Translate this page into:
Calotropis procera (Aiton) seeds fixed oil: Physicochemical analysis, GC–MS profiling and evaluation of its in-vivo anti-inflammatory and in-vitro antiparasitic activities
⁎Corresponding author at: Fats and Oils Department, Food Industries and Nutrition Research Institute, National Research Centre, 12622 Dokki, Cairo, Egypt. hazahran@hotmail.com (Hamdy A. Zahran),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
Calotropis procera (Aiton) is a wildly grown shrubbery that has been used by traditional healers in various remedies for centuries. Its different organs are reported to possess a variety of biological activities. However, a few reports chemically investigated the seed fixed oil, but they didn’t prospect its bioactivity. Accordingly, this study aimed to investigate the phytochemical composition, physicochemical properties, and biological activity of the seed oil. As well, exploration of the best extraction method that give the highest oil yield with the best composition. Physicochemical properties, total phenolic content and oxidative stability index of the oil were estimated. GC–MS analysis identified the oil content. Anti- inflammatory activity was examined using a carrageenan-induced rat paw edema assay. Immunomodulatory activity was determined by measuring the pro-inflammatory cytokines levels. Moreover, In-vitro anti-parasitic and antioxidant activities were studied. Soxhlet extraction method gives the highest yield of 40.5 ± 0.76% with no time interval. The oil shows a physicochemical property within the AOCS standards and low phenol content. GC-MS analysis reveals unsaturated fatty acids account for 75.25% of the oil, with an abundance of oleic acid (33.64%) and linoleic acid (31.95%). The oil exhibited potent anti-inflammatory and immunomodulatory activity. At various dose levels, paw edoema volume, pro-inflammatory cytokines, tumor necrosis factor alpha (TNF-α), interleukin-6 (IL-6), and myeloperoxidase (MPO) serum levels were reduced. The seed oil is a promising candidate for the treatment of inflammation and/or inflammatory associated diseases (as parasitic infections) besides its immunomodulatory activity.
Keywords
Seed oil
Calotropis procera
Blastocystis
Cytokines
Hydraulic press
Soxhlet extraction
1 Introduction
Calotropis procera (Aiton) [Apocynaceae] (C. procera) is a wildly grown evergreen large shrub or small tree (1–4 m) that tolerates drastic environmental conditions. It is native to North and tropical Africa, West and South Asia, and Indo-china. C. procera is recorded in Egypt and known as Oshar (Moustafa et al. 2010), with many other synonyms worldwide, such as Giant Indian Milkweed, Sodom Apple, and etc. (Parihar and Balekar 2016).
Although some researchers have reported that C. procera is a toxic plant (de Lima et al. 2011), different organs are extensively used in the traditional systems of Africa, Asia, and India owing to their ethnomedical importance. It is used to treat a variety of ailments, such as anti-inflammatory, anti-rheumatic, antifungal, skin diseases, anti-parasitic, dysentery, etc. C. procera has gained great interest from scientists. They reported that crude extracts and purified compounds obtained from different parts of C. procera possess a diversity of biological activities (antimicrobial, anthelmintic, antioxidant, antimalarial, nematocidal, anticancer, anti-inflammatory) (Kaur et al. 2021). C. procera seeds received little attention from scientists compared to other organs of the plant. Each fruit of C. procera encloses 350 to 500 seeds that are characterized by a high fixed oil content, about 23% (Pryde 1981; Gunstone et al. 1972) and about 0.23–0.47% cardenolides (Saber et al. 1969).
Parasitic infection is a universal health problem that can infect about one-third of the population (Herrick et al. 2020). Obstacles are exacerbated by parasite strain resistance to available drugs and/or the high cost of treatment. Blastocystis spp. is the most common parasitic infection. Its prevalence rates are higher in developing countries (63–100%) than in industrialized countries (0.5–24%) (Ajjampur et al. 2016). It triggers the inflammatory cascade and modulates the immune system (Poirier et al. 2012). Recently, many studies were performed in the hope of finding an alternative treatment for Blastocystis infection other than Metronidazole, which is the currently available treatment, yet reported to be often associated with failure or drug resistance (Mokhtar, El-Gayar, and Habib 2016). Not only is the parasitic infection that triggers the inflammatory cascade, but almost all critical diseases lead to or start with inflammation. Anti-inflammatory drugs lead to harmful adverse effects upon prolonged use. Thus, researchers are exploring medicinal plants rich in phytochemicals as a source for new medications (Wahid, Alqahtani, and Khan 2021).
Considering the wild growth of C. procera in Egypt and the seeds’ high yield of fixed oil, and the absence of research on C. procera fixed oil prospective bioavailability. It was deemed of interest to conduct a preliminary study for the first time on C. procera seed fixed oil. In this study, we analyze the phytochemical composition, physicochemical characteristics and investigate the potential bioavailability (antiparasitic and antioxidant activities (in-vitro), anti-inflammatory and immunomodulatory activities (in vivo) of seed oil.
2 Materials and methods
2.1 Materials
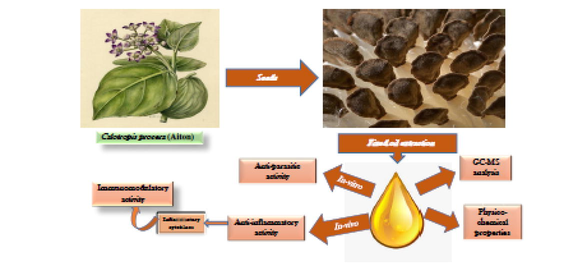
All solvents, chemicals and reagents were in chromatographic grade, and were purchased from Merck (Darmstadt, Germany). Fresh fruits of wild-grown Calotropis procera (Aiton) were collected from Obour City, Cairo, Egypt between March and June 2018. (Fig. 1). At fruit dehiscence, seventy-five grams of mature seeds were carefully separated from silky hair and dried in open air, then the seeds were grounded to mesh 0.2 mL, after that the grounded seeds were stored in the freezer for 2 days before investigation. The plant was identified by Dr. Trease Labib and a voucher specimen (No. 0108. CP.05.01.03) was deposited at the Herbarium of the Orman Botanical Garden, Giza, Egypt.
The Illustration of Calotropis procera (Aiton).
2.2 Methods
2.2.1 Fixed oil extraction
Seventy-five grams of C. procera seed powder were extracted by petroleum ether (40–60 °C), as an organic solvent, using two separate methods: first on was cold pressing by hydraulic press using pressure at 10 MPa, and the second was Soxhlet (for 6 h) till exhaustion, the ratio of solvent to the grounded seeds was 5:1 ratio, respectively. To obtain the oil, the filtrates after extraction were evaporated under reduced pressure using a rotary evaporator (Heidolph®, Germany). The yield of oil was calculated (g/kg of dry seeds).
2.2.2 Physicochemical properties of C. Procera seeds fixed oil
The AOCS Official Method AOCS Cd 3d-63 (AOCS 1993) was used to measure the acid value (AV) and peroxide value (PV). Iodine value (IV) was calculated according to the equation generated by (Kyriakidis and Katsiloulis 2000) and illustrated in Table 1.
Parameter
Egyptian (Obour city)
Brazil Serra Talhada*
Brazil, Mossoró**
Soxhlet extraction
Hydraulic press extraction
---
---
Acid value (mg/g)
1.45 ±0.11
0.74 ±0.03
1.86
5.54
Peroxide value (mEq. O2/Kg)
3.42 ±0.53
0.95 ±0.12
---
9.37
Iodine value (g/100 g)
99.83 ±2.43
95.27 ±1.15
---
43.01
Oxidative stability (h) by Rancimat
4.14 ±0.10
3.97 ±0.17
---
---
2.2.3 Total phenol content (TPC)
Polyphenols were extracted by the methanol/water solution at a ratio of 80:20 and TPC was determined by the Folin-Ciocalteu method (Naeem, Zahran, and Hassanein 2019). Extracted oil (2.5 g) was dissolved in 5 mL of hexane and extracted with a methanol/water solution (80:20, v/v). The aqueous phase was collected by centrifugation at 3500 rpm for 5 min, followed by vacuum drying at room temperature. The dried sample was dehydrated in 5 mL of the methanol solution, mixed with 2.5 mL of Folin reagent (10%) and 10 mL of sodium carbonate solution in a 50 mL volumetric flask, and adjusted to volume with deionized water. The absorbance was measured at 765 nm after 30 min. Gallic acid was used for calibration and the results were expressed as mg of gallic acid equivalent (GAE) per 100 g of oil sample. A triplicate test was performed for each sample.
2.2.4 Oxidative stability index
Approximately three grams of seed oil were heated in the Rancimat equipment 892 (Metrohm, Herisau, Switzerland) at 110 °C with an airflow rate of 20 L/h passing through the samples. The time from the beginning until oil oxidation (the deionized water (60 mL) conductivity rise caused by the adsorption of volatiles derived from oil oxidation) was recorded as the induction time in hours (Zahran and Najafi 2020).
2.2.5 Phytochemical composition
2.2.5.1 Preparation of fatty acids and sterols
The lipoidal matter of the extracted oil (3 g) was prepared by saponification. Briefly, crude oil was refluxed with 10% alcoholic potassium hydroxide in a boiling water bath for 5 h. After distilling off the solvent using a rotatory evaporator, 50 mL of water was added to the residue, and the mixture was transferred to a separating funnel and shaken with ether (4 × 80 mL). The combined ethereal extract was washed with distilled water until free of alkalinity, and then dehydrated over anhydrous sodium sulphate. The unsaponifiable matter, sterols and hydrocarbons, was obtained after evaporation of the solvent and analyzed using GC/MS spectroscopy (Khanzada et al. 2008).
2.2.5.2 Methylation of fatty acids
The fatty acid was methylated by adding 50 mL of methanol (HPLC grade) mixed with 1 mL of concentrated sulphuric acid. The solution was refluxed in a boiling water bath for 6 h and then evaporated. The residue was diluted with about 50 mL of distilled water and extracted several times with ether. The combined ether layers were washed until free of acidity and dehydrated over anhydrous sodium sulphate. The free fatty acid methyl ester (FAME) was obtained after evaporation of the solvent and analyzed using GC/MS spectroscopy (Hamed et al., 2020)
2.2.5.3 GC/MS analysis
A Finnigan MAT SSQ 7000 mass spectrometer coupled with a Varian 3200 gas chromatograph was used. DB-5 column, 30 m (long) × 0.32 mm (internal diameter) × 0.25 µm (film thickness), employed with helium as carrier gas (He pressure, 20 Mpa/cm2), injector temperature, 220 °C, volume injected: 1 μL, the oven temperature was programmed at 50 °C isothermal for 3 min, then heated to 300 °C at a rate of 5 °C/min, then isothermal at 300 °C for 5 min for USM while in the case of FAME isothermal at 150 °C for 4 min, then heated to 280 °C at a rate of 5 °C/min, then isothermal at 280 °C for 4 min. The mass spectra were recorded in electron ionization (EI) mode at 70 eV. Compounds were identified by comparing their retention times and mass fragmentation patterns with database libraries [Wiley (Wiley Int. USA) and NIST (Nat. Inst. St. Technol., USA)] and published data (Adams, 1995). The compounds identified were listed in Tables 2 and 3. ND, not detectable; SFA, Saturated fatty acids; UFA, Unsaturated fatty acids; MUFA, Mono-unsaturated fatty acids; PUFA, Poly-unsaturated fatty acids. Data are presented as mean ±SEM. Statistical analysis was carried out by one-way analysis of variance (ANOVA) followed by Tukey-Kramer test for multiple comparisons. *Significantly different from normal negative control group at p < 0.05.
Fatty acids
Area %
Common name
Abbreviation
Soxhlet extraction
Hydraulic press
Myristic acid
C14:0
1.65 ±0.12
ND
Palmitic acid
C16:0
11.88 ±0.32
11.47 ±0.75
Palmitoleic acid, ω-7
C16:1
2.98 ±0.17
2.08 ±0.27
Stearic acid
C18:0
9.95 ±0.25
11.64 ±0.94
Oleic acid, ω-9
C18:1
33.64 ±0.88
36.81 ±1.02
Linoleic acid, ω-6
C18:2
31.95 ±0.89
33.32 ±0.87
α- Linolenic acid, ω-3
C18:3, n3
5.89 ±0.31
0.85 ±0.07
γ-Linolenic acid, ω-6
C18:3, n6
0.80 ±0.05
ND
Arachidic acid
C20:0
0.78 ±0.13
1.81 ±0.11
Behenic acid
C22:0
0.48 ±0.02
1.16 ±0.35
Erucic acid
C22:1
ND
0.37 ±0.04
Lignoceric acid
C24:0
ND
0.49 ±0.10
Σ SFA
---
24.74
26.57
Σ UFA
---
75.26
73.43
Σ MUFA
---
36.62
39.26
Σ PUFA
---
38.64
34.17
Σ UFA/Σ SFA
---
3.04
2.76
Groups
Edema volume (% change from baseline)
1st hour
% Inhibition
2nd hour
% Inhibition
3rd hour
% Inhibition
4th hour
% Inhibition
24 h
% Inhibition
Control
92.99 ± 7.26
____
119.95 ± 8.51
____
140.28 ±5.67
____
164.66 ± 5.97
____
59.94 ± 6.16
____
Indomethacin (10 mg/kg)
21.90 ± 0.9
76.45
27.45 ± 2.87
70.48
39.98 ± 3.59*
57.00
44.63 ± 2.94*
52.01
8.01 ± 1.85
91.38
Dose 1 (70 mg/kg)
35.39 ± 1.01
61.94
45.06 ± 4.84
51.54
49.46 ± 4.08
46.81
65.80 ± 3.03
29.24
16.31 ± 1.41
82.46
Dose 2 (140 mg/kg)
33.99 ± 1.06
63.45
40.63 ± 5.22
56.30
45.84 ± 6.05
50.70
57.34 ± 4.75
38.34
19.50 ± 1.97
79.03
Dose 3 (280 mg/kg)
30.42 ± 2.83
67.29
38.46 ± 5.38
58.64
40.90 ± 3.76*
56.02
44.17 ± 4.86*
52.50
16.29 ± 1.63
82.49
2.2.6 Bioactivity evaluation
2.2.6.1 Antioxidant activity
The radical scavenging ability of the seed oil was determined using the stable DPPH free radical (2,2-diphenyl-1-picrylhydrazyl) according to (Wong et al. 2014). The previously prepared methanol solution (0.3 mL) was mixed with 2.7 mL of a freshly prepared DPPH solution (6 × 10−5 M in 95% methanol). The mixture was shaken vigorously and left in the dark at room temperature for 1 h until stable absorbance values were obtained. A negative control (methanol) was performed, and the absorbance was measured at 517 nm using a spectrophotometer. The IC50 (the concentration of the sample needed to inhibit 50% of the DPPH radicals) was obtained from the standard curve and compared to that of the standard sample, ascorbic acid.
2.2.6.2 Anti-inflammatory and immunomodulatory activities in-vivo
2.2.6.2.1 Animals
Adult male Sprague-Dawley rats weighing 200–250 g, aged 10 to 16 weeks, were purchased and maintained under standard laboratory conditions at the National Research Centre, Giza, Egypt. Rats were fed with basal diet pellets, supplied with water ad libitum, and kept in a temperature-controlled environment (20–22 °C). The experiment was performed in accordance with the National Research Centre's Medical Research Ethics Committee (MREC) ethical guidelines for regular experimental animal studies and the committee approved the experimental protocol which complied with the instructions provided by the Guide for Care and Use of Laboratory Animals (US - NIH Publication No. 85–23, revised 1996) and the ARRIVE guidelines. Anesthesia was used whenever applicable. The doses of the drugs were calculated accurately. Animals’ cadavers and parts of tissues were handled with care by following the principles of healthy hygiene; dead bodies were incinerated in the National Research Centre incinerator.
2.2.6.2.2 Acute toxicity study
The acute toxicity of the oil were evaluated in rats according to the Organization for Economic Cooperation and Development (OECD), guideline no. 423, 2001 (OECD 2001). Rats of either sex (five females and five males, weighing 100–120 g and aged 6–8 weeks old) were orally given the oil at a dose starting at 2000 mg/kg. The animals were observed for toxic symptoms continuously for the first 4 h after dosing. Finally, the number of survivors was noted after 24 h and then maintained for additional 13 days with daily observations.
2.2.6.2.3 Acute anti-inflammatory and immunomodulatory activities
Forty-two male Wistar rats (200 g each) were divided into seven groups as follows: group I received saline; group II received indomethacin (10 mg/kg body weight; p.o.); groups III-V received 70, 140, and 280 mg/kg of the extract (doses used as previously reported by (Sangraula, Dewan, and Kumar 2001). Anti-inflammatory activity was examined using a carrageenan-induced rat paw edema assay (Adeyemi, Okpo, and Ogunti 2002; Winter, Risley, and Nuss 1962). Edema was induced in all rats by the sub-plantar injection of 100 μL of a 1% freshly prepared solution of carrageenan in distilled water into the right hind paws of each rat. The paw thickness was measured using a Vernier Caliper before and after 1, 2, 3, 4, and 24 h after carrageenan injection and compared to its baseline.
Blood samples were taken through the retro-orbital sinus under Ketamine (50 mg/kg, i.p)–Xylazine (5 mg/kg, i.p) anesthesia (-). The Sera samples were collected and stored at 20 °C until needed for determination of inflammatory cytokines levels viz. interleukin-6 (IL-6), and tumor necrosis factor alpha (TNF-α), transforming growth factor beta 1 (TGF-β1) and myeloperoxidase (MPO) to determine the immunomodulatory activity (Jitta et al. 2019).
2.2.6.2.4 Determination of IL-6, TNF-α, TGF-β1 and MPO using ELISA
After 24 h of treatment, the rats in different groups were anaesthetized with chloral hydrate (0.33 mL/mg BW) and blood samples were collected. The serum was separated by centrifugation at 2,500 rpm/min for 10 min. The levels of inflammatory cytokines, TNF-α, IL-6, TGF-β1 and MPO in rat serum were determined by standard enzyme-linked immunosorbent assay (Matsuhiro et al. 2005) kits based on sandwich enzyme-linked immunosorbent assay technology and specific for murine cytokines according to the manufacturer’s instructions (Elabscience Biotechnology Inc, USA for TNF-α and IL-6, SunLong Biotech Co., Ltd., China for TGF-β1 and Wuhan Fine Biological Technology Co., Ltd., China for MPO).
2.2.6.3 Antiparasitic activity in-vitro
2.2.6.3.1 Isolation of Blastocystis spp.
Stool samples were collected from patients visiting outpatient clinics at Ain Shams University hospitals who complained of gastrointestinal manifestations. Consent is taken from the patients after approval by the Ethical Committee of the Faculty of Medicine, Ain Shams University. Samples were immediately examined for intestinal parasites by a wet smear stained with Lugol's iodine and followed by a formalin ethyl acetate concentration technique. Twelve stool samples were examined, two of which were positive for Blastocystis spp. Positive samples were cultured by the addition of 50 mg of positive stool samples into the NIH modification of the Boeck Drbohlav biphasic medium and incubated at 37 °C and examined for growth after 24, 48, and 72 h. The isolate with the best yield in culture was used. For maintenance of a positive culture, Blastocystis spp. were sub-cultured three times a week after peak growth was achieved (Elwakil and Hewedi 2010).
2.2.6.3.2 Antiparasitic activity
The susceptibility of Blastocystis spp to C. procera seed oil was tested in vitro. Dimethyl sulfoxide (DMSO) was used as a solvent at a concentration of 0.4% (v/v in distilled water) (Eldin and Sarhan 2014). Concentrations of 150, 120, 75 and 40 μg/mL of seed oil were tested. The culture tubes were prepared by subculture 2 days before the test. The initial inoculum was counted using a haemocytometer and adjusted to 60 × 105 parasite/mL. The seed oil was added to the culture tubes to reach the desired final concentrations. Besides, parasite control containing only culture media and parasite, and solvent control containing parasites in culture and DMSO (0.4%) were prepared. Metronidazole (MTZ) was used at a concentration of 0.1 mg/mL as a reference antiprotozoal drug. Each experiment was performed in triplicates. The activity was assessed after 24, 48 and 72 h of parasite exposure, where 100 μL from each test and control tube was transferred into 100 μL of the 0.4% Trypan blue media, left for 10 min and then counted by hemocytometer. Unstained parasites were viable and the stained were non-viable (Mokhtar, El-Gayar, and Habib 2016).
2.3 Statistical analysis
The data were presented as mean ±SEM. Results were analyzed by one-way ANOVA with Tukey’s post hoc test. A value of p < 0.05 was taken as a cutoff value for significance. All statistical analyses were done using GraphPad Prism™ software version 7.00 (GraphPad Software, Inc., La Jolla, CA, USA).
3 Results
3.1 Physicochemical properties of extracted oil
To determine the method that gives a higher yield of oil in this study, we compared two simple, applicable, and conventional methods: the Soxhlet and the hydraulic pressing extraction method. The Soxhlet extraction method shows a higher yield of oil than the hydraulic press method, 40.5 ±0.76% and 29.7 ±0.62% w/w, respectively. The acid number of C. procera seed oil was 1.45 ±0.11 and 0.74 ±0.03 mg KOH/g, PV was 3.42 ±0.53 and 0.95 ±0.12 mEq. O2/kg and IV recorded 99.83 ±2.43 and 95.27 ±1.15 g/100 g for oil extracted by Soxhlet and the hydraulic press, respectively. Both were within the AOCS standard limit. The Physicochemical properties of C. procera seed oil were compared to those in the published literature (Barbosa and de Oliveira 2010; Sousa et al. 2018) and are presented in Table 1. The Rancimat method reveals the oxidative stability of the oil with an induction time of 4.14 ±0.10 and 3.97 ±0.17 at 110 °C and an airflow rate of 20 L/h for oil extracted by Soxhlet and the hydraulic press, respectively (Table 1). The total phenolic content calculated was very low, 1.10 ±0.08 µg/g as gallic acid equivalent.
3.2 Phytochemical composition
GC/MS analysis (Table 2) revealed the abundance of four main long-chain fatty acids: two saturated fatty acids (SFA) palmitic, stearic and two unsaturated fatty acids (USF) oleic and linoleic acid, representing about 75% of the oil. Along with a minor amount of SFA: myristic, arachidic, and behenic acids, MUFA: palmitoleic acid, and PUFA: linolenic n-6, and linolenic n-3 acids.
The unsaponifiable matter (USM) analysis revealed the presence of a high content of hydrocarbons (>80%) with a chain length varying from n-C12-C32. Aromatic hydrocarbons were the most abundant, about 81% of total hydrocarbons. GC/MS analysis also detected three steroidal compounds: gamma-Sitosterol (5.42%), Campesterol (1.84%), Cholest-5-en-3-ol (1.37%), Stigmasterol (1.05%) and two triterpenes: Lupeol (0.82%) and 9,19-Cyclolanostane-3,7-diol (0.19) and revealed the low abundance of short-chain alkanes (<n-C20 %).
3.3 Bioactivity evaluation
3.3.1 Antioxidant activity
The IC50 of C. procera seed oil was calculated at 0.641 ±0.012 mg/mL compared to the IC50 of ascorbic acid as a standard material, which was 5.53 ±0.42 μg/mL.
3.3.2 Anti-inflammatory and immunomodulatory activities in vivo
No morbidity nor mortality were observed in the acute toxicity test animals over a period of 24 h at a dose level of up to 2000 mg/kg, indicating that this oil didn’t exhibit toxicity. The anti-inflammatory response of the seed oil was observed in the rat hind paw after injection of carrageenan. The oil significantly inhibited edema development at a dose of 200 mg/kg body weight, with an edema inhibition of 52% at 4 h after the induction of inflammation. These results were compared with the standard inflammatory drug (indomethacin 10 mg/kg body weight) as shown in Fig. 2 and Table 3. Rats given the oil as an anti-inflammatory treatment had a relatively high suppression of the cytokines, TNF-α and IL-6 serum levels (Fig. 3) and a significant reduction in the MPO level (Fig. 4). We observed that the TGF-β1 level did not significantly increase compared to the positive control group. It was slightly higher within groups 2 and 3 treated with doses 140 mg/kg and 280 mg/kg, respectively (Fig. 4).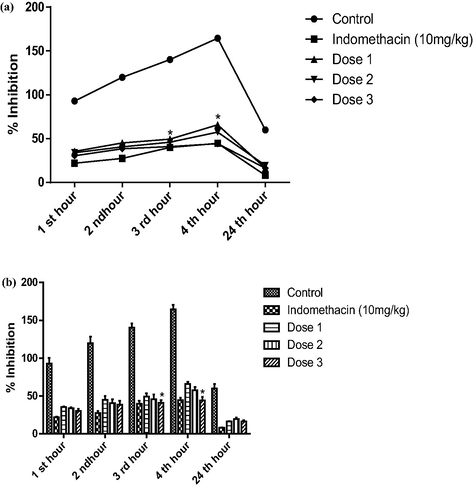
Anti-inflammatory activities in vivo.
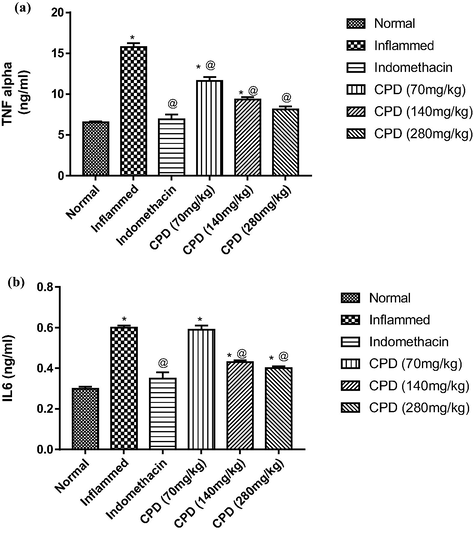
Anti-immunomodulatory activities in vivo.
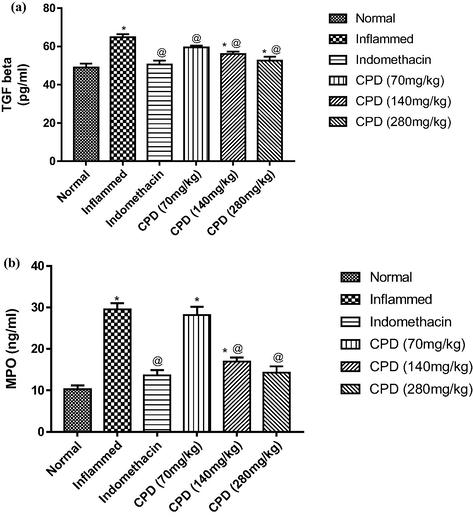
The reduction in MPO, TNF-α and IL-6 serum levels.
3.3.3 Antiparasitic activity in-vitro
C. procera seed fixed oil showed a noticeable decrease in the number of parasites, Blastocystis spp, at all concentrations used, 150, 120, 75, and 40 μg/mL compared to the parasite control. Whereas the concentrations of 150 and 120 μg/mL were more effective in decreasing the parasite count compared to the concentrations of 75 and 40 μg/mL. Besides, the concentrations of 150 and 120 μg/mL provided a complete clearance of the parasite after 72 h of exposure (Fig. 5), suggesting that it acts in a time-dependent manner and is comparable to the results of the reference drug metronidazole (MTZ).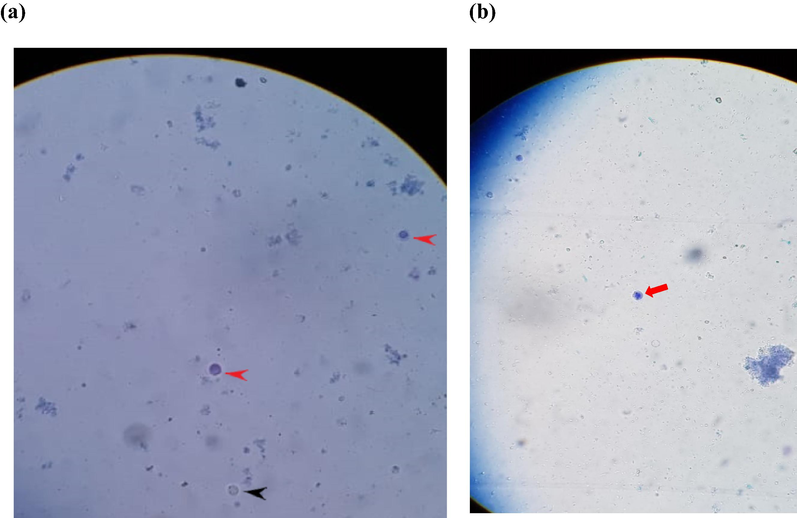
Antiparasitic activity in-vitro.
4 Discussion
The yield percentage and the chemical composition of the oil are interrelated to the environmental and geographical conditions under which the tree grows and its genotype (Barbosa et al. 2014). Nigerian specie show an oil yield percentage of 23% (Gunstone et al. 1972). Indian species give about 26% oil yield and abundance of unsaturated fatty acids (Pryde 1981). While, the Brazillin C. procera (invasive habitat) collected from different locations were severely studied. Authors represents a variation in the oil yield percentage ranging from 19.7 to 24.0% and its chemical composition (Barbosa et al. 2014; Barbosa and de Oliveira 2010; Sousa et al. 2018). In this study the yield ranged from 29.70 ±0.62% to 40.50 ±0.76% that is higher than any previous reported data. Soxhlet extraction was effectively performed for the separation of fixed oils from plum seed using five different solvents, according to Savic et al., (2020). Since non-polar solvents were used to extract fixed oils from plum seeds more efficiently (n-hexane and n-heptane). We speculate that the difference is retained to the unlimited set of hours used during extraction procedure (extraction till exhaustion). In the preceding studies, authors used to specify the extraction time ranging from 4 to 8 h only.
4.1 Physical and chemical characteristics of C. Procera seed oil
According to the AOCS standards cited by (Freire 2001), the AV should not exceed 4 mg KOH/g and the PV should not exceed 10 mEq/kg. The AV measures the free fatty acids in the oil and is considered one of the main indicators of the quality of the oil (Tasan, Gecgel, and Demirci 2011). In our study, two extraction method were used (Soxhlet and hydraulic press), the results showed that the AV of oil extracted by Soxhlet (1.45 mg/g) was higher than oil extracted by pressing (0.74 mg/g), due to the effect of heating temperature used for extraction. (Giuffrè et al. 2018) study the effect of heating on the quality parameters of different vegetable oils (olive “extra virgin and pomace”, palm and soybean), they concluded that the AV was directly proportional to the increase in the heating temperature and also with the increase in time.
The PV estimates the degree of oxidative rancidity. C. procera seed oil properties were within the AOCS standards that prove its stability with low AV and moderate PV. With the same manner, the PV was higher in case of extraction by Soxhlet in compared with pressing extraction method (Table 1). According to the previous study by (Giuffrè et al. 2018) the PV was affected the heating of vegetable oils with increasing the time of heating.
The moderately high IV of the oil extracted by the Soxhlet and pressing (99.83 ±2.43 and 95.27 ±1.15 g/100 g, respectively) reveals a high concentration of unsaturated fatty acids, which was confirmed by GC/MS analysis, 75.25% of the extracted oil was consisted as UFA (Table 2). The IV determines if an oil is non-siccative, semi-siccative, or siccative, depending on whether its value is below 100, between 100 and 140, or above 140. A high IV indicates that the oil is unfit for human consumption (Giuffrè et al. 2016).
The oxidative stability of the oil is directly related to the degree of unsaturation and the number and position of double bonds. SFA and MUFA are not prone to oxidation consequently elevates the oxidative stability of the oil (Przybylski and Eskin 2011). Moreover, The UFA oxidative stability was reported as follow: oleic acid was the most stable, followed by linoleic acid, and finally linolenic acid (Dwivedi and Sharma 2014). GC/MS analysis of the oil indicates that SFA and UFA resembles 24.75%, 75.76%, respectively for oil extracted by Soxhlet extraction, while the oil extracted by hydraulic press indicates that SFA and UFA were 26.57% and 73.43%, respectively. Moreover, the UFA, the analysis revealed that percent of 33.64% was oleic acid, followed by linoleic 31.95% and 5.89% of linolenic acid for sample extracted by Soxhlet method, in compare with the hydraulic press the results was in the same manner except the linolenic percent (0.85%). In a study by (Giuffrè and Capocasale 2016) they reported that the oxidative stability of oil extracted from tomato seeds from three industrial cultivars were ranged from 2.07 to 4.72 h, these results was in agreement with our results for both Soxhlet and pressing extracted oil, which contained the amount of unsaturated fatty acids (77.57%) are closed to our results. Moreover, the oxidative stability of oil extracted by Soxhlet was higher than oil extracted by pressing, that may be due to the oil extracted by solvent contained some phytochemicals which have an antioxidant effect.
Looking at the results obtained from both methods, it was found that there is no significant difference in the results of fatty acids content except the oil extracted by pressing has a lower percentage of linolenic acid (0.85%). On a study presented by (Jokić et al. 2013)) on the effect of extraction method (Soxhlet and supercritical carbon dioxide) on the fatty acid profile of soybean oil, the study found that SC-CO2 extraction may be used to fractionate soybean oil, lowering palmitic, linoleic, and linolenic fatty acids while increasing stearic and oleic acids.
The fatty acid composition of the oil was similar to that reported for the India (native habitat) and Brazil species (Sousa et al. 2018; Pryde 1981; Barbosa and de Oliveira 2010) in different ratios but with a predominance of oleic acid. On the contrary, (Gunstone et al. 1972; Barbosa et al. 2014) has stated the predominance of linoleic acid above the oleic acid in Nigerian and certain Brazilin species. Long-chain fatty acids (C18−C24); linolenic, arachidic, and behenic acids were detected in a very small percentage, which is uncommon for C. procera but has previously been reported for Caltropis giginata (Phoo et al. 2014). Thus, we speculate that these long-chain fatty acids are characteristic for the Egyptian C. procera species.
According to WHO (2008), the relation between PUFA to SFA and between n-6 to n-3 indicates the human health status. PUFA to SFA should be < 0.4, while the n-6/n-3 ratio should be 1–2. the n-6/n-3 ratio is directly proportional to the pathogenesis of diseases, while the PUFA/SFA ratio is inversely proportional to the high-risk diseases’ progression (Simopoulos 2002). Oil extracted by Soxhlet shows a PUFA/SFA and n-6/n-3 ratios of 1.56 and 5.69, respectively. While extraction by pressing shows ratio of 3.02 and 41.32, respectively. Thus, extraction using Soxhlet not only gives a higher yield of oil but also gives an oil of higher nutritional value. Further studies are required to evaluate the availability of consumption of the oil. Food with high content of MUF and PUFA viz. olives and avocados shows a beneficial medicinal activities. Olive oil is one of the most beneficial vegetables oil, it possess a medicinal, nutritional and cosmetic properties. As olive oil, C. procera oil shows a predominance of MUF and PUFA and the abundance of odd number chain alkanes over the even. But they differ in the chain length where C. procera oil exhibits short-chain (<n-C20) and the olive oil > n-C21 (Giuffrè 2021). Both phytosterol content exhibits the predominance of sitosterol, followed by campesterol then stigmasterol (Lukić, Lukić, and Moslavac 2021).
4.2 Bioactivity evaluation
4.2.1 Anti-inflammatory and immunomodulatory activities in vivo
An inflammatory response was observed in the rat hind paw after injection of carrageenan, which functions bi-phasically to control the various mediators’ actions, leading to the formation of edema (Haloui et al. 2011; Jitta et al. 2019). The initial inflammatory response to carrageenan (0–1 h) is attributed to histamine, serotonin, and bradykinin release (Morris 2003; Haloui et al. 2010). That is followed by a second accelerating phase of swelling (2–4 h), which is more associated with increased prostaglandin production (Vinegar et al. 1987; Ishola et al. 2014). Seed oil significantly inhibited edema development in both the initial and second phases, with an edema inhibition of 52% at 4 h after the induction of inflammation (Fig. 2 and Table3).
Overproduction of pro-inflammatory cytokines is a crucial step in the inflammatory process and inflammatory cell infiltration. TNF-α, and IL-6 trigger the immune system to propagate and prolong the inflammation (Chang et al. 2022; Sethi and Hotamisligil 2021). TNF-α, is synthesized and secreted by macrophages, lymphocytes, and polymorphonuclear cells of reactive oxygen species and oxidative stress-responsive genes. TGF-β1 is an important inflammatory mediator suppressive (Yoshimura, Wakabayashi, and Mori 2010). TGF-β plays a key role in tissue repair and remodelling (Brandsma et al. 2013). While MPO is a biomarker of skin irritation and inflammation, its level reflects the degree of neutrophil infiltration in inflammatory processes and catalyzes the formation of potent cytotoxic oxidants (Liu et al. 2008; Islam et al. 2008). The reduction in MPO, TNF-α and IL-6 serum levels (Figs. 3, 4) is a reliable indicator of the oil’s inflammation suppression and oxidative status improvement. Based on the aforementioned finding, C. procera oil can be used not only in the treatment of inflammation but also in inflammatory associated diseases. TNF-α and IL-6 inhibitors are used to treat rheumatoid arthritis, liver disorder, atherosclerosis, to improve liver profile, and are recently being under investigation for treatment of COVID-19 (Elahi et al. 2022; Feldmann et al. 2020; Verhoeven et al. 2020).
We observed no significant alteration in the TGF-β1 serum level compared to the positive control group. The short term follow-up of our study may rationalize the insignificant change in the TGF-β1 serum level, which reached its peak after 6–72 h (Dewald et al. 2004). Chloroform fraction of C. procera roots and latex have previously been reported to have potent anti-inflammatory activity (Sangraula, Dewan, and Kumar 2001; Chaudhary et al. 2016; Basu and Chaudhuri 1991).
4.2.2 Antiparasitic activity in-vitro
The Blastocystis isolate used in the present study was MTZ sensitive, which was shown by its ability to affect the number and viability of the parasites. Several studies (Eida et al. 2016; Zaman and Zaki 1996) found that different Blastocystis strains exhibited varying degrees of MTZ resistance. But, taking into consideration the hazardous effects of MTZ, as its mutagenic effects have been previously proven (Menéndez et al. 2001). Thus, a drug of natural origin would be preferred over MTZ. C. procera seeds fixed oil showed a noticeable decrease in the number of parasites at all concentrations used (Fig. 5) in a time-dependent manner.
Blastocystis spp. are the most common protozoan detected in human faecal samples and are the causative agent of inflammatory bowel disease. Infection is associated with inflammation through the release of inflammatory cytokines (Kaneda et al. 2001). Since the oil possesses potent anti-inflammatory activity and downregulates the production of cytokines, thus, this oil is a promising lead in the treatment of Blastocystis infection, through both its antiparasitic activity and its immunomodulatory and anti-inflammatory properties. The chemical composition of the oil also plays a role in its biological activity. Where fatty acids presents in higher concentration in the oil, oleic acid, linoleic acid, and palmitic acid were reported to exhibit antioxidant, anti-inflammatory activity and nematicide activity (Kumar, Karthik, and Rajakumar 2018; Pegoraro et al. 2021).
The antiparasitic effect of C. procera was previously studied on different plant organs, but first for seed oil. Ethanolic extracts of different parts of C. procera were effective against Plasmodium falciparum, the causative agent of deadly malaria transmitted by mosquito bites (Sharma and Sharma 2000). Flowers were found to possess good anthelmintic activity against nematodes. Their aqueous and methanolic extracts were tested on live Haemonchus contortus and the results showed an evident effect in the form of either mortality or temporary paralysis (Iqbal et al. 2005). Moreover, the effect of the latex ethyl acetate extract on adult Haemonchus contortus was studied by (Cavalcante et al. 2016), and the results proved its anthelmintic potential. Our findings conflict with the results of the previous studies and emphasis the antiparasitic effect of C. procera.
These promising results increase the interest in conducting the test on a larger scale of Blastocystis spp. isolates and studies on experimental animals to assess the therapeutic effect of C. procera seed oil in-vivo (further studies are carried out in our lab).
5 Conclusion
Egyptian C. procera seed fixed oil has a high UFA content 75.25% with oleic acid the most abundant followed by linoleic. GC–MS analysis revealed the presence of long-chain fatty acids, in a very small percentage, which is scarce for C. procera speculating that these fatty acids are characteristic for the Egyptian C. procera species and indicating its probability of being used as biodiesel (further studies are carried in our lab). The extraction by using Soxhlet not only gives a higher yield of oil but also gives an oil of higher nutritional value. Seed fixed oil shows weak antioxidant activity attributed to low phenolic content. Although it exhibits potent anti-inflammatory, immunomodulatory activities in-vivo though modulation of inflammatory cytokines, it also exhibits a significant antiparasitic activity in-vitro. The results emphasise the importance of C. procera seed fixed oil as a potential resource for pharmaceutical and industrial applications.
Acknowledgements
All experiments were performed in the labs of National Research Centre (NRC), Cairo, Egypt.
Authors’ contributions
WM., plant collection, designed the study, carried out the phytochemical study, writing and sequencing alignment, drafting of the manuscript. HZ., carried out the physicochemical analysis, evaluates the antioxidant activity and write its part. AK., performed the antiparasitic activity study and write its part. MA., carried out the immunomodulatory activity study and write its part. RA., participate in studying the immunomodulatory activity. DS., carried out the anti-inflammatory activity, statistical analysis and write its part. All authors read and approved the final manuscript.
Compliance with Ethical Standards
The experiments were conducted following the ethical guidelines for investigations in laboratory animals and complied with the National Institutes of Health guide for the care and use of laboratory animals (NIH Publications No. 8023, revised 1978).
Availability of data and material
The data are available within the manuscript and its supplementary data.
Funding
No Funder
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Analgesic and anti-inflammatory effects of the aqueous extract of leaves of Persea americana mill (lauraceae) Fitoterapia. 2002;73:375-380.
- [Google Scholar]
- Ex vivo and in vivo mice models to study Blastocystis spp. adhesion, colonization and pathology: Closer to proving Koch's postulates. PLoS ONE. 2016;11
- [Google Scholar]
- AOCS, Official. 1993. “Tentative Methods of the American Oil Chemists’ Society, vol. 1.” In.: AOCS Press, Champaign.
- Barbosa, Mariana Oliveira, Jarcilene Silva de Almeida-Cortez, Suzene Izídio Da Silva, and Antonio Fernando Morais de Oliveira. 2014. 'Seed oil content and fatty acid composition from different populations of Calotropis procera (Aiton) WT Aiton (Apocynaceae)', Journal of the American Oil Chemists' Society, 91: 1433-41.
- Barbosa, Mariana Oliveira, and Antonio Fernando Morais de Oliveira. 2010. “Caracterização físico-química e perfil de ácidos graxos do óleo de sementes de calotropis procera (apocynaceae).” In Embrapa Algodão-Artigo em anais de congresso (ALICE). In: congresso brasileiro de mamona, 4.; simpósio internacional de ….
- Basu, A, and AK Nag Chaudhuri. 1991. 'Preliminary studies on the antiinflammatory and analgesic activities of Calotropis procera root extract', Journal of Ethnopharmacology, 31: 319-24.
- Differential effects of fluticasone on extracellular matrix production by airway and parenchymal fibroblasts in severe COPD. Am. J. Physiol.-Lung Cell. Mol. Physiol.. 2013;305:L582-L589.
- [Google Scholar]
- Chemical composition and in vitro activity of Calotropis procera (Ait.) latex on Haemonchus contortus. Vet. Parasitol.. 2016;226:22-25.
- [Google Scholar]
- Interleukin-6 transiently promotes proliferation of osteoclast precursors and stimulates the production of inflammatory mediators. Mol. Biol. Rep. 2022
- [Google Scholar]
- Chaudhary, Priyanka, Marcio V Ramos, Mirele da Silveira Vasconcelos, and Vijay L Kumar. 2016. 'Protective effect of high molecular weight protein sub-fraction of Calotropis procera Latex in Monoarthritic Rats', Pharmacognosy magazine, 12: S147.
- Clinical and pathological effects of Calotropis procera exposure in sheep and rats. Toxicon. 2011;57:183-185.
- [Google Scholar]
- Of mice and dogs: species-specific differences in the inflammatory response following myocardial infarction. Am. J. Pathol.. 2004;164:665-677.
- [Google Scholar]
- Potential and limitation of straight vegetable oils as engine fuel–an Indian perspective. Renew. Sustain. Energy Rev.. 2014;33:316-322.
- [Google Scholar]
- In vivo and in vitro efficacy of nigella sativa aqueous extract on blastocystis hominis. J. Egypt. Soc. Parasitol.. 2016;240:1-8.
- [Google Scholar]
- An updated overview of recent advances, challenges, and clinical considerations of IL-6 signaling blockade in severe coronavirus disease 2019 (COVID-19) Int. Immunopharmacol. 2022:108536.
- [Google Scholar]
- Eldin, Hayam Mohamed Ezz, and Rania Mohamed Sarhan. 2014. 'Cytotoxic effect of organic solvents and surfactant agents on Acanthamoeba castellanii cysts', Parasitology research, 113: 1949-53.
- Pathogenic potential of Blastocystis hominis in laboratory mice. Parasitol. Res.. 2010;107:685-689.
- [Google Scholar]
- Trials of anti-tumour necrosis factor therapy for COVID-19 are urgently needed. The Lancet. 2020;395:1407-1409.
- [Google Scholar]
- 'Ricinoquímica', O agronegócio da mamona no Brasil. Embrapa Informação Tecnológica: Brasília; 2001. p. :295-335.
- Physicochemical composition of tomato seed oil for an edible use: the effect of cultivar. Int. Food Res. J.. 2016;23
- [Google Scholar]
- 'Effect of heating on chemical parameters of extra virgin olive oil, pomace olive oil, soybean oil and palm oil', Italian. J. Food Sci.. 2018;30
- [Google Scholar]
- N-Alkanes and n-alkenes in virgin olive oil from calabria (south Italy): The effects of cultivar and harvest date. Foods. 2021;10:290.
- [Google Scholar]
- Seed oil from ten Algerian peanut landraces for edible use and biodiesel production. J. Oleo Sci.. 2016;65:9-20.
- [Google Scholar]
- New tropical seed oils. IV—component acids of leguminous and other seed oils including useful sources of crepenynic and dehydrocrepenynic acid. J. Sci. Food Agric.. 1972;23:53-60.
- [Google Scholar]
- Pharmacological activities and chemical composition of the Olea europaea L. leaf essential oils from Tunisia. J. Food Agric. Environ.. 2010;8:204-208.
- [Google Scholar]
- Hydroxytyrosol and oleuropein from olive leaves: potent anti-inflammatory and analgesic activities. J. Food Agric. Environ.. 2011;9:128-133.
- [Google Scholar]
- Formulation of multi-functional omega-3 oil rich microcapsules by spray drying methodology. Egyptian Journal of Chemistry. 2020;63(12):5117-5136.
- [Google Scholar]
- Parasitic infections represent a significant health threat among recent immigrants in Chicago. Parasitol. Res.. 2020;119:1139-1148.
- [Google Scholar]
- Anthelmintic activity of Calotropis procera (Ait.) Ait. F. flowers in sheep. J. Ethnopharmacol.. 2005;102:256-261.
- [Google Scholar]
- Mechanisms of analgesic and anti-inflammatory properties of Annona muricata Linn. (Annonaceae) fruit extract in rodents. J Med Food. 2014;17:1375-1382.
- [Google Scholar]
- Anti-inflammatory effects of phytosteryl ferulates in colitis induced by dextran sulphate sodium in mice. Br. J. Pharmacol.. 2008;154:812-824.
- [Google Scholar]
- Jitta, Srinivasa Reddy, Prasanthi Daram, Karthik Gourishetti, CS Misra, Picheswara Rao Polu, Abhishek Shah, CS Shreedhara, Madhavan Nampoothiri, and Richard Lobo. 2019. 'Terminalia tomentosa bark ameliorates inflammation and arthritis in carrageenan induced inflammatory model and freund’s adjuvant-induced arthritis model in rats', Journal of Toxicology, 2019.
- Jokić, Stela, Rezica SudaR, Sandra Svilović, Senka Vidović, MATE Bilić, Darko Velić, and VLATKA Jurković. 2013. 'Fatty acid composition of oil obtained from soybeans by extraction with supercritical carbon dioxide', Czech Journal of Food Sciences, 31: 116-25
- Ribodemes of Blastocystis hominis isolated in Japan. Am. J. Trop. Med. Hygiene. 2001;65:393-396.
- [Google Scholar]
- 'An overview of the characteristics and potential of Calotropis procera from botanical, ecological, and economic perspectives' Front. Plant Sci.. 2021;12
- [Google Scholar]
- Khanzada, SAMINA KABIR, W Shaikh, TG Kazi, S Sofia, Amina Kabir, K Usmanghani, and AFTAB A Kandhro. 2008. 'Analysis of fatty acid, elemental and total protein of Calotropis procera medicinal plant from Sindh, Pakistan', Pak. J. Bot, 40: 1913-21.
- Kumar, Dinesh, M Karthik, and R Rajakumar. 2018. 'GC-MS analysis of bioactive compounds from ethanolic leaves extract of Eichhornia crassipes (Mart) Solms. and their pharmacological activities'.
- Calculation of iodine value from measurements of fatty acid methyl esters of some oils: comparison with the relevant American oil chemists society method. J. Am. Oil. Chem. Soc.. 2000;77:1235-1238.
- [Google Scholar]
- Changtai granule, a traditional Chinese drug, protects hapten-induced colitis by attenuating inflammatory and immune dysfunctions. J. Ethnopharmacol.. 2008;115:1-8.
- [Google Scholar]
- Sterols and triterpene diols in virgin olive oil: a comprehensive review on their properties and significance, with a special emphasis on the influence of variety and ripening degree. Horticulturae. 2021;7:493.
- [Google Scholar]
- Structural analysis and antiviral activity of a sulfated galactan from the red seaweed Schizymenia binderi (Gigartinales, Rhodophyta) Carbohydr. Res.. 2005;340:2392-2402.
- [Google Scholar]
- DNA breakage due to metronidazole treatment. Mutat. Res./Fund. Mol. Mech. Mutagene.. 2001;478:153-158.
- [Google Scholar]
- In vitro anti-protozoal activity of propolis extract and cysteine proteases inhibitor (phenyl vinyl sulfone) on Blastocystis species. J. Egypt. Soc. Parasitol.. 2016;240:1-12.
- [Google Scholar]
- Carrageenan-induced paw edema in the rat and mouse. Methods Mol. Biol.. 2003;225:115-121.
- [Google Scholar]
- Extraction and phytochemical investigation of Calotropis procera: effect of plant extracts on the activity of diverse muscles. Pharm. Biol.. 2010;48:1080-1190.
- [Google Scholar]
- Evaluation of green extraction methods on the chemical and nutritional aspects of roselle seed (Hibiscus sabdariffa L.) oil. OCL. 2019;26:33.
- [Google Scholar]
- Organization for Economic Cooperation and Development (OECD) Guidelines For Testing of Chemicals, no. 423. Acute Toxic Class Method: Acute Oral Toxic; 2001.
- 'Calotropis procera: a phytochemical and pharmacological review' Thai J. Pharm. Sci.. 2016;40
- [Google Scholar]
- Oleic acid exhibits an expressive anti-inflammatory effect in croton oil-induced irritant contact dermatitis without the occurrence of toxicological effects in mice. J. Ethnopharmacol.. 2021;267:113486
- [Google Scholar]
- Evaluation of Indian milkweed (Calotropis gigantea) seed oil as alternative feedstock for biodiesel. Ind. Crops Prod.. 2014;54:226-232.
- [Google Scholar]
- New insights into Blastocystis spp.: a potential link with irritable bowel syndrome. PLoS Pathog.. 2012;8:e1002545
- [Google Scholar]
- Pryde, Everett H. 1981. New sources of fats and oils (The American Oil Chemists Society).
- Przybylski, Roman, and NA Michael Eskin. 2011. 'Oil composition and properties.' in, Canola (Elsevier).
- Saber, AH, GH Maharan, MM Rizkallah, and AH Ste Saber. 1969. 'Sterols and pentacyclic triterpenes of Calotropis procera', Bull Fac Pharm Cairo Univ, 7.
- Evaluation of anti-inflammatory activity of latex of Calotropis procera in different models of inflammation. Inflammopharmacology. 2001;9:257-264.
- [Google Scholar]
- Physico-chemical properties and oxidative stability of fixed oil from plum seeds (Prunus domestica Linn.) Biomolecules. 2020;10(2):294.
- [Google Scholar]
- In-vitro schizonticidal screening of Calotropis procera. Fitoterapia. 2000;71:77-79.
- [Google Scholar]
- The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother.. 2002;56:365-379.
- [Google Scholar]
- Sousa, Lindeberg Ventura, Adriana Paula Batista Santos, Luiz Di Souza, Anne Gabriella Dias Santos, and Adilson Beatriz. 2018. 'Evaluation of the Properties of Calotropis procera Oil Aiming the Production of Biodiesel', Orbital: The Electronic Journal of Chemistry, 10: 147-52.
- Comparison of geometrical isomerization of unsaturated fatty acids in selected commercially refined oils. Grasas Aceites. 2011;62:284-289.
- [Google Scholar]
- Verhoeven F., Weil-Verhoeven D., Prati C., Di Martino V., Thevenot T., Wendling D., eds. Safety of TNF inhibitors in rheumatic disease in case of NAFLD and cirrhosis. Elsevier; 2020. p. :544-548.
- Pathway to carrageenan-induced inflammation in the hind limb of the rat. Fed. Proc.. 1987;46:118-126.
- [Google Scholar]
- Analgesic and anti-inflammatory effects and safety profile of Cucurbita maxima and Cucumis sativus seeds. Saudi J. Biol. Sci. 2021
- [Google Scholar]
- Carrageenin-induced edema in hind paw of the rat as an assay for antiinflammatory drugs. Proc. Soc. Exp. Biol. Med.. 1962;111:544-547.
- [Google Scholar]
- In vitro simulated digestion on the biostability of Hibiscus cannabinus L. seed extract. Czech J. Food Sci.. 2014;32:177-181.
- [Google Scholar]
- Cellular and molecular basis for the regulation of inflammation by TGF-β. J. Biochem.. 2010;147:781-792.
- [Google Scholar]
- Enhanced stability of refined soybean oil enriched with phenolic compounds of olive leaves. Egypt. J. Chem.. 2020;63:215-224.
- [Google Scholar]
- Resistance of Blastocystis hominis cysts to metronidazole. Trop. Med. Int. Health. 1996;1:677-678.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2022.104085.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1
Supplementary data 2
Supplementary data 2







