymus vulgaris
Lamiaceae
EO
39.5
Antioxidant
(Tohidi et al., 2017)
Thymus vulgaris, Thymus serpyllum, Thymus algeriensis
Lamiaceae
EO
38.5
48.8
56.0Antioxidant
Antimicrobial
Antitumor(Nikolić et al., 2014)
Thymus vulgaris
Lamiaceae
Commercial extract (leaves)
–
Antimicrobial
Anti-inflammatory(Oliveira et al., 2017)
Thymus vulgaris
Lamiaceae
EO
–
Anxiety
(Komaki et al., 2016)
Thymus vulgaris
Lamiaceae
EO
38.23–63.01
Antimicrobial
(Nezhadali et al., 2014)
Thymus vulgaris, Thymus tosevii
Lamiaceae
EO
48.9
10.4Antifungal
(Soković et al., 2009)
Thymus zygis
EO
19.5
Anti-inflammatory
(Rodrigues et al., 2019)
Thymus vulgaris
Lamiaceae
EO
35.4
Antioxidant antibacterial
(Dehghani et al., 2019)
Thymus vulgaris
Lamiaceae
EO
39.9
Feed additives for animals
(Oceľová et al., 2019)
Thymus vulgaris
Lamiaceae
EO
–
Mosquito repellents
(Park et al., 2005)
Carum copticum
Apiaceae
Leaves, Flowers, OE
17.4–72.3
Food additive
Traditional medicine(Boskabady et al., 2014)
Carum copticum
Thymus vulgaris
Apiaceae
LamiaceaeEO from dry fruits
EO36.7
Valued sensory characteristics
(Morsy, 2020)
Trachyspermum ammi
Apiaceae
EO
17.4
Antifungal Antibacterial
(Moein et al., 2015)
Oliveria decumbens
Apiaceae
EO
38.8
Antibacterial
Insecticidal
Anti-cholinesterase
and anti-butyrylcholinesterase activities
Cytotoxic(Eftekhari et al., 2019)
Satureja thymbra
Lamiaceae
EO
33.8
Antimicrobial
(Markovic et al., 2011)
Anemopsis californica
Saururaceae
roots and rhizomes EO
13.8
Anticancer
(Medina-Holguín et al., 2008)
Zataria multiflora Boiss
Lamiaceae
Various parts
15.6–64.9
Food additive
Traditional medicine(Sajed et al., 2013)
Zataria multiflora Boiss
Lamiaceae
EO
47.5
Antimicrobial
(Saei-Dehkordi et al., 2010)
Majorana syriaca
Lamiaceae
EO
42.9
Antioxidant
(Al‐Bandak and Oreopoulou, 2007)
Origanum glandulosum Desf
Lamiaceae
EO
41.6–81.1
Antimicrobial
Antifungal(Bendahou et al., 2008)
Lippia gracilis
Verbenaceae
EO
11
Acaricidal
(Born et al., 2018)
Lippia multiflora
Verbenaceae
EO
19
Analgesic Antipyretic
Anti-inflammatory(Abena et al., 2003)
Lippia chevalieri Lippia multiflora
Verbenaceae
EO
27.4
29.9Antibacterial
(Bassole, 2003)
In addition to the species T. vulgaris, thymol is also present in other plants, such as Carum copticum L. (Apiaceae) better known as “ajwain”. This species is planted in many parts of the world, Iran and India being one of its largest producers. Since ancient times it has been used to treat different ailments including bloating, fatigue, diarrhea, tumors and abdominal pain, and diseases of the respiratory system. Other interesting properties such as antifungal, antioxidant, antimicrobial, and hypolipidemic activity are also attributed to it (Boskabady et al., 2014). The amount of extractable oil of this species is between 2.5% and 5.0%, and its main constituent is thymol with percentages that vary between 35% and 60%. Other compounds present are p-cymene (50%–55%), limonene, γ-pinenes and β-pinene (30%–35%) (Asif et al., 2014). Food-damaging bacteria such as Salmonella Typhimurium, E. coli, Pseudomonas aeruginosa, Enteropathogenic and S. aureus have been the most frequently evaluated to determine the antibacterial activity of this oil, exhibiting significant results against all of them (Goudarzi et al., 2011). Morsy (2020) obtained Carum copticum L. (ajwain) and T. vulgaris L (thyme) oil, with thymol as the main component, by supercritical fluid CO2-extraction (SFE). The SFE extracts were made at 40 °C and two pressures, 10.4 and 16.7 MPa, thus substantially improving the relative amount of thymol in the ajwain and thyme extracts by 1.7 and 2.49 times, compared to the amount of thymol present in hydrodistilled oils, respectively. Chatterjee et al. (2017) reported the cloud point assisted extraction of thymol from water extract of ajwain. In it, a nonionic surfactant is added to the aqueous extract of Ajwain seeds, and heated beyond the temperature of the cloud point where it is separated into two phases, the aqueous phase and the surfactant-rich coagulated phase. The authors investigated the effects of different operating conditions, i.e., surfactant concentration, heating time, and temperature, on extraction efficiency, finding that maximum thymol extraction efficiency was achieved with 30% (v/v) of surfactant SPAN 80 (Sorbitan monooleate), 45 min of heating at 65 °C. Thymol recovery from the surfactant complex was optimal at a 1:3 vol ratio of coacervate phase to solvent (acetone).
Another plant that has a high content of thymol in its essential oil is Oliveria decumbens Vent (Apiaceae). This is an endemic plant in Iran, used in traditional medicine to treat indigestion, diarrhea, abdominal pain, and feverish conditions, and has been investigated to determine its components and antimicrobial and insecticidal activity (Eftekhari et al., 2019). In an investigation conducted by Amin et al. (2005), in which they studied the components of this oil, it was determined that the main compounds were thymol (47.06%) and carvacrol (23.31%). Additionally, the oil presented a high antibacterial and antifungal activity against all tested Gram(+) and Gram(−) bacteria and fungal strains.
Continuing with the plants that contain thymol, we can mention the species Satureja, which is an ornamental and pharmaceutical plant very common in the Mediterranean area. The most common Satureja specimen is Satureja thymbra L., to which antiseptic, gastrosedative and diuretic properties are attributed (Chorianopoulos et al., 2006). Marković et al. (2011) studied the chemical composition of the essential oils extracted from Satureja thymbra and Thymbra spicata, determining that the main components were thymol (33.8%), γ-terpinene (30.8%) and p-cymene (11.8%) for the S. thymbra oil, and carvacrol (74.5%) and γ-terpinene (11.2%) for the T. spicata oil.
On the other hand, the Anemopsis califórnica plant, which is cultivated in southwestern United States and in northern Mexico, is one of the five genera belonging to the Saururaceae family, commonly known as “yerba mansa”. Infusions made with leaves and roots of A. californica have been used to treat pain, inflammation, and infection (Medina et al., 2005). Medina-Holguín et al. (2008) determined the chemical variability of the main compounds present in the roots and rhizomes of A. californica, collected in different regions of New Mexico. They identified three different chemotypes, of which only one had high concentrations of piperitone and thymol.
From the Apiaceae family, there is the species Trachyspermum ammi (L.) Sprague, a common herb in some countries of South Asia, North Africa, and Europe. Due to its various chemical components, possible pharmacological applications have been studied, finding that it has stimulant, carminative, diuretic, anesthetic, antimicrobial, antiviral, antiulcer, antihypertensive, antitussive, bronchodilator, antiplatelet, and hepatoprotective effects (Zarshenas et al., 2014) and can be used as a food preservative (Paul et al., 2011; Raeisi et al., 2016). Moein et al. (2015) evaluated the chemical composition of the essential oil of the species Trachyspermum ammi (L.), determining that γ-terpinene (48.07%), p-cymene (33.73%) and thymol (17.41%) are the main components. In this research they also evaluated the antimicrobial activity of the total extract, of the extract fractions and of the standard thymol, finding as an outstanding fact that one of the analyzed oil fractions had a greater antibacterial effect than that obtained with the total essential oil and the standard thymol. This result was attributed to a synergistic effect between thymol and other compounds present in the oil fraction. The genus Lippia L., belonging to the family Verbenaceae, includes>200 species of herbs, shrubs, and small trees native to the tropical and subtropical regions of Africa and South America. One of the most abundant species in Brazil is Lippia gracilis Schauer, popularly known as “alecrim-da-chapada” (Cruz et al., 2018; dos Santos et al., 2019), which is used in traditional medicine to treat different respiratory diseases such as flu, sinusitis, bronchitis, nasal congestion, and others such as headache, jaundice, and paralysis (Veras et al., 2012). Its leaves are used to make ointments to treat skin wounds such as burns and ulcerations, and it is also used as an oral antiseptic (Riella et al., 2012). But its applications are not only medicinal, the bioactivity of its oil has also been proven against fungi, insects, mites, and ticks (Born et al., 2018; Cruz et al., 2013). On the other hand, Lippia multiflora and Lippia chevalieri are endemic species of West Africa. Traditional African medicine uses their leaves as tea and in the treatment of malaria, hypertension, boils, diarrhea, and as a mouth disinfectant (Abena et al., 2003; Bassole, 2003). The bioactivity of these plants is especially attributed to the fact that thymol is one of the major components of the essential oil of these species. Therefore, these oils and their major compounds have been studied to develop biopesticides, and anti-inflammatory and cicatrizing medications, among other applications. There are some oils that contain thymol to a lesser extent such as the extracts of the root of Uvaria chamae (Annonaceae) that present 8.7% thymol (Thomas and Essien, 2020) and Artemisia judaica (Asteraceae) with 3.5% thymol (Al-Wahaibi et al., 2020).
3 Bioavailability, composition and extraction of thymol
Throughout the evolution of plants, essential oils have had a transcendent role in the direct and indirect defenses of plants against possible predators and pathogens, in the processes of plant reproduction by attracting seed pollinators and disseminators, and in the thermotolerance of plants. This has made it feasible to think about isolating their components and studying them with a view to evaluating their antimicrobial, acaricidal, insecticidal, repellent activity, among others, and to know more about their synthetic routes and bioavailability. From these studies, it is now known that the biosynthesis and storage of oils are due to the presence of different structures in plant organs such as glandular trichomes responsible for secreting substances, and cavities and conduits responsible for transporting them. These substances are classified into two chemical groups based on the metabolic pathway of their synthesis: (i) terpenoids, mainly monoterpenes and sesquiterpenes; and (ii) phenylpropanoids with low molecular weight (Pavela and Benelli, 2016). Plants have developed a great capacity to synthesize compounds of different structures and functions, for example, there are>23,000 terpene structures identified in higher plants, which include hydrocarbons, alcohols, ethers, aldehydes, ketones, carboxylic acids, and esters. Within this large group, the monoterpenes thymol and carvacrol stand out as two of the molecules with the greatest biological activity (Trindade et al., 2018). The biosynthesis of monoterpenes begins with the synthesis of geranyl pyrophosphate (GPP), which is the precursor of all monoterpenes. Later, GPP generates the α-terpinyl cation, a highly unstable intermediate that, thanks to terpenoid synthase (TPS) enzymes, can be converted into specific monoterpenes. Thus, γ-terpinene and p-cymene are synthesized by the enzyme γ-terpinene synthase (Tps2) and these, in turn, are the precursors of the monoterpenes thymol and carvacrol (Fig. 2) (Lima et al., 2013; Tohidi et al., 2020b).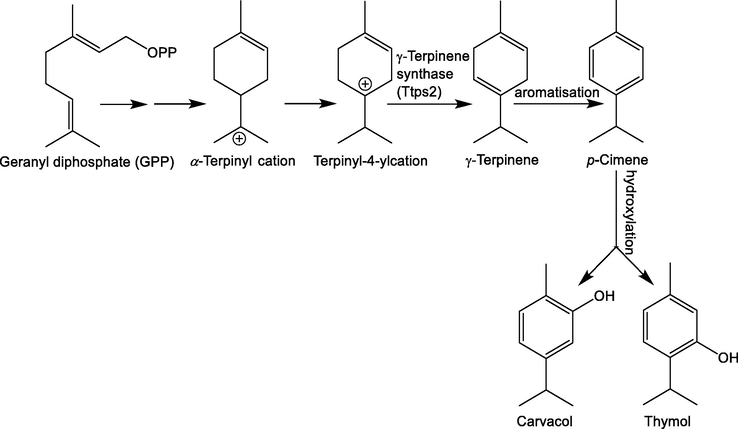
Biosynthetic pathway of thymol and carvacrol from geranyl pyrophosphate (GPP) as precursor (Tohidi et al., 2020b).
In addition to this biosynthetic route, the chemical composition of essential oils and the amount of thymol present in them depend on different factors such as species of the plant, geobotanical conditions (soil, light, humidity, altitude), cultivation method (fertilizers, agrochemicals, planting time, irrigation, pesticides), plant collection time, plant material storage method (fresh, dry, etc.), method of obtaining oil (distillation, maceration, pressing, extraction with or without solvents), type of solvent, age of the plant, genetic modifications, etc. For example, Nomani et al. (2019) genetically modified the Trachyspermum ammi species (Qom ecotype), to overexpress the Ttps2 gene using Agrobacterium tumefaciens and thus increase the thymol amount in the essential oil of the plant. For this, a gene construct called pBI121-TP harboring the neomycin phosphotransferase (nptII) gene as a plant-selected marker was designed. The factors studied included inoculation time (5, 10, 15, and 20 min) and co-cultivation time (1–3 days). The authors reported that the results of the polymerase chain reaction (PCR) determined that the TPS2 gene was expressed more in transgenic plants than in non-transgenic plants, which was reflected in a greater amount of thymol that varied between 6.12% and 18.36% in transgenic plants compared to non-transgenic plants. This study is the first of its kind to report an adequate and efficient method for overexpression of the TPS2 gene to increase the thymol biosynthesis in this plant genus.
Nezhadali et al. (2014) studied the chemical composition of the essential oil of T. vulgaris (Lamiaceae) species at different stages of plant growth. They determined that there was an inverse linear relationship between the lifetime and the total amount of oil extracted with values between 0.83% and 1.39% (w/w). In addition, the main essential oil components were thymol and o-cymene, with percentages that varied between 38.23% and 63.01%, and 5.56% and 15.47% respectively, finding that the amount of thymol decreased with the life of the plant, while the amount o-cymene increased. The authors also reported the composition of thymus essential oil from different countries, where thymol was the main component of the thymus species gathered from Morocco, Iran, and Algeria, while its concentration was very low in El-Asnam and Chrea National Park. A final important conclusion of this work was that in most of the cases studied, there was a linear relationship between the concentration of thymol and carvacrol. If thymol was in high concentration in the oil, the amount of carvacrol was low and vice versa. This was corroborated with another study that compared the variation of the composition of the oil of T. vulgaris L species, in different periods during the vegetative and life cycles of the plant, determining that the oil of the old plant (5 years) presented the minimum oil extract yield level recorded (0.15% v/w) and the minimum amount of thymol (19.38%) compared to the young plant (2 years), collected just before the end of the vegetative cycle, which provided the best oil yield (1.2%) with also the highest % of thymol (51.2%). This indicates that to obtain an essential oil with better quality and quantity, the best harvest time is when thyme plants are young (Hudaib et al., 2002).
As mentioned in the previous paragraph, the region where the crop is planted and the plant species also have a major role in the composition and properties of oils, which largely defines their best application, whether in the food or pharmaceutical industry. An example of this is a study conducted by Asensio et al. (2015) in which they evaluated the sensory, physicochemical profiles, and biological activities (antioxidant tests and lethality tests against intestinal worms) of four essential oils of Origanum spp. cultivated differently in central and southern Argentina, in the provinces of Córdoba, Rio Negro, and Neuquén. They found that the concentration of thymol varied between 12.1% and 17.4% and they had a lower amount of carvacrol, between 0.1% and 3.5%. The physicochemical and organoleptic properties of the oils showed that these parameters could be used to differentiate them. An antioxidant profile showed that oregano oils from the southern provinces were more active (9.2–10.4, arbitrary scale up to 12) than those from the center of the country, and these, in turn, showed aromas of better acceptance, compared to the southern ones. The same pattern was observed for lethality studies against yeast and intestinal worms. Thus oils from central Argentina that have better aroma have major applications in the food and fragrance industries for their taste and aroma, while oils from the southern provinces may have applications in the development of new pharmaceutical and veterinary products or in food preservation due to their antioxidant and biocidal activity. Similarly, Kokkini et al. (1997) analyzed the essential oils of Origanum vulgare hirtum from six locations in three different geographical areas of Greece, collected at the end of autumn, and analyzed them using GC/MS. The plants collected in northern Greece presented a high concentration of thymol (30.3%–42.8%), unlike those in the south that had carvacrol as the main component (57.4%–69.6%). In addition, when essential oils from the same regions but obtained in summer were compared, significant differences were observed in terms of the amount of oil obtained and the composition of its four main components. Bakha et al. (2020) studied the intraspecific chemical variability of the essential oils of Origanum elongatum L. (Lamiaceae), an endemic species of Morocco, comparing all its natural habitats in a biogeographic context. For this, they analyzed the essential oils of 168 individual plants collected in 30 populations that grow wild in two Moroccan mountains: Rif and Middle Atlas, collected by hydrodistillation (HD), with yields that varied between 0.81% and 3.12%. They found that carvacrol, thymol, and cymene constitute the main compounds of the essential oils, thymol being the most abundant compound in the samples taken in Rif unlike those taken in the Middle Atlas. Another agent that has been considered as one of the external factors that most affect the biosynthesis of secondary metabolites in recent times is light, intervening in the composition of the oil and in the amount of each metabolite (Silva et al., 2017). This is why Tohidi et al. (2020a) conducted an interesting study on the composition and antioxidant activity of four thymus species when exposed to five light spectra (namely, red, blue, red-blue, white, and greenhouse conditions). The main conclusion that they obtained, according to the analysis of variance, is that the effect of light was not the same for all the species studied. Thus, red light gave a higher yield in the production of oil in the species T. migricus (4.17%), while the lowest yield was found in the T. carmanicus species (1.05%) under greenhouse conditions. The highest amount of thymol (66%) was found in T. migricus under the action of blue light, and the lowest (1.69%) in T. kotschyanus under blue-red light.
The species also play an important role. Sefidkon et al. (2005) studied the chemical composition of essential oils of three Iranian species of Satureja, namely, S. mutica Fisch. & C. A. Mey., S. macrantha C. A. Mey., and S. intermedia C. A. Mey. that were extracted by HD and analyzed by GC/MS. They found a great variation between the components and the amounts of each of them in the oils, with S. intermedia oil being the one with the highest concentration of thymol (32.3%), other main components were γ-terpinene (29.3%) and p-cymene (14.7%). The second oil with the highest amount of thymol was S. mutica (26.5%), followed by γ-terpinene (14.9%) and p-cymene (10.3%). Finally, the low amount of thymol in S. macrantha oil (8.1%) caught the authors’ attention; the largest component in this oil was p-cymene (25.8%), followed by limonene (16.3%). Due to the high amounts of thymol and/or p-cymene in the oils of S. mutica and S. intermedia, the authors suggest that these oils can be used in medical and food applications. In another study, Mastelic et al. (2003) examined the content and composition of essential oils in the Satureja montana L species at different stages of plant development. Thirty-six compounds were identified in the essential oil, where the main compounds were thymol (30.88%–46.02%), p-cymene (7.10%–13.48%), γ-terpinene (7.57%–9.74%), and carvacrol (3.81%–6.86%). They also determined that the thymol content decreased with the maturation of the plant, and the contents of eugenol and geraniol increased at the same time.
Another factor that indirectly affects the composition of the oils is the method of extraction. Ebrahimzadeh et al. (2003) compared the extraction of oil from the Zataria multiflora Boiss, grown in Iran, using two techniques, steam distillation and SFE with CO2. The oils and extracts were analyzed by GC/MS. Parameters such as temperature, pressure, and extraction period were analyzed to determine the optimal conditions of SFE. Chemical analysis revealed that there was a great dependence between the extraction conditions and the composition of the oils, which varied in the following percentages: thymol (14.2%–67.6%), ʎ-terpinene (0.1%–19.5%), and p-cymene (3.6%–12.0%), and that SFE was more efficient to extract thymol than steam entrainment extraction, in which the amount of thymol extracted was 44.6%. Similarly, Khajeh et al. (2004) compared the SFE with CO2 method, this time with the HD method, in the extraction of the essential oil of Carum copticum grown in Iran. The characterization of the oils and the optimization of the parameters of the SFE method were the same as those reported by Ebrahimzadeh et al. (2003). The results showed that the SFE method offers many important advantages over HD such as shorter extraction times (30 min vs. 4 h for HD), lower energy expenditure, the possibility of manipulating the oil composition by changing the extraction parameters (pressure, temperature, modifier volume, and dynamic extraction time). Finally, it should be pointed out that although the compositions of the oils obtained by SFE and HD are not qualitatively different, they do differ quantitatively. By means of the HD method they identified six main compounds: thymol (49.0%), γ-terpinene (30.8%), p-cymene (15.7), β-pinene (2.1%), myrcene (0.8%), and limonene (0.7%). The extraction yield, based on HD, was 2.8% (w/w) and SFE varied in the range of 1.0–5.8% (w/ w) under the different conditions studied. Similar conclusions reached Morsy (2020). On the other hand, Abu-Lafi et al. (2008) compared the methods static headspace (HS) and steam distillation (SD), in the determination of secondary metabolites of the leaves of the Majorana syriaca (Zaatar in Arabic), belonging to the mint family, Labiates. The authors determined that main volatile and semivolatile metabolites were α-pinene, β-myrcene, o-cymene, p- cymene, γ-terpinene, thymol, and carvacrol, and that the results obtained by the HS method were similar to the percentages of the semivolatile phenols extracted using the conventional SD method. The authors also determined that the monoterpenes γ-terpinene and p-cymene, which are the biosynthetic precursors of thymol and carvacrol (through enzymatic hydroxylation), decrease their concentration in the month of May, which is consistent with the increase in thymol and carvacrol. Bendahou et al. (2008) studied essential oils obtained by HD, solvent-free microwave extraction (SFME), and the extract obtained by microwave-assisted extraction (MAE) of the species Origanum glandulosum Desf., and analyzed them by GC/MS. Thymol (41.6%–81.1%) was the majority compound extracted by the three methods. However, the research also highlighted that the SFME method was most selective for the extraction of thymol. Jain et al. (2018) did a great statistical work to evaluate the optimal process conditions for the aqueous extraction of phytonutrients and thymol from ajwain species (Trachyspermum ammi L.). They determined the following values: seed to water ratio: 0.30 g/mL, extraction time: 16 min, and extraction temperature: 52 °C. Under these conditions, 2.1 mg of thymol per kg of ajwain seed was extracted.
4 Chemical synthesis and reactions
There are several methods available for thymol synthesis that are classified into two categories: those that start from aromatic hydrocarbons such as p-cymene and those that begin from phenols such as m-cresol, the latter being the most common method. The first method was reported by Oskar Widman in 1882. This classic synthesis illustrates very well the complex procedure that should be performed at its beginning. It started from cuminaldehyde that, when treated with nitric acid, forms nitro-cuminal, the nitro group entering the meta position to the aldehyde group. This compound is then treated with phosphorus pentachloride generating nitro- cymyline chloride that, when reduced with zinc and hydrochloric acid, gives 3-aminocimene, and after diazotization and subsequent hydrolysis produces thymol (Widmann, 1882). Years later, in 1920, Max Phillips and H. D. Gibbs reported the synthesis of thymol from p-cymene. The process shown in Fig. 3 begins with the nitration of p-cymene by reacting with nitric acid, with the substitution largely in the ortho position to the methyl group (Phillips and Gibbs, 1920).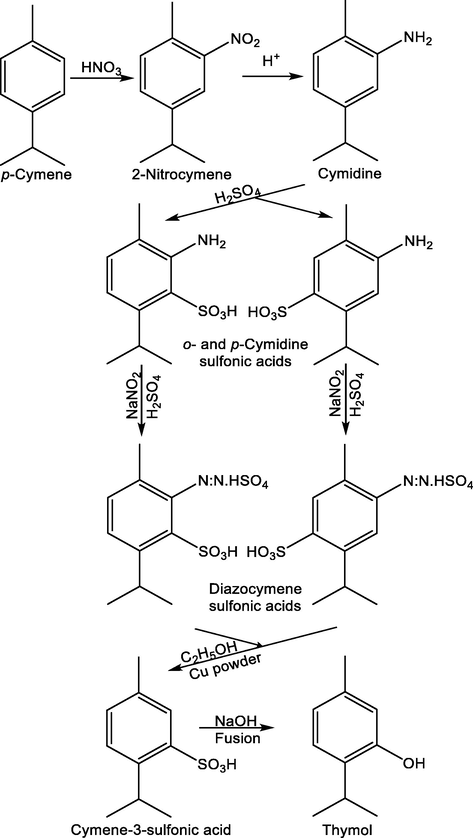
Synthesis of thymol from p-cymene using the method reported by Max Phillips and H. D. Gibbs (Phillips and Gibbs, 1920).
Then the reduction of this group by means of iron powder and hydrochloric acid generates amino acids. Then it is sulfonated and after different treatments with sodium nitrite/sulfuric acid, methanol/copper powder and fusion with sodium hydroxide, thymol is obtained (Phillips and Gibbs, 1920). In 1932 Joseph B. Niederl and Samuel Natelson obtained thymol by condensing propylene with m-cresol catalyzed by sulfuric acid, in which thymol sulfonic acid is formed, and later, the acid group is removed by means of superheated steam (Niederl and Natelson, 1932).
Since those first reported synthesis methods, which were quite complex, with low yields and with toxic and dangerous reagents for health and the environment, synthetic processes to obtain thymol have been much improved nowadays. In recent years, a large number of papers and patents that demonstrate this have been reported. The alkylation of m-cresol to produce thymol continues to attract considerable attention because it is a simple, high-yield process. It is the industrially used method to produce thymol known as the Bayer process in which the propylation of m-cresol is carried out in liquid phase at 280–365 °C and 5 MPa over activated alumina (Wedemeyer, 1976). Over the years, different catalysts have been studied in order to optimize the process in such a way that it generates minimal environmental threats and maximum economic benefits. In this order of ideas, different catalysts and reaction conditions have been used in the alkylation of m-cresol, with propylene in the presence of alumina or aluminum 3-methylphenoxide (Grabowska et al., 2004).
A patent published in 2016 uses m-cresol and propylene as raw materials, and an activated aluminum oxide catalyst prepared through a sol–gel method with cobalt doping as the main component; the structure of the activated aluminum oxide catalyst is Co&X@Al2O3, where X is family-VIII transition metal except for cobalt, and the content of X is smaller than that of cobalt. As advantages of the process the inventors/authors highlight the catalyst has high stability and long service life; besides, selectivity is very high, continuous production can be achieved, and industrialization is promoted (Zhang et al., 2016). Other proprietary catalysts are described as a composition of concentrated sulfuric acid/aluminum trichloride, polyphosphoric acid/aluminum chloride, concentrated sulfuric acid/zinc chloride or polyphosphoric acid/zinc chloride (Yang et al., 2014). The isopropylation of m-cresol with ZnAl-MCM-41 (Selvaraj and Kawi, 2008) and MgAl-MCM-41has also been reported (Vinu et al., 2004), as well as the alkylation of m-cresol with propene and zeolites H-ZSM-5 as catalysts (O’Connor et al., 2003). In the alkylation of m-cresol with various alkylating agents such as 1-propanol, 2-propanol, propane, and isopropyl acetate on H-Beta zeolite (Nie and Resasco, 2012; Yusuke;, 2014), the authors highlight that in general, large-pore zeolites, such as H-Beta and HY zeolites, are more effective in alkylating cresol and other substituted phenolic compounds than small-pore zeolites, such as HZSM-5 (Nie and Resasco, 2012). Modern methods such as microwave-assisted reaction and a carbonized sulfonic acidic (CSA) resin as catalyst have also been studied in m-cresol alkylation. The authors reported a complete conversion of m-cresol was obtained within a contact time of 3 min at 1:5 M ratio of m-cresol to isopropyl alcohol (Ali and Gaikar, 2011). Other procedures for synthesizing thymol include the dehydrogenation of menthone as raw material, under the action of an activated carbon loaded Ni/Na2SiO3 catalyst. The authors highlight the easy separation of the obtained product, the reduction of the production cost, the short reaction time, and that the method is environmentally friendly (Shuai and Zhang, 2014). Other more complex procedures with more steps have also been described, such as condensation reaction of m-cresol and ethyl acetoacetate to obtain 4,7- diformazan butylcoumariii, followed by decarboxylation under highly basic hot conditions to form 5-methyl-2-prop-1-en-2-ylphenol, which is finally reduced to obtain thymol, in the presence of palladium carbon and ammonium formate as reducing agent (Yu et al., 2018). Divakar et al. (2000) compared two methods to synthesize thymol from m-cresol. The first route includes the formation of 4,7-dimethylcoumarin, which is then heated in an alkaline medium followed by acidification to obtain (E)-3-(2-hydroxy-4-methylphenyl) but-2-enoic acid that, when it is finally heated, the acid easily loses CO, to give 5-methyl-2-(prop-1-en-2-yl) phenol and finally, thymol. The second route includes the heat treatment of 4,7-dimethylcoumarin at a higher temperature (190 °C) in alkaline medium, directly producing 5-methyl-2-(prop-1-en-2-yl) phenol and finally obtaining thymol by hydrogenation (Divakar et al., 2000). All these synthesis methods are represented in Fig. 4.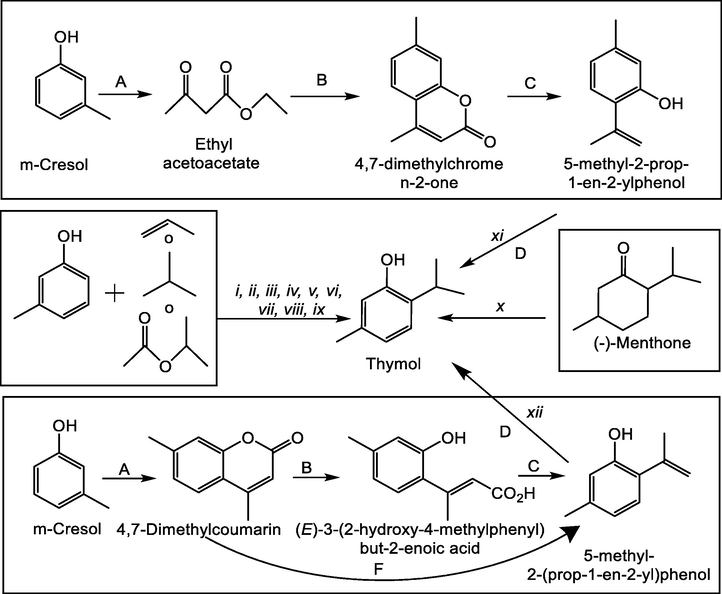
(i) γ-Al2O3, 250 °C/2h, 90% selectivity. (ii) Co&X@Al2O3, 210 °C/1h, >90% selectivity. (iii) Vitriol oil and aluminum chloride, 150 °C/2 ∼ 20 h, 98% selectivity. (iv) Zn–Al–MCM-41, 290 °C/2h, 100% selectivity. (v) MgAl-MCM-41, 300 °C/5h, >80% selectivity. (vi) H-ZSM-5, 250 °C/1h, 80% selectivity. (vii) H-ß zeolite, 200 °C/1h. (viii) ß-type zeolite, 180 °C/3h. (ix) Carbonized sulfonic acidic resin/microwave, 150 °C/3 min, 87% selectivity. (x) Ni/Na2SiO3, 360 °C, 83% selectivity. (xi) (A) Titanium tetrachloride, 120 °C/4 ∼ 6 h. (B) NaOH/KOH, 90 ∼ 186 °C/0.5 ∼ 6 h. (C) Palladium carbon, 0 ∼ 60 °C/2 ∼ 4 h. (D) Reducing agent is Ammonium formate as reducing agent, 80 ∼ 130 °C/0.5 ∼ 1.5 h. (xii). (A) Ethylacetoacetate, acidify, heat; (B) NaOH, heat/acidify; (C) heat; (D) H2. Raney Ni; (F) NaOH, ethanediol, heat/acidify.
Thymol is not only obtained on an industrial scale due to the large number of applications it has, but it is also a compound widely used in the synthesis of more complex molecules to improve their biological activity. This is why there are many publications on investigations of new synthetic routes through the chemical or biochemical transformation of thymol. Yoshiaki Noma et al. conducted research that includes biotransformations by using various kinds of bacteria and fungi, converting commercially available and cheap synthetic monoterpenoids, such as thymol, into other products with interesting properties (Fig. 5) (Noma and Asakawa, 2010).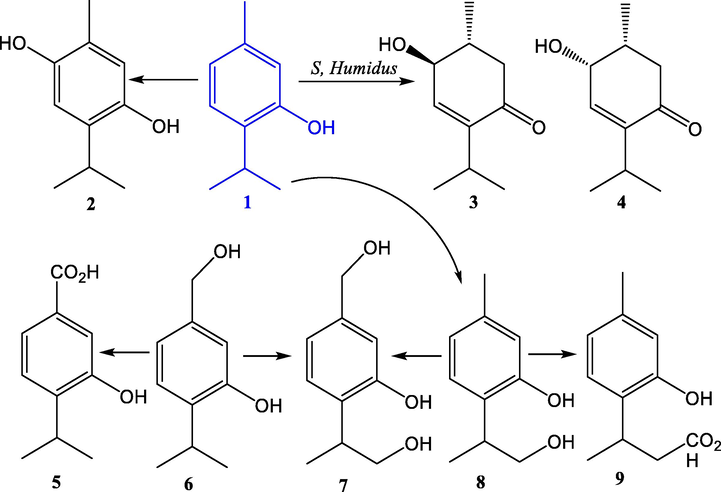
Biotransformation of thymol (1) to 6-hydroxythymol (2), (1R,2S)- (3) and (1R,2R)-2-hydroxy-3-p-menthen-5-one (4), thymol-7-oic acid (5), 7-hydroxythymol (6), 7,9-dihydroxythymol (7), 9-hydroxythymol (8), and thymol-9-oic acid (9), by the actinomycete strain Streptomyces humidus, Tu-1, and fungi Aspergillus niger, Mucor ramannianus, Rhizopus arrhizus, and Trichothecium roseum (Noma and Asakawa, 2010).
Another reaction of considerable commercial importance is the oxidation of thymol to thymoquinone, which has antitumor, hepatoprotective, chemotherapeutic, chemopreventive effects among many other applications in pharmaceutical chemistry (Badary et al., 2007; Kruk et al., 2000; Zhang et al., 2018). Skrobot et al. (2003) studied the oxidation of thymol to thymoquinone catalyzed by Y zeolite-entrapped manganese(III) tetra(4-N-benzylpyridyl)porphyrin and H2O2/ammonium acetate, at room temperature and atmospheric pressure, obtaining a conversion of 18% and 100% selectivity to thymoquinone. More recently Günay et al. (2016) studied the same reaction, this time in the presence of potassium peroxymonosulfate (KHSO5) and catalyzed by iron phthalocyanine tetrasulfonate (FePcTS), with a conversion of thymol to thymoquinone of 99% in 1 h at room temperature. Other compounds of interest include the synthesis of thymol/carvacrol derivatives with the carbamate moiety, whose inhibitory activity on acetylcholinesterase and butyrylcholinesterase was evaluated; these are two enzymes related to Alzheimer's disease. 5-Isopropyl-2-methylphenyl (3-fluorophenyl) carbamate and 5-isopropyl-2-methylphenyl (4-fluorophenyl) carbamate were found to be the two most potent inhibitors for each enzyme respectively (Kurt et al., 2017). Dhaneshwar et al. (2013) synthesized a co-drug of diacerein, an anthraquinone derivative with a marked effect on osteoarthritis (OA), with thymol, based on reports of the antioxidant and anti-inflammatory activity of thymol. The results of this study were promising for the treatment of OA. Brotzman et al. (2019) synthesized 4-oxobutanoate derivatives of carvacrol and thymol, and studied their inhibitory activity on the enzyme tyrosinase, finding that there is a strong relationship between the structure of the compound and the inhibitory activity, the derivatives that contained alkyl groups of three and four carbons being the most active. Havasi et al. (2020) synthesized heterocyclic sulfide derivatives containing thymol moiety and studied their activity to reduce cupric ions, and conducted tyrosinase coupling studies. Raghuvanshi et al. (2019) synthesized a group of thymol-based pyrazolines and chalcones, which were studied for antimalarial activity in both in vitro and in vivo tests. The results were positive, showing that all the analyzed compounds had activity against the strain of the human malaria parasite Plasmodium falciparum NF54.
The properties of thymol as disinfectant and/or odorant have also served as an inspiration to synthesize esters of alternating copolymers of maleic anhydride/styrene or vinyl acetate with thymol, eugenol, phenylethyl alcohol, and citronellol, in order to achieve controlled release of bioactive molecules. This research was carried out by Chitanu et al. (1999), who reported that by analyzing the different materials by TG, DSC and IR spectra showed that the active molecule is released at temperatures below 250 °C, followed by a renewal of anhydride rings by dehydration. On the other hand, one of the disadvantages of using thymol as a natural preservative in food and medicine is its low solubility in water and light decomposition. One way to counteract this is through glycosylation, which enables the conversion of unstable organic compounds with low solubility in polar solvents into soluble and stable compounds, thus improving their biological and pharmacological properties and expanding the range of applications (Mastelić et al., 2004). This is why Shimoda et al. (2006) studied the biotransformation of thymol and other compounds by cultured plant cells, turning them other more useful compounds (Fig. 6). Thus, 5-methyl-2-(1-ethylethyl)phenyl 6-O-(b-D-glucopyranosyl)-b-D-glucopyranoside was isolated from the suspension cells after the five-day incubation of thymol with a yield of 87%. On the other hand, Mastelic et al. (2004) studied the glycosylation of thymol and other molecules by chemical synthesis, by the Koenigs-Knorr-Zemplén method with yields between 19.5% and 52.2%.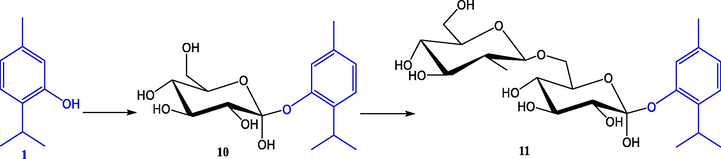
Glycosylation of thymol (1) 5-methyl-2-(1-methylethyl)phenyl b-D-glucopyranoside (10) and 5-methyl-2-(1-methylethyl)phenyl 6-O-(b-D-glucopyranosyl)-b-D-glucopyranoside (11) by the cultured cells of Eucalyptus perriniana (Shimoda et al., 2006).
5 Biological activities and applications
Since the Food and Drug Administration classified thymol as a GRAS, interest in investigating the possible biological applications of thymol in a great variety of fields such as medical, dentistry, veterinary, food, agrochemicals, among others, has increased. At the same time, the elucidation of its mechanism of action has received great interest from the scientific community. In this section we will first focus on the general pharmacological properties of thymol such as antioxidant and anti-inflammatory activity. Two properties of thymol that play an important role in several specific applications. In addition, the mechanism of antimicrobial action is briefly described. After this general introduction we will dedicate ourselves in depth to address some specific thymol applications.
5.1 General pharmacological remarks
5.1.1 Antioxidant activity
Natural antioxidants inhibit the spread of free radical reactions, thereby protecting the human body from disease and slowing oxidative rancidity of lipids in food, replacing potentially harmful synthetic additives (Tepe et al., 2007). Therefore, the antioxidant effect of thymol has also been studied both in disease and in food preservation. Deng et al. (2016) solubilized thymol in Tween 80 micelles to analyze the mechanism and effect of thymol solubilization on its antioxidant activity. The maximum solubilized concentration of thymol was 0.2% by weight, and no significant differences were observed between the average Z diameter of the empty micelles and the micelles solubilized with thymol. Tests of ferric reducing antioxidant potential (FRAP) and cupric ion reducing antioxidant capacity (CUPRAC) showed that the free thymol reducing antioxidant activity did not change after solubilization in Tween 80 micelles, but solubilized thymol did show greater activity to eliminate DPPH (2,2-diphenyl-1-picrylhydrazyl) and hydroxyl radicals than free thymol. These micelles can be used in food drinks. Similarly, Sedaghat Doost et al. (2019) developed thymol nanoemulsions, but they used Quillaja saponin (QS) biosurfactants (which are an eco-friendly alternative to the most widely used surfactants such as Tween 80, which is derived from petroleum) and edible oils such as high oleic sunflower oil (HOSO). The antioxidant activity of the free thymol and thymol nanoemulsions (4%) was tested with butylated hydroxytoluene and ascorbic acid, in raw chicken breast meat by means of the thiobarbituric acid reactive substance test, determining that nanoemulsions presented a significant improvement (p < 0.05) in AA compared to free thymol. This study suggests that thymol nanoemulsions can be used in the food industry, in cosmetic products, and for health care.
Considering that thymol will be present in food and food packaging, there is a need to study the extent to which thymol is safe for human health. In this context, Llana-Ruiz-Cabello et al. (2015) evaluated the pro-oxidant and antioxidant profiles of carvacrol, thymol, and their mixture (10:1), determining that carvacrol and its mixture with thymol (10:1) at low concentrations showed protection against induced oxidative stress. However, when high concentrations were analyzed, it was observed that they induced rather than prevented oxidative stress. These findings are important because they demonstrate the need to examine the concentration of thymol and other components such as carvacrol in food packaging and in food to prevent human overexposure to them, and thus ensure their safety, the authors concluded.
5.1.2 Anti-inflammatory
Inflammation is a defensive response to a foreign antigen or tissue injury that, if not treated properly, could cause irreversible damage (Arita et al., 2005). Riella et al. (2012) suggest that thymol may be a promising anti-inflammatory agent, since it significantly reduces edema and diminishes the affluence of leukocytes to the hurt area because it alters the cell membrane, inducing to a rapid leakage of intracellular components and deregulation of cellular function. Zhou et al. (2014) investigated the effects of thymol on allergic inflammation in asthma in ovalbumin (OVA) induced mice and explored its mechanism. The results revealed that thymol pretreatment reduced the level of OVA-specific immunoglobulin E, inhibited the recruitment of inflammatory cells in the respiratory tract, and decreased cytokine levels in bronchoalveolar lavage fluid. In addition, they noted a significant improvement in lung tissues. The authors suggest that the mechanism of action of thymol is possibly by inhibiting the activation of the transcription factor NF-κB, which has a great influence on the inflammatory process and other important processes such as survival, and cell development. The synergistic effect of thymol with other compounds on its anti-inflammatory activity has also been studied. A study by Liang et al. (2014) reports that thymol anti-inflammatory activity is associated with the descending inhibition of NF-κB signaling pathways and p-38 mitogen-activated protein kinases (MAPKs), in this case in mouse mammary epithelial cells. However, Gholijani et al. (2016) reported that thymol did not inhibit NF-κB expression and suggested that its anti-inflammatory effects could be due to the inhibition of other pro-inflammatory transcription factors, such as stress-activated protein kinases SAPK/JNK, signal transducer, and activator of transcription (STAT3), and several nuclear factors of activated T-cells (NFATs). The authors attribute these discrepancies between the results of Gholijani et al. (2016) and those of Liang et al. (2014) to the differences in the incubation time, the techniques and, in particular, the type of cells studied, which suggests the need to delve into these investigations to clarify the differences.
Some studies have also evaluated a possible synergy between thymol and other compounds. This is the case of Golbahari and Abtahi Froushani (2019) who, knowing the anti-inflammatory activity of nicotine and thymol, evaluated the effects of co-administering these two compounds on immunity in rheumatoid arthritis (RA) induced by Freund's complete adjuvant (FCA) in the Wistar rat. The authors reported that combined therapy at medium doses significantly reduced the effects of the RA and in a greater proportion than each drug only at full doses; in addition, the combined therapy did not show the immunosuppression side effect compared with using each of agents alone.
5.1.3 Antimicrobial mechanisms of action
In studies conducted by Wang et al. (2017) and Kwon et al. (2019) they wanted to investigate what the antibacterial mechanism of thymol is. For this, they evaluated its activity against Gram-positive S. aureus, finding that thymol antimicrobial activity results from its alteration of the lipid bilayer of the cytoplasmic membrane and the interaction with bacterial genomic DNA. When the bacteria were exposed to thymol-containing solutions, even at low concentrations, the composition of the fatty acids that form the lipid membrane was greatly affected, for example, the proportion of branched 12-methyltetradecanoic acid and 14-methylhexadecanoic acid decreased from 22.4% and 17.3% to 7.9% and 10.3%, respectively. At higher concentrations, thymol altered the integrity of the S. aureus cell membrane, which may reduce cell viability. Moreover, when studying the interaction of thymol with genomic DNA, it was found that a junction was formed between thymol and the minor groove of DNA with a constant binding value (Ka) of (1.22 ± 0.14) × 104M−1, which slightly destabilized the secondary structure of DNA, making it difficult for DNA molecules to aggregate (Wang et al., 2017). Chauhan and Kang (2014) determined the mechanism of action of thymol against Salmonella ser. Typhimurium confirming that it mainly alters the bacterial cell membrane, resulting in the uncontrolled release of intracellular materials such as potassium ions necessary for the normal metabolism and survival of bacteria. In addition, thymol significantly reduced the production of nitric oxide, as well as the level of glutathione, which helped the recovery of oxidative stress. Ferreira et al. (2019) investigated, in particular, the interaction of thymol with Langmuir lipid monolayers using dipalmitoylphosphotadylserine (DPPS) as a lipid that approximates a microbial and tumorigenic cell model. With this study, they determined that thymol expands DPPS monolayers and decreases their surface elasticity, thus changing the thermodynamic and rheological properties of membrane films. These changes in thymol-sensitive cell membranes increase the ability of thymol or other drugs to permeate the membrane by altering membrane fluidity, structural order, lipid packing density, and membrane morphology. A study by Boye et al. (2020) concluded that the high spectrum antibacterial activity of thymol is attributable to the hydroxyl moiety on C1 of the monoterpene nucleus, since when this hydroxyl group is etherified or esterified, they did not obtain significant antibacterial effects. However, they clarified that a greater exploitation of the chemical modification of the –OH on the C1 of the monoterpene thymol nucleus is necessary, possibly moving –OH to other carbons (C3 or C4).
5.2 Antimicrobial applications
5.2.1 Medical materials
For centuries, natural products have served as inspiration and raw material for the development of new drugs focused on treating microbial infections, essential oils being one of the main sources rich in secondary metabolites capable of inhibiting the growth of various pathogens. And added to the concern about the resistance of bacteria to traditional antibiotics, and given the relatively low frequency of infectious diseases in wild plants, the idea arises that these natural defense mechanisms can be very effective in treating diseases that affect humans. Many of those studies have dealt with thyme oil and other oils rich in thymol, carvacrol, menthol, and others (Kifer et al., 2016; Palaniappan and Holley, 2010; Pérez-Recalde et al., 2018). They focused on measuring their biological activity against a wide variety of pathogenic microorganisms and their mechanism of action, although most of the research published in recent years is related to the design of new materials for the treatment of wounds that prevent or reduce bacterial infection and have healing properties. Thus a wide variety of bandages based on biopolymeric materials have been designed with thymol as an additive (Kavoosi et al., 2013; Moeini et al., 2020). Walczak et al. (2020) investigated the antibacterial properties of films based on collagen and thymol, and evaluated them by measuring ATP level and enzymatic activity of S. aureus, E. coli, and Pseudomonas aeruginosa after the contact with materials. They found that collagen materials with thymol addition inhibit dehydrogenase activity and decrease ATP level of S. aureus, E. coli, and P. aeruginosa; furthermore, the materials were permeable for gaseous exchange and prevented secondary infection. Thymol addition increased hydrophobicity and roughness parameter, concluding that collagen with ≤ 0.5 mg thymol addition may be considered as a good alternative for traditional wound dressing. Michalska-Sionkowska et al. (2017) also studied the antimicrobial activity of collagen materials with the addition of thymol, reaching the same conclusion.
Koosehgol et al. (2017) prepared and characterized in situ chitosan/polyethylene glycol fumarate/thymol hydrogel as antibacterial wound dressing. These types of materials that combine different polymers have demonstrated better biological, mechanical, and biodegradable properties compared to the individual use of each component (Singh et al., 2017; Sionkowska, 2011). Khaldi et al. (2018) developed thymol and carvacrol based materials covalently bonded to kraft pulp fibers through triazine bonding and measured their antibacterial activity against E. coli and S. aureus. The authors stress that due to the covalent bonding, the leaching problem is avoided, and therefore the antibacterial properties of the material could be maintained for a longer period of time. In addition, thymol has a greater affinity with human skin cells compared to synthetic antibiotics, which makes it a better candidate to be an additive in this type of film, with the possibility of presenting fewer side effects in the affected area and promoting wound healing through natural treatment (Mollarafie et al., 2015). In the investigation carried out by Koosehgol et al. (2017), it was determined that the film with the highest amount of thymol (1.8% v/v) presented optimal properties that included acceptable mechanical characteristics, greater absorption of liquid water or water vapor, air permeability, more porous and rough structures and most importantly, excellent antibacterial activity against Gram-negative and Gram-negative bacteria. Nordin et al. (2020) prepared corn starch films with glycerol, thymol, and glycerol/thymol combination by solution molding. The results showed that the presence of glycerol and thymol led to synergistic effects such as better thermal stability and that the interaction between the compounds could be through hydrogen bonds. Jiji et al. (2019) developed hydrogels of thymol-enriched bacterial cellulose (BCT) and studied their antimicrobial activity against specific pathogens in burn wounds. The in vitro biocompatibility studies were carried out in mouse 3 T3 fibroblast cells. Through histopathological studies, the authors determined that the wound treated with the BCT hydrogel closed faster than the bacterial cellulose (BC) and the control groups (Fig. 7).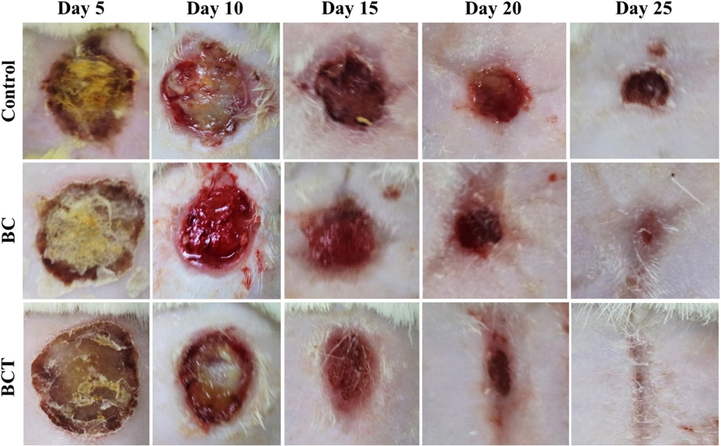
Representative images of burn wounds in control, BC, and BCT groups at days 5, 10, 5, 20, and 25 (Jiji et al., 2019).
On the other hand, Pieres et al. (2018) studied how to incorporate the active phase (thymol and beta-carotene) in a biopolymer composed of poly(dimethylsiloxane) on chitosan-alginate, analyzing two different methods: (1) immersion of the dried biopolymer in a solution containing the bioactive compound (IS) or (2) by supercritical carbon dioxide (scCO2) impregnation/deposition (SSI/D). The authors determined that, although by the two studied methods there was a low loading efficiency of thymol and beta-carotene in the films, it was a sufficient fraction for them to present biological activity, so they concluded that either method can be used to dope the biopolymer with the active phase. Finally, they highlighted that they used these two compounds because thymol acts as an anesthetic, antiseptic, and anti-inflammatory agent, and beta-carotene provides its antioxidant activity. It was studied the encapsulation of thymol in polylactic acid (PLA) (Marcet et al., 2018) and subsequently, its incorporation in gelatin films (Sáez-Orviz et al., 2020). The authors found that at the thymol concentrations studied, unlike the thymol encapsulated in PLA nanoparticles, all of the free thymol was completely evaporated during the film drying process. Additionally, gelatin films doped with PLA-thymol-nanoparticles showed ideal characteristics such as high transparency, a homogeneous microstructure, and antimicrobial activity.
But not only has the design of hydrogels and films been studied, but also the development of other materials such as nanoparticles consisting of metals, biopolymers, and thymol as an active phase with possible pharmacokinetic applications, antiproliferative activity of malignant cells, and route of drug administration (Alavi and Karimi, 2019; Venkatesan et al., 2017). In this direction, Manukumar et al. (2017) developed silver nanoparticles doped with thymol and chitosan (TC@AgNP) and evaluated them against a broad spectrum of microbial agents and antioxidant properties. The authors reported that TC@AgNP showed activity against Gram-positive S. aureus, S. epidermidis, S. haemolyticus, B. cereus, B. subtilis, and Gram-negative E. aerogenes, E. coli, S. typhimurium, S. flexneri, V. cholera, P. aeruginosa, and V. parahaemolyticus, all food-borne pathogens. They also had good antioxidant activity compared to the standard antibiotic Streptomycin.
5.2.2 Antimicrobial activity in the vapor phase
Although many reports have been described on the antimicrobial activity of thymol and its applications in different environments, industrial applications based on its volatility have been little developed, as well as few studies on its antimicrobial activity in vapor phase and there is no standard methodology for determining such activity. Even so, the few studies reported suggest possible applications, for example, in the production of new sprayable drugs used in respiratory and oral health therapies; disinfection products for the storage of agricultural products by controlled atmosphere; and in conservation and extension of the useful life of food products through active packaging (Houdkova and Kokoska, 2020; Reyes-Jurado et al., 2020). This is supported by some studies such as the one carried out by Mandras et al. (2016) who evaluated the antifungal activity (in the clinical strains of Candida albicans, Candida glabrata and Candida tropicalis) of some EO and its main components, including thymol, by means of two different methods: broth microdilution (BM) and vapour contact (VC) determining in most cases, more effective results with the VC method. Ács et al (2018) did a similar study, where they compared the antimicrobial activity of EO against pathogens that cause respiratory diseases such as Streptococcus pneumoniae, Streptococcus mutans, Streptococcus pyogenes, Haemophilus influenzae, Haemophilus parainfluenzae and Moraxella catarrhalis, by in vitro methods BM and VC, determining that EO of thyme was the most effective against S. mutans by the BM method and EO of cinnamon was the most effective against all pathogens by the VC method. The authors also highlight that although all the oils studied may be effective against diseases of the respiratory tract by the two methods studied, their activity is lower than that of commonly used antibiotics, which is why they suggest more studies on their possible use together. Netopilova et al. (2018) reached similar conclusions, who evaluated the synergistic antimicrobial activity between thymol and carvacrol in the liquid and vapor phase against twelve strains of S. aureus, finding an additive effect by the two methods studied against all strains evaluated when mixing the two compounds. For their part, López et al. (2007) studied the antimicrobial activity in the vapor phase of the main components of different EO against Gram-positive bacteria (Listeria monocytogenes) and Gram-negative bacteria (Salmonella choleraesuis), a mold (Aspergillus flavus) and a yeast (C. albicans), where cinnamaldehyde, thymol and carvacrol were the most effective compounds. To end this section, a very comprehensive study by Wang et al. (2016) evaluated the antibacterial activity in liquid and vapor phase of thymol and other phenolic compounds such as hinokithiol, carvacrol and menthol against the oral pathogens Aggregatibacter actinomycetemcomitans, S. mutans, S. aureus and E. coli under different conditions of temperature and pH, as well as synergistic effects between mixtures of compounds. The authors determined that the antibacterial activity of thymol is stable at room temperature both in liquid and vapor phases, but decreases at temperatures < 80 °C. There was no synergistic effect between carvacrol and thymol in the liquid phase and their activity in the vapor phase is inhibited in the presence of water and only Gram negative bacteria were sensitive to these two compounds in the vapor phase. As we can see, there is still vastly to investigate in this field.
5.2.3 Dentistry
Another health problem that attracts the attention of researchers is oral infections, where tooth decay and periodontitis represent the main oral infectious diseases worldwide, which are generated by bacteria such as S. aureus that adhere to biotic surfaces biotics (dental pieces), such as abiotic (dental implants) (Merghni et al., 2016). In addition, given the antibacterial properties of thymol, several studies have been carried out to analyze its efficiency against this type of bacteria and in the eradication of preformed bacterial biofilm both in implants and on the surface of teeth. Miladi et al. (2017) tested the antibacterial and antibiofilm activities of five components of essential oils: eugenol, carvacrol, thymol, p-cymene, and γ-terpinene (alone or in combination with tetracycline) against oral bacteria. The authors reported that the compounds analyzed induced selective antimicrobial activity and that there was a synergistic effect between the components studied and tetracycline (TET) with a reduction rate that varied from 2 to 8 times. On the other hand, an important antibiofilm activity (alone or in combination with antibiotics) was observed. Rezaeian et al. (2019) designed a novel system of dental resin doped with thymol to combat oral bacteria and evaluated its physico-mechanical characteristics, bonding strength, and antibacterial activity. The results showed a relevant antibacterial activity, as well as adequate cytocompatibility. In addition, this resin exhibited properties comparable to the control resin such as toughness and fracture resistance. The authors attributed this antibacterial activity and the prevention of biofilm formation to the great lipophilic nature of thymol, causing it to accumulate in the bacterial membrane, interfering with its proliferation. Botelho et al. (2007) evaluated the composition and antibacterial activity of the essential oil extracted from the species Lippia sidoides Cham. (Verbenaceae). Thymol (56.7%) and carvacrol (16.7%) were determined as major compounds. Antimicrobial activity was tested against cariogenic bacterial species of the genus Streptococcus and C. albicans, discovering that both the essential oil and the main compounds showed great antimicrobial activity against the organisms studied, C. albicans and S. mutans being the most sensitive microorganisms to the treatment.
To conclude this chapter concerning the applications of thymol in dentistry, it is important to review some literature on studies of commercially distributed thymol-containing products such as Listerine® antiseptic mouthwash and Cervitec® Plus protective varnish. Two products of common use for oral health and to prevent/treat diseases such as bacterial plaque, cavities, gingivitis and periodontitis, among others (Kokoska et al., 2019). In the case of Listerine®, it is mainly composed of a mixture of the major constituents of the essential oils of Eucalyptus spp., Gaultheria spp., Mentha piperita and Thymus vulgaris. Eucalyptol (0.0092%) and thymol (0.064%) are responsible for its antimicrobial activity, while menthol (0.042%) and methyl salicylate (0.06%) act as local anesthetic and cleaning agent, respectively (Kokoska et al., 2019). On the other hand, Cervitec® Plus varnish is composed of ethanol (90%), vinyl acetate copolymer, acrylate copolymer (8%), chlorhexidine (CHX, 1%) and thymol (1%) (Kokoska et al., 2019). A clinical study by Anand et al. (2012) on the efficacy of Cervitec® Plus in improving periodontal parameters, determined that although it is an adequate treatment for the control of subgingival infections, it works best when complemented with meticulous scaling and root planing in the treatment of chronic periodontitis. A study by Sachdeva et al. (2018) agrees with this conclusion and adds that periodic applications of the varnish generate a prolonged effect of its benefits. Similar conclusions were reached by George et al. (2010) who determined that Cervitec® Plus varnish significantly reduced the S. mutans count at the end of 1 month and Porphyromonas gingivalis at the end of 1 and 3 months compared to the placebo group, but this effect is lost after 3 months. For this reason they recommend its application at least once a month. They also highlight that no side effects were observed. For their part, Sehgal et al. (2018) evaluated the activity of Cervitec® Plus as a prophylactic and antibacterial product in patients with orthodontic treatment, observing a significant reduction of bacterial plaque in the CHX group at 3 and 6 months after the first visit compared to the initial value and with respect to the control group. However, there are studies that have not found evidence of anticaries prevention of Cervitec® Plus (Haukali and Poulsen, 2003; Tang et al., 2016), even so, it is known that chlorhexidine helps reduce the risk of tooth demineralization by influencing bacterial metabolism and reducing the amount of S. mutans, and that thymol is a strong antibacterial compound and these properties have a synergistic effect when applied with other anticariogenic agents such as fluoride-releasing chemically cured sealant and fluoride-releasing lightcured sealant (Park et al., 2019).
Now, referring to the studies reported on Listerine®, de Oliveira et al. (2018) evaluated the antibiofilm efficacy of different commercial mouthwashes, among them Listerine®, against S. aureus, E. faecalis, S. mutans, E. coli and P. aeruginosa bacteria, and C. albicans yeast; they also evaluated the cytotoxic effect of mouthwashes on gingival fibroblasts. They found that all the oral treatments analyzed presented a significant inhibition of biofilms and viable gingival fibroblasts and that there is no significant difference between them, being more effective against bacteria than with yeasts. Similarly, Erriu et al. (2013) reported the efficiency of Listerine® against A. actinomycetemcomitans after an exposure of at least 30 s. Excellent antibacterial activity of Listerine has also been reported against the pathogens P. gingivalis, Prevotella intermedia, Fusobacterium nucleatum, Eikenella corrodens (Richa et al., 2017; Vlachojannis et al., 2015). Regarding antifungal activity, a study by Shrestha et al. (2011) compared the antifungal effect of two commercial mouthwashes containing chlorhexidine and thymol (Hexidine® and Listerine®, respectively), determining that, although the two mouthwashes were effective in eliminating the strains of C. albicans and C. tropicalis, the Hexidine® mouthwash did so in time shorter and showed superior antifungal and fungicidal activities compared to Listerine®. Still, the authors recommend the use of both rinses as topical antifungal agents. De Andrade et al. (2009) evaluated the use of mouthwashes in patients in intensive care units, where Listerine® eliminated 90% of the evaluated strains. Finally, Milić et al. (2019) evaluated the possibility that Listerine® Cool Mint could cause cellular damage in the oral epithelial cells that are in direct contact with the rinse, determining that a statistically significant effect of differentiation and genomic stability of the oral cells was not observed, after two weeks of exposure to the product. All these studies show that commercial products such as Listerine® and Cervitec® Plus that contain thymol have great benefits for oral health and do not generate side effects in the amounts and times recommended by the manufacturers. Even so, more studies of the individual components and mixtures of them at different concentrations are needed, which could determine synergistic effects and further optimize the efficiency of these products, in concentrations that do not induce harm.
5.2.4 Veterinary
In the veterinary sector, research has also been carried out on possible applications of thymol to cure animal diseases. Arafa et al. (2020) studied the efficacy of thymol through in vitro and in vivo studies against coccidiosis in pigeons (Columba livia domestica), a disease caused by an apicomplexan protozoan parasite of the genus Eimeria. Different essential oils have shown oocystic activity, and specifically thymol (5 mg/mL) caused the destruction of chicken Eimeria oocysts in vitro (Remmal et al., 2013). The authors reported that in vitro studies showed that thymol solutions with concentrations ≥ 1.25% induced an important abnormality and nonsporulated Eimeria labbeana oocysts. In addition, biochemical parameters, including liver and kidney function tests, demonstrated the safety of thymol in pigeons, indicating that thymol can be safely used to control coccidiosis in pigeons. Similarly, Miró et al. (2020) studied the effect of thymol on the in vitro inhibition of hepatic S-oxygenation of the anthelmintic albendazole in sheep, since previous studies had shown thymol as a pharmacological alternative to treat gastrointestinal parasitic diseases of ruminants (André et al., 2017; Ferreira et al., 2016). The authors reported that in general, the results showed that thymol, in addition to having its own anthelmintic effect, can enhance ABZ anthelmintic activity by preventing its metabolic conversion into a less active metabolite (Miró et al., 2020). Soltani et al. (2014) studied the effect and mechanism of action of the essential oil of Shirazi thyme (Zataria multiflora) on the bacterium Lactococcus garvieae, a pathogen that affects various aquatic animals, including rainbow trout. The authors found that the oil inhibits the formation of the bacterium because it suppresses the expression of the epsD capsule gene in Lactococcus garvieae. This suggests that Z. multiflora oil may be used in the aquaculture industry to treat lactococcosis.
5.3 Food applications
5.3.1 Food preservative
One of the most common applications of thymol due to its antimicrobial activity is in the food industry as a natural preservative, since it can inhibit a variety of microorganisms that spoil food, prolonging its shelf life without compromising consumer health (Lee et al., 2020; Molva and Baysal, 2015; Tajkarimi et al., 2010; Tao et al., 2014). Cai et al. (2019) evaluated the effect of thymol on the survival and growth of Alicyclobacillus acidoterrestris, a non-pathogenic bacterium belonging to the Gram-positive species, which generates spores and spoils food. A. acidoterrestris has been found in many types of fruit juices, affecting the juice quality. The authors reported that thymol had good activity against this bacterium, both for vegetative cells (MIC = 0.25 mg/mL) and for spores (MIC = 0.5 mg/mL).
A drawback of essential oils as natural food preservatives is that their effective concentrations are usually high, altering the original flavor of the food. Therefore the need arises to find alternatives for their use, such as the combination of these molecules with other compounds that generate a synergistic effect, resulting in the same or better results, with a smaller amount of each compound (Wang et al., 2011). Thus Kim et al. (2020) determined the survival rate of different pathogens such as E. coli O157: H7, L. monocytogenes, and S. aureus in solutions containing highly concentrated thymol or carvacrol and NaCl (up to 15%, w/w), and compared it with the components separately, to investigate the synergistic interaction between NaCl and the main components of the essential oil of oregano against these target microorganisms. The authors determined that the combination of carvacrol or thymol (2.0 mM) plus NaCl (≥3%) completely inhibited all the bacteria analyzed. In contrast, individual treatments at equal concentrations had/showed minimal reduction in bacteria. These results were mainly attributed to the alteration of the membrane induced by carvacrol or thymol (19.6% of the cells were affected by treatment with 2.0 mM carvacrol for 10 min), since the presence of NaCl alters the osmotic cell balance.
Rivas et al. (2010) evaluated the antimicrobial activity of thymol and carvacrol against a selection of strains of verocytotoxigenic E. coli and other decomposition bacterial species using a model broth system. Several factors were found to affect the susceptibility of E. coli O157: H7 to carvacrol or thymol such as storage temperature, presence of water, pH, and inoculum size of E. coli O157: H7. Conversely, the antimicrobial activity of thymol or carvacrol was not affected by the presence of sodium chloride (0.5%–2.5%) or a cocktail of microflora. Another of the limitations of the use of thymol in food production is its low water solubility that inhibits uniform dispersion in aqueous media. For this reason, Li et al. (2017) studied the possibility of forming thymol nanoemulsions with high hydrophilic-lipophilic surfactants by spontaneous emulsification and assessed their activity as disinfectant washes on lettuce and blueberries inoculated with food-borne bacterial biofilms to simulate an application of food disinfection involving fresh products. They determined that while there was an antagonistic effect between thymol and surfactants, the best emulsion compositions showed/gave results comparable to chlorine efficiency at ∼50–200 ppm. Khan et al. (2020) investigated a control strategy combining solutions of natural substances such as 5% thymol and 2% chitosan and sealing under modified atmosphere packaging (MAP) using high permeability film and compared them with fruit treated only with distilled water and sealed under MAP conditions (MAPC) and others stored under air conditions (AIRC) used as controls. They concluded that the combined effects of the chemical and MAP treatments maintained fruit quality for 56 and 49 days with chitosan and thymol, respectively, compared to the MAPC and AIRC tests that lasted 28 days and 21 days respectively. Therefore, the preparation of food disinfectants that contain thymol is a suitable application for this compound. Myszka et al. (2016) evaluated the potential of the essential oil of T. vulgaris and its carvacrol and thymol components to prevent the formation of bacterial biofilms in the strain Pseudomonas fluorescens KM121. The authors reported that the analyzed compounds significantly inhibited biofilm formation on stainless steel surfaces (type 316L) and that only single bacterial cells were present on the surfaces.
The production of biodegradable packaging containing essential oils or the combination of their main components as antimicrobial agents has also been studied (Almasi et al., 2020; Guarda et al., 2011; Kazemi-Pasarvi et al., 2020; Liu et al., 2019). Li et al. (2020) developed gelatin films containing thymol nanoemulsions co-emulsified by gelatin and soy lecithin, which may have possible applications as new biodegradable GRAS packaging materials with the added benefit of prolonging the shelf life of food. These films showed effective inhibition against Gram-positive and Gram-negative bacteria. Tatlisu et al. (2019) manufactured and characterized thymol-loaded nanofiber (TLN) mats based on polyvinyl alcohol/whey protein, and evaluated their antifungal activity against Aspergillus parasiticus on the surface of kashar cheese, with in vivo and in situ tests, finding that the TLN mats had a greater activity against the mycelial growth of A. parasiticus than free thymol. Valero et al. (2006) evaluated the effect of combining a package doped with eugenol or thymol with MAP to prevent the deterioration of table grapes for 56 days. The authors determined that the grapes with the doped packaging and MAP presented insignificant damage in terms of sensorial, nutritional and functional properties, as well as a low presence of harmful microorganisms, compared to the control sample in which the deterioration was notable. Therefore, this technology is presented as an interesting and healthy alternative to the classic fungicide sulfur dioxide (SO2), to preserve the general quality of table grapes.
5.3.2 Feed additives in animal production
Due to the restriction of the prophylactic use of growth-promoting antibiotics in animal production, phytoadditives have become an important field of research studies, since it is proven that these substances can provide benefits on animal health and welfare, thanks its antioxidant and antibacterial properties, among others, but little is known about its mechanisms of action and metabolism in animals, as well as about the dose and time of administration in which the compound exhibits positive activity. Finally, there is also little information on its bioaccumulation in different organs, especially in animals for human consumption (Oceľová et al., 2019). One of these nutritional supplements is thyme EO, due to its high content of thymol. A study by Placha et al. (2014) evaluated the effects of thyme EO intake on the antioxidant status and the integrity of the intestinal barrier in broiler chickens, determining that the supply of a base diet supplemented with 0.5 g/kg of thyme oil, decreased the concentration of malondialdehyde (MDA) and increased the concentration of immunoglobulin A (IgA) in the duodenal mucosa, in the same way, it improved phagocytic activity in blood and the integrity of the intestinal barrier, which translates into a better antioxidant status and an improved immune response in the chicken. This same group evaluated the presence of thymol in the plasma, the duodenal wall and the breast muscle, as well as its influence on the antioxidant defense system, in broilers chickens, after ingesting different concentrations of EO thyme (0%, 0.05% and 0.1%, p/p) for 4 weeks. The authors determined that there is effective absorption of thymol through the digestive tract into the systemic circulation with 0.1% TEO supplements, as well as an increase in superoxide dismutase (SOD) activity and a significant decrease in malondialdehyde concentration. (MDA) in blood. Even so, only traces of thymol were found in the breast muscle. This means a marked antioxidant activity in blood but low lipid oxidation in muscles at this concentration (Placha et al., 2019). The research group of Haselmeyer et al. (2015) reached similar conclusions. In this case, broiler chickens were given thyme herb (leaves and flowers, without stem) instead of EO, as a dietary supplement at different concentrations (0, 0.1, 0.2, 0.3 and 1% w/w) for 35 days. They detected thymol in the 1% thyme group, in the intestine and blood plasma, and at concentrations close to the limit of quantification in the liver and muscle tissues. For their part, Ocel’ová et al. (2016) suggest that there is an intense metabolism of thymol in the liver of chickens and its accumulation in the kidney tissue, where this compound was found in greater quantity, even at concentrations of 0.5p/p of thyme EO as an additive and are consistent with a low accumulation in muscle tissue. The efficiency of thyme oil (35,40% thymol) at different concentrations (200, 300 and 400 ppm) with respect to the growth-promoting antibiotic flavophospholipol (100 ppm) in the diet of quail chicks, for 35 days, has also been compared. Significant benefits were found in quail fed 400 ppm EO, such as improved feed conversion index, decreased triglycerides, increased duodenal villi and muscle tone, and reduced oxidation rate during refrigerated storage compared to with control. Another benefit reported in this research was the inhibition of the growth of Gram positive bacteria and E. coli, even greater than with antibiotics (Dehghani et al., 2019, 2018). For its part, studies carried out by Fernández et al. (2019, 2017) evaluated the effect of feeding quail with thymol on the nutritional properties of their eggs and embryonic development, determining that a feeding with 4 g of thymol/kg of feed reduced the concentration of saturated fatty acids and increased the polyunsaturated fatty acids in the egg yolk after 14 days of administration and it was maintained after stopping consuming the supplement, which improves the nutritional quality of the eggs and is also beneficial for the embryos. In addition, no negative effects were determined in the hepatic histopathology of quail. When the effect of feeding supplemented with thymol was studied in rabbits, similar results were obtained (Bacova et al., 2020). These studies suggest that a diet supplemented with thymol directly or with substances containing thymol such as thyme (leaves, flowers and EO), provides nutraceutical benefits in animals such as poultry and rabbits without having an adverse effect on the animals or affecting their nutritional properties, at the concentrations studied.
Studies have also been conducted in pigs, which suggest that essential oil components such as thymol can promote the oxidative metabolism in the muscle and improve nutrient digestibility and gut health by regulating the gut microbiota and the immune system. However, the lack of standardized procedures for conducting research leads to variable results that are difficult to compare for consolidation of data (Luo et al., 2020; Peron, 2013). Even so, we will see some reports of studies on the effects of a diet supplemented with thymol on the health of pigs. One of the infections to which pigs are more prone to contract is Salmonella Typhimurium, a disease widely distributed in pig farms, and which represents a zoonotic risk. Janczyk et al. (2008) studied the effect of a high dose of thymol (1% w/w), added to the diet of weaned piglets (24 days old), on the microbial diversity of the small intestine in control animals and others exposed to the S. Typhimurium, determining that there was a slight effect of feeding supplemented with thymol on the pig jejunal microbial population, although it did not affect Salmonella infection. This shows that the effects determined in vitro may differ from in vivo studies. Similar conclusions were reached by Trevisi et al (2007) who, under the same study parameters (feeding supplemented with 1% w/w thymol, 24 days old), determined that the diet did not modify the fecal excretion of S. Typhimurium in pigs exposed to the bacteria, For their part, Anderson et al. (2012) observed the same effect of the ingestion of foods supplemented with thymol in the amount of Campylobacter spp. intestinal, suggesting this is due to the fact that at some point in the digestive process, thymol is absorbed, degraded or is no longer available and that this will probably be solved with encapsulation technologies that guarantee effective concentrations in the lower intestine and thus obtain in vivo, the results obtained in vitro. And precisely, Omonijo et al. (2018) conducted a study in which they developed thymol microparticles encapsulated in starch and alginate through a process of fusion granulation and evaluated them in in vitro tests in solutions that simulate salivary, stomach and intestinal fluids, determining a slow release of thymol. Now continue to perform in vivo tests. On the other hand, a study conducted by Panea and Ripoll (2020) determined that feeding pigs supplemented with thymol and other EO extracts affects the physicochemical characteristics of dry fermented sausages (DFS) and their acceptability by the consumer, especially when are combined with a diet low in salt, and suggest not supplying these supplements to pigs when the end goal is the production of DFS. As can be seen, much remains to be investigated in this field, especially in reference to standardized in vivo test procedures, which make it possible to unify results and reach solid conclusions on concentrations and ways of administering thymol that are effective in achieving the nutraceutical benefits desired in pig farms.
5.4 Other biological activities and applications
5.4.1 Natural agrochemical
One more application that has been given to thymol is in the field of natural agrochemicals, where the demand has increased considerably in recent years. Kumari et al. (2018) prepared a thymol nanoemulsion with saponin as a surfactant, due to the low solubility of thymol in water since laboratory experiments showed that nanoscale thymol has significantly higher antimicrobial activity compared to thymol in bulk, with the additional advantage that it does not have toxic effects on the soybean plant (Fig. 8).
Symptoms of bacterial pustule disease on soybean plants in pot experiments (a) lesions expanded and merged in control, (b) small yellow to brown lesions in soybean leaf at 0.06% v/v thymol nanoemulsion (Kumari et al., 2018).
Subsequently, the working group hypothesized that thymol nanoemulsion, in addition to functioning as a bactericide, could be a promising carrier of micronutrients in plants such as zinc, which is why they developed a zinc-functionalized thymol nanoemulsion (Zn- TNE), which showed a slow release of zinc and thymol, maintained antibacterial activity, and promoted plant growth. It is also presented as an option to provide other micronutrients and bioactive substances to plants (Kumari et al., 2019). Thymol can also help modulate plant physiology. A study by Cheng et al. (2020) concluded that thymol confers tolerance to salt stress by activating antioxidative defense and modulating Na+ homeostasis in rice root (Oryza sativa). Thymol treatment significantly decreased Na+ content in root cells upon salt stress, which might be ascribed to the upregulation of OsSOS1 (salt overly sensitive 1) facilitating Na+ exclusion. In addition, thymol stimulated the expression of genes encoding tonoplast OsNHX (Na+/H+ antiporter), which may help root cells to compartmentalize Na+ in vacuole. Thus, this study raises the possibility of carrying out thymol treatments in cultivated areas where salinity is high.
5.4.2 Insecticidal, acaricidal, and repellent properties
As already mentioned, essential oils are part of the defensive system of plants, but not only against minor organisms such as microorganisms but also against some more complex ones such as arthropod pests. Therefore, in recent years there has been growing interest in the study of the insecticidal, acaricidal, and repellent activity of different essential oils and their main components with promising results. Many of these studies showed high effectiveness, multiple mechanisms of action that make it more difficult to generate resistance to treatment, and low toxicity to nontarget vertebrates, which is the reason why they are considered as possible effective, cheap, and ecological mosquito and acarus larvicides (Pavela and Benelli, 2016; Regnault-Roger et al., 2012; Sertkaya et al., 2010). Tabari et al. (2017) studied the toxic and repellent activity of monoterpenes such as thymol, carvacrol and linalool against castor tick, Ixodes ricinus (Acari: Ixodidae), a species of veterinary and medical importance, which acts as vector for many pathogens that seriously affect human health such as Lyme disease. In all the parameters evaluated, unlike linalool, carvacrol and thymol showed promising results. In all larvae treated with carvacrol and thymol (1%, 2%, and 5%), mortality rates of 100% were reached after 24 h, representing greater larvicidal efficacy than permethrin. Similarly, at all concentrations tested carvacrol and thymol showed > 90% repellency in I. ricinus. Benelli et al. (2017) investigated the larvicidal toxicity of five essential oils (Pinus nigra, Hyssopus officinalis, Satureja montana, Aloysia citrodora, and Pelargonium graveolens) and the possible synergistic and antagonistic effects that could exist between them, against Culex quinquefasciatus Say (Diptera: Culicidae), a vector of lymphatic filariasis and of dangerous arboviral diseases, such as West Nile and St. Louis encephalitis. The authors analyzed the synergistic and antagonistic effects that might exist between the oils. The acute toxicity assays testing on C. quinquefasciatus larvae showed that. S. montana essential oil turned out to be the most toxic, with a LC50 value of 25.6 μLL−1. The authors attributed this result to the presence of phenolic compounds such as thymol (18.8%) and carvacrol (22.4%) and their precursors p-cymene (18.9%) and γ-terpinene (8.9%). The other oils analyzed did not present these compounds in their composition. The same compounds, analyzed independently, have also presented high toxicity against larvae of C. quinquefasciatus, with LD50 of 18 and 21 mgL−1, respectively (Pavela, 2015). Oliveira et al. (2018) studied the lethal and sublethal effects of the essential oil of Lippia sidoides and its main thymol compound against populations of Sitophilus zeamais (Coleoptera: Curculionidae), a weevil that affects corn plantations. To determine toxicity, they applied the treatments on/to five corn populations from different Brazilian regions and evaluated the lethal time and walking behavior for the most tolerant and susceptible populations. The authors found different levels of S. zeamais resistance, depending on the region, with LC50 ranging from 35.48 to 118.29 μLL−1 air for essential oil of L. sidoides, and from 65.00 to 91.23 μLL−1 air for thymol. Furthermore, the walking behavior of maize weevil showed that the essential oil of L. sidoides and thymol had a repellent effect. Tak et al. (2017) evaluated the acaricidal and repellent activity of 20 terpenes derived from plant essential oils and the effect of binary mixtures against Tetranychus urticae Koch (Acari: Tetranychidae). The four compounds with the highest toxicity were carvacrol, trans-cinnamaldehyde, α-terpineol, and thymol, and among these compounds, carvacrol showed the highest repellent activity (RC50 = 0.57 mg/mL), followed by thymol (1.86 mg/mL), α-terpineol (2.46 mg/mL) and trans-cinnamaldehyde (3.48 mg/mL). When evaluating the repellent activity of the combinations (1:1, w:w), several synergistic or antagonistic interactions were found for each compound in which thymol had the most synergistic interactions, in 6 of the 18 binary mixtures analyzed. Arena et al. (2020) studied the insecticidal and antibacterial effects of five essential oils, among which is Origanum vulgare, against Alphitobius diaperinus and its associated microorganisms. A. diaperinus is a global poultry pest that causes several problems, including the spread of pathogenic microorganisms. The authors reported that O. vulgare essential oil, whose main component is thymol (38.5%), presented high contact toxicity, with LC 50 = 0.128 µL/cm2; in addition, it strongly inhibited the growth of E. coli and S. aureus and significantly reduced the microbial load of the insect, indicating that it affected both insects and bacteria. This makes it a promising candidate to replace synthetic insecticides or to join current strategies for the management of A. diaperinus and its associated bacteria. Matos et al. (2019) evaluated the action of thymol in the cells and tissues of salivary glands of Rhipicephalus sanguineus sensu lato female (Acari: Ixodidae), a class of hard tick that attacks the brown dog. The authors concluded that exposure to thymol at concentrations between 1.25 and 5.0 mg/mL generated changes in the morphology and calcium levels in the cells and tissues of the singanglia and salivary glands, which makes thymol a possible acaricide in females partially infected with R. sanguineus.
For years, thymol and thymol mixtures with essential oils or essential oil components have been used to treat the parasitic mite Varroa destructor, which is a threat to beekeeping colonies, with mite mortality exceeding 90% (Imdorf et al., 1999). Although most studies suggest that thymol is not toxic to higher or nontarget species, a study by Glavan et al. (2020) reported that long-term consumption and high concentrations of thymol and carvacrol increase mortality on Carniolan honeybee workers (Apis carnellus mellifera). The authors determined that thymol and carvacrol caused mortality only in the highest concentrations tested (1% and 5%) and that both substances are effective against V. destructor in concentrations ten times lower than those causing significant honeybee mortality. However, prolonged exposure to carvacrol and thymol at concentrations of 0.05% causes accumulation in honeybees. The sublethal consequences were evidenced by increased activity of acetylcholinesterase (AChE), an enzyme involved in the control of neurotransmission, and the activity of the detoxifying enzyme glutathione S-transferase (GST) in heads and thoraxes. For these reasons, the authors suggest suspending treatment for detoxification times for bees, although this could have a lower effect on treatment (Glavan et al., 2020). It is also important to note that traces of thymol have been reported in products related to bees such as honey and pollen but below the threshold that would cause changes in taste and odor (Ares et al., 2020; Tananaki et al., 2014). Similarly, Zolfaghari and Vatanparast (2020) studied the possible toxic effects of thymol on the snail Caucasotachea atrolabiata, evaluating its action on the neuronal activity of the mollusk, and its mechanism of action. The authors found that thymol altered the property of action potentials and caused the epileptiform burst of their neurons; this effect was partially reversible after washing. They also determined that the mechanism of action of thymol involved the inhibition of calcium and potassium currents when interacting with cellular ion channels, emphasizing the vulnerability of the nervous system to this compound, which can be one of the possible causes of the biological activities of thymol in other organism and even in microorganisms.
5.4.3 Antifouling activity
All material immersed in sea water, whether natural or artificial such as maritime vessels, port structures, marine farms and others, are quickly covered with a macromolecular film, which favors colonization by prokaryotes (mostly bacteria), unicellular (microalgae, protozoans) and multicellular eukaryotes (barnacles, mussels, tubeworms, etc.), in a phenomenon called biofouling. Although biofouling is a natural process, when it occurs on artificial structures submerged by man it becomes a major environmental and economic problem, especially for maritime transport. Biofouling in ship hulls is the main source of cross-contamination of marine species on the world's coasts (Molnar et al., 2008). Moreover, it increases the weight of the boat, which makes it difficult to maneuver and increases fuel consumption, generating higher maintenance costs (M. P. Schultz and J. A. Bendick, 2010). To date, the most successful technique to prevent fouling settlement has been to coat ship hulls with paints containing an antifouling agent and in this context; only one work was found in which thymol has been studied as a possible additive of paints with antifouling activity. Thus, Pérez et al. (2015) investigated the antifouling activity of thymol, eugenol, and guaiacol against barnacle Balanus amphitrite in lab assays. Thymol affected naupliar activity (lost phototactic response and swimming movements) from low concentrations. Particularly, LC50 was determined at 4.41 µM for thymol. Cyprid settlement was also affected by thymol (EC50 2.26 µM). After 24 h exposure, nauplii and cyprids were transferred to fresh artificial seawater, and the percentage recovery was estimated. Larvae exposed (100%) to all thymol concentrations were able to recover their normal functions (metamorphosis and ability to settle), demonstrating that thymol has a temporary effect. In other words, it inhibits the larvae but does not kill them. It does not act as a classic biocide. In the same work, the authors studied an antifouling paint with a copper content (1.6%) 10 times lower than that of a traditional paint (16%) with the addition of 2% thymol. It could be concluded that both paints had a similar performance. In other words, thymol in combination with small quantities of copper could be considered as a promising environmentally friendly alternative for antifouling technology. Undoubtedly, further research on this combination will encouraged.
6 Conclusions
Throughout history, thyme and other thymol-containing plants have been used as medicinal herbs and food seasonings by different cultures. Today, due to extensive research, their pharmacological properties have been proven, and the wide spectrum of applications in other areas has been demonstrated. In structure, the thymol molecule is very simple, but there are studies that report that its activity is due to the hydroxyl moiety on C1 of the monoterpene nucleus. Reported studies on thymol include evaluation of extraction methods, its bioavailability, measurement of its biological activity, possible synergistic effects with other compounds, and mechanism of action, its toxicity, and possible side effects. Similarly, the increasing number of publications on the development of new materials containing thymol, such as biopolymers, metal nanospheres, nanoemulsions, and dental resins, with possible applications in the treatment of wounds, caries, disinfectants, and food packaging, or natural agrochemicals in the last five years is worth noting. Finally, it is a fact that all the studies carried out are difficult to pare and do not reach a single conclusion, since the data reported in the literature have been obtained with different methods and different amounts of evidence, and some are even contradictory. Even so, most of the reported studies agree that thymol has great potential as a bioactive component. Therefore, the research avenues have not been exhausted in any way and there are still new applications to study such as its possible antifouling activity. Studies that are more exhaustive are needed on concentrations of acute and chronic toxicity and teratogenicity, considering their pharmacological and food preservative applications. Although the FDA has classified it as GRAS in food products, it is necessary to be clear about the concentration at which it is safe. However, the toxicity of thymol to plants, animals, and other organisms, whether terrestrial or aquatic, should be thoroughly explored prior to the introduction of this compound into the environment.
Acknowledgements
This research work was supported by CONICET, ANPCyT, Universidad Nacional de La Plata and CICPBA.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Analgesic, antipyretic and anti-inflammatory effects of essential oil of Lippia multiflora. Fitoterapia. 2003;74:231-236.
- [CrossRef] [Google Scholar]
- Thymol and carvacrol production from leaves of wild Palestinian Majorana syriaca. Bioresour. Technol.. 2008;99:3914-3918.
- [CrossRef] [Google Scholar]
- Antibacterial activity evaluation of selected essential oils in liquid and vapor phase on respiratory tract pathogens. BMC Complement. Altern. Med.. 2018;18:1-9.
- [CrossRef] [Google Scholar]
- Comparative study on the essential oils of Artemisia judaica and A. herba-alba from Saudi Arabia. Arab. J. Chem.. 2020;13:2053-2065.
- [CrossRef] [Google Scholar]
- Antioxidant properties and composition of Majorana syriaca extracts. Eur. J. Lipid Sci. Technol.. 2007;109:247-255.
- [CrossRef] [Google Scholar]
- A review on the beneficial effect of thymol on health and production of fish. Rev. Aquac.. 2020;1–10
- [CrossRef] [Google Scholar]
- Biosynthesis of Ag and Cu NPs by secondary metabolites of usnic acid and thymol with biological macromolecules aggregation and antibacterial activities against multi drug resistant (MDR) bacteria. Int. J. Biol. Macromol.. 2019;128:893-901.
- [CrossRef] [Google Scholar]
- Microwave-assisted process intensification of synthesis of thymol using carbonized sulfonic acidic resin (CSA) catalyst. Ind. Eng. Chem. Res.. 2011;50:6543-6555.
- [CrossRef] [Google Scholar]
- Development and characterization of pectin films activated by nanoemulsion and Pickering emulsion stabilized marjoram (Origanum majorana L.) essential oil. Food Hydrocoll.. 2020;99 105338
- [CrossRef] [Google Scholar]
- Essential oil composition and antimicrobial activity of Oliveria decumbens. Fitoterapia. 2005;76:704-707.
- [CrossRef] [Google Scholar]
- Chlorhexidine-thymol varnish as an adjunct to scaling and root planing: A clinical observation. J. Oral Biol. Craniofacial Res.. 2012;2:83-89.
- [CrossRef] [Google Scholar]
- Effect of Thymol or Diphenyliodonium Chloride on Performance. Gut Fermentation Characteristics, and Campylobacter Colonization in Growing Swine. 2012;34(75):758-761.
- [CrossRef] [Google Scholar]
- Anthelmintic effect of thymol and thymol acetate on sheep gastrointestinal nematodes and their toxicity in mice. Rev. Bras. Parasitol. Veterinária. 2017;26:323-330.
- [CrossRef] [Google Scholar]
- Thymol efficacy against coccidiosis in pigeon (Columba livia domestica) Prev. Vet. Med.. 2020;176 104914
- [CrossRef] [Google Scholar]
- Arena, J.S., Merlo, C., Defagó, M.T., Zygadlo, J.A., 2020. Insecticidal and antibacterial effects of some essential oils against the poultry pest Alphitobius diaperinus and its associated microorganisms. J. Pest Sci. (2004). 93, 403–414. https://doi.org/10.1007/s10340-019-01141-5
- Simultaneous determination of carvacrol and thymol in bee pollen by using a simple and efficient solvent extraction method and gas chromatography-mass spectrometry. J. Pharm. Biomed. Anal.. 2020;181 113124
- [CrossRef] [Google Scholar]
- Resolvin E1, an endogenous lipid mediator derived from omega-3 eicosapentaenoic acid, protects against 2,4,6-trinitrobenzene sulfonic acid-induced colitis. Proc. Natl. Acad. Sci.. 2005;102:7671-7676.
- [CrossRef] [Google Scholar]
- Quality characters, chemical composition and biological activities of oregano (Origanum spp.) Essential oils from Central and Southern Argentina. Ind. Crop. Prod.. 2015;63:203-213.
- [CrossRef] [Google Scholar]
- A panoramic view on phytochemical, nutritional, ethanobotanical uses and pharmacological values of Trachyspermum ammi Linn. Asian Pac. J. Trop. Biomed.. 2014;4:S545-S553.
- [CrossRef] [Google Scholar]
- Effect of thymol addition and withdrawal on some blood parameters, antioxidative defence system and fatty acid profile in rabbit muscle. Animals. 2020;10:1248.
- [CrossRef] [Google Scholar]
- Anticlastogenic activity of thymoquinone against benzo(a)pyrene in mice. Food Chem. Toxicol.. 2007;45:88-92.
- [CrossRef] [Google Scholar]
- Intraspecific chemical variability of the essential oils of Moroccan endemic Origanum elongatum L. (Lamiaceae) from its whole natural habitats. Arab. J. Chem.. 2020;13:3070-3081.
- [CrossRef] [Google Scholar]
- Chemical composition and antibacterial activities of the essential oils of Lippia chevalieri and Lippia multiflora from Burkina Faso. Phytochemistry. 2003;62:209-212.
- [CrossRef] [Google Scholar]
- Antimicrobial activity and chemical composition of saccocalyx satureioides coss. et Dur. Essential oil and extract obtained by microwave extraction. Comparison with hydrodistillation. J. Essent. Oil Res.. 2008;20:174-178.
- [CrossRef] [Google Scholar]
- Acute larvicidal toxicity of five essential oils (Pinus nigra, Hyssopus officinalis, Satureja montana, Aloysia citrodora and Pelargonium graveolens) against the filariasis vector Culex quinquefasciatus : Synergistic and antagonistic effects. Parasitol. Int.. 2017;66:166-171.
- [CrossRef] [Google Scholar]
- Born, F. de S., da Camara, C.A.G., de Melo, J.P.R., de Moraes, M.M., 2018. Acaricidal property of the essential oil from Lippia gracilis against Tetranychus urticae and a natural enemy, Neoseiulus californicus, under greenhouse conditions. Exp. Appl. Acarol. 75, 491–502. https://doi.org/10.1007/s10493-018-0286-3
- Thymus vulgaris essential oil: chemical composition and antimicrobial activity. J. Med. Life. 2014;7(3):56-60.
- [Google Scholar]
- Carum copticum L.: A herbal medicine with various pharmacological effects. Biomed Res. Int.. 2014;2014:1-11.
- [CrossRef] [Google Scholar]
- Antimicrobial activity of the essential oil from Lippia sidoides, carvacrol and thymol against oral pathogens. Brazilian J. Med. Biol. Res.. 2007;40:349-356.
- [CrossRef] [Google Scholar]
- The hydroxyl moiety on carbon one (C1) in the monoterpene nucleus of thymol is indispensable for anti-bacterial effect of thymol. Heliyon. 2020;6 e03492
- [CrossRef] [Google Scholar]
- Synthesis and tyrosinase inhibitory activities of 4-oxobutanoate derivatives of carvacrol and thymol. Bioorg. Med. Chem. Lett.. 2019;29:56-58.
- [CrossRef] [Google Scholar]
- Buckle, J., 2014. How Essential Oils Work, in: Livingstone, C. (Ed.), Clinical Aromatherapy. Elsevier, Londres, pp. 15–36. https://doi.org/10.1016/B978-0-7020-5440-2.00002-4
- Antibacterial activity and mechanism of thymol against Alicyclobacillus acidoterrestris vegetative cells and spores. LWT. 2019;105:377-384.
- [CrossRef] [Google Scholar]
- Effect of different operating conditions in cloud point assisted extraction of thymol from Ajwain (Trachyspermum Ammi L.) seeds and recovery using solvent. J. Food Sci. Technol.. 2017;54:4353-4361.
- [CrossRef] [Google Scholar]
- Thymol disrupts the membrane integrity of Salmonella ser. typhimurium in vitro and recovers infected macrophages from oxidative stress in an ex vivo model. Res. Microbiol.. 2014;165:559-565.
- [CrossRef] [Google Scholar]
- Thymol confers tolerance to salt stress by activating anti-oxidative defense and modulating Na+ homeostasis in rice root. Ecotoxicol. Environ. Saf.. 2020;188 109894
- [CrossRef] [Google Scholar]
- Maleic copolymers with pendant disinfectant and/or odorant molecules II. Thermal and thermo-oxidative behaviour. Polym. Degrad. Stab.. 1999;65:75-85.
- [CrossRef] [Google Scholar]
- Characterization of the essential oil volatiles of satureja thymbra and Satureja parnassica: influence of harvesting time and antimicrobial activity. J. Agric. Food Chem.. 2006;54:3139-3145.
- [CrossRef] [Google Scholar]
- Effects of carvacrol, thymol and essential oils containing such monoterpenes on wound healing: a systematic review. J. Pharm. Pharmacol.. 2018;jphp.13054
- [CrossRef] [Google Scholar]
- Cruz, E.M. de O., Costa-Junior, L.M., Pinto, J.A.O., Santos, D. de A., Araujo, S.A. de, Arrigoni-Blank, M. de F., Bacci, L., Alves, P.B., Cavalcanti, S.C. de H., Blank, A.F., 2013. Acaricidal activity of Lippia gracilis essential oil and its major constituents on the tick Rhipicephalus (Boophilus) microplus. Vet. Parasitol. 195, 198–202. https://doi.org/10.1016/j.vetpar.2012.12.046
- Lippia gracilis Schauer essential oil nanoformulation prototype for the control of Thielaviopis paradoxa. Ind. Crops Prod.. 2018;117:245-251.
- [CrossRef] [Google Scholar]
- Action of mouthwashes on Staphylococcus spp. isolated in the saliva of community and hospitalized individuals. Brazilian J. Pharm. Sci.. 2009;45:551-557.
- [CrossRef] [Google Scholar]
- Chemical composition and antibacterial activity of essential oils from Thymus spinulosus Ten. (Lamiaceae) J. Agric. Food Chem.. 2003;51:3849-3853.
- [CrossRef] [Google Scholar]
- Mouthwashes: an in vitro study of their action on microbial biofilms and cytotoxicity to gingival fibroblasts. Gen. Dent.. 2018;66:28-34.
- [Google Scholar]
- In vitro and in vivo evaluation of thyme (Thymus vulgaris) essential oil as an alternative for antibiotic in quail diet1. J. Anim. Sci.. 2019;97:2901-2913.
- [CrossRef] [Google Scholar]
- Effect of pennyroyal, savory and thyme essential oils on Japanese quail physiology. Heliyon. 2018;4 e00881
- [CrossRef] [Google Scholar]
- Physical characterization and antioxidant activity of thymol solubilized Tween 80 micelles. Sci. Rep.. 2016;6:38160.
- [CrossRef] [Google Scholar]
- Studies on synthesis, stability, release and pharmacodynamic profile of a novel diacerein-thymol prodrug. Bioorg. Med. Chem. Lett.. 2013;23:55-61.
- [CrossRef] [Google Scholar]
- Evaluation of the environmental fate of thymol and phenethyl propionate in the laboratory. Pest Manag. Sci.. 2008;64:775-779.
- [CrossRef] [Google Scholar]
- A novel route to thymol from m-cresol. Org. Prep. Proced. Int.. 2000;32:92-94.
- [CrossRef] [Google Scholar]
- Bioactivity of essential oil from Lippia gracilis Schauer against two major coconut pest mites and toxicity to a non-target predator. Crop Prot.. 2019;125 104913
- [CrossRef] [Google Scholar]
- Dunn, B., 2013. A Brief History of Thyme [WWW Document]. A+E Networks. URL http://www.history.com/news/hungry-history/a-brief-history-of-thyme (accessed 6.13.16).
- Chemical composition of the essential oil and supercritical CO2 extracts of Zataria multiflora Boiss. Food Chem.. 2003;83:357-361.
- [CrossRef] [Google Scholar]
- Oliveria decumbens, a bioactive essential oil: Chemical composition and biological activities. Iran. J. Pharm. Res.. 2019;18:412-421.
- [Google Scholar]
- Oil essential mouthwashes antibacterial activity against aggregatibacter actinomycetemcomitans: A comparison between antibiofilm and antiplanktonic effects. Int. J. Dent.. 2013;2013
- [CrossRef] [Google Scholar]
- Effects of thymol and carvacrol, constituents of Thymus vulgaris L. essential oil, on the inflammatory response. Evidence-Based Complement. Altern. Med.. 2012;2012:1-10.
- [CrossRef] [Google Scholar]
- Dynamics of thymol dietary supplementation in quail (Coturnix japonica): Linking bioavailability, effects on egg yolk total fatty acids and performance traits. PLoS ONE. 2019;14:1-23.
- [CrossRef] [Google Scholar]
- Thymol feed supplementation in quail alters the percentages of nutritionally relevant egg yolk fatty acids: effects throughout incubation. J. Sci. Food Agric.. 2017;97:5233-5240.
- [CrossRef] [Google Scholar]
- Thymol in cellular membrane models formed by negative charged lipids causes aggregation at the air-water interface. Chem. Phys. Lett.. 2019;717:87-90.
- [CrossRef] [Google Scholar]
- Thymus vulgaris L. essential oil and its main component thymol: Anthelmintic effects against Haemonchus contortus from sheep. Vet. Parasitol.. 2016;228:70-76.
- [CrossRef] [Google Scholar]
- Antitussive effects of nasal thymol challenges in healthy volunteers. Respir. Physiol. Neurobiol.. 2013;187:104-107.
- [CrossRef] [Google Scholar]
- Chlorhexidine varnishes effectively inhibit Porphyromonas gingivalis and Streptococcus mutans - An in vivo study. J. Indian Soc. Periodontol.. 2010;14:178.
- [CrossRef] [Google Scholar]
- Modulatory effects of thymol and carvacrol on inflammatory transcription factors in lipopolysaccharide-treated macrophages. J. Immunotoxicol.. 2016;13:157-164.
- [CrossRef] [Google Scholar]
- Comparison of sublethal effects of natural acaricides carvacrol and thymol on honeybees. Pestic. Biochem. Physiol.. 2020;104567
- [CrossRef] [Google Scholar]
- Synergistic benefits of nicotine and thymol in alleviating experimental rheumatoid arthritis. Life Sci.. 2019;239 117037
- [CrossRef] [Google Scholar]
- Antibacterial activity and chemical composition of ajowan (Carum copticum Benth. & Hook) essential oil. J. Agric. Sci. Technol.. 2011;13:203-208.
- [Google Scholar]
- Vapour phase alkylation of ortho-, meta- and para-cresols with isopropyl alcohol in the presence of sol–gel prepared alumina catalyst. Appl. Catal. A Gen.. 2004;277:91-97.
- [CrossRef] [Google Scholar]
- The antimicrobial activity of microencapsulated thymol and carvacrol. Int. J. Food Microbiol.. 2011;146:144-150.
- [CrossRef] [Google Scholar]
- Oxidation of thymol and carvacrol to thymoquinone with KHSO5 catalyzed by iron phthalocyanine tetrasulfonate in a methanol-water mixture. Catal. Lett.. 2016;146:2306-2312.
- [CrossRef] [Google Scholar]
- Effects of thyme as a feed additive in broiler chickens on thymol in gut contents, blood plasma, liver and muscle. J. Sci. Food Agric.. 2015;95:504-508.
- [CrossRef] [Google Scholar]
- Effect of a varnish containing chlorhexidine and thymol (Cervitec®) on approximal caries in 13- to 16-year-old schoolchildren in a low caries area. Caries Res.. 2003;37:185-189.
- [CrossRef] [Google Scholar]
- Antioxidant and tyrosinase docking studies of heterocyclic sulfide derivatives containing a thymol moiety. Inorganica Chim. Acta. 2020;505:1-9.
- [CrossRef] [Google Scholar]
- Introduction of Thymus daenensis into cultivation: Analysis of agro-morphological, phytochemical and genetic diversity of cultivated clones. Ind. Crops Prod.. 2019;131:14-24.
- [CrossRef] [Google Scholar]
- Volatile antimicrobial agents and in vitro methods for evaluating their activity in the vapour phase: a review. Planta Med.. 2020;86:822-857.
- [CrossRef] [Google Scholar]
- GC/MS evaluation of thyme (Thymus vulgaris L.) oil composition and variations during the vegetative cycle. J. Pharm. Biomed. Anal.. 2002;29:691-700.
- [CrossRef] [Google Scholar]
- Use of essential oils for the control of Varroa jacobsoni Oud. in honey bee colonies. Apidologie. 1999;30:209-228.
- [CrossRef] [Google Scholar]
- Anticancer activity of thymol: A literature-based review and docking study with Emphasis on its anticancer mechanisms. IUBMB Life. 2019;71:9-19.
- [CrossRef] [Google Scholar]
- Thymus vulgaris essential oil and thymol inhibit biofilms and interact synergistically with antifungal drugs against drug resistant strains of Candida albicans and Candida tropicalis. J. Mycol. Med.. 2019;100911
- [CrossRef] [Google Scholar]
- Effect of process parameters on aqueous extraction of thymol and other phytonutrients from herbal seed Ajwain (Trachyspermum ammi L.) J. Appl. Res. Med. Aromat. Plants. 2018;11:27-36.
- [CrossRef] [Google Scholar]
- Effect of thymol on microbial diversity in the porcine jejunum. Int. J. Food Microbiol.. 2008;126:258-261.
- [CrossRef] [Google Scholar]
- Thymol enriched bacterial cellulose hydrogel as effective material for third degree burn wound repair. Int. J. Biol. Macromol.. 2019;122:452-460.
- [CrossRef] [Google Scholar]
- Mechanical, physical, antioxidant, and antimicrobial properties of gelatin films incorporated with thymol for potential use as nano wound dressing. J. Food Sci.. 2013;78:E244-E250.
- [CrossRef] [Google Scholar]
- Preparation, characterization, and permeability of novel poly (lactic acid)-based blends filled with thymol and ZnO. Polym. Test.. 2020;89 106550
- [CrossRef] [Google Scholar]
- Comparison of essential oil composition of Carum copticum obtained by supercritical carbon dioxide extraction and hydrodistillation methods. Food Chem.. 2004;86:587-591.
- [CrossRef] [Google Scholar]
- Synthesis and antibacterial properties of thymol and carvacrol grafted onto lignocellulosic kraft fibers. J. Bioact. Compat. Polym.. 2018;33:558-570.
- [CrossRef] [Google Scholar]
- Combined effects of natural substances and modified atmosphere packaging on reducing enzymatic browning and postharvest decay of longan fruit. Int. J. Food Sci. Technol.. 2020;55:500-508.
- [CrossRef] [Google Scholar]
- Antimicrobial potency of single and combined mupirocin and monoterpenes, thymol, menthol and 1,8-cineole against Staphylococcus aureus planktonic and biofilm growth. J. Antibiot. (Tokyo). 2016;69:689-696.
- [CrossRef] [Google Scholar]
- Sodium chloride significantly enhances the bactericidal actions of carvacrol and thymol against the halotolerant species Escherichia coli O157:H7, Listeria monocytogenes, and Staphylococcus aureus. LWT. 2020;122 109015
- [CrossRef] [Google Scholar]
- Short communication: Interaction of the isomers carvacrol and thymol with the antibiotics doxycycline and tilmicosin: In vitro effects against pathogenic bacteria commonly found in the respiratory tract of calves. J. Dairy Sci.. 2017;100:970-974.
- [CrossRef] [Google Scholar]
- Autumn essential oils of Greek oregano. Phytochemistry. 1997;44:883-886.
- [CrossRef] [Google Scholar]
- Plant-derived products as antibacterial and antifungal agents in human health care. Curr. Med. Chem.. 2019;26:5501-5541.
- [CrossRef] [Google Scholar]
- Study of the effect of extract of Thymus vulgaris on anxiety in male rats. J. Tradit. Complement. Med.. 2016;6:257-261.
- [CrossRef] [Google Scholar]
- Preparation and characterization of in situ chitosan/polyethylene glycol fumarate/thymol hydrogel as an effective wound dressing. Mater. Sci. Eng. C. 2017;79:66-75.
- [CrossRef] [Google Scholar]
- The effect of thymol and its derivatives on reactions generating reactive oxygen species. Chemosphere. 2000;41:1059-1064.
- [CrossRef] [Google Scholar]
- Thymus vulgaris. In: Press A., ed. Medicinal Spices and Vegetables from Africa: Therapeutic Potential Against Metabolic, Inflammatory, Infectious and Systemic Diseases. Cambridge, MA: Elsevier Inc.; 2017. p. :599-609.
- [CrossRef] [Google Scholar]
- Zinc-functionalized thymol nanoemulsion for promoting soybean yield. Plant Physiol. Biochem.. 2019;145:64-74.
- [CrossRef] [Google Scholar]
- Thymol nanoemulsion exhibits potential antibacterial activity against bacterial pustule disease and growth promotory effect on soybean. Sci. Rep.. 2018;8:6650.
- [CrossRef] [Google Scholar]
- Synthesis, anticholinesterase activity and molecular modeling study of novel carbamate-substituted thymol/carvacrol derivatives. Bioorg. Med. Chem.. 2017;25:1352-1363.
- [CrossRef] [Google Scholar]
- Inhibitory effects of thymol on the cytotoxicity and inflammatory responses induced by Staphylococcus aureus extracellular vesicles in cultured keratinocytes. Microb. Pathog.. 2019;134 103603
- [CrossRef] [Google Scholar]
- Synergistic antimicrobial activity of oregano and thyme thymol essential oils against Leuconostoc citreum in a laboratory medium and tomato juice. Food Microbiol.. 2020;90 103489
- [CrossRef] [Google Scholar]
- Thymol nanoemulsions formed via spontaneous emulsification: Physical and antimicrobial properties. Food Chem.. 2017;232:191-197.
- [CrossRef] [Google Scholar]
- Gelatin films incorporated with thymol nanoemulsions: Physical properties and antimicrobial activities. Int. J. Biol. Macromol.. 2020;150:161-168.
- [CrossRef] [Google Scholar]
- Thymol inhibits LPS-stimulated inflammatory response via down-regulation of NF-κB and MAPK signaling pathways in mouse mammary epithelial cells. Inflammation. 2014;37:214-222.
- [CrossRef] [Google Scholar]
- Genomic characterization, molecular cloning and expression analysis of two terpene synthases from Thymus caespititius (Lamiaceae) Planta. 2013;238:191-204.
- [CrossRef] [Google Scholar]
- Oregano essential oil loaded soybean polysaccharide films: Effect of Pickering type immobilization on physical and antimicrobial properties. Food Hydrocoll.. 2019;87:165-172.
- [CrossRef] [Google Scholar]
- In vitro pro-oxidant/antioxidant role of carvacrol, thymol and their mixture in the intestinal Caco-2 cell line. Toxicol. Vitr.. 2015;29:647-656.
- [CrossRef] [Google Scholar]
- Vapor-phase activities of cinnamon, thyme, and oregano essential oils and key constituents against foodborne microorganisms. J. Agric. Food Chem.. 2007;55:4348-4356.
- [CrossRef] [Google Scholar]
- Luo, P., Luo, L., Zhao, W., Wang, Leshan, Sun, L., Wu, H., Li, Y., Zhang, R., Shu, G., Wang, S., Gao, P., Zhu, X., Xi, Q., Zhang, Y., Wang, Lina, Jiang, Q., 2020. Dietary thymol supplementation promotes skeletal muscle fibre type switch in longissimus dorsi of finishing pigs 1–9. https://doi.org/10.1111/jpn.13269
- Schultz, M.P., Bendick, J.A., E.R., 2010. Economic impact of biofouling on a naval surface ship. J. Biofouling 87–98. https://doi.org/10.1080/08927014.2010.542809
- Thyme (Thymus vulgaris L.) oils. In: Press A., ed. Essential Oils in Food Preservation, Flavor and Safety. London: Elsevier Inc.; 2016. p. :825-834.
- [CrossRef] [Google Scholar]
- Liquid and vapour-phase antifungal activities of essential oils against Candida albicans and non-albicans Candida. BMC Complement. Altern. Med.. 2016;16:1-7.
- [CrossRef] [Google Scholar]
- Promising biocidal activity of thymol loaded chitosan silver nanoparticles (T-C@AgNPs) as anti-infective agents against perilous pathogens. Int. J. Biol. Macromol.. 2017;102:1257-1265.
- [CrossRef] [Google Scholar]
- Production and characterisation of biodegradable PLA nanoparticles loaded with thymol to improve its antimicrobial effect. J. Food Eng.. 2018;239:26-32.
- [CrossRef] [Google Scholar]
- Antibacterial and antifungal activities of thymol: A brief review of the literature. Food Chem.. 2016;210:402-414.
- [CrossRef] [Google Scholar]
- Chemical analysis and antimicrobial activities of the essential oils of Satureja thymbra L. and Thymbra spicata L. and their main components. Arch. Biol. Sci.. 2011;63:457-464.
- [CrossRef] [Google Scholar]
- Gas chromatography-mass spectrometry analysis of free and glycoconjugated aroma compounds of seasonally collected Satureja montana L. Food Chem.. 2003;80:135-140.
- [CrossRef] [Google Scholar]
- Synthesis of selected naturally occurring glucosides of volatile compounds. Their chromatographic and spectroscopic properties. Croat. Chem. Acta. 2004;77:491-500.
- [Google Scholar]
- Thymol action on cells and tissues of the synganglia and salivary glands of Rhipicephalus sanguineus sensu lato females (Acari: Ixodidae) Ticks Tick. Borne. Dis.. 2019;10:314-320.
- [CrossRef] [Google Scholar]
- Chemotypic variation of essential oils in the medicinal plant, Anemopsis californica. Phytochemistry. 2008;69:919-927.
- [CrossRef] [Google Scholar]
- Composition and antimicrobial activity of anemopsis californica leaf oil. J. Agric. Food Chem.. 2005;53:8694-8698.
- [CrossRef] [Google Scholar]
- High potential of adhesion to biotic and abiotic surfaces by opportunistic Staphylococcus aureus strains isolated from orthodontic appliances. Microb. Pathog.. 2016;91:61-67.
- [CrossRef] [Google Scholar]
- Antimicrobial activity of collagen material with thymol addition for potential application as wound dressing. Polym. Test.. 2017;63:360-366.
- [CrossRef] [Google Scholar]
- Synergistic effect of eugenol, carvacrol, thymol, p-cymene and γ-terpinene on inhibition of drug resistance and biofilm formation of oral bacteria. Microb. Pathog.. 2017;112:156-163.
- [CrossRef] [Google Scholar]
- Milić, M., Bolanča, I., Gjirlić, D., Benković, V., 2019. Assessment of Listerine Cool Mint mouthwash influence on possible DNA damage measured by buccal micronucleus cytome assay-preliminary results. Genet. Appl. 3, 24. https://doi.org/10.31383/ga.vol3iss1pp24-35
- Supercritical CO2-assisted production of PLA and PLGA foams for controlled thymol release. Mater. Sci. Eng. C. 2019;99:394-404.
- [CrossRef] [Google Scholar]
- In vitro inhibition of the hepatic S-oxygenation of the anthelmintic albendazole by the natural monoterpene thymol in sheep. Xenobiotica. 2020;50:408-414.
- [CrossRef] [Google Scholar]
- Trachyspermum ammi (L.) Sprague: Chemical composition of essential oil and antimicrobial activities of respective fractions. J. Evidence-Based Complement. Altern. Med.. 2015;20:50-56.
- [CrossRef] [Google Scholar]
- Wound healing and antimicrobial effect of active secondary metabolites in chitosan-based wound dressings: a review. Carbohydr. Polym.. 2020;115839
- [CrossRef] [Google Scholar]
- Carum copticum Benth. & Hook., essential oil chemotypes. Food Chem.. 2007;100:1217-1219.
- [CrossRef] [Google Scholar]
- Antibacterial and wound healing properties of thymol (Thymus vulgaris Oil) and its application in a novel wound dressing. J. Med. Plants. 2015;14:69-81.
- [Google Scholar]
- Assessing the global threat of invasive species to marine biodiversity. Front. Ecol. Environ.. 2008;6:485-492.
- [CrossRef] [Google Scholar]
- Antimicrobial activity of grape seed extract on Alicyclobacillus acidoterrestris DSM 3922 vegetative cells and spores in apple juice. LWT - Food Sci. Technol.. 2015;60:238-245.
- [CrossRef] [Google Scholar]
- Production of thymol rich extracts from ajwain (Carum copticum L.) and thyme (Thymus vulgaris L.) using supercritical CO2. Ind. Crops Prod.. 2020;145 112072
- [CrossRef] [Google Scholar]
- Inhibition of quorum sensing-related biofilm of Pseudomonas fluorescens KM121 by Thymus vulgare essential oil and its major bioactive compounds. Int. Biodeterior. Biodegradation. 2016;114:252-259.
- [CrossRef] [Google Scholar]
- Plants belonging to the genus Thymus as antibacterial agents: From farm to pharmacy. Food Chem.. 2015;173:339-347.
- [CrossRef] [Google Scholar]
- Pharmacological properties and molecular mechanisms of thymol: Prospects for its therapeutic potential and pharmaceutical development. Front. Pharmacol.. 2017;8:1-34.
- [CrossRef] [Google Scholar]
- Fumigant toxicity of essential oil from Artemisia sieberi Besser against three stored-product insects. J. Stored Prod. Res.. 2007;43:123-128.
- [CrossRef] [Google Scholar]
- Evaluation of in vitro growth-inhibitory effect of carvacrol and thymol combination against Staphylococcus aureus in liquid and vapour phase using new broth volatilization chequerboard method. Fitoterapia. 2018;129:185-190.
- [CrossRef] [Google Scholar]
- Chemical variation of leaf essential oil at different stages of plant growth and in vitro antibacterial activity of Thymus vulgaris Lamiaceae, from Iran. Beni-Suef Univ. J. Basic Appl. Sci.. 2014;3:87-92.
- [CrossRef] [Google Scholar]
- Improving carbon retention in biomass conversion by alkylation of phenolics with small oxygenates. Appl. Catal. A Gen.. 2012;447–448:14-21.
- [CrossRef] [Google Scholar]
- The synthesis of thymol, chlorothymol and homologs of thymol by the intramolecular rearrangement of meta-cresyl ethers. J. Am. Chem. Soc.. 1932;54:1063-1070.
- [CrossRef] [Google Scholar]
- Chemical composition, antimicrobial, antioxidant and antitumor activity of Thymus serpyllum L., Thymus algeriensis Boiss. and Reut and Thymus vulgaris L. essential oils. Ind. Crops Prod.. 2014;52:183-190.
- [CrossRef] [Google Scholar]
- Noma, Y., Asakawa, Y., 2010. 3.19 - Biotransformation of Monoterpenoids 669–801. https://doi.org/http://dx.doi.org/10.1016/B978-008045382-8.00742-5
- Overexpression of TPS2 gene to increase thymol content using Agrobacterium tumefaciens-mediated transformation in Trachyspermum ammi (Qom ecotype) Ind. Crops Prod.. 2019;130:63-70.
- [CrossRef] [Google Scholar]
- Effects of glycerol and thymol on physical, mechanical, and thermal properties of corn starch films. Food Hydrocoll.. 2020;106 105884
- [CrossRef] [Google Scholar]
- Alkylation of phenol and m-cresol over zeolites. Collect. Czechoslov. Chem. Commun.. 2003;68:1949-1968.
- [CrossRef] [Google Scholar]
- Ocel’ová, V., Chizzola, R., Pisarčíková, J., Novak, J., Ivanišinoviá, O., Faix, Š., 2016. Effect of Thyme Essential Oil Supplementation on Thymol Content in Blood Plasma, Liver, Kidney and Muscle in Broiler Chickens. Nat. Prod. Commun. 11, 1545–1550.
- Thymol in the intestinal tract of broiler chickens after sustained administration of thyme essential oil in feed. J. Anim. Physiol. Anim. Nutr. (Berl). 2019;103:204-209.
- [CrossRef] [Google Scholar]
- Essential oil of Lippia sidoides and its major compound thymol: Toxicity and walking response of populations of Sitophilus zeamais (Coleoptera: Curculionidae) Crop Prot.. 2018;112:33-38.
- [CrossRef] [Google Scholar]
- Thymus vulgaris L. extract has antimicrobial and anti-inflammatory effects in the absence of cytotoxicity and genotoxicity. Arch. Oral Biol.. 2017;82:271-279.
- [CrossRef] [Google Scholar]
- Omonijo, F.A., Kim, S., Guo, T., Wang, Q., Gong, J., Lahaye, L., Bodin, J., Nyachoti, M., Liu, S., Yang, C., 2018. Development of Novel Microparticles for E ff ective Delivery of Thymol and Lauric Acid to Pig Intestinal Tract. https://doi.org/10.1021/acs.jafc.8b02808
- Anticholinesterase and antioxidant activities of Savoury (Satureja thymbra L.) with identified major terpenes of the essential oil. Food Chem.. 2012;134:48-54.
- [CrossRef] [Google Scholar]
- Use of natural antimicrobials to increase antibiotic susceptibility of drug resistant bacteria. Int. J. Food Microbiol.. 2010;140:164-168.
- [CrossRef] [Google Scholar]
- Pig feedstuff effect on the physicochemical and sensory properties of low-salt, dry-fermented sausages. Anim. Sci. J.. 2020;91 e13458
- [CrossRef] [Google Scholar]
- Park, A.B., Choi, W., Kim, J., Kim, K., Lee, S., 2005. Monoterpenes from Thyme (Thymus vulgaris) as potential mosquito repellents 21, 80–83. https://doi.org/10.2987/8756-971X(2005)21[80:MFTTVA]2.0.CO;2
- Effectiveness of caries-preventing agents on initial carious lesions within the scope of orthodontic therapy. Korean J. Orthod.. 2019;49:246.
- [CrossRef] [Google Scholar]
- Trachyspermum ammi (L.) fruit essential oil influencing on membrane permeability and surface characteristics in inhibiting food-borne pathogens. Food Control. 2011;22:725-731.
- [CrossRef] [Google Scholar]
- Acute toxicity and synergistic and antagonistic effects of the aromatic compounds of some essential oils against Culex quinquefasciatus Say larvae. Parasitol. Res.. 2015;114:3835-3853.
- [CrossRef] [Google Scholar]
- Essential oils as ecofriendly biopesticides? Challenges and constraints. Trends Plant Sci.. 2016;21:1000-1007.
- [CrossRef] [Google Scholar]
- Could essential oils enhance biopolymers performance for wound healing? A systematic review. Phytomedicine. 2018;38:57-65.
- [CrossRef] [Google Scholar]
- Evaluation of low copper content antifouling paints containing natural phenolic compounds as bioactive additives. Mar. Environ. Res.. 2015;109:177-184.
- [CrossRef] [Google Scholar]
- The effect of essential oil compounds on performance and gut health. Asian poork Mag. 2013:3-6.
- [Google Scholar]
- A synthesis of thymol from p-cymene. J. Ind. Eng. Chem.. 1920;12:733-734.
- [CrossRef] [Google Scholar]
- Towards wound dressings with improved properties: Effects of poly(dimethylsiloxane) on chitosan-alginate films loaded with thymol and beta-carotene. Mater. Sci. Eng. C. 2018;93:595-605.
- [CrossRef] [Google Scholar]
- Effect of thymol on the broiler chicken antioxidative defence system after sustained dietary thyme oil application. Br. Poult. Sci.. 2019;60:589-596.
- [CrossRef] [Google Scholar]
- Effect of thyme essential oil and selenium on intestine integrity and antioxidant status of broilers. Br. Poult. Sci.. 2014;55:105-114.
- [CrossRef] [Google Scholar]
- The efficacy of techniques for the disinfection of artificial sub-surface dentinal caries lesions and their effect on demineralization and remineralization in vitro. J. Dent.. 2007;35:490-495.
- [CrossRef] [Google Scholar]
- Evaluation of antioxidant and antimicrobial effects of shallot (Allium ascalonicum L.) fruit and ajwain (Trachyspermum ammi (L.) Sprague) seed extracts in semi-fried coated rainbow trout (Oncorhynchus mykiss) fillets for shelf-life extension. LWT - Food Sci. Technol.. 2016;65:112-121.
- [CrossRef] [Google Scholar]
- Synthesis of thymol-based pyrazolines: An effort to perceive novel potent-antimalarials. Bioorg. Chem.. 2019;88 102933
- [CrossRef] [Google Scholar]
- Essential oils in insect control: low-risk products in a high-stakes world. Annu. Rev. Entomol.. 2012;57:405-424.
- [CrossRef] [Google Scholar]
- Oocysticidal effect of essential oil components against chicken eimeria oocysts. Int. J. Vet. Med. Res. Reports. 2013;1–8
- [CrossRef] [Google Scholar]
- Industrial Crops & Products Antimicrobial activity of Mexican oregano (Lippia berlandieri), thyme (Thymus vulgaris), and mustard (Brassica nigra) essential oils in gaseous phase. Ind. Crop. Prod.. 2019;131:90-95.
- [CrossRef] [Google Scholar]
- Essential oils in vapor phase as alternative antimicrobials: A review. Crit. Rev. Food Sci. Nutr.. 2020;60:1641-1650.
- [CrossRef] [Google Scholar]
- A novel thymol-doped enamel bonding system: Physico-mechanical properties, bonding strength, and biological activity. J. Mech. Behav. Biomed. Mater.. 2019;100 103378
- [CrossRef] [Google Scholar]
- Evaluation and comparison of the antimicrobial effect of two different mouthwashes on selected periodontal pathogens: An in vitro study. J. Curr. Res. Sci. Med.. 2017;3:40.
- [CrossRef] [Google Scholar]
- Anti-inflammatory and cicatrizing activities of thymol, a monoterpene of the essential oil from Lippia gracilis, in rodents. J. Ethnopharmacol.. 2012;143:656-663.
- [CrossRef] [Google Scholar]
- Inhibition of verocytotoxigenic Escherichia coli in model broth and rumen systems by carvacrol and thymol. Int. J. Food Microbiol.. 2010;139:70-78.
- [CrossRef] [Google Scholar]
- Development of an electrochemical method to determine phenolic monoterpenes in essential oils. Talanta. 2019;196:362-369.
- [CrossRef] [Google Scholar]
- Rodrigues, V., Cabral, C., Évora, L., Ferreira, I., Cavaleiro, C., Cruz, M.T., Salgueiro, L., 2019. Chemical composition, anti-inflammatory activity and cytotoxicity of Thymus zygis L. subsp. sylvestris (Hoffmanns. & Link) Cout. essential oil and its main compounds. Arab. J. Chem. 12, 3236–3243.
- Antimicrobial activity and chemical composition of Thymus vulgaris, Thymus zygis and Thymus hyemalis essential oils. Food Control. 2008;19:681-687.
- [CrossRef] [Google Scholar]
- Comparison of clinical effectiveness of single and multiple applications of 1% chlorhexidine varnish (Cervitec Plus) along with scaling and root planing in patients with chronic periodontitis. J. Indian Soc. Periodontol.. 2018;22:523.
- [CrossRef] [Google Scholar]
- Chemical composition of essential oils in Zataria multiflora Boiss. From different parts of Iran and their radical scavenging and antimicrobial activity. Food Chem. Toxicol.. 2010;48:1562-1567.
- [CrossRef] [Google Scholar]
- PLA nanoparticles loaded with thymol to improve its incorporation into gelatine films. J. Food Eng.. 2020;269 109751
- [CrossRef] [Google Scholar]
- Zataria multiflora Boiss. (Shirazi thyme)—An ancient condiment with modern pharmaceutical uses. J. Ethnopharmacol.. 2013;145:686-698.
- [CrossRef] [Google Scholar]
- Salehi, B., Mishra, A.P., Shukla, I., Sharifi-Rad, M., Contreras, M. del M., Segura-Carretero, A., Fathi, H., Nasrabadi, N.N., Kobarfard, F., Sharifi-Rad, J., 2018. Thymol, thyme, and other plant sources: Health and potential uses. Phyther. Res. 32, 1688–1706. https://doi.org/10.1002/ptr.6109
- Production of thymol nanoemulsions stabilized using Quillaja Saponin as a biosurfactant: Antioxidant activity enhancement. Food Chem.. 2019;293:134-143.
- [CrossRef] [Google Scholar]
- Chemical composition of the essential oil of three Iranian Satureja species (S. mutica, S. macrantha and S. intermedia) Food Chem.. 2005;91:1-4.
- [CrossRef] [Google Scholar]
- Efficacy of chlorhexidine varnish in patients undergoing multibracket fixed orthodontic treatment: A controlled clinical study. Biomed. Pharmacol. J.. 2018;11:945-950.
- [CrossRef] [Google Scholar]
- Comparison of mesoporous and microporous solid acid catalysts for highly selective synthesis of thymol by vapor phase isopropylation of m-cresol. Microporous Mesoporous Mater.. 2008;109:458-469.
- [CrossRef] [Google Scholar]
- Acaricidal activities of the essential oils from several medicinal plants against the carmine spider mite (Tetranychus cinnabarinus Boisd.) (Acarina: Tetranychidae) Ind. Crops Prod.. 2010;31:107-112.
- [CrossRef] [Google Scholar]
- Natural products. In: Science E., ed. Approaches to Design and Synthesis of Antiparasitic Drugs. New York: El Sevier; 1997. p. :347-383.
- [CrossRef] [Google Scholar]
- Biotransformation of thymol, carvacrol, and eugenol by cultured cells of Eucalyptus perriniana. Phytochemistry. 2006;67:2256-2261.
- [CrossRef] [Google Scholar]
- In vitro antifungal effect of mouth rinses containing chlorhexidine and thymol. J. Dent. Sci.. 2011;6:1-5.
- [CrossRef] [Google Scholar]
- Shuai Fangwen, Zhang Jiawei, W.X., 2014. Thymol synthesis method. CN104177233A.
- Effect of light and natural ventilation systems on the growth parameters and carvacrol content in the in vitro cultures of Plectranthus amboinicus (Lour.) Spreng. Plant Cell, Tissue Organ Cult.. 2017;129:501-510.
- [CrossRef] [Google Scholar]
- Acacia gum polysaccharide based hydrogel wound dressings: Synthesis, characterization, drug delivery and biomedical properties. Carbohydr. Polym.. 2017;165:294-303.
- [CrossRef] [Google Scholar]
- Current research on the blends of natural and synthetic polymers as new biomaterials: Review. Prog. Polym. Sci.. 2011;36:1254-1276.
- [CrossRef] [Google Scholar]
- Monoterpenes oxidation in the presence of Y zeolite-entrapped manganese(III) tetra(4-N-benzylpyridyl)porphyrin. J. Mol. Catal. A Chem.. 2003;201:211-222.
- [CrossRef] [Google Scholar]
- Chemical composition of essential oils of Thymus and mentha species and their antifungal activities. Molecules. 2009;14:238-249.
- [CrossRef] [Google Scholar]
- Shirazi thyme (Zataria multiflora) essential oil suppresses the expression of the epsD capsule gene in Lactococcus garvieae, the cause of lactococcosis in farmed fish. Aquaculture. 2014;433:143-147.
- [CrossRef] [Google Scholar]
- Toxic and repellent activity of selected monoterpenoids (thymol, carvacrol and linalool) against the castor bean tick, Ixodes ricinus (Acari: Ixodidae) Vet. Parasitol.. 2017;245:86-91.
- [CrossRef] [Google Scholar]
- Antimicrobial herb and spice compounds in food. Food Control. 2010;21:1199-1218.
- [CrossRef] [Google Scholar]
- Acaricidal and repellent activity of plant essential oil-derived terpenes and the effect of binary mixtures against Tetranychus urticae Koch (Acari: Tetranychidae) Ind. Crops Prod.. 2017;108:786-792.
- [CrossRef] [Google Scholar]
- Evaluation of the impact of Exomite ProTM on Varroa mite (Varroa destructor) populations and honeybee (Apis mellifera) colonies: efficacy, side effects and residues. Parasitol. Res.. 2014;113:1251-1259.
- [CrossRef] [Google Scholar]
- The antimicrobial effect of chlorhexidine varnish on mutans streptococci in patients with fixed orthodontic appliances: a systematic review of clinical efficacy. Int. J. Dent. Hyg.. 2016;14:53-61.
- [CrossRef] [Google Scholar]
- Synthesis and characterization of β-cyclodextrin inclusion complexes of thymol and thyme oil for antimicrobial delivery applications. LWT - Food Sci. Technol.. 2014;59:247-255.
- [CrossRef] [Google Scholar]
- Fabrication and characterization of thymol-loaded nanofiber mats as a novel antimould surface material for coating cheese surface. Food Packag. Shelf Life. 2019;21 100347
- [CrossRef] [Google Scholar]
- Antioxidative activity of the essential oils of Thymus sipyleus subsp. sipyleus var. sipyleus and Thymus sipyleus subsp. sipyleus var. rosulans. J. Food Eng.. 2007;66:447-454.
- [CrossRef] [Google Scholar]
- Antiglycation, antioxidant, and cytotoxic activities of Uvaria chamae root and essential oil composition. Nat. Prod. Res.. 2020;34:880-883.
- [CrossRef] [Google Scholar]
- Essential oil composition, total phenolic, flavonoid contents, and antioxidant activity of Thymus species collected from different regions of Iran. Food Chem.. 2017;220:153-161.
- [CrossRef] [Google Scholar]
- Thymol, carvacrol, and antioxidant accumulation in Thymus species in response to different light spectra emitted by light-emitting diodes. Food Chem.. 2020;307 125521
- [CrossRef] [Google Scholar]
- Sequencing and variation of terpene synthase gene (TPS2) as the major gene in biosynthesis of thymol in different Thymus species. Phytochemistry. 2020;169 112126
- [CrossRef] [Google Scholar]
- Antibacterial effect of essential oils from two medicinal plants against Methicillin-resistant Staphylococcus aureus (MRSA) Phytomedicine. 2010;17:142-145.
- [CrossRef] [Google Scholar]
- Effect of dietary addition of thymol on growth, salivary and gastric function, immune response, and excretion of Salmonella enterica serovar Typhimurium, in weaning pigs challenged with this microbe strain. Ital. J. Anim. Sci.. 2007;6:374-376.
- [CrossRef] [Google Scholar]
- Chemotypes and terpene synthase genes in Thymus genus: State of the art. Ind. Crops Prod.. 2018;124:530-547.
- [CrossRef] [Google Scholar]
- U.S. Food and Drug Administration (FDA), 2020. Substances Added to Food (formerly EAFUS) [WWW Document]. URL https://www.accessdata.fda.gov/scripts/fdcc/?set=FoodSubstances&sort=Sortterm&order=ASC&startrow=1&type=basic&search=thymol
- The combination of modified atmosphere packaging with eugenol or thymol to maintain quality, safety and functional properties of table grapes. Postharvest Biol. Technol.. 2006;41:317-327.
- [CrossRef] [Google Scholar]
- Antimicrobial and anticancer activities of porous chitosan-alginate biosynthesized silver nanoparticles. Int. J. Biol. Macromol.. 2017;98:515-525.
- [CrossRef] [Google Scholar]
- Synergistic antibiotic activity of volatile compounds from the essential oil of Lippia sidoides and thymol. Fitoterapia. 2012;83:508-512.
- [CrossRef] [Google Scholar]
- Synthesis of highly acidic and well ordered MgAl-MCM-41 and its catalytic performance on the isopropylation of m-cresol. Microporous Mesoporous Mater.. 2004;76:91-98.
- [CrossRef] [Google Scholar]
- A preliminary investigation on the antimicrobial activity of Listerine®, its components, and of mixtures thereof. Phyther. Res.. 2015;29:1590-1594.
- [CrossRef] [Google Scholar]
- Surface and antibacterial properties of thin films based on collagen and thymol. Mater. Today Commun.. 2020;100949
- [CrossRef] [Google Scholar]
- Preventive and therapeutic effects of thymol in a lipopolysaccharide-induced acute lung injury mice model. Inflammation. 2018;41:183-192.
- [CrossRef] [Google Scholar]
- Combination of microbiological, spectroscopic and molecular docking techniques to study the antibacterial mechanism of thymol against Staphylococcus aureus: membrane damage and genomic DNA binding. Anal. Bioanal. Chem.. 2017;409:1615-1625.
- [CrossRef] [Google Scholar]
- Synergistic, additive, and antagonistic effects of food mixtures on total antioxidant capacities. J. Agric. Food Chem.. 2011;59:960-968.
- [CrossRef] [Google Scholar]
- Evaluation of the antibacterial potential of liquid and vapor phase phenolic essential oil compounds against oral microorganisms. PLoS ONE. 2016;11:1-17.
- [CrossRef] [Google Scholar]
- Process for preparing thymol (US4086283A.). 1976.
- Ueber eine synthese von thymol aus cuminol. Berichte der Dtsch. Chem. Gesellschaft. 1882;15:166-172.
- [CrossRef] [Google Scholar]
- Yang Zhenghao, Ren Jing, Tang Liangping, Yang Dongsheng, G.G., 2014. Novel synthetic process of thymol. CN103951546A.
- Yu Kai, Jiang Yugang, Wang Yongguang, Ge Zhimin, Li Zhixiang, L.C., 2018. A kind of thymol and preparation method thereof and pharmaceutical composition. CN108911951A
- Yusuke;, G.H.S.K.K.Y.I.S.T.H.K., 2014. Production method of dialkylphenol. JP2015227289 (A).
- An overview on Ajwain (Trachyspermum ammi) pharmacological effects; modern and traditional. J. Nat. Remedies. 2014;14:98-105.
- [CrossRef] [Google Scholar]
- Thymoquinone induces apoptosis in bladder cancer cell via endoplasmic reticulum stress-dependent mitochondrial pathway. Chem. Biol. Interact.. 2018;292:65-75.
- [CrossRef] [Google Scholar]
- Zhang Yuhong, Xiao Tangxin, Hu Baiyan, Yu Ming, Zhang Long, Qi Yong, L.Q., 2016. Synthetic method for thymol. CN106008169A
- Thymol attenuates allergic airway inflammation in ovalbumin (OVA)-induced mouse asthma. Fitoterapia. 2014;96:131-137.
- [CrossRef] [Google Scholar]
- Thymol provokes burst of action potentials in neurons of snail Caucasotachea atrolabiata. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol.. 2020;228 108654
- [CrossRef] [Google Scholar]







