Discovery of conversion driven by β-glucuronidase from flavone glycoside to aglycone and application in identifying the raw Scutellariae Radix
⁎Corresponding authors. wangxinhuimaria@163.com (Xinhui Wang), wangyf0622@tjutcm.edu.cn (Yuefei Wang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
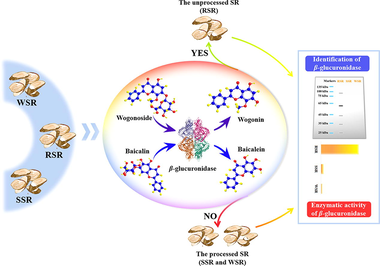
Scutellariae Radix (SR), the dried root of Scutellaria baicalensis Georgia, is a famous Chinese materia medica that has been widely employed. Raw Scutellariae Radix (RSR), steamed Scutellariae Radix (SSR), and wine Scutellariae Radix (WSR) are adopted for use in clinical practice. Because of their easily confused appearance, they are always misused. Aiming at this problem, an ultra performance liquid chromatography coupled with photodiode array detector (UPLC-PDA) method was established to survey misuse of the RSR and the processed SR (SSR and WSR) in the market by employing baicalin (BC), wogonoside (WS), baicalein (BN), and wogonin (WN) as quality indicators. Fortunately, β-glucuronidase, which mediates conversion from flavone glycoside to aglycone, was identified in the RSR samples by the sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) analysis. The significant production of BN and WN was witnessed in the RSR samples, which did not occur in the SSR and WSR samples in virtue of the inactivated β-glucuronidase. Besides, the different capacities of β-glucuronidase were evaluated in the tested samples. In general, we provided the first evidence to scientifically identify RSR from SSR and WSR.
Keywords
Scutellariae Radix
SDS-PAGE
UPLC-PDA
β-glucuronidase
- SR
-
Scutellariae Radix
- RSR
-
raw Scutellariae Radix
- SSR
-
steamed Scutellariae Radix
- WSR
-
wine Scutellariae Radix
- UPLC-PDA
-
ultra performance liquid chromatography coupled with photodiode array detector
- HPLC
-
high performance liquid chromatography
- BC
-
baicalin
- WS
-
wogonoside
- BN
-
baicalein
- WN
-
wogonin
- SDS-PAGE
-
sodium dodecyl sulfate polyacrylamide gel electrophoresis
- CMM
-
Chinese materia medica
- ChP
-
Chinese pharmacopoeia
- DMSO
-
dimethyl sulfoxide
- LOD
-
limit of detection
- LOQ
-
limit of quantitation
- IPA
-
ingenuity pathways analysis
- PCA
-
principal components analysis
- OPLS-DA
-
orthogonal partial least squares discriminant analysis
- VIP
-
variable important in projection
Abbreviations
1 Introduction
Scutellariae Radix (SR), the dried root of Scutellaria baicalensis Georgia, is one of the most popular Chinese materia medica (CMM), which was always employed in a variety of famous formulae, such as Xiaochaihu decoction, Gegen Qinlian decoction, and Huanglian Jiedu decoction. SR exerts effects of clearing heat and dampness, eliminating fire to detoxify, hemostasis and anti-abortion (National Commission of Chinese Pharmacopoeia, 2020), which has been widely used in clinical treatment of cardiovascular disease, cancer, and colitis (Chan et al., 2011, Liu et al., 2019, Cui et al., 2021). In order to enhance the potency, SR is usually steamed or stir-fried with wine to obtain steamed Scutellariae Radix (SSR) and wine Scutellariae Radix (WSR) respectively, which plays a significant role in clinical applications (Cui et al., 2017, Park et al., 2017). As the characteristic compounds of SR, flavonoids have been proved to have antitumor, antibacterial, antiviral, antioxidant, liver-protecting activities, etc. (Chen et al., 2017, Saralamma et al., 2017, Wang et al., 2017, Zhao et al., 2019, Liao et al., 2021), from which baicalin (BC) is stipulated as the indicator for evaluating the quality of SR by the Chinese pharmacopoeia (ChP) (National Commission of Chinese Pharmacopoeia, 2020). Also, several methods have been developed to elevate the level of quality control by determining more indicators in SR, including high performance liquid chromatography (HPLC) (Bai et al., 2020), ultra performance liquid chromatography tandem mass spectrometry (Ren et al., 2021), and ultra performance liquid chromatography (UPLC) (Ji et al., 2015). However, it is hard and infeasible to differentiate RSR, SSR, and WSR from each other through appearance identification or the quantified indicators due to the almost same appearance, giving rise to misuse and insufficient elaboration of efficacy. Therefore, it remains a challenge and urgent task to differentiate RSR from the processed SR (SSR and WSR).
As two typical forms, flavone aglycone (baicalein (BN), wogonin (WN), et al) and its glycoside (BC, wogonoside (WS), et al) were reported as active compounds in the root of S. baicalensis, whose abundance depends on the balance of glycosyltransferases and β-glucuronidase (Nagashima et al., 2000, Dikaya et al., 2018). In the process of growth, storage, and processing, the dynamic variation of enzyme activities brings about the transformation between aglycone and glycoside. In general, BN and WN show an increasing trend in RSR during storage, which is triggered by β-glucuronidase, leading to the green appearance of decoction pieces (Qi 1997). This phenomenon would be overcome to prevent the hydrolysis of flavone glycoside by killing β-glucuronidase through steaming or boiling, which is accepted in ChP (National Commission of Chinese Pharmacopoeia, 2020). Therefore, it is crucial to unveil the differences in enzyme activity and transformation rule of the focused compounds in processed and unprocessed SR.
In our study, by focusing on BC, WS, BN, and WN as quality indicators with the compounds-targets-diseases network, we established an ultra-performance liquid chromatography coupled with photodiode array detector (UPLC-PDA) method, then applied it in the quality evaluation of the tested SR samples. Importantly, the identification of β-glucuronidase was successfully performed. Meanwhile, the catalytic capacity of β-glucuronidase was systematically studied in the tested samples, leading to an exciting discovery that there is an obvious difference in the catalytic capacity to convert BC and WS into BN and WN. On this basis, we successfully discriminated the RSR samples from the processed SR samples (SSR and WSR).
2 Materials and methods
2.1 Reagents and materials
Chromatographic grade methanol was purchased from Sigma-Aldrich Co., Ltd. (St. Louis, MO, USA). Formic acid was bought from Shanghai Aladdin Bio-Chem Technology Co., Ltd. (Shanghai, China). Ethanol was obtained from Concord Technology Co., Ltd. (Tianjin, China). Dimethyl sulfoxide (DMSO) was provided by Tianjin Damao Chemical Reagent Factory (Tianjin, China). Water employed in this study was purified by a Milli-Q water purification system (Billerica, MA, USA). BC, WS, BN, and WN as reference standards were supplied by Shanghai Yuanye Biotechnology Co., Ltd. (Shanghai, China), with purity all above 98 %. Rice wine was offered by Zhejiang Guyuelongshan Shaoxing Wine Co., Ltd. (Zhejiang, China).
Collected from Anguo Traditional Chinese Medicine Digital Palace (Hebei, China), the fresh roots of S. baicalensis were sliced into pieces and divided into three groups, which were dried at 30 °C for 24 h to prepare RSR and numbered as R1, R2, and R3, respectively. A part of R1, R2, and R3 were individually steamed for 30 min to give SSR and designated as S1, S2, and S3. Meanwhile, A part of S1, S2, and S3 were mixed with rice wine at a weight ratio of 100 : 20 and then stir-fried at about 150 °C to produce WSR, respectively, and named as W1, W2 and W3. Additionally, 29 batches of SR were purchased from Anguo Traditional Chinese Medicine Digital Palace (Hebei, China), including ten batches of RSR, nine batches of WSR, and ten batches of SSR, whose detailed information was listed in Table S1. All SR samples were deposited in State Key Laboratory of Component-based Chinese Medicine, Tianjin University of Traditional Chinese Medicine (Tianjin, China).
2.2 Preparation of standard solution
Four reference standards were accurately weighed and dissolved in methanol, where BC and WS were co-solubilized with DMSO, to obtain stock solutions separately, which were then used to prepare a mixed standard solution with the final concentrations at 351.8 μg/mL BC, 60.06 μg/mL WS, 25.20 μg/mL BN, and 6.348 μg/mL WN. Nine standard solutions were obtained by serially diluting the mixed standard solution with 50 % methanol aqueous solution (v/v) for plotting the calibration curve. All solutions were stored at 4 °C until analysis.
2.3 Preparation of sample solution
The dried SR samples were pulverized and passed through a 65-mesh sieve to give homogeneous sample powders. Then, accurately weighed sample powders (0.3 g) were transferred into a 100 mL volumetric flask and ultrasonically extracted (600 W, 135 kHz) with 70 % ethanol aqueous solution for 30 min. After cooling down to room temperature, the sample solution was diluted to scale by 70 % ethanol aqueous solution, which was mixed and centrifuged at 18,213 g for 10 min. The supernatant (1 mL) was transferred into a 10 mL volumetric flask and diluted to scale by methanol immediately, followed by dilution of the solution with water at the volume ratio of 1 : 1. Moreover, according to the procedure for preparation of sample solution, only the extraction solvent was switched to 50 % or 25 % ethanol aqueous solution. Different sample solutions were prepared in the same way, which was employed to distinguish RSR from the processed SR.
In order to investigate the transformation from flavone glycoside to its aglycone in aqueous sample solution of RSR, accurately weighed RSR powder (1 g) was placed in a 50 mL centrifuge tube and subjected to ultrasonic treatment (600 W, 135 kHz) with 40 mL water for 5 min, followed by centrifugation at 18,213 g for 5 min. The obtained supernatant (1 mL) was immediately transferred into a 25 mL volumetric flask and incubated at 37 °C, which was promptly and respectively diluted to scale with methanol at 0, 15, 30, 45, 60, 75, 90 min to stop the hydrolysis of flavone glycoside by β-glucosidase. The sample solution was diluted six times by 50 % methanol aqueous solution before UPLC analysis.
2.4 UPLC analysis
Chromatographic analysis was performed on a Waters ACQUITY UPLC H class plus system (Milford, MA, USA) fitted with ACQUITY UPLC BEH C18 column (2.1 × 100 mm, 1.7 μm) at 40 °C. Mobile phase was employed with 0.2 % formic acid solution (A) and methanol (B) in a gradient elution (0–10 min, 40–63 % B) at a flow rate of 0.3 mL/min. The chromatogram was monitored at 280 nm and the injection volume was 2 μL.
2.5 Methodological validation
The method of quantitative analysis established in this study was validated in terms of linearity, limit of detection (LOD), limit of quantitation (LOQ), precision (intra- and inter-day), stability, repeatability, and recovery test. Calibration curves were constructed using the peak area of the tested analytes with nine different concentrations as y axis versus the corresponding concentrations as x axis. The LOD and LOQ of four compounds were determined by signal-to-noise ratio at about 3 and 10, respectively. The intra- and inter-day precisions were evaluated by six replicate injections of the same sample solution performed on the same day and three consecutive days, respectively. The stability of the sample solution stored at 10 °C was investigated by replicate injections at 0, 2, 4, 6, 8, 10, and 12 h. Repeatability was confirmed by analyzing six independently prepared sample solutions. To evaluate the accuracy of the method, the recovery test was carried out by adding a known amount of the mixed standard solution to 0.15 g sample powder, which was prepared and analyzed with the method described above.
2.6 Protein extraction from SR and separation by gel electrophoresis
In order to reveal the differences in enzyme composition between RSR and the processed SR (SSR, WSR), plant protein extraction kits (CWBIO, China) were used to extract the total protein of RSR, SSR, and WSR, respectively. According to the manufacturer’s instructions, the plant protein extraction reagent was added to protease inhibitor cocktail at a volume ratio of 99 : 1 to prepare a working solution. Then, SR powder (0.1 g) was incorporated into the obtained working solution (1 mL), and incubated in an ice bath for 30 min, followed by centrifugation at 13,400 g for 20 min at 4 °C to collect supernatant. The gained supernatant was placed on ice until no more precipitate appeared, then centrifuged at 18,213 g for 10 min at 4 °C to remove the precipitate, which was further centrifuged at 7500 g for 20 min at 4 °C by a 30 kDa ultrafiltration centrifuge tube to obtain about 200 μL concentrated protein extract.
The sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) was performed as described by Laemmli (Laemmli, 1970). The protein extract (16 μL) was mixed with 4 μL fivefold sample loading buffer (Biosharp, China), heated at 100 °C for 5 min, and then loaded onto 10 % separating gel with 5 % stacking gel. The gel electrophoresis was subsequently carried out at a constant voltage (120 V) by running buffer (2.5 mM Tris, 0.25 M glycine, 0.1 % (w/v) SDS). Coomassie blue fast staining and no-decoloring solution (Shanghai Epizyme Biomedical Technology Co., Ltd., Shanghai, China) was employed to locate protein and further washed by double distilled water. Protein ladders (10 to 180 kDa) were used for determining molecular mass.
2.7 Network pharmacology analysis
The molecular structures of BC, WS, BN, and WN were derived from Pubchem database (https://pubchem.ncbi.nlm.nih.gov/) and the targets corresponding to these compounds were obtained from SwissTargetPrediction database (https://www.swisstargetprediction.ch/). All targets were uploaded into ingenuity pathways analysis (IPA) software (version 2019, Redwood City, CA, US), where network and canonical pathway analysis were performed by “Core analysis” module, network among compounds, related targets, and candidate diseases was clarified by “Pathway designer” module, and top diseases were obtained from “Diseases and Functions” module.
2.8 Data processing
The bar and line graphs were drawn with origin 2019 software (OriginLab Ltd., Northampton, MA, USA) and Adobe Illustrator CC2015 (Adobe Systems Incorporated, San Jose, CA, USA). Principal components analysis (PCA) and orthogonal partial least squares discriminant analysis (OPLS-DA) were performed using SIMCA-P + 14.1 (Umetrics, Umea, Sweden).
3 Results and discussion
3.1 Exploration of potential pharmacological value of targeted compounds by IPA
As the main characteristic compounds in SR, BC and WS are always indirectly absorbed by the gastrointestinal tract, which are hydrolyzed into BN and WN by β-glucuronidase secreted by gut microbiota, respectively (Tao et al., 2017). The absorbed BN and WN are metabolized to BC and WS by UDP-glucuronosyltransferase and transferred into intestine through enterohepatic circulation (Noh et al., 2016). As the exposed compounds in vivo, it is meaningful to illustrate the potential pharmacological effects of BC, WS, BN, and WN via a components-targets-diseases network.
The structures of the focused compounds were obtained by Pubchem, and then their potential biological targets were found through SwissTargetPrediction databases, where the 233 targets related to these four compounds were imported into IPA for core analysis to locate a total of 474 canonical pathways (including metabolism and signaling) and 32 networks associated with these targets. Subsequently, these pathways and diseases were ranked respectively with p-value score to discover and distinguish their significance according to the Fisher’s exact test algorithm, as shown in Fig. 1A and 1B. Obtained by IPA canonical pathways analysis, the top pathways involved cancer, inflammatory disease, nutritional disease, cardiovascular disease, and so on. Four diseases highly correlated with the function of SR, including inflammatory response, lung cancer, hypertension, and liver lesion, were selected to establish the compounds-targets-diseases network for the demonstration of multi-targets biological process, as shown in Fig. 1C.
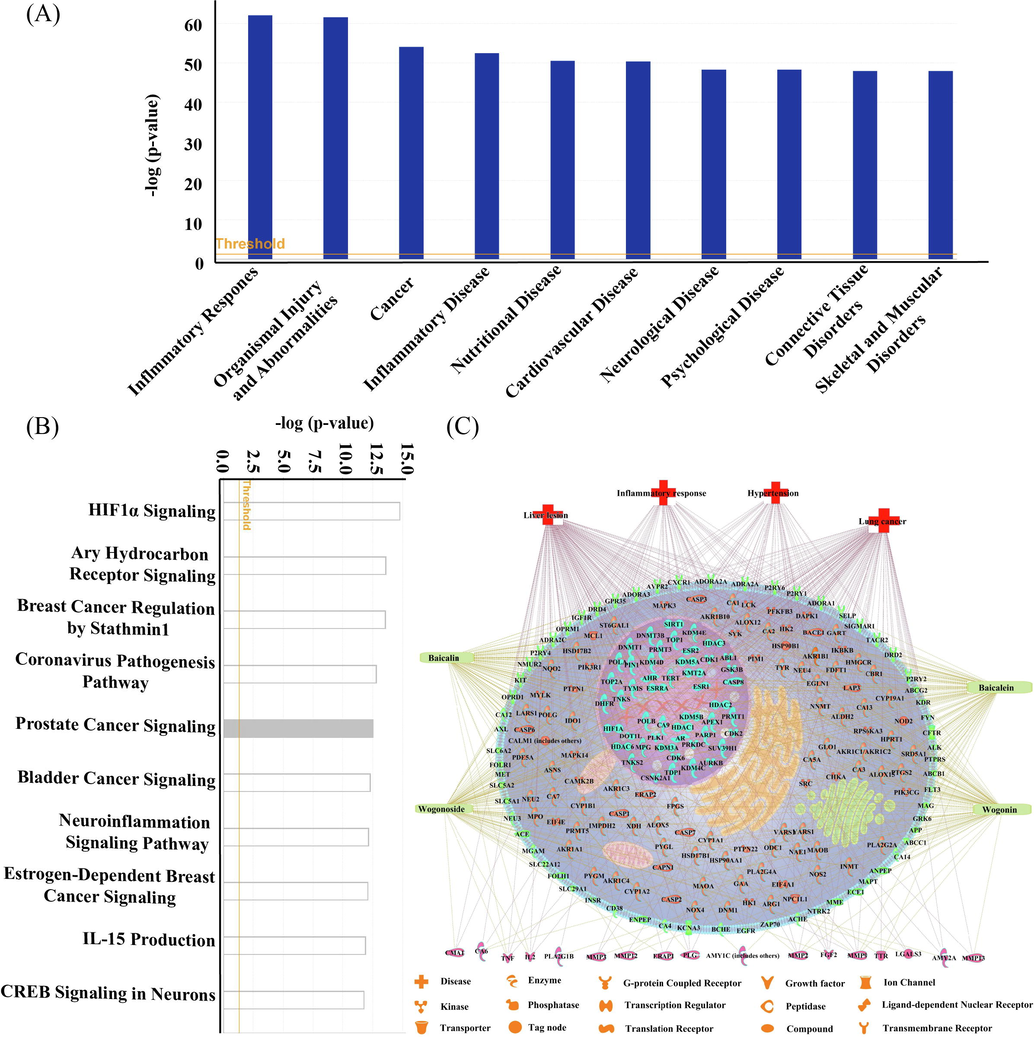
- Analysis of the compounds-targets-pathways-diseases network of four compounds in SR. The top 10 diseases (A) and top 10 pathways (B) related to BC, WS, BN and WN according to p-value score. The compounds-targets-diseases network (C).
3.2 Discovery of transformation from glycoside to aglycone driven by β-glucuronidase and establishment of the optimized method
The reported sample preparation methods (Xue et al., 2019, Bai et al., 2020) showed many limitations, including time-consuming preparation, complicated procedures, laborious operations, and incomplete extraction of interesting compounds, leading to a lack of synchronization between sample preparation and HPLC analysis. Thus, it is important to establish a suitable method for preparing sample solutions in CMM quality evaluation. Stipulated by the ChP, it takes three hours to prepare SR sample solution with 70 % ethanol aqueous solution by reflux extraction (National Commission of Chinese Pharmacopoeia, 2020), which greatly limits the analytical efficiency. To address this problem, ultrasonic extraction was adopted to accomplish the preparation of sample solution with 70 % ethanol aqueous solution in 30 min. It is worth noting that for RSR and SSR, the tested compounds can be excellently extracted by both the ChP and ultrasonic extraction methods. The ultrasonic extraction method surpassed the ChP method in terms of shorter time, simpler procedure, and multiple indicators. Interestingly, a surprising phenomenon was witnessed as shown in Fig. 2A. Along with the decreased ratio of ethanol in extracting reagent, the total content of focused compounds slightly decreased for SSR. But, for RSR, the total content of focused compounds declined obviously. Noticeably, a conspicuous conversion from flavone glycoside (BC and WS) to aglycone (BN and WN) occurred when sample was extracted by 50 % and 25 % ethanol aqueous solution or water, which was triggered by β-glucuronidase (Matsuda et al., 2000, Dikaya et al., 2018). The produced BN and WN with liposolubility cannot be satisfactorily extracted by extracting solvent with a decreased ratio of ethanol, especially by water. Moreover, it was further acknowledged by incubating the aqueous sample solution of RSR at 37 °C. As displayed in Fig. 2B, almost all BC and WS were converted into BN and WN within 90 min.
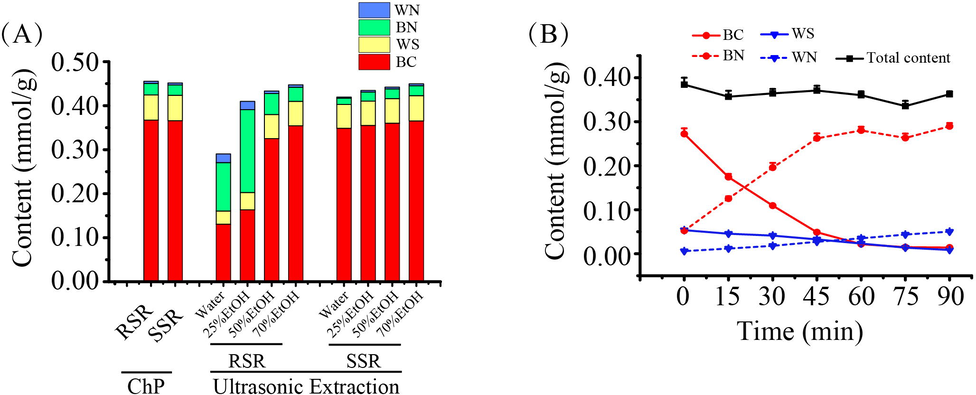
- The content of four active compounds in the tested samples treated by different methods. The histograms of BC, WS, BN, and WN content obtained by the different methods for SSR and RSR (A). The variation trend of four active compounds content in RSR aqueous extract incubated at 37 °C in 90 min (B).
In general, through our study, the focused indicators of both RSR and SSR can be completely extracted with 70 % ethanol aqueous solution in 30 min by ultrasonic extraction. On the account of β-glucuronidase in RSR, the conversion from flavone glycoside to aglycone was activated when the sample was treated with 50 % ethanol aqueous solution or a lower ratio of ethanol. Accordingly, the optimized extraction method paved the way for the methodological validation with the detailed results displayed in Table S2. As a result, all calibration curves showed good linearity with determination coefficients (r2 ≥ 0.9999) within the test ranges. The LOD and LOQ values were between 0.0083 – 0.051 μg/mL and 0.024 – 0.15 μg/mL, respectively. The intra- and inter-day precisions, repeatability, and stability were investigated by evaluating the RSD value below 2.7 %. The average recovery of the focused compounds ranged between 99.80 % and 105.6 % with the RSD value below 2.1 %. All the above results demonstrated that the established method was satisfactory for the simultaneous determination of BC, WS, BN, and WN in SR.
3.3 Distinguishment of RSR from the processed SR and identification of β-glucuronidase in RSR
In virtue of the similar appearance, it is difficult to distinguish RSR from the processed SR by appearance identification or the quantified indicators. The discovery of the difference in β-glucuronidase activity sheds the light on the distinction of RSR from the processed SR, which leads to the obvious discrepancy in the conversion from flavone glycoside to aglycone. In this study, 70 %, 50 %, and 25 % ethanol aqueous solutions were used as the extraction solvent for RSR (R1, R2, and R3) and SSR (S1, S2, and S3), respectively, as shown in Fig. 3A. The content of each targeted compound extracted by 70 %, 50 %, and 25 % ethanol aqueous solution was marked as Cf, C'f, and C″f (f represents for different compounds), respectively. The ratio of the content of each targeted compound extracted with 50 % and 25 % ethanol aqueous solution to that extracted with 70 % ethanol aqueous was individually assigned as N'f, and N″f. The calculation equations were listed as follows.
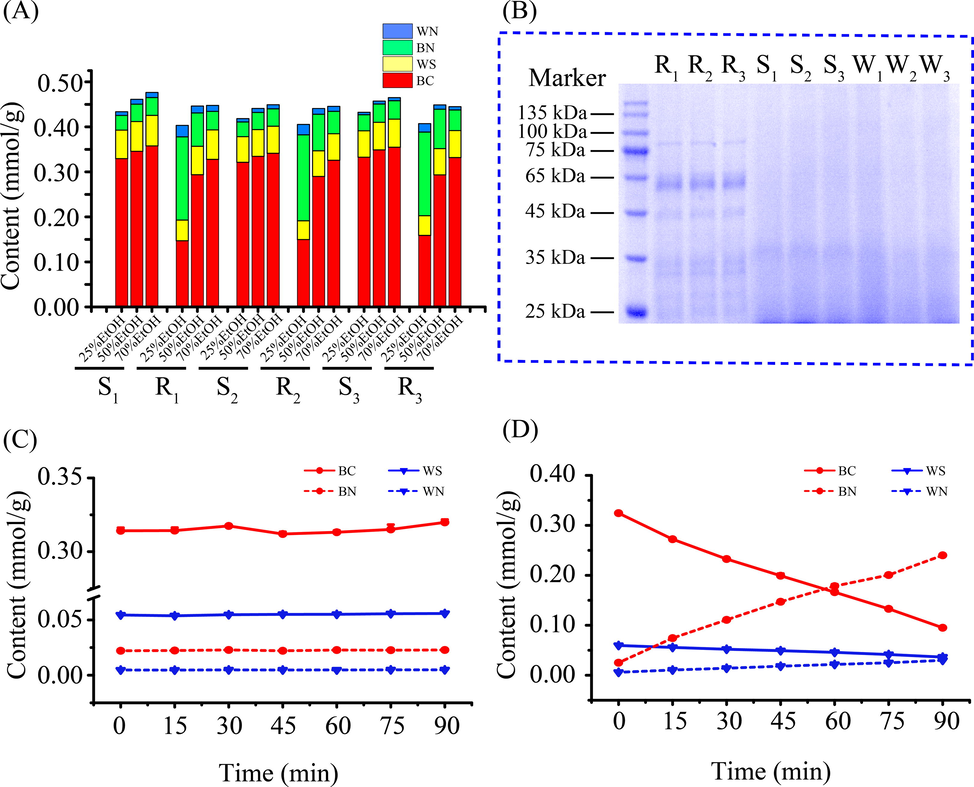
- The content of four active compounds in SR treated by the different extraction solution and identification of β-glucuronidase in RSR. The content of four active compounds obtained from RSR (R1, R2 and R3) and SSR (S1, S2 and S3) by the different extraction solution (25 %, 50 %, and 70 % ethanol aqueous solution) (A). Identification of β-glucuronidase in SR samples by SDS-PAGE (B). The variation trend of the interesting compounds content in water extract of SSR incubated at 37 °C in 90 min (C). The dynamic variation of the interesting compounds content in water extract of SSR incubated at 37 °C in 90 min by exposing to the enriched protein from RSR (D).
As displayed in Table 1, we found that N″f was more sensitive than N'f as indicators to distinguish RSR from SSR. Therefore, the tested samples with N″BC > 0.9 and N″BN < 1.1 or N″WS > 0.9 and N″WN < 1.1 were identified as the processed SR (SSR and WSR), whose β-glucuronidase was inactivated, or else the tested samples were adopted as RSR.
| Samples | N'BC | N'WS | N'BN | N'WN | N″BC | N″WS | N″BN | N″WN |
|---|---|---|---|---|---|---|---|---|
| S1 | 0.967 | 0.973 | 0.976 | 0.964 | 0.921 | 0.930 | 0.824 | 0.745 |
| S2 | 0.980 | 0.989 | 0.989 | 0.968 | 0.941 | 0.944 | 0.850 | 0.816 |
| S3 | 0.983 | 0.983 | 0.990 | 0.973 | 0.937 | 0.940 | 0.871 | 0.814 |
| R1 | 0.896 | 0.966 | 1.803 | 1.150 | 0.448 | 0.704 | 4.499 | 1.912 |
| R2 | 0.890 | 0.967 | 1.631 | 1.158 | 0.459 | 0.708 | 3.832 | 2.101 |
| R3 | 0.884 | 0.972 | 1.908 | 1.321 | 0.479 | 0.730 | 4.051 | 2.576 |
Furthermore, SDS-PAGE was employed to demonstrate the existence of β-glucuronidase in RSR. From Fig. 3B, the obvious difference was witnessed in the protein bands. The band at about 55 kDa was exclusively detected in RSR, which was proposed as β-glucuronidase according to the reported molecular mass of β-glucuronidase (Ikegami et al., 1995, Sasaki et al., 2000). Hardly had the protein extracted from RSR converted BC and WS to BN and WN when it was added to the water extract of SSR, as displayed in Fig. 3D. BC and WS were nearly transformed by the added proteins within 90 min. Nevertheless, BC and WS were very stable in the water extract of SSR, which were not transformed into BN and WN in Fig. 3C. The catalytic activity of β-glucuronidase in RSR was successfully approved, which plays an important role in the conversion from flavone glycoside to aglycone.
3.4 General survey for quality of SR samples from the different origins and identification of the false samples
As illustrated in Fig. 4, the established method was employed to perform a general survey for the quality of SR samples from different origins, whose detailed information was listed in Table S1. The total content of targeted compounds fluctuated narrowly from 0.3900 to 0.4806 mmol/g in 38 batches of SR samples (RSR, SSR, and WSR). As the quality indicator, BC was quantified to be 0.2984 to 0.3857 mmol/g, which significantly outweighed 0.2016 mmol/g (9 %) stipulated by the ChP. It indicated that consistency of quality is satisfactory. In contrast, the content of BN and WN were dispersed with RSDs above 40 %, which may attribute to the different capacity of β-glucuronidase for converting BC and WS into BN and WN. In order to identify the true samples from the false samples, 38 batches of SR samples were determined by N″f, including 13 batches of RSR, and 25 batches of the processed SR (SSR, and WSR). From 13 batches of RSR, R4, R8, R9, and R13 were identified as the false samples because N″f satisfied N″BC > 0.9 and N″BN < 1.1 or N″WS > 0.9 and N″WN < 1.1, which should be used as SSR or WSR. From 25 batches of the processed SR (SSR, and WSR), S5 was identified as RSR because S5 did not meet N″BC > 0.9 and N″BN < 1.1 or N″WS > 0.9 and N″WN < 1.1, which was misused as SSR.
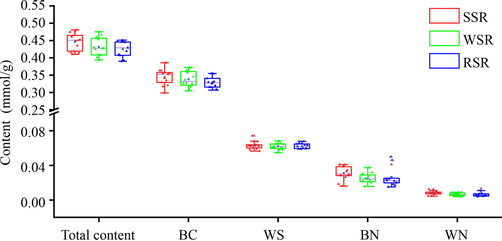
- The box plot of content of four active compounds in the tested SR samples.
PCA is an unsupervised multivariate data analysis method that can further retain the original information to make a holistic evaluation of the tested samples (Giuliani 2017). It is commonly used for analyzing, classifying and reducing the dimensionality of numerical datasets in multivariate problems (Guo et al., 2019), thus visualizing the classification trends among the SR samples. As shown in Fig. 5A, the tested samples are clearly divided into two groups by employing N″f as indicators. One group mainly includes the true RSR samples (9 batches), and the false SSR sample (S5), which locate on the right. The true SSR and WSR samples (24 batches), as well as the false RSR samples (R4, R8, R9, and R13) are clustered into another group, which are placed on the left. The key parameters of PCA were displayed as R2X = 0.989, and Q2 = 0.932, demonstrating the satisfactory explanatory and predictive ability of this model. In order to further reveal which variables contribute to the classification, the variable important in projection (VIP) values of N″f in OPLS-DA model were calculated. N″BN, and N″WN were recommended as the significantly preferential indicators with the VIP values above 1.0, as illustrated in Fig. 5B.
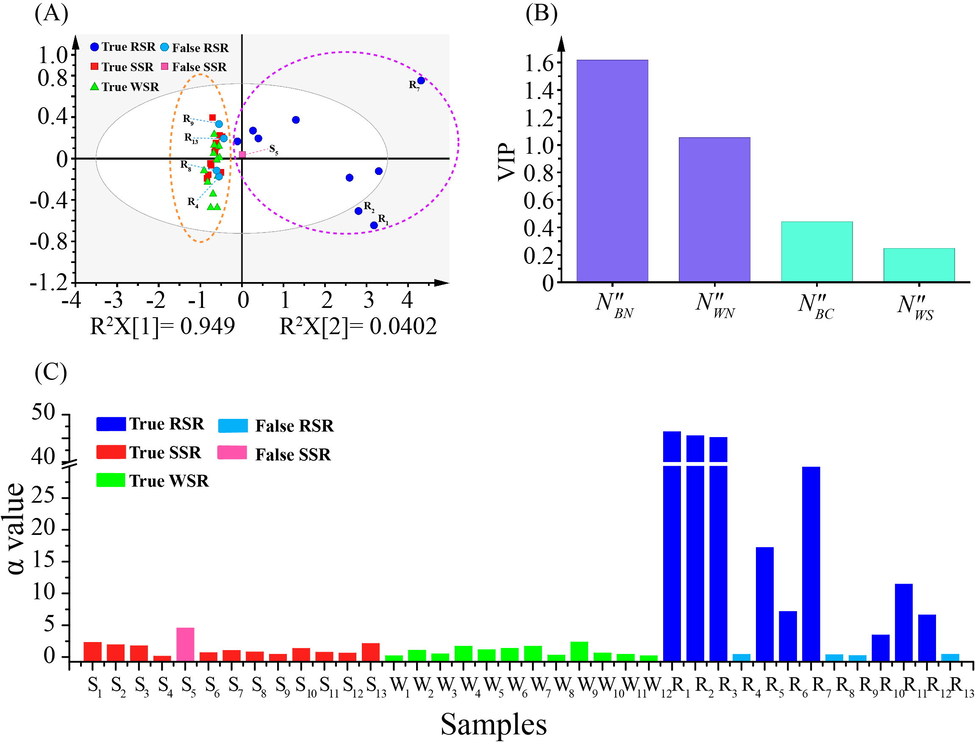
- Multivariate statistical analysis of 38 batches of SR. PCA scores plot of the tested SR samples (A). VIP plot of OPLS-DA for four variables (B). The α value of 38 batches of SR for evaluating β-glucuronidase activity (C).
In general, β-glucuronidase performs the catalysis from BC and WS to BN and WN, leading to fluctuations in the content of BN and WN in SR samples. Accordingly, we attempted to display the difference in the activity of β-glucuronidase in the tested SR samples by determining the conversed amount of BN, which was produced in the tested SR samples treated by 25 % ethanol aqueous solution. Focusing on this aim, we employed α value to predict the activity of β-glucuronidase, whose equation is as follows.
The amounts of BN in the true processed SR samples treated by 25 % and 70 % ethanol aqueous solution were tagged as
4 Conclusion
In our study, a UPLC-PDA method was successfully established by targeting BC, WS, BN, and WN as quality indicators with the compounds-targets-diseases network, which was applied to a general survey of the quality of SR samples. By unveiling the great difference in the catalytic performance of β-glucuronidase, the RSR samples were easily discriminated from the processed SR samples by determining conversion from BC and WS to BN and WN. This study provides a feasible method to identify the RSR samples from the processed samples, contributing to the scientific application of SR samples in clinics.
Acknowledgments
This work was supported by the Science and Technology Program of Tianjin (20ZYJDJC00070) and Innovation Team and Talents Cultivation Program of National Administration of Traditional Chinese Medicine (ZYYCXTD-D-202002).
References
- Growth years and post-harvest processing methods have critical roles on the contents of medicinal active ingredients of Scutellaria baicalensis. Ind. Crops Prod.. 2020;158:112985
- [Google Scholar]
- Extract of Scutellaria baicalensis Georgi root exerts protection against myocardial ischemia-reperfusion injury in rats. Am. J. Chin. Med.. 2011;39:693-704.
- [Google Scholar]
- Scutellaria baicalensis Ameliorates Acute Lung Injury by Suppressing Inflammation In Vitro and In Vivo. Am. J. Chin. Med.. 2017;45:137-157.
- [Google Scholar]
- Scutellaria baicalensis Georgi polysaccharide ameliorates DSS-induced ulcerative colitis by improving intestinal barrier function and modulating gut microbiota. Int. J. Biol. Macromol.. 2021;166:1035-1045.
- [Google Scholar]
- The enhancement mechanism of wine-processed Radix Scutellaria on NTG-induced migraine rats. Biomed. Pharmacother.. 2017;91:138-146.
- [Google Scholar]
- The Relationship Between Endogenous beta-Glucuronidase Activity and Biologically Active Flavones-Aglycone Contents in Hairy Roots of Baikal Skullcap. Chem. Biodivers.. 2018;15:e1700409.
- [Google Scholar]
- The application of principal component analysis to drug discovery and biomedical data. Drug. Discov. Today.. 2017;22:1069-1076.
- [Google Scholar]
- Identification of velvet antler and its mixed varieties by UPLC-QTOF-MS combined with principal component analysis. J. Pharm. Biomed. Anal.. 2019;165:18-23.
- [Google Scholar]
- Purification and properties of a plant beta-D-glucuronidase form Scutellaria root. Biol. Pharm. Bull.. 1995;18:1531-1534.
- [Google Scholar]
- Anti-H1N1 virus, cytotoxic and Nrf2 activation activities of chemical constituents from Scutellaria baicalensis. J. Ethnopharmacol.. 2015;176:475-484.
- [Google Scholar]
- Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680-685.
- [Google Scholar]
- The main bioactive compounds of Scutellaria baicalensis Georgi. for alleviation of inflammatory cytokines: A comprehensive review. Biomed. Pharmacother.. 2021;133:110917
- [Google Scholar]
- Total flavonoid aglycones extract in Radix Scutellariae induces cross-regulation between autophagy and apoptosis in pancreatic cancer cells. J. Ethnopharmacol.. 2019;235:133-140.
- [Google Scholar]
- Histochemical investigation of β-glucuronidase in culture cells and regenerated plants of Scutellaria baicalensis Georgi. Plant Cell Rep.. 2000;19:390-394.
- [Google Scholar]
- Purification and characterization of UDP-glucuronate: baicalein 7-O-glucuronosyltransferase from Scutellaria baicalensis Georgi. cell suspension cultures. Phytochemistry. 2000;53:533-538.
- [Google Scholar]
- Pharmacopoeia of the people’s Republic of China. Beijing, China: China Medical Science and Technology Press; 2020.
- Role of Intestinal Microbiota in Baicalin-Induced Drug Interaction and Its Pharmacokinetics. Molecules (Basel, Switzerland).. 2016;21:337.
- [Google Scholar]
- Heat-Processed Scutellariae Radix Protects Hepatic Inflammation through the Amelioration of Oxidative Stress in Lipopolysaccharide-Induced Mice. Am. J. Chin. Med.. 2017;45:1233-1252.
- [Google Scholar]
- On the causes and prevention of “turning green” of Scutellaria baicalensis. Anhui Medical Pharm. J.. 1997;38
- [Google Scholar]
- Investigation on the function tropism of Tiaoqin and Kuqin (different specification of Scutellaria baicalensis) by comparing their curative effect on different febrile disease model. J. Ethnopharmacol.. 2021;268:113596
- [Google Scholar]
- Korean Scutellaria baicalensis Georgi flavonoid extract induces mitochondrially mediated apoptosis in human gastric cancer AGS cells. Oncol. Lett.. 2017;14:607-614.
- [Google Scholar]
- Molecular characterization of a novel beta-glucuronidase from Scutellaria baicalensis Georgi. J. Biol. Chem.. 2000;275:27466-27472.
- [Google Scholar]
- Simultaneous determination of the bioactive components in rat plasma by UPLC-MS/MS and application in pharmacokinetic studies after oral administration of radix Scutellariae extract. Biomed. Chromatogr.. 2017;31:e3961.
- [Google Scholar]
- Baicalein protects tert-butyl hydroperoxide-induced hepatotoxicity dependent of reactive oxygen species removal. Mol. Med. Rep.. 2017;16:8392-8398.
- [Google Scholar]
- Rapid and Simultaneous Determination of Three Active Components in Raw and Processed Root Samples of Scutellaria baicalensis by Near-infrared Spectroscopy. Planta Med.. 2019;85:72-80.
- [Google Scholar]
- Scutellaria baicalensis Georgi. (Lamiaceae): a review of its traditional uses, botany, phytochemistry, pharmacology and toxicology. J. Pharm. Pharmacol.. 2019;71:1353-1369.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2022.104216.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







