Translate this page into:
Analysis and health risk assessment of heavy metals in some onion varieties
⁎Corresponding author. adosari@ksu.edu.sa (Ali Abdullah Aldosari),
⁎⁎Corresponding author. amabbasi@cuiatd.edu.pk (Arshad Mehmood Abbasi)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Quantification and risk assessment of metals was evaluated in nine onion varieties. Relatively higher levels in the onion’s bulb/leaves were found for Fe, Mn and Zn. Significant anthropogenic contamination was noted for the metals in present study. Metal levels in all onion varieties showed no significant cancer or no-cancer risk.
Abstract
Being a global concern, the current scenario of food chain contamination is one of the major threats to human health. Unrestrained human population, industrialization, urbanization, and extensive use of agrochemicals are among the major causes of heavy metals (HMs) contamination in the food chain. The present study was intended to quantify some HMs and to evaluate their sources and health risks in different varieties of onion using standard analytical approaches. Overall, nine HMs were quantified in the bulbs and leaves of nine varieties of onion. Ascending order of HMs’ concentration was as follow: Fe > Zn > Mn > Co > Pb > Cu > Cr > Cd > Li (bulbs) and Fe > Mn > Zn > Cu > Cr > Pb > Co > Li > Cd (leaves). Most of the measured levels were found to be relatively higher in the leaves than bulbs. Red Flame (V4), Super Sarhad (V3), Red Orb (V2), White Pearl (V7) and Zeus (V9) varieties showed elevated levels of Cu, Fe, and Zn; Cr and Mn; Co and Pb; Cd and Li, respectively in the bulbs. Likewise, Red Orb (V2) and White Pearl (V7) revealed elevated levels of the metals, while Golden Orb (V6) exhibited relatively lower metal levels. Highly significant positive correlations (≥90% at p ≤ 0.01) were found between Zn-Cu and Zn-Fe (in bulbs) and Cu-Co (in leaves), indicating their common origin. Principle component analysis indicated substantial anthropogenic contamination of the HMs in onion. In addition, hierarchal clustering revealed associations of different varieties based on similarities in the metal levels. The calculated values of health risk index (HRI), target hazard quotient (THQ), hazard index (HI), and target cancer risk (TCR) in the bulbs and leaves of all varieties were within the safe limit for both adults and children. Therefore, human consumption of these onion varieties is considered safe with respect to the HMs. However, currently used remediation approaches should focus on the reduction of health risks associated with HMs contamination in the food crops.
Keywords
Heavy metal
Health risk
Bulb
Leaves
Onion
Pakistan
1 Introduction
The diversity of environmental pollutants has amplified significantly due to the industrial revolution and economic globalization along with numerous anthropogenic sources (Toth et al., 2016). The food safety and security are among the topmost global concerns for sustainable development (Rai et al., 2019). Specifically, in recent decade antagonistic effects of unexpected pollutants have threatened both food security and human health (Shaheen et al., 2016; Selahvarzi and Ardakani, 2020). Various types of heavy metals (HMs) and metalloids can adversely affect the human metabolomics and may contribute significantly to the global morbidity and mortality (Rai et al., 2019). Precisely, some metals viz. As, Cd, Hg and Pb are lethal in various respects (Gall et al., 2015), and are listed in the top 20 dangerous substances by the “United States Environmental Protection Agency (USEPA)” and the “Agency for Toxic Substances and Disease Registry (ATSDR)” (Rai, 2018). Though, Cr (III), Cu, Fe, and Zn are indissolubly related to the metabolic functioning in living organisms, but they may cause harmful effects above threshold levels (Marschner, 2012). In most of the developing countries, use of sewage sludge, and industrial wastes as fertilizers and irrigation with inadequately treated wastewater are contributing expressively to the increasing levels of HMs in the food crops (Gebeyehu and Bayissa, 2020; Selahvarzi and Ardakani, 2020; Sobhanardakani et al., 2016).
Soil is essential for the nourishment to all food crops, and the soil–vegetable system is a typical instance of abiotic-biotic connections in the environment (Rai et al., 2019). In recent decades, soil has been significantly contaminated by HMs emanating both from point and non-point sources thereby adversely affecting the soil biota (Gall et al., 2015; Rai, 2018), beneficial soil insects, invertebrates, and small and large mammals (Gall et al., 2015; Bartrons and Peñuelas, 2017). Different types of HMs, such as, Al, As, Cd, Cr, Hg, and Pb are readily absorbed by the plant species via root system and significantly accumulated and magnified in their edible parts (Heidarieh et al., 2013; Taghizadeh, et al., 2017). Different plant species consumed as vegetables, vary extensively in their ability to absorb and accumulate HMs (Zhu et al., 2007), and such disparities even exists within varieties and cultivars of the same species (Säumel et al., 2012).
The HMs associated health risks to humans depends on the consumption rate of the contaminated vegetables and body weight of the consumer (Bortey-Sam et al., 2015; Huang et al., 2007). Moreover, toxicity of the HMs varies, such as, As, Cd, Cr, Pb are lethal even in at minor quantities in the human body (Hu et al., 2013; Rahman et al., 2014). However, Co, Cu, Fe, Mn, and Zn are required for regular body functions, development, and growth, but beyond permissible levels, they may cause deleterious effects (Oliveira et al., 2014). It is well established that Cd and Cr above the threshold levels (0.0004 and 150 mg/kg/day, respectively) cause chronic effects on the reproductive, nervous/immune systems, severe gastro-intestinal, endocrine and prostate disorders, cause osteoporosis, cardiac problem and various types of cancers (Barone et al., 2018; Dghaim et al., 2015; Liu et al., 2013). Likewise, elevated levels of Co results in heart problems, vomiting, and nausea, overproduction of red blood cells, and interrupt thyroid functioning (Parveen and Rafique, 2018). The neurotoxic effect of Pb leads to various adverse effects in almost every system or organ of the human body (Qin and Chen, 2010). Excessive exposure to Pb, damages reproductive system and kidneys and reduces intellectual performance and cognitive development in children (Rehman et al., 2017). Though, Mn acts as a co-factor in various enzymatic activities viz. pyruvate carboxylase (gluconeogenesis), astrocyte and glutamine (in the brain), and arginase (in the liver), but causes eternal neurodegenerative impairment if exceeds acceptable limit (Bocca et al., 2011). Similarly, adequate amount of Zn is necessarily required in the production of ˃300 different enzymes, but excessive concentration of this meal results in sideroblastic anemia, while its inadequacy causes anorexia, growth retardation, immune dysfunction, delayed sexual maturation, and mental retardation (Muhammad et al., 2011).
Vegetables are essential to human in order to meet the basic nutritional requirements and for healthy life. As a source of various nutrients, minerals, vitamins, dietary fibers, and secondary metabolites, vegetables hold promising bioactive properties (Siegel et al., 2014). Onion (Allium cepa L.) is among the most important and frequently consumed vegetable crops in the world (Mollavali et al., 2016). According to according to the statistics of Food and Agriculture Organization (FAO), the estimated production of onion in the world was around 100 million tons in 2019. Asia contributes the highest share (64%) in the global onion production, followed by America, America, Europe Africa, and Oceania at 12.7, 12.7, 11, and 0.4%, respectively (FAO STAT, 2019). In Pakistan, onion (Piyaz/Urdu) is cultivated in all parts of the country and according to the “Pakistan Economic Survey, 2019–2020” the total area of onion cultivation was 146.1 thousand hectares with estimated 2058.2 thousand tons of production in 2019–2020 (Ahmad, 2020). Various vegetables, including onion, are exposed to HMs contamination due to natural processes such as, volcanic outbreaks and discharges from soil and rocks etc. (Ali et al., 2021; Tchounwou et al., 2012), and diverse anthropogenic actions viz. extensive use of agrochemicals (Tong et al., 2014), dismantling of electrical and electronic waste (Zhang et al., 2019), mining and smelting (Zhou et al., 2018), sewage sludge, industrial wastes and use of contaminated irrigation water (Gebeyehu and Bayissa, 2020; Selahvarzi and Ardakani, 2020; Sobhanardakani et al., 2016). Both natural and anthropogenic activities can elevate HMs’ concentration in soil, water, air and food, and their accessibility is a crucial emblem of potential risks to the human health and environment (Barthwal et al., 2008).
Daily consumption of the vegetables and their role in human health, demands their proper monitoring and quality assessment, specifically with respect to the contamination of toxic pollutants and their adverse effects on consumers. Health risk assessment is a systematic investigation that elucidates the damage related with acquaintance to chemical substances. Appraisal of human health risk provides information on the associations between dosages, exposure, and health effects of various organic and inorganic toxins (Sobhanardakani et al., 2016; WHO, 2010). However, authentic information on the concentration of various toxins and their contrary effects is inadequate so far, particularly in the developing countries (Ferré-Huguet et al., 2008). For instance, in Pakistan few studies have been conducted on HMs contamination and their health risk assessment in different types of vegetables (Ashraf et al., 2021; Iqbal et al., 2020; Rehman et al., 2018; Mahmood and Malik, 2014; Jan et al., 2010; Khan et al., 2010; Jamali et al., 2009; Akbar et al., 2009). But to best of our knowledge, very little is known about the concentration and possible health effects of various HMs in onion bulbs and leaves, which are frequently consumed by the major populace of Pakistan. Therefore, present study was intended with the aim to (i) quantify the concentrations of HMs in the bulbs and leaves of onion varieties cultivated in different areas of Lahore and (ii) to estimate non-carcinogenic and carcinogenic risk assessment in the adults and children. The findings of this study will provide a consistent foundation for the quality evaluation and safety management of the vegetables, precisely the onion crop and create realization in consumers about toxic effects of the HMs.
2 Materials and methods
2.1 Sampling and sample preparation
Lahore, the capital city of Punjab province of Pakistan is located at 74° 21′ 31.49 E and 31° 31′ 13.33 N and occupies about 1772 km2 area. It is the second-largest city in the Pakistan and is the hub of various industries (>1000), including tanneries, pharmaceuticals, textile, and steel industries, etc. that are the main sources of various organic and inorganic toxic substances in the surrounding environment of the city (Mahmood and Malik, 2014).
A total of nine different varieties of onion as mentioned in Table 1, were cultivated in farmer fields at Pattoki Lahore during 2017–18. The experiment was conducted following Randomized Complete Block Design (RCBD) with three replications, where 12 cm plant to plant and 15 cm row to row distance was maintained. All the agronomic and cultural practices were adopted as a standard to raise the crop. Fresh leaves and bulbs were harvested randomly (at full maturity stage) from each plot in clean paper bags and labeled accordingly. Leaves of all varieties were collected in the month of April/May, while bulbs were harvested in late May and June. Composite samples (bulbs and leaves) of all varieties were transferred to the Analytical Chemistry laboratory at Quaid-i-Azam University Islamabad, Pakistan on the very next day. All samples were properly washed with tap water, followed by a rinse with distilled water. The sliced samples were kept in an electric oven at 70–80 °C for 48 h for drying (Arora et al., 2008; Yang et al., 2011). Oven-dried samples were crushed ground to a fine powder using ceramic mortar and pestle. Pulverized samples were stored in pre-cleaned and accurately labeled bottles and stored in desiccators at room temperature till further analysis. Different letters from a-g indicating significant difference in HMs’ concentration at p ≤ 0.05
Varieties
Code
Cd
Co
Cr
Cu
Fe
Li
Mn
Pb
Zn
Mustang
V1-B
0.028 ± 0.001c
0.184 ± 0.006c
0.181 ± 0.012c
0.290 ± 0.021de
4.483 ± 0.055e
bdl
1.138 ± 0.025b
0.088 ± 0.010d
1.060 ± 0.066 g
Red Orb
V2-B
0.046 ± 0.001b
0.576 ± 0.015a
0.066 ± 0.001e
0.297 ± 0.022def
3.573 ± 0.090f
0.017 ± 0.001d
1.420 ± 0.044a
0.576 ± 0.016a
1.426 ± 0.028de
Super Sarhad
V3-B
0.063 ± 0.001a
0.050 ± 0.001e
0.226 ± 0.005a
0.445 ± 0.017b
5.288 ± 0.144c
0.013 ± 0.001b
1.535 ± 0.028a
0.349 ± 0.018b
1.700 ± 0.052c
Red Flame
V4-B
0.017 ± 0.002e
0.195 ± 0.019c
0.200 ± 0.008b
0.568 ± 0.020a
7.537 ± 0.137a
0.004 ± 0.000c
1.476 ± 0.077a
0.087 ± 0.003d
2.616 ± 0.065a
Pulkara
V5-B
bdl
0.133 ± 0.006d
0.037 ± 0.001f
0.263 ± 0.008e
2.558 ± 0.039 g
0.002 ± 0.000d
0.638 ± 0.015e
0.055 ± 0.010d
0.892 ± 0.054 g
Golden Orb
V6-B
0.021 ± 0.001d
0.008 ± 0.001e
0.003 ± 0.000 g
0.365 ± 0.008c
3.383 ± 0.088f
0.004 ± 0.000d
0.977 ± 0.031c
bdl
1.239 ± 0.026f
White Pearl
V7-B
0.066 ± 0.001a
0.028 ± 0.003e
0.109 ± 0.002d
0.342 ± 0.022 cd
4.924 ± 0.115d
0.015 ± 0.001c
0.900 ± 0.035c
bdl
1.518 ± 0.080d
Amazon
V8-B
0.063 ± 0.002a
0.344 ± 0.039b
0.107 ± 0.004d
0.482 ± 0.008b
6.196 ± 0.107b
0.011 ± 0.001d
1.124 ± 0.058b
0.071 ± 0.001d
2.118 ± 0.074b
Zeus
V9-B
bdl
0.047 ± 0.001e
bdl
0.338 ± 0.019cde
3.341 ± 0.130f
0.020 ± 0.001a
0.763 ± 0.024d
0.232 ± 0.029c
1.282 ± 0.071ef
Max.
0.066
0.576
0.226
0.568
7.537
0.020
1.535
0.576
2.616
Min.
0.017
0.008
0.003
0.263
2.558
0.002
0.638
0.055
0.892
Mean
0.043
0.174
0.116
0.377
4.587
0.011
1.108
0.208
1.539
SE
0.016
0.058
0.041
0.126
1.529
0.004
0.369
0.085
0.513
Varieties
Code
Cd
Co
Cr
Cu
Fe
Li
Mn
Pb
Zn
Mustang
V1-L
0.016 ± 0.006c
bdl
0.573 ± 0.110f
1.588 ± 0.328c
112.6 ± 0.997a
0.346 ± 0.086e
10.83 ± 1.027bc
0.133 ± 0.008e
6.182 ± 0.614b
Red Orb
V2-L
0.017 ± 0.004c
2.913 ± 0.146a
2.453 ± 0.123d
13.90 ± 0.594a
28.51 ± 1.893d
0.897 ± 0.110a
12.47 ± 0.566b
0.564 ± 0.171c
6.262 ± 0.620b
Super Sarhad
V3-L
0.038 ± 0.008b
1.367 ± 0.108b
1.470 ± 0.094e
10.95 ± 0.552b
22.38 ± 0.952e
0.783 ± 0.073b
9.693 ± 0.542c
1.385 ± 0.184b
7.485 ± 0.371a
Red Flame
V4-L
bdl
0.410 ± 0.034c
3.952 ± 0.077b
2.135 ± 0.111c
17.53 ± 0.897f
0.831 ± 0.055b
10.75 ± 0.836bc
bdl
4.056 ± 0.327 cd
Pulkara
V5-L
0.175 ± 0.008a
bdl
3.086 ± 0.150c
2.324 ± 0.174c
21.18 ± 0.440e
0.606 ± 0.006bcd
11.98 ± 0.322b
2.217 ± 0.109a
4.517 ± 0.314c
Golden Orb
V6-L
bdl
0.411 ± 0.134d
0.394 ± 0.118f
1.443 ± 0.138c
12.73 ± 0.594 g
0.653 ± 0.075bc
7.211 ± 0.899d
0.458 ± 0.180 cd
3.956 ± 0.398 cd
White Pearl
V7-L
bdl
0.212 ± 0.008d
5.233 ± 0.086a
2.321 ± 0.261c
42.28 ± 0.537b
0.820 ± 0.049b
17.59 ± 0.405a
bdl
3.261 ± 0.247d
Amazon
V8-L
0.016 ± 0.007c
bdl
1.752 ± 0.163e
1.708 ± 0.231c
35.46 ± 1.375c
0.493 ± 0.209 cd
16.85 ± 0.697a
2.504 ± 0.141a
4.659 ± 0.341c
Zeus
V9-L
bdl
0.713 ± 0.034d
3.432 ± 0.162c
1.917 ± 0.126c
39.58 ± 0.545b
0.566 ± 0.038bcd
11.87 ± 0.380b
1.026 ± 0.157b
3.875 ± 0.384 cd
Max.
0.175
2.913
5.233
13.90
112.6
0.897
17.59
2.504
7.485
Min.
0.016
0.212
0.394
1.443
12.73
0.346
7.211
0.133
3.261
Mean
0.052
1.004
2.483
4.254
36.91
0.666
12.14
1.184
4.917
SE
0.023
0.410
0.828
1.418
12.30
0.222
4.046
0.447
1.639
2.2 Acid digestion
Onion samples were digested following the methods as reported earlier (Parveen et al., 2020) with some modifications. In short, triplicates of the bulb and leaves samples of each variety (∼1.0 g) were added in labeled conical flasks (50 mL), and 10 mL nitric acid (HNO3, Sigma 68.0–70.0% purity) was mixed. This mixture was kept overnight at room temperature and subsequently was heated on a hot plate until the appearance of brown fumes, then kept at room temperature to cool. Afterward, 15 mL perchloric acid (HCLO4, Sigma 70.0–72.0% purity), was added. This mixture was heated again until a clear solution was obtained. Then final volume (50 mL) was constituted with distilled water. A blank was prepared along with each batch of five samples while following the same procedure.
2.3 Instrumentation
Selected heavy metals viz. Cd, Co, Cr, Cu, Fe, Li, Mn, Pb, and Zn were quantified in the bulbs and leaves samples of nine onion varieties using atomic absorption spectrophotometer “AA-670 Shimadzu, Japan” under optimal analytical conditions as mentioned in Table S1, employing the calibration line method. Working standards of each metal were prepared from the standard stock solutions (1000 mg/L) just before the analysis. Triplicate measurements were performed for all the samples in this study. The precision of the quantitative results was assured using the internal standards/replicate analysis as well as quantification of these metals in certified/standard reference materials (NIST SRM 1515) which showed very good recoveries (98 to 100%) as shown in Table S1. Doubly distilled water was used for the preparation of the standards and reagent throughout this work. Blanks were routinely prepared with each batch of the samples and generally the blank contribution was < 5% of the measured metal levels. Few samples were also analyzed at an independent laboratory and the two results showed a maximum of ±2% difference.
2.4 Statistical analysis
Univariate and multivariate data analysis approaches were implemented to analyze the computed levels of HMs in the onion samples using STATISTICA (StatSoft Inc, 1999) and SPSS-V21.0 (IBM, Chicago USA). Ward’s and Varimax rotation methods were used in the cluster and principal components analyses, respectively. Sigma Plot-V12.5 and Bio-Vinci-1.1.5 software were used for the graphical presentation of analyzed data.
2.5 Health risk assessment
We adopted the United States Environmental Protection Authority (USEPA, 2005) model to evaluate HMs' associated health risk to consumers via ingestion of the onion bulbs and leaves.
Health Risk Index (HRI): The HRI was computed to estimate the health risk to consumers associated with intake of HMs via consumption of food i.e. vegetables and fruits using the reported relationship (Abbasi et al., 2013; Li et al., 2012). It is well established that HRI value < 1 reflects safe levels while > 1 indicates toxic effects.
Target Hazard Quotient (THQ): Non-carcinogenic risks to the consumers through ingestion of onion was calculated by THQ, and “hazard index” (HI) as explained earlier (Yang et al., 2011).
Hazard Index (HI): The HI was calculated as a total of all-hazard quotients for each HMs quantified in onion bulb and leaf samples and was intended as described by USEPA (2006) using the formula:
Target cancer risk (TCR): TCR was computed for Cd, Cr, and Pb that can most likely develop cancer in the consumers if ingesting food contains their high concentrations. TCR was calculated as reported formerly (USEPA, 2005), using the equation:
3 Results and discussion
3.1 Levels of HMs in the bulbs and leaves of onion varieties
Both bulbs and leaves were selected based on their utilization as food and for medicinal purposes. The onion bulb is eaten raw, and is also an important ingredient of various cooked foods. Moreover, the onion bulb is used in the treatment of many health disorders, precisely food poisoning, cholera, indigestion and to cure dermal infections because of its antimicrobial properties, which are mainly due to the presence of organo-sulphur compounds. Likewise, onion leaves are used in curries and soups and are a rich source of vitamins and health-beneficial secondary metabolites (Abbasi et al., 2012). A total of nine HMs were quantified in the bulbs and leaves (n = 54) of nine different varieties of onion cultivated in Lahore, Pakistan. In the bulbs of all varieties, ascending of HMs was: Fe > Zn > Mn > Co > Pb > Cu > Cr > Cd > Li as shown in Table 1. Cd is a toxic metal that is ingested via contaminated food and causes potential threats to human and animal health (USEPA, 2015). Mainly, Cd damages to the renal tubules in urinary system and disrupt the reabsorption of nutrients (Rubio et al., 2018). In our samples, Cd concentration was highest in the bulb of V7-B (0.066 ± 0.001 mg/kg), followed by V3-B and V8-B (0.063 ± 0.001 and 0.063 ± 0.002 mg/kg, respectively) on a fresh weight basis. But, these values were not significantly different (p ≤ 0.05). Moreover, in the bulbs of V5-B and V9-B varieties, Cd levels were below the detection limits. Average concentration of Cd in the bulbs of onion varieties cultivated in Lahore were analogous to those reported in different cultivars of onion from the Slovak Republic (Bystrická et al., 2015), Ghana (Ametepey et al., 2018) and North West of Nigeria (Yaradua et al., 2020), but were relatively less than reported from Bangladesh (Islam et al., 2017) and more than described from São Paulo Brazil (Guerra et al., 2010).
Measured levels of Co ranged from 0.008 ± 0.001 to 0.567 ± 0.045 mg/kg in the bulbs of all varieties (Table 1). The highest concentration of Co was estimated in V2-B, followed by V8-B and V4-B, while V6 had the lowest level. In our samples, measured levels of Co were higher than reported earlier (Din et al., 2013; Guerra et al., 2010). This revealed that long time exposure to Co via consumption of onion may cause cardiac, respiratory and gastric problems in consumers of the area (Parveen et al., 2020). In human, Cr3+ involves in the synthesis of nucleic acids and immune system functioning (Manore et al., 2009), however it has been reported that Cr6+ causes cancer, cardiac arrest and diabetes (Kabata-Pendias & Pendias, 2001). Comparatively, Cr levels were highest in the bulbs of V3-B, followed by V1-B and V7-B (0.0226 ± 0.005, 0.181 ± 0.012, 0.109 ± 0.002 mg/kg, respectively), while V6-B had the lowest level of Cr (0.003 ± 0.00 mg/kg). The bulb of V4 variety had elevated level of Cu (0.568 ± 0.020 mg/kg), followed by V8-B and V3-B, whereas V%-B had the lowest level (Table 1). Moderately, average concentrations of Cr and Cu were comparable to reported previously by Rehman et al. (2018). Concentrations of the same metals were higher than reported from Bangladesh and Brazil (Islam et al., 2017; Guerra et al., 2010), whereas Cr content was less than reported from Ghana (Ametepey et al., 2018). In addition, intake of excessive Cu through onion consumption may leads to respiratory and skin problems in consumers (Khan et al., 2010).
The highest concentration of Fe was quantified for the bulb of V4-B (7.537 ± 0.137 mg/kg), followed by V8-B (6.196 ± 0.107 mg/kg) and V3-B (3.573 ± 0.090 mg/kg). These values were expressively different at p ≤ 0.05 and were relatively higher than reported in onion bulbs from Ghana (Ametepey et al., 2018) and North West of Nigeria (Yaradua et al., 2020). Li concentration ranged from 0.002 ± 0.000 mg/kg to 0.020 ± 0.001 mg/kg with the highest level for V9-B, while lowest for V5-B. Measured levels of Mn in the bulbs of all varieties were in the following order: V3-B > V4-B > V2-B > V1-B > V8-B > V6-B > V7-B > V9-B > V9-B (Table 1). Fe, Mn and Zn are required for regular body functions, development, and growth, but when accumulate beyond the permissible levels cause deleterious effects on human health (Yousaf et al., 2016; Oliveira et al., 2014). Mean concentration of these metals in the bulbs of all varieties of onion were analogous to formerly reported levels by Ali and Al-Qahtani (2012) from Saudi Arabia but were higher than reported from Ghana (Ametepey et al., 2018).
It was noted that Pb concentration was maximum in V2-B (0.576 ± 0.016 mg/kg), followed by V3-B and V9-B (0.349 ± 0.018 and 0.232 ± 0.029 mg/kg, respectively), while the lowest concentration was calculated for V5-B (0.055 ± 0.010 mg/kg). These values were analogous to the reported levels from Bangladesh and Brazil (Islam et al., 2017; Guerra et al., 2010), however were lower than reported in the onion samples from the supermarkets in La Rochelle, France (Cherfi et al.,2014), Slovak Republic (Bystricka et al., 2016) and North West of Nigeria (Yaradua et al., 2020). Comparatively, Zn level was highest in the bulb of V4-B variety at 2.616 ± 0.065 mg/kg, followed by V8-B and V3-B, while the lowest concentration was calculated for the bulb of V5-B (0.892 ± 0.054 mg/kg). The average concentration of Zn in the bulbs of onion varieties was similar to reported previously in from Saudi Arabia (Ali & Al-Qahtani, 2012), but was comparatively higher than reported in onion from North West of Nigeria (Yaradua et al., 2020).
Measured levels of HMs in the leaves of all varieties are mentioned in Table 1. Comparatively, estimated level of Cd was highest in the leaves of V5-L (0.175 ± 0.008 mg/kg), followed by V3-L and V2-L (0.038 ± 0.008 and 0.017 ± 0.004 mg/kg, respectively), and these values were significantly different (p ≤ 0.05). However, same metal was below the detection limit in the leaves of V4, V6, and V7 varieties. Co concentration ranged from 2.913 ± 0.146 mg/kg to 0.017 ± 0.004 mg/kg, with a maximum level in V2-L, while minimum in V9-L. In the leaves of V1- V5 and V8 varieties, Co was below the detection limit. Cr is an essential micronutrient in living organisms, particularly in human beings for deoxyribonucleic acid transcription and insulin production. However, decreases the cellular response to insulin if Cr intake is < 0.02 mg/day (Kohlmeier, 2003). Elevated levels of Cr were estimated for V7-L, V4-L, and V9-L at 5.232 ± 0.086, 3.952 ± 0.077 and 3.432 ± 0.102 mg/kg, respectively (p < 0.05), whereas V6-L had the lowest concentration (0.392 ± 0.118 mg/kg). Comparatively, the mean concentration of Cr in the leaves of onion varieties was higher than reported from Mardan, Pakistan (Amin et al., 2013). Additionally, these values were above the acceptable limit of this metal in vegetables (FAO/WHO, 2001).
The estimated level of Cu was highest in the leaves of the V2-L variety (13.90 ± 0.594 mg/kg followed by V3-L and V5-L (10.95 ± 0.552 and 2.324 ± 0.174 mg/kg, respectively), which were considerably different at p < 0.05 from each other. However, these values were relatively higher than reported formally (Bedassa et al., 2017). In the leaves of all varieties, measured levels of Fe were in the subsequent order: V1-L > V7-L > V9-L > V8-L > V2-L > V3-L > V5-L > V4-L > V6-L (p ≤ 0.05). Fe values were ranged between 112.6 ± 0.992 mg/kg to 12.73 ± 0.594 mg/kg on fresh weight basis. V2-L, V4-L and V7-L varieties depicted considerable levels of Li (0.897 ± 0.110 mg/kg, 0.831 ± 0.055 mg/kg and 0.820 ± 0.049 mg/kg, respectively, whereas Mn was highest in V7-L (17.59 ± 0.405 mg/kg) and lowest inV6-L (7.211 ± 0.899 mg/kg). The highest concentration of Pb was quantified in the leaves of V8-L at 2.504 ± 0.141 mg/kg, followed by V5-L and V3-L at 2.217 ± 0.109 mg/kg and 1.385 ± 0.184 mg/kg, respectively. Measured levels of Zn varied from 7.485 ± 0.371 mg/kg to 3.261 ± 0.247 mg/kg in the leaves of V3-L and V7-L, in respective order (Table 1). It was noted that mean concentrations of Fe, Mn, and Zn in the leaves of onion varieties were less than reported previously by Bedassa et al. (2017).
3.2 Comparative assessment of HMs’ concentrations in bulbs and leaves
Overall, measured levels of HMs were high in the leaves samples than bulbs as illustrated in Fig. 1, and this trend was analogous to reported previously (Demirezen & Aksoy, 2006). Higher levels of HMs in leaves may be due to the fact that, aerial plant parts specifically leaves have more exposure routes than bulbs. Such as HMs may pass to leaves through stomata along with contaminated air in addition via transportation through xylem vessels from contaminated soil and water. Comparative assessment reveals that in the bulbs of all varieties, Fe was dominating with highest concentration, followed by Zn, Mn, and Cr. However, Co, Cu, and Pb were present in reasonable quantity, while Li and Cd were least in concentration. Though, Fe involves in various enzymatic activities and is an essential component of red blood cells, but its high concentration in onion may affects adversely on metabolic activities in human body and also leads to gastrointestinal and cardiac (Hashemi et al., 2017). Likewise, Zn in excessive amount causes anemia, obesity and diarrhea, harms reproductive, blood circulatory and immune systems (Dghaim et al., 2015; Singh et al., 2010). Mixed trends were noted with respected to measured levels of HMs in the bulbs of all varieties. Such as V4-B (Red Flame) depicted elevated levels of Cu, Fe, and Zn; V3-B (Super Sarhad) had the highest concentration of Cr and Mn; V2-B (Red Orb) contains maximum concentration of Co and Pb, while V7-B (White Pearl) and V9-B (Zeus) had the highest concentration of Cd and Li, respectively. Similarly, lowest concentrations of Cd, Cu, Fe, Li, Mn, Pb, and Zn metals were in the bulbs of V5-B (Pulkara) and that of Co and Cr concentrations were in the bulb of V6-B (Golden Orb). Based on these findings, ingestion of Pulkara and Golden Orb could be safer for the consumers.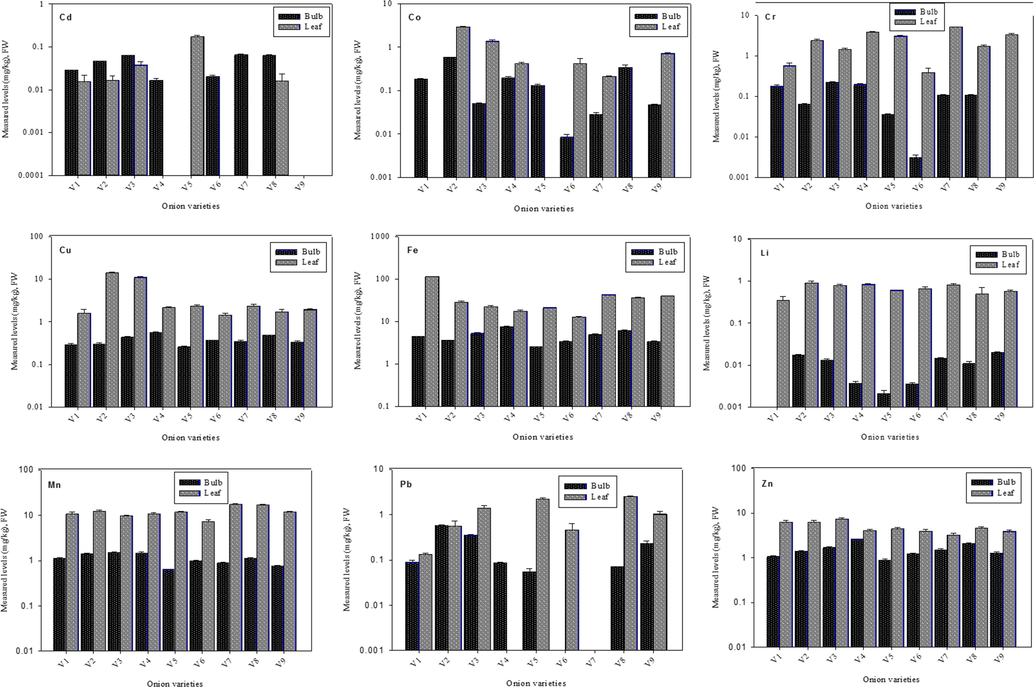
Average concentrations of heavy metals (mg/kg-FW) in the bulbs and leaves of onion varieties.
Conversely, in leaves samples, Red Orb (V2-L) contains maximum concentration of Co, Cu, and Li and White Pearl (V7-L) had elevated levels of Cr and Mn. However, leaves of Golden Orb (V6-L) contain minimum concentrations of Cd, Cr, Cu, Fe, and Mn, while that of White Pearl (V7-L) had lowest levels of Pb and Zn. In general, leaves contain elevated levels of Fe, Mn, Cu, Cr, and Zn along with a considerable amount of Co and Pb. Among all HMs, Fe was dominating with highest in concentration and was even more in leaves than bulbs. Fe is required in chlorophyll synthesis, therefore green leaves are considered as the main sink of iron (Ravet et al., 2009). Likewise, Cd and Li were least in concentration in the leaves of all varieties. Comparatively, Pb concentration was higher than the recommended levels. Variation in the concentration in HMs in bulbs and leaves of all varieties, may be attributed to the accessibility, uptake, and accumulation of these metals (Ahmed & Al-Swaidan, 1993). Furthermore, extensive use of pesticides results in more accumulation of Cu and Zn in the topsoil of agricultural lands that are taken up by the vegetables (Yang et al., 2011; Huang et al., 2010).
Comparative assessment exposes that onion varieties cultivated under similar growing conditions within the same area exhibiting significant variation in the concentration of different HMs that may mainly be attributed to the genetic factors. Because different vegetables possess extensively a wide disparity in their ability to uptake and accumulate the HMs and this difference even exists within varieties and cultivars of the same species (Säumel et al., 2012; Zhu et al., 2007). In addition, the quality of irrigated water and soil type may also be the contributing factors (Taghizadeh et al., 2017). Such as, soil pH plays an important role in the acceptability and mobility of the HMs in plants. As the soil of the study area is more alkaline, therefore may affects significantly on the uptake of HMs in onion because more acidic soil (<pH) accelerates mobility and bioavailability of the HMs in plants (Walker et al.,2006).
3.3 Relationships of HMs in onion
The mutual associations and probable sources of HMs contamination in the bulbs and leaves of onion varieties were explored by adopting the Pearson’s correlation coefficient matrix approach (Table 2). In the bulb samples of all varieties, highly significant positive associations were between Cu-Zn (r = 0.951), followed by Fe-Zn (r = 0.931) and Cu-Fe (r = 0.899). These relationships were statistically significant at p ≤ 0.01. Likewise, Cr depicted strong positive correlations with Fe and Mn at r = 0.742 and r = 0.730, respectively (p ≤ 0.05). In the case of leaves samples, Cu exhibited considerably strong positive relations with Co (r = 0.929) and Zn (r = 0.728) at p ≤ 0.01 and p ≤ 0.05, respectively. Strong positive associations between Cu, Zn, Fe, Cr and Mn (bulbs) and Cu, Co, and Zn (leaves) revealed their homogeneity and mutual origin, while negative correlations of Li with Cr, Cu, and Fe (in bulbs), and that of Cd with majority of the metals in leaves depicted predominant heterogeneity in their distribution and sources of the contamination in onion samples (Abbasi et al., 2020; Ametepey et al., 2018). In bulbs and leaves of onion, the synchronicity of Cu, Zn, Fe, and Mn revealed their common agronomic sources, such as, extensive use of agrochemicals (superphosphate fertilizers, pesticides, etc.) and contaminated irrigation water, not only in the onion cultivated areas but also in the adjacent agriculture lands (Guan et al.,2018).
Bulb
Cd
Co
Cr
Cu
Fe
Li
Mn
Pb
Zn
Cd
1.000
Co
0.227
1.000
Cr
0.466
0.039
1.000
Cu
0.259
−0.019
0.527
1.000
Fe
0.425
0.099
0.742*
0.899**
1.000
Li
0.345
0.160
−0.229
−0.019
−0.080
1.000
Mn
0.484
0.420
0.730*
0.577
0.633
0.056
1.000
Pb
0.187
0.605
0.072
−0.134
−0.156
0.565
0.528
1.000
Zn
0.336
0.204
0.527
0.951**
0.931**
0.093
0.623
−0.014
1.000
Leaf
Cd
Co
Cr
Cu
Fe
Li
Mn
Pb
Zn
Cd
1.000
−0.173
0.034
−0.001
−0.167
−0.123
−0.041
0.594
0.106
Co
1.000
−0.038
0.929**
−0.259
0.620
−0.161
−0.153
0.525
Cr
1.000
−0.100
−0.255
0.504
0.581
−0.178
−0.551
Cu
1.000
−0.224
0.592
−0.118
0.001
0.728*
Fe
1.000
−0.662
0.132
−0.272
0.243
Li
1.000
0.022
−0.312
−0.003
Mn
1.000
0.225
−0.312
Pb
1.000
0.137
Zn
1.000
3.4 Principal component analysis
Though, correlation analysis is an appropriate method to make interpretations about the possible sources of HMs contamination in soil, water, and food plants but due to its simplicity, we also applied PCA for better understanding and validation of the possible sources of HMs in onion bulbs and leaves. Results of PCA for HMs in bulbs and leaves of onion varieties are mentioned in Table 3. In the bulb samples, PCA of HMs revealed three components. The first component ‘PC 1′ accounted for 46.50% of the total variance with > 70% loading values of Fe, Zn, Cu, Mn, and Cr (0.931, 0.902, 0.853, 0.849, and 0.788, respectively), while Cd contribution was > 50%. This component revealed predominant anthropogenic contamination of HMs and these metals were mostly contributed by the fertilizers, pesticides and contaminations in the irrigation water. Percentage variance for PC 2 was 24.32% and that of PC 3 was 11.27%, with maximum loading values of 0.918% and 0.673% for Pb and Co (PC 2) and 0.712% for Li (PC 3). Second PC was mostly contributed by the industrial emissions, transportation sources and fossil fuel combustion while the third PC was mostly derived from lithogenic sources. On comparative basis, the PCA findings were consistent with the results of Pearson’s correlation analysis.
Variables
Onion bulbs
Variables
Onion leaves
PC1
PC2
PC3
PC1
PC2
PC3
PC4
Eigen value
4.186
2.189
1.014
Eigen value
2.959
2.298
1.751
1.127
Total variance (%)
46.50
24.32
11.27
Total variance (%)
32.88
25.53
19.46
12.52
Cumulative variance (%)
46.50
70.82
82.10
Cumulative variance (%)
32.88
58.41
77.87
90.39
Cd
0.570
0.311
0.237
Cu
0.956
-0.103
0.101
0.253
Co
0.279
0.673
-0.376
Co
0.941
0.019
-0.063
0.145
Cr
0.788
-0.148
-0.324
Li
0.722
0.649
0.007
-0.129
Cu
0.853
-0.330
0.225
Cr
-0.077
0.902
0.063
0.236
Fe
0.931
-0.303
0.076
Zn
0.610
-0.686
0.062
0.283
Li
0.062
0.674
0.712
Pb
-0.150
-0.232
0.884
0.146
Mn
0.849
0.307
-0.292
Cd
-0.105
-0.154
0.825
-0.040
Pb
0.171
0.918
-0.139
Mn
-0.287
0.500
0.154
0.766
Zn
0.902
-0.160
0.210
Fe
-0.381
-0.504
-0.493
0.530
Similarly, in the leaves samples, PCA also confirms the results of Pearson’s correlation analysis and four main components were observed (Table 3). The percentage variance of PC 1 was 32.88%, followed by 25.53%, 19.46%, and 12.52% for PC 2, PC 3, and PC 4, respectively. In PC 1, Cu, Co, and Li depicted the highest loading values at 0.956, 0.941, and 0.722, correspondingly. This PC showed mixed contributions of natural and anthropogenic sources particularly the industrial emissions. PC 2 was > 90% loaded with Cr along with>60% contribution of Li, while in PC 3, Pb and Cd were dominant with loading values of 0.884 and 0.825, respectively. These two PCs were mostly contributed by anthropogenic sources including wastewater, fossil fuel combustion and transportation activities. In the case of PC 4, Mn and Fe exhibited higher (>50%) loading values (0.766 and 0.530, respectively); these metals were mostly lithogenic in origin.
These findings specify that various anthropogenic activities in the sampling area (Lahore) are contributing significantly towards HMs contamination in the food chain, more specifically in the onion and other vegetables. Among these, extensive use of various chemical manures, specifically the phosphate fertilizers and pesticides could the possible sources of HMs contamination of Fe, Zn, Cu, Mn, and Cr in the onion bulbs and leaves (Yang et al., 2011). In addition, waste materials released from various industries in the study area i.e. tanneries, textile, cutlery, and steel, etc. could also be the major contributors (USEPA, 2015). Likewise, the use of Cd, Cr, Co, Li and Pb containing minerals in paint, steel, textile, ceramics, electronics, printing, glass, electroplating, petroleum and chemical industries, and in automobiles could be the possible sources of contamination of these metals (Kosiorek and Wyszkowski, 2019; Fang et al.,2016) in the onion samples collected from Lahore.
3.5 Hierarchal clustering
The hierarchal clustering of HMs in the bulbs and leaves of onion varieties is illustrated in heat maps (Fig. 2). Color scheme in the heat maps designates the concentrations of HMs in the studied samples. Based on the measured levels of HMs in the bulb samples, nine varieties of onion were classified into two main clusters comprising of four sub-groups. While moving from left to right in ‘heat map, V5-B, V6-B, V7-B, and V9-B varieties were placed in the first cluster, wherein V5-B and V9-B were more closely associated and placed in the same group while V6-B and V7-B were in the second group based on a close relationship in term of HMs levels. Likewise, the second cluster comprises five varieties including V1-B, V2-B, V3-B, V4-B, and V8-B, which were further categorized into two sub-clusters as shown in Fig. 2. Interpretation of V4-B variety in red color squares exposed elevated levels of Cu, Fe, and Zn, and also the same color indicates a high concentration of Co in the bulb of V2 variety. Hierarchal cluster analysis of HMs in the leaves samples categorized onion varieties into three main clusters (Fig. 2). Comparatively, V4-L, V7-L, and V9-L varieties were placed within the same group, V1-L, V8-L, and V8-L were in the second group, and V6-L, V3-L, and V2-L varieties were found in group three. Classification of different varieties in the same group indicates their close associations on the basis of HMs in their leaves. Furthermore, red color boxes on the heat map demonstrate elevated levels of Fe, Cd, Zn, and Cu in the leaves of V1-L, V5-L, V3-L, and V2-L varieties, respectively. Discrepancies in the trends of onion varieties as shown in the aforementioned clusters revealed differences in HMs’ intake capacity from the soil. As all varieties were harvested from the same location, therefore, such differences may be due to genetic variations in these varieties but the difference in leaves and bulbs may also be due to variation in the harvesting time. The varieties showing close associations may have the same HMs absorption capacity or there might be similarities in the source of contamination and distribution pattern of HMs in such varieties as reported formerly (Abbasi et al., 2020).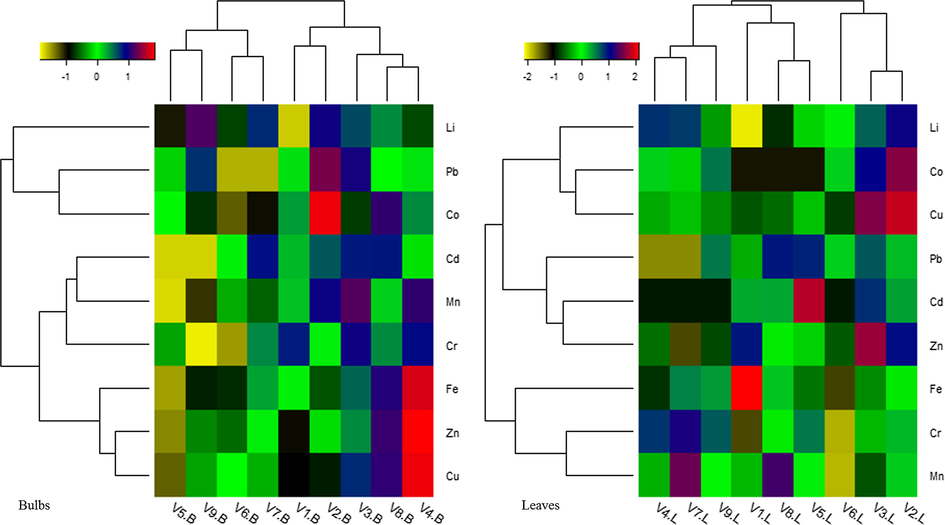
Hierarchal clusters of heavy metals (mg/kg-FW) distribution in the bulbs and leaves of onion varieties.
3.6 Health risk assessment
Ingestion of contaminated food, such as, vegetables is one of the most evidential routes of HMs exposure to humans (Ametepey et al., 2018; Muchuweti et al., 2006). Therefore, it is crucial to estimate the health risk to humans associated with HMs via consumption of vegetables. The majority of the populace in Pakistan ingest fresh vegetables, specifically, onion is consumed extensively as cook food and also eaten raw as salad. Health risk to the consumers was assessed on the basis of average concentrations of Cd, Co, Cr, Cu, Fe, Li, Mn, Pb, and Zn (on a fresh weight basis) in the bulbs and leaves of onion varieties cultivated in Lahore using different mathematical models viz. HRI, HI, THQ for non-carcinogenic and TCR for carcinogenic risk assessment.
Estimated values of HRI were computed in the bulbs and leaves of onion varieties using the formula as mentioned in eq. (1) and the results for adults and children are shown in Fig. 3. In the bulb samples, HRI was higher for children than adults. Both in adults and children HRI was in following order: Mn ˃ Pb ˃ Cd ˃ Cu ˃ Fe ˃ Zn ˃ Li ˃ Cr ˃ Co. Relatively, HRI for HMs was higher in the bulbs of V2, V3, V4 and V7 varieties. In the leaves, general trend of HRI was analogous to bulbs as measured levels were higher for children than adults. Increasing trend of HRI for HMs was: Mn ˃ Li ˃ Pb ˃ Cu ˃ Fe ˃ Cd ˃ Zn ˃ Cr ˃ Co. Particularly HRI of Mn in the leaves of V7 variety was 1.383 (in children), which is ≥ 1.0 (USEPA, 2006). However, on the whole, measured levels of HRI for adults and children were less than unity standard set by USEPA, (2006), hence consumption of the onion bulbs and leaves of all varieties can be considered safe for both adults and children.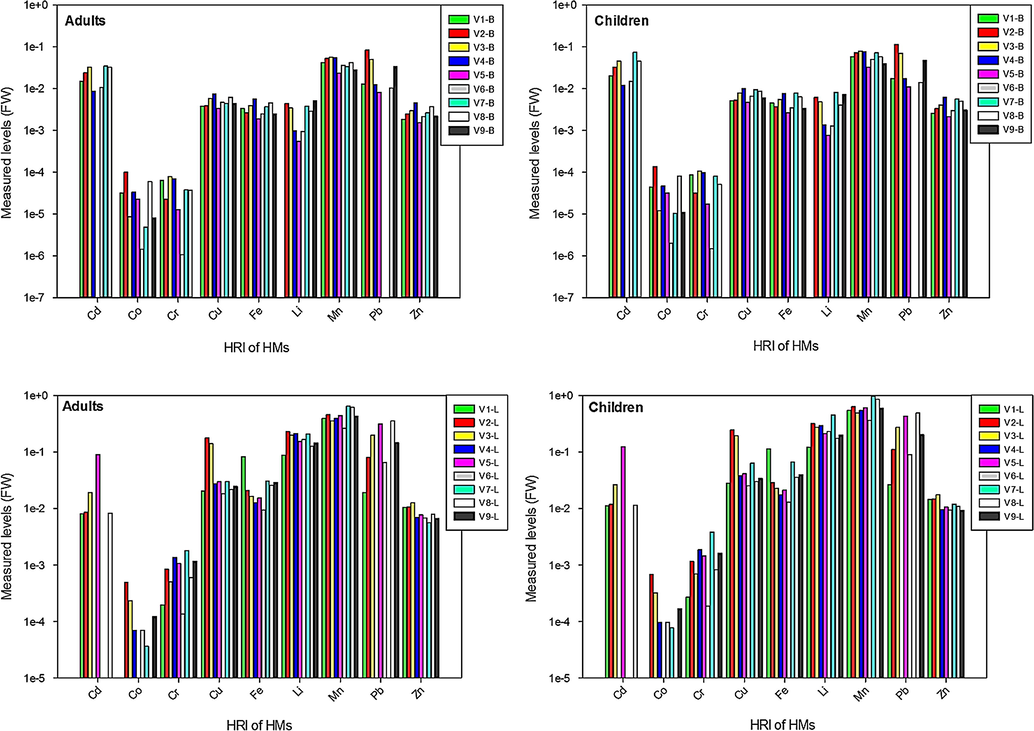
Appraisal of health risk index in the bulbs and leaves of onion varieties.
The THQ and HI have been proved as valid tools to stipulate the health hazard due to exposure to various toxins like HMs (Abbasi et al., 2020; Parveen et al., 2020; Khan et al., 2008; Wang et al., 2005), but could not provide a quantifiable approximation for the plausibility of facing an inverse health effect to the exposed populace, (Chary et al., 2008). Similar to Guerra et al. (2012), we estimated THQ and HI on the basis of exposure to HMs via ingestion of onion, while other routes viz. dermal contact, soil, and water were not considered. The THQ and HI values “≤ 1.0” considered safe that do not cause lifetime health risks to the consumers (USEPA, 2006). Measured levels of THQ and HI in the bulbs and leaves of all varieties are presented in Fig. 4. In general, THQ and HI trends were analogous to HRI; leaves again depicted higher values than the bulbs for all metals both in adults and children. In addition, estimated levels of THQ and HI were relatively higher in children compared to the adult consumers. In bulbs, Mn, Pb and Cd showed highest levels of THQ, whereas in leaves Mn, Li and Pb were dominating, however, Co and Cr showed lowest values in bulbs and leaves. Likewise, maximum THQ for HMs in the bulbs and leaves was calculated in V2, V3, V4 and V8 varieties both in adults and children. HI values demonstrates the collective risk due to the ingestion of various toxic metals in the foods (Gebeyehu and Bayissa, 2020). In the bulb samples, increasing order of HI in all varieties for children and adults was: V2-B > V8-B > V3-B > V9-B > V4-B > V7-B > V1-B > V6-B > V5-B, while in leaves it was as follow: V8-L > V9-L > V5-L > V2-L > V3-L > V7-L > V4-L > V1-L > V6-L. Variations in THQ and HI values in bulbs and leaves of different varieties may be attributed to the dissimilarity in the concentration of HMs’ in plant parts. Moreover, genetic variations may also be a potential factor affecting the uptake and accumulation of HMs in the bulbs and leaves of onion varieties and leads to fluctuations in health risk potential. In general, THQ and HI levels for all metals in various onion varieties were within the safe limit (<1) set by USEPA (2006). Moreover, THQ and HI for HMs in the bulb samples of all varieties were analogous to those reported previously from North West of Nigeria (Yaradua et al., 2020) and Tamale Metropolis, Ghana (Ametepey et al., 2018). However, to best of our knowledge THQ and HI in the leaves of onion were calculated for the first time.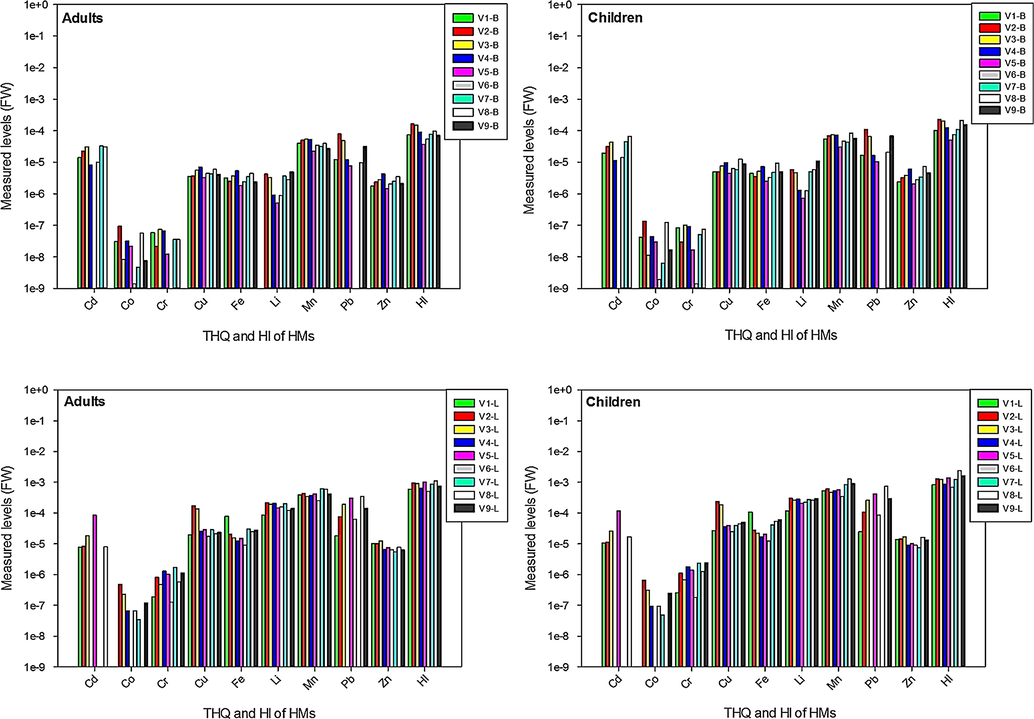
Comparison of target hazard quotient and health index in studied samples.
TCR was calculated using the values of daily intake and oral cancer slope factor (CSF) of the targeted metals to predict the possible cancer risk to the consumers. Measured values of TCR for Cd, Cr, and Pb as demonstrated in Fig. 5, were within the acceptable limit for these metals that is varied from 1✕10-4 to 1✕10-6 (USEPA, 2006). In leaves’ samples estimated levels of TCR for Cd, Cr and Pb were higher than bulbs. Likewise, children has more risk of HMs associated cancer risk compared to adults. TCR calculated for Cr was higher than other metals both in bulbs and leaves. Relatively, TCR for Cr was high in the bulbs of V3, V4, and V1 (in adults) and V1, V3 and V4 (in children), while in leaves V7, V4 and V9 depicted high levels of TCR in adults and children. Comparatively, TCR values for HMs in the bulbs and leaves of the onion varieties were less than reported from Nigeria and Ghana (Yaradua et al., 2020; Ametepey et al., 2018). Though, TCR for HMs in the bulbs and leaves of onion varieties was less than the threshold level set by USEPA (2006), but lifetime consumption the onion varieties, specifically V1, V3, V4 and V9 may be alarming for the consumers, particularly in children.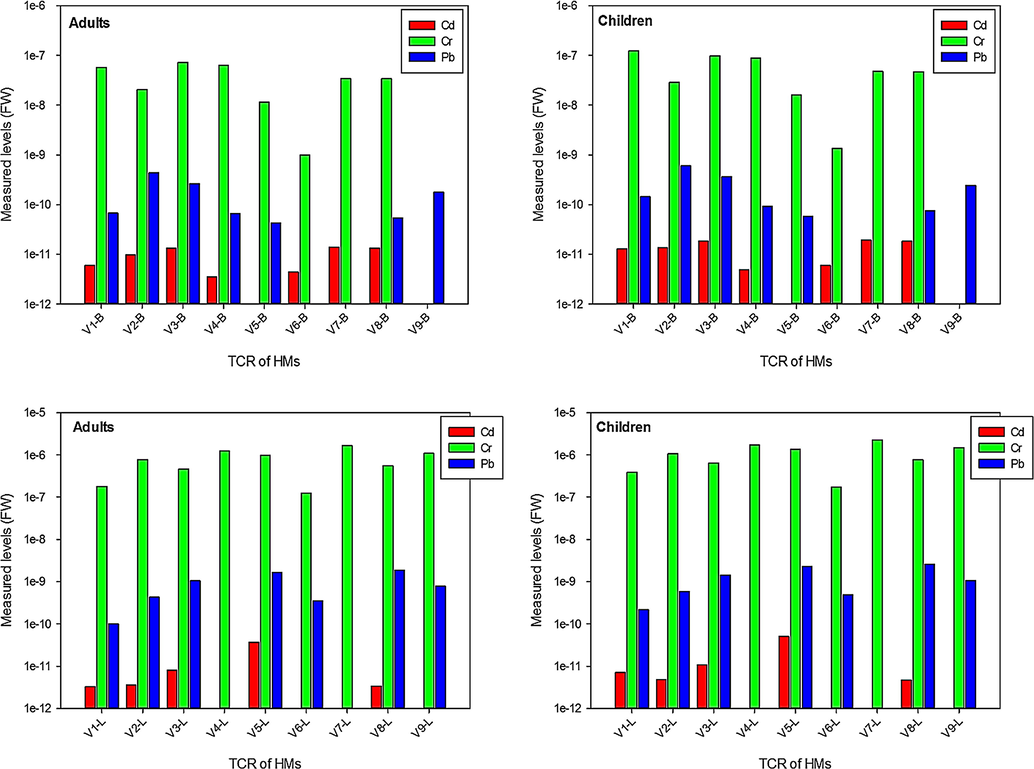
Carcinogenic risk assessment in the bulbs (VB) and leaves (VL) of onion varieties.
On the whole, HRI, THQ, HI and TCR values in bulbs and leaves of all varieties were within the safe limit set by USEPA. Hence, it could be assumed that consumption of the bulbs and leaves of these onion varieties has no potential health risk to the consumers. However, the consequence may vary with age factors in specific individuals that are more sensitive, having weak immunity, and live in a more polluted environment (Ametepey et al., 2018). Noteworthy, HRI, THQ, and HI values were computed on the basis of daily consumption of onion on a fresh weight basis in adults and children as reported earlier (Khan, 2015). In addition, we consider bulbs and leaves of the onion varieties only to evaluate the probable non-carcinogenic and carcinogenic health risks to the population of Lahore and its neighboring areas. Henceforth, these findings could not justify the total health risk to the population of the area associated with HMs via ingestion of onion bulbs and leaves because of different routes of exposure to HMs in humans. Moreover, intake of vegetables including onion as a whole is a proportion of various food consumed, specifically in the study area where meat, poultry, fish, rice, snacks, desserts, various types of fast foods, and street foods are extensively consumed that could also be the contributor of HMs accumulation in consumers.
4 Conclusion
Food safety/security and human health are strongly related with each other. Current study exposed significant disparities in the measured levels of HMs with respect to part(s) and varieties in onion. Relatively, leaves depicted elevated levels for most of the metals than bulbs that validate variation in the accessibility, uptake, and accumulation of HMs in different parts of the same species with diverse genetic makeup. Strong positive association and synchronicity between HMs revealed homogeneity in their source of contamination (particularly, extensive use of agrochemicals, industrial emissions, and contamination of irrigation water). Moreover, clustering of different varieties also revealed inconsistencies in HMs’ intake and accumulation capacity, and onion varieties within the same group might have comparable absorption capability and/or share common sources of contamination. Health risk assessment showed that the estimated values of health hazards indices viz. HRI, THQ, HI and TCR were<1, and perhaps consumption of bulbs and leaves of onion varieties exhibited no potential health risks to the adults and children. However, consequence may vary with consumer age, health condition, and habitat. Therefore, total health risk to the population of any area should be estimated via intake of various foods while taking into consideration all possible routes of HMs exposure. Furthermore, specific attentions are required to investigate the multi-metal toxicity, particularly in onion using appropriate epidemiological approaches and remediation options should be focused on the reduction of HMs in soil and food crops in order to prevent their health risks to the consumers.
CRediT authorship contribution statement
Nusrat Bibi: Formal analysis, Data curation, Investigation. Munir H. Shah: Conceptualization, Software, Resources, Validation, Project administration, Supervision. Nadeem Khan: Visualization, Project administration. Qaisar Mahmood: Visualization, Project administration. Ali Abdullah Aldosari: Funding acquisition, Visualization. Arshad Mehmood Abbasi: Conceptualization, Software, Resources, Validation, Project administration, Supervision.
Acknowledgments
The authors would like to extend their sincere appreciation to the Deanship of Scientific Re-search at King Saud University, through Research Group Project no. RGP-275. All authors are thankful to COMSATS University Islamabad, Abbottabad Campus, Pakistan, and Quaid-i-Azam University Islamabad, Pakistan for providing research facilities.
Funding
Deanship of Scientific Research at King Saud University, through Research Group Pro-ject no. RGP-275.
Data availability statement
All data is included in the manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Health risk assessment and multivariate apportionment of trace metals in wild leafy vegetables from Lesser Himalayas, Pakistan. Ecotoxicol. Environ. Saf.. 2013;92:237-244.
- [Google Scholar]
- Abbasi A.M., Khan M.A., Ahmad M., Zafar M., eds. Medicinal Plant Biodiversity of Lesser Himalayas-Pakistan. New York: Springer; 2012.
- Quantification of heavy metals and health risk assessment in processed fruits’ products. Arabian J. Chem.. 2020;13(12):8965-8978.
- [Google Scholar]
- Ahmad, I., 2020. Onion production in Pakistan. Pakistan Economic Survey, Finance division, Government of Pakistan. http://www.Finance.gov.pk/survey/chapter20/PES 2019-20.pdf.
- Lead and cadmium in urban dust of Riyadh, Saudi Arabia. Sci. Total Environ.. 1993;136(1–2):205-210.
- [Google Scholar]
- Multivariate statistical analysis of heavy metals pollution in industrial area and its comparison with relatively less polluted area: a case study from the City of Peshawar and District Dir Lower. J. Hazardous Mater.. 2009;176:609-616.
- [Google Scholar]
- Contamination and human health risk assessment of heavy metals in soil of a municipal solid waste dumpsite in Khamees-Mushait, Saudi Arabia. Toxin Rev.. 2019;1–14:466.
- [Google Scholar]
- Assessment of some heavy metals in vegetables, cereals and fruits in Saudi Arabian markets. Egypt. J. Aquatic Res.. 2012;38(1):31-37.
- [Google Scholar]
- Health risk assessment and heavy metal contamination levels in vegetables from Tamale Metropolis, Ghana. Int. J. Food Contaminat.. 2018;5(1):1-8.
- [Google Scholar]
- Accumulation of heavy metals in edible parts of vegetables irrigated with waste water and their daily intake to adults and children, District Mardan, Pakistan. Food Chem.. 2013;136(3–4):1515-1523.
- [Google Scholar]
- Heavy metal accumulation in vegetables irrigated with water from different sources. Food Chem.. 2008;111(4):811-815.
- [Google Scholar]
- Rice-spikelet-like copper oxide decorated with platinum stranded in the CNT network for electrochemical in vitro detection of serotonin. ACS Appl. Mater. Interfaces. 2021;13(5):6023-6033.
- [Google Scholar]
- Estimated dietary intake of trace metals from swordfish consumption: a human health problem. Toxics. 2018;6(2):22.
- [CrossRef] [Google Scholar]
- Heavy metal accumulation in medicinal plants collected from environmentally different sites. Biomed. Environ. Sci.. 2008;21(4):319-324.
- [Google Scholar]
- Pharmaceuticals and personal-care products in plants. Trends Plant Sci.. 2017;22(3):194-203.
- [Google Scholar]
- Assessment of selected heavy metals in onion bulb and onion leaf (Allium cepa L.), in selected areas of central rift valley of Oromia region Ethiopia. J. Horticulture. 2017;4:217.
- [Google Scholar]
- Assessment of reference ranges for blood Cu, Mn, Se and Zn in a selected Italian population. J. Trace Elem. Med Biol.. 2011;25(1):19-26.
- [Google Scholar]
- Human health risks from metals and metalloid via consumption of food animals near gold mines in Tarkwa, Ghana: Estimation of the daily intakes and target hazard quotients (THQs) Ecotoxicol. Environ. Saf.. 2015;111:160-167.
- [Google Scholar]
- The investigation of sensitivity of different types of onion to heavy metal intake from contaminated soil. Int. J. Environ. Res.. 2016;10(3):427-440.
- [Google Scholar]
- Intake of heavy metals in selected varieties of onion (Allium cepa l.) Grown in the different locations. Environ. Protect. Natl. Res.. 2015;26(65):17-21.
- [Google Scholar]
- Assessing risk of heavy metals from consuming food grown on sewage irrigated soils and food chain transfer. Ecotoxicol. Environ. Saf.. 2008;69:513-524.
- [Google Scholar]
- Food survey: levels and potential health risks of chromium, lead, zinc and copper content in fruits and vegetables consumed in Algeria. Food Chem. Toxicol.. 2014;70:48-53.
- [Google Scholar]
- Common hydrophytes as bioindicators of iron and manganese pollutions. Ecol. Ind.. 2006;6(2):388-393.
- [Google Scholar]
- Determination of heavy metals concentration in traditional herbs commonly consumed in the United Arab Emirates. J. Environ. Publ. Health. 2015;2015:1-6.
- [CrossRef] [Google Scholar]
- Assessment of heavy metals in onion and potato in imported and local variety of Pakistan and Afghanistan. Life Sci. J.. 2013;10(10s)
- [Google Scholar]
- Ambient air metallic elements (Mn, Fe, Zn, Cr, Cu, and Pb) pollutants sources study at a rural resident area near Taichung Thermal Power Plant and Industrial Park: 6-month observations. Environ. Earth Sci.. 2016;75:587.
- [Google Scholar]
- FAO/WHO, 2001. Food and Agricultural Organization (FAO)/World Health Organization (WHO). Report on the 32nd Session of the Codex Committee on Food Additives and Contaminants. In: Joint FAO/WHO Food Standard Programme Codex Alimentarius Commission, 24th Session. Geneva, Switzerland, pp. 2–7 July.
- FAOSTAT, 2019. Food and Agriculture Organization of the United State. http://www.fao.org/faostat/en/#home (accessed on 13th May, 2021).
- Risk assessment of metals from consuming vegetables, fruits and rice grown on soils irrigated with waters of the Ebro River in Catalonia, Spain. Biol. Trace Element Res.. 2008;123:66-79.
- [Google Scholar]
- Transfer of heavy metals through terrestrial food webs: a review. Environ. Monit. Assess.. 2015;187:201.
- [Google Scholar]
- Levels of heavy metals in soil and vegetables and associated health risks in Mojo area, Ethiopia. PloS One. 2020;15(1):e0227883.
- [Google Scholar]
- Source apportionment of heavy metals in agricultural soil based on PMF: a case study in Hexi Corridor, northwest China. Chemosphere. 2018;193:189-197.
- [Google Scholar]
- Heavy metals in vegetables and potential risk for human health. Scientia Agricola. 2010;69(1):54-60.
- [Google Scholar]
- Heavy metals in vegetables and potential risk for human health. Scientia Agricola. 2012;69(1):54-60.
- [Google Scholar]
- Contamination of toxic heavy metals in various foods in Iran: a review. J. Pharmac. Sci. Res.. 2017;9(10):1692-1697.
- [Google Scholar]
- Evaluate of heavy metal concentration in shrimp (Penaeus semisulcatus) and crab (Portunus pelagicus) with INAA method. Springer Plus. 2013;2(1):1-5.
- [Google Scholar]
- Accumulation and health risk of heavy metals in a plot-scale vegetable production system in a peri-urban vegetable farm near Nanjing, China. Ecotoxicol. Environ. Saf.. 2013;98:303-309.
- [Google Scholar]
- Survey of heavy metal pollution and assessment of agricultural soil in Yang Zhong district, Jiangsu Province, China. Chemosphere. 2007;67(11):2148-2155.
- [Google Scholar]
- Development of soft computing and applications in agricultural and biological engineering. Comput. Electron. Agric.. 2010;71(2):107-127.
- [Google Scholar]
- Heavy metal levels in vegetables cultivated in Pakistan soil irrigated with untreated wastewater: preliminary results. Sustainability. 2020;12:8891.
- [Google Scholar]
- Assessment of toxic metals in vegetables with the health implications in Bangladesh. Adv. Environ. Res.. 2017;6(4):241-254.
- [Google Scholar]
- Heavy metal accumulation in different varieties of wheat (Triticum aestivum L.) grown in soil amended with domestic sewage sludge. J. Hazard. Mater.. 2009;164(2–3):1386-1391.
- [Google Scholar]
- Multivariate statistical analysis of heavy metals pollution in industrial area and its comparison with relatively less polluted area: a case study from the City of Peshawar and district Dir Lower. J. Hazard. Mater.. 2010;176(1–3):609-616.
- [Google Scholar]
- Trace Elements in Soils and Plants (third ed.). Boca Raton: CRC Press; 2001.
- Technical efficiency of onion production in Pakistan, Khyber Pakhtunkhwa Province, District Malakand. J. Adv. Devel. Econ.. 2015;4(1):24-36.
- [Google Scholar]
- Human health risk from heavy metal via food crops consumption with wastewater irrigation practices in Pakistan. Chemosphere. 2013;93:2230-2238.
- [Google Scholar]
- Health risks of heavy metals in contaminated soils and food crops irrigated with wastewater in Beijing, China. Environ. Pollut.. 2008;152:686-692.
- [Google Scholar]
- Soil and vegetables enrichment with heavy metals from geological sources in Gilgit, northern Pakistan. Ecotoxicol. Environ. Saf.. 2010;73(7):1820-1827.
- [Google Scholar]
- Nutrient Metabolism: Structures, Functions, and Genetics (first ed.). San Diego, CA, USA: Elsevier; 2003.
- Effect of Cobalt on the environment and living organisms – a review. Appl. Biol. Environ. Res.. 2019;17(5):11419-11449.
- [Google Scholar]
- Health risk of heavy metals in food crops grown on reclaimed tidal flat soil in the Pearl River Estuary, China. J. Hazard. Mater.. 2012;227:148-154.
- [Google Scholar]
- Human health risk assessment of heavy metals in soil–vegetable system: a multi-medium analysis. Sci. Total Environ.. 2013;463:530-540.
- [Google Scholar]
- Human health risk assessment of heavy metals via consumption of contaminated vegetables collected from different irrigation sources in Lahore, Pakistan. Arab. J. Chem.. 2014;7(1):91-99.
- [Google Scholar]
- Mineral and Exercise. In: Sport Nutrition for Health and Performance. New York, USA: Thames and Hudson; 2009. p. :608.
- [Google Scholar]
- Marschner's Mineral Nutrition of Higher Plants (third ed.). London: Academic; 2012.
- Flavonol glucoside and antioxidant enzyme biosynthesis affected by mycorrhizal fungi in various cultivars of onion (Allium cepa L.) J. Agric. Food. Chem.. 2016;64(1):71-77.
- [Google Scholar]
- Heavy metal content of vegetables irrigated with mixtures of wastewater and sewage sludge in Zimbabwe: implications for human health. Agric. Ecosyst. Environ.. 2006;112(1):41-48.
- [Google Scholar]
- Health risk assessment of heavy metals and their source apportionment in drinking water of Kohistan region, northern Pakistan. Microchem. J.. 2011;98(2):334-343.
- [Google Scholar]
- Effects of arsenate, chromate, and sulfate on arsenic and chromium uptake and translocation by arsenic hyperaccumulator Pteris vittata L. Environ. Pollut.. 2014;184:187-192.
- [Google Scholar]
- Development of cobalt-doped alumina hybrids for adsorption of textile effluents. Adsorpt. Sci. Technol.. 2018;36(1–2):182-197.
- [Google Scholar]
- Accumulation of selected metals in the fruits of medicinal plants grown in urban environment of Islamabad, Pakistan. Arab. J. Chem.. 2020;13(1):308-317.
- [Google Scholar]
- Lead and copper levels in tea samples marketed in Beijing, China. Bullit. Environ. Contaminat. Toxicol.. 2010;78:128-131.
- [Google Scholar]
- Heavy metals in Australian grown and imported rice and vegetables on sale in Australia: health hazard. Ecotoxicol. Environ. Saf.. 2014;100:53-60.
- [Google Scholar]
- Heavy metal phyto-technologies from Ramsar wetland plants: green approach. Chem. Ecol.. 2018;34(8):786-796.
- [Google Scholar]
- Heavy metals in food crops: Health risks, fate, mechanisms, and management. Environ. Int.. 2019;125:365-385.
- [Google Scholar]
- Ferritins control interaction between iron homeostasis and oxidative stress in Arabidopsis. Plant J.. 2009;57:400-412.
- [Google Scholar]
- Lead and cadmium contamination and exposure risk assessment via consumption of vegetables grown in agricultural soils of five-selected regions of Pakistan. Chemosphere. 2017;168:1589-1596.
- [Google Scholar]
- Transfer of heavy metals from soils to vegetables and associated human health risks at selected sites in Pakistan. Pedosphere. 2018;28(4):666-679.
- [Google Scholar]
- Trace element and toxic metal intake from the consumption of canned mushrooms marketed in Spain. Environ. Monit. Assess.. 2018;190(4):237.
- [Google Scholar]
- How healthy is urban horticulture in high traffic areas? Trace metal concentrations in vegetable crops from plantings within inner city neighbourhoods in Berlin, Germany. Environ. Pollut.. 2012;165:124-132.
- [Google Scholar]
- Analysis and health risk assessment of toxic (Cd and Pb) and essential (Cu and Zn) elements through consumption of potato (Solanum tuberosum) cultivated in Iran. Int. J. Environ. Anal. Chem.. 2020;1–11
- [CrossRef] [Google Scholar]
- Presence of heavy metals in fruits and vegetables: Health risk implications in Bangladesh. Chemosphere. 2016;152:431-438.
- [Google Scholar]
- Do we produce enough fruits and vegetables to meet global health need? PLoS ONE. 2014;9(8):e104059.
- [CrossRef] [Google Scholar]
- Risk assessment of heavy metal toxicity through contaminated vegetables from wastewater irrigated areas in Varanasi, India. Tropical Ecool.. 2010;2:375-387.
- [Google Scholar]
- Health Risk Assessment of Heavy Metals (Cd, Cu, Pb and Zn) in Soybean Marketed in Hamedan City, Iran. Ann. Military Health Sci. Res.. 2016;14(3):e11464.
- [Google Scholar]
- Stat Soft, Inc., 1999. STATISTICA for windows, computer program manual. Stat Soft, Inc., Tulsa.
- Health risk assessment of heavy metals via dietary intake of five pistachio (Pistacia vera L.) cultivars collected from different geographical sites of Iran. Food Chem. Toxicol.. 2017;107:99-107.
- [Google Scholar]
- Heavy metal toxicity and the environment. Experientia Supplementum. 2012;101:133-164.
- [Google Scholar]
- Arsenic contamination of the soil–wheat system irrigated with high arsenic groundwater in the Hetao Basin, Inner Mongolia, China. Sci. Total Environ.. 2014;496:479-487.
- [Google Scholar]
- Heavy metals in agricultural soils of the European Union with implications for food safety. Environ. Int.. 2016;88:299-330.
- [Google Scholar]
- United State Environmental Protection Agency’s guidelines for carcinogen risk assessment, EPA/630/P-03/001F. In: Risk Assessment Forum. Washington, DC: US Environmental Protection Agency; 2005.
- [Google Scholar]
- United State Environmental Protection Agency’s III risk-based concentration table: Technical Background Information. Washington, DC: United States Environmental Protection Agency; 2006.
- USEPA IRIS, 2011. United State Environmental Protection Agency’s integrated risk information system. Environmental protection agency region I, Washington DC 20460. http://www.epa.gov/iris.
- USEPA, 2010. United State Environmental Protection Agency’s Toxicological Review of Hexavalent Chromium (CAS No. 18540-29-9): In 928 Support of Summary Information on the Integrated Risk Information System (IRIS), Washington, DC.
- USEPA, 2015. United State Environmental Protection Agency’s Quantitative Risk Assessment Calculations, pp. 7–9.
- Principles of Ecotoxicology (third ed.). New York: Taylor & Francis; 2006.
- Health risks of heavy metals to the general public in Tianjin, China via consumption of vegetables and fish. Sci. Total Environ.. 2005;350:28-37.
- [Google Scholar]
- WHO, 2010. World Health Organization human health risk assessment toolkit: chemical hazards (IPCS harmonizafion project document; no. 8.). Geneva: International Programme on Chemical Safety, 2010.
- Concentration and potential health risk of heavy metals in market vegetables in Chongqing, China. Ecotoxicol. Environ. Safe.. 2011;74(6):1664-1669.
- [Google Scholar]
- Human health risk assessment of heavy metals in onion bulbs cultivated in Katsina State, North West Nigeria. Arch. Curr. Res. Int.. 2020;20(2):30-39.
- [Google Scholar]
- The importance of evaluating metal exposure and predicting human health risks in urban-periurban environments influenced by emerging industry. Chemosphere. 2016;150:79-89.
- [Google Scholar]
- Heavy metals in human urine, foods and drinking water from an e-waste dismantling area: identification of exposure sources and metal-induced health risk. Ecotoxicol. Environ. Saf.. 2019;169:707-713.
- [Google Scholar]
- Exposure risk of local residents to copper near the largest flash copper smelter in China. Sci. Total Environ.. 2018;630:453-461.
- [Google Scholar]
- Heavy metal accumulations of 24 asparagus bean cultivars grown in soil contaminated with Cd alone and with multiple metals (Cd, Pb, and Zn) J. Agric. Food. Chem.. 2007;55(3):1045-1052.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2021.103364.
Appendix A
Supplementary material
The following are the Supplementary data to this article:







