Translate this page into:
Basic characterization and Alzheimer’s disease relieving property of a glucose riched polysaccharide from Cibotium barometz
⁎Corresponding author. yuanming@sicau.edu.cn (Ming Yuan)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
The water-soluble polysaccharides from plants have attracted ever-increasing attention in the field of food and drug due to their various activities and low toxicity. CBP50-1, as a purified fraction of polysaccharide from the rhizome of Cibotium barometz (CBP), mainly consisted of glucose (55.45%) and xylose (25.27%). CBP50-1 showed excellent antioxidant activity for scavenging 2,2‑Diphenyl‑1‑picryl‑hydrazy (DPPH) radical and hydroxyl radical, further inhibiting lipid peroxidation. CBP50-1 significantly improved the survival rate of Caenorhabditis elegans under thermal and oxidative stress. Furthermore, CBP50-1 reduced the paralysis and oxidative damage induced by amyloid-beta (Aβ) and increased the antioxidant enzyme activity in Alzheimer’s disease (AD) model C. elegans CL4176 through c-Jun N-terminal kinase (JNK) mitogen-activated protein kinase (MAPK) signal pathway. Thus, CBP50-1 had a potential application in health industries.
Keywords
Alzheimer’s disease
Cibotium barometz polysaccharide
Antioxidant
Aβ

- Aβ
-
amyloid-beta
- AD
-
Alzheimer’s disease
- ANOVA
-
analysis of variance
- BBD
-
Box-Behnken design
- BHT
-
2,6-Di-tert-butyl-p-cresol
- CAT
-
catalase
- CBP
-
Cibotium barometz polysaccharide
- DPPH
-
2,2‑Diphenyl‑1‑picryl‑hydrazy
- DTG
-
differential thermogravimetry
- DW
-
dry weight
- FT-IR
-
Fourier Transform infrared spectroscopy
- HPLC
-
High-performance Liquid Chromatography
- JNK
-
c-Jun N-terminal kinase Vc, Ascorbic acid
- MAPK
-
mitogen-activated protein kinase
- MDA
-
malonaldehyde
- RSM
-
response surface methodology
- SOD
-
superoxide dismutase
- UV
-
Ultraviolet
Abbreviations
1 Introduction
Alzheimer's disease (AD), or senile dementia, is a progressive chronic degenerative dementia of the central nervous system that occurs majority in the elderly. With the arrival of an aging society, the incidence of AD is increasing year by year. One in every 85 people will live with AD symptoms or a tendency to develop into AD by 2050 (Brookmeyer et al., 2018). The main hypotheses concerning AD include amyloid-beta (Aβ) peptides, dystrophic neuronal extensions, activated microglia, and reactive astrocytes (Folch et al., 2018). Additionally, much evidence proved that free radicals are related to AD (Jiang et al., 2016; Padurariu et al., 2013). The relationship between oxidative damage and amyloid plaques is interactive. Oxidative stress is indirectly involved in the amyloid genesis by inducing intralysosomal accumulation of Aβ (Zheng et al., 2006). Moreover, Aβ disturbed the redox balance and aggravated oxidative damage by precipitating lipid peroxidation and mitochondrial dysfunction, resulting in cell death (Zhou et al., 2014).
There is ample evidence that the intake of antioxidants could improve our resistance to diseases (Ribeiro et al., 2011). Natural polysaccharides showed many biological properties such as anti-oxidation, anti-aging, anti-tumor, and anti-inflammatory (Mzoughi et al., 2018). Owing to their safety and nontoxic properties, some of these polysaccharides could be antioxidants and drugs (Teng et al., 2020; Zheng et al., 2020). Consequently, there is growing attention to pursuing the isolation, characterization, and bioactivity of diverse polysaccharides from natural materials because of their potential application in functional food and medicine. Caenorhabditis elegans is often used to evaluate the activity of polysaccharides due to its short lifespan and easy operation (Feng et al., 2018; Zhang et al., 2018). C. elegans CL4176 expressed the human Aβ1-42 in the muscle when the culture temperature was over 23 °C, which caused progressive paralysis (Yen et al., 2021). This type of worm is often used to evaluate the effects of polysaccharides on AD (Li et al., 2018; Lin et al., 2020).
Cibotium barometz (L.) J. Sm, a great fern up to 2 meters, is widely distributed in Guangdong, Guangxi, Guizhou, Sichuan, and Yunnan in China (Zhang and Nishida,2013). It is a traditional food because of its abundant starch in the rhizome, and it is also a Chinese medicinal herb to relieve rheumatic arthralgia and cure traumatic hemorrhage (Huang et al., 2018). It is widely used as an ornamental plant today. Decoction was the most convenient method to obtain its effective constituents. Therefore, the polysaccharide was considered an active ingredient in the Cibotium barometz rhizome. It showed excellent immunoactivity and antioxidant properties in vitro (Huang et al., 2018; Shi et al., 2020). Previous research on fern polysaccharides mainly aimed at the extraction method or preliminary analysis of its antioxidant activity (Chiang and Lai, 2019; Xu et al., 2009). There was little report about its mitigation effect on AD. Based on the close relationship between oxidative damage and amyloid plaques, we evaluated the potential of polysaccharide from Cibotium barometz to relieve the oxidative damage and symptoms of paralysis in the AD model of C. elegans.
2 Materials and methods
2.1 Material and reagents
The C. barometz rhizome was purchased from Guangxi in China. The dried rhizome was pulverized and screened through 80-mesh sieves. Absolute ethyl alcohol, sodium chloride, n-butyl alcohol, chloroform, acetone, Ascorbic acid (Vc), Congo red, ethyl ether, and K2S2O4 were obtained from Chengdu Kelong Chemical CO., ltd (Chengdu, China). Monosaccharide standards, 2,2‑Diphenyl‑1‑picryl‑hydrazy (DPPH) were purchased from Solarbio Sci. & Tech. Co., ltd. (Beijing, China). All reagents were analytically pure.
2.2 Caenorhabditis elegans strains maintenance and treatment
Wild type N2 and CL4176 (pAF29(myo-3/A-Beta 1–42/let UTR) + pRF4 rol-6 (su1006)) were kept at 20 °C and 16 °C, respectively. All strains were cultured with Escherichia coli var. OP50 and maintained on standard nematode growth medium plates with CBP50-1 or not.
2.3 Optimation for polysaccharide extraction
2.3.1 Single-factor experiment
The effects of extraction time (30–70 min), ultrasonic power (150–270 W), extraction temperature (30–70 °C), and liquid to solid ratio (15–35 mL/g) on the yield of CBP were evaluated. The sugar content was assayed according to Zhao et al. (2019). The extraction yield was calculated by the formula:
2.3.2 Design of response surface methodology (RSM)
The design of RSM and statistical analysis were done by Design Expert Software (8.0.6). with each parameter at three levels: high (+1), medium (0), and low (-1). The Box-Behnken design (BBD) for the extraction of CBP were shown in Table S1. Each experiment was performed three times and the mean was taken as the response. Analysis of experimental data was performed using analysis of variance (ANOVA) and the results were fitted by the following full second-order polynomial equation:
2.3.3 Extraction and purification of polysaccharides
The degreased sample (200 g) was extracted under the optimized condition. The supernatant was condensed with a rotary evaporator, and the same volume of anhydrous ethanol was added, centrifuging and collecting the precipitates (CBP50). After freeze-drying, the crude polysaccharides were collected. Seveg solution was used for deproteinization. Deproteinated polysaccharides (CBP50) were separated by anion-exchange chromatography (DEAE-52 cellulose column, 2.5 × 30 cm) and eluted with 0–0.5 mol/L NaCl. The fraction washed with deionized water was named CBP50-1, and further purified with a Sephadex G-200 column. Then, followed by dialyzing (3500 Da) and lyophilizing, purified CBP50-1 was obtained.
2.4 Assay of total sugars, uronic acid, and protein
The uronic acid content and protein content were determined according to Zhao et al. (2019). The total sugar content was determined according to Zhao et al. (2019).
2.5 Characterization of CBP50-1
2.5.1 Molecular weight and monosaccharide composition
The molecular weight of CBP50-1 was determined by High-performance Gel Chromatography, and the standards curve was made with a series of dextrans.
The monosaccharide of CBP50-1 was determined through Agilent 1260 High-performance Liquid Chromatography (HPLC) system (Agilent, Santa Clara, CA, USA) coupled with an Agilent C-18 column (Ø 4.6 × 250 mm, 5 μm) (Wang et al., 2015). 0.05 mol/L phosphate buffer solution (pH 6.7) and acetonitrile worked as mobile phase with a 1.0 mL/min flow rate. The detection wavelength was 254 nm with the DAD detector.
2.5.2 The UV spectroscopy analysis and FT-IR
UV–visible absorption of CBP50-1 (100 μg/mL) was recorded with a spectrophotometer (Shimadzu UV-1800, Japan). CBP50-1 was compressed into a KBr pellet and scanned with FTIR-8400S (Shimadzu, Japan).
2.5.3 Thermal properties
Thermal properties were determined with a Q20 differential scanning calorimeter (DSC) (AT, USA) under N2 at 0.1 MPa. 3.0 mg of CBP50-1 was placed in a closed aluminum pan and heated from 50 to 350 °C.
2.5.4 Congo red assay
Congo red (1 mL, 0.2 mmol/L) and polysaccharide solution (1.5 mL, 2.0 mg/mL) were mixed, then 2 mol/L NaOH was added to the final concentration of 0–0.5 mol/L, followed by incubation for 30 min. The solution was scanned with a spectrophotometer (400–700 nm) (Shimadzu UV-1800 Japan).
2.6 Antioxidant activities in vitro
Hydroxyl radical scavenging activity and DPPH radical scavenging activity was performed as Zhao et al. described (2019), and Vc was taken as control. anti-lipid-peroxidation capacity was measured as Liu & Huang described (Liu and Huang, 2018), and 2,6-Di-tert-butyl-p-cresol (BHT) worked as a control.
2.7 Thermal stress and oxidative stress of N2
Synergized nematodes were cultured after lysis. The nematodes of the L4 stage were randomly transferred to new plates (containing 0.1 mg/mL CBP50-1 or not). For thermal stress, 30 nematodes were exposed to 35 °C and the survival worms were counted after 14 h. For oxidative stress, 30 nematodes were transferred onto a 24-well plate with 50 μmol/L of Juglone in 500 μL S-medium per well. The survival worms were counted after 20 h.
2.8 Determination of paralysis rate
Synchronized L3 larvae (CL4176) were treated with 0.1 mg/mL CBP50-1 for 48 h at 16 °C and then transferred to 25 °C. The number of paralysis nematodes was recorded after 36 h. Every plate contained 40 nematodes.
2.9 Fluorescent staining of Aβ deposits in C. elegans strain
Synchronized L3 larvae CL4176 was treated with 0.1 mg/mL CBP50-1 for 36 h at 16 °C and then transferred to 25 °C for 36 h. The fluorescent staining of Aβ deposits in CL4176 was performed according to Wang et al. (2022).
2.10 Measurement of antioxidant enzyme activity and MDA level
Synchronized L3 larvae CL4176 was treated with 0.1 mg/mL CBP50-1 for 36 h at 16 °C and then transferred to 25 °C for 36 h. Antioxidant enzyme activity superoxide dismutase (SOD) and catalase (CAT) and malonaldehyde (MDA) content were determined with corresponding kits (Nanjing, Jiancheng Cot).
2.11 Quantitative real-time PCR (qRT-PCR)
Total RNA was extracted using the RNA simple Total RNA Kit, and the quality and quantity of RNA were assessed with Nanodrop ND-1000 (NanoDrop Technologies Wilmington, DE) and formaldehyde gel electrophoresis. cDNA was synthesized following the RrimeScript TMRT regent kit’s instructions. Quantitative Real-Time PCR was performed on the CFXTM96 Real-time system (BIO-RAD, California, USA) following the instructions of the SYBR Premix Ex Taq kit. The PCR protocol was as follows: 95 °C for 2 min and then 45 cycles of 95 °C for 5 s, 55 °C for 10 s, and 72 °C for 20 s. The results were evaluated using the 2−ΔΔCT method relative to Actin. Primer information is shown in Table. S2.
2.12 Statistical analysis
All analyses were done with SPSS 22.0. All the experiments were conducted three times independently. Data were presented as the mean ± standard error of the mean (SEM). P < 0.05 was regarded as significant.
3 Results
3.1 Effect of single-factors on the yield of CBP
To measure the influence of ultrasonic power (150, 180, 210, 240, and 270 W) on the yield of CBP, the extraction temperature was 50 °C, the extraction time was 50 min, and the ratio of liquid to solid was 25 mL/g. Polysaccharide yield increased with ultrasonic power, and it came up to the maximum when the power was 210 W. Subsequently, the yield dropped with the power (Fig. S1 A). Therefore, 210 W was selected as the optimum ultrasonic power.
When evaluating the impact of extraction times (30, 40, 50, 60, and 70 min) on the yield of CBP, the temperature was 50 °C, the ultrasonic power was 210 W, and the ratio of liquid to sample was 25 mL/g. The yield kept escalating with the extraction time, and it reached the maximum when the time was 60 min (Fig. S1 B). Subsequently, the yield exhibited a slight decline. Hence, 60 min was chosen for optimum.
To assess the effect of the extraction temperature (30, 40, 50, 60, and 70 °C) on the yield of CBP, the ultrasonic power, time, and the ratio of liquid to solid were followed by 210 W, 50 min, and 25 mL/g. The yield reached the maximum when the temperature was 60 °C, then it declined with the temperature (Fig. S1 C). Thus, 60 °C was chosen for optimum.
When appraising the influence of the solvent to the material ratio (15, 20, 25, 30, and 35 mL/g) on the CBP yield, the ultrasonic power, time, and extraction temperature were followed by 210 W, 50 min, and 50 °C. The CBP yield increased with the ratio, and it reached the maximum when the ratio was 25 mL/g (Fig. S1 D). The redundant solvent increased the diffusion distance of compounds from sample tissue to liquid and inhibited their dissolution (Shang et al., 2020). Accordingly, 25 mL/g was adopted for further optimum.
3.2 Optimization of CBP extraction condition by Box-Behnken design
3.2.1 The RSM model fitting
According to the experimental conditions given by the BBD design, the corresponding yield was presented in Supplementary material Table S1. The simulated model was shown in the following equation:
Where A1 is the ultrasonic power, A2 is time, A3 is temperature, A4 is the ratio of liquid to solid, and Y is the yield of CBP.
The Model F (11.05) and P (<0.05) implied the model was reliable. In this case, the P of two linear terms (A1, A3, and A4), four interactive terms (A1A3, A1A4, A2A3, A2A4, and A3A4), and two quadratic terms (A12, A32, and A42) were lower than 0.05, suggesting these parameters significantly affected the yield of CBP. The P (1.20) of “Lack of Fit” was not appreciable, which is consistent with Model F. These results suggested that the model could guide the experimental design (Table S1).
The “R2” (0.92) was not close to the “Adj R2” (0.83). This might be caused by the low interaction between the ultrasonic power and extraction time as well as extraction temperature and the ratio of liquid to sample. The interactions between ultrasonic power and temperature, ultrasonic power and the ratio of liquid to sample, temperature and time, and time and the ratio of liquid to sample were notable (Fig. S2). These results were consistent with the ANOVA analysis (Table S1).
3.2.2 Overall optimization and model verification
Based on the model equation, the optimum variables for CBP extraction were followed by 26.5 mL/g of liquid to solid ratio, 67.6 °C of temperature, 69.6 min of time, and 238.5 W of ultrasonic power, and the maximum yield of CBP was 97.4 mg glucose/g DW. For operation convenience, the proved experiment was done under the 26.5 mL/g of liquid to solid ratio and 240 W of ultrasonic power at 68 °C for 70 min. The actual yield of CBP was 95.91 ± 1.33 mg glucose/g DW, which was very similar to the predicted yield, suggesting the model was suitable for CBP extraction.
3.3 Physicochemical properties
The CBP was precipitated by 50 % ethanol and named CBP50. CBP50 was fractioned with DEAE-52 and then CBP50-1 (eluted by water) was further purified with Sephadex G-200 (Fig. S3). Its molecular weight was 61.02 KDa (Fig. S4), and it contained 89.67 % of sugar. CBP50-1 contained 1.89 % of protein and had no uronic acid.
The polysaccharide CBP50-1 mainly contained glucose (55.45 %) and xylose (25.27 %), along with arabinose (7.30 %), fucose (6.36 %), mannose (3.67 %), and rhamnose (1.95 %) (Fig. 1). The kind of monosaccharide was agreed with previous reports (Huang et al., 2018; Shi et al., 2020), and the difference in the proportion of each monosaccharide might due to the different origin and extraction treatment of the C. barometz rhizome.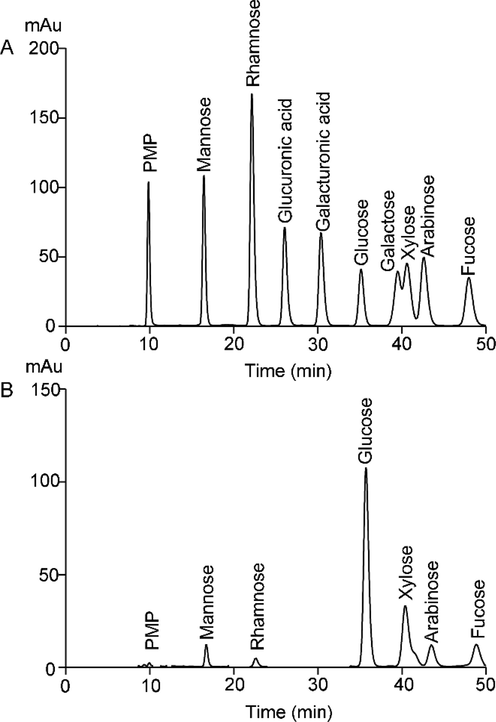
Monosaccharide composition of CBP50-1. (A) Standard monosaccharides. (B) Monosaccharides composition of CBP50-1.
3.4 The FT-IR and thermogravimetric analysis of CBP50-1
The FT-IR spectrum could provide information about the vibrations of atoms or functional groups in molecules, which is extensively used in the qualitative analysis of complex compounds. As shown in Fig. 2A, the bond at 3419 cm−1 was O—H, and the bond at 2937 cm−1 was C—H in the sugar ring. The peaks at 1611 cm−1 and 1417 cm−1 were attributed to the hydration peak. The band at 1253 cm−1 was the C—O—C bonds in acetyl esters and 1072 cm−1 was the C—O—H (Mzoughi et al., 2018). The characteristic peak at 894 cm−1 suggested the existence of a β-d-glucose pyranosyl ring in CBP50-1.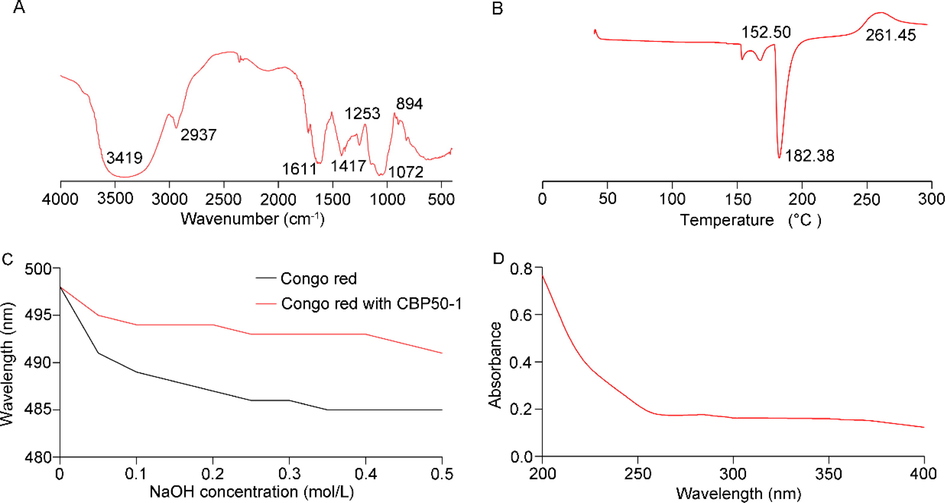
The property of CBP50-1. (A) FT-IR spectrum of CBP50-1. (B) Thermogravimetric analysis. (C) Congo red of CBP50-1. (D) UV spectrum of CBP50-1.
The differential thermogravimetry (DTG) curve of CBP50-1 was determined by TGA (Fig. 2B). A slight peak occurred at 152.50 °C due to the release of water from CBP50-1. Subsequently, an endothermic peak appeared at 182.38 °C indicted the exothermic decomposing and oxidation of the polysaccharides (Yu et al., 2019). Besides, the presence of an exothermic peak at 261.45 °C might be caused by the high thermal depolymerization and debranching of CBP50-1.
3.5 Congo red analysis and UV spectral of CBP50-1
The maximum absorption wavelength (λmax) change of polysaccharide and Congo red-complex in an alkaline solution could provide information about polysaccharide conformation. When Congo red forms a complex with a three-helix polysaccharide, the λmax will change. While NaOH exceeded 0.3 mol/L, its λmax decreased rapidly (Zhao et al, 2019). The λmax of control dropped with the concentration of the alkaline solution. The λmax of the mixture of Congo red and CBP50-1 showed the same tendency, indicating that CBP50-1 had no three-helix conformation (Fig. 2C).
No apparent peak occurred at 260 or 280 nm (Fig. 2D), suggesting CBP50-1 contained little nucleic acid and protein. This result confirmed the previous quantitative analysis of protein (3.3).
3.6 Antioxidant activity in vitro
Under normal physiological conditions, reactive oxygen species (ROS) are balanced by antioxidative defense systems. Once ROS overwhelms the cellular antioxidant system and causes oxidative damage, a series of untoward effects even neuron destruction occurs (Jiang et al., 2016). CBP50-1 showed a remarkable DPPH cleaning activity. It reached 82.57 % of the clearance rate at 1.0 mg/mL (Fig. 3A). Its hydroxyl radical scavenging rate was 65.51 % at 1.0 mg/mL (Fig. 3B). Additionally, CBP50-1 also showed strong anti-lipid-peroxidation capacity and its inhibition rate of lipid peroxidation was 51.83 % at 1.0 mg/mL (Fig. 3C).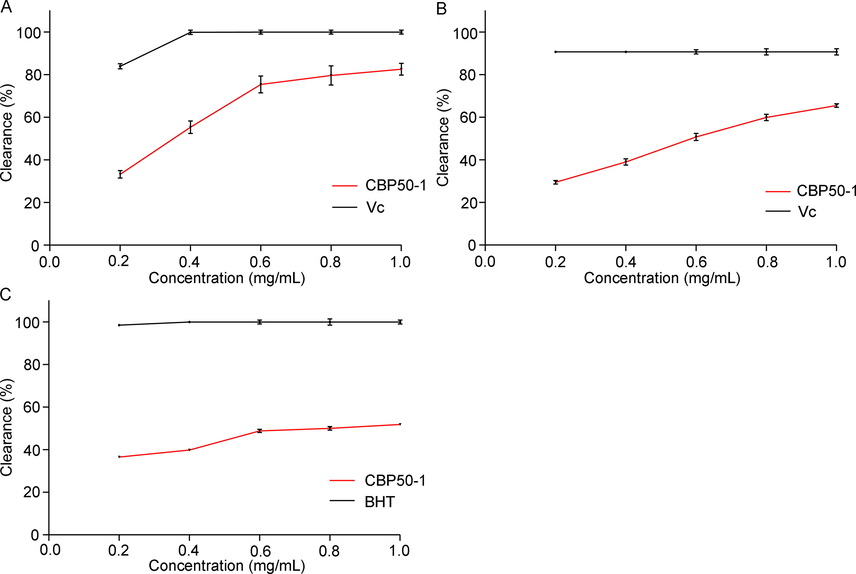
Antioxidant activity of CBP50-1. (A) DPPH radical cleaning activity. (B) hydroxyl radical cleaning activity. (C) anti-lipid peroxidation capacity.
3.7 Polysaccharide CBP50-1 improved the survival rate of N2 under heat and oxidative stress
The survival rate of the CBP50-1-treated worms was higher than the control under heat stress as well as acute oxidative stress (Fig. 4), indicating that CBP50-1 could improve the tolerance of N2 under adverse conditions. Other polysaccharides also played a similar role in N2 (Gu et al., 2020; Wang et al., 2019). Many pieces of research proved that oxidation was the main damage caused by heat stress (Hamilton et al., 2016; Kaldur et al., 2014). Therefore, CBP50-1 might be a good antioxidant in N2.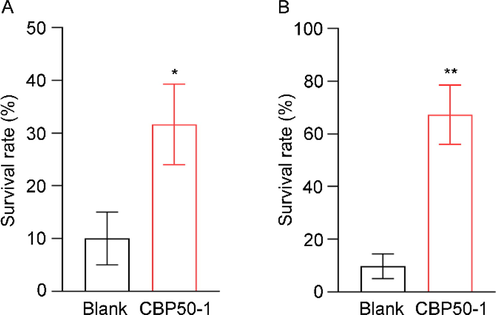
Effects of CBP50-1 on thermal stress and oxidative stress in N2. (A) Thermal stress. (B) Oxidative stress (* is significant at the 0.05 level, ** is significant at the 0.01 level).
3.8 Polysaccharide CBP50-1 relieved symptoms of paralysis and oxidative damage induced by Aβ in C. Elegans CL4176
Although the exact mechanism of AD has not been elucidated, Aβ was considered one of the major risk factors. It is effective for the treatment of AD by reducing or eliminating the negative consequences of Aβ (Sun et al., 2015). The expression of Aβ1-42 in the CBP50-1 group was higher than blank, but the mean fluorescent intensity was lower compared to the blank (Fig. 5A, B). These results indicated that CBP50-1 reduced the accumulation of Aβ1-42 in CL4176, which suggested CBP50-1 might work at the translational or posttranslational level. Besides, CBP50-1 could reduce the paralysis rate and the accumulation of MDA induced by Aβ (Fig. 5C, D).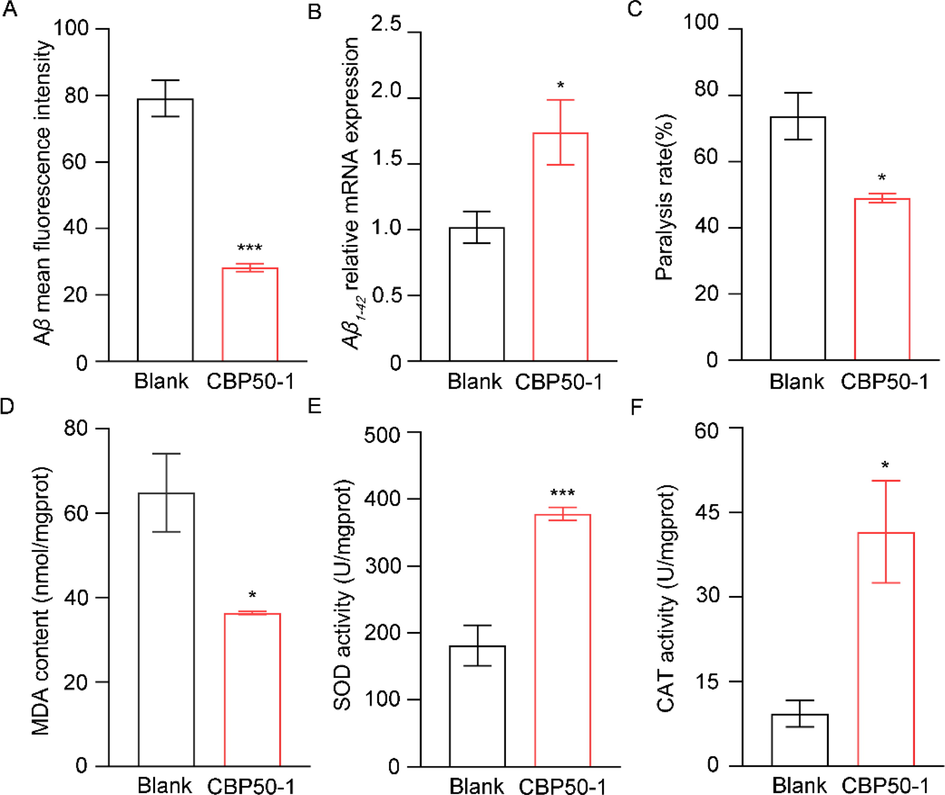
Effects of CBP50-1 on C. elegans CL4176. (A) Aβ deposits mean fluorescent intensity. (B) Aβ1-42 relative mRNA expression. (C) Paralysis rate. (D) MDA accumulation. (E) SOD activity. (F) CAT activity (* is significant at the 0.05 level, *** is significant at the 0.001 level).
Growing evidence indicated that Aβ-induced neurotoxicity was closely related to oxidative damage (Padurariu et al., 2013). Aβ induces oxidative damage to DNA, proteins, and lipids, and is one of the key reasons for neurological deterioration (Mancuso et al., 2010). CBP50-1 improved the capacity of SOD and CAT (Fig. 5E, F). These results indicated that CBP50-1 might promote the antioxidant system and reduce oxidative damage, and then relieve the symptom of paralysis.
3.9 Polysaccharide CBP50-1 could decrease the Aβ toxicity through the JNK MAPK pathway
The accumulation of Aβ will cause oxidative stress. c-Jun N-terminal kinase (JNK) mitogen-activated protein kinase MAPK signaling pathway, one of the MAPK signaling family, acts as a central signaling hub to regulate various important biological processes like movement, and stress resistance (Qu et al., 2020). Moreover, the activated C. elegans JNK MAPK can directly phosphorylate DAF-16 which plays an important role in stress resistance (Wolf et al., 2008). CBP50-1 could improve the expression of jnk-1 and up-regulate the transcription of daf-16 (Fig. 6).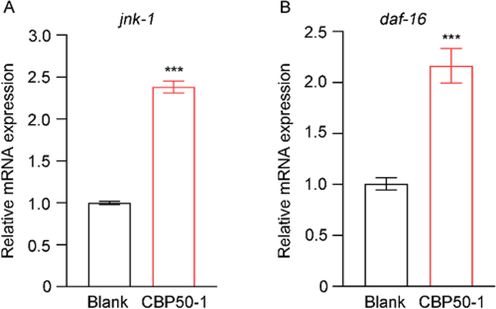
Relative mRNA expression. (A) jnk-1 expression. (B) daf-16 expression (*** is significant at the 0.001 level).
4 Discussion
Polysaccharide, as a native antioxidant, has been drawn more and more attention due to their low toxicity and stability in the foods or medical field. We optimized the process of hot water extraction of polysaccharide from C. barometz rhizome with a yield of 95.9 ± 1.3 mg glucose/g DW. Polysaccharides protect tissues primarily through antioxidant activity organisms. The main mechanism for its antioxidant activity is the riched hydroxyl which could scavenge free radicals. In addition, polysaccharide can reduce oxidative damage through the antioxidant system. CBP50-1 had strong antioxidant activity in vitro and could enhance the resistance of C. elegans to heat and acute oxidant stress.
The level of Aβ is in dynamic balance, and abnormal Aβ accumulation is one of the main etiologies in AD (Yan et al., 2022). Once the balance was upset, Aβ would accumulate, then a cascade reaction of inflammation, tau phosphorylation, and nerve fiber tangles occurred. These will exacerbate neurotoxicity. Antioxidant-based therapy is a potential pathway to relieve AD in mice (Dumont et al., 2009). C. elegans CL4176 expresses human Aβ1-42 in muscle cells when the temperature is higher than 23 °C, which exhibited paralysis symptoms related to AD. CBP50-1 could significantly reduce the accumulation of Aβ and paralysis of C. elegans CL4176. The polysaccharide from Coptis Franch and Cyclocarya paliurus also showed the same function (Li et al., 2018; Lin et al., 2020). To our astonishment, CBP50-1 up-regulated the expression of Aβ1-42, which should not be affected by the non-temperature factor. However, the result of fluorescent staining indicated a decrease in Aβ deposits treated by CBP50-1.
Oxidative stress is dangerous, and it is considered one of the main mechanisms which induce the neurodegenerative cascades of AD. Oxidative stress could lead to both the activation of AD-related pathways and the promotion of AD pathology. Oxidative stress promoted Aβ deposition in APP/PS1 mice, and reduced oxidative damage in mice (Lee et al., 2012; Dumont et al., 2009). CBP50-1 reduced the oxidative injury in CL4176 and N2 worms, which might explain why CBP50-1 decreased Aβ deposits but up-regulated the expression of Aβ1-42.
The JNK MAPK signaling was proven to regulate stress response in C. elegans (Mizuno et al., 2004; Zhao et al., 2015). The C. elegans JNK MAPK signaling pathway mainly contains JNK-1, a homolog of human JNK, and two MAP kinase kinases (MEK-1 and JKK-1). MEK-1 and JKK-1 act as activators of JNK. jnk-1 is expressed in the neurons and could impact daf-16 directly (Oh et al., 2005). Many natural compounds have proved that activated daf-16 could enhance the antioxidant activity of C. elegans under stress (Abdelfattah et al., 2022; Jabeen et al., 2020; Li et al., 2021). The deposition of Aβ would aggravate the injury in C. elegans CL4176, CBP50-1 could affect jnk-1 to activate daf-16, then the antioxidant enzyme activity was increased.
5 Conclusions
In this study, we extracted polysaccharides from the C. barometz root. The optimal parameters were ultrasonic power 234 W, liquid to solid ratio 26.5 mL/g, time 70 min, and temperature 68 °C. Under these conditions, the yield of CBP was 95.9 ± 1.3 mg glucose/g DW. CBP50-1 was a neutral polysaccharide with a molecular weight of 61.02 KDa, mainly consisting of glucose and xylose. CBP50-1 had notable antioxidant activities in vitro. Furthermore, it relieved the symptom of paralysis induced by the Aβ and enhanced the antioxidant system through the JNK MAPK pathway in the AD model of C. elegans. Taking together, CBP50-1 has a potential application in human health industries.
CRediT authorship contribution statement
Zhonghao Zhang: Conceptualization, Methodology, Investigation, Formal analysis, Data curation, Writing – original draft. Yujin Wang: Methodology. Tao Gao: Methodology. Zizhong Tang: Writing – review & editing. Lijun Zhou: Writing – review & editing. Tao Chen: Writing – review & editing. Shiling Feng: Methodology, Writing – review & editing. Chunbang Ding: Writing – review & editing. Shu Yuan: Validation. Ming Yuan: Conceptualization, Supervision, Resources, Funding acquisition, Project administration, Writing – review & editing.
Acknowledgments
This work was financially supported by Sichuan Agricultural University.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: [Ming Yuan reports financial support was provided by Sichuan Agricultural University.].
References
- Antioxidant and anti-aging effects of Warburgia salutaris bark aqueous extract: Evidences from in silico, in vitro and in vivo studies. J. Ethnopharmacol.. 2022;292:115187
- [CrossRef] [Google Scholar]
- Forecasting the prevalence of preclinical and clinical Alzheimer's disease in the United States. Alzheimers Dement: J. Alzheimer's Assoc.. 2018;14(2):121-129.
- [CrossRef] [Google Scholar]
- Effect of enzyme-assisted extraction on the physicochemical properties of mucilage from the fronds of Asplenium australasicum (J. Sm.) Hook. Int. J. Biol. Macromol.. 2019;124:346-353.
- [CrossRef] [Google Scholar]
- Reduction of oxidative stress, amyloid deposition, and memory deficit by manganese superoxide dismutase overexpression in a transgenic mouse model of Alzheimer's disease. FASEB J.. 2009;23(8):2459-2466.
- [CrossRef] [Google Scholar]
- Panax notoginseng polysaccharide increases stress resistance and extends lifespan in Caenorhabditis elegans. J. Funct. Foods. 2018;45:15-23.
- [CrossRef] [Google Scholar]
- Review of the advances in treatment for Alzheimer disease: strategies for combating β-amyloid protein. Neurologia (English Ed).. 2018;33(1):47-58.
- [CrossRef] [Google Scholar]
- Ultrasonic-assisted extraction of polysaccharides from Auricularia auricula and effects of its acid hydrolysate on the biological function of Caenorhabditis elegans. Int. J. Biol. Macromol.. 2020;167:423-433.
- [CrossRef] [Google Scholar]
- Evaluation of lasting effects of heat stress on sperm profile and oxidative status of ram semen and epididymal sperm. Oxid. Med. Cell. Longev.. 2016;2016:1687657.
- [CrossRef] [Google Scholar]
- Structural elucidation and osteogenic activities of two novel heteropolysaccharides obtained from water extraction residues of Cibotium barometz. Ind. Crop Prod.. 2018;121:216-225.
- [CrossRef] [Google Scholar]
- Date (Phoenix dactylifera) seed and syringic acid exhibits antioxidative effect and lifespan extending properties in Caenorhabditis elegans. Arab. J. Chem.. 2020;13(12):9058-9067.
- [CrossRef] [Google Scholar]
- Oxidative stress: A major pathogenesis and potential therapeutic target of antioxidative agents in Parkinson's disease and Alzheimer's disease. Prog. Neurobiol.. 2016;147:1-19.
- [CrossRef] [Google Scholar]
- Effects of heat acclimation on changes in oxidative stress and inflammation caused by endurance capacity test in the heat. Oxid. Med. Cell. Longev.. 2014;2014:1-8.
- [CrossRef] [Google Scholar]
- Early induction of oxidative stress in mouse model of Alzheimer disease with reduced mitochondrial superoxide dismutase activity. PLoS One. 2012;7(1):e28033.
- [Google Scholar]
- Neuroprotective effects of Coptis chinensis Franch polysaccharide on amyloid-beta (Aβ)-induced toxicity in a transgenic Caenorhabditis elegans model of Alzheimer's disease (AD) Int. J. Biol. Macromol.. 2018;113:991-995.
- [CrossRef] [Google Scholar]
- Trilobatin, a component from Lithocarpus polystachyrus Rehd., increases longevity in C. elegans through activating SKN1/SIRT3/DAF16 signaling pathway. Front. Pharmacol.. 2021;12:655045
- [CrossRef] [Google Scholar]
- Polysaccharide extracted from the leaves of Cyclocarya paliurus (Batal.) Iljinskaja enhanced stress resistance in Caenorhabditis elegans via skn-1 and hsf-1. Int J Biol Macromol.. 2020;143:243-254.
- [CrossRef] [Google Scholar]
- The derivatization and antioxidant activities of yeast mannan. Int. J. Biol. Macromol.. 2018;107(Pt A):755-761.
- [CrossRef] [Google Scholar]
- Clinical features and pathogenesis of Alzheimer’s Disease: Involvement of mitochondria and Mmitochondrial DNA. In: Ahmad S.I., ed. Diseases of DNA Repair. New York: Springer; 2010. p. :34-44.
- [Google Scholar]
- The Caenorhabditis elegans MAPK phosphatase VHP-1 mediates a novel JNK-like signaling pathway in stress response. EMBO J.. 2004;23(11):2226-2234.
- [CrossRef] [Google Scholar]
- Optimized extraction of pectin-like polysaccharide from Suaeda fruticosa leaves: characterization, antioxidant, anti-inflammatory and analgesic activities. Carbohyd Polym.. 2018;185:127-137.
- [CrossRef] [Google Scholar]
- JNK regulates lifespan in Caenorhabditis elegans by modulating nuclear translocation of forkhead transcription factor/DAF-16. P Natl Acad Sci USA. 2005;102(12):4494-4499.
- [CrossRef] [Google Scholar]
- The oxidative stress hypothesis in Alzheimer's disease. Psychiat Danub.. 2013;25(4):401-409.
- [Google Scholar]
- Exposure to low-dose nanopolystyrene induces the response of neuronal JNK MAPK signaling pathway in nematode Caenorhabditis elegans. Environ. Sci. Eur.. 2020;32(1):58.
- [CrossRef] [Google Scholar]
- N-acetylcysteine on oxidative damage in diabetic rats. Drug Chem. Toxicol.. 2011;34(4):467-474.
- [CrossRef] [Google Scholar]
- Extraction, purification, emulsifying property, hypoglycemic activity, and antioxidant activity of polysaccharides from comfrey. Ind. Crop Prod.. 2020;146:112183
- [CrossRef] [Google Scholar]
- The effect of polysaccharides from Cibotium barometz on enhancing temozolomide-induced glutathione exhausted in human glioblastoma U87 cells, as revealed by (1)H NMR metabolomics analysis. Int. J. Biol. Macromol.. 2020;156:471-484.
- [CrossRef] [Google Scholar]
- Beta-Amyloid: the key peptide in the pathogenesis of Alzheimer's disease. Front. Pharmacol.. 2015;6:221.
- [CrossRef] [Google Scholar]
- Structural characterization and antioxidant activity of alkali-extracted polysaccharides from quinoa. Food Hydrocolloid.. 2020;133:106392
- [CrossRef] [Google Scholar]
- Structural characterization and antioxidant activities of a novel fructan from Achyranthes bidentata Blume, a famous medicinal plant in China. Ind. Crop Prod.. 2015;70:427-434.
- [CrossRef] [Google Scholar]
- Chondroitin sulfate E alleviates β-amyloid toxicity in transgenic Caenorhabditis elegans by inhibiting its aggregation. Int. J. Biol. Macromol.. 2022;209:1280-1287.
- [CrossRef] [Google Scholar]
- The anti-aging effects of Gracilaria lemaneiformis polysaccharide in Caenorhabditis elegans. Int. J. Biol. Macromol.. 2019;140:600-604.
- [CrossRef] [Google Scholar]
- The MAP kinase JNK-1 of Caenorhabditis elegans: location, activation, and influences over temperature-dependent insulin-like signaling, stress responses, and fitness. J. Cell. Physiol.. 2008;214(3):721-729.
- [CrossRef] [Google Scholar]
- Antioxidant activity of a water-soluble polysaccharide purified from Pteridium aquilinum. Carbohyd. Res.. 2009;344(2):217-222.
- [CrossRef] [Google Scholar]
- The extraction, antioxidant and against β-amyloid induced toxicity of polyphenols from Alsophila spinulosa leaves. Arab. J. Chem.. 2022;15(4):103707
- [CrossRef] [Google Scholar]
- Early-life and chronic exposure to di(2-ethylhexyl) phthalate enhances amyloid-β toxicity associated with an autophagy-related gene in Caenorhabditis elegans Alzheimer’s disease models. Chemosphere. 2021;273:128594
- [CrossRef] [Google Scholar]
- Development of antibacterial pectin from Akebia trifoliata var. australis waste for accelerated wound healing. Carbohyd Polym.. 2019;217:58-68.
- [CrossRef] [Google Scholar]
- Zhang, X.C., Nishida, H., 2013. Cibotiaceae. In: Wu, Z.Y., Raven, P.H., Hong, D.Y. (Eds.), Flora of China. Science Press, Beijing, Missouri Botanical Garden Press, St. Louis, pp. 132-133.
- Constituent and effects of polysaccharides isolated from Sophora moorcroftiana seeds on lifespan, reproduction, stress resistance, and antimicrobial capacity in Caenorhabditis elegans. Chin. J. Nat. Med.. 2018;16:252-260.
- [CrossRef] [Google Scholar]
- Antioxidant and immunomodulatory activities of polysaccharides from the rhizome of Dryopteris crassirhizoma Nakai. Int. J. Biol. Macromol.. 2019;130:238-244.
- [CrossRef] [Google Scholar]
- A microRNAs–mRNAs network involved in the control of graphene oxide toxicity in Caenorhabditis elegans. RSC Adv.. 2015;5(112):92394-92405.
- [CrossRef] [Google Scholar]
- Oxidative stress induces intralysosomal accumulation of Alzheimer amyloid beta-protein in cultured neuroblastoma cells. Ann. N.Y. Acad. Sci.. 2006;1067:248-251.
- [CrossRef] [Google Scholar]
- Recent advances in plant polysaccharide-mediated nano drug delivery systems. Int. J. Biol. Macromol.. 2020;165:2668-2683.
- [CrossRef] [Google Scholar]
- Decreasing oxidative stress and neuroinflammation with a multifunctional peptide rescues memory deficits in mice with Alzheimer disease. Free Radical Bio Med.. 2014;74:50-63.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2023.104597.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1






