Translate this page into:
Physicochemical properties and antibacterial activity evaluation of new indigo extract from Baphicacanthus cusia (Nees) Bremek prepared by chemical conversion method
⁎Corresponding authors at: Institute of Chemical Industry of Forest Products, CAF, Nanjing 210042, Jiangsu, China. zhangcwlhs@sina.com (Changwei Zhang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
High-temperature and quick-drying technology can realize sustainable utilization of BCB. Chemical conversion process of preparing NIE was relatively green and efficient. NIE had high indigo content and similar physiochemical properties with TIN. NIE emulsion owned excellent stability and good inhibitory activity against S. aureus. NIE possessed great potential in the field of cosmetics and feed additives etc.
Abstract
The effect of acidolysis time, hydrochloric acid concentration, ozonization time and pH on the yield of indigo were investigated by single factor experiment using dried leaves of Baphicacanthus cusia (Nees) Bremek (DLB) as raw material. On this basis, the technological condition for preparing new indigo extract (NIE) by directional chemical conversion method was optimized by orthogonal experiment. Then, the physiochemical properties of NIE were analyzed by combustion assay, nitric acid assay, thin layer chromatography identification assay, moisture detection and water-soluble pigment observation assay. Moreover, UPLC-Q-TOF-MS/MS technology was utilized to compare chemical composition difference between the NIE and traditional indigo naturalis (TIN). Finally, NIE emulsion was prepared by ultrasound assisted high pressure homogenization method and its stability, antibacterial activity as well as antibacterial mechanism were evaluated. The experimental results showed that optimum process for preparing NIE were hydrochloric acid concentration of 2 %, ozonization time of 3 min, pH of 7 and acidolysis time of 1 h; Under this condition, the content and yield of indigo in the NIE were significantly improved, which could reach up to 42.6 ± 0.28 % and 2.26 ± 0.04 %, separately. NIE possessed similar physiochemical properties with TIN described in Chinese pharmacopoeia 2020, and had more diverse active ingredients than TIN. The prepared NIE emulsion demonstrated excellent stability, whether at dark or bright place, and no matter at low temperature (4 ℃) or room temperature (25 ℃), the particle size, PDI, pH and indigo content of which displayed little variation. In addition, the NIE emulsion owned good inhibitory activity against S. aureus, and its MIC and MBC values were 40 and 80 μg/mL, respectively. Moreover, NIE emulsion exhibited antibacterial effect by influencing cell membrane integrity, Ca2+-Mg2+-ATPase activity on the cell membrane and intracellular Ca2+ levels of S. aureus. All of above results will lay the foundation for the green and sustainable utilization of Baphicacanthus cusia (Nees) Bremek.
Keywords
Baphicacanthus cusia (Nees) Bremek
Chemical conversion
Indigo extract
Physicochemical properties
Antibacterial activity
1 Introduction
Baphicacanthus cusia (Nees) Bremek (BCB) is a characteristic undergrowth medicinal resource, which widely distributed in China, India and Myanmar (Xu et al., 2021, Zhou et al., 2017). The existing BCB planting area in Guizhou province of China is more than sixty thousand hm2, which is an important economic income for local minorities. Indole glycoside is a main active ingredient in the BCB, which is also a precursor substance that generates indigo and possesses good stability in the circumstance of liquid phase (Yu et al., 2008, Su et al., 2009). However, indole glycoside is easy to be transformed into unstable indoxyl by the hydrolytic action of endogenous enzyme in the BCB (Zheng et al., 2022). Traditionally, BCB were processed by drying in the shade or sun, or directly being soaked and fermented in the water, most of the indole glycosides were oxidized and inactivated under the action of endogenous enzymes (Garciamacias et al., 2004). Moreover, as the unstable and degradable characteristics of indoxyl, the content and yield of indigo will decrease if the BCB are not able to be processed in time, leading to they are difficult to be stored and used for a long time (Dong et al., 2008). Therefore, how to effectively stabilize the indole glycoside in the BCB, so as to solve the sustainable processing and utilization problem of BCB, is extremely urgent.
The traditional industry of BCB was mainly to produce indigo paste/indigo naturalis, and corresponding process were as follows: the BCB were naturally fermented in a fermentation tank, indigo and indirubin were formed after indole glycoside being hydrolyzed, reduced and condensed under the action of endogenous enzymes, and then coarse indigo paste and indigo naturalis were obtained by adding a large amount of lime for flower beating, foam flotation, adsorption and precipitation (Yan et al., 2009). However, lots of alkaline wastewater and smelly waste residue were also produced in the above production process, which seriously polluted the environment. At the same time, the conversion rate of indole glycoside was low, only reached 40–50 %. In addition, the quality of the gained indigo paste/indigo naturalis was poor which contained more than 90 % of calcium oxide, and the content of indigo in which only arrived at about 2 % (Saiki et al., 2021). As the added value of indigo paste/indigo naturalis is low, resulting in it cannot meet the application demand in terminal products such as daily chemicals and medicine etc..
Hsu et al prepared indigo by biotransformation of exogenous glucosidase using biosynthetic indole glycoside as raw material, the conversion rate and the content of indigo were both more than 85 % (Hsu et al., 2018). Japanese patent reported that the conversion rate of indole glycoside reached more than 90 % under collaborative transformation of endogenous enzyme and exogenous β-Glucosidase. However, these technologies are difficult to realize industrial production due to the high cost and harsh conditions of producing indigo through efficient conversion of exogenous and endogenous enzymes. So, how to realize the efficient and directional conversion of indole glycoside with low cost and simple way, and could reduce environmental pollution, is a key scientific problem that needs to be solved.
In view of the technical problems existing in the BCB industry, high-temperature and quick-drying technology was firstly adopted to obtain dried leaves of Baphicacanthus cusia (Nees) Bremek (DLB) with stable and high content of indole glycoside, and the technological condition for preparing new indigo extract (NIE) from DLB by directional chemical transformation method was optimized in this study. On this basis, the physiochemical properties of the NIE were analyzed by combustion test, nitric acid test, TLC identification test, moisture detection test, water-soluble pigment observation test, and the chemical compositions difference between the NIE and traditional indigo naturalis (TIN) were analyzed by UPLC-Q-TOF-MS/MS technology. Finally, NIE emulsion was prepared by ultrasound assisted high pressure homogenization method to solve the hydrophilicity issue of NIE, and its inhibitory activity and mechanism against Staphylococcus aureus (S. aureus) were evaluated by Oxford cup method, biofilm formation inhibition assay, mature biofilm disruption assay, Ca2+ mobilization assay and Ca2+-Mg2+-ATPase activities detection assay. All of above researches (Fig. 1) are very vital for developing green and sustainable processing technology, promoting industrial upgrading, as well as expanding the public demand and realizing high value-added application of BCB.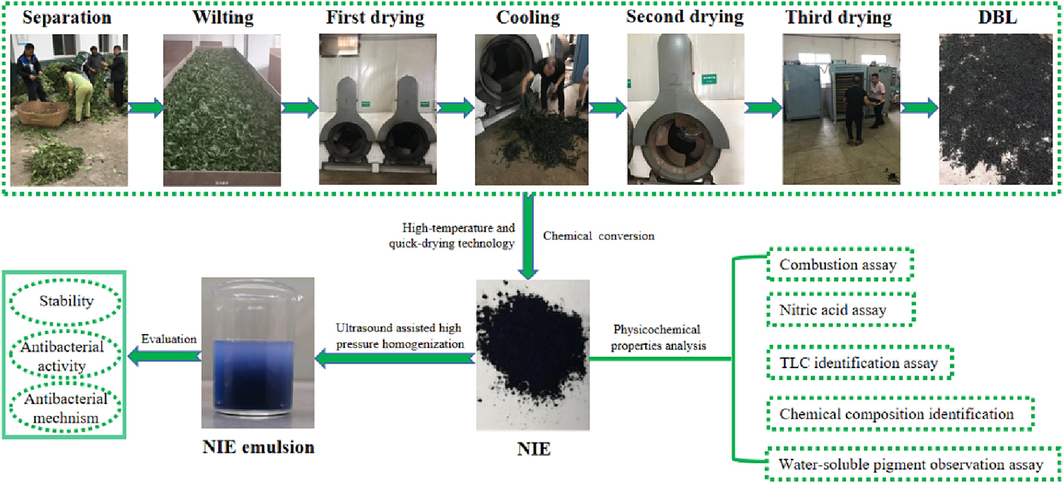
Graphical abstract.
2 Materials and methods
2.1 Chemical reagents and samples
S. aureus CGMCC 1.89 was obtained from China General Microbiological Culture Collection Center. Hydrochloric acid, sodium hydroxide, N, N-dimethylformamide, Tween-80 (T-80), caprylic/capric triglyceride (ODO), nutrient broth, Mueller-Hinton agar, gentamicin sulfate, miconazole nitrate, Tryptic Soytone Broth Medium, paraformaldehyde, crystal violet, phosphate buffer saline, glutaraldehyde, ultramicro-ATPase kits, indigo (97 %), indirubin (98 %) and nitric acid were all purchased from Aladdin Reagent (Shanghai) Co., Ltd.
2.2 Preparation of DLB
Firstly, 100 kg of fresh BCB (harvest time is August) were weighed and their branches and leaves were separated manually, then 60 kg of BCB leaves and 40 kg of BCB branches were individually gained. The fresh BCB leaves were put into wilting trough equipped with cold wind device for 16 h, and then withered BCB leaves were achieved. The withered BCB leaves were conducted stir-fly for 6 min in the 200 ℃ of tea drying machine, and then spreaded them on the ground and cooled for 10 min to accelerate water loss. The cooled BCB leaves were placed into 80 ℃ of tea drying machine to perform 50 min of fast drying, and then dried in the 65 ℃ of tea baking oven. Finally, 10 kg of DLB was obtained, and the content of indole glycosides (CAS:487–60-5) in which was analyzed by the following HPLC method: column Absolute® C18 (5 μm, 250 mm × 4.6 mm), the column temperature was 30℃, the detection wavenumber was 230 nm, the mobile phase was ultrapure water and methanol (v/v, 0.8/0.2), and the flow rate was 1.0 mL/min. Moreover, the contents of indole glycoside in DLB were singly detected after being stored for 2, 4, 6, 8, 10 and 12 months.
2.3 Determination of ozonization times by pre-experiment
50 g of DLB were weighed and put into 2 L of beaker, solid–liquid ratio was set as 1:20, temperature was set as 30 ℃, ultrasonic extraction time was set as twice, ultrasonic time was set as 30 min, and 2 L of extraction solution was gained. The extraction solution was divided into four equal parts, and named a, b, c, d, respectively. a was placed into 60 ℃ of water bath, and 10 mL of hydrochloric acid was added to perform acidolysis at 60 ℃ for 1 h, then pH of the solution was adjusted to 7 with sodium hydroxide solution. Finally, precipitation A was achieved after staying overnight, centrifugation and drying. b was placed into 60 ℃ of water bath, and ozone was introduced for 3 min after 10 mL of hydrochloric acid was added. The acidolysis was conducted at 60 ℃ for 1 h, then the pH of solution was adjusted to 7 with sodium hydroxide solution after acidolysis was carried out for 1 h. Finally, precipitation B was gained after staying overnight, centrifugation and drying. c was placed into 60 ℃ of water bath, and ozone was introduced for 3 min after 10 mL of hydrochloric acid was added, ozone was passed through again for 3 min after acidolysis was carried out for 30 min. Keeping acidolysis for another 30 min, then the pH of solution was adjusted to 7 with sodium hydroxide solution. Finally, precipitation C was obtained after staying overnight, centrifugation and drying. d was placed into 60 ℃ of water bath, and ozone was imported for 3 min after 10 mL of hydrochloric acid was added, ozone was brought into again for 3 min after acidolysis was carried out for 30 min. The ozone was introduced again for 3 min after acidolysis was performed for another 30 min, then the pH of solution was adjusted to 7 with sodium hydroxide solution. Finally, precipitation D was got after staying overnight, centrifugation and drying. The mass of precipitation A, B, C and D were weighed, and the content and total yield of indigo in which were calculated and analyzed by HPLC method as follows: column Thermo SCIENTIFIC (5 μm, 250 mm × 4.6 mm), the column temperature was 30 ℃, the detection wavenumber was 289 nm, the mobile phase was ultrapure water and methanol (v/v, 0.3/0.7), and the flow rate was 1.0 mL/min.
2.4 Process optimization of chemical conversion
The effect of ozonization time, acidolysis time, hydrochloric acid concentration and pH on the content and total yield of indigo were investigated using DLB as raw material. 50 g of DLB were weighed and put into 2 L of beaker, solid–liquid ratio was set as 1:20, temperature was set as 30 ℃, ultrasonic extraction time was set as twice, ultrasonic time was set as 30 min, and 2 L of extraction solution was gained. The extraction solution was divided into five equal parts. The ozonization time was set as 1 min, 2 min, 3 min, 4 min and 5 min, respectively. The hydrochloric acid concentration was set as 3 %, acidolysis time was set as 2 h, pH was set as 7, and the optimal ozonization time was determined using the content and total yield of indigo in the precipitation as index. Similarly, the best hydrochloric acid concentration (0.5 %, 1 %, 1.5 %, 2 % and 3 %), acidolysis time (0.5 h, 1.0 h, 2 h, 3 h, 4 h) and pH (5, 6, 7, 8, 9) were individually confirmed by following the above chemical conversion method. Based on this, orthogonal experiment was performed to obtain optimum chemical conversion process for preparing NIE.
2.5 Physicochemical properties analysis of NIE
According to the above optimal process of chemical conversion, the scale-up experiments (repeated for 5 times) were carried out to obtain NIE, and the following physicochemical properties were analyzed. Firstly, the shape, color and smell of the NIE were observed, and its properties were described according to the actual situation. Combustion test: a small amount of NIE was taken and put on the tinfoil to burn it with a small fire, then related phenomena were observed. Nitric acid test: a small amount of NIE was taken and placed into a small beaker, then nitric acid was added and corresponding phenomena were inspected. TLC identification test: indigo, indirubin and NIE solutions were prepared using chloroform as solvent, then the above three solutions were respectively sucked with capillary according to the thin layer chromatography (TLC) method, and pointed on the corresponding positions of the same silica gel G thin layer plate; Petroleum ether-trichloromethane-ethyl acetate (1:8:1, v/v/v) was utilized as the unfolding agent, and then the thin layer plate was placed in the chromatography cylinder, the Rf values of indigo, indirubin and NIE were calculated after the process of climbing plate, drying and color rendering being completed.
Water-soluble pigment observation test: 50.0 mg of NIE was weighed and put into a tube, and 10 mL of water was added, the tube was shacked and placed overnight, then related phenomena were recorded. Moisture detection test: five batches of NIE were separately weighed and laid in a flat weighing bottle which dried to constant weight, the bottle caps were opened and the samples were dried in a 100 ℃ of oven for 5 h, then the bottle caps were closed and the samples were transferred into a dryer; Finally, the samples were accurately weighed after being cooled at room temperature for 30 min, then being transferred to a oven for drying 1 h, cooled, weighed, and repeated the operation until the difference between the two consecutive weighing did not exceed 0.3 mg. At last, the water contents of the samples were calculated according to the reduced weight. In addition, the contents of indigo and indirubin for 5 batches of NIE were also determined by HPLC method.
2.6 Chemical components identification of NIE
0.40 g of TIN and NIE were individually dissolved ultrasonically in 100 mL mixed solution of methanol and water (5:2, v/v), and the chemical components in which were analyzed by UPLC-Q-TOF-MS/MS after being filtered by 0.25 μm filter membrane. Chromatographic conditions: UltraMate 3000 ultra-high pressure liquid phase; Chromatographic column (ACQUITY UPLC HSS T3 1.8 μm 2.1 × 100 mm); Column temperature was 40 ℃, sample loading was 3 μL; Mobile phase conditions: Negative ion mode: A: water (2 mM ammonium acetate); B: Acetonitrile. Gradient elution: (0 ∼ 2 min, 95 % A; 2 ∼ 30 min, 95 %∼7% A; 30 ∼ 40 min, 7 % A). Mass spectrometry conditions: AB 5600 Triple TOF mass spectrometer; Electric spray ion source: negative ion detection mode; Primary acquisition range (m/z): 50–1200; Bombing energy: 30 eV, 10 secondary spectra every 50 ms. ESI ion source parameters were set as follows: Atomization pressure (GS1): 60 Psi; Auxiliary air pressure: 60 Psi; Curtain air pressure: 35 Psi; Temperature: 650 ℃; Spray voltage: − 4000 V.
2.7 Fabrication and characterization of NIE emulsion
The NIE emulsion was prepared by high pressure homogenization method with some modifications (Shi et al., 2022). Firstly, T-80 and ODO with a mass ratio of 6:4 were uniformly mixed to gain a complex emulsifier. Then, NIE and the complex emulsifier (w/w, 1/2) were blended by a magnetic stirrer at room temperature for 20 min, and a dispersed phase was achieved. The deionized water and dispersed phase (w/w, 5/1) were regularly merged, and a crude emulsion was got after shearing at 10,000 r/min for 5 min utilizing a high speed shearing machine. The crude emulsion was homogenized 2 times by high pressure homogenizer, and its pH was adjusted to 7 by 0.5 mol/L citric acid solution. Finally, NIE emulsion (concentration of indigo was 2.56 mg/mL) was obtained. Similarly, TIN emulsion (concentration of indigo was 2.05 mg/mL), indigo standard (IS) emulsion (concentration of indigo was 2.56 mg/mL) and blank emulsion (without addition of NIE, TIN or IS, other steps as above) were also gained.
The appearance images of the IS emulsion, NIE emulsion and TIN emulsion were displayed in Fig. 2. The physical stability of the NIE emulsion was evaluated by a high speed centrifuge. Firstly, the NIE emulsion was pre-treated at a low speed of 4000 r/min for 15 min, and then at a high speed of 10,000 r/min for 15 min. In addition, NIE emulsion was stored at a bright place with light intensity of 4500 ± 500 lx and darkness place at 4 ℃ and 25 ℃ for 5 months, respectively. The particle size, pH value and polydispersity index (PDI) were measured at one month intervals, and the contents of indigo in all samples were also detected by HPLC method after the storage period.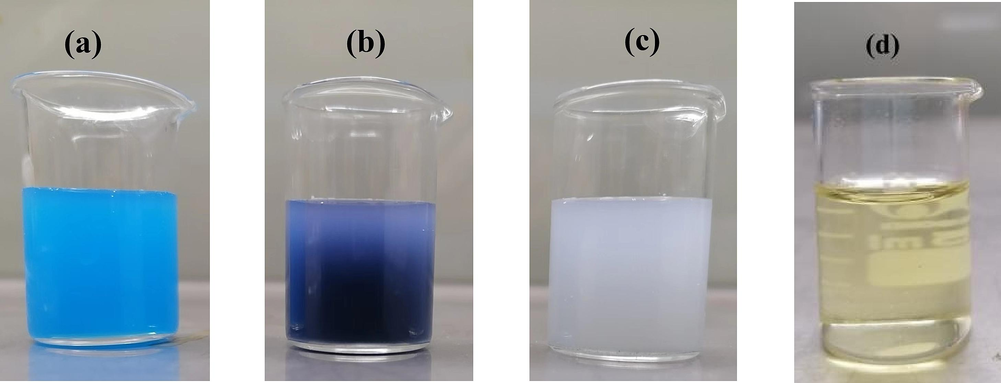
The appearance images of the IS emulsion (a), NIE emulsion (b), TIN emulsion (c) and blank emulsion (d).
2.8 Evaluation of antibacterial activity
Antibacterial activity experiments were carried out according to the method proposed by Zhang et al with some modifications (Zhang et al., 2020). A small piece of S. aureus were individually picked from bacterial tubes in the sterile environment with a sterile inoculating loop, and inoculated into 100 mL of nutrient broth (NA, HiMedia), then placed in a shaker at 30 ℃ and activated for 2 d. The final concentration of S. aureus was 2.3 × 109 colony forming units (CFU/mL). 100 μL of suspension of S. aureus was separately pipetted with a sterile pipette before coated on the Mueller-Hinton agar (MSA, Hi-Media). After the bacterial suspension were fully permeated into the media, three sterilized oxford cups were evenly placed in a petri dish with sterile forceps. Then, 100 μL of previously prepared NIE emulsion, TIN emulsion and IS emulsion were separately added to the oxford cups, and emulsion including gentamicin sulfate (GS) and emulsion containing miconazole nitrate (MN) were served as a positive control. The culture dishes were placed in an incubator (30℃, light irradiation and 36 % rh humidity) for a period of time so as to observe antibacterial effect of the samples and measure diameters of inhibition zone with a vernier caliper.
2.9 Measurement of MIC and MBC values
The minimum inhibitory concentration (MIC) and the minimum bactericidal concentration (MBC) values of GS, MN, IS, NIE and TIN emulsions were calculated according to the modified method proposed by Wang et al (Wang et al., 2021). 100 μL of nutrient broth was singly pipetted into every well of 96-well microtitration plate. Then 100 μL of NIE emulsion (concentration of indigo was 2.56 mg/mL), TIN emulsion (concentration of indigo was 2.05 mg/mL), IS emulsion (concentration of indigo was 2.56 mg/mL), emulsion including GS (0.5 mg/mL) and emulsion containing MN (0.5 mg/mL) were transferred into the first row of microtitre plates. These solutions were separately diluted (two-fold dilutions) in nutrient broth. At last, 100 μL of S. aureus suspension (adjusted to 0.5 McFarland, approximately 108 CFU/mL) was separately added in each well. All of the microtitre plates were incubated at 37 ℃ for 24 h. The MIC value was determined as the lowest concentration inhibiting the growth of bacteria. The final concentrations of indigo in the diluted solution of NIE (ranging from 5 to 320 μg/mL), TIN (ranging from 8 to 512 μg/mL) and IS (ranging from 10 to 640 μg/mL) emulsions were detected by HPLC method. Emulsion including GS (ranged from 0.5 to 128 μg/mL) and emulsion containing MN (ranged from 0.5 to 128 μg/mL) were used as a positive control in the assay. After broth microdilution tests, 10 μL of sample was pipetted from wells and subcultured on the new agar nutrient plates under 37 ℃ for 24 h to observe possible microbial development and also determine MBC values of GS, MN, IS, NIE and TIN emulsions.
2.10 Antibacterial mechanism of NIE emulsion
2.10.1 1. Biofilm formation inhibition assay
The effect of NIE emulsion on the biofilm formation of S. aureus was evaluated according to the modified method (Tao et al., 2022). S. aureus was cultured in Tryptic Soytone Broth Medium (TSB) at 37 ℃ for 15 h in the absence of NIE emulsion. Then, 100 μL of NIE emulsion (0.5 × MIC: 20 μg/mL, 1 × MIC: 40 μg/mL, 2 × MIC: 80 μg/mL) and 100 μL of the bacterial suspension (5.0 × 107 CFU/mL) were added to 96-well plates and incubated at 37 ℃ for 36 h to cultivate biofilm. TSB was utilized as a negative control. Removing the plate and discarding the suspension, then the cells were fixed for nearly 30 min after adding paraformaldehyde (4 %) solution to the plate. Afterwards, the paraformaldehyde solution was filtered out by suction, and a crystal violet (0.1 %) solution was added to stain the biofilm for 25 min and then washed with phosphate buffer saline (PBS) at room temperature. After the above treatment steps, the stained biofilm was exposed to 30 % acetic acid for 20 min, and the crystal violet solution was measured by absorbance at 550 nm. The amount of biofilm formation was proportional to the OD value of the crystal violet solution.
2.11 2. Mature biofilm disruption assay
To determine the influence of NIE emulsion on the mature biofilm, S.aureus was allowed to form biofilms on the surface of 96-well plates at 37 ℃ for 24 h according to a previous method (Li et al., 2022). After the supernatant was aspirated, 200 μL of TSB containing NIE emulsion (0.5 × MIC: 20 μg/mL, 1 × MIC: 40 μg/mL, 2 × MIC: 80 μg/mL) was added to the plates and incubated at 37 ℃ for another 24 h. TSB was adopted as a negative control. At last, crystal violet staining was carried out, and the OD value of the crystal violet solution was measured as described above.
2.12 3. Scanning electron microscopy (SEM) assay
S.aureus cell (1.0 × 108 CFU/mL in TSB) were seeded in 6-well plates, and 2 mL of TSB with 1 × MIC (40 μg/mL) or 2 × MIC (80 μg/mL) of NIE emulsion were added to the plates. After incubation at 37 ℃ for 24 h, the supernatant was aspirated and washed with PBS. Then, the samples were fixed with 2.5 % glutaraldehyde at 4 ℃ for 24 h. After washing with PBS again, the samples were dehydrated in different concentrations of ethanol (from 60 % to 100 %). The samples were attached to metallic stubs and sputter-coated with gold for 30 s. Finally, Images were achieved with SEM.
2.13 4. Ca2+ mobilization assay
The Ca2+ level in the cells of S.aureus was analyzed by Fluo-3/AM fluorescence staining in the light of the manufacturer’s protocols. Firstly, S.aureus cells (1.0 × 106 CFU/mL) were centrifuged twice at 5000 r/min for 10 min at 4 ℃ and resuspended in PBS. Intracellular calcium was marked through adding 5 μM Fluo-3/AM solution to the cell suspension. Then, S.aureus cells were incubated for 20 min at 25 ℃, centrifuged twice and suspended in PBS. The cell suspension was incubated in the dark for 10 min at 37 ℃. Every well of the 96-well plates was loaded with 100 μL (total volume) containing the prepared cell suspension and the tested NIE emulsion under 1 × MIC (40 μg/mL) or 2 × MIC (80 μg/mL) conditions. The S.aureus suspensions (1.0 × 106 CFU/mL) without NIE emulsion were served as controls. The fluorescence was recorded at 30-s intervals and measured with a Multi-Mode Microplate Reader (Thermo Scientific, Finland).
2.14 5. Ca2+-Mg2+-ATPase activities assay
The influence of NIE emulsion on the Ca2+-Mg2+-ATPase activities of S.aureus cell membranes were analyzed in accordance with the manufacture’s protocols (measured with ultramicro-ATPase kits). Firstly, S.aureus cells (1.0 × 106 CFU/mL) were incubated in 100 mL of nutrient broth and oscillated at 150 r/min at 37 ℃. Then 10 mL 1 × MIC (40 μg/mL) or 2 × MIC (80 μg/mL) of NIE emulsion and 90 mL suspension of tested S.aureus cells were merged and cultured at 37 ℃ for various times (1, 3, 5, 7 and 9 h). Then, the suspension was centrifuged for 20 min to gather the cells. The cells were washed and suspended in saline and then disrupted by ultrasonic waves to prepare cell homogenates. Finally, Ca2+-Mg2+-ATPase activities on the cell membrane of S.aureus affected by NIE emulsion were detected by means of ultramicro-ATPase kits. The S.aureus suspensions (1.0 × 106 CFU/mL) not including the NIE emulsion were adopted as controls.
3 Results and discussion
3.1 Optimization of chemical conversion process
The pretreatment and drying methods of BCB have an important impact on the content of indole glycoside, but few related researches have been reported. In this study, high-temperature and quick-drying technology was firstly applied to process the BCB, it not only could completely inactivate endogenous enzymes, but also quickly dehydrate the BCB, thereby preventing the encounter of indole glycoside and endogenous enzymes. The content of indole glycoside in DLB and fresh BCB (moisture content was 80 %) were measured as 9.26 ± 0.08 % and 1.87 ± 0.02 % (equivalent to 9.35 % in DLB) (w/w), respectively. This proved that the loss of indole glycoside in fresh BCB was minimal after being processed by the high-temperature and quick-drying technology. Moreover, indole glycoside in DLB presented little difference after being stored for 2, 4, 6, 8, 10 and 12 months (Table S1). This indicated that the DLB could be used as a stable raw material in the industrial production of indigo extract, thereby solving the problem of BCB sustainable utilization throughout the year. Indole glycoside will be transformed into indoxyl in the chemical conversion process, while indoxyl is an unstable ingredient and its stability will be affected by oxygen transfer in the liquid phase, leading to the yield and purity of indigo being influenced (Zheng et al., 2022). Based on this, the effect of ozonization times on the content of indigo and total yield of indigo were investigated and related results were demonstrated in the Table 1. It can be seen from Table 1 that both the content and total yield of indigo in the precipitates achieved by passing ozone once and twice were higher than other ozone introduced times. In comprehensive consideration, the ozonization times was selected as twice.
Ozonization times
0
1
2
3
Precipitate mass/g
0.40 ± 0.01
0.41 ± 0.01
0.63 ± 0.01
0.32 ± 0.01
Content of indigo/%
20.0 ± 0.15
43.2 ± 0.33
37.4 ± 0.20
29.0 ± 0.11
Total yield of indigo/%
0.64 ± 0.01
1.42 ± 0.02
1.89 ± 0.03
0.74 ± 0.01
As ozonization time, hydrochloric acid concentration, acidolysis time and pH are vital factors influencing directed generation of indigo and degradation efficiency of indole glycoside, the effect of these factors on the content and total yield of indigo were investigated, and related results were illustrated in the Fig. 3. It was obvious from Fig. 3a that the content of indigo in the precipitate firstly increased and then decreased with the prolongation of ozonization time, and the total yield of indigo displayed similar trend. This is because that the introduction of ozone can promote indoxyl to be oxidized and condensed to form indigo, but a portion of indoxyl will be transformed into indirubin with the extension of introduced ozone time. Therefore, the conversion rate of indigo was the best when ozonization time was 3 min. It can be seen from Fig. 3b that the content of indigo was highest when the hydrochloric acid concentration was 1.0 %, while the total yield of indigo reached maximum when the hydrochloric acid concentration arrived at 2.0 %. Overall consideration, 1.5 % was the optimal selection. As the higher the concentration of hydrochloric acid, the faster the degradation rate of indole glycosides. It is concluded that the concentration of indoxyl may be the main reason for the above results. From Fig. 3c, both the content of indigo and total yield of indigo were the highest when the acidolysis time was 2 h. It can be inferred that excessive acid hydrolysis time is not conducive to the generation of indigo. In Fig. 3d, indigo had the best directional conversion effect when pH was 6. It is indicated from above results that strong acidic and alkaline environments were not beneficial to the generation of indigo. In summary, the ozonization time, hydrochloric acid concentration, acidolysis time and pH were chosen as 3 min, 1.5 %, 2 h and 6, respectively.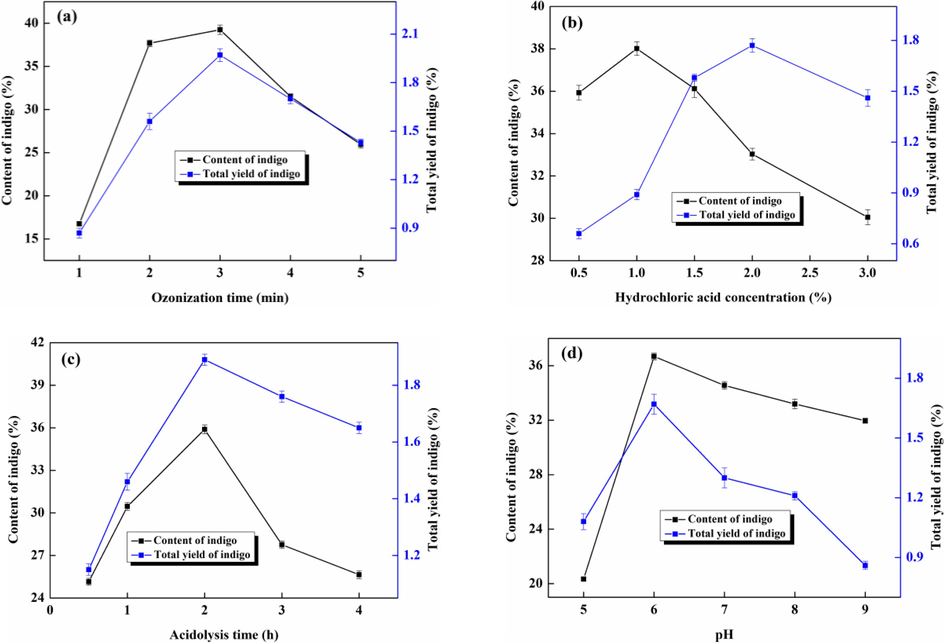
Effect of different factors on the content of indigo and total yield of indigo. a, b, c and d represent the ozonization time, hydrochloric acid concentration, acidolysis time and pH, respectively.
The phenomena of foam formation in the chemical conversion process were also observed and recorded. The longer the ozonization time was, the more foam would be generated. When the ozone introduced time was 1 min, the color of foam changed from blue to white mixed with yellow, indicating that too short ozonization time may affect the generation of indigo. When the ozonization time was 3 min, the color of foam was dark blue, while the ozonization time was more than 3 min, the color of foam was changed from blue to light blue mixed with yellow. It was reported the content and yield of indigo continue to reduce with the enhancement of ozone introduced time (Zhou et al., 2019). This result can also fully explain the experimental phenomenon mentioned above. Moreover, the higher the hydrochloric acid concentration was, the darker the color of the generated foam was. In addition, as the pH of solution after acidolysis was different, resulting in the color of foam produced by stirring demonstrated various, which presented light blue (pH = 5), light blue mixed with white (pH = 6), white mixed with light yellow (pH = 7), light green (pH = 8), dark green (pH = 9), and the larger the acidolysis pH was, the less foam produced by stirring. All of these phenomena will provide guidance for the quality control of indigo extract with different specifications.
The Factor level table was illustrated in Table S2, and the results of orthogonal experiment and variance analysis were showed in Table 2 and Table 3, singly. It can be seen from Table 2, the order of influence on the total indigo yield was B > D > A > C, that is, hydrochloric acid concentration > pH > ozonization time > acidolysis time. By comparing the k value, the optimal level of each factor was A2, B2, C3, D3. Therefore, A2B2C3D3 was the best combination for preparing indigo from DLB by directional chemical transformation method. It was suggested from Table 3 that ozonization time, hydrochloric acid concentration and pH had a significant impact on the total yield of indigo, while acidolysis time possessed no remarkable influence on the total yield of indigo. Therefore, the optimal process combination was adjusted to A2B2C1D3, that is, the ozonization time was 3 min, hydrochloric acid concentration was 2 %, acidolysis time was 1.0 h, and pH was 7. Under these conditions, the content and total yield of indigo could reach 42.6 % and 2.26 %, separately. Moreover, the conversion rate of indole glycosides was able to arrive at 90 %. Compared with traditional preparation process of indigo naturalis, the content of indigo and total yield of indigo in the NIE were increased to over 15.6 and 2.2 times, respectively (Pei et al., 2017). The mechanism of the new chemical conversion method proposed in this study were as following: Indole glycoside extracted from DLB was dissolved in aqueous solution, it conducted hydrolysis and converted to indoxyl when hydrochloric acid was added; After hydrolysis, ozone was introduced, it could produce oxygen molecules and oxygen atoms and create an oxidizing environment in the aqueous solution; At the same time, indoxyl was firstly oxidized to indolinone, then was condensed to form indigo (Zhou et al., 2019). The optimal chemical conversion process not only greatly decreased the time for producing indigo extract, but also reduced environmental pollution on account of quicklime not being used. Thus, it is a relatively green and efficient process which suitable for the industrial production of indigo with high quality.
No.
A ozonization time/min
B hydrochloric acid concentration/%
C acidolysis time/h
D pH
Total yield of indigo/%
1
1
1
1
1
1.43 ± 0.02
2
1
2
2
2
1.58 ± 0.03
3
1
3
3
3
1.76 ± 0.01
4
2
1
2
3
1.79 ± 0.02
5
2
2
3
1
1.95 ± 0.04
6
2
3
1
2
1.71 ± 0.02
7
3
1
3
2
1.52 ± 0.03
8
3
2
1
3
2.01 ± 0.05
9
3
3
2
1
1.72 ± 0.03
k1
1.59
1.58
1.72
1.70
k2
1.82
1.85
1.70
1.60
k3
1.75
1.73
1.74
1.85
R
0.23
0.27
0.04
0.25
Source of
varianceDegree of
freedomSum of
squaresMeans
quareF value
F0.05
F0.01
Signifificance
A
2
0.083
0.028
28.00
19
99
*
B
2
0.110
0.037
37.00
*
C
2
0.002
0.001
1.000
D
2
0.095
0.032
32.00
*
Error
2
0.002
Total
8
3.2 Physical and chemical properties of NIE
The physical and chemical properties of the NIE were observed and analyzed. As shown in Fig. 4(a), the NIE presented properties of blue-black powder, light and easy to fly, mild gas and tasteless. It can be seen from Fig. 4(b) that purplish red smoke was appeared in burning test. It was obvious from Fig. 4(c) that the water layer demonstrated the color of light blue. From Fig. 4(d), bubbles occurred and presented the color of yellowish brown. All of the above experimental phenomena were similar with TIN described in Chinese Pharmacopoeia (2020 Edition). In the TLC chromatogram, the same light blue and purplish red spots exhibited at the positions corresponding to the standard color spectrum of indigo and indirubin, as shown in Fig. 4(e), wherein the Rf values of indigo and indirubin was 0.64 and 0.38, respectively. The contents of indigo, moisture and indirubin for five batches of NIE were detected, and the corresponding results were exhibited in Fig. 5. It can be seen from the Fig. 5 that the content of moisture for five batches of NIE were all not more than 5.79 %, and the contents of indigo and indirubin were not less than 42.3 % and 2.19 %, separately. Moreover, the contents of indigo, moisture and indirubin among the five batches of NIE displayed little difference. Thereby, the above chemical conversion process for preparing the NIE was stable and reliable.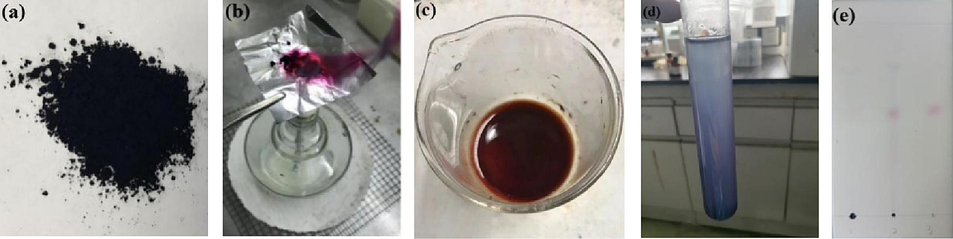
The phenomena of appearance (a), burning test (b), nitric acid test (c), water soluble pigment inspection test (d) and TLC test (e) for NIE. 1, 2 and 3 represented indigo standard, NIE and indirubin standard, respectively.
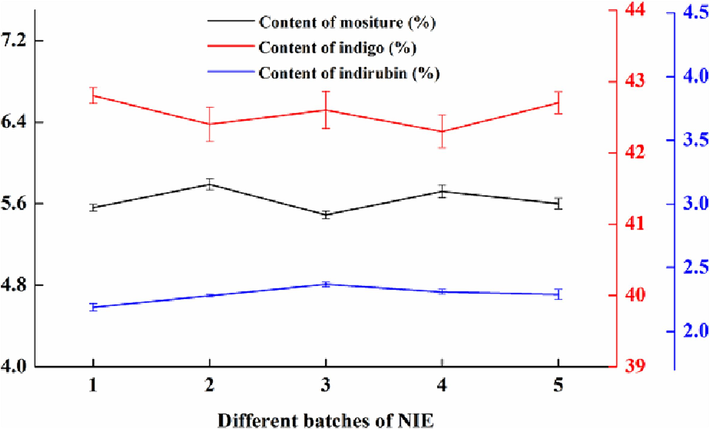
Contents of indigo, moisture and indirubin for five batches of NIE.
The UPLC-Q-TOF-MS/MS spectrum of TIN and NIE were illustrated in Fig. 6, and their chemical compositions statistical analysis were demonstrated in Table 4. From Table 4, it can be seen that TIN was mainly composed of six components: organic acids, polysaccharides, flavonoids, alkaloids, polyphenols and esters, in which organic acids and alkaloids accounted for 51.7 % and 38.7 % respectively; Polysaccharides, flavonoids, polyphenols and esters accounted for a relatively small proportion of 3.11 %, 2.12 %, 2.77 %, and 1.60 %, singly. The NIE was mainly made up of nine ingredients: organic acids, polysaccharides, flavonoids, alkaloids, glycosides, polyphenols, aldehydes, proteins and esters. Among them, organic acids and polyphenols accounted for a relatively high proportion of 86.9 % and 8.15 %, separately, followed by flavonoids and alkaloids, accounting for 3.05 % and 1.35 %, individually; Polysaccharides, glycosides, aldehydes, proteins, and esters accounted for the lowest proportion of 0.05 %, 0.14 %, 0.25 %, 0.04 %, and 0.07 %, respectively.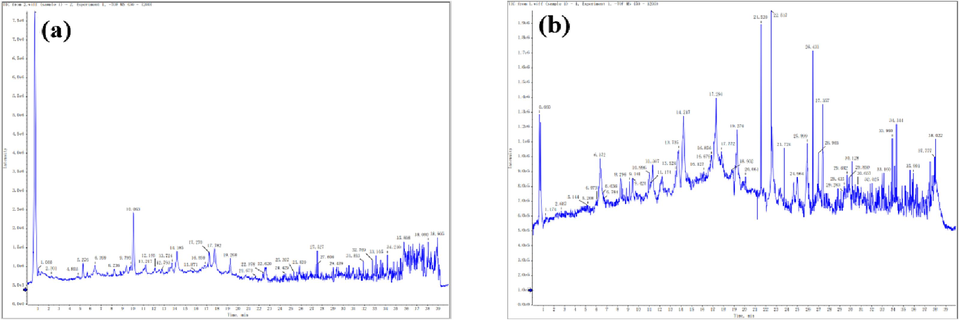
UPLC-Q-TOF-MS/MS spectrum of TIN (a) and NIE (b).
NO.
Category
TIN
NIE
1
Organic acids
31
97
2
Polysaccharides
4
4
3
Flavonoids
4
19
4
Alkaloids
15
35
5
Glycosides
0
6
6
Polyphenols
4
8
7
Aldehyde
0
8
8
Proteins
0
2
9
Esters
3
5
Total
61
184
Compared with TIN, the NIE possessed more kinds of organic acids, and mainly were unsaturated fat acids (Table S3). It was reported that unsaturated fat acid had a good effect in improving feed utilization and meat quality of livestock and poultry (Tartrakoon et al., 2016). The type and proportion of flavonoids and polyphenols in the NIE were higher than TIN (Table S3). Due to the antioxidant and antibacterial activities of flavonoids and polyphenols, the NIE may has higher added value and greater potential in the fields of feed additives and cosmetics etc. In addition, the proportion of alkaloids in the NIE was lower than TIN (Table S3). This was because that some alkaloids reacted with hydrochloric acid during the chemical conversion process, but the contents of the main alkaloid markers such as indigo and indirubin were not affected, and on the contrary, they were significantly higher than TIN. Researches showed that alkaloid can easily cause animal diarrhea, so the NIE with fewer types of alkaloids will be more safety when it was applied to the fields of feed additive and food (Zhang et al., 2012).
3.3 Stability of NIE emulsion
Physical stability experiments revealed that the appearance of NIE emulsion did not change and there was no precipitation phenomenon after it was respectively treated by low-speed centrifuge and high-speed centrifuge. Moreover, it can be seen in Fig. 7, whether at dark or bright place, and no matter at low temperature (4 ℃) or room temperature (25 ℃), the particle size, PDI, pH and indigo content of NIE emulsion demonstrated little variation during 5 months. These experiment results showed that the fabricated NIE emulsion possessed excellent physical and chemical stability. Indigo had the function of anti-ultraviolet, anti-oxidation, anti-inflammatory and immunosuppressive activities (Lin et al., 2019). Thus, the prepared NIE emulsion containing indigo will present outstanding development potential in the cosmetic field.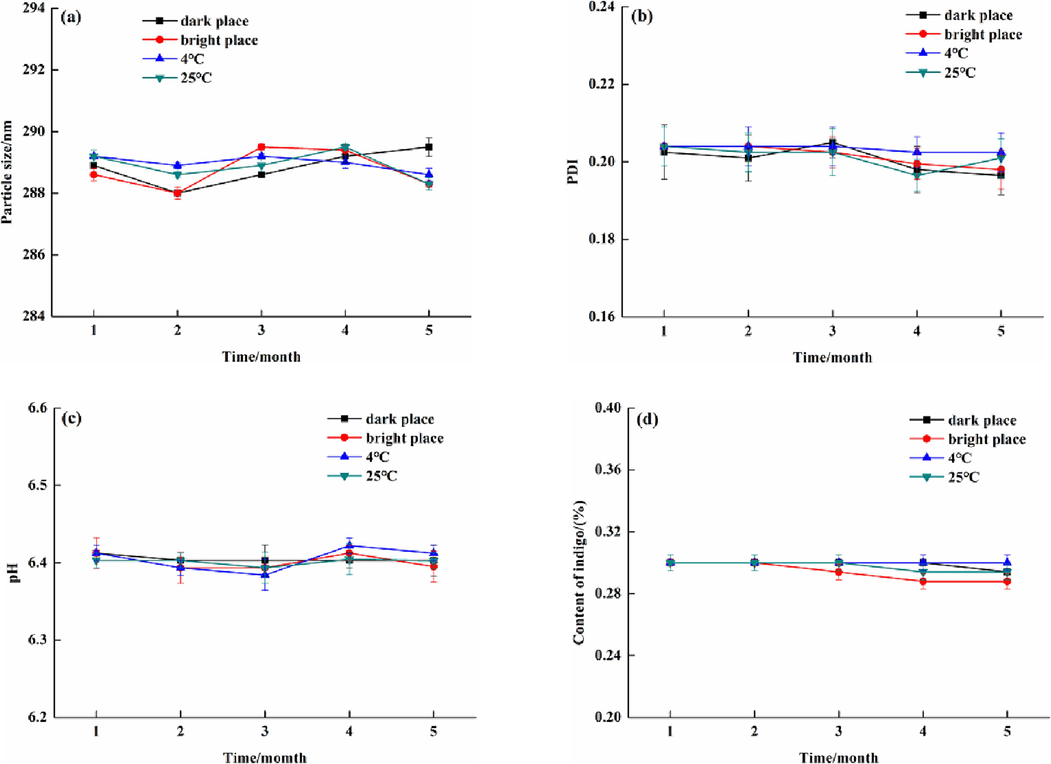
Particle size (a), PDI (b), pH (c) and indigo content (d) of NIE emulsion at different months.
3.4 Inhibitory activity of NIE emulsion against S. Aureus
It was reported that indigo possessed antibacterial activity (Zeng et al., 2016, Andreazza et al., 2015). Besides, flavonoids and polyphenols in the NIE also have antibacterial potential. Therefore, it is meaningful to explore whether have synergistic antibacterial effects among indigo, flavonoids and polyphenols in the NIE. Inhibition zone pictures of IS, TIN, NIE GS and MN emulsions against S. aureus were demonstrated in Fig. 8, and indigo concentrations in those emulsions were 80, 128, 160, 32, 32 μg/mL, respectively. It was obvious that IS, TIN and NIE emulsions had inhibitory effect on the S. aureus. From Table 5, it was indicated that the inhibition zone values range, MBC and MIC value ranges of TIN emulsion were 12.0–20.4 mm, 128 μg/mL and 64 μg/mL, respectively; The inhibition zone values range, MBC and MIC values range of NIE emulsion were 11.3–22.6 mm, 80 μg/mL and 40 μg/ mL, separately; The inhibition zone values range, MBC and MIC values range of IS emulsion were 8.3–15.3 mm, 160 μg/mL and 80 μg/ mL, separately. From an overall perspective of above results, the inhibitory effect of NIE emulsion on S. aureus were higher than IS and TIN emulsions. According to the UPLC-Q-TOF-MS/MS results in Table S3, NIE possessed more plentiful ingredients (organic acids, flavonoids, alkaloids, polyphenols) than TIN. Moreover, some of these ingredients have good antibacterial activity, such as kaempferol, genistein, sinomenine, terpineol etc.. Thus, high content of indigo and synergistic effects of multiple antibacterial ingredients may be the main reasons of the above results.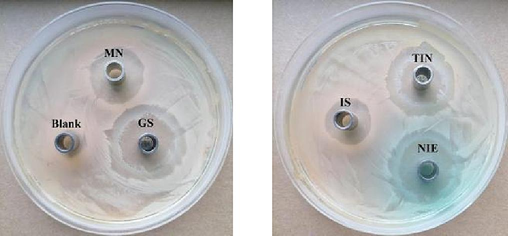
Inhibition zone pictures of IS, TIN and NIE emulsions against S. aureus.
TIN
NIE
IS
GS
MN
Values of MIC (μg/mL)
64
40
80
4
16
Values of MBC (μg/mL)
128
80
160
8
31
3.5 Effect of NIE emulsion on the biofilm formation
To know the antibacterial mechanism of the NIE, it is important to study the formation and damage of bacterial biofilms because these two factors have close relationship with bacterial growth and apoptosis (Zhang et al., 2020). Therefore, absorbance measurements and microscopic observations were firstly adopted to confirm the effect of NIE emulsion on the biofilm formation of S.aureus. As shown in the Fig. 9, the absorbance values in the 96-well plate explained that the amount of biofilm displayed not much difference with the enhancement of the NIE emulsion concentration. This also could be verified from the microscopic images of Fig. 10. As a result, it was concluded that the inhibitory activity of NIE emulsion against S. aureus was not achieved by inhibiting the biofilm formation. Inhibition of biofilm formation can not be easy to kill bacterial with mature biofilm, but be able to inhibit bacterial proliferation from the root cause. Thus, NIE emulsion needs to exert its maximized antibacterial effect under specific conditions.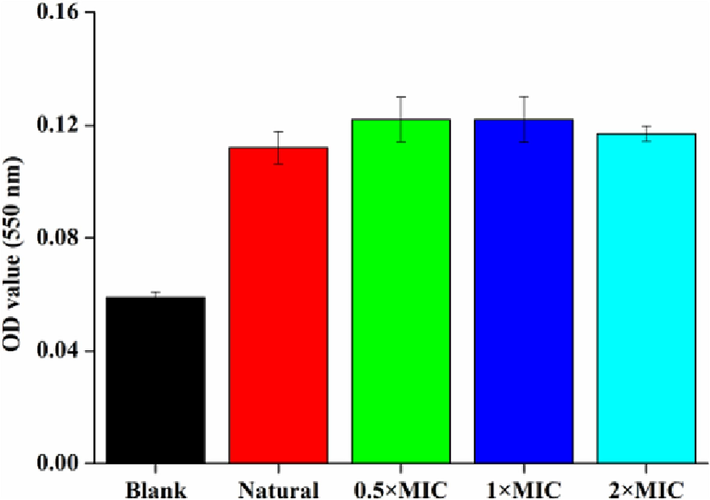
Aborbance values of crystal violet solution dealt with different MIC of NIE emulsion in the biofilm formation inhibition assay. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
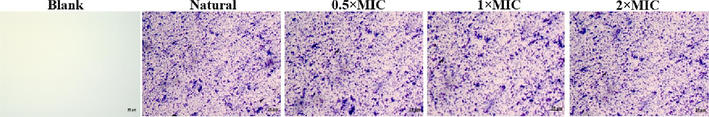
The biofilm inhibition micrographs of S.aureus processed by different MIC of NIE emulsion.
3.6 Effect of NIE emulsion on biofilm disruption
The mature biofilms of S.aureus is vital for the persistence of chronic infections (Lister et al., 2014). Researching the influence of antimicrobial agents on the mature biofilms disruption of bacterial is an essential part of investigating their efficacy (Maria et al., 2018). The antibiofilm efficacy of the NIE emulsion on the mature biofilms of S.aureus were assessed by a mature biofilm disruption assay. As shown in the Fig. 11, the biofilm amount of S.aureus decreased with the 20 and 40 μg/mL NIE emulsion treatments. Meanwhile, 80 μg/mL of NIE emulsion presented extremely destructive effect on the preformed biofilms of S.aureus. In conclusion, the effect of NIE emulsion on the biofilm disruption of S.aureus emerge concentration dependence, this trend is consistent with the research result of Tao et al (Tao et al., 2022). Furthermore, similar results were also illustrated in the micrographs of Fig. 12. In order to further verify the damage effect of NIE emulsion on the biofilm of S.aureus, SEM was utilized to observe the related phenomena and corresponding results were presented in Fig. 13. From Fig. 13, it was obvious that the mature biofilms of S.aureus were destroyed more seriously with the enhancement of the NIE emulsion concentration, and invagination, deformation and rupture were also observed in the NIE emulsion-treated group.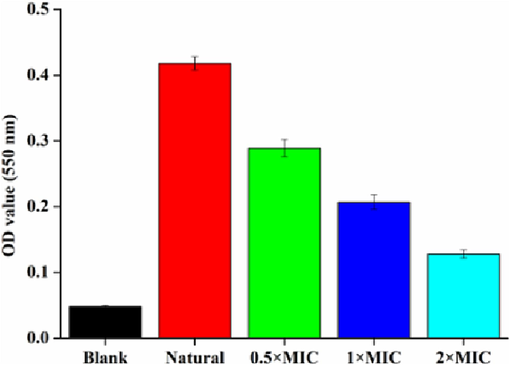
Aborbance values of crystal violet solution processed by different MIC of NIE emulsion in the mature biofilm disruption assay. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
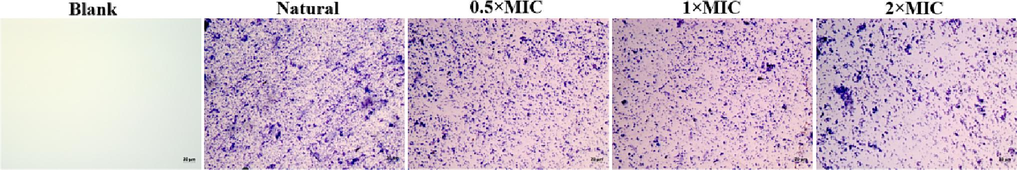
The biofilm disruption micrographs of S.aureus dealt with different MIC of NIE emulsion.
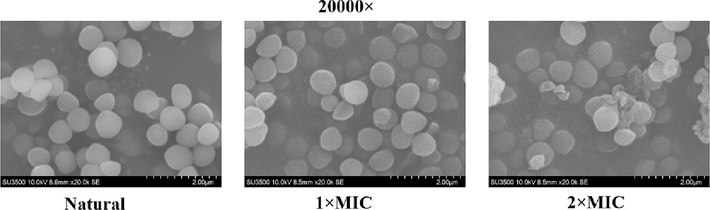
SEM images of S.aureus biofilm disruption for NIE emulsion with different MIC.
3.7 Ca2+ mobilization assay treated with NIE emulsion
Fig. 14 demonstrated the cell membrane permeability utilizing the Ca2+ mobilization assay in S.aureus cells processed by different MIC of NIE emulsion. It was obvious that both the 1 × MIC (40 μg/mL) and 2 × MIC (80 μg/mL) concentrations of NIE emulsion dramatically raised the fluorescence intensity in contrast to the control in S.aureus cells. This meant that S.aureus cells processed by NIE emulsion indicated an apparent enhancement in intracellular Ca2+ concentrations compared with the control. In addition, intracellular calcium levels (the fluorescence intensity value range) in the 2 × MIC (80 μg/mL) group were always higher than 1 × MIC (40 μg/mL) group at the same time. It was concluded that NIE emulsion could improve the intracellular calcium levels of S.aureus, and possessed a positive correlation with its concentration within the time frame of this experimental design.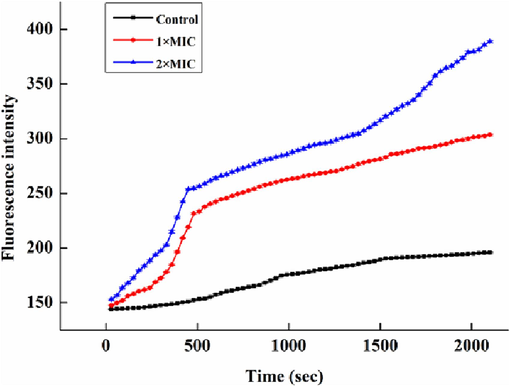
Effect of NIE emulsion on cell cmembrane Ca2+ mobilization.
3.8 Ca2+-Mg2+-ATPase activities treated with NIE emulsion
Ca2+-Mg2+-ATPase is a Ca2+ pump on the cell membrane and is involved in regulating intracellular Ca2+ concentration (Yang et al., 2021). It can hydrolyze ATP to pump intracellular Ca2+ to the extracellular space to keep relatively low intracellular Ca2+ concentrations, so as to guarantee the general function of cells. From Fig. 15, it was suggested that Ca2+-Mg2+-ATPase activity on the cell membrane of S.aureus emerged a descending trend with the extended processing time by NIE emulsion. Moreover, it was obvious that NIE emulsion could inhibit Ca2+-Mg2+-ATPase activities on the cell membrane of S.aureus in the antibacterial process, and the inhibitory effect presented a positive correlation with NIE emulsion concentrations that showed as an inhibitory effect of 2 × MIC group higher than 1 × MIC group. Ca2+-Mg2+-ATPase activity was inhibited meant that NIE emulsion could induce S.aureus to produce high intracellular Ca2+ level. Furthermore, the cell wall permeability of S.aureus was changed, leading to its death.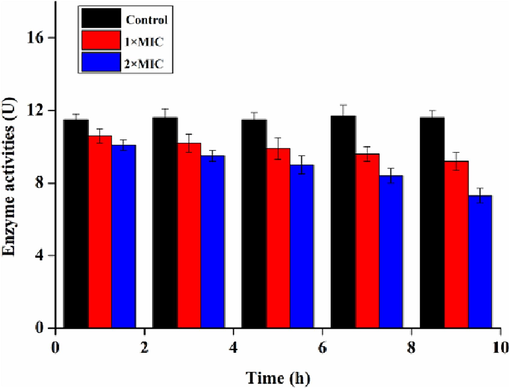
Ca2+-Mg2+-ATPase activities of S.aureus processed by different MIC of NIE emulsion.
4 Conclusion
In this study, DLB with stable and high content of indole glycoside within a year was firstly achieved by high-temperature and quick-drying technology, solving the long-term utilization problem of BCB. Then, the optimal chemical conversion condition for efficiently and greenly preparing NIE from DLB was determined and conducted as follows: Hydrochloric acid concentration was 2 %, ozonization time was 3 min, pH was 7, acidolysis time was 1 h; Under these conditions, the content and total yield of indigo in the NIE were greatly improved, which could reach 42.6 % and 2.26 %, separately. These improvements of NIE make the preparation of natural indigo monomer with high purity become more easy and simple. The prepared NIE demonstrated similar physiochemical properties with TIN described in Chinese pharmacopoeia 2020, and possessed more diverse chemical compositions than TIN. In addition, the NIE emulsion prepared by high pressure homogenization method displayed excellent stability, and possessed good antibacterial activity against S. aureus. Moreover, the NIE emulsion realized antibacterial effect by influencing cell membrane integrity, Ca2+-Mg2+ −ATPase activity on the cell membrane and intracellular Ca2+ levels of S. aureus. All of these systematic researches could pave the way for the green, sustainable and high-value development and application of BCB.
CRediT authorship contribution statement
Changwei Zhang: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Writing – original draft, Writing – review & editing. Hua Yuan: Formal analysis, Investigation, Writing – review & editing. Hong Shen: Formal analysis, Resources, Software, Visualization. Chuan Li: Investigation, Writing – review & editing. Jianzhong Ye: Data curation, Validation. Huaxing Zhang: Resources, Investigation. Chengzhang Wang: Project administration, Supervision, Validation, Writing – review & editing.
Acknowledgments
The work supported by Guizhou Provincial Key Technology R&D Program (No.2023244) and Guizhou Provincial Key Technology R&D Program (No.2022159).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Photodynamic antimicrobial effects of bis-indole alkaloid indigo from Indigofera truxillensis Kunth (Leguminosae) Lasers. Med. Sci.. 2015;30(4):1315-1324.
- [CrossRef] [Google Scholar]
- Effect of drying method and extracting temperature on contents of bioactive constituents in Radix Isatidis and Folium Isatidis. Chin. Trad. Herb. Med.. 2008;39(1):111-114.
- [CrossRef] [Google Scholar]
- Garciamacias, P., John, P. Formation of natural indigo derived from woad (Isatis tinctoria L.) in relation to product purity. 2004. J. Agric. Food. Chem. 52(26):7891. doi:https://doi.org/10. 1021/jf0486803.
- Hsu, T. M., Welner, D. H., Russ, Z. N., et al. 2018. Employing a biochemical protecting group for a sustainable indigo dyeing strategy. Nat. Chem. Biol. 14:256-261. doi:10.1038/NCHEMBI O.2552.
- Membrane-active amino acid-coupled polyetheramine derivatives with high selectivity and broad-spectrum antibacterial activity. Acta. Biomater.. 2022;142:136-148.
- [CrossRef] [Google Scholar]
- Advances in biological activity of indigo. Dyestuffs. Coloration.. 2019;56(4):16-18.
- [Google Scholar]
- Staphylococcus aureus biofilms: recent developments in biofilm dispersal. Front. Cell. Infect. Microbiol.. 2014;4:178.
- [CrossRef] [Google Scholar]
- Poly(silsesquioxanes) and poly(siloxanes) grafted with N-acetylcysteine for eradicating mature bacterial biofilms in water environment. Colloid. Surface. b.. 2018;172:S0927776518306283-
- [CrossRef] [Google Scholar]
- Pei, Y., Huang, Z. J., Huo, Z. P., et al. 2017. Simultaneous determination of indigo and indirubin in indigo naturalis by HPLC with multiple UV wavelength detection. Tianjin. Pharm. 29(2): 14-18. doi:10.3969/j.issn.1006-5687.2017.02.006.
- Treatment-refractory ulcerative colitis responsive to indigo naturalis. BMJ. Open. Gastroenter.. 2021;8(1):e000813.
- [Google Scholar]
- Nano-emulsion prepared by high pressure homogenization method as a good carrier for Sichuan pepper essential oil: Preparation, stability, and bioactivity. LWT-Food. Sci. Technol.. 2022;154:112779
- [CrossRef] [Google Scholar]
- Study on the soaking principle in the processing of indigo naturalis. Chin. Tradit. Pat. Med.. 2009;31(11):1719-1721. CNKI: SUN: ZCYA.0.2009- 11-023
- [Google Scholar]
- Characterization and antibacterial activity of ruthenium-based shikimate cross-linked chitosan composites. Int. J. Biolo. Macromol.. 2022;217:890-901.
- [CrossRef] [Google Scholar]
- Effects of the ratio of unsaturated fatty acid to saturated fatty acid on the growth performance, carcass and meat quality of finishing pigs. Anim. Nutr.. 2016;2:79-85.
- [CrossRef] [Google Scholar]
- Ginkgo biloba exocarp extracts inhibit S. aureus and MRSA by disrupting biofilms and affecting gene expression. J. Ethnopharmacolo.. 2021;6(113895)
- [CrossRef] [Google Scholar]
- Optimization of extraction of bioactive compounds from Baphicacanthus cusia leaves by hydrophobic deep eutectic solvents. Molecules.. 2021;26(6):1729.
- [CrossRef] [Google Scholar]
- Study on optimization for traditional preparation technique of natural indigo in China. Guizhou. Chem. Ind.. 2009;34(3):1-3.
- [Google Scholar]
- Preparation of three-layer flaxseed gum/chitosan/flaxseed gum composite coatings with sustained-release properties and their excellent protective effect on myofibril protein of rainbow trout. Int. J. Biol. Macromol.. 2021;194:510-520.
- [CrossRef] [Google Scholar]
- Study on the content determination and extraction process of indole glycoside from Baphicacanthus cusia (Nees) Bremek. Xinjiang. Med. J.. 2008;38:130-132.
- [CrossRef] [Google Scholar]
- Secondary metabolites of Baphicacanthus cusia (Nees) Bremek. Chin. Agri. Sci. Bull.. 2016;32(20):30-34. CNKI: SUN: ZNTB.0.2016-20-007
- [Google Scholar]
- Research progress of the effect of alkaloid of Sophora alopecuroides on Cattle bacterial diarrhea. J. Agri. Sci.. 2012;33(2):73-76.
- [Google Scholar]
- Physiochemical property and antibacterial activity of formulation containing polyprenol extracted from Ginkgo biloba leaves. Ind. Crop. Prod.. 2020;147:112213
- [CrossRef] [Google Scholar]
- Synergistic chemotherapy, physiotherapy and photothermal therapy against bacterial and biofilms infections through construction of chiral glutamic acid functionalized gold nanobipyramids. Chem. Eng. J.. 2020;393:124778
- [CrossRef] [Google Scholar]
- Integrated process for the production of indigo and indirubin in an anaerobic environment at laboratory and pilot level. J. Clean. Prod.. 2022;364:132610
- [CrossRef] [Google Scholar]
- Box-Behnken response surface methodology to optimize the processing conditions of indigo naturalis. Chin. J. Mod. Appl. Pharm.. 2019;36(23):2881-2887.
- [Google Scholar]
- Progress on germplasm resources of Baphicacanthus cusia (Nees) Bremek. J. Pharm. Pract.. 2017;6(1–4):16.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2024.105801.
Appendix A
Supplementary data
The following are the Supplementary data to this article:Supplementary Data 1
Supplementary Data 1







