Translate this page into:
Theoretical insight and experimental exploration of indole extraction from wash oil with deep eutectic solvents
⁎Corresponding authors. lan@wust.edu.cn (Lan Yi), ying@tyut.edu.cn (Wen-Ying Li)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The extraction of indole from wash oil is of economic value and practical significance. In this study, deep eutectic solvents (DESs) are investigated to extract indole from wash oil. Initially, density functional theory calculations were used to explore the association of indole with aromatic compounds in wash oil, and the feasibility of indole extraction with a tetraethylammonium chloride/p-toluenesulfonic acid (TEAC/2TsOH) DES. Then, the influence of the temperature, stirring time, DESs dose, initial indole concentration, and amount of water added were investigated, followed by discussions on the comparison with other extractants and the performance of extracting of indole from real wash oil. The results show that indole associates with aromatic compounds in wash oil through π−π interactions, but that a large electrostatic interaction energy of −52.8 kJ/mol between indole and the DESs is the main driving force for the extraction process. Under optimal conditions, TEAC/2TsOH extracts more than 63.5 ± 3% of indole from wash oil, and its extraction capacity is superior to other extractants.
Keywords
Solvent extraction
Indole
Association structure
Intermolecular interactions
Deep eutectic solvents
Nomenclature
- BSSE
-
basis set superposition error
- ChCl
-
choline chloride
- DESs
-
deep eutectic solvents
- DFT
-
density functional theory
- EDA-FF
-
energy decomposition analysis based on forcefield
- ESP
-
electrostatic potential
- FA
-
formic acid
- GC
-
gas chromatography analysis
- IGMH
-
independent gradient model based on Hirshfeld partition
- KOH
-
potassium hydroxide
- LOLIPOP
-
localized orbital locator integrated π over plane
- OA
-
oxalic acid
- Sign(λ2)ρ
-
the product of the sign (λ2) function, which is the sign of the second largest eigenvalue λ2 of the electron density Hessian matrix, multiplied by the value of the electron density
- TEAC
-
tetraethylammonium chloride
- TsOH
-
p-toluenesulfonic acid
Abbreviation
1 Introduction
Indole is an important organic raw chemical widely used in the production of spices, dyes, amino acids, and pesticides (Ji et al., 2018; Jiao et al., 2022; Zhang et al., 2022). Coal tar, which is the liquid product of coal pyrolysis, contains 0.1 ∼ 0.2 wt% of indole (Ma et al., 2021) and the extraction of indole from coal tar not only obtains valuable indole resources, but also reduces the hydrogen consumption of direct hydrogenation of coal tar. At present, the conventional method to extract indole from coal tar is to first distill the coal tar and enrich the indole in the wash oil fraction (230 ∼ 300 °C) in indole, and then recover indole from the wash oil by alkali fusion or acid polymerization (Gao, 2010). This process leads to issues such as the discharge of dangerous wastewater, and the use of a large amount of KOH as solvent (1.2 kg to extract 1 kg of indole (Gao, 2010), which increases its environmental burden and economic cost.
To overcome the above-mentioned disadvantages, supercritical fluid extraction (Yanagiuchi et al., 1993), high-pressure crystallization (Yamamoto et al., 1991), and inclusion in cyclodextrin (Makoto and Akinori, 1992) have been applied to extract indole from wash oil. These methods bring other challenges in extracting indole: Supercritical fluid extraction requires expensive equipment, high-pressure crystallization is carried out under harsh conditions, and cyclodextrin solutions are difficult to prepare. Compared with the above methods, liquid–liquid extraction is a common separation method in the chemical industry, and due to its non-reactive physical nature and mild operating conditions, it is widely present in the extraction of indole. The separation efficiency in liquid–liquid extraction depends on the design and the selection of solvents. Thus, it is critical to develop new green extraction agents for liquid–liquid extraction.
Recently, the development of ionic liquid-based indole separation technologies (Xu et al., 2019; Zhang et al., 2022; Gao et al., 2023; Zhang et al., 2021) has made remarkable progress. For example, 1,1,3,3-tetramethylguanidine lactate (Zhang et al., 2021) and 1-butyl-1-methyl morpholinium thiocyanate (Gao et al., 2023) have been widely used to extract indole. Optimal indole extraction conditions were determined, but the selectivity for indole was not high; the relatively high cost and complex synthesis of ionic liquids also limit the large-scale implementation of these processes (Jiao et al., 2022; Zhang et al., 2022). At the same time, deep eutectic solvents (DESs) (Abbott et al., 2003), acknowledged as ionic liquid analogues, have emerged and attracted momentous attention. DESs are composed of hydrogen bond donors (e.g., amides, glycols, polyols, organic acid) and hydrogen bond acceptors (e.g., amino acids, betaines, quaternary ammonium or phosphine salts) (Makoś-Chełstowska, 2023), and they can be designed for specific purposes. In addition to some of the characteristics of ionic liquids (including renewability, non-flammability, etc.), DESs also have the advantages of a low ecological footprint, attractive price, and simple preparation process (Makoś-Chełstowska, 2023). Therefore, the applications of DESs in various fields proliferate like bamboo shoots after a spring rain, among others in catalysis, separation, organic synthesis, and electrochemistry (Prabhune and Dey, 2023). In the upsurge of applications of DESs, it was discovered that DESs exhibit excellent performance in extracting various aromatic compounds from coal tar or fuel, such as heterocyclic nitrogen/sulfur compounds (Wang et al., 2022; Mohammed et al., 2022), aromatic hydrocarbons (Ge et al., 2022; Ren et al., 2022), and phenolic compounds (Yi et al., 2019; Liu and Zhang, 2022). In contrast, there are few studies on the extraction of indole from wash oil by DESs, including the formation of DESs from quaternary ammonium salts and indole (Ji et al., 2018; Jiao et al., 2022), and the direct extraction of indole with ethylene glycol-based DESs (Zhang et al., 2022). To the best of our knowledge, little attention has been paid to association structures formed by indole and wash oil or extraction agents, and the extraction mechanism for indole was generally assumed to be hydrogen bond between indole and the extraction agent.
The components present in wash oil are complex, and indole and other aromatic compounds can associate via C−H···π or π−π interactions (Faisal et al., 2021). The solvent-based liquid–liquid extraction of indole is a phase transfer process in which association structures formed by indole and wash oil molecules are destroyed and replaced with association structures of indole and the extractant. The reorganization of association structures in the separation process is bound to be accompanied by changes in electrostatic interaction energy and dispersion interaction energy for the system. Clarifying the intrinsic nature and the evolution of these structure, and the intermolecular interactions present is the key to achieving the efficient extraction of indole. To explore the rules governing the formation and reorganization of structures in the extraction of indole, and to achieve high efficiency and selectivity of indole extraction, it is urgent to explore the mechanism of solvent extraction of indole in wash oil from the perspective of intermolecular interactions.
Quaternary ammonium salts, such as tetraethylammonium chloride (TEAC) are readily available and degradable. p-Toluenesulfonic acid (TsOH) contains a benzene ring and a strong acid group, which may form π−π and hydrogen bonds with indole, respectively. Therefore, the TEAC/2TsOH DESs system was selected to extract indole from wash oil in this study. We first explored the association structures formed by indole with aromatic compounds in wash oil, and analyzed the interactions between indole and TEAC/2TsOH with quantum chemical calculations, to investigate the possibility of extracting indole from wash oil using a TEAC/2TsOH DESs. After confirming feasibility, the influence of the conditions including the temperature, stirring time, DESs dose, initial indole concentration, and amount of water added, on the extraction performance were investigated to optimize the conditions. The reutilization of the DESs and the comparison of its extraction capacity with other methods were also considered. Finally, the extraction of indole from real wash oil by the DESs was examined. This efficient extraction system has great potential for applications in the extraction of heterocyclic nitrogen-containing compounds.
2 Experimental procedures
2.1 Reagents
Analytically pure indole, naphthalene, quinoline, acenaphthylene, choline chloride (ChCl), tetraethylammonium chloride (TEAC) and anhydrous formic acid (FA) were provided by the Shanghai Aladdin Biochemical Technology Co. Analytically pure toluene, anhydrous oxalic acid (OA), and p-toluenesulfonic acid (TsOH) monohydrate were provided by the China Pharmaceutical Group Chemical Reagent Co. The 20 wt% ammonium bisulfate aqueous solution was provided by the Tianjin Zhiyuan Chemical Reagent Co. With the exception of the ammonium bisulfate solution, the materials were dried before use. The water used was deionized. The real wash oil at 230 ∼ 250 °C was supplied by one steel factory, Wuhan, China, and its composition was detected by GC–MS (TRACE1300 ISQ Series, Thermo Fisher Scientific (China) Co). The composition of real wash oil is shown in Table S1.
2.2 Preparation of DESs and model wash oil
Preparation of DESs. The DESs systems used herein were prepared from TEAC or ChCl as hydrogen bond acceptor, and TsOH, FA, or OA as hydrogen bond donor. The preparation of TEAC/2TsOH is described herein as an example. TEAC and TsOH were added to a covered flask in a molar ratio of 1:2, and magnetically stirred at 90 °C until a uniform liquid was obtained. 1H NMR results indicated the formation of hydrogen bonding between TEAC and TsOH (Fig. S1).
The TEAC/2FA, TEAC/OA, and ChCl/2TsOH DESs were prepared in the same way. All the prepared DESs were kept in a desiccator to prevent the absorption of moisture from the air.
Preparation of model wash oil. The composition of wash oil is complex, with bicyclic and tricyclic aromatic hydrocarbons, and nitrogen-containing compounds as main components. Herein, indole, naphthalene, acenaphthylene, and quinoline were selected as representative components to prepare the model wash oil. With the exception of quinolone, all the three compounds are solids at room temperature. Toluene is one of the important components of coal tar and has a very small solubility in DES (Larriba et al., 2018). Therefore, toluene was chosen as the solvent to dissolve the above four compounds for the preparation of the model wash oil. To this end, 10 g of indole, 30 g of naphthalene, 35 g of acenaphthylene, and 5 g of quinoline were added to a beaker and dissolved in a small amount of toluene until the mixture was clear. The mixture was transferred to a 250-mL volumetric flask and diluted with toluene to obtain a 40 g/L indole solution. Model oils with indole concentrations of 15, 25, and 60 g/L were prepared by the same method.
2.3 Separation process
Extraction of indole. A separation experiment was completed as follows: Equal mass of DESs was loaded in a graduated test tube with the model wash oil and a magnetic stirring rotor. The tube was placed in a water bath at a set temperature and stirred for 30 min. The tube was then allowed to settle at the water bath, and soon two layers were clearly visible. The upper layer was wash oil with the indole removed, and the lower layer was the DESs containing the indole. The volumes of upper and lower layer were recorded as VU and VL, respectively. These two layers were separated in a separatory funnel, and a small aliquot of the upper layer was removed for further analysis. The extraction process used was identical when TEAC alone was used to extract indole. In addition to replacing the tube with a beaker, the extraction process of indole from the real wash oil was the same as that of the model wash oil. A small amount of the upper layer was taken in a beaker and the pH was measured followed by the addition of AgNO3. No change in pH or white precipitation was found, indicating that tetraethylammonium chloride and p-toluenesulfonic acid were not present in the residue of model or real wash oil.
Regeneration of DESs. Deionized H2O was applied to regenerate the DESs. Twice the mass of deionized water was added to the DESs phase and the eutectic mixture was stirred at 45 °C for 20 min. The yellowish-brown crude indole settled at the test tube bottom. The DESs aqueous solution was assembled and subjected to rotary evaporation at 60 °C. The regenerated DESs and recovered deionized H2O were stored for reuse.
Refining of indole. The yellowish-brown crude indole may contain a small amount of quinoline, which was removed by adding an equal volume of 20 wt% ammonium bisulfate aqueous solution. The mixture, stirred magnetically at room temperature for 30 min, then separated into two layers after standing for 10 min. The upper layer contained the refined indole, which was dried and analyzed. The same refining process was applied when using TEAC alone to extract the indole.
2.4 Analysis methods
The indole content in the model wash oil was measured by gas chromatography analysis (GC, PerkinElmer Clarus 580), using an HP-5 capillary column (60 m × 0.25 mm × 0.25 mm) and a flame ionization detector. High purity nitrogen served as carrier gas. The operating temperature was 280 °C for the injector and 300 °C for the detector, and the split ratio was 5:1. The heating program was to maintain an initial temperature of 80 °C for 1 min, then raise it to 220 °C at a rate of 10 °C/min and hold for 3 min. o-Nitrotoluene was an internal standard substance, and the calibration curve was shown in Fig. S2.
The separation performace for indole was represented by the extraction efficiency and the distribution coefficient, calculated from equations (1) and (2), respectively.
FTIR spectroscopy (Bruker Tensor 27 spectrometer) was conducted in the wavenumber range of 400–4000 cm−1. 1H NMR spectroscopy (Agilent 600 spectrometer) was conducted at 25 °C with a 1H sensitivity of ≥ 500:1.
2.5 DFT calculations
Density functional theory (DFT) calculations were performed using the Gaussian 09 program package (Frisch et al., 2009). The optimization of the structure and frequency analysis for the compounds and the conformations were performed at the M06-2X/6–31+G(d,p) level. The optimized structures without imaginary frequencies corresponded to the minimum energy structures. The single point energy was calculated at the level of M06-2X/6–311++G (d,p) with consideration of the counterpoise procedure (Yi et al., 2021) (basis set superposition error, BSSE). The interaction energy (
E) was calculated with equation (3), where E(A⋅⋅⋅B), E(A) and E(B) represent the energies of complex AB, compound A and compound B, respectively.
The localized orbital locator integrated π over plane (LOLIPOP) index (Lu and Chen, 2020) was used to quantify the π-stacking ability of aromatic compounds, including indole, toluene, naphthalene, quinoline, and acenaphthylene. The independent gradient model based on Hirshfeld partition (IGMH) (Lu and Chen, 2022) was used to visualize the interactions between indole and the other aromatic compounds or TEAC/2TsOH. Energy decomposition analysis based on forcefield (EDA-FF) (Lu et al., 2021) was used to analyze the non-bonded interactions between indole and the other aromatic compounds or TEAC/2TsOH. Electrostatic potential (ESP) (Qu et al., 2022) analysis was performed to determine the electrophilic and nucleophilic sites of indole and TEAC/2TsOH. The wave function was analyzed using Multiwfn 3.8 (Lu and Chen, 2012).
3 Results and discussion
3.1 Association structures of indole with aromatic compounds
The components in wash oil are mostly aromatic compounds, with the electrons in the aromatic rings delocalized to form a conjugated system. Bicyclic and tricyclic aromatic hydrocarbons (naphthalene, acenaphthylene) are isoelectronic conjugated systems; quinoline is also an isoelectronic conjugated system, but the lone pair electrons of nitrogen participate in the conjugated system, which increases its electron density. In contrast, indole is a multi-electron conjugated system. The π−π interactions are one of the most important intermolecular interaction in aromatic compounds with a conjugated system. The localized orbital locator integrated π over plane (LOLIPOP) (Lu and Chen, 2020) method is useful to measure the π-stacking ability of aromatic systems. A ring with a smaller LOLIPOP value has stronger π-depletion (namely lower π-delocalization), and hence shows stronger π-stacking ability. The calculated LOLIPOP values and delocalized π electron orbitals for indole, toluene, naphthalene, quinoline, and acenaphthylene are compared in Fig. 1. There are three disconnections (shown with the red arrows in Fig. 1a) in the π-electron delocalized orbital of the indole six-membered ring, while that in the five-membered ring is continuous, indicating that the six-membered ring has lower π-delocalization than the five-membered ring. The calculated LOLIPOP values for indole also illustrate this result: The value for the six-membered ring (4.1) is lower than for the five-membered ring (4.6), showing that the six-membered ring of indole has better π-stacking ability than the five-membered ring. According to their LOLIPOP values, the π-stacking ability decreases in the order quinoline (2.7 ∼ 4.0) > naphthalene (3.7) > indole (4.1 ∼ 4.6) > acenaphthylene (6.9 ∼ 8.0) > toluene (8.5).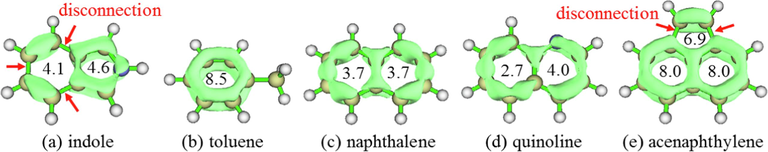
LOLIPOP analysis of indole and other aromatic compounds (: H,: C).
The independent gradient model based on Hirshfeld partition (IGMH) (Lu and Chen, 2022), an important graphical method to display intermolecular interactions, was used to visualize the interactions of indole with toluene, quinoline, naphthalene, and acenaphthylene, and the colored isosurface results of IGMH analysis are shown in Fig. 2. The isosurfaces represent regions where interactions occur. Since toluene contains a methyl group and a benzene ring, it forms C−H···π and π−π interactions with indole (Fig. 2a). The three other aromatic compounds, namely naphthalene, quinoline, and acenaphthylene, form face-to-face π−π interactions with indole (Fig. 2b to 2d). Obviously, indole mainly forms association structures with aromatic compounds in wash oil through π−π interactions. According to the isosurface area representing the interaction strength, the interactions between indole and toluene are weakest and those between indole and acenaphthylene are strongest.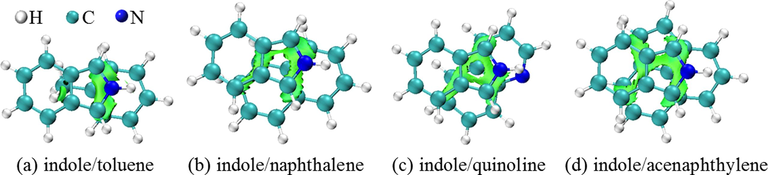
IGMH analysis of interactions between indole and other aromatic compounds (Isovalue = 0.009).
The nature of intermolecular interactions is divided into electrostatic, dispersion and repulsive interactions, among which electrostatic and dispersion interactions are attractive (Emamian et al., 2019). Energy decomposition analysis based on force field (EDA-FF) (Lu et al., 2021) was used to reveal the nature of interactions between indole and the aromatic compounds present in model wash oil, and the results are presented in Fig. 3. The electrostatic interaction energy between indole and the aromatic compounds (toluene, naphthalene, quinoline, and acenaphthylene) is less than −5.0 kJ/mol, and the dispersion interaction energy is distributed from −47.7 to −73.5 kJ/mol, indicating that association between indole and the aromatic compounds in wash oil is mainly induced by dispersion interactions. Therefore, the extraction of indole should overcome these dispersion interactions.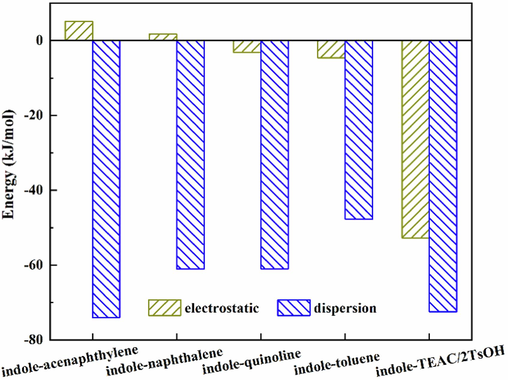
Energy decomposition analysis of interactions between indole and aromatic compounds.
3.2 Interactions between indole and TEAC/2TsOH
Molecular electrostatic potential (ESP) analysis is a useful tool to predict the nucleophilic and electrophilic sites in molecules (Qu et al., 2022). The strength and orientation of intermolecular interactions, such as hydrogen bonds and π-π interactions, can be well-predicted and explained by analyzing the magnitude and positions of minimum and maximum ESP. The ESP analysis of indole and TEAC/2TsOH was performed, as shown in Fig. 4. For indole, the minimum ESP (−100.1 kJ/mol) and maximum ESP (225.0 kJ/mol) sites appear on the benzene ring and the H atom connected to the N atom, respectively, indicating that the electron-rich benzene ring and the electron-deficient H in indole molecule can interact easily with other molecules. For TEAC/2TsOH, there are three ESP minimum sites (−172.4, −180.1, and −182.1 kJ/mol) and three ESP maximum sites (147.3, 149.5, and 153.3 kJ/mol) that can interact with other molecules. These results indicate that the contact of indole with TEAC/2TsOH may be most favorable when the benzene ring of indole is close to the choline cation of TEAC/2TsOH, and when the −NH of indole is close to the Cl or O atoms of TEAC/2TsOH. This was confirmed by structural optimization of the conformation (Fig. 5), that is, the benzene ring approaching the choline cation and −NH approaching Cl atom make the complex most stable. The indole-TEAC/2TsOH complex has five hydrogen bonds, including four C−H···C (ranging from 2.53 to 2.87 Å) and one N−H···Cl (2.44 Å), which are illustrated by black dashed lines in Fig. 5. Incidentally, the sum of the Bondi radii of C and H is 2.90 Å, while that of H and Cl is 2.95 Å (Yi et al., 2019). To visualize graphically the interactions between indole and TEAC/2TsOH, IGMH analysis was performed and the result is shown in Fig. 6. The interactions between −NH and the Cl ion are stronger than those between the benzene ring and the choline cation, because the value of sign(λ2)ρ between −NH and the Cl ion ranges from −0.02 to −0.01 a.u, while that between the benzene ring and the choline cation is near −0.01 a.u. The farther the value of sign(λ2)ρ is from zero, the stronger the interactions (Lu and Chen, 2022).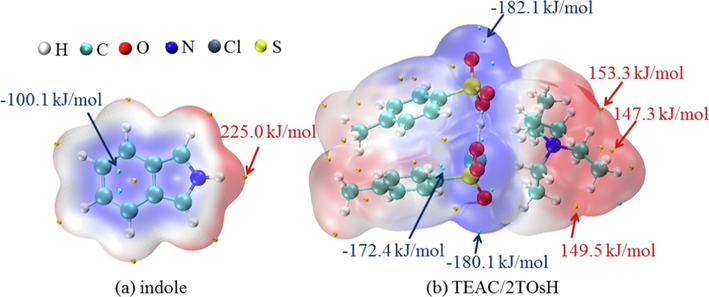
ESP analysis of indole and TEAC/2TsOH.
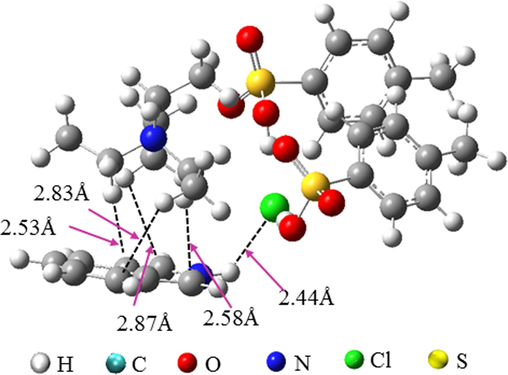
Optimized complex structure of indole with TEAC/2TsOH.
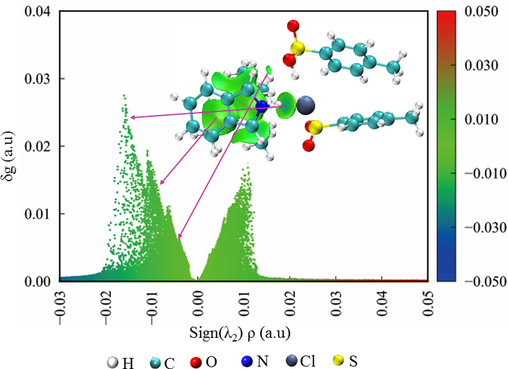
IGMH analysis of interaction between indole and TEAC/2TsOH (Isovalue = 0.005).
There are more hydrogen bonds and C−H···π interactions between indole and TEAC/2TsOH than between indole and the aromatic compounds in wash oil. Accordingly, the results of energy decomposition analysis (Fig. 6) show that the electrostatic interaction energy between indole and TEAC/2TsOH is −52.8 kJ/mol, which is 10 ∼ 30 times that between indole and the other compounds in wash oil. The dispersion interaction energy is −72.5 kJ/mol, which is equivalent to the maximum dispersion interaction energy (−73.1 kJ/mol) between indole and the compounds in wash oil. The multiple interaction sites and larger interaction energy imply that it is feasible to extract indole from wash oil with TEAC/2TsOH DES.
3.3 Extraction performance of indole by TEAC/2TsOH
Temperature. Temperature is an important parameter in liquid–liquid extraction. Five operation temperatures were selected herein to assess the influence of temperature on the extraction of indole, and the results are provided in Fig. 7a. As shown, the extraction efficiency of indole was not sensitive to temperature and remained almost constant at 96.8 ± 2% in the temperature range studied. This temperature-independent extraction efficiency indicates that the separation process is nonreactive, in agreement with results for the separation of nitrogen/sulfur/oxygen-containing compounds in coal-based liquids or fuel by DESs (Yi et al., 2019; Xu et al., 2022; Wang et al., 2022). Meanwhile, the concentration of indole in wash oil remained low (1.2 ± 1 g/L) at the different temperatures. From an economic viewpoint, a temperature of 30 °C would be optimal and was used in subsequent investigations.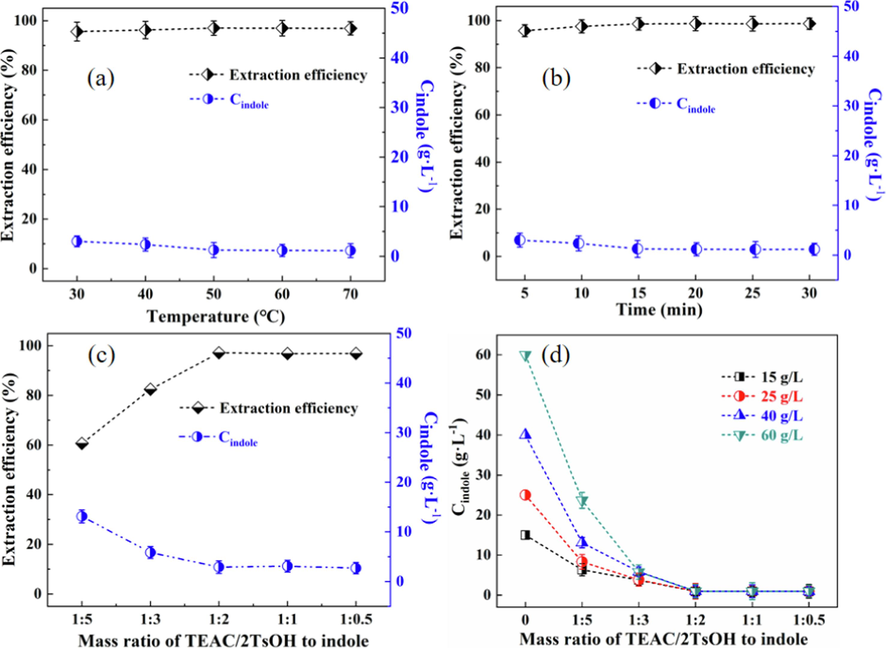
Effects of experimental conditions on the extraction performance of indole: (a) extraction temperature; (b) extraction time; (c) mass ratio of TEAC/2TsOH to indole; (d) initial concentration of indole in the wash oil.
Stirring time. The stirring time is another important parameter in liquid–liquid extraction. The shorter the time to reach equilibrium, the more conducive is the process to large-scale use. To assess the influence of time, the stirring time was varied from 5 to 30 min, and the results obtained are summarized in Fig. 7b. As can be seen in the figure, there was only a slight increase in indole extraction efficiency for increasing stirring time, indicating that indole can be transferred quickly from the wash oil to the TEAC/2TsOH DES. This result is due to the stronger interactions between indole and TEAC/2TsOH than between indole and the aromatic compounds in wash oil (Fig. 3). Correspondingly, the content of indole in wash oil only decreased slightly from 3.0 to 1.2 ± 1 g/L as the stirring time increasing from 5 to 30 min. To separate indole quickly and maintain a balanced separation procedure, the optimal stirring time was set to 20 min.
Mass ratio of TEAC/2TsOH to indole. The influence of the mass ratio of TEAC/2TsOH to indole on the separation performance was investigated in the range of 1:5 ∼ 1:0.5 and the results are presented in Fig. 7c. As expected, increasing the mass of TEAC/2TsOH used increased the extraction efficiency of indole; when a mass ratio of TEAC/2TsOH to indole below 1:2, the amount of TEAC/2TsOH was insufficient to extract all the indole. For mass ratios from 1:2 to 1:0.5, the extraction efficiency remained almost unchanged at 97.2 ± 2%, and the content of indole in wash oil only decreased from 13.1 to 2.2 ± 1 g/L. On the basis of the good extraction efficiency and the favorable distribution coefficient, a 1:2 mass ratio was deemed optimal.
Initial indole concentration. To assess the extraction performance of TEAC/2TsOH for wash oil with different indole concentrations, the influence of the initial indole concentration on the extraction efficiency was analyzed in a range from 15 to 60 g/L. The results presented in Fig. 7d show that when the amount of TEAC/2TsOH was insufficient, that is, the mass ratio of TEAC/2TsOH to indole was below 1:2, the residual indole content in the oil phase after extraction increased with the increasing of initial indole concentration in the wash oil. However, when the mass ratio of TEAC/2TsOH to indole was above 1:2, the wash oil with different initial indole contents eventually contained the same residual concentration of indole, around 1.0 ± 1 g/L. These results indicate that when the amount of TEAC/2TsOH used is sufficient, the extraction performance is not correlated with the initial indole concentration, demonstrating that TEAC/2TsOH can be applied to wash oils with different initial indole concentrations.
Adding water to TEAC/2TsOH. The influence of H2O on the extraction of indole using TEAC/2TsOH is illustrated in Fig. 8. It can be seen that the extraction efficiency of anhydrous TEAC/2TsOH is highest, reaching 97.2 ± 2%. For increasing amounts of H2O added from 2 ∼ 10 wt%, the indole extraction efficiency decreased rapidly from 91.4 to 37.6 ± 1%. For 10 ∼ 60 wt% of H2O addition, the extraction efficiency decreased more slowly but was essentially lost at 60 wt%. Kaur et al. (Kaur et al., 2020) suggested that H2O has a great influence on hydrogen-bonded DESs systems. The native hydrogen-bonded structure of the pure DESs is destructed upon dilution, and more or less destroyed to form an aqueous solution when the H2O content reaches a certain level (> 40%). Because indole is insoluble in water, the TEAC/2TsOH aqueous solution cannot extract it from the wash oil, resulting in the indole extraction efficiency dropping to zero at 60 wt% of H2O content.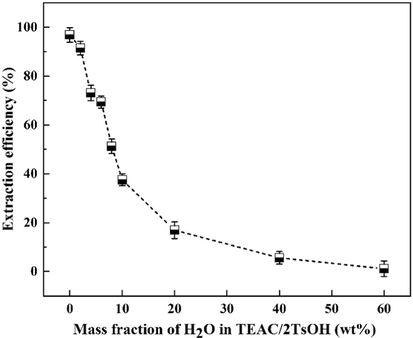
Influence of water addition on the extraction efficiency of TEAC/2TsOH.
3.4 DESs recycling
DESs regeneration is usually performed with organic solvents, such as ethyl acetate (Yi et al., 2019) and diethyl ether (Qu et al., 2022), both toxic and highly flammable. The experimental results reported above demonstrated that TEAC/2TsOH has no indole extraction ability when the H2O content in TEAC/2TsOH reaches 60 wt%. The TEAC/2TsOH DES regenerated with H2O instead of organic solvents was applied to separate indole under the same conditions for three additional cycles. The separation performance of TEAC/2TsOH in the successive cycles, provided in Fig. 10a, decreased slightly from 97.2 ± 2% to 95.3 ± 2, 94.7 ± 2, and 93.2 ± 1% for cycles 2 ∼ 4. The reason for this may lie in that when TEAC/2TsOH was regenerated with H2O, some of it dissolved in H2O, which reduced the amount of TEAC/2TsOH left for extracting indole, resulting in a gradual decrease in extraction efficiency. When comparing the fresh and recycled TEAC/2TsOH by FTIR analysis, there were no obvious changes observed in the spectra (Fig. 10b), indicating that TEAC/2TsOH can be regenerated without structural changes. The good stability of the extraction capacity and the structure are important from an economic perspective for the implementation of TEAC/2TsOH in commercial processes.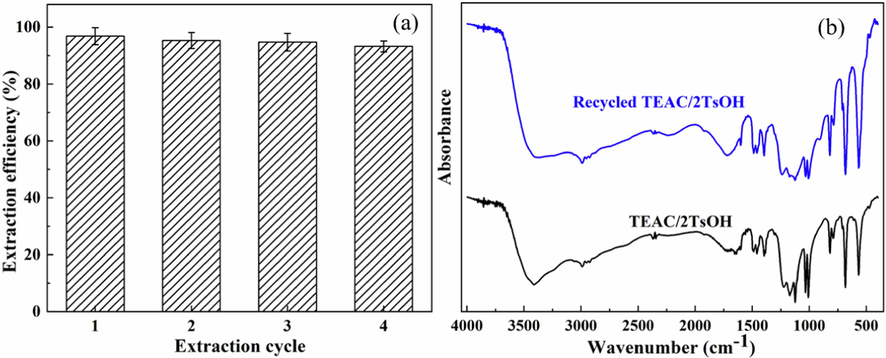
Extraction efficiency in successive indole extraction cycles (a) and FTIR spectra of fresh and regenerated TEAC/2TsOH (b).
3.5 Comparison with other extraction systems
Other acidic DESs. Formic acid (FA) or oxalic acid (OA), and choline chloride (ChCl) are widely used as hydrogen bond donors and hydrogen bond acceptor (Lemaoui et al., 2021), respectively. They were also used herein to prepare three additional DESs for the separation of indole, and their separation performance is compared with TEAC/2TsOH in Fig. 11. The extraction efficiency and distribution coefficient were highest for TEAC/2TsOH among the investigated DESs, and lowest for TEAC/OA, which demonstrates that the combination of HBA and HBD selected for the preparation of DESs influences their extraction performance for indole. This may be due to the benzene ring in TsOH being more conducive to the formation of intermolecular interactions between the DES and indole than the alkyl carbon chains in FA and OA, while the –OH group in the ChCl cation increases the polarity of the DESs, which may adversely affect the phase transfer of indole.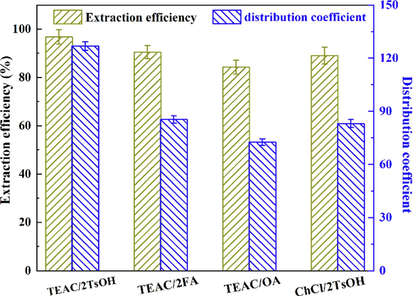
Separation of indole with different acidic deep eutectic solvents.
Other reported extractants. For comparison, a quaternary ammonium salt (TEAC) was applied to separate indole from wash oil, as first deliberated by Wu’s group (Ji et al., 2018). The experimental procedures used were the same as described for TEAC/2TsOH. The separation performance of TEAC and TEAC/2TsOH is compared in Fig. 12. There is no obvious difference in indole extraction efficiency when using TEAC (97.5 ± 3%) and TEAC/2TsOH (97.2 ± 2%). The similar extraction efficiencies may be due to the same driving forces for separation in both systems, namely hydrogen bond and van der Waals interactions, and the comparable interaction energies of indole and both extraction agents. The interaction energy between indole and TEAC/2TsOH is −83.0 kJ/mol, and that between indole and TEAC is −84.6 kJ/mol. The main difference between the two processes lies in the purity level of the isolated indole, which was lower for TEAC (90.2 ± 2%) than for TEAC/2TsOH (98.5 ± 1%). The lower indole purity for TEAC extraction may be ascribed to the fact that TEAC forms DESs with indole during its separation from the oil phase, and the DESs formed in situ acts as a solvent interacting and dissolving the other aromatic compounds in the wash oil, including naphthalene and acenaphthylene, resulting in more aromatic compounds being transferred to the extraction phase. These aromatic compounds lowered the purity level of the indole product.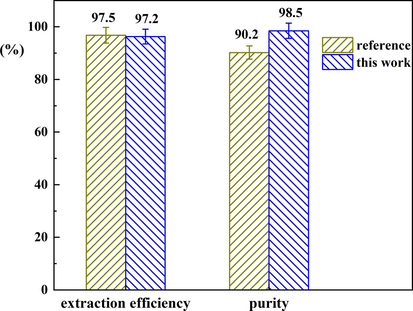
Separation performance of indole with TEAC and TEAC/2TsOH.
3.6 Extraction of indole from real wash oil
The extraction experiment on real wash oil via TEAC/2TsOH DESs was carried out. The amount of indole in the original wash oil was 5.2 wt%. TEAC/2TsOH with a 0.5 time mass amount of indole was added to wash oil, and result showed that the amount of indole in the extracted wash oil was 1.9 wt%. The extraction efficiency of indole in real wash oil was around 63.5% (The uncertainty of this extraction efficiency was ± 3%). The GC–MS results of refined indole product are shown in Table S2. It can be seen that the area percentage of aromatic compounds entrapped in the indole product was 42.3%, indicating that the purity of the indole product is not high. The main reason for the low purity is the very complex composition of the real wash oil (Table S1), with compounds such as pyridines, phenolic compounds, benzene or naphthalene derivatives (with unsaturated side chains), indanol, biphenyl, hexadecane, and chamazulene being entrained during the extraction process (Table S2). Designing DESs with higher selectivity is a future endeavor.
4 Conclusions
A new method for the extraction of indole from wash oil with a TEAC/2TsOH DES was proposed. Indole has moderate π-stacking ability, allowing it to associate with aromatic compounds in wash oil through π−π interactions, and with the DES through a combination of hydrogen bond and C−H···π interactions, respectively. The electrostatic interaction energy between indole and the DES (−52.8 kJ/mol) was larger than between indole and the aromatic compounds in wash oil (−4.6 ∼ 5.1 kJ/mol), thereby promoting the extraction process. Among the conditions investigated, the initial indole concentration had no effect on extraction by the DES, and optimal extraction performance was obtained at 30 °C with stirring for 20 min, using anhydrous DES and a mass ratio of DES to indole of 1:2. These conditions resulted in an indole extraction efficiency, distribution coefficient, and indole purity of 97.2 ± 2%, 131.8 ± 4, and 98.5 ± 1%, respectively. The indole extraction efficiency was higher than achieved with other acid DESs systems, and the purity level was higher than when using TEAC alone. The TEAC/2TsOH DES could be regenerated with no structural changes nor a significant reduction in separation performance. Impressively, this DES can achieve effective indole extraction from real wash oil with an extraction efficiency of 63.5 ± 3%. It is noteworthy that to obtain pure indole from the crude products, an aqueous ammonium bisulfate solution could be used to remove quinoline. These results pave the way to further studies on the design of DESs systems, for example to further improve the selectivity of the separation process so as to make the removal of quinoline from the crude indole product unnecessary or to target the extraction of selected compounds from complex mixtures.
CRediT authorship contribution statement
Lan Yi: Methodology, Investigation, Writing – original draft. Xiaoqin Wu: Resources. Shuguang Ouyang: Conceptualization. Li Guo: Data curation. Jialing Chen: Validation. Mario Gauthier: Writing – review & editing. Wen-Ying Li: Funding acquisition, Project administration, Supervision.
Acknowledgements
This work was financially supported by Key Laboratory of Hubei Province for Coal Conversion and New Carbon Material (grant number: WKDM202207), Research Project of Hubei Province Department of Education (grant number: Q20221102), and National Natural Science Foundation of China (grant number: 22038008). Numerical calculation is supported by High-Performance Computing Center of Wuhan University of Science and Technology.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003:70-71.
- [CrossRef] [Google Scholar]
- Exploring nature and predicting strength of hydrogen bonds: a correlation analysis between atoms-in-molecules descriptors, binding energies, and energy components of symmetry-adapted perturbation theory. J. Comput. Chem.. 2019;40:2868-2881.
- [CrossRef] [Google Scholar]
- Formation of noncovalent complexes between complex mixtures of polycyclic aromatic hydrocarbons (asphaltenes) and substituted aromatics studied by fluorescence spectroscopy. Energy Fuels. 2021;35:8742-8755.
- [CrossRef] [Google Scholar]
- Gaussian 09, Revision D. 01. Wallingford, CT: Gaussian, Inc.; 2009.
- Coal Pyrolysis, Coking and Coal Tar Processing. Beijing: Chemical Industial Press; 2010.
- Experimental and quantum chemical calculations investigations of morpholine-based ionic liquids as extractants for efficient extraction of nitrogen heterocyclic neutral compounds. Fuel. 2023;333:126446
- [CrossRef] [Google Scholar]
- Mechanism of extractive separation of light cycle oil using a deep eutectic solvent composed of tetrabutylphosphonium bromide and levulinic acid. Energy Fuels. 2022;36:1854-1862.
- [CrossRef] [Google Scholar]
- Efficient extraction of indole from wash oil by quaternary ammonium salts via forming deep eutectic solvents. Fuel. 2018;215:330-338.
- [CrossRef] [Google Scholar]
- The extraction mechanism research for the separation of indole through the formation of deep eutectic solvents with quaternary ammonium salts. J. Mol. Liq.. 2022;347:118325
- [CrossRef] [Google Scholar]
- How hydration affects the microscopic structural morphology in a deep eutectic solvent. J. Phys. Chem. B. 2020;124:2230-2237.
- [CrossRef] [Google Scholar]
- Choline chloride-based deep eutectic solvents in the dearomatization of gasolines. ACS Sustain. Chem. Eng.. 2018;6:1039-1047.
- [CrossRef] [Google Scholar]
- Simultaneous dearomatization, desulfurization, and denitrogenation of diesel fuels using acidic deep eutectic solvents as extractive agents: a parametric study. Sep. Purif. Techn.. 2021;256:117861
- [CrossRef] [Google Scholar]
- Systematic method of screening deep eutectic solvents as extractive solvents for m-cresol/cumene separation. Sep. Purif. Technol.. 2022;291:120853
- [CrossRef] [Google Scholar]
- Multiwfn: a multifunctional wavefunction analyzer. J. Comput. Chem.. 2012;33:580-592.
- [CrossRef] [Google Scholar]
- A simple method of identifying π orbitals for non-planar systems and a protocol of studying π electronic structure. Theor. Chem. Acc.. 2020;139:25-37.
- [CrossRef] [Google Scholar]
- Independent gradient model based on Hirshfeld partition: A new method for visual study of interactions in chemical systems. J J. Comput. Chem.. 2022;43:539-555.
- [CrossRef] [Google Scholar]
- Comment on “18 and 12 - Member carbon rings (cyclo[n]carbons) - A density functional study”. Mater. Sci. Eng.. 2021;273:115425
- [CrossRef] [Google Scholar]
- Value-added utilization of high-temperature coal tar: a review. Fuel. 2021;292:119954
- [CrossRef] [Google Scholar]
- VOCs absorption from gas streams using deep eutectic solvents – a review. J. Hazard. Mater.. 2023;448:130957
- [CrossRef] [Google Scholar]
- Makoto, T., Akinori, M., 1992. Method for recovery of indole by formation of inclusion compound with cyclodextrin. JP04217953, Japan.
- Choline chloride-based deep eutectic solvents for ultrasonic-assisted oxidative desulfurization of actual heavy crude oil. Chem. Eng. Res. Des.. 2022;182:659-666.
- [CrossRef] [Google Scholar]
- Green and sustainable solvents of the future: deep eutectic solvents. J. Mol. Liq.. 2023;379:121676
- [CrossRef] [Google Scholar]
- Removal intensification of basic and non-basic nitrides from liquid fuels by the optimization design of quaternary ammonium salt green solvents. Fuel. 2022;326:125093
- [CrossRef] [Google Scholar]
- Effective separation of toluene from n-heptane with imidazolium-based deep eutectic solvents. Fuel. 2022;326:124992
- [CrossRef] [Google Scholar]
- Intermolecular interactions induced desulfurization/denitrification of oil with deep eutectic solvents. J. Mol. Liq.. 2022;366:120159
- [CrossRef] [Google Scholar]
- Molecular mechanism and extraction explorations for separation of pyridine from coal pyrolysis model mixture using protic ionic liquid [Hnmp][HSO4] Fuel. 2022;309:122130
- [CrossRef] [Google Scholar]
- Rational design of caprolactam-based deep eutectic solvents for extractive desulfurization of diesel fuel and mechanism study. ACS Sustain. Chem. Eng.. 2022;10:4551-4560.
- [CrossRef] [Google Scholar]
- Separation of heterocyclic nitrogen compounds from coal tar fractions via ionic liquids: COSMO-SAC screening and experimental study. Chem. Eng. Commun.. 2019;206:1199-1217.
- [CrossRef] [Google Scholar]
- Separation of high purity indole from coal tar by high pressure crystallization. Fuel. 1991;70:565-566.
- [CrossRef] [Google Scholar]
- Separation and purification of indole from coal tar by supercritical fluid extraction. J. Chem. Eng. Jpn. 1993;26:153-158.
- [CrossRef] [Google Scholar]
- High-performance separation of phenolic compounds from coal-based liquid oil by deep eutectic solvents. ACS Sustain. Chem. Eng.. 2019;7:7777-7783.
- [CrossRef] [Google Scholar]
- Effect of the addition of deep eutectic solvent to the anthracene separation. J. Mol. Liq.. 2021;339:116762
- [CrossRef] [Google Scholar]
- Extraction and multi-scale mechanism explorations for separating indole from coal tar via tetramethylguanidine-based ionic liquids. J. Environ. Chem. Eng.. 2021;9:105255
- [CrossRef] [Google Scholar]
- Extraction performance and interaction analysis of 2-pyrrolidone-based brønsted acidic ionic liquids on removal of quinoline from coal-based products. J. Mol. Liq.. 2022;366:120320
- [CrossRef] [Google Scholar]
- Highly efficient extraction of indole from model wash oil by using environmentally benign deep eutectic solvents. Sep. Purif. Technol.. 2022;285:120381
- [CrossRef] [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2023.105341.
Appendix A
Supplementary data
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1
Supplementary data 2
Supplementary data 2







