Translate this page into:
A stereo, regioselective synthesis and discovery of antimycobaterium tuberculosis activity of novel β-lactam grafted spirooxindolopyrrolidine hybrid heterocycles
⁎Corresponding author. anatarajan@ksu.edu.sa (Natarajan Arumugam)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
A stereo- and regioselective synthesis of hitherto unexplored novel class of β-lactam embedded spirooxindolopyrrolidine hybrid heterocycles have been accomplished via ionic liquid accelerated [3 + 2]-cycloaddition reaction process. The expected unusual lactonization/lactamization product could not be observed even in traces. The in vitro antimycobacterium tubercular activity of the synthesized spiroheterocyclic hybrids were assessed against Mycobacterium tuberculosis H37Rv. Among them, the compounds with no substitution and chlorosubstitution on the oxindole ring showed the most potent activity with a MIC 0.78 μg/mL and 1.56 μg/mL, respectively which were two-fold and equal activity than the standard drug, ethambutol (MIC = 1.56 μg/mL).
Keywords
Spirooxindolopyrrolidines
β-lactam
1,3-Dipolar cycloaddition reaction
Ionic liquids
DFT study
Mycobacterium tuberculosis activity
1 Introduction
Tuberculosis (TB) is lethal communicable diseases caused mainly by the pathogenic aerobic bacteria Mycobacterium tuberculosis (Mtb) which usually form an infection in the lung of the host and is one of the leading inauspicious health issues globally since prehistoric times (Fogel, 2015). According to World Health Organization (WHO) statistics in 2018, around 10.0 million people fell ill and 1.2 million deaths from TB. The pathogenic synergy of TB is lifelong risk for immune compromised individual and human immunodeficiency virus (HIV) infected patients (Koul et al., 2011). Although, the current therapies include a combination of available anti-tuberculosis drugs that inhibits both metabolic and non-metabolic targets, these therapies will inevitably become less effective, poor efficacy in eliminating latent pathogens, prolong treatment (Somoskovi and Parson, 2001) inducing hepatotoxicity (ATDH) besides triggering substantial morbidity and mortality (Ramappa and Aithal, 2013). In addition, emergency multidrug resistant (MDR) and extensively drug resistant (XRD) strain of Mtb is another challenge towards curbing TB. Hence, the search of new natural or synthetic compounds having high efficient, fast acting, capable to shortening and simplify the actual treatment with unique mechanism of action, low toxicity profiles, active against MDR-TB and XDR-TB including suitable with anti-HIV treatment in TB-HIV individuals to efficiently combat this disease is imperative (see Schemes 1 and 2).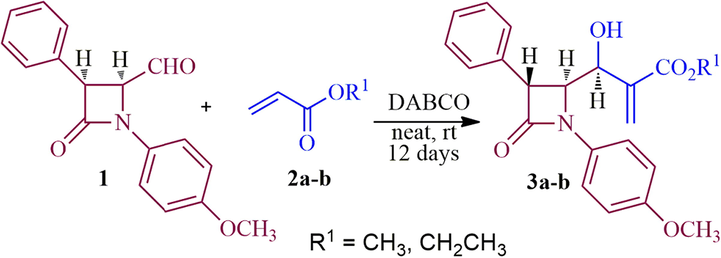
Synthesis of β-lactam alkene, 3a-b.
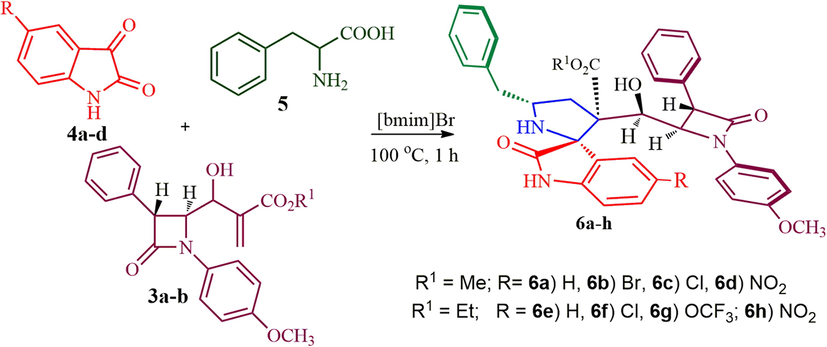
β-lactam integrated spirooxindolopyrrolidine hybrid heterocycles, 6a-h.
Spirocycles are privileged class of heterocyclic motif as they have been employed both as core unit and adjoined to the periphery of molecules in drug discovery. Such spiro compounds are expected to interact more proficiently with active site of protein more easily than flat aromatic ring systems due to their unique three-dimensional structural properties. Ultimately, spirocycle embedded heterocycles are of interest to modulate log P, metabolic stability and have sp3 hybridization which probably favors water solubility, a crucial property in the course of drug development. Spirooxindolopyrrolidines are very attractive in this connection as they are ubiquitous in many natural products and biological relevant synthetic analogs, which showed multifarious biological and pharmaceutical activities (Yu et al., 2015; Rajesh et al., 2011; Bhaskar et al., 2012; Rajanarendar et al., 2013; Kornet and Thio, 1976; Arumugam et al., 2019; Kia et al., 2014).
β-Lactam is another important class of heterocyclic moiety as these structural motifs prevalent in most commonly existing antibiotics such as penicillin, cephalosporins, carbapenems, nocardicins and monobactams which has unveiled a prominent place in protection against bacterial infections (Hubbard and Walsh, 2003). Moreover, β-lactam moiety is relatively highly reactive structural unit owing to their strained four membered ring system which makes them diverse chiral analogs (Ojima and Delaloge, 1997; Deshmukh et al., 2004; Alcaide and Almendros, 2004). In recent past years, we have been engaged in the construction of spirooxindolopyrrolidine and β-lactam employing domino multicomponent reaction sequence (Arumugam et al., 2013; Malathi et al., 2015). Among them, 1,3-dipolar cycloaddition reaction (Suresh Kumar et al., 2018) is a protuberant methodology for the construction of structurally intriguing hybrid heterocycles including spirooxoindolopyrrolidines as this synthetic strategy allows the creation of multiple bonds in a single synthetic cascade process with the following advantages such as (i) non-isolation of intermediates, (ii) reduction in number of synthetic steps and work ups, (iii) minimization of waste, (iv) facile automization (v) operational simplicity, and (vi) atom economy.
Taking into consideration the above notable biological precedents, we postulate that heterocycles comprising spirooxindolopyrrolidine and β-lactam motifs in a single molecule would be of great value in medicinal chemistry. Besides, integration of β-lactam unit into spirohybrid heterocycles has often brought about the intensification of biological activity of the molecules. Keeping in mind the above-mentioned facts, herein we report an easy access to β-lactam fused spirooxindolopyrrolidine hybrid heterocycles via a one-pot three component synthetic protocol employing an ionic liquid accelerated 1,3-dipolar cycloaddition strategy and their antimycobacterium activity against Mycobacterium tuberculosis H37Rv. It is noteworthy to mention that the β-lactam integrated spirooxindolopyrrolidine hybrids are relatively meagre in the literature (Rajesh and Raghunathan, 2013, Rajesh and Raghunathan, 2014) and this is the first report on the synthesis and biological intervention of spirooxindolopyrrolidine embedded β-lactam from the combination of 1,3-dipole component derived from isatin and L-phenylalanine and Baylis-Hillman adduct of β-lactam through 1,3-dipolar cycloaddition process. The synthetic approach for the spiroheterocyclic hybrids has been described in Fig. 1. The expected latonization and lactomization product could not be observed, a probable reason for the non-formation of these expected products has also been investigated through computational study. In addition, the biological intervention of these spirooxindolopyrrolidine heterocyclic hybrids has also been explored in this article.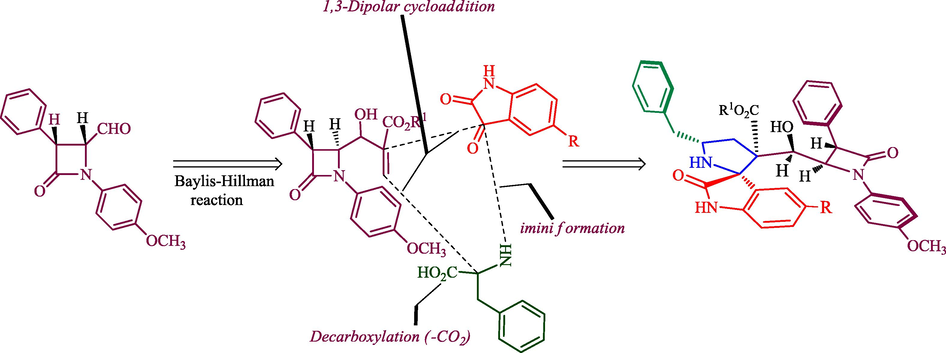
Synthetic strategy for the formation of β-lactam grafted spiropyrrolidine hybrid heterocycles.
2 Material and methods
2.1 Preparation of β-lactam integrated spirooxindolopyrrolidines, 6a-h
A mixture of Baylis-Hillman adduct of azetidinone 3a (0.20 g, 0.545 mmol), substituted isatin 4a (0.080 g, 0.545 mmol), and L-phenylalanine 5 (0.090 g, 0.545 mmol) were heated in [bmim]Br (3 mL) for 1 h at 100 °C. After completion of the reaction (TLC), the reaction mixture was diluted with EtOAc (2 × 5 mL) and water (15 mL). The ethyl acetate layer was extracted and dried over anhydrous sodium sulfate. The solvent was removed under reduced temperature. The crude product was purified by column chromatography in EtOAc: petroleum ether (3:7 v/v). Similarly, the same reaction protocol was used for the compounds 3b, 4b-d and 5.
Methyl 5′-benzyl-3′-(hydroxy(1-(4-methoxyphenyl)-4-oxo-3-phenylazetidin-2-yl)methyl)-2-oxospiro[indoline-3,2′-pyrrolidine]-3′-carboxylate, 6a: White solid; Mp: 147–149 °C; 1H NMR: 2.00–2.06 (dd, J = 15.0, 10.5 Hz, 1H), 2.16–2.21 (dd, J = 14.5, 6.5 Hz, 1H), 2.55–2.60 (dd, J = 13.5, 6.5 Hz, 1H), 2.68–2.72 (dd, J = 13.0, 6.0 Hz, 1H), 3.32–3.35 (m, 1H), 3.45 (s, 3H), 3.70 (s, 3H), 3.77 (d, J = 2.0 Hz, 1H, NH), 4.47 (d, J = 2.0 Hz, 1H), 4.73 (d, J = 2.5 Hz, 1H), 4.83 (m, 1H), 6.79–6.85 (m, 3H, ArH), 7.07–7.12 (m, 5H, ArH), 7.21–7.29 (m, 9H, ArH), 7.69 (d, J = 7.5 Hz, 1H), 8.79 (s, NH, 1H): 13C NMR: 37.5, 41.9, 50.8, 54.7, 55.3, 55.6, 58.7, 59.7, 70.3, 76.0, 114.9, 116.3, 116.5, 119.8, 124.9, 126.9, 127.8, 128.1, 128.3, 128.7, 128.8, 128.9, 129.5, 130.8, 133.5, 135.1, 137.8, 157.0, 164.4, 169.8, 176.4. Mass: m/z = 617 (M+); Anal. Calcd for C37H35N3O6: C, 71.94; H, 5.71; N, 6.80; Found C, 72.02; H, 5.82; N, 6.91%.
Methyl 5′-benzyl-5-bromo-3′-(hydroxy(1-(4-methoxyphenyl)-4-oxo-3-phenylazetidin-2-yl)methyl)-2-oxospiro[indoline-3,2′-pyrrolidine]-3′-carboxylate, 6b: White solid; Mp: 175–177 °C; 1H NMR: 1.87–1.92 (dd, J = 14.4, 5.6 Hz, 1H), 2.17–2.24 (m, 1H), 2.53–2.61 (m, 2H), 2.87–2.92 (dd, J = 13.2, 4.8 Hz, 1H), 3.06 (s, 3H), 3.49 (s, 1H, NH), 3.62 (s, 3H), 4.34 (m, 1H), 4.65 (m, 1H), 5.27 (d, J = 3.6 Hz, 1H), 6.63–6.78 (m, 6H, ArH), 6.97–7.11 (m, 9H, ArH), 7.18–7.22 (m, 2H, ArH), 10.49 (s, NH, 1H): 13C NMR: 35.7, 44.2, 51.9, 54.6, 55.3, 61.1, 62.0, 67.1, 69.6, 74.5, 111.1, 114.2, 118.9, 124.2, 126.4, 127.3, 127.5, 128.2, 128.5, 128.7, 128.9, 129.0, 129.6, 134.3, 134.6, 138.3, 139.0, 156.0, 165.4, 171.1, 179.9; Mass: m/z: = 696 (M+); Anal. Calcd for C37H34BrN3O6: C, 63.80; H, 4.92; N, 6.03; Found C, 63.97; H, 5.01; N, 6.11%.
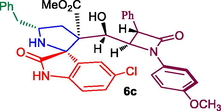
Methyl 5′-benzyl-5-chloro-3′-(hydroxy(1-(4-methoxyphenyl)-4-oxo-3-phenylazetidin-2-yl)methyl)-2-oxospiro[indoline-3,2′-pyrrolidine]-3′-carboxylate, 6c: White solid; Mp: 188–190 °C; 1H NMR: 1.86–1.91 (dd, J = 14.4, 5.6 Hz, 1H), 2.16–2.23 (m, 1H), 2.51–2.59 (m, 2H), 2.86–2.91 (dd, J = 13.2, 4.8 Hz, 1H), 3.05 (s, 3H), 3.48 (s, 1H, NH), 3.60 (s, 3H), 4.23 (brs, 1H, OH), 4.33 (m, 1H), 4.64 (m, 1H), 5.25 (d, J = 3.6 Hz, 1H), 6.60–6.77 (m, 6H, ArH), 6.96–7.10 (m, 9H, ArH), 7.13–7.21 (m, 2H, ArH), 10.48 (s, NH, 1H): 13C NMR: 35.5, 44.0, 51.7, 54.5, 55.1, 60.9, 61.8, 67.0, 69.5, 74.3, 110.9, 114.1, 118.7, 124.0, 126.2, 127.2, 127.3, 128.2, 128.3, 128.6, 128.7, 128.8, 129.4, 134.1, 134.4, 138.1, 138.8, 155.8, 162.2, 170.9, 179.7. Mass: m/z = 652 (M+); Anal. Calcd for C37H34ClN3O6: C, 68.14; H, 5.26; Cl, 5.44; N, 6.44; Found C, 72.11; H, 5.94; N, 6.97%.
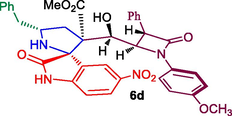
Methyl 5′-benzyl-3′-(hydroxy(1-(4-methoxyphenyl)-4-oxo-3-phenylazetidin-2-yl)methyl)-5-nitro-2-oxospiro[indoline-3,2′-pyrrolidine]-3′-carboxylate, 6d: White solid; Mp: 204–206 °C; 1H NMR:1.92–1.98 (dd, J = 13.6, 9.2 Hz, 1H), 2.08–2.13 (dd, J = 14.8, 6.8 Hz, 1H), 2.47–2.52 (dd, J = 13.2, 6.4 Hz, 1H), 2.60–2.64 (dd, J = 13.2, 6.8 Hz, 1H), 3.23–3.28 (m, 1H), 3.38 (s, 3H), 3.65 (s, 3H), 4.39 (d, J = 2.4 Hz, 1H), 4.65 (d, J = 2.4 Hz, 1H), 4.76 (m, 1H), 6.72–6.77 (m, 3H, ArH), 6.99–7.26 (m, 13H, ArH), 7.61 (d, J = 8.4 Hz, 1H), 8.59 (s, NH, 1H): 13C NMR: 37.8, 42.2, 51.2, 55.1, 55.7, 56.0, 59.0, 60.0, 70.6, 76.3, 115.3, 116.6, 116.9, 119.7, 120.1, 125.2, 127.3, 128.2, 128.5, 128.7, 129.0, 129.2, 129.8, 131.2, 133.8, 135.5, 138.2, 142.9, 157.3, 164.7, 170.1, 176.8. Mass: m/z = 662 (M+); Anal. Calcd for C37H34N4O8: C, 67.06; H, 5.17; N, 8.45; Found C, 67.17; H, 5.26; N, 8.52%.
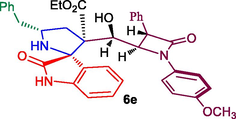
Ethyl 5′-benzyl-3′-(hydroxy(1-(4-methoxyphenyl)-4-oxo-3-phenylazetidin-2-yl)methyl)-2-oxospiro[indoline-3,2′-pyrrolidine]-3′-carboxylate, 6e: White solid; Mp: 156–158 °C; 1H NMR: 0.82 (t, J = 7.5 Hz, 3H), 2.08–2.12 (dd, J = 14.5, 6.0 Hz, 1H), 2.39–2.44 (m, 1H), 2.66–2.70 (dd, J = 13.5, 8.5 Hz, 1H), 3.03–3.07 (dd, J = 12.5, 5.5 Hz, 1H), 3.47–3.52 (m, 1H), 3.69–3.71 (m, 2H), 3.75 (s, 3H), 4.38–4.41 (brs, 1H), 4.56 (d, J = 2.0 Hz, 1H), 4.86 (d, J = 4.5 Hz, 1H), 5.16 (d, J = 3.5 Hz, 1H), 6.76–6.84 (m, 5H, ArH), 6.97–7.00 (m, 1H, ArH), 7.06–7.08 (m, 1H, ArH), 7.15–7.25 (m, 9H, ArH), 7.29–7.36 (m, 2H), 7.92 (s, NH, 1H): 13C NMR: 13.5, 36.2, 44.6, 55.0, 55.6, 61.4, 61.7, 62.1, 67.5, 70.0, 74.4, 110.0, 114.5, 119.2, 123.6, 124.5, 126.6, 127.6, 127.9, 128.9, 129.0, 129.3, 129.6, 130.0, 133.4, 134.7, 138.6, 139.3, 156.3, 165.8, 171.0, 180.5. Mass: m/z = 631 (M+); Anal. Calcd for C38H37N3O6: C, 72.25; H, 5.90; N, 6.65; Found C, 72.36; H, 5.97; N, 6.73%.
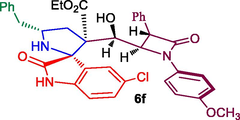
Ethyl 5′-benzyl-5-chloro-3′-(hydroxy(1-(4-methoxyphenyl)-4-oxo-3-phenylazetidin-2-yl)methyl)-2-oxospiro[indoline-3,2′-pyrrolidine]-3′-carboxylate 6f: White solid; Mp: 196–197 °C; 1H NMR: 0.86 (t, J = 7.0 Hz, 3H), 2.08–2.13 (dd, J = 13.5, 5.5 Hz, 1H), 2.34–2.39 (m, 1H), 2.68–2.72 (dd, J = 12.5, 8.0 Hz, 1H), 3.00–3.04 (dd, J = 13.0, 5.5 Hz, 1H), 3.46 (s, 1H, NH), 3.58–3.62 (m, 1H), 3.74 (s, 3H), 3.76–3.79 (m, 2H), 4.41–4.41 (brs, 1H), 4.55 (m, 1H), 4.84 (d, J = 4.5 Hz, 1H), 5.05 (d, J = 4.0 Hz, 1H), 6.67 (d, J = 8.5 Hz, 1H, ArH), 6.80–6.86 (m, 4H, ArH), 6.95 (s, 1H, ArH), 7.13–7.25 (m, 9H, ArH), 7.32–7.38 (m, 2H, ArH), 8.26 (s, 1H, NH); 13C NMR: 13.5, 35.9, 44.4, 54.9, 55.6, 61.4, 61.9, 62.1, 67.5, 70.2, 74.6, 111.1, 114.6, 119.1, 124.9, 126.8, 127.7, 127.8, 128.8, 128.9, 129.1, 129.4, 130.0, 134.5, 135.0, 137.9, 138.3, 156.3, 165.8, 170.9. 180.2. Mass: m/z: m/z = 666 (M+); Anal. Calcd for C38H36ClN3O6: C, 68.51; H, 5.45; N, 6.31; Found C, 68.63; H, 5.53; N, 6.40%.
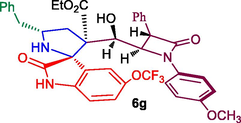
Ethyl 5′-benzyl-3′-(hydroxy(1-(4-methoxyphenyl)-4-oxo-3-phenylazetidin-2-yl)methyl)-2-oxo-5-(trifluoromethoxy)spiro[indoline-3,2′-pyrrolidine]-3′-carboxylate, 6 g: White solid; Mp: 146–148 °C; 1H NMR: 0.84 (t, J = 6.8 Hz, 3H), 2.11–2.16 (dd, J = 14.0, 6.0 Hz, 1H), 2.34–2.41 (m, 1H), 2.68–2.73 (dd, J = 13.2, 8.4 Hz, 1H), 2.98–3.03 (dd, J = 13.2, 6.0 Hz, 1H), 3.60–3.63 (m, 1H), 3.73 (s, 3H),3.68–3.70 (m, 2H), 4.41 (brs, 1H, OH), 4.39–4.44 (brs, 1H), 4.58 (d, J = 1.2 Hz, 1H), 4.86 (d, J = 5.2 Hz, 1H), 5.10–5.11 (m, 1H), 6.72 (d, J = 8.4 Hz, 1H, ArH), 6.79–6.82 (m, 2H, ArH), 6.86–6.88 (m, 3H, ArH), 7.02–7.05 (m, 1H), 7.18–7.25 (m, 8H, ArH), 7.31–7.37 (m, 2H, ArH), 8.72 (s, 1H, NH); 13C NMR: 13.3, 35.8, 44.4, 54.8, 55.4, 61.1, 61.7, 62.0, 67.3, 70.0, 74.5, 110.7, 114.5, 114.9, 118.4, 119.0, 122.5, 126.7, 127.7, 128.8, 129.0, 129.2, 129.8, 134.4, 134.8, 138.2, 144.9, 156.3, 165.8, 170.8, 180.6. Mass: m/z = 715 (M+); Anal. Calcd for C39H36F3N3O7: C, 65.45; H, 5.07; N, 5.87; Found C, 65.56; H, 5.18; N, 5.95%.
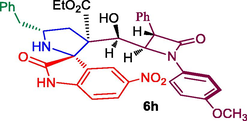
Ethyl 5′-benzyl-3′-(hydroxy(1-(4-methoxyphenyl)-4-oxo-3-phenylazetidin-2-yl)methyl)-5-nitro-2-oxospiro[indoline-3,2′-pyrrolidine]-3′-carboxylate, 6h: White solid; Mp: 211–213 °C; 1H NMR: 0.87 (t, J = 7.5 Hz, 3H), 2.13–2.17 (dd, J = 14.5, 6.0 Hz, 1H), 2.44–2.49 (m, 1H), 2.71–2.75 (dd, J = 13.5, 8.5 Hz, 1H), 3.07–3.11 (d, J = 12.5, 5.5 Hz, 1H), 3.51–3.56 (m, 1H), 3.71–3.78 (m, 2H), 3.79 (s, 3H), 4.41–4.50 (brs, 1H), 4.60 (d, J = 2.0 Hz, 1H), 4.90 (d, J = 4.5 Hz, 1H), 5.20 (d, J = 3.5 Hz, 1H), 6.81–6.89 (m, 5H, ArH), 7.02–7.05 (m, 1H, ArH), 7.11–7.12 (m, 1H, ArH), 7.19–7.41 (m, 10H, ArH), 7.97 (s, NH, 1H): 13C NMR: 13.7, 36.5, 44.8, 55.2, 55.8, 61.7, 61.9, 62.4, 67.7, 70.2, 74.6, 110.2, 114.8, 119.4, 123.8, 124.8, 126.9, 127.8, 128.1, 129.1, 129.3, 129.5, 129.8, 130.3, 133.6, 134.9, 138.8, 142.5, 156.6, 171.3, 166.6, 180.8. Mass: m/z = 676 (M+); Anal. Calcd for C38H36N4O8: C, 67.44; H, 5.36; N, 8.28; Found C, 67.57; H, 5.44; N, 8.40; %.
3 Results and discussion
3.1 Chemistry
The highly functionalized β-lactam Baylis-Hillman adducts 3a/3b (Scheme 1) were prepared from 4-formyl azetidinone 1 (Alcaide et al., 1992) and methyl/ethylacrylate in presence of DABCO according to the literature report (Prasanna et al., 2011). It is pertinent to note that the utilization of Baylis-Hillman adducts of β-lactam as dipolarophile for the multicomponent process has not been explored much. With dipolarophiles 3a/3b in hand, we initiated our investigation on the 1,3-dipolar cycloaddition process of Baylis-Hillman adducts 3a/3b with azomethine ylide derived from isatin/substituted isatin and α-amino acid under heating at 100 °C in [bmim]Br, which led to the construction of β-lactam integrated spirooxindolopyrrolidine hybrid heterocycles 6 as a single diastereoisomer in good to excellent yields (85–95%) after 1 h (Scheme 2). Firstly, the solvent optimization was studied with an equimolar mixture of 3a, isatin 4a and L-phenylalanine 5 in various organic solvents viz., CH3OH, EtOH, CH3CN, 1,4-dioxane including mixture of solvents viz. acetonitrile:1,4-dioxane (1:1 v/v) afforded 62, 65, 58, 60 and 63% (Table 1). Alternatively, the reaction was also carried out with an ionic liquid, [bmim]br. An equimolar mixture of 4, 5 and 3a in [bmim]br at 100 °C for 1 h. The formation of the product was observed by TLC, the reaction mixture was diluted with EtOAc and water. The organic layer was evaporated under reduced pressure and the crude product obtained was purified by column chromatography to afford the product in 87% yield (see Fig. 3).
Entry
Solvents
Time (h)
Yield (%)
1
MeOH
2
62
2
EtOH
2
65
3
MeCN
2
58
4
1,4-Dioxane
2
60
5
1.4-Dioxane: MeOH
2
63
5
[bmim]Br
30 min
87
The structure of proposed compounds was in agreement with their one- and two-dimensional NMR spectroscopic data as evidenced for a representative example 6a (Fig. 2). The 1H NMR spectrum of 6a has a doublet at δ 4.47 (J = 2.0 Hz) ppm ascribable to H-3′ hydrogen of β-lactam which showed (i) H, H-COSY correlation with the multiplet at δ 4.72–4.73 ppm assignable to H-4′ hydrogen of β-lactam (ii) HMBCs (Fig. 3) with carbon signal C-4′ at 59.7 ppm, besides showing a correlation with β-lactam ring carbonyl (C-2′) at δ 164.4 ppm. H-4′ hydrogen showed (i) HMBCs with C-3′, C-7, methyl ester carbonyl at δ 55.3, 76.0 and 169.8 ppm respectively, (ii) H, H-COSY correlation with the multiplet at δ 4.83 ppm assignable to H-7 hydrogen. H-7 hydrogen showed HMBCs with C-3 (quaternary carbon), C-6, C-2′′ (oxindole ring carbonyl) respectively at 54.7, 37.5 and 176.4 ppm. The two doublet of doublets at δ 2.00–2.06 (J = 15.0, 10.5 Hz) and 2.16–2.21 (J = 14.5, 6.0 Hz) ppm related by H, H-COSY correlation were assigned to H-6 hydrogens, which shows (i) H, H-COSY correlations with the multiplets at δ 3.32–3.35 ppm due to H-5 hydrogen (ii) HMBCs with spirocarbon (C-3′′), C-4, C-5, at δ 70.3, 41.9 and 58.7 ppm, respectively. The two doublets of doublets at 2.55–2.60 and 2.68–2.72 (J = 13.0, 6.0 Hz) can be assigned to H-4 hydrogens as both hydrogens coupled with each other. H-4 hydrogen showed HMBCs with C-5 and C-6 at 58.7, 37.5 ppm, respectively. Further, methoxy, methylene, methine hydrogens, spiro and quaternatry carbon were assigned based on the DEPT-135 spectrum. Finally, the cycloadduct was undoubtedly determined by single crystal X-ray (deposition CCDC no.1949551) diffraction analysis (Fig. 4).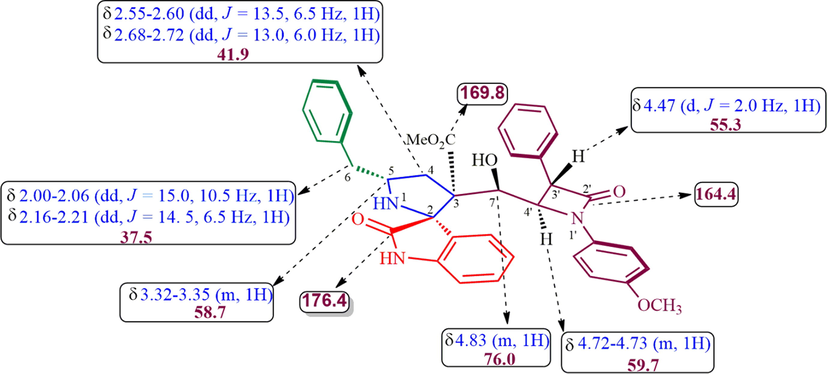
1H and 13C Chemical shift of 6a.
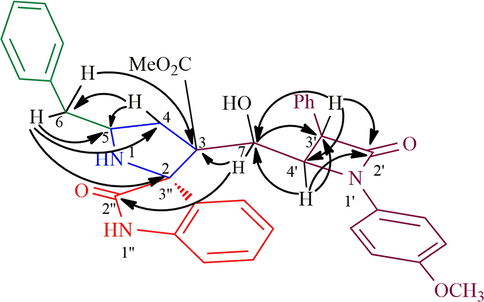
Selected HMBCs of 6a.
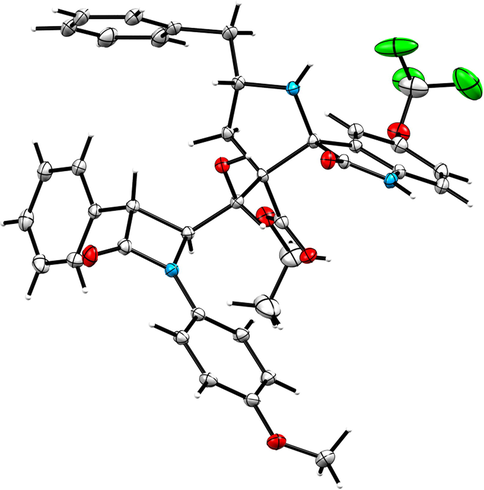
ORETP diagram of 6g.
The persuasive mechanism for the formation β-lactam integrated spiroheterocyclic hybrids 6 is described in Scheme 3. The reaction of acrylate and DABCO furnished the intermediate 8 which further reacts with active carbonyl carbon of 4-formyl azetidinone 1 to furnish Baylis-Hillman adduct of β-lactam 3 by elimination of DBACO via intermediate 9. Three component cycloaddition process was initiated by the reaction of ketone function of 4 and 5 afforded spiro intermediate 11 via 10 followed by the in situ generation of highly reactive 1,3-dipole component 12 via decarboxylative condensation. The subsequent reaction of 12 with electron deficient alkene of β-lactam 3 may occur via path A or path B. However, the exclusive formation of β-lactam grafted spirooxindolopyrrolidines 6 disclose that path A is favored over path B. Presumably, due to the electrostatic repulsion wielded between the oxindole and ester carbonyls present in the same face during the approach of 1,3-dipole 12 over the alkene 3 which led to unfavored formation of compound 16. In path A, the carbonyls of ylide 12 and ester carbonyl of dipolarophile 3 in the opposite face which overcomes possible electrostatic repulsion. The above dispute is further proved by the X-ray structure of a representative compound 6g (Fig. 4). In addition, the three component cycloadditions were found to be regioselective and the regioisomer of spirooxindolopyrrolidines 6 was not found in the reaction (Scheme 3). Possibly, this may be occurred from the electron rich carbon of the azomethine ylide 12 adds to the more electron deficient β-carbon of the α, β-unsaturated 3. Also, the above cycloaddition process creates up to six adjacent stereogenic carbon in a single transformation. Further, the expected lactonization 14 /lactamization product 15 was not observed and this has been investigated through energy minimization calculations using the Density functional theory (DFT) study. For the purpose, we employed Gaussian 09 quantum chemistry software. The optimized structure of both compounds (6 and 15) are presented in Fig. 5. It is clear from the optimized energies of the compounds that the compound 6 is nearly 155 Hartree energy lesser than that of the compound 15, and therefore the formation of former compound 6 is found to be highly favored than 15.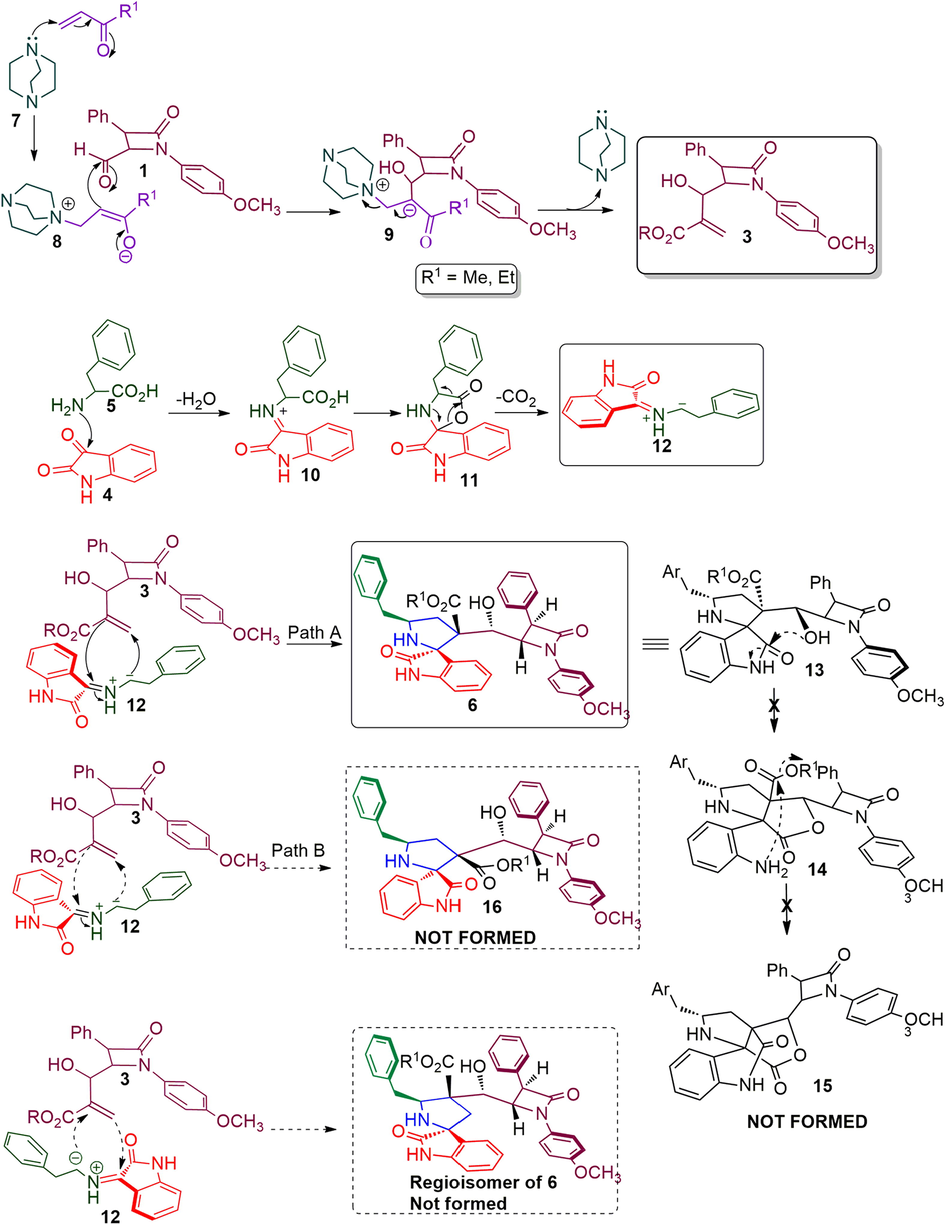
The plausible pathway for the construction of β-lactam integrated spirooxindolopyrrolidine hybrid heterocycles, 6.
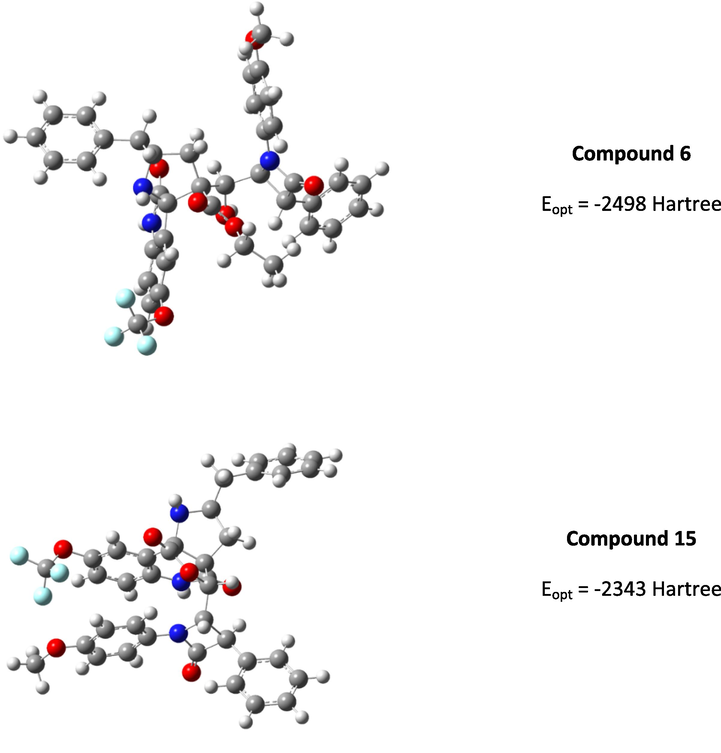
Optimized structure of the compounds 6 and 15.
3.2 Biology
In recent years, we have reported the usefulness of spiro moiety embedded hybrid heterocycles as anticancer, antimicrobial, cholinesterase inhibitors, anti-inflammatory and antimycobaterium agents [27–31]. Some of the spiroheterocycles, exhibited significant activity comparable to or even higher activity than the respective reference standards [31,32]. In this perspective, in the present work, the synthesized Baylis-Hillman adduct of β-lactam 3a/3b and β-lactam embedded spirooxindolopyrrolidines 6 were assessed for their tubercular activity against Mycobacterium tuberculosis H37Rv. Compounds 6a, 6c, 6e and 6f displayed good to excellent activity against Mtb with MIC values 0.78, 1.56, 1.56 and 3.25 μg/mL, respectively (Table 2). Among them, compound 6a (MIC 0.78 μg/mL) possessing unsubstituted oxindole ring displayed higher potency which disclose comparable or even higher activity than that of the standard drug, ethambutol (MIC = 1.56 μg/mL). Compound 6c (MIC 1.56 μg/mL) and 6e (MIC 1.56 μg/mL) bearing chloro and unsubstituted oxindole ring showed excellent activity which are equipotent that of standard drug ethambutol (MIC 1.56 μg/mL). Whereas compound 6f (MIC 3.25 μg/mL) carrying chloro subsititutent on the oxindole ring exhibited good activity. The most active compounds 6a, 6c and 6e were evaluated for their toxicity effect and these compounds displayed minimal toxicity on Raw 264.7 macrophage cell lines at 50 μg/mL concentration. With respect to structure-MTB activity relationship disclosed that β-lactam tethered spiropyrrolidine carrying unsubstituted and chloro substituent on oxindole ring is more potent than the other substituted derivatives. In this series, chloro and unsubstituted on the oxindole ring displayed good to excellent activities with the order being 6a > 6c ≥ 6e when compared to the standard drug.
Entry
Compound
Yield (%)
MIC (µg/mL)
Toxicity (% inhibition when tested at 50 µg/mL)
1
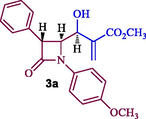
72
>25
–
2
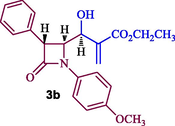
70
>25
–
3
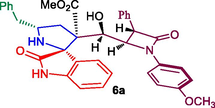
84
0.78
27.94
4
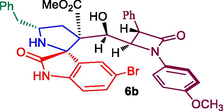
79
>25
–
5
85
1.56
34.02
6
81
12.5
–
7
88
1.56
1.05
8
84
3.25
–
9
87
6.25
–
10
85
12.5
–
81
>25
–
11
Isoniazid
–
0.05
12
Rifampicin
–
0.1
13
Ethambutol
–
1.56
4 Conclusion
A stereo- and regioselective synthesis of structurally intriguing novel β-lactam integrated spirooxindolopyrrolidine hybrid heterocycles in excellent yields employing multicomponent cycloaddition reaction sequence. These spirooxindolopyrrolidine hybrid heterocycles possess six adjacent stereocenter out of which one is one spirocarbon via two C-C bonds and one C-N bonds in single-pot transformation. The synthesized compounds were elucidated by spectroscopic analysis and the regio-/stereochemistry outcome was further determined by X-ray diffraction studies. Anti-tubercular activity of these heterocyclic hybrids revealed that compounds 6a, 6c, 6e showed potent activity against mycobacterium tuberculosis and displayed less toxicity at 50 µg concentration.
Acknowledgment
The authors acknowledge the Deanship of Scientific Research at King Saud University for funding this work through the Research grant RG-1438-052.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- β-Lactams as Versatile Synthetic Intermediates for the Preparation of Heterocycles of Biological Interest. Curr. Med. Chem.. 2004;11:1921-1949.
- [Google Scholar]
- Stereoselective preparation of mono- and bis-β-lactams by the 1,4-diaza-1,3-diene - acid chloride condensation: scope and synthetic applications. J. Org. Chem.. 1992;57(22):5921-5931.
- [Google Scholar]
- Dispiropyrrolidinyl-piperidone embedded indeno[1,2-b]quinoxaline heterocyclic hybrids: Synthesis, cholinesterase inhibitory activity and their molecular docking simulation. Bioorg. Med. Chem.. 2019;27:2621-2628.
- [Google Scholar]
- A 1,3-dipolar cycloaddition–annulation protocol for the expedient regio-, stereo- and product-selective construction of novel hybrid heterocycles comprising seven rings and seven contiguous stereocentres. Tetrahedron Lett.. 2013;54:2515-2519.
- [Google Scholar]
- Synthesis of novel spirooxindole derivatives by one pot multicomponent reaction and their antimicrobial activity. Eur. J. Med. Chem.. 2012;51:79-91.
- [Google Scholar]
- Azetidin-2-ones, Synthon for Biologically Important Compounds. Curr. Med. Chem.. 2004;11:1889-1920.
- [Google Scholar]
- Synthesis and discovery of highly functionalized mono- and bis-spiro-pyrrolidines as potent cholinesterase enzyme inhibitors. Bioorg. Med. Chem. Lett.. 2014;24:1815-1819.
- [Google Scholar]
- Oxindole-3-spiropyrrolidines and piperidines. Synthesis and local anesthetic activity. J. Med. Chem.. 1976;19:892-898.
- [Google Scholar]
- Multicomponent [3+2] cycloaddition strategy: stereoselective synthesis of novel polycyclic cage-like systems and dispiro compounds. Tetrahedron Lett.. 2015;56:6132-6135.
- [Google Scholar]
- Asymmetric synthesis of building-blocks for peptides and peptidomimetics by means of the β-lactam synthon method. Chem. Soc. Rev.. 1997;26:377-386.
- [Google Scholar]
- 2-{Hydroxy[1-(4-methoxyphenyl)-4-oxo-3-phenylazetidin-2-yl]methyl}acrylonitrile. Acta Crystallogr. Sect. E. 2011;67:02340.
- [Google Scholar]
- A facile synthesis, anti-inflammatory and analgesic activity of isoxazolyl-2,3-dihydrospiro[benzo[f]isoindole-1,3'-indoline]-2',4,9-triones. Bioorg. Med. Chem. Lett.. 2013;23:3954-3958.
- [Google Scholar]
- Antimycobacterial activity of spirooxindolo-pyrrolidine, pyrrolizine and pyrrolothiazole hybrids obtained by a three-component regio- and stereoselective 1,3-dipolar cycloaddition. Med. Chem. Commun.. 2011;2:626-630.
- [Google Scholar]
- Synthesis of β-lactam-Tethered polycyclic fused heterocycles through rearrangement by a one-pot tandem [3+2] cycloaddition reaction. Eur. J. Org. Chem. 2013:2597-2607.
- [Google Scholar]
- A tactical approach for the synthesis of novel β-lactam substituted-polycyclic-fused isoxazolidine derivatives via an intramolecular [3+2]-cycloaddition. Tetrahedron Lett.. 2014;55:699-705.
- [Google Scholar]
- Hepatotoxicity related to anti-tuberculosis drugs: mechanisms and management. J. Clin. Exp. Hepatol.. 2013;3:37-49.
- [Google Scholar]
- The Molecular Basis of Resistance to Isoniazid, Rifampin, and Pyrazinamide in Mycobacterium tuberculosis. The molecular basis of resistance to isoniazid, rifampin, and pyrazinamide in Mycobacterium tuberculosis. Respir. Res.. 2001;2:164-168.
- [Google Scholar]
- Dipolar cycloaddition based multi-component reaction: Synthesis of spiro tethered acenaphthylene–indolizine–pyridinone hybrids. Tetrahedron Lett.. 2018;29:3336-3340.
- [Google Scholar]
- Global Tuberculosis Report 2018. http://www.who.int/tb/publications/global_report/en/.
- Spirooxindoles: Promising scaffolds for anticancer agents. Eur. J. Med. Chem.. 2015;97:673-698.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2020.102938.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







