Translate this page into:
Aqueous scavenging of polycyclic aromatic hydrocarbons using epichlorohydrin, 1,6-hexamethylene diisocyanate and 4,4-methylene diphenyl diisocyanate modified starch: Pollution remediation approach
⁎Corresponding author. Tel.: +86 18701336534. guoqj@igsnrr.ac.cn (Qingjun Guo) nonsokoli@gmail.com (Qingjun Guo)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Cross linking of starch molecules with suitable reagents provides opportunity for incorporation of relevant functional groups, thereby tuning the adsorption properties of the starch polymer adsorbent towards target pollutants, as a function of the nature of the cross linking agent and the chemical identity of the pollutants. In this vein, this study assessed the applicability of epichlorohydrin cross-linked starch (EPIS), 1,6-hexamethylene diisocyanate cross-linked starch (HDIS), and 4,4-methylene diphenyl diisocyanate cross-linked starch (MDIS) polymer adsorbents for treatment of aqueous Polycyclic Aromatic Hydrocarbon (PAHs) pollution in environmental water samples. Cross-linking process led to improvement in surface characteristics, level of hydrophobicity, and incorporation of relevant functional groups. Adsorption capacities of the adsorbents were in the order of MDIS > HDIS > EPIS. PAHs molecules were adsorbed in multi-layer, in a flat-wise orientation, while external and internal diffusion dominated the adsorption kinetics. Adsorption mechanisms suggested that these adsorbents spontaneously adsorb PAHs molecules driven mainly by enthalpy change, and the adsorption process was attributed to physisorption involving hydrophobic, van der Waals and π–π interactions between adsorbent and adsorbates. The thermodynamic evaluation indicated that the adsorption process was majorly endothermic and spontaneous. Desorption studies indicated that the adsorbents can be efficiently regenerated. The adsorbents were effective in treating simulated and real environmental water samples, and hence, are promising cost-effective alternative for control of aqueous PAHs pollution.
Keywords
Polycyclic Aromatic Hydrocarbons (PAHs)
Cross-linked starch polymer
Adsorption mechanism
Adsorbent regeneration
Water remediation
1 Introduction
The presence of PAHs in drinking, surface and waste water has generated much public health concern and wide reportage in the literature (Sun et al., 2009; Wang et al., 2009; Wu et al., 2011; Zhao et al., 2011; Adedayo et al., 2012). Aside being hydrophobic, PAHs exhibit mutagenic and carcinogenic toxicities even at ultra-trace level. As a result of their persistence in the environment, they have been classified as priority pollutants with well-established restrictive limits by most environmental regulatory agencies (Diggs et al., 2011). Humans are exposed to PAHs through several means – air, water, food, skin contact, and occupation. However, it has been established that one of the main routes of human exposure is via water. PAHs find their way into water bodies through effluent discharges, leaching from waste dumps, accidental discharges, atmospheric deposition, and through diffuse sources. In this vein, the potential health risk of exposure to these chemicals is higher in the developing countries due to the fact that water for domestic activities is collected directly from streams with little or no treatment (Bhatnagar and Sillanpää, 2010).
Conventional physicochemical processes such as flocculation, coagulation, sedimentation and filtration have proved to be ineffective for removal of PAHs (Chen et al., 2011). Adsorption, which involves the scavenging of atoms, ions, or molecules from aqueous media onto a solid surface is one of the most economical and effective techniques for removing PAHs and other organic pollutants from aqueous media (Aksu, 2005). However, high cost and difficult regeneration of active carbon (AC), the most commonly used conventional adsorbent, limit its application in large-scale wastewater treatment. In search of potential alternatives, dead microorganisms such as algae, bacteria, yeasts and fungi (Chung et al., 2007), industrial wastes such as fly ash (Sasithorn et al., 2010), nanomaterials such as grapheme oxides (Sun et al., 2013), plant residue materials such as cork waste, bamboo leaves, bio chars and orange peels, (Chen et al., 2011), pure and modified naturally occurring minerals (Vidal et al., 2011), and synthetic materials such as Amberlite XAD resin, High Density Polyethylene (HDP) and Macronet (Valderrama et al., 2007; Fries and Zarfl, 2012), have been applied for removal of PAHs from aqueous media. However, most of these potential options suffered limitations due to non-uniform, and consequently unpredictable physical and/or chemical characteristics, low sorption capacity for biomass based adsorbents, and disposal problem due to non-biodegradable nature on the part of synthetic adsorbents.
Modified starch has demonstrated high potential as adsorbent for removal of pollutants from aqueous media (Crini, 2005). Starch is of particular interest as a precursor because of its ready availability, specific structure, chemical properties, biodegradability, and excellent selectivity to aromatic compounds and metals. Modifications of starch by cross linking of the glucose chains of starch polymer not only increase hydrophobicity, but also provide opportunity for tuning the sorption properties of the adsorbent as a function of the nature of the cross linking agent as well as degree of cross linking (Imam et al., 2012). However, despite these sterling qualities and potentials, no study has been carried out to develop starch based adsorbents for PAHs.
This study was therefore set out to utilize, for the first time, starch based adsorbents for remediation of aqueous PAHs pollution.
2 Materials and methods
2.1 Materials
The acenaphthylene and phenanthrene PAH standards were supplied by Dr. Ehrenstorfer GmbH, Augsburg, Germany. Fluorene, epichlorohydrin, 1,6-hexamethylene diisocyanate (HDI), 4,4-methylene diphenyl diisocyanate (MDI), N,N-dimethyl formamide (DMF), and aqueous ammonia, were sourced from Aladdin Chemistry Company Limited, Shanghai, China. Soluble starch was purchased from Guangfu Research Institute, Tianjin, China. Acetonitrile (HPLC grade) was sourced from Fisher Scientific, USA.
2.2 Preparation and characterization of adsorbents
2.2.1 Preparation
The cross-linked starch polymers were prepared in one step by reticulation of starch using different cross-linking agents. The epichlorohydrin cross-linked starch (EPIS), hexamethylene diisocyanate cross-linked starch (HDIS) and methylene diphenyl diisocyanate (MDIS) adsorbents were prepared in one step by cross-linking starch (15.0 g) using epichlorohydrin (20.0 mL), 1,6-hexamethylene diisocyanate (5.9 mL) and 4,4-methylene diphenyl diisocyanate (17.6 g) respectively. Reactant ratios applied for the synthesis were confirmed to give the best adsorption performance via preliminary studies. The details of procedure for the preparation have been reported elsewhere (Okoli et al., 2014a).
A similar procedure for the synthesis of EPIS adsorbent was adopted for the synthesis of epichlorohydrin aminated cross-linked starch (EPIAS) and epichlorohydrin methyl aminated cross-linked starch (EPIMAS) by introduction of 20 mL each of aqueous ammonia and mono-methyl amine, respectively, into the reaction mixture.
2.2.2 Characterization
Scanning Electron Micrographs (SEM images) were taken using Hitachi S4800 model Scanning Electron Microscope. ASAP 2020 Model (Micromeritics) surface and porosity analyzer was used to study the surface characteristics using N2 adsorption, and Brunauer–Emmett–Teller (BET) method was adopted for the surface area and pore analysis. Perkin Elmer model Thermogravimetric and Differential Thermal Analysis (TGA/DTA) was used to investigate the thermal behavior, phase changes and decomposition pattern of the prepared adsorbents. Infra-red (FT-IR) spectra of the synthesized adsorbent were obtained using Perkin Elmer Spectrum 1 FTIR spectrophotometer, by scanning from 4000 to 450 cm−1. Dry weight-based carbon, hydrogen and nitrogen (CHN) contents of the synthesized adsorbents were determined by Elementar (Germany) Vario Macro Cube elemental analyzer (see section SM 1 of the supplementary material for full procedure of the characterization study).
2.3 Adsorption and desorption studies
2.3.1 Adsorption setup
Batch adsorption method was adopted for this study using simulated single solute PAH polluted water samples (except for competitive adsorption study where both single and binary PAHs solutions were applied). The samples were prepared in synthetic ground water (deionized water with 44 mg/L CaCl2·H2O) and 200 mg/L Sodium azide (Na3N) to mimic environmental water and prevent PAHs biodegradation, respectively, using acenaphthylene, fluorene, and phenanthrene. Because of low water solubility, PAH stock solutions were made at high concentrations in methanol and then the experimental solutions of desired concentrations were obtained by serial dilution. The carrier solvent (methanol) was assumed to have no effect on solute equilibrium behavior due to its low concentration (<0.1% v/v) in the final experimental solution. The working concentration was chosen with due consideration on the solubility of the respective polycyclic aromatic hydrocarbons.
Adsorption experiments were carried out by adding aliquots of simulated PAHs polluted water samples into 50/100 ml capacity glass stopped Erlenmeyer flasks containing known dose of adsorbent. Aluminum foil was used to wrap the flasks to minimize possible losses by photo-degradation. The flasks were equilibrated in the dark by shaking at 170 rpm for 24 h at room temperature (25 ± 2 °C), using a horizontal shaker water bath, except where otherwise stated. After equilibrium was attained, the PAHs solution was separated from the adsorbent by centrifugation at 4000 rpm for 15 min. Thereafter, equilibrium levels of PAHs were quantified using PerkinElmer series 200 (USA) High Performance Liquid Chromatography (HPLC) system with Brownlee Analytical (PerkinElmer, USA) reverse phase C18 column (150 × 3.2 mm2, 5 μm, 110 Å) equipped with Series 200 UV and fluorescence detectors. PAHs were analyzed using isocratic elution with acetonitrile and water in the ratio of 70:30. Acenaphthylene was monitored with UV detector at UV absorbance of 254 nm, while fluorene and phenanthrene were monitored with fluorescence detector at the excitation wavelength of 224 and 252 nm, and emission wavelength of 320 and 370 nm, respectively. All experiments were done in duplicates, and the mean values were used for data analysis.
2.3.2 Adsorption experiments
Preliminary screening adsorption study was carried out using 20 mg of adsorbent (MDIS, HDIS, EPIS, EPIAS, and EPIMAS) and 50 ml aliquots of 3.0 mg/L acenaphthylene and 1.0 mg/L phenanthrene, after which the selected adsorbents (MDIS and HDIS) were subsequently subjected to detailed adsorption studies. The rest adsorption experiments applied adsorbent/solution ratio of 10 mg/50 ml, except the rate determination experiments (effect of contact time, particle size, temperature and competition) where 10 mg/100 ml was applied, and effect of adsorbent dose where variable doses were applied. The study of effect of contact time was carried out in a rate determination experiment with aliquots of 2.5 mg/L acenaphthylene, 1.0 mg/L phenanthrene and 1.5 mg/L fluorene solution, while the residual PAHs concentration was monitored at 10, 30, 90, 180, 360, 720 and 1440 min. The effect of adsorbent dose was studied by agitating 10, 20, 30, 40, 50, 70, and 100 mg of adsorbents with 50 ml aliquot of 1.0 mg/L phenanthrene solution. The effect of pH was studied using aliquots of 1.0 mg/L phenanthrene solutions of 3.5, 5.0, 6.5, 8.0, 9.5 and 11.0 pH values, while the effect of ionic strength (salinity) was studied using solutions of different ionic strengths (0, 0.01 0.05, 0.10, 0.25, and 0.5 M of Na+) that were prepared using NaCl. The effect of water hardness was carried out with aliquot of 1.0 mg/L phenanthrene solution having varying aqueous CaCO3 concentration of 50.0, 75.0, 100.0, 125.0, and 150.0 mg/L. The effect of particle size on both the rate and adsorption capacity of the adsorbents was studied in a rate determination experiment with 1.9 mg/L fluorene solution using adsorbents of particle sizes of less than 0.125 mm, 0.125–12.7 mm, 12.7–25.4 mm and above 25.4 mm, designated as sizes 1, 2, 3, and 4, respectively. The effect of initial PAHs concentration was studied by varying the initial concentrations through 1.0, 1.5, 2.0, 2.5, 3.0 and 3.5 mg/L for acenaphthylene, and 0.2, 0.4, 0.6, 0.8, 1.0 and 1.2 for phenanthrene. Study of the effect of temperature was carried out by repeating the rate determination experiment for the effect of contact time at temperatures of 25, 45, and 60 °C. The effect of competition was also investigated in a rate determination experiment using single (2.5 mg/L acenaphthylene and 1.5 mg/L fluorene) and binary solute solution system (1.0/2.5 mg/L phenanthrene/acenaphthylene and 1.0/1.5 phenanthrene/fluorene).
A blank study was done by adding a known mass of the adsorbent to a given volume of Milli-Q water free of organic traces to check whether the adsorbent could release PAHs in the aqueous solution. For every experiment, adsorbent free PAHs solutions were subjected to the same experimental conditions to evaluate the loss of PAHs to factors, other than adsorption.
2.3.3 Desorption and regeneration study
100 mg of dried spent adsorbents (adsorbed with acenaphthylene, phenanthrene and fluorene under conditions of the equilibrium experiment using 2.5, 1.0, and 1.5 mg/L respectively) was shaken at 180 rpm with a 100-fold volume of a suitable desorption solvent, and the desorbed concentration ( ), was measured after 2 h. The effect of the nature of desorption solvent was studied using acetone, methanol, dichloromethane, and hexane, while desorption kinetics was also studied by determining the desorbed quantity (and consequently desorption efficiency) at time of 2, 5, 10, 20, 30, 60, and 120 min. To evaluate the regeneration efficiency, the adsorbents were regenerated five consecutive times, and their adsorption performances were compared to that of the fresh adsorbents.
2.3.4 Application of the developed adsorbents in real environmental water treatment
The applicability of the developed adsorbents for treatment of real water samples was tested with water samples collected from Sahe river (40°7′43.36″N, 116°19′9.43″E) and Shangzhuang (40°5′59.6″N, 116°12′17.3″E) reservoir of Beijing, China. The initial PAHs concentration was first determined following standard procedure, after which aliquots of the samples were spiked with 1.0 mg/L mixed concentrations of acenaphthylene, phenanthrene, and fluorine. Adsorption performance of the developed adsorbents was studied by applying 10 mg of the adsorbents on 100 ml of both spiked and un-spiked water samples following standard adsorption procedure.
2.4 Data treatment
Quantity of PAHs adsorbed by the adsorbent after the specified incubation period was calculated using Eq. (2.1)
The sorption data were explained using four kinetics models: Lagergren (1898) pseudo-first and -second order, Morris–Weber intra-particle diffusion and liquid film diffusion models (Okoli et al., 2014b); three adsorption isotherm models: Langmuir, Freundlich, and BET models (Febrianto et al., 2008); as well as the thermodynamic parameters: free energy change (ΔG), enthalpy change (ΔHo), and entropy change (ΔSo) (see section SM2 of the supporting materials for details of the models and computation).
Desorption and regeneration studies applied Eqs. (2.2) and (2.3) for the evaluation of Desorption Efficiency (DE) and Regeneration Efficiency (RE) respectively.
3 Result and discussion
3.1 Characterization of the adsorbents
The result of surface and pore analysis of the adsorbents is shown in Table 1. The values of BET specific surface area of the synthesized adsorbents were higher than those of un-modified starch (0.980 m2/g); hence, it can be concluded that cross-linking process led to improvement in the surface and porosity characteristics of the cross-linked polymer adsorbents. This observation laid credence to the established principle that cross-linking and amination processes increase the polymer network of the adsorbents, thus increasing the surface area (Delval et al., 2005). The values of the pore surface/total surface area ratio of the adsorbents, which reflect the extent of adsorption that takes place on the exterior, as well as the interior surfaces of the adsorbents, indicate that MDIS might likely have the slowest adsorption rate, despite having the highest surface area, since majority of MDIS adsorption is likely to occur on the interior surfaces of the adsorbents.
Adsorbent
EPIS
EPIAS
EPIMAS
HDIS
MDIS
BET surface area (m2/g)
4.3091
5.7891
7.5713
3.2804
40.3858
BJH adsorption surface area of pores (m2/g)a
2.9023
4.0237
5.8931
1.9820
40.0851
Pore surface/total surface area
0.6735
0.6950
0.7783
0.6042
0.9925
Total pore volume (cm3/g)
0.017281
0.01149
0.02047
0.007463
0.129811
BJH adsorption pore volume (cm3/g)a
0.03241
0.03562
0.05830
0.02361
0.188124
BET pore width (4V/A) (Å)
104.3561
110.6350
99.8130
91.0011
128.5709
BJH pore width (4V/A) (Å)a
503.2710
497.4724
602.4732
476.473
187.7271
The scanning electron micrograph (Fig. 1) revealed that the surface of the starch precursor (Fig. 1c and d) was relatively smooth, while the surface of the cross linked polymer counterparts (MDIS – Fig. 1a, HDIS – Fig. 1b) exhibited noticeable pores, thus corroborating higher BET surface areas observed for these adsorbents. Also, the SEM images revealed that cross-linking process leads to agglomeration in physical terms, as the particles of starch were globular in shape and of different sizes while the cross linked polymer counterparts were agglomerates of irregular shapes and sizes, indicating the formation of bulkier polymer units emanating from the cross linking process.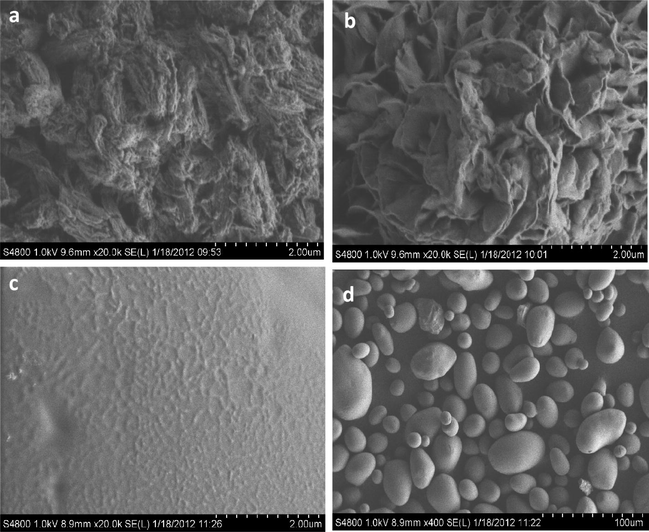
Scanning electron micrographs of (a) MDIS, (b) HDIS adsorbents, (c) pristine starch (20,000×) and (d) pristine starch (400×).
The TGA and DTA plots of MDIS and HDIS polymer adsorbents are shown in Fig. 2. TGA showed mass losses in three steps while the DTA revealed the thermal events corresponding to these losses. For MDIS, the first mass loss was between 30 – 104 °C (3%) and corresponding to the peak at 47 °C (Fig. 2a), which is attributed to the dehydration that occurs in a single step. Once dehydrated, the compound is stable up to 260 °C and above this temperature the thermal decomposition occurs in two consecutive and/or overlapping steps between 260 and 670 °C. The first mass loss (39.5%) of the anhydrous compound was observed between 260 – 366 °C corresponding to the sharp exothermic peak at 350 °C with oxidative process. The last mass loss (57.5%) was between 366 – 513 °C corresponding to the exothermic peak at 520 °C, ascribed to the oxidation of the organic matter. The trend for HDIS (Fig. 2b) was similar to that of MDIS, except that the phase changes occurred at different temperatures.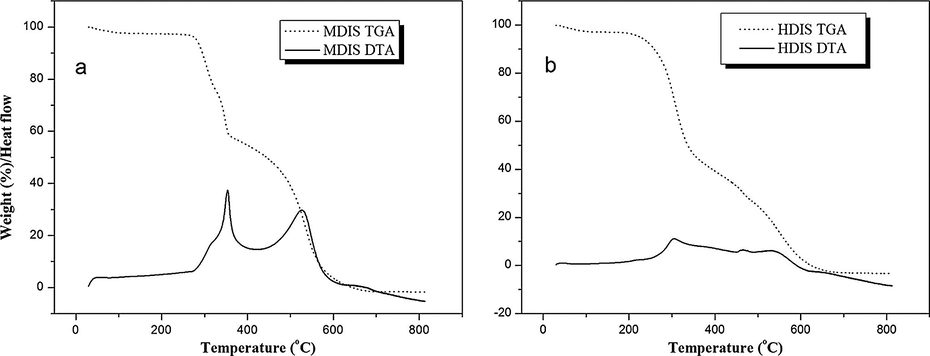
TGA/DTA plots of (a) MDIS and (b) HDIS adsorbents.
The presence of stretching vibration of C–H bonds of glucose units (2905 cm−1), C–O (1320–1000 cm−1) and O–H (3500–3200 cm−1) stretching vibrations of polymeric compounds especially polysaccharides, in addition to the characteristic broad peaks of anomeric C–H ring deformations (554, 596, 662, 567 and 580 cm−1 for EPIS, HDIS, MDIS, EPIAS and EPIMAS, respectively) in the infrared spectra of the polymers (Fig. S1), showed that the starch structural backbone was retained in the cross-linked polymer adsorbents. The discussion of infra-red characterization of EPIS, HDIS and MDIS has been reported in our previous study (Okoli et al., 2014a). The presence of starch C–N vibrations (1719 and 1712 cm–1 for EPIAS and EPIMAS, respectively), aliphatic C–N stretch (1167 and 1151 cm–1 for EPIAS and EPIMAS, respectively), and N–H wag (754 cm−1 for EPIAS alone) in the spectra of the for EPIAS and EPIMAS confirmed the successful introduction of nitrogen atom into the starch polymer matrix. This is a desirable property since Density Functional Theorem (DFT) model studies (Okoli et al., 2014a) have shown that nitrogen being an electron rich center, will increase the polarizability of the cross linked starch polymer, which is subsequently expected to enhance the sorption properties of the polymers.
The analysis of elemental composition data revealed that the cross-linking process led to improvement in the level of hydrophobicity and aromaticity (for MDIS alone) of the synthesized polymers. The data for EPIS, HDIS, and MDIS polymer adsorbents have been previously reported (Okoli et al., 2014a). The elemental composition of EPIAS and EPIMAS polymers (Table S1) showed a positive linear correlation between the nitrogen content and the quantities of amination reagents applied in the synthesis. The values of (O + N)/C atomic ratios, which are an important parameter for predicting sorption, ranged from 1.08–1.14 and 1.16–1.20 for EPIAS and EPIMAS, respectively. These values are not favorable when compared to the range of some commercial lignin (0.33–0.94), which is a reference adsorbent for aromatic pollutants reported in the literature (Wang and Xing, 2007). The N/C value (which is also an important factor that reflects the polarizability α, of the polymer adsorbents), for all the aminated adsorbents, showed a positive correlation with the degree of amination. The C/H atomic ratio, an indication of hydrophobicity, for the EPIAS adsorbents showed no significant change, while EPIMAS showed little increment with a progressive increase in the quantity of amination agent. This observation is in consonance with the fact that aqueous ammonia had no carbon, and thus did not contribute to increment in carbon content, while monomethyl amine incorporated the carbon atom of its methyl group into the starch polymer matrix.
3.2 Adsorption studies
3.2.1 Screening adsorption studies
Preliminary screening study gave the mean adsorption capacities of EPIS, HDIS, MDIS, EPIAS, and EPIMAS as 0.77, 1.11, 2.24, 1.22 and 1.27 for acenaphthylene, and 0.59, 0.70, 1.03, 0.24, and 0.35 mg/g for phenanthrene, respectively. The result showed that MDIS showed the highest adsorption capacity for both PAHs. This can be attributed to the higher affinity in the form of π–π interaction between the aromatic ring of the MDIS polymer matrix and that of PAH molecules, which does not exist in other adsorbents because of non-existence of aromatic rings, as well as high surface and pore characteristics of MDIS polymer as revealed by the BET surface study. The HDIS adsorbent showed moderate adsorption capacity while the EPIS had the least adsorption capacity for acenaphthylene. The aminated cross-linked starch (EPIAS and EPIMAS) set of polymers were second in performance for the small molecular size PAHs as represented by acenaphthylene, but not as good for the relatively higher molecular size PAHs as represented by phenanthrene. The HDIS displayed poor sorption performance in terms of sorption of small size acenaphthylene, and this can be justified on the fact that HDIS exhibited the least surface area.
However, HDIS showed better sorption for relatively larger molecular size phenanthrene, than EPIS polymer. This observation can be explained on the principle of steric hindrance. It has been established that adsorption of high molecular mass PAHs occurs on the sorption sites created by the functional groups of the interfacial spaces of the polymer network of cross linked starch adsorbents, in addition to adsorption at the polymer surfaces (Okoli et al., 2014a). Since the size of the interfacial spaces of the starch polymer network is regulated by the spacer arm length of the cross-link unit, the size of interfacial space for HDIS is expectedly bigger than that of the EPIS polymers due to the fact that the length of HDI cross-link unit is longer than that of EPI polymers. Hence, the effect of steric hindrance on adsorption will be more in EPIS than in HDIS set of polymers.
From the foregoing, MDIS and HDIS were selected for detailed adsorption studies because of their relatively high sorption capacity and wide spectrum of adsorption for the target analytes. The application of EPIS is limited by its very low adsorption capacity, while the aminated counterparts, EPIAS and EPIMAS were not considered because of the relatively more complex synthetic procedure, as well as their low adsorption capacity.
3.2.2 Effect of time and kinetic study
The plot of quantity adsorbed at given time, qt against time, t (Fig. 3) shows the observed trend of the PAHs uptake rate. For phenanthrene, it was observed that there was a gradual increase in pollutant uptake from 42.7% and 33.5% at 10 min to a maximum of 87.6% and 63.0% at 720 min, and then the least increase to 91.50% and 63.4% at 1440 min for MDIS and HDIS, respectively. Acenaphthylene and fluorene also exhibited similar trends observed for phenanthrene. This observation can be attributed to the fact that a large number of vacant surface sites were available for adsorption during the initial stage, and with lapse of time, the remaining vacant sites would continually reduce. From the study therefore, the time required to attain equilibrium was 720 min. However, a contact time of 1440 min has been employed for all other studies just to monitor the possibility of further adsorption after this time.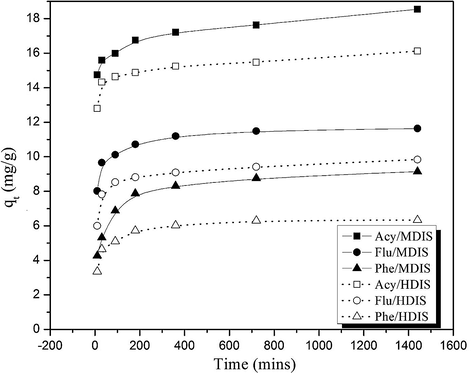
Plots for adsorption of PAHs on (a) MDIS and (b) HDIS adsorbents.
Table 2 showed the comparative fit of the various models. In elucidating the kinetic mechanism, it is generally known that a typical liquid/solid adsorption involves three consecutive steps: (1) diffusion across the liquid film surrounding the adsorbent particles, i.e., external or film diffusion; (2) diffusion in the liquid contained in the pores and/or along the pore walls, which is the internal or intra-particle diffusion; and (3) adsorption and desorption between the adsorbate and active sites of the adsorbent, otherwise known as mass action. One of these steps usually offers much greater resistance than the others and may thus be considered as the rate-limiting step of the process (Qiu et al., 2009; Zhang et al., 2014). In practice however, unless chemical modifications occur during the sorption, the third stage is assumed to be too fast to contribute significantly to the overall sorption rate. It is thus generally understood that slow sorption kinetics are caused by rate-limiting diffusive mass transfer (Ball and Roberts, 1991).
PAHs adsorbates
Phenanthrene
Acenaphthylene
Fluorene
Starch adsorbents
MDIS
HDIS
MDIS
HDIS
MDIS
HDIS
Experimental qe
9.1450
6.3380
18.5480
16.1220
11.6370
9.8410
Pseudo-first order model
K1 (/min)(×10−3)
2.4415
2.4905
3.5407
4.6597
2.6013
3.2407
qe calc (mg/g)
3.2144
1.4940
4.1915
3.4613
2.8289
1.8387
R2
0.9276
0.8099
0.9171
0.8739
0.9376
0.2240
Pseudo-second order model
K2 (g/mg min)(×10−3)
3.8372
2.0842
3.4371
5.3434
7.4959
5.8582
qe calc (mg/g)
9.2593
6.4103
18.5632
16.1238
11.7054
9.8746
h (mg/g min)
0.3292
0.8564
1.1844
1.3883
1.0271
0.5709
R2
0.9994
0.9999
0.9992
0.9994
0.9999
0.9993
Morris-Weber intra-particle diffusion model
Kid (g/mg min1/2)(×10−2)
12.9590
7.2540
10.0490
7.4290
8.6620
8.5250
C
5.0870
4.1630
14.9859
13.5619
8.9806
7.0983
R2
0.7311
0.6388
0.9212
0.7265
0.6596
0.6278
Liquid film diffusion
Ri (/min)(×10−3)
2.4400
2.4900
3.5400
4.6600
2.5700
2.5804
R2
0.9276
0.8099
0.9171
0.8739
0.9036
0.9393
The good correlation between the calculated qe values and the experimental values, as well as the correlation coefficient (r2) of pseudo-second order model for the kinetic data indicated that the mass action aspect of the sorption mechanism of PAHs onto cross-linked adsorbent surfaces is governed by pseudo-second order kinetics model. This consistency of data indicates that PAHs adsorption on the sorption sites of the adsorbents followed pseudo-second order kinetics and is controlled by physical adsorption (physisorption). This indicates that the interaction mechanism that took place between the adsorbents and PAHs molecules is weak interactions such as hydrophobic, van der Waals and π–π interactions (for MDIS only). The existence of the weak interactions can be explained on the basis of chemical structural properties of the polymer adsorbents as elucidated in the FTIR and elemental analysis data, and the PAH molecules. For instance, the presence of phenyl ring on the adsorbents, as revealed by the FTIR spectrum of MDIS, is favorable to increase the adsorption capacity and affinity of the aromatic rings on PAH molecules by π–π interaction (Shao et al., 2010), while the increased hydrophobicity that resulted from the cross-linking process, as revealed by the elemental analysis data, increases the potential for exhibition of hydrophobic interaction. It is, thus, assumed that adsorption capacity is proportional to the number of active sites occupied on the sorbent surface. Table 2 also showed that liquid film diffusion and intra-particle diffusion models both quite described the sorption data. Since the intra-particle diffusion plot does not pass through the origin ( ), it confirmed that intra-particle diffusion is not the only rate-controlling step (Zhang et al., 2014). Hence, both liquid film diffusion and intra-particle diffusion contributed to the rate limiting step of the entire kinetic mechanism of the sorption process.
3.2.3 Effect of adsorbent dose
The amount of adsorbent used in adsorption unit process is crucial for economic reasons. It is therefore necessary, that the effects of adsorbent dose are analyzed for the optimization and selection of the best required dose for scale-up and designing large scale equipments (Shao et al., 2011). It was observed that increasing the dosage from a range 0.005 g through 0.050 g increased the percentage removal of phenanthrene from aqueous solution from 35.70% to 86.47%, and 83.78% to 97.10%, for HDIS and MDIS polymers respectively, while on the other hand, the equilibrium adsorption capacity, qe, per unit mass of these adsorbents was found to decrease (3.57 mg/g to 0.86 mg/g and 8.38 mg/g to 0.97 mg/g for HDIS and MDIS polymers respectively) with increment in adsorbent dose (Fig. S2). It is apparent that by increasing the amount of adsorbent, available sorption sites for sorbent–solute interaction are increased due to increased available surface functional group, thus leading to observable increment in percentage PAHs removal from aliquot solution. The qe decrease with mass is attributed to the decreasing total surface area of the adsorbent and an increase in diffusion path length due to aggregation of cross linked starch polymer particles. As the weight of the polymer increased, the aggregation becomes increasingly significant. Therefore, it is apparent that the choice of dose for these adsorbents will depend on which of the parameters (adsorption capacity or efficiency) the operator wants to maximize. However, the plots (Fig. S2) revealed that the optimum values were ≈9 mg and 15 mg per 100 ml for MDIS and HDIS, respectively. However, this study adopted 10 mg to maintain uniformity.
3.2.4 Effect of particle size
Difficulties in phase separation after adsorption have limited the application of powdered adsorbents in water decontamination (Olu-owolabi et al., 2012). Particles with bigger size are relatively easier to prepare than those of finer particles. However, for adsorbents with moderate porosity like the ones under study, increase in particle size reduces the available surface sorption sites. In some cases, adsorbents with low density float on water when the particles are too small. Particle size is therefore an important factor that needed to be moderated. The study showed that the particle size had negative correlation to both the equilibrium sorption capacity and sorption rate for the two adsorbents under study (Fig. S3 and Table S2). The experimental adsorption capacities (qe expt.) for sizes 1, 2, 3, and 4 were 8.57, 8.43, 7.62, and 5.96 mg/g respectively for MDIS, while those of HDIS for sizes 1, 2, 3, and 4 were 7.20, 6.69, 5.81, and 5.22 mg/g respectively. Also, the rate constant k2 values for sizes 1, 2, 3, and 4 were 0.013, 0.0053, 0.0012, and 0.0005 for MDIS, while those of HDIS for sizes 1, 2, 3, and 4 were 0.0124, 0.0100, 0.0030, and 0.0024 mg/g respectively. Considering the fact that the values for qe and k2 were very close for both sizes 1 and 2 of the adsorbents, size 2 was considered to be suitable as the optimum size.
3.2.5 Effect of pH, water hardness and salinity
The result of the study (Table S3) showed that pH changes did not significantly affect adsorption of phenanthrene onto MDIS adsorbent from very low to moderately high pH values. The adsorption capacity (qe values) of the adsorbent was relatively constant at 4.53 mg/g from the pH of 3.5 to 8.0, and thereafter rose slightly from 4.53 to 4.88 mg/g as the pH rose from 8.0 to 11.0. This observation is expected, and stems from the fact that both the surfaces of the adsorbents and the PAHs molecules are not charged. Hence, change in hydrogen ion concentration is expected to have minimum effect on the adsorbent–PAHs interaction. However, at very high pH values, there was a significant increment in adsorption performance. This can be explained on the basis that increasing pH induced deprotonation of the OH functional group on adsorbent surface and enhanced the π-electron-donor ability of the surface and thus strengthened π–π electron-donor–acceptor interactions of the aromatics.
Though Ca2+ was incorporated in all the working solutions used for the adsorption studies to simulate environmental waters, independent study was necessary to investigate the effect of these ions on the PAHs removal. The results showed that the adsorption efficiency of phenanthrene on the MDIS adsorbent was not significantly affected at any of the five different Ca2+ concentrations as the adsorption capacity slightly increased from 4.55 to 4.75 mg/g. The adsorption capacity was relatively steady (4.51–4.49 mg/g) as the alkalinity increased from 0.0 to 0.1 mol/L, but increased to 4.97 mg/g as the alkalinity rose to 0.5 mol/L. This indicated that alkalinity has no significant effect on the sorption of PAHs by cross linked starch adsorbents. This observation can be explained by the simple fact that neither the adsorbent nor the adsorbate had charged moieties. However, at relatively high values, increment in salinity led to resultant increment in adsorption performance. This situation can be attributed to the principle of “salting out” which is predicated on the fact that solubility of non-polar organics decreases with increment in ionic strength.
3.2.6 Effect of initial PAHs concentration and isotherm study
The data for this study (Fig. 4) showed that PAHs sorption was concentration dependent and increased with an increase in initial PAHs concentration. Increment in adsorbate uptake capacity with the increment in initial concentration is generally expected due to higher availability of the adsorbates in solution which provided increased driving force to overcome all mass transfer resistance of the adsorbates between the aqueous and solid phases. This situation resulted in higher probability of collision between PAHs and active sites of the adsorbent leading to higher pollutant uptake.
Effect of initial concentration on the adsorption of (a) phenanthrene and (b) acenaphthylene by MDIS and HDIS adsorbents.
The adsorption capacity and surface properties of the two adsorbents were assessed using the Langmuir, Freundlich, and Brunauer–Emmet–Teller (BET) sorption isotherms. An isotherm is said to fit the adsorption data if the correlation coefficient (r2) value is closer to unity than any other isotherm as well as the estimated adsorption capacity (qe calc.) being close to the experimental value. The model parameters (Table S3) showed that Langmuir model gave better r2 values (0.8223 ⩽ r2 ⩽ 0.9669) than Freundlich (0.7464 ⩽ r2 ⩽ 0.9254) and BET (0.2295 ⩽ r2 ⩽ 0.8728), whereas the values of qe calc. were better correlated with the experimental qe values for other isotherm models. The complexity of simultaneously comparing these variables is made simpler by solving for qe calc. from the non-linear forms of these models using the model parameters obtained from their linear forms (Foo and Hameed, 2010). The non-linear plots of these isotherm models (Fig. 5) revealed that the experimental adsorption process for phenanthrene for both adsorbents was best described by BET isotherm and seconded by Freundlich, while that of acenaphthylene was best described by Freundlich and seconded by BET isotherms. However, both cases are still in tandem with the established fact that adsorption of PAHs occurs via multilayer process (Olivella et al., 2011). Thus, adsorption of PAHs onto the polymer surfaces which occurred via multilayer adsorption can be explained on the basis of π–π stacking interaction that is exhibited by PAHs.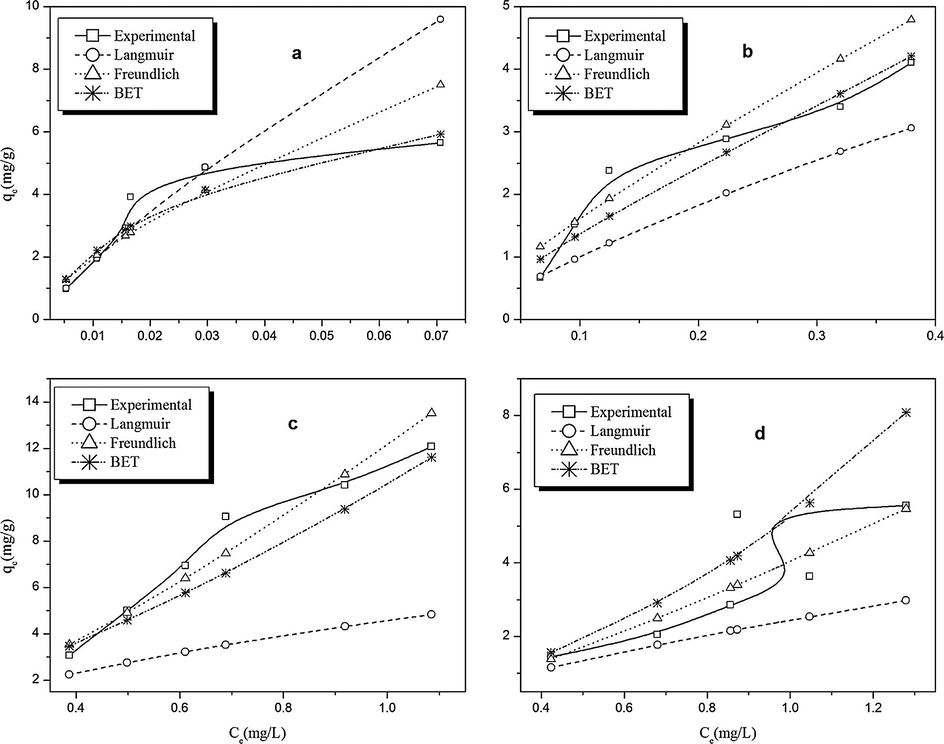
Graphical representation of the fitting of the experimental data (solid lines) into the isotherm models (dotted lines) for the following adsorbent/adsorbate pairs: (a) MDIS/Phenanthrene, (b) HDIS/Phenanthrene, (c) MDIS/Acenaphthylene and (d) HDIS/Acenaphthylene.
The experimental adsorption isotherms of the studied PAHs for MDIS and HDIS adsorbents are shown in Fig. 5 (solid lines). According to the classification of Giles et al. (1960), initial convex curve of the adsorption isotherms indicates an L-type isotherm profile, while initial concave curve indicates an S-type of isotherm profile. The plots of Fig. 5 showed that the equilibrium sorption curves for MDIS/Phenanthrene, HDIS/Phenanthrene, and MDIS/Acenaphthylene were of the L-type profile, while those of HDIS/Acenaphthylene were borderline of L-type and S-type isotherm profile.
According to Giles classification, the initial curvature of the L-type isotherm profile shows that as more sites in the substrate are filled it becomes increasingly difficult for a bombarding solute molecule to find a vacant site available. This implies either that the adsorbed solute molecule is not vertically oriented (as against the S curve) or that there is no strong competition from the solvent. Since PAHs are non-polar and the solvent is polar, there is no likelihood of strong competition between the adsorbates (PAHs) and the solvent. Hence, the only explanation for the exhibition of the L-type isotherm is that the PAH molecules were adsorbed flat. Though the current study is the first study on the adsorption of PAHs by cross-linked starch polymer adsorbents, previous studies on PAHs adsorption unto cork waste adsorbent by Olivella et al. (2011) had reported that PAH molecules were adsorbed flat.
For the S-type adsorption isotherm, the initial direction of curvature shows that adsorption becomes easier as concentration rises. In practice, the S-type usually appears when the following three conditions are fulfilled: the solute molecule (a) is monofunctional, (b) has moderate inter-molecular attraction, causing it to pack vertically in regular array in the adsorbed layer, and (c) meets strong competition for substrate sites, from molecules of the solvent or of another adsorbed species. The definition of “monofunctional’’ in this context is that the solute molecule has a fairly large hydrophobic residue (>C5), a marked localization of the forces of attraction for the substrate over a short section of its periphery, and that it is adsorbed as a single unit and not in the form of a micelle (Giles et al., 1960). Since PAHs are non-polar and the solvent is polar, there is no likelihood of strong competition between the adsorbates (PAHs) and the solvent. Hence, only two conditions were met by the PAHs/adsorbent system; consequently, the HDIS/Acenaphthylene system did not exhibit complete S-type isotherm.
On first approximation, all the observed isotherm profiles approach linear isotherm, otherwise known as the C-type isotherm profile, which is characterized by a constant partitioning of the adsorbate in the adsorbent and is obtained for solutes that penetrate into the solid more readily than the solvent. Overall, the adsorption isotherm of PAHs for the studied adsorbents, was the combinations of S-type and L-type isotherms, with elements of C-type in all the cases. This stems from the fact that the PAHs/adsorbent system did not completely meet all the conditions for any of the isotherm types. Hence, the implication of the partial fulfillment of the requirements for complete manifestation of these isotherms resulted in the exhibition of the borderline types of these principal isotherms. This observation indicated that (i) the adsorbates (PAHs) are monofunctional, (ii) PAHs have moderate inter-molecular attraction, causing it to pack flat in regular array in the adsorbed layer and (iii) there is no strong competition from the solvent.
3.2.7 Effect of temperature and thermodynamic study
The experiment was designed to investigate the effect of temperature on both the rate and adsorption capacity of the adsorbents. The result showed that increase in temperature led to a corresponding increment in the adsorption rate as evidenced in the values of rate constant, k2 and initial reaction rate, h (Table S5). This effect may be due to the fact that at higher temperatures, the increase in heat and the subsequent kinetic energy led to increased mobility of the solute, which results in higher adsorption rates. Also, the observed positive relationship between the temperature and k2, and h still confirmed the fact that pseudo second order is not the rate limiting step (Boparai et al., 2011).
Since solubility of PAHs increases with increase in temperature, the quantity adsorbed at equilibrium qe is expected to have a negative correlation with temperature. However, the data showed that for phenanthrene, there was no significant change in equilibrium sorption capacity for MDIS polymer, whereas there was a noticeable increment in the sorption capacity values for HDIS. The trend became clearer with sorption data of acenaphthylene where the sorption capacity increased from 1.18 to 2.79 mg/g and 1.39 to 5.72 mg/g for MDIS and HDIS polymer adsorbents respectively. This observation can simply be explained by the fact that at higher temperature, starch based adsorbents swell in aqueous solution thus increasing access to more sorption sites that were hitherto inaccessible at lower temperatures. This increment in adsorption is in tandem with the principle of maximization of the surface area. According to Erto et al. (2010), maximization of surface area is a valid criterion for design/synthesis and selection of a suitable sorbent for adsorption of non-ionic organic compounds from waters. The marked difference in the magnitude of the increment of qe for MDIS and that of HDIS can be explained in terms of the difference in their swellability as shown by a preliminary swellability test (result not shown). This study also showed that the linearity of this observed trend was more visible for acenaphthylene than phenanthrene. This can as well be attributed to molecular size screening/steric effect, due to the fact that even with the swelling and the subsequent increase in pore size, the solutes with smaller molecular size will have more access to the hitherto inaccessible sorption sites than their bigger counterparts. This observation is another merit of these set of noble adsorbents, since it confers the adsorbents, the capacity to be applied for treating industrial effluents without compulsorily cooling them to ambient temperature unlike other adsorbents.
Investigations of the thermodynamic parameters of an adsorption process are necessary to ascertain the spontaneity of the process. The values of the thermodynamic parameters: free energy change (ΔG), enthalpy (ΔH) and entropy (ΔS) are shown in Table 3. The ΔG values for all the adsorbent–adsorbate systems were negative, indicating that the sorption process is spontaneous. It was also observed that as the temperature increases, ΔG decreases indicating the feasibility of adsorption at higher temperatures. ΔH values were positive, indicating endothermic process, except for adsorption of phenanthrene on MDIS which exhibited exothermic process with negative ΔH values (Table 3). ΔS values of sorption process were positive, which indicated increased randomness at the adsorbent/solution interface.
Adsorbate/adsorbent
ΔH (kJ/mol)
ΔS (J/mol/K)
ΔG (kJ/mol)
298 K
318 K
333 K
Phenanthrene
MDIS
−6.80
19.83
−12.71
−13.11
−13.4
HDIS
11.56
63.43
−7.34
−8.61
−9.56
Acenaphthylene
MDIS
13.49
73.95
−8.55
−10.02
−11.13
HDIS
15.7
77.38
−7.36
−8.91
−10.07
3.2.8 Effect of competition
Knowledge of competitive sorption characteristics is critical for the environmental application of these adsorbents in wastewater treatment because real environmental water samples always contain potpourri of organic pollutants. The study showed that the presence of other aromatic compounds in the aqueous media enhanced the adsorption of PAHs, as evidenced by the values of the adsorption capacities in single and binary solutions (Table 4). This observation is predicated on π–π electron interaction between the PAH molecules. Recalling the fact that isotherm studies had confirmed that PAH molecules are adsorbed flat on the surfaces of these adsorbents, it is obvious that since these molecules are not chemically bonded to atoms of the adsorbents, the aromatic core of the adsorbed PAH molecules will exhibit stronger π–π interaction than free surface of the adsorbents. Hence, adsorbed PAHs molecules will act as better adsorption sites than vacant sites on the surfaces of the adsorbents. The resultant effect is that PAHs will prefer to adsorb in multi-layers than maintaining a monolayer. This is in consonance with the findings of the isotherm studies which confirmed that PAHs adsorption was best described by Freundlich and BET isotherm models, which are characteristics of multi-layer adsorption.
Adsorbents
PAHs solution
k2
h
qe
MDICS
Fluorene single
0.0075
1.0271
0.9999
11.7055
Fluorene binary
0.0042
0.6368
0.9993
12.2594
HDICS
Fluorene single
0.0059
0.5709
0.9993
9.8746
Fluorene binary
0.0055
0.5535
0.9992
10.0030
MDICS
Acya single
0.0034
1.1844
0.9992
18.5632
Acy binary
0.0021
0.8666
0.9989
20.4165
HDICS
Acy single
0.0053
1.3890
0.9995
16.1238
Acy binary
0.0052
1.4619
0.9998
16.7757
3.2.9 Evaluation of sorption performance
One of the suitable ways of evaluating sorption performance of adsorbents for a particular pollutant is by comparing the sorption coefficient of the adsorbent under investigation, to those of other adsorbents reported in the literature. Sorption coefficient, Kd, was calculated from the slope of a plot of quantity adsorbed at equilibrium, qe, against equilibrium concentration, Ce, (
). The values of sorption coefficients for phenanthrene using different adsorbents as reported in the literature, and the values from this study are shown in Table 5. It has been observed from the data that MDIS, one of the adsorbents developed from this study and which is equally considered as cheap alternative to more costly materials such as synthetic resins and activated carbons, is only second to activated carbon in terms of the values of their sorption coefficient. The value for HDIS showed that its sorption performance, though lower than the value for MDIS, is as good as that of lignin, which is equally accepted as good adsorbent for aromatic compounds.
Sorbents
log Kd
Source
Cross linked starch adsorbents
HDIS
3.97
Present study
MDIS
4.79
Algae
Botryococcus braunii
4.13
Salloum et al. (2002)
Sargassum hemiphyllum
3.83
Chung et al. (2007)
Fungi
White-rot fungi
3.83
Chen et al. (2010)
Plant based materials
Wood chip
3.40
Chen et al. (2011)
Orange peel
3.47
Plant roots
3.32–3.70
Zhu et al. (2007) and Chen et al. (2011)
Plant cuticles
4.21–4.73
Li and Chen (2009)
Leaves
3.52–4.05
Lin et al. (2007) and Zhu et al. (2007)
Pine bark
3.53
Li et al. (2010)
Natural organic matter
Cellulose
2.98
Collagen
4.47
Salloum et al. (2002)
Lignin
4.03
Modified biosorbents
Aspen wood fiber
3.60–3.67
Huang et al. (2006)
Bleached wood
3.24–3.26
Temperature hydrolyzed wood fibers
4.03–4.75
Surfactant modified fibric peat
4.42
Tang et al. (2010)
Acid hydrolysis of pine bark
4.23
Li et al. (2010)
Geosorbents and abiotic sorbents
Humic acid
4.45
Salloum et al. (2002)
Pula kerogen
4.76
Nature chars
4.83–6.31
Fibric peat
4.11
Tang et al. (2010)
Artificial wood chars
5.19–7.20
James et al. (2005)
Active carbon
5.70–5.90
3.2.10 Regeneration of spent adsorbent (desorption study)
Fig. 6 shows desorption efficiencies for acenaphthylene, phenanthrene, and fluorene from MDIS and HDIS adsorbents using various desorption solvents. The trend for all the studied nature of desorption solvent was acetone > DCM > hexane > methanol. This trend can be explained on the basis of polarity, except for methanol which had the least desorption ability, despite having same polarity index value of 5.1 with acetone. This observation might likely be linked to the presence of different functional groups in acetone and methanol, and their associated interactions with the adsorbents. Hence, acetone which is an aldehyde (R-CHO) will likely interact more with the adsorbent than methanol which is an alcohol (R-OH). It was equally observed that HDIS had poor desorption efficiency, hence cannot be effectively regenerated. Also, the rate plot showed that desorption process was fast and adjudged to be complete in less than 10 min (Fig. S4). The regeneration efficiency tests showed that the adsorption performance of the regenerated MDIS adsorbent was still more than 70% of the original performance, even after five consecutive regenerations (Fig. S5). This implied that these adsorbents could be advantageously be reused several times, hence a very big comparative cost advantage over other common adsorbents for PAHs.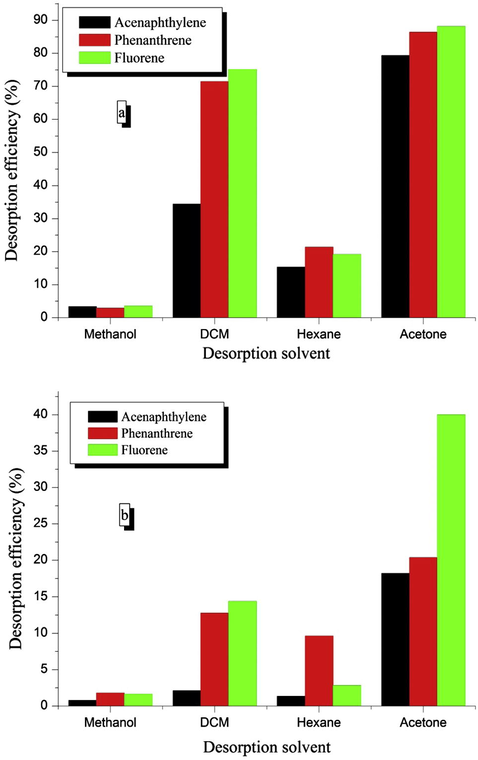
Desorption efficiency of PAHs on (a) MDIS and (b) HDIS using different desorption solvents.
3.2.11 Application of the developed adsorbents for treatment of real environmentally polluted water samples
The adsorption capacities for acenaphthylene, phenanthrene, and fluorene for the spiked environmental water samples were 6.04, 8.59, and 6.81 mg/g for MDIS, and 4.91, 6.82, and 5.04 mg/g for HDIS, respectively. Though the values were slightly lower than the values obtained for simulated water samples (7.57, 9.14, and 7.79 mg/g for MDIS, and 6.43, 7.03, and 6.6 mg/g for HDIS, respectively), the adsorbents can be said to have exhibited good adsorption performance for the treatment of spiked environmentally polluted water samples. The drop in performance can be attributed to the presence of numerous other competing pollutants in real environmental water samples. PAHs were not detected after adsorption treatment of the un-spiked environmental water samples, which is adjudged to contain ambient (environmentally obtainable) levels of these pollutants, and thus confirmed the effectiveness of the adsorbents.
4 Conclusion
This study demonstrated the treatment of aqueous PAHs pollution by adsorption using cross-linked starch adsorbents. The results obtained show that MDIS and HDIS adsorbents, easily synthesized starch based polymers, can be applied for the treatment of PAHs polluted water. Adsorption is affected by a number of factors, especially contact time, PAHs concentrations, adsorbents dose and particle size, temperature of aqueous media, and to a less extent pH, water harness and salinity. Adsorption was deemed to be complete in reasonable time. Efficient desorption of PAHs and regeneration of adsorbents using acetone (relatively non-toxic and cheap organic solvent) made the developed adsorbents a viable alternative to the common adsorbents available for treatment of aqueous PAHs pollution. Application of these adsorbents in real environmental water samples confirmed their effectiveness and efficiency, and consequently the relevance of this study. Considering their good adsorption capacity, ease of synthesis, as well as availability of starch precursor at relatively low cost, the adsorbents are promising cost-effective adsorbent, and thus recommended for control of aqueous PAHs pollution.
Acknowledgements
The authors of this work acknowledge the support of The World Academy of Sciences (TWAS) and the Chinese Academy of Sciences (CAS) for providing fellowship to Peter Chukwunonso Okoli (FR number: 3240240233) at the Institute of Geographic Sciences and Natural Resources Research (IGSNRR), CAS, Beijing, China, where this research was carried out. The research was financially supported by the One Hundred Talents Program of the Chinese Academy of Sciences, 863 Program (2013AA06A211-2). We are equally grateful to Mr. Jianli Wang, of Centre for Environmental Remediation Unit, for his assistance in the laboratory instrumentation.
References
- Evaluation of the levels of polycyclic aromatic hydrocarbons in surface and bottom waters of Lagos Lagoon. Afr. J. Pharmaceut. Sci. Pharm.. 2012;3:1.
- [Google Scholar]
- Application of biosorption for the removal of organic pollutants: a review. Process Biochem.. 2005;40:997-1026.
- [Google Scholar]
- Long-term sorption of halogenated organic chemicals by aquifer material. Environ. Sci. Technol.. 1991;25:1223-1249.
- [Google Scholar]
- Utilization of agro-industrial and municipal waste materials as potential adsorbents for water treatment: a review. Chem. Eng. J.. 2010;157:277-296.
- [Google Scholar]
- Kinetics and thermodynamics of cadmium ion removal by adsorption onto nanozerovalent iron particles. J. Hazard. Mater.. 2011;186:458-465.
- [CrossRef] [Google Scholar]
- Biosorption and biodegradation of polycyclic aromatic hydrocarbons in aqueous solutions by a consortium of white-rot fungi. J. Hazard. Mater.. 2010;179:845-851.
- [Google Scholar]
- Removal of polycyclic aromatic hydrocarbons from aqueous solution using plant residue materials as a biosorbent. J. Hazard. Mater.. 2011;188:436-442.
- [Google Scholar]
- Removal of aqueous phenanthrene by brown seaweed Sargassum hemiphyllum: sorption-kinetic and equilibrium studies. Sep. Purif. Technol.. 2007;54:355-362.
- [Google Scholar]
- Recent developments in polysaccharide-based materials used as adsorbents in wastewater treatment. Prog. Polym. Sci.. 2005;30:38-70.
- [Google Scholar]
- Preparation, characterization and sorption properties of cross-linked starch-based exchangers. Carbohyd. Polym.. 2005;60:67-75.
- [Google Scholar]
- Polycyclic aromatic hydrocarbons and digestive tract cancers: a perspective. J. Environ. Sci. Health, Part C. Environ. Carcinog. Ecotoxicol. Rev.. 2011;29:324-357.
- [Google Scholar]
- Factors affecting the adsorption of trichloroethylene onto activated carbons. Appl. Surf. Sci.. 2010;256:5237-5242.
- [Google Scholar]
- Equilibrium and kinetic studies in adsorption of heavy metals using biosorbent: a summary of recent studies. J. Hazard. Mater. 2008
- [Google Scholar]
- Insights into the modeling of adsorption isotherm systems. Chem. Eng. J.. 2010;156:2-10.
- [Google Scholar]
- Sorption of polycyclic aromatic hydrocarbons (PAHs) to low and high density polyethylene (PE) Environ. Sci. Pollut. Res.. 2012;19:1296-1304.
- [CrossRef] [Google Scholar]
- Studies in adsorption. Part XI: A system of classification of solution adsorption isotherms, and its use in diagnosis of adsorption mechanisms and in measurement of specific surface areas of solids. J. Chem. Soc.. 1960;111:3279-3284.
- [Google Scholar]
- Sorption of PAHs by aspen wood fibers as affected by chemical alterations. Environ. Sci. Technol.. 2006;40:3279-3284.
- [Google Scholar]
- Starch chemistry microstructure processing and enzymatic degradation. In: Ahmed Jasim, Tiwari Brijesh K., Imam Syed H., Rao M.A., eds. Starch-Based Polymeric Materials and Nanocomposites Chemistry, Processing, and Applications. CRC Taylor and Francis Group; 2012.
- [Google Scholar]
- Evaluating phenanthrene sorption on various wood chars. Water Res.. 2005;39:549-558.
- [Google Scholar]
- About the theory of so-called adsorption of soluble substances. Kungl. Svenka Vetenskapsakademiens, Handlingar. 1898;24:1-39.
- [Google Scholar]
- Phenanthrene sorption by fruit cuticles and potato periderm with different compositional characteristics. J. Agric. Food Chem.. 2009;57:637-644.
- [Google Scholar]
- Enhanced sorption of polycyclic aromatic hydrocarbons from aqueous solution by modified pine bark. Bioresour. Technol.. 2010;101:7307-7313.
- [Google Scholar]
- Characterization and phenanthrene sorption of tea leaf powders. J. Agric. Food Chem.. 2007;55:5718-5724.
- [Google Scholar]
- Application of quantum descriptors for predicting adsorption performance of starch and cyclodextrin adsorbents. Carbohyd. Polym.. 2014;101:40-49.
- [Google Scholar]
- Mechanism of dialkyl phthalates removal from aqueous solution using γ-cyclodextrin and starch based polyurethane polymer adsorbents. Carbohyd. Polym.. 2014;114:440-449.
- [Google Scholar]
- The use of cork waste as a biosorbent for persistent organic pollutants – study of adsorption/desorption of polycyclic aromatic hydrocarbons. J. Environ. Sci. Health, Part A. 2011;46:824-832.
- [Google Scholar]
- Mechanism of Pb2+ removal from aqueous solution using a nonliving moss biomass. Chem. Eng. J.. 2012;195–196:270-275.
- [Google Scholar]
- Critical review in adsorption kinetic models. J. Zhejiang Univ. Sci. A. 2009;10:716-724.
- [Google Scholar]
- Phenanthrene sorption by aliphatic-rich natural organic matter. Environ. Sci. Technol.. 2002;36:1953-1958.
- [Google Scholar]
- Utilization of fly ash from power plant for adsorption of hydrocarbon contamination in water. J. Metals Mater. Min.. 2010;20:5-10.
- [Google Scholar]
- Removal of polychlorinated biphenyls from aqueous solutions using β-cyclodextrin grafted multiwalled carbon nanotubes. Chemosphere. 2010;79:679-685.
- [Google Scholar]
- Plasma induced grafting multiwall carbon nanotubes with chitosan for 4,4-dichlorobiphenyl removal from aqueous solution. Chem. Eng. J.. 2011;170:498-504.
- [Google Scholar]
- Distribution of polycyclic aromatic hydrocarbons (PAHs) in Henan reach of the Yellow River, Middle China. Ecotoxicol. Environ. Safety. 2009;72:1614-1624.
- [Google Scholar]
- Adsorption of polycyclic aromatic hydrocarbons on graphene oxides and reduced graphene oxides. Chem. Asian J.. 2013;8:2755-2761.
- [Google Scholar]
- Sorption of polycyclic aromatic hydrocarbons from aqueous solution by hexadecyltrimethyl-ammonium bromide modified fibric peat. J. Chem. Technol. Biotechnol.. 2010;85:1084-1091.
- [Google Scholar]
- Kinetics of sorption of polyaromatic hydrocarbons onto granular activated carbon and macronet hypercross-linked polymers (MN200) J. Colloid Interface Sci.. 2007;310:35-46.
- [Google Scholar]
- Adsorption of polycyclic aromatic hydrocarbons from aqueous solutions by modified periodic mesoporous organosilica. J. Colloid Inter. Sci.. 2011;357:66-473.
- [Google Scholar]
- Importance of structural make up of biopolymers for organic contaminant sorption. Environ. Sci. Technol.. 2007;4:3559-3565.
- [Google Scholar]
- Characterization, ecological risk assessment and source diagnostics of polycyclic aromatic hydrocarbons in water column of the Yellow River Delta, one of the most plenty biodiversity zones in the world. J. Hazard. Mater.. 2009;169(1):460-465.
- [Google Scholar]
- Health risk assessment of polycyclic aromatic hydrocarbons in the source water and drinking water of China: Quantitative analysis based on published monitoring data. Sci. Total Environ.. 2011;410–411:112-118.
- [Google Scholar]
- Porous magnetic carbon sheets from biomass as an adsorbent for the fast removal of organic pollutants from aqueous solution. J. Mater. Chem. A. 2014;2:4391-4397.
- [Google Scholar]
- Sulfonated graphene for persistent aromatic pollutant management. Adv. Mater.. 2011;23:3959-3963.
- [Google Scholar]
- Improved Approaches for Modeling the Sorption of Phenanthrene by a Range of Plant Species. Environ. Sci. Technol.. 2007;41:7818-7823.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.arabjc.2015.06.004.
Appendix A
Supplementary material
Supplementary data 1
Supplementary data 1







