Translate this page into:
Structure-based design, synthesis and biological evaluation of N-substituted 6H-thiochromeno[2,3–c]quinolin-12(12H)-one as potential breast cancer drugs
⁎Corresponding authors. chaw1211@tmu.edu.tw (Alexander T.H Wu), huanghs99@tmu.edu.tw (Hsu-Shan Huang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
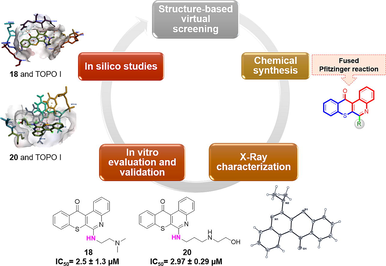
Tetraheterocyclic compounds, derived from natural sources and contemporary pharmaceuticals, have shown promise as multitarget therapeutic agents. However, their mechanisms of action remain partially understood. In this study, we synthesized a series of 6H-thiochromeno[2,3-c]quinolin-12(12H)-one derivative, totaling 26 compounds, and assessed their potential for therapeutic application. We evaluated their effects on cell proliferation and conducted NCI-60 cell panel assays. MTT assays revealed that select compounds exhibited notable antiproliferative activity against two breast cancer cell lines (MCF-7 and MDA-MB-468). Notably, compounds 17 and 18 displayed the highest cytotoxicity against these cell lines. Furthermore, one-dose assays of the NCI-60 human tumor cell line screening program identified compounds 6, 7, 16, 18, 20, 24, 25, and 30 for further investigation.
Subsequent five-dose cytotoxicity studies focused on compounds 18 and 20, which met the threshold inhibition criteria across a panel of cell lines. Our study highlights the effectiveness of compounds 18 and 20 in targeting breast cancer cell lines. Molecular docking simulations revealed that these compounds bind to the active sites of topoisomerase I (TOPO I). Our findings suggest that these novel compounds are promising anticancer agents, particularly against breast cancer, and are worthy of consideration as lead pharmacological candidates.
Keywords
Breast cancer
NCI 60-cell panel assay
Molecular docking
Tetraheterocyclic
1 Introduction
Breast cancer is the second most prevalent and leading cause of cancer deaths in women globally (Siegel, 2023; Fares et al., 2023). Based on molecular biological techniques and gene expression profiles, breast cancer can be classified into five clinical characteristics and treated depending on whether estrogen receptor (ER)-, progesterone receptor (PR)-, or human epidermal growth factor receptor (HER)-2-positive, respectively (Orrantia-Borunda et al., 2022; Tang, 2018). Despite the exploitation of genes and immunotherapy, chemotherapy is still the most effective strategy in clinical practice for most cancer patients. In addition, DNA topoisomerases are essential enzymes that manipulate the topology of DNA in cells and play essential roles in DNA replication, transcription, chromosome segregation, and recombination (McKie et al., 2021). Topoisomerase inhibitors are still widely used as first-line anticancer drugs due to their high activity and high expression in tumor cells. Natural product-derived agents have greatly contributed to the development of novel treatments for cancer, such as camptothecin (Ghanbari-Movahed et al., 2021), topotecan (Jaeckle et al., 2020), and irinotecan (Wang et al., 2020), are classical topoisomerase inhibitors (Fig. 1). Targeting of the topoisomerase for cancer research continues to be a highly active area of the development of new anticancer drugs, especially in breast cancer (Hevener et al., 2018).![Rational design of 6H-thiochromeno[2,3-c] quinoline-12 (12H)-one derivatives as anticancer drugs.](/content/184/2024/17/1/img/10.1016_j.arabjc.2023.105423-fig2.png)
Rational design of 6H-thiochromeno[2,3-c] quinoline-12 (12H)-one derivatives as anticancer drugs.
Accordingly, many small active molecules with different chemical scaffolds have emerged as small molecule inhibitors. Single-targeted chemotherapy strategies are often hampered by limited efficacy, toxic side effects, and drug resistance (Min and Lee, 2022). Multitarget therapeutics have become a popular area that can act on two or more targets simultaneously, with better therapeutic advantages and potentially synergistic effects (Raevsky, 2018; Zhong et al., 2021). Although multitarget small-molecule inhibitors have several different mechanisms of action, this therapy still faces issues, such as drug resistance and undesirable side effects, which need to be resolved for their application to breast cancer treatment (Liu et al., 2020; Yuan et al., 2020).
As part of our ongoing efforts to develop potent and selective small molecule inhibitors for cancer therapy, we have employed a computational fragment-based drug discovery (FBDD) method to obtain a series of tetraheterocyclic derivatives because of their adaptability and distinctive qualities in different biological and medicinal applications (Abdella et al., 2020; Jampilek, 2019). Although heterocyclic anthracycline and its derivatives are well-known drugs with several therapeutic applications, their pharmacological characteristics can still be improved (Karthikeyan et al., 2023; Martins-Teixeira and Carvalho, 2020).
The innovation in our approach lies in our efforts to deliver multitarget therapies that can simultaneously address multiple critical cellular processes involved in cancer progression. This strategy aims to enhance treatment efficacy and minimize drug resistance, offering a potential breakthrough in breast cancer therapy. In our previous study, compound 11 (NSC763967), which contains thiadiazoles, was discovered to have broad-spectrum cytotoxicity against several cancer cells among our collection of small-molecule inhibitors. However, our candidate exhibited promising results, which may reveal important details about the cytotoxic, cytostatic, and selectivity characteristics of cells (Ali et al., 2021). Additionally, significant attention has been paid to developing techniques that lower the toxicity of parent compounds of anthracycline derivatives, such as metixene (Fares et al., 2023), xanthones (Kurniawan et al., 2021), and thioxanthones (Lima et al., 2018) (Fig. 1). In addition to our ongoing efforts to develop potent and selective small-molecule inhibitors for cancer therapy, we have explored quinoline, a unique and promising chemical scaffold in our research (Abdolmohammadi et al., 2020). Quinoline is a chemical building block of a large number of heterocyclic compounds with a broad range of biological and pharmacological properties such as antibacterial (Bakır and Lawag, 2020), antimicrobial (Senerovic, 2020), antitumor (Lauria et al., 2021; El Rhabori et al., 2022; Iqbal et al., 2019), and anti-HIV (Chokkar et al., 2019). These promising results encouraged us to identify the most favorable chemical modifications based on our previous candidate, which is required for the development of novel drugs with potential applications in cancer therapy.
Herein, we designed and synthesized a novel series of compounds based on the 6H-thiochromeno[2,3–c] quinoline-12 (12H)-one scaffold using different N-substitutions on the 3-position as depicted in Scheme 1. Their cytotoxicity against two human breast cancer cell lines (MCF7 and MDA-MB-468) using MTT assays is assessed (Table 1). Moreover, compounds 6 (NSC784437), 7 (NSC784438), 16 (NSC784445), 18 (NSC784447), 20 (NSC784449), 24 (NSC784440), 25 (NSC784442), 28 (NSC784441), and 30 (NSC784444) were tested by the NCI, using single-dose cytotoxicity experiments against a panel of 60 human cancer cell lines (Table 2). All these results characterize 18 (NSC784447) and 20 (NSC784449) satisfied the predetermined threshold of growth-inhibition criteria of the NCI. Accordingly, they were evaluated using cytotoxicity studies with five doses against a panel of 60 cancer cell lines (Table 3). We discovered these two compounds had significant multi-log differential activity patterns, with 50 % growth inhibition (GI50) values against several cancer cell lines in the sub-micromolar range (Table 3).![Synthesis routes of 6H-thiochromeno [2,3-c] quinolin-12(12H)-one derivatives. Reagents and conditions: (i) NaOAc, 150 °C, 1 h; (ii) POCl3, 150 °C, 48 h; (iii) DMSO, appropriate secondary amines, Na2CO3, 150 °C, 10 h.](/content/184/2024/17/1/img/10.1016_j.arabjc.2023.105423-fig3.png)
Synthesis routes of 6H-thiochromeno [2,3-c] quinolin-12(12H)-one derivatives. Reagents and conditions: (i) NaOAc, 150 °C, 1 h; (ii) POCl3, 150 °C, 48 h; (iii) DMSO, appropriate secondary amines, Na2CO3, 150 °C, 10 h.
No.
R substitutions
MTT assay
No.
R substitutions
MTT assay
MCF-7 (μM)
MDA-MB-468 (μM)
MCF-7 (μM)
MDA-MB-468 (μM)
5
2.99 ± 0.35
1.84 ± 0.33
19
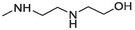
3.68 ± 0.49
2.6 ± 0.07
6

> 20
> 20
20
3.5 ± 0.19
2.97 ± 0.29
7
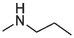
> 20
> 20
21
18.35 ± 1.18
> 20
8

> 20
> 20
22
> 20
> 20
9
> 20
2.65 ± 0.11
23
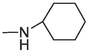
> 20
2.3 ± 0.28
10

> 20
2.46 ± 0.13
24

19.29 ± 1.24
2.45 ± 0.27
11
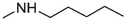
> 20
> 20
25

> 20
> 20
12
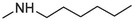
> 20
> 20
26
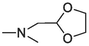
> 20
> 20
13

> 20
> 20
27

> 20
> 20
14

> 20
> 20
28

> 20
> 20
15

> 20
2.72 ± 0.04
29
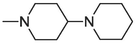
> 20
> 20
16
> 20
> 20
30
> 20
> 20
17
2.3 ± 0.28
2.07 ± 0.57
–
Doxorubicin
0.41 ± 0.01
1.96 ± 0.52
18
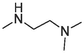
2.94 ± 0.19
2.5 ± 1.3
–
Camptothecin
0.54 ± 0.11
2.23 ± 0.32
Compound
Cell lines
MCF-7
MDA-MB-468
IC50 (μM)
Selectivity index b
IC50 (μM)
Selectivity index b
5
2.99
1.93
1.84
1.30
17
2.3
2.51
2.07
1.16
18
2.94
1.96
2.5
0.96
19
3.68
1.57
2.6
0.92
20
3.5
1.65
2.97
0.80
24
19.29
0.29
2.45
0.98
Total mean a
5.78
2.40
Compound
Physicochemical Properties
Lipophilicity and Water Solubility
Drug likeliness
Medicinal chemistry
M.W
HBA
HBD
TPSA
XLOGP3
Consensus Log Po/w
Log S
Lipinski’s rules
Veber’s rules
Ghose’s rules
PAINS
Synthetic accessibility
5
292.35
2
1
70.23
4.09
3.68
−4.80
Yes
Yes
Yes
0 alert
2.84
17
335.42
3
2
82.26
3.66
3.52
−4.52
Yes
Yes
Yes
0 alert
3.12
18
349.45
3
1
73.47
4.13
3.74
−4.88
Yes
Yes
Yes
0 alert
3.23
19
365.45
4
3
102.49
2.97
3.06
−4.09
Yes
Yes
Yes
0 alert
3.20
20
379.48
4
3
102.49
3.88
3.49
−4.67
Yes
Yes
Yes
0 alert
3.28
21
332.42
2
1
70.23
4.83
4.41
−5.30
Yes
Yes
Yes
0 alert
2.98
24
391.49
4
1
82.70
3.75
3.59
−4.84
Yes
Yes
Yes
0 alert
3.35
Doxorubicin
543.52
12
6
206.07
1.27
0.44
−3.91
No
No
No
1 alert
5.81
Camptothecin
348.35
5
1
81.42
1.74
2.20
−3.49
Yes
Yes
Yes
0 alert
3.84
Using comparative correlations of the cytotoxic activities of drugs featured in the NCI database, we performed COMPARE analytical studies to identify compounds with similar targets and mechanisms of action to our test compounds and gain insights into potential targets and mechanisms of our drugs (Haji et al., 2023). We discovered that certain inhibitors, including aurora kinase A (AURKA), poly(ADP ribose) polymerase (PARP), cyclin-dependent kinase (CDK) 4/6, and topoisomerase (TOPO) I and II, were strongly correlated with the cytotoxic profiles of 18 (NSC784447) and 20 (NSC784449). Additionally, based on our molecular modeling investigations, we discovered that compounds 18 (NSC784447) and 20 (NSC784449) are effective TOPO-1 inhibitors. As shown in this publication, our data present a novel series of potential multitarget anticancer drugs that represent interesting candidates for further therapeutic development.
2 Material and methods
2.1 Chemistry
2.1.1 General experimental procedures
All reagents and solvents were purchased from Merck (Germany) and Sigma-Aldrich (USA) and were used without further purification. All reactions were monitored using precoated silica gel 60 F254 (Merck), and thin-layer chromatography (TLC) plates were observed under UV light. Melting points were determined with a Büchi Melting Point B-545 apparatus (National Defense Medical Center, Taiwan). 1H nuclear magnetic resonance (NMR) spectra were recorded using GEMINI300 MHz (National Defense Medical Center, Taiwan) and AM-500 MHz (Bruker) instruments. Chemical shift (d) values were in ppm ranges relative to tetramethylsilane (TMS) as an internal standard. Mass spectra were obtained by Finnigan (National Taiwan University, Taiwan) MAT 95 XL high-resolution mass spectroscopy (HRMS) and Finnigan/Thermo Quest MAT HRMS. Typical experiments illustrating the general procedures for preparing the anthraquinone derivatives are described below.
2.1.1.1 General method for synthesis of compound 3
A mixture of isatin (1) (0.44 g, 2.99 mmol), (phenylthio)acetic acid (2) (0.78 g, 4.64 mmol), and sodium acetate (0.05 g) was heated to 150 °C in a miniclave for 1 h monitored by TLC (Thin Layer Chromatography). After cooling, 10 mL of acetic acid was added to the mixture, and the gray precipitate was collected and washed with acetic acid, water, and n-hexane to obtain a light-purple compound (3).
2.1.1.2 General method for synthesis of compound 4
A mixture of compound 3 (84 mol) and POCl3 (200 mL) was heated to 150 °C for 48 h. After the reaction was complete, the mixture was cooled to room temperature and poured into a water bath at 0 °C. It was then filtered by suction to collect a green precipitate, and the precipitate was added to a 10 % NaHCO3 solution (50 mL) and stirred for 1 h. The precipitate was collected and washed with water. The crude product was recrystallized in dichloromethane to give a yellowish product (4).
2.1.1.3 General method for synthesis of compounds 5-30
Method 1: Preparation of compounds 5, 7–12, 16, 21, 23, 25, 28, and 29.
A mixture of compound 4 (2 mmol), alkylamine, and sodium carbonate (2.5 mmol) was dissolved in DMSO (9 mL) and heated in a miniclave (to 150 °C) for 2 h. After the reaction was completed, the mixture was poured into water (250 mL) and allowed to stand for about 5–10 min. At this time, a yellow precipitate had precipitated out. The precipitate was collected by suction and filtered. The precipitate was recrystallized with dichloromethane to obtain compounds 5, 7–12, 16, 21, 23, 25, 28, and 29.
Method 2: Preparation of compounds 6, 13–15, 17–19, 22, 24, 26, 27, and 30.
A mixture of compound 4 (1 mmol), alkylamine, and sodium carbonate (1.5 mmol) in DMSO (8 mL) was refluxed for 4 h. After the reaction was completed, the mixture was poured into water (250 mL) and filtered to collect the resulting precipitate. The precipitate was extracted with dichloromethane and water to collect the organic material. The layers were concentrated and drained under reduced pressure. The precipitate was recrystallized with dichloromethane to afford compounds 6, 13 ∼ 15, 17–19, 22, 24, 26, 27, and 30.
Method 3: Preparation of compound 20.
To a solution of compound 4 (2 mmol) in DMSO was added alkylamine and sodium carbonate (2.5 mmol), and then the mixture was refluxed for 6 h (at 100 °C). After the reaction was completed, the material was poured into 250 mL of water. The precipitate appeared at this time and was filtered to collect the precipitate. The crude precipitate was purified by column chromatography (hexane: dichloromethane 1:1) to afford compound 20.
2.1.1.3.1 2-hydroxy-3-(phenylthio)quinoline-4-carboxylic acid (3)
The pure compound was obtained as a purple solid (yield 86 %), Rf = 0.8 at MeOH, Mp 304–305 °C. 1H NMR (400 MHz, DMSO‑d6): δ (ppm) 7.16–7.32 (6H, m), 7.38 (1H, d, J = 8.4 Hz), 7.45 (1H, d, J = 8.4 Hz), 7.61 (1H, t, J = 8.4 Hz), 12.20 (1H, s). 13C NMR (100 MHz, DMSO‑d6): δ (ppm) 115.17, 115.78, 120.26, 122.73, 125.74, 126.28, 128.05, 128.97, 132.00, 134.84, 139.64, 151.18, 159.07, 166.44. HRMS (ESI) m/z calcd. for C16H11NO3S+ [M]+: 297.0460; found [M + H]+: 298.0520, [M + Na]+: 320.0340, [M−H]+: 296.0392.
2.1.1.3.2 6-chloro-12H-thiochromeno[2,3–c]quinolin-12-one (4)
The pure compound was obtained as a yellow solid (yield 74 %), Rf = 0.8 at CH2Cl2, Mp 212–213 °C. 1H NMR (400 MHz, DMSO‑d6): δ (ppm) 7.74 (1H, t, J = 8 Hz), 7.85–7.92 (3H, m), 8.08–8.11 (2H, m), 8.49 (1H, dd, J = 8, 0.8 Hz), 9.62 (1H, dd, J = 8.4, 1.6 Hz). 13C NMR (100 MHz, DMSO‑d6): δ (ppm) 124.92, 126.39, 127.43, 128.81, 129.25, 129.57, 130.34, 130.63, 130.84, 131.49, 133.76, 134.44, 144.94, 146.79, 181.52, 166.44. HRMS (ESI) m/z calcd. for C16H7ClNOS+ [M]+: 295.9931; found [M + H]+: 298.0088, [M + H + 2]+: 300.0063.
2.1.1.3.3 6-(Methylamino)-12H-thiochromeno[2, 3-c]quinolin-12-one (5)
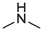
Compound 5 was prepared from 4 and methylamine (20 mmol). The compound was obtained in a 71 % yield, Rf = 0.2 at CH2Cl2, Mp 184–186 °C. 1H NMR (400 MHz, DMSO‑d6): δ (ppm) 3.24 (3H, d, J = 4.4 Hz), 4.94 (1H, s), 7.43 (1H, t, J = 7.2 Hz), 7.54–7.66 (4H, m), 7.84 (1H, d, J = 8.4 Hz), 8.56 (1H, d, J = 8 Hz), 9.46 (1H, d, J = 8.8 Hz). 13C NMR (100 MHz, DMSO‑d6): δ (ppm) 29.35, 120.89, 123.88, 124.45, 125.95, 126.01, 127.09, 127.62, 129.29, 129.76, 129.98, 131.65, 132.10, 132.84, 145.60, 151.38, 182.18. HRMS (ESI) m/z calcd. for C17H11N2OS+ [M]+: 291.0587; found [M + H]+: 293.0744.
2.1.1.3.4 6-(Ethylamino)-12H-thiochromeno[2, 3-c]quinolin-12-one (6)
Pure compound 6 was prepared from 4 and ethylamine (10 mmol). The compound was obtained as a yellow solid (yield 83 %), Rf = 0.3 at CH2Cl2, Mp 162–163 °C. 1H NMR (400 MHz, DMSO‑d6): δ (ppm) 1.41 (3H, t, J = 7.2 Hz), 3.75 (2H, sep, J = 4.4 Hz), 4.84 (1H, s), 7.43 (1H, td, J = 8.4, 1.2 Hz), 7.55–7.68 (4H, m), 7.82 (1H, d, J = 8.4 Hz), 8.57 (1H, d, J = 8 Hz), 9.46 (1H, dd, J = 8.4 Hz, 1.2 Hz). 13C NMR (100 MHz, DMSO‑d6): δ (ppm) 14.80, 37.32, 120.84, 123.70, 124.35, 125.92, 125.97, 127.09, 127.58, 129.22, 129.73, 129.97, 131.60, 132.08, 132.86, 145.62, 150.69, 182.19. HRMS (ESI) m/z calcd. for C18H13N2OS+ [M]+: 305.0743; found [M + H]+: 307.0895.
2.1.1.3.5 6-(Propylamino)-12H-thiochromeno[2, 3-c]quinolin-12-one (7)
Compound 7 was prepared from 4 and propylamine (6 mmol). The compound was obtained in a 69 % yield, Rf = 0.37 at CH2Cl2, Mp 133–134 °C. 1H NMR (400 MHz, DMSO‑d6): δ (ppm) 1.09 (3H, d, J = 7.6 Hz), 1.75–1.85 (2H, m), 3.65–3.70 (2H, m), 4.88 (1H, t, J = 4.8 Hz), 7.41 (1H, t, J = 7.2 Hz), 7.53–7.66 (4H, m), 7.81 (1H, dd, J = 8 Hz, 0.8 Hz), 8.55 (1H, dd, J = 8.4 Hz, 1.2 Hz), 9.45 (1H, dd, J = 8.8 Hz, 1.2 Hz). 13C NMR (100 MHz, DMSO‑d6): δ (ppm) 11.69, 22.65, 44.09, 120.81, 123.73, 124.29, 125.90, 125.94, 127.06, 127.54, 129.20, 129.68, 129.92, 131.54, 132.04, 132.81, 145.59, 150.75, 182.15. HRMS (ESI) m/z calcd. for C19H15N2OS+ [M]+: 319.0900; found [M + H]+: 321.1057.
2.1.1.3.6 6-(Butylamino)-12H-thiochromeno[2, 3-c]quinolin-12-one (8)
Compound 8 was prepared from 4 and n-butylamine (5 mmol). The compound was obtained in a 69 % yield, Rf = 0.43 at CH2Cl2, Mp 104–106 °C. 1H NMR (400 MHz, DMSO‑d6): δ (ppm) 0.94 (3H, t, J = 7.6 Hz), 1.40 (2H,J = 7.6 Hz), 1.69 (2H, quin, J = 7.2 Hz, –CH2-), 3.58 (2H, q, J = 6.4 Hz, –CH2-), 7.08 (1H, t, J = 5.2 Hz), 7.34 (1H, td, J = 7.2 Hz, 1.2 Hz), 7.57 (1H, t, J = 8.4 Hz), 7.67 (2H, t, J = 7.2 Hz), 7.82 (1H, t, J = 8.4 Hz), 7.91 (1H, d, J = 8 Hz), 8.45 (1H, d, J = 8 Hz), 9.34 (1H, d, J = 8.8 Hz). 13C NMR (100 MHz, DMSO‑d6): δ (ppm) 13.98, 20.37, 31.57, 42.07, 120.83, 123.73, 124.31, 125.92, 125.98, 127.08, 127.59, 129.23, 129.75, 130.00, 131.63, 132.08, 132.86, 145.64, 150.78, 182.21. HRMS (ESI) m/z calcd. for C20H17N2OS+ [M]+: 333.1056; found [M + H]+: 335.1212.
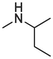
2.1.1.3.7 6-(sec-butylamino)-12H-thiochromeno[2, 3-c]quinolin-12-one (9)
Compound 9 was prepared from 4 and sec-butylamine (5 mmol). The compound was obtained in a 35 % yield, Rf = 0.53 at CH2Cl2, Mp 139–141 °C. 1H NMR (400 MHz, DMSO‑d6): δ (ppm) 1.05 (3H, t, J = 7.2 Hz), 1.36 (3H, d, J = 6.4), 1.67–1.80 (2H, m), 4.46–4.52 (1H, m), 4.72 (1H, d, J = 7.2 Hz), 7.42 (1H, t, J = 8.4 Hz), 7.56–7.63 (2H, m), 7.66 (2H, d, J = 4 Hz), 7.81 (1H, dd, J = 8.4 Hz), 8.58 (1H, d, J = 8 Hz), 9.46 (1H, dd, J = 8.8 Hz, 1.2 Hz). 13C NMR (100 MHz, DMSO‑d6): δ (ppm) 10.39, 20.28, 29.57, 48.66, 120.69, 123.68, 124.15, 125.88, 125.90, 127.08, 127.52, 129.14, 129.67, 129.98, 131.53, 132.02, 132.80, 145.65, 150.20, 182.19. HRMS (ESI) m/z calcd. for C20H17N2OS+ [M]+: 333.1056; found [M + H]+: 365.1324.
2.1.1.3.8 6-(Isobutylamino)-12H-thiochromeno[2, 3-c]quinolin-12-one (10)
Compound 10 was prepared from 4 and isobutylamine (5 mmol). The compound was obtained in a 67 % yield, Rf = 0.4 in CH2Cl2, Mp 151–153 °C. 1H NMR (400 MHz, DMSO‑d6): δ (ppm) 1.09 (6H, d, J = 6.4 Hz), 2.11 (1H, sep, J = 6.8 Hz), 3.57 (2H, t, J = 5.6 Hz), 4.98 (1H, t, J = 4.8 Hz), 7.42 (1H, td, J = 7.2, 1.2 Hz), 7.57–7.63 (2H, m), 7.67 (2H, d, J = 3.6 Hz), 7.82 (1H, d, J = 8.4 Hz), 8.59 (1H, d, J = 8 Hz), 9.46 (1H, dt, J = 8.8 Hz, 0.8 Hz). 13C NMR (100 MHz, DMSO‑d6): δ (ppm) 20.48, 28.15, 49.65, 120.78, 123.71, 124.24, 125.87, 125.91, 127.02, 127.52, 129.17, 129.66, 129.92, 131.51, 132.02, 132.75, 145.54, 150.78, 182.12. HRMS (ESI) m/z calcd. for C20H17N2OS+ [M]+: 333.1056; found [M + H]+: 335.1212.
2.1.1.3.9 6-(Pentylamino)-12H-thiochromeno[2, 3-c]quinolin-12-one (11)
Compound 11 was prepared from 4 and amylamine (4.5 mmol). The compound was obtained in a 69 % yield, Rf = 0.4 in CH2Cl2, Mp 105–107 °C. 1H NMR (400 MHz, DMSO‑d6): δ (ppm) 0.96 (3H, t, J = 6.8 Hz), 1.41–1.51 (4H, m), 1.77(2H, quint, J = 7.2 Hz), 3.68 (2H, q, J = 6.4 Hz), 4.85 (1H, t, J = 4.8 Hz), 7.41 (1H, t, J = 8 Hz), 7.52–7.65 (4H, m), 7.80 (1H, dd, J = 8.4, 0.4 Hz), 8.54 (1H, d, J = 8.4 Hz), 9.45 (1H, dd, J = 8.8, 0.8 Hz). 13C NMR (100 MHz, DMSO‑d6): δ (ppm) 14.06, 22.49, 29.08, 29.33, 42.32, 120.77, 123.71, 124.24, 125.88, 125.90, 127.03, 127.50, 129.15, 129.64, 129.86, 131.50, 132.00, 132.79, 145.58, 150.70, 182.10. HRMS (ESI) m/z calcd. for C21H19N2OS+ [M]+: 347.1213; found [M + H]+: 349.1369.
2.1.1.3.10 6-(Hexylamino)-12H-thiochromeno[2, 3-c]quinolin-12-one (12)
Compound 12 was prepared from 4 and hexylamine (4 mmol). The compound was obtained in a 96 % yield, Rf = 0.47 in CH2Cl2, Mp 89–91 °C. 1H NMR (400 MHz, DMSO‑d6): δ (ppm) 0.93 (3H, t, J = 6.8 Hz), 1.33–1.42 (4H, m), 1.49 (2H, quin, J = 7.2 Hz), 1.76 (2H, quin, J = 7.2 Hz), 3.68 (2H, q, J = 6.4 Hz), 4.84 (1H, t, J = 4.8 Hz), 7.41 (1H, t, J = 8 Hz), 7.52–7.64 (4H, m), 7.80 (1H, dd, J = 8, 0.8 Hz), 8.54 (1H, d, J = 8.4 Hz), 9.45 (1H, dd, J = 8.4, 0.8 Hz). 13C NMR (100 MHz, DMSO‑d6): δ (ppm) 14.09, 22.64, 26.87, 29.37, 31.63, 42.38, 120.79, 123.74, 124.25, 125.90, 125.92, 127.05, 127.51, 129.17, 129.65, 129.86, 131.50, 132.01, 132.81, 145.59, 150.72, 182.11. HRMS (ESI) m/z calcd. for C22H21N2OS+ [M]+: 361.1369; found [M + H]+: 363.1531.
2.1.1.3.11 6-(2-hydroxyethylamino)-12H-thiochromeno[2, 3-c]quinolin-12-one (13)
Compound 13 was prepared from 4 and ethanolamine (10 mmol). The compound was obtained in a 45 % yield, Rf = 0.57 in EA:CH2Cl2 (2:3), Mp 185–187 °C. 1H NMR (400 MHz, DMSO‑d6): δ (ppm) 3.66–3.70 (4H, m), 4.85 (1H, s), 6.95 (1H, d, J = 4.4 Hz), 7.37 (1H, td, J = 8.4, 1.2 Hz), 7.60 (1H, t, J = 8.4 Hz), 7.69 (2H, t, J = 6 Hz), 7.84 (1H, t, J = 8.4 Hz), 7.94 (1H, d, J = 8 Hz), 8.46 (1H, d, J = 8 Hz), 9.35 (1H, d, J = 8 Hz). 13C NMR (100 MHz, DMSO‑d6): δ (ppm) 44.76, 59.84, 120.46, 124.08, 125.19, 125.93, 127.01, 127.12, 128.46, 129.32, 129.45, 129.65, 131.08, 133.32, 133.51, 145.54, 151.61, 181.89. HRMS (ESI) m/z calcd. for C18H13N2O2S+ [M]+: 321.0692; found [M + H]+: 323.0851.
2.1.1.3.12 6-(3-hydroxypropylamino)-12H-thiochromeno[2, 3-c]quinolin-12-one (14)
Compound 14 was prepared from 4 and 3-amino-1-propanol (6 mmol). The compound was obtained in a 91 % yield, Rf = 0.57 in EA:CH2Cl2 (2:3), Mp 166–168 °C. 1H NMR (400 MHz, DMSO‑d6): δ (ppm) 1.87 (2H, quin, J = 6.4 Hz), 3.58 (2H, q, J = 5.6 Hz), 3.64 (2H, q, J = 6.4 Hz), 4.69 (1H, t, J = 5.2 Hz), 7.07 (1H, t, J = 5.2 Hz), 7.33 (1H, t, J = 8.4 Hz), 7.56 (1H, td, J = 8, 1.2 Hz), 7.64 (1H, t, J = 8 Hz), 7.78 (1H, td, J = 8, 1.2 Hz), 7.88 (1H, d, J = 7.6 Hz), 8.42 (1H, dd, J = 8, 0.8 Hz), 9.33 (1H, dd, J = 8.8, 0.8 Hz). 13C NMR (100 MHz, DMSO‑d6): δ (ppm) 31.63, 59.20, 119.90, 123.44, 124.74, 125.46, 126.49, 126.54, 127.87, 128.71, 128.92, 129.06, 130.55, 132.73, 133.06, 145.13, 151.09, 181.34. HRMS (ESI) m/z calcd. for C19H15N2O2S+ [M]+: 335.0849; found [M + H]+: 337.1005.
2.1.1.3.13 6-(5-hydroxypentylamino)-12H-thiochromeno[2, 3-c]quinolin-12-one (15)
Compound 15 was prepared from 4 and 5-amino-1-pentanol (3 mmol). The compound was obtained in a 71 % yield, Rf = 0.53 in EA:CH2Cl2 (2:3), Mp 139–141 °C. 1H NMR (400 MHz, DMSO‑d6): δ (ppm) 1.37–1.44 (2H, m), 1.47–1.54 (2H, m), 1.71 (2H, quin, J = 7.2 Hz), 3.42 (2H, q, J = 6 Hz), 3.57 (2H, q, J = 6.4 Hz), 4.36 (1H, t, J = 5.2 Hz), 7.07 (1H, t, J = 5.2 Hz), 7.34 (1H, td, J = 8.4 Hz, 1.6 Hz), 7.57 (1H, td, J = 8.8, 1.6 Hz), 7.64–7.69 (2H, m), 7.81 (1H, t, J = 8.4 Hz), 7.90 (1H, d, J = 8 Hz), 8.44 (1H, dd, J = 8, 1.2 Hz), 9.34 (1H, dd, J = 8.4, 1.2 Hz). 13C NMR (100 MHz, DMSO‑d6): δ (ppm) 23.66, 28.88, 32.83, 42.10, 61.20, 120.35, 123.86, 125.26, 125.91, 127.06, 128.37, 129.20, 129.41, 129.53, 131.05, 133.24, 133.63, 145.70, 151.53, 181.88. HRMS (ESI) m/z calcd. for C21H19N2O2S+ [M]+: 363.1162; found [M + H]+: 365.1324.
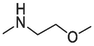
2.1.1.3.14 6-(2-methoxyethylamino)-12H-thiochromeno[2, 3-c]quinolin-12-one (16)
Compound 16 was prepared from 4 and 2-methoxyethylamine (3 mmol). The compound was obtained in a 69 % yield, Rf = 0.9 in EA:CH2Cl2 (2:3), Mp 133–135 °C. 1H NMR (400 MHz, DMSO‑d6): δ (ppm) 3.46 (3H, s), 3.72 (2H, t, J = 5.2 Hz), 3.88 (2H, q, J = 5.2 Hz), 5.34 (2H, t, J = 4.8 Hz), 7.40 (1H, t, J = 8 Hz), 7.37–7.59 (4H, m), 7.76 (1H, d, J = 8.4 Hz), 8.48 (1H, d, J = 8 Hz), 9.42 (1H, dd, J = 8.8 Hz).). 13C NMR (100 MHz, DMSO‑d6): δ (ppm) 41.75, 58.84, 70.89, 120.86, 124.00, 124.30, 125.82, 125.89, 126.92, 127.37, 129.05, 129.46, 129.71, 131.29, 131.87, 132.77, 145.28, 150.59, 181.89. HRMS (ESI) m/z calcd. for C19H15N2O2S+ [M]+: 335.0849; found [M + H]+: 337.1011.
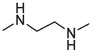
2.1.1.3.15 6-(2-(Methylamino)ethylamino)-12H-thiochromeno[2, 3-c]quinolin-12-one (17)
Compound 17 was prepared from 4 and N-methyl ethylenediamine (6 mmol). The compound was obtained in a 47 % yield. Rf = 0.47 in MeOH: CH2Cl2 (1:4), Mp 155–157 °C. 1H NMR (400 MHz, DMSO‑d6): δ (ppm) 2.54 (3H, s), 3.01 (2H, t, J = 6 Hz), 3.80 (2H, s), 5.72 (1H, s), 7.43 (1H, t, J = 8.4), 7.57–7.67 (4H, m), 7.81 (1H, dd, J = 8.4, 0.8 Hz), 8.58 (1H, d, J = 8 Hz), 9.47 (1H, dd, J = 8.4, 0.8 Hz). 13C NMR (100 MHz, DMSO‑d6): δ (ppm) 36.13, 41.26, 50.37, 120.89, 124.21, 124.33, 125.96, 126.03, 126.97, 127.53, 129.21, 129.70, 129.92, 131.59, 132.04, 133.09, 145.57, 151.02, 182.20. HRMS (ESI) m/z calcd. for C19H16N3OS+ [M]+: 334.1009; found [M + H]+: 336.1172.
2.1.1.3.16 6-(2-(Dimethylamino)ethylamino)-12H-thiochromeno[2, 3-c]quinolin-12-one (18)
Compound 18 was prepared from 4 and N, N-dimethylethylenediamine (4.5 mmol). The compound was obtained in a 52 % yield. Rf = 0.67 in MeOH: CH2Cl2 (1:4), Mp 157–159 °C. 1H NMR (400 MHz, DMSO‑d6): δ (ppm) 2.36 (6H, s), 2.69 (2H, t, J = 6.0 Hz), 3.75 (2H, q, J = 5.2 Hz), 5.85 (1H, s), 7.42 (1H, t, J = 7.2), 7.55–7.70 (4H, m), 7.81 (1H, d, J = 8.4 Hz), 8.58 (1H, d, J = 8 Hz), 9.48 (1H, d, J = 8.8 Hz). 13C NMR (100 MHz, DMSO‑d6): δ (ppm) 39.53, 45.30, 57.62, 120.84, 124.24, 124.32, 125.96, 126.04, 126.92, 127.49, 129.18, 129.68, 129.83, 131.59, 132.00, 133.0, 145.63, 151.01, 182.21. HRMS (ESI) m/z calcd. for C20H18N3OS+ [M]+: 348.1165; found [M + H]+: 350.1328.
2.1.1.3.17 6-(2-(2-hydroxyethylamino)ethylamino)-12H-thiochromeno[2,3-c]quinolin-12-one (19)
Compound 19 was prepared from 4 and N-(2-hydroxyethyl)ethylenediamine (4.9 mmol). The compound was obtained in a 49 % yield. Rf = 0.43 in MeOH:CH2Cl2 (1:4), Mp 158–160 °C. 1H NMR (400 MHz, DMSO‑d6): δ (ppm) 2.15 (1H), 2.66 (2H, t, J = 6 Hz), 2.89 (2H, t, J = 6.4 Hz), 3.48 (2H, s), 3.66 (2H, t, J = 6.4 Hz), 4.49 (1H, s), 7.01 (1H, s), 7.37 (1H, t, J = 8.4 Hz), 7.59 (1H, td, J = 8.4, 1.2 Hz), 7.67–7.71 (2H, m), 7.84 (1H, t, J = 8.4 Hz), 7.95 (1H, d, J = 8 Hz), 8.46 (1H, dd, J = 8 Hz, 0.8 Hz), 9.36 (1H, d, J = 8.4 Hz). 13C NMR (100 MHz, DMSO‑d6): δ (ppm) 42.06, 48.27, 51.85, 60.94, 120.40, 124.01, 125.20, 125.89, 127.04127.10, 128.42, 129.23, 129.41, 129.58, 131.04, 133.27, 133.49, 145.59, 151.56, 181.86. HRMS (ESI) m/z calcd. for C20H18N3O2S+ [M]+: 364.1114; found [M + H]+: 366.1274.
2.1.1.3.18 6-(3-(2-hydroxyethylamino)propylamino)-12H-thiochromeno[2,3-c]quinolin-12-one (20)
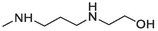
Compound 20 was prepared from 4 and N-(2-hydroxyethyl)ethylenediamine (4.2 mmol). The compound was obtained with an 84 % yield. Rf = 0.25 in MeOH:AcOH (9:1), Mp 149–151 °C. 1H NMR (400 MHz, DMSO‑d6): δ (ppm) 1.84 (2H, quin, J = 6 Hz), 2.65 (2H, t, J = 6 Hz), 2.73 (2H, t, J = 6.4 Hz), 3.57 (2H, t, J = 5.6 Hz), 3.64 (2H, t, J = 6.4 Hz), 4.58 (1H, s), 7.35 (1H, t, J = 8 Hz), 7.57 (1H, t, J = 7.6 Hz, Ar-H9), 67–7.69 (2H, m, Ar-H3,10), 7.83 (1H, t, J = 7.6 Hz, Ar-H8), 7.88 (1H, d, J = 8 Hz, Ar-H1), 8.45 (1H, d, J = 8 Hz, Ar-H11), 9.35 (1H, d, J = 8.4 Hz). 13C NMR (100 MHz, DMSO‑d6): δ (ppm) 28.40, 41.98, 48.56, 52.28, 60.89, 120.31, 123.82, 125.31, 125.93, 127.02, 128.39, 129.13, 129.44, 129.53, 131.06, 133.26, 133.60, 145.78, 151.56, 181.87. HRMS (ESI) m/z calcd. for C21H20N3O2S+ [M]+: 378.1271; found [M + H]+: 380.1431.
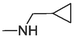
2.1.1.3.19 6-(Cyclopropylmethylamino)-12H-thiochromeno[2, 3-c]quinolin-12-one (21)
Compound 21 was prepared from 4 and cyclopropanemethylamine (7 mmol). The compound was obtained in a 56 % yield. Rf = 0.77 in CH2Cl2, Mp = 169–171 °C. 1H NMR (400 MHz, CDCl3): δ (ppm) 0.39 (2H, q, J = 4.8 Hz, –CH2-), 0.62–0.66 (2H, m, –CH2-), 1.23–1.29 (1H, m, –CH-), 3.56 (2H, q, J = 5.2 Hz, –CH2-), 5.02 (1H, t, J = 4.8 Hz, –NH-), 7.41–7.45 (1H, m, Ar-H2), 7.55–7.66 (4H, m, Ar-H3,8,9,10), 7.80 (1H, dd, J = 8.4, 0.8 Hz, Ar-H1), 8.57 (1H, d, J = 8 Hz, Ar-H11), 9.46 (1H, dd, J = 8.4, 0.8 Hz, Ar-H4). 13C NMR (100 MHz, CDCl3): δ (ppm) 3.65, 10.73, 47.49, 120.86, 123.73, 124.36, 125.92, 125.96, 127.02, 127.56, 129.22, 129.71, 129.94, 131.57, 132.07, 132.89, 145.55, 150.71, 182.16. HRMS (ESI) m/z calcd. for C20H15N2OS+ [M]+: 331.0900; found [M + H]+: 333.1057.
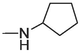
2.1.1.3.20 6-(Cyclopentylamino)-12H-thiochromeno[2, 3-c]quinolin-12-one (22)
Compound 22 was prepared from 4 and cyclopentylamine (5 mmol). The compound was obtained in a 50 % yield. Rf = 0.8 in CH2Cl2, Mp = 153–155 °C. 1H NMR (400 MHz, CDCl3): δ (ppm) 1.59–1.64 (2H, m, –CH2-), 1.69–1.86 (4H, m, –CH2-), 2.27 (2H, J = 6 Hz, –CH2-), 4.66 (1H, J = 6.4 Hz, –CH-), 4.87 (1H, d, J = 6 Hz, –NH-), 7.42 (1H, t, J = 8.4 Hz, Ar-H2), 7.54–7.65 (4H, m, Ar-H3,8,9,10), 7.82 (1H, dd, J = 8.4, 0.8 Hz, Ar-H1), 8.56 (1H, d, J = 7.6 Hz, Ar-H11), 9.46 (1H, dd, J = 8.4, 0.8 Hz, Ar-H4). 13C NMR (100 MHz, CDCl3): δ (ppm) 24.03, 33.53, 53.82, 120.76, 123.75, 124.27, 125.89, 125.95, 127.21, 127.56, 129.17, 129.74, 129.94, 131.62, 132.07, 132.90, 145.71, 15.51, 182.23. HRMS (ESI) m/z calcd. for C21H17N2OS+ [M]+: 345.1056; found [M + H]+: 347.1223.
2.1.1.3.21 6-(Cyclohexylamino)-12H-thiochromeno[2, 3-c]quinolin-12-one (23)
Compound 23 was prepared from 4 and cyclohexylamine (4 mmol). The compound was obtained in a 57 % yield. Rf = 0.83 in CH2Cl2, Mp = 128–130 °C. 1H NMR (400 MHz, CDCl3): δ (ppm) 1.15–1.24 (1H, m, –CH2-), 1.35–1.53 (4H, sep, J = 11.6 Hz, –CH2-), 1.65 (2H, quin, J = 13.2 Hz, –CH2-), 1.78 (2H, q, J = 12.8 Hz, –CH2-), 2.02 (2H, q, J = 11.2 Hz, –CH2-), 4.18–4.26 (1H, m, –CH-), 6.57 (1H, d, J = 7.6 Hz, –NH-), 7.35 (1H, td, J = 9.2, 1.2 Hz, Ar-H2), 7.58 (1H, t, J = 8.8 Hz, Ar-H9), 7.68 (2H, t, J = 8 Hz, Ar-H3,10), 7.83 (1H, t, J = 8 Hz, Ar-H8), 7.93 (1H, d, J = 8 Hz, Ar-H1), 8.45 (1H, d, J = 8 Hz, Ar-H11), 9.34 (1H, d, J = 8.4 Hz, Ar-H4). 13C NMR (100 MHz, CDCl3): δ (ppm) 25.58, 25.99, 32.45, 50.72, 120.38, 123.98, 125.36, 125.86, 127.05, 127.10, 128.39, 129.35, 129.41, 129.57, 131.03, 133.28, 133.72, 145.61, 150.86, 181.93. HRMS (ESI) m/z calcd. for C22H19N2OS+ [M]+: 359.1213; found [M + H]+: 361.1375.
2.1.1.3.22 6-(2-morpholinoethylamino)-12H-thiochromeno[2, 3-c]quinolin-12-one (24)
Compound 24 was prepared from 4 and 4-(2-aminoethyl)morpholine (4 mmol). The compound was obtained in a 49 % yield. Rf = 0.27 in EA:CH2Cl2 (2:3), Mp = 136–138 °C. 1H NMR (400 MHz, CDCl3): δ (ppm) 2.60 (4H, t, J = 4.4 Hz, –CH2-), 2.79 (2H, t, J = 6 Hz, –CH2-), 3.77–3.82 (6H, m, –CH2-), 5.91 (1H, s, –NH-), 7.44 (1H, t, J = 8.4 Hz, Ar-H2), 7.58–7.63 (2H, m, Ar-H9,10), 7.67–7.73 (2H, m, Ar-H3,8), 7.81 (1H, d, J = 8 Hz, Ar-H1), 8.60 (1H, d, J = 8.4 Hz, Ar-H11), 9.48 (1H, dd, J = 8.4, 1.6 Hz, Ar-H4). 13C NMR (100 MHz, CDCl3): δ (ppm) 38.30, 53.29, 56.47, 67.25, 120.88, 124.10, 124.35, 125.97, 126.06, 126.94, 127.58, 129.24, 129.73, 129.94, 131.62, 132.07, 133.03, 145.62, 150.90, 182.16. HRMS (ESI) m/z calcd. for C22H20N3O2S+ [M]+: 390.1271; found [M + H]+: 392.1426.
2.1.1.3.23 6-(Dimethylamino)-12H-thiochromeno[2, 3-c]quinolin-12-one (25)
Compound 25 was prepared from 4 and dimethylamine (4.5 mmol). The compound was obtained in a 96 % yield. Rf = 0.87 in CH2Cl2, Mp = 141–142 °C. 1H NMR (400 MHz, CDCl3): δ (ppm) 3.07 (6H, s, –CH3), 7.54–7.70 (5H, m, Ar-H2,3,8,9,10), 7.98 (1H, dd, J = 8.4, 1.2 Hz, Ar-H1), 8.59 (1H, dt, J = 8.4, 0.8 Hz, Ar-H11), 9.61 (1H, dd, J = 8.4, 1.2 Hz, Ar-H4). 13C NMR (100 MHz, CDCl3): δ (ppm) 42.96, 123.52, 125.74, 126.18, 126.96, 127.13, 128.37, 129.13, 129.44, 130.56, 130.96, 131.25, 132.03, 136.02, 144.75, 158.34, 182.68. HRMS (ESI) m/z calcd. for C18H13N2OS+ [M]+: 305.0743; found [M + H]+: 307.0903.
2.1.1.3.24 6-(((1,3-dioxolan-2-yl)methyl)(methyl)amino)-12H-thiochromeno[2, 3-c]quinolin-12-one (26)
Compound 26 was prepared from 4 and 2-methylaminomethyl-1,3-dioxolane (4.4. mmol). The compound was obtained in a 51 % yield. Rf = 0.57 in CH2Cl2, Mp = 127–128 °C. 1H NMR (400 MHz, CDCl3): δ (ppm) 3.13 (3H, s, –CH3), 3.65 (2H, d, J = 4.4 Hz, –CH2-), 3.82–3.90 (2H, m, –CH2-), 3.94–4.00 (2H, m, –CH2-), 5.26 (1H, q, J = 4.4 Hz, –CH-), 7.50 (1H, t, J = 8 Hz, Ar-H2), 7.56–7.68 (4H, m, Ar-H3,8,9,10), 7.96 (1H, dd, J = 8, 0.8 Hz, Ar-H1), 8.54 (1H, dd, J = 8, 0.8 Hz, Ar-H11), 9.60 (1H, dd, J = 8.8, 0.8 Hz, Ar-H4). 13C NMR (100 MHz, CDCl3): δ (ppm) 42.26, 56.84, 64.87, 103.14, 123.65, 125.68, 126.17, 126.99, 127.14, 128.37, 128.98, 129.26, 130.91, 131.03, 131.87, 135.91, 144.46, 157.87, 182.418. HRMS (ESI) m/z calcd. for C21H17N2O3S+ [M]+: 377.0954; found [M + H]+: 379.1114.
2.1.1.3.25 6-(Piperidin-1-yl)-12H-thiochromeno[2, 3-c]quinolin-12-one (27)
Compound 27 was prepared from 4 and piperidine (5 mmol). The compound was obtained in a 69 % yield. Rf = 0.93 in CH2Cl2, Mp = 168–169 °C. 1H NMR (400 MHz, CDCl3): δ (ppm) 1.72 (2H, d, J = 4.8 Hz, –CH2-), 1.88 (4H, t, J = 5.2 Hz, –CH2-), 3.33 (4H, s, –CH2-), 7.53–7.71 (5H, m, Ar-H2,3,8,9,10), 7.98 (1H, d, J = 8 Hz, Ar-H1), 8.59 (1H, d, J = 8 Hz, Ar-H11), 9.63 (1H, d, J = 8.4 Hz, Ar-H4). 13C NMR (100 MHz, CDCl3): δ (ppm) 24.32, 25.91, 52.29, 123.58, 125.81, 126.27, 127.01, 127.03, 128.47, 128.99, 129.42, 130.81, 131.20, 131.63, 131.92, 136.35, 144.90, 158.55, 182.69. HRMS (ESI) m/z calcd. for C21H17N2OS+ [M]+: 345.1056; found [M + H]+: 347.1218.
2.1.1.3.26 6-(4-hydroxypiperidin-1-yl)-12H-thiochromeno[2, 3-c]quinolin-12-one (28)
Compound 28 was prepared from 4 and 4-hydroxypiperidine (3 mmol). The compound was obtained in a 29 % yield. Rf = 0.57 in EA:CH2Cl2 (2:3), Mp = 209–210 °C. 1H NMR (400 MHz, CDCl3): δ (ppm) 1.64 (1H, s, –OH), 1.89–1.98 (2H, m, –CH2-), 2.16–2.20 (2H, m, –CH2-), 3.19 (2H, t, J = 12.8 Hz, –CH2-), 3.63–3.68 (2H, m, –CH2-), 4.01 (1H, sep, J = 4.4 Hz, –CH-), 7.55–7.72 (5H, m, Ar-H2,3,8,9,10), 7.98 (1H, d, J = 8 Hz, Ar-H1), 8.60 (1H, d, J = 8.8 Hz, Ar-H11), 9.64 (1H, d, J = 8.8 Hz, Ar-H4). 13C NMR (100 MHz, CDCl3): δ (ppm) 34.52, 48.87, 67.90, 123.73, 125.88, 126.32, 127.17, 127.30, 128.55, 129.13, 129.52, 130.96, 131.26, 131.32, 132.08, 136.14, 144.84, 157.89, 182.67. HRMS (ESI) m/z calcd. for C21H17N2O2S+ [M]+: 361.1005; found [M + H]+: 363.1163.
2.1.1.3.27 6-(1,4′-bipiperidin-1′-yl)-12H-thiochromeno[2, 3-c]quinolin-12-one (29)
Compound 29 was prepared from 4 and 4-piperidinopiperidine (2.5 mmol). The compound was obtained in a 38 % yield. Rf = 0.7 in MeOH:CH2Cl2 (1:4), Mp = 185–187 °C. 1H NMR (400 MHz, CDCl3): δ (ppm) 1.50–1.53 (2H, m, –CH2-), 1.66 (4H, quin, J = 5.6 Hz, –CH2-), 1.78 (2H, s, –CH2-), 1.93 (2H, qd, J = 13.6, 4 Hz, –CH2-), 2.06 (2H, d, J = 10.8 Hz, –CH2-), 2.48–2.56 (1H, m, –CH-), 2.61–2.66 (4H, m, –CH2-), 3.04 (2H, t, J = 11.2 Hz, –CH2-), 3.76 (2H, d, J = 12.8 Hz, –CH2-), 7.55–7.71 (5H, m, Ar-H2,3,8,9,10), 7.98 (1H, dd, J = 8.4, 0.8 Hz, Ar-H1), 8.60 (1H, d, J = 8.4 Hz, Ar-H11), 9.64 (1H, dd, J = 8.4, 1.2 Hz, Ar-H4). 13C NMR (100 MHz, CDCl3): δ (ppm) 24.83, 26.43, 28.28, 50.47, 51.13, 62.45, 123.67, 125.85, 126.29, 127.10, 127.17, 128.51, 129.07, 129.48, 130.86, 131.23, 131.45, 132.02, 136.25, 144.86, 158.00, 182.67. HRMS (ESI) m/z calcd. for C26H26N3OS+ [M]+: 428.1791; found [M + H]+: 430.1946.
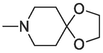
2.1.1.3.28 6-(1,4-dioxa-8-azaspiro[4.5]decan-8-yl)-12H-thiochromeno[2,3-c]quinolin-12-one (30)
Compound 30 was prepared from 4 and 4-piperidone-ethylene ketal (4 mmol). The compound was obtained in a 40 % yield. Rf = 0.83 in EA:CH2Cl2 (2:3), Mp = 201–203 °C. 1H NMR (400 MHz, CDCl3): δ (ppm) 2.05 (4H,t, J = 5.6 Hz, –CH2-), 3.50 (4H,t, J = 5.6 Hz, –CH2-), 4.05 (4H,s, –CH2-), 7.55–7.72 (5H, m, Ar-H2,3,8,9,10), 7.98 (1H, dd, J = 8,1.2 Hz, Ar-H1), 8.60 (1H, dd, J = 8, 0.8 Hz, Ar-H11), 9.64 (1H, dd, J = 8.4, 1.2 Hz, Ar-H4). 13C NMR (100 MHz, CDCl3): δ (ppm) 35.06, 49.26, 64.44, 107.11, 123.66, 125.82, 126.30, 127.14, 127.22, 128.62, 129.09, 129.49, 130.90, 131.12, 131.24, 132.04, 136.10, 144.84, 157.63, 182.67. HRMS (ESI) m/z calcd. for C23H19N2O3S+ [M]+: 403.1111; found [M + H]+ = 405.1277.
2.2 Biological evaluation
2.2.1 Initial in vitro cytotoxicity screening of compounds
To investigate the potential cytotoxic effects of synthesized compounds 5 ∼ 30, MTT assays were performed to determine the 50 % inhibitory concentration (IC50) values of each compound against the MCF-7 and MDA-MB-468 human breast carcinoma cell lines. These cell lines were obtained from American Type Culture Collection (USA) and cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10 % heat-inactivated fetal bovine serum (FBS) at 37 °C in a humidified atmosphere containing 5 % CO2 co.
MCF-7 and MDA-MB-468 cells were seeded (at 3000 cells/well) in 96-well microplates containing DMEM supplemented with 10 % FBS and treated with various concentrations of the compounds for 72 h. The microplates were rinsed three times with phosphate-buffered saline (PBS) after treatment, and 100 µL of an MTT solution (at a final concentration of 0.5 mg/mL in the medium) was added to each well, followed by incubation at 37 °C for 1 h. Mitochondrial succinate dehydrogenase was used to convert MTT to blue formazan crystals. The microplates were washed again with PBS and then solubilized with 100 µL of DMSO per well. The absorbance at 540 nm was measured using an enzyme-linked immunosorbent assay (ELISA) microplate reader to determine the effects of the synthetic compounds on cell viability as relative activities.
2.2.2 One-dose and five-dose assays of the NCI-60 human tumor cell lines screening program
We conducted experiments to test the growth-inhibition capabilities of our selected compounds against a panel of 60 human cancer cell lines under the NCI drug screening program. Initially, the compounds were tested at a single high dose concentration of 10 µM. Subsequently, those compounds that demonstrated significant growth-inhibitory capabilities and fulfilled the predetermined threshold inhibition criteria of the NCI were selected to proceed to the five-dose assay. The methodology used in this study was described in detail in our previous publications (Chen et al., 2019; Chen et al., 2019).
2.3 In silico study
2.3.1 Drug-likeness, ADME, and toxicity predictions
The drug-likeness, ADME (Absorption, Distribution, Metabolism, and Excretion), and toxicity properties of all synthesized compounds were predicted using the online software tools swissADME (http://www.swissadme.ch/) (Daina et al., 2017; Daina et al., 2014) and ADMETlab 2.0 (https://admetmesh.scbdd.com/) (Xiong et al., 2021; Dong et al., 2018).
2.3.2 Identification of drugs with similar profiles to our compounds by a COMPARE analysis
Results from the NCI five-dose cytotoxicity studies of compounds 18 (NSC784447) and 20 (NSC784449) were used as a “seed” in COMPARE algorithms to correlate with investigational drugs and standard drugs in NCI databases using Pearson's correlation coefficient calculations. At the same time, GI50, TGI, and LC50 were set as endpoints (Ali et al., 2016; Lawal et al., 2021).
2.3.3 Molecular modeling
Crystal structures of recombinant human topoisomerase (TOPO) I (PDB ID: 1EJ9) (Redinbo et al., 2000), TOPO II (PDB ID: 4FM9) (Wendorff et al., 2012), CDK4 (PDB ID: 2W9Z) (Day et al., 2009), AURKA (PDB ID: 5ORY) (McIntyre et al., 2017), and CDK6 (PDB ID: 3NUP) (Cho et al., 2010) were downloaded from the protein data bank (PDB) site (https://www.rcsb.org/). Pymol was used to prepare protein structures (Schrödinger and DeLano, 2020). Avogadro optimized the ligand structure, bond length, and bond angle after the 2D ligand structure was created with ChemDraw Ultra 12.0 software (Hanwell et al., 2012). The ligand and protein receptor were both saved as PDB files.
Auto dock vina software (Eberhardt et al., 2021; Trott and Olson, 2010) was used for docking investigations after the above procedures were finished. Auto-dock was used to import the prepared PDB files (proteins and ligands) and save them in pdbqt file format. Further, amino acids from the active sites were also targeted to correct the grid parameters. The Discovery Studio Visualizer 2021 client was used to examine and visualize these docking conformations (in both 2D and 3D).
3 Result and discussion
3.1 Chemistry
Compound 3 was sequentially synthesized by the Pfitzinger reaction using isatin and (thiophenyl) acetic under alkaline conditions. The acid reaction resulted in 2-hydroxy-3-(phenylthio) quinoline-4-carboxylic acid (1). This is because the amide bond is easily hydrolyzed in alkaline conditions, opening the ring and forming ketoacids. When the loop is opened, the intermediate is easily converted to quinoline-4-carboxylic acid compounds by reacting with (thiophenyl) acetic acid (carbonyl compound) (Komatsu et al., 2023). Chlorination and a cyclization reaction were used to obtain 6-chloro-12H-thiochromeno [2,3–c] quinoline-12-one (compound 4) using POCl3. The synthesis of 5–30 was carried out via amination of 4 with various suitable primary amines, secondary amines, and sodium bicarbonate. The appropriate primary and secondary amines and 4 were reacted in DMSO to form 5–30 (35 %–96 %), respectively. The detailed reaction process is shown in Scheme 2. 1H nuclear magnetic resonance (NMR), 13C NMR, and high-resolution mass spectroscopy (HRMS) spectra were used to determine their chemical compositions.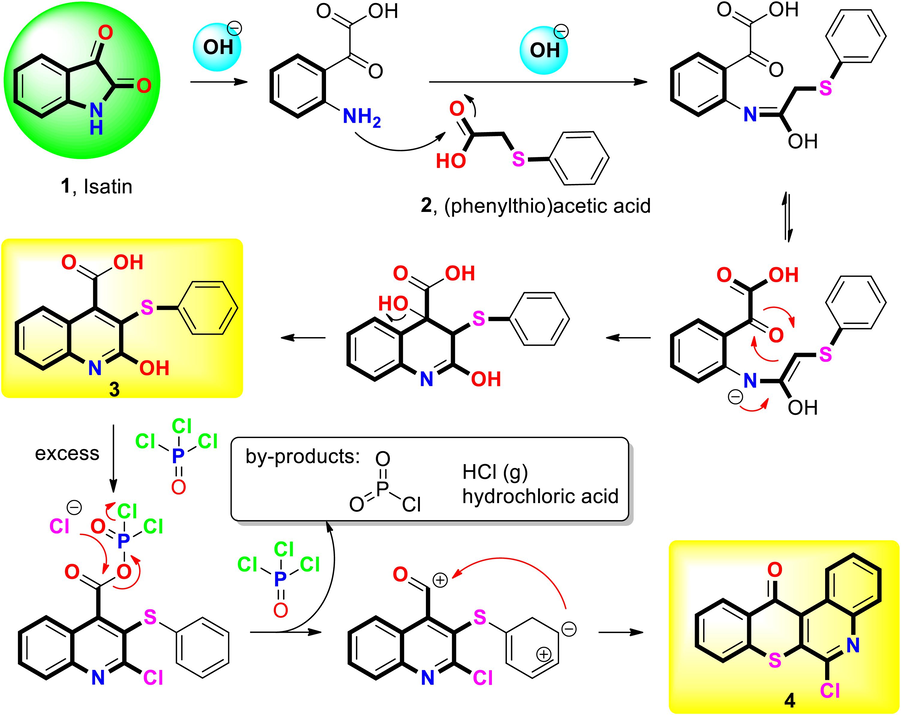
Mechanism and plausible catalytic cycle during Pfitzinger reaction and cyclization.
Compound 3 structurally contains an acid group (–COOH) according to 1H NMR results, where a peak was found at 11–13 ppm, and according to 13C NMR results, where the acid group (–COOH) was located at 166.44 ppm. However, the carbonyl group (C = O) of compound 4 in the 13C NMR signal was found at 181.52 ppm, demonstrating the peak that resulted from the substitution of a carbonyl group (C = O) for an acid group (–COOH) on the structure. The sixth position of compounds 4, 5, and 25 were utilized based on 13C NMR results. After additional functional groups replaced the position of chlorine in the primary structure, the direction of the low magnetic field chemically shifted due to carbon atoms (downfield). When the substituted group was a primary amine (compound 5), the peak showed its location in both 1H NMR and 13C NMR results, and the corresponding position was demonstrated to be a substitution of secondary amines (compound 25) biased toward a strong magnetic field (upfield) (Table S1).
The conditions, times, and yields of reactions differed depending on the amine type. The reaction yield could be decreased, and in some cases, the reaction proceeded only under controlled temperature conditions. Therefore, in these kinds of processes, temperature control is essential (Table S4). Furthermore, due to limited yield, it was challenging to obtain crystalline material for the most promising compounds, specifically compounds 18 and 20. However, compound 25 yielded large crystals when crystallized in hot CH2Cl2 with a slow evaporation process, allowing its structural determination by SC-XRD (Fig. 2, Table S2).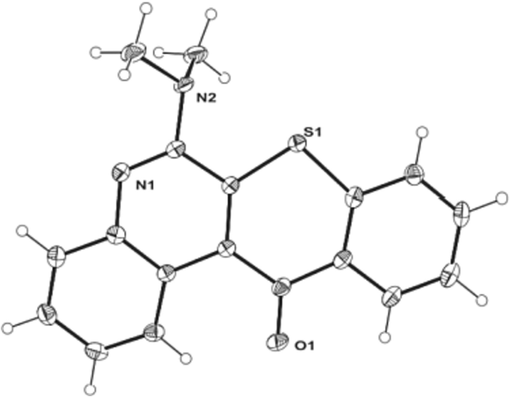
ORTEP diagram of compound 25.
3.2 Biological evaluation
3.2.1 In vitro cytotoxicity screening of compounds and structure–activity relationship (SAR) studies
We tested the toxicity of active substances against MCF-7 and MDA-MB-468 human breast cancer cells. SAR data revealed that most of the compounds had potent cytotoxic effects and that primary amine substitutions (IC50 values of 2.3 to > 20 mM) had stronger anticancer activity than secondary or tertiary amine substitutions. Additionally, an alkylamine substitution showed that the carbon side chain length of the 6H-thiochromeno [2,3-c] quinoline-12 (12H)-one scaffold was essential for enhancing the cytotoxic effects of our synthesized compounds.
Compounds 5, 17, 18, 19, 20, and 24 exhibited the lowest IC50 values against MCF7 and MDA-MB-468 cells out of all the synthesized compounds (Table 1). However, antiproliferative results for compounds 9, 10, 15, and 23 only demonstrated anticancer activities in MDA-MB-468 cells, while compound 21 only demonstrated such activities in MCF-7 cells (other compounds showed IC50 values of > 20 μM in both cell lines).
As shown in Table 2, we further investigated the selectivity index (SI) to analyze the sensitivities of compounds 5, 17, 18, 19, 20, and 24 against both MCF7 and MDA-MB-468 cells. Compounds 5, 17, 18, 19, and 20 exhibited high sensitivity (SI > 1) toward MCF-7 cells, while only compounds 5 and 17 showed high sensitivity toward MDA-MB-468 cells. Compounds 18, 19, and 20 displayed no sensitivity (SI < 1) toward MDA-MB-468 cells. However, compound 24 showed no selectivity (SI < 1) toward either cell line. Overall, compounds 17 and 18 were the most active and sensitive among these TC-S-1 derivatives against both MCF7 and MDA-MB-468 cells.
3.2.2 Evaluation of the cytotoxic activities of compounds by one-dose assays of the NCI-60 human tumor cell line screening program
Nine compounds, including 6 (NSC784437), 7 (NSC784438), 16 (NSC784445), 18 (NSC784447), 20 (NSC784449), 24 (NSC784440), 25 (NSC784442), 28 (NSC784441), and 30 (NSC784444), were selected by the one-dose assays of the NCI to be evaluated against various types of cancer, including leukemia, non-small cell lung cancer (NSCLC), colon cancer, central nervous system (CNS) cancers, melanomas, ovarian cancers, renal cancers, prostate cancer, and breast cancer (Martorana et al., 2022). We primarily evaluated these nine compounds for their antiproliferative and cytotoxic effects against the NCI-60 cell lines. The results of each drug were expressed as the ratio of the growth percentage of cells treated with a compound concentration at 10 µM to the growth percentage of untreated control cells. Among the evaluated compounds, 18 (NSC784447) and 20 (NSC784449) demonstrated anticancer activities against these cancer cell lines.
The most inhibited cell lines after treatment with compound 18 (NSC784447) were LOX IMVI, COLO 205, M14, SK-MEL-5, MCF7, SW-620, MDA-MB-468, and K-562 which showed respective growth percentages of −91.59 %, −9.58 %, −54.67 %, −83.13 %, −46.92 %, −54.03 %, –23.62 %, and −35.06 %. For 20 (NSC784449), the most affected cell lines were MCF7, MDA-MB-468, COLO 205, SW-620, LOX IMVI, M14, SK-MEL-5 and HCT-116 which showed respective growth percentages of −72.93 %, −57.26 %, −78.95 %, −62.45 %, −87.42 %, −61.84 %, 64.67 %, and −57.48 % (Fig. 3). Compound 24 (NSC784440) showed inhibitory effects against the K-562 and SF295 cell lines and reduced their growth percentages to −11.67 % and −7.24 %.
Growth percentages of the NCI-60 human cancer cell lines after treatment with a single dose of 10 µM of each of our selected compounds 6 (NSC784437), 7 (NSC784438), 16 (NSC784445), 18 (NSC784447), 20 (NSC784449), 24 (NSC784440), 25 (NSC784442), 28 (NSC784441), and 30 (NSC784444).
Multiple cancer cell lines responded effectively to the initial single doses of 18 (NSC784447) and 20 (NSC784449) of 10 µM, indicating the need for further research into the dose-dependent effects. However, for this investigation, we primarily focused on data related to breast cancer.
3.2.3 Compounds 18 (NSC784447) and 20 (NSC784449) exhibit dose-dependent anticancer activities tested by five-dose assays of NCI-60 human cancer cell
Further investigation was carried out since NCI-60 one-dose screening parameters for threshold inhibition were met by 18 (NSC784447) and 20 (NSC784449) in equal measure. Five dosages of our compounds were evaluated in each cell line. Compound 18 (NSC784447) and 20 (NSC784449) dose–response curves were plotted using the percentages of growth of each tested cell line following treatment with various concentrations of those compounds, and percentages of the results relative to the values of the untreated control cells are shown as the 50 % growth inhibitory concentration (GI50), the 50 % lethal concentration (LC50), and the total growth inhibition (TGI). However, this time, we concentrated on treating breast cancer.
Results demonstrated that the T-47D, HS 578 T, MDA-MB-231/ATCC, and MCF7 breast cancer cell lines were more responsive in dose-dependent manners to 18 (NSC784447) than to 20 (NSC784449). Two other cell lines, MDA-MB-468 and BT-549, were more responsive to 20 (NSC784449) treatment (Fig. 4).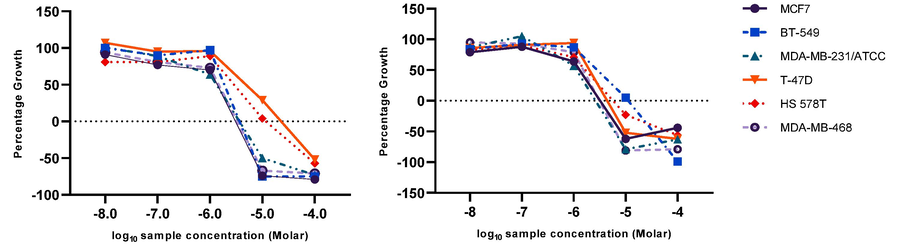
Graphs illustrate the relationship between the dose and the effect of compounds 18 (NSC784447) and 20 (NSC784449) on breast cancer cell lines. A growth percentage value of 100 means that the cells grew at the same rate as untreated cells, while a value of 0 indicates no growth during the experiment, and a value of −100 signifies that all the cells were dead by the end of the experiment.
Compound 20 showed higher activity against breast cancer (GI50 range of 1.13–2.81 µM) than compound 18 (GI50 range of 1.34–4.91 µM) (Fig. 5). These findings confirmed our initial screening findings and highlighted these potential compounds as potential future anticancer medications. Therefore, in this study, we aimed to characterize better and comprehend the biological actions of these two compounds.
Cytotoxic activities of 18 (NSC784447) and 20 (NSC784449) against breast cancer cell lines. GI50, 50% growth inhibition; TGI, total growth inhibition; LC50, 50% loss of cells.
3.3 In-silico studies
3.3.1 Analysis of drug-likeness, absorption, distribution, metabolism and excretion (ADME), and toxicity parameters
Synthesized compounds 5, 17, 18, 19, 20, and 24 were analyzed using the SwissADME (Daina et al., 2017) and ADMETlab 2.0 tools (Xiong et al., 2021). These compounds met the criteria for drug properties as outlined by Lipinski's rule of five (Chen et al., 2020), Veber's rules (Yadav et al., 2021), and Ghose's rules (Morak-Młodawska et al., 2023). Log p values, which show the hydrophobicity or lipophilicity of a molecule, indicate that the compounds have acceptable values for absorption and permeability (Chen et al., 2023). The total polar surface area (TPSA), a measure of the polar surface area of a molecule, was also in a good range for oral bioavailability (TPSA values of < 140 Å) (Yukawa and Naven, 2020). The ADME analysis revealed that all of the compounds have the ability to be absorbed by the human intestine, and some even have the ability to cross the blood–brain barrier. Toxicity predictions showed that these compounds have no potential for carcinogenicity and a low risk for cardiotoxicity (Table 3, Table S3).
3.3.2 Identification of drugs with similar profiles to our compounds by a COMPARE analysis
The DTP-COMPARE analysis showed that 18 (NSC784447) and 20 (NSC784449) have antitumor characteristics that resembled those of investigated drugs and standard agents, with p values of 0.11–0.47 for 18 (NSC784447) and 0.21–0.52 for 20 (NSC784449). Table 4 presents the target descriptors, mechanisms, cell counts, and p values for the investigated drugs and standard agents.
Compound
Investigational drug
Standard agent
Rank
r
CCLC
Target descriptor
Mechanism
Rank
r
CCLC
Target descriptor
Mechanism
18 (NSC784447)
1
0.47
59
Anlotinib
RTK inhibitor
1
0.17
58
Mitindomide
Topo II inhibitor
2
0.41
58
CT-XL228
Bcr-Abl inhibitor
2
0.16
58
Aclacinomycin
Topo I and II inhibitor
3
0.40
50
AMG900
AURKA inhibitor
3
0.16
59
Echinomycin
HIF1 inhibitor
4
0.37
56
Alisertib
AURKA inhibitor
4
0.15
41
Maytansine
Tubulin inhibitor
5
0.36
59
Flavopiridol
CDK inhibitor
5
0.15
58
ICRF-159
Topo II inhibitor
6
0.36
56
AZD-4205
JAK inhibitor
6
0.14
59
Thioguanine
PARP inhibitor
7
0.34
56
X396
ALK inhibitor
7
0.13
59
Carboplatin
PARP inhibitor
8
0.31
59
Foretinib
MET inhibitor
8
0.13
49
Menogaril
Topo II inhibitor
9
0.31
59
Bafetinib
Bcr-Abl inhibitor
9
0.11
58
ICRF-187
Topo II inhibitor
10
0.29
59
Danusertib
AURKA inhibitor
10
0.11
59
VP-16
Topo II inhibitor
20(NSC784449)
1
0.52
58
XR-11576
Topo I and II inhibitor
1
0.50
58
Amonafide
Topo II inhibitor
2
0.49
59
Narazaciclib
CDK4/6 inhibitor
2
0.50
58
Pyrazoloacridine
HER2 inhibitor
3
0.48
58
XR-5000
Topo I and II inhibitor
3
0.44
58
ICRF-187
Topo II inhibitor
4
0.46
51
AS-703569
AURKA inhibitor
4
0.44
49
Deoxydoxorubicin
Topo II inhibitor
5
0.46
56
XL-019
JAK inhibitor
5
0.43
58
Menogaril
Topo II inhibitor
6
0.45
58
CT-XL228
Bcr-Abl inhibitor
6
0.37
59
Doxorubicin
Topo II inhibitor
7
0.43
56
Alisertib
AURKA inhibitor
7
0.33
56
Tamoxifen
Nonsteroidal agent
8
0.43
57
Pamiparib
PARP1/2 inhibitor
8
0.30
59
5-fluorouracil
cell-cycle inhibitor
9
0.41
59
Vosaroxin
Topo I and II inhibitor
9
0.28
58
Etoposide
Topo II inhibitor
10
0.31
57
Pacritinib
JAK inhibitor
10
0.21
58
Topotecan
Topo I inhibitor
Interestingly, our analysis of correlation patterns of 20 (NSC784449) correlated with amonafide, ICRF-187, deoxydoxorubicin, menogaril, and doxorubicin, which have been mechanistically reported to exhibit anticancer activities via TOPO II inhibition. Compound 18 (NSC784447), on the other hand, shared similar (r = 0.11–0.17) standard agent fingerprints with known inhibitors of TOPO II, such as mitindomide, ICRF-159, menogaril, ICRF-187, and VP-16. However, the investigated drug fingerprints showed the highest correlations in both 18 (NSC784447) and 20 (NSC784449) with multiple mechanisms of action, such as inhibition of AURKA, Janus kinase (JAK), cyclin-dependent kinase 4/6 (CDK4/6), poly(ADP ribose) polymerase 1/2 (PARP1/2), and receptor tyrosine kinase (RTK) (r = 0.41–0.52).
3.3.3 Molecular modeling
Etoposide, topotecan, ICRF-187, and doxorubicin have molecular structures similar to those of compounds 18 (NSC784447) and 20 (NSC784449) and are powerful inhibitors of TOPO I and II by interfering with TOPO-DNA complexes, resulting in DNA damage and cell death (Bali et al., 2018; Li et al., 2017; Marinello et al., 2018). Notably, amine-substituted derivatives of anthra[2,1–c] [1,2,5]thiadiazole-6,11-dione showed strong TOPO I inhibitory effects (Ali et al., 2021), indicating that our compounds might have related activities.
To investigate whether our series of compounds are potential inhibitors, compounds 18 (NSC784447) and 20 (NSC784449) were docked in predicted active target sites, including AURKA, CDK4, CDK6, TOPO I, and TOPO II. They exhibited binding energies ranging from −6.1 to −8.8 kcal/mol and interacted with neighboring amino acid residues (Table 5). Ligand-protein binding interactions are illustrated in 2D and 3D figures (Fig. 6).
Protein
18 (NSC784447)
20 (NSC784449)
ΔG = (kcal/mol)
Type of interaction
Interacting AA (distance (Å))
ΔG = (kcal/mol)
Type of interaction
Interacting AA (distance (Å))
AURKA
−8.5
Pi-Alkyl
LEU:139 (3.98)ALA:273
(4.43)−8.4
Pi-Alkyl
LEU:139 (3.94)ALA:273
(4.31)
H-Bond
GLU:260 (2.65)
H-Bond
GLU:260 (2.72)GLY:145
(2.20)
Pi-Sigma
LEU:263 (3.76)
Pi-Sigma
LEU:263 (3.72)
CDK4
−8.1
H-Bond
ASP:158 (3.22)
−7.4
H-Bond
LYS:35 (2.62)ILE:12
(1.86; 4.95; 5.14)
Pi-Anion
GLU:144 (4.99)
C–H-Bond
THR:102 (3.66)
Pi-Alkyl
VAL:20 (5.28)LEU:147
(4.58)Pi-Alkyl
LEU:147 (4.91)VAL:20
(4.65)
CDK6
−6.6
H-Bond
LEU:281 (2.29)
−6.1
H-Bond
LYS:279 (2.56)
C–H-Bond
GLY:236 (3.53)ILE:235
(3.58)C–H-Bond
THR:282 (3.48; 3.70)PHE:283
(2.85)
Pi-Sigma
LEU:278 (3.72)
Pi-Cation
LYS:287 (3.73;5.27;5.05)
TOPO I
−8.8
H-Bond
DG:6 (2.29)DA:7
(2.37)−8.7
H-Bond
DA:114 (1.93; 4.67; 2.25)
C–H-Bond
GLN:421 (3.47)GLY:422
(3.54)C–H-Bond
ILE:424 (3.14)
Pi-Alkyl
LYS:493 (5.08)
Pi-Sigma
DT:116 (3.42; 5.89)
TOPO II
−8.6
H-Bond
ASN:779
ARG:929−7.3
H-Bond
ARG:727 (2.56)GLU:712
(2.33)
C–H-Bond
SER:778
GLY:777C–H-Bond
GLY:1007 (3.37)
Pi-Anion
DG:10
Pi-Anion
GLU:839 (4.22; 4.43)
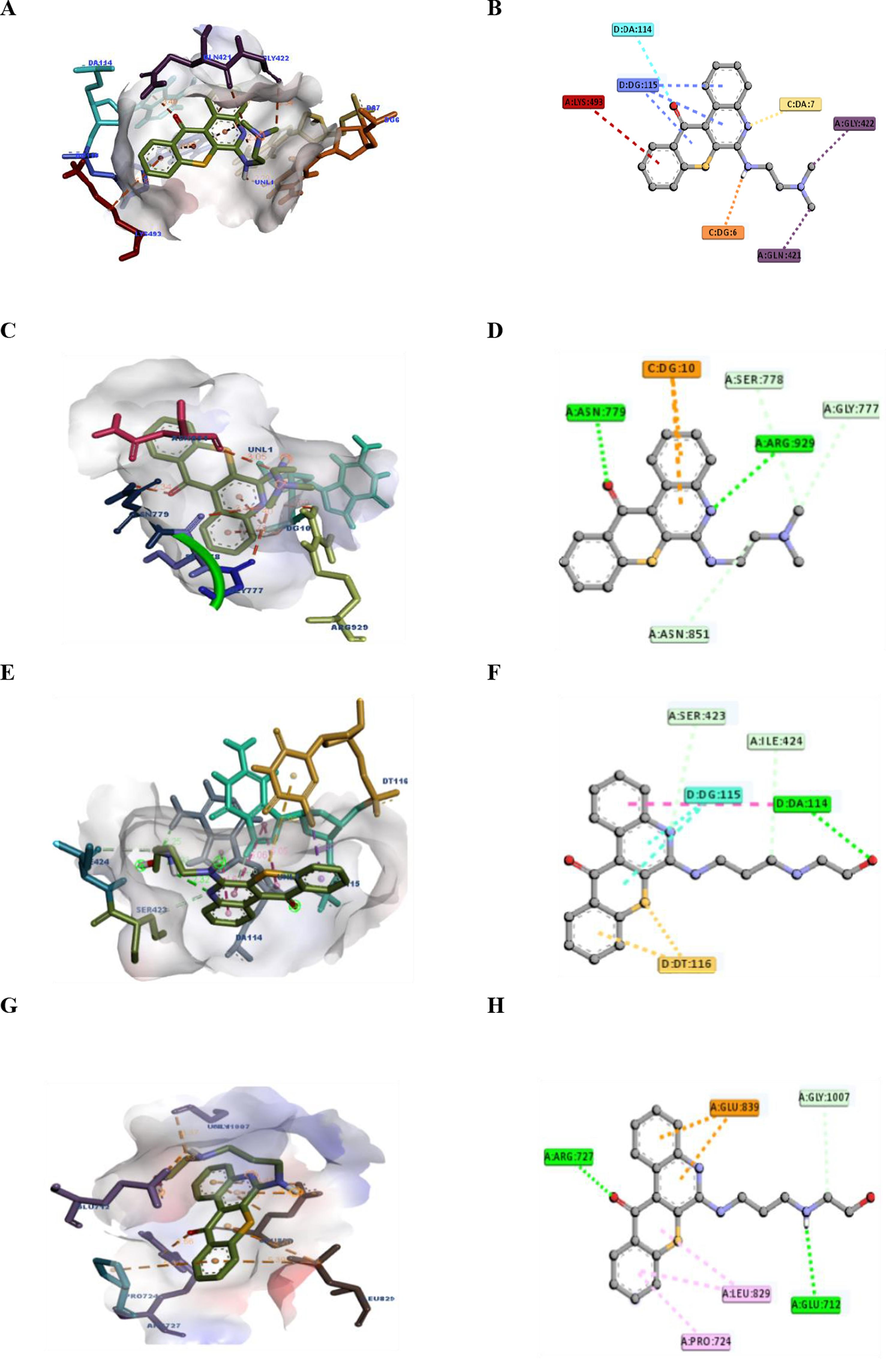
Binding interactions of compounds 18 and 20 at topoisomerase (TOPO) I and TOPO II active sites. (A) 3D structures of 18 and the TOPO I (PDB ID: 1EJ9) receptor and distances (left). (B) 2D analysis of the amino acids and bonding interactions within the complex formed by 18 and TOPO I using Discovery Studio software. (C) 3D structures of 20 and the TOPO II (PDB ID: 4FM9) receptor and distances (left). (D) 2D analysis of the amino acids and bonding interactions within the complex formed by 20 and TOPO II using Discovery Studio software.
The highest binding score was for compound 18 (NSC784447), with a binding score of −8.8 to TOPO I, followed by other proteins, including TOPO II, AURKA, and CDK4, with respective binding scores of −8.6, −8.5, and −8.1. In contrast, compound 20 (NSC784449) showed the highest binding score to the same proteins as compound 18 (NSC784447), such as TOPO I, TOPO II, AURKA, and CDK4, with respective binding scores of −8.7, −7.3, −8.4, and −7.4. The binding affinities were consistent with the observed cytotoxic activities and IC50 values, indicating that the binding of compounds to TOPO I was strongly associated with their cytotoxic activities. However, a few exceptions were observed.
4 Conclusions
In summary, a computational fragment-based drug discovery (FBDD) approach to develop and synthesize 26 compounds of tetraheterocyclic derivatives based on the 6H-thiochromeno[2,3–c]quinoline-12(12H)-one scaffold. This particular scaffold was selected because of its adaptability and distinctive moieties for medicinal applications, particularly in targeting DNA topoisomerases, which play a key role in addressing the demand for new anticancer drugs in breast cancer therapy. We investigated their cytotoxic and biochemical activities as multitarget small-molecule inhibitors through experiments involving two human breast cancer cell lines (MCF-7 and MDA-MB-468) and a panel of NCI-60 cancer cell lines. Structure-activity relationship (SAR) studies revealed that primary amine substitutions had stronger anticancer activity than secondary or tertiary amine substitutions. Compounds 5, 17, 18, 19, 20, and 24 showed promising cytotoxicity profiles against breast cancer cell lines. Computational analysis indicated that the synthesized compounds exhibited drug-like properties, with acceptable log P values, polar surface areas (TPSA), and the potential for intestinal absorption. The compounds demonstrated no potential for carcinogenicity and low risk of cardiotoxicity.
Further, we aimed to understand the mechanisms of action of our compounds by performing in-silico studies using a COMPARE analysis and molecular modeling experiments.
Overall, the study presents a promising series of compounds with potential multitarget anticancer properties, particularly in breast cancer treatment. The compounds showed favorable drug-like properties and demonstrated significant cytotoxicity against breast cancer cell lines. These findings suggest that compounds 18 (NSC784447) and 20 (NSC784449) warrant further investigation as potential anticancer agents. The work contributes to the development of novel drugs for cancer therapy and underscores the importance of interdisciplinary approaches that combine chemistry, biology, and computational modeling in drug discovery.
Acknowledgments
The authors thank the NCI Developmental Therapeutics Program (DTP) for the 60-cancer-cell-line screening of selected compounds described in this paper, funded by the National Cancer Institute and National Institutes of Health (NIH-NCI).
Funding
The National Science and Technology Council, Taiwan (NSTC112-2314-B-038-006) and the Shin Kong Wu Ho-Su Memorial Hospital (SKH-TMU-112-02) awarded to H.-S. Huang. ATH Wu was also funded by Taipei Medical University (TMU111-AE2-I14-3) and the National Science and Technology Council (112-2314-B-038 -019-).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Synthesis of heterocyclic compounds via Michael and Hantzsch reactions. J. Heterocycl. Chem.. 2020;57(4):1476-1523.
- [Google Scholar]
- Aqueous-Mediated green synthesis of novel spiro[indole-quinazoline] derivatives using kit-6 mesoporous silica coated Fe3O4 nanoparticles as catalyst. J. Heterocycl. Chem.. 2020;57(7):2729-2737.
- [Google Scholar]
- Novel Anthra[1,2-c][1,2,5]thiadiazole-6,11-diones as promising anticancer lead compounds: Biological evaluation, characterization & molecular targets determination. PLoS One. 2016;11(4):e0154278.
- [Google Scholar]
- Structure-based strategies for synthesis, lead optimization and biological evaluation of N-substituted anthra[1,2-c][1,2,5]thiadiazole-6,11-dione derivatives as potential multi-target anticancer agents. Arab. J. Chem.. 2021;14(2):102884
- [Google Scholar]
- Preparation, characterization, antioxidant properties of novel Schiff bases including 5-chloroisatin-thiocarbohydrazone. Res. Chem. Intermed.. 2020;46(5):2541-2557.
- [Google Scholar]
- Activity of topotecan toward the DNA/topoisomerase I complex: A theoretical rationalization. Biochemistry. 2018;57(9):1542-1551.
- [Google Scholar]
- Synthesis and biological evaluation of anthra [1, 9-cd] pyrazol-6 (2H)-one scaffold derivatives as potential anticancer agents. Arab. J. Chem.. 2019;12(8):2864-2881.
- [Google Scholar]
- Synthesis and evaluation of new 3-substituted-4-chloro-thioxanthone derivatives as potent anti-breast cancer agents. Arab. J. Chem.. 2019;12(8):3503-3516.
- [Google Scholar]
- Analysis of the physicochemical properties of acaricides based on Lipinski's rule of five. J. Comput. Biol.. 2020;27(9):1397-1406.
- [Google Scholar]
- Chen, Y.-F., et al. In Vitro and In Silico Biological Studies of 4-Phenyl-2-quinolone (4-PQ) Derivatives as Anticancer Agents. Molecules, 2023. 28, DOI: 10.3390/molecules28020555.
- 4-(Pyrazol-4-yl)-pyrimidines as Selective Inhibitors of Cyclin-Dependent Kinase 4/6. J. Med. Chem.. 2010;53(22):7938-7957.
- [Google Scholar]
- A Review on Quinoline Derived Scaffolds as Anti-HIV Agents. Mini Rev. Med. Chem.. 2019;19(6):510-526.
- [Google Scholar]
- iLOGP: A Simple, Robust, and Efficient Description of n-Octanol/Water Partition Coefficient for Drug Design Using the GB/SA Approach. J. Chem. Inf. Model.. 2014;54(12):3284-3301.
- [Google Scholar]
- SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep.. 2017;7:42717.
- [Google Scholar]
- SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep.. 2017;7(1):42717.
- [Google Scholar]
- Crystal structure of human CDK4 in complex with a D-type cyclin. Proc. Natl. Acad. Sci.. 2009;106(11):4166-4170.
- [Google Scholar]
- ADMETlab: a platform for systematic ADMET evaluation based on a comprehensively collected ADMET database. J. Cheminf.. 2018;10(1):29.
- [Google Scholar]
- AutoDock Vina 1.2.0: New Docking Methods, Expanded Force Field, and Python Bindings. J. Chem. Inf. Model.. 2021;61(8):3891-3898.
- [Google Scholar]
- Design of novel quinoline derivatives as antibreast cancer using 3D-QSAR, molecular docking and pharmacokinetic investigation. Anticancer Drugs. 2022;33(9):789-802.
- [Google Scholar]
- Metixene is an incomplete autophagy inducer in preclinical models of metastatic cancer and brain metastases. J. Clin. Invest. 2023
- [Google Scholar]
- Recent advances in improved anticancer efficacies of camptothecin nano-formulations: A systematic review. Biomedicines. 2021;9(5)
- [Google Scholar]
- Heterocyclic Iminoquinones and Quinones from the National Cancer Institute (NCI, USA) COMPARE Analysis. Molecules. 2023;28(13)
- [Google Scholar]
- Marcus D Hanwell, D.E.C., David C Lonie, Tim Vandermeersch, Eva Zurek and Geoffrey R Hutchison, Avogadro: An advanced semantic chemical editor, visualization, and analysis platform. Journal of Cheminformatics, 2012. 4: p. 17.
- Recent developments in topoisomerase-targeted cancer chemotherapy. Acta Pharm. Sin. B. 2018;8(6):844-861.
- [Google Scholar]
- Exploration of quinolone and quinoline derivatives as potential anticancer agents. Daru. 2019;27(2):613-626.
- [Google Scholar]
- Intra-CSF topotecan in treatment of breast cancer patients with leptomeningeal metastases. Cancer Med.. 2020;9(21):7935-7942.
- [Google Scholar]
- A review on medicinally important heterocyclic compounds and importance of biophysical approach of underlying the insight mechanism in biological environment. J. Biomol. Struct. Dyn. 2023:1-21.
- [Google Scholar]
- Three-component synthesis of quinoline-4-carboxylic acids based on doebner hydrogen-transfer reaction. J. Org. Chem.. 2023;88(17):12816-12820.
- [Google Scholar]
- An update on the anticancer activity of xanthone derivatives: A review. Pharmaceuticals (Basel). 2021;14(11)
- [Google Scholar]
- Quinoline anticancer agents active on DNA and DNA-interacting proteins: From classical to emerging therapeutic targets. Eur. J. Med. Chem.. 2021;220:113555
- [Google Scholar]
- Pharmacoinformatics and Preclinical Studies of NSC765690 and NSC765599, Potential STAT3/CDK2/4/6 Inhibitors with Antitumor Activities against NCI60 Human Tumor Cell Lines. Biomedicines. 2021;9(1)
- [Google Scholar]
- Camptothecin (CPT) and its derivatives are known to target topoisomerase I (Top1) as their mechanism of action: Did we miss something in CPT analogue molecular targets for treating human disease such as cancer? Am. J. Cancer Res.. 2017;7(12):2350-2394.
- [Google Scholar]
- The Antitumor Activity of a Lead Thioxanthone is Associated with Alterations in Cholesterol Localization. Molecules. 2018;23(12)
- [Google Scholar]
- Small molecule STAT3 inhibitor, 6Br-6a suppresses breast cancer growth in vitro and in vivo. Biomed. Pharmacother.. 2020;121:109502
- [Google Scholar]
- Anthracyclines as topoisomerase II poisons: From early studies to new perspectives. Int. J. Mol. Sci.. 2018;19(11):3480.
- [Google Scholar]
- Antitumour anthracyclines: Progress and perspectives. ChemMedChem. 2020;15(11):933-948.
- [Google Scholar]
- Antiproliferative activity predictor: A new reliable in silico tool for drug response prediction against NCI60 panel. Int. J. Mol. Sci.. 2022;23(22):14374.
- [Google Scholar]
- Characterization of three druggable hot-spots in the aurora-A/TPX2 interaction using biochemical, biophysical, and fragment-based approaches. ACS Chem. Biol.. 2017;12(11):2906-2914.
- [Google Scholar]
- DNA topoisomerases: Advances in understanding of cellular roles and multi-protein complexes via structure-function analysis. Bioessays. 2021;43(4):2000286.
- [Google Scholar]
- Molecular targeted therapy for anticancer treatment. Exp. Mol. Med.. 2022;54(10):1670-1694.
- [Google Scholar]
- Study of Lipophilicity and ADME Properties of 1,9-Diazaphenothiazines with Anticancer Action. Int. J. Mol. Sci.. 2023;24(8)
- [Google Scholar]
- Orrantia-Borunda, E., et al., Subtypes of Breast Cancer, in Breast Cancer, H.N. Mayrovitz, Editor. 2022, Exon Publications Copyright: The Authors.; The authors confirm that the materials included in this chapter do not violate copyright laws. Where relevant, appropriate permissions have been obtained from the original copyright holder(s), and all original sources have been appropriately acknowledged or referenced.: Brisbane (AU).
- Applications of multi-target computer-aided methodologies in molecular design of CNS drugs. Curr. Med. Chem.. 2018;25(39):5293-5314.
- [Google Scholar]
- Novel insights into catalytic mechanism from a crystal structure of human topoisomerase I in complex with DNA. Biochemistry. 2000;39(23):6832-6840.
- [Google Scholar]
- Schrödinger, L., & DeLano, W., Pymol. 2020.
- Quinolines and quinolones as antibacterial, antifungal, anti-virulence, antiviral and anti-parasitic agents. Adv. Exp. Med. Biol.. 2020;1282:37-69.
- [Google Scholar]
- Genetic association between HER2 and ESR2 polymorphisms and ovarian cancer: a meta-analysis. Onco Targets Ther.. 2018;11:1055-1066.
- [Google Scholar]
- AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem.. 2010;31(2):455-461.
- [Google Scholar]
- The Impact of Chemotherapy Completion on the Efficacy of Irinotecan in the Preoperative Chemoradiotherapy of Locally Advanced Rectal Cancer: An Expanded Analysis of the CinClare Phase III Trial. Clin. Colorectal Cancer. 2020;19(2):e58-e69.
- [Google Scholar]
- The Structure of DNA-Bound Human Topoisomerase II Alpha: Conformational Mechanisms for Coordinating Inter-Subunit Interactions with DNA Cleavage. J. Mol. Biol.. 2012;424(3):109-124.
- [Google Scholar]
- ADMETlab 2.0: an integrated online platform for accurate and comprehensive predictions of ADMET properties. Nucleic Acids Res.. 2021;49(W1):W5-W14.
- [Google Scholar]
- Virtual screening, ADMET prediction and dynamics simulation of potential compounds targeting the main protease of SARS-CoV-2. J. Biomol. Struct. Dyn.. 2021;39(17):6617-6632.
- [Google Scholar]
- Utility of physicochemical properties for the prediction of toxicological outcomes: Takeda perspective. ACS Med. Chem. Lett.. 2020;11(2):203-209.
- [Google Scholar]
- Small molecules in targeted cancer therapy: advances, challenges, and future perspectives. Signal Transduct. Target. Ther.. 2021;6(1):201.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2023.105423.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







