Translate this page into:
Enhancement of dye-sensitized solar cell efficiency through co-sensitization of thiophene-based organic compounds and metal-based N-719
⁎Corresponding author at: Department of Chemistry, College of Applied Sciences, Umm Al-Qura University, 21955 Makkah, Saudi Arabia. nmmohamed@uqu.edu.sa (Nashwa El-Metwaly)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Synthesis of five novel thiophene-based metal-free organic dyes, named IS1-5, to study their photophysical, electrochemical properties and photovoltaic performance as sensitizers and co-sensitizers for DSSCs. Dyes IS-5 are cyanoacetanilide derivatives, which are distinguished by their small size, the simple preparation method, and the presence of multiple anchoring species (C⚌O and CN) within their molecular structure. From their UV/Vis. spectra, IS-4 and IS-5 displayed the highest molar extinction coefficient with a bathochromic-shift to the intramolecular charge transfer band. In addition, to recognize the potential of HOMO level and the potential of LUMO level among all IS1-5 dyes, the cyclic voltammetry technique must be used. Their characteristics assert that all dyes possess the qualifications essential to be used as a sensitizer for dye-sensitized solar cells (DSSCs). Interestingly, over co-sensitization, all dyes showed better photovoltage values (VOC) than the standard dye N-719. Among Dyes IS1-5, co-sensitizer IS-5 showed the highest photoconversion efficiency (η) of (8.09%) compared to the standard dye N-719 (7.61%). Moreover, electrochemical impedance spectroscopy (EIS) asserts the impact of this dye on improving charge transfer in TiO2. Finally, density functional theory (DFT) has been studied for dyes IS1-5 using Guassian09 software and the outcomes comfortably agree with experimental results.
Keywords
Thiophene
Photoconversion efficiency
Co-sensitization
DFT
1 Introduction
Environmental changes caused by gases resulting from burning fuels have increased interest in alternative and sustainable energy sources, which will be of interest sooner rather than the latter to achieve economic development worldwide (Abbasi et al., 1991). Proceeding from this view, the use of solar radiation by dye-sensitized solar cells (DSSCs) to produce electricity is being the introductory solution for meeting energy demands (Grätzel, 2001; Wu et al., 2015). In DSSCs, the dye molecule is necessary to absorb solar energy and inject electrons into the semiconductor's conduction band. The most promising dye sensitizers are ruthenium (Ru) complexes such as N3, N-719, and black dyes (Nazeeruddin et al., 1993; Chen et al., 1996; Tang et al., 2015). Organic sensitizer of alkoxysilyl-anchoring moiety has so far registered powerful conversion efficiencies (14.3%) (Kakiage et al., 2015). However, there are some disadvantages of using metal-based dye-sensitized solar such as many synthesis procedures, difficulty in purification and expensive materials used. As a result, several investigators have studied metal-free dyes because of their low-cost preparation, high molar extinction, the versatility of structural designs, and tunable optical and electrochemical properties (Hagfeldt et al., 2010; Wang et al., 2017). Abundant aromatic heterocyclic dyes have been used as an effective sensitizer when used in DSSCs, such as carbazole, indole, phenoxazine, phenothiazine, and coumarin (Mishra et al., 2009; Zhang et al., 2020; Nesheli et al., 2020). In addition, BODIPY and porphyrins have been used as an efficient sensitizer for DSSC to tune their optoelectronic properties (Shah et al., 2020; He et al., 2017; Islam et al., 2018; Zou et al., 2020; Lu et al., 2019; Kurumisawa et al., 2019; Mathew et al., 2014; Zeng et al., 2020).
To gain the merits of both metal-free and metal-based dyes, the co-sensitization technique involving the mixing of various types of dyes in the same manufactured cell has been used as a promising method for enhancing the cell's photovoltaic efficiency as the dye's light-harvesting ability increases (Sharma et al., 2014; Elmorsy et al., 2020; Lee et al., 2018; Younas and Harrabi, 2020). Hence, our research project focused on the synthesis of small metal-free dyes IS1-5 as sensitizers and co-sensitizers with the standard N-719 Ru (II) complex for DSSC applications. The newly IS1-5 dyes possess a thiophene moiety, which has been identified as potential entities because it has good tunable spectroscopic and electrochemical characteristics (Sharmoukh et al., 2020; Giri et al., 2020). Also, dyes IS-5 are cyanoacetanilide derivatives, which are distinguished by their small size, the simple preparation method, and the presence of multiple anchoring species (C⚌O and CN) within their molecular structure. Using multiple anchoring moieties within the molecular structure enhances the photovoltaic performance of the fabricated dye (Ambrosio et al., 2012; Beni et al., 2015). The chemical structure of the fabricated dyes IS1-5 and N-719 is depicted in Fig. 1. The synthesized pathways have been displayed according to Scheme 1. All dyes IS1-5 were characterized by 1H NMR, 13C NMR, FTIR, and mass spectroscopy. Their optical studies were investigated using UV–Vis absorption, the molecular orbital energies were calculated from cyclic voltammetric (CV) studies, and density functional theory studies (DFT) using guassian09 software. Finally, the fabrication of novel dyes IS1-5 as sensitizers and co-sensitizers along with N-719 (Fig. 1) for studying their photovoltaic performance as DSSCs.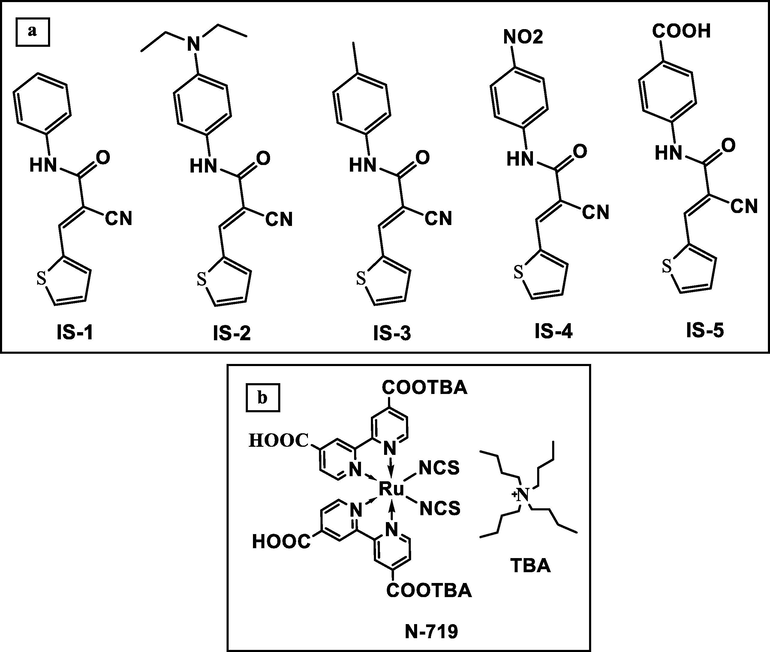
Molecular structure of dyes IS1-5 (a) and N-719 (b).
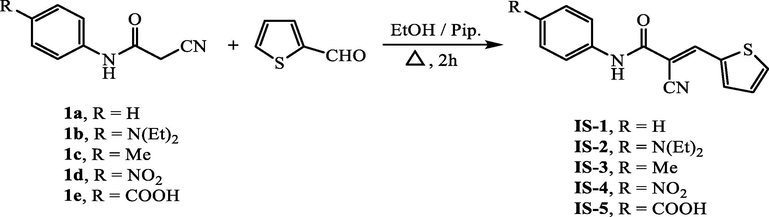
Synthetic pathway of cyanoacetanilides IS1-5.
2 Experimental
2.1 Synthesis and characterization
All organic solvents and reagents were acquired from TCI America and Sigma-Aldrich. The recorded melting points (°C) were measured using Gallenkamp apparatus and are uncorrected. Spectroscopic analysis such as IR and NMR spectra were detected using Thermo Scientific Nicolet iS10 FTIR spectrometer and JEOL’s NMR spectrometer in DMSO‑d6 (500 MHz for 1H NMR and 125 MHz for 13C NMR), respectively. The high-performance double beam spectrophotometer (T80 series) has been used for measuring UV–Visible spectrophotometer. Elemental analyses (C, H, and N) were estimated on Perkin Elmer 2400 analyzer. Fabrication of DSSC devices and measuring photovoltaic parameters have been performed at Damietta University (Egypt). Moreover, cyclic voltammetry measurements, impedance spectroscopy techniques, solar cell fabrication devices, and solar cell characterization can be found in detail in the supplementary file.
2.1.1 Synthesis of intermediates 1a-e
Intermediates 1a-e has been investigated before, as per a known protocol (Elmorsy et al., 2020).
2.1.2 General synthesis of N-aryl-2-cyano-3-(thiophen-2-yl)acrylamide sensitizers IS1-5
To a solution of each cyanoacetanilide derivative 1a-e (4 mmol) in 25 mL ethanol, 2-formylthiophene (0.45 mL, 4 mmol) and piperidine (0.1 mL) were added. The reaction contents were refluxed for 2 h followed by cooling to 25 °C. The precipitate that produced was collected and dried to furnish the targeted thiophene-based co-sensitizers IS1-5.
2.1.2.1 2-Cyano-N-phenyl-3-(thiophen-2-yl)acrylamide (IS-1)
Red solid (83%), m.p. = 177–179 °C. IR (νmax, cm−1): 3352 (—NH stretch), 2212 (—CN stretch), 1678 (—CO stretch). 1H NMR (δ, ppm): 7.12 (t, J = 7.25 Hz, 1H), 7.33–7.37 (m, 3H), 7.65 (d, J = 7.50 Hz, 2H), 7.93 (d, J = 3.50 Hz, thiophene-H), 8.13 (d, J = 5.00 Hz, thiophene-H), 8.51 (s, 1H, C⚌CH), 10.28 ppm (s, 1H, NH). 13C NMR (δ, ppm): 102.59, 116.48, 120.59 (2C), 124.26, 128.68, 128.73 (2C), 135.34, 135.70, 138.10, 138.29, 143.88, 160.34 ppm. Mass analysis (m/z, %): 254 (34.23), 246 (66.11), 228 (52.46), 193 (36.01), 188 (42.15), 176 (37.56), 154 (70.20), 134 (67.00), 128 (100.00), 95 (77.30), 89 (52.89). Anal. calcd for C14H10N2OS (254.05): C, 66.12; H, 3.96; N, 11.02%. Found: C, 66.02; H, 3.98; N, 11.09%.
2.1.2.2 2-Cyano-N-(4-(diethylamino)phenyl)-3-(thiophen-2-yl)acrylamide (IS-2)
Violet solid (65%), m.p. = 181—183 °C. IR (νmax, cm−1): 3419 (—NH stretch), 2212 (—CN stretch), 1663 (—CO stretch). 1H NMR (δ, ppm): 1.06 (t, J = 7.00 Hz, 6H), 3.30 (q, J = 7.00 Hz, 6H), 6.63 (d, J = 9.00 Hz, 2H), 7.32 (dd, J = 4.00, 4.00 Hz, thiophene-H), 7.41 (d, J = 9.00 Hz, 2H), 7.90 (d, J = 4.00 Hz, thiophene-H), 8.10 (d, J = 4.00 Hz, thiophene-H), 8.44 (s, 1H, C⚌CH), 9.94 (s, 1H, NH). 13C NMR (δ, ppm): 12.42 (2C), 43.72 (2C), 102.82, 111.50 (2C), 116.64, 122.51 (2C), 126.61, 128.59, 134.89, 135.92, 137.73, 143.17, 144.67, 159.38 ppm. Mass analysis (m/z, %): 325 (46.63), 316 (52.71), 305 (25.64), 271 (100.00), 265 (51.50), 257 (53.33), 228 (55.36), 208 (37.56), 152 (41.40), 107 (25.35), 78 (39.48). Anal. calcd for C18H19N3OS (325): C, 65.43; H, 4.89; N, 11.91%. Found: C, 66.51; H, 5.85; N, 11.85%.
2.1.2.3 2-Cyano-3-(thiophen-2-yl)-N-(p-tolyl)acrylamide (IS-3)
Red solid (88%), m.p. = 191–192 °C. IR (νmax, cm−1): 3349 (—NH stretch), 2213 (—CN stretch), 1674 (—CO stretch). 1H NMR (δ, ppm): 2.26 (s, 3H), 7.14 (d, J = 8.00 Hz, 2H), 7.33 (dd, J = 4.50, 4.00 Hz, thiophene-H), 7.53 (d, J = 8.00 Hz, 2H), 7.91 (d, J = 3.00 Hz, thiophene-H), 8.12 (d, J = 5.00 Hz, thiophene-H), 8.49 (s, 1H, C⚌CH), 10.19 ppm (s, 1H, NH). 13C NMR (δ, ppm): 20.55, 102.69, 116.38, 120.70 (2C), 128.71, 129.17 (2C), 133.41, 135.26, 135.78, 135.84, 138.05, 143.75, 160.18 ppm. Mass analysis (m/z, %): 268 (20.38), 241 (15.33), 202 (21.80), 175 (24.66), 133.63 (30.82), 82 (56.41), 55.22 (100.00). Anal. calcd for C15H12N2OS (268.07): C, 67.16; H, 4.11; N, 10.95%. Found: C, 67.35; H, 3.98; N, 11.08%.
2.1.2.4 2-Cyano-N-(4-nitrophenyl)-3-(thiophen-2-yl)acrylamide (IS-4)
Black solid (70%), m.p. = 203–205 °C. IR (νmax, cm−1): 3323 (—NH stretch), 2218 (—CN stretch), 1689 (—CO stretch). 1H NMR (δ, ppm): 7.36 (dd, J = 5.00, 4.00 Hz, thiophene-H), 7.93 (d, J = 8.50 Hz, 2H), 7.96 (d, J = 3.00 Hz, thiophene-H), 8.18 (d, J = 4.50 Hz, thiophene-H), 8.26 (d, J = 9.00 Hz, 2H) 8.58 (s, 1H, C⚌CH), 10.75 ppm (s, 1H, NH). 13C NMR (δ, ppm): 102.02, 116.24, 120.18 (2C), 124.85 (2C), 128.86, 135.63, 136.10, 138.71, 142.85, 144.64, 144.88, 161.19 ppm. Mass analysis (m/z, %): 300 (60.40), 292 (56.27), 279 (42.65), 246 (53.08), 231 (100.00), 185 (90.90), 176 (63.45), 163 (66.46), 157 (82.42), 145 (41.32), 90 (52.73), 57 (56.86). Anal. calcd for C14H9N3O3S (299.04): C, 56.18; H, 3.03; N, 14.04%. Found: C, 56.28; H, 3.00; N, 14.11%.
2.1.2.5 4-(2-Cyano-3-(thiophen-2-yl)acrylamido)benzoic acid (IS-5)
Black solid (62%), m.p. = 239–241 °C. IR (νmax, cm−1): 3332 (—NH stretch), 2218 (—CN stretch), 1677 (—CO stretch). 1H NMR (δ, ppm): 7.35 (dd, J = 5.00, 3.50 Hz, thiophene-H), 7.78 (d, J = 9.00 Hz, 2H), 7.93 (d, J = 9.00 Hz, 2H), 7.94 (d, J = 3.00 Hz, thiophene-H), 8.15 (d, J = 5.00 Hz, thiophene-H), 8.55 (s, 1H, ⚌CH), 10.58 (s,1H, NH), 12.86 (s, 1H, COOH). 13C NMR (δ, ppm): 102.38, 116.37, 119.75 (2C), 126.14, 128.77, 130.29 (2C), 135.71 (2C), 138.39, 142.41, 144.33, 160.80, 166.89 ppm. Mass analysis (m/z, %): 298 (10.77), 292 (80.58), 270 (50.43), 242 (18.75), 173 (8.01), 82 (41.13), 77 (64.76), 54 (100.00). Anal. calcd. for C15H10N2O3S (298.04): C, 60.38; H, 3.37; N, 9.96%. Found: C, 60.23; H, 2.29; N, 10.08%.
3 Result and discussion
3.1 Structural designing and synthesis
Cyanoacetanilide derivatives 1a-e have been synthesized through refluxing 1-cyanoacetyl-3,5-dimethylpyrazole with N, N-diethyl-p-phenylenediamine, aniline, 4-toluidine, 4-aminobenzoic acid, and 4-nitroaniline, respectively, as reported in the literature. Then, IS1-5 precursors were achieved by following Knoevenagel reaction conditions between different 1a-e cyanoacetanilides and 2-formyl thiophene in presence of piperidine as a catalyst in a boiling solution of EtOH (Scheme 1).
3.2 UV–visible studies
Fig. 2 showed the UV–visible absorptance spectra for IS1-5 in chloroform medium (2 × 10−5 M) and their related spectral data are stated in Table 1.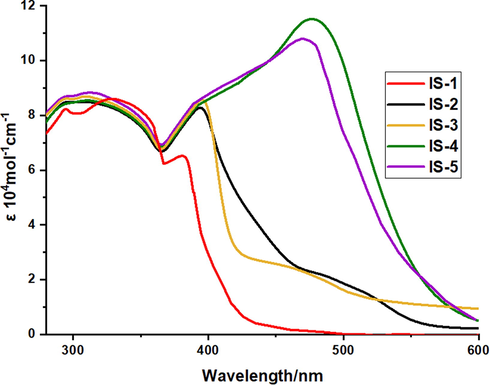
UV–Vis absorption of IS1-5 in CHCl3 (2 × 10−5 M).
Dyes
λ (nm)
ε (104 M−1cm−1)
E0-0 Experimental (λonset)
E0-0 (theoretical)
IS-1
336, 381
8.52, 6.51
2.96
2.95
IS-2
393, 390
5.23, 8.47
2.58
2.55
IS-3
396, 394
8.56, 8.25
2.74
2.81
IS-4
396, 477
8.71, 11.51
2.49
2.43
IS-5
396, 471
8.41, 10.74
2.53
2.47
All dyes IS1-5 showed two obvious absorption bands. The first band related to π → π* appeared in the region from 330 to 400 nm while a second band from 400 to 570 nm reflected the internal charge transfer from the donor to the acceptor.
Further, Dyes IS-4 and IS-5 containing —NO2 and —COOH anchoring species, respectively showed broadening in their spectra with a red-shift in their lowest energy band due to the extension of the conjugation within molecular frames. Moreover, band energy gaps (E0-0) were measured from their UV/Vis. spectra showed an agreement with theoretical values calculated from DFT studies (Al-Eid et al., 2014). From the results, dye IS-4 exhibited the least band gap value that can be attributed to the nitro group's good withdrawal capacity.
Moreover, the absorption spectra of products IS1-5 adsorbed onto TiO2 film are shown in Fig. 3. The spectra were broadened due to the interaction between sensitizers and TiO2 (Li et al., 2006). The broadening in the spectra indicated that most sensitizers were adsorbed on the TiO2 surface. Besides, dye IS-5 bearing carboxyl group —COOH as an anchoring moiety is found to be even more adsorbed on TiO2 than other compounds IS1-4, providing a good indication of this dye's better photovoltaic efficiency.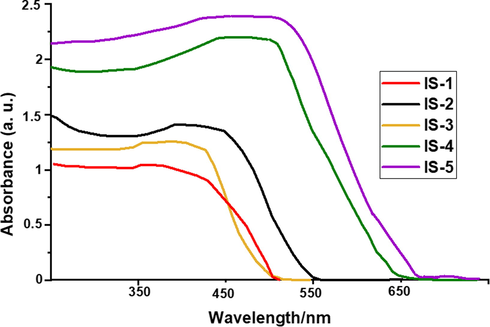
UV–Vis absorption of IS1-5 anchored onto nonporous TiO2.
3.3 Computational studies
Optimization of the ground state geometries and frontier molecular orbitals ((FMOs) of dyes IS1-5 were performed using density functional theory (DFT) by Guassian09 software at B3LYP/6-311G (d, p) level (Frisch et al., 2017). The calculated E0-0 and orbital energies agreed well with cyclic voltammetry results. Fig. 4 depicts the simulated FMOs of dyes IS1-5.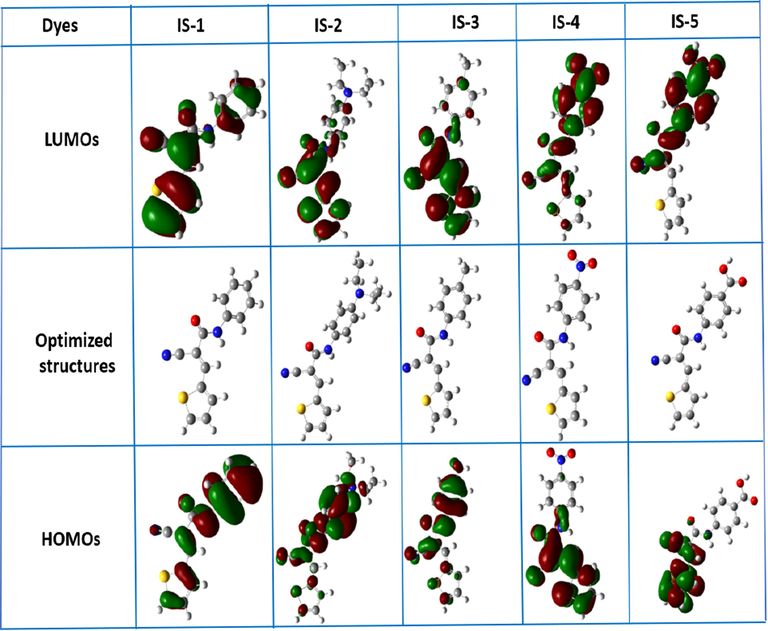
Optimized structures and simulated FMOs of IS1-5.
The outcomes showed variations in electron distributions in their HOMO-Level, which may be attributed to their structural nature. For dye IS-1, there is symmetry in the electron distribution for the GSOP/HOMO and ESOP/LUMO which may be attributed to the absence of strong donating/accepting moiety. This observation indicates a poor charge transfer of the former dye. For Dyes IS-2 and IS-3, which contain N, N-diethyl, and methyl moiety, respectively, the electron distribution is concentrated on the phenyl ring for their HOMO levels, while in their LUMO levels, the electron is concentrated in the thiophene moiety. In contrast, Dyes IS-4 and IS-5 show opposite behavior, in their HOMO levels, the electron distribution mainly concentrated on the thiophene ring, while in their LUMO levels, the electron concentrated on the phenyl ring due to the presence of strong accepting moieties ability (—NO2 and —COOH) beside the benzene ring. From the aforementioned, Dyes IS2-5 are expected to show good charge transfer from The HOMO to LUMO, which could be translated to better performance.
3.4 Cyclic voltammetry experiments (CV)
Excited state oxidation potentials (ESOPs) and ground state oxidation potentials (GSOPs) of all dyes were measured by cyclic voltammetry techniques (Fig. 5).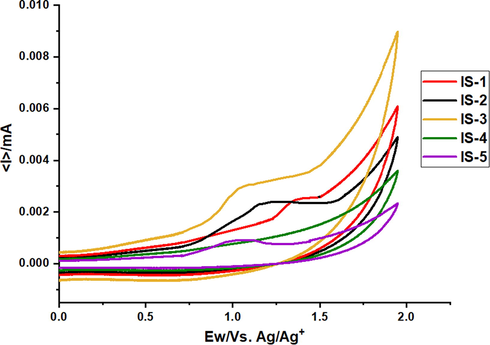
Cyclic voltammetry traces for IS1-5.
First of all, GSOPs were calculated from oxidation onset of cyclic voltammogram as shown in equation 1:
Then, ESOPs was derived from the values of E0-0 and GSOP as shown in equation 2:
An ideal dye should include some thermodynamic criteria for being used as DSSC; The GSOP levels should be below the redox potential of the electrolyte (−5.2 eV) and the ESOP levels should be above the conduction band of nanocrystalline TiO2 layer (−4.2 eV) (Qu and Meyer, 2001; Oskam et al., 2001) as shown in Fig. 6.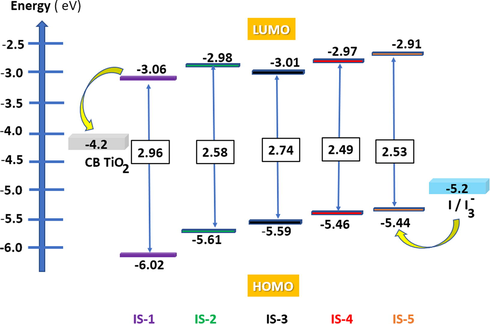
Energy level diagram of IS1-5 based on the experimental values.
From the results in Table 2, the estimated values of GSOP for IS1-5 were found to be; IS-1 (−6.02 eV), IS-2 (−5.61 eV), IS-3 (−5.59 eV) and IS-4 (−5.46 eV) and IS-5 (−5.44), below the redox potential of the electrolyte for better dye regeneration. Also, the measured ESOPs were found to be; IS-1 (−3.06 eV), IS-2 (−2.98 eV), IS-3 (−3.01 eV), IS-4 (−2.97 eV) and IS-5 (−2.91 eV), above the conduction band potential of the TiO2 for better electron injection.
Structure
Experimental (eV)
Theoretical (eV)
Eox (V)
GSOP
ESOP
GSOP
ESOP
IS-1
1.32
−6.02
−3.06
−6.12
−3.17
IS-2
0.91
−5.61
−2.98
−5.52
−3.09
IS-3
0.89
−5.59
−3.01
−5.70
−3.15
IS-4
0.76
−5.46
−2.97
−5.53
−3.06
IS-5
0.74
−5.44
−2.91
−5.72
−2.91
Further, IS-5 possess the highest negative free energy of (1.29 eV), which represents a good indication of the best injection of electron to TiO2 band and so IS-5 is expected to give a good photovoltaic performance compared to other dyes IS1-4.
3.5 Photovoltaic parameters measurements
DSSCs were fabricated using five novel metal free dyes IS1-5 alone and co-sensitized with N-719. The fabrication procedure and instruments were discussed briefly in the supplementary file. Chenodeoxycholic acid “CDCA” was added into the dye solution as a co-adsorbent to diminish the dye aggregation and suppress recombination reactions, which ultimately leads to enhanced VOC values as well as total efficiency of the fabricated devices (Neale et al., 2005; Ito et al., 2008). Fig. 7 displays the J-V curves of the fabricated cells IS1-5 alone. Their corresponding photovoltaic parameters are presented in Table 3. Under standard global AM 1.5 solar conditions, the cells employing IS1-5 sensitizers yielded PCE of 0.49, 0.89, 0.64, 1.69 and 2.72%, respectively. The IS-5 sensitized cell showed better photovoltaic parameters values: Jsc = 6.15 mA/cm2, Voc = 0.646 V and FF = 68.30 compared to other dyes IS1-4. Increasing JSC value of IS-5 can be attributed to an efficient anchoring moiety (—COOH). The better anchoring moiety (—COOH) led to better charge transfer from The HOMO to the LUMO which, allowing electron injection to the TiO2 edge conduction band (Koops et al., 2009). On the other hand, the low performances of dyes IS1-3 can be related to the absence of good anchoring moieties as observed from the low adsorption of these dyes to TiO2 nanoparticles (Fig. 3) (Cheema et al., 2009). Besides this reason, there is symmetry in electron distribution of the HOMO/LUMO of dye IS-1 leading to poor charge transfer and hence the lowest performance (0.49%) (Montiel et al., 2019).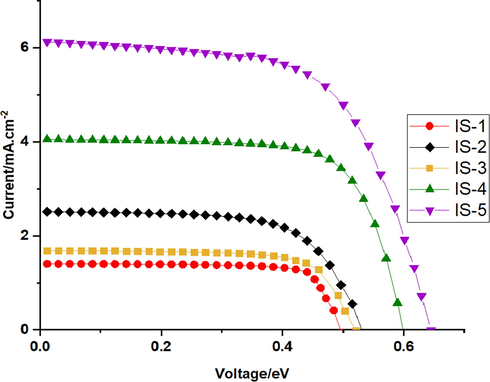
J-V curves of the fabricated devices IS1-5.
Sensitizer (0.2 mM)
Dye (0.2 mM)
CDCA (mM)
VOC (V)
JSC (mA cm−2)
FF (%)
Ƞ (%)
N-719
–
20
0.658
18.59
62.15
7.61
IS1
20
0.660
10.62
62.74
4.35
IS2
20
0.668
15.45
63.67
6.61
IS3
20
0.663
17.20
61.84
7.08
IS4
20
0.671
18.77
60.73
7.69
IS5
20
0.680
19.23
61.72
8.09
–
IS1
10
0.496
1.38
73.52
0.49
–
IS2
10
0.530
2.49
66.98
0.89
–
IS3
10
0.521
1.68
73.07
0.64
–
IS4
10
0.599
4.00
71.49
1.69
–
IS5
10
0.646
6.15
68.30
2.72
In order to study the effect of co-sensitization of IS1-5 along with the standard Ru based N-719 dye, the devices were fabricated using 0.2 mM of N-719 dye and 0.2 mM of co-sensitizer IS1-5. Fig. 8 displays the J-V curves of the photovoltaic devices co-sensitized with dyes IS-5. Their corresponding photovoltaic parameters are tabulated in Table 3. From the results, dyes IS-4 and IS-5 exhibited better photovoltaic performance than the N-719 alone. As the results indicate, the cells employing N-719 along with co-sensitizers IS4 and IS-5 5 yielded PCE of 7.69% and 8.09%, respectively, while N-719 alone showed 7.61%. The improved performance of the former dyes can be attributed to their maximum Jsc and Voc values compared to N-719 dye. It is interesting to note that, IS-4 and IS-5 displayed improved photocurrent density, Jsc of 18.77 mA.cm−2 and 19.23 mA.cm−2, respectively, whereas N-719 alone sensitized device showed 18.59 mA.cm−2. The higher Jsc values can be attributed to the additional anchoring moieties (—NO2 and —COOH), resulting in increasing the amount of dye adsorbed in the surface of the TiO2 layer compared to other devices IS1-3.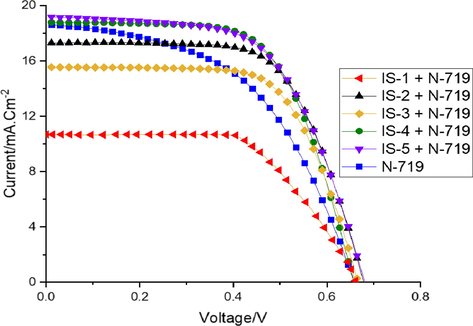
J-V curves of the fabricated devices IS1-5 co-sensitized with N-719.
Additionally, it is clear from the results obtained that photovoltage values are decreasing in the order of IS-5 (0.680 V) > IS-4 (0.671 V) > IS-2 (0.668 V) > IS-3 (0.663 V) > IS-1 (0.660 V) upon co-sensitization with N-719 (0.658). The enhanced VOC values for all co-sensitizers IS1-5 can be due, when the bulky N-719 dye adsorbed on TiO2 surface, it has left unfilled gaps behind, now the relatively small organic dyes IS1-5 can cover these unfilled voids. Therefore, the co-sensitization technique leads to improved VOC values (Su et al., 2019).
3.6 Electrochemical impedance study (EIS)
Moreover, electrochemical impedance spectroscopy (EIS) is a powerful technique used to estimate the charge recombination in DSSCs. Fig. 9 shows the Nyquist plots of the fabricated co-sensitizers IS1-5 along with standard N-719 and their equivalent circuit (inset Fig. 9). A typical EIS spectrum of a cell exhibited three semicircles in the Nyquist plots. Generally, the first semicircle at high frequencies represents the redox charge transfer resistances at the Pt/electrolyte interface (RCE). The second semicircle at intermediate frequencies is related to the charge transfer resistances at the interface between TiO2/dye/electrolyte (RCT). The third semicircle in the low frequency range represents Warburg diffusion impedance (RW). Usually, a larger RCT value indicates a smaller dark current and an increased charge recombination resistance and therefore a larger open-circuit photovoltage (Qu and Meyer, 2001; Oskam et al., 2001; Hua et al., 2013). The charge recombination resistance of these dyes (RCT) corresponding to the diameter of the middle frequency semicircle was calculated to decrease in the order of IS-5 + N-719 (52.64 Ω), IS-4 + N-719 (50.15 Ω), IS-2 + N-719 (48.17 Ω), IS-3 + N-719 (36.24 Ω), IS-1 + N-719 (28.14 Ω) and N-719 (25.19 Ω), in good agreement with the order photovoltage data.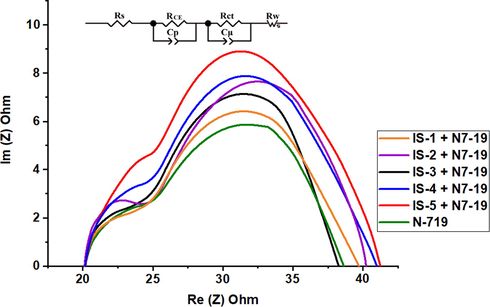
Nyquist plots for IS1-5 co-sensitized with N-719.
4 Conclusions
Five organic compounds IS1-5 have been engineered as sensitizers for DSSCs. Further, the performance of a DSSC using co-sensitization was improved in this study. IS1-5 co-sensitizers were used with N-719, a ruthenium-based dye (II). Dyes (IS1-5) showed better VOC values over co-sensitization with N-719 of using individual N-719. IS-5 showed the best impact on N-719′s photovoltaic efficiency with a PCE of 8.09%, JSC (19.23) and VOC (0.680) compared to PCE of 7.61%, JSC (18.59) and VOC (0.658 eV) of N-719. Dyes IS-4 and IS-5 containing —NO2 and —COOH anchoring species, respectively showed broadening in their spectra with a red-shift in their lowest energy band due to the extension of conjugation within molecular frames. Finally, in accordance with the experimental data, simulation studies based on DFT studies were used.
Acknowledgements
The authors would like to thank Deanship of Scientific Research at Umm Al-Qura University, Saudi Arabia (project ID: 19-SCI-1-01-0001) for the financial support to this research extracted from the project.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- The return to renewables: Will it help in global warming control. Renew. Sustain. Energy Rev.. 1991;15:891-894.
- [CrossRef] [Google Scholar]
- Facile synthesis of metal-free organic dyes featuring a thienylethynyl spacer for dye sensitized solar cells. Dyes Pigm.. 2014;104:197-203.
- [CrossRef] [Google Scholar]
- Effect of the anchoring group on electron injection: theoretical study of phosphonated dyes for dye-sensitized solar cells. J. Phys. Chem. C. 2012;116:2622-2629.
- [CrossRef] [Google Scholar]
- Synthesis and characterization of organic dyes bearing new electron-withdrawing group for dye-sensitized solar cells. Electrochim. Acta. 2015;186:504-511.
- [CrossRef] [Google Scholar]
- Influence of number of benzodioxan-stilbazole-based ancillary ligands on dye packing, photovoltage and photocurrent in dye-sensitized solar cells. ACS Appl. Mater. Interfaces. 2009;6:11617-11624.
- [CrossRef] [Google Scholar]
- Improved electroluminescence performance of poly (3-alkylthiophenes) having a high head-to-tail (HT) ratio. J. Mater. Chem.. 1996;6:1763-1766.
- [CrossRef] [Google Scholar]
- Co-sensitization of the HD-2 complex with low-cost cyanoacetanilides for highly efficient DSSCs. Photochem. Photobiol. Sci.. 2020;19:281-288.
- [CrossRef] [Google Scholar]
- Molecular geometry, synthesis and photovoltaic performance studies over 2-cyanoacetanilides as sensitizers and effective co-sensitizers for DSSCs loaded with HD-2. J. Photochem. Photobiol. A.. February 2020;389(15):112239
- [CrossRef] [Google Scholar]
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, Eur. J. Inorg. Chem. 2017, 3690–3697 www.eurjic.org 3697 © 2017 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, Ö. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski, D. J. Fox, Gaussian 09, Revision A.02, Gaussian, Inc., Wallingford CT, 2009.
- Diketopyrrolopyrrole/perylene-diimide and thiophene based D-π-A low bandgap polymer sensitizers for application in dye sensitized solar cells. Dyes Pigments. 2020;174:108032
- [CrossRef] [Google Scholar]
- Sisters together: co-sensitization of near-infrared emission of ytterbium (III) by BODIPY and porphyrin dyes. Chem. Commun.. 2017;53:10120-10123.
- [CrossRef] [Google Scholar]
- Bulky dendritic triarylamine-based organic dyes for efficient co-adsorbent-free dye-sensitized solar cells. J. Power Sources. 2013;237:195-203.
- [CrossRef] [Google Scholar]
- Panchromatic absorption of dye sensitized solar cells by co-Sensitization of triple organic dyes. Sustain. Energy Fuels. 2018;2:209-214.
- [CrossRef] [Google Scholar]
- High-conversionefficiency organic dye-sensitized solar cells with a novel indoline dye. Chem. Commun. 2008:5194-5196.
- [CrossRef] [Google Scholar]
- Highly-efficient dye-sensitized solar cells with collaborative sensitization by silyl-anchor and carboxy-anchor dyes. Chem. Commun.. 2015;51:15894-15897.
- [CrossRef] [Google Scholar]
- Parameters influencing the efficiency of electron injection in dye-sensitized solar cells. J. Am. Chem. Soc.. 2009;131:4808-4818.
- [CrossRef] [Google Scholar]
- Renaissance of Fused Porphyrins: Substituted Methylene-Bridged Thiophene-Fused Strategy for High-Performance Dye-Sensitized Solar Cells. J. Am. Chem. Soc.. 2019;141(25):9910-9919.
- [CrossRef] [Google Scholar]
- Co-sensitization of metal free organic dyes in flexible dye sensitized solar cells. Org. Electron.. 2018;52:103-109.
- [CrossRef] [Google Scholar]
- Novel organic dyes for efficient dye-sensitized solar cells. Chem. Commun. 2006:2792-2794.
- [CrossRef] [Google Scholar]
- Multiply Wrapped Porphyrin Dyes with a Phenothiazine Donor: A High Efficiency of 11.7% Achieved through a Synergetic Coadsorption and Cosensitization Approach. ACS Appl. Mater. Interfaces.. 2019;11(5):5046-5054.
- [CrossRef] [Google Scholar]
- Dye-sensitized solar cells with 13% efficiency achieved through the molecular engineering of porphyrin sensitizers. Nature Chem.. 2014;6:242-247.
- [CrossRef] [Google Scholar]
- Metal-free organic dyes for dyesensitized solar cells: from structure: property relationships to design rules. Angew. Chem. Int. Ed.. 2009;48:2474-2499.
- [CrossRef] [Google Scholar]
- Theoretical Study of the Effect of Different π Bridges Including an Azomethine Group in Triphenylamine-Based Dye for Dye-Sensitized Solar Cells. Molecules. 2019;24(21):3897.
- [CrossRef] [Google Scholar]
- Conversion of light to electricity by cis-X2bis (2,2’-bipyridyl-4,4’-dicarboxylate)ruthenium(II) charge-transfer sensitizers (X = Cl-, Br-, I-, CN-, and SCN-) on nanocrystalline titanium dioxide electrodes. J. Am. Chem. Soc.. 1993;115:6382-6390.
- [CrossRef] [Google Scholar]
- Effect of a coadsorbent on the performance of dye-sensitized TiO2 solar Cells: shielding versus band-edge movement. J. Phys. Chem. B. 2005;109:23183-23189.
- [CrossRef] [Google Scholar]
- Design, Synthesis and Photophysical Analysis of New Unsymmetrical Carbazole-Based Dyes for Dye-Sensitized Solar Cells. J. Photochem. Photobiol. A.. June 2020;397(15):11252.
- [CrossRef] [Google Scholar]
- pseudohalogens for dye-sensitized Ti photoelectrochemical cells. J. Phys. Chem. B. 2001;105:6867-6873.
- [CrossRef] [Google Scholar]
- Pseudohalogens for dye-sensitized TiO2 photoelectrochemical cells. J. Phys. Chem. B. 2001;105:6867-6873.
- [CrossRef] [Google Scholar]
- Proton-controlled electron injection from molecular excited states to the empty states in nanocrystalline TiO2. Langmuir. 2001;17:6720-6728.
- [CrossRef] [Google Scholar]
- Cross-conjugated BODIPY pigment for highly efficient dye sensitized solar cells. Sustain. Energy Fuels. 2020;4:1908-1914.
- [Google Scholar]
- Stepwise co-sensitization as a useful tool for enhancement of power conversion efficiency of dye-sensitized solar cells: the case of an unsymmetrical porphyrin dyad and a metal-free organic dye. Org. Electron.. 2014;15:1324-1337.
- [CrossRef] [Google Scholar]
- Comparison between Benzothiadizole–Thiophene-and Benzothiadizole–Furan-Based D-A− π–A Dyes Applied in Dye-Sensitized Solar Cells: Experimental and Theoretical Insights. ACS Omega. 2020;5:16856-16864.
- [CrossRef] [Google Scholar]
- Su, R., Lyu, L., Elmorsy, M., El-Shafei, A., 2019. Novel metal-free organic dyes constructed with the D-D|A-π-A motif: Sensitization and co-sensitization study 194, 400-414. https://doi.org/10.1016/j.solener.2019.10.061.
- Porphyrins containing atriphenylamine donor and up to eight alkoxy chains for dye-sensitized solar cells: a high efficiency of 10.9%. ACS Appl. Mater. Interfaces. 2015;7:27976-27985.
- [CrossRef] [Google Scholar]
- Multimolecular assemblies on high surface area metal oxides and their role in interfacial energy and electron transfer. Chem. Soc. Rev.. 2017;47:104-148.
- [CrossRef] [Google Scholar]
- Electrolytes in dyesensitized solar cells. Chem. Rev.. 2015;115:2136-2173.
- [CrossRef] [Google Scholar]
- Performance enhancement of dye-sensitized solar cells via co-sensitization of ruthenium (II) based N749 dye and organic sensitizer RK1. Sol. Energy. 2020;203:260-266.
- [CrossRef] [Google Scholar]
- Efficient Solar Cells Based on Concerted Companion Dyes Containing Two Complementary Components: An Alternative Approach for Cosensitization. J Am Chem Soc.. 2020 Mar 18;142(11):5154-5161.
- [CrossRef] [Google Scholar]
- Phenothiazine (or phenoxazine) based (D–π–A)-L2-(A–π–D–π–A)2-type organic dyes with five anchors for efficient dye-sensitized solar cells. Sol. Energy. 2020;212:220-230.
- [CrossRef] [Google Scholar]
- Light-Absorbing Pyridine Derivative as a New Electrolyte Additive for Developing Efficient Porphyrin Dye-Sensitized Solar Cell. ACS Appl. Mater. Interfaces.. 2020;12(51):57017-57024.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2021.103080.
Appendix A
Supplementary material
The following are the Supplementary data to this article:







