Translate this page into:
Water vapor molecule adsorption onto 'Ajwa' dates: Analytical investigation via infinite multilayer statistical physics model
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
A gravimetric technique was used to examine the water activity onto the ‘Ajwa’ dates. The equilibrium adsorption isotherms of water molecules were carried out at three temperatures (between 303 K and 323 K). A theoretical method was developed using statistical physics treatment to describe the experimental data at the ionic scale. The date’s isotherms were analyzed via the infinite multilayer adsorption model (formation of a high number of adsorbed layers) which is established based on the ideal gas law (there are no lateral interactions influences on the adsorption mechanism). The chosen model gave significant interpretation of the adsorption of water on the Ajwa dates based on the physicochemical model’s parameters (the density of binding sites (Dm), the number of water molecules per site (n) and the energetic parameters (a1) and (a2)). The physicochemical interpretation of the appropriate model indicated that the adsorption of water on the Ajwa dates occurred via a multi-anchorage process since the n values are lower than 1 for the three tested temperatures. The Ajwa dates adsorption was found typical to an exothermic process by the intermediate of the steric parameter Dm (Dm (303 K) = 0.58 kg/kg˃ Dm (323 K) = 0.33 kg/kg). Moreover, the energies values |−ε1| and |−ε2|, which varied from 27.8 KJ/mol to 51.2 KJ/mol, confirmed that the ‘Ajwa’ dates adsorption was a chemical process presenting covalent bonds between the water molecules and the dates’ sites.
Keywords
Static gravimetric setup
‘Ajwa’ dates adsorption, water activity
Advanced modeling
Energetic analysis
1 Introduction
Dates are considered as basic nutrition to the human body in many countries around the world, especially in the Gulf region, including the Kingdom of Saudi Arabia (Bakri et al., 2020; Zhang et al., 2015). Generally, the dates have important health benefits, especially the Ajwa dates, known as Ajwa Almadinah (Adeline et al., 2020). The Kingdom of Saudi Arabia has topped the first place in the production of Ajwa, and is accounted for 17% of the global production of dates, with more than 75 varieties; ranking second around the world. Al-Ajwa represents a large percentage of the number of date palms in Al-Madinah Al-Munawwarah region and its governorates. In this region, the number of palm trees is approximately 4 million, ranking third in the Kingdom. There are 14 dates factories in Al-Madina, which constitutes 9% of the total production in the Kingdom (Adeline et al., 2020; Myhara et al., 1998; Farahnaky et al., 2016).
The Ajwa date contains high amount of nutrients compared to other types of dates (Seerangurayar et al., 2017). In fact, it is rich in carbohydrates, proteins, minerals, fibers, vitamins, and fats. It also contains a large group of phytochemicals, such as flavonoids, glycosides, phytosterols, and polyphenols (Farahnaky et al., 2016; Seerangurayar et al., 2017). The medical laboratory studies have proven what was mentioned in the prophetic guidance that it is considered as an excellent natural laxative which prevents constipation. It strengthens muscles, treats anemia, strengthens hearing and eyesight, and calms nerves (Fasina et al., 1999). Ajwa dates contain a high amount of dietary fiber and minerals necessary for a healthy body, such as potassium and magnesium.
The estimation of the potential period time and the safety characterization of the Ajwa dates production is fundamental in controlling the physical and chemical processes which occur during food storage (Borchani et al., 2012; Sahari et al., 2008; Koc et al., 2011). The accurate determination of the potential period time and safety characterization presents a relevant role in the field of food industry and the forecast of both physical and chemical procedures occurring during the drying process. This can be performed by controlling the humid air that influences the food product behavior (Koc et al., 2011). In this paper, the control of the humid air of the ‘Ajwa’ dates product is carried out through the sorption isotherms data which describes the relationship between the total moisture content (Qa) and the water activity (aw) at various sorption temperatures (Alyousef et al., 2020). The parameter (aw) which describes the partial pressure of water vapor (P) per water vapor saturation (Pvs) has been used by many researchers to control the quality of food production by extracting some physical concepts that contribute to the product preservation (Sahari et al., 2008; Koc et al., 2011; Alyousef et al., 2020). Indeed, many authors encourage the description of water activity data in the domain of food engineering science in order to explain the water vapor behavior on foods (Pathare et al., 2013). Thus, in accordance with the literature, many analytical models were adopted by many authors for the modeling of water activity isotherms to discuss the water adsorption in the field of food science (Koc et al., 2011; Alyousef et al., 2020; Pathare et al., 2013).
Although many experimental techniques were adopted to obtain the water sorption isotherms (Gal et al., 1981; Wolf et al., 1985). For example, the manometric technique was successfully used to measure the water vapor pressure in the space vapor surrounding the food at constant temperature (Gal et al., 1981). The measurement of the equilibrium relative humidity of air in contact with a food substance at determined moisture content was also performed by the hygrometric technique (Gal et al., 1981). However, the gravimetric technique was found to be the best tool to perform the experimental data of water sorption on foods including the control of weight changes (Aouaini et al., 2014; Bahloul et al., 2008). This method is applied in the current work to measure the experimental sorption data of the Ajwa dates which were derived from the ‘phoenixdactylifera L’ date fruit (Koc et al., 2011).
Recently, it was found that the water sorption data could be investigated through physical models determined via the innovative statistical physics theory (Aouaini et al., 2014; Knani et al., 2020; Ben et al., 2021) which gave an advanced description of the water activity on foods at the molecular level. The equations of the advanced models provided interesting microscopic results of the sorption curves. For example, the number of water molecules per date sites and the density of dates sites will be evaluated. The interaction type between the water molecules and the Ajwa dates can be also described.
2 Experiments
The morphology of the adsorbent presented in Fig. 1 is investigated by a scanning electron microscopy (SEM). This image shows the surface of the dates’ pores which adsorbed the water molecules.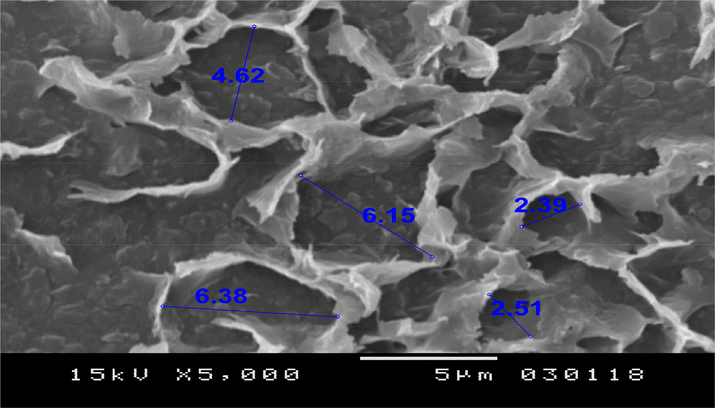
Scanning electronic microscopy (SEM) analyzes of the ‘Ajwa’ dates used as adsorbent in the experiments.
Fig. 2 showed the adopted static-gravimetric technique (Aouaini et al., 2014) for the determination of water adsorption isotherms on the Ajwa dates at 303, 313, and 323 K.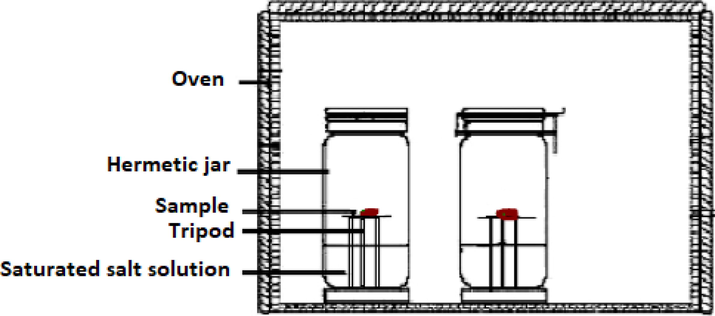
Experimental setup of the static gravimetric technique devoted for the determination of experimental adsorption isotherms of water molecules on the ‘Ajwa’ dates.
First of all, 11 saturated solution of salts (LiCl, NaCl, KCl, CaCl2, BaCl2 MgCl2, Mg (NO3)2, NaNO3, NaBr, KI, and K2SO4) were prepared to maintain a constant vapor pressure. The salts solutions were poured into eleven glass jars which were filled to one quarter depth, resulting in water activity values between 0.116 and 0.97 during the experimental measurements.
Secondly, the prepared salt solutions were placed in an oven and took at least 24 h in order to reach the working temperature. Then, the Ajwa date sample was put in small crucibles on the tripod placed in each jar.
Thirdly, the Ajwa dates samples were introduced in the hermetically sealed bottles at the equilibrium temperatures (303–323 K). Note that Mettler AT 400 balance (Bahloul et al., 2008; Boudhrioua et al., 2008) was used to measure the dates weights every three days and that the equilibrium weight was considered after three nearly equal measurements (Boudhrioua et al., 2008). The dried weight of the dates sample was also noted for every measurement.
Finally, the moisture content at the equilibrium Qa (kg water vapour / kg dry matter) can be measured via the relationship between the equilibrium measured weight meq (kg) and the dried measured weight md (kg) through the next formula (Aouaini et al., 2014):
The experimental adsorbed quantities of water vapor molecules on the Ajwa dates are plotted in Fig. 3 at 303–323 K.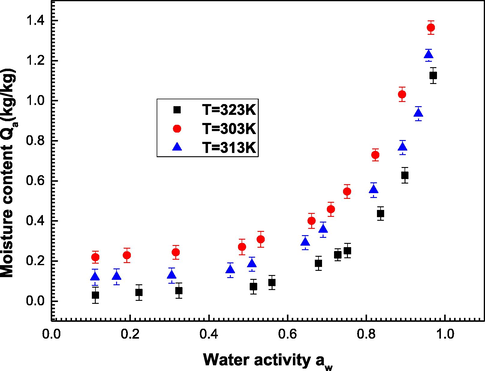
Equilibrium adsorption isotherms of water molecules on the ‘Ajwa’ dates obtained at three adsorption temperatures (303–323 K).
According to the literature, the profile of the water sorption data indicates that a multi-layer process occurred during the dates sorption. The classical models that can describe this sorption process are the PELEG model, the GAB model and the BET model (Brunauer et al., 1940; Dural and Hines, 1993; Peleg, 1993). However, the equations of these models describe the isotherms curves with empirical manner without considering the steric aspect or the energetic aspect of the sorption mechanism. For this reason, the adopted descriptive models of this investigation are established on the basis of the statistical physics assumptions.
Observing the experimental data of the three temperatures, we can observe the following order of adsorption capacity: Qa (303 K) ˃ Qa (313 K) ˃ Qa (323 K). The adsorbed quantities decrease with the temperature expansion demonstrating the exothermic character of the water molecules adsorption. In the following, the theoretical examination of the experimental data behavior is performed by the numerical modeling of the experimental data.
3 Theoretical modeling
3.1 Adsorption models
In order to elaborate an advanced modeling of water sorption data, we first develop the expressions of the multi-layer models based on some assumptions of the statistical physics theory. Next, a numerical adjustment of the measured sorption data with the fitting models is performed to choose the best descriptive model. Lastly, the steric and energetic investigation of the water sorption process on the Ajwa dates are carried out via the analysis of the selected model’s parameters.
According to the adsorption isotherms of dates in Fig. 3, we can notice a multi-layer adsorption phenomenon. In this case, the measured data are interpreted using four physical analytical models which are divided in to two categories. The first category characterizes a finite number of formed adsorbed layers giving rise to two adsorption models: the expression of the first FMMPG model is developed via the perfect gas assumption (Brunauer et al., 1940) and the expression of the second FMMRG model is obtained based on the real gas assumption (Knani et al., 2020). The second category reflects the formation of an infinite number of adsorbed layers involving also two adsorption models: the IMMPG model (perfect gas) and the IMMRG model (real gas).
Note that during the models’ development, we consider certain hypotheses:
At first, the adsorption phenomenon should be described through the grand-canonical ensemble of Gibbs disclosed in (Brunauer et al., 1940; Dural and Hines, 1993). Therefore, the adsorption reaction implicating the free phase (water vapor molecules) and the tested adsorbent (Ajwa dates) are summarized in Eq. (2) (Brunauer et al., 1940; Dural and Hines, 1993):
W: the water molecule (adsorbate).
D: the date (adsorbent).
(W)n-D: the water- date product.
n: the stoechiometric coefficient of the sorption reaction. It describes the number of binding molecules per site. Generally, this parameter identifies the property of the adsorption mechanism (n ≥ 1 multi-molecular phenomenon, n ≤ 0.5: multi-anchorage phenomenon).
The grand-canonical system is characterized by the chemical potential (μ), the sorption energy (−εi) and the temperature (T) enforcing from the exterior towards the examined system. The total expression of the partition-function of the grand-canonical ensemble (zgc) including these variables presents the departure idea for models development (Brunauer et al., 1940; Dural and Hines, 1993).
For the multi-layer process of water sorption, two energies (−ε1) and (−ε2) are considered for this mechanism. Note that the first energy (−ε1) characterizes the first layer formation; and the second energy (−ε2) is in relation with the formation of the supplementary adsorbed layers (Brunauer et al., 1940; Dural and Hines, 1993). This hypothesis is taken into account because the first layer is directly interacted with the surface of adsorbent so it is suggested that it is adsorbed with a particular energy level which should be greater than the additional layers. The grand canonical partition function of each system is then given as:
For the finite multi-layer process:
For the infinite multi-layer process:
The following step of this physical modeling requires the calculation of the average number (N0) of similar dates sites (Dm) (Ben and Bouazra, 1997; Sellaoui et al., 2018):
Moreover, the adsorption quantity expression (Qa) of the analytical models is given by the following formula (Souissi and Ben, 2021; Ben and Yahia, 2020; Prola et al., 2013; Marquardt, 1963):
Note here that the analytical equation of the moisture content (Qa) corresponding to the four multi-layer adsorption models is calculated from the product of the number of water molecules per adsorbing site n and the average occupation number N0 of each model (Souissi and Ben, 2021; Ben and Yahia, 2020; Prola et al., 2013; Marquardt, 1963).
The average number (N0) and the adsorbed amount expression (Qa) can be determined by inserting the chemical potential of perfect gas (μp) in the mathematical development of the FMMPG model and the IMMPG model, using its expression determined according to the partition-function of translation per unit of volume (zTr), the number of adsorbates (N), the partial pressure of the water vapor (P) and the gas volume (V) (Dural and Hines, 1993; Peleg, 1993; Ben and Bouazra, 1997; Sellaoui et al., 2018; Souissi and Ben, 2021):
The translation partition function per unit of volume zTr is written versus the vaporization energy of one adsorbed mole of molecules ΔEv and the vapor pressure at saturation Pvs (Aouaini et al., 2014; Yazidi et al., 2020):
Here, we suppose that there are no interactions between the adsorbed molecules and that only the translation degrees of freedom are taking into account because the others degrees (vibrational, rotational, electronic and nuclear degrees of freedoms) act only at high temperature (Aouaini et al., 2014).
Using Eqs. (8) and (9) at equilibrium, energies can be expressed as:
The water activity aw is the ratio of P and Pvs and, a1 and a2 include the molar sorption energies (−ΔE1) and (−ΔE2):
Then, we obtain the expression of the moisture content corresponding to the FMMPG model and the IMMPG model established through the ideal gas assumption:
FMMPG model:
IMMPG model:
The energetic variables a1 and a2 are given by Eqs. (13) and (14).
The modeling work was also executed using the chemical potential (µr) of real gas to establish the expressions of the FMMRG model and the IMMRG model. In this case, the lateral interactions involving the adsorbates at free state are taken into account by means of the parameters a (the pressure of cohesion) and b (the co-volume) (Ben and Bouazra, 1997; Sellaoui et al., 2018):
Then, the theoretical equations of the moisture content of the FMMRG model and the IMMRG model are:
FMMRG model:
IMMRG model:
In this paper, the four developed multi-layer models are advanced equations which can be used for the modeling of multi-layer isotherms curves. The two models established through the ideal gas law (FMMPG and IMMPG) were developed and published in previous papers (Alyousef et al., 2020; Sellaoui et al., 2018). The two models established by taking account of the lateral interactions between the molecules based on the real gas approach (FMMRG and IMMRG) were developed for the first time in this paper and they were not previously published in our knowledge. Both real gas and ideal gas are assumptions of the statistical physics theory devoted for the development of advanced descriptive models’ equations which are useful for giving new physical investigation of water sorption isotherms on foods. The difference between the two assumptions can be seen in the parameters number in the analytical expression of the model but they include in their expressions parameters that have physical meaning, contrary to the empirical forms (Brunauer et al., 1940; Dural and Hines, 1993; Peleg, 1993), whose parameters usually have no physical presentation.
3.2 Selection of the descriptive model
The four multi-layer models were adjusted with the water sorption isotherms using a numerical fitting program (Sellaoui et al., 2018; Ben and Yahia, 2020). The criterions to adopt a descriptive model are the RMSE (Marquardt, 1963; Hadi et al., 2010; Rêgo et al., 2013; Kapoor and Yang, 1989) error coefficient, the well-known AIC (Sellaoui et al., 2018; Ben and Yahia, 2020) coefficient and the determination coefficient R2 (Prola et al., 2013; Marquardt, 1963).
The Numerical adjustment of the isotherms indicated the best fitting multi-layer model showing the highest values of R2 which should be close to the unit and the lowest values of AIC and RMSE comparing to the other models (Sellaoui et al., 2018). These conditions are necessary to adopt a descriptive model because they indicate that the gap between the measured isotherms and the estimated values of the theoretical model is minimized to a confidence level of 95% (Prola et al., 2013; Marquardt, 1963). Thus, the fitted values of parameters given by the appropriate multi-layer model are presented with scattering (error interval). So, any value of the parameters outside the error interval depreciate the accuracy of the fit. Consequently, the model (by different other parameters) cannot approximate the experimental data with good values of errors coefficients (AIC, RMSE and R2).
Table 1 shows the fitting coefficients values.
Fitting model
Adjustment coefficient
Isotherm temperature: 303 K
Isotherm temperature: 313 K
Isotherm temperature: 323 K
FMMPG model
R2
0.91
0.89
0.91
RMSE
6.1
6.7
6.2
AIC
20.3
21.1
20.9
FMMRG model
R2
0.92
0.92
0.91
RMSE
4.2
4.5
5.1
AIC
18.4
19.2
19.1
IMMPG model
R2
0.98
0.99
0.98
RMSE
1.12
1.4
0.33
AIC
14.5
15.4
16.3
IMMRG model
R2
0.97
0.96
0.96
RMSE
2.14
2.5
2.6
AIC
16.4
17.3
17.2
From Table 1, the experimental isotherms of the Ajwa dates were analyzed by the infinite multi-layer model (ideal gas theory) since the adsorption isotherms present the best adjustment coefficients with the IMMPG model. This shows that an infinite number of water molecules layers are adsorbed onto the Ajwa dates and that the adsorption mechanism is performed without lateral interaction impacts (the lateral interactions at free state are only considered in the real gas models (FMMRG and IMMRG) where the analytical expressions of the models include the parameters a and b).
It should be pointed out that the IMMPG adsorption model (Eq. (16)) contains four physicochemical parameters, Dm and n (steric aspect) and a1 and a2 (energetic aspect), which can be used for the microscopic analysis of the adsorbate-adsorbent system.
In the following part, the adjusted values of these parameters are discussed and analyzed versus temperature to interpret the sorption reaction at the molecular degree.
4 Physicochemical explanation of the adsorption process
In Table 2, the adjustment values of the energetic and steric parameters affecting the adsorption of water on dates at the three temperatures is summarized:
Fitting model
Model parameters
Isotherm temperature: 303 K
Isotherm temperature: 313 K
Isotherm temperature: 323 K
Infinite multi-layer model (perfect gas): IMMPG
n
0.89 (±0.018)
0.71 (±0.019)
0.62 (±0.016)
Dm
0.58 (±0.012)
0.41 (±0.011)
0.33 (±0.012)
a1
0.21 (±0.009)
0.25 (±0.007)
0.24 (±0.008)
a2
0.83 (±0.021)
0.86 (±0.019)
0.82 (±0.02)
4.1 Steric study
The steric aspect of the water sorption process can be understood from the behaviors of the variables n and Dm (Marquardt, 1963; Hadi et al., 2010). The first variable describes the number of water molecule per date site and the second one characterizes the amount of occupied dates sites.
According to Table 2, all n values of the water molecules adsorption are inferior to 1 for the three sorption temperatures which demonstrates that the water molecules adopt a multi-anchorage process during the Ajwa dates adsorption (Rêgo et al., 2013; Kapoor and Yang, 1989; Almogait et al., 2020; Yahia et al., 2019; Yazidi et al., 2020).
According to the IMMPG model, the fitting values of n were found to be between 0.62 and 0.89. The fact that n never exceeds the value of 1, indicates that no aggregation occurs prior to the sorption phenomenon. As a fundamental definition, an n value superior to 1 depicts the number of particles per site, while an n value inferior to 1 depicts the fractions of particles per site (Rêgo et al., 2013; Kapoor and Yang, 1989; Almogait et al., 2020; Yahia et al., 2019; Yazidi et al., 2020). Depending on this hypothesis, n'=1/n depicts the anchorage numbers of one particle on various receptor sites. In fact, the adsorbed water molecule has numerous ways to be bonded on the receptor date site depending on its angle of incidence and its geometry with the date surface but if we simplify this multitude, it can be supposed that the adsorbed water molecule has only two kinds of anchorages.
For instance, the n value is equal to 0.71 at 313 K. This value is specified as the weighted average between 1/2 and 1, presenting an anchorage number n' ranging from 1 to 2. The value of n is given as an average between two ensembles of water molecules presenting one anchorage (n1′ = 1) and two anchorages (n2′ = 2). x is indicated as the percentage of particles with a single anchorage, the percentage of water molecules presenting two anchorages is then (1 − x). Consequently, it can be written as 0.71 = x × 1 + (1 − x) × 0.5, which rules out that 42% of water molecules are adsorbed with a single anchorage and 56% of water molecules are anchored with two dates sites.
In addition, it is noted from Fig. 3 that once the temperature rises, the adsorption amounts decrease. This is proved from Fig. 4 which indicates that the values of the coefficients Dm and n decrease with the rise of the temperature from 303 K to 323 K. It can be concluded that the thermal agitation effect disfavors the adsorption dynamics which was an exothermic process (Elqahtani et al., 2021; Ben et al., 2016; Aouaini et al., 2017).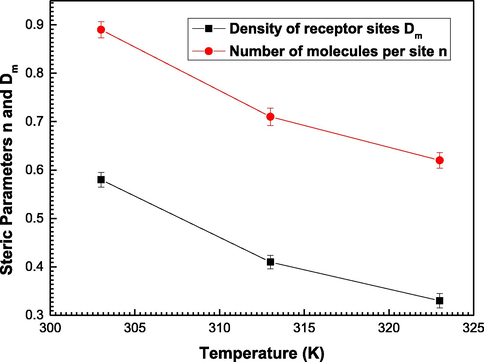
Variations of the adjusted values of the steric parameters number of molecules per site ‘n’ and density of receptor sites ‘Dm’ as a function of temperature.
4.2 Energetic study
The molar adsorption energies (−ΔE1) and (−ΔE2) (kJ.mol−1) can be calculated by means of the next formula including the saturation vapor energy (−ΔEv) (kJ.mol−1) and the energetic coefficients a1 and a2, which are determined from numerical simulation of the IMMPG model with the experimental data (Aouaini et al., 2017):
According to Table 3, it is clearly observed that the determined values of |−ΔE1| which defines the water-date interaction are higher than those of |−ΔE2| (interaction between the formed layers (Bouzid et al., 2018).
Adsorption temperature (K)
303 K
313 K
323 K
|−ΔE1| (kJ/mol)
51.2 (±0.6)
48.6 (±0.7)
42.3 (±0.7)
|−ΔE2| (kJ/mol)
32.1 (±0.7)
30.4 (±0.6)
27.8 (±0.5)
We observe also that |−ΔE2| are below 40 kJ.mol−1 which characterizes physical bindings between the adsorbed layers (Wjihi et al., 2021; Sun and Wang, 2010). This leads to conclude that the interaction between the adsorbed layers of water molecules is weak and can be cut leading to a reversible phenomenon (desorption). Thus, these water molecules layers can be easily removed from the adsorbing surface of dates. Whereas, the adsorption energies |−ΔE1| are above 40 kJ/mol. For this system, chemical process of adsorption is carried out through covalent bonds between the water and the dates (Peleg, 1993). One can conclude that the interaction between the first layer of water molecules and the dates surface is strong because the chemical process is an irreversible phenomenon and the chemical bonds involve the change in the structures of the adsorbate and the adsorbent (Wjihi et al., 2021; Yon et al., 1991). Then, the first layer of water molecule cannot be easily removed from the Ajwa dates surface contrary to the other adsorbed layers.
A final deduction from Fig. 5: It can be observed that all the calculated energies decline with the growth of the temperature from 303 K to 323 K. It can be noticed that the Ajwa dates adopt an exothermic adsorption mechanism of water vapor molecules (Kapoor and Yang, 1989).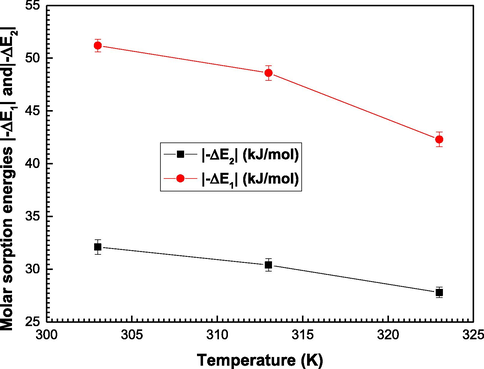
Evolution of the molar sorption energies|-ΔE1| and |-ΔE2| as a function of adsorption temperature.
5 Conclusion
The goal of this paper is to interpret the water-Ajwa date interaction through the gravimetric technique which is used to measure the experimental adsorption isotherms of water activity on dates. Based on statistical physics modeling, it was discovered that an infinite multi-layer model (perfect gas) is utilized for the theoretical presentation of date’s adsorption showing that there is no lateral interactions effect on the system.
Theoretically speaking, the steric study demonstrated the multi-anchorage aspect of the water adsorption on dates since the number of bonded molecules per site n was found between 0.62 and 0.89. Thus, 42% of water molecules were anchored with a single date site and 56% of water molecules were anchored with two dates sites. The number of occupied dates sites was the highest at 303 K, leading to conclude the exothermic criterion of the adsorption mechanism. The values of the energy |-ΔE1| which ranged between 42.3 KJ/mol and 51.2 KJ/mol showed that the interaction water-date can be an ionic or covalent bonds (chemical process) while the adsorption of the others layers in the multilayer region occurred through a physisorption process since the values of the energy |-ΔE2| were found between 27.8 KJ/mol and 32.1 KJ/mol. This indicates that all the adsorbed layers of water molecules can be removed from the dates surface expect the first layer which is chemically bonded to the dates pores surface.
The results of the present investigation can be improved in future papers by some characterization of the surface morphology of the dates surface after the water adsorption like scanning force microscopy (SFM) and scanning electron microscopy (SEM). The Ajwa dates sorption can be also investigated using the density functional theory (DFT) method in which the electron localization function (ELF) and electron densities plots are provided and analyzed in detail. The advanced multilayer model can be also performed to study the sorption energy distribution (AED) and the pore size distribution (PSD).
Acknowledgements
This research was funded by the Deanship of Scientific Research at Princess Nourah bint Abdulrahman University through the Fast-track Research Funding Program to support publication in the top journal (Grant no. 42-FTTJ-51).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Synthesis, characterization, adsorption isotherm, and kinetic study of oil palm trunk-derived activated carbon for tannin removal from aqueous solution. ACS Omega. 2020;5:28673-28683.
- [Google Scholar]
- Advanced statistical physics modeling of a chiral molecular tweezer of silver ion: Microscopic investigation of adsorption of silver(I) on hexahelicene and heptahelicene. AIP Adv.. 2020;10 105229-105229
- [Google Scholar]
- New insights on physico-chemical investigation of water adsorption isotherm into seed of dates using statistical physics treatment: Pore size and energy distributions. J. Mol. Liq.. 2020;298:112041
- [Google Scholar]
- Application of statistical physics on the modeling of water vapor desorption isotherms. Dry. Tech.. 2014;32:1905-1922.
- [Google Scholar]
- Application of statistical physics on the modeling of water vapor desorption isotherms dry. Tech.. 2014;32:1905-1922.
- [Google Scholar]
- Statistical physics studies of multilayer adsorption isotherm in food materials and pore size distribution. Phys. A. 2017;432:373-390.
- [Google Scholar]
- Moisture desorption–adsorption isotherms and isosteric heats of sorption of Tunisian olive leaves (Olea europaea L) Indust. Crops Prod.. 2008;28:162-176.
- [Google Scholar]
- Bakri, H., Mustapha, Abdullateef T., Al-Awaadh, Alhussein M., Ahmed, Khaled A.M., 2020. Physical and moisture sorption thermodynamic properties of Sukkari date (Phoenix dactylifera L.) powder. J. Food, 18, 264–273.
- Application of advanced statistical physics modeling for the physicochemical analysis of adsorption isotherms of manganese (II) on porphyrins H2TPP and H2TTP. Braz. J. Chem. Eng. 2021
- [Google Scholar]
- Application of statistical thermodynamics to the olfaction mechanism. Chem. Senses.. 1997;22:67-75.
- [Google Scholar]
- Modeling of muscone enantiomers olfactory response by an adsorption process onto the mouse muscone receptor MOR215-1. J. Mol. Liq.. 2016;221:896-903.
- [Google Scholar]
- New insights in the physicochemical investigation of the vitamin B 12 nucleus using statistical physics treatment: interpretation of experiments and surface properties. RSC Adv.. 2020;10:21724-21735.
- [Google Scholar]
- Influence of oven drying temperature on physicochemical and functional properties of date fibre concentrates. Food Bioprocess Technol.. 2012;5(5):1541-1551.
- [Google Scholar]
- Sorption isotherms and isosteric heats of sorption of olive leaves (Chemlali variety): experimental and mathematical investigations food. Bioproducts Process. 2008;86:167-175.
- [Google Scholar]
- New insight in adsorption of pyridine on the two modified adsorbents types MN200 and MN500 by means of grand canonical ensemble. J. Mol. Liq.. 2018;263:413-421.
- [Google Scholar]
- On a theory of Van der Waals adsorption of gases. J. Am. Chem. Soc.. 1940;62:1723-1732.
- [Google Scholar]
- A new theoretical isotherm equation for water vapor food systems: Multilayer adsorption on heterogeneous surfaces. J. Food Eng.. 1993;20:75-96.
- [Google Scholar]
- Statistical physics modeling of sorption isotherms of aluminum, iron and indium on tetraphenylporphyrin (H2TPP) and tetrakis(4-tolylphenyl)porphyrin (H2TTPP): Phenomenological investigation of metalloporphyrins at the molecular level. Ads. Sci. Tech.. 2021;2021:1-14.
- [Google Scholar]
- Physicochemical and sorption isotherm properties of date syrup powder: Anti-plasticizing effect of maltodextrin. Food Bioprod. Process.. 2016;98:133-141.
- [Google Scholar]
- Thermodynamics of moisture sorption in winged bean seed and gari. J. Food Process Eng.. 1999;22(6):405-418.
- [Google Scholar]
- Techniques for obtaining complete sorption isotherms. In: Rockland L.B., Stewart G.F., eds. Water Activity: Influences on Food Quality. New York: Academic Press; 1981. p. :89-154.
- [Google Scholar]
- Equilibrium two-parameter isotherms of acid dyes sorption by activated carbons: study of residual errors. Chem. Eng. J.. 2010;160:408-416.
- [Google Scholar]
- Correlation of equilibrium adsorption data of condensable vapours on porous adsorbents, Gas Sep. Purif.. 1989;3:187-192.
- [Google Scholar]
- Statistical physics study of the interaction of the 5, 10, 15, 20-tetrakis (4-tolylphenyl) porphyrin (H2TTPP) with magnesium ion: New microscopic interpretations. Arab. J. Chem.. 2020;13:4374-4385.
- [Google Scholar]
- Koc, M., Koc, B., Susyal, G., Sakin-Yilmazer, M., Kaymak-Ertekin, F., Bağdatlioğlu, N., 2011. Functional and physicochemical properties of whole egg powder: Effect of spray drying conditions. J. Food Sci. Technol., 48(2), 141–149.
- An algorithm for least-squares estimation of nonlinear parameters. J. Soc. Ind. Appl. Math.. 1963;11:431-441.
- [Google Scholar]
- Water Sorption Isotherms of Dates: Modeling Using GAB Equation and Artificial Neural Network Approaches. 1998;31:699-706.
- Colour measurement and analysis in fresh and processed foods: A review. Food Bioprocess Technol.. 2013;6(1):36-60.
- [Google Scholar]
- Assessment of a semi-empirical four parameter general model for sigmoid moisture sorption isotherms. J. Food Process Eng.. 1993;16:21-37.
- [Google Scholar]
- Comparison of Jatropha curcas shells in natural form and treated by non-thermal plasma as biosorbents for removal of Reactive Red 120 textile dye from aqueous solution. Ind. Crops Prod.. 2013;46:328-340.
- [Google Scholar]
- Rêgo, T.V., Cadaval, T.R.S. Jr., Dotto, G.L., Pinto, L.A.A., 2013. Statistical optimization, interaction analysis and desorption studies for the azo dyes adsorption onto chitosan films, J. Colloid Interface Sci. 411 27–33.
- Optimization of vacuum drying characteristics of date powder. Drying Technol.. 2008;26(6):793-797.
- [Google Scholar]
- Effect of carrier agents on flowability and microstructural properties of foam-mat freeze-dried date powder. J. Food Eng.. 2017;215:33-43.
- [Google Scholar]
- Insights on the statistical physics modeling of the adsorption of Cd2+ and Pb2+ ions on bentonite-chitosan composite in single and binary systems. Chem. Eng. J.. 2018;354:569-576.
- [Google Scholar]
- Application of innovative analytical modeling for the physicochemical analysis of adsorption isotherms of silver nitrate on helicenes: phenomenological study of the complexation process. Ads. Sci. Thec. 2021:1-14.
- [Google Scholar]
- Estimation on the intramolecular hydrogen-bonding energies in proteins and peptides by the analytic potential energy function. J. Mol. Struct.. 2010;956:38-43.
- [Google Scholar]
- Advanced interpretation of CO2 adsorption thermodynamics onto porous solids by statistical physics formalism. Chem. Eng. J.. 2021;406:126669
- [Google Scholar]
- Standardization of isotherm measurements. In: Simatos D., Multon J.L., eds. Properties of Water in Foods. The Netherlands: Martinus Nijhoff; 1985. p. :661-679.
- [Google Scholar]
- Theoretical investigation of the chlorophyll nucleus adsorption monitored with Quartz Crystal Microbalance technique: New insights on physicochemical properties. J. Mol. Liq.. 2019;289:111188
- [Google Scholar]
- Physicochemical interpretation of the adsorption of 4-Bromophenol and 4-Chloroaniline on an activated carbon. J. Envi. Chem. Eng.. 2020;8 104542-104542
- [Google Scholar]
- Sorption of nonpolar and polar compounds to soils: Processes, measurements and experience with the applicability of the modified OECD-guideline 106. Chemosphere. 1991;22:285-304.
- [Google Scholar]
- Determination of the variability of sugars in date fruit varieties. J. Plantation Crops. 2015;43(1):53-61.
- [Google Scholar]







