Translate this page into:
Unveiling the anticancer, antimicrobial, antioxidative properties, and UPLC-ESI-QTOF-MS/ GC–MS metabolite profile of the lipophilic extract of siam weed (Chromolaena odorata)
⁎Corresponding author. fredrickeze@rocketmail.com (Fredrick Nwude Eze)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
C. odorata aerial part lipophilic extract (COLE) was prepared via a facile green chemistry approach. COLE demonstrated potent and selective anticancer activity against human breast and colon cancer cells. Compared to the hydrophilic extract, COLE displayed superior antibacterial properties. COLE also exhibited potent antioxidant and metal chelation activities. UPLC-ESI-QTOF-MS and GC–MS analysis revealed that COLE was predominantly endowed with phenolic and terpene metabolites.
Abstract
There has been growing interest in the biological and pharmacological potential of C. odorata in recent times. While a number of reports had highlighted the beneficial attributes of various extracts prepared from C. odorata, the biological properties of the lipophilic extract remained unexplored. This work aimed at examining the anticancer, antimicrobial and antioxidant properties of C. odorata aerial part lipophilic extract (COLE). The results revealed that COLE possessed potent and selective anticancer properties against human breast and colon cancer cells. The extract presented strong cytotoxic activity against MCF-7 cells (IC50 of 9.18 µg/mL), MDA-MB-231 (IC50 of 12.19 µg/mL), HT-29 (IC50 of 19.48 µg/mL), and HCT-116 cells (IC50 of 14.12 µg/mL). Meanwhile, COLE was found to be non-toxic at the concentrations tested against normal (HEK-293 and CCD-841 CoN) cells. In addition, COLE demonstrated significant antibacterial activity against B. cereus (MIC value of 0.157 mg/mL), and moderate activity against L. monocytogenes (MIC value of 0.63 mg/mL) and E. coli (MIC value of 1.06 mg/mL). Furthermore, COLE effectively scavenged free radical species, including ABTṠ+ (IC50 of 46.80 µg/mL), DPPḢ (IC50 of 27.78 µg/mL), and superoxide anion (IC50 of 64.07 µg/mL), likely due to the high phenolic (262.48 mg gallic acid equivalent/g) and flavonoid content (70.20 mg quercetin equivalent/g). UPLC-MS analysis revealed the putative identities of 85 secondary metabolites whereas the GC–MS analysis detected 36 volatile constituents in COLE. Also, it was revealed that terpenes and phenolic compounds were the predominant specialized metabolites present in COLE, and putatively responsible for the remarkable biological properties. Taken together, these results revealed the richness of C. odorata lipophilic extract in terms of its metabolite profile and biological activities. In conclusion, COLE is worthy of further investigation to take advantage of its good anticancer, antioxidative and antimicrobial properties in pharmacological or nutraceutical application.
Keywords
Chromolaena odorata lipophilic extract
Phytochemical profiling
UPLC-QTOF-MS
Antioxidant activity
Anticancer activity
Nutraceuticals
1 Introduction
Cancer is a leading cause of global morbidity and mortality, accounting for a massive 10 million deaths worldwide in 2020 (Sung et al., 2021). For most countries, cancer remains the first or second cause of death and represents a major hindrance in increasing life expectancy in every country (Bray et al., 2021). According to recent estimates, about 19.3 million new cases were recorded with female breast cancer and colon cancer representing the first and the third most commonly diagnosed cases (Sung et al., 2021). A number of therapeutic strategies are frequently deployed to combat these cancers, including hormone therapy, radiation therapy, chemotherapy or various combinations of the afore-mentioned. Despite recorded advances in screening and therapeutic development, achieving successful anticancer treatment outcomes remain a challenge. Increasing incidence of high tumor relapse and recurrences, anticancer drug resistance, as well as drug-induced adverse side effects have been highlighted as contributing factors limiting therapeutic success (Skarkova et al., 2019). Thus, it is necessary to continue the search for more effective, safe, and affordable therapeutic alternatives.
Over the years, natural products of plant origin continue to attract strong interest for the identification and development of anti-cancer agents. The presence of specialized metabolites such as phenolics, alkaloids, xanthones, lignans, terpenoids, fatty acids, coumarins, anthraquinones, etc., with almost limitless structural diversity and biological activity afford huge possibilities for plant-based anticancer drug discovery (Hashem et al., 2022). Besides, phytocomplexes such as plant extracts and essential oils could provide improved clinical efficacy via synergism of the individual specialized metabolites and simultaneous effects on multiple pharmacological targets (Gordaliza, 2007; Schmidt et al., 2007). For instance, fermented dried pericarp water extracts of Camellia japonica L. reported exhibited anticancer activity against FaDu cells via regulation of IGFBP-2/mTOR pathway (Cho et al., 2021) whereas the C. japonica oil and distillate fractions abrogated the spontaneous metastasis of mouse melanoma BL6 cells (Miura et al., 2007). Similarly, a number of reports in literature have noted the cytotoxic properties of C. odorata and its chemical constituents against HeLa cervical cancer cells (Nath et al., 2015; Yusuf et al., 2022a), LLC and L-60 cancer cells (Hung et al., 2011), Cal51, and MCF7 breast cancer cells (Kouamé et al., 2013), underscoring its anticancer potentials.
Chromolaena odorata (L) R.M. King & H. Rob. is a perennial shrub of neotropical origin (Muniappan et al., 2005). C. odorata belongs to Asteraceae family. The plant produces a scrambling thicket and grows to about three meters. It colonizes vacant lands, pastures, disturbed forests, and riverbanks. In many regions of the world including several countries in Africa and Asia, the plant is widely abundant. Furthermore, the ethnomedicinal application of C. odorata has been steadily increasing over the previous six decades. Traditionally, C. odorata has been applied for wound healing, for treatment of diabetes, stomach ache, urinary tract infections and cancer (Tun et al., 2022). Notable chemical constituents present in C. odorata include flavanones, flavonoids, phenolic acids, terpenoids, chalcones, alkaloids, and fatty acids. Complex mixtures of lipophilic aglycones, viz; chalcones, flavonols, flavanones, and flavones, as well as phenolic acids with powerful antioxidant properties were implicated in the effective wound healing action associated with C. odorata (Phan et al., 2001). Eupolin, a herbal remedy based on aqueous extract of C. odorata enhanced the proliferation of fibroblast and endothelial cells (Phan et al., 1998). Meanwhile, methyl cellulose encapsulated C. odorata methanol extract was found to inhibit the growth of E. coli and S. aureus (Azmi et al., 2019). The essential oil from C. odorata aerial part was found to modulate inflammation via its effect on cyclooxygenase function of prostaglandin-H synthase activity (Bedi et al., 2015). Nonetheless, there had not been any published account on the biological properties C. odorata aerial part lipophilic extract prepared without noxious organic solvents.
This work aimed to examine the biological attributes of C. odorata lipophilic extract focusing on the anticancer, antimicrobial and antioxidant activities. Furthermore, UPLC-ESI-QTOF-MS and GC–MS analysis were employed to profile the various individual secondary metabolites found in the extract. The results generated from this work could provide for the first time an overview of the secondary metabolites present in the lipophilic extract of C. odorata aerial parts as well as delineate its anticancer and antioxidative potential.
2 Materials and methods
2.1 Preparation of C. odorata lipophilic extract
C. odorata lipophilic extract was prepared according to the procedure previously described by (Yanping Huang et al., 2022) with minor modifications. Fresh aerial part of C. odorata was collected from the botanical garden of the Faculty of Pharmaceutical Sciences, Prince of Songkla University, Hat Yai, Songkhla, Thailand, with coordinates of 7°00′39″ N and 100°29′49″ E. The sample was authenticated by Asst. Prof. Dr. Opeyemi Joshua Olatunji, Faculty of Traditional Thai Medicine, Prince of Songkla University, Hat Yai 90110, Thailand and specimen was deposited at the herbarium with voucher F.N.2 (PSU). The plant sample was air-dried under a shade for five days and then ground into fine powder using WF-10B Thai grinder (Bangkok, Thailand) electric grinder. The powder (50 g) was subsequently extracted with 80 % (v/v) ethanol (1 L) using an overhead stirrer at ambient conditions for 2 h. The mixture was filtered to separate the extract from the residue. The residue was re-extracted following the same procedure. The extracts from the two rounds of extraction were combined and concentrated under vacuum.
using a rotary evaporator at 35 °C to one-fifth of the initial volume. The extract was then kept at 4 °C for 12 h to allow it to fractionate. Thereafter, the clear hydrophilic upper layer was carefully removed, and the congealed lipophilic bottom layer was collected and further dried in a rotational vacuum concentrator (RVC 2–18 CDplus, Martin Christ Gefriertrocknungsanlagen GmbH, Osterode, Germany) to give a dark green paste of C. odorata lipophilic extract (COLE). The percentage yield of COLE obtained in terms of C. odorata aerial part dry weight was 5.28 %. COLE was stored in airtight amber vials at 4 °C awaiting further investigations.
2.2 Chemical characterization of COLE
2.2.1 Uplc-esi-qtof-ms analysis of cole
Qualitative metabolite profile of the individual compounds present in COLE was obtained by UPLC-ESI-QTOF-MS analysis. COLE (40 mg) was dissolved in 1 mL of 70 % (v/v) methanol and vortexed for 10 min. The COLE sample was then centrifuged at 8000 rpm for 10 min and the supernatant was collected. The COLE solution was further filtered through a 0.2 μm nylon membrane syringe filter to obtain a clear solution. This solution was then subjected to UPLC-ESI-QTOF-MS analysis as detailed previously (Eze and Tola, 2020; Yanping Huang et al., 2022). The analysis was performed on Liquid chromatograph-quadrupole time-of-flight mass spectrometer (LC-QTOF MS), 1290 Infinity II LC-6545 Quadrupole-TOF (Agilent Technologies, USA) equipped with MassHunter Workstation Software, Qualitative Analysis Workflow, Version B.08.00 (Agilent Technologies, Inc. USA). The mass spectrometer consisted of a model G6545A MS QTOF, fitted with a Dual AJS ESI ion source and operated in a negative ionization mode at 325 °C drying gas temperature, 13 L/min drying gas flow rate, 35 psig nebulizer pressure, 4000 V capillary voltage, 175 V fragmentor voltage, 750 V OctopoleRFPeak, and 10 eV, 20 eV, and 40 eV fixed collision energies. The LC component was equipped with a model G7129B autosampler, a model G7120A binary pump, a model G7116B column compartment and a model G7117A diode array detector. Sample separation was accomplished with a reversed phase Zorbax Eclipse Plus C18 2.1 × 150 mm, 1.8 μm column (Agilent Technologies, USA) as the stationary phase while the mobile phase consisted of ultrapure water + 0.1 % formic acid (solvent A) and acetonitrile (solvent B). The sample (2.0 μL) was injected and eluted at a flow rate of 0.2 mL/min using a gradient elution of 0.50 min, 100% A; 16.50 min, 0 % A; 17.50 min, 0 % A; 20.00 min, 100 % A; 22.00 min, 100 % A.
2.2.2 Gc–ms analysis of cole
GC-electron ionization mass spectrometry (GC-EI-MS) was used to profile the volatile and semi-volatile constituents in COLE. The analysis was performed on GC/MS GC 7890, MSD 5977B (Agilent Technologies, USA) with helium as the carrier gas and Agilent HP-5MS column (30 m × 250 μm × 0.25 μm). The flow rate, pressure, average velocity, holdup time and post run were 1 mL/min, 7.0699 psi, 36.262 cm/s, 1.3789 and 1 mL/min, respectively. One microliter of the sample was injected in split mode. The total run time was 72 min consisting of an initial oven temperature of 40 °C, held for 1 min, increased to 250 °C at 5 °C/min (held for 5 min), again increased further to 320 °C at 5 °C/min (held for 10 min). The tentative identities of the compounds present in COLE was established with the aid of Wiley 10 and NIST14 (National Institute of Standards and Technology 14 (NIST14) chemical libraries. Match score ≥ 90 % was considered acceptable (Yanping Huang et al., 2022).
2.2.3 Total phenolic and flavonoid content of COLE
The total phenolic content of COLE was determined using Folin-Ciocalteu assay as previously described. COLE solution (100 µL) or gallic acid (standard) was introduced into 2-mL Eppendorf tubes. Into the sample solution was added 200 µL of 10 % (v/v) Folin-Ciocalteu reagent. The tube was vortexed and incubated for 5 min. Thereafter, 800 µL of Na2CO3 solution was added to the sample, vortexed and incubated for 2 h in the dark. The absorbance of the blue reaction mixture was read at 765 nm. The total phenolic content of COLE was determined from calibration curve prepared using gallic acid and expressed as mg gallic acid equivalent per gram of COLE.
The total flavonoid content was determined using aluminum chloride colorimetric assay with quercetin serving as standard. Succinctly, the reaction mixture consisting of COLE solution or quercetin solution (30 µL), methanol (160 µL), freshly 10 % (w/v) aluminum chloride in methanol (30 µL), 1 M sodium acetate solution (30 µL) and distilled water (850 µL) was vortexed and incubated at room temperature in the dark for 30 min. The absorbance of the solution was recorded at 415 nm. The total flavonoid content was calculated from the calibration curve prepared using quercetin and presented as mg quercetin equivalent per gram of COLE.
2.2.4 Total saponin content of COLE
The total saponin content of COLE was estimated by vanillin-sulfuric acid colorimetric assay with asiatic acid serving as standard (Le et al., 2018). Briefly, solution of COLE or asiatic acid (200 µL) was introduced into a screw cap glass test tube. Vanillin solution (8 % w/v in methanol; 200 µL) was added to the sample and vortexed. Then sulfuric acid (72 %; 2 mL) was added into the solution. The solution was vortexed and incubated at 60 °C for 10 min. After cooling at room temperature, the absorbance was read at 544 nm. The total saponin content was calculated from the calibration curve prepared using asiatic acid and expressed as mg asiatic acid equivalent per gram of COLE.
2.3 Color analysis of COLE
Hydrophilic and lipophilic fractions of C. odorata ethanol extract (5 mg/mL) were prepared in 50% aqueous ethanol and absolute ethanol, respectively. Color analysis of the extracts were performed using total transmittance mode (TTRAN) on HunterLab UltraScan pro (HunterLab, USA). The color parameters L (Lightness), a (redness to greenness), b (yellowness to blueness) were obtained from the sample, taking into account their corresponding blanks, while the hue angle, chroma and total color change ΔE were calculated as previous reported (Acero et al., 2019).
2.4 Determination of anticancer activity of COLE
2.4.1 Cell lines and culture conditions
Human breast cancer (MCF-7: ATCC HTB-22 and MDA-MB-231: ATCC HTB-26), colonal cancer (HT-29: ATCC HTB-38 and HCT-116: ATCC CCL-247) and non-cancer (human embryonic kidney, HEK-293: ATCC CRL-1573, and human normal colon epithelial, CCD-841 CoN: ATCC CRL-1790) cell lines were procured from American Type Culture Collection (ATCC, Manassas, VA, USA). All cells were cultured in a humidified incubator with 5 % CO2 at 37 °C and nourished with DMEM (Dulbecco's Modified Eagle Medium) medium (Sigma-Aldrich, USA) supplemented with 10% fetal bovine serum (Gibco, Thermo Fisher, USA) and 100 units/mL of penicillin and 100 µg/mL of streptomycin (Gibco, Thermo Fisher, USA).
2.4.2 In vitro cytotoxicity
The cytotoxic activity of COLE was determined via MTT assay according to previous report (Hrubik et al., 2012). Initially, the cells were seeded at a density of 2 × 104 cells/well into 96-well microplates and allowed to attach. Thereafter, the cells were exposed to different concentrations of COLE (1 – 800 µg/mL) for 72 h. Negative control consisted of wells treated only with the diluent. After cell viability (%) was calculated, the IC50 values of COLE on the various cell lines were determined using GraphPad prism 7.0.
2.5 Determination of antimicrobial activity of COLE
The antimicrobial property of the lipophilic (COLE) and hydrophilic (COHE) fractions of C. odorata was determined against the following bacteria viz: Escherichia coli O157:H7 (ATCC 43890), Listeria monocytogenes F2365 (Nelson et al., 2004), and one clinical isolate of Bacillus cereus from the culture collection of the Division of Biological, Prince of Songkla University, Hat Yai. Vancomycin hydrochloride and gentamicin sulfate (Sigma-Aldrich Pte Ltd., Singapore) were used as positive control. The antimicrobial activity of the samples was evaluated using broth microdilution assay as previously described (CLSI, 2020).
Pure bacterial inoculums grown in tryptic soy broth (TSB) (Difco, Le Port de claix, France) at early log phase were adjusted to 106 CFU mL−1 using MHB (Müller Hinton broth) to obtain bacterial suspensions. The respective bacterial suspensions (100 µL) were introduced into wells of 96-well plates containing serial dilutions of COLE or COHE. The plates were subsequently incubated at 37 °C for 24 h. The lowest concentrations of C. odorata sample that completely inhibited bacterial growth was regarded as the minimum inhibitory concentration (MIC) value. In addition, the MBC (minimum bactericidal concentration) values of the samples were also estimated. The MBC values were ascertained using the spot plot technique by seeding 10 μL aliquots from the wells that did not display any microbial growth onto TSA plates followed by incubation for 18 h at 37 °C. The MBC value of each sample is taken from the lowest concentration that showed no microbial growth on the TSA plates. All experiments were performed in triplicates for two independent studies.
2.6 Determination of antioxidant activity of COLE
2.6.1 DPPH radical scavenging activity
The radical scavenging activity of COLE against DPPḢ was determined spectrophotometrically as previously described by (Lesjak et al., 2018) with minor modifications. Succinctly, the samples diluted with methanol (100 µL) were added into the wells of 96-well plates. Wells containing only methanol (100 µL) were used as the control. DPPH solution in methanol (0.1 mM,100 µL) was added to samples. In the blank wells, methanol (100 µL) was added to the samples instead of DPPH solution. Ascorbic acid was used as a reference antioxidant compound. The reaction solutions were allowed to incubate in the dark for 30 min. Thereafter, absorbance was recorded at wavelength of 515 nm. After correcting for the blank, the percentage radical scavenging activity (RSA %) was calculated thusly:
Where AC was absorbance of the control solution and AS was absorbance of the sample solution. The antioxidant activity was estimated from the curve of RSA vs. concentration, plotted using GraphPad Prism version 7.0, and expressed as IC50 i.e., the concentration that scavenged the DPPH radical by 50%.
2.6.2 Abts radical scavenging activity
Firstly, the ABTṠ+ stock solution was made by mixing 2 mL of ABTS solution (7.4 mM) and 2 mL of potassium persulfate solution (2.6 mM) followed by incubation for 12 h. The working solution was prepared by diluting the stock solution with ethanol to achieve an absorbance value of 0.70 ± 0.01 at 734 nm. Then COLE solution (15 µL) was added into 96-well microplate followed by 180 µL of the ABTṠ+ working solution. Ascorbic acid was used as a reference antioxidant compound. After 10 min of incubation, the absorbance was measured at 734 nm. The ABTṠ+ radical scavenging activity of COLE was calculated thusly:
Where AC was absorbance of the control solution and AS was absorbance of the sample solution. The IC50 value of the sample against ABTS was estimated as described in the DPPH assay supra.
2.6.3 Determination of superoxide anion radical scavenging
Briefly, 2.52 mM nitrobluetetrazolium chloride (50 µL), 624 mM NADH (50 µL) were mixed to generate the superoxide anion. COLE solution (50 µL) with final concentration ranging from 10 to 100 µg/mL, was added to the radical solution. Ascorbic acid was used as a reference antioxidant compound. To initiate the reaction, 120 µg/mL of phenazine methosulfate solution (50 µL) was added to the reaction mixture. After 5 min of incubation at room temperature, absorbance of the solutions were recorded at 560 nm (Sannasimuthu et al., 2018). The radical scavenging activity (RSA) was calculated as follows:
The IC50 value of the sample against superoxide anion was estimated as described in the DPPH assay supra.
2.6.4 Metal chelation activity
The ability of COLE to chelate ferrous ions was estimated using a colorimetric assay with EDTA-Na2 (ethylenediaminetetraacetic acid disodium salt) serving as positive control (Santos et al., 2017). The reaction mixture consisted of COLE or EDTA-Na2 solution (50 µL), ultrapure water (160 µL), and 0.30 mM FeSO4 solution (20 µL). After 5 min, 0.8 mM Ferrozine solution (30 µL) was added. The reaction mixture was incubated for 30 min after which absorbance was read at 562 nm. The metal chelating effect as a percentage was obtained thus:
Where, AS and AC are the absorbance of the sample and control, respectively.
2.7 Statistical analysis
All sample data were analyzed using ANOVA followed by post hoc analysis using Tukey test. The statistical analysis was performed on GraphPad Prism version 7.0 (GraphPad Software, Inc., San Diego, CA). Values with p < 0.05 were considered statistically significant.
3 Results and discussion
3.1 Chemical composition of C. odorata lipophilic extract
Plant bioactives, especially polyphenols such as flavonoids are widely regarded as powerful sources of natural antioxidants and the health benefits of consuming fruits and vegetables has largely been attributed to these specialized metabolites. Consequently, there has been a consistent interest in identifying new sources rich in bioactive components as well as new approaches for their preparation. Herein, C. odorata lipophilic extract (COLE) from the aerial part was prepared in a simple green chemistry approach (Fig. 1). The only organic solvent used in the extraction process was ethanol, which is deemed to be generally recognized as safe (GRAS) in the food industry. This was to ensure that if the extract exhibits desirable attributes, then valorizing it into useful phytomedicinal or nutraceutical products would not be challenging. In particular, the challenge arising from solvent-associated toxicity would not be a serious issue of concern. The crude C. odorata extract obtained using food grade ethanol was portioned via cold fractionation into an upper hydrophilic component (COHE) and a lower lipophilic component (COLE). Various classes of specialized metabolites from the C. asiatica extracts were initially evaluated via UV–vis spectroscopy and the results are presented in Table 1. The lipophilic extract was found to possess high amounts of total phenolics, flavonoids and saponins. The high phenolic content of COLE was found to be in line with previous reports on C. odorata extract (Srinivasa Rao et al., 2010). Interestingly, comparison of the phytochemical content of COLE with the hydrophilic fraction revealed that the COLE contained higher amounts of phytochemicals.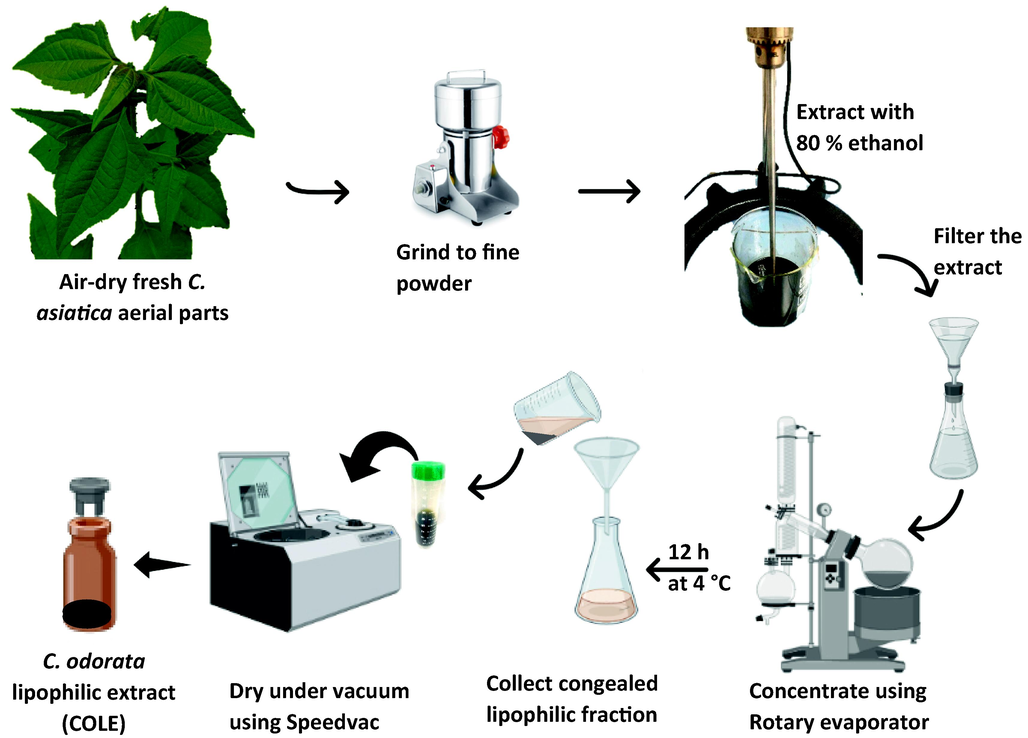
Schematic representation of the preparation of C. odorata lipophilic extract (COLE) from aerial part of the plant.
Sample
Phenolic and saponin contents
Color analysis
Total phenolics (mg gallic acid/g)
Total flavonoids (mg quercetin/g)
Total saponin (mg asiatic acid/g)
L
a
b
ΔE
COHE
189.96 ± 1.25a
22.82 ± 0.87b
55.07 ± 1.28c
91.57 ± 0.01
−4.07 ± 0.01
29.38 ± 0.01
96.25 ± 0.01
COLE
262.48 ± 1.66
70.20 ± 22.60
129.56 ± 18.39
16.73 ± 0.03
6.54 ± 0.03
10.91 ± 0.02
21.02 ± 0.04
Besides, both components of C. odorata were also different in their physical appearance. The hydrophilic layer was clear orange in appearance while the lipophilic layer presented a dark green appearance. The color of the two samples were examined in terms of the following chromatic parameters viz; L, lightness: white/ black (100/0), a: red/green (+/-), b: yellow/blue (+/-), and ΔE, color difference. The L values confirmed that that COHE (91.57) was lighter than COLE (16.73). Also, it was observed that the chroma values were markedly different in both fractions. The chroma value of COHE was more than twice that of COLE. Contrariwise, the values for all the colorimetric parameters for COLE, with the exception of a, were lower relative to those observed for COHE. This therefore justified the darker appearance COLE.
3.1.1 Secondary metabolites profile of C. odorata lipophilic extract by UPLC-MS analysis
In this study, an extensive qualitative phytochemical profile of the individual bioactive constituents present in COLE was established via UHPLC-ESI-QTOF-MS analysis in negative ionization mode. Prior preliminary analysis indicated that the negative ionization mode was more sensitive than the positive ionization mode. The total ion chromatogram of the extract in the negative ionization mode is presented in Fig. 2 and features several peaks at different retention times spread across the length of the chromatogram, suggestive of phytoconstituents belonging to different families (Fig. S1). The individual compounds in the lipophilic extract of C. odorata that were tentatively identified via QTOF-MS analysis in the negative ionization mode and with an accuracy error below 5 ppm (Table 2). In addition, other parameters that facilitated the characterization of the bioactive compounds such as retention times (Rt), detected accurate mass ([M−H]-), mass error (ppm), and molecular formula of each chemical component are also provided (Eze et al., 2019). Rt: Retention time in minutes.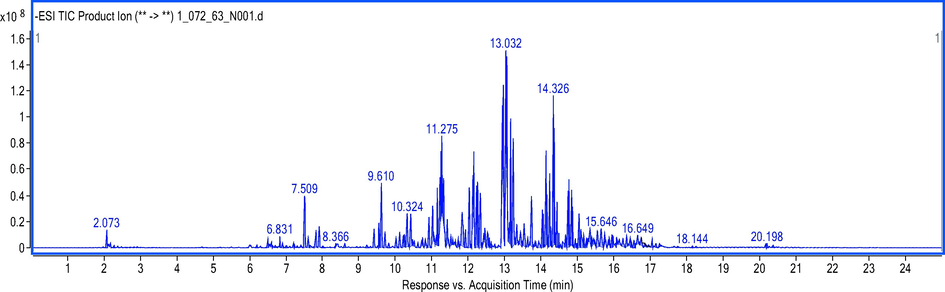
Total ion chromatogram of secondary metabolites found in C. odorata lipophilic extract by UPLC-ESI-QTOF-MS in negative ionization mode.
S/N
RT (min)
Precursor (m/z)
Accurate Mass
Diff (ppm)
Score (DB)
Formula
Tentative ID
1
2.01
373.0924
374.0999
0.77
97.7
C19H18O8
Quercetagetin 6,7,3′4′-tetramethyl ether
2
2.19
191.0563
192.0636
−1.06
99.43
C7H12O6
Quinic acid
3
4.75
271.0819
272.0891
1.81
98.55
C12H16O7
Arbutin
4
5.25
169.0141
170.0215
0.38
99.56
C7H6O5
Gallic acid
5
5.63
315.0717
270.0737
1.08
98.33
C12H14O7
Phenyl glucuronide
6
5.70
359.0981
360.1054
0.76
99.3
C15H20O10
6′-Methoxypolygoacetophenoside
7
5.93
323.1345
264.1206
1.13
97.89
C11H20O7
(Z)-2-Methyl-2-butene-1,4-diol 4-O-β-D-Glucopyranoside
8
5.95
479.2491
480.2563
1.5
97.4
C22H40O11
3-O-(α-L-Rhamnopyranosyl-(1 → 2)-α-L-rhamnopyranosyl)-3-hydroxydecanoic acid
9
6.06
353.0877
354.095
0.25
99.86
C16H18O9
Chlorogenic acid
10
6.33
153.0193
154.0266
−0.06
99.89
C7H6O4
3,4-Dihydroxybenzoic acid
11
6.36
285.0615
286.0687
0.51
99.78
C12H14O8
Uralenneoside
12
6.43
349.1863
350.1935
1.59
98.74
C16H30O8
(1S,2R,4R,8S)-p-Menthane-2,8,9-triol 9-glucoside
13
6.46
477.2335
432.2351
1.95
74.39
C21H36O9
Glucosyl (2E,6E,10x)-10,11-dihydroxy-2,6-farnesadienoate
14
6.56
191.0563
192.0636
−0.95
99.46
C7H12O6
Quinic acid
15
6.81
771.1985
772.2058
0.56
98.54
C33H40O21
Quercetin 3-glucosyl-(1 → 2)-[rhamnosyl-(1 → 6)-galactoside]
16
6.93
431.1925
386.1942
−0.3
81.48
C19H30O8
Sonchuionoside C
17
6.96
299.0771
300.0844
0.44
99.64
C13H16O8
Salicylic acid β-D-glucoside
18
7.01
755.2038
756.2109
0.5
98.85
C33H40O20
Kaempferol 3-sophoroside 7-rhamnoside
19
7.03
433.2079
388.2103
−1.44
78.62
C19H32O8
5α,6α-Epoxy-7E-megastigmene-3β,9ξ-diol 9-glucoside
20
7.06
415.1245
416.1318
0.12
98.73
C18H24O11
2-Hydroxybenzaldehyde O-[xylosyl-(1 → 6)-glucoside]
21
7.13
593.2812
534.2674
0.49
99.11
C25H42O12
3-Hydroxy-β-ionol 3-[glucosyl-(1 → 6)-glucoside]
22
7.16
547.2755
488.2617
0.93
98.43
C24H40O10
α-Ionol O-[arabinosyl-(1 → 6)-glucoside]
23
7.31
581.1877
582.1949
−0.11
98.91
C27H34O14
10-Acetoxyligustroside
24
7.34
461.2391
462.2464
0.27
97.26
C22H38O10
Neryl rhamnosyl-glucoside
25
7.36
335.0772
336.0845
0.07
84.73
C16H16O8
5-O-Caffeoylshikimic acid
26
7.41
367.1035
368.1108
−0.13
98.78
C17H20O9
3-O-Caffeoyl-4-O-methylquinic acid
27
7.46
177.0195
178.0268
−0.93
87.22
C9H6O4
Esculetin
28
7.565
435.1294
436.1367
0.56
99.14
C21H24O10
Phloridzin
29
7.566
609.1492
610.1565
−5.06
99.06
C27H30O16
Rutin
30
7.69
917.2353
918.2425
0.57
98.57
C42H46O23
Kaempferol 3-(2-p-coumaroylsophoroside) 7-glucoside
31
7.74
225.113
226.1204
0.61
99.6
C12 H18 O4
Tuberonic acid
32
7.81
547.2397
502.2415
−0.11
99.4
C24H38O11
3-Oxo-α-ionol 9-[apiosyl-(1 → 6)-glucoside]
33
7.93
593.1524
594.1592
−1.28
96.82
C27H30O15
Vitexin 4′-O-galactoside
34
7.94
901.2401
902.2472
0.93
99.02
C42H46O22
Kaempferol 3-[2′'-(6′''-coumaroylglucosyl)-rhamnoside] 7-glucoside
35
8.16
549.255
504.2567
0.8
96.8
C24H40O11
Blumenol C O-[apiosyl-(1 → 6)-glucoside]
36
8.26
755.1821
756.1895
0.85
98.95
C36H36O18
2′'-(6′'-p-Coumaroylglucosyl)quercitrin
37
8.46
353.088
354.0951
−0.02
98.57
C16H18O9
5Z-Caffeoylquinic acid
38
8.645
303.051
304.0582
0.28
98.56
C15H12O7
(±)-Taxifolin
39
8.74
623.1612
624.1678
2.01
81.21
C28H32O16
Isoscoparin 2′'-O-glucoside
40
8.77
161.0244
162.0317
−0.06
86.95
C9H6O3
7-Hydroxycoumarin
41
8.846
457.2438
458.2512
0.75
84.52
C23H38O9
[6]-Gingerdiol 5-O-β-D-glucopyranoside
42
9.14
447.2232
448.2305
0.75
99.31
C21H36O10
Kenposide B
43
9.22
317.0666
318.0739
0.24
98.7
C16H14O7
Demethylsulochrin
44
9.34
497.1086
498.1159
0.55
99.1
C25H22O11
3,4-Dicaffeoyl-1,5-quinolactone
45
9.44
259.0612
260.0684
0.17
99.36
C14H12O5
Khellin
46
9.474
125.0246
126.0318
−1.08
87.6
C6H6O3
(Z)-Tamarindienal
47
9.474
287.0575
288.0646
−4.16
91.94
C15H12O6
3′,4′,5,7-Tetrahydroxyisoflavanone
48
9.54
315.0874
316.0947
0.1
85.11
C17H16O6
7-O-Methylpeltogynol
49
9.574
577.2863
518.2724
0.6
97.43
C25H42O11
Blumenol C O-[rhamnosyl-(1 → 6)-glucoside]
50
9.963
135.0451
136.0523
0.61
99.81
C8H8O2
3-(3-Furanyl)-2-methyl-2-propenal
51
10.0
287.0565
288.0638
−1.37
98.74
C15H12O6
Eriodictyol
52
10.252
345.0979
346.1052
0.22
97.68
C18H18O7
2,8-Dihydroxy-3,9,10-trimethoxypterocarpan
53
10.29
327.2178
328.225
−0.23
84.66
C18H32O5
9,12,13-Trihydroxy-10,15-octadecadienoic acid
54
10.528
289.0719
290.0792
−0.53
99.56
C15H14O6
(±)-Catechin
55
10.553
635.1405
636.1478
0.2
98.98
C32H28O14
Kaempferol 3-(3′'-acetyl-6′'-p-coumaroylglucoside)
56
10.629
207.0664
208.0737
−0.65
98.27
C11H12O4
2,5-Dimethoxycinnamic acid
57
10.729
301.0719
302.0792
−0.59
98.4
C16H14O6
Hesperetin
58
10.817
329.2335
330.2407
−0.37
98.14
C18H34O5
11,12,13-Trihydroxy-9-octadecenoic acid
59
10.83
269.0458
270.053
−0.83
97.39
C15H10O5
Apigenin
60
10.93
271.0634
272.0707
−0.17
99.42
C15H12O5
(±)-Naringenin
61
11.081
285.0426
286.0498
−0.34
98.97
C15H10O6
Luteolin
62
11.181
327.2176
328.2249
0.29
98.78
C18H32O5
9,12,13-Trihydroxy-10,15-octadecadienoic acid (isomer)
63
11.381
283.0611
284.0683
0.73
99.19
C16H12O5
Biochanin A
64
11.557
315.051
316.0584
−0.28
98.77
C16H12O7
Isorhamnetin
65
11.909
603.1511
544.1371
−0.33
99.21
C30H24O10
(2S,2′'S,3S,3′'R,4S)-3,4′,5,7-Tetrahydroxyflavan(2 → 7,4 → 8)-3,4′,5,7-tetrahydroxyflavan
66
12.122
165.0193
166.0267
−0.3
99.32
C8H6O4
3,4-Methylenedioxybenzoic acid
67
12.511
359.0773
360.0846
−0.1
98.97
C18H16O8
Chrysosplenol F
68
12.599
345.0981
346.1053
−0.05
99.2
C18H18O7
2,8-Dihydroxy-3,9,10-trimethoxypterocarpan isomer
69
12.763
329.0666
330.0743
−0.92
98.69
C17H14O7
7,3′,4′-Trihydroxy-3,8-dimethoxyflavone
70
13.001
283.0625
284.0696
−3.84
98.72
C16H12O5
1,5-Dihydroxy-2-methoxy-6-methylanthraquinone
71
13.516
329.0672
330.0744
−1.43
98.35
C17H14O7
7,3′,4′-Trihydroxy-3,8-dimethoxyflavone
72
13.792
299.0562
300.0636
−0.63
97.95
C16H12O6
Diosmetin
73
13.943
627.3537
568.3403
−0.59
88.6
C34H48O7
(24E)-3α,15α-Diacetoxy-23-oxo-7,9(11),24-lanostatrien-26-oic acid
74
14.47
579.3688
534.3707
0.4
99.33
C35H50O4
Pyrohyperforin
75
14.733
611.3599
612.367
−1.3
98.3
C36H52O8
(24E)-3β,15α,22S-Triacetoxylanosta-7,9(11),24-trien-26-oic acid
76
14.896
243.1756
244.1829
−0.72
84.52
C17H24O
Falcarinol
77
15.75
593.3487
548.3513
0.37
72.24
C36H44N4O
Manzamine A
78
16.089
593.3483
594.3556
0.07
73.97
C36H50O7
Glycosyl-4,4′-diaponeurosporenoate
79
16.302
291.1965
292.2037
0.33
85.61
C18H28O3
13-epi-12-oxo Phytodienoic Acid
80
16.804
647.3801
648.3873
0.1
99.61
C36H56O10
2α-Hydroxygypsogenin 3-O-β-D-glucoside
81
16.867
575.3379
530.3413
−3.27
58.43
C35H46O4
(-)-Neolinderatin
82
17.608
881.5201
836.5231
−0.47
69.12
C53H72O8
Amitenone
83
17.96
263.2014
264.2087
0.72
99.24
C17H28O2
10E-Heptadecen-8-ynoic acid
84
20.306
271.228
272.2353
−0.4
99.4
C16H32O3
16-Hydroxyhexadecanoic acid
85
20.42
441.3587
382.3448
−0.19
99.73
C24H46O3
3-Oxo-tetracosanoic acid
3.1.2 Terpene and terpenoid derivatives
It has been previously noted that terpenoids could be vital in the management of cancer (Ben Sghaier et al., 2016; Dahham et al., 2015; Hui et al., 2015), type 1 diabetes via the preservation and rejuvenation of β cells (Liu et al., 2010), and in type 2 diabetes through activation of the transcription factor Nrf2 (Castellano et al., 2013). Also widely reported is the fact that terpenoid compounds could stimulate wound healing and antimicrobial actions via multiple mechanisms (Agra et al., 2015). Some studies have alluded to the presence of terpenoids in C. odorata aerial parts, but very few have actually mentioned the individual compounds from this group (Joshi, 2013; Vijayaraghavan et al., 2018). Thus, it was essential to establish the identities of the terpenoid compounds present in C. odorata hydrophilic extract. Mass spectral data from COLE revealed the presence of the following putative terpene glycosides; (1S,2R,4R,8S)-p-menthane-2,8,9-triol 9-glucoside (Rt 6.436), glucosyl (2E,6E,10x)-10,11-dihydroxy-2,6-farnesadienoate (Rt 6.461 min), sonchuionoside C (Rt 6.938 min), 3-hydroxy-β-ionol 3-[glucosyl-(1 → 6)-glucoside] (Rt 7.139 min), 10-acetoxyligustroside (Rt 7.315 min), neryl rhamnosyl-glucoside (Rt 7.34 min), kenposide B (Rt 9.147 min). The terpenoids, (24E)-3α,15α-diacetoxy-23-oxo-7,9(11),24-lanostatrien-26-oic acid (Rt 13.943 min), (24E)-3β,15α,22S-triacetoxylanosta-7,9(11),24-trien-26-oic acid (14.733 min), glycosyl-4,4′-diaponeurosporenoate (Rt 16.089 min), monoterpenoid, pyrohyperforin (Rt 14.47 min), diterpenoid, amitenone (Rt 17.608 min) and triterpene saponin, 2α-hydroxygypsogenin 3-O-β-D-glucoside (Rt 16.804 min). Many of these terpene compounds are being reported in C. odorata aerial parts for the first time and therefore requires further characterization with other spectral techniques to confirm their presence. The group of compounds tentatively identified places terpenes and terpenoid derivatives as another major class of specialized metabolites present in C. odorata.
3.1.3 Phenolic compounds
Phenolic compounds constitute one of the predominant groups of specialized metabolites in plants and continue to engender strong research interest due to the important roles they play in both plant and animal protection. Natural phenolic compounds have been associated with numerous pharmacological and health benefits including antioxidant, anti-inflammatory, immunomodulatory, antimicrobial, neuroprotective, and anticancer properties. The genus, Chromolaena, is noted for the presence of diverse phenolic compounds represented in phenolic acids, flavonoids as well as numerous derivatives.
3.1.4 Simple phenolics and derivatives
Free phenolic acids in the form of hydroxybenzoic acid, hydroxycinnamic acid, as well as their derivatives were the most prominent simple phenolic compounds in C. odorata lipophilic extract. Compound #4 (Rt 5.256 min) furnished a precursor ion at m/z 169.0141 and was assigned as gallic acid (Yunle Huang et al., 2022), a hydroxybenzoic acid derivative. The MS/MS fragment ion at m/z 125.0243 (Fig. S2) was deemed to arise from the characteristic neutral-loss of CO2 from the precursor ion [M−H−44]- (Khallouki et al., 2015; Newsome et al., 2016). Gallic acid is one of the most widely distributed and studied plant phenolic compounds. Gallic acid is known to protect biomolecules from oxidative damage by scavenging of reactive chemical species. It is also reported to exert antifungal, antimicrobial, antioxidant, and anticancer activities (Sagdicoglu Celep et al., 2022). Meanwhile, the compound eluted at Rt 6.336 min was putatively identified on the basis of its precursor ion (m/z 153.0193) as 3,4-dihydroxybenzoic acid, which is often referred to as protocatechuic acid (Kumar et al., 2017). The putative identity of the compound was confirmed on the basis of the spectral data and fragment ion at m/z 136.9368, which corresponds to dihydroxybenzoic acid with lost hydroxyl group [M−H−17]-. Literature search also confirmed the presence of high levels of 3,4-dihydroxybenzoic acid in C. odorata aerial parts extracted with diethyl ether (Phan et al., 2001). Hydroxycinnamic acids are another group of phenolic acids commonly found in plant materials. According to Chaowuttikul et al., C. odorata leaves contain large amounts of chlorogenic acids, albeit the identities of the individual metabolites were not enunciated (Chaowuttikul et al., 2020). Herein, several hydroxycinnamates were identified in COLE. QTOF-MS data revealed a characteristic fragment ion at m/z 191.0559 for compounds number #9, #26, and #37. This fragment (also present as compound #2) is likely derived from deprotonated quinic acid [M−H]- moiety (Kumar et al., 2017). Considering the molecular ion at m/z 353.0877 as well as the quinic acid fragment (m/z 191), which is likely due to the liberation of a caffeoyl moiety [M−H−162]-, compound #9 was tentatively assigned as chlorogenic acid (Long et al., 2012). Similarly, compound #26 was ascribed to 3-O-caffeoyl-4-methylquinic acid because of the parent ion being featured at m/z 367.1035 and base peak at m/z 191.0559 corresponding to the release of caffeoyl and methyl moieties from the molecular ion [M−H−162−14]. The identity of compound #37 was established as 5Z-caffeoylquinic acid, a chlorogenic acid isomer based on database search of the parent ion m/z 353.088 and MS2 fragments. Besides the aforementioned quinic acid derivatives, a coumaric acid derivative with m/z 497.1086 [M−H]- eluted at Rt 9.348 min was identified as 3,4-dicaffeoyl-1,5-quinolactone.
3.1.5 Flavonoid derivatives
Several flavonoids were putatively identified in C. odorata lipophilic extract. These included many derivatives of quercetin and kaempferol. For example, compound #15 was putatively identified as quercetin 3-glucosyl –(1 → 2)-[rhamnosyl-(1 → 6)-galactoside] while compound #29 was established as rutin. The flavonoid presented a parent ion at m/z 609.1492 and daughter ion at 300.0273, corresponding to the deprotonated aglycone ([Y-H]-̇) following cleavage of the rutinose moiety [M−H−308]- from the parent ion (Kumar et al., 2017). The QTOF MS data for compound #36 (Rt 8.268 min) featured a precursor ion at m/z 755.1821 and a product ion at m/z 609.1447, likely due to the loss of a coumaroyl moiety [M−H−146]- leaving behind a glucosylquercitrin fragment. With reference to the deprotonated molecular ion peak at m/z 755.1822, compound #36 was assigned as 2″-(6″-p-coumaroylglucosyl)quercitrin whereas compound #64 with pseudomolecular peak at m/z 273.0769 was assigned as the flavonoid aglycone isorhamnetin (Im et al., 2018). Regarding compound #28 (Rt 7.565 min), a deprotonated molecular ion was observed at m/z 435.1294. The MS/MS data featured a prominent product ion at m/z 273.0763 caused by the loss of 162 amu. By matching the MS data with database as well as comparing with previous literature reports, this compound was identified as phloridzin or phloretin-2-glucoside (Prakash et al., 2019). Additionally, several kaempferol derivatives were putatively identified including compound #18, kaempferol 3-sophoroside 7-rhamnoside (Rt 7.01 min), compound #30 kaempferol 3-(2-p-coumaroylsophoroside) 7-glucoside (Rt 7.69 min), compound #34 kaempferol 3-[2′'-(6′''-coumaroylglucosyl)-rhamnoside] 7-glucoside (Rt 7.94 min), and compound #55 kaempferol 3-(3′'-acetyl-6′'-p-coumaroylglucoside) (Rt 10.55 min). For compound #54, a deprotonated parent ion peak at m/z 289.0719 was observed and the compound was assigned as (±)-catechin. MS/MS data yielded a characteristic product peak at m/z 139 which likely originated from ring A as a result of cleavage of two bonds in ring C (i.e., O-C of position 1 and 2, and C–C of position 3 and 4) of the molecule (Spáčil et al., 2010). Besides, compound #72 (Rt 13.792 min) was found as the methoxylated flavonoid aglycone, diosmetin which yielded pseudomolecular ion at m/z 299.0562 and a strong fragment peak at m/z 284.0767. The fragment at m/z 284 is thought to arise due to loss of.CH3 from the deprotonated molecular ion [M−H−CH3]-· (Justesen, 2000). Compound #59 presented a deprotonated ion at m/z 269.0458, with prominent MS/MS fragments at m/z 151.0034 and m/z 117.0346. On the basis of previously reported literature and database search, the compound was identified as apigenin (Sánchez-Rabaneda et al., 2004). Other flavonoid aglycones, many of which had been previously found in C. odorata were also identified from the QTOF-MS data of COLE. These included phloridzin (Rt 7.565 min), (±)-taxifolin (Rt 8.645), eriodictyol (Rt 10.026 min), hesperetin (Rt 10.729), apigenin (Rt 10.83 min), (±)-naringenin (10.93 min) and luteolin (Rt 11.081 min), as well as biochanin A (Rt 11.381 min) and (-)-neolinderatin (Rt 16.867 min) (Yuan et al., 2007).
3.1.6 Other compounds
Fatty acids and fatty acyl glycosides were noticed as another important group of compounds based on the number of constituents identified in the aerial part of C. odorata lipophilic extract. Seven fatty acyl glycosides were tentatively identified in COLE, including (Z)-2-methyl-2-butene-1,4-diol 4-O-α-D-glucopyranoside (Rt 5.934), 3-O-(α-L-rhamnopyranosyl-(1 → 2)-α-L-rhamnopyranosyl)-3-hydroxydecanoic acid (Rt 5.959), 5α,6α-Epoxy-7E-megastigmene-3β,9ξ-diol 9-glucoside (Rt 7.038 min), α-Ionol O-[arabinosyl-(1 → 6)-glucoside] (Rt 7.164 min), 3-oxo-α-ionol 9-[apiosyl-(1 → 6)-glucoside] (Rt 7.817 min), and blumenol C O-[apiosyl-(1 → 6)-glucoside] (Rt 8.168 min). In all, a total of 85 secondary metabolites were putatively identified in COLE by UPLC-MS.
3.1.7 GC–MS analysis of C. odorata lipophilic extract
A number of previous reports have highlighted the beneficial roles of volatile and semi-volatile constituents from C. odorata. Most of these reports however focused on the essential oil as the source of volatile compounds. COLE presents an opportunity to further extend the coverage of specialized metabolites in C. odorata aerial parts. The volatile and semi-volatile constituents of COLE were profiled by GC–MS analysis. A total of thirty six compounds were tentatively identified. These volatile metabolites can be broadly categorized into hydrocarbons, sesquiterpene and derivatives, terpenoids, fatty acids and derivatives, as well as flavonoids (Table 3). The major composition of volatile compounds were found to be sesquiterpene and their derivatives, which made up 55.38 % of the total. Rt: retention time; CAS RN: CAS Registry Number.
S/N
RT (min)
Name (Tentative ID)
Formula
CAS RN
% of Total
Nature of Compound
1
11.4127
Undecane
C11H24
1120–21-4
1.23
Hydrocarbon
2
12.5354
Geijerene
C12H18
6902–73-4
1.15
Hydrocarbon
3
14.2355
Tridecane
C13H28
629–50-5
0.22
Hydrocarbon
4
17.9179
Bicycloelemene
C15H24
2000216–46-8
0.27
Elemane sesquiterpenoids
5
18.9188
α-Copaene
C15H24
3856–25-5
1.94
Hydrocarbon
6
20.0158
(-)-β-Caryophyllene
C15H24
87–44-5
9.15
Bicyclic sesquiterpene
7
20.2532
β-Cubebene
C15H24
13744–15-5
3.51
Tricyclic sesquiterpene
8
20.3815
γ-Elemene
C15H24
29873–99-2
0.37
Sesquiterpene
9
20.869
α-Humulene
C15H24
6753–98-6
2.72
Monocyclic sesquiterpene
10
21.4464
γ-Muurolene
C15H24
30021–74-0
1.61
Sesquiterpene
11
21.5491
Germacrene D
C15H24
23986–74-5
3.38
Sesquiterpene
12
21.671
Aromadendrene
C15H24
489–39-4
0.58
5,10-Cycloaromadendrane sesquiterpenoids
13
21.8121
cis-Muurola-4(15),5-diene
C15H24
157477–72-0
0.38
Carbobicyclic compound
14
21.8891
Cubebol
C15H26O
23445–02-5
2.14
Sesquiterpene alcohol
15
22.0302
α-Muurolene
C15H24
31983–22-9
1.11
Sesquiterpene
16
22.5755
(+)-δ-Cadinene
C15H24
483–76-1
8.75
Sesquiterpene
17
23.0374
α-Calacorene
C13H16
30364–38-6
0.09
Sesquiterpene
18
23.185
α-Elemol
C15H26O
639–99-6
1.43
Sesquiterpene
19
23.5827
τ-Cadinol
C15H26O
11/1/5937
1.76
Sesquiterpene
20
23.8329
(-)-Spathulenol
C15H24O
77171–55-2
1.64
Tricyclic sesquiterpene
21
23.9613
Caryophyllene oxide
C15H24O
1139–30-6
3.2
Bicyclic sesquiterpene
22
24.1601
Viridiflorol
C15H26O
552–02-3
0.6
Sesquiterpene
23
24.5643
(-)-Humulene epoxide II
C15H24O
19888–34-7
0.55
Sesquiterpenoid
24
25.0134
τ-Cadinol acetate
C17H28O2
149197–48-8
0.95
Sesquiterpene
25
25.0711
(+)-γ-Eudesmol
C15H26O
1209–71-8
1.42
Oxygenated sesquiterpene
26
25.4753
β-Eudesmol
C15H26O
473–15-4
4.38
Oxygenated sesquiterpene
27
25.5523
α-Eudesmol
C15H26O
473–16-5
5.18
Oxygenated sesquiterpene
28
28.946
Cryptomeridiol
C15H28O2
2000334–99-5
0.59
Sesquiterpene alcohol
29
31.2556
Methyl palmitate
C17H34O2
112–39-0
0.68
Fatty acid methyl ester
30
32.5643
Ethyl palmitate
C18H36O2
628–97-7
2.92
Fatty acid ethyl ester
31
34.7199
Phytol
C20H40O
150–86-7
2.71
Hydrogenated diterpene alcohol
32
35.0022
Methyl isostearate
C19H38O2
5129–61-3
0.33
Fatty acid methyl ester
33
35.5988
Methyl linolelaidate
C19H34O2
2566–97-4
1.06
Fatty acid methyl ester
34
35.7143
Ethyl Linoleate
C20H36O2
544–35-4
2.28
Fatty acid ethyl ester
35
49.8923
2′-Hydroxy-4,4′,5′,6′-tetramethoxychalcone
C19H20O6
41929–26-4
1.8
Flavanone
36
56.8017
Lupeol
C30H50O
545–47-1
4.9
Pentacyclic terpenoid
Besides, COLE also presented the following compounds with considerably high amounts, viz; γ-eudesmol (1.42 %), β-eudesmol (4.38 %), α-eudesmol (5.18 %), lupeol (4.9 %), (+)-δ-cadinene (8.75 %), (-)-β-caryophyllene (9.15 %). Interestingly, some of these compounds have been reported to demonstrate desirable biological and pharmacological properties. For instance, the oxygenated sesquiterpenoid compound, β-eudesmol was previously found to stimulate appetite (Ohara et al., 2017), inhibit glucosyltransferase and reduce dental caries (Shruthi et al., 2021), demonstrate antiangiogenic (Tsuneki et al., 2005), and antitumor properties (Ma et al., 2008). Similarly, δ-cadinene reportedly suppressed the growth of ovarian cancer cells through caspase-dependent apoptosis and cell cycle arrest (Hui et al., 2015), whereas the triterpenoid, lupeol was reported to exhibit potent anti-inflammatory and anticancer activities (Liu et al., 2021). Considering that β-caryophyllene constituted almost one-tenth of the total volatiles in COLE, it is only germane to mention that the sesquiterpene has been reported to possess antioxidant, antimicrobial, anticarcinogenic and anticancer potentiating effects (Legault and Pichette, 2007). The presence of these specialized metabolites in COLE in significant amounts is a strong pointer that it could be effectively valorized for biomedical and pharmacological purposes.
3.2 C. odorata lipophilic extract demonstrated potent anticancer activities
The cytotoxic activity of COLE against human breast cancer (MCF-7 and MDA-MB-231), colon cancer (HT-29 and HCT-116), as well as non-cancer (HEK-293 and CCD-841 CoN) cells was investigated using MTT assay. Cells were exposed to various concentrations of COLE and after 72 h, the cytotoxic activity was determined. The results of the cytotoxic effect of COLE are summarized in Table 4 and Fig. 3. It can be seen that COLE exhibited potent cytotoxic effect against the cancer cell lines with IC50 in the range of 9.18 – 19.48 µg/mL. Also, it can be observed that COLE was more active against the breast cancer cells than the colon cancer cells, and the most potent effect was noticed against MCF-7 cells. Regarding the cytotoxic effect of COLE against normal cell lines, COLE was found to induce some cytotoxic effect. But when compared to the effect of COLE against cancer cells, the cytotoxic effect against normal cells were significantly lower. In fact, according to the recommendations of the US National Cancer Institute for in vitro screening of anti-cancer agents, botanicals such as plant extract are expected to display IC50 values below 20 µg/mL to be considered cytotoxic (Geran et al., 1972).
Cell line
Classification
COLE (IC50 µg/mL)
COLE Selectivity Indexb
MCF-7
Breast cancer cell
9.18 ± 0.21
21.59 – 41.86
MDA-MB-231
Breast cancer cell
12.19 ± 0.43
16.26 – 31.53
HT-29
Colon cancer cell
19.48 ± 0.11
10.17 – 19.73
HCT-116
Colon cancer cell
14.12 ± 0.50
14.04 – 27.22
HEK-293
Kidney normal cell
198.2 ± 2.89
–
CCD-841 CON
Colon normal cell
384.3 ± 4.14
–
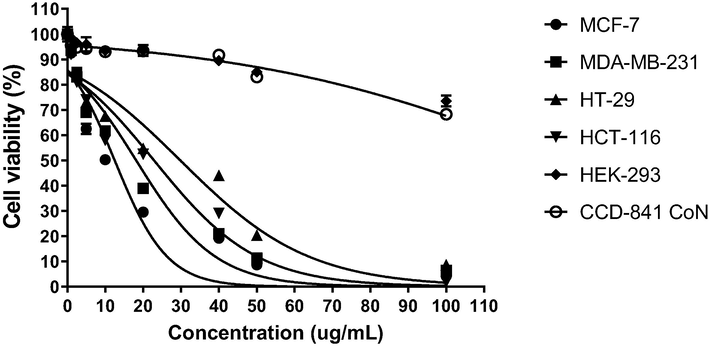
Impact of various concentrations of C. odorata lipophilic extract on viability of cells after 72 h determined by MTT assay.
In this context, the obtained large IC50 values of COLE against normal cells (≥198 µg/mL) suggests that the extract was not cytotoxic on these cells. At the same time, it must be emphasized that while it is important for prospective anticancer extracts to display potent effects against cancer cells, such cytotoxic effects should not be extended against normal healthy cells. At most, the cytotoxic effect against normal healthy cells should be minimal. For in vitro anti-cancer drug screening, the relative effect of anticancer agents against normal cells vis-à-vis cancer cells can be estimated using the selectivity index (SI). Anticancer agents with high SI values (≥10) tend to be more selective towards cancer cells and less active against normal cells (Peña-Morán et al., 2016). As shown in Table 4, COLE displayed substantially high SI values in the range of 10.17 – 41.86, indicating that the anticancer effect of the extract is likely to be restricted only to the cancerous cells.
Previous studies on the anticancer effects of C. odorata extracts revealed some notable findings. Yusuf and co-workers recently reported that crude ethanol extract from C. odorata leaves inhibited the proliferation of HepG2 cancer cells and induced cell cycle arrest. The authors also found that following 24 h period of cell exposure to the samples, the crude extract was more potent with IC50 of 23.44 µg/mL compared to the hexane (84.54 µg/mL), ethyl acetate (167.49 µg/mL) and ethanol (88.59 µg/mL) fractions. Meanwhile, both the extract and fractions were less cytotoxic to normal Vero cells with IC50 > 950 µg/mL (Yusuf et al., 2022b). In the same vein, while evaluating the anticancer effect of C. odorata, Singh noticed that the crude ethanol extract demonstrated modest cytotoxic effects against HCT116 (IC50 60.18 µg/mL) and HeLa cells (IC50 71.74 µg/mL) (Singh, 2012). When crude ethanol extract was fractionated, Yusuf et al. found that the soluble ethyl acetate fraction was able to inhibit the proliferation of HeLa cervical cancer cells after 24 h with an IC50 value of 82.41 µg/mL (Yusuf et al., 2022a). Two points are worth noting from these earlier studies. Firstly, they underscore the anticancer potential of C. odorata extract. Secondly, although not clearly stated, the authors implicitly identified the need to improve the anticancer property of the crude extract. This was accomplished via further fractionation using organic solvents. However, success of the fractionation process, that is, collection of the active fraction from the crude extract is not guaranteed. In other cases, the collection of the active fraction may involve the use of toxic organic solvents which poses serious health and environmental concerns and complicates the downstream formulation processes (Singh, 2012; Yusuf et al., 2022a). Although we agree with the need to target the preparation of extracts with high anticancer activity, we also bore in mind the necessity to reduce/eliminate potentially noxious organic solvents during the preparation process; thus, the method adopted. Compared to the afore-mentioned previous studies, COLE displayed superior anticancer activity in vitro and acceptable selectivity. The presence and prominence of specialized metabolites with known anticancer attributes, including, β-caryophyllene and caryophyllene oxide, (Fidyt et al., 2016; Lei et al., 2021), δ-cadinene (Hui et al., 2015), β-eudesmol (Ma et al., 2008), and lupeol (Liu et al., 2021) in the lipophilic extract further supports the anticancer properties of COLE. For instance, β-caryophyllene was reported to affect the growth and proliferation of many cancer cells. In non-small cell lung cancer cells, treatment with the natural sesquiterpene was reported to enhance apoptotic rate, inhibit growth, elevate the level of antioxidant enzymes, miR-659-3p, apoptotic factors, and reduce the level of oxidative stress and SphK1 (Lei et al., 2021). Similarly, according to Dahham et al., treatment of HCT-116 and HT-29 (colon cancer) cells, as well as PANC-1 (pancreatic cancer) cells with β-caryophyllene resulted in strong growth inhibition of the cancer cell lines (Dahham et al., 2015). Whereas it was revealed that when multiple myeloma cells were exposed to β-caryophyllene treatment, the sesquiterpene caused antiproliferative effects via the induction of apoptosis and modulation of cell cycle (Mannino et al., 2021). Moreover, β-caryophyllene oxide was previously found to inhibit the growth of human prostate cancer (PC-3) and breast cancer (MCF-7) cells. Treatment of the cancer cells with the compound reportedly induce ROS-mediated MAPKs activation, inhibit PI3K/AKT/mTOR/S6K1 signaling cascades, and induce apoptosis via suppression of gene products that mediate proliferation, angiogenesis, metastasis, and tumor cell survival. α-Humulene, an isomer of β-caryophyllene was equally observed to exert anticancer activity. It was revealed that the sesquiterpene exhibited significant antiproliferative effect against CaCo-2 intestinal cancer cells (Ambrož et al., 2015). Beside, β-caryophyllene and β-caryophyllene oxide were also shown to synergistically potentiate the anticancer activity of other natural metabolites such as α-humulene and the synthetic anticancer agent, doxorubicin (Park et al., 2011). Meanwhile, β-eudesmol, another compound present in COLE, was reported to inhibit superoxide production, proliferation, adhesion and migration of lung cancer (A549) and colon cancer (HT29) cells. The sesquiterpene was also found to inhibit tumor growth via the suppression of tumor neovascularization and tumor cell proliferation (Ben Sghaier et al., 2016; Ma et al., 2008). Similarly, it has been noted that lupeol suppressed the proliferation and migration of MDA-M−231 breast cancer cells through a crosstalk mechanism between EMT and autophagy (Zhang et al., 2022). As a phytocomplex harboring all these bioactive constituents, it is reasonable to deduce that the mode of COLE anticancer activity probably involves a number of the aforementioned mechanisms working together in synergy or addition. To the best of our knowledge, this is the first report on the anticancer properties of the lipophilic fraction of C. odorata aerial part extract prepare in an innocuous manner, and the findings highlight the potential therapeutic benefits of COLE in the management of cancer disease.
3.3 Antimicrobial activities of COLE
Plant extracts remain an indispensable and invaluable resource for the discovery and development of important antimicrobial formulations. In many parts of the world, formulations based on plant extracts are still very much relied upon for countering various forms of microbial infections. Pertaining to the antimicrobial activity of extracts from edible parts of plants, it has been postulated that highly active extracts exhibit MIC values < 100 µg/mL, significantly active extracts feature 100 ≤ MIC values ≤ 512 µg/mL, those with moderate activity present 512 ≤ MIC values ≤ 2048 µg/mL, low activity if the MIC values > 2048 µg/mL, and inactive if the MIC values > 10 mg/mL (Tamokou et al., 2017). The antimicrobial property of two different fractions prepared from C. odorata extract was evaluated via broth microdilution technique. The MIC and MBC values are presented in Table 5. From the MIC values, it is apparent that both fractions demonstrated antimicrobial activity, although the lipophilic fraction was comparatively more potent. The lipophilic fraction was significantly active against B. cereus (MIC value of 0.16 mg/mL) and moderately active against E. coli (MIC value of 1.06 mg/mL) and L. monocytogenes (MIC value of 0.63 mg/mL). Meanwhile, the hydrophilic extract presented low activity towards all the microbial pathogens. These findings were in accord with previous reports by Naidoo et al., who observed that the aqueous, but not methanolic or ethyl acetate extract did not inhibit B. cereus and the other microbes investigated (Naidoo et al., 2011). Similarly, Omokhua et al. noted that the non-polar extract of C. odorata was more enriched with antimicrobial agents compared to the polar extracts (Omokhua et al., 2017). Comparatively, the results also seemed to suggest that COLE exhibited better antimicrobial action against the gram-positive microbial strains relative to the gram negative microbial strain. This may be due to the presence of the outer membrane on the gram-negative bacteria which affords them an additional layer of protection against antibiotic agents. As presented in Table 2, COLE is endowed with several notable phenolic compounds, such as (±)-catechin, luteolin, isorhamnetin, gallic acid, chlorogenic acid, quinic acid, hesperetin, apigenin, naringenin, rutin, amongst others. These phenolic compounds have been reported to exert good antibacterial activities against many different bacteria, inclusive of those used in the current study (Adamczak et al., 2019; Fu et al., 2016). Similarly, terpenes and their derivatives such as β-caryophyllene, phytol, lupeol, (+)-δ-cadinene, germacrene D, and β-eudesmol, were present in large amounts in COLE as evidenced in the GC–MS results (Table 3). It is worthy to mention that these compounds, especially β-caryophyllene, the dominant volatile constituent, are also known to strongly inhibit the growth and/or cause the death of microbial pathogens such as fungi and bacteria (Dahham et al., 2015; Selestino Neta et al., 2017). Interestingly, it was previously reported that amongst the volatile constituents, those with hydroxyl groups (phenolic and alcohol), compared to the hydrocarbons, were far more efficacious (Guimarães et al., 2019). Thus, the antimicrobial activity of COLE can be attributed to the presence of not just a single compound, but rather the ensemble of different groups of compounds present in the extract including phenolics, flavonoids, terpenes, and terpenoids. Pertaining to the mode of antimicrobial action, it is probable that COLE exerts its antimicrobial effect via a multipronged manner which could be linked to those of phenolics, flavonoids and terpenes. This may involve binding to adhesins, damage to microbial cell wall, destabilization of cell membrane, as well as the permeation and interruption of vital intracellular functions through protein binding, enzyme inactivation, metal ions complexation, or induction of oxidative stress by constituents of the extract (Borges et al., 2013; Lee et al., 2016; Pérez Zamora et al., 2018). The good antibacterial activity of the lipophilic fraction of C. odorata, but not the hydrophobic fraction, suggest that it could be valorized toward the control of various pathogenic microbes. This could be of tremendous value as a cost-effective natural antibiotic in certain parts of the world with meagre economic means but abundant supply of C. odorata.
Antimicrobial activity
Bacterial isolates
C. odorata lipophilic fraction
C. odorata hydrophilic fraction
Vancomycin/ Gentamicina
MIC (mg/mL)
MBC (mg/mL
MIC (mg/mL)
MBC (mg/mL)
MIC (mg/mL)
MBC (mg/mL)
B. cereus
0.16
0.31
3.13
6.25
0.02<
0.02<
E. coli
1.06
2.01
6.25
12.5
0.02<
0.02<
L. monocytogenes
0.63
2.01
6.25
12.5
0.02<
0.02<
3.4 C. odorata lipophilic extract demonstrated potent antioxidant and metal chelating activities
There is ample evidence from many epidemiological studies suggesting an inverse relationship between the consumption of fruits and vegetable (reputable sources of dietary antioxidants) and reduced risk of cardiovascular, non-cardiovascular, cancer, and premature mortality (Aune et al., 2017; Liu et al., 2000). Consequently, studies involving plant extracts for food and nutraceutical applications routinely determine their antioxidant content. DPPH and ABTS assays are well established in vitro techniques for evaluating the properties of compounds or extracts in vitro. While the ABTS assay measures the ability of the extract to donate electrons (SET: single electron transfer mechanism), the DPPH assay is based on the capacity of the analyte to donate electrons and hydrogen atoms to the radicals. Most natural product extracts employ more than a single radical scavenging mechanism to exert their antioxidant action and thus the need for multiple radical scavenging assays.
The in vitro antioxidant capacity of COLE was evaluated based on their ability to scavenge the cationic ABTS, anionic DPPH, and superoxide radicals. As shown in Fig. 4, COLE and ascorbic acid (the antioxidant standard) demonstrated potent radical scavenging properties and their effects were concentration dependent. The IC50 value of COLE against ABTS, DPPH and superoxide radicals were 46.80 ± 3.11, 27.78 ± 0.98 and 64.07 ± 2.36 µg/mL. These values were expectedly higher than those obtained for ascorbic acid (antioxidant standard) with IC50 values of 4.98 ± 0.32, 2.06 ± 0.02, 8.35 ± 0.43 µg/mL for inhibition of ABTS, DPPH and superoxide anion radicals, respectively, considering that the standard is a pure compound whereas COLE is a crude mixture of phytochemicals. However, it must be stated that when COLE was compared to literature reports of some common natural extracts with known antioxidant activities, such as guava leaves extract (Sampath Kumar et al., 2021), essential oil of guava leaves (Lee et al., 2012), polyphenol-rich extract from leaves (Nivedha et al., 2020), and Eucalyptus camaldulensis lipophilic extract (Yanping Huang et al., 2022), the IC50 values of COLE presented comparable, and in some cases superior antioxidant activity. The strong antioxidant effect of COLE can be attributed to the presence of multiple secondary metabolites with potent antioxidant properties, such as gallic acid, rutin, chlorogenic acid, (±)-catechin, hesperetin, and lupeol.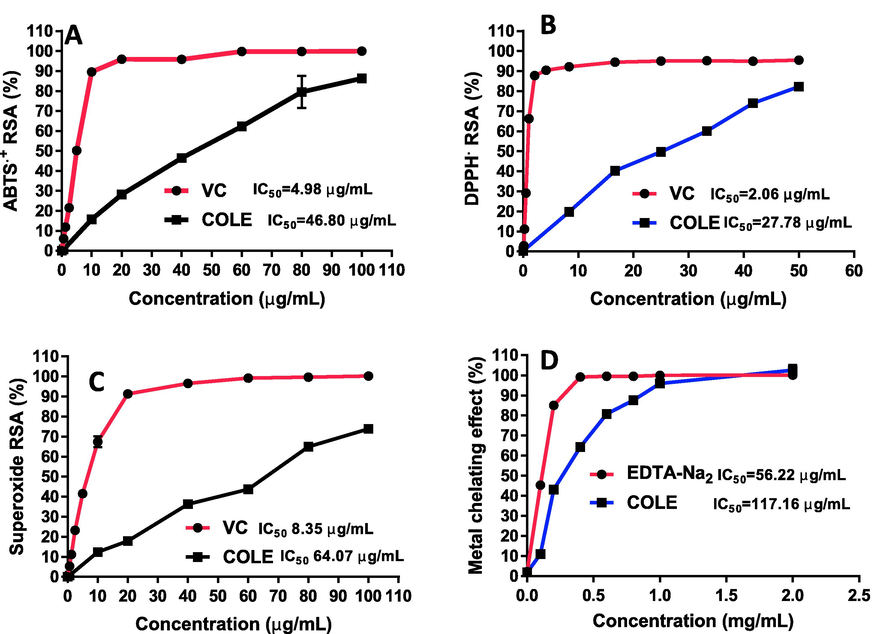
Antioxidant activity of COLE against (A) ABTS, (B) DPPH, (C) superoxide anion and (D) metal-ion chelating effect.
Furthermore, metal ions such as iron and copper are known to be actively involved in free radical initiation. (Duthie et al., 1997). Thus, it was worthwhile to determine the metal chelating activity of COLE. It was revealed that the lipophilic extract of C. odorata exhibited strong metal ion chelating capacity with an IC50 value of 117.16 ± 4.30 µg/mL (Fig. 4D) towards ferrous ions. This may be due to the high flavonoid content and presence of individual metabolites such as catechin, rutin, apigenin, and naringenin, known to be effective metal ion chelators (Cherrak et al., 2016; Jahanshahi et al., 2021; Spiegel and Sroka, 2023). Iron accumulation has been implicated in the development of degenerative diseases such as Parkinson’s disease, Alzheimer’s disease and cardiovascular disease. In cancers, excess iron has been tightly linked to tumorigenesis via a number of mechanisms. Excess iron may induce the generation of reactive oxygen species (ROS) via Fenton reaction. Elevated levels of ROS can cause severe damage to proteins, lipids, and DNA, consequently triggering tumorigenesis. Besides, the proliferating tumor cells require iron as an essential nutrient (Brown et al., 2020). Authors have documented in literature the close association between elevated levels of iron and development of multiple human cancer types, such as breast cancer, lung cancer, prostate cancer, pancreatic cancer, hepatocellular cancer, and colorectal cancer. Thus, multiple therapeutic approaches based on the deprivation of iron has been proposed (Islam et al., 2022; Zhang and Zhang, 2015). Taken together, the good iron chelation activity, strong antioxidant activity, and selective anticancer property suggest that COLE could have positive implications in the amelioration of these conditions.
4 Conclusions
Herein, we report for the first time the chemical and biological characterization of C. odorata aerial part lipophilic extract (COLE) prepared via a facile green chemistry approach. COLE was successfully prepared without the use of any innocuous organic solvent and found to contain a wide range of bioactive metabolites. Also, it was revealed that COLE was enriched predominantly with valuable terpenes, terpenoids, and phenolic constituents, contributing to its remarkable biological and pharmacological properties. In particular, the lipophilic extract was found to exhibit potent and selective anticancer properties towards breast and colon cancer cell lines, whereas over the range of concentrations tested, the extract was non-toxic to normal cell lines. The results also indicated that COLE possessed significant activity against B. cereus and moderate activity against L. monocytogenes and E. coli. Furthermore, the extract was found to exert impressive radical scavenging and metal chelating activities, highlighting its capacity to neutralize free radical and oxidizing species. With the current global challenge of increasing incidence of chronic diseases associated with oxidative stress and cancers, the antioxidative and anticancer effects of COLE is a good indication that the extract could be considered as a promising cost-effective and efficacious pharmacological resource for combating these conditions. Thus, to maximize the potential health benefits of COLE, further studies aimed at elucidating its potency, potential adverse effects, precise mechanistic underpinnings, bioavailability, amongst others, in animal models are highly encouraged.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Comparison of phenolic compounds profile and antioxidant properties of different sweet cherry (Prunus avium L.) varieties. Food Chem.. 2019;279:260-271.
- [CrossRef] [Google Scholar]
- Antibacterial activity of some flavonoids and organic acids widely distributed in plants. J. Clin. Med.. 2019;9:109.
- [CrossRef] [Google Scholar]
- Triterpenes with healing activity: a systematic review. J. Dermatolog. Treat.. 2015;26:465-470.
- [CrossRef] [Google Scholar]
- The influence of Sesquiterpenes from Myrica rubra on the antiproliferative and pro-oxidative effects of doxorubicin and its accumulation in cancer cells. Molecules. 2015;20:15343-15358.
- [CrossRef] [Google Scholar]
- Fruit and vegetable intake and the risk of cardiovascular disease, total cancer and all-cause mortality—a systematic review and dose-response meta-analysis of prospective studies. Int. J. Epidemiol.. 2017;46:1029-1056.
- [CrossRef] [Google Scholar]
- Encapsulation of C. odorata extracts for antimicrobial activity. J. Phys.: Conf. Ser.. 2019;1372:012046
- [CrossRef] [Google Scholar]
- Bedi, G., Tonzibo, Z.F., Oussou, K.R., Choppard, C., Mahy, J.P., N???Guessan, T.Y., 2015. Effect of essential oil of Chromoleana odorata (Asteraceae) from Ivory Coast, on cyclooxygenase function of prostagladin-H synthase activity. International Journal of Pharmacy and Pharmacology 5, 1–4
- β-eudesmol, a sesquiterpene from Teucrium ramosissimum, inhibits superoxide production, proliferation, adhesion and migration of human tumor cell. Environ. Toxicol. Pharmacol.. 2016;46:227-233.
- [CrossRef] [Google Scholar]
- Antibacterial activity and mode of action of ferulic and gallic acids against pathogenic bacteria. Microb. Drug Resist.. 2013;19:256-265.
- [CrossRef] [Google Scholar]
- The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer. 2021;127:3029-3030.
- [CrossRef] [Google Scholar]
- Altered iron metabolism and impact in cancer biology, metastasis, and immunology. Front. Oncol.. 2020;10:476.
- [CrossRef] [Google Scholar]
- Biochemical basis of the antidiabetic activity of oleanolic acid and related pentacyclic triterpenes. Diabetes. 2013;62:1791-1799.
- [CrossRef] [Google Scholar]
- Quantification of chlorogenic acid, rosmarinic acid, and caffeic acid contents in selected Thai medicinal plants using RP-HPLC-DAD. Braz. J. Pharm. Sci.. 2020;56
- [CrossRef] [Google Scholar]
- In vitro antioxidant versus metal ion chelating properties of flavonoids: a structure-activity investigation. PLoS One. 2016;11:e0165575.
- [CrossRef] [Google Scholar]
- Anticancer properties of dried-pericarp water extracts of Camellia japonica L. fermented with Aspergillus oryzae through regulation of IGFBP-2/mTOR pathway. Sci. Rep.. 2021;11:21527.
- [CrossRef] [Google Scholar]
- CLSI, 2020. Performance Standards for Antimicrobial Susceptibility Testing. 30th ed. CLSI supplement M100. Wayne, PA: Clinical and Laboratory Standards Institute.
- The anticancer, antioxidant and antimicrobial properties of the Sesquiterpene β-Caryophyllene from the essential oil of Aquilaria crassna. Molecules. 2015;20:11808-11829.
- [CrossRef] [Google Scholar]
- The effect of dietary flavonoids on DNA damage (strand breaks and oxidised pyrimdines) and growth in human cells. Mutat. Res./Genet. Toxicol. Environ. Mutagen.. 1997;390:141-151.
- [CrossRef] [Google Scholar]
- Chromolaena odorata (Siam weed): a natural reservoir of bioactive compounds with potent anti-fibrillogenic, antioxidative, and cytocompatible properties. Biomed. Pharmacother.. 2021;141:111811
- [CrossRef] [Google Scholar]
- Protein glycation and oxidation inhibitory activity of Centella asiatica phenolics (CAP) in glucose-mediated bovine serum albumin glycoxidation. Food Chem.. 2020;332:127302
- [CrossRef] [Google Scholar]
- Centella asiatica phenolic extract-mediated bio-fabrication of silver nanoparticles: characterization, reduction of industrially relevant dyes in water and antimicrobial activities against foodborne pathogens. RSC Adv.. 2019;9:37957-37970.
- [CrossRef] [Google Scholar]
- β-caryophyllene and β-caryophyllene oxide—natural compounds of anticancer and analgesic properties. Cancer Med.. 2016;5:3007-3017.
- [CrossRef] [Google Scholar]
- Phenolic compounds and in vitro antibacterial and antioxidant activities of three tropic fruits: persimmon, guava, and sweetsop. Biomed. Res. Int.. 2016;2016:4287461.
- [CrossRef] [Google Scholar]
- Protocol for screening chemical agenst and natural product against animal tumors and other biogical system. Cancer Chemother. Rep.. 1972;3:51-61.
- [Google Scholar]
- Natural products as leads to anticancer drugs. Clin. Transl. Oncol.. 2007;9:767-776.
- [CrossRef] [Google Scholar]
- Antibacterial activity of terpenes and terpenoids present in essential oils. Molecules. 2019;24:2471.
- [CrossRef] [Google Scholar]
- Targeting cancer signaling pathways by natural products: exploring promising anti-cancer agents. Biomed. Pharmacother.. 2022;150:113054
- [CrossRef] [Google Scholar]
- Myrtus comunis and Eucalyptus camaldulensis cytotoxicity on breast cancer cells. Zbornik Matice srpske za prirodne nauke 2012:65-73.
- [Google Scholar]
- Antiproliferative activities of the lipophilic fraction of eucalyptus camaldulensis against MCF-7 breast cancer cells, UPLC-ESI-QTOF-MS metabolite profile, and antioxidative functions. ACS Omega. 2022;7:27369-27381.
- [CrossRef] [Google Scholar]
- Characterisation of catechins and their oxidised derivatives in Ceylon tea using multi-dimensional liquid chromatography and high-resolution mass spectrometry. J. Chromatogr. A. 2022;1682:463477
- [CrossRef] [Google Scholar]
- δ-Cadinene inhibits the growth of ovarian cancer cells via caspase-dependent apoptosis and cell cycle arrest. Int. J. Clin. Exp. Pathol.. 2015;8:6046-6056.
- [Google Scholar]
- Flavonoid glycosides from chromolaena odorata leaves and their in vitro cytotoxic activity. Chem. Pharm. Bull.. 2011;59:129-131.
- [CrossRef] [Google Scholar]
- Im, A.-R., D, A.-R., M, A.-N., I, W., A, S.-C., 2018. Untargeted metabolite profiling and phytochemical analysis of Micromeria fruticosa L. (Lamiaceae) leaves. Food Chem 279, 128–143. https://doi.org/10.1016/j.foodchem.2018.11.144
- Iron overload and breast cancer: iron chelation as a potential therapeutic approach. Life. 2022;12:963.
- [CrossRef] [Google Scholar]
- Naringin chelates excessive iron and prevents the formation of amyloid-beta plaques in the hippocampus of iron-overloaded mice. Front. Pharmacol.. 2021;12:651156
- [CrossRef] [Google Scholar]
- Chemical composition of the essential oils of aerial parts and flowers of Chromolaena odorata (L.) R. M. King & H. Rob. from Western Ghats Region of North West Karnataka, India. J. Essent. Oil Bearing Plants. 2013;16:71-75.
- [CrossRef] [Google Scholar]
- Negative atmospheric pressure chemical ionisation low-energy collision activation mass spectrometry for the characterisation of flavonoids in extracts of fresh herbs. J. Chromatogr. A. 2000;902:369-379.
- [CrossRef] [Google Scholar]
- Identification of polyphenolic compounds in the flesh of Argan (Morocco) fruits. Food Chem.. 2015;179:191-198.
- [CrossRef] [Google Scholar]
- Phytochemicals isolated from leaves of chromolaena odorata: impact on viability and clonogenicity of cancer cell lines. Phytother. Res.. 2013;27:835-840.
- [CrossRef] [Google Scholar]
- Identification and characterization of phenolics and terpenoids from ethanolic extracts of Phyllanthus species by HPLC-ESI-QTOF-MS/MS. J. Pharm. Anal.. 2017;7:214-222.
- [CrossRef] [Google Scholar]
- Le, A., E. Parks, S., H. Nguyen, M., D. Roach, P., 2018. Improving the Vanillin-Sulphuric Acid Method for Quantifying Total Saponins. Technologies 6, 84. https://doi.org/10.3390/technologies6030084
- Lee, W.C., Mahmud, R., Pillai, S., Perumal, S., Ismail, S., 2012. Antioxidant Activities of Essential Oil of Psidium Guajava L. Leaves. APCBEE Procedia, 3rd International Conference on Biotechnology and Food Science (ICBFS 2012), April 7-8, 2012 2, 86–91. https://doi.org/10.1016/j.apcbee.2012.06.016.
- Lee, W., Woo, E.-R., Lee, D.G., 2016. Phytol has antibacterial property by inducing oxidative stress response in Pseudomonas aeruginosa. Free Radic Res 50, 1309–1318. https://doi.org/10.1080/10715762.2016.1241395
- Potentiating effect of β-caryophyllene on anticancer activity of α-humulene, isocaryophyllene and paclitaxel. J. Pharm. Pharmacol.. 2007;59:1643-1647.
- [CrossRef] [Google Scholar]
- Beta-caryophyllene from chilli pepper inhibits the proliferation of non-small cell lung cancer cells by affecting miR-659-3p-targeted Sphingosine kinase 1 (SphK1) IJGM. 2021;14:9599-9613.
- [CrossRef] [Google Scholar]
- Antioxidant and anti-inflammatory activities of quercetin and its derivatives. J. Funct. Foods. 2018;40:68-75.
- [CrossRef] [Google Scholar]
- Asiatic acid preserves beta cell mass and mitigates hyperglycemia in streptozocin-induced diabetic rats. Diabetes Metab. Res. Rev.. 2010;26:448-454.
- [CrossRef] [Google Scholar]
- Fruit and vegetable intake and risk of cardiovascular disease: the Women’s Health Study. Am. J. Clin. Nutr.. 2000;72:922-928.
- [CrossRef] [Google Scholar]
- Lupeol and its derivatives as anticancer and anti-inflammatory agents: Molecular mechanisms and therapeutic efficacy. Pharmacol. Res.. 2021;164:105373
- [CrossRef] [Google Scholar]
- Long, H.S., Stander, M.A., Van Wyk, B.-E., 2012. Notes on the occurrence and significance of triterpenoids (asiaticoside and related compounds) and caffeoylquinic acids in Centella species. South African Journal of Botany, Quality Control 82, 53–59. https://doi.org/10.1016/j.sajb.2012.07.017
- Beta-eudesmol suppresses tumour growth through inhibition of tumour neovascularisation and tumour cell proliferation. J. Asian Nat. Prod. Res.. 2008;10:159-167.
- [CrossRef] [Google Scholar]
- Beta-caryophyllene exhibits anti-proliferative effects through apoptosis induction and cell cycle modulation in multiple myeloma cells. Cancers (Basel). 2021;13:5741.
- [CrossRef] [Google Scholar]
- Camellia oil and its distillate fractions effectively inhibit the spontaneous metastasis of mouse melanoma BL6 cells. FEBS Lett.. 2007;581:2541-2548.
- [CrossRef] [Google Scholar]
- Muniappan, R., Reddy, G.V.P., Lai, P.-Y., 2005. Distribution and biological control of Chromolaena odorata, in: Inderjit (Ed.), Invasive Plants: Ecological and Agricultural Aspects. Birkhäuser, Basel, pp. 223–233. https://doi.org/10.1007/3-7643-7380-6_14.
- Screening of Chromolaeana odorata (L.) King and Robinson for antibacterial and antifungal properties. JMPR. 2011;5:4859-4862.
- [Google Scholar]
- Kaempferide, the most active among the four flavonoids isolated and characterized from Chromolaena odorata, induces apoptosis in cervical cancer cells while being pharmacologically safe. RSC Adv.. 2015;5:100912-100922.
- [CrossRef] [Google Scholar]
- Whole genome comparisons of serotype 4b and 1/2a strains of the food-borne pathogen Listeria monocytogenes reveal new insights into the core genome components of this species. Nucleic Acids Res.. 2004;32:2386-2395.
- [CrossRef] [Google Scholar]
- Improved quantification of free and ester-bound gallic acid in foods and beverages by UHPLC-MS/MS. J. Agric. Food Chem.. 2016;64:1326-1334.
- [CrossRef] [Google Scholar]
- In vitro studies on antioxidant and cyto-protective activities of polyphenol-rich fraction isolated from Mangifera indica leaf. S. Afr. J. Bot.. 2020;130:396-406.
- [CrossRef] [Google Scholar]
- β-Eudesmol, an oxygenized sesquiterpene, stimulates appetite via TRPA1 and the autonomic nervous system. Sci Rep. 2017;7:15785.
- [CrossRef] [Google Scholar]
- A comparison of the antimicrobial activity and in vitro toxicity of a medicinally useful biotype of invasive Chromolaena odorata (Asteraceae) with a biotype not used in traditional medicine. S. Afr. J. Bot.. 2017;108:200-208.
- [CrossRef] [Google Scholar]
- β-Caryophyllene oxide inhibits growth and induces apoptosis through the suppression of PI3K/AKT/mTOR/S6K1 pathways and ROS-mediated MAPKs activation. Cancer Lett.. 2011;312:178-188.
- [CrossRef] [Google Scholar]
- Cytotoxicity, post-treatment recovery, and selectivity analysis of naturally occurring podophyllotoxins from bursera fagaroides var. fagaroides on breast cancer cell lines. Molecules. 2016;21:1013.
- [CrossRef] [Google Scholar]
- Antimicrobial activity and chemical composition of essential oils from verbenaceae species growing in South America. Molecules. 2018;23:544.
- [CrossRef] [Google Scholar]
- Enhanced proliferation of fibroblasts and endothelial cells treated with an extract of the leaves of Chromolaena odorata (Eupolin), an herbal remedy for treating wounds. Plast. Reconstr. Surg.. 1998;101:756-765.
- [CrossRef] [Google Scholar]
- Phenolic compounds of chromolaena odorata protect cultured skin cells from oxidative damage: implication for cutaneous wound healing. Biol. Pharm. Bull.. 2001;24:1373-1379.
- [CrossRef] [Google Scholar]
- Characterization, quantification of free, esterified and bound phenolics in Kainth (Pyrus pashia Buch.-Ham. Ex D.Don) fruit pulp by UPLC-ESI-HRMS/MS and evaluation of their antioxidant activity. Food Chem.. 2019;299:125114
- [CrossRef] [Google Scholar]
- Antioxidant and anticancer activities of gallic acid loaded sodium alginate microspheres on colon cancer. Curr. Appl. Phys.. 2022;40:30-42.
- [CrossRef] [Google Scholar]
- Extraction of bioactive compounds from Psidium guajava leaves and its utilization in preparation of jellies. AMB Express. 2021;11:36.
- [CrossRef] [Google Scholar]
- Qualitative analysis of phenolic compounds in apple pomace using liquid chromatography coupled to mass spectrometry in tandem mode. Rapid Commun. Mass Spectrom.. 2004;18:553-563.
- [CrossRef] [Google Scholar]
- Radical scavenging property of a novel peptide derived from C-terminal SOD domain of superoxide dismutase enzyme in Arthrospira platensis. Algal Res.. 2018;35:519-529.
- [CrossRef] [Google Scholar]
- High-throughput assay comparison and standardization for metal chelating capacity screening: a proposal and application. Food Chem.. 2017;214:515-522.
- [CrossRef] [Google Scholar]
- Revisiting the ancient concept of botanical therapeutics. Nat. Chem. Biol.. 2007;3:360-366.
- [CrossRef] [Google Scholar]
- Effects of β-caryophyllene and Murraya paniculata essential oil in the murine hepatoma cells and in the bacteria and fungi 24-h time–kill curve studies. Pharm. Biol.. 2017;55:190-197.
- [CrossRef] [Google Scholar]
- Shruthi, N., R, N., Elumalai, E., Gupta, K.K., 2021. Eudesmol-A promising inhibitor for glucosyltransferase: Docking and Molecular dynamics stud.
- Singh, A., 2012. Phytochemical Characterisation and in Vitro Anticancer Screeening of Ethanol Extract of Chromolaena odorata Linn. (M. Pharm). THE TAMILNADU Dr. M.G.R. MEDICAL UNIVERSITY, Chennai, India
- Selected aspects of chemoresistance mechanisms in colorectal carcinoma—a focus on epithelial-to-mesenchymal transition, autophagy, and apoptosis. Cells. 2019;8:234.
- [CrossRef] [Google Scholar]
- Comparison of positive and negative ion detection of tea catechins using tandem mass spectrometry and ultra high performance liquid chromatography. Food Chem.. 2010;123:535-541.
- [CrossRef] [Google Scholar]
- Quantum-mechanical characteristics of apigenin: antiradical, metal chelation and inhibitory properties in physiologically relevant media. Fitoterapia. 2023;164:105352
- [CrossRef] [Google Scholar]
- Evaluation of anti-oxidant activities and total phenolic content of Chromolaena odorata. Food Chem. Toxicol.. 2010;48:729-732.
- [CrossRef] [Google Scholar]
- Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin.. 2021;71:209-249.
- [CrossRef] [Google Scholar]
- Chapter 8 - Antimicrobial Activities of African Medicinal Spices and Vegetables. In: Kuete V., ed. Medicinal Spices and Vegetables from Africa. Academic Press; 2017. p. :207-237.
- [CrossRef] [Google Scholar]
- Antiangiogenic activity of beta-eudesmol in vitro and in vivo. Eur. J. Pharmacol.. 2005;512:105-115.
- [CrossRef] [Google Scholar]
- Cytotoxic activity of secondary metabolite compounds from myanmar medicinal plants. IntechOpen 2022
- [CrossRef] [Google Scholar]
- Phytochemical screening, free radical scavenging and antimicrobial potential of Chromolaena odorata leaf extracts against pathogenic bacterium in wound infections– a multispectrum perspective. Biocatal. Agric. Biotechnol.. 2018;15:103-112.
- [CrossRef] [Google Scholar]
- Anti proliferative and apoptotic effect of soluble ethyl acetate partition from ethanol extract of chromolaena odorata linn leaves against hela cervical cancer cell line. Asian Pac. J. Cancer Prev.. 2022;23:183-189.
- [CrossRef] [Google Scholar]
- Inhibitory effects on HepG2 cell proliferation and induction of cell cycle arrest by Chromolaena odorata leaf extract and fractions. Pharmacia. 2022;69:377-384.
- [CrossRef] [Google Scholar]
- Lupeol inhibits the proliferation and migration of MDA-MB-231 breast cancer cells via a novel crosstalk mechanism between autophagy and the EMT. Food Funct.. 2022;13:4967-4976.
- [CrossRef] [Google Scholar]
- Iron homeostasis and tumorigenesis: molecular mechanisms and therapeutic opportunities. Protein Cell. 2015;6:88-100.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2023.104834.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







