Translate this page into:
Antioxidant and lipid lowering effects of dried fruits oil extract of Pterodon emarginatusin Caenorhabditis elegans
⁎Corresponding author at: Universidade Federal do Pampa – UNIPAMPA, Programa de Pós-Graduação em Bioquímica, BR 472 – km 592 – Caixa Postal 118, CEP 97500-970 Uruguaiana, Rio Grande do Sul, Brazil. avilads1@gmail.com (Daiana Silva Avila)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
The aim of this study was to evaluate the antioxidant and lipid-lowering effects of Pterodon emarginatus Vog. Oil obtained from the dried fruits by using Caenorhabditis elegans as an experimental model. Worms were exposed to different concentrations of P. emarginatus oil for 30 min. Following treatments, survival, life span, reproduction, lipid accumulation and oxidative stress endpoints were assessed. The oil composition was analyzed by GC–MS. The oil extract (0.2, 0.5, 1.0 mg/mL) increased worms longevity without affecting their reproduction. Under oxidative stress conditions, the oil provided protection against pro-oxidants, decreased reactive oxygen species production and increased antioxidant enzymes levels, thus indicating that increased survival against the pro-oxidants might be associated with the increase in antioxidant response, possibly by modulation of gene expression. Furthermore, we observed that the oil extract reduced lipid accumulation and triglycerides. We determined flavonoids and total phenolic content, which revealed a small amount of these phytochemicals. The GC–MS analysis demonstrated the presence of different components such as sesquiterpenes, which in association with flavonoids may be contributing to the antioxidant activity and to the reduction in lipid accumulation observed in C. elegans, thus indicating that this oil should be further studied as a functional food.
Keywords
C. elegans
Oil extract
Lipid accumulation
Oxidative stress
1 Introduction
In the last decades, the interest in antioxidant and lipid-reducing molecules from natural origin has increased. Their pharmacological and therapeutic properties have been attributed to different chemical constituents isolated from their crude extracts (Surveswaran et al., 2010). Nevertheless, some studies reported that whole extracts have higher efficacy when compared to the isolated molecules. Of particular importance, chemical constituents from essential oils with antioxidant activity can be found at high concentrations in plants and are responsible for their preventive effects against cancer, neurological and cardiovascular diseases (Bartikova et al., 2014; Vinholes et al., 2014a,b). Moreover, these constituents, such as sesquiterpenes, can alter lipid metabolism, thus reducing triglycerides and total cholesterol, for instance (Fotschki et al., 2015; Lai et al., 2014; Tzeng et al., 2014). Thus, the antioxidant and lipid reducing properties of the plants have depicted a wide range of promising applications in human healthcare (Pietrovski et al., 2006). On the other hand, the indiscriminate use of the natural products has led to cases of poisoning (Boubaker et al., 2013). Hence, a safety assessment of the usage of natural products should be previously carried on.
In this context, the oil obtained from the fruits of trees from the genus Pterodon (Fabaceæ) has been widely used without proper toxicological screenings. This genus comprises four species native to Brazil: Pterodon abruptus Benth, Pterodon apparicioi Pedersoli, Pterodon polygalaeflorus Benth and Pterodon emarginatus Vogel (Alves et al., 2013; Hansen et al., 2010). P. emarginatus Vogel, known in Brazil as “Sucupira-branca” or “Faveiro”, is a native tree species widely distributed throughout central area. Chemical studies on the genus Pterodon have shown the presence of alkaloid compounds in the bark, isoflavones and some triterpenes in the wood and diterpenes and isoflavones in the dried fruit oil (Dutra et al., 2008). The hydroalcoholic infusions of the grinded fruits are used in the folk medicine for their anti-rheumatic, analgesic and anti-inflammatory properties (Nucci et al., 2012). Studies found that these plants possess significant cytotoxic and antibacterial activities; however, no toxicological studies in vivo have been conducted with dried fruits oil.
Several animal models have been used to screen the toxicology and pharmacology of medicinal plants. The nematode Caenorhabditis elegans has a number of features that make it not just relevant but quite powerful as a model for this type of research (An and Blackwell, 2003). First of all, C. elegans is easy and inexpensive to maintain in laboratory conditions with a diet of Escherichia coli. The short, hermaphroditic life cycle (∼3 days) and large number (300) of offspring of C. elegans allow large-scale production of animals within a short period of time. The transparent body also allows clear observation of all cells in mature and developing animals. C. elegans shows a strong conservation in molecular and cellular pathways in relation to mammals, and comparison between human and C. elegans genomes confirmed that the majority of human genes and pathways disease are present in the worm (Gonzalez-Manzano et al., 2012).
Hence, this study aimed to evaluate the possible toxic effects of the fruits oil of P. emarginatus, in order to determine the safety of its use. Furthermore, we aimed to verify its antioxidant potential to elucidate its pharmacological mechanism as described by the population. We hypothesized that the oil would have low toxicity and it would protect against different toxicants due to the presence of antioxidants in its phytochemical composition. In addition, due to its oil characteristic, we intend to evaluate the effect on the lipid accumulation in worms.
2 Materials and methods
2.1 Plant material
Dried fruits were obtained from Instituto Brasileiro de Florestas, Londrina, Paraná, Brazil. Commercially, these dried fruits are named seeds, however seeds are inside the dried fruits, which was botanically confirmed. All the reagents were of analytical grade and obtained from Sigma or from local suppliers.
2.1.1 Fruit oil from P. emarginatus
To obtain the oil 800 mL of n-hexane was applied to 70 g of fruits previously grinded and macerated at room temperature. The extract obtained was filtered and concentrated to eliminate the solvent in a bath at 60 °C (Dutra et al., 2008). We obtained a yield of 25% (w/w). We determined three final concentrations to experiments: 0.2, 0.5 and 1.0 mg/mL.
2.2 Antioxidant activity – DPPH assay
The antioxidant activity of P. emarginatus was evaluated in the fruits oil by their ability to scavenge DPPH (diphenyl-2-picrylhydrazyl hydrate) free radicals (Brand-Williams et al., 1995; Dutra et al., 2008). The DPPH molecule is characterized as stable-free radical which decolorizes when scavenged. Its absorbance is observed at 517 nm.
2.3 Determination of total phenolic and flavonoid contents
Total phenolic content of fruits oil was determined by the Folin–Ciocalteu micromethod (Swain and Hillis, 1959) with slight modification. Briefly, 100 μL of solution (10 mg/mL) was mixed with 1.6 mL distilled water and 100 μL of Folin–Ciocalteu reagent. Reaction solution was shaken and allowed to stand for 3 min. Afterward, it was added 200 μL of Na2CO3 solution (15%) and solution was shaken for 30 s. Subsequently, reaction solution was incubated for 30 min and its absorbance was measured at 725 nm. Gallic acid was used as a standard for calibration curve. The phenolic content was expressed as mg of gallic acid equivalents (GAE) per mL of oil.
The P. emarginatus fruits oil total flavonoid content was determined using the aluminum chloride colorimetric method (Marinova et al., 2005) with slight modification. The flavonoid content was calculated using a standard calibration of quercetin solution and expressed mg quercetin equivalents (Que) per mL of oil.
2.4 C. elegans strains and handling of the worms
C. elegans Bristol N2 (wild type) were handled and maintained at 20 °C on E. coli OP50/NGM (nematode growth medium) plates. All strains were provided by the Caenorhabditis Genetics Center (University of Minnesota, Twin Cities, MN, USA). Synchronous L1 populations were obtained by isolating embryos from gravid hermaphrodites using bleaching solution (1% NaOCl; 0.25 M NaOH), followed by flotation on a sucrose gradient to segregate eggs from dissolved worms and bacterial debris, according to standard procedures. All experiments were carried out at 22 °C in a humidified-controlled environment.
2.5 Dose–response curves for acute P. emarginatus oil exposure
The lethal dose 50% (LD50) of P. emarginatus fruits oil in C. elegans was determined with doses ranging from 0.1 to 5 mg/mL. 1,500 synchronized L1 worms per dose were treated at 22 °C for 30 min by constant agitation in a rotator with each of the compounds, followed by three washes with 85 mM NaCl solution at the end of the incubation. Worms were placed on OP50-seeded NGM plates and the dose–response curves were plotted from scoring the number of surviving worms on each dish at 24 h post exposure. LD50 values were obtained from the logarithm curve plotted in Microsoft Excel.
2.6 Lifespan assay
Synchronized L1 worms were treated as above described. Live and healthy looking worms (20 per condition; in duplicates) were collected on the same day at the late L4 stage and transferred every 4 days to new OP50-seeded NGM plates. Survival was assessed each day until all the worms died. Each experiment was performed three times.
2.7 Brood size
After a treatment with P. emarginatus fruits oil in different concentration, L4 larvae were transferred individually to new NGM/OP50 plates and the number of offspring per animal was counted until the end of the reproductive period.
2.8 Determination of ROS
ROS was determined as described previously (Gonzalez-Manzano et al., 2012) with modifications. Synchronized L1 worms were acutely pre-treated for 30 min with P. emarginatus fruits oil. Next, worms were washed three times in M9 buffer (KH2PO4, Na2HPO4, NaCl). 2′,7′-Dichlorodihydrofluorescein diacetate (DCF-DA) at a final concentration of 1 mM was added for 1 h and the worms were maintained in the dark. The worms were then washed four times in M9 buffer. Worms were frozen and thawed twice and homogenized by sonication and then centrifuged. The supernatants were transferred to a 96-well plate and their fluorescence levels (excitation 485 nm; emission 535 nm). The fluorescence from each well was measured. The experiments were repeated three times in independent worm preparations.
2.9 Stress resistance challenges
2.9.1 t-BOOH and FeSO4
To assay the stress resistance to t-BOOH (tert-butyl hydroperoxide- 5 mM) and to FeSO4 (Iron (II) sulfate- 1 mM) worms were pretreated with P. emarginatus fruits oil for 30 min. Next, worms were washed three additional times in M9 buffer. t-BOOH or FeSO4 were added for 30 min. Worms were then washed four times in M9 buffer and were placed on OP50-seeded NGM plates and curves were plotted from scoring the number of surviving worms on each at 24 h post exposure.
2.9.2 Heat shock sensitivity
The worms exposed to P. emarginatus fruits oil grown to L4 stage on OP50-seedes NGM plates. Then 20 worms from each plate were transferred to new plates and placed on NGM/OP50 heat 37 °C for 2 h. After the heat treatment the worms were allowed to recover at 20 °C for 24 h and then live and dead animals were counted.
2.9.3 Superoxide dismutase (SOD) and catalase levels
To assay the expression of these important antioxidant enzymes, 1500 L1 stage worms strains CF 1553 (SOD-3::GFP) and GA 800 (CATALASE 1, 2 and 3::GFP) were exposed to P. emarginatus fruits oil as previously described. After the last wash chip removal treatment, the pellet containing the animals was pipetted into each well (96 well plate) containing 200 μL NaCl 0.5%. The total GFP fluorescence was measured with excitation at 485 nm and emission filters of 530 nm on plate reader (CHAMELEON™ V Hidex Model 425-106).
2.10 Fluorescence analysis
To confirm the expression, L1 worms from the strains CF 1553 (sod-3p::GFP + rol-6) and GA 800 (ctl-1 + ctl-2 + ctl-3 + myo-2::GFP) were exposed to P. emarginatus fruits oil as previously described. After 48 h the animals were transferred to agarose (2%) pads with M9 with 22.5 μM of levamisole. Image acquisitions were carried out with an epifluorescence microscope in an appropriated room (20–22 °C).
2.11 Measurement of lipids in C. elegans
The worms were previously treated with P. emarginatus fruits oil at the L1 stage and incubated at 22 °C on E. coli OP50-seeded NGM plates. Lipids from worms treated as above were measured when they reached the L4 stage by using AdipoRed™ Assay Reagent (Lonza) (Nile Red dye -5H-benzo[α]phenoxazine-5-one-9-diethylamino). 50 worms were transferred to 96 well microplate containing NaCl 0.5% and 5 μL of the Nile red reagent. Worms were incubated at 20 °C for 1 h in the dark and thereafter the fluorescence was measured in a microplate reader (CHAMELEON™ V Hidex Model 425-106) by excitation at 485 nm and emission at 540 nm.
2.12 Triacylglycerol measurement in C. elegans
The lipid content was quantified by measuring the triacylglycerol content. Worms were treated with P. emarginatus fruits oil at the L1 stage and incubated at 22 °C on E. coli OP50-seeded NGM plates until they reached the L4 stage. After ∼48 h, worms were washed off from the plates and centrifuged at 2500 rpm for 2 min. Worms were washed until removing all existing bacteria. Then, worms were frozen twice, sonicated and centrifuged. The supernatant (50 μL) was transferred to a 96-well plate and triacylglycerol assay was assessed using a BioVision triglyceride assay kit (Mountain view, USA). The optical density of each of the incubated samples (5 min at 37 °C) was measured at 500 nm. For normalization of the data, protein was measured using Bradford colorimetric method.
2.13 Analyses of volatile compounds
Sample preparation – Volatile composition was analyzed from headspace of oil sample. About 200 ± 1 mg of sample was weighted in 4 mL vials and immediately sealed with a septum of an internal face of PTFE. Solid phase microextraction (SPME) technique was used for volatile compound extraction. A Carboxen/Polydimethylsiloxane fiber (Car/PDMS) 10 mm–75 μm (Supelco, USA) was used in a manual holder for extractions. Volatile compounds were extracted at 45 °C using water bath for 60 min. Before the exposure to the headspace, the flask containing the samples was maintained at same temperature as the extraction for 15 min to equilibrate. The volatile compounds were thermally desorbed by inserting the fiber into the injection port of a gas chromatograph. The sample was analyzed in triplicate.
Gas Chromatography – volatile fraction was analyzed in a gas chromatograph coupled to a mass spectrometer (GC/MS), Shimadzu model GC/MS QP-2010Plus (Shimadzu Corporation, Kyoto, Japan). The thermal desorption of the SPME was carried out at 250 °C in split mode (20:1) using a liner of 0.75 mm internal diameter. The separation of volatiles was performed on a polar capillary column phase of polyethyleneglycol (PEG), Chrompack-WAX 52 CB (Chrompack Middelburg, The Netherlands) of 60 m × 0.25 mm of i.d. × 0.25 μm of stationary phase thickness. Helium was employed as carrier gas, at a constant pressure of 30 psi. The oven temperature held at 35 °C for 5 min, raised to 80 °C at 2 °C min−1, increased to 250 °C at 4 °C min−1, and then held at this temperature for 5 min. The GC/MS interface and the ionization source were maintained at 250 °C. The instrument was run in the electron ionization mode with the ion source at +70 eV. Mass spectra were collected over a 35–350 m/z range. Identification of the volatile compounds was carried out by matching the unknown mass spectra with those provided by the library mass spectra (National Institute of Standards & Technology library — NIST 05), comparison of the experimental and retention indices (RI) from the literature and by the elution order of compounds. A series of n-alkanes (C6–C18) was analyzed under the same conditions to obtain the RI values, according to Shimoda et al. (1995); Sumitani et al. (1994); Tóth (1983).
2.14 Fatty acids analysis
The oil fraction was derivatizated to fatty acid methyl esters (FAME) according to Hartman and Lago (1973). Around 50 mg of samples was submitted firstly to alkaline catalysis, using potassium hydroxide methanolic solution (0.4 M) under water bath at 100 °C for 10 min. Then an acid catalysis was carried out using sulfuric acid methanolic solution (1 M) following by water bath at 100 °C for 10 min. FAME were partitioned in hexane. Samples were analyzed in a gas chromatograph equipped with flame ionization detector (GC-FID), Varian Star 3400CX model (Varian INC, USA) and automatic sampler, Varian model 4200. 1 μL of sample was injected in the injection port in split mode (ratio 1:50) and temperature of 250 °C. The carrier gas used was hydrogen at constant pressure of 25 psi. FAME were separated by capillary column SP 2560 (Agilent Technologies, USA) (100 m × 0.25 mm × 0.20 μm). The initial oven temperature was 80 °C, where it remained 1 min, and increased to 240 °C with a rate of 3 °C/min, keeping in isotherm for 15 min, and detector at 240 °C. The identification of FAME was performed by comparison of retention times of analytes with authentic mixture of standards, 37 Component FAME Mix (P/N 47885-U Supelco, Bellefont, USA).
2.15 Statistics
Dose–response lethality curves, longevity curves and ROS content measurements were generated with GraphPad Prism (GraphPad Software Inc.). Statistical analysis was carried out by one-way analysis of variance (ANOVA) for the dose–response curves, and ROS content, followed by post-hoc Tukey test when the overall p value was less than 0.05. For lifespan assay, a repeated measures analysis (MANOVA) was applied.
3 Results
3.1 Safety assessment
The increase in the concentration of P. emarginatus fruits oil showed slight toxicity compared to untreated worms. The LD50 estimated was 9.89 mg/mL, which was not tested once the concentration of 3 mg/mL already reduced significantly survival after 30 min of exposure to the fruits oil (Fig. 1A). Furthermore, none of the tested concentrations caused reproductive toxicity in the worms (Fig. 1B). Hence, we considered that concentrations up to 1 mg/mL would be safe for further assays.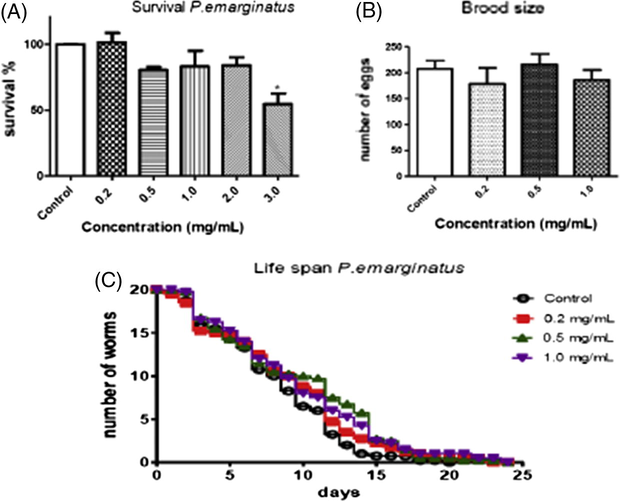
(A) Analysis of P. emarginatus on C. elegans (N2) survival after exposure. The higher concentration used (3 mg/mL) was considered significant with a *p < 0.05 when compared to control group. (B) Brood size. Data are expressed as mean of three different experiments. (C) Analysis of lifespan treated with P. emarginatus fruits oil in different concentration. The errors represent the SEM.
3.2 Antioxidant potential of P.emarginatus fruits oil
In vitro DPPH assay demonstrated that the oil does not present scavenger activity (Data not show). On the other hand, we observed in vivo that the oil increased worms life span at all tested concentrations, which indicated antioxidant activity (p < 0.05) (Fig. 1C). We also observed that the oil decreased, per se, the fluorescence of DCF from 0.5 mg/mL to 1.0 mg/mL indicating again antioxidative potential (Fig. 2A). In order to confirm this effect, we challenged the oil against different pro-oxidants: tert-butyl hydroperoxide (a classic pro-oxidant), iron (a metal) and heat shock stress. The results showed that the treatment with the oil protected C. elegans from t-BOOH and Fe-induced mortality (Fig. 2B and C). Notably, all tested concentrations significantly reduced toxicity by increasing worms survival. At the higher concentration, the oil decreased worms mortality under thermal stress (Fig. 2D).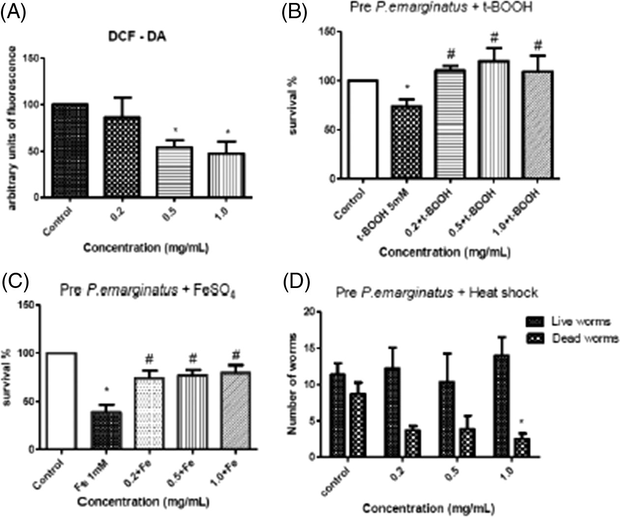
Analysis of antioxidant and stress resistance on C. elegans. The analysis of antioxidant with (A) DCF. The worms pre-treated with different concentrations of P. emarginatus fruits oil against: (B) t-BOOH (5 mM), (C) FeSO4 (1 mM), and (D) heat shock stress. Data are expressed as mean of 3 different experiments. The error represents the SEM. *Indicates p < 0.05 when compared to control group.
3.3 Antioxidant enzyme levels
The fluorescence levels from CAT-1,2,3::GFP were higher at the concentration of 1.0 mg/mL of P. emarginatus fruits oil. Catalase is an enzyme responsible for antioxidant defenses, acting by scavenging hydrogen peroxide. Therefore, the increase in this protein may suggest that the P. emarginatus fruits oil operates by modulating antioxidant enzyme expression (Fig. 3). An increase in SOD-3 levels was also observed (Fig. 4). The images confirmed the increased expression in both CAT-1,2,3::GFP and SOD-3::GFP.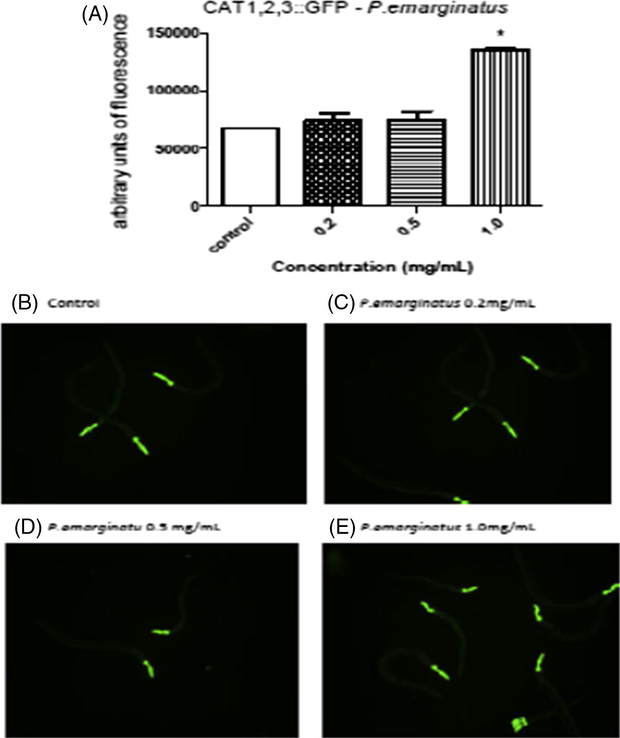
GFP fluorescence in C. elegans treated with P. emarginatus fruits oil (A) levels CATALASE 1,2,3::GFP; The fluorescence images, (B) representative image of a control worm; (C) representative image of a worm treated with P. emarginatus fruits oil 0.2 mg/mL; (D) representative image of worm treated with P. emarginatus fruits oil 0.5 mg/mL; (E) representative image of a worm treated with P. emarginatus fruits oil 1.0 mg/mL. Data are expressed as percent of control from 3 different experiments. The error represents the SEM. *Indicates p < 0.05 when compared to control group.
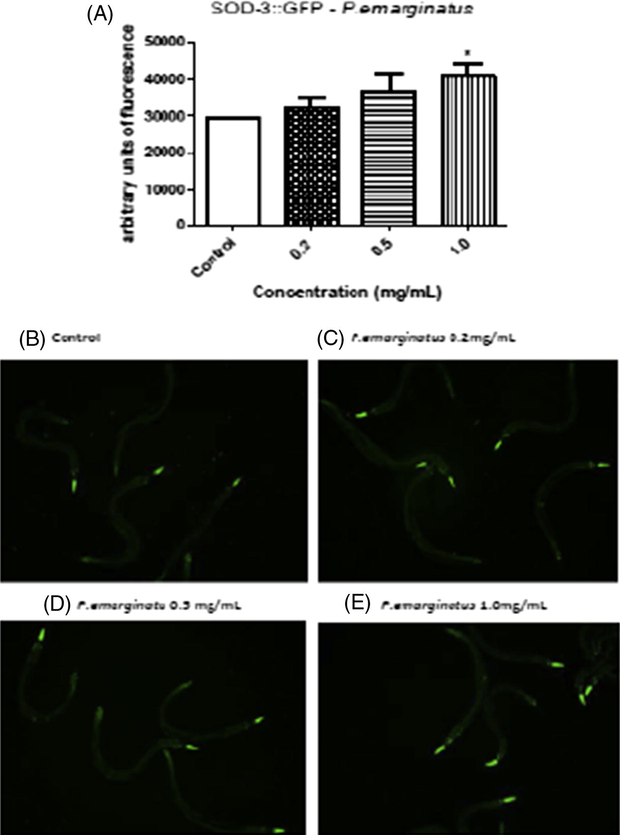
GFP fluorescence in C. elegans treated with P. emarginatus fruits oil (A) levels SOD-3::GFP; the fluorescence images, (B) representative image of a control worm; (C) representative image of a worm treated with P. emarginatus fruits oil 0.2 mg/mL; (D) representative image of worm treated with P. emarginatus fruits oil 0.5 mg/mL; (E) representative image of a worm treated with P. emarginatus fruits oil 1.0 mg/mL. Data are expressed as percent of control from 3 different experiments. The error represents the SEM. *Indicates p < 0.05 when compared to control group.
3.4 P. emarginatus fruits oil on lipid accumulation in C. elegans
All concentrations of P. emarginatus fruits oil were effective in reducing the accumulation of lipids, showed by reduction in lipid droplets when compared to control group (Fig. 5A). The higher concentration of P. emarginatus fruits oil caused a significant decrease in triglycerides levels (Fig. 5B). The decrease in these levels could be resultant of a possible fat metabolism modulation by the oil.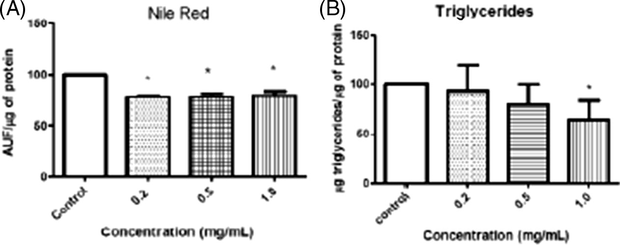
(A) Analysis of lipid accumulation in C. elegans (N2) after 30 min treatment with P. emarginatus fruits oil. They were exposed to a Nile Red dye. (B) Analysis of the triacylglycerol content in C. elegans (N2) after 30 min treatment. Data are expressed as mean of 3 different experiments. The error represents the SEM. *Significant p < 0.05.
3.5 Phytochemical analysis
To characterize the P. emarginatus fruits oil, a GC–MS analysis was performed. The chromatogram demonstrated the presence of several components (S1), mainly terpenoids and sesquiterpenoids, such as β-caryophyllene and α-humulene (Table 1), as well as the presence of fatty acids (Table 2). We also determined the total phenolics and flavonoid content (Table 3), which has shown the significative presence of these important constituents in the oil. Indices calculated by retention time and confirmed by the literature, NIST 05, Shimoda et al. (1995), Sumitani et al. (1994) and Tóth (1983). SD – Standard deviation Data are expressed as mean ± SEM (n = 3 independent experiments).
Compounds
RIexp
RILit
Area (×105)
%
α-Cubebene
1357
1365
37
0.13
α-Copaene
1434
1438
224
0.79
Prehnitene (1,2,3,4-tetramethylbenzene)
1464
1460
18
0.07
Tetranap (naphthalene, 1,2,3,4-tetrahydro-)
1481
1462
13
0.04
β-Copaene <–>
1503
1517
52
0.18
β-Caryophyllene
1507
1550
3472
12.26
Alloaromadendrene
1560
1570
116
0.41
α-Humulene
1602
1606
1375
4.85
Germacrene-d
1632
1638
618
2.18
Bicyclogermacrene
1676
1676
68
0.24
γ-Cadinene
1700
1706
160
0.56
Calamenene
1728
1730
50
0.17
Caryophyllene oxide
1803
1826
29
0.10
Guaia-6,9-dien-4-ol
>2000
2023
28
0.10
Compounds
Mean (%)
SD
C16:0 (Palmitic acid)
6.0
0.5
C18:1n9c (Oleic acid)
32.1
2.4
C18:2n6c (Linoleic acid)
37.0
1.9
C22:0 (Behenic acid)
17.7
1.0
C24:0 (Lignoceric acid)
7.1
0.2
Extract
Total phenolics content (mg GAE/mL oil)
Total flavonoids content (mg Quer/mL oil)
Pterodon emarginatus
1158.96 ± 56.01
2.68 ± 0.14
4 Discussion
In the present study, we examined some toxicological and pharmacological parameters in C. elegans following treatment with P. emarginatus oil extracted from its dried fruits. We observed that the treatment with this oil protected from mortality induced by different stressors and that this protection may be provided by the modulation of antioxidant gene expression, as observed by increased SOD-3::GFP and CAT 1,2,3::GFP levels. Related to that, we have found that the oil extract reduced lipid accumulation per se in the worms, which has been never reported in the literature.
There are no studies reporting P. emarginatus dried fruit oil toxicity; then, initially, we determined the oil LD50. After observing its low toxicity, we evaluated the antioxidant activity of the oil in vitro, however we did not observe significant results on DPPH scavenging radical assay, probably because of the small amount of phenolic compounds (Table 3), which would be scavengers of reactive species. On the other hand, when we tested the extract in vivo, in the worms, we observed a significant reduction in ROS levels. Corroborating to its antioxidant effect, we observed that the treatment prolonged worms life span, another strong parameter of antioxidant potential. Hence, we tested the antioxidant ability of P. emarginatus fruits oil against several pro-oxidants though survival assay. tert-Butyl hydroperoxide (t-BOOH), a precursor in the formation of malondialdehyde, among other hydroperoxides, is a membrane-permeate oxidant that has been extensively used as model of oxidative stress, in different systems (Garcia-Cohen et al., 2000). Here, the pre-treatment with P. emarginatus fruits oil protected from t-BOOH mortality at all tested concentrations. The same occurred against Fe, as the oil caused protection at all tested concentrations against the mortality induced by the exposure to this metal. It is well documented that Fe can induce the generation of free radicals by the Fenton reaction, which attacks important biomolecules and thus causes oxidative damage (Zhang et al., 2003). In this context, the increase in SOD-3::GFP and CAT 1,2,3::GFP levels may be responsible for the protection afforded by the fruits oil. SOD converts superoxide radical into hydrogen peroxide and molecular oxygen, while catalase converts this hydrogen peroxide into water and oxygen. As we did not observe this antioxidant potential in vitro, we believe that in vivo antioxidant extract effect might be due to the metabolization of the bioactive compounds.
Antioxidant activity of P. emarginatus fruits oil is important to the properties that have been described to the plant. In folk medicine, wine infusions from the seeds of Pterodon genus are used in the treatment of rheumatoid arthritis, an auto-immune disease characterized by chronic inflamed joints and exacerbated functions of macrophages and T and B lymphocytes (Cardoso et al., 2008). Further analysis revealed the anti-inflammatory and anti-nociceptive effects of ethanolic extracts of Pterodon pubescens seeds, confirming the rationale behind the use of this plant in popular medicine to treat pain disorders (Coelho et al., 2005; Silva et al., 2004). The essential oil from the seeds exhibited significant protection against ulcers induced by ethanol, indometacin and HCl/ethanol. The essential oil caused a marked reduction in the exudate volume and inhibited leukocyte and neutrophil influx in carrageenan-induced pleurisy. Moreover, the essential oil significantly decreased nitric oxide (NO) and interleukin-1 (IL-1) levels, without affecting tumor necrosis factor-alpha production (Dutra et al., 2009). Furthermore, previous studies with rats have shown that the extract of P. emarginatus abolished oxidative stress induced by acute exercise stress (Paula et al., 2005). The results showed that the crude extract of the P. emarginatus dried fruits depicted anti-inflammatory, antioxidant and anti-nitrosative activities in vivo in various organs following acute exercise (Carvalho et al., 1999; Paula et al., 2005). Usually, extracts are obtained by using solvent mixes that contain ethanol, which is a good flavonoid extractor. In fact, (Dutra et al., 2008) quantified phenolic constituents of P. emarginatus oil obtained from different extraction procedures, however, could not detect phenolic compounds in hexanic extracts. In the present study, we found small amounts of phenolic compounds and flavonoids in the oil extracted with hexane, probably because our trituration method was more efficient and because we controlled temperature when evaporating hexane (temperature was never above 60 °C)
Phenolic compounds can be classified as simple phenols, with a single aromatic ring bearing at least one hydroxyl group. Flavonoids are the largest group of plants phenols and the most studied. They comprise compounds of low molecular weight that usually occur bound to sugar molecules (King and Young, 1999). They have been proposed to exert beneficial effects in a multitude of disease states, including cancer, cardiovascular disease, and neurodegenerative disorder. Many of the biological actions of flavonoids have been attributed to their antioxidant properties. Flavonoids possess scavenging ability due to their hydroxyl groups, which can interact with reactive species and scavenge them (Williams et al., 2004). Thus, the presence, even though small, of phenolic and flavonoid content can be contributing to the antioxidant activity of the fruits oil. However, the oil is richer in other important phytochemicals.
Previous phytochemical analysis has shown that the fruits hexanic extract contains sesquiterpenic compounds such as trans-β-caryophyllene (35.9%), β-elemene (15.3%), germacrene D (9.8%), spathulenol (5.9%), α-humulene (6.8%) and bicyclogermacrene (5.5%) (Dutra et al., 2008) and another analysis found sesquiterpenics such as α-caryophyllene, β-caryophyllene, mircene, α-pirene and diterpenics in the hexanic extract (Teixeira, 2003). In our analysis the major components found were sesquiterpenes such as β-caryophyllene (12.26%), α-humulene (4.85%) as other minor components such as germacreme D, α-cubebene, α-copaene, which have also been found in previous studies (Pant et al., 2014). In fatty acid analysis we found linolenic acid (an omega-6 unsaturated fatty acid) and oleic acid (omega-9 unsaturated fatty acid). Many of these compounds, particularly omega 3 and omega 6 fatty acids, can cause resistance to free radical attack and reduce lipid peroxidation (Ashrafi et al., 2003).
Besides, we observed that higher tested concentrations of P. emarginatus fruits oil reduced significantly triacylglycerol levels (Fig. 5B). Meanwhile, there was also a decrease in lipid droplets compared to control, as visualized by the Nile red dye (Fig. 5A). We suggest that the bioactive compounds found in the oil may modulate fat metabolism because most of the lipid species in C. elegans (triglycerides, phospholipids, sphingolipids) are composed of fatty acids directly absorbed from the bacterial food source (Houthoofd et al., 2002). The results are in agreement with findings with the methanolic extract of Vernonia amygdalina that has shown lipid-lowering effects in rats fed with high cholesterol diet (Adaramoye et al., 2008). In addition, the phytochemical screening of V. amygdalina also revealed the presence of saponins, sesquiternes and flavonoids (Igile et al., 1994; Erasto et al., 2006), which may be a hint regarding the lipid-reducing potential of this combination of biomolecules. Zerumbone, a natural cyclic sesquiterpene obtained from Zingiber zerumbet S., has been described by modulating lipolytic and lipogenic pathways, thus reducing the hypercholesterolemia induced by high fat diets (Tzeng et al., 2013, 2014). Hence, this reduction in lipid accumulation per se may indicate that the sesquiterpenes, PUFAS and flavonoids present in P. emarginatus fruits oil can also modulate lipid metabolism in worms.
Another hypothesis is the link between oxidative stress and lipid metabolism. In mammals, oxidative stress interferes with hepatic lipid metabolism at various levels, ranging from lipid accumulation to inflammation activation (Lanaspa et al., 2012; Wheeler et al., 2001). Interestingly, it has been shown that fish oil, resveratrol and isoquercetin, for instance, are able to protect from oxidative stress caused by high fat diets (Espinosa et al., 2013; Hassan et al., 2014; Wang et al., 2013). Worms do not have liver, however it has been shown that lipid accumulation is related to aging, and aging is dependent on the oxidative/antioxidant homeostasis (Ackerman and Gems, 2012). Furthermore, C. elegans SOD-3 is regulated by the transcription factor DAF-16. Once activated by xenobiotics, DAF-16 migrates to the nucleus and activates not only SOD-3, but also lipases that mobilize fat acids from their stores (Wang et al., 2008). In agreement, daf-16 mutants accumulate more fat and have a short life span (Murphy et al., 2003; Oh et al., 2006). Hence, it is possible that the antioxidant activity and the lipid lowering potential of the P. emarginatus extract observed in the present study are united by a common pathway which is modulated by the treatment with the oil.
5 Conclusion
In conclusion we observed that P. emarginatus fruits oil has an antioxidant activity in C. elegans, caused an increase in life span and promotes a protection against oxidative damage at all concentrations tested. We identified the presence of phenolic compounds, sesquiterpenoids and PUFAS in the dried fruits oil. We also conclude the P. emarginatus fruits oil can modulate lipid metabolism in worms, which mechanism will be further studied by our group.
Contributors
All authors approved the final version of this manuscript. AHCDF designed and performed the experiments and wrote the manuscript; DFC performed experiments; BP performed experiments; CFR performed experiments; JJS performed analytical analysis; RW performed analytical analysis; SRR performed analytical analysis; VF designed the experiments and revised the manuscript; RP designed the experiments and revised the manuscript; SEH idealized the study, designed the experiments and revised the manuscript; FMF idealized the study, designed the experiments and revised the manuscript; ELGD performed analytical analysis; CCD idealized the study, designed the experiments and revised the manuscript; DSA idealized the study, designed the experiments and revised the manuscript.
Acknowledgments
Authors acknowledge the financial support provided by FAPERGS and CNPq. Strains were provided by the CGC, which is funded by NIH Office of Research Infrastructure Programs (P40 OD010440). AHCDF and DFC received scholarships from FAPERGS and CNPq.
References
- The mystery of C. elegans aging: an emerging role for fat. Distant parallels between C. elegans aging and metabolic syndrome? BioEssays. 2012;34(6):466-471.
- [Google Scholar]
- Lipid-lowering effects of methanolic extract of Vernonia amygdalina leaves in rats fed on high cholesterol diet. Vasc. Health Risk Manage.. 2008;4(1):235-241.
- [Google Scholar]
- Chemical variability of the essential oils from fruits of Pterodon emarginatus in the Brazilian Cerrado. Revista Brasileira De Farmacognosia-Brazilian Journal of Pharmacognosy. 2013;23(2):224-229.
- [Google Scholar]
- SKN-1 links C. elegans mesendodermal specification to a conserved oxidative stress response. Genes Dev.. 2003;17(15):1882-1893.
- [Google Scholar]
- Genome-wide RNAi analysis of Caenorhabditis elegans fat regulatory genes. Nature. 2003;421(6920):268-272.
- [Google Scholar]
- Antioxidant, pro-oxidant and other biological activities of sesquiterpenes. Curr. Top. Med. Chem.. 2014;14(22):2478-2494.
- [Google Scholar]
- Acute renal failure by ingestion of Euphorbia paralias. Saudi J. Kidney Dis. Transplant.. 2013;24(3):571-575.
- [Google Scholar]
- Use of free radical method to evaluate antioxidant activity. Lebensm Wiss Technol.. 1995;28:25-30.
- [Google Scholar]
- Suppression of T and B cell responses by Pterodon pubescens seeds ethanolic extract. Pak. J. Biol. Sci.. 2008;11(19):2308-2313.
- [Google Scholar]
- Anti-inflammatory activity of the crude extract from the fruits of Pterodon emarginatus Vog. J. Ethnopharmacol.. 1999;64(2):127-133.
- [Google Scholar]
- Antinociceptive properties of ethanolic extract and fractions of Pterodon pubescens Benth. seeds. J. Ethnopharmacol.. 2005;98(1-2):109-116.
- [Google Scholar]
- Antiulcerogenic and anti-inflammatory activities of the essential oil from Pterodon emarginatus seeds. J. Pharm. Pharmacol.. 2009;61(2):243-250.
- [Google Scholar]
- Quantification of phenolic constituents and antioxidant activity of Pterodon emarginatus vogel seeds. Int. J. Mol. Sci.. 2008;9(4):606-614.
- [Google Scholar]
- Bioactive sesquiterpene lactones from the leaves of Vernonia amygdalina. J. Ethnopharmacol.. 2006;106(1):117-120.
- [Google Scholar]
- Prevention of liver steatosis through fish oil supplementation: correlation of oxidative stress with insulin resistance and liver fatty acid content. Arch. Latinoam Nutr.. 2013;63(1):29-36.
- [Google Scholar]
- Dietary supplementation with raspberry seed oil modulates liver functions, inflammatory state, and lipid metabolism in rats. J. Nutr.. 2015;145(8):1793-1799.
- [Google Scholar]
- Oxidative stress induced by tert-butyl hydroperoxide causes vasoconstriction in the aorta from hypertensive and aged rats: role of cyclooxygenase-2 isoform. J. Pharmacol. Exp. Ther.. 2000;293(1):75-81.
- [Google Scholar]
- Oxidative status of stressed Caenorhabditis elegans treated with epicatechin. J. Agric. Food Chem.. 2012;60(36):8911-8916.
- [Google Scholar]
- Pharmaceutical properties of ’sucupira’ (Pterodon spp.). Brazilian. J. Pharm. Sci.. 2010;46(4):607-616.
- [Google Scholar]
- Rapid preparation of fatty acid methyl esters from lipids. Lab. Pract.. 1973;22(6):1.
- [Google Scholar]
- Reduced oxidative stress contributes to the lipid lowering effects of isoquercitrin in free fatty acids induced hepatocytes. Oxid. Med. Cell. Longev.. 2014;2014:313602.
- [Google Scholar]
- Axenic growth up-regulates mass-specific metabolic rate, stress resistance, and extends life span in Caenorhabditis elegans. Exp. Gerontol.. 2002;37(12):1371-1378.
- [Google Scholar]
- Flavonoids from Vernonia-amygdalina and their antioxidant activities. J. Agric. Food Chem.. 1994;42(11):2445-2448.
- [Google Scholar]
- Characteristics and occurrence of phenolic phytochemicals. J. Am. Diet. Assoc.. 1999;99(2):213-218.
- [Google Scholar]
- Garlic essential oil protects against obesity-triggered nonalcoholic fatty liver disease through modulation of lipid metabolism and oxidative stress. J. Agric. Food Chem.. 2014;62(25):5897-5906.
- [Google Scholar]
- Uric acid induces hepatic steatosis by generation of mitochondrial oxidative stress: potential role in fructose-dependent and -independent fatty liver. J. Biol. Chem.. 2012;287(48):40732-40744.
- [Google Scholar]
- Total phenolics and total flavonoids in Bulgarian fruits and vegetables. J. Univ. Chem. Technol. Metall.. 2005;40(3):5.
- [Google Scholar]
- Genes that act downstream of DAF-16 to influence the lifespan of Caenorhabditis elegans. Nature. 2003;424(6946):277-283.
- [Google Scholar]
- Oleaginous extract from the fruits Pterodon pubescens Benth induces antinociception in animal models of acute and chronic pain. J. Ethnopharmacol.. 2012;143(1):170-178.
- [Google Scholar]
- Identification of direct DAF-16 targets controlling longevity, metabolism and diapause by chromatin immunoprecipitation. Nat. Genet.. 2006;38(2):251-257.
- [Google Scholar]
- Beta-caryophyllene modulates expression of stress response genes and mediates longevity in Caenorhabditis elegans. Exp. Gerontol.. 2014;57:81-95.
- [Google Scholar]
- Protective action of a hexane crude extract of Pterodon emarginatus fruits against oxidative and nitrosative stress induced by acute exercise in rats. BMC Complement Altern. Med.. 2005;5:17.
- [Google Scholar]
- Antinociceptive properties of the ethanolic extract and of the triterpene 3beta,6beta,16beta-trihidroxilup-20(29)-ene obtained from the flowers of Combretum leprosum in mice. Pharmacol. Biochem. Behav.. 2006;83(1):90-99.
- [Google Scholar]
- Comparison of volatile compounds among different grades of green tea and their relations to odor attributes. J. Agric. Food Chem.. 1995;43:4.
- [Google Scholar]
- Acute and topic anti-edematogenic fractions isolated from the seeds of Pterodon pubescens. J. Pharm. Pharmacol.. 2004;56(1):135-141.
- [Google Scholar]
- Changes in composition of volatile compounds in high pressure treated peach. J. Agric. Food Chem.. 1994;42:5.
- [Google Scholar]
- Antioxidant properties and principal phenolic phytochemicals of Indian medicinal plants from Asclepiadoideae and Periplocoideae. Nat. Prod. Res.. 2010;24(3):206-221.
- [Google Scholar]
- The phenolic constituents of Prunus domestica I. – The quantitative analysis of phenolic constituents. J. Sci. Food Agric.. 1959;10(1):5.
- [Google Scholar]
- Estudo química e avaliação biológica de Attalea excelsa Mart. ex Spreng. (urucuri) e Pterodon emarginatus Vog. (sucupira-branca) em Aedes aegypti Dissertação de Mestrado. Rio de Janeiro: Universidade Federal do Rio de Janeiro; 2003.
- Use of capillary gas chromatogrphy in collecting retention and chemical information for the analysis of complex petrochemical mixtures. J. Chromatogr.. 1983;278:9.
- [Google Scholar]
- Zerumbone, a natural cyclic sesquiterpene of Zingiber Zerumbet Smith, attenuates nonalcoholic fatty liver disease in hamsters fed on high-fat diet. Evid. Based Complement Alternat. Med.. 2013;2013:303061.
- [Google Scholar]
- Lipid-lowering effects of zerumbone, a natural cyclic sesquiterpene of Zingiber zerumbet Smith, in high-fat diet-induced hyperlipidemic hamsters. Food Chem. Toxicol.. 2014;69:132-139.
- [Google Scholar]
- Assessment of the antioxidant and antiproliferative effects of sesquiterpenic compounds in in vitro Caco-2 cell models. Food Chem.. 2014;156:204-211.
- [Google Scholar]
- Hepatoprotection of sesquiterpenoids: a quantitative structure-activity relationship (QSAR) approach. Food Chem.. 2014;146:78-84.
- [Google Scholar]
- Resveratrol prevents suppression of regulatory T-cell production, oxidative stress, and inflammation of mice prone or resistant to high-fat diet-induced obesity. Nutr. Res.. 2013;33(11):971-981.
- [Google Scholar]
- Fat metabolism links germline stem cells and longevity in C. elegans. Science. 2008;322(5903):957-960.
- [Google Scholar]
- The role of Kupffer cell oxidant production in early ethanol-induced liver disease. Free Radic. Biol. Med.. 2001;31(12):1544-1549.
- [Google Scholar]
- Flavonoids: antioxidants or signalling molecules? Free Radic. Biol. Med.. 2004;36(7):838-849.
- [Google Scholar]
- Iron-induced oxidative damage and apoptosis in cerebellar granule cells: attenuation by tetramethylpyrazine and ferulic acid. Eur. J. Pharmacol.. 2003;467(1–3):41-47.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.arabjc.2016.04.001.
Appendix A
Supplementary material
Supplementary data 1
Supplementary data 1







