Translate this page into:
Bisphenol A and bisphenol AF co-exposure induces apoptosis in human granulosa cell line KGN through intracellular stress-dependent mechanisms
⁎Corresponding author at: Inflammation & Allergic Diseases Research Unit, The Affiliated Hospital of Southwest Medical University, Luzhou 646000, China. youngm1@126.com (Meng Yang)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Bisphenol A (BPA) is a chemical substance used in large amounts in the production of epoxy resins, phenolic resins and polycarbonate plastics. Although its toxicity to the female reproductive system has been extensively studied, little is known about the combined toxicity of BPA and its analogues. In this study, the human granulosa cell line KGN was co-exposed to BPA and bisphenol AF (BPAF), and we subsequently examined the effect of these chemicals on cell apoptosis and their mechanism. We found that co-exposure to BPA and BPAF induced intracellular stress in KGN cells, including significantly elevated levels of reactive oxygen species (ROS) and calcium ions (Ca2+). Then, apoptosis and its associated biological events, including increased caspase-3 activity, and decreased Bcl-2/Bax protein expression ratio, were measured under the combined exposure. Notably, we confirmed that the intracellular stress and activation of the signaling axis of the downstream ASK1-JNK signaling pathway involved in the apoptosis of KGN cells was induced by the mixture of BPA and BPAF. In addition, we verified with pretreatment inhibitors that the BPA and BPAF mixture promoted the co-induction of KGN cell apoptosis via this stress pathway. Our work provides novel insights into the combined cytotoxicity and molecular toxicity mechanism of bisphenol mixtures.
Keywords
Bisphenol A
Bisphenol AF
Co-exposure
Apoptosis
Oxidative stress
Granulosa cells
1 Introduction
Bisphenol A (BPA) is used in the manufacture of epoxy resins, phenolic resins, and polycarbonate plastics, and large quantities of this chemical are produced worldwide (Rubin, 2011; Flint et al., 2012). Widespread use of BPA-containing products leads to ubiquitous BPA exposure. Previous reports indicated that BPA is present in various human body fluids (Kuruto-Niwa et al., 2007; He et al., 2009; Minatoya et al., 2017). In addition, elevated levels of BPA are closely related to the occurrence of various diseases and health conditions (Fenichel et al., 2013; Mirmira and Evans-Molina, 2014; Rezg et al., 2014).
Considering the adverse health effects of BPA, its use is being limited and gradually replaced by other bisphenols (Rosenmai et al., 2014; Eladak et al., 2015). Bisphenol AF (BPAF; fluorine-containing BPA) is one of the most important substitutes for BPA and is widely used in industry and to manufacture daily necessities (Chen et al., 2016). Although BPAF is considered a safe alternative to BPA, studies have shown that it has stronger female reproductive toxicity than BPA. It has been established that it interferes with the synthesis of steroid hormones, and also causes ovarian follicular atresia characterized by granulosa cell apoptosis, which ultimately leads to follicular development disorders (Shi et al., 2015; Yang et al., 2016; Jones et al., 2018). Besides, BPAF also shows a stronger ability to induce apoptosis as compared to BPA (Ding et al., 2017; Maćczak et al., 2017; Mokra et al., 2018; Meng et al., 2019; Huang et al., 2020c).
Granulosa cells are the main component of the ovarian follicle, and their survival or death plays a crucial role in determining whether the follicle develops normally or not (Albertini et al., 2001; Buratini and Price, 2011). The primary mechanism of follicular atresia and degeneration of the corpus luteum involves the physiological apoptosis of granulosa cells (Hughes and Gorospe, 1991; Matsuda et al., 2012; Regan et al., 2018). Empirical studies have highlighted that under pathological conditions, such as non-physiological apoptosis of granulosa cells caused by exposure to exogenous chemicals, follicular development disorders can occur (Jia et al., 2011; Lee et al., 2013; Migliaccio et al., 2018). In our previous studies, we have elucidated that BPA and BPAF exposure alone can cause granulosa cell apoptosis, while recent animal studies have shown that BPA or BPAF exposure can lead to the development of abnormal follicular atresia characterized by granulosa cell apoptosis in mice (Jones et al., 2018; Huang et al., 2020a; Huang et al., 2020b).
Although the toxicity of bisphenols, especially BPA, to the female reproductive tract has been extensively studied, human beings are usually simultaneously exposed to multiple bisphenols due to their contaminating presence in the natural environment. Previous measurements indicate that BPA and BPAF coexist in different environmental matrices and human body fluids, but little is known about their combined reproductive toxicity, and the underlying mechanism remains poorly understood. Hence, the aim of this study is to examine the effects of co-exposure of BPA and BPAF on the apoptosis of the human granulosa cell line KGN and its mechanism. We found that co-exposure to BPA and BPAF induced intracellular stress in KGN cells, including significantly elevated levels of reactive oxygen species (ROS) and calcium ions (Ca2+). In addition, we further confirmed that BPA and BPAF co-exposure promoted the apoptosis of KGN cells, which was regulated by the apoptosis signal-regulating kinase 1-c-Jun N-terminal kinase (ASK1-JNK) pathway and mediated by intracellular stress.
2 Materials and methods
2.1 Reagents
BPA and BPAF (analytical purity, 99–99.5%) were purchased from Sigma-Aldrich (Darmstadt, Germany). The fluorescent probe for calcein AM, DCFH-DA, Fluo-3/AM and FITC-Annexin V were purchased from US Everbright Inc. (Suzhou, China). N-acetyl cysteine (NAC) was purchased from Beyotime Biotechnology (Shanghai, China). GS-4997 (selonsertib) was purchased from Apex Biotechnology (Houston, USA). SP600125 and BAPTA-AM were purchased from AbMole (Houston, TX, USA). YF®488-Annexin V apoptosis kit and caspase-3 assay kit were purchased from US Everbright Inc. (Suzhou, China). Anti-Bcl-2, Bax and ASK1 antibodies were purchased from Abcam (Cambridge, USA). Anti-JNK, β-actin and anti-ASK1 and JNK phosphorylation antibodies were purchased from Cell Signaling Technology (Danvers, MA, USA).
2.2 Cell culture and treatment
KGN cells were purchased from Cellcook, Ltd. (Guangzhou, China). Short tandem repeat (STR) profiling was performed to ensure authenticity. Cells were cultured in Dulbecco’s modified Eagle’s medium/Nutrient Mixture F-12 (DMEM/F-12) cell culture media supplemented with 10% fetal bovine serum (FBS), 100 IU/mL penicillin/streptomycin, at 37 °C in an atmosphere of 95% humidified air and 5% CO2. Cultured KGN cells were treated with BPA and/or BPAF for 24 h and then tested according to the experimental design. The concentrations of BPA used in this study were 30 μM and 50 μM, the concentrations of BPAF were 3 μM and 5 μM, and their combined exposure concentrations were BPA 30 μM + BPAF 3 μM and BPA 50 μM + BPAF 5 μM.
2.3 Cell viability measurement
Cell Counting Kit-8 (CCK-8, AbMole, USA) was used to measure changes in cell viability after BPA and/or BPAF exposure according to our previous description (Huang et al., 2020c). In addition, we further used the calcein AM probe to detect changes in cell viability. After the cells seeded in 12-well plates were exposed to BPA and/or BPAF for 24 h, the culture media was aspirated, followed by washing the cells once with PBS. Then 500 μL of calcein AM detection working solution was added per well, and the plates were incubated for 30 min at 37 °C in the dark. After incubation, the cells were observed under a laser confocal microscope (Olympus, Japan).
2.4 Intracellular ROS level detection
The cells were cultured in a 12-well plate and trypsinized after exposure to BPA and/or BPAF for 24 h. After centrifuging the digested cells, the DCFH-DA (Ex/Em: 488 nm/530 nm) fluorescent probes were loaded. After incubating for 30 min at 37 °C, the cells were washed twice with serum-free cell culture media to fully remove the DCFH-DA that did not enter the cells. The changes in the intracellular levels of ROS were measured using flow cytometry (ACEA Biosciences, USA).
2.5 Intracellular Ca2+ level detection
The cells were cultured in a 12-well plate and trypsinized after exposure to BPA and/or BPAF for 24 h. Afterward, the media was removed and the cells were washed three times with PBS. Then, the Fluo-3/AM working solution was added to the cell suspension, which was incubated at 37 °C for 60 min. After removing the working solution and washing the cells three times with PBS, a suspension of 1 × 105 cells/mL was created. Thereafter, the cells were incubated further at 37 °C for 10 min to ensure complete de-esterification of AM in the cells. Lastly, the changes in intracellular Ca2+ levels were detected using flow cytometry (ACEA Biosciences, USA). The excitation wavelength and emission wavelength of Fluo-3/AM were 506 nm and 526 nm, respectively.
2.6 Antioxidant capacity detection
Cells were cultured in a 6-well plate and treated with BPA and/or BPAF for 24 h. Then, the antioxidant capacity was measured according to the instructions of the corresponding kit (Jiancheng Bioengineering Institute, Nanjing, China), including that of superoxide dismutase (SOD), catalase (CAT), and glutathione (GSH).
2.7 Western blotting analysis
Cells were cultured in a 6-well plate and treated with BPA and/or BPAF for a specified time. The cells were lysed with RIPA lysis buffer and centrifuged to obtain the supernatant. After determining the protein concentration using the BCA method, the denatured protein (20 μg) was resolved by SDS/PAGE using a 12% gel and then transferred to a PVDF membrane. After a blocking step, the membrane was incubated with the primary antibody overnight at 4 °C. The membrane was then rinsed with TBST and incubated with the corresponding secondary antibody at room temperature for 1 h. After sufficient rinsing, the immune complexes were visualized using the enhanced chemiluminescence reaction (NCM Biotech, China) (additional information regarding the antibodies used in this study is described in the supplemental material).
2.8 Apoptosis analysis
After 24 h of BPA and/or BPAF exposure, the cells were cultured in 12-well plates and digested with trypsin without EDTA. Briefly, cells were washed twice with prechilled PBS and centrifuged at 300 × g for 5 min at 4 °C. Subsequently, approximately 1 × 105 cells were collected and resuspended with 100 μL of 1 × binding buffer. Following this, 5 μL of Annexin V and 5 μL of PI working solution were added to each tube. Eventually, after incubation at room temperature for 15 min, 400 μL of PBS was added to each tube. Flow cytometry was used to detect the apoptosis of granulosa cells (Annexin V: excitation wavelength, 490 nm; emission wavelength, 515 nm; PI: excitation wavelength, 535 nm; emission wavelength, 617 nm).
2.9 Caspase-3 activity detection
After exposure to BPA and/or BPAF for 24 h, the cells were trypsinized. Then the cells were resuspended in culture media at a density of 106 cells/mL. We detected changes in caspase-3 activity according to the kit instructions (Cat. No. S6007, US Everbright Inc., Suzhou, China). Thereafter, 0.2 mL of cell suspension was pipetted into a flow cytometry tube, 5 μL of 0.2 mM substrate was added, and the solution was immediately mixed to form a substrate concentration of 5 μM. In addition, the cells were incubated for 30 min in the dark at room temperature. After adding 300 μL of media, the caspase activity was analyzed with flow cytometry (ACEA Biosciences, USA). The excitation wavelength was 500 nm, and emission wavelength was 530 nm.
2.10 Statistical analysis
The data are presented as the mean ± SD from at least three independent experiments. Comparison of multiple groups was performed using one-way ANOVA, followed by Tukey’s multiple comparison test. A value of P < 0.05 was deemed statistically significant.
3 Results
3.1 BPA and/or BPAF exposure reduced the cell viability of KGN cells
We used CCK-8 to measure the changes in cell viability after exposure to BPA, BPAF and their mixtures. Compared with the control group, exposure to high concentrations of BPA (50 μM) and BPAF (5 μM) significantly reduced the viability of KGN cells. In particular, the combined exposure of BPA and BPAF significantly reduced cell viability at both low (30 μM + 3 μM) and high (50 μM + 5 μM) concentrations (Fig. 1). Likewise, using calcein AM probe labeling, we also found that the viability of KGN cells was considerably reduced after co-exposure to BPA and BPAF (Fig. 2).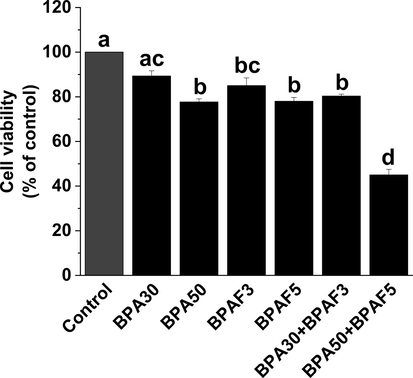
BPA and/or BPAF exposure reduces the cell viability of KGN cells. KGN cells were treated with BPA and/or BPAF for 24 h. We used the CCK-8 method to evaluate cellular viability. The data was expressed as the mean ± SD (n = 6). Different lowercase letters indicate significant differences between the treatment groups (P < 0.05).
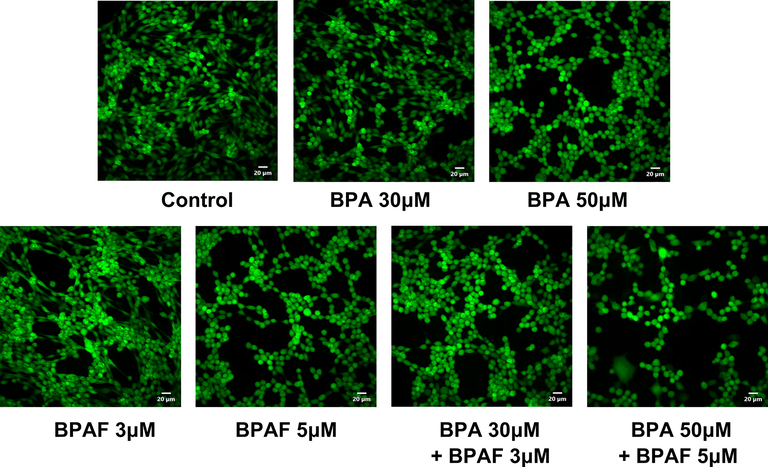
Cell viability changes in KGN cells after exposure to BPA and/or BPAF. Images of cell viability in KGN cells after exposure to BPA and/or BPAF. KGN cells were labeled using calcein AM probes. Scale bar = 20 μm.
3.2 BPA and/or BPAF exposure elevates levels of ROS and Ca2+ and induces apoptosis of KGN cells
The use of fluorescent probe labeling demonstrated that ROS and Ca2+ levels in KGN cells markedly increased after exposure to high concentrations of BPA (50 μM) or BPAF (5 μM) alone, and their mixture (50 μM + 5 μM) (Fig. 3A and 3B). In contrast, the antioxidant capacities of CAT, SOD, and GSH were significantly decreased under combined exposure to BPA and BPAF (50 μM + 5 μM) (Fig. 3C).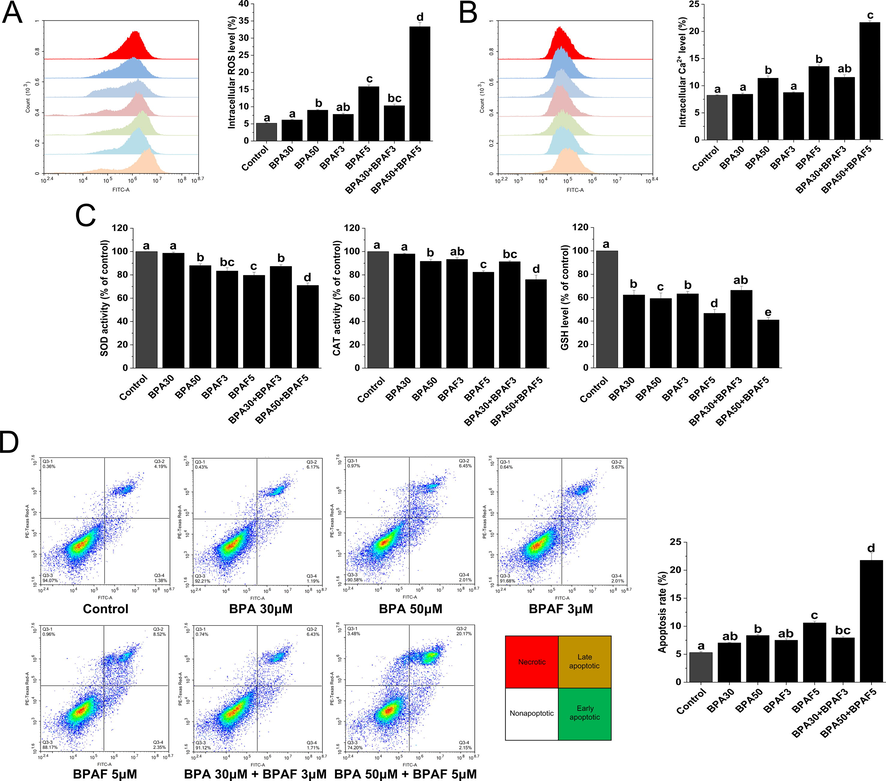
BPA and/or BPAF exposure induces intracellular stress generation and apoptosis in KGN cells. (A) Intracellular ROS was assessed using flow cytometry. (B) Intracellular Ca2+ was analyzed using flow cytometry after Fluo-3 labeling. (C) Cellular antioxidant capacity, via such agents as SOD, CAT and GSH were evaluated using the corresponding kit. (D) Flow cytometry was used to detect apoptosis. The data was expressed as the mean ± SD (n = 3). Different lowercase letters indicate significant differences between the treatment groups (P < 0.05).
We used flow cytometry to detect the effects of exposure to BPA, BPAF and their mixtures on KGN cell apoptosis. We found that exposure to high concentrations of BPA (50 μM) or BPAF (5 μM) alone, and their mixture (50 μM + 5 μM) can significantly induce apoptosis in KGN cells, while the apoptosis levels exhibited no significant difference after exposure to low concentrations of BPA (30 μM), BPAF (3 μM) or their mixtures (30 μM + 3 μM) (Fig. 3D).
3.3 BPA and/or BPAF exposure decreases the Bcl-2/Bax ratio and increases caspase-3 activity in KGN cells
Using the western blotting method, we measured the effect of BPA, BPAF, and their mixture on the reduction of Bcl-2 protein expression and the promotion of Bax protein expression. The expression ratio of Bcl-2/Bax protein indicated that the mixture exerted a more significant reduction effect on it (Fig. 4A).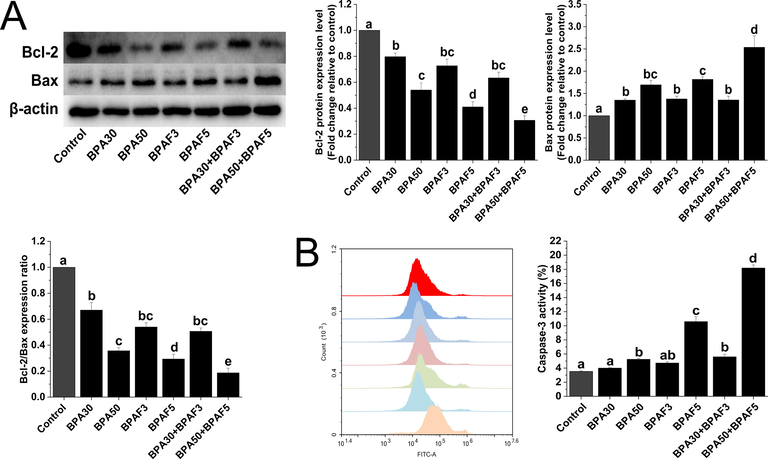
BPA and/or BPAF exposure decreases the Bcl-2/Bax ratio and increases caspase-3 activity in KGN cells. (A) Western blot analyses of Bcl-2 and Bax proteins. β-actin was used as an internal control. (B) Flow cytometry was used to analyze the changes in caspase-3 activity. The data was expressed as the mean ± SD (n = 3). Different lowercase letters indicate significant differences between the treatment groups (P < 0.05).
Caspase-3 is an enzyme necessary for a mitochondrial pathway to induce apoptosis. Our findings showed that the caspase-3 activity significantly increased after co-exposure to BPA and BPAF (Fig. 4B).
3.4 Intracellular stress is involved in KGN cell apoptosis induced by BPA and BPAF co-exposure
We herein further validated the role of ROS and Ca2+ in the induction of apoptosis in KGN cells by BPA and BPAF co-exposure. Using flow cytometry assays, we found that pre-treatment with NAC (an antioxidant) and BAPTA-AM (a calcium chelator) significantly attenuated apoptosis induced by the BPA and BPAF mixture (Fig. 5A). In addition, we recorded a concomitant decrease in caspase-3 activity (Fig. 5C). The attenuating effect of NAC and BAPTA-AM on the apoptosis of KGN cells induced by the combination of BPA and BPAF was also observed by fluorescence microscopy (Fig. 5D).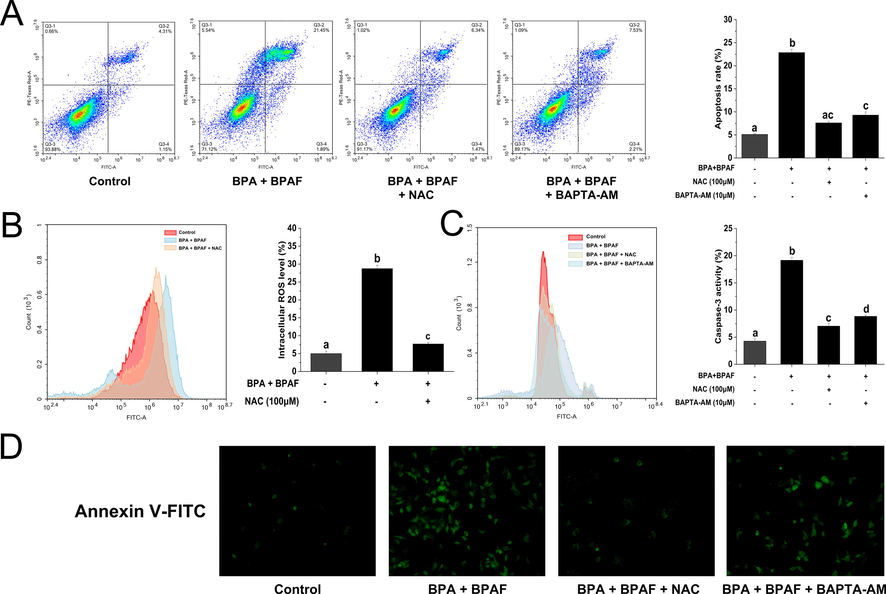
Intracellular stress is involved in KGN cell apoptosis induced by BPA and BPAF co-exposure. (A) After pretreatment with NAC and BAPTA-AM, flow cytometry was used to detect apoptosis. (B) After NAC pretreatment, the changes in intracellular ROS were detected by DCFH-DA probe labeling. (C) Caspase-3 activity was measured after NAC and BAPTA-AM pretreatment. (D) Apoptosis was detected using FITC-Annexin V labeling after NAC and BAPTA-AM pretreatment. Scale bar = 20 μm. The data was expressed as the mean ± SD (n = 3). Different lowercase letters indicate significant differences between the treatment groups (P < 0.05).
3.5 BPA and/or BPAF exposure induces downstream signal activation of intracellular stress
ASK1 is a family member of MAP3K that activates both the JNK and p38 MAPK signaling cascades in response to an array of stresses. Here, we used the western blotting technique to measure the activation of ASK1 and JNK proteins after co-exposure to BPA and BPAF. The results illustrated that compared with BPA or BPAF exposure alone, their co-exposure significantly increased the phosphorylation levels of ASK1 and JNK proteins (Fig. 6A). After pretreatment with GS-4997 (ASK1 inhibitor, 5 μM) and SP600125 (JNK inhibitor, 5 μM) for 1 h, we co-exposed KGN cells to BPA and BPAF for 24 h. We recorded that the apoptosis of KGN cells induced by co-exposure to BPA and BPAF was significantly weakened (Fig. 6B). These findings suggest that the ASK1-JNK signal axis was involved in granulosa cell apoptosis induced by the mixture of BPA and BPAF.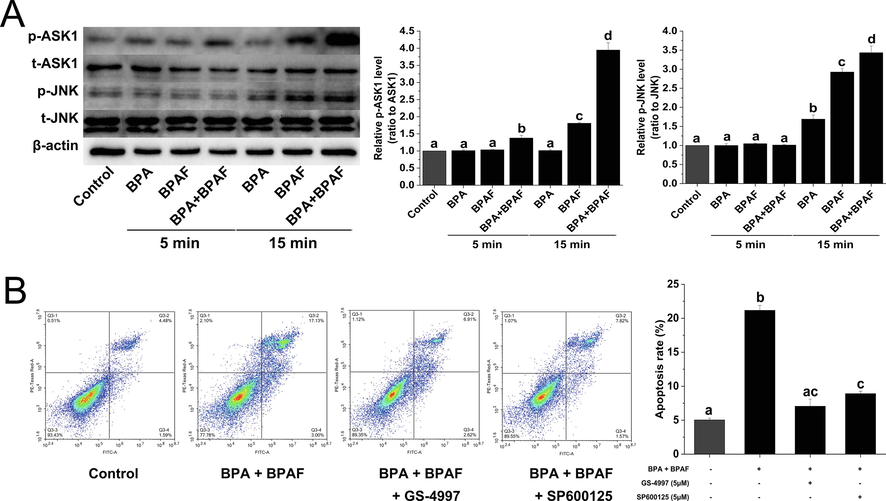
BPA and/or BPAF exposure induces downstream signal activation of intracellular stress. (A) The changes in the phosphorylation levels of ASK1 and JNK proteins were analyzed by western blot. β-actin was used as an internal control. (B) Flow cytometry was used to detect apoptosis after pretreatment with GS-4997 (ASK1 inhibitor) and SP600125 (JNK inhibitor). The data was expressed as the mean ± SD (n = 3). Different lowercase letters indicate significant differences between the treatment groups (P < 0.05).
4 Discussion
To date, bisphenols have been detected in many ordinary items, indoor dust, surface water, sediment and sewage (Loganathan and Kannan, 2011; Liao et al., 2012a; Liao et al., 2012b; Yamazaki et al., 2015). However, little is known about their combined toxicity to the female reproductive system. In this context, we herein used KGN cells that maintained the physiological functions of ovarian granulosa cells to evaluate the effect of co-exposure to BPA and BPAF on cell apoptosis and its underlying mechanism.
In the present study, we demonstrated that the combined exposure of BPA and BPAF can significantly induce intracellular stress and activate its downstream signaling pathway ASK1-JNK in KGN cells.
A large number of studies have confirmed that BPA exposure can cause oxidative stress in different types of cells (Babu et al., 2013; Xin et al., 2014; Barbonetti et al., 2016; Yuan et al., 2019). We previously reported that exposure to BPA or BPAF alone significantly induced the production of oxidative stress in granulosa cells. In this study, we observed that the co-exposure to BPA and BPAF significantly increased the levels of intracellular reactive oxygen species (ROS) in KGN cells. In contrast, the antioxidant capacity, via such agents as SOD, CAT, and GSH, was significantly reduced. Oxidative stress is the main mechanism that causes follicular developmental disorders (Sabuncu et al., 2001; Yeon Lee et al., 2010; Murri et al., 2013). Studies have found that in the follicular fluid of patients with polycystic ovary syndrome, which is one of diseases that induces female infertility, the amount of BPA was significantly higher than that in healthy women (Kandaraki et al., 2011; Rutkowska and Rachoń, 2014; Vahedi et al., 2016). Our results suggest that as a major component of intracellular stress, oxidative stress induced by the combination of BPA and BPAF may be involved in granulosa cell damage and female reproductive toxicity.
In addition to ROS, Ca2+ is another important second messenger that has a well-appreciated role in the transmission of biological information in the cytoplasm, thereby activating many enzymes or proteins with physiological activities (Cerella et al., 2010; Bagur and Hajnóczky, 2017). In the present investigation, we found that co-exposure to BPA and BPAF caused an increase in the levels of intracellular Ca2+. Previous reports, including ours, have confirmed that BPA and its analogues, such as bisphenol S (BPS), bisphenol F (BPF), and BPAF alone, can substantially increase intracellular calcium levels (Maćczak et al., 2016; Huang et al., 2020a; Huang et al., 2020c). Nevertheless, to our knowledge, this is the first report that shows that co-exposure to BPA and BPAF can modulate the increase in Ca2+ concentration in granulosa cells.
Apoptosis is the main mechanism of follicular development disorder and even abnormal follicle atresia. Elsewhere, it has been reported that follicular atresia in mice was caused by BPA or BPAF exposure alone (Jones et al., 2018). Likewise, in our previous studies, we also found that BPA or BPAF exposure can cause apoptosis of granulosa cells cultured in vitro (Huang et al., 2020a; Huang et al., 2020b). In the present study, we noted that co-exposure to BPA and BPAF also induces apoptosis of granulosa cells. Finally, the attenuation of granulosa cell apoptosis was further detected after pretreatment with NAC (an antioxidant) or BAPTA-AM (a calcium chelator). Our study revealed that intracellular stress, including ROS overproduction and Ca2+ overload, play a decisive role in BPA and BPAF co-induced apoptosis of granulosa cells.
The generation of intracellular ROS and the increase in Ca2+ levels are the main sources of intracellular stress, which regulate the occurrence of biological events including apoptosis (Görlach et al., 2015). ASK1 is preferentially activated in response to various types of stress, and plays a key role in various cellular responses such as apoptosis, differentiation, and inflammation (Takeda et al., 2003; Hayakawa et al., 2006). Here, we observed that co-exposure to BPA and BPAF significantly induced activation of ASK1 and its downstream pathway JNK compared to BPA or BPAF exposure alone. The important role of ASK1-JNK, a stress-initiated signaling axis, in the co-induction of granulosa cell apoptosis by BPA and BPAF was confirmed by the application of the corresponding inhibitors.
In summary, our present study demonstrates that combined exposure to BPA and BPAF significantly induced the generation of intracellular stress including elevated levels of ROS and Ca2+ in KGN cells. We further confirmed that intracellular stress and the activation of the signal axis of the downstream signaling pathways of ASK1-JNK involved in the apoptosis of KGN were induced by the mixture of BPA and BPAF. Although the concentrations of BPA or BPAF used in this study were higher than those detected in the environment, studies on female reproductive toxicity in mice showed that environmental or occupational exposure doses of BPA or BPAF could still lead to the follicular atresia characterized by granulosa cell apoptosis. Therefore, our work provides novel insights into the combined cytotoxicity and molecular toxicity mechanism of bisphenol mixture exposure.
Acknowledgments
This research was supported by PhD Scientific Research Start-up Fund of The Affiliated Hospital of Southwest Medical University (16235, 19033 and 16236), and Natural Science Foundation of Southwest Medical University (2017-ZRQN-144, 2019-ZRQN-136 and 2017-ZRQN-048).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Cellular basis for paracrine regulation of ovarian follicle development. Reproduction. 2001;121:647-653.
- [Google Scholar]
- Prooxidant actions of bisphenol A (BPA) phenoxyl radicals: implications to BPA-related oxidative stress and toxicity. Toxicol. Mech. Methods. 2013;23:273-280.
- [Google Scholar]
- Intracellular Ca Sensing: Its Role in Calcium Homeostasis and Signaling. Mol. Cell. 2017;66:780-788.
- [Google Scholar]
- In vitro exposure of human spermatozoa to bisphenol A induces pro-oxidative/apoptotic mitochondrial dysfunction. Reprod. Toxicol.. 2016;66:61-67.
- [Google Scholar]
- Follicular somatic cell factors and follicle development. Reprod. Fertil. Dev.. 2011;23:32-39.
- [Google Scholar]
- The dual role of calcium as messenger and stressor in cell damage, death, and survival. Int. J. Cell Biol.. 2010;2010:546163
- [Google Scholar]
- Chen, D., Kannan, K., Tan, H., Zheng, Z., Feng, Y.-L., Wu, Y., Widelka, M., 2016. Bisphenol analogues other than BPA: environmental occurrence, human exposure, and toxicity a review. Environ. Sci. Technol. 50, 5438-5453.
- Bisphenol AF negatively affects oocyte maturation of mouse in vitro through increasing oxidative stress and DNA damage. Chem.-Biol. Interact.. 2017;278:222-229.
- [Google Scholar]
- A new chapter in the bisphenol A story: bisphenol S and bisphenol F are not safe alternatives to this compound. Fertil. Steril.. 2015;103:11-21.
- [Google Scholar]
- Bisphenol A: an endocrine and metabolic disruptor. Ann. Endocrinol. (Paris): Elsevier; 2013. p. :211-220.
- Bisphenol A exposure, effects, and policy: a wildlife perspective. J. Environ. Manage.. 2012;104:19-34.
- [Google Scholar]
- The ASK1-MAP kinase pathways in immune and stress responses. Microb. Infect.. 2006;8:1098-1107.
- [Google Scholar]
- Bisphenol A levels in blood and urine in a Chinese population and the personal factors affecting the levels. Environ. Res.. 2009;109:629-633.
- [Google Scholar]
- Bisphenol A induces apoptosis through GPER-dependent activation of the ROS/Ca2+-ASK1-JNK pathway in human granulosa cell line KGN. Ecotoxicol. Environ. Saf.. 2020;208:111429
- [Google Scholar]
- Bisphenol AF induces apoptosis via estrogen receptor beta (ERβ) and ROS-ASK1-JNK MAPK pathway in human granulosa cell line KGN. Environ. Pollut.. 2020;116051
- [Google Scholar]
- Bisphenol A and its analogues bisphenol S, bisphenol F and bisphenol AF induce oxidative stress and biomacromolecular damage in human granulosa KGN cells. Chemosphere. 2020;253:126707
- [Google Scholar]
- Biochemical identification of apoptosis (programmed cell death) in granulosa cells: evidence for a potential mechanism underlying follicular atresia. Endocrinology. 1991;129:2415-2422.
- [Google Scholar]
- Quercetin attenuates cadmium-induced oxidative damage and apoptosis in granulosa cells from chicken ovarian follicles. Reprod. Toxicol.. 2011;31:477-485.
- [Google Scholar]
- Use of a Mouse Model of Experimentally Induced Endometriosis to Evaluate and Compare the Effects of Bisphenol A and Bisphenol AF Exposure. Environ. Health Perspect.. 2018;126:127004
- [Google Scholar]
- Endocrine disruptors and polycystic ovary syndrome (PCOS): elevated serum levels of bisphenol A in women with PCOS. The Journal of Clinical Endocrinology & Metabolism. 2011;96:E480-E484.
- [Google Scholar]
- Measurement of bisphenol A concentrations in human colostrum. Chemosphere. 2007;66:1160-1164.
- [Google Scholar]
- Bisphenol A exposure during adulthood causes augmentation of follicular atresia and luteal regression by decreasing 17β-estradiol synthesis via downregulation of aromatase in rat ovary. Environ. Health Perspect.. 2013;121:663-669.
- [Google Scholar]
- Occurrence of eight bisphenol analogues in indoor dust from the United States and several Asian countries: implications for human exposure. Environ. Sci. Technol.. 2012;46:9138-9145.
- [Google Scholar]
- Bisphenol analogues in sediments from industrialized areas in the United States, Japan, and Korea: spatial and temporal distributions. Environ. Sci. Technol.. 2012;46:11558-11565.
- [Google Scholar]
- Occurrence of bisphenol A in indoor dust from two locations in the eastern United States and implications for human exposures. Arch. Environ. Contam. Toxicol.. 2011;61:68-73.
- [Google Scholar]
- Eryptosis-inducing activity of bisphenol A and its analogs in human red blood cells (in vitro study) J. Hazard. Mater.. 2016;307:328-335.
- [Google Scholar]
- Bisphenol A, bisphenol S, bisphenol F and bisphenol AF induce different oxidative stress and damage in human red blood cells (in vitro study) Toxicol. In Vitro. 2017;41:143-149.
- [Google Scholar]
- Follicular growth and atresia in mammalian ovaries: regulation by survival and death of granulosa cells. J Reprod Develop. 2012;58:44-50.
- [Google Scholar]
- Effects of perinatal exposure to BPA, BPF and BPAF on liver function in male mouse offspring involving in oxidative damage and metabolic disorder. Environ. Pollut.. 2019;247:935-943.
- [Google Scholar]
- Characterization of Follicular Atresia Responsive to BPA in Zebrafish by Morphometric Analysis of Follicular Stage Progression. Int. J. Endocrinol.. 2018;2018:4298195.
- [Google Scholar]
- Cord blood bisphenol A levels and reproductive and thyroid hormone levels of neonates: The Hokkaido Study on Environment and Children’s Health. Epidemiology. 2017;28:S3-S9.
- [Google Scholar]
- Bisphenol A, obesity, and type 2 diabetes mellitus: genuine concern or unnecessary preoccupation? Translational research. 2014;164:13-21.
- [Google Scholar]
- Low-concentration exposure to BPA, BPF and BPAF induces oxidative DNA bases lesions in human peripheral blood mononuclear cells. Chemosphere. 2018;201:119-126.
- [Google Scholar]
- Circulating markers of oxidative stress and polycystic ovary syndrome (PCOS): a systematic review and meta-analysis. Hum. Reprod. Update. 2013;19:268-288.
- [Google Scholar]
- Granulosa Cell Apoptosis in the Ovarian Follicle-A Changing View. Front. Endocrinol. (Lausanne). 2018;9:61.
- [Google Scholar]
- Bisphenol A and human chronic diseases: current evidences, possible mechanisms, and future perspectives. Environ. Int.. 2014;64:83-90.
- [Google Scholar]
- Are structural analogues to bisphenol a safe alternatives? Toxicol. Sci.. 2014;139:35-47.
- [Google Scholar]
- Bisphenol A: an endocrine disruptor with widespread exposure and multiple effects. The Journal of steroid biochemistry and molecular biology. 2011;127:27-34.
- [Google Scholar]
- Bisphenol A (BPA) and its potential role in the pathogenesis of the polycystic ovary syndrome (PCOS) Gynecol. Endocrinol.. 2014;30:260-265.
- [Google Scholar]
- Oxidative stress in polycystic ovary syndrome and its contribution to the risk of cardiovascular disease☆. Clin. Biochem.. 2001;34:407-413.
- [Google Scholar]
- Long-term effects of bisphenol AF (BPAF) on hormonal balance and genes of hypothalamus-pituitary-gonad axis and liver of zebrafish (Danio rerio), and the impact on offspring. Chemosphere. 2015;128:252-257.
- [Google Scholar]
- Roles of MAPKKK ASK1 in stress-induced cell death. Cell Struct. Funct.. 2003;28:23-29.
- [Google Scholar]
- Metabolic and endocrine effects of bisphenol A exposure in market seller women with polycystic ovary syndrome. Environ. Sci. Pollut. Res.. 2016;23:23546-23550.
- [Google Scholar]
- Bisphenol A induces oxidative stress-associated DNA damage in INS-1 cells. Mutation Research/Genetic Toxicology and Environmental Mutagenesis. 2014;769:29-33.
- [Google Scholar]
- Bisphenol A and other bisphenol analogues including BPS and BPF in surface water samples from Japan, China, Korea and India. Ecotoxicol. Environ. Saf.. 2015;122:565-572.
- [Google Scholar]
- Exposure to Bisphenol AF disrupts sex hormone levels and vitellogenin expression in zebrafish. EnTox. 2016;31:285-294.
- [Google Scholar]
- Role of oxidative stress in polycystic ovary syndrome. Current women's health reviews. 2010;6:96-107.
- [Google Scholar]
- Bisphenol A-induced apoptosis, oxidative stress and DNA damage in cultured rhesus monkey embryo renal epithelial Marc-145 cells. Chemosphere. 2019;234:682-689.
- [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2021.103399.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







