Translate this page into:
Zinc oxide nanoparticles fabrication using Eriobotrya japonica leaves extract: Photocatalytic performance and antibacterial activity evaluation
⁎Corresponding authors. anmalik77@gmail.com (Arif Nazir), Nmtamam@pnu.edu.sa (Nissren Tamam)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
In the present investigation, ZnO NPs was fabricated using Eriobotrya japonica leaves extract. The E. Japonica leaves powder was extracted and mixed with 0.2 M zinc nitrate in 2:4 ratio and pH was adjusted using ammonia solution. Different techniques including UV–Visible spectroscopy (U.V-Vis), X-ray diffraction (XRD), Energy dispersive X-ray (EDX), Fourier transform infrared (FTIR) and Scanning electron microscope (SEM) techniques were employed to characterize the synthesized ZnO NPs. The peak at 375 nm was observed for ZnO NPs, while the average particle size was 13.0 nm. The elemental composition analysis revealed the NPs was highly pure having irregular platelets shape with aggregates tendency. The as-prepared ZnO NPs was analyzed for their photocatalytic, antibacterial and antioxidant properties. The ZnO NPs exhibited a promising DPPH scavenging activity and was highly active against S. aureus, P. multocida, E. coli and B. subtilis strains. The photocatalytic activity (PCA) was appraised against the removal of methylene blue (MB) dye and we found 72% degradation within 160 min of treatment. Since the synthesized NPs have shown promising bioactivity and PCA, the synthesis of these NPs using E. japonica leaves extracts is suggested for different applications.
Keywords
Nanoparticles
Green synthesis
Plant mediated
Photocatalytic
Antibacterial
Antioxidant activity
1 Introduction
Nanotechnology provides a progressive pathway for innovative development that concern with material at nanoscale. Nanoparticles show very unique and enhanced physicochemical properties due to their sizes at nanoscale. The nanomaterials have been proved to be highly efficient in catalytic application in diverse fields (Awwad et al., 2021a; Chen et al., 2021; Khan et al., 2020, 2019; Li et al., 2021; Mathew et al., 2020; Mukhtar et al., 2019; Numrah et al., 2019). A range of state-of-arts like carbon dots, nanotubes, magnetic NPs, nanorods, nanoflowers, nanorods, nano-rings, nanowires, nano-walls, nanospheres and core–shell structures etc. have been prepared using different techniques (Chundattu et al., 2016; Jabeen et al., 2019; Jamila et al., 2020; Kamran et al., 2019). Interestingly, the applications of nanomaterials depend on specific size, dimensions and physical properties and researchers used different strategies to enhance the chemical and physical properties, which include chemical, physical and biological approaches (Arshad et al., 2018; Ismail et al., 2021; Jamil et al., 2020; Sohail et al., 2020). The physical approaches include vapor deposition, laser ablation, microwave-assisted, sono-chemical reduction, gamma radiation-assisted and arc discharge, while chemical approaches includes polyol route, electrochemical, micro-emulsion and thermal decomposition etc. (Sanda et al., 2021; Sönmezoğlu et al., 2014). On the other hand, biological methods are either plant or microbe facilitated. Preparation of metal NPs via biological approach is very economical in term of energy, reaction time, safety, simplicity and yield of the product (Yedurkar et al., 2016). Before, different biological agents were employed for the fabrication of ZnO NPs, which showed promising photocatalytic and biological activities (Table 1). E. japonica (also known as Loquat locally) belongs to Rosaceae family. It has been generally employed as a medicinal material in Asian countries for chest related problems. Phytochemical analysis revealed the presence of many useful compounds including terpanes, flavonoid, tannin, glycoside and essential oil in E. japonica extracts (Li et al., 2020). However, the extract can be further employed for the fabrication of NPs.
S. No
Plants
Parts
Particle size and shape
Functional groups
Applications
References
1
Z. jujube
Fruit
21–37 nm, spherical
Hydroxyl, carboxyl, carbonyl
Photocatalytic activity (PCA)
(Golmohammadi et al., 2020)
2
Z. nummularia
Leaves
12.47–26.97 nm, spherical
Hydroxyl, alkyne, amine,
Antifungal activity (ANA)
(Padalia and Chanda, 2017)
3
Z. mays
Peel
300–550 nm, flowers
Alkene, hydroxyl, ether,
Antibacterial activity (ABA)
(Quek et al., 2020)
4
S. cumini
Leaves
64–78 nm, spherical
Amide, hydroxyl
PCA
(Rafique et al., 2020)
5
S. baicalensis
Roots
50 nm, spherical
Hydroxyl, alkene, alkane
PCA
(Chen et al., 2019)
6
Quince seed
Mucilage
25 nm, spherical
Hydroxyl
PCA
(Tabrizi Hafez Moghaddas et al., 2020)
7
P. caerulea L.
Leaves
70 nm, spherical
Alkene, amide, amine, carbonyl, alkyl halide, alkane
ABA
(Santhoshkumar et al., 2017)
8
L. esculentum
Peel
9.7 nm, Polyhedral
Aromatic rings
PCA
(Nava et al., 2017)
9
L. nobilis
Leaves
20–30 nm, Spherical and hexagonal
Hydroxyl, amine, alkene, alkane, carboxyl
PCA
(Chemingui et al., 2019)
10
L. nobilis
Leaves
20–30 nm, Spherical and hexagonal
Hydroxyl, alkane, amine, alkene, carboxyl
ABA
(Chemingui et al., 2019)
11
J. regia L.
Leaves
45–65 nm, Spherical
Hydroxyl, alkene, alkane
ABA
(Darvishi et al., 2019)
12
C. citratus
Leaves
6.6–42.9, spherical
Amide, hydroxyl
PCA
(Sidik et al., 2020)
13
C. sinensis
Peel
9.7 nm, Polyhedral
Aromatic rings
PCA
(Nava et al., 2017)
14
C. colocynthis (L.)
Fruit, seed and pulp
20–100 nm, Irregular polygon, flowers, Hexagonal
Hydroxyl, alkane, carbonyl
ABA
(Azizi et al., 2017)
15
J. sambac
Leaves
13.4 nm, platelets, irregular
Hydroxyl, alkane, carbonyl
PCA, ABA
Present study
So far, the green synthetic approaches gained much attention due to their eco-benign nature (Al Banna et al., 2020; Awwad et al., 2020b; Igwe and Nwamezie, 2018; Remya et al., 2017; Shammout and Awwad, 2021). On the other hand, the population growth, water resources contamination, climate change demands the green and clean approaches to avoid the environmental issues. Among water pollution sources, the textile industry is one of major contributors, hence, there is need to remediate dyes in effluents to ensure the environmental integrity is an important concern of the present rea (Golmohammadi et al., 2020). The dyes are considered damaging to the breathing organisms due to their poisonous characteristics. It is indispensable to remove these dyes from any source including wastewater. Out of the various approaches, photocatalysis is one of major and most valid approach for removal of dyes through the process of degradation. The NPs prepared via green route showed excellent photocatalytic activity for the elimination of dyes. Earlier studies stated that NPs synthesized via green route have been studied for their PCA and biological profiling and responses were promising, i.e., sea buckthorn fruit was used for the synthesis of NPs and used as a photocatalysts for the elimination of malachite green, eosin yellow, congo red and methylene blue dyes and up to 99% degradation was achieved under UV light irradiation (Rupa et al., 2019). Similarly, Murex plant juice was employed for synthesis of NPs and exhibited excellent photocatalysis against MB dye under UV (Babitha et al., 2019) and the ZnO prepared using C. ramiflora also exhibited PCA for the removal of RhB, which was 98% in 200 min under UV (Varadavenkatesan et al., 2019). To date, the ZnO NPs have been prepared from these plants, i.e., Olea europaea, C. sinensis, M. oleifera, E. globulus, A. indica, R. officinalis, O. basilicum, C. gigantea, H. sabdariffa, C. procera, P. odorifer, S. torvum, G. wallichianum, A. Sativum, L. esculentum, C. papaya, A. esculentus, C. sinensis, C. aurantifolia, C. woodsonii and S. glauca (Weldegebrieal, 2020). Based on literature survey, The E. japonica has not been investigated for the fabrication of ZnO NPs.
Hence, this investigation was undertaken to fabricate ZnO NPs using E. japonica leaves extract. The synthesized NPs were studied for physico-chemical properties using EDX, XRD, FTIR, SEM and UV–vis techniques. The antibacterial (S. aureus, P. multocida, E. coli and B. subtilis strains), antioxidant (TPC, DPPH and TFC) and photocatalytic activities was also evaluated. The catalytic performance was evaluated against MB dye under UV irradiation.
2 Material and methods
The study was conducted for the fabrication and characterization of ZnO NPs using analytical grade chemicals. These were acquired from Sigma Aldrich. The fresh E. Japonica plant leaves were obtained from fields, when plants started flowering. Leaves were collected from same plant at different branches from Choa saidan shah. The leaves were washed with deionized water, which were dried under shadow. The dried leaves were pulverized to obtained fine particles. The powder was kept in an air tight container for further use.
2.1 Extract preparation
The leaves powder (20 g) was mixed with deionized water (100 mL). The mixture was heated for 60 min at 80 ℃ with continues stirring. Finally, extract is cooled down to 25 ℃ and filtered. The filtrate was kept at 4 ℃ and employed for subsequent NPs fabrication.
2.2 Preparation of ZnO NPs
The zinc nitrate solution (0.2 M) was prepared in deionized water. Now, the zinc nitrate solution was mixed with E. Japonica leaf extract (2:4 ratio) in 250 mL conical flask. The mixture is heated with constant stirring for 10 min at 60 ℃. The pH level of the mixture was adjusted using NaOH (2 M) until pH reached to 12 and stirred for 150 min (Conter checked it is ok). The obtained precipitates were separated by centrifuging at 5000 rpm, which was washed with water and ethanol many times. The synthesized NPs were kept at 60 ℃ in an oven overnight for drying. For characterization and PCA, the NPs were calcined at 300 0C for 2 h and for biological activity, the NPs were dried at 80 0C for 2 h.
2.3 Characterization
We have prepared ZnO NPs using plant extract and then these NPs was further characterized by using various advanced techniques including UV–visible, XRD, FTIR, SEM and EDX techniques. The Schimadzu UV-1800 (spectrophotometer) was employed for UV–visible analysis and Agilent Cary 630 was used for FTIR analysis. The structure and fundamental arrangement were investigated by SEM analysis by employing Nova Nano SEM 450 and XRD analysis was performed with the help of instrument named as Philips-x pert PRO 3040/60 (diffractometer) at 298 K in range 2θ = 20-80° using Cu Kα (λ = 0.15405 nm). Scherer’s formula given in Eq. (1) was applied for crystallite size determination.
2.4 Evaluation of PCA of ZnO NPs
Fig. 1 demonstrates the PCA of NPs examined by removal of MB dye under the effect of UV irradiations. The lamp of mercury, 500 W was employed as a UV light source (intensity 100 mW/cm2). For the procedure to carry out, 15 mg catalyst and MB dye solution (10 mg/L; 50 mL) was assorted. The mixture was agitated for half an hour in dark to obtain equilibrium and furthermore, it was irradiated with UV light for 160 min. During this procedure, the stirring was continuously performed. 2 mL solution was withdrawn after certain regular time interval. It was then filtered and concentration was assessed at 660 nm. The efficiency (%) was calculated by employing Eq. (2). Where, C0 and Cf are the absorbance values before irradiation and at time “t”, respectively.
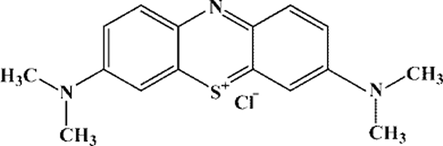
Structure of MB dye used for PCA evaluation of ZnO NPs.
2.5 Evaluation of antibacterial activity (ABA)
The ABA of ZnO NPs was appraised versus a set of bacterial strains. Fresh cultures of bacterial strains were prepared using nutrient broth as culture media. All cultures were stored at 4 ℃ and spores (1.2 × 108 CFU/mL) were used. Nutrient Agar media was prepared and sterilized the media by autoclaving the media at 121 ℃ for 15 min. Wick’s paper discs as a loading agent of ZnO NPs were prepared of 9 mm size and were also sterilized and then prior to the transfer of medium to petri plates; inoculums of different bacterial strains (100 μL/100 mL) were added to the warm agar medium and mix it for homogeneity of bacterial culture with media. Then (20 mL) nutrient agar media were poured in each petri plates and left for solidification of media, for preparation of uniform bed of media in petri plates, avoid to shaking the plates. Then, 500 μL of ZnO NPs (10 mg/L) in 10 to 40 mg/mL range was loaded on disc. These discs were then dried for further use. By using sterile forceps, discs containing different concentration of controls and ZnO NPs were laid down on media plates marks them. Rifampicin with the concentration of 10 mg/mL was kept a control (positive control for comparison). The petri plates were placed for 24 h at 37 ℃. The ZnO NPs displayed ABA by inhibiting the bacterial growth. All the tests were run in a set of three runs and data obtained was calculated as a mean and SD (Brudzynski and Kim, 2011).
For the estimation of MIC of ZnO NPs, a 96 well plate’s method was used in aseptic environment. In 96 well plate, ZnO NPs 100 µL in 10–40 mg/mL concentration range were poured into the first row of the plate and nutrient broth (50 µL) was added in all other wells, and 50 µL of (Zno NPs) from first well transferred into next wells and then serial dilutions of tested (Zno NPs) were performed. Resazurin indicator (10 µL) was supplied in each well. Later, bacterial suspension (10 µL with 5x106 CFU/mL) were transferred. A positive control (antibiotic) was run in parallel to the sample. Plates were covered to avoid dehydrated and plates were kept for 24 h at 37 °C. Elisa reader was used to measure absorbance at 500 nm. Any color change (observed) at most diluted concentration was observed taken as least inhibitory concentration value (Sarker et al., 2007).
2.6 Evaluation of antioxidant activity (AOA)
The AOA was appraised by DPPH (2, 2-diphenyl-1-picryl hydrazyl) test following method reported elsewhere (Bozin et al., 2006). The scavenging effect (%) was measured using Eq. (3). The assay was carried out in a set of three runs and data was averaged.
3 Results and discussion
3.1 UV–Visible and FTIR analysis
The UV–Vis analysis of ZnO NPs was performed, results are depicted in Fig. 2 and a band at 375 nm exposed the presence of ZnO NPs. The absorption of radiant energy is an essential for a photocatalyst to carry out the photocatalytic reaction. The ZnO displayed a higher captivation capacity in the UV light range. Previous studies also revealed similar observation that pure ZnO is active UV light (Mahmud et al., 2020). The FTIR is used to find the presence of bioactive functional groups attached with the NPs. In FTIR analysis (Fig. 3), the bands were observed at 3505, 1560, 1507, 1388, 1034 and 885 (cm−1). The band at 3505 cm−1 corelates to the O-H stretching and a band at 1560 cm−1 is due to the aromatic C = C group (Awwad et al., 2020a). The C-C (aromatic) was recorded 507 cm−1. The band at 1388 cm−1 is due to C-H vibration functional group. The C-N (aliphatic amine) was detected at 1034 cm−1 and a band at 885 cm−1 is due to the C-H (aromatic) group. The FTIR analysis of extracts was also performed and the functional groups detected in NPs was also found to be in the extract used for ZnO NPs preparation (Fig. 4).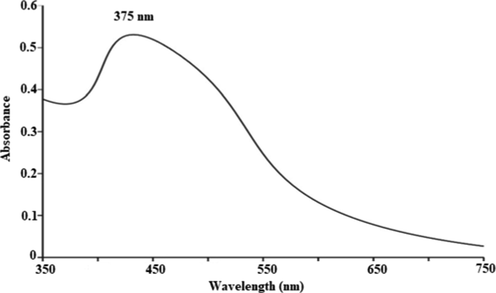
UV–Visible response of Zn NPs synthesized fabricated by employing E. japonica leaves extract.
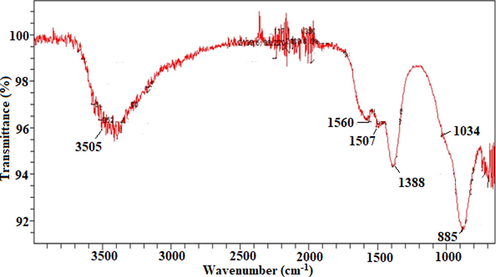
FTIR analysis of ZnO NPs fabricated by employing E. japonica leaves extract.
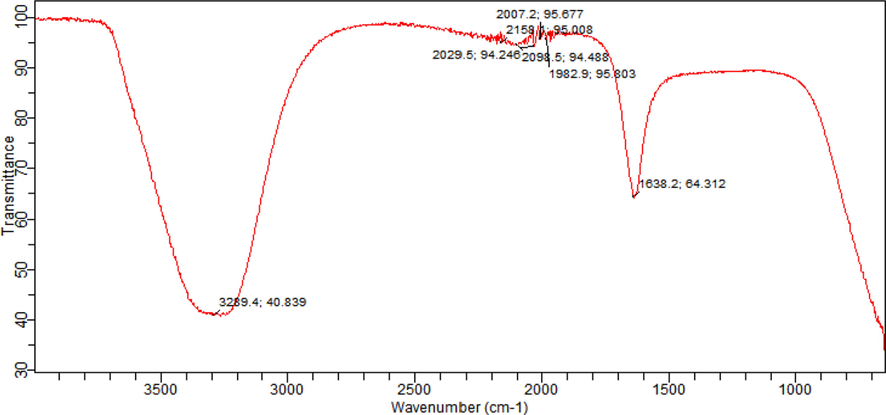
FTIR analysis of E. japonica leaves extract used for the synthesis of ZnO NPs.
3.2 Morphology and elemental composition
The morphology of ZnO NPs synthesized via green route was examined by SEM analysis. SEM gave the evidence of surface characteristics of NPs and any interaction between ZnO NPs and bioactive agents. The response of SEM analysis is depicted in Fig. 5. The ZnO particles was in the form of platelets and irregular in shape. Moreover, the ZnO NPs were in aggregates form, which is due to the interface among biomolecules caping and stabilizing the NPs. Elemental analysis of the prepared ZnO NPs was also performed for the assessment of purity and composition of the prepared ZnO NPs and response is depicted Fig. 6. The oxygen and zinc were detected in the prepared sample with 19.87 and 74.95 (%) ratio, which indicates that ZnO NPs are highly pure.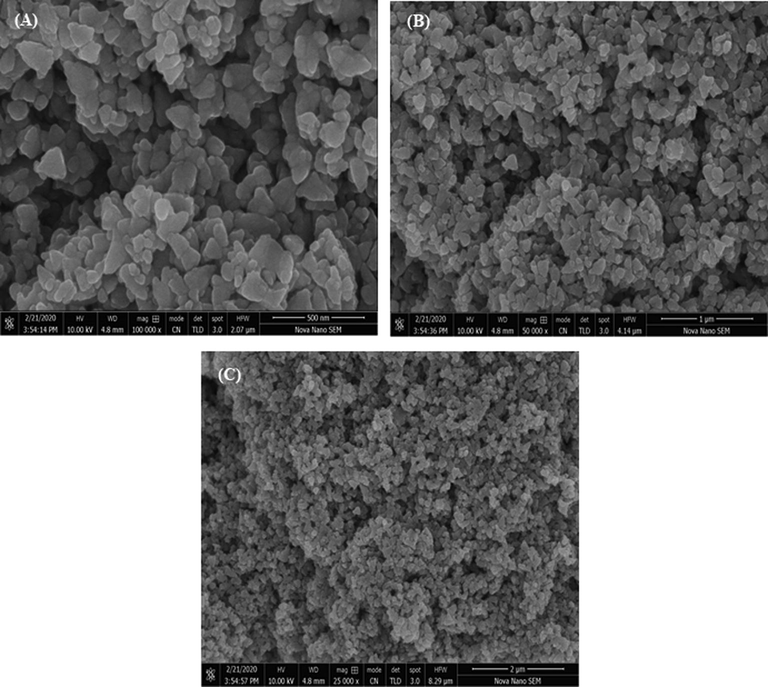
SEM images at of ZnO NPs fabricated using E. japonica leaves extracts.
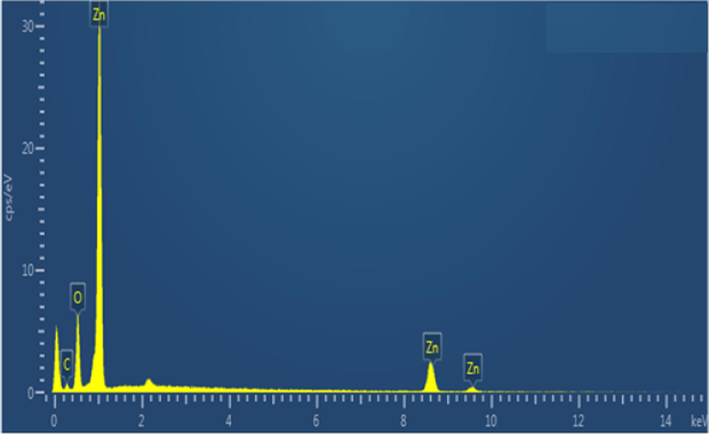
EDX spectrum of ZnO NPs fabricated using E. japonica leaves extracts.
3.3 Structural analysis
The size and crystallinity of the ZnO NPs was determined by XRD analysis and responses thus observed are presented in Fig. 7. The ZnO NP was face centered cubic (FCC) crystals with (1 0 0), (0 0 2), (1 0 1), (1 0 2), (1 1 0), (1 0 3) and (1 1 2) planes, which is in line with the standard (JCPDS # 36–1451) (Jamdagni et al., 2018). The Scherer formula was employed to approximate the particles size of ZnO NPs.
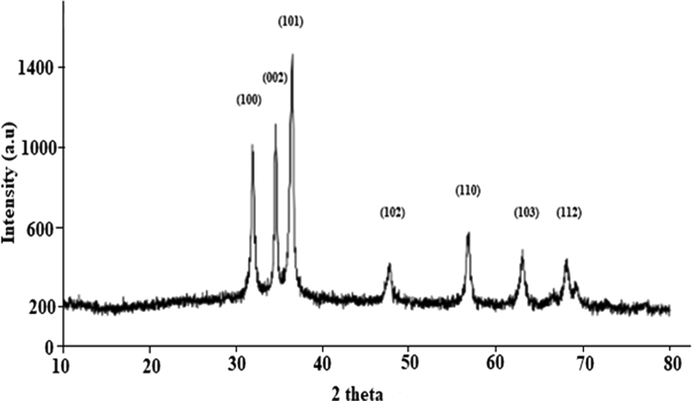
XRD pattern of ZnO NPs fabricated using E. japonica leaves extracts.
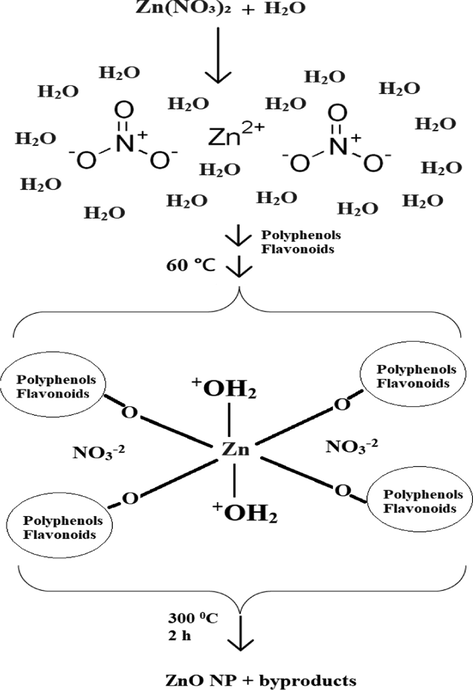
Proposed ZnO NPs formation mechanism using E. japonica leaves extract.
3.4 Photocatalytic activity
The PCA of ZnO NP was scrutinized by removing MB dye under the impact of UV irradiation and the PCA response of ZnO NPs was highly promising. A peak at 681 nm decreased quickly with irradiation time, which show the removal of MB dye (Fig. 9). The dye removal was recorded to be 18, 32, 41, 50, 62 and 72 (%) for the irradiation time of 5, 10, 20, 40, 80 and 160 (min), respectively. The enhanced dye degradation is corelated with generation of highly reactive species on irradiation of ZnO NPs, like hydroxyl radical, which degrade the dye oxidatively to low molecular moieties. The degradation mechanism is presented in Eqs. (5)–(9). On irradiation, an e− and h+ pair is formed by excitation of electrons from VB to CB. The e− (CB) reacts with oxygen to produce superoxide radical and h+ react with water to produce hydroxyl radical. The hydroxyl radical are reactive species that degrades the MO dye oxidatively into intermediate of low molecular weight and finally, in to H2O, CO2 and inorganic ions (Eqs. (5)–(9)). The PCA of CuO NPs was auspicious for the removal of MO dye under the exposure of visible light, which is considered economical for the redress of MB dyes. Also, previous studies support the findings of the present investigation regarding fabrication of ZnO via eco-benign approaches and their promising PCA, i.e., jujube fruit extract was employed for the fabrication of ZnO NPs, which were studied employing TEM, XRD, FTIR, SEM and EDX techniques. The ZnO NPs were employed for the photocatalytic removal of MB and Eriochrome black T dyes under direct solar energy expoure. The ZnO NPs displayed auspicious PCA for both dyes removal and up to 92% and 86% efficiency was achieved within 5 h@8.7 × 10−3 and 6.7 × 10−3 (min−1) for MB and ECBT, respectively (Golmohammadi et al., 2020). Similarly, S. cumini leaves extracts is employed for the fabrication of ZnO NPs and NPs were in pure crystalline hexagonal and spherical shapes. The ZnO NPs were employed for the removal of RhB dye and under optimum conditions, up to 98% removal of RhB dye was achieved (Rafique et al., 2020). Also, S. baicalensis root extract was employed for ZnO NPs fabrication, which were studied employing UV–vis, FE-TEM, FTIR, EDX, and XRD approaches. The ZnO NPs displayed auspicious competence for the removal of dye under UV effect with degradation rate of 0.016 min−1, up to 98.6% MB dye was degraded in 210 min (Chen et al., 2019). On the same line, Quince seed mucilage was also employed for the fabrication of ZnO NPs, whose properties were studied by employing FESEM, EDX, FTIR, XRD, UV–Visible techniques. The PCA was appraised for the removal of MB dye and 80% removal was observed within 2 h following the Langmuir–Hinshelwood kinetics model (Tabrizi Hafez Moghaddas et al., 2020). In another study C. sinensis, L. esculentum, C. aurantifolia and C. paradisi peels extracts were tested for the fabrication of ZnO NPs, which were studied by employing XRD, FTIR and HRTEM techniques. The MB dye removal was up to 97% within 180 min irradiation (Nava et al., 2017). Also, L. nobilis plant extract furnished ZnO NPs (Wurtzite hexagonal) within 20 and 35 nm range. The ZnO NPs showed promising PCA for RBR-F3B and RR180 dyes under UV light irradiation and up to 99% was achieved in 60 min (Chemingui et al., 2019). Hence, the ZnO prepared utilizing eco-benign approach furnished extremely dynamic photocatalysts and have potential for the remediation of pollutants in the effluents (Abbas et al., 2021; Adetutu et al., 2020; Alaqarbeh et al., 2020; Awwad et al., 2021b; Chham et al., 2018; Elsherif et al., 2021; Shindy et al., 2021; Ukpaka and Eno, 2020) since green route is safer versus other physicochemical approaches (Amer and Awwad, 2021).
ZnO NPs + irradiation → catalyst(h+(VB) + e−(CB)(5)
h+ (VB) + H2O → •OH + H+(6)
ē (CB) + O2 → O•2−(7)
O•2− +2H+ → HO•2− + H+ → 2OH•(8)
OH•/•O2−∙ + MB dye → CO2 + H2O2 + inorganic ions(9)
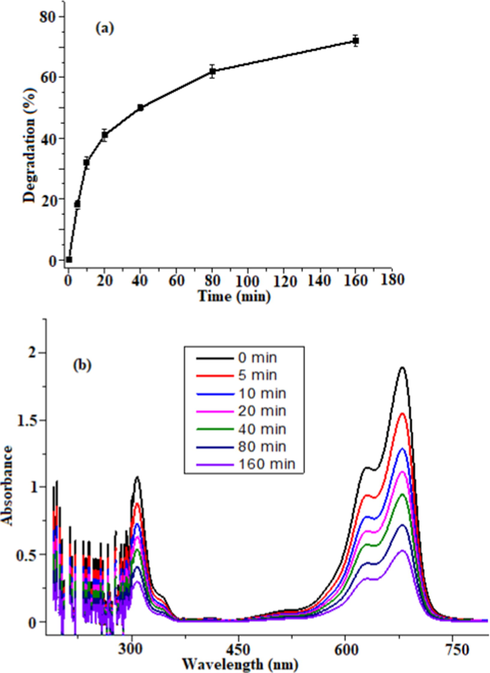
(a) Degradation (%) of MB w.r.t UV exposure time and (b) UV–visible analysis of MB dye treated isng ZnO NPs catalyst under UB light irradiation.
3.5 Antibacterial activity
The ABA of ZnO NPs (10–40 mg/L) fabricated using green approach were tested against bacterial strains and responses thus obtained are presented in Fig. 10. The antibacterial in the term of ZOI was recorded to be 17, 19, 21 and 20 (mm) against E. coli, P. multocida, B. subtilis and S. aureus, respectively at the rate of 10 mg/L ZnO NPs. The ABA was increased significantly with ZnO NPs concentration and ZOI were recorded to be 25 mm (E. coli), 24 mm (P. multocida), 26 mm (B. subtilis) and 26 mm (S. aureus). The ZOI in case of standard drug (Rifampicin) were 33 mm (E. coli), 34 mm (P. multocida), 36 mm (B. subtilis) and 35 mm (S. aureus). In comparison to the standard the ZnO NPs showed promising antibacterial activity. The MIC was also recorded of ZnO NPs against bacterial strains and response this observed are shown in Fig. 11. The MIC values were recorded to be 364, 347, 311 and 321 (µg/L) against E. coli, P. multocida, B. subtilis and S. aureus, respectively at the rate of 10 mg/L ZnO NPs. The MIC values were decreased as the ZnO NPs concentration was increased and these values were, 215 µg/L (E. coli), 231 µg/L (P. multocida), 194 µg/L (B. subtilis) and 197 µg/L (S. aureus) at the rate of 40 mg/L ZnO NPs. The ZnO NPs interactive bacterial cell wall that cause the destruction of cell wall due to ions and ROS formation (Sharmila et al., 2019).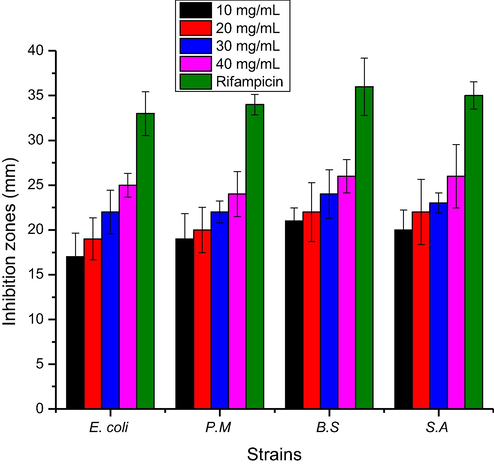
Zones of inhibition against bacterial strains of ZnO NPs fabricated using E. japonica leaves extracts.
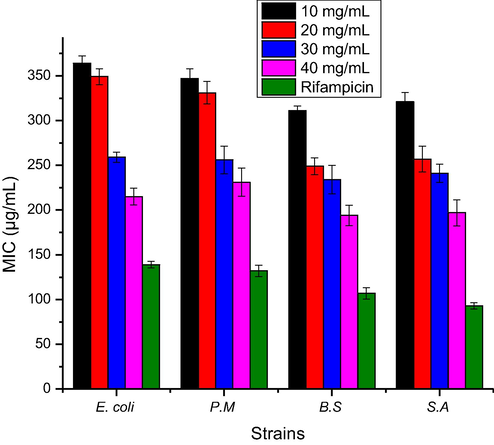
MIC against bacterial strains of ZnO NPs fabricated using E. japonica leaves extracts.
3.6 Antioxidant activity
The ZnO NPs concentrations (10–40 mg/mL) were employed for DPPH assay to weigh the DPPH (free radical) scavenging activity of ZnO NPs. Ascorbic acid was used as standard for comparison of percentage inhibition of DPPH radical by ZnO NPs. The ZnO NPs displayed excellent inhibition of DPPH radical, which increased as the ZnO NPs increases. The DPPH is a source of reliable information for antioxidant nature of a substance. High absorbance exhibited high reducing ability (Ahalya et al., 2013). Antioxidants activity measured can be related to bioactive in the extract that intricate the NPs formation from metal ions in the reaction media (Awwad et al., 2020a). The results of DPPH scavenging effect of ZnO NPs (10–40 mg/mL) are given in Fig. 12. The ZnO NPs showed the percentage inhibition of DPPH radical of 36.43 ± 2.46, 56.71 ± 1.35, 68.18 ± 0.76 and 83.23 ± 1.63 for ZnO NPs concentrations (10–40 mg/L), respectively. The TPC and TFC was also evaluated and the outcomes observed are presented in Fig. 13. Both the TPC and TFC contents were increased with concentration and the values were recorded to be in the range of 90.51–315.86 μg GAE/g. DW (TPC) and 48.72–209.34 μg CAE/g. DW (TFC), which revealed a promising antioxidant activity, which is due to bioactive components, which enables the formation NPs (atom) form the metal ions by the reduction process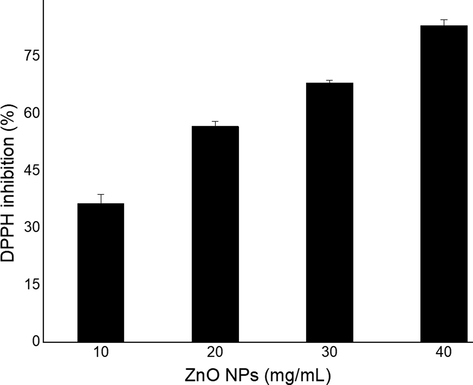
DPPH radical scavenging activity of ZnO NPs fabricated using E. japonica leaves extracts.
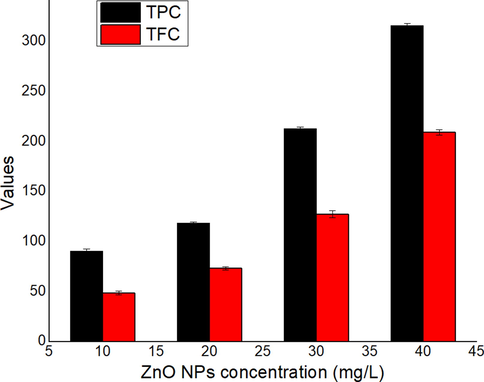
Value of TPC (total phenolic contents) and TFC (total flavonoid contents).
The observations are in accordance with reported studies that are documented for the ZnO NPs fabrication utilizing eco-benign approaches and their bioactivities, i.e., the ZnO NPs were prepared using Zea may husk, jackfruit peel and pomegranate peel, which are characterized and their antimicrobial response was evaluated. The ZnO NPs verified excellent antibacterial activities against E. faecalis (Quek et al., 2020). Similarly, P. caerulea fresh leaf extract was employed for ZnO NPs fabrication, which are characterized advanced approaches like FT-IR, SEM, XRD, UV–vis, EDAX and AFM techniques. The ABA was assessed against urinary tract infection causing microbes and ZnO NPs showed promising antibacterial activity (Santhoshkumar et al., 2017). On the same line, ZnO NPs was prepared using L. nobilis plant extract. The ZnO NPs indicated promising applications as an active biological agents (Chemingui et al., 2019). The J. regia L. leaf extract has also been used for the fabrication of ZnO NPs as a function of process variable and resultantly, a variables structures and sizes were observed. The antimicrobial effect of ZnO NPs was evaluated against E. coli, P. aeruginosa and A. baumannii. The antimicrobial effect of ZnO NPs obtained in green route was significantly higher versus chemically synthesized. Also, ZnO NP synthesized using green route was biocompatible versus chemically synthesized (Darvishi et al., 2019). In another study, C. colocynthis (L.) extracts were used for ZnO NPs synthesis. The NPs with hexagonal wurtzite structure was in 27–85 nm size range and same were tested for antioxidant and amicrobial activity. The ZnO NPs showed promising antioxidant activity through scavenging of DPPH free radical. In vitro cytotoxicity was evaluated against T3 cells and up to 0.26 mg/mL concentration, no toxic effect was observed. The ZnO NPs were highly active B. subtilis, S. aurous, P. aeruginosa and E. coli bacterial strains. Hence, the present investigation and previous studies proved that green route is efficient method to prepare the ZnO NPs with promising biological activities that might have potential applications in biomedical field (Azizi et al., 2017).
4 Conclusion
The ZnO NPs was prepared successfully using an eco-benign route, which were studied for different physicochemical properties. The ZnO NPs was highly pure having irregular platelets shape with aggregates tendency and average size was recorded to be 13.0 nm. The antibacterial, antioxidant and photocatalytic activities were also evaluated. The ZnO NPs showed promising DPPH scavenging activity, which was dependent to ZnO NPs concentration. The ZnO NPs was highly active against E. coli, S. aureus, P. multocida, B. subtilis strains. The ZnO NPs showed excellent PCA and 160 min of UV irradiation exhibited 72% MB dye degradation. In view of eco-benign and cost effectiveness of green route, the use E. japonica extracts is suggested to prepare ZnO NPs for different applications.
Acknowledgements
This research was funded by the Deanship of Scientific Research at Princess Nourah bint Abdulrahman University through the Fast-track Research Funding Program.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Wound healing potential of curcumin cross-linked chitosan/polyvinyl alcohol. Int. J. Biol. Macromol.. 2019;140:871-876.
- [Google Scholar]
- Phytoremediation potential of Typha latifolia and water hyacinth for removal of heavy metals from industrial wastewater. Chem. Int.. 2021;7:103-111.
- [Google Scholar]
- Carcinogenicity of dioxin-like polychlorinated biphenyls in transformer soil in vicinity of University of Port Harcourt, Choba. Nigeria. Chem. Int.. 2020;6:144-150.
- [Google Scholar]
- Evaluation of in vitro anti-oxidant activity of Annona muricata bark. Int. J. Pharma. Chem. Biol. Sci.. 2013;3:406-410.
- [Google Scholar]
- Green synthesis of sulfur nanoparticles using Rosmarinus officinalis leaves extract and nematicidal activity against Meloidogyne javanica. Chem. Int.. 2020;6:137-143.
- [Google Scholar]
- Nano platelets kaolinite for the adsorption of toxic metal ions in the environment. Chem. Int.. 2020;6:49-55.
- [Google Scholar]
- Green synthesis of copper nanoparticles by Citrus limon fruits extract, characterization and antibacterial activity. Chem. Int.. 2021;7:1-8.
- [Google Scholar]
- Zn-doped SiO2 nanoparticles preparation and characterization under the effect of various solvents: Antibacterial, antifungal and photocatlytic performance evaluation. J. Photochem. Photobiol. B. 2018;185:176-183.
- [Google Scholar]
- Green synthesis of zinc oxide nanoparticles (ZnO-NPs) using Ailanthus altissima fruit extracts and antibacterial activity. Chem. Int.. 2020;6:151-159.
- [Google Scholar]
- Fe(OH)3/kaolinite nanoplatelets: Equilibrium and thermodynamic studies for the adsorption of Pb (II) ions from aqueous solution. Chem. Int.. 2021;7:90-102.
- [Google Scholar]
- Adsorptive removal of Pb (II) and Cd (II) ions from aqueous solution onto modified Hiswa iron-kaolin clay: Equilibrium and thermodynamic aspects. Chem. Int.. 2021;7:139-144.
- [Google Scholar]
- Green synthesis, characterization of silver sulfide nanoparticles and antibacterial activity evaluation. Chem. Int.. 2020;6:42-48.
- [Google Scholar]
- Green microwave-assisted combustion synthesis of zinc oxide nanoparticles with Citrullus colocynthis (L.) Schrad: characterization and biomedical applications. Molecules. 2017;22:301.
- [Google Scholar]
- Enhanced antibacterial activity and photo-catalytic properties of ZnO nanoparticles: pedalium murex plant extract-assisted synthesis. J. Nanosci. Nanotechnol.. 2019;19:2888-2894.
- [Google Scholar]
- Characterization of the volatile composition of essential oils of some Lamiaceae spices and the antimicrobial and antioxidant activities of the entire oils. J. Agric. Food Chem.. 2006;54:1822-1828.
- [Google Scholar]
- Storage-induced chemical changes in active components of honey de-regulate its antibacterial activity. Food Chem.. 2011;126:1155-1163.
- [Google Scholar]
- Facile green synthesis of zinc oxide nanoparticles (ZnO NPs): Antibacterial and photocatalytic activities. Mater. Res. Exp.. 2019;6:1050b1054.
- [Google Scholar]
- Green synthesis, characterization, cytotoxicity, antioxidant, and anti-human ovarian cancer activities of Curcumae kwangsiensis leaf aqueous extract green-synthesized gold nanoparticles. Arab. J. Chem.. 2021;14:103000
- [Google Scholar]
- Green synthesis of zinc oxide nanoparticles from root extract of Scutellaria baicalensis and its photocatalytic degradation activity using methylene blue. Optik. 2019;184:324-329.
- [Google Scholar]
- The use of insoluble mater of Moroccan oil shale for removal of dyes from aqueous solution. Chem. Int.. 2018;4:67-77.
- [Google Scholar]
- Phytochemical investigation of Calotropis procera. Arab. J. Chem.. 2016;9:S230-S234.
- [Google Scholar]
- Comparison of different properties of zinc oxide nanoparticles synthesized by the green (using Juglans regia L. leaf extract) and chemical methods. J. Mol. Liq.. 2019;286:110831
- [Google Scholar]
- Adsorption of crystal violet dye onto olive leaves powder: Equilibrium and kinetic studies. Chem. Int.. 2021;7:79-89.
- [Google Scholar]
- A green approach to synthesis of ZnO nanoparticles using jujube fruit extract and their application in photocatalytic degradation of organic dyes. Spectrochim. Acta A. 2020;229:117961
- [Google Scholar]
- Green synthesis of iron nanoparticles using flower extract of Piliostigma thonningii and antibacterial activity evaluation. Chem. Int. 2018;4:60-66.
- [Google Scholar]
- Kaolin and bentonite catalysts efficiencies for the debutylation of 2-tert-butylphenol. Chem. Int.. 2021;7:21-29.
- [Google Scholar]
- Statistical Modeling for the Extraction of Dye from Natural Source and Industrial Applications. Polish J. Environ. Stud.. 2019;28:2145-2150.
- [Google Scholar]
- Green synthesis of zinc oxide nanoparticles using flower extract of Nyctanthes arbor-tristis and their antifungal activity. J. King Saud Uni.- Sci.. 2018;30:168-175.
- [Google Scholar]
- Photocatalytic degradation of disperse dye Violet-26 using TiO2 and ZnO nanomaterials and process variable optimization. J. Mater. Res. Technol.. 2020;9:1119-1128.
- [Google Scholar]
- Piper longum catkin extract mediated synthesis of Ag, Cu, and Ni nanoparticles and their applications as biological and environmental remediation agents. Arab. J. Chem.. 2020;13:6425-6436.
- [Google Scholar]
- Green Synthesis of Metal Nanoparticles and their Applications in Different Fields: A Review. Z. Phys. Chem.. 2019;233:1325-1349.
- [Google Scholar]
- Kinetic Study of Degradation of Basic Turquise Blue X-GB and Basic Blue X-GRRL using Advanced Oxidation Process. Z. Phys. Chem.. 2020;234:1803-1817.
- [Google Scholar]
- Decolorization of Basic Turquise Blue X-GB and Basic Blue X-GRRL by the Fenton’s Process and its Kinetics. Z. Phys. Chem.. 2019;233:361-373.
- [Google Scholar]
- Eriobotrya japonica leaf triterpenoid acids ameliorate metabolic syndrome in C57BL/6J mice fed with high-fat diet. Biomed. Pharmacother.. 2020;132:110866
- [Google Scholar]
- Green synthesis of gold nanoparticles using aqueous extract of Mentha Longifolia leaf and investigation of its anti-human breast carcinoma properties in the in vitro condition. Arab. J. Chem.. 2021;14:102931
- [Google Scholar]
- Graphene nanoplatelets with low defect density as a synergetic adsorbent and electron sink for ZnO in the photocatalytic degradation of Methylene Blue under UV–vis irradiation. Mater. Res. Bull.. 2020;128:110876
- [Google Scholar]
- Biosynthesis of silver nanoparticle using flowers of Calotropis gigantea (L.) W.T. Aiton and activity against pathogenic bacteria. Arab. J. Chem.. 2020;13:9139-9144.
- [Google Scholar]
- Kinetics and equilibrium studies of eriobotrya japonica: a novel adsorbent preparation for dyes sequestration. Z. Phys. Chem.. 2019;233:1469-1484.
- [Google Scholar]
- Fruit peel extract mediated green synthesis of zinc oxide nanoparticles. J. Mol. Struct.. 2017;1147:1-6.
- [Google Scholar]
- A novel approach for modification of biosorbent by silane functionalization and its industrial application for single and multi-component solute system. Z. Phys. Chem.. 2019;233:1603-1623.
- [Google Scholar]
- Characterization, antifungal and cytotoxic evaluation of green synthesized zinc oxide nanoparticles using Ziziphus nummularia leaf extract. Artificial Cells. Nanomed. Biotechnol.. 2017;45:1751-1761.
- [Google Scholar]
- Bioinspired green synthesis of ZnO structures with enhanced visible light photocatalytic activity. J. Mater. Sci.: Mater. Electron.. 2020;31:1144-1158.
- [Google Scholar]
- Plant-mediated green synthesis of zinc oxide nanoparticles from Syzygium Cumini for seed germination and wastewater purification. Int. J. Environ. Anal. Chem.. 2020;26:1-16.
- [Google Scholar]
- Synthesis of a zinc oxide nanoflower photocatalyst from sea buckthorn fruit for degradation of industrial dyes in wastewater treatment. Nanomaterials. 2019;9:1692.
- [Google Scholar]
- Preparation and antioxidant properties of extracts of Japanese persimmon leaf tea (kakinoha-cha) Food Chem.. 2005;89:569-575.
- [Google Scholar]
- Development of TiO2-based dye-sensitized solar cells using natural dyes extracted from some plant-based materials. Chem. Int.. 2021;7:9-20.
- [Google Scholar]
- Synthesis of zinc oxide nanoparticles using plant leaf extract against urinary tract infection pathogen. Resourc. Efficient Technol.. 2017;3:459-465.
- [Google Scholar]
- Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods. 2007;42:321-324.
- [Google Scholar]
- A novel route for the synthesis of copper oxide nanoparticles using Bougainvillea plant flowers extract and antifungal activity evaluation. Chem. Int.. 2021;7:71-78.
- [Google Scholar]
- Green synthesis of ZnO nanoparticles using Tecoma castanifolia leaf extract: Characterization and evaluation of its antioxidant, bactericidal and anticancer activities. Microchem. J.. 2019;145:578-587.
- [Google Scholar]
- Solvatochromism and halochromism of some furo/pyrazole cyanine dyes. Chem. Int.. 2021;7:39-52.
- [Google Scholar]
- The potential control strategies of membrane fouling and performance in membrane photocatalytic reactor (MPR) for treating palm oil mill secondary effluent (POMSE) Chem. Eng. Res. Design. 2020;162:12-27.
- [Google Scholar]
- Polyamidoamine (PAMAM) dendrimers synthesis, characterization and adsorptive removal of nickel ions from aqueous solution. J. Mater. Res. Technol.. 2020;9:498-506.
- [Google Scholar]
- Fast production of ZnO nanorods by arc discharge in de-ionized water and applications in dye-sensitized solar cells. J. Alloy. Comp.. 2014;586:593-599.
- [Google Scholar]
- Biosynthesis of pure zinc oxide nanoparticles using Quince seed mucilage for photocatalytic dye degradation. J. Alloy. Comp.. 2020;821:153519
- [Google Scholar]
- Modeling of Azadirachta indica leaves powder efficiency for the remediation of soil contaminated with crude oil. Chem. Int.. 2020;7:62-70.
- [Google Scholar]
- Photocatalytic degradation of Rhodamine B by zinc oxide nanoparticles synthesized using the leaf extract of Cyanometra ramiflora. J. Photochem. Photobiol. B. 2019;199:111621
- [Google Scholar]
- Synthesis method, antibacterial and photocatalytic activity of ZnO nanoparticles for azo dyes in wastewater treatment: A review. Inorgan. Chem. Commun.. 2020;120:108140
- [Google Scholar]
- Biosynthesis of zinc oxide nanoparticles using ixora coccinea leaf extract—a green approach. Open J. Synth. Theor. Appl.. 2016;5:1-14.
- [Google Scholar]







