Translate this page into:
Ultrasonic extraction, structural modification and gastric mucosal cells protective activity of a polysaccharide from Dendrobium denneanum
⁎Corresponding authors. yijfan@163.com (Yijun Fan), aoxueluo@sicau.edu.cn (Aoxue Luo)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Optimize the ultrasonic extraction process of polysaccharides from Dendrobium denneanum by Response surface design (RSM). The main polysaccharide (DP40) from was separated by anion exchange chromatography, and its structure was modified by low-temperature plasma. The results showed that the polysaccharide extraction rate of ultrasonic was 22.98 ± 0.97%, which was significantly higher than water extraction method(P < 0.05). FT-IR showed that low-temperature plasma modification could improve the water solubility and —OH content. NMR showed that the 1 → 6 glycosidic bonds of DP40 are transformed into 1 → 4 glycosidic bonds and 1 → 3 glycosidic bonds, and the α-configuration sugar ring is transformed into β-configuration after plasma treatment. SEM and TEM analysis showed that the morphology of modified DP40 changed from layered porous network structure to smooth morphology, and bulged into semicircular particles, and the degree of cross-linking of surface molecules increased. The results of biological activity experiments indicated that the modified polysaccharide (500–1000 μg·mL−1) can significantly improve the survival rate of GES-1 cells (p < 0.01). Compared with the untreated group, the high concentration of 1000 μg·mL−1 DP40-plasma could not only reduce ROS by 14.5% (p < 0.05) and MDA by 27.45% (p < 0.05), increase SOD activity by 28.27%, but also reduce cytokines (IL-8, TNF-α, IL-1β) by 31.82% (p < 0.05), 33.23% (p < 0.05) and 21.97% (p < 0.05), respectively. At the same time, low-temperature plasma modification can effectively reduce the caspase 3 activity, prevent cell apoptosis, and protect gastric mucosal cells from ethanol damage. Therefore, low-temperature plasma modification can improve the biological activity of DP40 polysaccharide by altering its structural characteristics. The results provide a new idea for the application of low-temperature plasma modification in biomacromolecules.
Keywords
Ultrasonic extraction
Dendrobium denneanum
Polysaccharide
Low-temperature plasma
Structural characteristics
GES-1 cells
1 Introduction
Dendrobium is a perennial epiphytic herb of Orchidaceae. It is a valuable traditional Chinese medicine recorded in the Chinese Pharmacopoeia (Fan et al., 2022a, 2022b, 2022c). Dendrobium has the function of “Generating saliva and benefiting stomach”. The polysaccharide is an important active component of Dendrobium and has the function of Treating gastritis (Zeng et al., 2017), Anti-inflammatory effect (Wen et al., 2021), Liver protection (Yang et al., 2020), Antioxidant (Li et al., 2022), Immune regulation (Dong et al., 2022), Anti-cancer (Tao et al., 2021), Hypoglycemic (Peng et al., 2023) and other pharmacological effects. The polysaccharide is also one of the main active components of D. denneanum, which has antioxidant (Fan et al., 2009), immune promoting and other activities (Wang et al., 2017).
However, the polysaccharide of D. denneanum has poor water solubility, poor adhesion to target organs, which greatly reduces its effect, resulting in the limited application of these active polysaccharides. Chemical modification often changes the primary molecular structure and pharmacological activity of polysaccharides (Borchers et al., 1999), so they cannot be widely used in polysaccharides. Therefore, finding effective modification methods is an urgent difficulty in the research of active polysaccharides. Plasma is a partially ionized gaseous substance, which mainly contains ions, electrons, metastable molecules, atoms, free radicals and photons. Because they are high-energy active particles in frequent collisions, especially —OH radicals, can trigger chemical reactions that cannot be carried out under normal conditions. These highly active particles are also closely related to the surface modification of polymer materials (Prasertsung et al., 2013). Plasma is divided into normal-temperature plasma and low-temperature plasma. Treating biological macromolecules with normal-temperature plasma can induce the required chemical groups, change the surface micro morphology of substances, and improve the hydrophilicity and adhesion of polymer surface (Wang et al.,2019; Boenig et al., 1988), However, the high temperature required to initiate plasma is easy to destroy the chemical structure of biological macromolecules, resulting in the loss of their pharmacological activity. Low-temperature plasma is a kind of plasma balanced at low temperature. There is almost no energy loss in the collision between electrons and ions or neutral particles. Using it to treat materials is not only easy to operate, efficient and safe, but also can effectively avoid the damage to the structure of biological macromolecules under high temperature conditions. At present, low-temperature plasma modification has been applied to the biological-macromolecules such as protein and polysaccharide (Cui et al., 2022; Fan et al., 2020).
Plant polysaccharides, which are biological macromolecules with certain biological activity, have significantly lower efficacy and application compared to other small molecule compounds due to their large molecular weight and poor water solubility. To overcome this deficiency, new nanotechnology and molecular modification techniques have been applied to plant polysaccharides (Mosleh-Shirazi et al., 2012; 2022). In this study, we will search for an active polysaccharide from D. denneanum, and isolate, purify and analyze its structural characteristics. At the same time, the structure of polysaccharides was modified by low-temperature plasma, and the changes of structure and biological activity of polysaccharides before and after modification were compared and analyzed. This study will optimize the ultrasonic preparation technology of D. denneanum polysaccharides and provided structural modification techniques to enhance their biological activity. This provides new ideas for the application and development of polysaccharide compounds, as well as new insights for the application of low-temperature plasma modification in biomacromolecules.
2 Materials and methods
2.1 Materials and reagents
Dextrans (Glucan Standards, 2,500 Da − 2,000,000 Da) were purchased from Pharmacia Co. (Uppsala, Sweden). D2O, CCK − 8 and DEAE − cellulose were purchased from Haoboyou Co. (Chengdu, China), RPMI-1640, Fetal bovine serum, Penicillin-streptomycin mixture were purchased from Hyclone (Logan, USA). Trypsin was purchased from Gibco·H2O2 was purchased from Huagong Co. (Beijing, China). Caspase3 assay Kit, IL-8 assay Kit, TNF-α assay Kit, IL-1β assay Kit, MDA assay Kit were purchased from Haoboyou Co. (Chengdu, China). Ethanol, petroleum ether, pyridine, hydroxylamine hydrochloride, and all other chemicals and reagents were purchased from Kelong Co. (Chengdu China).
2.2 Preparation of polysaccharide from D. Denneanum
The fresh stems of D. denneanum were purchased from Miaoling Traditional Chinese Medicine Company (Sichuan, China). The stems were dried at 60 ℃ and crushed. 100.0 g D. denneanum powder was accurately weighed, and 500 mL petroleum ether (60–90 ℃) was added for extraction 30 min by ultrasonic. After the dried petroleum ether was volatilized at room temperature, 500 mL ethanol (95%) was added for ultrasonic extraction for 30 min. Filter and discard the filtrate, add deionized water into the filter residue, extracted the polysaccharides from D. denneanum in accordance with the following extraction conditions, and investigated the effects of different levels of ultrasonic power (150, 200, 250, 300, 350; W), ultrasonic temperature (50 ℃, 60 ℃, 70 ℃, 80 ℃, and 90 ℃), material solution ratio (1:50, 1:100, 1:150, 1:200, 1:250), and ultrasonic time (10, 20, 30, 40, 50; min) on the yield of polysaccharides from D. denneanum. Collected the extract obtained under various conditions, filtered and collected the filtrate. The protein was extracted with Sevage reagent (n-butanol: trichloromethane = 1:4). The supernatant was collected, extracted for 4 times and combined with the supernatant collected for 4 times concentrate to 100 mL. Anhydrous ethanol was slowly added into the concentrated solution, until the volume of ethanol reached 40%. The precipitate was collected by centrifugation and labeled as C-DP40.
2.3 Box-Behnken design
Based on single factor experiment, three groups of experimental factors with the largest standard deviation were selected as the next experimental factors. Three factors were ultrasonic power, temperature and extraction time. The RSM analysis of 3-factors and 3 − levels was used to design the central composite test. The conditions for the maximum polysaccharide extraction rate in these three factors were marked as “0″, The conditions around the maximum value are recorded as ”−1″ and “1″ (Fan et al., 2022a, 2022b, 2022c) (as shown in Table S1). The response surface design of 3 − factors and 3 − levels was obtained, and the response surface design test generated by Design Expert 8.0.6 software was carried out.
2.4 Separation and purification of polysaccharides
The protein in the polysaccharides extracted by the above method was removed, and the crude polysaccharides were obtained by ethanol precipitation. The precipitates were washed with anhydrous ethanol and centrifuged. After centrifugation, the precipitate was dissolved in deionized water and put into dialysis bag (7000 Da). Dialysis was performed with deionized water (magnetic stirring) for 72 h, and the water was changed every 8 h. The dialyzed solution was collected and added to DEAE − cellulose (2.6 × 30 cm) chromatographic column, wash with ddH2O and 0.1 M, 0.2 M NaCl respectively, the flow rate was 1.0 mL·min−1, the column temperature was room temperature, 10.0 mL·Tub-1, collected by computer automatic partial collector, and the absorbance value was measured at 490 nm by phenol sulfuric acid method.
2.5 Polysaccharide structural modification by plasma
The plasma source was designed to use argon as carrier gas. The flow of carrier gas was controlled by mass flow controller through discharge chamber, placeed the sample on the warehouse platform, used the vacuum pump to adjust the air pressure to 1.33 Pa, then passed the carrier gas to remove the residual gas, repeated 5 times, finally adjusted the pressure to 40 Pa, turned on the power supply discharge, and generated the plasma jet (Fan et al., 2022a, 2022b, 2022c). The processing conditions were as follows: the processing time was 60 s, output current was 8 mA, ionized gas was argon. After treatment, the samples were collected and put into sealed bags and stored at 4 ℃.
2.6 Analysis of structural characteristics of polysaccharides
2.6.1Analysis of Gel permeation chromatograph (GPC).
5.0 mg·mL−1 was dissolved in ddH2O for GPC analysis. The detection conditions were: column temperature was room temperature, waters ultrahydraulic linear column (300 × 7.8 mm), eluted with 0.2 M phosphoric acid buffer, the flow rate was 0.70 mL·min−1, and the standard reference substance was different dextran (2500 Da − 2,000,000 Da) (luo et al., 2009).
2.6.1 Fourier transform infrared Spectrum(FT-IR) analysis
2.0 mg polysaccharide sample, grinded and mixed with KBr, then pressed the tablet, and using nexus 670 Fourier transform infrared spectrometer to scan and analyze, the scanning range is from 500 cm−1 to 4000 cm−1 (Chen et al., 2023).
2.6.2 Nuclear magnetic resonance spectroscopy (NMR) analysis
Weighed 30.0 mg of polysaccharide, exchanged it with D2O for three times, and finally dissolved it in 0.4 mL of 99.9% D2O, and placed it in NMR to collect data (Liu et al.,2016; Hao et al., 2023).
2.6.3 Water contact angle (WCA) analysis
The WCA of polysaccharides was measured by VCA 2500XE optical contact angle meter. The hydrophilicity of polysaccharides was evaluated by detecting the change of water contact angle before and after plasma treatment, and image information was collected (Abe et al., 2022).
2.6.4 Scanning electron microscope (SEM) analysis
Dissolved an appropriate amount of polysaccharide sample in ultrapure water, took 1–2 drops of polysaccharide solution and dropped it on the cover glass, and put it in a 30 ℃ oven for drying, sputter with Pt/At powder, and observed the surface morphology of the sample under high vacuum mode (Singh et al., 2023).
2.6.5 Transmission electron microscope (TEM) analysis
Weighed 1.0 mg polysaccharide sample and prepared it into 0.5 mg·mL−1 concentration. dipped it in a copper mesh coated with carbon film for 15 min, then dyed it with uranium acetate for 15 min, and kept it away from light for 25 min, and observed and take photos under the transmission electron microscope. (Pal et al., 2023).
2.7 Gastric mucosal protective activity of polysaccharides
2.7.1 Cell culture and establishment of human gastric mucosal epithelial cells (GES-1) injury model
GES-1 cell line was purchased from the Chinese Academy of Sciences. GES-1 was cultured in RPMI-1640 medium containing 10% FBS at 37° C, saturated humidity and 5% CO2 concentration. GES-1 cells grown in logarithmic phase were treated with different concentrations of ethanol (0.3 M, 0.5 M, 0.7 M, 0.9 M, 1.1 M, 1.3 M, 1.5 M) for 8 h. The optimal ethanol treatment concentration was selected to establish GES-1 cell injury model (Ma et al., 2022).
2.7.2 Analysis of intracellular reactive oxygen species (ROS) and inflammatory factors
Each treatment was divided into control group (normal GES-1 cell), model group (EtOH 0.9 M) and low concentration treatment (250 μg·mL−1 + EtOH), medium concentration treatment (500 μg·mL−1 + EtOH), high concentration treatment (1000 μg·mL−1 + EtOH). Cells with 1 × 105 cells/well were spread in 96 wells (100 μL per well). After 8 h, discarded the supernatant and added 20 μL CCK − 8 solution, continue to culture for 4 h, discarded the medium and added 150 μL DMSO, the OD was measured at the wave-length of 490 nm. IL-1β, IL-8 and TNF-α in the supernatant of GES-1 cells were analyzed by ELISA. At the same time, the cells were centrifuged after ultrasonic crushing. SOD and MDA in each group were measured according to the instructions of the kit. The ROS of cells in each group was analyzed by DCFH-DA method.
2.7.3 Apoptosis analysis
The GES-1 cells were placed in 6-well plates with 2.0 mL medium per well. Drug treatment was carried out according to the above groups, and the cells of each group were collected for detection after 8 h of action. Prepared Annexin V/PI dyeing working solution. Discarded the supernatant and added 200 μL Annexin V/PI staining solution, incubated in dark for 15 min and then detected. At the same time, the activity of Caspase 3 was used to analyze by the kit in cells.
2.8 Statistics analysis
The test results were expressed by mean ± SD. Statistical analysis was conducted with SPSS 23.0 and significant difference and all data analysis of variance (ANOVA) was carried out using LSD test, *p < 0.05, **p < 0.01.
3 Results
3.1 Optimization of ultrasonic extraction by RSM
Based on single factor experiment results, it was found that three factors including temperature, time and power had a significant impact on the extraction rate. Therefore, three factors were selected to further optimize the extraction process of polysaccharides (Design expert. 8.0.6). Investigated the interaction between various factors and the impact on the extraction rate of polysaccharides from D. denneanum, as shown in Table S2. At the same time, the software automatically performs variance analysis on the response surface obtained, as shown in Table S3. The regression equation of polysaccharide extraction rate of D. denneanum was obtained as follows: Extraction rat = 21.40 + 1.99 × A + 1.54 × B + 0.17 × C + 0.12 × AB − 0.25 × AC + 0.35 × BC − 1.51 × A2 − 0.063 × B2 − 0.94 × C2 (A: temperature, B: power, C: time).
According to the regression equation obtained, response surface figures (Fig. 1) were drawn for the three factors of ultrasonic power, temperature and time. Each figure represents the influence of two of the three factors on the extraction rate of D. denneanum polysaccharides, which can reflect the interaction between the factors. The yellow part at the bottom of the figure was a contour line. From the three response surface graphs, it can be seen that each factor has an impact on the extraction rate. From the analysis of the graph, temperature has the most significant impact on the extraction rate. The main effect relationship of the three factors was: temperature (A) > ultrasonic power (B) > time (C), in which the response surface of temperature and ultrasonic power was the steepest, which proved that the interaction between temperature and ultrasonic power was the strongest.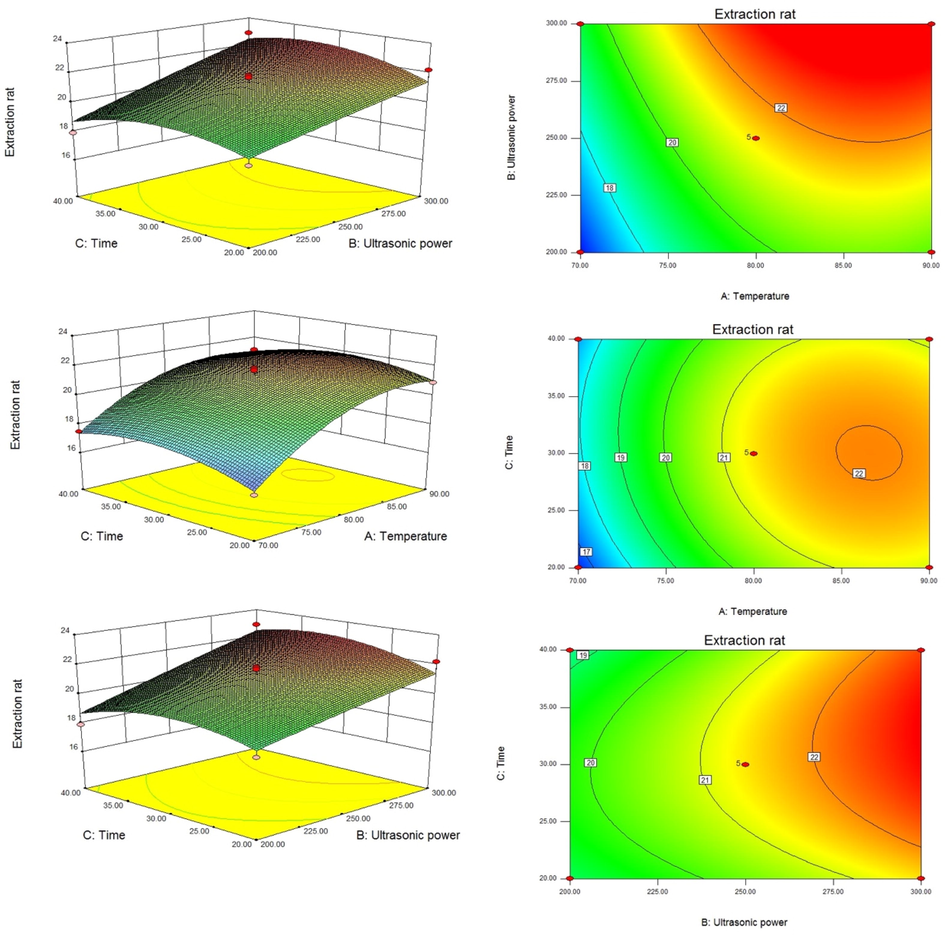
Box-Behnken experimental results.
The ultrasonic extraction conditions of polysaccharide were obtained by using Design Expert 8.0.6 software as follows: temperature was 83.74 ℃, power was 283 W, time was 32.33 min. Under these conditions, the predicted theoretical extraction rate of polysaccharides was 22.97 ± 1.05%. The results of repeated validation experiments showed that the average extraction rate of polysaccharide was 22.98 ± 0.97% (P > 0.05 vs predicted value), which was significantly higher than water extraction method(P < 0.05) (Luo et al.,2005).
3.2 Isolation and purification of polysaccharides from D. Denneanum
It can be seen from the Figure (Fig. 2) that after stage elution, two polysaccharide components were obtained, named DP40 and DP40-NaCl respectively. DP40 was obtained by washing with deionized water, and DP40-NaCl was obtained by washing with 0.1 M NaCl. It was also known from the Figure that DP40 accounts for the highest proportion and was the main polysaccharide of D. denneanum. Therefore, DP40 will be further studied in this project.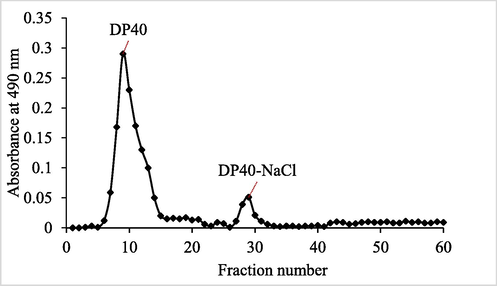
Chromatography of eluted crude polysaccharide (C-DP40) on DEAE-Cellulose column (26 mm × 300 mm). DP40 eluted with distilled water; DP40-NaCl eluted with 0.1 M NaCl.
3.3 Comparative analysis of structural characteristics of polysaccharide before and after low temperature plasma modification
3.3.1 Molecular weight analysis
Generally, the molecular weight (Mw) distribution can only be represented by the average. The Mw of polysaccharide samples was determined by GPC, and analyzed by Empower software (Fig. 3). From the Figure, the molecular weight of DP40 before treatment was 3.71 × 104 Da, while the molecular weight modified by low temperature plasma was 5.97 × 104 Da. There was no significant change in Mw of DP40 before and after low-temperature plasma treatment, indicating that the main chain structure of polysaccharides was not changed. However, compared with the untreated, the average molecular weight increased slightly, which indicating that the low-temperature plasma modified part of the structure of the polysaccharide, it was estimated that many hydroxyl groups were added, which was also verified in the later infrared experiments.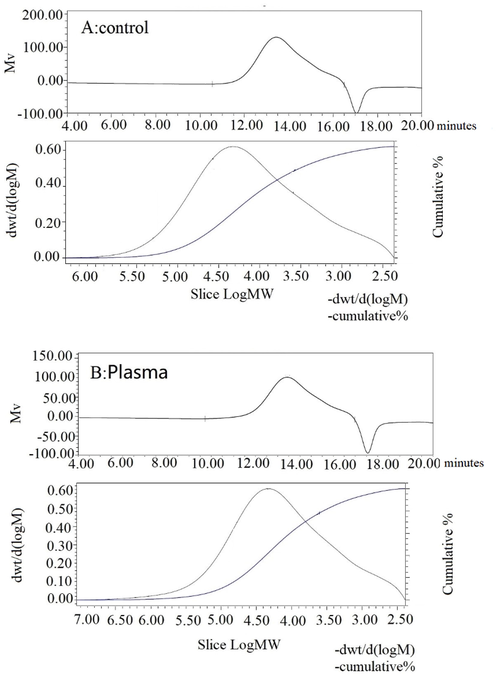
Analysis of polysaccharide by GPC. A for control and B for plasma treatment.
3.3.2 Infrared spectrum analysis
FT-IR spectroscopy is an indispensable tool in the study of organic chemistry and polymer chemistry. We can use infrared spectroscopy and quantitative analysis of the existence of some special chemical compounds. Fig. 4 was the FT-IR spectrum of DP40 before and after plasma modification. A broad peak at 3500–3200 cm−1 indicated stretching-vibration of sugar molecules –OH and N–H, and there were intermolecular and intramolecular hydrogen bonds. The peak of DP40 after plasma treatment was significantly higher than that of untreated polysaccharide, which indicating that plasma treatment can increase the –OH content and water solubility of the polysaccharide. In the treatment group, the peak height near 2917 cm−1 was increased, and the C–H stretching vibration representing carbohydrate was significantly enhanced. The peak near 1735 cm−1 did not change after plasma treatment, which indicated that the C = O stretching vibration of polysaccharides did not change. The peaks from 1032 cm−1 to 1251 cm−1 also increased slightly, indicating the increase of C–H and C-O in the polysaccharide (Fan et al., 2022a, 2022b, 2022c). Compared with the untreated, DP40-plasma has an absorption peak at 885 cm−1, indicating that part of the DP40 after plasma treatment appears β-configuration of sugar ring (Luo et al., 2011).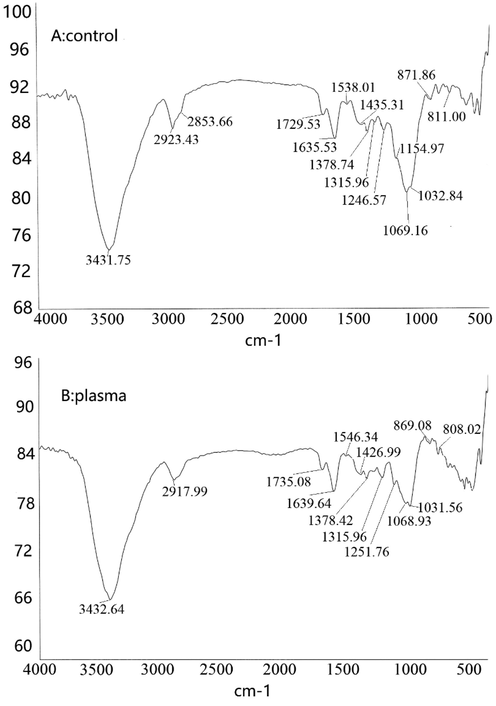
FTIR analysis of polysaccharide from D. denneanum.A for control and B for plasma treatment.
3.3.3 Nuclear magnetic resonance (NMR) analysis
The DP40-plasma has no absorption peak between 61 ppm −68 ppm, indicating that it does not contain 1 → 6 glycosidic bonds, indicating that after plasma treatment, the 1 → 6 glycosidic bonds in DP40 may have changed into 1 → 4 glycosidic bonds and 1 → 3 glycosidic bonds. Therefore, plasma treatment may break some of the original glycosidic bonds and reassemble the links. After plasma treatment, DP40 has an absorption peak near 174 ppm, suggesting that the treated polysaccharide has been acetylated (Fig. 5). After plasma treatment, absorption peaks appeared in the hydrogen spectrum of DP40 at 4.12 ppm and 4.42 ppm, indicating that glucose residue was β-configuration (1H NMR and 13CNMR chemical shifts see Table S4, Table S5).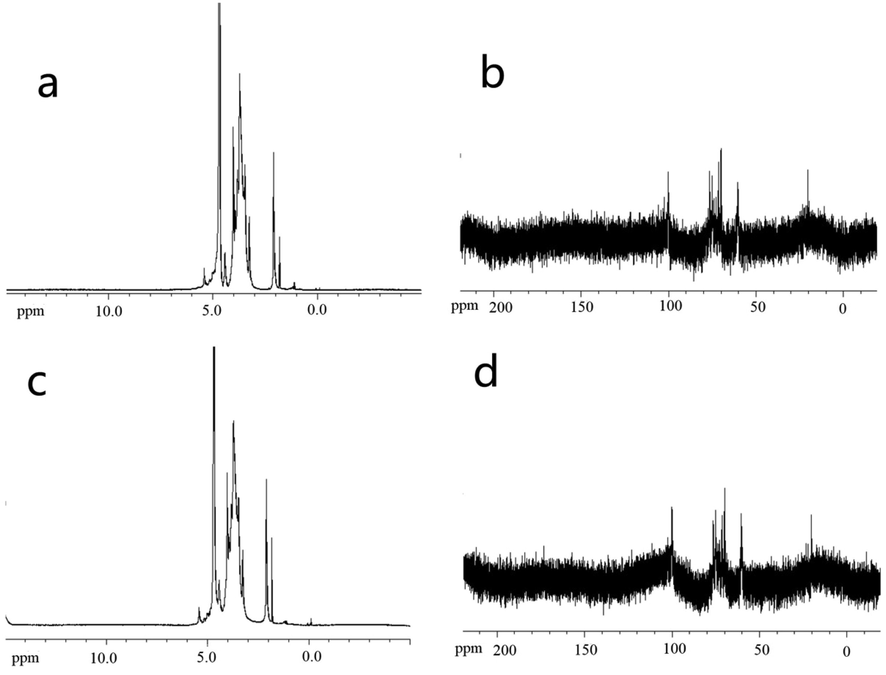
NMR analysis results. a is the 1H NMR of DP40, b is 13C NMR of DP40, c is the 1H NMR of DP40- plasma, d is 13C NMR of DP40- plasma.
3.3.4 Water contact angle (WCA) analysis
Production of highly active particles was the important characteristics of plasma treatment, especially —OH radicals, which were related to the surface modification of polymer materials (Prasertsung et al., 2013). At the same time, plasma can change the hydrophilic property of the material, so as to change the hydrophobicity to hydrophilicity (Jeon et al., 2016). In this experiment, the hydrophilicity of DP40 was analyzed by water contact angle detection (Fig. 6). The smaller the contact angle, the better the water solubility. It can be seen from the figure that the WCA of the ck was 70.67 ± 1.19° (n = 5) (Fig. 6 control), and that of the low-temperature plasma treatment was 26.51 ± 1.33° (n = 5) (Fig. 6 plasma) (p < 0.01). It was proved that the hydrophilicity of polysaccharides can be obviously improved by low-temperature plasma treatment, which may be due to the interaction between high active particles produced by low-temperature plasma and polysaccharides, which can increase the number of hydroxyl groups or other oxygen-containing groups in the polysaccharide chain, so as to improve its hydrophilicity.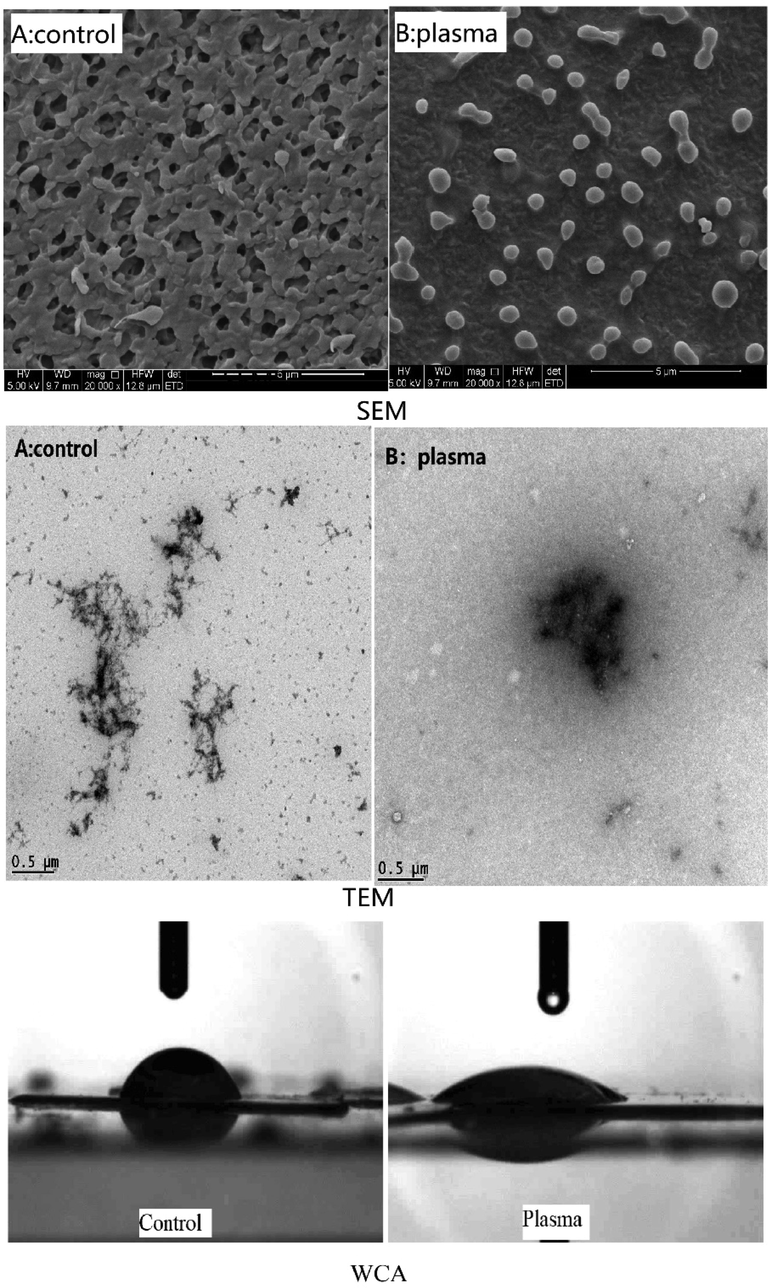
Morphology and Hydrophilicity changes of DP40 before and after low temperature plasma treatment. A for control and B for plasma treatment.
3.3.5 SEM analysis results
The SEM results of DP40 (Fig. 6) showed that the surface of polysaccharides without low-temperature plasma treatment was relatively smooth, with large-scale layered structure and regular porous network structure, which indicated that the branched monomers of polysaccharides were interwoven with each other, and most of them existed in the state of aggregation and winding. However, after low-temperature plasma treatment, D. denneanum polysaccharide surface morphology was smooth, convex form semicircular particles, structure was more regular, molecules are more dispersed. This phenomenon may be caused by the reduction of intermolecular forces between polysaccharides caused by high-energy active particle bombardment, radiation, and free radical oxidation in the plasma. The main chain and substituent groups of polysaccharides were depolymerized, and the degree of molecular cross-linking was reduced (Shen et al., 2011). As a result, the degree of intramolecular polymerization increased, causing the network structure of polysaccharides to become smooth morphology. Therefore, low-temperature plasma can change the surface morphology and structural characteristics of polysaccharides.
3.3.6 TEM analysis
TEM analysis results (Fig. 6) showed that the polysaccharide molecules without low-temperature plasma treatment were in the form of cypress branches, with obvious branches on the main chain, while the polysaccharide molecules after treatment showed spiral coiled morphology. The branches of polysaccharide chain were closely arranged and cross-linked. This phenomenon may be due to the van der Waals force interaction between molecules and the hydrogen bond between sugar chains. It was also possible that the intermolecular interaction starts to form micelles of multi stranded molecules, and the close contact between hydroxyl groups on the molecular chain of micellar polysaccharides was conducive to the formation of molecular colloidal aggregation structure under the interaction. Therefore, low-temperature plasma can increase the crosslinking degree of Dendrobium polysaccharide, and make the branch connection of polysaccharide more closely.
3.4 Comparative analysis of the biological activity of polysaccharide before and after low temperature plasma modification
3.4.1 Establishment of GES-1 cell injury model
In order to establish the model of ethanol injury to GES-1 cells and analyze its effect on the proliferation. GES-1 cells were treated with different concentrations ethanol (0 mol·L-1, 0.3 mol·L-1, 0.5 mol·L-1, 0.7 mol·L-1, 0.9 mol·L-1, 1.1 mol·L-1, 1.3 mol·L-1 and 1.5 mol·L-1). After 8 h of treatment, the proliferation activity was detected by MTT assay. Results as shown in Fig. 7a, low concentration of ethanol had no significant effect on the proliferation activity (p > 0.05). With the increase of ethanol concentration, the proliferation activity showed a significant downward trend. At 0.9 mol/L, its proliferation activity was only 40.49% of that of the control (p < 0.05). The activity decreased not significantly with the increase of ethanol concentration when>0.9 mol·L-1. Therefore, 0.9 mol·L-1 ethanol was selected as the concentration to establish GES-1 cell injury model.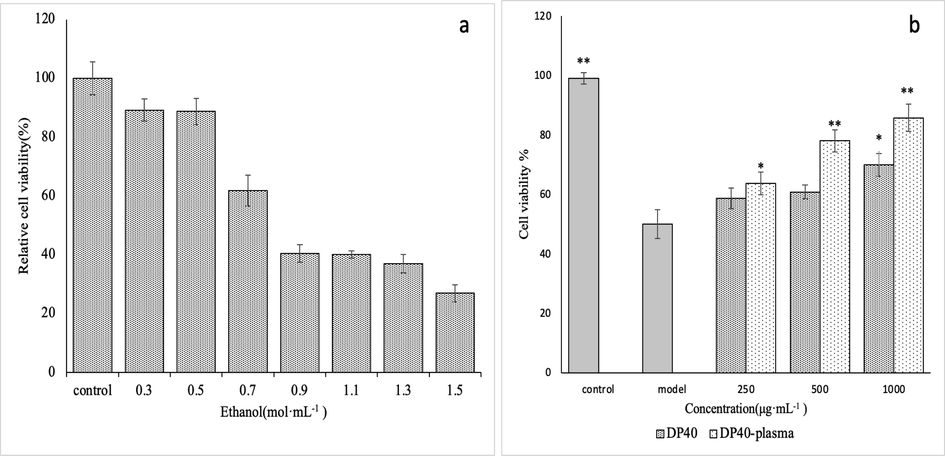
Establishment of GES-1 injured cell model. a is the injured of GES-1 cells induced by different concentrations of ethanol, and b is the effect of different concentrations of polysaccharides (DP40 is the untreated group and DP40-plasma is the low temperature plasma treatment group) on the viability of GES-1 cells induced by ethanol.
In order to study the effect of polysaccharide DP40 on the cell viability before and after low-temperature plasma treatment, the GES-1 cell injury model was established by 0.9 mol·L-1 ethanol induction, and the low concentration (250 μg·mL−1), medium concentration (500 μg·mL−1) and high concentration (1000 μg·mL−1) was investigated through the model. Results as shown in Fig. 7b, ethanol could reduce the cell viability (p < 0.05). Polysaccharide DP40 can improve the cell viability in a concentration dependent manner. The polysaccharide treated by low-temperature plasma has a more significant effect on the increase of cell viability, especially at high concentration (500–1000 μg·mL−1) could significantly increase the cell viability (p < 0.01). At 500 μg·mL−1 and 1000 μg·mL−1, compared with the ck, the cell viability of the low-temperature plasma treatment group increased by 28.28% (p < 0.05) and 22.63% (p < 0.05), respectively. Therefore, the activity of DP40 could been significantly improved by low-temperature plasma.
3.4.2 Effect of polysaccharide on ROS in injured GES-1 cells
In order to test whether low-temperature plasma treatment increase the antioxidant activity of polysaccharides, this study compared the effects of DP40 on ROS, SOD and MDA in GES-1 cells before and after low-temperature plasma treatment. As shown in Fig. 8, ethanol treatment can significantly increase the concentration of ROS in GES-1 cells, while polysaccharide DP40 can reduce ROS in cells in a concentration dependent manner. At medium dose (500 μg·mL−1) and high dose (1000 μg·mL−1), the polysaccharide DP40 after low-temperature plasma treatment could significantly reduce ROS in damaged cells (p < 0.05). Compared with the polysaccharide without low-temperature plasma treatment, the treatment group decreased by 38.2% and 14.5%, respectively.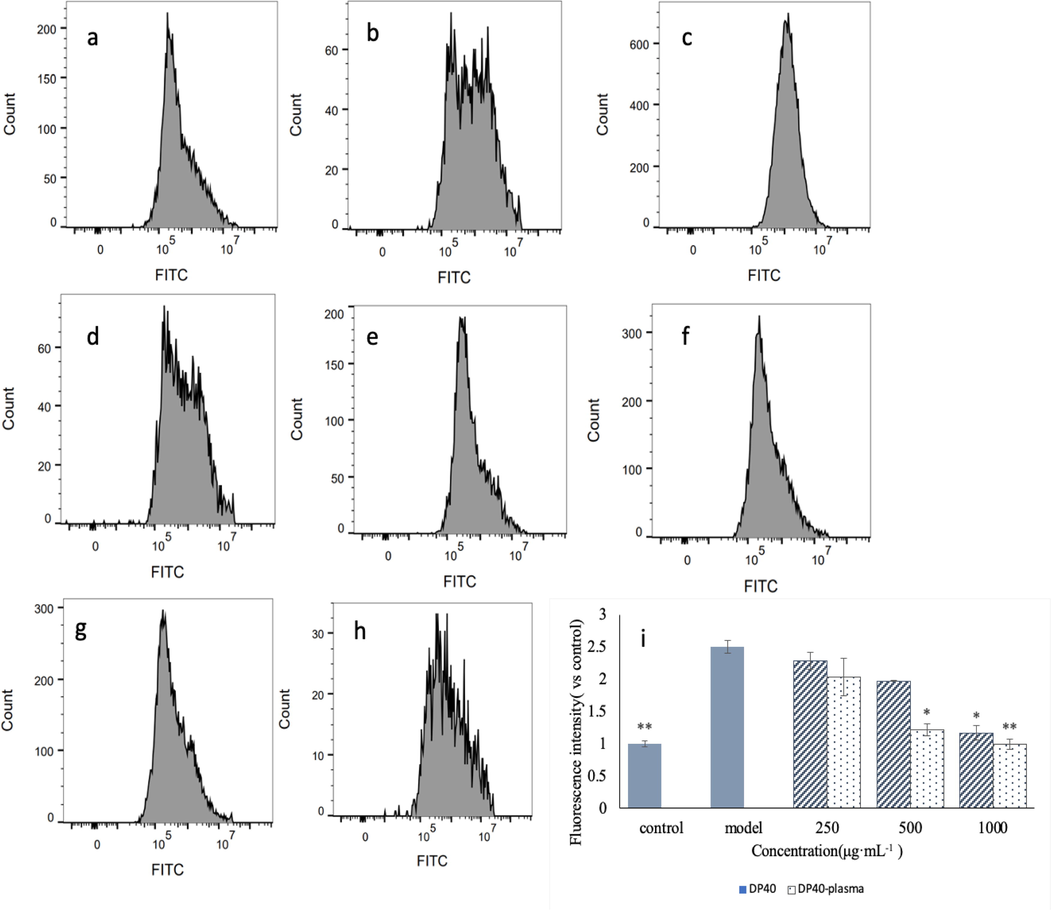
Effect of polysaccharide on ROS in injured GES-1 cells. a is DP40-L group (250 μg·mL−1), b is DP40-M group (500 μg·mL−1), c is DP40-H group (1000 μg·mL−1), d is DP40-plasma-L group, e is DP40-plasma-M group, f is DP40-plasma-H group, g is normal control group, h is model group, i is the ROS intensity of each treatment group, and j is the MDA content of cells in each group. k is the SOD enzyme activity of cells in each group.
Ethanol treatment could also increase MDA and reduce SOD activity (p < 0.01). Polysaccharide could alleviate this situation. Compared with the model group, low-temperature plasma can effectively reduce MDA in cells, especially at medium concentration and high concentration, which decreased by 23.37% and 27.45% compared with the untreated (p < 0.05). Meanwhile, low-temperature plasma can also increase the SOD activity. At medium and high concentrations, compared with the untreated, low-temperature plasma treatment can increase the SOD activity by 7.35% and 28.27% respectively (p < 0.05). It showed that low-temperature plasma treatment can effectively reduce ROS and MDA, improve cell SOD activity, and protect GES-1 cells from oxidative injured.
3.4.3 Effect of DP40 on the secretion of inflammatory factors by injured GES-1 cells
As shown in Table 1, ethanol can significantly enhance the level of inflammatory factors in GES-1 cells (p < 0.01), while polysaccharide can reduce the level of inflammatory factors in varying degrees. At high concentration (1000 μg·mL−1), DP40 can significantly reduce TNF-α, IL-8 and IL-1β. At the same time, low-temperature plasma could significantly improve the activity of polysaccharides and reduce the inflammatory factors level at high concentration (p < 0.01). Compared with the untreated group, IL-1β, IL-8 and TNF-α in DP40-plasma decreased by 31.82% (P < 0.05), 33.23% (p < 0.05) and 21.97% (p < 0.05), respectively. Therefore, low-temperature plasma can increase the abilities of DP40 to reduce the level of inflammatory factors in damage GES-1 cells. Note: Data were expressed as mean ± SD from three independent experiments, * p < 0.05, ** p < 0.01when compared with Model group.
Inflammatory factors(pg·mg−1 prot)
Groups(μg·mL−1)
control
model
250
500
1000
IL-8 DP40
43.8 ± 3.86**
139.6 ± 9.06
105.7 ± 8.55
80.3 ± 3.95
75.6 ± 5.11*
DP40-plasma
43.03 ± 1.67**
137.18 ± 6.95
100.46 ± 9.75
70.27 ± 5.96*
57.35 ± 3.06**
TNF-α DP40
10.7 ± 2.13**
49.3 ± 3.79
38.5 ± 2.51
30.9 ± 3.01*
16.8 ± 1.96**
DP40-plasma
11.15 ± 2.88**
48.74 ± 2.85
35.75 ± 3.14
21.05 ± 1.79*
12.61 ± 1.86**
IL-1β DP40
20.6 ± 1.18**
40.9 ± 2.87
38.8 ± 3.27
35.9 ± 1.07
26.7 ± 1.64*
DP40-plasma
20.62 ± 2.59**
41.03 ± 3.75
38.26 ± 3.53
36.08 ± 3.07
21.89 ± 1.96**
3.4.4 Effect of polysaccharide on apoptosis of injured GES-1 cells
Reducing apoptosis is an effective way for cells to resist oxidative damage. In order to verify whether low-temperature plasma treatment can reduce the degree of apoptosis of injured cells, the apoptosis and caspase 3 enzyme activity of each treatment group were analyzed by flow cytometry. As shown in Fig. 9, the apoptosis rate of ethanol treatment was significantly higher than ck (p < 0.01). Low concentration of polysaccharide had no significant effect on the apoptosis of injured GES-1 cells. With the increase of polysaccharide dose, the apoptosis level decreased gradually. At high dose (1000 μg·mL−1), the apoptosis level and caspase 3 enzyme activity of polysaccharide treatment group were the lowest (p < 0.05). Compared with DP40 group, the polysaccharide activity after low-temperature plasma treatment was higher, at 1000 μg·mL-1, the apoptosis rate and caspase 3 enzyme activity in DP40-plasma group were 52.64% (p < 0.01) and 16.03% (p < 0.05) lower than those in DP40 group, respectively. Therefore, low-temperature plasma can reduce the apoptosis rate of damaged GES-1 cells by reducing caspase 3 enzyme activity.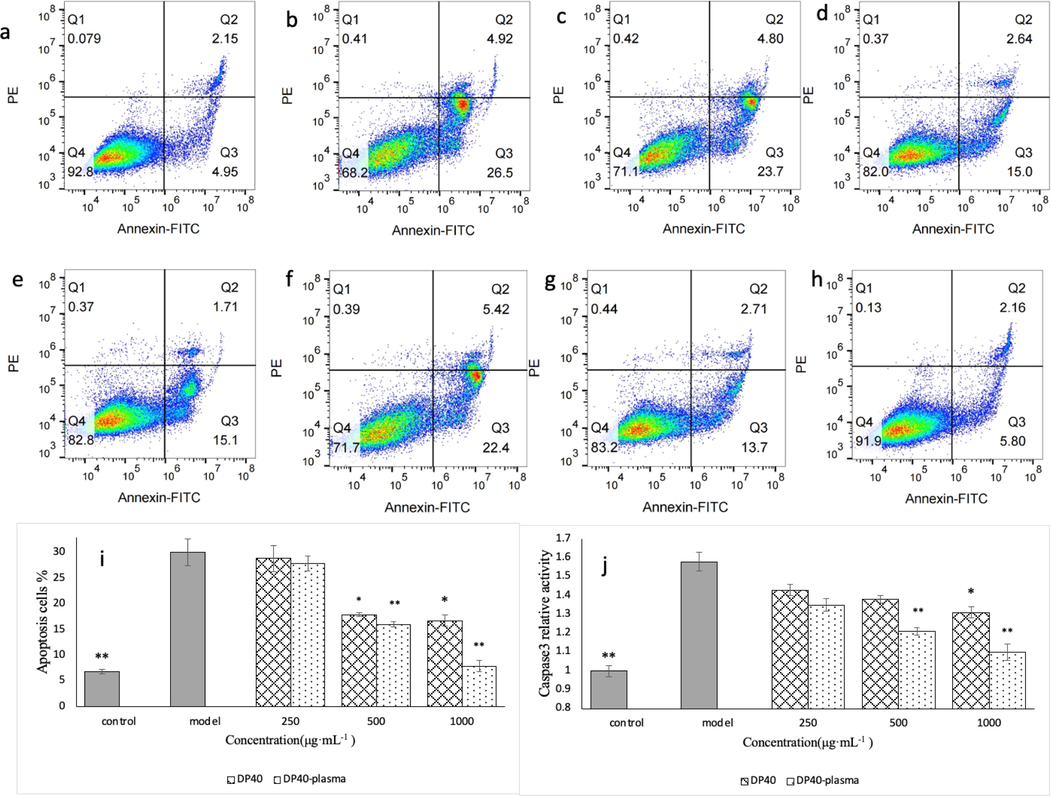
Effect of polysaccharide on apoptosis of injured GES-1 cells. a is the normal control group, b is the model group, and c is the DP40-L group (250 μg·mL−1), d is DP40-M group (500 μg·mL−1), e is DP40-H group (1000 μg·mL−1), f is DP40-plasma-L group, g is DP40-plasma-M group, h is DP40-plasma-H group, i is the apoptosis rate of each treatment group, and j is the caspase 3 enzyme activity in cells of each group.
4 Discussions
Polysaccharides are a kind of important biological macromolecules, which have pharmacological activities such as immune regulation, hypoglycemia, antioxidation, etc. The biological activity is directly related to their structure. Therefore, it is one of the important directions to modify the structure of polysaccharides by appropriate methods. The chemical modification of polysaccharides structure included acetylation and phosphorylation. However, the traditional modification often changes the primary molecular structure and pharmacological activity of polysaccharides (Borchers et al., 1999). Compared with chemical modification, low-temperature plasma modification has the advantage of not changing the main structure of biological macromolecules. At present, low-temperature plasma modification has been successfully applied to some biological macromolecules. For example, Delaux et al. (Delaux et al., 2016) treated cellulose with low-temperature plasma to obtain soluble dextran with branched chain, and found the way to produce dextran directly from cellulose. Tantiplapol et al. (Tantiplapol et al., 2015) found that low Mw chitosan can be obtained by plasma treatment of chitosan, so plasma technology may be a potential method for the preparation of low molecular weight chitosan and chitosan oligosaccharide.
However, we found that there were a lot of hydrogen bonds in the main chain of D. denneanum polysaccharide, which made it difficult to dissolve in conventional solvents. Moreover, due to the complex molecular structure and tight arrangement of polysaccharides, the reagents were not easy to penetrate the interior of the particles in the chemical reaction process, and the reaction often only occurs on the surface of polysaccharide particles, resulting in low chemical reaction activity and reaction efficiency. Therefore, in addition to the traditional use methods, the application of D. denneanum polysaccharide has not made a breakthrough. Therefore, we have been looking for an effective method to increase the water solubility and promote the biological activity of polysaccharides. In this study, low-temperature plasma was used to modify D. denneanum polysaccharide. It was found that under this condition (ionized gas is argon, current is 8 mA, treatment time is 60 s), the hydrophilicity of the polysaccharide was significantly improved, and the WCA was decreased from 70.67 ± 1.19° to 26.51 ± 1.33° (n = 5). Compared with the ck, the WCA of the low temperature plasma treatment was decreased (p < 0.01). Infrared spectrum (IR) exhibited that —OH on the polysaccharide increased significantly.
Compared with synthetic polymers, polysaccharides have more complex structures, so their structures are more complex and changeable, mainly related to chain structure, intramolecular and intermolecular forces (including hydrogen bond, dipole interaction, hydrophobic force, electrostatic force and other non-covalent forces) and solvents (Rinaudo et al., 2006). The possible conformational characteristics of polysaccharide molecular chain in solution mainly include random coil chain, single helix, double helix, triple helix, worm like, rod-shaped and aggregate (Ranieri et al., 2003). These structures have important effects on the bioactivity of polysaccharides. In this study, low-temperature plasma treatment of D. denneanum polysaccharide, SEM observation found that the polysaccharides morphology after plasma-treatment changed from layered porous network structure to smooth morphology, protruding to form semicircular particles. TEM detection exhibited that the ultrastructure of polysaccharides had changed, the degree of surface molecular crosslinking increased, and the arrangement of polysaccharides was more orderly, which indicated that the high-level structure of polysaccharide from D. denneanum had changed significantly.
Ethanol will promote the excessive secretion of ROS, which will aggravate the degree of gastric injury (Beiranvand et al., 2020). Because ROS attack gastric mucosa and reduce the antioxidant defense capacity, GPx and its substrate GSH were over formed (Zhou et al., 2020). In addition, ROS triggered mucosal cell apoptosis also played an important role in protecting gastric mucosal injury. At this time, Bax protein expression was up-regulated, Bcl2 was down-regulated, and caspase-3 activity was correspondingly increased (Arab et al., 2019). This study also found that ethanol treatment could increase the concentration of ROS in gastric mucosal cell, increase the activity of caspase 3 and the apoptosis rate. When treated by polysaccharides, the concentration of ROS, MDA, caspase 3 activity gradually decreased, resulting in the decrease of apoptosis level, and the effect of DP40-plasma was significantly higher than DP40 without plasma treatment. Similar results have also appeared in other studies, such as Ma et al. (2018) found that after plasma treatment, the thermal stability of chitosan decreased and its crystalline state was disrupted, but the functional groups were not affected, and the antioxidant and antibacterial properties of the product were improved. Tantiplapol et al. (2015) found through research that plasma treatment of chitosan resulted in a higher degradation rate of chitosan using Fe electrodes compared to W and Cu electrodes. The main structure of the degradation products remained unchanged and the crystallinity decreased.
Cytokine is synthesized and secreted by immune cells and non-immune cells. It is an efficient functional protein existing between cells, which can regulate immune function, mediate inflammatory response, participate in tissue repair and improve body resistance. It can feel the stimulation of external factors on the body and transmit information to the body. According to literature reported that human gastric mucosal epithelial GES-1 cells will also release a variety of cytokines that can induce inflammation, such as TNF-α, IL-6 et al. (Mei et al., 2012; Zhao et al., 2015), The experiment also confirmed that DP40 could reduce inflammatory factors in varying degrees. Fan et al. (2020) also found in vitro cell experiments that polysaccharides treated with low-temperature plasma significantly increased the phagocytic ability of RAW264.7 and promoted its secretion of cytokine(TNF-α,IL-6, IL-1) expression. Low-temperature plasma can enhance the biological activity of DP40, which may be related to its modification effect. The main chain and substituent groups of DP40 were depolymerized, and the degree of molecular cross-linking was reduced, the network structure of polysaccharides to become smooth morphology. At the same time, low-temperature plasma modification brought more - OH groups to DP40, which greatly increased its water solubility and the binding capacity of polysaccharide on target cells, thus increasing its biological activity. Therefore, low-temperature plasma treatment can increase the ability of DP40 to reduce the level of inflammatory factors that injured GES-1 cells, so as to protect gastric mucosal cells from ethanol injured (Fig. 10).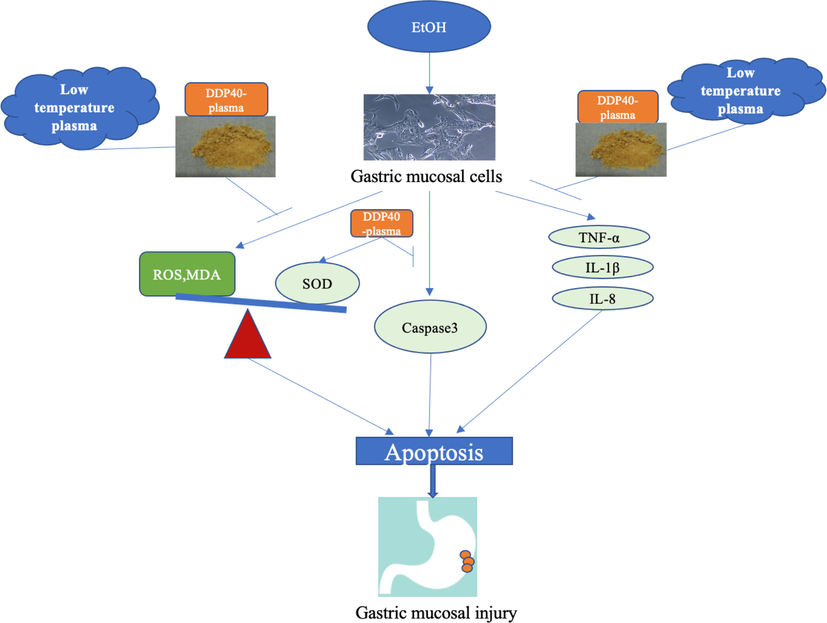
Low-temperature plasma improves the protective process of DP40 against ethanol induced gastric mucosal injury (→: activation, ┤: inhibition),
5 Conclusion
In this study, the optimal ultrasonic extraction conditions of D. denneanum polysaccharides were obtained through response surface design optimization: extraction temperature was 83.74 ℃, the ultrasonic power was 283 W, and the extraction time was 32.33 min. The extraction rate of polysaccharide was 22.98 ± 0.97%, which was significantly higher than water extraction method(P < 0.05). A major polysaccharide (DP40) of D. denneanum, was obtained by anion exchange chromatography, and was treated by Low-temperature plasma. The results exhibited that low-temperature plasma treatment could increase the content of —OH and water solubility of DP40, and make the polysaccharide better combine with receptors in vivo and exert their biological activities. At the same time, low-temperature plasma treatment did not significantly change the molecular weight and monosaccharide structure of D. denneanum polysaccharide, indicating that the treatment can protect the main chain structure of D. denneanum polysaccharide. However, the high-level structure of polysaccharides and the morphology of molecules changed. In the biological activity experiment, we found that the polysaccharide treated by low temperature plasma can significantly improve the activity of GES-1 cells, reduce the concentration of ROS and MDA damaged by ethanol, and increase the activity of SOD. At the same time, the polysaccharide after low-temperature plasma treatment can effectively reduce the caspase 3 enzyme activity and cytokine (IL-8, TNF-α and IL-1β) secretion of damaged cells to prevent apoptosis, so as to protect gastric mucosal cells from ethanol damage. However, Further research is needed on the mechanism of low-temperature plasma modification of polysaccharides and the structure–activity relationship of the increased activity of polysaccharides after modification.
CRediT authorship contribution statement
Yijun Fan: Investigation, Conceptualization, Writing – original draft. Jie Ma: Methodology, Software, Data curation. Gang Wang: Visualization, Resources. Xuebing Li: Investigation, Methodology. Yuanyuan Liu: Software, Data curation. Erya Xu: Software, Data curation. Aoxue Luo: Writing – review & editing, Conceptualization, Supervision.
Acknowledgement
This research was funded by Sichuan Science and Technology Program (2022YFH0036, 2023YFH0017, 2021YFYZ0012).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Hydrophobic polysaccharides: partially 2-deoxygenated amyloses. Asian J. Org. Chem.. 2022;11(2):286-290.
- [Google Scholar]
- Targeting mapks, NF-κb, and PI3k/Akt pathways by methyl palmitate ameliorates ethanol-induced gastric mucosal injury in rats. J. Cell. Physiol.. 2019;234(2):22424-22438.
- [Google Scholar]
- Ameliorating and protective effects mesalazine on ethanol-induced gastric ulcers in experimental rats. Eur. J. Pharmacol.. 2020;888:173573
- [Google Scholar]
- Fundamentals of Plasma Chemistry and Technology. Laneaster: Technmic Publishing; 1988. p. :417.
- A polysaccharide from mycelia of Metarhizium taii: Structural characterization, inhibition on α-glucosidase and improvement of insulin resistance in HepG2 cells. Process Biochem.. 2023.A;125:212-221.
- [Google Scholar]
- Improving packing performance of lily polysaccharide based edible films via combining with sodium alginate and cold plasma treatment. Int. J. Biol. Macromol.. 2022;206:750-758.
- [Google Scholar]
- Impact of nonthermal atmospheric plasma on the structure of cellulose: access to soluble branched glucans. Chemistry. 2016;22(46):16522-16530.
- [Google Scholar]
- Modulating effects of a functional food containing Dendrobium officinale on immune response and gut microbiota in mice treated with cyclophosphamide. J. Funct. Foods. 2022;94:105102
- [Google Scholar]
- Composition analysis and antioxidant activity of polysaccharide from Dendrobium denneanum. Int. J. Biol. Macromol.. 2009;45:169-173.
- [Google Scholar]
- Effects of Low temperature plasma treatment on the polysaccharide from Dendrobium nobile Lindl. And its immune activities in vitro. Int. J. Biol. Macromol.. 2020;153:942-950.
- [Google Scholar]
- Extraction, characterization and antioxidant activities of an acidic polysaccharide from Dendrobium devonianum. J. Food Meas. Charact.. 2022;16:867-879.
- [Google Scholar]
- Screening and characterization of an acid polysaccharide with antioxidant activity in vitro and in vivo from Dendrobium aurantiacum var. denneanum (Kerr) Phcog. Mag.. 2022;18:44-51.
- [Google Scholar]
- Transcriptome analysis reveals the effects of red and blue light on the physiological and medicinal components of Dendrobium denneanum. Ind. Crop. Prod.. 2022;180:114798
- [Google Scholar]
- Sulfation of the extracellular polysaccharide from the edible fungus Stropharia rugosoannulata with its antioxidant activity. J. Future Foods. 2023;3(1):37-42.
- [CrossRef] [Google Scholar]
- Nanostructured surface of electrospun PCL/dECM fibres treated with oxygen plasma for tissue engineering. Rsc. Advances. 2016;6(39):32887-32896.
- [Google Scholar]
- Dendrobium nobile Lindl. Polysaccharides protect fibroblasts against UVA-induced photoaging via JNK/c-Jun/MMPs pathway. J. Ethnopharmacol.. 2022;298(115590)
- [Google Scholar]
- Isolation, purification and structural characterization of a water-soluble polysaccharide HM41 from Halenia elliptica D. Don. Chinese Chem. Lett.. 2016;27:979-983.
- [Google Scholar]
- vitro antioxidant of a water-soluble polysaccharide from Dendrobium fimhriatum Hook.var.oculatum Hook. Int. J. Mol. Sci.. 2011;12:4068-4079.
- [Google Scholar]
- Orthogonal design was used to optimize the extraction process of polysaccharides from Dendrobium denneanum. Study J. Traditional Chinese Med.. 2005;11:1991-1992.
- [CrossRef] [Google Scholar]
- vitro antioxidant activities of a water-soluble polysaccharide derived from Dendrobium nobile Lindl. Extracts. Int. J. Biol. Macromol.. 2009;45:359-363.
- [Google Scholar]
- Effect of solution plasma process with hydrogen peroxide on the degradation of water-soluble polysaccharide from Auricularia auricula. II: Solution conformation and antioxidant activities in vitro. Carbohyd. Polym.. 2018;198:575-580.
- [Google Scholar]
- Mechanisms of Dendrobium officinale polysaccharides in repairing gastric mucosal injuries based on mitogen-activated protein kinases (MAPK) signaling pathway. Bioengineered. 2022;13(1):71-82.
- [Google Scholar]
- Novel role of Zn(II)-curcumin in enhancing cell proliferation and adjusting proinflammatory cytokine- mediated oxidative damage of ethanol-induced acute gastric ulcers. Chem. Biol. Interact.. 2012;197(1):31-39.
- [Google Scholar]
- Nanotechnology Advances in the Detection and Treatment of Cancer: An Overview. Nanotheranostics. 2022;6(4):400-423.
- [Google Scholar]
- Investigation of physical and chemical properties of polypropylene hybrid nanocomposites. Materials & Design. 2012;34:474-478.
- [Google Scholar]
- Green synthesis of silver nanoparticles using polysaccharide-based guar gum. Mater. Today: Proc. 2023
- [CrossRef] [Google Scholar]
- Characterization of antidiabetic effects of Dendrobium officinale derivatives in a mouse model of type 2 diabetes mellitus. Food Chem.. 2023;399:133974
- [Google Scholar]
- Degradation of β-chitosan by solution plasma process. Polym. Degrad. Stabil.. 2013;98:2089-2093.
- [Google Scholar]
- Chain Conformations of Polysaccharides in Different Solvents. ChemInform. 2003;34(23):1-8.
- [Google Scholar]
- Non-covalent interactions in polysaccharide system. Macromol. Biosci.. 2006;6:590-610.
- [Google Scholar]
- H2O2-induced surface modification: a facile, effective and environmentally friendly pretreatment of chitosan for dyes removal. Chem. Eng. J.. 2011;166(2):474-482.
- [Google Scholar]
- Polysaccharide gum based network hydrogels for controlled drug delivery of ceftriaxone: synthesis, characterization and biomedical evaluations. Results Chem.. 2023;5:100695
- [Google Scholar]
- Influences of solution plasma conditions on degradation rate and properties of chitosan. Innovative Food Sci. Emerg. Tech.. 2015;32:116-120.
- [Google Scholar]
- Effect of the polysaccharides derived from Dendrobium officinale stems on human HT-29 colorectal cancer cells and a zebrafish model. Food Biosci.. 2021;41:100995
- [Google Scholar]
- Low-temperature plasma technology for electrocatalysis. Chinese Chem. Lett.. 2019;30(4):826-838.
- [Google Scholar]
- Structural Analysis and Immuno-Stimulating Activity of an Acidic Polysaccharide from the Stems of Dendrobium nobile Lindl. Molecules.. 2017;22(4):611.
- [Google Scholar]
- Polysaccharides from Dendrobium officinale ameliorate colitis-induced lung injury via inhibiting inflammation and oxidative stress. Chem-Biol. Interact.. 2021;347:109615
- [Google Scholar]
- Dendrobium officinale polysaccharides protected against ethanol-induced acute liver injury in vivo and in vitro via the TLR4/NF-κB signaling pathway. Cytokine. 2020;130:155058
- [Google Scholar]
- Polysaccharides of Dendrobium officinale Kimura & Migo protect gastric mucosal cell against oxidative damage-induced apoptosis in vitro and in vivo. J. Ethnopharmacol.. 2017;208:214-224.
- [Google Scholar]
- Effect and mechanism of evodia- mine against ethanol-induced gastric ulcer in mice by suppressing Rho/NF-κB pathway. Int. Immunopharmacol.. 2015;28(1):588-595.
- [Google Scholar]
- Gastroprotective effect of gallic acid against ethanol-induced gastric ulcer in rats: involvement of the Nrf2/HO-1 signaling and anti-apoptosis role. Biomed. Pharmacother.. 2020;126:110075
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2023.105033.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







