Translate this page into:
Fermented whey as natural descaling agent: Electrochemical and microscopical analysis
⁎Corresponding author. enrico.marsili@nu.edu.kz (Enrico Marsili)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
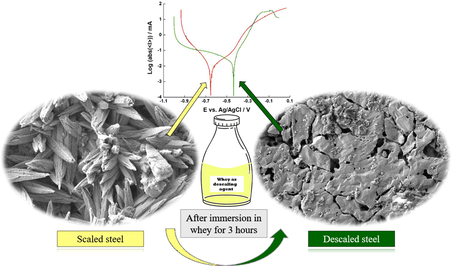
Electroanalysis was used to characterize the scaling and descaling of steel coupons. Short exposure time removes scaling without causing strong corrosion. Fermented whey can be used as low-cost descaling product in rural areas.
Abstract
Tap water in Kazakhstan is rich in carbonate due to contamination of surface water and insufficient drinking water treatment. This leads to scaling in home appliances such as cooking ware. Fermented whey is a by-product of small-scale farming, and is rich in lactic acid, with a pH ∼ 2.8. Thus, it can be used to formulate a low-cost, environmentally friendly descaling agent. In this work, mild steel coupons were scaled through carbonate precipitation in boiling water. Scale removal with immersion in fermented whey, DI water, and food-grade vinegar was then carried out and monitored through electrochemical analysis and scanning electron microscopy (SEM). While both fermented whey and vinegar removed the fouling layer, they caused pitting corrosion after prolonged immersion. The corrosion potential and current for mild steel coupons in 0.1 M KCl changed from −360 ± 26 mV and 49.3 ± 11.8 µA to −652 ± 1 mV and 16.2 ± 3.8 µA, upon scaling, indicating partial protection from corrosion and the formation of a non-conductive carbonate layer. Following treatment with fermented whey for 3 h, the corrosion potential increased to −427 ± 11 mV, indicating a significant removal of the scale layer comparable to that obtained with commercial vinegar. However, the corrosion current increased only slightly to 24.7 ± 6.2 µA, indicating that the fermented whey treatment did not cause rapid corrosion of the coupons. SEM images confirmed the removal of scale layer upon treatment with fermented whey, relative to the control coupons treated with DI water. These results show that fermented whey can be used as environmentally friendly descaling agent. However, prolonged treatment with whey causes damages to the steel surface and a corrosion inhibitor must be included in the formulation. The availability of whey in small farming communities, in addition to the simple and low-cost methodology outlined here, show promises for practical application and adoption of this green technology in rural areas.
Keywords
Descaling
Fermented whey
Electroanalysis
1 Introduction
Steel fouling and corrosion result in expensive process downtime and structural damage, which account for 1–3% of the global GDP, depending on the calculation methods and the database adopted (Bowman et al., 2016). Inorganic fouling following normal operation of steel equipment (e.g., heaters and heat exchangers) decreases the thermal conductivity and the lumen of the pipes, which, in turn, increases head losses. Further, inorganic fouling layer provides nutrients and support for microbial growth, which enhances both fouling and corrosion rate. The growth of pathogens in the fouling layer is relevant to food processes and bioprocesses, as it affects the quality of the products (Barish and Goddard, 2013).
Metal corrosions comprise several electrochemical reactions, which occur at the interface between the metal and the electrolyte solution. Metal oxidizes and metal ions migrate from the anodic regions of the metal surface to the solution; the resulting electrons are consumed in cathodic reactions, where they reduce oxidized species in the cathodic regions of the metal surface. Electron acceptors such as oxygen (at neutral or basic pH) and H+ (at acidic pH) are required to complete the redox reaction (McCafferty, 2010).
Electroanalysis is widely used to monitor fouling and corrosion processes of metal surfaces, being highly sensitive and non-destructive analytical method if used near the corrosion potential (Mansfeld, 1990). Integration with scanning electron microscopy (SEM) provide further information on the morphology and chemistry of the fouling layer (Javaherdashti, 2008). Among electroanalytical methods, linear sweep voltammetry (LSV) and electrochemical impedance spectroscopy (EIS) near the open circuit potential (OCP) are the most used to determine the anodic and cathodic corrosion current through Tafel’s equation and the resistance or capacitance of the fouling layer, respectively (Sancy et al., 2010; Grgur et al., 2015; Caporali et al., 2008; Bandarenka, 2013). Here, LSV and EIS were used to determine changes in the surface electrochemistry with time and surface treatment.
The characterization of scaling process through electrochemical impedance methods has been reported by Zhao et al. (2019) and Marín-Cruz et al. (2007); however, the effect of descaling treatment on corrosion needs to be established for each specific descaling agent (Atapour et al., 2019). Scaling is often associated with calcium (Ca) and magnesium (Mg) salts that form in presence of concentrated anions like
,
,
. Calcium carbonate appears to be the most prevailing scaling component in heat transfer systems as reported by Marín-Cruz et al. (2004). It forms when hard water is processed and evaporated in heat transfer equipment (Eq. (1)):
Overall, three crucial polymorphs of calcium carbonate have been described in the study of Andritsos et al. (1997): calcite, aragonite and vaterite, albeit the latter is semi-occasional.
Most water sources in Kazakhstan are being polluted with industrial wastewater as effective wastewater treatment is not available in rural and less-urbanized areas as reported by Bayekenova and Bazarbayev (2008). Generally, the quality of surface water is good, however water in Kazakhstan is often hard due to contamination of Ca and Mg ions based on the study of Abuduwaili et al. (2019).
Descaling treatments such as acid pickling, hydraulic descaling with high pressure nozzles, electromagnetic and electrolytic descaling are commonly used for fouling removal and control (Abdul-Latif et al., 1988). However, acid pickling is harmful to health and environment because of spent pickle liquor generated during process as recorded by Binder (1989) and Regel-Rosocka (2010). On the other hand, mechanical cleaning of metal surfaces results in surface damage due the aggressive metal brushes used as reported by Gillström and Jarl (2006). Alternative approaches based on natural products can be investigated.
Citric acid, a natural component of fermented whey, is regarded as a green inhibitor of scale formation, hence, it is suggested for descaling various household appliances by Basso et al. (2017). However, instead of citric acid, fermented whey is commonly used to clean metal utensils in West Kazakhstan region, where restricted access to commercial descaling products prompted residents to seek low-cost solutions.
Whey is a by-product of cheese production, mainly consisting of whey proteins (Kosikowski, 1979). Since lactose is the main whey constituent along with soluble vitamins and proteins, whey is a feedstock for production of lactic acid (Panesar et al., 2007). Lactic acid is an effective descaling agent, and it is frequently added to commercial decalcifying products used in coffee making and ship maintenance industries (Wee et al., 2006). Nevertheless, 47% of cheese whey from dairy industry is discarded, leading to serious environmental pollution as reported by Jelen (1979) and Martín-del-Campo et al. (2019). Thus, alternative uses of whey and its fermentation products can be beneficial.
To the best of our knowledge, the use of fermented whey as descaling agent has not been reported, except for few minor notes on descaling ability of natural lactic acid as reported by Wee et al. (2006). In this work, we validate the descaling properties of fermented whey on mild steel coupons using both electrochemical and electron microscopy techniques. The results were then compared with minimal treatment (deionized water) and aggressive treatment (acetic acid). Fermented whey can be used as component of descaling products but should be mixed with environment-friendly corrosion inhibitors and applied only for short time as suggested by Popoola (2019).
2 Materials and methods
2.1 Surface preparation: clean coupons
Mild steel was obtained from local supplier and cut in-house into coupons. The composition of mild steel is shown in Table 1 (KV Steel, 2020). The specimens analyzed in this study were coupons with dimensions with 2 × 2 × 0.2 cm (surface area 5.6 cm2), which were drilled to obtain a 2 mm hole. The surface of drilled steel coupons was abraded with fine grit sandpaper (P400) to remove any macroscopic irregularity on the metal surface, followed by polishing with ultra-fine sandpaper (P800). Subsequently, metal coupons were polished with super ultra-fine waterproof abrasive paper (P1200) to obtain a smooth surface, and then washed with deionized (DI) water. Prior to experiments, metal specimens were briefly degreased in ethanol and acetone, rinsed with DI water, and dried at room temperature.
Element
Composition (wt%)
Carbon
0.17
Silicon
0.4
Manganese
0.8
Sulfur
0.04
Phosphorous
0.04
2.2 Scaling tests
Typical hard water utilized in household appliances contains approximately 300 mg L−1 of CaCO3 as reported by Surendran et al. (2016). Scaling tests were performed by preparing ‘very hard’ water, hardness of which was 11 times higher (3300 mg L−1) than tap water. CaCO3 (calcium carbonate) was dissolved in cold tap water (3 g L−1) and mixed thoroughly. Drilled and polished steel specimens were placed in a metal pot, and 100 mL of prepared 3000 mg L−1 CaCO3 solution was poured into the pot. The solution was boiled until it evaporated completely, and the next portion of hard water was added and boiled based on the same procedure. After boiling procedure, the steel coupons were left to dry in fume hood for 24 h. Scaled coupons were examined under Scanning Electron Microscopy (SEM) by applying an accelerated voltage of 2 kV (Core facilities, NU).
2.3 Descaling procedures
All the experiments were carried out at room temperature (25 ± 1 °C). Fermented cheese-whey exhibits lower pH compared to fresh whey, due to fermentation process as suggested by Shraddha and Nalawade (2015). Hence, descaling process of scaled coupons was performed by immersing scaled steel coupons in 200 mL of fermented whey solution (pH ∼ 2.8) obtained from local dairy company for 3, 5, 10 and 15 h. After immersion, coupons were washed gently with DI water and dried in fume hood for 24 h. It should be noted that there were no mechanical cleaning efforts made during descaling procedure. Control experiments with two reference solutions (vinegar (pH ∼ 2.8) and DI water) were also carried out using the same procedure.
2.4 Electrochemical analysis
Typical three-electrode system was applied for electrochemical studies of the mild steel coupons: clean, scaled, immersed in whey, vinegar, and DI water. Electrochemical impedance spectroscopy (EIS) and Linear Sweep Voltammetry (LSV) methods were conducted to evaluate the performance of whey as descaling agent. The electrodes were soldered to copper wire for connection to the potentiostat and insulated with epoxy resin. Coiled titanium wire was employed as counter electrode. Ag/AgCl (KCl saturated) with standard potential of 230 ± 10 mV vs. standard hydrogen electrode (SHE) served as reference electrode (BAS Inc., Japan). The three electrodes were placed into a 50 mL electrochemical cell containing 0.1 M KCl solution, which was then covered with parafilm. A VSP multichannel potentiostat (Bio-Logic, France) was used to carry out EIS and LSV experiments. The experimental results were interpreted through the EC-Lab software (Bio-Logic, France).
2.5 Electrochemical impedance spectroscopy
EIS was performed at OCP with a potential amplitude of 10 mV in the frequency range from 100 kHz to 0.1 Hz. The impedance of the system was modeled with an equivalent circuit comprising two-time constants in series (Scheme 1). The Equation (2) was used for data fitting.
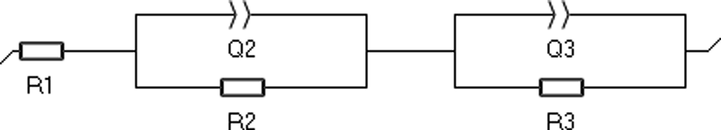
Equivalent electrical circuit for a mild steel coupon in electrolyte solution corrosion interphase. R1: electrolyte resistance, Q2, R2 high-frequency capacitance and resistance; Q3 and R3 low-frequency capacitance and resistance.
The equivalent circuit parameters R2, and R3 were obtained from the fitting. The high-frequency parameters Q2 and R2 did not show any pattern and were likely to be related to non-idealities in the electrical connection electrode-coupon and to the electrical double layer. The low-frequency parameters Q3 and R3 showed a recognizable pattern and were used for further analysis. The low-frequency, non-ideal capacitance
was expressed in terms of effective capacitance with the equation (3) as suggested by Bharatula et al. (2020):
2.6 Linear sweep voltammetry (LSV)
Linear potential sweeps were carried out from −0.5 V to 0.5 V vs. OCP, with scan rate of 0.5 mV s−1. Three samples for each treatment were measured. The overall flow diagram of experiments is represented in Fig. 1.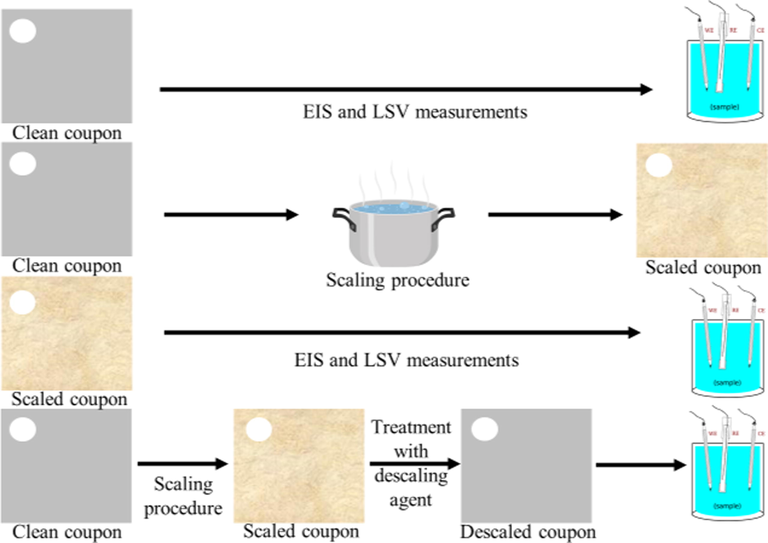
Flow diagram of the experiments.
2.7 Statistical analysis
Analysis of variance (ANOVA) at a confidence level of 95% was used to quantify the statistical significance of low-frequency, non-ideal capacitance .
3 Results and discussion
3.1 Scaling and descaling tests
Scaled and descaled coupons were imaged with SEM (Fig. 2). After scaling, the coupons showed stick-shaped crystals formed by dendritic growth arrangement, which is common for aragonite crystals (CaCO3) (Fig. 2b). After immersion in fermented whey (pH ∼ 2.8) for 15 h, the aragonite crystals were largely removed, and pitting corrosion was observed (Fig. 2f). Immersion in vinegar for 15 h showed a similar effect, with strong pitting corrosion and removal of most fouling layer (Fig. 2h). In the control experiment, scaled coupons were immersed in DI water for 15 h, which result in partial damage to the fouling layer, but without visible pitting corrosion (Fig. 2g). The descaling process was repeated with a shorter immersion time, 3 h, for fermented whey, DI water and vinegar (Fig. 2c, 2d, 2e). The effects were similar, but less pitting corrosion was observed in whey and vinegar. The effect of treatment with DI water for 3 h and 15 h on the coupon surface appeared similar, with a partial removal of the CaCO3 crystals in both cases.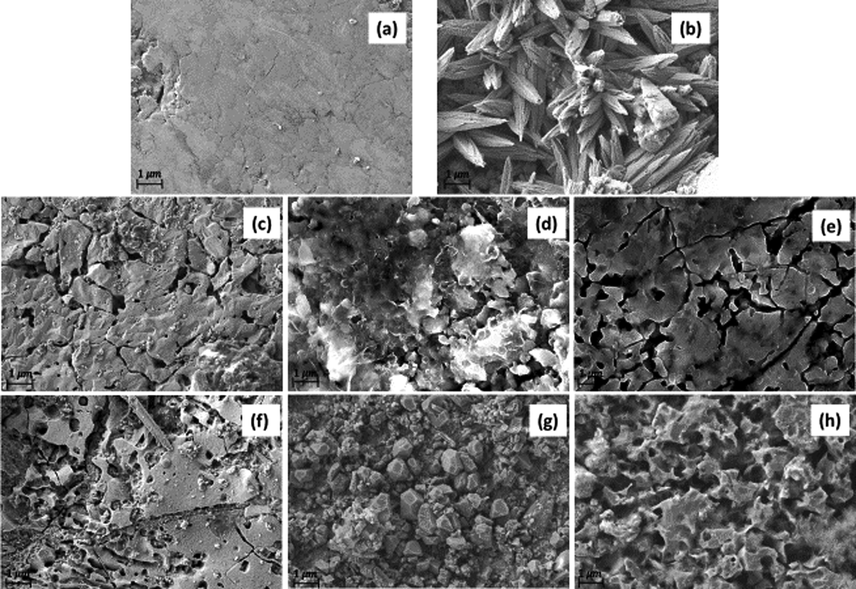
SEM images mild steel coupons clean (a), scaled (b), immersed in whey for 3 and 15 h (pH ∼ 3) (c), (f), immersed in DI water for 3 and 15 h (d), (g), immersed in vinegar for 3 and 15 h (pH ∼ 3) (e), (h).
3.2 Corrosion assessments
3.2.1 Open circuit potential
The equilibrium potential of the working electrode when no potential or current is forced between the working electrode and the reference electrode is referred to as OCP. For mild steel coupons in conductive electrolyte, OCP usually decreases with time and then stabilizes as demonstrated by Hassan et al. (2007). The OCP values depend on the surface and/or structural features, electrolyte concentration, and other variables of the system. Mild steel does not show ennoblement, a phenomenon commonly observed for stainless steel, in which OCP increases because of the formation of a passivation layer. Scaled electrodes achieve higher OCP (Fig. 3A), as the fouling layer protect partially the surface from anodic corrosion as reported by Zhu et al. (2018) and Zhu et al. (2019).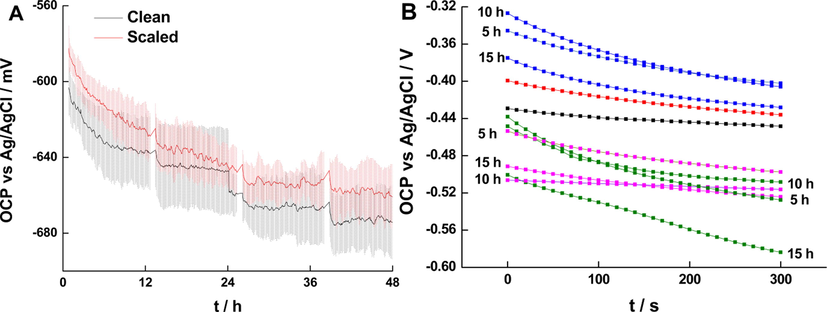
(A) Averaged OCP vs time for clean (black) and scaled (red) electrodes. The shades indicate the standard deviation of the 3 independent samples; (A) Variation of short-term OCP for clean (black), scaled (red), immersed in whey (green), vinegar (purple) and DI water (blue) for 5, 10,15 h.
The short-term variations of OCP prior to EIS for steel coupons in 0.1 M KCl after treatment for 5, 10, and 15 h are presented in Fig. 3b for independent steel specimens. The coupons scaled and descaled with DI water showed higher OCP for all treatment times compared to bare metal samples. The high values of OCP for samples treated in DI water indicate that DI water removed only partially the fouling layer (ArunKumar et al., 2017). The OCPs for whey and vinegar treatment showed a much lower value at all treatment times, which was lowest for whey treatment at 15 h. These results confirmed the strong corrosion particularly for fermented whey at 15 h treatment time. Overall, both fermented whey and vinegar effectively removed the scaling layer, but they caused strong corrosion at long treatment times.
3.2.2 LSV measurement
Fig. 4 illustrates the Tafel plots of five coupons in 0.1 M KCl: clean, scaled, and descaled with DI water, fermented whey, and vinegar for 3 h, respectively. The corrosion potential (Ecorr) and corrosion current (Icorr) are given in Table 2. The Ecorr decreases following the scaling treatment and descaling with DI water did not affect Ecorr. On the other hand, immersion in fermented whey and vinegar nearly restore the original Ecorr, indicating the removal of the scale layer. The Icorr showed a similar pattern but the difference between clean and treated coupons were not statistically significant, except for scaled coupons (Fig. S1).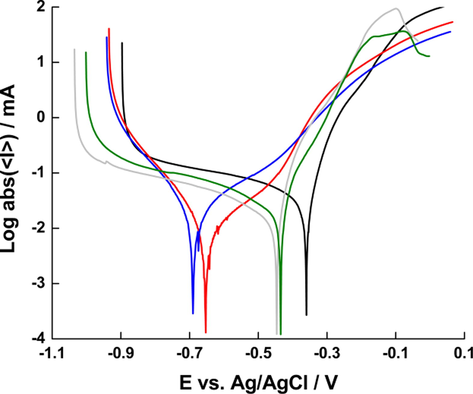
Polarization curves for clean (black), scaled (red), descaled in DI water (blue), whey (green) and vinegar (gray) (pH ∼ 3) for 3 h mild steel samples in 0.1 M KCl.
Sample
Ecorr / mV
Icorr / µA
Clean
−360 ± 26
49.3 ± 11.8
Scaled
−652 ± 1
16.2 ± 3.8
Cleaned with DI water
−687 ± 4
15.4 ± 15.1
Cleaned with vinegar
−448 ± 1
19.3 ± 11.8
Cleaned with whey
−427 ± 11
24.7 ± 6.2
The Icorr for scaled and DI-treated coupons were lower than that of clean specimen, which might be due to the blocking of the active regions of the mild steel surface, hence calcium carbonate scale acting as corrosion inhibitor. Similar effect of plant extracts as corrosion inhibitors was reported by Al-Otaibi et al. (2014). In other words, presence of calcium carbonate scale provided protection to mild steel as it was concluded by Tavares et al. (2015). The Icorr for whey- and vinegar-treated descaled samples was significantly lower than clean samples and slightly higher than scaled and DI-treated coupons, although the increase was not statistically significant. This may have been caused by the elimination of fouling layer which reduce the corrosion inhibition, thus allowing for faster metal dissolution. A more detailed analysis of the cathodic and anodic slope in the Tafel plot was not possible due to the poor reproducibility of the results, which is common when dealing with large steel coupons. The similarity of Tafel plots for both vinegar and fermented suggest that the descaling and corrosion mechanisms are similar in the two reagents, which has similar pH values. Whey seems to perform slightly better than vinegar, as shown by the Icorr values, however, the difference is not statistically significant.
4 Electrochemical impedance
The estimated values of the effective capacitance
are plotted in Fig. 5.
increased following the formation of the scale layer and decreases again following whey treatment, confirming that fermented whey effectively removes the scale layer, as previously shown in the OCP analysis (Fig. 5). ANOVA analysis confirmed that
was significantly different between clean, scaled and whey-descaled coupons (Table S2). The non-ideality of the low-frequency capacitance, described by the parameter a (Table S1), did not change significantly with the scaling and de-scaling treatments.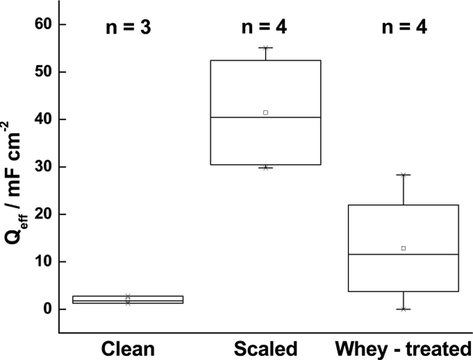
Effective capacitance
for clean, scaled, and descaled (immersed in fermented whey for 15 h) coupons. n = number of independent replicates.
It should also be noted that EIS returns the average impedance of the whole sample, without accounting for the local scale distribution. Therefore, it is likely that the of scaled coupon varies across independent replicates because of the inconsistency of the scale layer on the metal surface. The small value of effective capacitance for the clean coupons indicates that the steel surface is fully accessible and subject to continuous corrosion (Marín-Cruz et al., 2004). The effective capacitance of scaled electrodes describes the combined properties of two interphases, the metal/electrolyte, and the scale layer/electrolyte, in which the scale layer has lower conductivity and higher capacitance than the metal surface. As expected, EIS results confirm the formation of a scale layer as observed in the SEM analysis.
Overall, EIS results are consistent with the formation of a porous, poorly conductive layer of calcium carbonate on the metal surface and its removal following weak acid treatment. The difference between effective capacitance of clean and descaled electrodes can be explained with the appearance of pitting corrosion caused by descaling process and confirmed through SEM (Fig. 2). While the simultaneous characterization of corrosion and descaling processes requires further experiments, this work shows the potential of simple electroanalysis for rapid and low-cost monitoring of physicochemical processes at steel/electrolyte interface. Shorter treatment time will likely minimize pitting corrosion, while achieving effective removal of the scale layer. On the other hand, the release of toxic impurities from low-quality steel coupons following weak acid treatment as reported by Tang et al. (2012) should be considered for practical applications of fermented whey as descaling agent.
5 Conclusion
Mild steel coupons scaled with calcium carbonate were descaled through immersion in fermented whey. The scaling and descaling process was characterized with SEM and electroanalytical methods. The OCP was lower for clean steel coupons than scaled coupons, indicating lower corrosion rate for scaled coupons. The effective capacitance was higher for the scaled coupons, showing the formation of a poorly conductive calcium carbonate layer. decreased after the immersion in fermented whey, due to the partial removal of the scale layer, as confirmed by SEM images and LSV analysis. However, the treatment with fermented whey led to pitting corrosion. Shorter treatment time (3 h) effectively remove the scale layer while minimizing the pitting corrosion. Overall, the results show that fermented whey can be used to formulate low-cost and eco-friendly descaling products. The formulation should be complemented with a corrosion-protecting agent. Dissolution of allergenic and toxic impurities from steel surface following descaling treatment should be also considered in further studies.
Although the economic viability and scale-up of the proposed technology is yet to be investigated, we believe that the simplicity of the methodology outlined here and the ready availability of whey in small farming communities will favor applications in rural areas, where access to household cleaning products for descaling of home appliances is limited by poor availability and high costs. Further, partial reuse of whey in formulation of cleaning products will reduce the overall environmental impact of small-scale faming.
Acknowledgements
This work was funded by School of Engineering and Digital Sciences, Nazarbayev University, Kazakhstan, under the Faculty Development Competitive Research Grant Program (Grant number 110119FD4537). The authors would also like to acknowledge Dr. Almagul Mentbayeva for the support and contribution to this study.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Removal of scale deposited on heat-transfer surfaces using chemical methods. Ind. Eng. Chem. Res.. 1988;27(8):1548-1551.
- [CrossRef] [Google Scholar]
- Abuduwaili, G.J, Issanova, G., Saparov, 2019. Water resources in Kazakhstan, in: Hydrol. Limnol. Cent. Asia, Springer, Singapore, pp. 11–46.
- Corrosion inhibitory action of some plant extracts on the corrosion of mild steel in acidic media. Arab. J. Chem.. 2014;7(3):340-346.
- [CrossRef] [Google Scholar]
- Morphology and structure of CaCO3 scale layers formed under isothermal flow conditions. Langmuir. 1997;13(10):2873-2879.
- [CrossRef] [Google Scholar]
- Evaluating the performance of MoS2 based materials for corrosion protection of mild steel in an aggressive chloride environment. RSC Adv.. 2017;7(28):17332-17335.
- [CrossRef] [Google Scholar]
- Metal release from stainless steel 316L in whey protein - And simulated milk solutions under static and stirring conditions. Food Control. 2019;101:163-172.
- [CrossRef] [Google Scholar]
- Exploring the interfaces between metal electrodes and aqueous electrolytes with electrochemical impedance spectroscopy. Analyst. 2013;138:5540-5554.
- [CrossRef] [Google Scholar]
- Anti-fouling surface modified stainless steel for food processing. Food Bioprod. Process.. 2013;91(4):352-361.
- [CrossRef] [Google Scholar]
- Study of chemical environments for washing and descaling of food processing appliances: an insight in commercial cleaning products. J. Ind. Eng. Chem.. 2017;53:23-36.
- [CrossRef] [Google Scholar]
- Ecology and modern socio-economic conditions in Kazakhstan. In: Qi J., Evered K.T., eds. Environmental Problems of Central Asia and Their Economic, Social and Security Impacts. Netherlands, Dordrecht: Springer; 2008. p. :209-214.
- [Google Scholar]
- Impedimetric detection of Pseudomonas aeruginosa attachment on flexible ITO-coated polyethylene terephthalate substrates. Electrochim. Acta. 2020;332:135390.
- [CrossRef] [Google Scholar]
- Deaths, injuries, and evacuations from acute hazardous materials releases. Am. J. Public Health. 1989;79(8):1042-1044.
- [CrossRef] [Google Scholar]
- International measures of prevention, application, and economics of corrosion technologies study. NACE Int. 2016:1-216.
- [Google Scholar]
- Aluminium electroplated from ionic liquids as protective coating against steel corrosion. Corros. Sci.. 2008;50(2):534-539.
- [CrossRef] [Google Scholar]
- Mechanical descaling of wire rod using reverse bending and brushing. J. Mater. Process. Technol.. 2006;172(3):332-340.
- [CrossRef] [Google Scholar]
- Corrosion of mild steel with composite polyaniline coatings using different formulations. Prog. Org. Coatings. 2015;79:17-24.
- [CrossRef] [Google Scholar]
- Inhibition of mild steel corrosion in hydrochloric acid solution by triazole derivatives. Part I. Polarization and EIS studies. Electrochim. Acta. 2007;52(22):6359-6366.
- [CrossRef] [Google Scholar]
- Microbiologically Influenced Corrosion: An Engineering Insight. Engineering Materials and Processes. Springer; 2008.
- Industrial whey processing technology: an overview. J. Agric. Food Chem.. 1979;27(4):658-661.
- [CrossRef] [Google Scholar]
- Whey utilization and whey products. J. Dairy Sci.. 1979;62(7):1149-1160.
- [CrossRef] [Google Scholar]
- Electrochemical impedance spectroscopy (EIS) as a new tool for investigating methods of corrosion protection. Electrochim. Acta. 1990;35(10):1533-1544.
- [CrossRef] [Google Scholar]
- EIS characterization of the evolution of calcium carbonate scaling in cooling systems in presence of inhibitors. J. Solid State Electrochem.. 2007;11(9):1245-1252.
- [CrossRef] [Google Scholar]
- Characterization of different allotropic forms of calcium carbonate scales on carbon steel by electrochemical impedance spectroscopy. J. Appl. Electrochem.. 2004;34(3):337-343.
- [CrossRef] [Google Scholar]
- Production of antioxidant and ACEI peptides from cheese whey discarded from Mexican white cheese production. Antioxidants. 2019;8:1-10.
- [CrossRef] [Google Scholar]
- McCafferty, E., 2010. I. to corrosion science. S.S. & B.M., 2010. Introduction to corrosion science. Springer Science & Business Media.
- MILD STEEL [WWW Document], n.d. URL https://kvsteel.co.uk/steel/mild-steel.html (accessed 11.4.20).
- Bioutilisation of whey for lactic acid production. Food Chem.. 2007;105(1):1-14.
- [CrossRef] [Google Scholar]
- Progress on pharmaceutical drugs, plant extracts and ionic liquids as corrosion inhibitors. Heliyon. 2019;5(2):e01143.
- [CrossRef] [Google Scholar]
- A review on methods of regeneration of spent pickling solutions from steel processing. J. Hazard. Mater.. 2010;177(1-3):57-69.
- [CrossRef] [Google Scholar]
- Mechanism of corrosion of cast iron covered by aged corrosion products: application of electrochemical impedance spectrometry. Corros. Sci.. 2010;52(4):1222-1227.
- [CrossRef] [Google Scholar]
- Shraddha, R.C., C.R., Nalawade T, K.A., 2015. Whey based beverage: its functionality, formulations, health benefits and applications. J. Food Process. Technol. 6. https://doi.org/10.4172/2157-7110.1000495.
- The impacts of magnetic treatment of irrigation water on plant, water and soil characteristics. Agric. Water Manag.. 2016;178:21-29.
- [CrossRef] [Google Scholar]
- Minimizing the creation of spent pickling liquors in a pickling process with high-concentration hydrochloric acid solutions: mechanism and evaluation method. J. Environ. Manage.. 2012;98:147-154.
- [CrossRef] [Google Scholar]
- Effect of calcium carbonate on low carbon steel corrosion behavior in saline CO 2 high pressure environments. Appl. Surf. Sci.. 2015;359:143-152.
- [CrossRef] [Google Scholar]
- Biotechnological production of lactic acid and its recent applications. Food Technol. Biotechnol.. 2006;44:163-172.
- [Google Scholar]
- Fundamental nanoscale surface strategies for robustly controlling heterogeneous nucleation of calcium carbonate. J. Mater. Chem. A. 2019;7(29):17242-17247.
- [CrossRef] [Google Scholar]
- The role of corrosion inhibition in the mitigation of CaCO3 scaling on steel surface. Corros. Sci.. 2018;140:182-195.
- [CrossRef] [Google Scholar]
- Local scaling of CaCO3 on carbon steel surface with different corrosion types. Powder Technol.. 2019;356:990-1000.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2021.103065.
Appendix A
Supplementary material
The following are the Supplementary data to this article:







