Translate this page into:
Exploring sustainable corrosion inhibition of copper in saline environment: An examination of hydroquinazolinones via experimental and ab initio DFT simulations
⁎Corresponding authors. hlgaz@hanyang.ac.kr (Hassane Lgaz), ercleehs@hanyang.ac.kr (Han-seung Lee)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Copper's potential in various applications is constrained due to environmental degradation, particularly in high-salinity environments, representing a sustainability concern. Herein, two novel hydroquinazolinones, namely 1-((4-hydroxynaphthalen-1-yl)methyl)-2-phenyl-2,3-dihydroquinazolin-4(1H)-one (DQ-H) and 1-((4-hydroxynaphthalen-1-yl)methyl)-2-(p-tolyl)-2,3-dihydroquinazolin-4(1H)-one (DQ-CH3), were analyzed for their ability to inhibit copper corrosion in a 3.5 % NaCl solution. The compounds, characterized by 1H and 13C NMR, were evaluated via potentiodynamic polarization curves, electrochemical impedance spectroscopy, and weight loss measurements. Comprehensive analyses utilizing scanning electron microscopy, Fourier Transform Infrared, and UV–vis spectroscopy have revealed insights into the surface morphology and the interactive nature of inhibitor molecules with the copper surface. Our findings highlight the formation of a strong, sustainable inhibitor film on the copper surface due to the addition of hydroquinazolinones, thereby exhibiting an enhanced polarization resistance and decreased double-layer capacitance. Both DQ-H and DQ-CH3 demonstrated a considerable inhibition effect, with efficiencies of 92 % and 94 % respectively, illustrating their potential for sustainable copper protection. Electrochemical impedance spectroscopy (EIS) results indicated a significant increase in polarization resistance from 605.7 (blank) to 9403 and 12861 Ω cm2, after the addition of DQ-H and DQ-CH3, respectively. Furthermore, the adsorption attributes of the compounds on the Cu(1 1 1) surface were examined using first-principles density functional theory simulation, revealing several covalent bonds formation. Our work aims to contribute to sustainability efforts in materials science by offering a corrosion-protective solution that is less harmful to the environment and more efficient in preserving copper's durability, particularly in saline environments.
Keywords
Hydroquinazolinones
Copper corrosion
Saline environment
Density functional theory simulation
Electrochemical methods
1 Introduction
Corrosion is a widespread and pervasive problem that affects a wide range of industries and infrastructures, posing considerable economic and safety concerns worldwide. The detrimental effects of corrosion include structural damage and alterations in the chemical and physical properties of metals, leading to diminished mechanical strength and premature failure (Khalaf et al., 2020; Sun et al., 2021; Tantawy et al., 2021; White et al., 2020). Among the metals utilized in numerous applications, copper stands out for its widespread use in critical sectors such as petrochemical, marine engineering, shipbuilding, and chemical industry (Mousavi and Baghgoli, 2016; Xia et al., 2019). Despite its exceptional electrical and thermal conductivity, as well as moderate strength, copper is susceptible to corrosion, particularly in aggressive environments containing substances like HNO3, H2SO4, HCl, and NaCl (Aabid et al., 2021; He et al., 2020). Addressing the imperative need to prolong the service life of copper and its alloys while averting premature deterioration, the utilization of corrosion inhibitors has emerged as a prominent strategy (Al Jahdaly et al., 2022; Medupin et al., 2023; Thomas et al., 2021; Wei et al., 2020). In recent literature, the focus has been on the synthesis of a new generation of organic corrosion inhibitors that are proving remarkably effective in preventing metal corrosion. These inhibitors, particularly those containing elements such as oxygen, nitrogen, sulfur and conjugated aromatic systems, have attracted attention for their powerful corrosion control capabilities (Chaouiki et al., 2020b; Shaw et al., 2019; Tan et al., 2023a). Numerous relevant and up-to-date references highlight the synthesis and performance of these innovative inhibitors, demonstrating their potential to effectively combat corrosion (Benbouguerra et al., 2018; Bourzami et al., 2019; Chafai et al., 2017, 2019; Djenane et al., 2019; Mammeri et al., 2021; Moumeni et al., 2020; Ouksel et al., 2020). Among them, the nitrogen-based heterocyclic compounds are widely used to mitigate corrosion in aqueous environments. Basically, these N-heterocyclic compounds adhere to metal surfaces using the non-bonding electrons of nitrogen and the π electrons of their side chains or aromatic rings, thus forming a protective film against corrosion (Galai et al., 2021; Gao et al., 2020). Quinazolinones, a type of N-heterocyclic compound, are easy to synthesize and boast excellent corrosion inhibiting properties as well as remarkable biological activities (Khan et al., 2014). In the field of pharmaceuticals, quinazolinones are essential building blocks for over 150 natural alkaloids derived from various plants, micro-organisms and animals. The reactivity, stability and flexibility of these compounds have made them essential intermediates in organic chemistry, facilitating their integration into medicinal applications (Alsibaee et al., 2023). These applications cover a wide range of anti-inflammatory, antidepressant, anticancer, anti-allergy, hypotensive, anticonvulsant, and antimicrobial functions (Giri et al., 2009; Jafari et al., 2016; Patel et al., 2013; Reddy and Sivaramakrishna, 2020). On the other side, the corrosion-inhibiting potential of quinazolinones has been recognized, playing a role in protecting metals and extending the service life of engineered materials. For example, in a study by Zhang et al. (Zhang et al., 2016), 2-(quinolin-2-yl)quinazolin-4(3H)-one was investigated as corrosion inhibitor for Q235 steel in 1 mol/L HCl solution by means of electrochemical impedance spectroscopy (EIS), potentiodynamic polarization curves, scanning electron microscopy (SEM)/energy dispersive X-ray spectroscopy (EDX), Fourier- transform infrared (FTIR) spectroscopy and X-ray photoelectron spectroscopy (XPS) methods. The obtained results demonstrate a remarkable performance of the investigated product in terms of corrosion inhibition as it reached 91 % at 0.185 mmol/L. In another study (Ayati et al., 2011), the inhibitive effect of synthesized 2-(3-pyridyl)-3,4-dihydro-4-quinazolinone (PDQ) as a new corrosion inhibitor for mild steel in HCl (0.1 M) solution was tested employing electrochemical and thermodynamic assessments. The test data show that PDQ efficiently protects steel from corrosion by adsorption on its surface covering its active sites. Further, Aldana-González and co-workers (Aldana-gonzález et al., 2020) provided an in-depth discussion of the corrosion protection offered by a quinazoline derivative, specifically 2-bromo-6(2′,6′-dichlorophenyl)dihydrobenzo[4,5]imidazo[1,2-c]quinazoline, for API 5L X52 steel in an HCl medium. Their study revealed that the inhibitor studied effectively prevented steel degradation by adsorbing onto its surface, according to the Langmuir adsorption isotherm model. Given the previous successful applications of quinazolinone derivatives developed in our laboratory for corrosion protection of steel in acidic medium (Galai et al., 2021; Oubaaqa et al., 2021; Rbaa et al., 2018; Rbaa et al., 2019a), it is worthwhile to examine the performance of this class of organic compounds for copper corrosion in chloride environments. While experimental evaluations provide crucial insights into the corrosion inhibition performance of organic compounds, theoretical approaches offer complementary perspectives by elucidating molecular-level interactions between inhibitors and metal surfaces. Experimental evaluation of the corrosion inhibition performance of organic compounds is so far the first approach that provides a clear answer whether a given organic material is an excellent corrosion inhibitor or not. Weight loss experiments, for instance, are essential to estimate the variation of corrosion rate of metals in a corrosive medium and after adding corrosion inhibitors. A deeper insight into the corrosion inhibition mechanism and mode of action of inhibitors can be obtained from electrochemical measurements (Ahmad, 2006; Tait, 2018). Besides, surface analytical methods are used to characterize the morphology and chemical states of metal surfaces, providing evidence of the influence of inhibitors on the anti-corrosion ability of metals (Ahmad, 2006; Umoren et al., 2022). However, it is still very challenging to characterize the corrosion inhibition process due to its complexity and the influence of several factors on its performance. This can be overcome to some extent by implementing theoretical tools to assess the nature of interactions between organic compounds and metal surface, thus providing molecular-level physical insights that would explain structure/property relationships and binding mechanisms. Using computational techniques like DFT, researchers are seeking to elucidate the relationship between the molecular structure of these compounds and their inhibition efficacy. One of the main objectives is to determine the electronic properties crucial to the inhibitory action of organic compounds (Ebenso et al., 2021). In addition to electronic property calculations, molecular dynamics simulations have become a powerful tool for studying the adsorption of inhibitors to metal surfaces at the molecular level. These simulations provide detailed information on the adsorption mode of inhibitor molecules and facilitate the determination of the adsorption energy between the organic inhibitor and the metal surface (Verma et al., 2018a). However, several works have been published on the applications, advantages, and limitations of these methods in corrosion inhibition studies (Ebenso et al., 2021; Obot et al., 2019). Recent studies on the application of these theoretical models have demonstrated that their limitations are greater than their advantages (Kokalj, 2021, 2022). First-principles (ab initio) density functional theory (DFT) simulations are one of the most promising alternatives that can ease the understanding of complex interactions between organic molecules and metal surfaces (Kokalj, 2021, 2022). It allows the characterization of adsorption geometries, bond formation and breaking upon adsorption, and changes in chemical states before and after adsorption on the metal surface (Ebenso et al., 2021; Lgaz and Lee, 2022).
Given the abovementioned facts, this study reports the synthesize of two 8-hydroxyquinoline based quinazolinone namely, 1-((4-hydroxynaphthalen-1-yl)methyl)-2-phenyl-2,3-dihydroquinazolin-4(1H)-one (DQ-H) and 1-((4-hydroxynaphthalen-1-yl)methyl)-2-(p-tolyl)-2,3-dihydroquinazolin-4(1H)-one (DQ-CH3) to explore their corrosion inhibition performance, adsorption characteristics and structure–activity relationship for copper in 3.5 % NaCl solution using potentiodynamic polarization curves (PPC), electrochemical impedance spectroscopy (EIS) and weight loss (WL) measurements. The nature of interactions and surface morphology of copper were experimentally evaluated using scanning electron microscope (SEM), FT-IR spectrometer, and UviLine spectrophotometer methods. Besides, a comprehensive theoretical calculation was carried out to investigate the interaction and binding mechanisms of tested compounds on the copper surface. To this end, quantum chemical parameters, first-principles DFT simulations of inhibitor adsorption geometries, and projected densit y of states were reported and discussed.
2 Experimental and technical details
2.1 Synthesis of hydroquinazolinones
General procedure: These organic compounds were synthesized by the reaction of 8-hydroxyquinoline with quinazolinone derivatives. In the first, we dissolved two moles of 5-chloromethyl-8-hyroxyquinoline (5-CMHq) in a polar solvent tetrahydrofuran (THF) for a few minutes (25 min) in one mole of a weak base (NaHCO3), after which two moles of quinazolinone derivatives (P1, P4) are added under stirring (50 °C). The reaction was brought to reflux for 12 h (Scheme 1). All chemicals were purchased from Sigma Aldrich.
Molecular structures of investigated quinazolinone derivatives DQ-CH3 and DQ-H.
1-((4-hydroxynaphthalen-1-yl)methyl)-2-(p-tolyl)-2,3-dihydroquinazolin-4(1H)-one (P1 = DQ-CH3): Chemical Formula: C26H22N2O2, molecular Weight: 394.47.
1-((4-hydroxynaphthalen-1-yl)methyl)-2-phenyl-2,3-dihydroquinazolin-4(1H)-one (P2 = DQ-H): Chemical Formula: C25H20N2O2.
Molecular Weight: 380.44.
Spectra 13C and 1H NMR for synthesized compounds are reported in the supplementary material.
2.2 Materials and solutions
In this work, copper (99.9 % purity) was used to investigate the inhibition performance of the studied inhibitors. The metal was cut into small-sized rectangular coupons of dimensions 2 × 2 × 0.2 cm3 for gravimetric studies, while a cylindrical rod (8 mm diameter) of the same metal was used for electrochemical tests. The rod was covered with Teflon to only leave a surface area of 0.5 cm2 in contact with the solution. Before and after each experimental manipulation, the coupons were abraded with emery paper of graded grift from 600 to 2000 degrees successively, washed with deionized water then degreased with acetone, and finally dried with hot air.
The corrosive medium of 3.5 wt% NaCl was prepared using analytical grade sodium chloride dissolved in bi-distilled water. Given the structural characteristics of DQ-H and DQ-CH3, their appreciable solubility in a sodium chloride solution is unlikely. We therefore chose to dissolve them first in dimethylformamide (DMF), then introduce them into the test electrolyte. The concentration range of the tested products varied from 10−6 mol/L to 10−3 mol/L.
2.3 Electrochemical tests
The electrochemical behavior of copper in sodium chloride has been investigated with potentiodynamic polarization and electrochemical impedance spectroscopy tests. For this purpose, an assembly of the three-electrode cell was used to carry out the electrochemical experiments with a saturated calomel electrode (SCE) as a reference electrode, a platinum plate as a counter electrode, and the working electrode was a copper rod placed near the reference electrode. These tests were performed using a potentiostat/galvanostat/ PGZ100 controlled with analytics software (Voltamaster 4). The copper electrode was plunged in the examined solution for 40 min to reach the steady state open circuit potential (EOCP) at the temperature of 298 K. PPC measurements were recorded with a scan rate of 1 mV/s in the range from −600 to 600 mV of the corrosion potential. This wide range of potentials was chosen to provide a comprehensive investigation into the influence of inhibitory species on the observed phenomena, with a particular focus on diffusion-controlled copper dissolution.
Impedance spectra were conducted in the frequency range 100 kHz − 10 mHz by applying a sinusoidal voltage with 10 mV amplitude (peak to peak).
2.4 Weight loss method
The weight loss measurements were conducted under the approved method “ASTM G 31-72” (Abbasov et al., 2013; Verma et al., 2019). The copper sheets were mechanically polished, cleaned, dried, and carefully weighed before immersion into the corrosive medium. The specimens were kept in 50 mL of 3.5 % NaCl with and without DQ-H and DQ-CH3 for 10 days at 298 K. Thereafter, the samples were removed from the solution, rinsed with distilled water, degreased in acetone, dried, and then reweighed. Experiments were carried out in triplicate and average results were reported. Following equations (1 and 2) were used to calculate the corrosion rate Wcorr and the inhibition efficiency IE (%) respectively:
2.5 Solutions and surface morphology characterization
The morphology of the copper surface was investigated by scanning electron microscopy (SEM) instrument (Hirox SH4000M) after immersion for 72 h in free and inhibited solutions at 298 K. In addition, an FT-IR spectrometer was used to evaluate the probable protective film formed on the metal surface while an analytics UVILINE spectrophotometer was used to characterize the interactions between hydroquinazolinone derivatives and Cu+.
2.6 Computational studies
2.6.1 Quantum chemical calculations
Full geometry optimizations of tested compounds were carried out using the density functional theory (DFT), GGA functional and DNP basis set (Grimme, 2006) along with COSMO solvation model using DMol3 code (Delley,2000, 1990). Computed quantum chemical parameters include the energy of the highest occupied molecular orbital (EHOMO), the energy of the lowest unoccupied molecular orbital (ELUMO), energy band gap (ΔEg), and the fraction of transferred electrons (ΔN) among others. These quantum chemical reactivity indices are calculated via the following relationships as previously reported in literature (Ansari et al., 2022):
2.6.2 First-principles DFT simulations
Inhibitor-iron interactions were fully optimized by spin polarized first-principles DFT, including dispersion interaction (DFT-D3) and ultra-soft pseudopotentials using the CASTEP code (Clark et al., 2005). The generalized gradient approximation (GGA) within its PBE formulation was used for the electron exchange and correlation (Perdew et al., 1996). The adsorption systems were fully optimized using a self-consistent field (SCF) tolerance of 10−6 eV/atom, and plane-wave basis set with an energy cut-off of 30 Ry. All other convergence tolerance values were set according to fine quality. Meanwhile, the bulk lattice parameters optimization was carried out using (8x8x8) k-point grid, which was reduced to (2x2x1) k-point grid for adsorption models. Inhibitor-iron adsorption models were generated by constructing Cu(1 1 1) copper surface consisting of a (5x5) supercell and a vacuum spacing of 20 Å along the z-direction separating periodic image in each direction. Then, inhibitor molecules were placed on the top side of the slab and all atoms were allowed to relax except the two bottom-most atomic layers. Optimization of standalone molecules was conducted on a cubic box of 30 Å in size. Origin 2016 and Inkscape software were used for plotting and post-processing of all figures. The total energies of isolated molecules (noted Emol), Cu(1 1 1) copper surface (noted Esurf), and molecules/Cu(1 1 1) adsorption systems (noted Emol/surf) were used to determine the adsorption binding energy as:
The selection of the high-density Cu (1 1 1) surface model for the study of DQ-H, DQ-CH3 adsorption is based on its characteristics as the most stable surface among low-Miller index copper surfaces and on its predominance. This dual attribute underlines its relevance as a representative model for the study of these compounds (Sun et al., 2012; Vitos et al., 1998).
3 Results and discussion
3.1 Weight loss measurements
The experimental data derived from weight loss measurements for copper in 3.5 % NaCl medium without and with various doses of DQ-H and DQ-CH3 are represented in Fig. 1 and Table 1.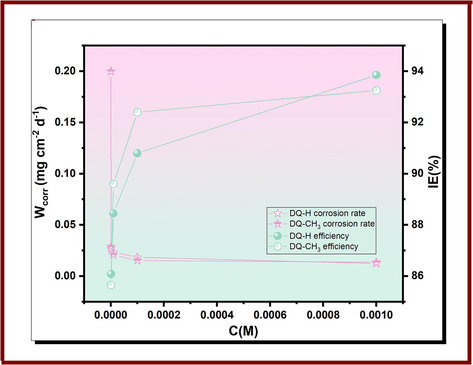
Weight loss results of copper in 3.5 % NaCl with and without various concentrations of DQ-H and DQ-CH3 at 298 K.
Inhibitor
Concentration(mol/L)
(mg cm−2 d−1) × 10−2
Surface coverage
(
)
Blank
–
20
–
–
10−6
2.64
0.8608
86.08
10−5
2.31
0.8845
88.45
10−4
1.84
0.9080
90.80
10−3
1.23
0.9385
93.85
3
10−6
2.87
0.8585
85.85
10−5
2.08
0.8960
89.60
10−4
1.52
0.9240
92.40
10−3
1.35
0.9325
93.25
Gravimetric analysis results indicate a significant decrease in corrosion rate with increasing compound concentrations in the 3.5 % NaCl solution, demonstrating the inhibitors' effectiveness in mitigating copper corrosion. Besides, high inhibition efficiencies were observed at all concentrations for DQ-H and DQ-CH3. Notably, at a concentration of 10−3 mol/L, DQ-H and DQ-CH3 showed remarkable inhibition efficiencies of 93.85 % and 93.25 %, respectively, underlining the strong inhibitory properties of hydroquinazolinones under these experimental conditions. These high inhibition levels result mainly from the adsorption of inhibitor molecules onto the metal surface, which reduces metal dissolution by blocking the active sites (Zhang et al., 2018b). From a mechanistic perspective, these compounds have the ability to form coordination complexes with copper in its various oxidation states (0, I, II). The possibility of forming chelated complexes arises when these molecules engage in donor–acceptor interactions, in which the free electron pairs of the heteroatoms (O, N) interact with the vacant d-orbitals of copper. (Otmacic Curkovic et al., 2010). Generally, the corrosion-inhibiting attributes of these molecules derive from their structural features, in particular the presence of lone and π electrons, crucial for their adsorption to the target metal surface. Changes in the chemical structure of organic molecules, including variations in heteroatoms, functional groups, phenyl rings, their positions and natures, significantly influence the adsorption strength and stability of the adsorbed film (Ouici et al., 2015; Tan et al., 2023b). The following sections of this research will look at relevant cases illustrating these phenomena.
3.2 Electrochemical impedance spectroscopy studies
EIS, known for its effectiveness in revealing precise aspects of corrosion mechanisms, was used in this study (Sukul et al., 2018). The impedance plots of copper in 3.5 % NaCl without and with different concentrations of DQ-H and DQ-CH3 were carried out automatically after a stabilization period of 2400 s at 298 K and shown in Fig. 2. The Bode diagrams shown in Fig. 2 (a) and (b) reveal a significant increase in impedance values over the entire frequency spectrum with increasing DQ-H and DQ-CH3 concentration. Also, analysis of the Bode phase diagrams reveals the presence of two relaxation time constants governing the corrosion process occurring at the electrode surface. One is linked to the relaxation of the electric double-layer capacitor, while the other is associated with the relaxation process of the adsorbed inhibitors (Sherif et al., 2008). Furthermore, it can be seen that an increase in quinazolinones’ concentration leads to a corresponding increase in the maximum phase angle shown in Fig. 2 (a) and (b), indicating an inhibition action of the corrosion process (Hong et al., 2012).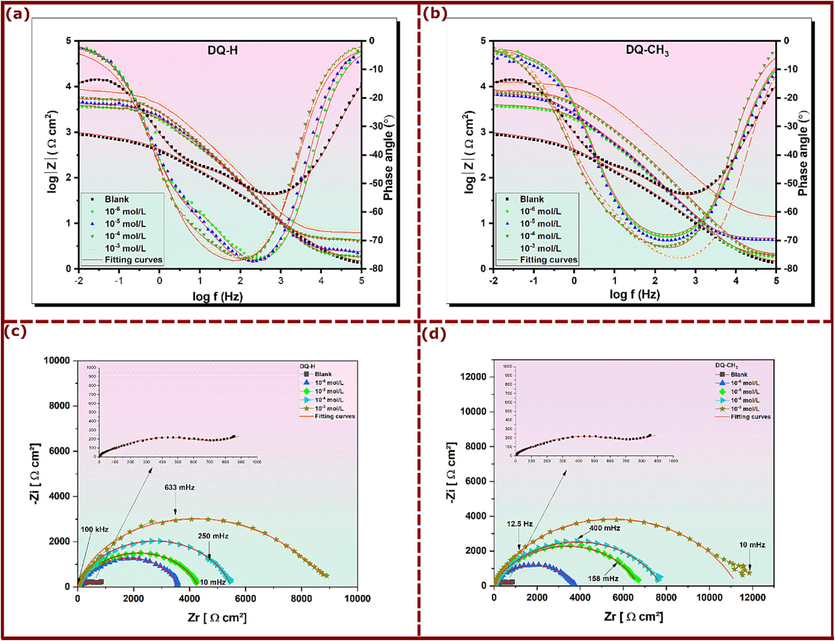
Bode and Nyquist plots for copper in 3.5% NaCl with and without DQ-H and DQ-CH3 concentrations; (a)-(b) Bode, and (c)-(d) Nyquist plots.
In the presence of quinazolinones, the impedance spectra for the Nyquist plots (Fig. 2 (c) and (d)) clearly show a depressed semicircle in the high frequency region. This depressed semicircle is attributed to the charge transfer and double-layer capacitance (Deslouis et al., 1988; Hong et al., 2012; Tribollet, 1994). While in the absence of DQ-H and DQ-CH3, the depressed semicircle of Nyquist plot is followed by a straight line at low frequency region. Generally, the presence of a diffusion layer around the electrode surface, characterized by an ion concentration gradient due to electrochemical reactions, is a common phenomenon when an electrode is immersed in an ion-containing solution. More specifically, in the case of copper dissolution in NaCl solution, the appearance of a Warburg line on the blank Nyquist diagram indicates that the copper dissolution process is mainly controlled by diffusion. This suggests that the rate of copper dissolution is limited by the transport of copper ions through the diffusion layer around the electrode (Chen et al., 2018; Liao et al., 2011; Yao et al., 2021). In the present circumstances, one may suggest that copper undergoes various electrochemical reactions, leading to the formation of complexes such as CuCl and . At the interface between the copper electrode and the solution, adsorption of ions from the solution onto the electrode surface results in the formation of an electrical double layer. This later functions as a capacitor, capable of storing charges and thus influencing the impedance of the system (Sukul et al., 2018). It is also clear from Fig. 2 (b) and (c) that the diameter of the Nyquist semicircle grows with increasing concentration of the inhibitors accompanied with a complete disappearance of Warburg line. The absence of Warburg line in the Nyquist plots of the inhibited solutions implies that the DQ-H and DQ-CH3 have attenuated the electrochemical reactions associated with mass transfer, indicating protective ability of quinazolinones by adsorption on the copper surface covering its active sites (Dafali et al., 2003; Sukul et al., 2018).
The impedance characteristics were analyzed using the equivalent circuit models shown in Fig. 3. This circuit models were also reported in various studies for Cu/NaCl system (Hong et al., 2012).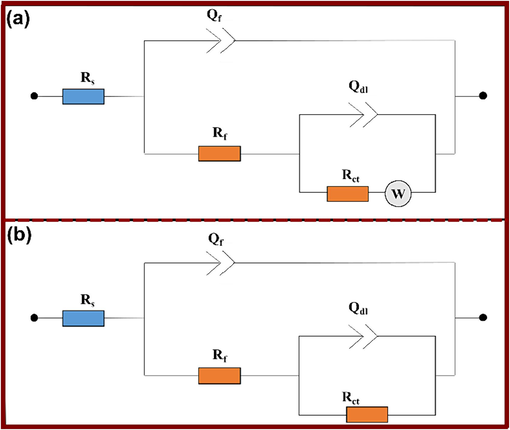
Equivalent circuits models that have been used to fit EIS data of uninhibited (a) and inhibited (b) solutions.
The parameters determined by fitting the equivalent circuits, along with the calculated inhibition efficiencies, are presented in Table 2. Here, Rs represents the resistance of the studied medium, Rf is the resistance of the formed film on the copper surface, Qf and Qdl refer to constant phase elements (CPE), Rct is the charge transfer resistance, and W signifies the Warburg element (Kannan et al., 2018). According to the roughness of the electrode surface and frequency dispersion, the capacitive loops of Nyquist diagrams were not perfectly semicircular. As a result, the use of CPE instead of pure capacitance is more suitable to fit the obtained EIS data. The double-layer capacitance Cdl and film capacitance Cf were determined by equations 13 and 14 respectively (Tasić et al., 2021):
Inhibitor
Conc (mol/L)
Blank
–
1.019
54.3
0.6907
144.6
174.66
0.6504
605.7
750.3
49.15
0.690
–
10−6
1.876
23.02
0.9178
570
48.57
0.7527
3020
3590
–
0.302
79.10
10−5
2.404
23.00
0.9227
795
43.21
0.7095
3529
4324
–
0.319
82.65
10−4
4.353
22.86
0.9242
1011
23.66
0.7062
4493
5504
–
0.264
86.37
10−3
6.696
16.00
0.9029
1464
9.1
0.4878
7939
9403
–
6.359
92.02
DQ-CH3
10−6
1.831
21.79
0.8699
508.1
28.57
0.5789
3303
3811
–
0.181
80.31
10−5
4.292
20.77
0.8960
862
26.19
0.7097
5842
6704
–
0.379
88.80
10−4
1.863
10.64
0.8898
1320
20.13
0.6465
6479
7799
–
6.793
90.38
10−3
2.979
4.13
0.9138
1454
18.22
0.6765
11,407
12,861
–
0.380
94.17
The Inhibition efficiencies, derived from Rp, show an upward trend with increasing inhibitor concentration. The highest value recorded was 92.02 % for DQ-H and 94.17 % for DQ-CH3 at 10−3 mol/L. The strong inhibitory capacity of these inhibitors is attributed to their association with high electron density, particularly notable in the π electrons of aromatic rings, as well as in the non-bonding electrons of oxygen and nitrogen atoms (Verma et al., 2020).
The introduction of an additional methyl group into the molecular structure of quinazolinones appears to have a slight effect on their inhibition efficacy against copper corrosion in NaCl solution. The observed inhibition efficiencies of 94.17 % for DQ-CH3 and 92.02 % for DQ-H underline the impact of structural modifications on corrosion inhibition properties. Although the improvement in inhibition efficiency with the addition of a methyl group may not be significant, it could be attributed to several factors. Firstly, the presence of the methyl group may lead to changes in the electronic structure of the molecule, influencing its interaction with the metal surface and facilitating stronger adsorption to the copper substrate. In addition, steric effects introduced by the methyl group could alter the spatial arrangement of inhibitor molecules at the metal/solution interface, potentially enhancing their coverage and effectiveness in corrosion inhibition (Verma et al., 2018). Similar trends have been observed in recently published studies on the effect of structural modifications on corrosion inhibition performance. For example, a study by El-Haddad et al. (El-Haddad and Fouda, 2015) investigated the corrosion inhibition efficacy of imidazole derivatives, both with and without alkyl groups, on aluminum in a 0.5 M HCl solution. Their findings demonstrated that methyl imidazole exhibited superior corrosion inhibition compared to regular imidazole. Interestingly, the methyl substituent did not alter the mechanism of action of imidazole; instead, it enhanced the compound's coverage and adsorption on the aluminum surface. Similarly, research by Chaouiki et al. (2020a) reported improved inhibition efficacy with the addition of methyl group to quinoxaline derivatives for mild steel corrosion inhibition in acidic medium. Specifically, 8-Hydroxyquinoline, namely 1-((8-hydroxyquinolin-5-yl)methyl)-3,6-dimethylquinoxalin-2(1H)-one (Q1) achieved an efficacy of 96 %, whereas 1-((8-hydroxyquinolin-5-yl)methyl)quinoxalin-2(1H)-one (Q2) reached 89 %. This phenomenon extends beyond the substitution of the methyl group. Numerous papers have also examined the impact of substituents such as –OCH3, –NH2, –OH and others on the inhibition efficacy of organic N-heterocyclic inhibitors. Gad Allah et al. (Gad Allah et al., 1989) outlined the corrosion inhibition properties of various pyrazole derivatives on zinc corrosion in hydrochloric acid solution through capacitance and polarization measurements. Their findings revealed the following order of inhibition efficiencies attributed to different substituents within the molecules: -H (77 %) < –CH3 (85 %) < –OCH3 (87.8 %). For mild steel corrosion in 2 M HCl solution, double Schiff base derivative with –OCH3 and –OH substituents showed better corrosion protection as compared to non-substituted one (Soltani et al., 2010). Similar observation has been reported for pyridine derivatives, where for acidic corrosion of mild steel methyl and methoxy substituents manifest better protection ability as compared to non-substituted compound (Noor and Al-Moubaraki, 2008). What these groups have in common, then, is their nature as electron-donating moieties. It has been reported that electron-donating substituents such as –OH, –NH2, –CH3 and –OCH3 increase the electron density at the adsorption center of the inhibitor, thereby intensifying interactions between the inhibitor molecule and the metal surface (Belghiti et al., 2022). These results underline the importance of molecular design and structural optimization in tailoring the corrosion inhibition properties of organic compounds, with the addition of methyl groups a promising strategy for enhancing their effectiveness in protecting metals against corrosion in chloride-containing environments. But what about other groups of the opposite nature, i.e. electron-attracting substituents?
3.3 Potentiodynamic polarization measurements
The cathodic and anodic polarization curves of copper in 3.5 % NaCl solution at 298 K were analyzed in the absence and presence of varying concentrations of DQ-CH3 and DQ-H, as shown in Fig. 4. Electrochemical parameters, including corrosion potential (Ecorr), corrosion current density (icorr), cathodic and anodic Tafel constants (βc and βa), shown in Table 3, were obtained by extrapolating current–potential (i-E) curves. Inhibition efficiency was calculated using equation (21), where icorr and iinh are the corrosion current density values without and with DQ-CH3 and DQ-H, respectively (Abd El-Lateef et al., 2020, 2022).
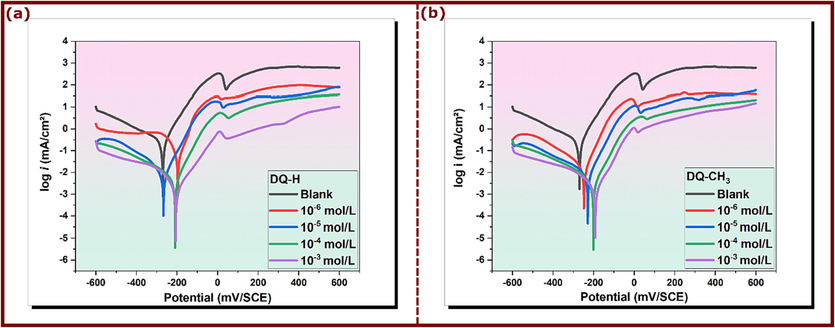
PP curves of copper/3.5 % NaCl in the presence and absence of DQ-H (a) and DQ-CH3 (b) at 298 K.
Medium
Concentration(mol/L)
−
(mV vs. SCE)
(mV dec−1)−
(mV dec−1)
(μA cm−2)
(%)
Blank
–
267.8
49.6
78
131.4
–
10−6
194
44.7
43.9
36.8
71.99
10−5
264.8
56.8
84.7
8.46
93.56
10−4
207
58.5
131.2
4.04
96.65
10−3
204.7
94.6
100.6
2.17
98.34
3
10−6
245.2
48.1
84.2
10.1
92.31
10−5
225.7
49.8
93.3
5.11
96.11
10−4
198.9
59.8
115.4
2.62
98.00
10−3
189.8
72
100.2
1.67
98.73
Fig. 4a and 4b show that the inclusion of quinazolinone derivatives reduces both cathodic and anodic currents, preventing chloride attack on the copper electrode in 3.5 % NaCl. Generally, adsorption of inhibitors can significantly influence corrosion rates through two main mechanisms. Firstly, inhibitors can reduce the available reaction zone, a phenomenon known as the geometric blocking effect. This reduction limits the access of corrosive agents to the metal surface, preventing the initiation and propagation of corrosion. Secondly, inhibitors can modify the activation energy required for cathodic and anodic reactions, thus affecting the kinetics of the corrosion process (M. E. Belghiti et al., 2016). It has been discussed that in the case of the first mode, inhibition arises from the reduction of the reaction zone on the surface of the corroded metal, whereas for the other two modes, inhibition effects arise from changes in the average activation energy barriers of the anodic and cathodic reactions of the corrosion process (Muralidharan et al., 1995). The values of the cathodic Tafel slope (βc) shows slight changes when DQ-H and DQ-CH3 are added, indicating that the inhibitory action occurs by simply blocking the available cathodic sites on the metal surface. Furthermore, the values of the anodic Tafel slopes (βa) also change with the addition of DQ-H and DQ-CH3, suggesting that these inhibitors are initially adsorbed onto the metal surface and hinder by simply blocking the reaction sites on the metal surface without affecting the mechanism of the anodic reaction (Ouakki et al., 2020). Moreover, the displacement in Ecorr value was less than 85 mV between blank, and inhibited solution. On the basis of these results, the DQ-H and DQ-CH3 derivatives can be classified as mixed-type inhibitors in 3.5 % NaCl, delaying both anodic and cathodic corrosion reactions with a predominant effect on the anodic reaction (Abdelsattar et al., 2020; Belhadi et al., 2023). Table 3 also shows that current density undergoes a noticeable decrease with increasing DQ-H and DQ-CH3 concentration. In the absence of inhibitors, current density is measured at 131.4 μA/cm2, while in the presence of 10−3 mol/L DQ-H and DQ-CH3, it decreases significantly to 2.17 μA/cm2 and 1.67 μA/cm2, respectively. This observation indicates that the quinazolinone derivatives studied show increasing adsorption to the copper surface as their concentration increases. This adsorption leads to coverage of the active sites on the copper surface, forming a protective layer that decreases the reactivity of copper dissolution (Verma et al., 2017).
As previously indicated, the size and active centers within the molecular structure of organic compounds play a crucial role in determining their inhibitory efficacy (Huang and Guo, 2020). Consequently, the inhibition efficacy of quinazolinones, as assessed by potentiodynamic polarization measurements, aligns with the results obtained by electrochemical impedance spectroscopy (EIS) tests. Specifically, the inhibition efficiency for DQ-CH3 is calculated at 98.73 %, which is slightly higher than the 98.34 % measured for DQ-H. The corrosion inhibition effect is attributed to the presence of several π-systems of the aromatic rings and several nitrogen atoms in the main molecular structure of both DQ-H and DQ-CH3 that would act as adsorption centers. Functional groups would have a great influence on the corrosion inhibition performance of compounds when they are directly contributing to the binding and affinity to metal surfaces. In the present case, the core of both compounds is the main responsible for the adsorption of molecules on the copper surface and that is why the influence of the additional methyl group is negligible. It should be noted that, although the introduction of a –CH3 group into the molecular structure does not significantly affect corrosion performance in the current scenario, some research papers in references (Bentiss et al., 2007; Isin and Karakus, 2015; Zaafarany, 2009) demonstrated that the addition of certain substituents to the molecular skeleton of an inhibitor significantly enhances its corrosion inhibition capability. In our previously published article (Oubahou et al., 2023), we studied the efficacy of three isomers, namely: 2-(4-chlorophenyl)-1-((4-hydroxynaphthalen-1-yl)methyl)-2,3-dihydroquinazolin-4(1H)-one (DQ-Cl), 2-(4-bromophenyl)-1-((4-hydroxynaphthalen-1-yl)methyl)-2, 3-dihydroquinazoline-4(1H)-one (DQ-Br), and 2-(2-chlorophenyl)-1-((4-hydroxynaphthalen-1-yl)methyl)-2,3-dihydroquinazoline-4(1H)-one (DQ-Cl'), under the same experimental conditions as the current study. Comparing the results of our previous research with those of the present study, we observed that DQ-H and DQ-CH3 showed higher inhibition efficiencies than halogenated quinazolinones. The order of efficacy is as follows: DQ-CH3 > DQ-H > DQ-Br > DQ-Cl > DQ-Cl'. The improved performance of DQ-CH3 and DQ-H compared with DQ-Br, DQ-Cl and DQ-Cl' can be explained by the fact that halogen substituents withdraw electrons. As established, electron-donating substituents increase the electron density at the adsorption center of the inhibitor, thus promoting its affinity for the metal surface. Conversely, electron-withdrawing substituents such as -Br, -Cl, –NO2, –CN, –COOH, and- COOC2H5 decrease the electron density at the adsorption center, thus reducing the interaction between inhibitor molecules and the metal surface (Harvey et al., 2011; Li et al., 1999). Table S3 (supplementary material) compares the corrosion inhibition performance of several other quinazolinones previously used for metal corrosion protection (Ayati et al., 2011; Chen et al., 2021a; Errahmany et al., 2020; Fouda et al., 2010; Rbaa et al., 2019b; Zhang and Zhang, 2021). It is evident that DQ-H and DQ-CH3 exhibit reasonable corrosion inhibition efficiencies compared with other quinazolinones listed in Table S3. Furthermore, the data in Table S3 underline that the nature and/or position of substituents can, in some cases, influence inhibitor efficacy (Arrousse et al., 2022; Faydy et al., 2019; Fouda and Hassan, 2013; Khan et al., 2017; Kumar et al., 2020).
3.4 Temperature effect
It is acknowledged that the temperature has a significant impact on the corrosion process resulting in a modification in the mechanism of the inhibitor’s action on a metallic surface (Boudjellal et al., 2020; Damej et al., 2021; Galai et al., 2020; Ouici et al., 2013). To study the effect of temperature on the protective power of the investigated hydroquinazolinones, potentiodynamic polarization measurements were conducted for copper in 3.5 % NaCl in the presence and absence of 10−3 mol/L of DQ-CH3 and DQ-H in the temperature range of 298–328 K. The obtained curves are shown in Fig. 5. In addition, a summary of electrochemical parameters is listed in Table 4.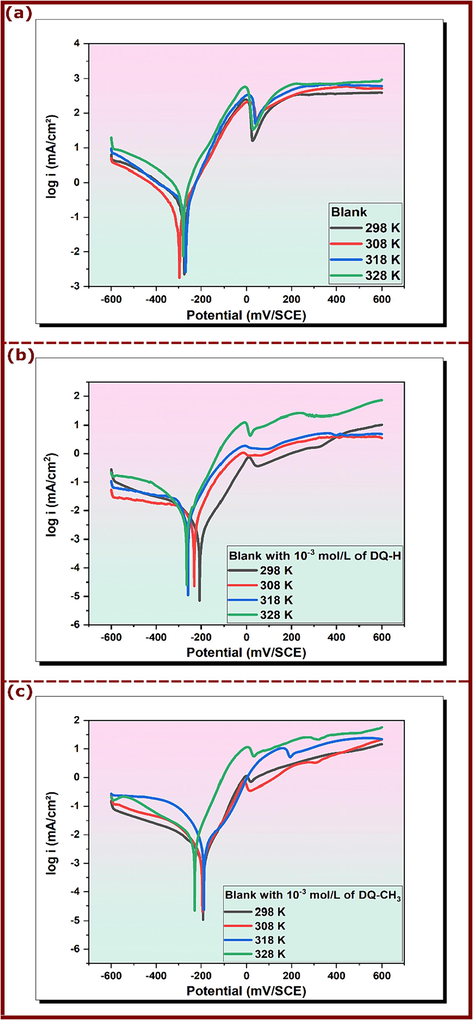
Tafel plots of the copper electrode in 3.5 NaCl with and without 10−3 mol/L of DQ-H and DQ-CH3 at different temperatures; (a) Blank, (b) DQ-H and (c) DQ-CH3.
Medium
Temperature(K)
−Ecorr(mV vs. SCE)
βa
(mV dec−1)−βc
(mV dec−1)
(μA cm−2)
(%)
Blank
298
267.8
49.6
78.0
131.40
–
308
296.6
81.9
132.1
234.70
–
318
275.1
69.7
127.0
436.0
–
328
277.6
72.4
157.5
732.80
–
DQ-H
298
204.7
94.6
100.6
2.17
98.34
308
228.7
54.5
104.1
11.43
95.13
318
256.4
67.8
78.8
40.80
90.64
328
262.5
66.8
113.2
97.10
86.75
DQ-CH3
298
189.8
72.0
100.2
1.67
98.73
308
192.6
81.0
103.3
9.15
96.10
318
185.4
124.4
78.2
48.36
88.91
328
225.7
58.1
135.6
89.97
87.72
The rise in temperature leads to the modification of Ecorr values in both uninhibited and inhibited solutions. At the same time, the anodic and cathodic current densities increased especially for uninhibited medium indicating that the corrosion rate of copper is influenced by the temperature (Hrimla et al., 2021). The changes observed in Ecorr and icorr coincide with a decrease in inhibitory efficiency, showing a slight decline to 86.75 % for DQ-H and 87.72 % for DQ-CH3 at 328 K, respectively. This trend can be attributed to the intensification of corrosion activity leading to surface roughening of copper (El Mouaden et al., 2018). More specifically, the acceleration of various corrosion processes, including electrochemical and chemical reactions, as well as the transfer of reactive species to the metal surface, is enhanced at higher temperatures (Desimone et al., 2011). Despite these difficulties, it should be noted that DQ-H and DQ-CH3 compounds retain strong inhibition capabilities even at elevated temperatures, underscoring their effectiveness in corrosion mitigation.
For further details on the copper corrosion process, activation energy Ea for the adsorption of DQ-CH3 and DQ-H on copper in the absence and presence of 10−3 mol/L is calculated using the Arrhenius equation (Eq.28) (El-Hajjaji et al., 2018).
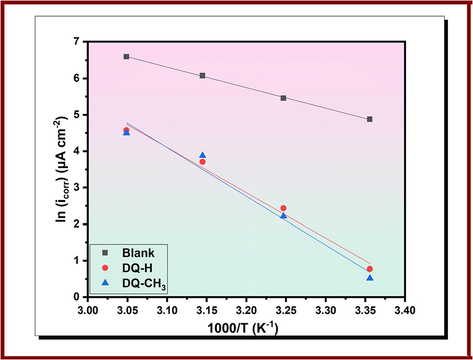
Arrhenius plots for copper corrosion in 3.5 % NaCl in the absence and presence of 10−3 mol/L of DQ-H and DQ-CH3.
The addition of DQ-CH3 and DQ-H to the blank solution moves significantly the Ea from being 46.93 kJ mol−1 in the free solution to a higher value in the presence of inhibitors where it reaches 103.35 and 111.21 kJ mol−1 for 10−3 mol/L of DQ-H and DQ-CH3 respectively. Hence, there is less metal dissolution, which justifies the rise in activation energy (Rouifi et al., 2020). Also, the higher value of activation energy in the presence of DQ-H and DQ-CH3 indicates that the inhibitor molecules adsorb on the copper surface in a strong way (Hamdy and El-Gendy, 2013).
3.5 Adsorption isotherms
Inhibitors based on organic molecules adsorb on the metal/solution interface and create protective films (Lgaz et al., 2020). To get a better understanding of the adsorption mechanism of DQ-H and DQ-CH3 on the copper surface, it is vital to determine which form of adsorption, whether physical or chemical, has taken place during that process. For this purpose, Langmuir (Eq. 29) (El et al., 2023), Frumkin (Eq. 30) (Souza and Spinelli, 2009), Temkin (Eq. 31) (Akinbulumo et al., 2020), Freundlich (Eq. 32) (Belhadi et al., 2023), and El-Awady (Eq.33) (El-Awady et al., 1990; El‐Awady et al., 1992) isotherms were used to simulate the changes in the coverage rate (θ) obtained from PPC measurements, with the concentration of the inhibitor. These models were calculated using the following equations and were plotted in Fig. 7.
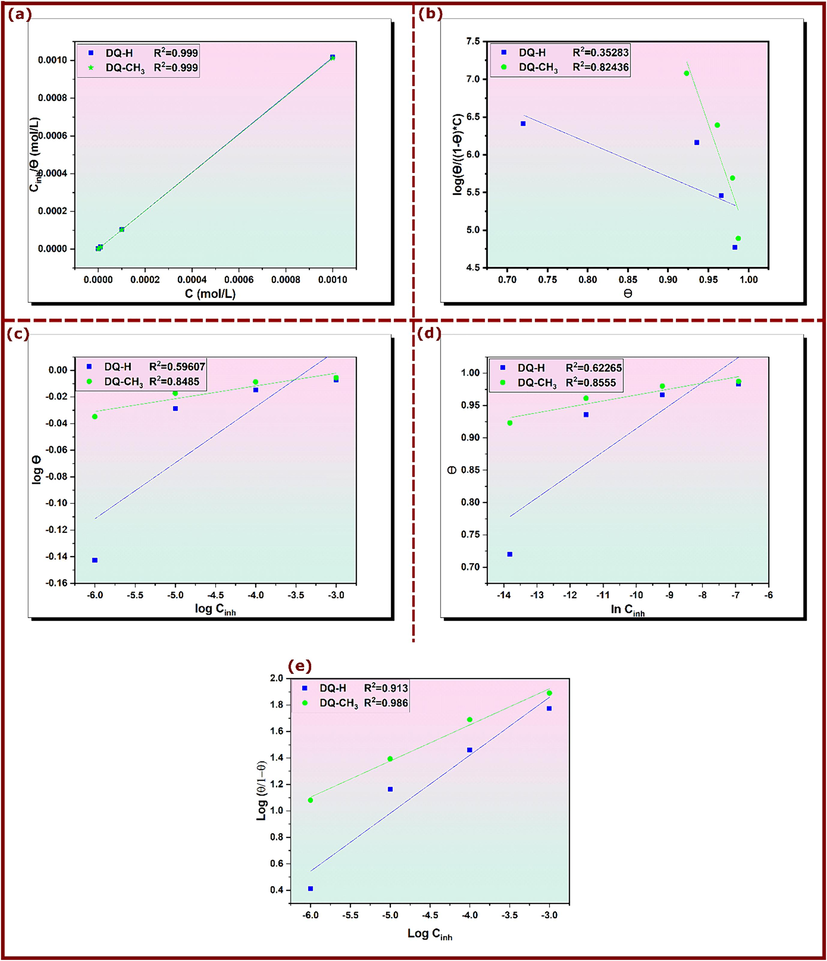
Langmuir (a), Frumkin (b), Temkin (c), Freundlich (d) and El-Awady (e) adsorption isotherm models of DQ-H and DQ-CH3 for copper in 3.5% NaCl.
The comparison of the correlation coefficient R2 of the listed models shows that the Langmuir isotherm presents the highest R2 value and thus, it is the suitable model to describe the adsorption of DQH and DQCH3 on the copper surface (Galai et al., 2021). On the other side, high values of Kads illustrated in Table 5 suggest that the inhibitors under examination interact strongly with the surface of the metal (Al-Azawi et al., 2018). The standard free energy is directly related with Kads by the following equation (27) (Lgaz et al., 2017):
Inhibitor
Slope
(L.mol−1)
(kJ.mol−1)
DQ-H
1.02
112.55 × 104
−44.47
DQ-CH3
1.01
273.71 × 104
−46.67
It has been reported that values of around −20 kJ.mol−1 or less negative, characterize an electrostatic adsorption process (physisorption), whereas values around −40 kJ mol−1 or more negative correspond to chemisorption (Alaoui et al., 2019; Yildiz et al., 2014; Zhang et al., 2018a). In corrosion inhibition studies, many previously published works use the fitting of isotherm models to experimental data to determine some thermodynamical parameters like values, then making decisive conclusions about an inhibitor’s adsorption mechanism on metal surfaces (Dutta et al., 2017; W. Guo et al., 2020; Tang et al., 2018; Verma et al., 2018b). However, recent studies indicated that this approach is not always true and should be considered with strict precautions (Kokalj, 2022; Walczak et al., 2019). Therefore, adsorption isotherm data are not reported in the present studies and no decisive conclusions about adsorption mechanisms can be made based on adsorption isotherms.
3.6 Surface morphology characterization
The SEM micrographs shown in Fig. 8 provide visual evidence of the effects of quinazolinones on the surface morphology of copper immersed in 3.5 % chloride medium for 72 h at 298 K. Initially, Fig. 8a shows the abraded copper surface, which exhibits a relatively clear surface with minimal scratches resulting from mechanical polishing. This serves as a reference for comparison. In Fig. 8b, where the copper sample was immersed in the blank chloride solution, a significant amount of thick corrosion products is observed, indicating pronounced dissolution of copper in the chloride medium, in line with the results reported by Guo et al. (Guo et al., 2021).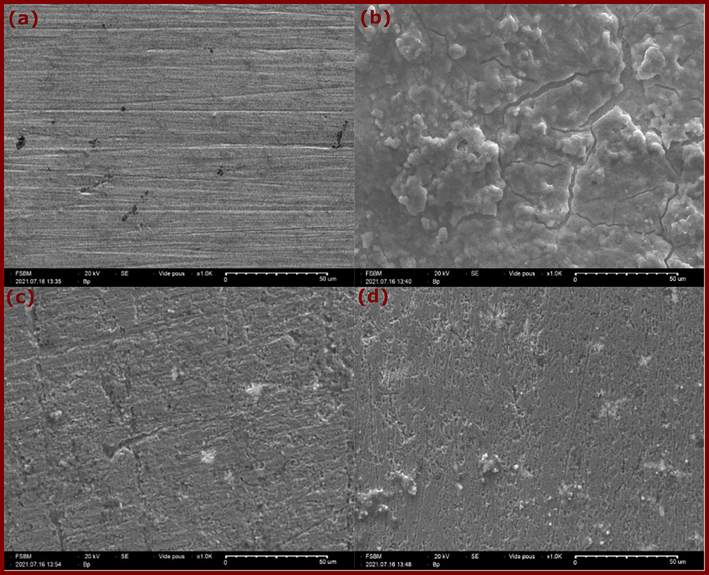
SEM images of copper (a) polished, (b) after 3 days of immersion in 3.5 % NaCl, (c) and (d) after immersion in 3.5 % NaCl with 10−3 mol/L of DQ-H and DQ-CH3, respectively.
This observation underlines the aggressive nature of the chloride environment on the copper surface, leading to corrosion. However, after addition of DQ-H and DQ-CH3, Fig. 8c&8d show a noticeable alteration in copper surface morphology. Here, copper dissolution is noticeably reduced and the metal surface appears smoother compared to the sample immersed in the blank solution. The observed decrease in copper dissolution and smoother surface can be attributed to the formation of a layer of adsorbed inhibitor molecules on the copper surface. This layer acts as a barrier, preventing interaction between the copper surface and the aggressive chloride ions present in the medium (H. Li et al., 2021). Consequently, the inhibitory action of DQ-H and DQ-CH3 molecules attenuates the corrosion process, preserving the integrity of the copper surface to some extent. While SEM analysis provides visual information on surface morphology and the effects of inhibitors on copper corrosion, complementary spectroscopic techniques such as UV–vis and FT-IR allow further characterization of molecular interactions between inhibitors and the metal surface.
3.7 UV–visible spectroscopy
Complementing the SEM observations, UV–vis spectroscopy provides additional insights into copper-inhibitor interactions in solution. Solutions containing 10−3 mol/L of DQ-H and DQ-CH3 are analyzed before and after immersion of copper for three days, and results are shown in Fig. 9. Before immersion of the metallic sample, the UV–vis spectra of DQ-H and DQ-CH3 solutions show absorption peaks at 266 nm and 278 nm for DQ-H and DQ-CH3, respectively. These absorption bands characterize π-π* transitions in aromatic rings that are present in the molecular structure of the inhibitors (Singh et al., 2011). However, a displacement in the absorption bands was observed after immersion of copper samples in solutions containing inhibitors, leading to a shift from 266 nm to 279.34 nm for DQ-H and from 278 nm to 284 nm for DQ-CH3. Furthermore, a decrease in the intensity of the absorption bands accompanies this shift, suggesting the formation of an inhibitor film on the copper surface (H. Li et al., 2021). In other words, the shift in spectral wavelength position (λ) in solutions of these compounds is often interpreted as an indication of the formation of Cu-inhibitor complexes (Yadav and Quraishi, 2012).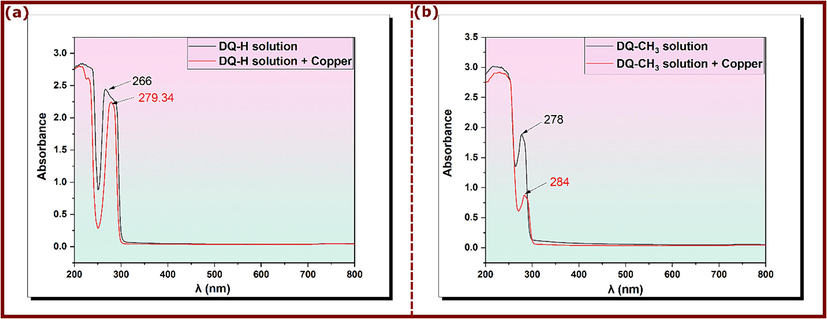
UV–Vis spectra of 3.5 % NaCl solution with 10−3 mol/L of (a) DQ-H and (b) DQ-CH3 before and after immersion of copper sample for 72 h at 298 K.
3.8 FT-IR spectroscopic measurements
FT-IR spectroscopic assessments have been carried out to ensure the existence of inhibitor film on the copper surface. Fig. 10 represents FT-IR results of pure DQ-H and DQ-CH3 which have likely the same plots with a slight difference in some band’s positions. O–H stretching vibration appears at 3420 cm−1 and 3438 cm−1 for DQ-H and DQ-CH3, respectively. The adsorption bands at 2985–2937 and 2833 cm−1 are assigned to C–H stretching vibration for DQ-CH3, while the same bands appear at 2929 cm−1 and 2854 cm−1 for DQ-H. The broad bands 1679 cm−1 and 1685 cm−1 are attributed to C = O. The peaks at 1371 cm−1 and 1380 cm−1 are ascribed to the bending vibration of C–H (Hrimla et al., 2021). A comparison of the FTIR spectra of pure products with those of the scraped material obtained from the copper surface after being submerged in 3.5 % NaCl in the presence of DQ-H and DQ-CH3 shows that the absorption bands of hydroxyl are shifted from 3420 to 3434 cm−1 and from 3438 to 3463 cm−1 for DQ-H and DQ-CH3, respectively. Also, the frequency for C = O is moved from 1679 cm−1 to 1675 cm−1 and from 1685 cm−1 to 1632 cm−1, respectively, for DQ-H and DQ-CH3. Thus, FTIR spectra of pure DQ-H and DQ-CH3 and their scratches from the copper surface have the same groups and differ only in wavenumbers indicating that the
electrons of these groups are involved in the inhibitor adsorption process (Kathiravan et al., 2021). These results reinforce what has been obtained by the above techniques about the existence of a chemical interaction between copper and the molecules of the inhibitor (Zhang et al., 2018c).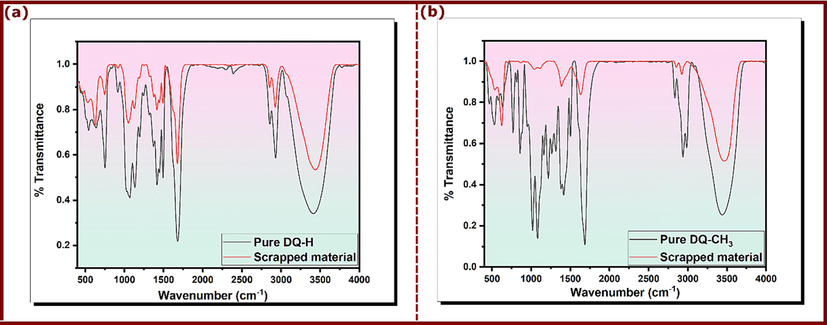
FT-IR spectra of pure (a) DQ-H and (b) DQ-CH3 and their scratches from the copper surface in 3.5% NaCl.
3.9 Quantum chemical calculations
Quantum chemical (QC) calculations have been changing the traditional research in corrosion inhibition studies in recent decades (Obot et al., 2015). QC calculations can effectively predict the electronic features and reactivity of compounds, which can clarify the mechanism underlying their interactions with other chemical species. In this regard, global reactivity descriptors such as HOMO, LUMO, and energy gap have been extensively used to predict the adsorption strength of corrosion inhibitors (Lgaz et al., 2021). Although these parameters have limited prediction accuracy because it provides information about the reactivity of individual molecules, they still can be of benefit in terms of predicting adsorption sites and electronic behavior of compounds, particularly when complemented with advanced simulation models such as first-principles DFT calculations. From experimental tests, it has been demonstrated that investigated compounds exhibit similar corrosion inhibition behavior when added to 3.5 wt% NaCl, maintaining higher protection of copper. Numerically, both compounds showed almost equal inhibition efficiency values from weight loss and electrochemical measurements. Therefore, we suppose that the additional methyl group in DQ-CH3 has no significant effect on the adsorption abilities of tested compounds. In this section, it would be interesting to investigate the effect of this small structural difference on the electronic and charge distributions of DQ-H and DQ-CH3 compounds. Fig. 11 represents the optimized molecular structure of DQ-H and DQ-CH3 and iso-surfaces of HOMO and LUMO densities. It can be observed from optimized molecular structures that both molecules have almost the same geometry and no serious structural changes are noticed. Moving to HOMO and LUMO densities, it can be said that the quinazolinone core is the main part where electron-donating and electron-accepting power of molecules exist. It is well-known that HOMO density is related to the electron-donating ability of molecules while the LUMO density is associated with electron-accepting tendency (Ebenso et al., 2021). Quinazolinone moiety with its heteroatoms and π-systems of aromatic rings can effectively participate in interactions with accepting chemical species such as vacant d-orbitals of copper, which if happened, would play a crucial role in the stabilization of molecules-copper interactions. Numerical values of HOMO and LUMO energies and other related quantum chemical parameters are listed in Table 6. Unsurprisingly, numerical values are in line with conclusions made from graphical representation. That is the reactivity descriptors of DQ-H and DQ-CH3 show almost identical values with no significant differences. It highlights the excellent corrosion inhibition effect of both compounds on the copper corrosion in 3.5 wt% NaCl that is attributed to their electron-rich molecular structures.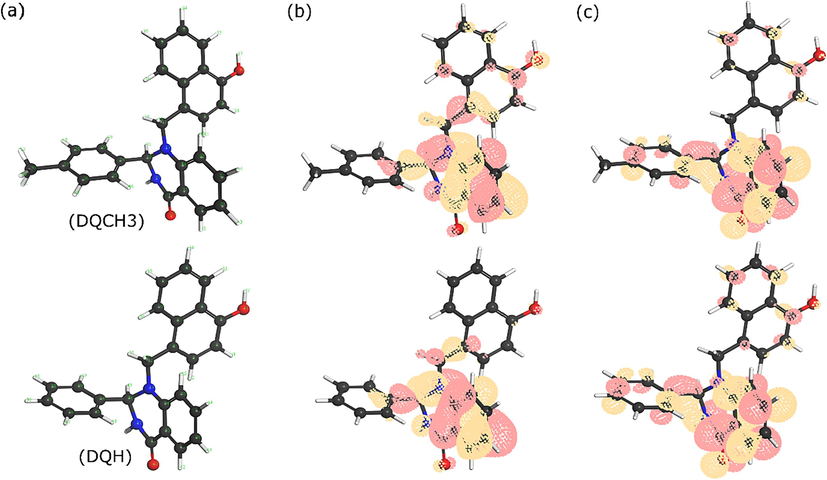
(a) optimized molecular structures, (b) HOMO, and (c) LUMO iso-surfaces of investigated compounds obtained by DFT/GGA method.
Molecule
EHOMO
ELUMO
ΔE
Ƞ
ω
σ
ΔN
ε
DQ-CH3
−5.013
−2.24
2.773
1.386
4.742
0.721
0.430
0.210
DQ-CH
−5.115
−2.243
2.872
1.436
4.712
0.696
0.397
0.212
To get more insights into the reactivity and the most reactive sites of investigated molecules, Fukui functions are computed and visualized by iso-surfaces in Fig. 12. Fukui functions (FFs) are one of the most useful DFT-derived reactivity indices (Thanikaivelan et al., 2002). FFs have been successfully used to characterize the capacity of molecules, and particularly atoms of molecules to accept or donate electrons; in other words, the tendency of an atom to have a nucleophilic or electrophilic character (De Proft, 2022; Gannouni et al., 2023). The site where the
is maximum indicates a high electrophilic property (susceptible to nucleophilic attacks) while the highest
means that the atomic site has a high nucleophilic character (susceptible to electrophilic attacks) (Saha et al., 2016). From the graphical representation of FFs in Fig. 12, it can be observed that nucleophilic and electrophilic sites are distributed on the entire molecular structure of DQ-H and DQ-CH3 compounds. However, an inspection of data in Table S1 (supplementary material) reveals that C(16), C(21), O(23) in DQ-CH3 and N(13), C(20), and C(22) in DQ-H exhibit the highest electrophilic Fukui index f+k, thus acting as preferred atomic sites to accept electrons during a nucleophilic attack (Pareek et al., 2019).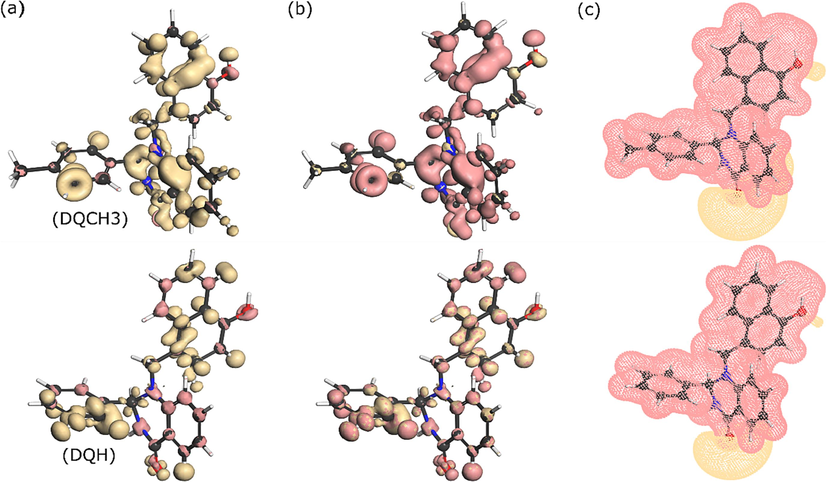
Iso-surfaces of Fukui functions plotted on molecular structures of DQ-H and DQ-CH3 (a) f+, (b) f- and (c) molecular electrostatic potential maps.
Besides, the electrostatic potential plotted on the optimized molecular structure of molecules is shown in Fig. 12c. It is obvious from the electrostatic potential map that carbonyl and hydroxyl groups represent the more electron-rich regions compared to other parts of the molecules, which indicates that they can be highly reactive adsorption sites when interacting with copper atoms.
3.10 First-principles DFT simulation
3.10.1 Geometries and interaction energies
First-principles calculations are increasingly taking place in different research fields thanks to the significant development of computer power. An unprecedented level of accuracy can now be achieved for complex simulation systems (Li et al., 2021). In corrosion inhibition research, first-principles DFT calculations are one of the most accurate tools to investigate the adsorption characteristics of molecule–metal systems (Kokalj, 2022).
Herein, first-principles DFT calculations are carried out to assess the adsorption geometries and electronic properties of DQ-H-Cu(1 1 1) and DQ-CH3-Cu(1 1 1) adsorption systems. It is well-documented that large-size corrosion inhibitors mostly adopt a parallel adsorption configuration on metal surfaces. In the case of hydroquinazolinones, it would be difficult to guess the most stable adsorption geometries given the presence of three cyclic systems in each molecule: two with two rings and one with phenyl (DQ-H) and methylphenyl (DQ-CH3). In this situation, three different initial configurations were optimized to get an overall view of the most probable stable adsorption geometries. Fig. 13&14 represent the DFT optimized adsorption geometries of DQ-CH3 and DQ-H on Cu(1 1 1) surface, respectively. Considering DQ-CH3-Cu(1 1 1) adsorption geometries, it can be observed that in the three configurations, DQ-CH3 molecule forms bonds with Cu atoms through its carbon and oxygen atoms. Fig. 13a (DQ-CH3-I) shows an adsorption configuration where the DQ-CH3 molecule lies parallel to the Cu(1 1 1) surface via its methylphenyl and 4-hydroxynaphthalene parts. Carbon atoms of these two moieties form three bonds with Cu atoms with bond distances between 2.285 and 2.327 Å. The adsorption configuration shown in Fig. 13b (DQ-CH3-II) indicates that only one bond is formed between the oxygen atom of the carbonyl group and Cu atom, with a bond distance of 2.024 Å whereas two bonds involving the oxygen atom of the carbonyl group (O-Cu = 1.974 Å) and one carbon atom of the methylphenyl moiety (C-Cu = 2.297 Å) are observed in adsorption configuration shown in Fig. 13c (DQ-CH3-III). Moving to DQ-H-Cu(1 1 1) adsorption configurations, it can be noticed that opposite to DQ-CH3-Cu(1 1 1) configuration, only 4-hydroxynaphthalene moiety is involved in bonding with Cu atoms as shown in Fig. 14a (DQ-H-I). Two carbons of 4-hydroxynaphthalene moiety are involved in interactions with copper atoms with bond distances of 2.279 and 2.296 Å. Opposite to DQ-CH3, the second optimized adsorption geometry of DQ-H-Cu(1 1 1) represented in Fig. 14b (DQ-H-II) shows no bonding with copper atoms, while the third adsorption configuration in Fig. 14c (DQ-H-III) reveals the formation of one bond between the oxygen atom of the carbonyl group and copper atoms, having a distance of 2.006 Å. It has been reported that the sum of the covalent radii values for Fe-C (rC + rFe) and Fe-O (rO + rFe) are 2.08 and 1.98 Å, respectively (Cordero et al., 2008).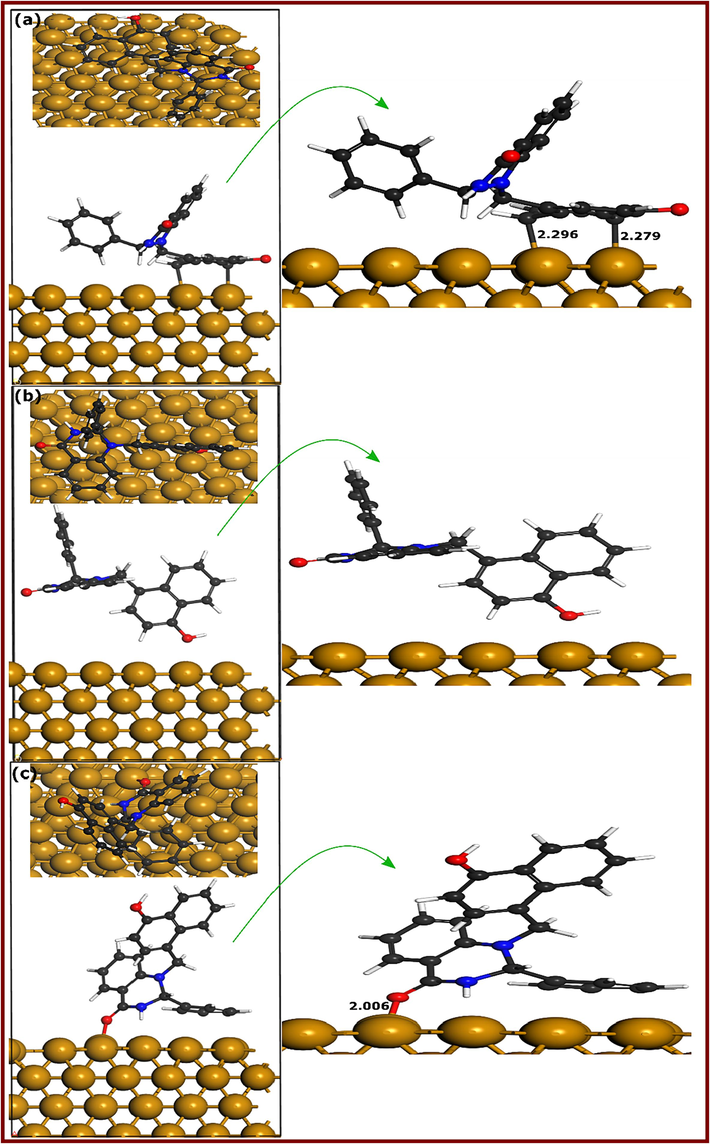
DFTB-optimized adsorption geometries of DQ-CH3 at different configurations; (a) Conf.1, (b) Conf. 2, and (c) Conf. 3.
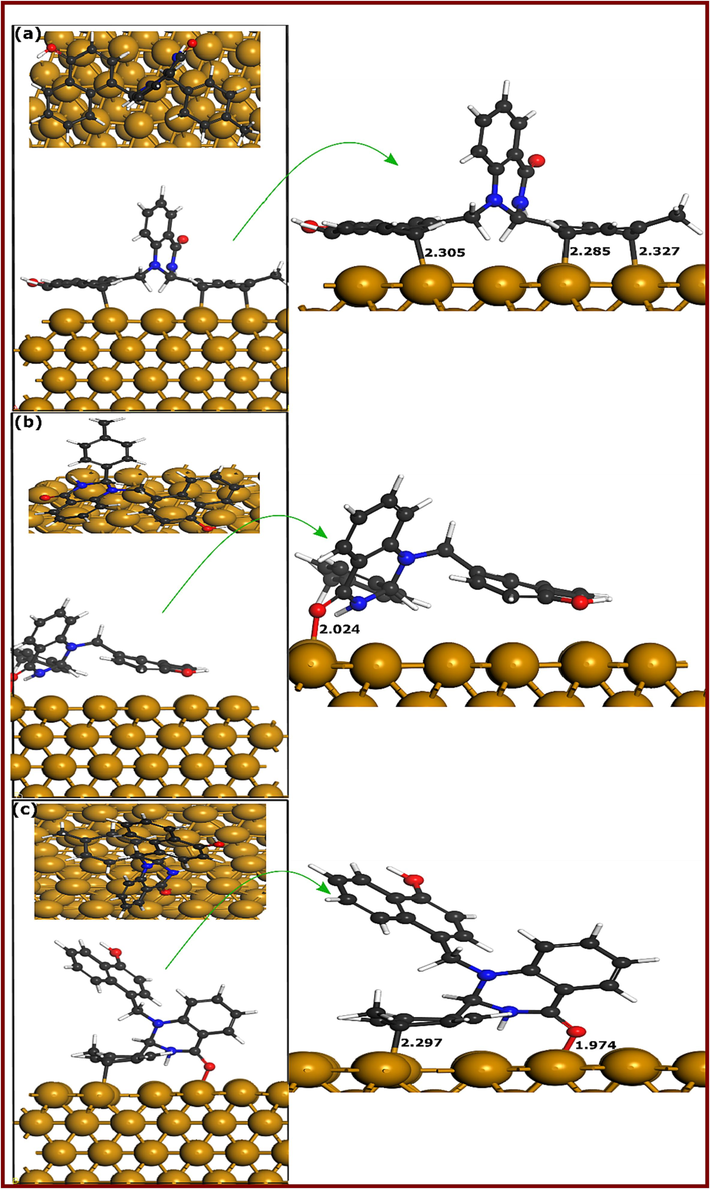
DFTB-optimized adsorption geometries of DQ-H at different configurations; (a) Conf.1, (b) Conf. 2, and (c) Conf. 3.
It suggests that bonds formed between hydroquinazolinones and Cu(1 1 1) surface are covalent.
Despite the fact that experimental evaluations showed similar corrosion inhibition performance, first-principles DFT simulations reveal that the small structural changes resulting from the addition of methyl group have a remarkable effect on the interaction strength with the copper surface. Energetically speaking, analysis of adsorption geometries indicates that DQ-CH3 configurations have interaction energies of −2.54, −1.06, and −2.24 eV for DQ-CH3-I, DQ-CH3-II, and DQ-CH3-III, respectively. Considering DQ-H-Cu(1 1 1) adsorption geometries, DQ-H-I, DQ-H-II, and DQ-H-III have interaction energies of −2.07, −0.033, and −1.64 eV, respectively. These results further confirm conclusions from optimized adsorption geometries, highlighting the significant effect of the additional methyl group on the adsorption behavior of investigated compounds.
3.10.2 Projected density of states
Deeper insights into the stability of inhibitors’ adsorption geometries on Cu(1 1 1) can be obtained by electronic structure analysis of inhibitor-Cu(1 1 1) in terms of the projected density of states. Projected density of states analysis is a useful tool to get information on the changes in the electronic structure and dominating interactions upon adsorption of DQ-H and DQ-CH3 on Cu(1 1 1) surface (Benbella et al., 2023; Matanou et al., 2023). Figs. 15 & 16 show the projected density of states on d-orbitals of Cu atoms and s,p-orbitals of DQ-CH3 and DQ-H adsorbed molecules on Cu(1 1 1) surface. By comparison with PDOSs of isolated molecules shown in Figs. 15a and 16a, a slight distortion of states is observed for the three adsorption configurations of DQ-CH3 represented in Figs. 15b-d and 16b-d. After the adsorption of DQ-CH3 molecules, states are shifted slightly to negative energies, which indicates a strong hybridization of the Cu d-states with adsorbate s,p-states of DQ-H molecules (Benbella et al., 2023). Concerning adsorption configurations of DQ-H-Cu(1 1 1), the same conclusions can be made for DQ-H-I and DQ-H-III adsorption configurations, where the energy shift and distortion of states are noticeable. However, a close inspection of PDOS plots of DQ-H-II reveals no hybridization of states (Benbella et al., 2023). These results strengthen the conclusions made from DFT-optimized adsorption geometries and confirm the chemical action between inhibitors’ orbitals and vacant d-orbitals of copper as a main mechanism of the corrosion inhibition effect.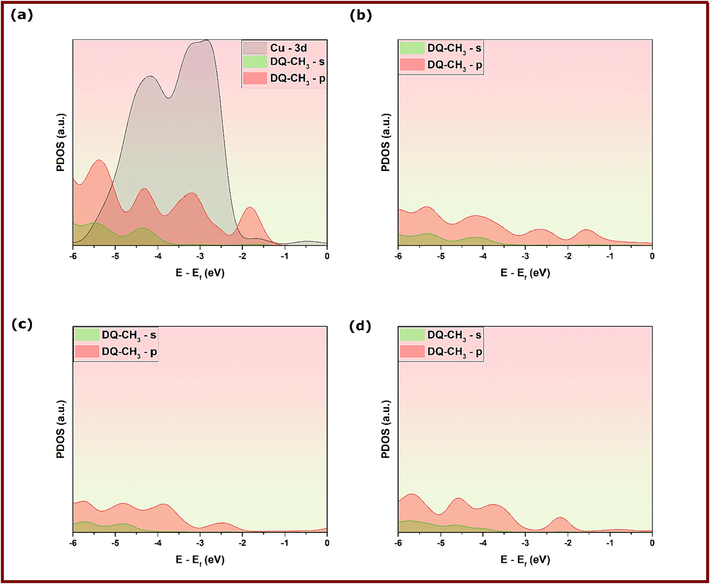
PDOS analysis of DFTB-optimized adsorption geometries of DQ-CH3 at different configurations; (a) Before adsorption, (b) Conf. 1, (b) Conf. 2 and (c) Conf. 3.
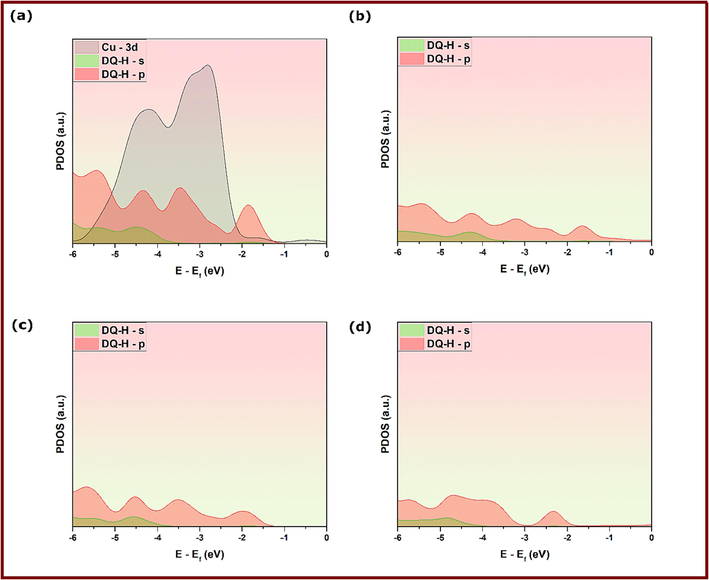
PDOS analysis of DFTB-optimized adsorption geometries of DQ-H at different configurations; (a) Before adsorption, (b) Conf. 1, (b) Conf. 2 and (c) Conf. 3.
4 Conclusions
In the present study, a comprehensive experimental and theoretical characterization of the corrosion inhibition performance and adsorption characteristics of two novel hydroquinazolinones derivatives for copper in 3.5 wt% NaCl. On the basis of the experimental and theoretical results, it could be concluded that:
Gravimetric analysis, potentiodynamic polarization curves (PPC) and electrochemical impedance spectroscopy (EIS) collectively confirm the robust inhibition capabilities of the quinazolinones studied, as evidenced by inhibition efficiency values exceeding 90 % at a concentration of 10−3 mol/L according to all techniques.
Electrochemical evaluation of corrosion inhibition abilities of DQ-CH3 and DQ-H compounds revealed an outstanding anti-corrosion effect with inhibition efficiencies reaching 92 % and 94 % for DQ-H and DQ-CH3, respectively.
The increase in polarization resistance (Rp) from 3590 to 9403 Ω cm2 for DQ-H and from 3811 to 12861 Ω cm2 for DQ-CH3, as the concentration increased from 10−6 to 10−3 mol/L in the EIS, along with the decrease in corrosion current density (icorr) from 36.8 to 2.17 µA cm−2 for DQ-H and from 10.1 to 1.67 µA cm−2 for DQ-CH3 in the PPC, illustrate the preventive properties of DQ-H and DQ-CH3 against copper corrosion.
The influence of temperature was further examined by PPC test, and a slight decrease in inhibition efficiency and an increase in corrosion rate were observed with increasing temperature.
Despite the incorporation of an additional methyl group in the DQ-CH3 compound, there was no remarkable effect on the corrosion inhibition performance, and both compounds showed a strong inhibition effect on copper corrosion in 3.5 wt% NaCl.
SEM analysis revealed a significant improvement in copper surface morphology after the addition of DQ-CH3 and DQ-H compounds to 3.5 wt% NaCl solutions.
Conclusions made from FTIR analysis suggested that inhibitor molecules strongly adsorbed on the copper surface, which was further confirmed by UV–vis spectroscopy measurements.
The DFT optimization of adsorption geometries was carried out for three different configurations of each molecule. It revealed the formation of several covalent bonds between the DQ-CH3 molecule and copper surface whereas DQ-H showed the formation of covalent bonds only for two adsorption configurations.
The electronic structure analysis of inhibitor-Cu(1 1 1) adsorption systems affirmed the existence of strong hybridization of the Cu d-states with adsorbate s,p-states of DQ-H and DQ-CH3 molecules.
Outcomes from the present study provided deeper insights into the corrosion inhibition mechanisms of hydroquinazolinones for copper in 3.5 wt% NaCl, which is believed to fill a gap in the literature on the application of hydroquinazolinones.
CRediT authorship contribution statement
Mohammed Oubahou: Conceptualization, Methodology, Formal analysis, Investigation, Data curation, Writing – original draft. Mohamed Rbaa: Conceptualization, Methodology, Formal analysis, Investigation, Data curation, Writing – original draft. Hassane Lgaz: Conceptualization, Methodology, Formal analysis, Investigation, Data curation, Writing – original draft. Driss Takky: Investigation, Supervision, Visualization, Writing – review & editing. Youssef Naimi: Investigation, Supervision, Visualization, Writing – review & editing. Awad A. Alrashdi: Project administration, Funding acquisition, Supervision, Writing – review & editing. Han-seung Lee: Project administration, Funding acquisition, Supervision, Writing – review & editing.
Acknowledgment
“This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. NRF-2018R1A5A1025137).”
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- A. Aabid, A., A. Jafar, S., Obaid Ahmed, M., & Humadi, J. I. (2021). Inhibitory effect of ceftriaxone sodium on corrosion of copper in 1M nitric acid. Mater. Today: Proc., xxxx. Doi: 10.1016/j.matpr.2021.05.345.
- A study of the corrosion inhibition of mild steel C1018 in CO2-saturated brine using some novel surfactants based on corn oil. Egypt. J. Pet.. 2013;22(4):451-470.
- [CrossRef] [Google Scholar]
- Corrosion inhibition of carbon steel in hydrochloric acid solution using newly synthesized urea-based cationic fluorosurfactants: experimental and computational investigations. New J. Chem.. 2020;44(41):17791-17814.
- [CrossRef] [Google Scholar]
- Novel natural surfactant-based fatty acids and their corrosion-inhibitive characteristics for carbon steel-induced sweet corrosion: detailed practical and computational explorations. Front. Mater.. 2022;9(February):1-18.
- [CrossRef] [Google Scholar]
- Corrosion control of carbon steel in watert-based mud by nanosized meallo-cationic surfactant complexes during drilling operations. ACS Omega. 2020;5(48):30881-30897.
- [CrossRef] [Google Scholar]
- CHAPTER 1 - introduction to corrosion. In: Ahmad Z., ed. Principles of Corrosion Engineering and Corrosion Control. Butterworth-Heinemann; 2006. p. :1-8.
- [CrossRef] [Google Scholar]
- Akinbulumo, O. A., Odejobi, O. J., & Odekanle, E. L. (2020). Thermodynamics and adsorption study of the corrosion inhibition of mild steel by Euphorbia heterophylla L. extract in 1.5 M HCl. Results in Materials, 5(October 2019), 100074. Doi: 10.1016/j.rinma.2020.100074.
- Role of green chemistry in sustainable corrosion inhibition: a review on recent developments. Mater. Today Sustain.. 2022;20:100242
- [CrossRef] [Google Scholar]
- Molecular dynamics, Monte-Carlo simulations and atomic force microscopy to study the interfacial adsorption behaviour of some triazepine carboxylate compounds as corrosion inhibitors in acid medium. J. Bio- and Tribo-Corrosion. 2019;5(1)
- [CrossRef] [Google Scholar]
- Experimental and quantum chemical simulations on the corrosion inhibition of mild steel by 3-((5-(3,5-dinitrophenyl)-1,3,4-thiadiazol-2-yl)imino)indolin-2-one. Results Phys.. 2018;9:278-283.
- [CrossRef] [Google Scholar]
- Experimental and theoretical study on the corrosion inhibition of API 5L X52 steel in acid media by a new quinazoline derivative. J. Mol. Liq.. 2020;320:114449
- [CrossRef] [Google Scholar]
- Quinazolinones, the winning horse in drug discovery. Molecules. 2023;28(3)
- [CrossRef] [Google Scholar]
- Experimental, theoretical modeling and optimization of inhibitive action of Ocimum basilicum essential oil as green corrosion inhibitor for C38 steel in 0.5 M H2SO4 medium. chemistry. Africa. 2022;5(1):37-55.
- [CrossRef] [Google Scholar]
- Experimental and theoretical study of xanthene derivatives as corrosion inhibitor for mild steel in hydrochloric acid solution. J. Appl. Electrochem.. 2022;52(8):1275-1294.
- [CrossRef] [Google Scholar]
- Corrosion inhibition of mild steel by some schiff base compounds in hydrochloric acid. Appl. Surf. Sci.. 2005;239(2):154-164.
- [CrossRef] [Google Scholar]
- Inhibitive effect of synthesized 2-(3-pyridyl)-3,4-dihydro-4-quinazolinone as a corrosion inhibitor for mild steel in hydrochloric acid. Mater. Chem. Phys.. 2011;126(3):873-879.
- [CrossRef] [Google Scholar]
- Performance of triazole derivatives as potential corrosion in-hibitors for mild steel in a strong phosphoric acid medium: combining experimental and computational (DFT, MDs & QSAR) approaches. J. Mol. Struct.. 2022;1256:132515
- [CrossRef] [Google Scholar]
- Anti-corrosive properties of 4-amino-3,5-bis(disubstituted)-1,2,4-triazole derivatives on mild steel corrosion in 2 M H3PO4 solution: experimental and theoretical studies. J. Mol. Liq.. 2016;216:874-886.
- [CrossRef] [Google Scholar]
- A comprehensive assessment of carbon steel corrosion inhibition by 1,10-phenanthroline in the acidic environment: insights from experimental and computational studies. Environ. Sci. Pollut. Res. 2023
- [CrossRef] [Google Scholar]
- Synthesis, spectroscopic characterization and a comparative study of the corrosion inhibitive efficiency of an α-aminophosphonate and Schiff base derivatives: experimental and theoretical investigations. J. Mol. Struct.. 2018;1157:165-176.
- [CrossRef] [Google Scholar]
- The influence of some new 2,5-disubstituted 1,3,4-thiadiazoles on the corrosion behaviour of mild steel in 1 M HCl solution: AC impedance study and theoretical approach. Electrochim. Acta. 2007;52(24):6865-6872.
- [CrossRef] [Google Scholar]
- Experimental and theoretical approach to the corrosion inhibition of mild steel in acid medium by a newly synthesized pyrazole carbothioamide heterocycle. J. Mol. Struct.. 2020;1199:127051
- [CrossRef] [Google Scholar]
- Synthesis, spectral analysis, theoretical studies, molecular dynamic simulation and comparison of anticorrosive activity of an ester and an acid α-hydroxyphosphonates. J. Mol. Struct.. 2019;1195:839-849.
- [CrossRef] [Google Scholar]
- Synthesis, characterization and the inhibition activity of a new α-aminophosphonic derivative on the corrosion of XC48 carbon steel in 0.5 M H2SO4: experimental and theoretical studies. J. Taiwan Inst. Chem. Eng.. 2017;70:331-344.
- [CrossRef] [Google Scholar]
- Synthesis, spectral analysis, anti-corrosive activity and theoretical study of an aromatic hydrazone derivative. J. Mol. Struct.. 2019;1181:83-92.
- [CrossRef] [Google Scholar]
- New 8-hydroxyquinoline-bearing quinoxaline derivatives as effective corrosion inhibitors for mild steel in HCl: electrochemical and computational investigations. Coatings. 2020;10(9)
- [CrossRef] [Google Scholar]
- Comprehensive assessment of corrosion inhibition mechanisms of novel benzimidazole compounds for mild steel in HCl: an experimental and theoretical investigation. J. Mol. Liq.. 2020;320
- [CrossRef] [Google Scholar]
- Electrochemical, ToF-SIMS and computational studies of 4-amino-5-methyl-4H-1,2,4-triazole-3-thiol as a novel corrosion inhibitor for copper in 3.5% NaCl. J. Mol. Liq.. 2019;289
- [CrossRef] [Google Scholar]
- Mitigation effect of quinazolin-4(3H)-one derivatives on the corrosion behaviour of mild steel in HCl. Colloids Surf. A Physicochem. Eng. Asp.. 2021;627
- [CrossRef] [Google Scholar]
- Synthesis and surface characterization of self-assembled monolayers of thiazoles incorporating hydrocarbon and fluorocarbon chains on copper substrates. Appl. Surf. Sci.. 2018;456(May):25-36.
- [CrossRef] [Google Scholar]
- First principles methods using CASTEP. Z. Kristallogr.. 2005;220(5–6):567-570.
- [CrossRef] [Google Scholar]
- Substituted uracils as corrosion inhibitors for copper in 3% NaCl solution. Corros. Sci.. 2003;45(8):1619-1630.
- [CrossRef] [Google Scholar]
- Damej, M., Kaya, S., EL Ibrahimi, B., Lee, H. S., Molhi, A., Serdaroğlu, G., Benmessaoud, M., Ali, I. H., EL Hajjaji, S., & Lgaz, H. (2021). The corrosion inhibition and adsorption behavior of mercaptobenzimidazole and bis-mercaptobenzimidazole on carbon steel in 1.0 M HCl: Experimental and computational insights. Surfaces and Interfaces, 24(December 2020). Doi: 10.1016/j.surfin.2021.101095.
- Caffeic acid as a green corrosion inhibitor for mild steel. Corros. Sci.. 2009;51(3):642-649.
- [CrossRef] [Google Scholar]
- Electrochemical behaviour of copper in neutral aerated chloride solution. II. impedance investigation. J. Appl. Electrochem.. 1988;18(3):384-393.
- [CrossRef] [Google Scholar]
- Synthesis, spectral properties and corrosion inhibition efficiency of new ethyl hydrogen [(methoxyphenyl) (methylamino) methyl] phosphonate derivatives: experimental and theoretical investigation. J. Mol. Struct.. 2019;1175:398-413.
- [CrossRef] [Google Scholar]
- Effect of substitution on corrosion inhibition properties of 2-(substituted phenyl) benzimidazole derivatives on mild steel in 1 M HCl solution: a combined experimental and theoretical approach. Corros. Sci.. 2017;123(April):256-266.
- [CrossRef] [Google Scholar]
- Molecular modelling of compounds used for corrosion inhibition studies: a review. Phys. Chem. Chem. Phys.. 2021;23(36):19987-20027.
- [CrossRef] [Google Scholar]
- Expired amoxicillin as an eco – friendly corrosion inhibitor for cast steel in sulfuric acid environment : electrochemical, surface and thermodynamic studies. J. Solid State Electrochem.. 2023;0123456789
- [CrossRef] [Google Scholar]
- Kinetics and thermodynamics of the inhibition of the acid corrosion of steel by some macrocyclic ligands. Int. J. Chem. 1990;1(4):169-179.
- [Google Scholar]
- Electroanalytical, quantum and surface characterization studies on imidazole derivatives as corrosion inhibitors for aluminum in acidic media. J. Mol. Liq.. 2015;209:480-486.
- [CrossRef] [Google Scholar]
- Adsorption and corrosion inhibition effect of 2-mercaptobenzimidazole (surfactant) on a carbon steel surface in an acidic medium: experimental and Monte Carlo simulations. Port. Electrochim. Acta. 2018;36(3):197-212.
- [CrossRef] [Google Scholar]
- Electrochemical and theoretical insights on the adsorption and corrosion inhibition of novel pyridinium-derived ionic liquids for mild steel in 1 M HCl. J. Mol. Liq.. 2020;314:113737
- [CrossRef] [Google Scholar]
- Experimental, DFT calculations and MC simulations concept of novel quinazolinone derivatives as corrosion inhibitor for mild steel in 1.0 M HCl medium. J. Mol. Liq.. 2020;312:113413
- [CrossRef] [Google Scholar]
- Faydy, M. El, Rbaa, M., Lakhrissi, L., Lakhrissi, B., Warad, I., Zarrouk, A., & Obot, I. B. (2019). Corrosion protection of carbon steel by two newly synthesized benzimidazol-2-ones substituted 8-hydroxyquinoline derivatives in 1 M HCl: Experimental and theoretical study. Surfaces and Interfaces, 14(October 2018), 222–237. Doi: 10.1016/j.surfin.2019.01.005.
- Inhibition of copper corrosion by 1,2,3-benzotriazole: a review. Corros. Sci.. 2010;52(9):2737-2749.
- [CrossRef] [Google Scholar]
- Some quinazoline derivatives as corrosion inhibitors for copper in HNO3 solution. Desalin. Water Treat.. 2010;22:340-348.
- [Google Scholar]
- Quinazoline derivatives as green corrosion inhibitors for carbon steel in hydrochloric acid solutions. Int. J. Electrochem. Sci.. 2013;8(4):5866-5885.
- [CrossRef] [Google Scholar]
- The role of frontier orbitals in chemical reactions (nobel lecture) Angew. Chem. Int. Ed. Eng.. 1982;21(11):801-809.
- [CrossRef] [Google Scholar]
- Corrosion inhibition of zinc in HCl solution by several pyrazole derivatives. Corrosion. 1989;45(7):574-578.
- [CrossRef] [Google Scholar]
- Chemically functionalized of 8-hydroxyquinoline derivatives as efficient corrosion inhibition for steel in 1.0 M HCl solution: experimental and theoretical studies. Surf. Interfaces. 2020;21(September):100695
- [CrossRef] [Google Scholar]
- Effect of alkyl group position on adsorption behavior and corrosion inhibition of new naphthol based on 8-hydroxyquinoline: electrochemical, surface, quantum calculations and dynamic simulations. J. Mol. Liq.. 2021;335:116552
- [CrossRef] [Google Scholar]
- Evaluation of corrosion inhibition performance of a novel ionic liquid based on synergism between cation and anion. New J. Chem.. 2020;44(19):7802-7810.
- [CrossRef] [Google Scholar]
- Design, synthesis and characterization of novel 2-(2,4-disubstituted-thiazole-5-yl)-3-aryl-3H-quinazoline-4-one derivatives as inhibitors of NF-κB and AP-1 mediated transcription activation and as potential anti-inflammatory agents. Eur. J. Med. Chem.. 2009;44(5):2184-2189.
- [CrossRef] [Google Scholar]
- The inhibition mechanism and adsorption behavior of three purine derivatives on the corrosion of copper in alkaline artificial seawater: structure and performance. Colloids Surf. A Physicochem. Eng. Asp.. 2021;622(February):126644
- [CrossRef] [Google Scholar]
- Corrosion inhibition of carbon steel by three kinds of expired cephalosporins in 0.1 M H2SO4. J. Mol. Liq.. 2020;320:114295
- [CrossRef] [Google Scholar]
- Thermodynamic, adsorption and electrochemical studies for corrosion inhibition of carbon steel by henna extract in acid medium. Egypt. J. Pet.. 2013;22(1):17-25.
- [CrossRef] [Google Scholar]
- The effect of inhibitor structure on the corrosion of AA2024 and AA7075. Corros. Sci.. 2011;53(6):2184-2190.
- [CrossRef] [Google Scholar]
- Insight into the anti-corrosion mechanism of 2-aminobenzenethiol as the inhibitor for copper in acid environment. J. Mol. Liq.. 2020;320:114494
- [CrossRef] [Google Scholar]
- Inhibition effect of 4-amino-antipyrine on the corrosion of copper in 3wt.% NaCl solution. Corros. Sci.. 2012;57:270-278.
- [CrossRef] [Google Scholar]
- Corrosion inhibition performance of a structurally well-defined 1,2,3-triazole derivative on mild steel-hydrochloric acid interface. J. Mol. Struct.. 2021;1231:129895
- [CrossRef] [Google Scholar]
- The relationship between the inhibition performances of three benzo derivatives and their structures on the corrosion of copper in 3.5 wt.% NaCl solution. Colloids Surf. A Physicochem. Eng. Asp.. 2020;598(January):124809
- [CrossRef] [Google Scholar]
- Quantum chemical study on the inhibition efficiencies of some sym-triazines as inhibitors for mild steel in acidic medium. J. Taiwan Inst. Chem. Eng.. 2015;50:306-313.
- [CrossRef] [Google Scholar]
- Quinazolinone and quinazoline derivatives: recent structures with potent antimicrobial and cytotoxic activities. Res. Pharm. Sci.. 2016;11(1):1-14.
- [Google Scholar]
- Improvement in the corrosion resistance of carbon steel in acidic condition using naphthalen-2-ylnaphthalene-2-carboxammide inhibitor. J. Colloid Interface Sci.. 2018;512:618-628.
- [CrossRef] [Google Scholar]
- Inhibitory action of aqueous Ruellia Tuberosa L leaves extract on the corrosion of copper in HCl solution. J. Indian Chem. Soc.. 2021;98(11):100207
- [CrossRef] [Google Scholar]
- Cationic gemini-surfactants based on waste cooking oil as new ‘green’ inhibitors for N80-steel corrosion in sulphuric acid: a combined empirical and theoretical approaches. J. Mol. Struct.. 2020;1203:127442
- [CrossRef] [Google Scholar]
- Journal of colloid and Interface science electrochemical investigation on the corrosion inhibition of mild steel by quinazoline Schiff base compounds in hydrochloric acid solution. J. Colloid Interface Sci.. 2017;502:134-145.
- [CrossRef] [Google Scholar]
- Recent advances in the structural library of functionalized quinazoline and quinazolinone scaffolds: synthetic approaches and multifarious applications. Eur. J. Med. Chem.. 2014;76:193-244.
- [CrossRef] [Google Scholar]
- Molecular modeling of organic corrosion inhibitors: calculations, pitfalls, and conceptualization of molecule–surface bonding. Corros. Sci.. 2021;193:109650
- [CrossRef] [Google Scholar]
- Corrosion inhibitors: physisorbed or chemisorbed? Corros. Sci.. 2022;196:109939
- [CrossRef] [Google Scholar]
- Kumar, C. B. P., Prashanth, M. K., Mohana, K. N., Jagadeesha, M. B., Raghu, M. S., Lokanath, N. K., and Kumar, K. Y. (2020). Protection of mild steel corrosion by three new quinazoline derivatives : experimental and DFT studies. Surf. Interfaces, 18(October 2019), 100446. Doi: 10.1016/j.surfin.2020.100446.
- Effect of clozapine on inhibition of mild steel corrosion in 1.0 M HCl medium. J. Mol. Liq.. 2017;225:271-280.
- [CrossRef] [Google Scholar]
- Evaluation of 2-mercaptobenzimidazole derivatives as corrosion inhibitors for mild steel in hydrochloric acid. Metals. 2020;10(3):1-14.
- [CrossRef] [Google Scholar]
- Computational methods of corrosion inhibition assessment. In ACS Symposium Series 2021
- [CrossRef] [Google Scholar]
- First-principles based theoretical investigation of the adsorption of alkanethiols on the iron surface: a DFT-D3 study. J. Mol. Liq.. 2022;348:118071
- [CrossRef] [Google Scholar]
- Effects of two fungicides on the corrosion resistance of copper in 3.5% NaCl solution under various conditions. Corros. Sci.. 2011;53(2):735-745.
- [CrossRef] [Google Scholar]
- Some aspects of quantum chemical calculations for the study of Schiff base corrosion inhibitors on copper in NaCl solutions. Corros. Sci.. 1999;41(9):1769-1782.
- [CrossRef] [Google Scholar]
- Corrosion retardation effect of a green cauliflower extract on copper in H2SO4 solution: electrochemical and theoretical explorations. J. Mol. Liq.. 2021;321:114450
- [CrossRef] [Google Scholar]
- Inhibition of copper corrosion in sodium chloride solution by the self-assembled monolayer of sodium diethyldithiocarbamate. Corros. Sci.. 2011;53(5):1999-2005.
- [CrossRef] [Google Scholar]
- Protection of steel against corrosion in acid medium using dihydropyrimidinone derivatives: experimental and DFT study. Iran. J. Sci. Technol., Trans. A: Sci.. 2021;45(5):1607-1619.
- [CrossRef] [Google Scholar]
- Sustainable approach for corrosion control in mild steel using plant-based inhibitors: a review. Mater. Today Sustain.. 2023;22:100373
- [CrossRef] [Google Scholar]
- Synthesis, structural and anticorrosion properties of diethyl (phenylamino) methyl) phosphonate derivatives: experimental and theoretical study. J. Mol. Struct.. 2020;1206:127693
- [CrossRef] [Google Scholar]
- Application of interaction energy in quantitative structure-inhibition relationship study of some benzenethiol derivatives on copper corrosion. Corros. Sci.. 2016;105:170-176.
- [CrossRef] [Google Scholar]
- The effect of molecular structure on hydrogen permeation and the corrosion inhibition of mild steel in acidic solutions. Corros. Sci.. 1995;37(11):1739-1750.
- [CrossRef] [Google Scholar]
- Thermodynamic study of metal corrosion and inhibitor adsorption processes in mild steel/1-methyl-4[4′(-X)-styryl pyridinium iodides/hydrochloric acid systems. Mater. Chem. Phys.. 2008;110(1):145-154.
- [CrossRef] [Google Scholar]
- Density functional theory (DFT) as a powerful tool for designing new organic corrosion inhibitors: part 1: an overview. Corros. Sci.. 2015;99:1-30.
- [CrossRef] [Google Scholar]
- Atomistic simulation: a unique and powerful computational tool for corrosion inhibition research. Arab. J. Sci. Eng.. 2019;44(1):1-32.
- [CrossRef] [Google Scholar]
- The influence of pH value on the efficiency of imidazole based corrosion inhibitors of copper. Corros. Sci.. 2010;52(2):398-405.
- [CrossRef] [Google Scholar]
- Electrochemical, thermodynamic and theoretical studies of some imidazole derivatives compounds as acid corrosion inhibitors for mild steel. J. Mol. Liq.. 2020;319:114063
- [CrossRef] [Google Scholar]
- Novel triphenyl imidazole based on 8-hydroxyquinoline as corrosion inhibitor for mild steel in molar hydrochloric acid: experimental and theoretical investigations. J. Appl. Electrochem.. 2021;52:413-433.
- [Google Scholar]
- Elucidating the role of novel halogenated hydroquinazolinone derivatives in mitigating copper corrosion in saline conditions: a joint assessment of experimental outcomes and computational analysis. J. Mol. Liq.. 2023;390(PA):122966
- [CrossRef] [Google Scholar]
- Inhibition of mild steel corrosion in 5 % HCl solution by 5-(2-hydroxyphenyl)-1,2,4-triazole-3-thione. Res. Chem. Intermed.. 2013;39(6):2777-2793.
- [CrossRef] [Google Scholar]
- Corrosion inhibition of mild steel in acidic media using newly synthesized heterocyclic organic molecules: correlation between inhibition efficiency and chemical structure. AIP Conf. Proc.. 2015;1653
- [CrossRef] [Google Scholar]
- Solvent and catalyst-free synthesis, corrosion protection, thermodynamic, MDS and DFT calculation of two environmentally friendly inhibitors: bis-phosphonic acids. J. Mol. Struct.. 2020;1222:128813
- [CrossRef] [Google Scholar]
- Synthesis and biological evaluation of cationic fullerene quinazolinone conjugates and their binding mode with modeled mycobacterium tuberculosis hypoxanthine-guanine phosphoribosyltransferase enzyme. J. Mol. Model.. 2013;19(8):3201-3217.
- [CrossRef] [Google Scholar]
- Generalized gradient approximation made simple. Phys. Rev. Lett.. 1996;77(18):3865-3868.
- [CrossRef] [Google Scholar]
- Synthesis, characterization and corrosion inhibition studies of novel 8-hydroxyquinoline derivatives on the acidic corrosion of mild steel: experimental and computational studies. Mater. Discover. 2018;12(August):43-54.
- [CrossRef] [Google Scholar]
- Two new 8-hydroxyquinoline derivatives as an efficient corrosion inhibitors for mild steel in hydrochloric acid: synthesis, electrochemical, surface morphological, UV–Visible and theoretical studies. J. Mol. Liq.. 2019;276:120-133.
- [CrossRef] [Google Scholar]
- Synthesis and investigation of quinazoline derivatives based on 8-hydroxyquinoline as corrosion inhibitors for mild steel in acidic environment: experimental and theoretical studies. Ionics. 2019;25(7):3473-3491.
- [CrossRef] [Google Scholar]
- Remarkably flexible quinazolinones—synthesis and biological applications. J. Heterocycl. Chem.. 2020;57(3):942-954.
- [CrossRef] [Google Scholar]
- Synthesis, characterization and corrosion inhibition potential of newly benzimidazole derivatives: combining theoretical and experimental study. Surf. Interfaces. 2020;18(January)
- [CrossRef] [Google Scholar]
- Evaluating electronic structure of quinazolinone and pyrimidinone molecules for its corrosion inhibition effectiveness on target speci fi c mild steel in the acidic medium : a combined DFT and MD simulation study. J. Mol. Liq.. 2016;224:629-638.
- [CrossRef] [Google Scholar]
- Functionalized 2-hydrazinobenzothiazole with carbohydrates as a corrosion inhibitor: electrochemical, XPS, DFT and Monte Carlo simulation studies. Mater. Chem. Front.. 2019;3(5):931-940.
- [CrossRef] [Google Scholar]
- Inhibition of copper corrosion in acidic chloride pickling solutions by 5- (3-aminophenyl) -tetrazole as a corrosion inhibitor. Corros. Sci.. 2008;50(12):3439-3445.
- [CrossRef] [Google Scholar]
- Isolation and characterization of exopolysaccharides from seaweed associated bacteria Bacillus licheniformis. Carbohydr. Polym.. 2011;84(3):1019-1026.
- [CrossRef] [Google Scholar]
- Corrosion inhibition of mild steel in hydrochloric acid solution by some double Schiff bases. Corros. Sci.. 2010;52(4):1351-1361.
- [CrossRef] [Google Scholar]
- Electrochemical behaviour of uncoated and phosphatidylcholine coated copper in hydrochloric acid medium. J. Mol. Liq.. 2018;249:930-940.
- [CrossRef] [Google Scholar]
- Density functional theory study of imidazole, benzimidazole and 2-mercaptobenzimidazole adsorption onto clean Cu(111) surface. Corros. Sci.. 2012;63:140-147.
- [CrossRef] [Google Scholar]
- The effect of regional selectivity on the corrosion inhibition properties of synthetic ionic liquid via the synergistic action of the cation and anion. New J. Chem.. 2021;45(43):20201-20213.
- [CrossRef] [Google Scholar]
- Electrochemical corrosion basics. Handbook of Environmental Degradation of Materials: Third Edition. 2018;97–115
- [CrossRef] [Google Scholar]
- Passiflora edulia Sims leaves extract as renewable and degradable inhibitor for copper in sulfuric acid solution. Colloids Surf. A: Physicochem. Eng. Aspects. 2022;645(February):128892
- [CrossRef] [Google Scholar]
- Insight into the anti-corrosion performance of two food flavors as eco-friendly and ultra-high performance inhibitors for copper in sulfuric acid medium. J. Colloid Interface Sci.. 2022;609:838-851.
- [CrossRef] [Google Scholar]
- Insight into anti-corrosion mechanism of Dalbergia odorifera leaves extract as a biodegradable inhibitor for X70 steel in sulfuric acid medium. Ind. Crop. Prod.. 2023;194(December 2022):116106
- [CrossRef] [Google Scholar]
- Revealing the correlation between molecular structure and corrosion inhibition characteristics of N-heterocycles in terms of substituent groups. Materials. 2023;16(6):2148.
- [CrossRef] [Google Scholar]
- Experimental and theoretical study on the synergistic inhibition effect of pyridine derivatives and sulfur-containing compounds on the corrosion of carbon steel in CO2-saturated 3.5 wt.% NaCl solution. Molecules. 2018;23(12)
- [CrossRef] [Google Scholar]
- Experimental and computational approaches of sustainable quaternary bisammonium fluorosurfactants for corrosion inhibition as protective films at mild steel/H2SO4 interface. Colloids Surf. A Physicochem. Eng. Asp.. 2021;614(October 2020):126141
- [CrossRef] [Google Scholar]
- Experimental and theoretical studies of paracetamol as a copper corrosion inhibitor. J. Mol. Liq.. 2021;327
- [CrossRef] [Google Scholar]
- Thomas K.V., Thomas K.J., Rapheal P.V., Sabu, A.S., Ragi, K., Johnson, R. (2021). Tinospora cordifolia extract as an environmentally benign green corrosion inhibitor in acid media: electrochemical, surface morphological, quantum chemical, and statistical investigations. Mater. Today Sustain., 13, 100076. Doi: 10.1016/J.MTSUST.2021.100076.
- Anodic dissolution of metal coated by a formed salt film. In: Trethewey K.R., Roberge P.R., eds. Modelling Aqueous Corrosion: from Individual Pits to System Management. Netherlands: Springer; 1994. p. :141-159.
- [CrossRef] [Google Scholar]
- Mechanism of corrosion inhibition by polymers. Polym. Mater. Corrosion Inhibition. 2022;565–589
- [CrossRef] [Google Scholar]
- Corrosion inhibitors for ferrous and non-ferrous metals and alloys in ionic sodium chloride solutions: a review. J. Mol. Liq.. 2017;248:927-942.
- [CrossRef] [Google Scholar]
- Substituents effect on corrosion inhibition performance of organic compounds in aggressive ionic solutions: a review. J. Mol. Liq.. 2018;251:100-118.
- [CrossRef] [Google Scholar]
- A green and sustainable approach for mild steel acidic corrosion inhibition using leaves extract: experimental and DFT studies. J. Bio- and Tribo-Corrosion. 2018;4(3)
- [CrossRef] [Google Scholar]
- Adsorption and anticorrosion behaviour of mild steel treated with 2-((1H-indol-2-yl)thio)-6-amino-4-phenylpyridine-3,5-dicarbonitriles in a hydrochloric acid solution: experimental and computational studies. J. Mol. Liq.. 2019;283:491-506.
- [CrossRef] [Google Scholar]
- Quinoline and its derivatives as corrosion inhibitors: a review. Surf. Interfaces. 2020;21(June)
- [CrossRef] [Google Scholar]
- Determining Gibbs energies of adsorption from corrosion inhibition efficiencies: is it a reliable approach? Corros. Sci.. 2019;155(May):182-185.
- [CrossRef] [Google Scholar]
- Wei, H., Heidarshenas, B., Zhou, L., Hussain, G., Li, Q., & Ostrikov, K. (Ken). (2020). Green inhibitors for steel corrosion in acidic environment: state of art. Mater. Today Sustain., 10, 100044. Doi: 10.1016/J.MTSUST.2020.100044.
- Towards materials discovery: assays for screening and study of chemical interactions of novel corrosion inhibitors in solution and coatings. New J. Chem.. 2020;44(19):7647-7658.
- [CrossRef] [Google Scholar]
- Enhanced corrosion resistance of a Cu–10Ni alloy in a 3.5 wt% NaCl solution by means of ultrasonic surface rolling treatment. Surf. Coat. Technol.. 2019;363(November 2018):390-399.
- [CrossRef] [Google Scholar]
- Application of some condensed uracils as corrosion inhibitors for mild steel: gravimetric, electrochemical, surface morphological, UV-visible, and theoretical investigations. Ind. Eng. Chem. Res.. 2012;51(46):14966-14979.
- [CrossRef] [Google Scholar]
- 5,5′-dithiobis-(2-nitrobenzoic acid) self-assembled monolayer for corrosion inhibition of copper in sodium chloride solution. J. Mol. Liq.. 2021;343:117535
- [CrossRef] [Google Scholar]
- Experimental studies of 2-pyridinecarbonitrile as corrosion inhibitor for mild steel in hydrochloric acid solution. Corros. Sci.. 2014;82:125-132.
- [CrossRef] [Google Scholar]
- Phenyl phthalimide as corrosion inhibitor for corrosion of C-steel in sulphuric acid solution. Port. Electrochim. Acta. 2009;27(5):631-643.
- [CrossRef] [Google Scholar]
- Comparative studies of two benzaldehyde thiosemicarbazone derivatives as corrosion inhibitors for mild steel in 1.0 M HCl. Results Phys.. 2018;11(September):554-563.
- [CrossRef] [Google Scholar]
- Electrochemical and surface analysis studies of 2-(quinolin-2-yl)quinazolin-4(3H)-one as corrosion inhibitor for Q235 steel in hydrochloric acid. J. Mol. Liq.. 2016;222:671-679.
- [CrossRef] [Google Scholar]
- Gravimetric{,} electrochemical and surface studies on the anticorrosive properties of 1-(2-pyridyl)-2-thiourea and 2-(imidazol-2-yl)-pyridine for mild steel in hydrochloric acid. New J. Chem.. 2018;42(15):12649-12665.
- [CrossRef] [Google Scholar]
- Halogen-substituted acridines as highly effective corrosion inhibitors for mild steel in acid medium. J. Phys. Chem. C. 2018;122(44):25349-25364.
- [CrossRef] [Google Scholar]
- Study of novel quinazolinone derivatives with different chain lengths as corrosion inhibitors for copper in 0.5 M sulfuric acid medium. Int. J. Electrochem. Sci.. 2021;16(7):1-17.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2024.105716.
Appendix A
Supplementary data
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







