Translate this page into:
Topical application of berberine ameliorates imiquimod-induced psoriasis-like dermatitis in BALB/c mice via suppressing JAK1/STAT1 signaling pathway
⁎Corresponding author at: Hospital of Dermatology, Chinese Academy of Medical Sciences and Peking Union Medical College, Nanjing 210042, China. xinyusli609@163.com (Xinyu Li)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Psoriasis is a common chronic inflammatory skin disease, which has seriously affected human health. Berberine is a plant alkaloid with significant anti-inflammatory effect. In this study, we aimed to determine whether topical application of berberine could ameliorate skin inflammation in psoriasis and explore the potential molecular mechanism. Imiquimod (IMQ)-induced psoriasis-like dermatitis in mice was firstly used to reveal the potential pathogenic mechanism. The transcriptome analysis showed that Janus kinase (JAK)-Signal transducer and activator of transcription (STAT) signaling pathway was significantly enriched in IMQ-induced dermatitis, which included the key genes such as Janus kinase 1 (JAK1), Interleukin-2 (IL2), Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (PIK3CA), Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform (PIK3CB) and Signal transducer and activator of transcription 1 (STAT1). Network pharmacology and molecular docking then predicted that topical berberine may treat psoriasis by JAK-STAT signaling pathway, especially act on JAK1, IL2, PIK3CA and PIK3CB. Experimental studies in vivo further demonstrated that topical application of berberine could ameliorate IMQ-induced psoriasis-like skin inflammation by suppressing JAK-STAT signaling pathway. In addition, experimental studies in vitro showed that berberine could directly act on and enter into human immortalized keratinocytes (HaCaT cells). Meanwhile, berberine may inhibit the hyperproliferation and proinflammatory functions of HaCaT cells induced by Interferon-gamma (IFN-γ) via suppressing JAK1/STAT1 signaling pathway. In conclusion, this study suggested that berberine may be a promising topical agent to ameliorate skin inflammation in psoriasis.
Keywords
Berberine
Topical application
Psoriasis
Dermatitis
Keratinocytes
JAK1/STAT1
- DEGs
-
Differentially expressed genes
- GSEA
-
Gene set enrichment analysis
- HaCaT
-
Human immortalized keratinocytes
- HE
-
Hematoxylin-Eosin
- IFN-γ
-
Interferon-gamma
- IHC
-
Immunohistochemistry
- IL2
-
Interleukin-2
- IL-10
-
Interleukin-10
- IL-17A
-
Interleukin-17A
- IL-23
-
Interleukin-23
- IMQ
-
Imiquimod
- JAK
-
Janus kinase
- JAK1
-
Janus kinase 1
- PASI
-
Psoriasis Area and Severity Index
- PIK3CA
-
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform
- PIK3CB
-
Phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit beta isoform
- SOCS
-
Suppressors of cytokine signaling
- STAT
-
Signal transducer and activator of transcription
- STAT1
-
Signal transducer and activator of transcription 1
- TNF-α
-
Tumour necrosis factor-α
Abbreviations
1 Introduction
Psoriasis, a common chronic inflammatory skin disease, is characterized by erythema, scales, inflammatory infiltration and epidermal thickening in skin lesions (Ru et al., 2020). Epidemiological studies have shown that the prevalence of psoriasis in adult was approximately 0.51 % to 11.43 % in the worldwide (Michalek et al., 2017), which has been listed as one of the important diseases affecting human health by World Health Organization (Mehrmal et al., 2021).
At present, the pharmacological interventions for the treatment of psoriasis includes systemic drugs, biological agents and topical drug therapies (Johnson and Armstrong, 2013). Approximately 80 % of psoriatic patients are usually mild-to-moderate, which can be treated with topical treatments (Stein Gold, 2016; Menter et al., 2009) and current guidelines recommend topical therapies as first-line treatment (Burden et al., 2010; Samarasekera et al., 2012). Furthermore, topical therapies can be used alone or as adjunctive agents with systemic treatment in order to enhance therapeutic outcomes (Segaert et al., 2022). Therefore, topical drug therapies play significant roles in the treatment of psoriasis. In clinical practice, ameliorating the skin lesions by topical drug therapies is the preferred solution of treating psoriasis (Samarasekera et al., 2013; Svendsen et al., 2021; Segaert et al., 2022). It has been known that topical application of retinoids, vitamin D3 analogues and glucocorticoids can effectively alleviate the skin lesions of psoriasis, but inherent side effects such as skin irritation and limitation of medication time also exists (Bos and Spuls, 2008). In particular, long-term use of glucocorticoids will cause skin atrophy, telangiectasia and other problems (Oray et al., 2016). Thus, it is necessary to develop the topical drugs with positive therapeutic efficacy and reduced toxicity. Herbal medicinal products have the characteristics of relatively low toxicity and less side effects, and are suitable for long-term use, especially in the treatment of chronic inflammatory diseases such as psoriasis (Gendrisch et al., 2021). Phytochemical agents with less toxicity may show more significant therapeutical effects on psoriasis.
Berberine, a natural pentacyclic isoquinoline alkaloid, is abundantly found as a principal constituent of many medicinal plants, especially the stems and roots of Berberis species (Habtemariam, 2020). Berberine has various pharmacological effects such as anti-cancer (Gong et al., 2020), anti-diabetes (Zhang et al., 2008), anti-obesity and anti-hyperlipidemia (Kim et al., 2009), cardioprotection (Derosa et al., 2012), notably anti-inflammation (Zeng et al., 2020). Due to the significant anti-inflammatory effect of berberine, it has been studied in various inflammatory diseases such as chronic respiratory inflammatory diseases (Tew et al., 2020), chronic ulcerative colitis (Li et al., 2020) and rheumatoid arthritis (Huang et al., 2021). Traditional Chinese medicine Coptis chinensis, with berberine as the main active ingredient, has been used in the external treatment of inflammatory skin diseases such as eczema (Gu et al., 2014). The above studies suggested that berberine has significant therapeutic potential for skin inflammation, especially for topical application. Therefore, the effect and molecular mechanism of topical berberine treating dermatitis in psoriasis were then studied in vivo and in vitro.
In this study, imiquimod (IMQ) is used to induce psoriasis-like dermatitis in mice that closely resembles the skin lesions of psoriasis patients in phenotype and histopathology (Flutter and Nestle, 2013). Firstly, potential pathogenic mechanism of IMQ-induced psoriasis-like dermatitis in mice was investigated by transcriptome analysis. Therapeutic targets and pathways of topical berberine treating psoriasis were then predicted by network pharmacology. Meanwhile, IMQ-induced mice were used to study the effects and potential mechanisms of topical berberine treating psoriasis-like dermatitis. Finally, the effect of berberine acting on HaCaT cells and potential molecular mechanism were further studied in vitro.
2 Materials and methods
2.1 Chemicals and reagents
Berberine hydrochloride (HPLC 98 %) and Curcumin (HPLC 98 %) were obtained from Macklin (Shanghai, China). Octadecanoic acid and Glycerol were bought from Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). Glyceryl monostearate, Vaseline, Triethanolamine, Ethyl 4-hydroxybenzoate and Azone were provided by Shanghai Aladdin Biochemical Technology Co., Ltd. (Shanghai, China). IMQ cream (5 %) was purchased from iNova Pharmaceuticals (Singapore). Halometasone cream (0.05 %) was obtained from Bright Future Pharmaceuticals (Hong Kong, China). BCA protein assay kit was bought from Beyotime (Shanghai, China). Mouse ELISA kits of Tumour necrosis factor-α (TNF-α), Interleukin-10 (IL-10), Interleukin-17A (IL-17A) and Interleukin-23 (IL-23) were supplied from Nanjing SenBeiJia Biological Technology Co., Ltd. (Nanjing, China). Anti-CD68 Rabbit pAb was obtained from Servicebio Technology (Wuhan, China).
MTT kit was purchased from Sigma-Aldrich (Merck, Germany). Recombinant Human IFN-γ was supplied from PeproTech (Cranbury, NJ, USA). JAK1 (6G4) Rabbit mAb, Phospho-JAK1(Tyr1034/1035) (D7N4Z) Rabbit mAb, STAT1 (D1K9Y) Rabbit mAb and Phospho-STAT1 (Tyr701) (58D6) Rabbit mAb were obtained from Cell Signaling Technology (Danvers, MA, USA). GAPDH Rabbit pAb was provided by GOODHERE BIOTECH (Hangzhou, China). Goat Anti-Rabbit IgG-HRP was bought from Santa Cruz Biotechnology (Dallas, Texas, USA).
2.2 Study of the potential mechanism of IMQ-induced psoriasis-like dermatitis in mice
2.2.1 Evaluation of phenotype in skin lesion
8 weeks old female BALB/c mice (16–19 g) from Zhejiang Vital River Laboratory Animal Technology Co., Ltd. (Jiaxing, China) (Certification NO. 20211015Abzz0619000573) were raised in standardized laboratory animal center with free access to forage and water. The experimental manipulations were performed according to the National Institutes of Health Guidelines on Laboratory Research and approved by Animal Ethics Committee of Hospital of Dermatology, Chinese Academy of Medical Sciences & Peking Union Medical College. As shown in the previous study, IMQ-induced psoriasis-like dermatitis in mice was used in this study (Sangaraju et al., 2021). 16 mice were evenly divided into Normal control group (Control) and IMQ-induced model group (IMQ). 5 % IMQ cream with a dose of 62.5 mg/d was topically applied on the shaved back (2 × 3 cm) of each mouse to induce psoriasis-like dermatitis. The experimental processes required 6 consecutive days. The severity of skin lesions was firstly evaluated using Psoriasis Area and Severity Index (PASI) score system (Zhao et al., 2016). The skin lesion tissues were then fixed, sectioned and stained with Hematoxylin-Eosin (HE) for histopathological observation by upright fluorescence microscope (OLYMPUS, Japan).
2.2.2 Rna-seq of skin lesion tissues
Total RNA were respectively isolated from skin lesion tissues in Control and IMQ groups. Three biological replications were adopted in each group. Firstly, Standard Sensitivity RNA Analysis Kit (15 nt) (DNF-471) was used to detect the quality of RNA samples by Fragment Analyzer (Agilent Technologies). Library construction and Transcriptome sequencing were then performed on a DNBSEQ-T7 platform by BGI-Wuhan (Wuhan, China). Raw reads were obtained from sequencing. Quality control (QC) was further performed on raw reads to determine whether the sequencing data were suitable for subsequent analysis. After filtering low quality, joint contamination and reads with unknown base N content, clean reads were generated from raw reads. The filtered clean reads were then compared to the reference sequence. The comparison rate and the distribution of clean reads on the reference sequence were collected to analyze the results of the second quality control (QC of alignment). Meanwhile, new transcript prediction was also performed. To form a complete reference sequence, it added a new transcript with the potential ability of protein coding into the reference gene sequence. The level of gene expression was then calculated. Finally, quantitative analysis of gene and various analysis based on the level of gene expression such as principal component analysis, correlation analysis, differential gene screening were performed, respectively. Differentially expressed genes (DEGs) were screened out by the following criteria with |log2 Ratio| ≥ 1 and Q-value ≤ 0.05. GO function enrichment analysis, KEGG pathway enrichment analysis and other analysis for the DEGs were also further analyzed. Data analysis above was supported by Dr.Tom system.
2.3 Prediction of targets and pathways of topical berberine treating psoriasis by network pharmacology analysis
2.3.1 Targets collection
PharmMapper database (https://www.lilab-ecust.cn/pharmmapper/), SEA database (https://sea.bkslab.org/) and SIB database (https://www.swisstargetprediction.ch/) were used to obtain these targets of berberine. Meanwhile, GeneCards database (https://www.genecards.org/), DisGeNET database (https://www.disgenet.org/) and MalaCards database (https://www.malacards.org/) were applied to the targets collection of psoriasis.
2.3.2 Network construction of the interaction between compound and target
Venn diagram analysis of the collected targets between berberine and psoriasis was firstly performed to screen out the potential targets of berberine treating psoriasis. TiGER database (https://bioinfo.wilmer.jhu.edu/tiger/), a database for tissue-specific gene expression and regulation (Liu et al., 2008), was then used to verify the skin tissue-specific gene expression and regulation of these potential targets. STRING database (https://string-db.org/) was used to obtain the interaction relationship between these targets filtered by TiGER database. The compound-target network was then constructed by Cytoscape 3.7.2 software.
2.3.3 Enrichment analysis
GO molecular function enrichment analysis was firstly performed by FunRich software to analyze the gene function of topical berberine treating psoriasis. The significant functional annotation was determined with corrected p-value less than 0.05. KEGG pathway enrichment analysis was then performed to study the biological function by KOBAS database (https://kobas.cbi.pku.edu.cn/). The significant functional annotation was also confirmed with corrected p-value less than 0.05. Finally, omicshare cloud platform ((https://www.omicshare.com/) was used to visualize KEGG pathway enrichment analysis.
2.3.4 Molecular docking
To generate 3D chemical structure of berberine and minimize energy, Chem 3D (v15.0) software was firstly used. RCSB Protein Data Bank (https://www.rcsb.org/) was then used to obtain the crystal structures of candidate target proteins. The solvent and organic of target proteins were removed by PyMOL software. Meanwhile, AutoDock (v4.2.6) software was used to add hydrogen, optimize and patch amino acids. Autodock vina was then used to perform the molecular docking between berberine and candidate target proteins. The results of molecular docking were visualized by PyMOL software.
2.4 Experimental validation of topical berberine treating IMQ-induced psoriasis-like dermatitis in vivo
2.4.1 Preparation of cream containing 0.5 % or 1 % berberine
In the preparation of Berberine cream, multi-factor orthogonal test was performed to optimize the best preparation processes. Based on the evaluation criteria of appearance quality, pH, centrifugal stability, heat-resistant stability and cold-resistant stability, the content of Triethanolamine (factor A) and Glyceryl monostearate (factor B), emulsification temperature (factor C) and emulsification time (factor D) were taken as investigation factors to optimize the preparation of Berberine cream. Finally, an optimized Berberine cream with the best centrifugal stability, heat-resistant stability and cold-resistant stability was then prepared for this study.
The cream formulation consists of oil phase and aqueous phase. The oil phase contains the following ingredients: Octadecanoic acid (10 %), Glyceryl monostearate (5 %) and Vaseline (4 %). The aqueous phase includes the following ingredients: Triethanolamine (5 %), Glycerol (6 %), Azone (2 %), Berberine (0.5 % or 1 %), Ethyl 4-hydroxybenzoate (0.1 %) and purified water. Berberine can be completely dissolved in the aqueous phase under the condition of heat. Finally, the cream preparation is completed with purified water.
The preparation of cream is performed by adding the oil phase into the aqueous phase to form an emulsion. Specifically, the ingredients of oil phase are firstly mixed at 80℃ until complete melting. The blend is then added into the aqueous phase, which is also heated at 80℃. Firstly, the mixture continually stirs for 15 min. After the mixture is cooled to 40℃, it continues to stir for 15 min to form the emulsion. After the emulsion is cooled to room temperature, a cream containing 0.5 % or 1 % berberine is finished. Vehicle cream is carried out in the same way, but without adding berberine into the aqueous phase.
2.4.2 Animal experiment and drug treatment
8 weeks old female BALB/c mice (16–19 g) from Zhejiang Vital River Laboratory Animal Technology Co., Ltd. (Jiaxing, China) (Certification NO. 20220602Abzz0619000465) were raised in standardized laboratory animal center with free access to forage and water. The experimental manipulations were performed according to the National Institutes of Health Guidelines on Laboratory Research and approved by Animal Ethics Committee of Hospital of Dermatology, Chinese Academy of Medical Sciences & Peking Union Medical College.
Six experimental groups with 8 mice in each group were set as follows: Normal control group (Control), IMQ-induced model group (IMQ), Vehicle control group (Vehicle), Halometasone treatment group (0.05 % Halometasone) and Berberine treatment groups (0.5 % Berberine and 1 % Berberine). As shown above, IMQ-induced psoriasis-like dermatitis in mice was performed in these groups except for Control group. According to the previous studies (Li et al., 2018a; Li et al., 2018b), creams of Vehicle, 0.05 % Halometasone, 0.5 % Berberine and 1 % Berberine were then applied with a dosage as same as IMQ cream after topically administered with IMQ cream for 4 h. All treatments continued for 6 days. Meanwhile, the assessment of the severity of skin lesions was also performed by PASI score system everyday. The skin lesion tissues were then collected for further study.
2.4.3 Histology and immunohistochemistry
The skin lesion tissues in each group were fixed in 4 % paraformaldehyde and embedded in paraffin wax. The paraffin sections (5 μm) were stained with Hematoxylin-Eosin (HE), and observed by upright fluorescence microscope (OLYMPUS, Japan). Image-Pro Plus 6.0 software was then used to measure the epidermal thickness. Meanwhile, the macrophage infiltration in these skin lesion tissues was also detected by immunohistochemistry (IHC) staining of Anti-CD68 Rabbit pAb.
2.4.4 Biochemical test by ELISA
Firstly, the skin lesion tissues were removed fat parts, weighed and cut into pieces. These tissues were homogenized according to the ratio of 1: 9 (tissue weight: PBS volume), and then centrifuged at 4℃, 4000 r/min for 10 min. The supernatant was collected and diluted to measure the protein concentration by BCA method. According to the instructions of ELISA kits, the level of TNF-α, IL-17A, IL-23 and IL-10 in skin lesion tissues were measured by microplate reader (TECAN, Switzerland).
2.4.5 Real-time qPCR
Total RNA was extracted from skin lesion tissues in Control, IMQ, Vehicle and 1 % Berberine groups, and then transcribed into cDNAs. Finally, Real-time qPCR was performed by Roche Light Cycler 480 QPCR System (Roche, Switzerland) with a thermal profile of 95℃ for 30 s and 40 cycles of 95℃ for 5 s, 60℃ for 30 s. The primer sequences were listed in Table 1. The primers were synthesized by Sangon Biotech (Shanghai) Co.,Ltd. (Shanghai, China). The data were standardized to the expression level of β-actin. Relative mRNA expression levels were calculated using the 2-△△CT method.
Gene symbol
Forward primer (5′ to 3′)
Reverse primer (5′ to 3′)
β-actin
AGCCATGTACGTAGCCATCC
CTCTCAGCTGTGGTGGTGAA
JAK1
CTCCGAACCGAATCATCACT
GCCGTTTTTCTGCTTCTTTG
IL2
AACCTGAAACTCCCCAGGAT
TCCACCACAGTTGCTGACTC
PIK3CA
ACTGTTCAGAGAGGCCAGGA
CGGTTGCCTACTGGTTCAAT
PIK3CB
AGCTGGTCTTCGTTTCCTGA
TCCACCACGACTTGACACAT
EGFR
ACACTGCTGGTGTTGCTGAC
CCCAAGGACCACTTCACAGT
SOCS3
CCTTTGACAAGCGGACTCTC
GCCAGCATAAAAACCCTTCA
SOCS1
CTTAACCCGGTACTCCGTGA
GAGGTCTCCAGCCAGAAGTG
BCL2
AGGAGCAGGTGCCTACAAGA
GCATTTTCCCACCACTGTCT
CCND2
CTGAGTCTGGTTGGTGCTGA
ACACCCGAGACCACAGAAAC
CCND1
GCGTACCCTGACACCAATCT
ATCTCCTTCTGCACGCACTT
IFNGR1
TCCTCCACCCTGATCATCTC
AGACTTACGGCTGGCTTTGA
IFNAR2
GACTGAGCAGGTAGCCTTGG
CCCACAAGGGTTTCTTTTGA
STAT1
TGGTGAAATTGCAAGAGCTG
TGTGTGCGTACCCAAGATGT
IL23A
GACTCAGCCAACTCCTCCAG
GGCACTAAGGGCTCAGTCAG
IL10
CCAAGCCTTATCGGAAATGA
TTTTCACAGGGGAGAAATCG
IL2RA
AGAACACCACCGATTTCTGG
CTGTGGGTTGTGGGAAGTCT
2.5 Study of berberine acting on HaCaT cells in vitro
2.5.1 Cell culture
HaCaT cell lines were obtained from American Type Culture Collection (ATCC, Manassas, VA, USA). HaCaT cells were cultured in DMEM medium (KeyGEN BioTECH, Nanjing, China) supplemented with 10 % fetal bovine serum at 37℃, 5 % CO2. Cell digestion and passage were performed using 0.2 % trypsin solution, and these cells were passaged every 2–3 days.
2.5.2 Cell viability assay
To determine the effect of berberine on HaCaT cells, cell viability of HaCaT cells treated with berberine was tested by MTT assay. Curcumin, a natural anti-psoriatic compound from Curcuma longa L., was then selected as the positive control in the cell experiments of this study (Varma et al., 2017). HaCaT cells were seeded into 96-well plates at a density of 5 × 105 cells/mL and 0.1 mL/well. After cultured overnight, the medium was replaced with various concentrations of berberine ranging from 6.25 to 200 μg/mL (0.2 mL/well) and various concentrations of curcumin ranging from 1.563 to 50 μg/mL (0.2 mL/well). After incubation for 2 h or 24 h, 20 μL of MTT solution (5 mg/mL) was added to each well, and the cells were incubated for another 4 h at 37℃. Finally, the medium was removed and then 150 μL DMSO was added into each well. The absorbance was measured at 550 nm using a microplate reader (TECAN, Switzerland).
2.5.3 Flow cytometry
Due to the natural fluorescence characteristic of berberine and curcumin, flow cytometry could be used to measure the cellular uptake of berberine and curcumin in HaCaT cells (Serafim et al., 2008; Priyadarsini, 2014). 4 × 105 cells/well were seeded into 6-well plates. After cultured overnight, the medium was removed, and various concentrations of berberine (6.25, 12.5, 25, 50 μg/mL) and curcumin (3.125, 6.25, 12.5, 25 μg/mL) were then added into each well. After 0.5, 1, 2, 6, 24 h of incubation, the cells were washed with PBS for 2 times and then digested using a Trypsin-EDTA solution (Trypsin: EDTA = 1: 4). The cell suspension was collected and centrifuged at 300 × g for 5 min. The supernatant was discarded, and then 0.5 mL PBS was added. After filtered by 300 mesh nylon mesh, it was immediately measured using a flow cytometer (BD FACSVERSE, USA) with FITC filter.
2.5.4 Confocal microscopic observation
Confocal microscopy was used to further visualize the intracellular uptake of berberine and curcumin in HaCaT cells. 4 × 105 cells/well were seeded into 6-well plates. After cultured overnight, the medium was replaced with 25 μg/mL berberine and 6.25 μg/mL curcumin. After 0.5, 2, 24 h of incubation, the cells were washed with PBS and then fixed with 4 % paraformaldehyde for 10 min. After treated with mounting medium, it was viewed and photographed using a confocal microscope (OLYMPUS FV1000, Japan) with a FITC filter as same as the methods described in the previous study (Floriano et al., 2021).
2.5.5 Western blot
HaCaT cells were seeded into 100 mm petri dishes at a density of 3 × 106 cells/mL. After incubation for 6 h, serum-free medium was then used for culture overnight. After pre-treated with berberine (6.25, 12.5, 25 μg/mL) for 24 h, the proinflammatory function of HaCaT cells was stimulated by IFN-γ at a concentration of 500 U/mL for 24 h of incubation (Ishimaru et al., 2013; Li et al., 2023). Total protein from cells were extracted by cell lysis buffer. Protein concentrations were determined by BCA method. Protein samples were separated by 10 % SDS-PAGE and transferred to a nitrocellulose membrane. After blocking at room temperature in 5 % bovine serum albumin for 2 h, the membrane was probed with various primary antibodies (GAPDH, JAK1, p-JAK1, STAT1, p-STAT1) at 4℃ overnight. Membranes were then washed three times in TBST buffer, followed by incubation with secondary antibody at room temperature for 2 h, and then washed three times in TBST. Visualization was performed using a high-sig ECL western blotting substrate (Tanon, China). The density of the bands on western blots was quantified by densitometry analysis of the scanned blots using ImageJ software.
2.6 Statistical analysis
Data are expressed as mean ± SD. Data from multiple groups were assessed by one-way ANOVA followed by Sidak's multiple comparison test using GraphPad Prism. Student's t test was used to analyze the data between two groups. P < 0.05 was considered statistically significant.
3 Results
3.1 Potential pathogenic mechanism of IMQ-induced psoriasis-like dermatitis in mice
The phenotype of skin lesions of IMQ-induced psoriasis-like dermatitis in mice was firstly evaluated. As shown in Fig. 1A and B, the typical features of erythema, scales and thickness were significantly observed in IMQ-induced skin lesions as compared to Control group. Meanwhile, histopathological analysis of skin lesions showed that the epidermal thickness of mice in IMQ group was obviously increased compared to that in Control group (P < 0.001), and the inflammatory cell infiltration was also observed in IMQ-induced psoriasis-like dermatitis (Fig. 1C).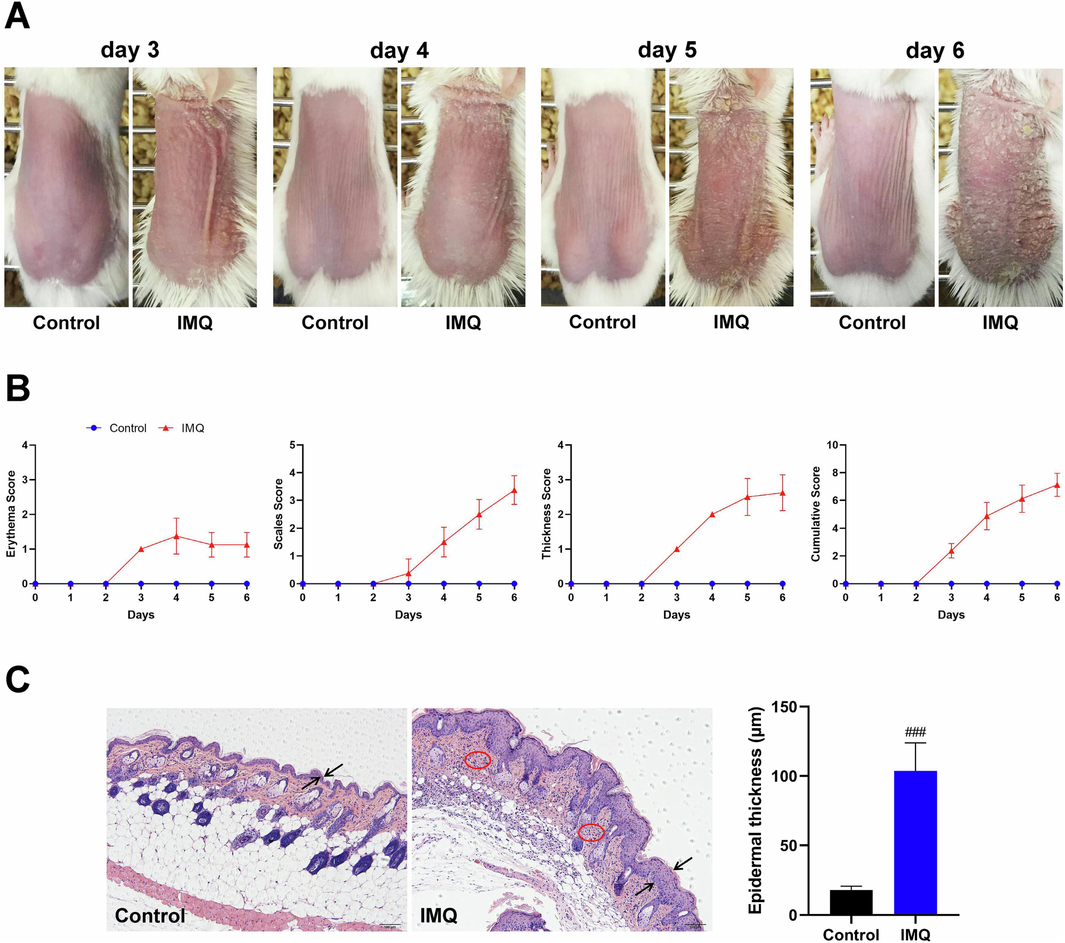
The phenotype of skin lesions in mice with IMQ-induced psoriasis-like dermatitis. (A) Representative pictures of dorsal skin between Control and IMQ groups during the experimental period from day 3 to day 6 when IMQ-induced skin lesions occurred (n = 8). (B) Evaluating the severity of skin lesions by PASI score including erythema score, thickness score, scales score and cumulative score (n = 8). (C) Histopathological observation of skin lesions by HE staining (scale bar, 100 μm) and morphometric analysis by Image-Pro Plus 6.0 software (n = 8). Two opposite arrows indicated the epidermis and red circle indicated the inflammatory cell infiltration. Data are expressed as mean ± SD. ###P < 0.001 versus Control group according to Student's t test.
The transcriptome analysis of skin lesion tissues from Control and IMQ groups was then performed to reveal the potential pathogenic mechanism of IMQ-induced psoriasis-like dermatitis. As shown in Fig. 2A, principal components analysis showed the obvious differentiation of the samples between Control and IMQ groups. Meanwhile, there were 3,118 up-regulated genes and 3,057 down-regulated genes between Control and IMQ groups by Scatter Plot analysis (Fig. 2B). Molecular function analyzed by GO functional enrichment of DEGs between Control and IMQ groups showed that the molecular function such as protein binding, kinase activity, protein kinase binding and protein tyrosine kinase activity were significantly enriched in IMQ-induced psoriasis-like dermatitis (Fig. 2C). KEGG pathway enrichment analysis of DEGs between Control and IMQ groups was also performed (Fig. 2D). JAK-STAT signaling pathway was significantly enriched in IMQ-induced psoriasis-like dermatitis. Furthermore, Gene set enrichment analysis (GSEA) showed the enrichment profile of JAK-STAT signaling pathway, which indicated the significance of JAK-STAT signaling pathway in IMQ-induced psoriasis-like dermatitis (Fig. 2E). In addition, the relative expression of genes such as JAK1, IL2, PIK3CA, PIK3CB and STAT1 in JAK-STAT signaling pathway between Control and IMQ groups was also showed in Fig. 2F.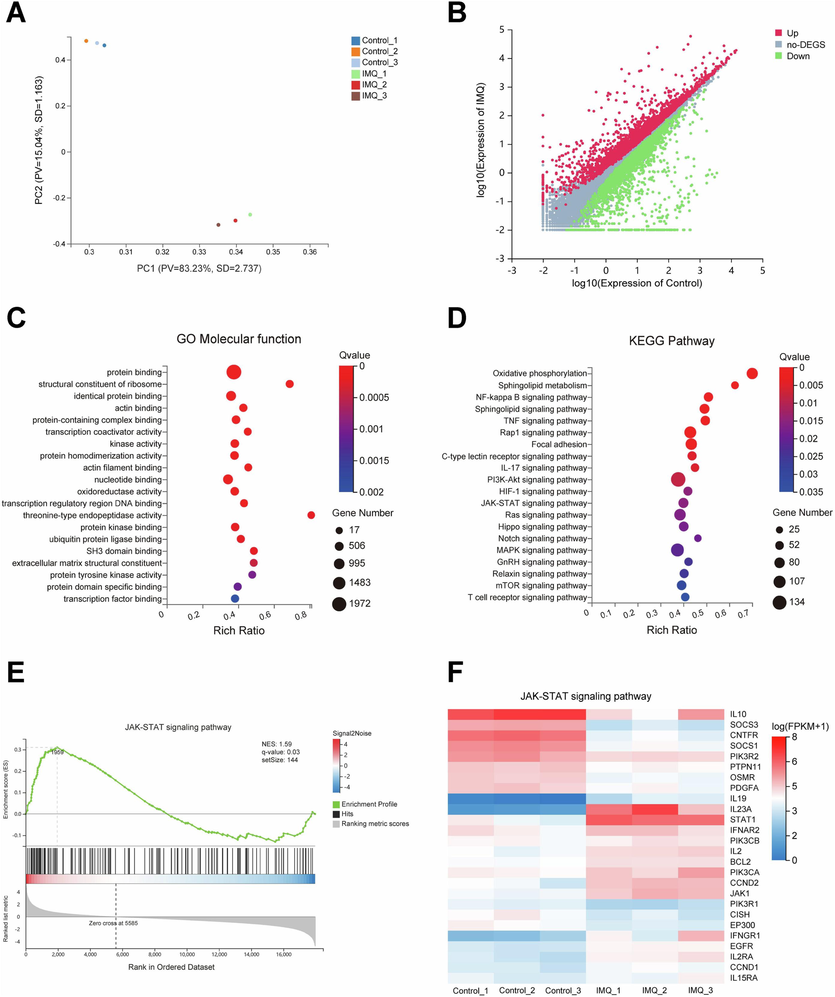
The transcriptome analysis of skin lesion tissues from Control and IMQ groups. (A) Score plot for discriminating the samples from Control and IMQ groups by principal components analysis. (B) Scatter plot for showing the difference of gene expression from DEGs between Control and IMQ groups. (C) Molecular function analyzed by GO functional enrichment analysis of DEGs. (D) KEGG pathway enrichment analysis of DEGs. (E) Gene set enrichment analysis of JAK-STAT signaling pathway. (F) Heatmap of gene expression in JAK-STAT signaling pathway between Control and IMQ groups.
3.2 Prediction of therapeutic targets and pathways of topical berberine treating psoriasis by network pharmacology and molecular docking
Berberine (Fig. 3A), a natural plant alkaloid, has the significant effect of anti-inflammation. SwissADME, a tool to predict ADME parameters, pharmacokinetic properties, druglike nature and medicinal chemistry friendliness of compounds, showed that the skin permeation coefficient (logKp) of berberine was −5.78 cm/s. It indicated that berberine was suitable for topical application according to the predicted skin permeability (Ghafourian et al., 2010). Therefore, the effect of topical application of berberine on IMQ-induced psoriasis-like dermatitis was then explored in this study. Before the experimental studies, network pharmacology and molecular docking were firstly performed to explore the potential of topical berberine treating psoriasis.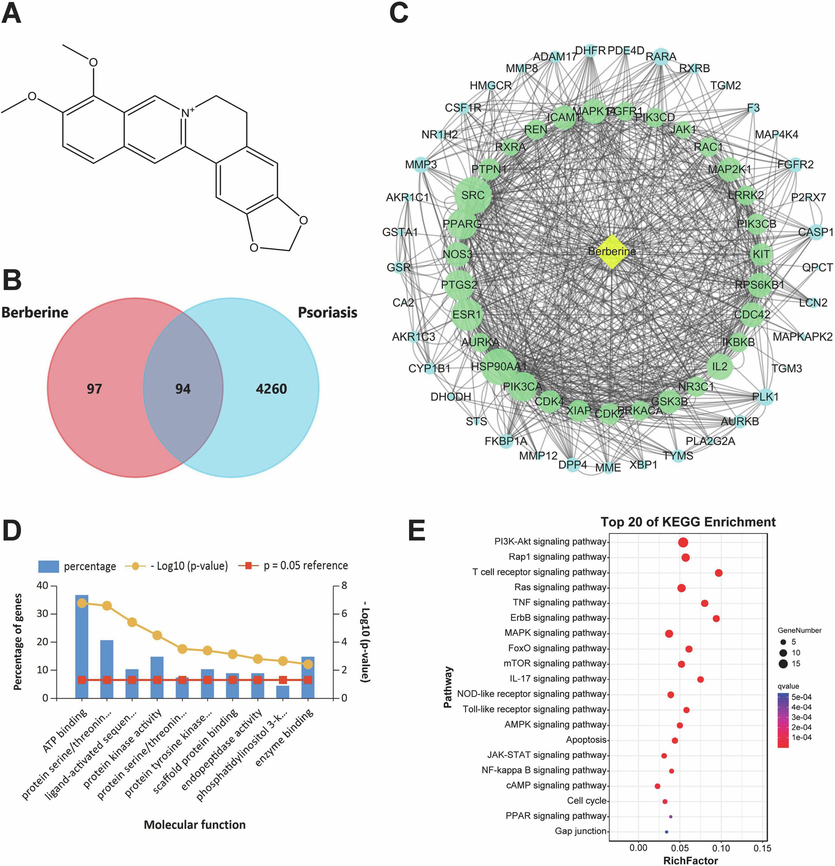
Prediction of therapeutic targets and pathways of topical berberine treating psoriasis by network pharmacology. (A) Chemical structure of berberine. (B) Venn diagram analysis for disease targets of psoriasis and active targets of berberine. (C) Network construction of berberine and these screened therapeutic targets with skin tissue-specific gene expression and regulation. The size of the node represents the node degree and the green nodes are the top 31 targets with the highest node degree and relevance. (D) Molecular function analyzed by GO functional enrichment of these screened therapeutic targets. (E) Top 20 pathways of KEGG enrichment analysis of these screened therapeutic targets.
191 active targets of berberine and 4,354 disease targets of psoriasis were obtained from databases, respectively. After processed by Venn diagram analysis, 94 targets were shared by berberine and psoriasis (Fig. 3B). TiGER database was then used to verify the skin tissue-specific gene expression and regulation of these shared targets. 68 targets with skin tissue-specific gene expression and regulation were finally screened out (Table S1). The screened 68 targets were imported into STRING database, and the network construction of interactions between berberine and 68 active targets was then performed by Cytoscape 3.7.0 software (Fig. 3C). According to the size of node degree, it showed that the top 31 targets such as PIK3CA, IL2, PIK3CB and JAK1 were significantly associated with topical berberine treating psoriasis. GO functional enrichment analysis of the screened 68 targets was performed by FunRich version 3.1.3 software. As shown in Fig. 3D, molecular function predicted by network pharmacology mainly included protein kinase activity, protein tyrosine kinase activity and phosphatidylinositol 3-kinase activity. These molecular functions were similar to that analyzed by transcriptome analysis mentioned above. KEGG pathway enrichment analysis of the screened 68 targets was then performed by KOBAS database. In the top 20 pathways of KEGG enrichment, JAK-STAT signaling pathway was also significantly enriched by network pharmacology (Fig. 3E). Notably, PIK3CA, IL2, PIK3CB and JAK1 were also involved in JAK-STAT signaling pathway. The molecular docking of berberine binding to these targets was then performed, respectively (Fig. 4). Berberine could bind to JAK1 by hydrogen bond. Meanwhile, berberine may bind to IL2 by hydrogen bond and hydrophobic interaction. Berberine may also bind to PIK3CA by hydrogen bond, hydrophobic interaction and π-cation interaction. Furthermore, berberine could bind to PIK3CB by hydrogen bond and hydrophobic interaction. According to the value of binding affinity, it showed that berberine could effectively act on JAK1, IL2, PIK3CA and PIK3CB.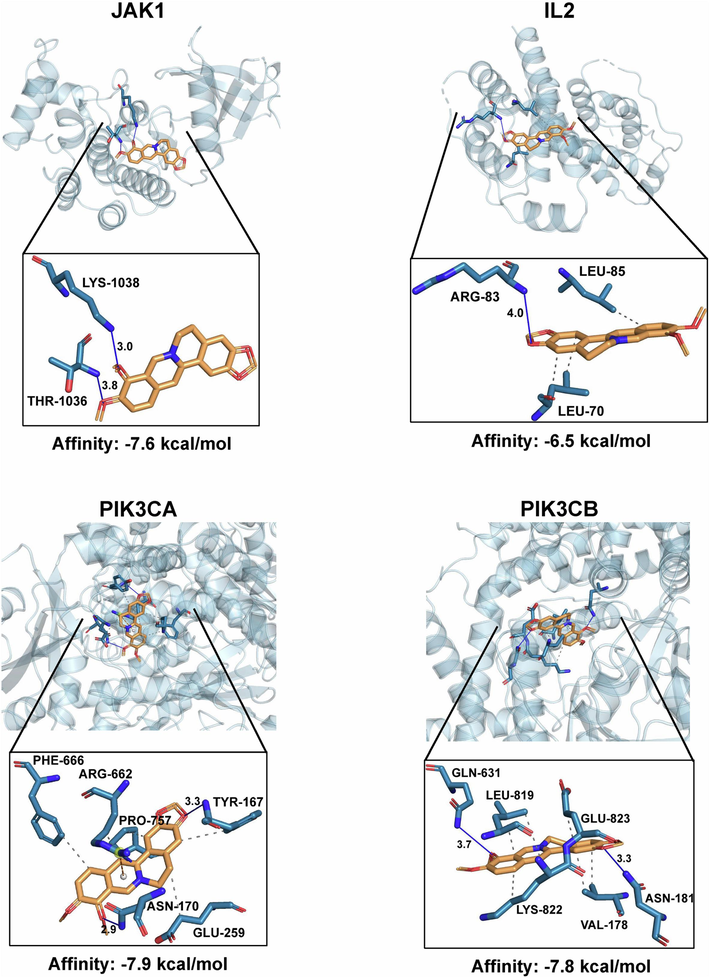
The virtual docking analysis of berberine acting on JAK1, IL2, PIK3CA and PIK3CB. These four targets were from the top 31 targets and also involved in JAK-STAT signaling pathway according to the analysis of network pharmacology.
3.3 Topical application of berberine ameliorated IMQ-induced psoriasis-like dermatitis in mice
Before the formal experiment, the skin safety of Vehicle cream and 0.5 %, 1 %, 2 %, 4 % Berberine creams for topical administration of 6 days has been evaluated in the pre-experiment. As shown in Fig. S1A and B, the dorsal skin tissues of mice in Vehicle group and 0.5 %, 1 % Berberine groups were normal as the same as Control group. The dorsal skin tissues of mice in 2 % and 4 % Berberine groups had abnormal epidermal thickening as compared to Control group, which showed a certain degree of skin irritation. Therefore, 0.5 % and 1 % Berberine creams were then used in this study.
0.5 % Berberine cream and 1 % Berberine cream were prepared as the described methods mentioned above, which has been optimized by multi-factor orthogonal test. As shown in Fig. 5A and B, IMQ-induced skin lesions were significantly observed in IMQ and Vehicle groups. Topical application of Halometasone cream or Berberine cream could obviously ameliorate the phenotype of skin lesions including erythema, scales and thickness induced by IMQ and significantly decrease the cumulative score as compared to IMQ and Vehicle groups (P < 0.05, P < 0.01, P < 0.001). Meanwhile, histological observation and morphometric analysis of skin lesion tissues also showed that halometasone and berberine could alleviate the inflammatory cell infiltration and decrease the epidermal thickness as compared to IMQ and Vehicle groups (P < 0.001) (Fig. 5C). IHC staining of skin lesion tissues indicated that halometasone and berberine could obviously reduce the inflammatory infiltration of macrophage in skin lesions as compared to IMQ and Vehicle groups (Fig. 6A). Furthermore, biochemical test of skin lesion tissues was also performed. As shown in Fig. 6B, the level of TNF-α, IL-17A and IL-23 in skin lesion tissues was significantly increased (P < 0.001) and the content of IL-10 was obviously decreased (P < 0.001) in IMQ and Vehicle groups as compared to Control group. Compared with IMQ and Vehicle groups, topical application of halometasone and berberine could decrease the content of TNF-α, IL-17A and IL-23 in skin lesion tissues (P < 0.05, P < 0.01, P < 0.001) and increase the level of IL-10 to a certain degree. These results showed that topical application of berberine could ameliorate IMQ-induced psoriasis-like skin inflammation in mice.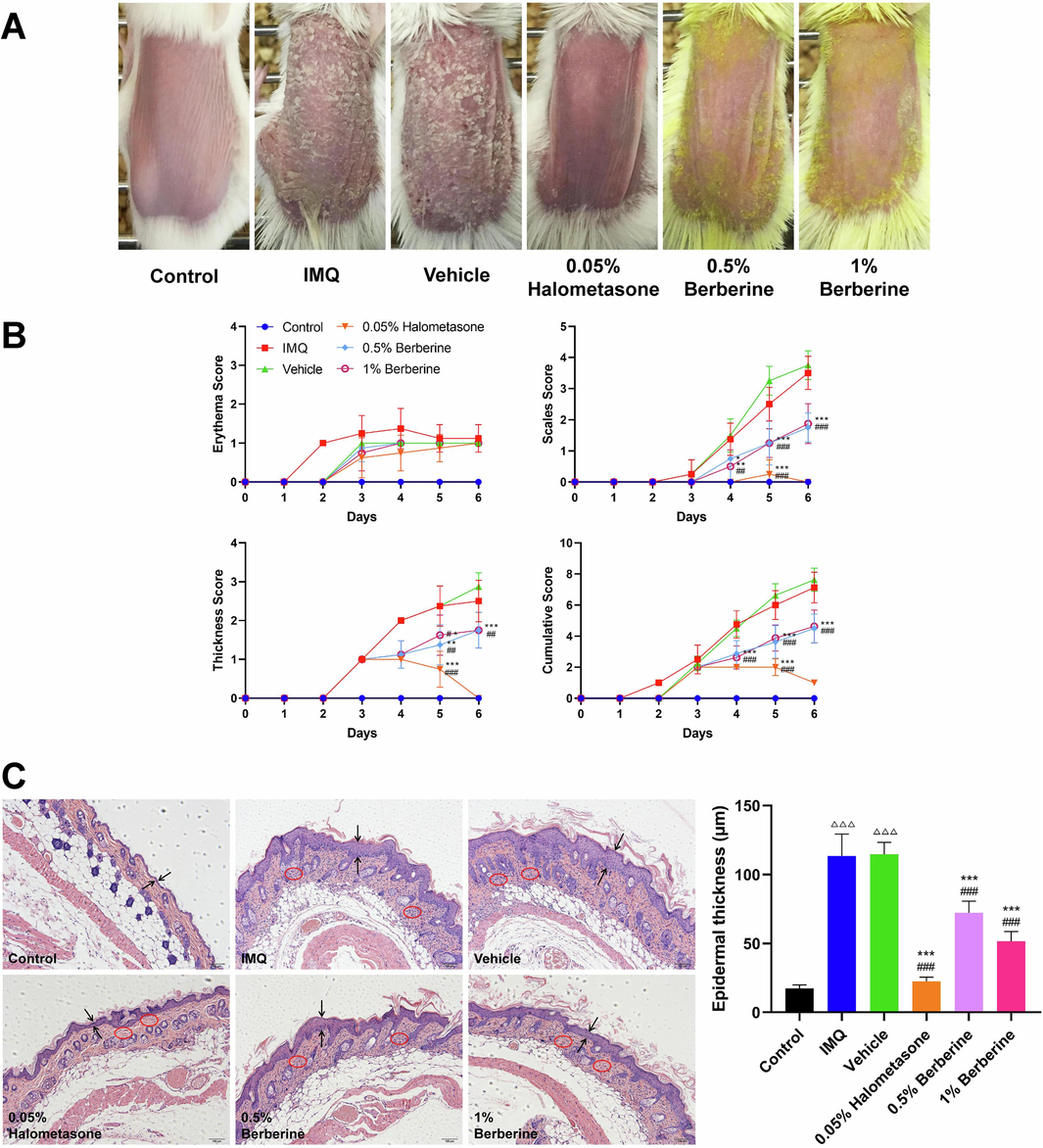
Topical application of berberine ameliorated IMQ-induced skin lesions. (A) Representative photographs of dorsal skin in IMQ-induced mice after 6 days for topical application of berberine (n = 8). (B) Assessment of the severity of skin lesions by PASI score including erythema score, thickness score, scales score and cumulative score (n = 8). (C) Topical application of berberine could decrease epidermal thickness and alleviate inflammatory cell infiltration in skin lesions (scale bar, 100 μm) (n = 8). Two opposite arrows indicated the epidermis and red circle indicated the inflammatory cell infiltration. Data are represented as mean ± SD. △△△P < 0.001 versus Control group, ###P < 0.001 versus IMQ group and ***P < 0.001 versus Vehicle group according to one-way ANOVA analysis followed by Sidak's multiple comparison test.
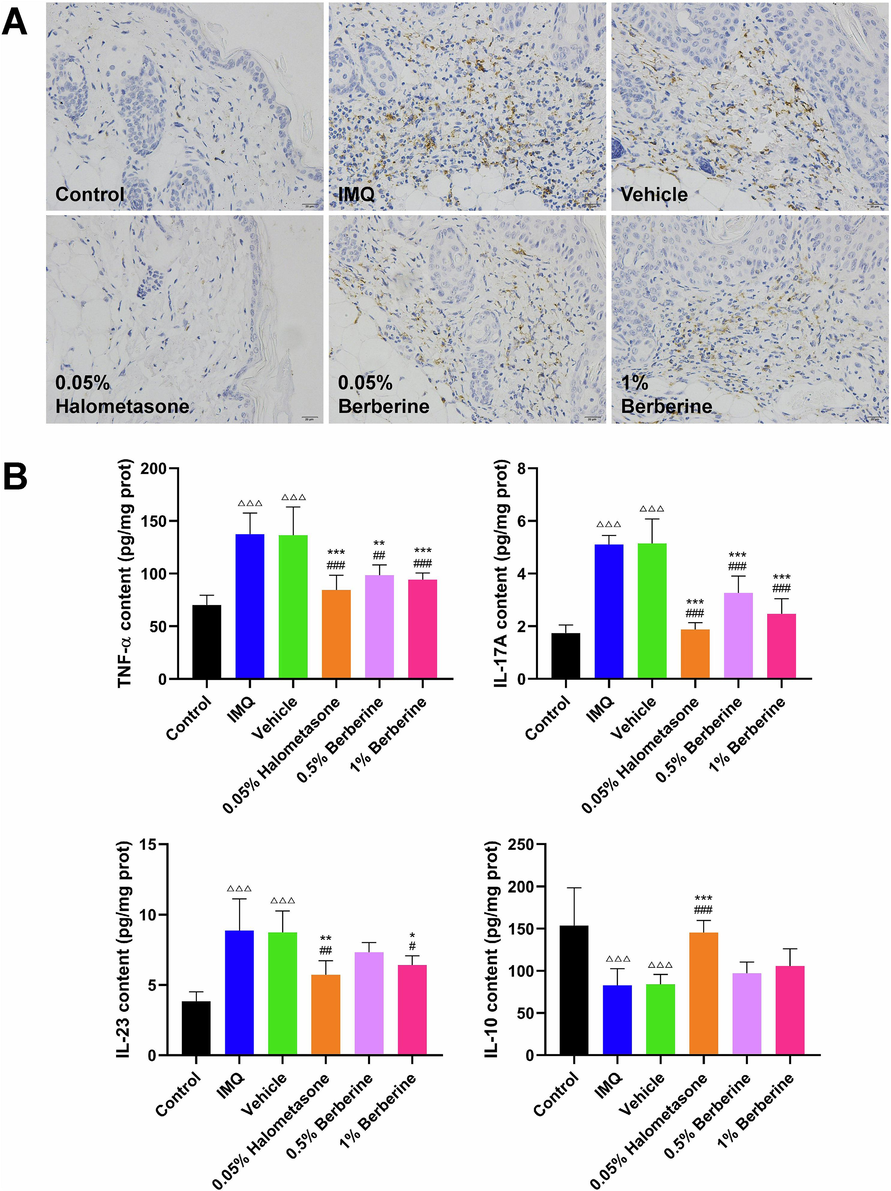
Topical application of berberine decreased the macrophage infiltration, reduced the release of inflammatory factor and increased the level of anti-inflammatory cytokine in skin lesion tissues in IMQ-induced mice. (A) Representative pictures of IHC-stained skin lesions with antibody against CD68 (scale bar, 20 μm) (n = 6). (B) Topical application of berberine decreased the level of TNF-α, IL-17A, IL-23 and increased the content of IL-10 in skin lesion tissues (n = 6). Data are expressed as mean ± SD. △△△P < 0.001 versus Control group, #P < 0.05, ##P < 0.01, ###P < 0.001 versus IMQ group and *P < 0.05, **P < 0.01, ***P < 0.001 versus Vehicle group according to one-way ANOVA analysis followed by Sidak's multiple comparison test.
3.4 Berberine suppressed JAK-STAT signaling pathway in IMQ-induced mice
Numerous studies have indicated that JAK-STAT signaling pathway could be a potential target for the treatment of inflammatory diseases such as inflammatory bowel disease and inflammatory skin disease (Banerjee et al., 2017; Welsch et al., 2017; Wang et al., 2021). In this study, JAK-STAT signaling pathway was significantly enriched in IMQ-induced psoriasis-like dermatitis by transcriptome analysis, and JAK-STAT signaling pathway was also significantly enriched in the biological function of topical berberine treating psoriasis predicted by network pharmacology. To prove the reliability of transcriptome analysis and study the regulation of JAK-STAT signaling pathway for topical berberine treating IMQ-induced psoriasis-like dermatitis, the relative mRNA expressions of 16 key genes involved in JAK-STAT signaling pathway were then measured by qPCR (Fig. 7). Berberine could potently reverse the mRNA expression of these key genes induced by IMQ, which indicated that berberine could ameliorate IMQ-induced psoriasis-like skin inflammation by suppressing JAK-STAT signaling pathway in IMQ-induced mice.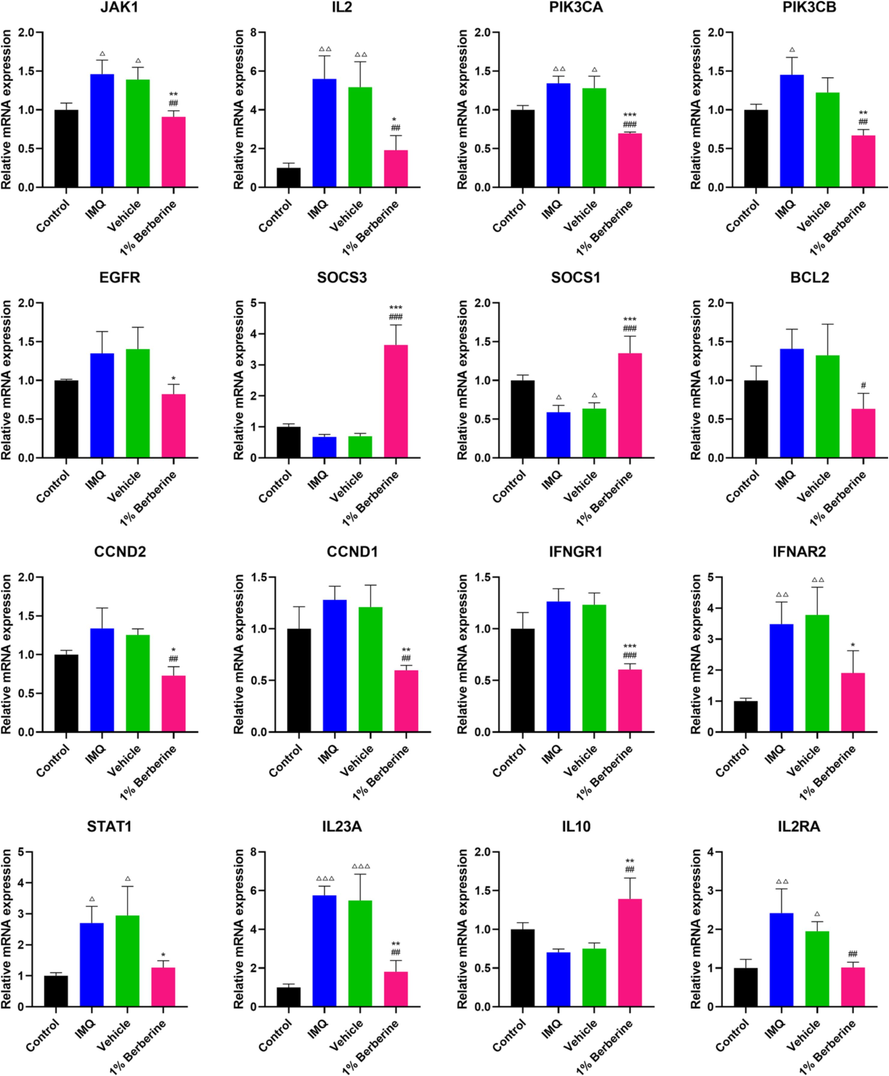
Berberine regulated the mRNA expression of key genes in JAK-STAT signaling pathway in IMQ-induced mice. JAK-STAT signaling pathway was significantly enriched in IMQ-induced psoriasis-like dermatitis in mice by transcriptome analysis. To prove the reliability of transcriptome analysis, qPCR validation for 16 key genes involved in JAK-STAT signaling pathway was then performed. Berberine could potently reverse the mRNA expression of these key genes induced by IMQ. Data are represented as mean ± SD. △P < 0.05, △△P < 0.01, △△△P < 0.001 versus Control group, #P < 0.05, ##P < 0.01, ###P < 0.001 versus IMQ group and *P < 0.05, **P < 0.01, ***P < 0.001 versus Vehicle group according to one-way ANOVA analysis followed by Sidak's multiple comparison test.
3.5 Cell viability of HaCaT cells treated by berberine and curcumin
To determine the cytotoxic effect of berberine and curcumin on HaCaT cells, cell viability was assessed by MTT assay. HaCaT cells were treated with various concentrations of berberine ranging from 6.25 to 200 μg/mL and various concentrations of curcumin ranging from 1.563 to 50 μg/mL (Fig. 8). The cell viability of 6.25 μg/mL berberine on HaCaT cells for 24 h of incubation was obviously decreased as compared to 2 h of incubation (P < 0.01). There was no significant difference of the cell viability of 12.5 μg/mL berberine on HaCaT cells between 2 h of incubation and 24 h of incubation, as the same as 25 μg/mL berberine. However, the cell viability of 50, 100, 200 μg/mL berberine on HaCaT cells for 24 h of incubation was significantly decreased as compared to 2 h of incubation (P < 0.05, P < 0.001), which indicated the occurrence of cytotoxic effect of 50, 100, 200 μg/mL berberine on HaCaT cells for 24 h of incubation. In addition, there was no significant difference of the cell viability of 1.563 μg/mL curcumin on HaCaT cells between 2 h of incubation and 24 h of incubation, as the same as 3.125 μg/mL and 6.25 μg/mL curcumin. Furthermore, the cell viability of 12.5, 25, 50 μg/mL curcumin on HaCaT cells for 24 h of incubation was significantly decreased as compared to 2 h of incubation (P < 0.01, P < 0.001), which indicated the occurrence of cytotoxic effect of curcumin on HaCaT cells.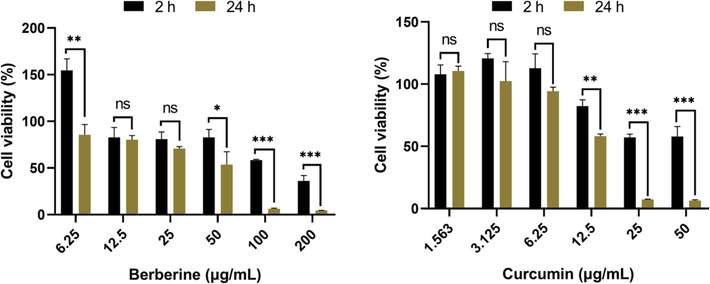
Cytotoxic effect of berberine and curcumin on HaCaT cells after 2, 24 h of incubation. The cells were respectively treated with different concentrations of berberine and curcumin, and the cell viability was assessed by MTT assay (n = 3). Data are expressed as mean ± SD. *P < 0.05, **P < 0.01, ***P < 0.001 indicated the significance between two groups according to Student's t test.
3.6 Intracellular uptake of berberine and curcumin in HaCaT cells
Because berberine and curcumin are all fluorescent compounds that can be detected by both flow cytometry and epifluorescence microscopy, the intracellular uptake of berberine and curcumin in HaCaT cells was then measured using these methods. As shown in Fig. 9A, measurement of total cellular fluorescence was firstly performed by flow cytometry. During 0.5, 1, 2 h of incubation treated with berberine, the fluorescence levels of 6.25, 12.5, 25, 50 μg/mL berberine demonstrated that the uptake of berberine in HaCaT cells was increased in a dose-dependent manner. After 6 h of incubation, the fluorescence levels of different concentrations of berberine in HaCaT cells were all accumulated to a maximum level. The maximum fluorescence levels in HaCaT cells were then slightly decreased between 6 h and 24 h of incubation. Particularly, the fluorescence levels of different concentrations of curcumin in HaCaT cells all have reached to a maximum level after 0.5 h of incubation. The fluorescence level of 3.125 μg/mL curcumin in HaCaT cells remained stable between 0.5 h and 1 h of incubation, and then this level was gradually decreased during 1, 2, 6, 24 h of incubation. After treated with 6.25, 12.5, 25 μg/mL curcumin, the fluorescence levels in HaCaT cells also remained stable during 0.5, 1, 2, 6 h of incubation. The fluorescence level of 6.25 μg/mL curcumin in HaCaT cells was then decreased at least half between 6 h and 24 h of incubation, and the fluorescence levels of 12.5, 25 μg/mL curcumin in HaCaT cells still remained stable between 6 h and 24 h of incubation. Representative flow cytometric readout pictures of 25 μg/mL berberine and 6.25 μg/mL curcumin during 0.5, 1, 2, 6, 24 h of incubation were also showed in Fig. 9A.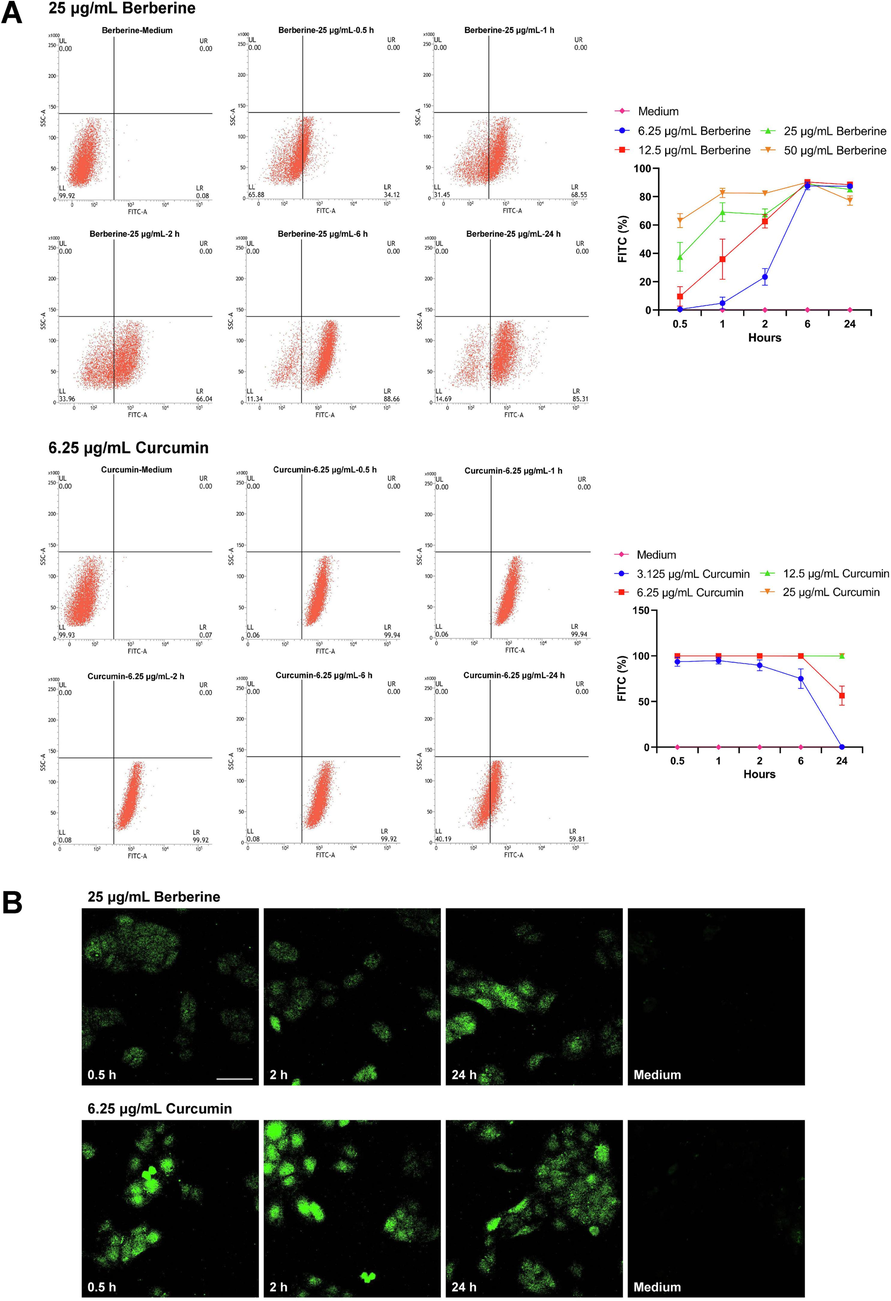
Intracellular uptake of berberine and curcumin after treated on HaCaT cells. (A) Uptake of different concentrations of berberine and curcumin in HaCaT cells measured by flow cytometry after 0.5, 1, 2, 6, 24 h of incubation (n = 3). (B) Confocal microscopy images of HaCaT cells incubated with berberine and curcumin. The photographs were taken after 0.5, 2, 24 h of incubation with 25 μg/mL berberine or 6.25 μg/mL curcumin using a confocal microscope (scale bar, 50 μm) (n = 3). Data are represented as mean ± SD.
Confocal microscopy was further used to visualize the intracellular uptake of berberine and curcumin in HaCaT cells. After 0.5, 2, 24 h of incubation, representative confocal microscopy images of HaCaT cells treated with 25 μg/mL berberine or 6.25 μg/mL curcumin were showed in Fig. 9B. The fluorescence of 25 μg/mL berberine in HaCaT cells was gradually noticeable during 0.5, 2, 24 h of incubation, which was corresponding to the increase of fluorescence signal detected by flow cytometry. The fluorescence of 6.25 μg/mL curcumin in HaCaT cells was significantly observed between 0.5 h and 2 h of incubation and then the fluorescence was gradually decreased between 2 h and 24 h of incubation, which was also consistent with the changes of fluorescence signal measured by flow cytometry. In summary, the uptake of berberine in HaCaT cells was increased to a maximum level as the incubation time extended, and then the uptake of berberine was slightly decreased or remained stable. Furthermore, the uptake of curcumin in HaCaT cells immediately reached to a maximum level at the beginning of incubation and remained stable, and finally the uptake of curcumin was gradually decreased.
3.7 Berberine suppressed JAK1/STAT1 signaling pathway in HaCaT cells induced by IFN-γ
In the present study, it has indicated that JAK-STAT signaling pathway played an important role in IMQ-induced psoriasis-like dermatitis and the treatment of topical application of berberine. Among of the molecules in JAK-STAT signaling pathway, JAK1/STAT1 were essential in the regulation of proliferation and inflammation of keratinocytes, which was closely associated with the pathogenesis of psoriasis (Huang et al., 2022). To explore whether berberine affected HaCaT cells induced by IFN-γ via suppressing JAK1/STAT1 signaling, western blot assay was then performed (Fig. 10). The increased phosphorylation and non-phosphorylation of JAK1 and STAT1 was significantly observed in IFN-γ-induced HaCaT cells (P < 0.05, P < 0.01, P < 0.001). After treated with 25 μg/mL berberine, the levels of both the phosphorylated and non-phosphorylated forms of these proteins were obviously decreased (P < 0.05, P < 0.01, P < 0.001). These results suggested that berberine may inhibit the hyperproliferation and proinflammatory functions of HaCaT cells induced by IFN-γ via suppressing JAK1/STAT1 signaling pathway.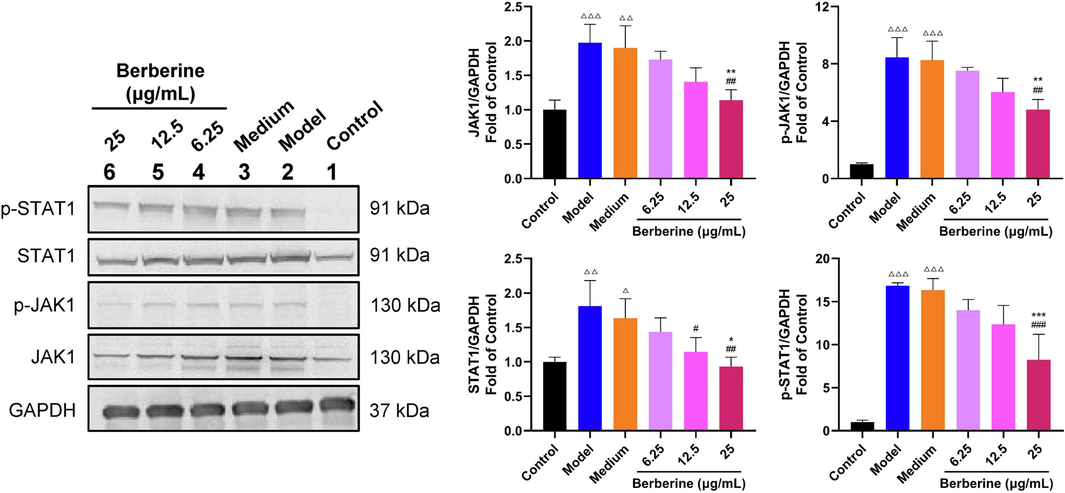
Berberine inhibited the activation of JAK1/STAT1 signaling pathway in HaCaT cells induced by IFN-γ. Berberine significantly decreased the protein level of JAK1, p-JAK1, STAT1 and p-STAT1 in HaCaT cells stimulated by IFN-γ. Data are expressed as mean ± SD. △P < 0.05, △△P < 0.01, △△△P < 0.001 versus Control group, #P < 0.05, ##P < 0.01, ###P < 0.001 versus Model group and *P < 0.05, **P < 0.01, ***P < 0.001 versus Medium group according to one-way ANOVA analysis followed by Sidak's multiple comparison test.
4 Discussion
Psoriasis is a immune-mediated inflammatory skin disease with typical skin lesions induced by skin inflammation (Kamiya et al., 2019). It has been reported that immune-mediated skin inflammation plays an important role in the development of psoriasis (Li et al., 2023). In this study, IMQ-induced psoriasis-like dermatitis was used to resemble the skin lesions of psoriasis and explore the potential pathogenic mechanism. Previous studies have indicated that JAK-STAT signaling pathway is closely associated with the pathogenesis of inflammatory and autoimmune diseases including rheumatoid arthritis, psoriasis and inflammatory bowel disease (Banerjee et al., 2017). As a pathway of intracellular signal transduction in response to cytokines, JAK-STAT signaling pathway is essential for skin inflammation (Welsch et al., 2017). In psoriatic skin lesions, many cytokines such as IL-19, IL-20, IL-22 and IL-23 signal via JAK-STAT signaling pathway (Witte et al., 2014; Cochez et al., 2016). In the present study, JAK-STAT signaling pathway was also significantly enriched in IMQ-induced psoriasis-like dermatitis by transcriptome analysis, which further indicated the importance of JAK-STAT signaling pathway in psoriatic skin inflammation.
To search for the potential agents treating psoriatic dermatitis, many studies have been performed. In recent years, multiple natural products have been used to explore the therapeutic potential in the treatment of psoriasis-like skin inflammation (Li et al., 2018a; Thatikonda et al., 2020). Berberine, a natural plant alkaloid, has the significant effect of anti-inflammation (Li et al., 2019). It has been reported that berberine can treat inflammatory diseases by inhibiting the activation of NLRP3 inflammasome (Sarbadhikary et al., 2021). Furthermore, berberine can regulate inflammatory signaling pathways in the immune system via NF-κB, JAK-STAT and MAPK signaling pathways (Haftcheshmeh et al., 2022). Therefore, the potential effect and molecular mechanism of topical berberine treating skin inflammation in psoriasis were then investigated in this study.
Network pharmacology and molecular docking were firstly performed to explore the potential of topical berberine treating psoriasis. It predicted that topical berberine may treat psoriasis by regulating JAK-STAT signaling pathway, especially act on these potential key targets such as JAK1, IL2, PIK3CA and PIK3CB involved in JAK-STAT signaling pathway. Meanwhile, the following experimental studies demonstrated that topical application of berberine could significantly ameliorate IMQ-induced psoriasis-like dermatitis in mice. Furthermore, the potential mechanism may be associated with the regulation of JAK-STAT signaling pathway in IMQ-induced mice by topical application of berberine.
JAK-STAT signaling pathway is mainly composed of Janus kinase (JAK), Tyrosine kinase-related receptor and Signal transducer and activator of transcription (STAT) (Wang et al., 2021). Tyrosine kinase-related receptors are also known as cytokine receptors in JAK-STAT signaling pathway, including class I cytokine (IL2 family, IL3 family, IL6 family, IL12/IL23) receptors and class II cytokine (IL10 family, interferon family) receptors (Hu et al., 2021). JAKs (JAK1, JAK2, TYK2 and JAK3) are non-receptor tyrosine kinase, which mediates cytokine-related signal transmission through coupling with Tyrosine kinase-related receptors (Shivaji et al., 2020). STATs including STAT1, 2, 3, 4, 5a, 5b and 6 are the downstream molecules of JAKs, which can couple with the phosphorylated and activated JAKs and then play an important role in the transcriptional activation and regulation of target genes such as BCL2 (Xin et al., 2020). Furthermore, the activation of receptor tyrosine kinase such as EGFR may lead to JAK-independent tyrosine phosphorylation of STATs (Liang et al., 2020). As an important pathway of signal transduction, JAK-STAT signaling pathway is widely involved in various pathophysiological processes such as inflammatory immune regulation, proliferation, differentiation and apoptosis (Xin et al., 2020). The activation of JAK-STAT signaling pathway can cause multiple immune inflammatory responses in cells and tissues (Yan et al., 2018). It has been reported that the target genes of JAK-STAT signaling such as IFNGR1, IL2, CCND1, CCND2 were up-regulated and IL10 was down-regulated under the activation of JAK-STAT signaling pathway (Xiong et al., 2022). PIK3CA, participating in PI3K-AKT-mTOR pathway (an indirect pathway of JAK-STAT signaling pathway), mainly regulates the physiological functions of cell proliferation and differentiation (Lang and Ling, 2012). PIK3CA is inactive in normal conditions. When PIK3CA is abnormally activated, it will lead to the excessive proliferation of cells. In addition, suppressors of cytokine signaling (SOCS) is also involved in JAK-STAT signaling pathway. It is also the target gene of STAT (STAT promotes the transcription and expression of SOCS), but SOCS can inhibit the phosphorylation of STAT (Durham et al., 2019). SOCS has been considered as the inhibitor of the classic JAK-STAT signaling pathway. It has also been reported that SOCS1 and SOCS3 are the most important inhibitors of JAK-STAT signaling pathway (Liau et al., 2018). In the present study, berberine could potently reverse the mRNA expression of these key genes of JAK-STAT signaling pathway induced by IMQ, which indicated that topical berberine may ameliorate IMQ-induced psoriasis-like skin inflammation by suppressing JAK-STAT signaling pathway.
The inflammation, abnormal proliferation and differentiation of keratinocytes are typical pathological features of psoriasis (Lowes et al., 2007). Keratinocytes can be stimulated by multiple cytokines such as IL-17, IL-22, IL-23 and IFN-γ that produced by dendritic cells and T cells in skin lesions (Lowes et al., 2014). Meanwhile, multiple proinflammatory cytokines such as TNF-α, IL-1, IL-18 and chemokines can be released by activated keratinocytes to sustain psoriatic dermatitis (Albanesi et al., 2018). Thus, it can be a effective therapy for the treatment of psoriasis by targeting these pathways of proliferation and inflammation in keratinocytes. In this study, the cell viability of HaCaT cells treated by 6.25, 12.5, 25 μg/mL berberine was higher than that treated by 50, 100, 200 μg/mL berberine. Furthermore, the intracellular uptake of berberine in HaCaT cells was gradually increased to a maximum level during 24 h of incubation. These results indicated that berberine could directly act on and enter into keratinocytes, and then potently affect the biological functions of keratinocytes.
Previous studies have shown that the proinflammatory functions of keratinocytes can be potently activated by IFN‐γ binding to constitutively expressed IFN‐γR on keratinocytes, and then the development of psoriasis is promoted (Albanesi et al., 2001; Federici et al., 2002). In addition, the hyperproliferation of keratinocytes can be stimulated by autocrine action of IFN-γ (Konur et al., 2005). It has been reported that JAK-STAT signaling pathway can be activated by IFN‐γ stimulating HaCaT cells (Tu et al., 2011). In particular, the key molecules of JAK-STAT signaling pathway such as JAK1 and STAT1 play an important role in IFN‐γ-induced JAK-STAT signaling pathway in HaCaT cells (Li et al., 2023). In the present study, berberine could significantly decrease the levels of both phosphorylated and non-phosphorylated forms of JAK1 and STAT1. It indicated that berberine may inhibit the proliferation and inflammation of IFN-γ-induced HaCaT cells by suppressing JAK1/STAT1 signaling pathway. In addition, 13-hexylberberine, a berberine derivative, also showed positive effects on regulating the proliferation and inflammation pathways of HaCaT cells in the previous studies (Jiang et al., 2009; Song et al., 2012), which was worthy of further study.
In the present study, the uptake of berberine was mainly investigated in HaCaT cells in vitro. Although the skin permeation coefficient (logKp) of berberine (-5.78 cm/s) was predicted by SwissADME, the actual transdermal permeability of berberine and concentration changes of berberine in skin tissues after topical application of Berberine cream were still not clear in this study. Thus, in vitro permeation test of berberine and quantitative measurement of berberine in skin tissues in vivo are required to elucidate the relationships of transdermal absorption and therapeutic effect of berberine in the following research. Meanwhile, further experimental studies in HaCaT cells, primary keratinocytes and skin tissues are also needed to illustrate the molecular mechanism related to JAK1/STAT1 signaling pathway in the future.
5 Conclusion
This study showed that topical application of berberine could inhibit the development of skin lesions and alleviate the inflammatory response of skin tissues in IMQ-induced mice. The potential mechanism may be associated with the inhibition of JAK1/STAT1 signaling pathway in keratinocytes after treated by berberine. Furthermore, this study also indicated that berberine may be a promising topical agent to ameliorate skin inflammation in psoriasis in the future.
Ethics statement
The animal study was reviewed and approved by Animal Ethics Committee of Hospital of Dermatology, Chinese Academy of Medical Sciences & Peking Union Medical College. The application of approval number is No. 2022-DW-011.
CRediT authorship contribution statement
Yi Chen: Conceptualization, Methodology, Formal analysis, Data curation, Writing – original draft, Visualization, Investigation. Shasha Song: Visualization, Investigation, Formal analysis. Yongfang Wang: Visualization, Investigation. Xiaoli Zhang: Visualization, Investigation. Jiafen Zhang: Visualization, Investigation. Lili Wu: Visualization, Investigation. Jianbing Wu: Visualization, Investigation. Xinyu Li: Conceptualization, Methodology, Data curation, Writing – original draft, Supervision, Validation, Writing – review & editing.
Acknowledgments
This work was supported by the grant from Chinese Academy of Medical Sciences (CAMS) Innovation Fund for Medical Sciences (CAMS-2017-I2M-1-011).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- A cytokine-to-chemokine axis between T lymphocytes and keratinocytes can favor Th1 cell accumulation in chronic inflammatory skin diseases. J. Leukocyte Biol.. 2001;70(4):617-623.
- [CrossRef] [Google Scholar]
- The interplay between keratinocytes and immune cells in the pathogenesis of psoriasis. Front. Immunol.. 2018;9:1549.
- [CrossRef] [Google Scholar]
- JAK–STAT signaling as a target for inflammatory and autoimmune diseases: current and future prospects. Drugs. 2017;77:521-546.
- [CrossRef] [Google Scholar]
- Topical treatments in psoriasis: today and tomorrow. Clin. Dermatol.. 2008;26(5):432-437.
- [CrossRef] [Google Scholar]
- Diagnosis and management of psoriasis and psoriatic arthritis in adults: summary of SIGN guidance. BMJ. 2010;341:c5623
- [CrossRef] [Google Scholar]
- AhR modulates the IL-22-producing cell proliferation/recruitment in imiquimod-induced psoriasis mouse model. Eur. J. Immunol.. 2016;46(6):1449-1459.
- [CrossRef] [Google Scholar]
- Berberine on metabolic and cardiovascular risk factors: an analysis from preclinical evidences to clinical trials. Expert Opin. Biol. Th.. 2012;12(8):1113-1124.
- [CrossRef] [Google Scholar]
- Targeting SOCS proteins to control JAK-STAT signalling in disease. Trends Pharmacol. Sci.. 2019;40(5):298-308.
- [CrossRef] [Google Scholar]
- Impaired IFN-γ-dependent inflammatory responses in human keratinocytes overexpressing the suppressor of cytokine signaling 1. J. Immunol.. 2002;169(1):434-442.
- [CrossRef] [Google Scholar]
- Effect of berberine nanoemulsion Photodynamic therapy on cervical carcinoma cell line. Photodiagn. Photodyn.. 2021;33:102174
- [CrossRef] [Google Scholar]
- TLRs to cytokines: mechanistic insights from the imiquimod mouse model of psoriasis. Eur. J. Immunol.. 2013;43(12):3138-3146.
- [CrossRef] [Google Scholar]
- The effect of herbal medicinal products on psoriasis-like keratinocytes. Biomolecules. 2021;11(3):371.
- [CrossRef] [Google Scholar]
- Validated models for predicting skin penetration from different vehicles. Eur. J. Pharm. Sci.. 2010;41(5):612-616.
- [CrossRef] [Google Scholar]
- Berberine inhibits proliferation and migration of colorectal cancer cells by downregulation of GRP78. Anti-Cancer Drug.. 2020;31(2):141-149.
- [CrossRef] [Google Scholar]
- Topical application of Chinese herbal medicine for atopic eczema: a systematic review with a meta-analysis. Dermatology. 2014;228(4):294-302.
- [CrossRef] [Google Scholar]
- Berberine pharmacology and the gut microbiota: A hidden therapeutic link. Pharmacol. Res.. 2020;155:104722
- [CrossRef] [Google Scholar]
- Berberine as a natural modulator of inflammatory signaling pathways in the immune system: Focus on NF-κB, JAK/STAT, and MAPK signaling pathways. Phytother. Res.. 2022;36(3):1216-1230.
- [CrossRef] [Google Scholar]
- The JAK/STAT signaling pathway: From bench to clinic. Signal Transduct. Tar.. 2021;6(1):402.
- [CrossRef] [Google Scholar]
- Efficacy of berberine in treatment of rheumatoid arthritis: From multiple targets to therapeutic potential. Pharmacol. Res.. 2021;169:105667
- [CrossRef] [Google Scholar]
- OAS1, OAS2, and OAS3 contribute to epidermal keratinocyte proliferation by regulating cell cycle and augmenting IFN-1-induced Jak1-Signal transducer and activator of transcription 1 phosphorylation in psoriasis. J. Invest. Dermatol.. 2022;142(10):2635-2645.
- [CrossRef] [Google Scholar]
- Involvement of P2Y11 receptor in IFN-γ-induced IL-6 production in human keratinocytes. Eur. J. Pharmacol.. 2013;703:67-73.
- [CrossRef] [Google Scholar]
- Effects of 13-hexyl-berberine hydroehloride and 13-hexyl-palmatine hydroehloride on the activation of nuclear factor-kappa B and phosphorylation of p38 mitogen-activated protein kinase (MAPK) in a human keratinooyte cell line (HaCaT) stimulated by tumor necrosis factor alpha. Chinese J. Dermatol.. 2009;42(5):339-342.
- [CrossRef] [Google Scholar]
- Clinical and histologic diagnostic guidelines for psoriasis: a critical review. Clin. Rev. Allerg. Immu.. 2013;44:166-172.
- [CrossRef] [Google Scholar]
- Risk factors for the development of psoriasis. Int. J. Mol. Sci.. 2019;20(18):4347.
- [CrossRef] [Google Scholar]
- Berberine improves lipid dysregulation in obesity by controlling central and peripheral AMPK activity. Am. J. Physiol. Endoc. m.. 2009;296(4):E812-E819.
- [CrossRef] [Google Scholar]
- Interferon (IFN)-γ is a main mediator of keratinocyte (HaCaT) apoptosis and contributes to autocrine IFN-γ and tumour necrosis factor-α production. Brit. J. Dermatol.. 2005;152(6):1134-1142.
- [CrossRef] [Google Scholar]
- MiR-124 suppresses cell proliferation in hepatocellular carcinoma by targeting PIK3CA. Biochem. Bioph. Res. Co.. 2012;426(2):247-252.
- [CrossRef] [Google Scholar]
- Intervention of oncostatin M-driven mucosal inflammation by berberine exerts therapeutic property in chronic ulcerative colitis. Cell Death Dis.. 2020;11(4):271.
- [CrossRef] [Google Scholar]
- Glabridin, an isoflavan from licorice root, ameliorates imiquimod-induced psoriasis-like inflammation of BALB/c mice. Int. Immunopharmacol.. 2018;59:243-251.
- [CrossRef] [Google Scholar]
- Berberine suppresses IL-33-induced inflammatory responses in mast cells by inactivating NF-κB and p38 signaling. Int. Immunopharmacol.. 2019;66:82-90.
- [CrossRef] [Google Scholar]
- Kaempferol modulates IFN-γ induced JAK-STAT signaling pathway and ameliorates imiquimod-induced psoriasis-like skin lesions. Int. Immunopharmacol.. 2023;114:109585
- [CrossRef] [Google Scholar]
- Aromatic-turmerone ameliorates imiquimod-induced psoriasis-like inflammation of BALB/c mice. Int. Immunopharmacol.. 2018;64:319-325.
- [CrossRef] [Google Scholar]
- Natural tyrosine kinase inhibitors acting on the epidermal growth factor receptor: Their relevance for cancer therapy. Pharmacol. Res.. 2020;161:105164
- [CrossRef] [Google Scholar]
- The molecular basis of JAK/STAT inhibition by SOCS1. Nat. Commun.. 2018;9(1):1558.
- [CrossRef] [Google Scholar]
- TiGER: a database for tissue-specific gene expression and regulation. BMC Bioinf.. 2008;9(1):1-7.
- [CrossRef] [Google Scholar]
- The global, regional, and national burden of psoriasis in 195 countries and territories, 1990 to 2017: a systematic analysis from the Global Burden of Disease Study 2017. J. Am. Acad. Dermatol.. 2021;84(1):46-52.
- [CrossRef] [Google Scholar]
- American Academy of Dermatology. Guidelines of care for the management of psoriasis and psoriatic arthritis. Section 3. Guidelines of care for the management and treatment of psoriasis with topical therapies. J. Am. Acad. Dermatol.. 2009;60(4):643-659.
- [CrossRef] [Google Scholar]
- A systematic review of worldwide epidemiology of psoriasis. J. Eur. Acad. Dermatol.. 2017;31(2):205-212.
- [CrossRef] [Google Scholar]
- Long-term side effects of glucocorticoids. Expert Opin. Drug Saf.. 2016;15(4):457-465.
- [CrossRef] [Google Scholar]
- The chemistry of curcumin: from extraction to therapeutic agent. Molecules. 2014;19(12):20091-20112.
- [CrossRef] [Google Scholar]
- Role of keratinocytes and immune cells in the anti-inflammatory effects of Tripterygium wilfordii Hook. f. in a murine model of psoriasis. Phytomedicine. 2020;77:153299
- [CrossRef] [Google Scholar]
- Assessment and management of psoriasis: summary of NICE guidance. BMJ. 2012;345:e6712.
- [Google Scholar]
- Topical therapies for the treatment of plaque psoriasis: systematic review and network meta-analyses. Br. J. Dermatol.. 2013;168(5):954-967.
- [CrossRef] [Google Scholar]
- Galangin ameliorates imiquimod-induced psoriasis-like skin inflammation in BALB/c mice via down regulating NF-κB and activation of Nrf2 signaling pathways. Int. Immunopharmacol.. 2021;96:107754
- [CrossRef] [Google Scholar]
- Inhibitory role of berberine, an isoquinoline alkaloid, on NLRP3 inflammasome activation for the treatment of inflammatory diseases. Molecules. 2021;26(20):6238.
- [CrossRef] [Google Scholar]
- Long-term topical management of psoriasis: the road ahead. J. Dermatolog. Treat.. 2022;33(1):111-120.
- [CrossRef] [Google Scholar]
- Different concentrations of berberine result in distinct cellular localization patterns and cell cycle effects in a melanoma cell line. Cancer Chemoth. Pharm.. 2008;61(6):1007-1018.
- [CrossRef] [Google Scholar]
- Small molecule oral targeted therapies in ulcerative colitis. Lancet Gastroenterol.. 2020;5(9):850-861.
- [CrossRef] [Google Scholar]
- Effects of 13-hexyl-berberine hydrochloride on the activation of T lymphocytes and the expression of cytokines in human keratinocytes. J. Med. Res.. 2012;41(12):58-61.
- [CrossRef] [Google Scholar]
- Topical therapies for psoriasis: improving management strategies and patient adherence. Semin. Cutan. Med. Surg.. 2016;35(Suppl 2):S36-S44.
- [CrossRef] [Google Scholar]
- Psoriasis patient preferences for topical drugs: a systematic review. J. Dermatolog. Treat.. 2021;32(5):478-483.
- [CrossRef] [Google Scholar]
- Immunological axis of berberine in managing inflammation underlying chronic respiratory inflammatory diseases. Chem. Biol. Interact.. 2020;317:108947
- [CrossRef] [Google Scholar]
- Piperlongumine regulates epigenetic modulation and alleviates psoriasis-like skin inflammation via inhibition of hyperproliferation and inflammation. Cell Death Dis.. 2020;11(1):21.
- [CrossRef] [Google Scholar]
- Triptolide inhibits IFN-γ signaling via the Jak/STAT pathway in HaCaT keratinocytes. Phytother. Res.. 2011;25(11):1678-1685.
- [CrossRef] [Google Scholar]
- Imiquimod-induced psoriasis-like inflammation in differentiated Human keratinocytes: Its evaluation using curcumin. Eur. J. Pharmacol.. 2017;813:33-41.
- [CrossRef] [Google Scholar]
- Targeting JAK/STAT signaling pathways in treatment of inflammatory bowel disease. Inflamm. Res.. 2021;70:753-764.
- [CrossRef] [Google Scholar]
- Targeting JAK/STAT signalling in inflammatory skin diseases with small molecule inhibitors. Eur. J. Immunol.. 2017;47(7):1096-1107.
- [CrossRef] [Google Scholar]
- IL-19 is a component of the pathogenetic IL-23/IL-17 cascade in psoriasis. J. Invest. Dermatol.. 2014;134(11):2757-2767.
- [CrossRef] [Google Scholar]
- The role of JAK/STAT signaling pathway and its inhibitors in diseases. Int. Immunopharmacol.. 2020;80:106210
- [CrossRef] [Google Scholar]
- Cannabis suppresses antitumor immunity by inhibiting JAK/STAT signaling in T cells through CNR2. Signal Transduct. Tar.. 2022;7(1):99.
- [CrossRef] [Google Scholar]
- Role of the JAK/STAT signaling pathway in regulation of innate immunity in neuroinflammatory diseases. Clin. Immunol.. 2018;189:4-13.
- [CrossRef] [Google Scholar]
- Berberine directly targets the NEK7 protein to block the NEK7–NLRP3 interaction and exert anti-inflammatory activity. J. Med. Chem.. 2020;64(1):768-781.
- [CrossRef] [Google Scholar]
- Treatment of type 2 diabetes and dyslipidemia with the natural plant alkaloid berberine. J. Clin. Endocr. Metab.. 2008;93(7):2559-2565.
- [CrossRef] [Google Scholar]
- Paeoniflorin inhibits imiquimod-induced psoriasis in mice by regulating Th17 cell response and cytokine secretion. Eur. J. Pharmacol.. 2016;772:131-143.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2024.105612.
Appendix A
Supplementary data
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







