Translate this page into:
UV induced synthesis of starch capped CdSe quantum dots: Functionalization with thiourea and application in sensing heavy metals ions in aqueous solution
⁎Corresponding author. madhab@barc.gov.in (M.C. Rath)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Herein, a highly facile, rapid and one-pot approach has been applied for the synthesis of water soluble starch capped CdSe quantum dots (QDs) by using UV irradiation in the aqueous solution containing cadmium sulphate, sodium selenosulfate, acetone and 2-propanol. No external reducing agents were added to the solution, as the radicals generated in situ upon photoirradiation i.e., 2-hydroxy-2-propyl radicals, (CH3)2C•OH could reduce the precursor ions for the synthesis of these QDs. These QDs were characterised by various techniques such as UV–Vis absorption, XRD, Raman, FTIR, TEM and SEM measurements. The presence of strong quantum confinement effects could be realized from their very small size i.e., ∼3 nm as revealed by TEM studies. Besides, these QDs were found to exhibit photoluminescence (PL) in the longer wavelength region. The PL intensity as well as the charge carrier lifetime values could be conveniently tuned by simply varying the Cd to Se precursor ratio during the synthesis. Furthermore, a relatively novel approach has been adopted to extract these QDs from the colloidal solution by freezing it to 0 °C followed by de-freezing to room temperature. The extracted QDs were functionalized with thiourea in order to increase the PL quantum yield and the stability of the QDs. The effects of pH and temperature on the PL intensity of functionalized CdSe QDs were also investigated to explore their applicability in sensing of heavy metal ions. Interestingly, the QDs displayed highly selective PL quenching in the presence of Cu2+, Cr6+ and Hg2+ metal ions. The limits of detection for these metal ions have been determined and a probable PL quenching mechanism is postulated.
Keywords
CdSe quantum dot
UV radiation
Starch
Surface functionalization
Sensor
1 Introduction
Cadmium selenide (CdSe) quantum dots (QDs) are one of the most important and extensively studied II–VI semiconductors. The quantum confinement behaviour arises when size becomes less than Bohr radius of exciton (5.7 nm in the case of CdSe), which is responsible for its versatile optoelectronic properties leading to applications in various areas (Zorman et al., 1995; Bera et al., 2010). CdSe QDs have been synthesised using different methods like organometallics, electrochemical, sonochemical, radiation and photochemical routes, etc. Out of these methods, photochemical synthesis route is very important because there is no use of additional reducing agents, no requirement of stringent laboratory conditions like inert atmosphere and high temperature (Sakamoto et al., 2009). Various capping agents have been used to control the shape, size and surface morphology of the CdSe QDs. The capping agents play important role in determining properties of QDs. Capping the QDs with biomolecules like protein, amino acids, carbohydrates etc. provide an extra edge by reducing the cytotoxicity of these QDs (Medintz, 2013; Sapsford et al., 2013; Singh et al., 2017). Furthermore, the synthesis in aqueous solution is very crucial because most of the time these QDs are used in applications involving the aqueous solution (Jing et al., 2016). After Murray et al. (1993) synthesised CdSe QDs in organic solvents, various successful attempts have been made to synthesise CdSe QDs in aqueous solution by different routes (Jing et al., 2016; Kalasad et al., 2009; Fernandez-Arguelles et al., 2005; Qian et al., 2005; Li et al., 2013; Su et al., 2009). These QDs have been widely used in the area of photovoltaics, light emitting devices, photocatalysis, photodynamic therapy. cell imaging, biological sensors, gas sensors and metal ion sensors.
Metal ion sensing is very crucial due to rapid economic development and other anthropogenic activities which cause mingling of various metal ions with the ground water and other consumables. According to WHO, half of the human population is suffering from water borne diseases and heavy metal ions are one of the most important sources for the contamination of water (WHO, 2018). Heavy metal ions, Cu2+, Cr6+ and Hg2+ have several adverse effects on human as well as environment. Copper (Cu) is one of the essential micro nutrients that is required for plant, animals and human health but in excess, it causes many abnormalities inside the biological system like, stomach upset, headache, abdominal pain, nausea, diarrhoea etc. Severe toxicity of copper causes Wilson disease, respiratory difficulty, haemolytic anaemia, haematuria, massive gastrointestinal bleeding, liver and kidney failure, and death (Gaetke and Chow, 2003). Mercury (Hg) is a widely known toxic metal and has adverse effect on biological systems even in very low concentration. Hg poisoning has effects like, loss of hair, teeth, nails, hypotonia (muscle weakness), kidney dysfunction, memory impairments and brain damage, etc. (Bernhoft, 2012). Chromium (Cr) is also one of the potent toxic metals, which is generally found in 3+ and 6+ oxidation states. Cr3+ compounds and Cr metal are not toxic but Cr6+ is severely toxic and carcinogen too (Baruthio, 1992). The Cr3+ has specific transport mechanism so only very small amount of Cr3+ enters in the cell but Cr6+ is in higher oxidation state and hence is more reactive and having strong oxidative properties which causes DNA damage (Cohen et al., 1993). The major sources of these metal ions are coal based power plants, mineral, steel and cement industry, waste disposal, biomass burning, agricultural chemicals, rocks and volcanos (Tchounwou et al., 2012).
Hence, sensing of these toxic metal ions are very essential and fluorimetric sensing is one of the most effortless, reliable, rapid and efficient method with low detection limit (Valeur and Leray, 2000). Using QDs as a fluorimetric sensor provide many advantages over conventional organic fluorophores like its size dependent optical and physical properties, broad absorption and narrow emission band, high molar absorption coefficient, high quantum yield and resistance against photo bleaching (Murphy, 2002). So, synthesising QDs in aqueous solution using a facile root at ambient conditions for the sensing of different metal ions is very important as well as essential. Various researchers have synthesized different QDs using different methods and used them in sensing of metal ions (Cai et al., 2014; Jing et al., 2014; Lou et al., 2014; Silvi and Credi, 2015; Wu and Yan, 2010; Wu et al., 2014; Zhang et al., 2016). Koneswaran and Narayanaswamy (2009) have synthesized L-cysteine capped ZnS QDs at high temperature for photoluminescence (PL) sensor of Cu2+. Bu et al. (2013) have synthesized CdSe QDs in aqueous solution using bioinspired approach and used it in detection of Cu(II) ions. However, the sensing of heavy metal ions through PL quenching process by using the CdSe QDs synthesized at room temperature is still to be investigated. We are reporting the detection of heavy metal ions by thiourea functionalised CdSe QDs synthesized using UV irradiation which is a rapid, one-pot and facile approach of synthesis.
In this study, we have synthesised nearly monodispersed starch capped CdSe QDs in aqueous solution through UV irradiation using 300 nm UV lamps in a photoreactor at ambient condition. The colour of these QDs depends upon the stoichiometric ratio of Cd and Se precursors taken in the reaction mixture. These QDs were extracted from the colloidal solutions using a novel technique developed by us and characterised by TEM, SEM, XRD, Raman and FTIR spectroscopic measurements. The extracted QDs were functionalised with thiourea and were used for the sensing of Cu2+, Cr6+ and Hg2+ions via PL quenching.
2 Experimental
2.1 Materials
High purity chemicals CdSO4, Na2SO3, Se powder, 2-propanol, acetone, and starch were obtained from sigma and used without further purification. Ammoniated cadmium sulphate, Cd(NH3)4SO4 was used as the Cd precursor and freshly prepared sodium selenosulphate, Na2SeSO3 was used as the Se precursor. Sodium selenosulfate was synthesized by following the reported literature; typically, a mixture of 5 g of Na2SO3 and 0.5 g of Se powder was refluxed at 70 °C for 7 h to obtain a clear transparent solution, which contains Na2SeSO3 (250 mM) and unreacted Na2SO3 (Singh et al., 2011a,b). It is to be noted here that the excess Na2SO3 present in the solution neither take part nor hinder the reactions during the formation of CdSe QDs (Singh et al., 2011a,b). Zn(SO4)·7H2O, HgCl2, Hg2Cl2, NiSO4·6H2O, MnSO4·7H2O, CoSO4·7H2O, CrCl3, K2CrO4, CuCl2, FeCl3, PbCl2 salts were used in the metal ion sensing experiments. Nanopure water obtained from a Millipore water purifying system (resistivity >18 mega ohm cm) was used for preparation of solutions.
2.2 Synthesis of CdSe quantum dots
5 mg/mL of potato starch solution was prepared by taking the required amount of potato starch in 25 mL of water and heated to 60οC for 20 min. The initial turbid solution of starch became clear after heating. This process is termed as gelatinization of starch (Jenkins and Donald, 1998). Reaction mixture with different amount of CdSO4 and Na2SeSO3 were taken in 10 mL flask containing 0.5 mg/mL of starch solution. 2% v/v of acetone and 2-propanol were added to it. The reaction mixture was irradiated in a quartz cell sealed with rubber septum, inside a Rayonet Photoreacter equipped with sixteen 300 nm UV lamps.
The concentrations of CdSO4 and Na2SeSO3 were different in the case of different stoichiometric ratios, (i) 0.5:0.5 mM, (ii) 0.5:1.0 mM, (iii) 1.0:0.5 mM, (iv) 1.5:0.5 mM and (v) 2.0:0.5 mM. QDs extracted from the colloidal solution synthesized with 1.5:0.5 mM of precursors were functionalized with thiourea by taking 20 mg of QDs with 10 mM of thiourea in 10 mL water and refluxing it at 85 °C for 7 h. Metal ion sensing experiments were performed by diluting ten times the stock solution of the as functionalized QDs followed by addition of 10 mM of different metal ions. Interference study was performed by adding 0.1 mM of Cu2+, 1.0 mM of Cr6+ and 2.0 mM of Hg2+ along with equal and tem times higher concentration of interfering metal ions.
2.3 Characterization
UV–visible absorption spectra were recorded using spectrophotometer (Jasco V-650). Steady state PL spectra were recorded at room temperature using a spectrofluorometer (Hitachi F-450). PL lifetime measurements were carried out by time-correlated single photon counting (ICSPC) technique using a time resolved spectrofluorometer (Horiba Jobin Yvon IBH 400). X-Ray diffraction (XRD) measurements were carried out using panalytic (X-Pert pro) instrument. Raman spectral studies were carried out using Raman spectrometer (Seki’s STR300). FTIR measurements were carried out with the sample on KBr pallet using SHIMADZU IR- Affinity-1 FTIR instrument in diamond ATR mode. TEM images were recorded using a Libra 200FE instrument. SEM images were recorded using JEOL JSM-T330 SEM instrument.
3 Results and discussion
3.1 Synthesis of starch capped CdSe QDs
The reaction mixture in one of the cases was prepared by taking 0.5 mM of CdSO4, 0.5 mM of Na2SeSO3, 0.5 mg/mL of starch solution, 2% v/v of acetone and 2% v/v of 2-propanol. After photoirradiation, the colourless solution changed to orange coloured colloidal solution (Fig. 1). Acetone absorbs UV photons and in the excited state it abstracts an H atom from 2-propanol molecule to form two 2-hydroxy-2-propyl radicals, (CH3)2C•OH which are highly reducing in nature (E0 = −1.5 V vs NHE) (Wardman, 1989). These radicals reduce Cd2+ and release Se− which eventually react together to form CdSe QDs. The complete reaction mechanism is discussed below:

(a) Unirradiated reaction mixture containing Cd:Se precursors as 0.5:0.5 mM along with 0.5 mg/ml starch in the presence of 2% (v/v) acetone and 2-propanol, (b) orange-yellow coloured colloidal solution obtained after photoirradiation with 300 nm UV lamp and (c) camera-ready picture of the same colloidal solution under UV light.
The growth of CdSe QDs was monitored by recording the absorption spectra at different time of irradiation. Fig. 2 shows the absorption spectra of a solution containing 1.5 mM of CdSO4 and 0.5 mM of Na2SeSO3at different time of photoirradiation (For other Cd:Se ratios see the supporting information Figs. S1–S4). After around 10 min of photoirradiation, the absorbance did not increase further indicating the synthesis of CdSe QDs has been completed. In the absence of acetone, the colour of the reaction mixture does not change, which indicates the reaction is initiated by the excited state of acetone only. The excitonic peak position as well as particle size (calculated from Brus equation) were plotted at different time of photoirradiation (insets of Fig. 2). The PL spectra of as synthesized QDs (with different stoichiometric ratio of CdSO4 and Na2SeSO3) were recorded (Fig. 3a).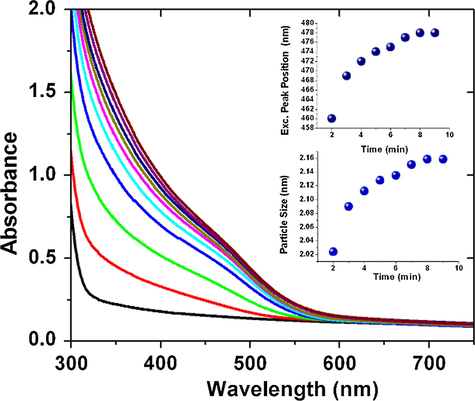
Absorption spectra of reaction mixture containing Cd and Se precursor with starch at different time of photoirradiation (inset1 excitonic peak position and inset 2 particle size vs. time).
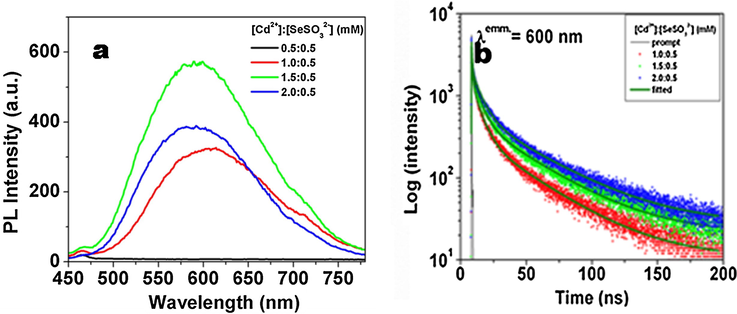
(a) steady-state PL spectra and (b) time-resolved PL decay profiles of CdSe QDs synthesized with different Cd:Se precursor ratios.
It was found that the emission intensity increases with increase in Cd:Se ratio and was maximum for 1.5:0.5 mM. The QDs synthesized with Cd:Se ratio 0.5:0.5 mM and 0.5:1.0 mM were found to be non fluorescent in nature. The PL lifetimes of the CdSe QDs with Cd:Se ratio 1.0:0.5, 1.5:0.5 and 2.0:0.5 mM (Fig. 3b) have two components (τ1 and τ2), which is expected to be originated from the band gap (τ1) and trap states (τ2) (Javier et al., 2003). The band gap (τ1) e-h pair recombine rapidly and have short life time (<10 ns), while the trap state (τ2) e-h pair recombine slowly having lifetime larger than 30 ns. It was observed that the average lifetime value of the CdSe QDs increases with the increase in the Cd content in the reaction mixture (see Table 1). The QDs containing higher Cd to Se ratio get better capped by starch molecules and hence have better PL and therefore the QDs synthesized with lower Cd:Se ratio 0.5:0.5 and 0.5:1.0 mM have very poor PL.
[Cd2+]:[Se2−] (mM)
Lifetime (ns)
τ1
A1
τ2
A2
〈τ〉
1.0:0.5
6.16
0.38
38.3
0.62
26.1
1.5:0.5
5.93
0.31
39.6
0.69
29.2
2.0:0.5
6.95
0.30
43.4
0.70
32.5
The QDs were extracted by following a novel colloidal processing method developed in this study. The room temperature colloidal solution of QDs was first freezed at 0 °C and then was allowed to de-freeze to room temperature. The amylose and amylopectin residues present in starch got sedimented after freezing (Fig. 4a) (Spoehr et al., 1936). The colloidal solution of CdSe QDs was also found to get sedimented along with the amylose and amylopectin residues of starch in the bottom of the beaker (Fig. 4b). The sedimented starch along with QDs could be easily extracted out in the powder form (Fig. 4c–e).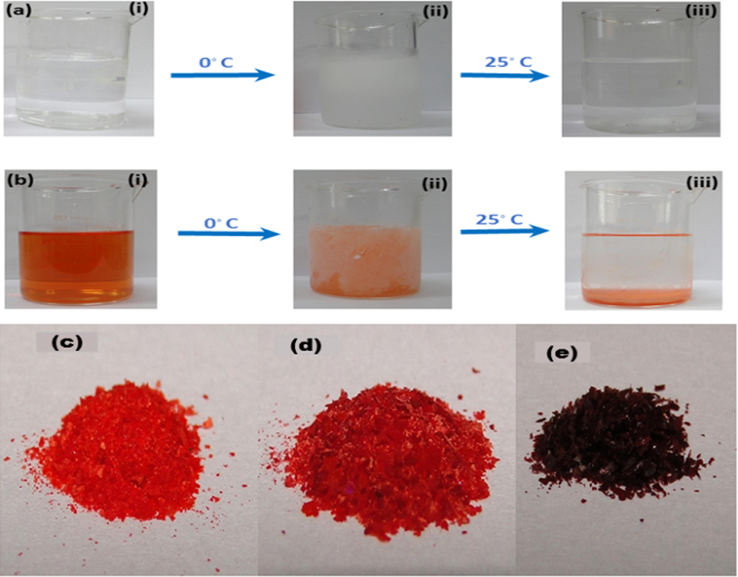
(a) Colloidal solution of starch; at room temperature (i), after freezing (ii), and again after attaining room temperature (iii), (b) colloidal solution of CdSe QDs capped with starch (i), after freezing (ii) and again after attaining room temperature (iii), Solid CdSe QDs extracted from the reaction mixture synthesizing with (c) 1.5:0.5 mM, (d) 1.0:0.5 mM and (e) 0.5:0.5 mM of Cd:Se precursors.
3.2 Characterization of CdSe QDs
The XRD pattern of CdSe QDs (Fig. 5a) confirms cubic zinc blende crystal of CdSe (Murray et al., 1993). The crystallite size was calculated by the Scherrer formula and was found to be around 2.0 nm. Raman spectra of the CdSe QDs (Fig. 5b) shows two prominent peaks at 204 and 409 cm−1 which corresponds to 1st and 2nd order longitudinal modes of CdSe nanocrystals (Dzhagan et al., 2008).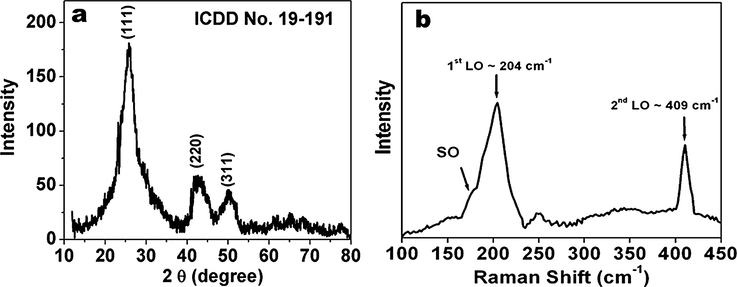
(a) XRD pattern and (b) Raman spectra of CdSe QDs.
The TEM image of the particle is shown in Fig. 6a. The image showed that the particles are well dispersed and spherical in size having maximum size around 3 nm. The FFT pattern (inset Fig. 6a) shows the wave vector corresponding to (1 1 1) and (2 0 0) planes with 540 of angle between them. The HRTEM image clearly showed the (1 1 1) and (2 0 0) planes with inter planar distance of 3.5 Å and 2.9 Å respectively, which matches very well with the cubic zinc blende crystal structure of CdSe. The SEM image shows spherical shapes of relatively bigger size, which indicates that these QDs exist in agglomerated form (Fig. 6b).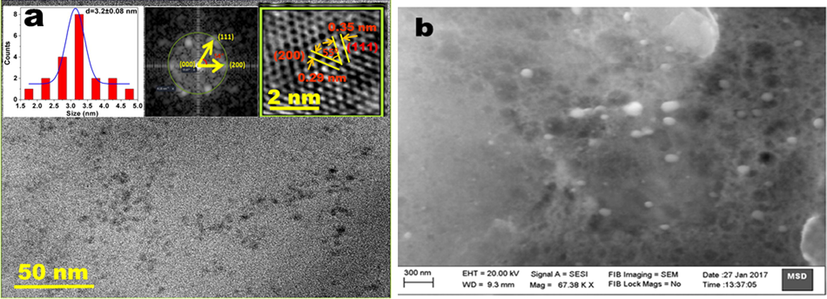
(a) TEM image of CdSe QDs inset 1; particle size distribution, inset 2 FFT pattern and inset 3 HRTEM image showing inter planar distances corresponding to (1 1 1) and (2 0 0) planes, (b). SEM image showing aggregated morphology of CdSe QDs.
In the FTIR spectra (Fig. 7) the OH stretching peak present in starch (3650–3000 cm−1) is very broad shows the presence of several OH groups, inter and intra molecular hydrogen bonding in starch. The other peaks are at 2900 cm−1 (asymmetric C—H stretching), 1640 cm−1 (O—H bending of water in starch), 1340 cm−1 (angular deformation of C—H bond), 1150 cm−1 (C—O and C—C stretching), 1075 cm−1 (C—O—H bending), 998 cm−1 (skeletal vibration of α 1–4 glyosidic linkage (C—O—C), 926 cm−1 (C—C stretching) and 850 cm−1 (C2 deformation) (Kizil et al., 2002). The starch capped CdSe samples give distinct peaks which indicate the existence of starch on CdSe surface. The extracted QDs were modified by surface functionalized with thiourea to increase its PL quantum yield and stability. In the FTIR spectra of thiourea functionalized CdSe QDs, the characteristics peaks of thiourea at 3365 cm−1, 3275 cm−1, 3150 cm−1 (symmetric and asymmetric stretching of NH, NH2), 1600 cm−1 (NH2 bending), 1400 cm−1 (CN stretching) 1075 cm−1 (NH2 rocking), 734 cm−1 (CS asymmetric stretching) and 620 cm−1 (CS symmetric stretching) (Stewart, 1957) vanishes in thiourea functionalized CdSe QDs, which confirmed the binding of thiourea on the surface of the QDs. Functionalization with thiourea reduces the spectral broadness of the PL spectrum as well as increases the PL quantum yield (see the supporting information Fig. S5). It is worth to mention here that before functionalization with thiourea the PL spectrum is broad having emission mainly from trap states, however, after functionalization the PL spectrum becomes narrow with very low stokes shift and therefore it could be mainly arising from the band gap recombination of the charge carriers. Further to confirm the functionalization, we recorded the DLS (dynamic light scattering) measurements of starch capped CdSe QDs both before and after functionalization with thiourea. We found that the hydrodynamic diameter of QDs increases after functionalization from 193 nm to 267 nm (see the supporting information Fig. S6). Such an increase in the hydrodynamic diameter clearly indicates the functionalization of QDs (Wenger et al., 2017).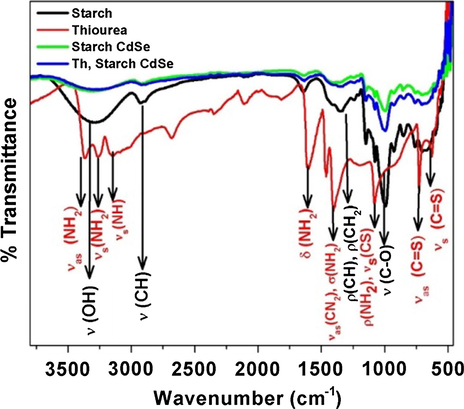
FTIR spectra of starch, thiourea, starch CdSe and starch CdSe with thiourea.
3.3 Effect of temperature and pH on the PL properties of CdSe QDs
The effect of temperature on the PL properties of thiourea functionalized CdSe QDs was studied by recording the temperature dependent PL spectra from 5 °C to 80 °C (Fig. 8a). From these spectra it is clearly evident that QDs have maximum PL at 20 °C. Further on increasing or decreasing temperature the PL yield decreases. Also the PL peak position shifts from 592 nm at room temperature to 600 nm at 80 °C. This red shift can be attributed to minute change in particle size after ligand detachment from the surface of QDs at higher temperature (Valerini et al., 2005). On increasing the temperature, the surface capped molecules get detached from the surface of the QDs and create surface defects which enhance the non-radiative recombination of charge carriers and hence there is a reduction in PL Intensity (Zhao et al., 2012) as well as an increase in broadness (Mohamed et al., 2014). It is a well-known fact that the loss of capping ligands from the surface of QDs creates surface defects.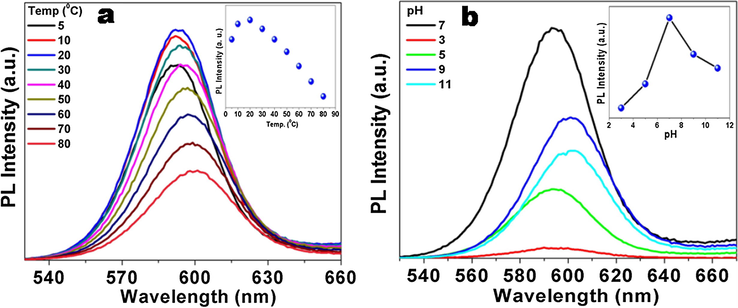
Temperature dependent (a) and pH dependent (b) PL spectra of thiourea functionalized CdSe QDs.
The PL spectra of thiourea functionalized CdSe QDs were also recorded after changing the pH of the colloidal solution from 3 to 11 (Fig. 8b). It was found that the PL yield decreased on either decreasing or increasing from the neutral pH. At lower pH, the thiourea and starch gets protonated and the QDs become destabilised, hence the PL intensity decreases. At higher pH, the formation of hydrated product on the surface of QDs might cause a reduction in the PL intensity. Furthermore, the metal ions also get precipitated as their corresponding hydroxides at higher pH causing a reduction in the PL intensity/yield (Gore et al., 2011).
3.4 Metal ion sensing studies
3.4.1 Selectivity
Thiourea functionalized CdSe QDs were used in the sensing of various metal ions. 10.0 μM concentration of Hg+, Hg2+, Co2+, Cr3+, Cr6+, Zn2+, Pb2+, Cd2+, Ni2+, Mn2+, Fe3+ or Cu2+ were added to the aqueous QDs solution. Out of these metal ions only Cu2+, Cr6+ and Hg2+were able to selectively quench the PL intensity of QDs. (Fig. 9). Others metal ions were not able to quench the PL intensity appreciably.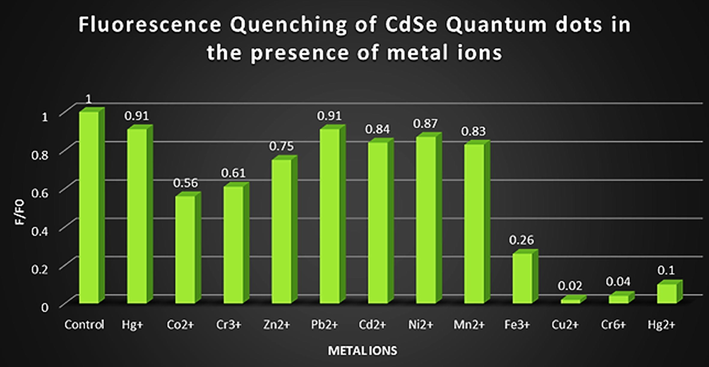
The PL quenching effect of different metal ions on the PL of CdSe QDs.
3.4.2 PL quenching by Cu2+, Cr6+ or Hg2+ ions
The effect of Cu2+, Cr6+ or Hg2+ ions on the PL quenching of CdSe QDs among various metal ions is established from their PL investigations (see Fig. 10). So in order to understand the PL quenching behaviour of CdSe QDs in the presence of these ions different concentration of Cu2+, Cr6+or Hg2+ions were added to the aqueous colloidal solution of CdSe QDs. It was found that the quenching of PL linearly depends upon the concentration of these metal ions (Fig. 10a–c). Therefore, this system can be used as systematic and selective method in sensing of Cu2+, Cr6+ and Hg2+ ions as well as the 3+ and 6+ oxidation states of Cr and 1+ and 2+ oxidation state of Hg can be differentiated. The PL quenching data were analysed using Stern-Volmer equation (Lakowicz, 2006) which can be given as follows.
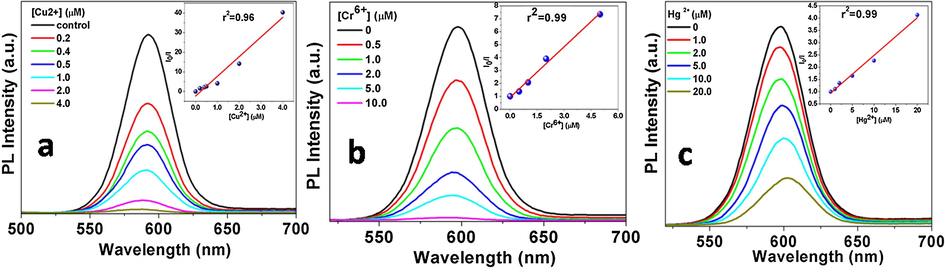
PL quenching of CdSe QDs in presence of different metal ions (a) Cu2+, (b) Cr6+ and (c) Hg2+, inset- Stern Volmer plot.
Metal ion
LOD (μM)
kq (M−1 s−1)
Cu2+
27.6
4.66 × 1014
Cr6+
196.0
8.53 × 1013
Hg2+
279.2
1.32 × 1014
Further, to confirm it, we recorded the time-resolved PL decay profiles and determined the lifetime values of the QDs in the absence as well as in the presence of these metal ions (see the supporting information Fig. S7a–c). It was found that with all these metal ions the PL lifetime decreases with increasing the concentration of metal ions which reaffirm the dynamic nature of the PL quenching of the QDs in the presence of these metal ions.
The limit of detection (LOD) is evaluated by using the formula 3σ/k, (listed in Table 2) where, σ is the standard deviation of the blank signal and k is the slope of the linear calibration plot (validation of analytical procedures: text and methodology Q2(R1), 2015). It is to be mentioned here that LOD for Cu2+ ions is lower than that for Cr6+ and Hg2+ ions.
3.4.3 PL quenching mechanism
Several quenching mechanisms have been proposed by various researchers to explain how metal ions quenches the PL of functionalized QDs. Non radiative recombination pathway, inner filter effect, electron transfer process, aggregation induced quenching, metal ion replacement, and ion binding interaction are the possible quenching mechanism to explain the PL quenching phenomena (Du et al., 2017). As we have discussed in the previous section, from the lifetime analysis it is clear that there could be a dynamic PL quenching of the QDs because the PL lifetime values decrease on increasing the metal ion concentration (Lakowicz, 2006). Further to confirm it, we have recorded the absorption spectra of thiourea functionalized CdSe QDs both in absence and the presence of Cu2+, Cr6+ and Hg2+ (see the supporting information Fig. S8). The presence of Cu2+ and Cr6+ has no effect on the absorption spectra the QDs but in the presence of Hg2+, the excitonic peak position has been slightly red shifted. This slight red shift in the absorption spectra may be due to nominal increase in the size of QDs in the presence of Hg2+ which causes PL quenching (Du et al., 2017). The quenching rate constant, kq (Table 2) for all the three metal ions are almost 103–104 times higher than the diffusion-controlled quenching rate constant. This shows that the quenching could also be static in nature even though there is no significant change in the absorption spectra of the QDs in the presence of these metal ions (Ding et al., 2015). So from the decrease in the PL lifetime values of QDs with increase in metal ion concentration and the high quenching rate constant obtained in this case, we suggest that the PL quenching could be assigned as both static as well as dynamic in nature. In previous literature, it is reported that the metal ions can interact with QDs through the capping layer and can quench their PL (Wu et al., 2008; Yu et al., 2012). Thiourea has two binding sights through which it can bind with the QDs through the (i) S atom and (ii) two N atoms. It is already confirmed by the FTIR spectra that both N and S atoms of thiourea are involved in the functionalization of CdSe QDs, but the S atom will be predominantly bound to Cd atom of the QDs (according to HSAB principle: soft acid (Cd) and soft base (S)), leaving the N uncoordinated.
Scheme 1 depicts the possible bindings of thiourea with QDs and metal ions. Being a borderline acid Cu2+ can bind with both S and N atom while Hg2+ and Cr6+ can only bind with S and N atom, respectively. Thus, Cu2+ has a strong affinity towards thiourea and hence a lower limit of detection and higher quenching rate constant as compare to Cr6+ and Hg2+. It is seen that Cu2+, Cr6+ and Hg2+ ions predominantly form complexes with thiourea on the CdSe QDs surface, which results in the imperfection of their surface and facilitate the non-radiative e/h recombination. The higher quenching rate constant also favours the association of the metal ions directly to the surface of QDs and photo-induced e transfer from the conduction band of QD to the metal ions.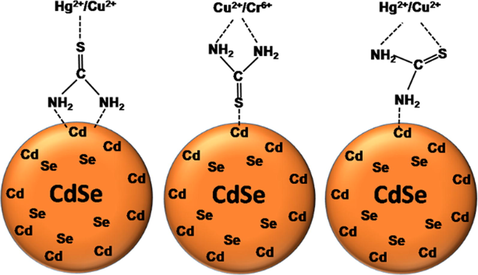
Possible bindings metal ions with thiourea attached to the surface of CdSe QDs.
3.4.4 Interference effect of Pb2+, Cd2+and Fe3+ ions
In order to adopt the QDs as a probe for these metal ions it is very essential to study the interference effect of certain other ions. This interference study is focused on the cations which are naturally found along with these metal ions or have adverse effect on human being or environment. Pb2+ and Cd2+are one of the most hazardous heavy metals along with Hg2+. Fe3+ is the constituent metal ion which is found along with copper (chalcopyrite; CuFeS2) and chromium (chromite; FeCrO4). So interference of Pb2+, Cd2+ and Fe3+were studied by taking the equal or ten times higher concentration of these interfering ions. From Fig. 11 it is clear that the Pb2+ is showing very little interference with Cu2+ and Hg2+ while it is showing adequate interference with Cr6+. On the other hand, Cd2+ is showing very little interference with Cu2+ and Cr6+ but showing good interference with Hg2+. Fe3+ is showing good interference with Cu2+ and Hg2+.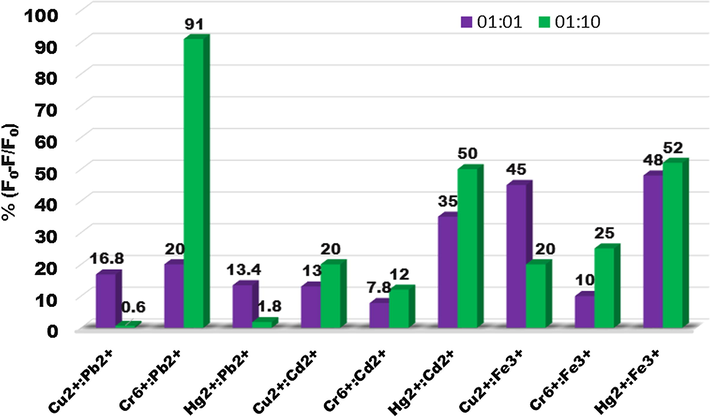
Histograms showing the change in PL of CdSe QDs in the presence of Pb2+, Cd2+ or Fe3+ with Cu2+, Cr6+ and Hg2+(equal concentration and ten times higher concentration of interfering metal ions).
4 Conclusion
In summary, starch capped monodispersed CdSe QDs were successfully synthesized in aqueous solution via photochemical route using 300 nm UV irradiation in solution containing cadmium sulphate, sodium selenosulfate, acetone and 2-propanol in the presence starch. This is a rapid, one-pot, facile alternative method to synthesize monodispersed CdSe QDs. Their colour, PL intensity and PL lifetime strongly depend upon the Cd to Se precursor ratio used during the synthesis. A new method has been demonstrated for the extraction of these QDs from the colloidal solution. The extracted QDs after further surface functionalization with thiourea were found to exhibit maximum PL intensity at room temperature and the neutral pH. It was also demonstrated that the PL intensity of CdSe QDs can be quenched by Cu2+, Cr6+ and Hg2+ ions with high sensitivity and selectivity. The mechanism of PL quenching in the presence of Cu2+, Cr6+ and Hg2+ could be attributed to the (i) non-radiative charge carrier recombination on the thiourea capping layer as well as (ii) photo induced electron transfer from QDs to metal ions. The different oxidation states of Cr (III and IV) and Hg (I and II) can also be differentiated by the PL quenching behaviour of these QDs. The thiourea functionalised starch capped CdSe QDs have a potential application in sensing heavy metal ions present in aqueous solutions.
Acknowledgements
Mr. Avinash Singh acknowledges BARC, DAE for providing him a research fellowship. The authors would like to acknowledge Dr. S. N. Achary, ChD, BARC for his help in XRD and Mr. Amit Kanjilal, RPCD for his help FTIR measurements. The authors gratefully acknowledge Dr. P. D. Naik, Head, RPCD and Associate Director, Chemistry Group, BARC for his support and encouragement during this study.
References
- Toxic effects of chromium and its compounds. Biol. Trace Elem. Res.. 1992;32:145-153.
- [Google Scholar]
- Quantum dots and their multimodal applications: a review. Materials. 2010;3:2266-2345.
- [Google Scholar]
- Mercury toxicity and treatment: a review of the literature. J. Environ. Public Health. 2012;2012:1-10.
- [Google Scholar]
- Bioinspired, direct synthesis of aqueous CdSe quantum dots for high-sensitive Copper(II) ion detection. Dalton Trans.. 2013;42:15411-15420.
- [Google Scholar]
- Mercaptosuccinic acid modified CdTe quantum dots as a selective PL sensor for Ag+ determination in aqueous solutions. 2014. RSC Adv.. 2014;4:59157-59163.
- [Google Scholar]
- Mechanisms of chromium carcinogenicity and toxicity: critical reviews. Toxicol.. 1993;23:255-281.
- [Google Scholar]
- A highly selective and simple fluorescent sensor for mercury (II) ion detection based on cysteamine-capped CdTe quantum dots synthesized by the reflux method. Lumin.. 2015;30:465-471.
- [Google Scholar]
- Aqueous synthesis of functionalized copper sulphide quantum dots as near-infrared luminescent probes for detection of Hg2+, Ag+ and Au3+. Nat. Sci. Rep.. 2017;7:11451-11462.
- [Google Scholar]
- Size effects on Raman spectra of small CdSe nanoparticles in polymer films. Nanotechnology. 2008;19:305707-305712.
- [Google Scholar]
- Surface-modified CdSe quantum dots for the sensitive and selective determination of Cu(II) in aqueous solutions by luminescent measurements. Anal. Chim. Acta. 2005;549:20-25.
- [Google Scholar]
- Copper toxicity, oxidative stress and antioxidant nutrients. Toxicol.. 2003;189:147-163.
- [Google Scholar]
- A novel method for ranitidine hydrochloride determination in aqueous solution based on PL quenching of functionalised CdS QDs through photoinduced charge transfer process: Spectroscopic approach. Analyst. 2011;136:2606-2612.
- [Google Scholar]
- Nanosecond exciton recombination dynamics in colloidal CdSe quantum dots under ambient conditions. Appl. Phys. Lett.. 2003;83:1423-1425.
- [Google Scholar]
- Gelatinisation of Starch: a combined SAXS/WAXS/DSC and SANS study. Carbohydr. Res.. 1998;308:133-147.
- [Google Scholar]
- Magnetically engineered semiconductor quantum dots as multimodal imaging probes. Adv. Mater.. 2014;26:6367-6386.
- [Google Scholar]
- Ambient synthesis and characterization of high-quality CdSe quantum dots by an aqueous route. Langmuir. 2009;25:12729-21273.
- [Google Scholar]
- Characterization of irradiated starches by using FT-Raman and FTIR spectroscopy. J. Agric. Food Chem.. 2002;50:3912-3918.
- [Google Scholar]
- L-cysteine capped ZnS quantum dots based PL sensor for Cu2+ ion. Sens. Act. B Chem.. 2009;139:104-109.
- [Google Scholar]
- Principles of PL Spectroscopy (3rd ed.). Springer; 2006.
- Aqueous synthesis of highly monodispersed thiol-capped CdSe quantum dots based on the electrochemical method. Mater. Sci. Semicond. Process.. 2013;16:149-153.
- [Google Scholar]
- Metal ions optical sensing by semiconductor quantum dots. J. Mater. Chem. C. 2014;2:595-613.
- [Google Scholar]
- Functionalizing nanoparticles with biological molecules: developing chemistries that facilitate nanotechnology. Chem. Rev.. 2013;113:1904-2074.
- [Google Scholar]
- Time resolved and temperature dependence of the radiative properties of thiol-capped CdS nanoparticles films. J Nanopart Res. 2014;16:2242-2258.
- [Google Scholar]
- Synthesis and characterization of nearly monodisperse CdE (E = sulfur, selenium, tellurium) semiconductor nanocrystallites. J. Am. Chem. Soc.. 1993;115:8706-8715.
- [Google Scholar]
- One-step and rapid synthesis of high quality alloyed quantum dots (CdSe–CdS) in aqueous phase by microwave irradiation with controllable temperature. Mater. Res. Bull.. 2005;40:1726-1736.
- [Google Scholar]
- Light as a construction tool of metal nanoparticles: synthesis and mechanism. J. Photochem. Photobiol. C: Photochem. Rev.. 2009;10:33-56.
- [Google Scholar]
- Functionalizing nanoparticles with biological molecules: developing chemistries that facilitate nanotechnology. Chem. Rev.. 2013;113:1904-2074.
- [Google Scholar]
- Luminescent sensors based on quantum dot–molecule conjugates. Chem. Soc. Rev.. 2015;44:4275-4289.
- [Google Scholar]
- Saccharide capped CdSe quantum dots grown via electron beam irradiation. Mater. Chem. Phys.. 2017;199:609-615.
- [Google Scholar]
- Investigation of dynamics of radiolytic formation of CdSe nanoparticles in aqueous solutions. J. Phys. Chem. A. 2011;115:13251-13258.
- [Google Scholar]
- CdSe nanoparticles grown via radiolytic methods in aqueous solutions. Radiat. Phys. Chem.. 2011;80:736-741.
- [Google Scholar]
- The starch isolated from plant material by the freezing method. J. Biol. Chem.. 1936;116:493-502.
- [Google Scholar]
- Infrared absorption spectra of urea, thiourea, and some thiourea-alkali halide complexes. J. Chem.Phys.. 1957;26:248-254.
- [Google Scholar]
- The cytotoxicity of cadmium based, aqueous phase synthesized, quantum dots and its modulation by surface coating. Biomaterials. 2009;30:19-25.
- [Google Scholar]
- Temperature dependence of the photoluminescence properties of colloidal CdSe/ZnS core/shell quantum dots embedded in a polystyrene matrix. Phys. Rev. B. 2005;71:235409-235414.
- [Google Scholar]
- Design principles of fluorescent molecular sensors for cation recognition. Coord. Chem. Rev.. 2000;205:3-40.
- [Google Scholar]
- WHO, 2018. <http://www.who.int/mediacentre/factsheets/fs391/en/>.
- Reduction potentials of one-electron couples involving free radicals in aqueous solution. J. Phys. Chem. Ref. Data. 1989;18:1637-1755.
- [Google Scholar]
- Functionalization of cadmium selenide quantum dots with poly(ethyleneglycol): Ligand exchange, surface coverage, and dispersion stability. Langmuir. 2017;33:8239-8245.
- [Google Scholar]
- A novel method for the determination of Pb2+ based on the quenching of the fluorescence of CdTe quantum dots. Microchim. Acta. 2008;161:81-86.
- [Google Scholar]
- A simple chemical etching strategy to generate ‘‘ion-imprinted’’ sites on the surface of quantum dots for selective PL turn-on detecting of metal ions. Chem. Comm.. 2010;46:7046-7048.
- [Google Scholar]
- One-Pot aqueous synthesis of PbS quantum dots and their Hg2+sensitive properties. J. Nanosci. Nanotech.. 2012;12:2783-2790.
- [Google Scholar]
- Fluorescent nanoprobes for sensing and imaging of metal ions: recent advances and future perspectives. Nano Today. 2016;11:309-329.
- [Google Scholar]
- High-temperature luminescence quenching of colloidal quantum dots. ACS Nano. 2012;6:9058-9067.
- [Google Scholar]
- Quantum confinement effects in CdSe quantum dots. J. Phys. Chem.. 1995;99:7649-7653.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2018.09.006.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







