Translate this page into:
Synthesis, X-ray crystal structures and anticancer studies of four Pd(II) dithiocarbamate complexes
⁎Corresponding author. ajibadep@ukzn.ac.za (Peter A. Ajibade)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Bis(piperidinyldithiocarbamato)palladium(II) ([Pd(Pipdtc)2]), bis(1-phenylpiperazinyldithiocarbamato)palladium(II) [Pd(1-PhPzdtc)2], bis(phenyldithiocarbamato)palladium(II) ([Pd(Phdtc)2]) and bis(4-benzylpiperidinyl)palladium(II) ([Pd(4-BenzPipdtc)2]) were prepared and characterized by elemental analysis, spectroscopic techniques and single crystal X-ray crystallography. Molecular structures of the Pd(II) complexes revealed square planar PdS4 geometry in which each Pd(II) is coordinated to two chelating dithiocarbamato anions. [Pd(Pipdtc)2] and [Pd(1-PhPzdtc)2] crystallized in monoclinic P21/c space group while [Pd(Phdtc)2] and [Pd(4-BenzPipdtc)2] crystallized in triclinic P-1 space group. Cytotoxic studies of the dithiocarbamate ligands and corresponding Pd(II) dithiocarbamate complexes were tested against three human cell lines: Cervical cancer, breast adenocarcinoma, and epithelial colorectal adenocarcinoma at four different concentrations. All the compounds displayed high to moderate potency against the cell lines. [Pd(Pipdtc)2] and [Pd(Phdtc)2] are very potent against the three cell lines and are more active than the corresponding dithiocarbamate ligands whereas the anticancer potency of 1-PhPzdtc-Na and 4-BenzPipdtc-Na are generally higher than the corresponding Pd(II) complexes.
Keywords
Dithiocarbamate
Pd(II)
Molecular structure
Cytotoxic evaluation
High potency
1 Introduction
Dithiocarbamate (RHCNS2- or R2CNS2-) belong to the class of monoanionic 1,1-dithiolate ligands. Dithiocarbamates are well known for their ability to coordinate through one or both sulphur atoms to form stable complexes with transition metals in a diversity of oxidation states (Chaudhari et al. 2019, Ferreira et al. 2016, Manar et al. 2017, Paca and Ajibade, 2017, Tiekink, 2018, Saeidifar et al., 2016). This class of compounds are strong metal chelators and bind the metal ions in various coordination modes to form metal complexes with various stereochemistry (Jamaluddin et al., 2014, Hou et al., 2014). This is due to their soft nature and small bite-angle, which allows them to stabilize metal ions in lower or higher oxidation states (El-Samanody et al. 2019, Rai et al., 2006, Siddiqi et al. 2006). Besides stabilizing metal cations, dithiocarbamate and their metal complexes are acknowledged scaffolds for the development of novel therapeutic agents. It has also been shown that dithiocarbamates are inhibitors of nephrotoxicity associated with cisplatin anticancer drug (Amir et al., 2016). Other studies have shown that platinum(II) dithiocarbamates could prevent platinum from binding to intracellular renal sulphur containing enzymes or reverse platinum–sulphur bonding and thus reduce nephrotoxicity (Fregona, et al., 2003, Nardon et al., 2014).
In search of novel chemotherapeutic agents, recent studies on platinum(II) and palladium(II) complexes indicate they are less toxic in comparison to cisplatin and its analogs (Eslami et al., 2016, Li et al., 2017). However, Pd(II) complexes are highly labile and their high reactivity results in rapid hydrolysis and isomerization (Fanelli, et al., 2016, Noodeh, et al. 2018). This limits the pharmacological basis of their reactive species. Thus, coordination of Pd(II) to ligands with soft donor atoms such as dithiocarbamates could be used to overcome undesirable reactivity. Pd(II) complexes containing nitrogen and sulphur donor ligands have been studied as alternative to reduce the inherent toxicity associated with platinum-based drugs (Khan et al., 2016a, Khan et al., 2016b). Heterocyclic compounds possess a high degree of structural flexibility and could be used as scaffolds for the development of therapeutic agents with biological activity (Hemmerling and Hahn, 2016, Mohammad et al., 2009). The biological activity of these compounds is inherently linked with their structural properties (Prasad et al. 2013). Nitrogen containing heterocycles have received considerable for the development of biologically active compounds (Abrigach et al., 2018, Atobe et al., 2017, Blanchard et al., 2010, Dou et al. 2012, Singh, et al. 2017). Recently, metal complexes with functionalized –NHC moiety were investigated for their biological properties (Onar et al., 2019).
Alias et al. investigated the in vitro cytotoxic effect of [Pd((5-(pnitro phenyl)-4-phenyl-1,2,4-triazole-3-dithiocarbamato hydrazide)2] on rhabdomyosarcoma cell lines and discovered that the inhibition rate of this complex is almost equivalent to that of cis-platin, but they could not establish the mechanism of action (Alias et al., 2016). Shaheen and colleagues (Shaheen et al., 2007) prepared six palladium(II) dithiocarbamate complexes. The in vitro cytotoxicity of the compounds was not potent (Shaheen et al., 2007) but thus indicate that Pd(II) complexes have better solubility compared to Pt(II) complexes and could lead to compound with better activity. Other study has shown that Pd(II) complexes containing dithiol group has low side effects particularly on the kidney (Hadizadeh, et al., 2014). In this study, we report the preparation of four dithiocarbamate ligands and their corresponding palladium(II) dithiocarbamate complexes. The compounds were characterized by elemental analysis, spectroscopic techniques, and single crystals X-ray crystallography. The anticancer potential of the four dithiocarbamate ligands and corresponding Pd(II) dithiocarbamate complexes were evaluated against cervical cancer (HeLa), breast adenocarcinoma (MCF-7) and epithelial colorectal adenocarcinoma (Caco-2).
2 Materials and methods
2.1 Materials
All chemicals and solvents used were of analytical grades purchased from Merck and used as received with no further purification. All cell culture media and reagents were obtained from Lonza Biowhittaker (Walkersville, USA), and all sterile plastic ware from Corning Inc. (NY, USA). MCF-7 and HEK293 cells were originally sourced from the American Type Culture Collection (ATCC, Manassas, VA, USA).
2.2 Physical measurements
1H and 13C NMR spectra of the ligands were recorded on a Bruker Biospin 600 MHz spectrometer and were referenced internally using residual solvent signals of D2O (4.79 ppm for 1H NMR) or (CD3)2CO (2.05 and 206.26 ppm for 1H and 13C NMR) or (CD3)2SO (3.31 and 39.52 for 1H and 13C NMR) at room temperature. Thermoscientific Flash 2000 elemental analyser was used to examine the elemental composition of each of the compounds. Melting points of the ligands and complexes were measured by Stuart SMP3 Digital Melting Point apparatus using melting point capillary tubes. The molar conductivity of the complexes was measured by an Electric Conductivity apparatus (Jenway 4510 Conductivity Meter) at room temperature. The ligands were dissolved in methanol while the complexes were dissolved in DMSO. The FTIR spectra were recorded on a PerkinElmer Spectrum 100 FTIR spectrometer in the 4000–650 cm−1 range. Electronic spectra of the ligands were measured by a PerkinElmer Lambda 25 spectrometer from 200 to 700 nm in distilled water at room temperature. Mass spectra were recorded on Waters Micromass LCT Premier TOF-MS.
2.3 Synthesis of dithiocarbamate ligands
The ligands: sodium piperidinedithiocarbamate (Pipdtc-Na), sodium 1-phenylpiperazinedithiocarbamate (1-PhPzdtc-Na), ammonium phenyldithiocarbamate (Phdtc-NH4) and sodium 4-benzylpiperidinedithiocarbamate (4-BenzPip-Na) were prepared as detailed in literature (Andrew and Ajibade, 2018; Andrew and Ajibade, 2019). In a typical synthesis, 0.05 mol of piperidine, 1-phenylpiperizine, aniline or 4-benzylpiperidine was added to a cold aqueous sodium hydroxide (0.05 mol) or concentrated aqueous ammonia (30 mL). The mixture was stirred rapidly for 30 min at 4 °C, and cold carbon disulphide (0.05 mol, 3.01 mL) was then added dropwise with constant stirring until a precipitate formed. The reaction was maintained for 4 h at 4 °C, after which, the precipitate was filtered, rinsed with diethyl ether, and desiccated.
Pipdtc-Na: White powder, yield (%): 94.23, m.p (°C): 289, Λm (μS): 215, Mw (g/mol): 160.03, 1H NMR (D2O, 400 MHz, ppm): 4.24–4.27 (d, 8H), 1.59–1.62 (d, 4H), 1.65–1.68 (t, 2H), 13C NMR (D2O, 400 MHz, ppm): 53.7 (N-CH2), 25.6, 23.8 (—C5H10), 205.16 (C—S). Selected FTIR (cm−1): 1417 (N—C), 963 (C-S), 1216 (C⚌S). Molecular mass (m/z): Calc for C6H10NS2 [M]+: 160.03, Found: 160.00. Ana. Calc. for C6H12NNaOS2: C, 35.80; H, 6.01; N, 6.96. Found: C, 35.29; H, 6.43; N, 6.57.
1-PhPzdtc-Na: Cream solid, yield (%): 78.12, m.p (°C): 295, Λm (μS): 234, Mw (g/mol): 237.05, 1H NMR (D2O, 400 MHz, ppm): 3.23 (t, 4H), 4.51 (t, 4H), 7.04–7.42 (m, —C6H5), 13C NMR (D2O, 400 MHz, ppm): 49.7–50.5 (N—CH2), 118.0–150.34 (C6H5), 209.0 (C—S). Molecular mass (m/z): Calc for C11H13N2S2 [M]+: 237.05, Found: 237.00. Selected FTIR (cm−1): 1416 (N—C), 995 (C-S), 1205 (C⚌S). Ana. Calc. for C11H17N2NaO2S2: C, 44.58; H, 5.78; N, 9.45. Found: C, 44.37; H, 5.73; N, 9.40.
Phdtc-NH4: White powder, yield (%): 58.96, m.p (°C): 109, Λm (μS): 146.1, Mw (g/mol): 168.00, 1H NMR (D2O, 400 MHz, ppm): 7.44–7.48(t, 2H), 7.20–7.23 (t, 1H), 7.14–7.12 (d, 2H), 4.27 (s, RN-H), 13C NMR (D2O, 400 MHz, ppm): 126.1–140.7 (C6H5), 213.9 (C—S). Molecular mass (m/z): Calc for C11H13N2S2 [M]+: 167.99, Found: 168.00. Selected FTIR (cm−1): 3078 (N—H), 1401 (N—C), 994 (C—S), 1280 (C⚌S). Ana. Calc. C7H10N2S2: C, 45.13; H, 5.41; N, 15.04. Found: C, 45.81; H, 5.40; N, 14.72.
BenzPip-Na: White powder, yield (%): 49.25. m.p (°C): 116, Λm (μS): 254, Mw (g/mol): 250.00, 1H NMR (D2O, 400 MHz, ppm): 1.36–1.35 (t, 4H), 1.96–2.01 (m, 1H), 2.64–2.66 (s, —CH2—), 3.13–3.20 (t, 4H), 7.42–7.32 (C6H5), 13C NMR (D2O, 400 MHz, ppm): 50.4 (N—CH2), 29.9 (—CH2—), 35.6 (—CH—), 40.0 (—CH2—), 125.5–139.5 (C6H5), 204.0 (C-S). Molecular mass (m/z): Calc for C13H16NS2 [M]+: 250.07, Found: 250.00. Selected FTIR (cm−1): 1462 (N—C), 958 (C—S), 1164 (C⚌S). Ana. Calc. for C13H20NNaO2S2: C, 50.46; H, 6.51; N, 4.53. Found: C, 50.31; H, 6.51; N, 4.28.
2.4 Synthesis of bis(acetonitrile)dichloropalladium(II) precursor
Palladium(II) chloride (2.82 mmol, 0.5 g) dispersed in acetonitrile (10 mL) was refluxed for 1 h at 80 °C under nitrogen flow. The yellow residue was filtered, washed with acetonitrile, and dried under vacuum (Bego, et al, 2009, Dai et al., 2011).
Mw: 259.43 g/mol, Yield (%): 75.6. 1H NMR (ppm): 2.30 (s, —CH3), 13C NMR (ppm): 0.51 (—CH3), 117.3 (—C≡N). Ana. Calc. for PdCl2∙(CH3CN)2: C, 18.52; H, 2.33; N, 10.80. Found: C, 18.11; H, 2.31; N, 10.67.
2.5 Synthesis of the Pd(II) dithiocarbamate complexes
Complexes of palladium(II) dithiocarbamate (Fig. 1) were prepared using a method reported in literature (Marcheselli, et al., 1993, Mukherjee et al., 2012, Bobinihi et al., 2019) Bis(acetonitrile)dichloropalladium(II) (0.4 g, 1.56 mmol) dissolved in acetone (50 mL) was refluxed for 30 min at 60 °C. To this, dithiocarbamate ligand (3.12 mmol) dissolved in acetone (50 mL) was added. The reaction was carried out for 4 h at 60 °C and thereafter at room temperature overnight. The resultant yellow precipitate was filtered, washed, and dried under vacuum.
Structures of the prepared palladium(II) dithiocarbamate complexes.
[Pd(Pipdtc)2]: Yellow powder, yield (%): 61.32, m.p (°C): 299, Λm (μS): 3.67. 1H NMR (ppm): 4.34–4.36 (t, 8H), 1.79–1.76 (m, 4H), 1.75–1.72 (m, 8H), 13C NMR (ppm): 54.6 (N—CH2), 27.2, 25.4 (—C5H10), 206.9 (C—S). Selected FTIR (cm−1): 1505 (N—C), 995 (C—S). Ana. Calc. for C12H24N2O2PdS4: C, 31.13; H, 5.22; N, 6.05. Found: C, 31.21; H, 5.58; N, 5.72.
[Pd(1-PhPzdtc)2]: Rusty-yellow solid, yield (%): 58.73, m.p (°C): 214. Λm (μS): 2.59. 1H NMR (ppm): 2.51–2.52 (t, 8H), 3.34-0.32 (t, 8H), 6.84–7.29 (C6H5), 13C NMR (ppm): 52.7–53.5 (N—CH2), 120.9–153.4 (C6H5), 212.03 (C—S). Molecular mass (m/z): Calc for C22H26N4PdS4 [M]+: 581.15, Found: 580.03. Selected FTIR (cm−1): 1478 (N—C), 1009 (C—S). Ana. Calc. for C22H28N4OPdS4: C, 44.10; H, 4.71; N, 9.35. Found: C, 43.77; H, 4.36; N, 9.08.
[Pd(Phdtc)2]: Dark brown powder, yield (%): 31.06, m.p (°C): 164. Λm (μS): 1.98. 1H NMR (ppm): 7.52–7.54 (d, 4H), 7.45–7.47 (t, 4H), 7.33–7.37 (t, 2H), 13C NMR (ppm): 123.5–136.8 (C6H5), 210.8 (C—S). Molecular mass (m/z): Calc for C14H12N2PdS4 [M]+: 442.94, Found: 442.00. Selected FTIR (cm−1): 3142 (N—H), 1507 (N—C), 974 (C—S). Ana. Calc. for C14H12N2PdS4: C, 39.96; H, 3.73; N, 6.32. Found: C, 39.53; H, 3.43; N, 6.34.
[Pd(BenzPipdtc)2]: Orange powder, yield (%): 29.93. m.p (°C): 207 (decomposed). Λm (μS): 4.05. 1H NMR (ppm): 1.14–1.24 (t, 4H), (m, 1H), 2.50–2.51 (s, —CH2—), 3.13–3.20 (t, 4H), 7.18–7.30 (C6H5), 13C NMR (ppm): 54.5 (N-CH2), 34.0 (—CH2—), 39.7 (—CH—), 44.14 (—CH2—), 128.6–143.7 (C6H5), 208.16 (C-S). Molecular mass (m/z): Calc for C27H36N2OPdS4 [M]+: 639.27, Found: 638.07. Selected FTIR (cm−1): 1500 (N—C), 963 (C—S). Ana. Calc. for C26H36N2O2PdS4: C, 48.55; H, 5.64; N, 4.35. Found: C, 48.33; H, 5.24; N, 4.37.
2.6 X-ray crystallography data collection
Single crystals of the four Pd(II) dithiocarbamate complexes were obtained by slow evaporation of the compound in dimethyl sulfoxide (DMSO) at room temperature. The crystals were isolated under oil and fixed on a MITIGEN crystal mounter. The data was gathered by a Bruker APEX-II CCD diffractometer fitted with an Oxford Cryostems low temperature instrument, running at T = 100(2) K. The structure was resolved by the She1XS-2013 software and refined using She1XL 2016/6 version (Sheldrick, 2015a, b).
2.7 Anticancer screening
The cytotoxic effect of the dithiocarbamate ligands and corresponding Pd(II) dithiocarbamate complexes on three human cell lines: Cervical cancer (HeLa), breast adenocarcinoma (MCF-7) and epithelial colorectal adenocarcinoma (Caco-2), were assessed by 3-[(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium (MTT) assay (Ajibade et al., 2020b; Scudiero et al., 1988). Cells were seeded at a density of 2 × 103 cells/well in 96-well microplates in growth medium (100 μL) and incubated for 24 h at 37 °C. Thereafter, the growth medium was removed and substituted with fresh medium containing the test compounds prepared in DMSO at four different concentrations (50, 30, 20 and 10 μg/ μL), and cells were incubated with the test compounds for 48 h at 37 °C. A cell control containing no test compounds was included. Following the incubation, growth medium (100 μL) containing 10 μL 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) salt solution (5 mg/mL in PBS) was added to each well and cells incubated for 4 h at 37 °C. The medium-MTT mixture was then removed and 100 μL of DMSO was added to each well to solubilize the formazan crystals produced. The absorbance of each well at was measured at 540 nm, and cell survival calculated using the equation (Moodley and Singh, 2019):
Each experiment was done in triplicate and the IC50 values were obtained using GraphPad Prism software.
3 Results and discussion
3.1 Molecular structures of four palladium(II) dithiocarbamate complexes
Single crystals of [Pd(Pipdtc)2], [Pd(1-PhPzdtc)2], [Pd(Phdtc)2] and [Pd(4-BenzPipdtc)2] complexes were obtained by slow evaporation of DMSO solution of the compounds. Crystallographic and refinement information are given in Table 1. Selected bond lengths and angles are provided in Table 2. The molecular structures with the atom numbering schemes are illustrated in Fig. 2 and the unit cell packing diagrams are shown in Fig. 3. [Pd(Pipdtc)2] formed a monoclinic crystal system with space group P21/c (Andrew and Ajibade, 2018), with molecules occupying the unit cell (Z = 2). We have found that the Pd-S bond lengths are slightly shorter compared to those of the same structure reported by Shaheen et. al (Shaheen et al. 2006).
Complex
[Pd(Pipdtc)2]
[Pd(1-Phpzdtc)2]
[Pd(Phdtc)2]· (CH3)2SO
[Pd(4-BenzPipdtc2)]
Empirical formula
C12H20N2PdS4
C22H26N4PdS4
C16H18N2PdS5O
C26H32N2PdS4
Formula weight (g/mol)
426.94
581.11
519.07
607.17
Temperature (K)
100(2) K
100(2)
100(2)
100(2)
Wavelength (Å)
1.54178
0.71073
1.54178
1.54178
Crystal system
Monoclinic
Monoclinic
Triclinic
Triclinic
Space group
P21/c
P21/c
P−1
P−1
Unit cell dimensions
a (Å)
α (°)
b (Å)
β (°)
c (Å)
γ (°)
6.0289(2)
90
8.4846(2)
95.5360(10)
15.3040(5)
90
7.1932(3)
90
6.1253(3)
93.439(2)
25.4715(12)
90
8.3286(12)
105.494(8)
8.9561(13)
100.021(8)
9.7047(14)
112.186(10)
7.9829(2)
68.6270(10)
11.9550(3)
78.0280(10)
15.6176(4)
75.4140(10)
Volume (Å3)
779.19(4)
1120.27(9)
614.52(16)
1332.13(6)
Z
2
4
2
2
Calculated density (Mg/m3)
–
1.723
1.619
1.514
Absorption coefficient (mm−1)
1.820
1.220
11.002
8.675
F(000)
432
592
304
624
Crystal size (mm3)
? x ? x ?
0.200 × 0.120 × 0.080
0.205 × 0.155 × 0.105
0.100 × 0.085 × 0.025
Theta range for data collection (°)
5.809–68.262
1.602–28.270
4.971–70.318
4.047–69.947
Limiting indices
−7≤h≤7, −10≤k≤10, −2≤l≤18
−9≤h≤9, −8≤k≤7, −33≤l≤33
−10≤h≤10, −10≤k≤10, −11≤l≤11
−7≤h≤9, −14≤k≤14, −19≤l≤19
Reflections collected / unique
1427/ 1427 [R(int) = ?]
19,058 / 2770 [R(int) = 0.0311]
10,838
28,192
Completeness to theta = 25.242 (%)
99.9
100.0
97.3
97.2
Absorption correction
–
Semi-empirical from equivalents
Semi-empirical from equivalents
–
Max. and min. transmission
–
0.7457 and 0.6726
0.7533 and 0.5287
–
Refinement method
Full-matrix least-squares on F2
Full-matrix least-squares on F2
Full-matrix least-squares on F2
Full-matrix least-squares on F2
Data/ restraints/ parameters
1427/ 0/ 89
2770 / 0 / 142
2248 / 0 / 139
4893 / 0 / 301
Goodness-of-fit on F2
1.249
1.076
1.014
1.029
Final R indices [I > 2sigma(I)]
R1 = 0.0318, wR2 = 0.0841
R1 = 0.0267, wR2 = 0.0538
R1 = 0.0406, wR2 = 0.1036
R1 = 0.0211, wR2 = 0.0544
R indices (all data)
R1 = 0.0329, wR2 = 0.0843
R1 = 0.0312, wR2 = 0.0555
R1 = 0.0454, wR2 = 0.1073
R1 = 0.0252, wR2 = 0.0570
Largest diff. peak and hole (e.A3)
0.655 and −0.542
0.839 and −0.652
1.075 and −1.185
0.368 and −0.216
Bond lengths (Å) and angles (°) for [Pd(Pipdtc)2]
Bond length (Å)
Bond angles (°)
Pd(1)-S(1)#1
2.3186 (11)
S(1)#1-Pd(1)-S(1)
180.00 (4)
Pd(1)-S(1)
2.3186(11)
S(1)#1-Pd(1)-S(2)
104.59(4)
Pd(1)-S(2)
2.3291(12)
S(1)-Pd(1)-S(2)
75.41(4)
Pd(1)-S(2)#1
2.3292(12)
S(1)#1-Pd(1)-S(2)#1
75.41(4)
S(1)-C(6)
1.730(5)
S(1)-Pd(1)-S(2)#1
104.59(4)
S(2)-C(6)
1.721(5)
S(2)-Pd(1)-S(2)#1
180.0
Bond lengths (Å) and angles (°) for [Pd(1-Phpzdtc)2]
Pd(1) - S(1)#1
2.3236(5)
S(1)#1-Pd(1)-S(1)
180.000
Pd(1) - S(1)
2.3236(5)
S(1)#1-Pd(1)-S(2)#1
75.458(18)
Pd(1) - S(2)#1
2.3300(5)
S(1)-Pd(1)-S(2)#1
104.542(18)
Pd(1) - S(2)
2.3300(5)
S(1)#1-Pd(1)-S(2)
104.542(18)
S(1) - C(1)
1.7180(2)
S(1)-Pd(1)-S(2)
75.458(18)
Bond lengths (Å) and angles (°) for [Pd(Phdtc)2]
Pd(1)-S(1)
2.3232(9)
S(1)-Pd(1)-S(1)#1
180.0
Pd(1)-S(1)#1
2.3232(9)
S(1)-Pd(1)-S(2)
75.66(3)
Pd(1)-S(2)
2.3308(10)
S(1)#1-Pd(1)-S(2)
104.34(3)
Pd(1)-S(2)#1
2.3309(10)
S(1)-Pd(1)-S(2)#1
104.34(3)
S(1S)-O(1S)
1.517(3)
S(1)#1-Pd(1)-S(2)#1
75.66(3)
S(1S)-C(2S)
1.778(4)
S(2)-Pd(1)-S(2)#1
180.0
Bond lengths (Å) and angles (°) for [Pd(4-BenzPipdtc)2]
Pd(1)-S(1)
2.3239(5)
S(1)-Pd(1)-S(1)#1
180.00(3)
Pd(1)-S(1)#1
2.3240(5)
S(1)-Pd(1)-S(2)#1
104.630(17)
Pd(1)-S(2)#1
2.3325(5)
S(1)#1-Pd(1)-S(2)#1
75.369(17)
Pd(1)-S(2)
2.3325(5)
S(1)-Pd(1)-S(2)
75.370(17)
Pd(2)-S(4)
2.3138(5)
S(1)#1-Pd(1)-S(2)
104.630(17)
Pd(2)-S(4)#2
2.3138(5)
S(2)#1-Pd(1)-S(2)
180.00(2)
Pd(2)-S(3)
2.3339(5)
S(4)-Pd(2)-S(4)#2
180.0
Pd(2)-S(3)#2
2.3339(5)
S(4)-Pd(2)-S(3)
75.736(17)
S(1)-C(1)
1.7258(19)
S(4)#2-Pd(2)-S(3)
104.264(17)
S(2)-C(1)
1.7196(19)
S(4)-Pd(2)-S(3)#2
104.264(17)
![Molecular structure of [Pd(Pipdtc)2], [Pd(1-PhPzdtc)2], [Pd(Phdtc)2] and [Pd(4-BenzPipdtc)2] showing 50 % probability displacement ellipsoid and atom labelling. The hydrogen atoms are excluded for clearness.](/content/184/2021/14/9/img/10.1016_j.arabjc.2021.103326-fig2.png)
Molecular structure of [Pd(Pipdtc)2], [Pd(1-PhPzdtc)2], [Pd(Phdtc)2] and [Pd(4-BenzPipdtc)2] showing 50 % probability displacement ellipsoid and atom labelling. The hydrogen atoms are excluded for clearness.
![Unit cell packing diagram of [Pd(Pipdtc)2], [Pd(1-PhPzdtc)2], [Pd(Phdtc)2] and [Pd(4-BenzPipdtc)2].](/content/184/2021/14/9/img/10.1016_j.arabjc.2021.103326-fig3.png)
Unit cell packing diagram of [Pd(Pipdtc)2], [Pd(1-PhPzdtc)2], [Pd(Phdtc)2] and [Pd(4-BenzPipdtc)2].
[Pd(1-PhPzdtc)2] complex also formed a monoclinic crystal system with space group P21/c (Andrew and Ajibade, 2018; Paca and Ajibade, 2021), with four molecules in the unit cell (Z = 4). [Pd(Phdtc)2] and [Pd(4-BenzPipdtc)2] crystallized in a triclinic crystal system with space group P-1 with a Z value of 2. The [Pd(Phdtc)2] complex is similar to those reported by Bobinihi et. al (Bobinihi, et al., 2019) which crystallized in an orthorhombic crystal system which might be due to the solvent and method used for crystallization.
In all the complexes, the Pd(II) ion is coordinated to four sulphur atoms from two bidentate dithiocarbamato anions. The Pd(II) ion lies within the center of symmetry, hence each asymmetric unit consist of half distinct molecule. The palladium(II) ion is coordinated to four sulphur atoms to form a distorted square planar geometry. Though the equatorial PdS4 fraction is planar, distortion is a result of the small bite angle of S(1)-Pd-(S2) of 75.41 (4), 75.458 (18), 75.66 (33) and 75.369 (17) for [Pd(Pipdtc)2], [Pd(1-PhPzdtc)2], [Pd(Phdtc)2] and [Pd(4-BenzPipdtc)2], respectively which are approximately 75° and therefore hindering the formation of a perfect square planar geometry (Ehsan, et al., 2014, Gupta et al. 2014). The S1-Pd-S2 bond angles are 180(4), 104.59(4) and 75.41(18)° for [Pd(Pipdtc)2], 180, 104.542(18) and 75.458(18)° for [Pd(1-Phpzdtc)2], 180, 104.34(3) and 75.66(4)° for [Pd(Phdtc)2] and 180.00(3), 104.64300(17) and 75.369(17)° and 180.00(2), 104.736(17) and 75.736(17)°. These bond angles are comparable to those of previously reported related compounds (Mukherjee et al., 2012, Poirier et al., 2018) [43, 44]. The Pd-S bond lengths in all the complexes are almost equidistance and are similar to related complexes retrieved from the CSD (Bonamico et al., 1977, Eslami et al. 2016, Ferreira et al., 2014, Konarev et al., 2005, Phadnis et al., 2005, Poirier et al. 2014). Even though the four dithiocarbamate ligands are all coordinated to Pd(II) ion, the bond lengths and bond angles are not exactly the same. This might be ascribed to the nature of the dithiocarbamato anions, and their electronic influence on the stereochemistry of the Pd(II) dithiocarbamate complexes which resulted in the variations observed in the Pd-S bond lengths. It was observed that the bulkier the dithiocarbamate ligand, the longer the bond distance.
The C—S bond lengths are intermediate between the C—S and C⚌S bond lengths suggesting a partial double bond character because of delocalization of the CS2 electron density in the dithiocarmate moiety. The thioureide N(1)-C(1) bond lengths are 1.471(6), 1.327(3), 1.328(5) and 1.321(2) Å for [Pd(Pipdtc)2], [Pd(1-PhPzdtc)2], [Pd(Phdtc)2] and [Pd(4-BenzPipdtc)2], respectively. These values suggested a partial (N ≅ C) double-bond because of the higher electronic density expected on coordination of the dithiocarbamato anions to the Pd(II) ions which indicate sp2 hybridization and delocalization of the electron density in the CS2 group (Ferreira et al., 2014).
3.2 FTIR spectra studies
The FTIR spectra of the dithiocarbamate ligands and corresponding Pd(II) dithiocarbamate complexes were assigned after careful comparisons. Dithiocarbamates have three mains characteristic FTIR bands that can be used to determine the mode of coordination between the dithiocarbamato anions and the metal ions. The FTIR spectra of the ligands showed ʋ(C—N) stretching bands at 1417, 1416, 1401 and 1462 cm−1 for Pipdtc-Na, 1-PhPzdtc-Na, Phdtc-NH4 and BenzPipdtc-Na, respectively. These bands were observed in the complexes at 1505, 1478, 1507 and 1500 cm−1 for [Pd(Pipdtc)2], [Pd(1-PhPzdtc)2], [Pd(Phdtc)2] and [Pd(BenzPipdtc)2], respectively. The shifts to higher frequencies observed in the spectra of the Pd(II) complexes are ascribed to the coordination of the dithiocarbamato anions and the Pd(II) ions that causes delocalization of the thioureide electrons within the dithiocarbamate moiety (Ajibade et al., 2020a,c, Oluwalana and Ajibade, 2020). This was confirmed by the single crystal X-ray crystal structure bond lengths in the complexes. The binding mode of the dithiocarbamate ligand to the metal center was established based on the Bonati-Ugo method (Mbese and Ajibade, 2017; Bonati and Ugo, 1967). Two bands in the region 940–1060 cm−1 in the FTIR spectra of the dithiocarbamate ligands are assigned to the v(C⚌S) stretching vibrations. In the spectra of the Pd(II) complexes, only one peak was observed that signifies the symmetrical bidentate coordination of the dithiocarbamate ligand to the Pd(II) ion (Sathiyaraj et al., 2015). The v(M—S) band normally appears in the far-IR region but could not be detected by the FTIR instrument used in this study.
3.3 Electronic spectra studies
Relevant electronic spectra data of the dithiocarbamate ligands and their corresponding Pd(II)complexes are summarized in Table 3. Three absorptions bands were observed for each ligand as shown in Fig. 4. The first band observed around 264, 258, 257 and 254 nm for Pipdtc-Na, 1-PhPzdtc-Na, Phdtc-NH4 and 4-BenzPipdtc-Na, respectively are assigned to the π-π* transition of N—C⚌S fragment (Ajibade et al., 2020; Al-Hasani and Al-Taie, 2015). The second absorption band at 282 nm for pipdtc-Na, 286 nm for 1-phpzdtc-Na, 295 nm for Phdtc-NH4 and 301 nm for 4-BenzPipdtc-Na are due to the π-π* transitions of the S—C⚌S system (Mbese and Ajibade, 2014). The band at about 443 nm in the spectrum of (Pipdtc-Na), 430 nm in (1-PhPzdtc-Na), 429 nm in (Phdtc-NH4) and 435 nm in (4-BenzPipdtc-Na) are due to the n-π* transition of the lone pair of electrons found on the dithiocarbamate sulphur atoms.
Compounds
λmax (nm)
1st band
2nd band
3rd band
Pipdtc-Na
264
282
443
[Pd(Pipdtc)2]
283
305
340
1-PhPzdtc-Na
258
286
430
[Pd(1-PhPzdtc)2]
262
308
Phdtc-NH4
257
295
429
[Pd(Phdtc)2]
317
350
4-BenzPipdtc-Na
254
301
435
[Pd(4-BenzPipdtc)2]
306
343
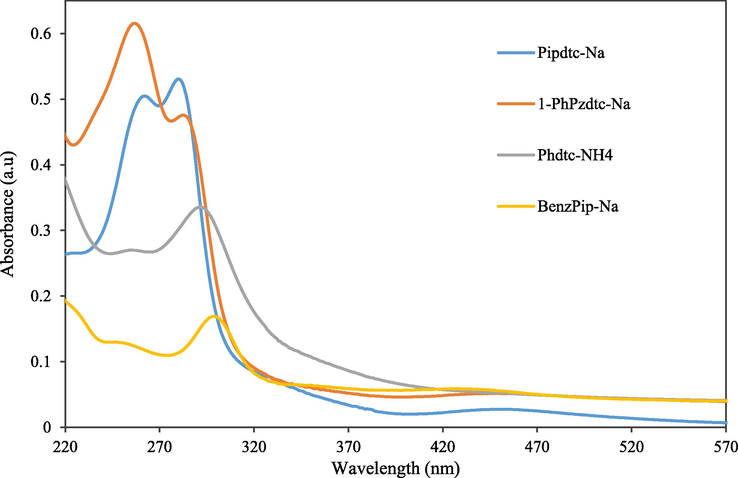
Electronic spectra of the dithiocarbamate ligands.
The electronic spectrum of [Pd(Pipdtc)2] exhibited three absorption bands around 283, 305 and 340 nm. The bands observed at 283 and 305 nm are due to the inter-ligand π-π* transitions of the dithiocarbamate moiety. The broad band at about 340 nm could be due to the charge transfer transitions. The spectrum of [Pd(1-PhPzdtc)2] complex exhibited two absorption peaks at 262 and 308 nm which are ascribed to the π-π* transitions. The electronic spectra of [Pd(Phdtc)2] and [Pd(4-BenzPipdtc)2] exhibited two absorption bands. The first set of bands ascribed to the inter-ligand transitions due to the dithiocarbamate moiety. The second bands are attributed to the metal–ligand-charge-transfer (MLCT) transitions, a consequence of the coordination of the dithiocarbamate ligands to the Pd(II) ion. The stability of the Pd(II) complexes were investigated under physiological conditions in dimethyl sulfoxide (DMSO). Absorption spectrum for each complex was recorded at different intervals as shown in Fig. 5. The results indicate that all the Pd(II) complexes were stable in DMSO for 48 h.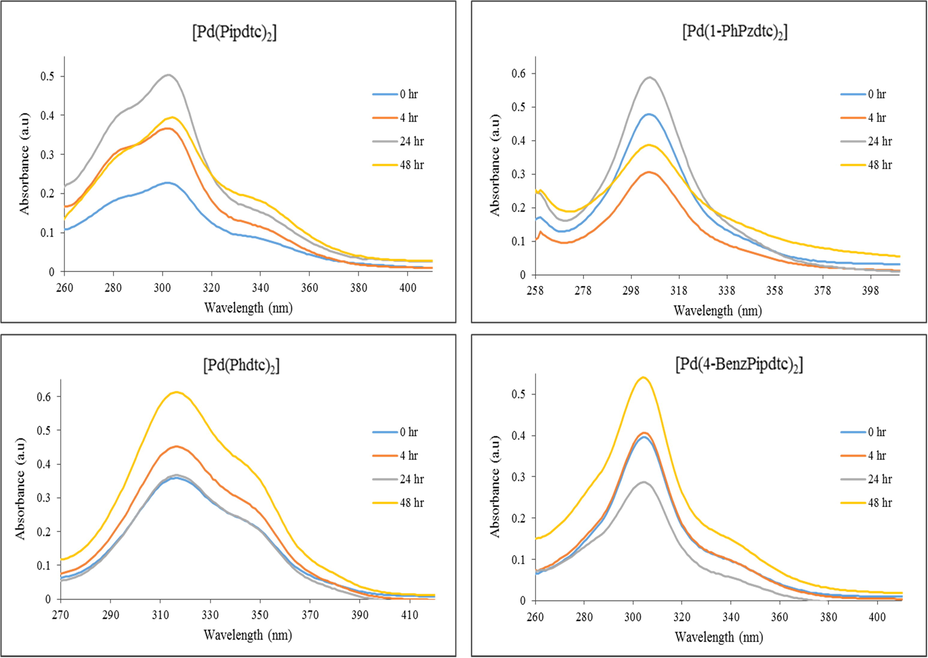
Electronic spectra of palladium(II) dithiocarbamate complexes in DMSO over time.
3.4 1H and 13C NMR of the ligands
The 1H and 13C NMR spectra of the dithiocarbamate ligands corresponded with the suggested formulation of the compounds. Pipdtc-Na ligand showed an overlapped triplet-like doublet of doublet at 4.24–4.27 ppm for the –CH2N protons due to the germinal proton attached to the same carbon atom. These protons were deshielded due to the existence of the electron withdrawing cloud towards the nitrogen atom. The other –CH2 protons shifted upfield in the region 1.29–1.68 ppm and appeared a triplet-like multiplet. In the 13C NMR, C-S bond was observed at 205.16 ppm for the free dithiocarbamate ligands. The spectrum of 1-PhPzdtc-Na showed protons of the piperazine group at 4.51 and 3.23 ppm as doublet of doublet of doublets (Andrew and Ajibade, 2018). The protons of the phenyl ring appeared at 7.04–7.42 ppm. Upon coordination to the palladium(II) ion, the protons shifted upfield. For the Phdtc-NH4 ligand, the protons due to the phenyl ring appeared at 7.48–7.14 ppm. The proton of the RNH group appeared at 4.27 ppm. Once coordinated to the Pd(II) ion, a downfield shift was observed for the phenyl ring but the C-S bond shifted from 213.92 to 210.77 ppm. In the spectrum of 4-BenzPip-Na, the –CH2 protons of the piperidine ring appeared at 1.34–1.188 and 2.91–3.01 ppm for the protons near the nitrogen atom and methyl group, respectively. The methyl protons appeared at 2.54 ppm and the benzyl ring appeared at 7.27–7.40 ppm. In the spectrum of [Pd(4-BenzPipdtc)2], a shift was observed on the proton and carbon NMR spectra confirming the formation of the palladium(II) dithiocarbamate complex.
3.5 Cytotoxicity studies
In vitro cytotoxic activities of the dithiocarbamate ligands and their corresponding Pd(II) complexes were evaluated against cervical cancer (HeLa), breast adenocarcinoma (MCF-7), and epithelial colorectal adenocarcinoma (Caco-2) cell lines and their IC50 values presented in Table 4. The tested compounds showed highly potent cytotoxic activity against MCF-7 cells with cytotoxic activity in the sequence [Pd(1-PhPzdtc)2] > [Pd(Phdtc)2] > [Pd(4-BenzPipdtc)2] > [Pd(Pipdtc)2] with IC50 values of 8.09, 0.151, 0.11, and 0.032 μM, respectively. The cytotoxic activities of the Pd(II) complexes are higher than that of the dithiocarbamate ligands. This proved that the coordination of the dithiocarbamate ligands to the Pd(II) enhances the activity of the compounds in MCF-7 cells. Andrew and Ajibade, 2018 prepared Cu(II), Zn(II) and Pt(II) complexes of 1-phenylpiperazine dithiocarbamate (1-PhPzdtc) and assessed their activity against MCF-7 cell line. Their results showed that the [Pt(1-PhPzdtc)2] complex is only active at concentration higher than 100 μM. The IC50 values of 17.52 and 8.42 μM were obtained for the [Cu(1-PhPzdtc)2] and [Zn2(μ-1-PhPzdtc)2(1-PhPzdtc)2] complexes, respectively. Comparing their results with what we obtained for the [Pd(1-PhPzdtc)2] against MCF-7 cell line, one can deduce that palladium(II) complexes have better cytotoxicity compared to Cu(II), Zn(II) and Pt(II). Three of the Pd(II) complexes are highly potent against the MCF-7 cancer cell line and their potency is more than that of cis-platin against the same cancer cell line. When these four compounds were screened against HeLa cell, only [Pd(Pipdtc)2] and [Pd(Phdtc)2] complexes showed higher potency than their corresponding dithiocarbamate ligands. The potency of [Pd(Pipdtc)2] against the cell line is higher than that of cis-platin while the potency of [Pd(Phdtc)2] is comparable to that of cis-platin against the cancer cell line. [Pd(Phdtc)2] and the ligand showed excellent cytotoxicity against Caco-2 cells but that of the Pd(II) is better than that of the free ligand which indicate that the coordination of the Pd(II) to the dithiocarbamate ligand enhances the potency of the complex. While this is the case in most instances, but in others, the potency of the Pd(II) complexes are lower than that of the free ligands. It was deduced that the presence of the Pd(II) ion does not have much significant cytotoxicity in HeLa and Caco-2 cells. However, the four Pd(II) dithiocarbamate compounds exhibited potent anticancer activity against the breast cancer cells (MCF-7), with lower activity when compared to their respective free ligands. This cancer cell specificity in vitro is encouraging and interesting, warranting their further optimization and screening in additional cells lines to confirm their potential as future anticancer agents. After optimization, it will also be necessary to carry out structure activity relationship studies on the active compound for further derivatization.
Compound
IC50 (μM)
HeLa
MCF-7
Caco-2
Pipdtc-Na
1.85
1.02
3.02 × 10−10
[Pd(Pipdtc)2]
0.105
0.032
2.63 × 10−8
1-PhPzdtc-Na
24.5
15.1
3.222
[Pd(1-PhPzdtc)2]
190
8.09
10.96
Phdtc-NH4
39.8
13.8
10.1
[Pd(Phdtc)2]
3.33
0.151
1.12
4-BenzPipdtc-Na
0.52
0.425
0.27
[Pd(4-BenzPipdtc)2]
28.2
0.11
6.04
Cisplatin
3.2
2.5
4 Conclusion
We report the synthesis, spectroscopic characterization, single crystal X-ray crystallography and anticancer studies of four palladium(II) dithiocarbamate complexes. FTIR spectra data confirmed that the dithiocarbamato anions coordinate bidentately to the Pd(II) ion. This was further confirmed by the single X-ray crystal structures of the palladium(II) complexes. [Pd(Pipdtc)2] and [Pd(1-PhPzdtc)2] crystallized in monoclinic P21/c space group while [Pd(Phdtc)2] and [Pd(4-BenzPipdtc)2] crystallized in triclinic P-1 space group. Molecular structures of the Pd(II) complexes revealed square planar PdS4 geometry in which each Pd(II) is coordinated to two chelating dithiocarbamato anions to form a square planar geometry around the Pd(II) ions. The anticancer potential of the dithiocarbamate ligands and corresponding Pd(II) complexes were evaluated against cervical cancer (HeLa), breast adenocarcinoma (MCF-7) and epithelial colorectal adenocarcinoma (Caco-2). The results shows that piperidinyl dithiocarbamate sodium salt and 4-benzylpiperidinyl dithiocarbamate sodium salt ligands were more active than cis-platin against the cancer cell lines. Two palladium(II) complexes, ([Pd(Pipdtc)2] and [Pd(Phdtc)2]) are highly potent in comparison to their corresponding free ligands. The potency of [Pd(Pipdtc)2] against the cell line is higher than that of cis-platin while the potency of [Pd(Phdtc)2] is comparable to that of cis-platin against the cancer cell lines. [Pd(Phdtc)2] and the ligand showed excellent cytotoxicity against Caco-2 cells. Most of the Pd(II) complexes are more potent than free dithiocarbamate ligands which indicate that the coordination of the Pd(II) to the dithiocarbamate ligand enhances their anticancer potency.
Acknowledgements
The authors are grateful for the financial support of National Research Foundation (grant number 129275) and Sasol, South Africa.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- In vitro screening, homology modeling and molecular docking studies of some pyrazole and imidazole derivatives. Biomed. Pharmacother.. 2018;103:653-661.
- [Google Scholar]
- Synthesis, characterization and anticancer studies of Mn(II), Cu(II), Zn(II) and Pt(II) dithiocarbamate complexes-crystal structures of the Cu(II) and Pt(II) complexes. Inorg. Chim. Acta. 2020;504:119431
- [Google Scholar]
- Structural, photocatalytic and anticancer studies of hexadecylamine capped ZnS nanoparticles. Chem. Phys. Lett.. 2020;137813
- [Google Scholar]
- Synthesis, crystal structures and anticancer studies of morpholinyldithiocarbamato Cu(II) and Zn(II) complexes. Molecules. 2020;25:3584.
- [Google Scholar]
- Synthesis, structural and antibacterial study of some metal ion dithiocarbamate-azo complexes. Al-Nahrain J. Sci.. 2015;18:1-12.
- [Google Scholar]
- Synthesis and cytotoxic effect on RD cell line of Pd(II) and Cu(II) J. Saudi Chem. Soc.. 2016;20:24-32.
- [Google Scholar]
- Synthesis, characterization, and electrochemical studies of Co(II, III) dithiocarbamate complexes. J. Coord. Chem.. 2019;72:1171-1186.
- [Google Scholar]
- Synthesis, characterization and anticancer studies of bis(1-phenylpiperazine dithiocarbamato) Cu(II), Zn(II) and Pt(II) complexes: crystal structures of 1-phenylpiperazine dithiocarbamato-S, S′ zinc(II) and Pt(II) J. Mol. Struct.. 2018;1170:24-29.
- [Google Scholar]
- Anticancer activity, DNA-binding and DNA-denaturing aptitude of palladium(II) dithiocarbamates. Inorg. Chim. Acta. 2016;451:31-40.
- [Google Scholar]
- A series of novel indazole derivatives of Sirt 1 activator as osteogenic regulators. Bioorg. Med. Chem. Lett.. 2017;27:4828-4831.
- [Google Scholar]
- Immunomodulatory effects of palladium(II) complexes of 1, 2, 4-triazole on murine peritoneal macrophages. J. Braz. Chem. Soc.. 2009;20:437-444.
- [Google Scholar]
- Synthesis and evaluation of alkenyl indazoles as selective Aurora kinase inhibitors. Bioorg. Med. Chem. Lett.. 2010;20:2443-2447.
- [Google Scholar]
- Group 10 metal complexes of dithiocarbamates derived from primary anilines: Synthesis, characterization, computational and antimicrobial studies. Polyhedron. 2019;158:296-310.
- [Google Scholar]
- Structural studies of metal complexes with sulphur-containing bidentate ligands. Part 2. Evidence for a metal–metal bond from the molecular structures of bis(phenyldithioacetato)-nickel(II) and -palladium(II) J. Chem. Soc., Dalton Trans.. 1977;23:2315-2319.
- [Google Scholar]
- Organotin(IV) N, N-disubstituted dithiocarbamates. J. Organomet. Chem.. 1967;10:257-268.
- [Google Scholar]
- Synthesis, structure, photoluminescence and electrochemical properties of mononuclear Ag (I) and polymeric Zn(II) complexes of potassium 4-methyl piperazine-1-carbodithioate. J. Mol. Struct.. 2019;1177:260-268.
- [Google Scholar]
- Pd-catalysed decarboxylative Suzuki reactions and orthogonal Cu-based O-arylation of aromatic carboxylic acids. Chem. Commun.. 2011;47:677-679.
- [Google Scholar]
- Inhibition of noroviruses by piperazine derivatives. Bioorg. Med. Chem. Lett.. 2012;22:377-379.
- [Google Scholar]
- Vysotskite structured photoactive palladium sulphide thin films from dithiocarbamate derivatives. New J. Chem.. 2014;38:4083-4091.
- [Google Scholar]
- Synthesis, crystal structure, spectral and thermal investigations of morpholinyldithiocarbamate complexes: a novel coordinated precursors for efficient metal oxide nanophotocatalysts. Inorg. Chim. Acta. 2019;487:307-315.
- [Google Scholar]
- Synthesis, cytotoxicity assessment, and interaction and docking of novel palladium(II) complexes of imidazole derivatives with human serum albumin. J. Biomol. Struct. Dyn.. 2016;34:1751-1762.
- [Google Scholar]
- New trends in platinum and palladium complexes as antineoplastic agents. Coord. Chem. Rev.. 2016;310:41-79.
- [Google Scholar]
- Study of metal dithiocarbamate complexes, Part V. Metal complexes of [S2CN(CH2CH(OMe)2]: a standard dimeric zinc dithiocarbamate structural motive, a rare cadmium dithiocarbamate coordination polymer, and a hydrated sodium dithiocarbarmate complex, with a [Na2O2] core and chain. Inorg. Chim. Acta. 2016;441:137-145.
- [Google Scholar]
- Synthesis, characterization and antifungal activity of new dithiocarbamate-based complexes of Ni(II), Pd(II) and Pt(II) Inorg. Chim. Acta. 2014;423:443-449.
- [Google Scholar]
- Pt(II) and Pd(II) derivatives of ter-butylsarcosinedithiocarbamate: synthesis, chemical and biological characterization and in vitro nephrotoxicity. J. Inorg. Biochem.. 2003;93:181-189.
- [Google Scholar]
- Intermolecular anagostic interactions in group 10 metal dithiocarbamates. Cryst. Eng. Comm.. 2014;16:9299-9307.
- [Google Scholar]
- Cytotoxic effects of newly synthesized palladium(II) complexes of diethyldithiocarbamate on gastrointestinal cancer cell lines. Biochem. Res. Int.. 2014;2014:1-9.
- [Google Scholar]
- Biosynthesis of oxygen and nitrogen-containing heterocycles in polyketides. Beilstein J. Org. Chem.. 2016;12:1512.
- [Google Scholar]
- Synthesis, characterization and antitumor activities of new palladium(II) complexes with 1-(alkyldithiocarbonyl)-imidazoles. J. Coord. Chem.. 2014;67:461-469.
- [Google Scholar]
- Synthesis, structural and antibacterial studies of new dithiocarbamate complexes of Sb(III) and Bi(III) Malaysian J. Anal. Sci. 2014;18:251-259.
- [Google Scholar]
- New heteroleptic palladium(II) dithiocarbamates: synthesis, characterization, packing and anticancer activity against five different cancer cell lines. Appl. Organomet. Chem.. 2016;30:392-398.
- [Google Scholar]
- New 3D and 2D supramolecular heteroleptic palladium(II) dithiocarbamates as potent anticancer agents. J. Coord. Chem.. 2016;69:2999-3009.
- [Google Scholar]
- Neutral and ionic complexes of C60 with metal dibenzyldithiocarbamates. Reversible dimerization of C60•- in ionic multicomponent complex [CrI(C6H6)2•+]·(C60•)·0.5[Pd(dbdtc)2] Inorg. Chem. 2005;44:9547-9553.
- [Google Scholar]
- Synthesis, crystal structures and antitumor activity of two platinum(II) complexes with methyl hydrazinecarbodithioate derivatives of indolin-2-one. Eur. J. Med. Chem.. 2017;127:137-146.
- [Google Scholar]
- Effect of functionalities on the crystal structures of new zinc(II) dithiocarbamates: a combined anti-leishmanial and thermal decomposition study. Cryst. Eng. Comm.. 2017;19:2660-2672.
- [Google Scholar]
- Synthesis, characterization and evaluation of biological activity of palladium(II) and platinum(II) complexes with dithiocarbamic acids and their derivatives as ligands. Eur. J. Med. Chem.. 1993;28:347-352.
- [Google Scholar]
- Synthesis, spectroscopic, structural, and optical studies of Ru2S3 nanoparticles prepared from single-source molecular precursors. J. Mol. Struct.. 2017;1143:274-281.
- [Google Scholar]
- Synthesis, characterization and antifungal activities of 3d-transition metal complexes of 1-acetylpiperazinyldithioc arbamate, M (acpdtc) 2. Spectrochim. Acta, Part A. 2009;73:20-24.
- [Google Scholar]
- Polymeric mesoporous silica nanoparticles for enhanced delivery of 5-fluorouracil in vitro. Pharmaceutics. 2019;11:1-21.
- [Google Scholar]
- Palladium(II) complexes of dithiocarbamic acids: synthesis, characterization, crystal structure and DNA binding study. Met. Chem.. 2012;37:155-161.
- [Google Scholar]
- Beyond platinums: gold complexes as anticancer agents. Anticancer Res.. 2014;34:487-492.
- [Google Scholar]
- Antiproliferative and interaction studies of a synthesized palladium(II) complex with human hemoglobin. J. Mol. Liq.. 2018;249:265-271.
- [Google Scholar]
- Synthesis and crystal structures of Pb(II) dithiocarbamates complexes: precursors for PbS nanophotocatalyst. J. Sulfur Chem.. 2020;41:182-199.
- [Google Scholar]
- Palladium(II) and ruthenium(II) complexes of benzotriazole functionalized N-heterocyclic carbenes: cytotoxicity, antimicrobial, and DNA interaction studies. J. Organomet. Chem.. 2019;886:48-56.
- [Google Scholar]
- Synthesis and structural studies of iron sulphide nanocomposites prepared from Fe(III) dithiocarbamates single source precursors. Mater. Chem. Phys.. 2017;202:143-150.
- [Google Scholar]
- Bis-(N-ethylphenyldithiocarbamato)palladium(II) as molecular precursor for palladium sulfide nanoparticles. J. Mol. Struct.. 2021;12435:130777
- [Google Scholar]
- Synthesis, spectroscopy, structure and photophysical properties of dinaphthylmethylarsine complexes of palladium(II) and platinum(II) Inorg. Chim. Acta. 2005;358:2609-2617.
- [Google Scholar]
- Variation of M···H–C interactions in square-planar complexes of Nickel(II), Palladium(II), and Platinum(II) probed by luminescence spectroscopy and X-ray diffraction at variable pressure. Neutral and ionic complexes of C60 with metal dibenzyldithiocarbamates. Reversible dimerization of C60•- in ionic multicomponent complex [CrI(C6H6)2•+]·(C60•-)·0.5[Pd(dbdtc)2] Inorg. Chem.. 2018;57:7713-7723.
- [Google Scholar]
- Why do the luminescence maxima of isostructural palladium(II) and platinum(II) complexes shift in opposite directions? Can. J. Chem.. 2014;92:958-965.
- [Google Scholar]
- Palladium(II) complexes as biologically potent metallo-drugs: synthesis, spectral characterization, DNA interaction studies and antibacterial activity. Spectrochim. Acta, Part A. 2013;107:108-116.
- [Google Scholar]
- Lanthanum(III) and praseodymiu(III) derivatives with dithiocarbamates derived from α-amino acids. Spectrochim. Acta A. 2006;64:789-794.
- [Google Scholar]
- Investigation of the binding behavior between the S-heterocyclic aromatic palladium(II) complex and human serum albumin: spectroscopic approach. Polycycl. Aromat. Comp.. 2016;36:40-57.
- [Google Scholar]
- Nickel(II) dithiocarbamate complexes containing the pyrrole moiety for sensing anions and synthesis of nickel sulfide and nickel oxide nanoparticles. New J. Chem.. 2015;39:5336-5349.
- [Google Scholar]
- Evaluation of a soluble tetrazolium/formazan assay for cell growth and drug sensitivity in culture using human and other tumor cell lines. Cancer Res.. 1988;48:4827-4833.
- [Google Scholar]
- Synthesis, characterization, antibacterial and cytotoxic activity of new palladium(II) complexes with dithiocarbamate ligands: X-ray structure of bis (dibenzyl-1-S: S′-dithiocarbamato) Pd(II) J. Organomet. Chem.. 2007;692:3019-3026.
- [Google Scholar]
- Bis(piperidine-1-dithiocarbamato-κ2S, S′)palladium(II) Acta Crystallogr., Sect. E: Struct. Rep. Online. 2006;62:329-330.
- [Google Scholar]
- Crystal structure refinement with SHELXL. Acta Crystallogr. C Struct. Chem.. 2015;71:3-8.
- [Google Scholar]
- SHELXT–Integrated space-group and crystal-structure determination. Acta Crystallogr. A Found Adv.. 2015;71:3-8.
- [Google Scholar]
- Template synthesis of symmetrical transition metal dithiocarbamates. J. Braz. Chem. Soc.. 2006;17:107-112.
- [Google Scholar]
- Electrochemical, surface and quantum chemical studies of novel imidazole derivatives as corrosion inhibitors for J55 steel in sweet corrosive environment. J. Alloys Compd.. 2017;712:121-133.
- [Google Scholar]
- Perplexing coordination behaviour of potentially bridging bipyridyl-type ligands in the coordination chemistry of zinc and cadmium 1, 1-dithiolate compounds. Crystals. 2018;8:1-29.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2021.103326.
Appendix A
Supplementary material
The following are the Supplementary data to this article:







