Translate this page into:
CCDC167 promotes hepatocellular carcinoma cell proliferation and is associated with the immune microenvironment of hepatocellular carcinoma
⁎Corresponding authors at: Shihezi University School of Medicine, Shihezi 832000, China (Wu. Chen). wxwshz@126.com (Xiangwei Wu), chenxueling@shzu.edu.cn (Xueling Chen)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Coiled-coil domain-containing (CCDC)family proteins play an important role in tumour progression, however, the role of CCDC167 in hepatocellular carcinoma remains unclear. To investigate the role of coiled-coil structural domain 167 (CCDC167) in hepatocellular carcinoma progression, we downloaded three hepatocellular carcinoma datasets (TCGA-LIHC, ICGC-LIRI and GSE76427) and analysed the mechanisms by which CCDC167 regulates hepatocellular carcinoma progression using MetaScape, CPTAC, HPA, TISCH and TISIDB.“ pRRophetic” package predicted the chemotherapeutic agents associated with CCDC167. The results showed that CCDC167 was highly expressed in hepatocellular carcinoma tissues and was associated with poor prognosis in patients with hepatocellular carcinoma. Knockdown of CCDC167 inhibits the proliferative capacity of hepatocellular carcinoma cells. CCDC167 was involved in the regulation of cell cycle, DNA replication and oxidative phosphorylation. Hepatocellular carcinoma samples with high expression of CCDC167 showed higher immune scores and C4 (lymphocyte depleted) subtypes. CCDC167 was associated with a variety of immune molecules (CD274, IL6R, and KDR), not only that, but CCDC167 was positively correlated with Treg infiltration and negatively correlated with T cells CD4 memory resting. High expression of CCDC167 increased immune escape in hepatocellular carcinoma patients. CCDC167 was associated with sensitivity to cisplatin in hepatocellular carcinoma patients. CCDC167 promoted the proliferative capacity of hepatocellular carcinoma cells. Our study suggests that CCDC167 is a novel and potentially valuable prognostic marker for hepatocellular carcinoma, and that CCDC167 iCCDC167 not only promotes hepatocellular carcinoma cell proliferation but is also associated with immune function in hepatocellular carcinoma patients.
Keywords
Bioinformatics
CCDC167
Immune infiltration
Drug sensitivity
Cell proliferation
1 Introduction
Hepatocellular carcinoma (HCC) is a significant cause of cancer-related deaths and is one of the most common cancers worldwide. The incidence of liver cancer is increasing, making it a serious threat to human health (Chakraborty and Sarkar, 2022). Various risk factors are associated with the pathogenesis of HCC, such as alcohol consumption, NAFLD, chronic HBV and HCV infections, aflatoxin B1 intake, and infection (Sun et al., 2020). Although there are multiple treatment options for HCC, including immunotherapy, partial hepatectomy, hepatic artery chemoembolization, liver transplantation, and targeted therapy, their therapeutic efficacy is limited due to the high recurrence and metastasis rate characteristics of liver cancer (Bruix et al., 2017; Chang et al., 2016; Fan et al., 2022; Kokudo et al., 2016).
The CCDC family of proteins with coiled-coil structures has a wide range of physiological functions(Li et al., 2017; Modjtahedi et al., 2016), such as regulation of gene expression, cell division, delivery of drugs, and membrane fusion(Occhionorelli et al., 2011; Radulovich et al., 2015; Rai et al., 2019). Some members of the CCDC family have been found to regulate cancer progression, and CCDC67, CCDC6, and CCDC34 have been associated with apoptosis and invasion of thyroid, lung, and gastric cancer cells (Cheng et al., 2022; Gong et al., 2015; Park et al., 2012). Additionally, CCDC137 and CCDC134 have been linked to the tumor immune microenvironment (Guo et al., 2021; Huang et al., 2022). However, the role of CCDC167 in hepatocellular carcinoma remains unclear.
In our experimental study, we found that CCDC167 was highly expressed in hepatocellular carcinoma tissues, and its high expression was associated with poor prognosis and worse clinical stage in hepatocellular carcinoma patients. Further analysis showed that CCDC167 is associated with immune function and chemotherapy sensitivity in hepatocellular carcinoma patients. Our cellular-level experiments also revealed that CCDC167 can affect the proliferation of hepatocellular carcinoma cells. In conclusion, our study provides insights into the potential mechanism of action of CCDC167 in hepatocellular carcinoma and offers direction for further exploration of new drug targets to improve the prognosis of hepatocellular carcinoma patients.
2 Methods and materials
2.1 Data acquisition
RNA sequencing data and corresponding clinical information for TCGA-LIHC were downloaded from the TCGA database (https://portal.gdc.cancer.gov/) and GSE76427 (based on the GPL10588 platform) from GEO (https://www.ncbi.nlm.nih.gov/geo); RNA expression data and corresponding clinical information for ICGC-LIRI in Japan were downloaded from ICGC (https://dcc.icgc.org/).From CPTAC (https://gdc.cancer.gov/about-gdc/contributed-genomic-data-cancer-research/clinical-proteomic-tumor-analysis-consortium-cptac), proteomic data of HCC were obtained.
2.2 Biological function analysis
GO and KEGG: 500 genes associated with CCDC167 were obtained from TISCH (https://tisch.comp-genomics.org/search-gene/) and MetaScape (https://metascape.org/gp/index.html) was used for enrichment analysis. ssGSEA analysis was performed using CAOMIP (https://www.camoip.net/) and GSEA was performed using “ClusterProfiler” package.
2.3 Analysis of immune function and chemotherapy sensitivity
The relationship between the expression of CCDC167 and the level of immune cells and immune molecules in patients with hepatocellular carcinoma was analysed using “cibersort” package. The “estimate” package was used for the immune infiltration analysis. The TIDE (https://tide.dfci.harvard.edu/) was used to calculate TIDE scores for HCC samples. TISIDB (https://cis.hku.hk/TISIDB/) was used to analyse the correlation between CCDC167 and immune molecules.
2.4 Cell culture and cell transfection
Hep3B and Huh7 hepatocellular carcinoma cells were obtained from the Cell Collection Committee of Chinese Academy of Sciences (Shanghai, China), and MHCC97H hepatocellular carcinoma cells were obtained from Pronox Life Sciences (Wuhan, China). All cells were cultured using complete medium (90 % DMEM and 10 % FBS) in an incubator at 37 °C with 5 % CO2, and all experiments were carried out when cell fusion reached 60 %-80 %. Cell transfection: Experiments were performed using siRNA (Table S1) and plasmids synthesized by Gene Pharma Co. Lip3000 dissolved in 100 μL DMEM, 3 μL of siRNA reagent dissolved in 100 μL DMEM, both mixed and incubated at room temperature for 10 min-15 min, to which 800 μL DMEM was added and incubated at 37 °C for 24 h–48 h for other experiments. Plasmid transfection: P3000 reagent should be added at the same time when the plasmid and DMEM are mixed. Other operation steps are the same as those for siRNA transfection.
2.5 Detection of cell proliferation ability
CCK-8: Cells were spread into 96-well plates and 100 μL of cell suspension containing 5000 cells was added to each well and incubated at 37℃. 10 μL of CCK-8 reagent (Apex BIO, Houston, USA) was added to each well at 0 h, 24 h, 48 h, and 72 h, and incubated for 2 to 4 h. The absorbance values were measured at 450 nm using an enzyme marker (Thermo, American). EdU assay: The Edu kit (Apex BIO, USA) method was used to detect the cells by After operating according to the reagent instructions, images were taken by fluorescence microscope (OLYMPUS, Japan) and image J was used for analysis. Colony formation: Inoculate 1000 cells per well in a six-well plate, incubate in an incubator for 14 days, fix with formaldehyde, and then add crystalline violet dye for observation.
2.6 Cell cycle
The experiments were performed using cell Cycle Staining Kit (MULTI SCIENCES, Guangzhou, China). 1 × 106 cells washed with PBS were collected, 10 μL of membrane breaker and 1 mL of DNA staining solusion were added to each sample, shaken, vortexed for 10 s, and incubated at room temperature and protected from light. The cells were incubated for half an hour and then subjected to cell cycle assay (NovoCyte, California, USA).
2.7 IC50
The “pRRophetic” package (Geeleher et al., 2014) was used to assess the IC50 of chemotherapeutic agents in liver cancer samples from TCGA-LIHC by classifying liver cancer samples into high and low expression groups based on the median value of CCDC167 expression, and the “ggpplot2″ package was used for visualization. PubChem (https://pubchem.ncbi.nlm.nih.gov/) was used for 2D visualisation of chemotherapeutic drugs.
Sorafenib was purchased from APExio, and Cisplatin was purchased from MCE. Cells were spread into 96-well plates in advance, drug concentration gradient was set (fold dilution), and cells were wall-adhered with drug and placed in the incubator at 37 °C for 24 h. The absorbance of the cells was detected using an enzyme marker at 450 nm for 2 h after the addition of CCK-8 reagent (10 ul per well, Biosharp).
2.8 RT-PCR
Total RNA was extracted using Total RNA Kit I (OMEGA bio-Tek, USA) and complementary DNA (cDNA) was synthesized using a reverse transcription kit (Thermo Fisher Scientific, Waltham, Massachusetts, EUA). Afterwards, cDNA samples were subjected to quantitative real-time PCR on a CFX96 system (Bio-Rad). primers for quantitative real-time PCR (GenePharma Co. Ltd., Shanghai, China) were as follows: β-Actin: For: 5′- AAGCTCATTTCCTGGTATGACA −3′, Rev: 5′- GGAGATGCTCAGTGTTGGGG −3′; CCDC167 For: 5′- AGATGACCCTTTCTCATGCTGC-3′, Rev: 5′- CTCTCCAGGAGTGAACACGG-3′.
2.9 Western blot
Equal amounts of protein extracts were separated by SDS-PAGE gel and transferred to PVDF membrane, and incubated with the internal reference antibody and the target antibody as follows: Tubulin (A01857-1, rabbit monoclonal antibody, 1:1000, BOSTER); CCDC167 (ab126337, rabbit monoclonal antibody, 1:1000, Abcam); using an automated exposure machine (Clinx ChemiScope, China) for exposure.
2.10 Statistical analysis
Statistical analyses were performed using GraphPad Prism (8.0.2) and R software (4.2.0). *P < 0.05, ** P < 0.01, *** P < 0.001, **** P < 0.0001.
3 Results
3.1 1. CCDC167 is highly expressed in hepatocellular carcinoma and associated with worse clinical grade
Paired sample analysis from GSE76427 (Fig. 1A) and TCGA-LIHC (Fig. 1B) showed higher expression of CCDC167 in hepatocellular carcinoma than in paired normal tissues. Analysis of the ICGC-LIRI dataset (Japanese hepatocellular carcinoma) (Fig. 1C) also revealed that higher expression of CCDC167 in tumor. Furthermore, analysis of pan-cancer using TIMER 2.0 indicated that CCDC167 was differentially expressed in various cancers, including hepatocellular carcinoma, stomach adenocarcinoma, and lung adenocarcinoma (Fig. 1D). To analyze the relationship between CCDC167 and hepatocellular carcinoma progression, we utilized TCGA-LIHC and ICGC-LIRI databases with clinical data for univariate cox and multivariate cox analyses (Table S2, Table S3). Our analysis suggests that CCDC167 expression may be an independent prognostic factor in patients with hepatocellular carcinoma. Additionally, CCDC167 expression correlated with worse clinical grade and stage of hepatocellular carcinoma patients (Fig. 1 E-H).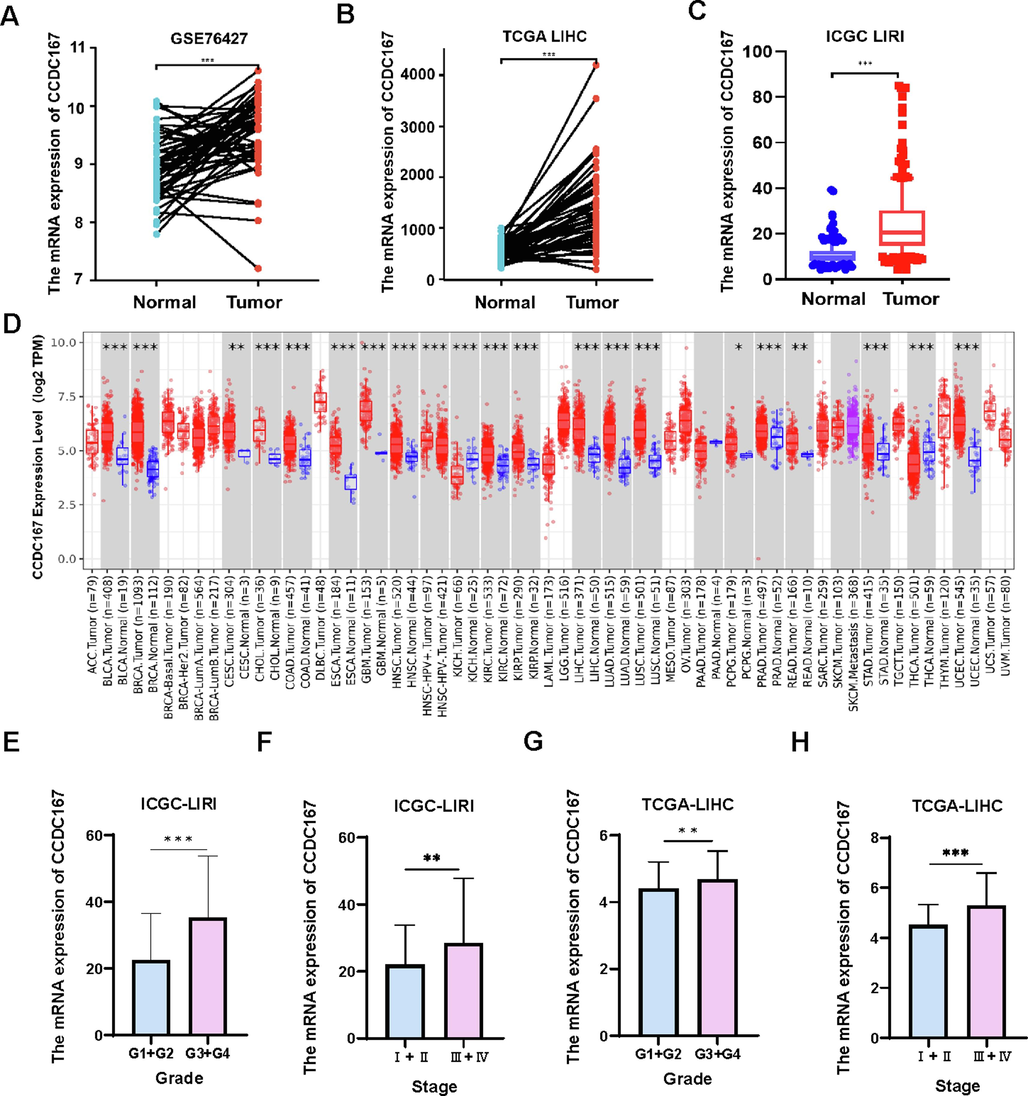
Expression of CCDC167 and its relationship with clinical features of hepatocellular carcinoma. (A) CCDC167 expression in paired normal and hepatocellular carcinoma tissues (GSE76427). (B) Expression of CCDC167 in paired normal and liver cancer tissues (TCGA-LIHC). (C) Expression of CCDC167 in the ICGC-LIRI liver cancer dataset. (D) CCDC167 expression in multiple cancers from TIMER 2.0. (E-H) Relationship between CCDC167 in ICGC-LIRI and TCGA-LIHC and clinical grade and stage of patients with hepatocellular carcinoma. ***P < 0.001, **P < 0.01, *P < 0.05.
We also analysed the expression of CCDC167 at the protein level, HPA (immunohistochemistry database) analysis showed that the expression of CCDC167 protein was higher in hepatocellular carcinoma tissues than in normal tissues (Fig. 2A), however, CPTAC (protein sequencing database) analysis concluded that the expression of CCDC167 protein was lower in hepatocellular carcinoma tissues than in paired normal tissues(Fig. 2B), further OS and RFS survival analyses indicated that hepatocellular carcinoma patients with high expression of CCDC167 protein possessed worse survival outcomes (Fig. 2C-D).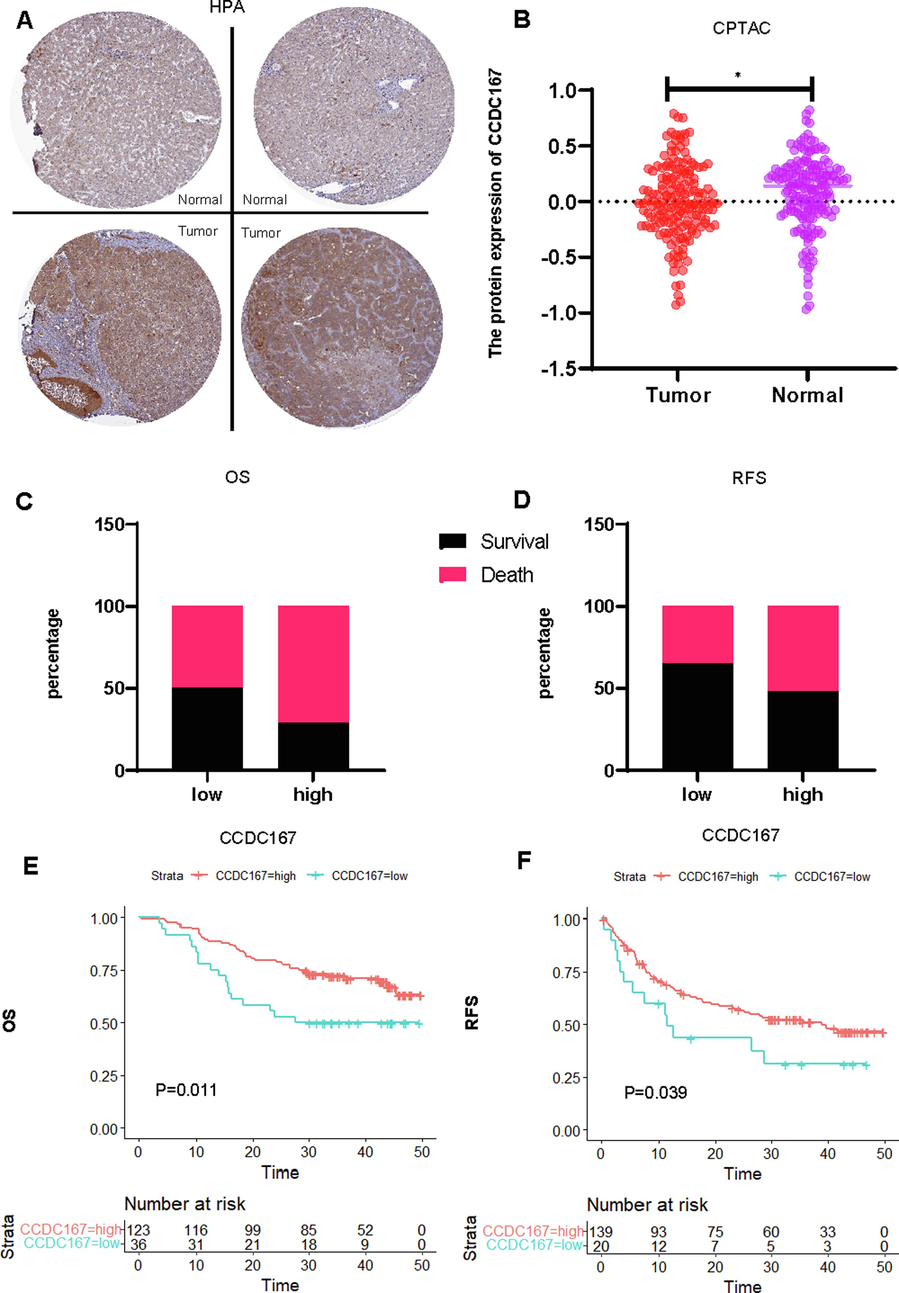
Comprehensive analysis of the expression of CCDC167 at the protein level and its relationship with the prognosis of hepatocellular carcinoma. (A) Histochemical staining of CCDC167 in normal and hepatocellular carcinoma tissues (HPA). (B) Expression analysis of CCDC167 protein in normal and hepatocellular carcinoma tissues (CPTAC),*P < 0.05. (C, D) Distribution of survival outcome and recurrence-free outcome between high and low CCDC167 protein expression groups (CPTAC). (E, F) Survival analysis and and recurrence-free survival analysis (CPTAC).
3.2 Biological function of CCDC167
The 500 genes co-expressed with CCDC167 in the single cell dataset of hepatocellular carcinoma (GSE140228) were obtained from the TISCH database (Table S4), and MetaScape analysis revealed that CCDC167 is mainly involved in cell cycle, DNA repair and RNA metabolism (Fig. 3A).TRRUST database suggests that CCDC167 may be transcriptionally regulated by multiple oncogenes including TP53, YY1 (Kelley et al., 2016; Yang et al., 2019) (Fig. 3B). The hepatocellular carcinoma samples were divided into two groups of high and low expression according to the expression of CCDC167 in TCGA-LIHC, and GSEA analysis was performed, which showed that CCDC167 was involved in cell cycle, DNA replication, oxidative phosphorylation and other pathways related to tumor progression (Fig. 3C). ssGSEA analysis showed that cell cycle activation was greater in hepatocellular carcinoma samples with high CCDC167 expression than in hepatocellular carcinoma samples with low CCDC167 low expression (Fig. 3D), and correlation analysis from GEPIA also showed that CCDC167 was significantly correlated with key genes of cell cycle (CCNB1, CCNB2) (Fig. 3E).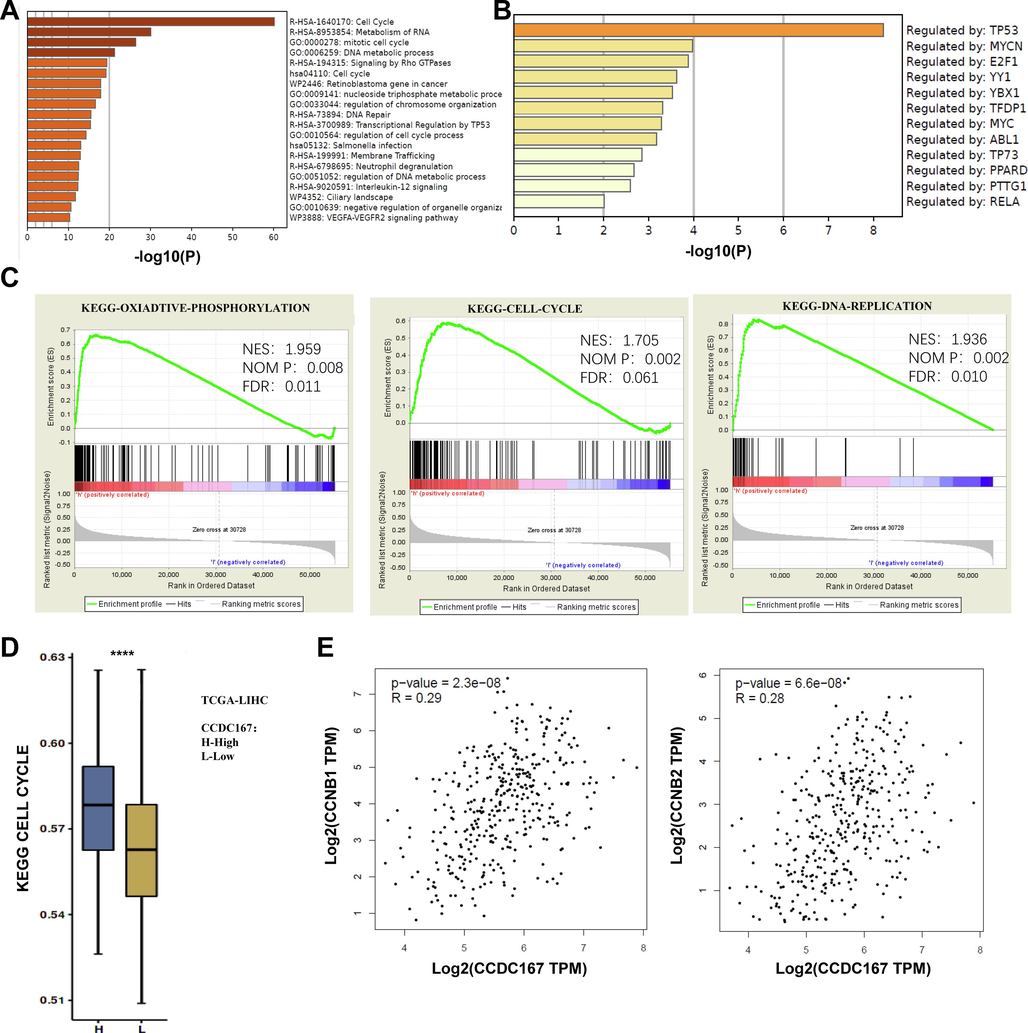
Biological functional analysis of CCDC167. (A) GO and KEGG analysis of the function of CCDC167. (B) Enrichment of transcription factors regulating CCDC167. (C) GSEA enrichment to the pathways involved in CCDC167. (D) ssGSEA comparison of the degree of cell cycle enrichment between high and low CCDC167 expressing liver cancer samples. (E-F) GEPIA 2.0 analysis of CCDC167 correlation with CCNB1, CCNB2. ***P < 0.001, **P < 0.01, *P < 0.05.
3.3 CCDC167 is associated with immune function in hepatocellular carcinoma patients
Recent studies have highlighted the importance of CCDC137, a member of the same protein family, in the regulation of tumour immune function (Bai et al., 2022). so we also analysed the possible relationship between CCDC167 and immune function. Analysis at the single cell level (GSE140228) showed that CCDC167 was expressed mainly on Tprolif, Treg and mast cells (Fig. S1A–C). We observed that hepatocellular carcinoma samples with high expression of CCDC167 exhibited higher immune scores, whereas hepatocellular carcinoma samples with low expression of CCDC167 exhibited higher stromal scores and estimation scores (Fig. 4A), meanwhile, hepatocellular carcinoma samples with high expression of CCDC167 exhibited more C4 (lymphocyte-poor) subtypes, which are usually associated with a poorer prognosis of the tumour and an immune-suppressed closely associated with poorer tumour prognosis and immunosuppression(Thorsson et al., 2018), while hepatocellular carcinoma samples with low expression of CCDC167 showed more C3 (inflammatory) subtype (Fig. 4B). In addition, we analysed the correlation between CCDC167 and immune molecules (immunosuppressive and immune-enhancing molecules) using TISIDB, and found that CCDC167 was associated with a variety of immune molecules (CD274, IL6R and KDR) (Fig. 4C–D). In additional, CIBESORT was used to estimate the content of 28 immune cells in liver cancer samples, and the results showed that the expression of CCDC167 was strongly correlated with the content of various immune cells (P < 0.05) (Fig. 4E), and the scatterplot demonstrated the relationship between CCDC167 and two immune cells(Treg and T cells CD4 memory resting) that were closely correlated (|R|>0.2, P < 0.05)(Fig. 4F).In addition, the CCDC167 high expression group had higher TIDE and MDSC scores (Fig. S2A), implying that patients with hepatocellular carcinoma with high CCDC167 expression may respond less well to immunotherapy, and the (immunotherapy cohort) survival curves also demonstrated that patients with high CCDC167 expression did not do well when faced with immunotherapy (Fig. S2B–E).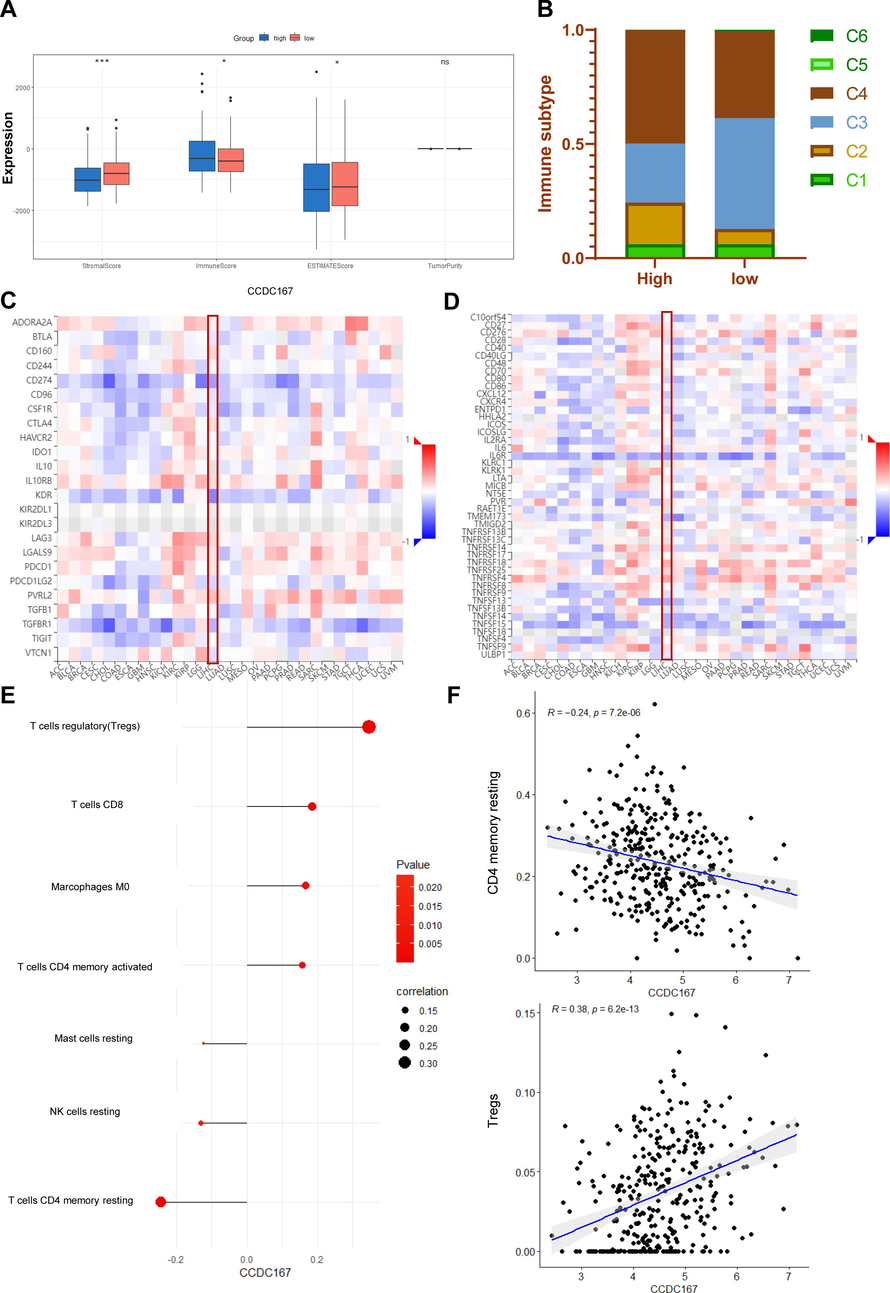
Relationship between CCDC167 and immune function in patients with hepatocellular carcinoma. (A) Box-and-line plot demonstrating the immunity scores of the CCDC167 high and low expression groups. (B) Stacked plots demonstrating the immune subtypes of CCDC167 high and low expression groups. (C) Heatmap of correlation between CCDC167 and immunosuppressants (TISIDB). (D) Heatmap of correlation between CCDC167 and immune enhancers (TISIDB). (E) Bubble diagram showing the correlation of CCDC167 with immune cells. (F) Scatterplot showing the correlation of CCDC167 with immune cells, the top panel shows the correlation scatterplot of CCDC167 with Treg, and the bottom panel shows the correlation scatterplot of CCDC167 with T cells CD4 memory resting. ***P < 0.001, **P < 0.01, *P < 0.05, ns: not significantly.
3.4 CCDC167 correlates with the sensitivity of several hepatocellular carcinoma chemotherapeutic agents
Chemotherapy is a widely used treatment modality for hepatocellular carcinoma (HCC) and can greatly improve patient prognosis. We investigated the potential role of CCDC167 in modulating the sensitivity of HCC to commonly used chemotherapeutic agents and Multi-kinase inhibitors (Carr, 2006; Ding et al., 2015; He et al., 2021). Our results demonstrated significant differences in sensitivity to Sorafenib (P < 0.01) and Cisplatin (P < 0.01) between the high- and low-risk groups (Fig. 5A, D). To facilitate clinical dosing, we obtained 2D structures of the three chemotherapeutic drugs from PubChem (Fig. 5B, E).Further experiments showed that CCDC167 knockdown hepatocellular carcinoma cells had elevated IC50 to cisplatin, which was consistent with the predicted results, however, the drug sensitivity experiments of sorafenib did not yield the desired results, and the above mentioned Bioinformatics predicts results and experimental results could further deepen our understanding of the underlying mechanisms of the differences in drug sensitivities between the high-risk and the low-risk CCDC167-expressing groups.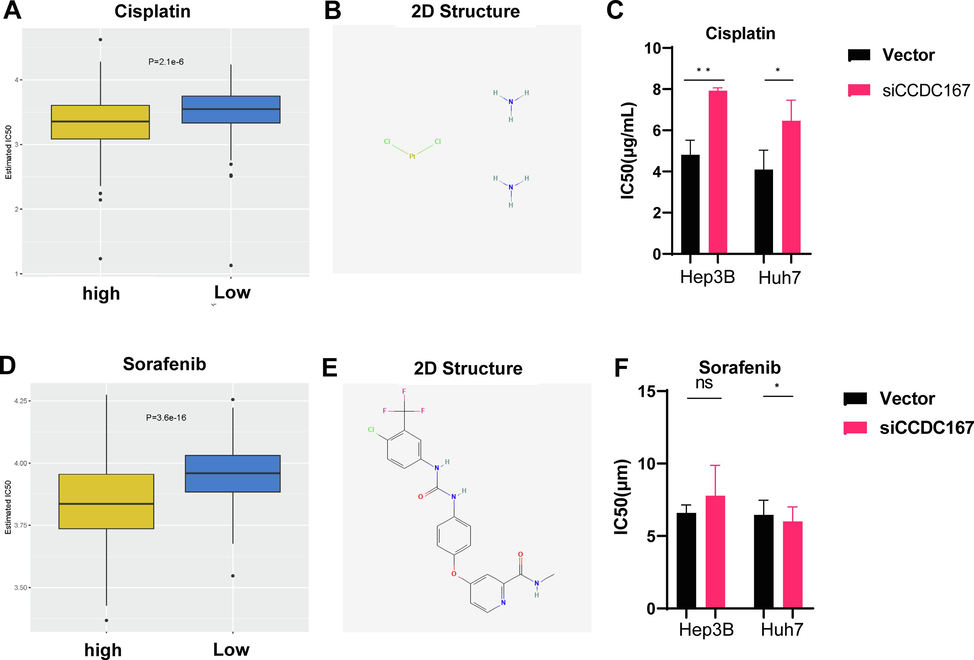
Sensitivity analysis of CCDC167 with chemotherapeutic drugs. (A, D) Box plot demonstrating the sensitivity of CCDC167 high and low expression groups with sorafenib and cisplatin. (B, E) 2D structure of cisplatin and sorafenib(Pubchem).(C, F) Detection of IC50 of hepatocellular carcinoma cells to cisplatin and sorafenib. ***P < 0.001, **P < 0.01, *P < 0.05, ns: not significantly.
3.5 CCDC167 promotes proliferation of hepatocellular carcinoma cells
The CCDC family is thought to be involved in regulating various aspects of tumour development, including cell proliferation, metastasis and migration. To explore the potential role of CCDC167 in hepatocellular carcinoma (HCC), we selected two high expressing CCDC167 cell lines (Huh7 and Hep3B) and one low expressing CCDC167 cell line (MHCC97H) for knockdown or overexpression assays (Fig. S3A–D). Using CCK-8 proliferation, EdU and cell colonization assays, we observed that knockdown of CCDC167 significantly reduced the proliferation of the Huh7 and Hep3B cell lines (Fig. 6A, C, E), indicating its potential role in promoting HCC cell growth, and conversely, overexpression of CCDC167 promoted the proliferation of hepatoma cells (Fig. 6B, D, F). Surprisingly, however, neither knockdown nor overexpression of CCDC167 affected the migration and invasive ability of HCC cells (Fig. S4A–F). Given the critical involvement of cell cycle regulators in cancer development, we further investigated whether CCDC167 plays a role in cell cycle regulation (Otto and Sicinski, 2017).Our cell cycle assay showed that knockdown of CCDC167 increased the number of HCC cells in G0/G1 phase and decreased the number of cells in G2/M phase (Fig. 6G–H), consistent with the results of bioinformatic analysis. Conversely, overexpression of CCDC167 decreased the number of HCC cells in G0/G1 phase and increased the number of cells in G2/M phase (Fig. 6I), further supporting the involvement of CCDC167 in cell cycle regulation and HCC cell proliferation.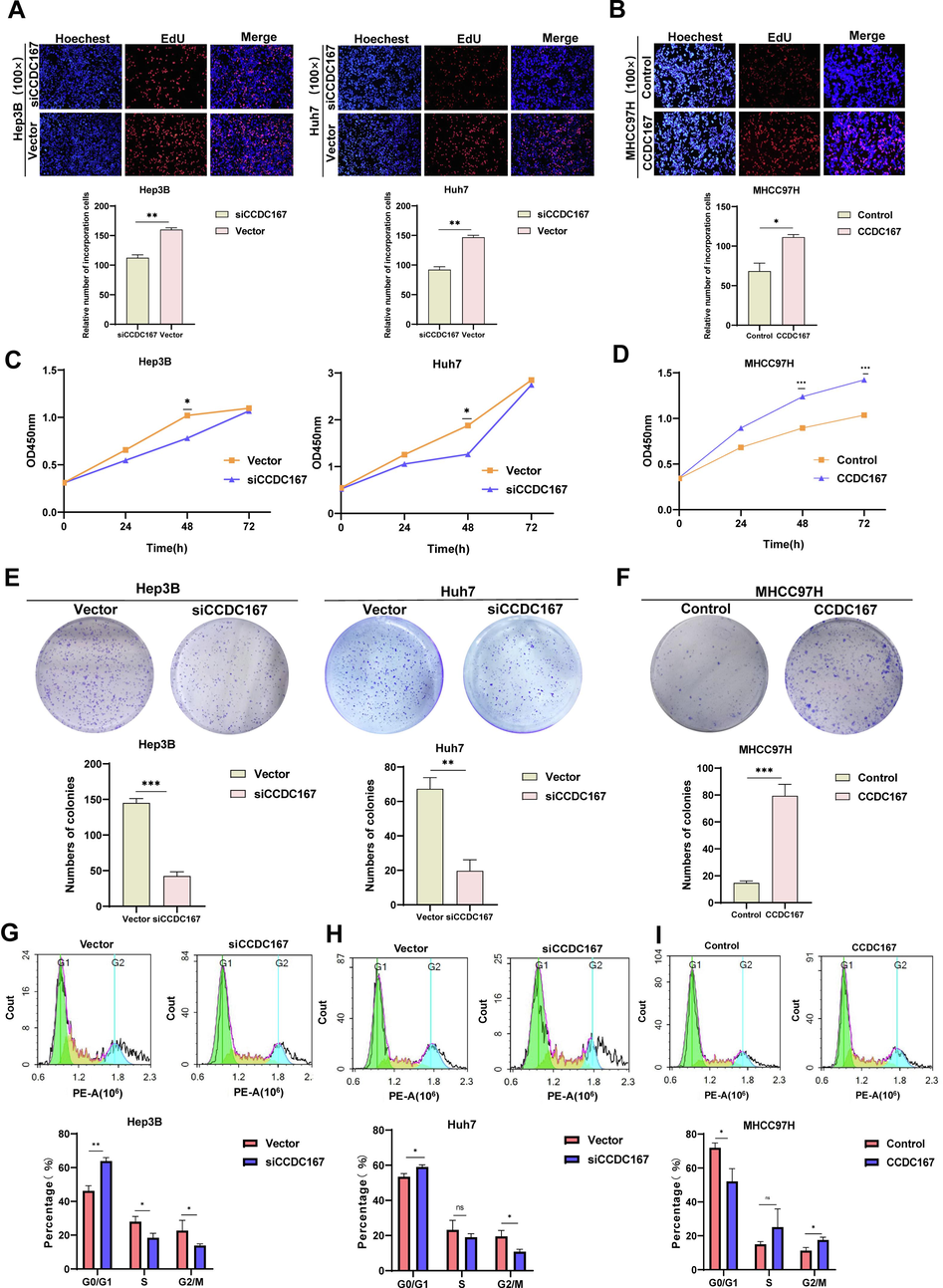
CCDC167 promotes proliferation of hepatocellular carcinoma cells. (A, B) Detection of proliferation ability of hepatocellular carcinoma cells after knockdown or overexpression of CCDC167 by EDU. (C, D) CCK-8 assay for proliferation of hepatocellular carcinoma cells after knockdown or overexpression of CCDC167. (E, F) Cell colony assay to detect the sphere-forming ability of hepatocellular carcinoma cells after knockdown or overexpression of CCDC167. (G, H, J) Cell cycle assay to detect cell cycle changes in hepatocellular carcinoma cells after knockdown or overexpression of CCDC167. ***P < 0.001, **P < 0.01, *P < 0.05, ns: not significantly.
4 Discussion
Aberrant expression of various proteins in the curly helix family has been associated with the progression of a number of cancer types, including lung, bladder, thyroid and pancreatic cancers (Wang et al., 2020).However, the role of CCDC167 in hepatocellular carcinoma (HCC) has not been fully investigated. Our analysis showed that the expression level of CCDC167 was significantly associated with the prognosis of HCC patients and acted as an independent prognostic factor for HCC patients.
Biological functional analysis has revealed that the CCDC167 protein plays a crucial role in several important pathways, including DNA replication, cell cycle regulation, and oxidative phosphorylation. Interestingly, our experimental results also indicate that manipulating the expression of CCDC167 does not appear to have any significant impact on the migratory or invasive properties of hepatocellular carcinoma cells. However, we have observed that changes in CCDC167 expression levels do significantly affect the proliferative capacity of these cells.
Exciting new findings from single-cell analyses have revealed that CCDC167 exhibits high levels of expression in both regulatory T cells (Treg) and cell proliferating T cells. Given the crucial role that Tregs play in suppressing anti-tumor immunity (Chakraborty and Zappasodi, 2021), these results suggest that targeting CCDC167 may represent a promising avenue for modulating Treg status in patients with hepatocellular carcinoma. By disrupting the expression or function of CCDC167, it may be possible to shift the balance of immune cell populations within the tumor microenvironment in favor of cytotoxic T cells, thus enhancing the efficacy of immunotherapeutic interventions against this deadly disease.
There is mounting evidence to suggest that the tumor immune microenvironment (TME) plays a critical role in the development and progression of hepatocellular carcinoma (HCC) (Guizhen et al., 2023), and can significantly impact the efficacy of therapeutic interventions. Recent immunoassays have revealed strong correlations between CCDC167 expression levels and various immune cell types, including Tregs, M2 macrophages, and CD8 T cells, as well as key immune molecules like CD274, CD8A, and CD276. Moreover, In terms of immunosubtyping, HCC samples with high CCDC167 expression showed more C4 immunosubtyping. Interestingly, drug sensitivity analyses indicate that patients with HCC who have high levels of CCDC167 expression may be more responsive to standard chemotherapy agents such as sorafenib, cisplatin, and gemcitabine. These findings could have major implications for the development of personalized treatment strategies that account for the complex interplay between tumor cells and the immune system in patients with HCC.
To summarize, our study has uncovered several important insights into the role of CCDC167 in hepatocellular carcinoma. Specifically, we have demonstrated that high levels of CCDC167 expression in HCC tissues are strongly associated with poor prognosis and more advanced clinical stage. Furthermore, we have shown that CCDC167 promotes the proliferative capacity of HCC cells, providing a potential target for therapeutic intervention. Importantly, our findings also indicate that CCDC167 expression is closely linked to immune function and chemotherapy sensitivity in HCC patients, opening up exciting new possibilities for personalized treatment strategies. Taken together, these results highlight the critical importance of CCDC167 in the pathogenesis of HCC, and underscore its potential as a biomarker and therapeutic target in this deadly disease.
Despite the progress we have made in elucidating the role of CCDC167 in hepatocellular carcinoma, our study is not without its limitations. Firstly, the precise mechanisms underlying CCDC167 function require further exploration using animal models to better understand their implications for human disease. Secondly, while our findings suggest a correlation between CCDC167 and immune function in HCC patients, additional experiments are needed to validate these results in larger cohorts and across different populations. Lastly, although our drug sensitivity analyses indicate that CCDC167 may be a useful predictive biomarker for chemotherapy response, further experimental validation is required to fully establish its clinical utility. Moving forward, we plan to address these shortcomings in future studies, with the ultimate goal of developing more effective treatments for HCC and improving outcomes for patients suffering from this devastating disease.
5 Conclusion
Taken together, our findings suggest that CCDC167 represents a promising new prognostic marker for liver cancer, with potential applications in predicting both immune status and chemotherapy sensitivity. Moreover, our cellular experiments provide compelling evidence linking CCDC167 expression to increased proliferative capacity in liver cancer cells, raising the possibility of CCDC167 as a novel therapeutic target. These results have important implications for the development of personalized treatment strategies that take into account the complex interplay between tumors and the immune system in liver cancer patients, underscoring the need for further research to fully elucidate the role of CCDC167 in this disease.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Funding
This research was supported by Corps guiding science and technology plan project(2022ZD041) and the Non-profit Central Research Institute Fund of Chinese Academy of Medical Sciences(2020-PT330-003).
Authors' contributions
Qianwen Wang.: Create study concept, Perform cell culture, analyze and interpret data, and write the manuscript. Wenhua Li.: statistical analysis, WB and PCR. Junxia Lu: Assist in WB and cell experiments. Bin Zhao.: Assist in data collection; Yuqing Geng.: Assist in Perform cell culture. Xiangwei Wu.: Interpret data and provide technical or material support. Xueling Chen.: Propose project concepts, supervise projects and experiments, interpret data, and write manuscripts. All authors read and approved the final manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Prognostic significance of CCDC137 expression and its association with immune infiltration in hepatocellular carcinoma. Dis. Markers 2022
- [Google Scholar]
- Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389:56-66.
- [Google Scholar]
- Gemcitabine hepatic arterial chemoembolization (TACE) in the treatment of unresectable HCC. J. Clin. Oncol.. 2006;24:4141.
- [Google Scholar]
- To Go or Not to Go?—Targeting tregs traveling in tumors. Cancer Res.. 2021;81:2817-2819.
- [Google Scholar]
- Long-term effects of hepatitis B immunization of infants in preventing liver cancer. Gastroenterology. 2016;151(472–480):e1.
- [Google Scholar]
- Tumor-associated macrophages in liver cancer: From mechanisms to therapy. Cancer Commun.. 2022;42:1112-1140.
- [Google Scholar]
- Overexpression of osteopontin promotes resistance to cisplatin treatment in HCC. Oncol. Rep.. 2015;34:3297-3303.
- [Google Scholar]
- Systemic therapy for hepatocellular carcinoma: current updates and outlook. J. Hepatocellular Carcinoma 2022:233-263.
- [Google Scholar]
- pRRophetic: an R package for prediction of clinical chemotherapeutic response from tumor gene expression levels. PLoS One. 2014;9:e107468
- [Google Scholar]
- CCDC34 is up-regulated in bladder cancer and regulates bladder cancer cell proliferation, apoptosis and migration. Oncotarget. 2015;6:25856
- [Google Scholar]
- The tumor microenvironment of hepatocellular carcinoma and its targeting strategy by CAR-T cell immunotherapy. Complexity of Tumor Microenvironment: a Major Culprit in Cancer Development. 2023;16648714:141.
- [Google Scholar]
- CCDC137 is a prognostic biomarker and correlates with immunosuppressive tumor microenvironment based on pan-cancer analysis. Front. Mol. Biosci.. 2021;8:674863
- [Google Scholar]
- Hsa-miR-4277 decelerates the metabolism or clearance of sorafenib in HCC cells and enhances the sensitivity of HCC cells to sorafenib by targeting cyp3a4. Front. Oncol.. 2021;11:735447
- [Google Scholar]
- CCDC134 as a prognostic-related biomarker in breast cancer correlating with immune infiltrates. Frontiers. Oncology. 2022;12
- [Google Scholar]
- Next-generation sequencing (NGS) in an advanced hepatocellular carcinoma (HCC) cohort: Analyses of TP53 and CTNNB1. American Society of Clinical Oncology; 2016.
- Survival benefit of liver resection for hepatocellular carcinoma associated with portal vein invasion. J. Hepatol.. 2016;65:938-943.
- [Google Scholar]
- Transmembrane and Coiled-Coil Domain 1 Impairs the AKT Signaling Pathway in Urinary Bladder Urothelial Carcinoma: A Characterization of a Tumor SuppressorTMCO1 Is a Tumor Suppressor in UBUCs. Clin. Cancer Res.. 2017;23:7650-7663.
- [Google Scholar]
- Mitochondrial proteins containing coiled-coil-helix-coiled-coil-helix (CHCH) domains in health and disease. Trends Biochem. Sci.. 2016;41:245-260.
- [Google Scholar]
- The self-association coiled-coil domain of PML is sufficient for the oncogenic conversion of the retinoic acid receptor (RAR) alpha. Leukemia. 2011;25:814-820.
- [Google Scholar]
- Cell cycle proteins as promising targets in cancer therapy. Nat. Rev. Cancer. 2017;17:93-115.
- [Google Scholar]
- Epigenetic alteration of CCDC67 and its tumor suppressor function in gastric cancer. Carcinogenesis. 2012;33:1494-1501.
- [Google Scholar]
- Coiled-coil domain containing 68 (CCDC68) demonstrates a tumor-suppressive role in pancreatic ductal adenocarcinoma. Oncogene. 2015;34:4238-4247.
- [Google Scholar]
- The ATG5-binding and coiled coil domains of ATG16L1 maintain autophagy and tissue homeostasis in mice independently of the WD domain required for LC3-associated phagocytosis. Autophagy. 2019;15:599-612.
- [Google Scholar]
- Purification of HCC-specific extracellular vesicles on nanosubstrates for early HCC detection by digital scoring. Nat. Commun.. 2020;11:4489.
- [Google Scholar]
- The CCDC43-ADRM1 axis regulated by YY1, promotes proliferation and metastasis of gastric cancer. Cancer Lett.. 2020;482:90-101.
- [Google Scholar]
- YY1 promotes endothelial cell-dependent tumor angiogenesis in hepatocellular carcinoma by transcriptionally activating VEGFA. Front. Oncol.. 2019;9:1187.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2023.105385.
Appendix A
Supplementary material
The following are the Supplementary data to this article: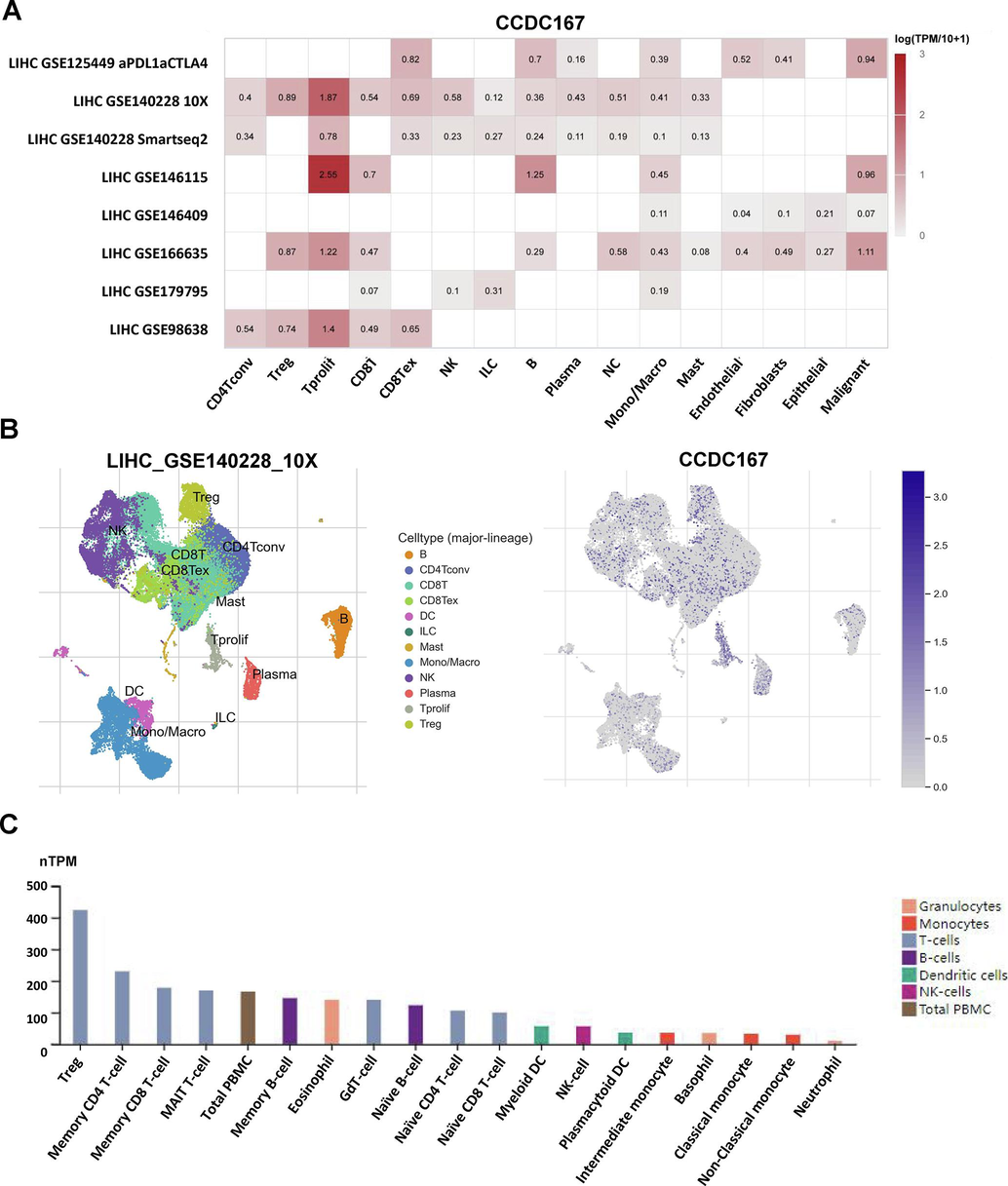
Expression of CCDC167 in different cells of hepatocellular carcinoma. (A) Heat map presenting the expression of CCDC167 in different cells of hepatocellular carcinoma. (B) Descending plots presenting the expression of CCDC167 in different immune cells. (C) Expression of CCDC167 in different immune cells from HPA database.
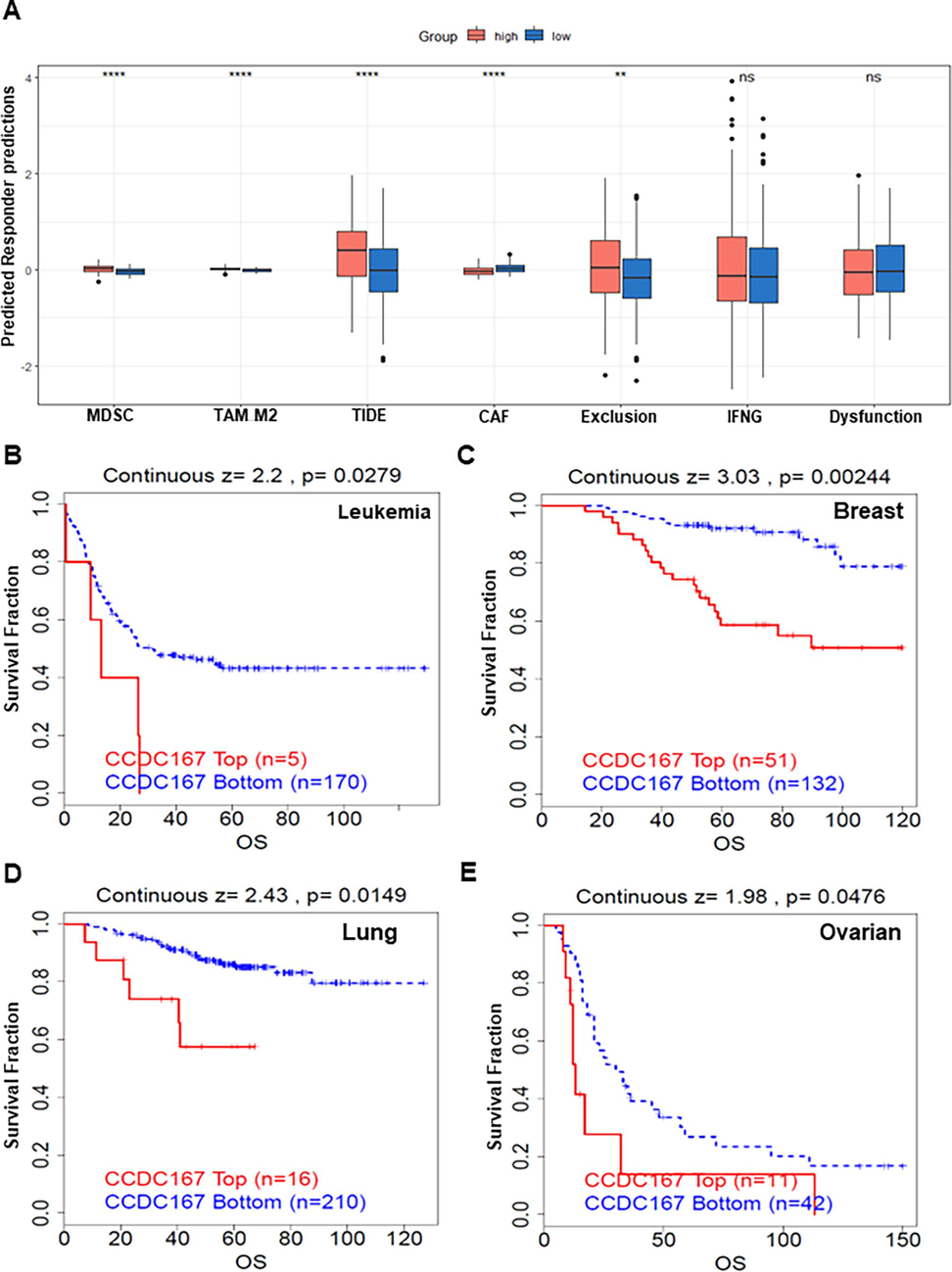
High expression of CCDC167 is associated with poor immunotherapy outcomes. (A) Box plot showing TIDE scores between high and low CCDC167 expression groups. (B-D) Survival curve analysis of the immunotherapy cohort.
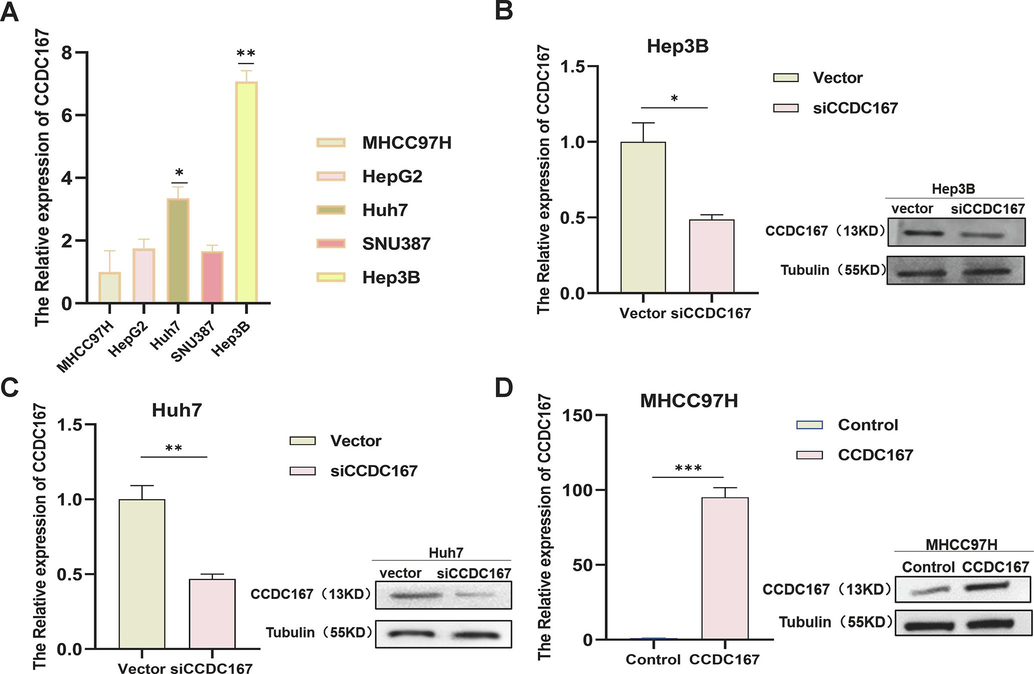
PCR and WB to detect the expression of CCDC167. (A) PCR to detect CCDC167 expression in five hepatocellular carcinoma cell lines. (B-D) PCR and WB to detect transfection efficiency.
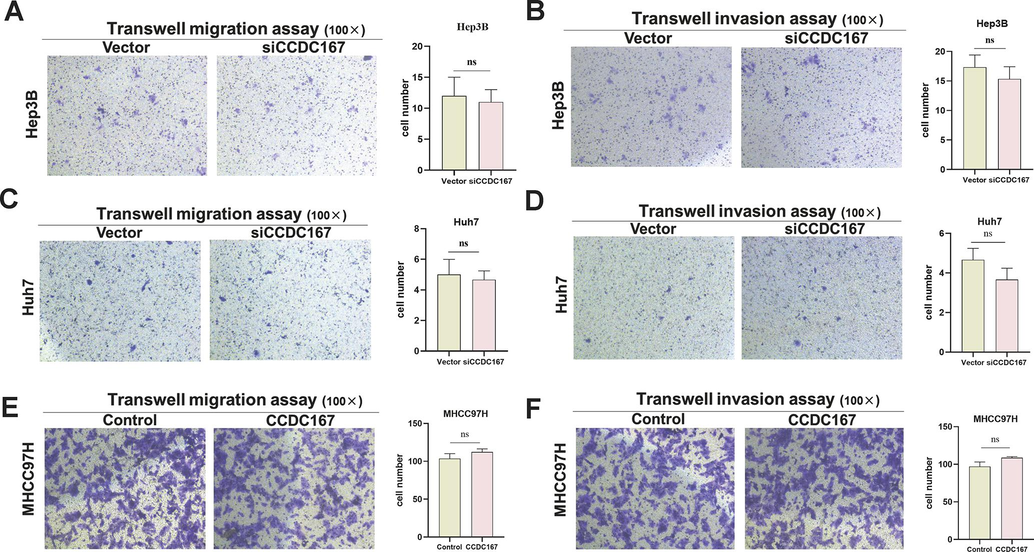
CCDC167 does not affect the migration and invasive ability of hepatocellular carcinoma cells. (A, C, E) Transwell migration assay for cell migration ability. (B, D, F) Transwell invasion assay for cell invasion ability.
Supplementary data 1
Supplementary data 1
siRNA sequences.
Supplementary data 2
Supplementary data 2
Univariate COX and multivariate COX analysis of prognostic parameters in the TCGA database.
Supplementary data 3
Supplementary data 3
Univariate COX and multivariate COX analysis of prognostic parameters in the ICGC database.
Supplementary data 4
Supplementary data 4
The 500 most relevant genes for CCDC167 in GSE140228.







