Translate this page into:
Synthesis, in vitro analysis and molecular docking study of novel benzoxazole-based oxazole derivatives for the treatment of Alzheimer’s disease
⁎Corresponding authors. sono_waj@yahoo.com (Wajid Rehman)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Alzheimer's disease (AD) is treated by targeting cholinesterase enzymes like acetylcholinesterase and butyrylcholinesterase, and these enzymes' inhibitors serve as important tools for treatment of alzheimer diseases. Hybrid analogues with a 1,3-oxazole moiety based on benzoxazole were designed, developed, and then tested for their cholinesterase inhibition. All the newly synthesized analogues showed moderate to good inhibitory potentials having IC50 values raging between 0.90 ± 0.05 µM to 35.20 ± 0.70 µM against acetylcholinesterase and 1.10 ± 0.10 µM to 37.70 ± 0.60 µM against butyrylcholinesterase enzymes. Among the series, the analogue 11 (IC50 = 0.90 ± 0.05 µM), (IC50 = 1.10 ± 0.10 µM) and 18 (IC50 = 1.20 ± 0.05 µM), (IC50 = 2.10 ± 0.10 µM) being the strongest acetylcholinesterase and butyrylcholinesterase inhibitors as compared to standard donepezil drug. Nonetheless, the remaining analogues also displayed better inhibition profile against both these targeted enzymes. Furthermore, the structures of all the synthesized analogues were confirmed by using HREI-MS, 1HNMR and 13CNMR spectroscopy. Additionally, molecular docking experiments were conducted to determine the potential mode of interaction between the majority of active analogues and the enzyme active site. The findings corroborated the experimental data.
Keywords
Synthesis
AChE & BuChE inhibitors
1,3-Oxazole
Benzoxazole
Molecular docking
SAR
1 Introduction
Alzheimer’s disease is recognized as the progressive neurodegenerative disorder with multiple symptoms such as behavioral disturbance, disorientation, memory loss and cognitive dysfunction (Millard and Broomfield 1995; Yang and Dettbarn 1998; Weinstock and Groner2008). In the basal forebrain, it is associated in connection to the deficiency of cholinergic function of the cortex (Massoulié et al., 1993; Jann 1998; Rapanelli et al., 2023; Zhang et al.,2023). Thus, in alzheimer patients the loss of memory and learning skill is adversely affected by shortening of cholinergic neurotransmission. It has been reported that the global number of people with dementia has increased to 43.8 million in 2016, an increase of 117% compared with 20.3 million in 1990. It is estimated that, by 2050, there will be 152 million people with Alzheimer's disease and other dementias (Li et al., 2022). A review of the literature found that Alzheimer's disease could be treated more successfully and clinically better by suppressing cholinesterase enzymes (Aisen and Davis 1997). Acetylcholinesterase (AChE) and butyrylcholinesterase (BuChE), two types of cholinesterase (ChEs), are thought to be the fundamental building blocks of cholinergic transmission since they hydrolyze acetylcholine neurotransmitter (Whitehouse et al., 1982). Cholinergic neurons, muscle, and the brain all contain acetylcholinesterase and some of its subunits. It is a membrane-bound enzyme, similar to those found in mammalian brains, and is mostly found in the G4 form; however, as the number of degenerating neurons increases, so does the quantity of this enzyme (Jann 1998). Additionally, by converting the neurotransmitter acetylcholine at cholinergic synapses, this enzyme also plays a critical role in the control of several physiological processes (Adams et al., 1986; Schetinger et al., 2000; Kanwar et al., 2023; Liu et al., 2023; Nimgampalle et al., 2023). Neuralgias express the butyrylcholinesterase enzyme, which is also found in the liver, lungs, gut, serum, and kidneys. This enzyme's primary function is to catalyze molecules with an ester moiety (Prandota2018). BuChE functions similarly to AChE, although its level does not fluctuate and occasionally even rises (Mesulam et al., 2002). AChE predominates in the brain and either remains constant or declines, whereas BuChE activity increases in AD patients (Greig et al., 2001). Due to this major issue, it may be preferable to use a specific medication to block both AchE and BuChE action. Synthetic inhibitors, such as galantamine, donepezil, tacrine and rivastigmine, have recently gained popularity as effective treatments for AD (Fig. 1). However, the likelihood of complications from their use is still low due to their insufficient activity and a variety of side effects, such as hepatotoxicity and gastrointestinal disturbance (Smallet al., 1997, Melzer 1998, Schulz 2003). Due to the unfavorable side effects of these medications, researchers have become more interested in creating powerful ChEs inhibitors in place of these inhibitors. As a result, numerous natural bioresources have been used to extract several natural AchE inhibitors (Kang et al., 2000, Savelev et al., 2003, Ahmed et al., 2006, Al-Rajhi et al., 2023, Chaudhary et al., 2023, Rani et al., 2023, Takahashi 2023).(See Table 1)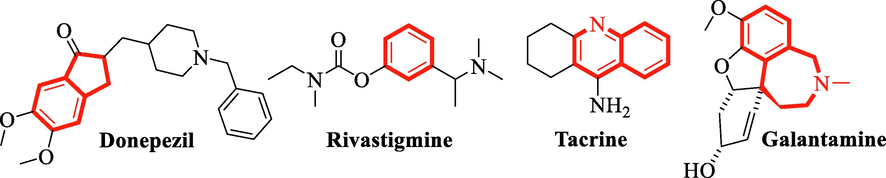
Structures of drugs used against Alzheimer’s disease.
S. No.
Ring-B
Ring-C
AChE IC50 ± SEMa (µM)
BuChE IC50 ± SEMa (µM)
1
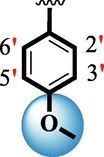

5.80 ± 0.10
7.50 ± 0.20
2

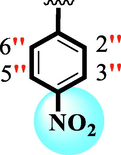
13.40 ± 0.30
10.50 ± 0.20
3
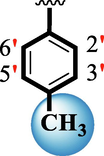
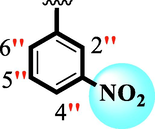
10.60 ± 0.20
6.80 ± 0.10
4
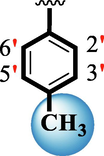
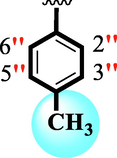
18.60 ± 0.40
25.50 ± 0.30
5
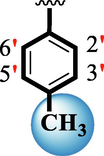
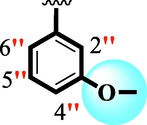
15.60 ± 0.10
18.70 ± 0.20
6
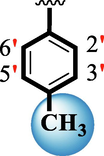
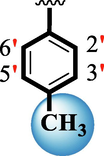
1.70 ± 0.05
2.50 ± 0.10
7
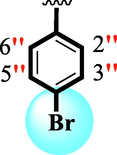
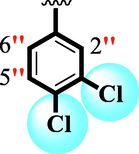
24.80 ± 0.30
32.40 ± 0.40
8
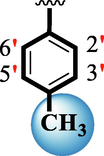

16.40 ± 0.20
20.40 ± 0.30
9
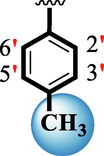
4.10 ± 0.10
7.20 ± 0.20
10
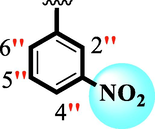
6.10 ± 0.10
9.30 ± 0.20
11
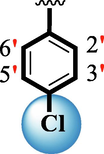
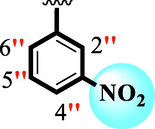
0.90 ± 0.05
1.10 ± 0.10
12
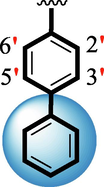
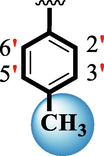
7.70 ± 0.20
11.30 ± 0.30
13
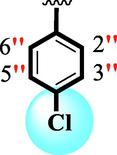
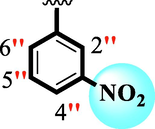
12.10 ± 0.20
14.50 ± 0.30
14
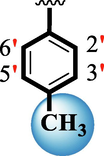
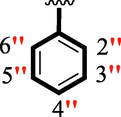
16.50 ± 0.30
19.40 ± 0.40
15
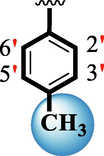
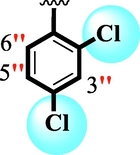
2.10 ± 0.10
3.20 ± 0.20
16
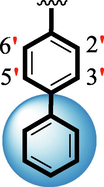
35.20 ± 0.70
37.70 ± 0.60
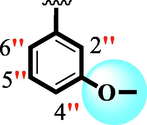
17
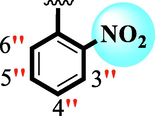
8.20 ± 0.20
11.20 ± 0.30
18
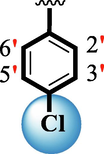
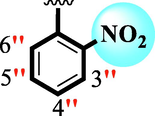
1.20 ± 0.05
2.10 ± 0.10
19
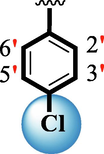
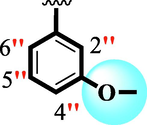
10.20 ± 0.20
14.30 ± 0.30
Standard donepezil drug
0.016 ± 0.12
4.5 ± 0.11 µM
Benzoxazole derivatives serve as “privileged scaffolds” to access compounds with an intriguing biological profile in numerous therapeutic areas, such as antibacterial (Sirgamalla et al., 2020), antifungal (Baldisserotto et al., 2020), antiinflammatory activities (Tariq et al., 2018)and anticancer (Aswathy et al., 2017, Aboul Wafa et al., 2023, Elkady et al., 2023, Jiang et al.,2023). In addition, boxazomycin B, flunoxaprofen, benoxaprofen, boxazomycin A, calcimycin, nataxazole, chloroxazole, nakijinol B, and nocarbenzoxazole G are all naturally occurring and biologically active chemicals that use benzoxazole-based scaffolds as basic ingredients (Demmer and Bunch 2015, Kakkar et al., 2018a, 2018b, Guzow et al., 2021).
Oxazole scaffolds serve as valuable and essentials building blocks in designing and development of numerous biologically active pharmaceutical drugs of interesting biological profile, ligand frameworks, functional materials and natural products (Zhou et al., 2017, Zhang et al.,2018, Wong and Yeong 2021). Oxazole is biologically active scaffolds on which pharmacophore are arranged to provide potent, selective drugs and were commonly found in remarkably bioactive molecules and numerous natural products, such as AD-5061 (antidiabetic agent), peptide alkaloid (−)-muscoride A, JTE-5229 COX-2 inhibitors (anti-inflammatory drug),aristoxazole (anti-inflammatory drugs),aleglitazar (antidiabetic),ditazole (platelets aggregation inhibitor) mubritinib (tyrosine kinase inhibitor) and oxaprozin (Zhou et al., 2017, Kakkar et al., 2018a, 2018b).
Our research group had reported various classes of heterocyclic compounds in search of biologically active drugs. In recent past, we had investigated different category of nitrogen and sulphur bearing heterocyclic compounds such as benzimidazole bearing thiazole (Hussain et al., 2022a, 2022b), thiazole-sulphonamide (Khan et al., 2023), triazole (Rahim et al., 2022) and thiazolidinone (Hussain et al., 2022a, 2022b) as potent inhibitors of acetylcholinesterase and butyrylcholinesterase enzymes (Fig. 2).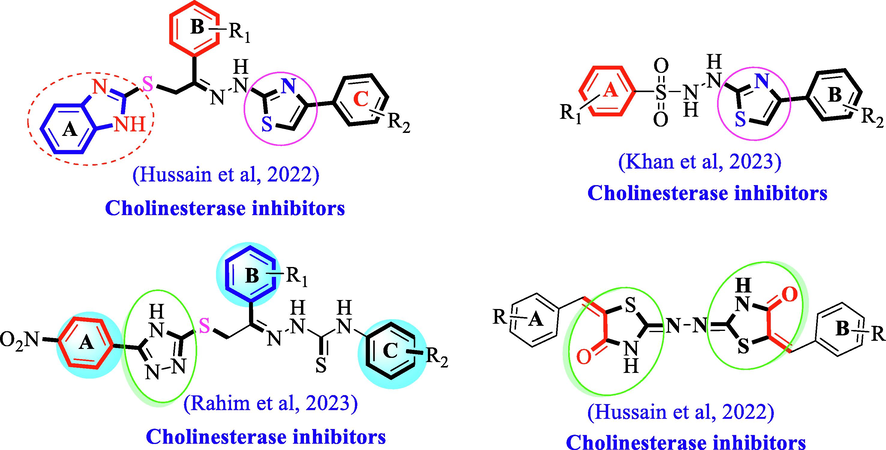
Our reported N-containing heterocyclic analogs as potent inhibitors of acetylcholinesterase and butyrylcholinesterase enzymes.
Keeping in view, the biological significance of oxazole (Šagud et al., 2020) and benzoxazole (Temiz-Arpaci et al., 2016, Srivastava et al., 2019, Hussain et al., 2023) scaffolds, herein this study, we have designed and synthesized hybrid scaffolds incorporating both oxazole and benzoxazole rings in the same molecules to enhance the anti-cholinesterase activity in search of lead candidate. We found that our designed benzoxazole based oxazole hybrids analogues showed remarkable anti-cholinesterase activity than individual benzoxazole and oxazole analogues (Fig. 3).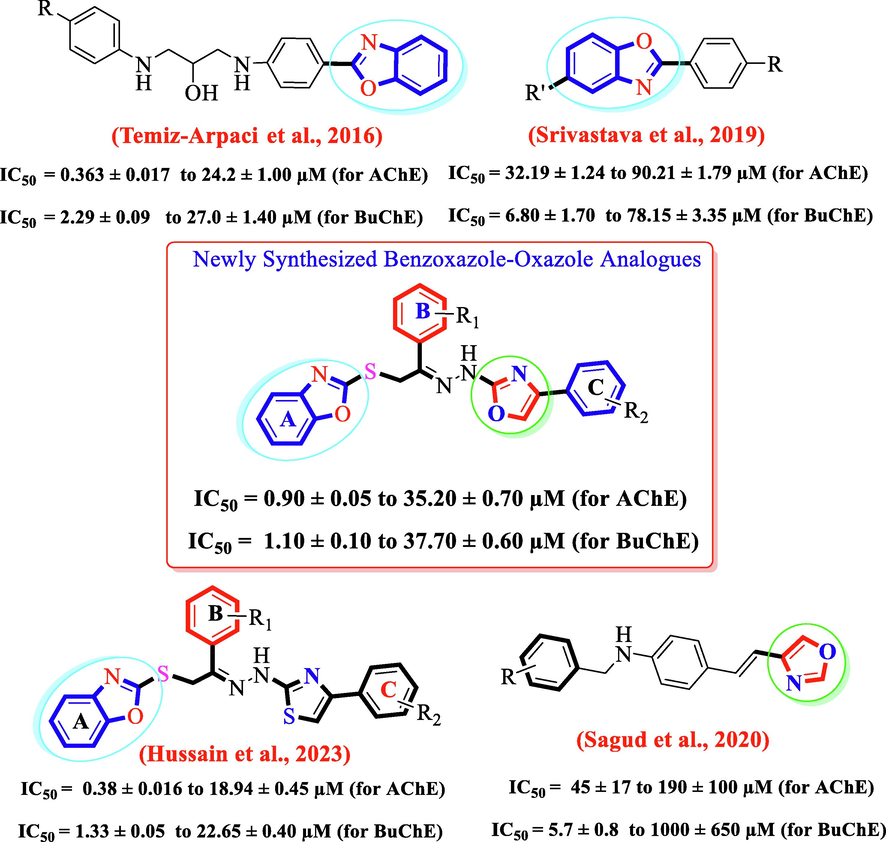
Rational of the current study.
2 Results and discussion
2.1 Chemistry
The synthesis of 2-marcaptobenzoxazole was offered as a substrate (II) by the addition of 2-aminophenol solution (I) and carbon disulphide (CS2) in acetone. The resultant residue was stirred at refluxing temperature for 4 hrs under the catalytic action of triethylamine (Et3N). When the reaction was completed, the solvent was evaporated under decreased pressure and resulting solid residue (II) was further treated with various substituted phenacyl bromide in ethanol (EtOH) and triethylamine (Et3N) to afford S-substituted benzoxazole (III) substrate which was further re-dissolved in ethanol followed by addition of semicarbazide in ethanol and glacial acetic acid. Upon completion, the solvent was evaporated under reduced pressure and the resultant substrate (IV) underwent cyclization on refluxing with various substituted phenacyl bromide in ethanol and triethylamine while being stirred for 12hrs at refluxing temperature to afford targeted benzoxazole-based oxazole derivatives (1–20) (Scheme 1).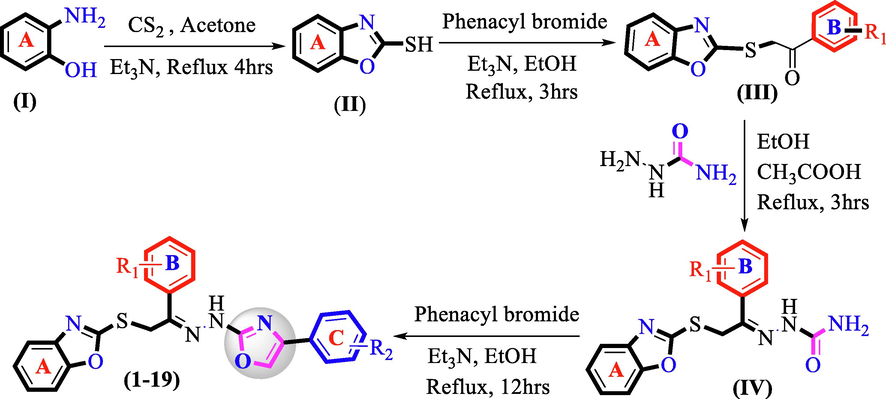
Preparation of benzoxazole-bearing 1,3-oxazole derivatives (1–19).
The precise structures of all the newly synthesized compounds were confirmed using variety of spectroscopic tools including 1H NMR, 13C NMR and HREI-MS. The proton NMR spectrum of compound 13 confirmed the formation of new entity. The most de-shielded proton attached to hydrazine-linked NH require lesser applied magnetic field to achieve resonance and thus giving a singlet resonating at chemical shift values of 11.69 ppm. The more singlets were also observed; one for two chemically equivalent methylene proton (—SCH2—) resonating at chemical shift values of 4.01 ppm, while other for three chemically equivalent methyl proton appearing at chemical shift values of 3.91 ppm and third for oxazole proton resonating at 7.81 chemical shift values. Furthermore, in order to NMR spectrum of compound 13 in better way we have splitted the aromatic protons into three different categories including aromatic-ring A proton represented by numbers without prime; aromatic ring B proton represented by numbers with single prime and aromatic ring-C protons represented by numbers with double prime as given in figure-4. The two protons that are ortho- to —Cl group of aromatic ring-C are chemically equivalent and appeared as doublet with chemical shift values of 8.40 ppm. Similarly, the two protons that are meta- to —Cl group are also chemically equivalent coupled to its neighboring ortho-protons to give doublet appearing at 8.17 ppm chemical shift values. On the other hand, the fours protons of para-disubstituted aromatic ring-B also observed as two different doublet; one for two chemically equivalent protons that are ortho- to —CH3 group with chemical shift values of 7.86 ppm, while other for two protons that are meta- to —CH3 group resonating at chemical shift values of 7.91 ppm. Finally, taking into account the fours protons of aromatic ring-A. The two protons which are present at 4- and 7-postion of aromatic ring-A coupled to its neighboring protons simultaneously to give multiplet appearing at chemical shift values of 7.78 ppm. Similarly, the other two protons which are present at 5- and 6-position of aromatic ring-A also give multiplet with chemical shift values of 7.46 ppm.
2.2 Biological activity
2.2.1 In vitro acetylcholinesterase and butyrylcholinesteraseinhibitory potentials
This study was aimed to synthesize the hybrid analogues of benzoxazole containing 1,3-oxazole moiety and then illustrated for their cholinesterase inhibition profile. All the analogues were found to possess substantial potency with IC50 values ranging between 0.90 ± 0.05 µM to 35.20 ± 0.70 µM against acetylcholinesterase and 1.10 ± 0.10 µM to 37.70 ± 0.60 µM against butyrylcholinesterase enzymes when compared to donepezil (0.016 ± 0.12 µM), (4.5 ± 0.11 µM). It was noteworthy that the analogues bear either powerful electron withdrawing or electron donating groups on the both rings B & C were identified as excellent inhibitors of cholinesterase enzymes. Among the series, analogues 6, 11, 15 and 18 were recognized as the most active against acetylcholinesterase and butyrylcholinesterase enzymes. Other derivatives showed remarkable inhibition as such, but these are also found to be less effective than standard medication. As shown in (Fig. 4), we divided basic structure into the four parts: the aryl portion B, benzoxazole ring A, the aryl part C and the 1,3-oxazole ring, in order to further explore the structure–activity-relationship. By introducing various substitutions at various places of these rings respectively, an interesting pattern in activity was demonstrated (Fig. 4).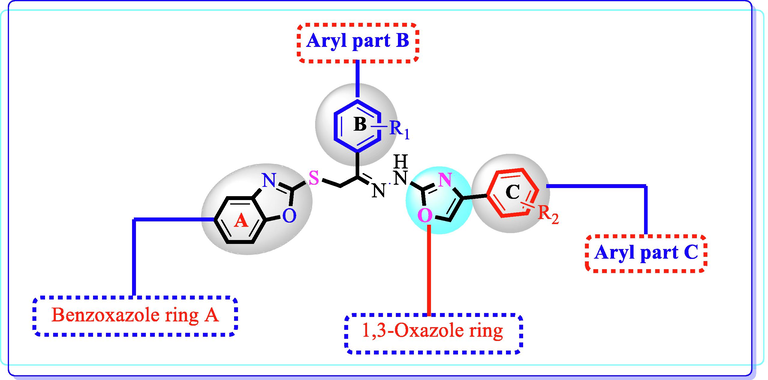
Represent general skeleton of hybrid analogues of oxazole carrying benzoxazole moiety.
2.2.2 Structure-activity relationship (SAR) studies
SAR studies suggested that presence of groups of strong electron withdrawing nature such as —Cl and —NO2 on both rings B and C of benzoxazole based oxazole hybrid scaffolds (1–19) are of immense importance. Therefore, scaffolds mainly with —Cl and —NO2 groups showed superior activity than other analogues. Analogue (11) having —Cl substitution at para-position of ring B and —NO2 groups attached to meta-position of ring C, was found to be the most active inhibitors of cholinesterase enzymes among the series. However, its structurally similar analogue (18) bearing the same –Cl and –NO2 substituents, but holds —NO2 group at ortho-position of ring C was found as the second most active scaffold of this series. This analogue (18) showed one-folds less potency than analog (11). The elevation in potency of these analogues (11) and (18) against cholinesterase enzymes might be due to the attached —Cl & —NO2 moieties on the both rings, where these groups withdraw most of the electronic density from the phi-system resulting partial positive charge over both rings B and C, and further these phi-rings try to regain the stability by possessing several interactions with enzymes active site, and hence enhance the enzymatic potency. The substitution of NO2 group at various position of ring C like as 2-nitro or 4-nitro were proved to be enhancement for inhibition profile of these enzymes. Compound (2) with –OCH3 group at 4th position of ring B and para-NO2 substitution on ring C demonstrated good inhibition profile, but 2-folds less potent than its counterpart (1) having ortho-NO2 substitution on ring C along with para-hydroxyl moiety at 4-position of ring B. This discrepancy in activity of these scaffolds (1) and (2) might be owing to different orientation of nitro group around ring C toward the hydrophilic residue of both targeted cholinesterase enzymes (Fig. 5).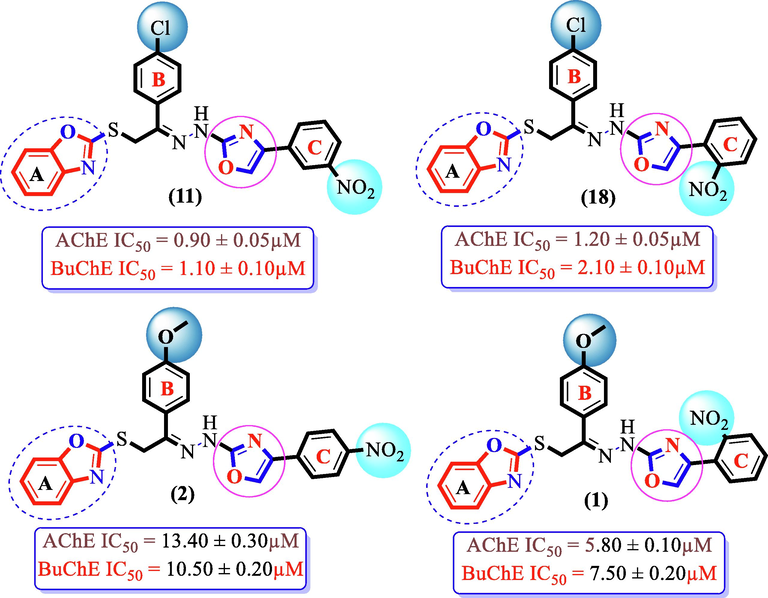
SAR study of chloro- and methoxy-substitutedanalogues11, 18, 1 and 2.
Analogues having —CH3 group at the para-postion of ring B and —Cl moiety on ring C, were knwon as enhancement for inhibition of both targeted AchE and BuChE enzymes. Analogue (14) being un-substituted ring C along with para-methyl substitution on ring B, showed moderate potency against both AchE and BuChE enzymes, even somewhat less inhibitory potential than standard donepezil drug. However, the inhibition profile of analogue (14) was enhanced to many folds by adding di-chloro groups either at 2, 4-position or 3,4-position of ring C as in analogues (15) and (6). If we compared analogue (15) bearing –CH3 group at para-position of ring B and 2,4-di-Cl moieties on ring C with analog (13) having para-Cl group on ring C along with para-CH3 moeity on ring B, the analogue (15) displayed better activity than analogue (13) owing to higher number of attached EWG groups such as –Cl on 2,4-position of ring C. The declined in activity of both analogues (13) and (15) were observed by replacing either mono–Cl or di-Cl moieties with hydrogen atom as in analogue (14) showing that substitution of electron withdrawing groups boost up the enzymatic activity. In addition, analogue (6) having di-Cl moieties on 3,4-position of ring C showed enhanced enzymatic activity than its structurally similar analogue (15) (bearing the same substituents but holds the di-Cl moieties around ring C at 2,4-positions), even tough both these scaffolds demonstrated higher potency than standard donepozil drug against BuChE and somewhat less potency than standard against AchE enzymes (Fig. 6).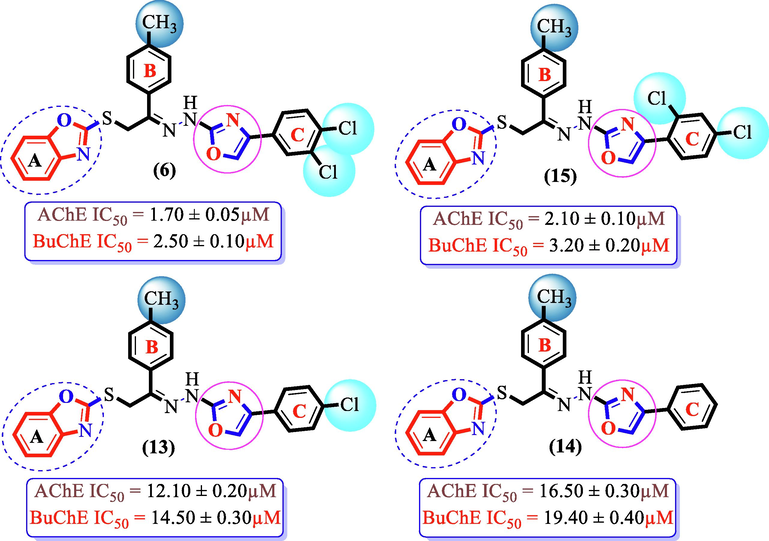
SAR study of analogues6, 13, 14 and 15.
Analogues (5) and (8) bearing —OCH3 group on different position of ring C along with para-methyl substituent on ring B, respectively, displayed variable inhibition profile which showed that the change in position of –OCH3 around ring C shown a various binding interaction site for enzyme-ligand interaction. Analogue (5) having meta-methoxy group on ring C and methyl moiety at 4-position of ring B, respectively, exhibited better inhibitory activity. However, it structurally similar analogue (8) bearing meta-methoxy substituent on ring C showed activity comparable to analogue (5). Nonetheless, analogues (9) bearing para-hydroxy moiety on ring C and methyl group at 4-position of ring B and (19) having para-Cl moiety on ring B and meta-methoxy group at 3-position of ring C, respectively, showed better potency when compared to analogues (5) and (8). Therefore, it can be concluded that addition of either hydroxyl group on para-position of ring C or –Cl group at 4-position of ring B leads to the enhanced enzymatic activity. In addition, analogue (9) showed superior activity than analogue (19) owing to involvement of hydroxyl group in H-bonding with hydrophilic residue of amino acid of targeted cholinesterase enzymes (Fig. 7).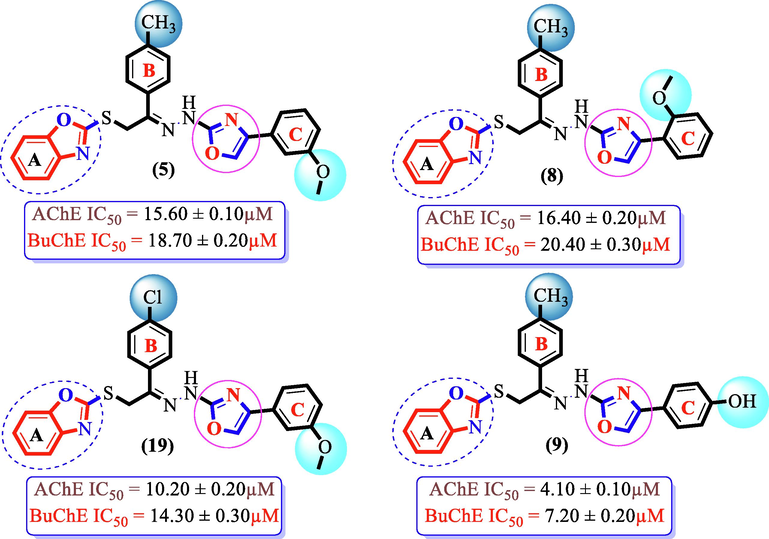
SAR study of analogues 5, 8, 9 and 19.
Here in this study, it was clearly indicated through the addition of either electron donating group (EDG) or electron withdrawing group (EWG) on both rings B &C, respectively, enhanced the enzymatic activity. Furthermore, the position as well as the number/s of substituents on both rings B and C also greatly affects the inhibition profile of targeted cholinesterase enzymes. The binding affinity between most active analogues and active site of enzymes were confirmed through molecular docking study.
2.3 Docking study
Molecular docking studies were done in order to investigate the better binding interaction of molecule in the protein complex. The protein was downloaded from the online source RCSB protein data bank (PDB) for both AchE and BchE. Prepared the protein and ligand in Auto dock tool where water molecules were removed, polar hydrogen and charges were added then the docking procedure were done by using command prompt. After completion different poses were obtained having varied binding affinity among the smallest one was chosen for visualization. Docking was performed against acetylcholinesterase and butyrylcholinesterase enzymes for biologically potent analogues 18, 15 and 6. In order to evaluate binding position on these analogues and how these analogs interact, molecular docking had been conducted. Molecular docking result for compound (18) showed that it can be interact and bind with acetylcholinesterase and butyrylcholinesterase enzymes and also compound (18) showed several key interactions with acetylcholinesterase including Trp286, Phe297, Tyr124, Tyr337, Phe338 and Tyr342 in (Fig. 8). Benzoxazole bearing compound stabilized the ligand-enzyme complex by forming pi-pi T-shaped interaction with tyr337 and tyr124. Besides that, both extended chlorobenzene and nitrobenzene moieties were stabilized through pi-alkyl and interacted with Tyr341, Trp286 and Leu289. Another interaction which further improves the torsional strain data is the interaction of sulfur with Phe338 and Phe297 which takes place through pi-sulphur. On the other hand, Trp286 forms pi-pi T-shaped interaction with oxazole moiety and pi-alkyl showed interaction with extended chlorobenzene ring which reduce the torsional strain and allows extended chlorobenzene molecule well fit in the cavity. Oxazole moiety is also being stabilized through carbon hydrogen bond interaction with Val294 and Ser293 in (Fig. 8). As for butyrylcholinesterase enzyme, hydrophobic π-π T-shaped and pi-pi stacking can be observing for compound (18) between amino acid residueTyr332 and benzoxazole moiety in (Fig. 10). Oxazole moiety forms a C—H bond with on the other hand, Ala199 and Pro285. The amino (NH) group acts as a H bond donor and forms conventional H bond donor shown interaction with Thr120 and Gly116. This amino (NH) linkage between oxazole and benzoxazole/chlorobenzene minimize the torsional strain and thus improving the flexibility of enhanced chlorobenzene moiety. The enhanced chlorobenzene moiety is being through pi-alkyl interaction with Try82. One of the oxygen of nitro group extended over the benzene ring can act as hydrogen bond donor to form H-bonding with Gly113, His438 and Gly116 and therefore enhances the butyrylcholinesterase inhibition profile. Besides that, extended nitrobenzene ring was stabilized by pi-pi T-shaped interaction with Trp231 and Phe329. The extended nitrobenzene ring stabilized through pi-alkyl interaction with Leu286 in (Fig. 8).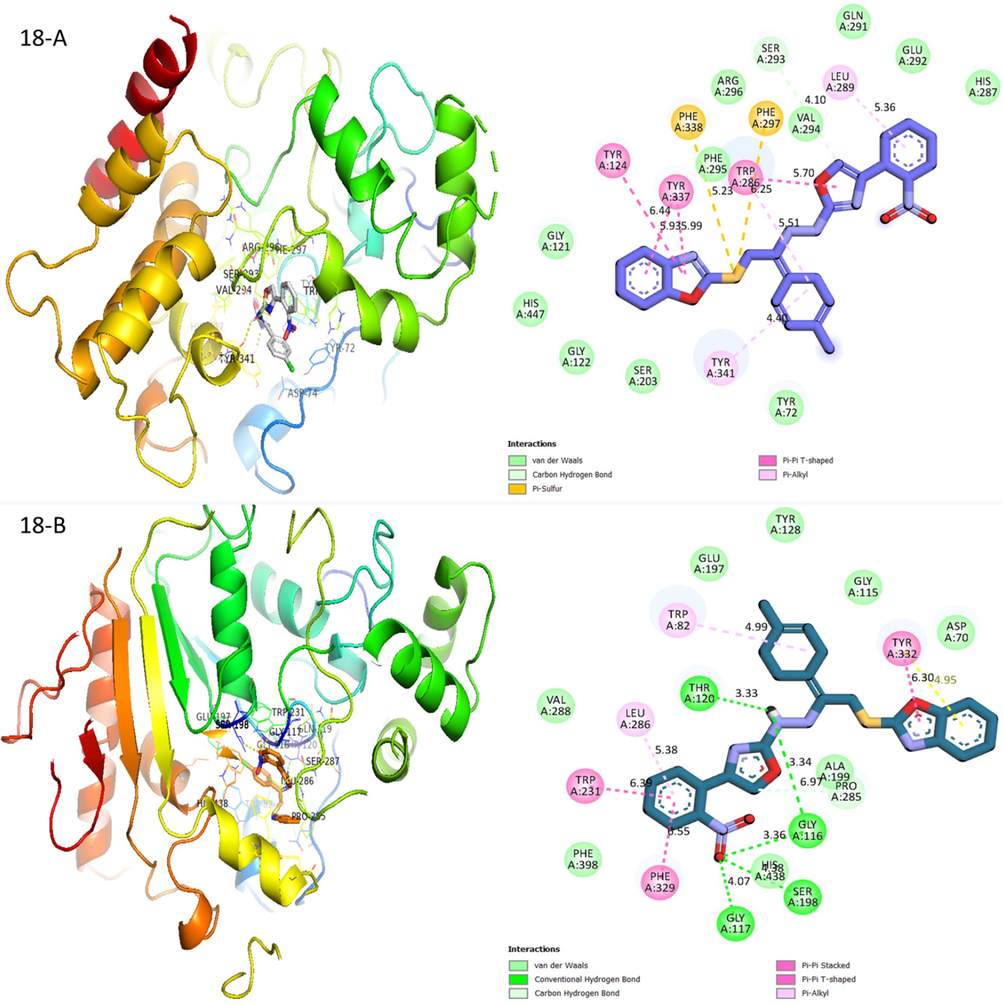
Protein-ligand interactions (PLI) profile of analogue-18: (A) for analogue-18 against acetylcholinesterase, while (B) against butyrylcholinesterase enzymes.
Showed less similar interaction against acetylcholinesterase, compound (15) showed minimum interaction when it compared with compound (18). Unlike compound (15), the sulfur linkage for compound (18) does not form interaction with any residue in the active site. The key skeleton of compound (15) which is the benzoxazole and oxazole moieties stabilized by two hydrophobic interactions that includes pi-pi stacking interaction with Trp341 and Trp286, while being further stabilized through Leu276 and Val294 through a hydrophobic π-alkylation interaction. Besides that, the extended 2,4-dichlorobenzene ring was stabilized through two separate hydrophobic pi-pi stacking interactions with Try341 and Trp154. One of chlorine atom of extended 2,4-dichlorobenzene ring forms alkyl interactions with Tyr347 and Tyr341. The Leu289 residue was also observed to form an alkyl interaction with para-methyl moiety of extended methyl substituted benzene. The oxazole ring is also stabilized through carbon hydrogen showed interaction with Val 294 in (Fig. 9). Likewise, docking result for compound (15) against butyrylcholinesterase (Fig. 9) displayed that binding orientation of the benzoxazole moiety is quite like compound (18). The benzoxazole moiety of compound (15) is also stabilized through various hydrophobic interactions that include pi-pi stacking interaction between benzoxazole moiety with Phe329 and amide-π stacking interaction that took place between fused oxazole rings of benzoxazole moiety with Gly116. The benzoxazole moiety is also stabilized through pi-alkylation interacted with Leu286. The extended para-substituted chlorobenzene is also stabilized through pi-pi stacking with Tyr332. Besides that, other extended 2,4-dichlorobenzene ring interact with Trp82 through π-π stacking interaction. Para-substituted chlorine on 2,4-dichlorobenzene ring further stabilizes the complex by forming a π-sigma interaction with Trp82 as shown in (Fig. 9).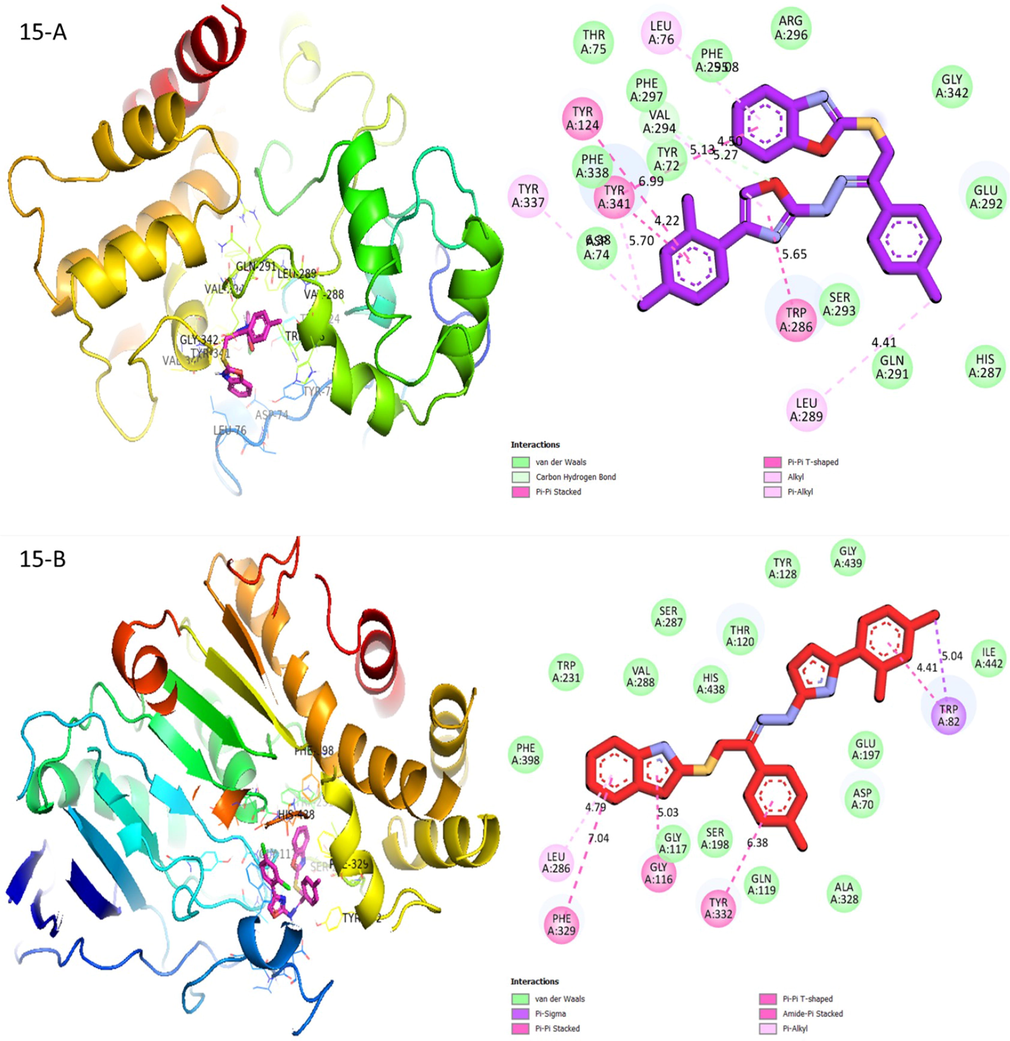
Protein-ligand interactions (PLI) profile of analogue-15: (A) for analogue-15 against acetylcholinesterase, while (B) for same analogue-15 against butyrylcholinesterase enzymes.
In the case of compound (6), amino linkage (NH) acts as hydrogen bond donor and forms a conventional hydrogen bond with amino acid residue Tyr341. Benzoxazole moiety is stabilized by two hydrophobic interactions that include π-π stacking interaction with Val294, while being further stabilized by Leu76 through a hydrophobic π-alkylation interaction. On the other hand, there is Leu289 which forms an alkyl interaction with the methyl group on para-position of extended benzene ring. Besides that, the extended 3,4-dichloro benzene ring is stabilized by π-π T shaped interactions with two various amino acid residue Trp286 and Tyr124. This extended ring is also being stabilized by π-alkylation interactions with Tyr341 and Tyr337 in (Fig. 10). As for compound (6) against butyrylcholinesterase, benzoxazole moiety forms π-π T shaped interactions with two various amino acid residues Trp82 and His438, while nitrogen of benzoxazole moiety is being further stabilized by conventional hydrogen bonding with residue His438 and therefore enhances the butyrylcholinesterase inhibition activity. The amino (NH) linkage between oxazole and benzoxazole moieties involves an electrostatic attractive charge interaction with Asp70. Oxazole moiety on the other hand, forms carbon hydrogen bond interaction with amino acid residue Gly117. The para-methyl group over extended benzene ring forms alkyl interaction with two separate amino acid residue Tyr332 and Pro285. The amino acid residue Tyr332 further stabilized the extended methylbenzene ring through π-π stacking interaction. The extended 3,4-dichlorobenzene ring is stabilized by hydrophobic interaction that include π-π stacking interaction with Phe329 and Gly116. The two chlorine atoms on meta- and para-position formed a π-π T shaped interaction with Leu286, Val288, Phe398 and His438. Para-chlorine is further stabilized by Trp231 through π-sigma interaction in (Fig. 10).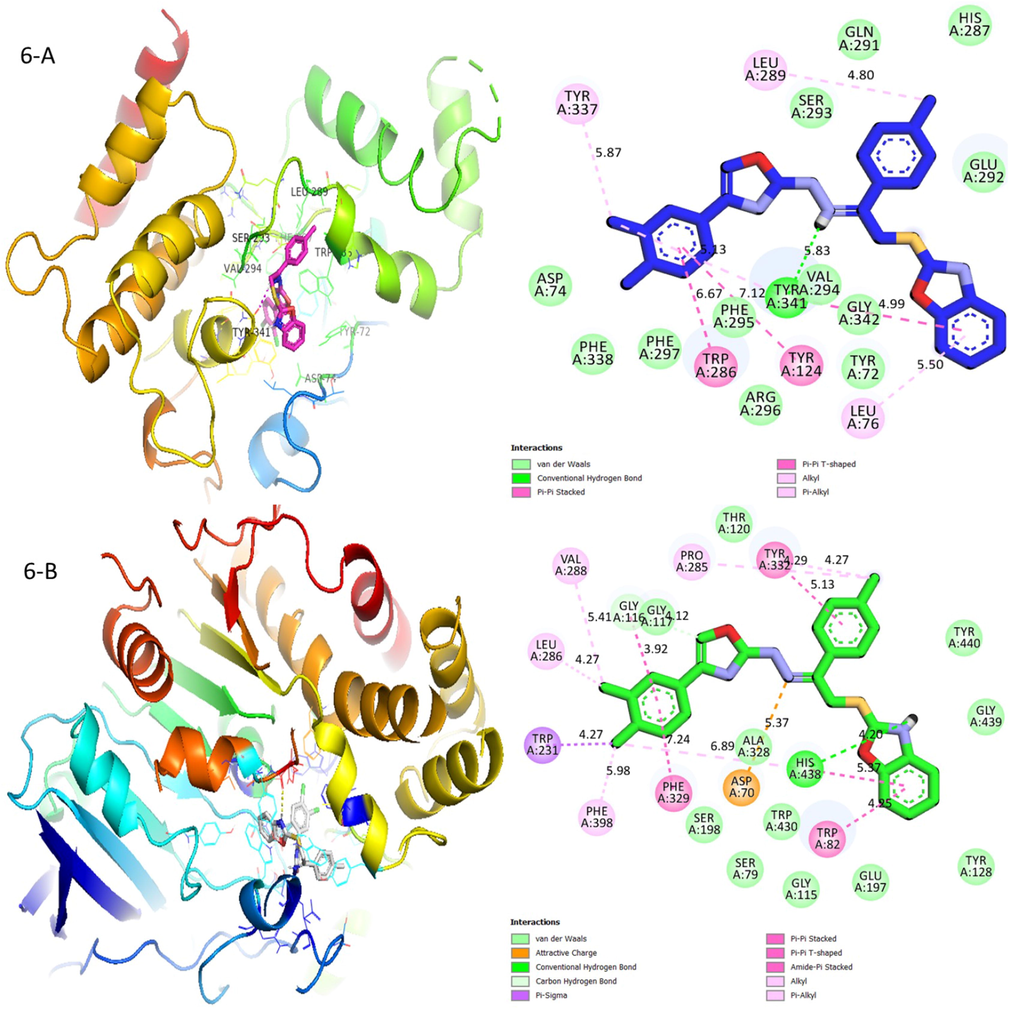
Protein-ligand interactions (PLI) profile of analogue-6: (A) for analogue-6 against acetylcholinesterase, while (B) for same analogue-6 against butyrylcholinesterase enzymes.
In addition, the docking results of selected compounds in comparison to Donepezil as standard drug, the binding energy was few folds better than standard drug and further these compounds (18, 15 and 6) established some additional interactions than interactions established by standard Donepezil drug. The interaction of standard drug against both AChE and BuChE enzymes are shown in 2d structures (Fig. 11). Binding interaction of subjected analogs were found with different values but the most selective one was analog-18 showing excellent binding affinity toward the targeted sites in both AchE and BchE cases. Whereas, the remaining compounds 6 and 15 were also found with better binding modes of interaction might be the attachment of substituents on varied position as shown in Table 2.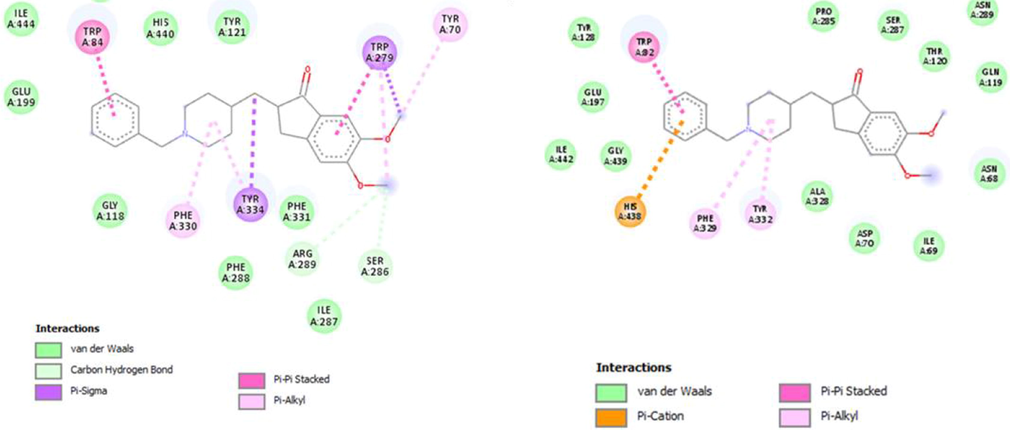
Binding interactions of standard Donepezil drug with acetylcholinesterase (left) and butyrylcholinesterase (right) enzymes and its 2D interaction diagram.
Active
AnaloguesTargeted enzymes
Receptors
Types ofInteractions
Bindingaffinities
Analogue-18
AChE
TYR-124
Pi-Pi T Shaped
−12.43Kcal/mol
TYR-334
Pi-Pi T Shaped
PHE-338
Pi-Sulphur
PHE-297
Pi-Sulphur
TRP-286
Pi-Pi T shaped
LEU-289
Pi-Alkyl
TYR-341
Pi-Alkyl
SER-293
Carbon HB
BuChE
TRP-231
Pi-Pi Stacked
−13.93Kcal/mol
LEU-286
Pi-Alkyl
PHE-329
Pi-Pi Stacked
GLY-117
HB
SER-198
HB
GLY-116
HB
THR-120
HB
TYR-332
Pi-Pi T Shaped
TRP-82
Pi-alkyl
Analogue-15
AChE
LEU-289
Pi-alkyl
−11.32Kcal/mol
TRP-286
Pi-Pi Stacked
TYR-341
Pi-Pi T Shaped
TYR-124
Pi-Pi Stacked
TYR-337
Pi-alkyl
TYR-341
Pi-alkyl
VAL-294
Carbon HB
LEU-76
Pi-alkyl
BuChE
LEU-286
Pi-alkyl
−10.96Kcal/mol
PHE-329
Pi-Pi T Shaped
GLY-116
Amide-Pi Stacked
TYR-332
Pi-Pi Stacked
TRP-92
Pi-Sigma
TRP-92
Pi-Pi Stacked
Analogue-6
AChE
TYR-337
Pi-Alkyl
−9.89Kcal/mol
TRP-286
Pi-Pi T Shaped
TYR-124
Pi-Pi T Shaped
TYR-341
Pi-Pi Stacked
TYR-341
HB
LEU-76
Pi-Alkyl
LEU-389
Pi-Alkyl
BuChE
VAL-288
Pi-Alkyl
−10.28Kcal/mol
LEU-286
Pi-Alkyl
TRP-231
Pi-Sigma
PHE-398
Pi-Alkyl
PHE-329
Pi-pi T shaped
GLY-116
Pi-pi T shaped
GLY-117
Carbon HB
ASP-70
Attractive Charge
HIS-438
HB
HIS-438
Pi-pi T shaped
TRP-82
Pi-pi T shaped
TYR-332
Pi-pi Stacked
PRO-285
Pi-alkyl
TYR-332
Pi-alkyl
Standarddonepezil
AChE
TRP-84
Pi-Pi Stacked
−8.33Kcal/mol
PHE-330
Pi-Alkyl
TYR-334
Pi-Alkyl and Pi-Sigma
TRP-279
Carbon HB
TYR-70
Carbon HB
ARG-289
Carbon HB
SER-286
Carbon HB
TYR-334
Alkyl and Pi-Sigma
BuChE
TRP-92
Pi-Pi Stacked
−8.93Kcal/mol
HIS-438
Pi-Cation
PHE-329
Pi-Alkyl
TYR-332
Pi-Alkyl
3 Material and methods
3.1 General information
Acetylcholinesterase (E.C.3.1.1.7) (Type VI-S, from electric eel and recombinant human enzyme)—and butyrylcholinesterase (E.C. 3.1.1.8) from equine serum were purchased from Sigma–Aldrich (Steinheim, Germany). Sigma-Aldrich was source from which the analytical-grade solvents and reagents, which were then applied without further purification. On the MAT 113D and MAT 312 mass spectrometers, electron impact (EI) was used to record mass spectra. The 1H and 13C NMR spectra on Advance Bruker (AM) spectrometers operating at 500 and 125 MHz were recorded. The coupling constants (J) are given in Hz, and the chemical shift values are given in ppm, relative to tetramethylsilane (TMS) as an internal reference. Singlet (s), doublet (d), triplet (t), doublet of doublets (dd), doublet of triplets (dt), quartet (q), or multiplet are the terms used to describe multiples (m). Aluminum plates with precoated silica gel were used for the thin-layer chromatography (TLC) procedure (Kieselgel 60, 254, E. Merck, Germany). TLC chromatograms were seen at 254 and 366 nm in ultraviolet light.
3.2 General procedure for the synthesis of benzoxazole based 1,3-oxazole analogues
In demand to synthesize the hybrid scaffolds of benzoxazole bearing 1,3-oxazole, initially, we took carbon disulphide (2 mL) and was added drop wise to stirred solution of 2-aminophenol (I) (1 equivalent) in ethanol (10 mL) under catalytic amount of triethylamine (0.5 mL) and resulting residue was allowed to put on stirring until the consumption of starting substrates was complete (monitored by TLC, 4hrs) to give 2-marcaptobenzoxazole (II). In the second step, the mixture of 2-marcaptobenzoxazole (II) (1 equivalent), the corresponding phenacyl bromide (1 equivalent) and Et3N (few drops) in ethanol (10 mL) was stirred for 3hrs at 25 °C to afford the synthesis of substrate (III). In the next step, the substrate (III) (1 equivalent) was subjected to dehydrative condensation upon sequential addition of semicarbazide ((1 equivalent) and glacial acetic acid (few drops) in ethanol (10 mL) and resulting mixture was stirred magnetically at 25 °C. After the completion of the reaction (conversion was monitored by TLC, 3hrs reflux), the solvent was evaporated to give benzoxazole based semicarbazone as intermediate product (IV).The intermediate product (IV) (1 equivalent) was subsequently converted to cyclic-oxazole on reaction with phenacyl bromide (1 equivalent) in ethanol (10 mL) while being stirred overnight at refluxing temperature. The finished product was dried over anhydrous sodium sulphate, concentrated, and purified by washing with n-hexane (15 mL), allowing for the manufacture of required hybrid benzoxazole scaffolds carrying 1,3-oxazole (1–19) in good to outstanding yield.
3.3 Spectral analysis
Provided in supplementary information.
3.4 Assay protocol for acetylcholinesterase inhibition
It has been carried out according to our previously reported work (Hussain et al., 2022a, 2022b).
3.5 Assay protocol for butyrylcholinesterase inhibition
It has been carried out according to our previously reported work (Hussain et al., 2022a, 2022b).
3.6 Assay protocol for molecular docking study
It has been carried out according to our previously reported work (Hussain et al., 2022a, 2022b, Khan et al., 2022).
4 Conclusion
In conclusion, we have afforded nineteen hybrids 1,3-oxazole-based benzoxazole analogues and evaluated them for their ability to inhibit acetylcholinesterase and butyrylcholinesterase. When compared to the standard donepezil, all analogues displayed varying degrees of acetylcholinesterase and butyrylcholinesterase inhibition. Among the series, analogues 6, 11, 15 and 18 having IC50 values1.70 ± 0.05, 0.90 ± 0.05, 2.10 ± 0.10 and 1.20 ± 0.05 µM respectively were identified as the most potent inhibitors of acetylcholinesterase enzyme. Six analogues 1, 3, 6, 11, 15, 18 having IC50 values 7.50 ± 0.20, 6.80 ± 0.10, 2.50 ± 0.10, 1.10 ± 0.10, 3.20 ± 0.20, 2.10 ± 0.10 µM respectively also displayed remarkable potency against butyrylcholinesterase enzyme. The remaining analogues also exhibited moderate to weak potency against cholinesterase enzymes. Spectroscopic techniques like HREI-MS, 1H NMR, and 13C NMR spectroscopy were used to characterize the newly synthesized analogues. In order to determine their potential mechanism of binding contacts with the enzyme active site, molecular docking studies were conducted on the most active analogues.
Acknowledgments
The authors extend their appreciation to the Researchers Supporting Project number (RSPD2023R628), King Saud University, Riyadh, Saudi Arabia for supporting this research, the authors also extend their appreciation to Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2023R342), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia for supporting this research.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Benzoxazole-appended piperidine derivatives as novel anticancer candidates against breast cancer. Bioorg. Chem.. 2023;134:106437
- [Google Scholar]
- Isolation and cholinesterase-inhibition studies of sterols from Haloxylon recurvum. Bioorg. Med. Chem. Lett.. 2006;16:573-580.
- [Google Scholar]
- The search for disease-modifying treatment for Alzheimer's disease. Neurology. 1997;48:35S-41S.
- [Google Scholar]
- Anti-helicobacter pylori, antioxidant, antidiabetic, and anti-Alzheimer’s activities of laurel leaf extract treated by moist heat and molecular docking of its flavonoid constituent, naringenin, against Acetylcholinesterase and Butyrylcholinesterase. Life. 2023;13:1512.
- [Google Scholar]
- Modification of benzoxazole derivative by bromine-spectroscopic, antibacterial and reactivity study using experimental and theoretical procedures. J. Mol. Struct.. 2017;1141:495-511.
- [Google Scholar]
- Synthesis and evaluation of antioxidant and antiproliferative activity of 2-arylbenzimidazoles. Bioorg. Chem.. 2020;94:103396
- [Google Scholar]
- High-performance thin layer chromatography method development, validation, and assessment of enzymatic inhibition potential of Potentilla fulgens hydroalcoholic roots extract. Separation Science Plus.. 2023;6:2300023.
- [Google Scholar]
- Benzoxazoles and oxazolopyridines in medicinal chemistry studies. Eur. J. Med. Chem.. 2015;97:778-785.
- [Google Scholar]
- Novel promising benzoxazole/benzothiazole-derived immunomodulatory agents: design, synthesis, anticancer evaluation, and in silico ADMET analysis. Arch. Pharm.. 2023;e2300097
- [Google Scholar]
- A new therapeutic target in Alzheimer's disease treatment: attention to butyrylcholinesterase. Curr. Med. Res. Opin.. 2001;17:159-165.
- [Google Scholar]
- Biological activity of 3-(2-benzoxazol-5-yl) alanine derivatives. Amino Acids. 2021;53:1257-1268.
- [Google Scholar]
- Synthesis and Molecular Docking of New Bis-Thiazolidinone-Based Chalcone Analogs as Effective Inhibitors of Acetylcholinesterase and Butyrylcholinesterase. Chem. Biodivers.. 2022;19:e202200323.
- [Google Scholar]
- Multipotent cholinesterase inhibitors for the treatment of Alzheimer’s disease: Synthesis, biological analysis and molecular docking study of benzimidazole-based thiazole derivatives. Molecules. 2022;27:6087.
- [Google Scholar]
- Benzoxazole based thiazole hybrid analogs: synthesis, in vitro cholinesterase inhibition, and molecular docking studies. Comput. Toxicol.. 2023;25:100253
- [Google Scholar]
- Preclinical pharmacology of metrifonate. Pharmacother.: J. Hum. Pharmacol. Drug Ther.. 1998;18:55-67.
- [Google Scholar]
- In silico studies of a novel scaffold of benzoxazole derivatives as anticancer agents by 3D-QSAR, molecular docking and molecular dynamics simulations. RSC Adv.. 2023;13:14808-14824.
- [Google Scholar]
- Design, synthesis and biological evaluation of 3-(2-aminooxazol-5-yl)-2 H-chromen-2-one derivatives. Chem. Cent. J.. 2018;12:1-13.
- [Google Scholar]
- Benzoxazole derivatives: design, synthesis and biological evaluation. Chem. Cent. J.. 2018;12:1-16.
- [Google Scholar]
- Coumarines isolated from angelica gigas inhibit acetylcholinesterase. Structure-Activity Relationships 대한약학회학술대회 2000:2263-2264.
- [Google Scholar]
- Effect of dietary zinc deficiency on acetylcholinesterase activity in the male wistar rat brain sub-regions. J. Experimental Zool. India. 2023;26
- [Google Scholar]
- New biologically potent benzimidazole-based-triazole derivatives as acetylcholinesterase and butyrylcholinesterase inhibitors along with molecular docking study. J. Heterocycl. Chem.. 2022;59:2225-2239.
- [Google Scholar]
- Synthesis, DFT studies, molecular docking and biological activity evaluation of thiazole-sulfonamide derivatives as potent Alzheimer’s inhibitors. Molecules. 2023;28:559.
- [Google Scholar]
- Global, regional, and national burden of Alzheimer's disease and other dementias, 1990–2019. Front. Aging Neurosci.. 2022;14:937486
- [Google Scholar]
- α7 Nicotinic acetylcholine receptor: a key receptor in the cholinergic anti-inflammatory pathway exerting an antidepressant effect. J. Neuroinflammation. 2023;20:84.
- [Google Scholar]
- Molecular and cellular biology of cholinesterases. Prog. Neurobiol.. 1993;41:31-91.
- [Google Scholar]
- New drug treatment for Alzheimer's disease: lessons for healthcare policy. BMJ. 1998;316:762-764.
- [Google Scholar]
- Widely spread butyrylcholinesterase can hydrolyze acetylcholine in the normal and Alzheimer brain. Neurobiol. Dis.. 2002;9:88-93.
- [Google Scholar]
- Anticholinesterases: medical applications of neurochemical principles. J. Neurochem.. 1995;64:1909-1918.
- [Google Scholar]
- Neurotransmitter systems in the etiology of major neurological disorders: emerging insights and therapeutic implications. Ageing Res. Rev. 2023101994
- [Google Scholar]
- Unexplained liver damage, cryptogenic liver cirrhosis, and steatohepatitis may be caused by latent chronic toxoplasmosis. J. Gastroenterol. Hepatol. Res.. 2018;8:2824-2840.
- [Google Scholar]
- Synthesis of new triazole-based thiosemicarbazone derivatives as anti-alzheimer’s disease candidates: evidence-based in vitro study. Molecules. 2022;28:21.
- [Google Scholar]
- Plant essential oils and their constituents for therapeutic benefits. Essential Oils: Extraction Methods and Applications 2023:977-1008.
- [Google Scholar]
- Cholinergic neurons in the basal forebrain are involved in behavioral abnormalities associated with Cul3 deficiency: role of prefrontal cortex projections in cognitive deficits. Transl. Psychiatry. 2023;13:22.
- [Google Scholar]
- Design, synthesis and cholinesterase inhibitory properties of new oxazole benzylamine derivatives. J. Enzyme Inhib. Med. Chem.. 2020;35:460-467.
- [Google Scholar]
- Synergistic and antagonistic interactions of anticholinesterase terpenoids in Salvia lavandulaefolia essential oil. Pharmacol. Biochem. Behav. 2003;75:661-668.
- [Google Scholar]
- New benzodiazepines alter acetylcholinesterase and ATPDase activities. Neurochem. Res.. 2000;25:949-955.
- [Google Scholar]
- Ginkgo extract or cholinesterase inhibitors in patients with dementia: what clinical trials and guidelines fail to consider. Phytomedicine. 2003;10:74-79.
- [Google Scholar]
- Cupper-catalyzed an efficient synthesis, characterization of 2-substituted benzoxazoles, 2-substituted benzothiazoles derivatives and their anti-fungal activity. Chem. Data Collect.. 2020;27:100362
- [Google Scholar]
- Diagnosis and treatment of Alzheimer disease and related disorders: consensus statement of the American Association for Geriatric Psychiatry, the Alzheimer's Association, and the American Geriatrics Society. J. Am. Med. Assoc.. 1997;278:1363-1371.
- [Google Scholar]
- Design and development of some phenyl benzoxazole derivatives as a potent acetylcholinesterase inhibitor with antioxidant property to enhance learning and memory. Eur. J. Med. Chem.. 2019;163:116-135.
- [Google Scholar]
- Isolation, structure elucidation and biological activity of natural products. MDPI. 2023;28:5392.
- [Google Scholar]
- 1, 2, 4-Triazole-based benzothiazole/benzoxazole derivatives: Design, synthesis, p38α MAP kinase inhibition, anti-inflammatory activity and molecular docking studies. Bioorg. Chem.. 2018;81:630-641.
- [Google Scholar]
- Biological evaluation and docking studies of some benzoxazole derivatives as inhibitors of acetylcholinesterase and butyrylcholinesterase. Zeitschrift für Naturforschung C.. 2016;71:409-413.
- [Google Scholar]
- Rational design of a drug for Alzheimer's disease with cholinesterase inhibitory and neuroprotective activity. Chem. Biol. Interact.. 2008;175:216-221.
- [Google Scholar]
- Alzheimer's disease and senile dementia: loss of neurons in the basal forebrain. Science. 1982;215:1237-1239.
- [Google Scholar]
- A patent review on the current developments of benzoxazoles in drug discovery. ChemMedChem. 2021;16:3237-3262.
- [Google Scholar]
- Prevention of tolerance to the organophosphorus anticholinesterase paraoxon with carboxylesterase inhibitors. Biochem. Pharmacol.. 1998;55:1419-1426.
- [Google Scholar]
- Cortical connectivity of cholinergic basal forebrain in Parkinson’s disease with mild cognitive impairment. Quant. Imaging Med. Surg.. 2023;13:2167.
- [Google Scholar]
- Recent advance in oxazole-based medicinal chemistry. Eur. J. Med. Chem.. 2018;144:444-492.
- [Google Scholar]
- Acid-Promoted multicomponent tandem cyclization to synthesize fully substituted oxazoles via Robinson–Gabriel-type reaction. J. Org. Chem.. 2017;82:6450-6456.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2023.105244.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary Data 1
Supplementary Data 1







