Translate this page into:
Gallic acid-derived carbon dots intercalated 3D porous Ti3C2Tx MXene for high-capacity supercapacitors
* Corresponding authors: E-mail addresses: wukeliang@nyist.edu.cn (K. Wu), 15709932986@163.com (N. Gu)
-
Received: ,
Accepted: ,
Abstract
Ti3C2Tx Mxene shows good promise as an energy storage material. However, two-dimensional MXene materials are easily stacked, which negatively affects the energy density and limits the application of MXene-based supercapacitors. In this paper, a strategy for embedding carbon dots is proposed to address this issue. A three-dimensional structure is formed by cross-linking Ti3C2Tx MXene nanosheets with gallic acid (GA), and a subsequent carbonization process is then utilized to prepare a three-dimensional porous carbon dot intercalated Ti3C2Tx MXene material. The hydroxyl structures in GA are cross-linked with the hydroxyl structures on the surface of Ti3C2Tx under the action of Zn2+ to form a three-dimensional structure. The carbon dots formed by carbonizing the GA are embedded within the Ti3C2Tx nanosheets, which increases the Ti3C2Tx nanosheet layer spacing. The increase of layer spacing of Ti3C2Tx nanosheets is conducive to the diffusion and transport of electrolyte ions in Ti3C2Tx MXene. Consequently, at a scan rate of 2 mV s-1, a Ti3C2Tx@Celectrode achieves a high electrochemical gravimetric pecific capacitance (Cg) of 393.7 F g-1. At a higher scan rate of 200 mV s-1, 83.7% capacitance retention is obtained. Moreover, this electrode also shows 90.3% performance retention after 5000 cycles, demonstrating its good cycle stability. Finally, a symmetric supercapacitor was built using carbon cloth and Ti3C2Tx@C, and at a power density of 153.25Wkg-1, a superb energy density of 21.28Whkg-1is attained. This study offers a facile strategy for effectively preparing MXene electrodes with desirable electrochemical performance.
Keywords
3D porous MXene
Carbon dots
Fast electronic transmission
Flexible electrode
Supercapacitors
1. Introduction
MXenes are two-dimensional (2D) materials that have been widely investigated in many fields due to their compelling surface properties and unique structures. For instance, MXene-based materials exhibit high conductivity and good hydrophilicity, indicating their promising potential for catalysis, energy storage, shielding electromagnetic waves, and sensing applications [1,2]. However, the van der Waals forces between the monolayers in MXene structures lead to layer stacking or undesirably narrow layer spacing. When MXenes are used as electrochemical materials, this results in poor electrochemical performance because ion intercalation/deintercalation is inhibited [3-5].
To address this daunting challenge, researchers have investigated a variety of interesting approaches. For instance, a guest material can be introduced as an intermediate layer to generate a porous structure between the MXene layers [6-8]. Jiang’s team reports an innovative restricted hydrothermal strategy that combines chemical crosslinking to construct three-dimensional layered porous MXene/reduced graphene oxide (RGO) composite fibers. This well-designed architecture effectively prevents MXene stacking while creating efficient ion transport channels. It is worth noting that thiourea serves as both a chemical crosslinking agent and selective reducing agent, while reduced graphene oxide nanosheets have the physical barrier effect, which synergically enhances the oxidation resistance of the composite. The optimized composite fiber has excellent electrical conductivity (862.2 S cm−1), mechanical strength (93.1 MPa), and oxidation resistance (90.5% conductivity after 60 days) [9]. Assembling 2D sheets into three-dimensional (3D) morphologies is another effective method for preventing MXene layer stacking [10,11]. Moreover, the self-assembly of 3D porous structures generates an abundance of electrochemically active sites that can be accessed by electrolyte ions [12,13]. Yang’s team designed and implemented a layered heterostructure of conductive mesoporous hollow carbon spheres (MHCS) and MXene composite electrodes through simple vacuum filtration without further template removal processes. This direct preparation strategy not only effectively improves the specific surface area of the electrode, but also enhances the abundant surface pores and good electrical conductivity of MHCS, improves the permeability of electrolyte solution, shortens the ion transport path, and thus significantly improves the specific capacitance (395F g-1 at 2 mVs-1) and rate performance (70.9% at 1000 mV s-1) of the electrode. The introduction of carbon nanotubes (CNTs) further improves electrical conductivity and stability while maintaining good flexibility. As a result, MXene/MHCS/CNT films offer high specific capacitance, excellent magnification performance, and excellent cycle stability [11]. However, although three-dimensional structures are conducive to electrolyte ion transport in Ti3C2Tx MXene electrodes, these structures are not conducive to electron transport between the Ti3C2Tx MXene nanosheets. Due to these factors, new strategies should be developed to prepare three-dimensional porous MXene structures that inhibit the interlayer stacking of Ti3C2Tx MXene nanosheets while guaranteeing rapid electron transport between the nanosheet layers. Due to their small size, carbon dots formed in situ can be easily embedded into the interlayer of MXene, playing a pillar-like role and thus inhibiting the interlayer stacking of MXene. At the same time, due to its high conductivity, it can also serve as an electron transmission channel between MXene layers, thereby enhancing the electron transmission efficiency between MXene layers. Zhang’s team proposed a carbon dot-intercalated strategy to prepare flexible MXene thin film electrodes with large ion-accessible active surfaces and high density by gelating and carbonizing calcium alginate (CA) within MXene nanosheets. CA hydrogels are formed in MXene nanosheets and accompanied by evaporation drying, resulting in high density of MXene/CA films. During the carbonization process, CA-derived carbon dots can be embedded into MXene nanosheets, increasing the layer spacing and promoting electrolyte diffusion within MXene films. Thus, MXene films embedded with carbon dots exhibit high capacitance at 3 M H2SO4 (1244.6F cm−3 at 1 A g−1). Excellent rate performance (662.5 F cm−3 at 1000 A g−1) and excellent cycle stability (93.5% capacitance maintained after 30000 cycles). However, it is difficult for polymer materials to completely enter the interlayer of MXene, resulting in an uneven distribution of carbon dots between the MXene layers, and carbon dots derived from small molecular materials can solve this problem [14].
GA is a natural compound containing a catechol structure based on mussel adhesion protein, which shows great potential in the surface coating, modification, and functionalization of different substrates. The small molecule compounds containing phenolic hydroxyl groups can form intermolecular hydrogen bonds with the functional groups (-O,-OH, -F) on the surface of MXene nanosheets. Meanwhile, the phenolic hydroxyl groups on the surface of small molecule compounds and the hydroxyl groups on the surface of MXene nanosheets can be physically cross-linked under the action of divalent metal cations. Therefore, GA coating can effectively maintain the structural stability of MXene and achieve controllable surface functionalization.
In this paper, a carbon dot intercalation strategy is proposed for preparing three-dimensional porous carbon dot-intercalated MXene materials. First, the MXene nanosheets are cross-linked with GA. The hydroxyl groups of GA and the MXene surface are cross-linked under the action of Zn2+, which forms a three-dimensional structure. Then, a carbonization process is employed to generate carbon dots from the GA. These carbon dots are embedded into the MXene nanosheet structure, which increases the layer spacing and promotes electrolyte ion diffusion into the MXene. Thus, the Ti3C2Tx@C electrode achieves excellent electrochemical performance, good retention of capacitance at higher scanning rate values, and excellent cycle stability. Moreover, a symmetric supercapacitor prepared using carbon cloth and the Ti3C2Tx@C electrode also demonstrates superb energy density. The results in this paper offer a compelling and efficient pathway for obtaining strong electrochemical performance.
2. Materials and Methods
2.1. Materials
Analytical-grade chemicals were used in all experiments without being purified further. Zinc chloride (ZnCl2), concentrated hydrochloric acid (HCl), and carbon cloth were purchased from China Sinophosphate Chemical Reagent Co., Ltd. The 600 meshTi3AlC2 powder was provided by 11 Technology Co., Ltd. (Jilin, China). Gallic acid (C7H6O5) and lithium fluoride (LiF) were obtained from Macklin, Inc.
2.2. Ti3C2Tx MXene colloid suspension
The Ti3C2Tx MXene was formed by removing the Al from Ti3AlC2 powder via selective etching. First, a 9 mol/L HCl solution was generated by slowly combining 29.8 mL of concentrated HCl with 10.2 mL of water. Then, 40 mL of this 9 mol/L HCl solution was employed to dissolve 2 g LiF, and this solution was combined with 1 g Ti3AlC2 powder. Next, this mixture was stirred at 40°C for 24 hrs to obtain a slurry. The temperature was maintained with an oil bath. The pH of this slurry was then adjusted to about 6 via repeated washing and centrifugation at 7000 rpm. Finally, a Ti3C2Tx solution was obtained by ultrasonicating the obtained supernatant under Ar gas for 1 hour and centrifuging at 3500 rpm [15]. The Ti3C2Tx colloid solution concentration was 5 mg mL-1, as confirmed by freeze-drying.
2.3. Three-dimensionalporous Ti3C2Tx@C aerogel
First, 19 mL of the MXene solution (5 mg mL-1) and 1 mL of gallic acid (5 mg mL-1) were combined in a beaker. This combined solution was continuously stirred for 30 minutes. Then, 1 mL ZnCl2(1 mol/L) was added to the beaker, and stirring was continued for another hour. As a crosslinking agent, Zn2+ makes Ti3C2Tx and GA physically cross-link, so GA intercalates between the layers of Ti3C2Tx. Next, centrifugation with deionized water was performed thrice. Finally, Ti3C2Tx@GA aerogel was acquired by freeze-drying. The Ti3C2Tx@GA was calcined in a tube furnace under Ar gas (500°C, 10°C min-1, 2 hour dwell time). The Ti3C2Tx@C aerogel was obtained after natural cooling.
2.4. Flexiblesymmetric supercapacitor
Symmetric supercapacitor devices are prepared by using Ti3C2Tx@C/carbon cloth as both positive and negative electrodes. Two Ti3C2Tx@C/carbon cloth electrodes (3 cm × 3 cm) were packaged in polyethylene terephthalate (PET), with 3 mol/L polyvinyl alcohol (PVA)/H2SO4 gel as electrolyte and water microporous filter membrane as diaphragm material. The Ti3C2Tx@C/carbon cloth electrode is prepared as follows: 8 mg Ti3C2Tx@C and 80 µL Nafion (as a binder) are dispersed in water/ethanol solution to prepare the electrode, which is then dripped onto a carbon cloth and dried. The preparation of PVA/H2SO4 electrolyte is as follows: Take 20 g of PVA aqueous solution with a mass fraction of 2% (wt), add 3.92 g of sulfuric acid to stir and cool, and then add 5 mg of ammonium persulfate and stir well.
2.5. Characterization
A scanning electron microscope (SEM, Gemini 300, ZEISS) and high-resolution transmission electron microscope (HRTEM, JEOL JEM 2100F) were utilized to view the morphology and microstructure. The surface chemical state and composition were studied with an X-ray photoelectron spectrometer (XPS, AXIS Ultra DLD and AXIS Supra, Kratos). Nitrogen adsorption–desorption analysis was utilized to study the textural properties of each sample (ASAP2460, Micromeritics). Crystal structures were evaluated with an X-ray diffractometer (XRD, Rigaku Ultima IV, Cu Kα, 5°/min scan speed).
2.6. Electrochemical tests
Electrode materials were prepared by dispersing 8 mg of active substance with 80 µL Nafionbinder in a water/ethanol solution. Each obtained slurry was coated on a 10 mm glass carbon electrode. The mass of the active material coated on the working electrode is 0.5 mg. Then, a three-electrode Chenhua Chi 760e (Shanghai Chenhua) was used to analyze each prepared electrode. The analysis was performed using working, counter, and reference electrodes (the prepared Ti3C2Tx@C electrode, a graphite rod, and Ag/AgCl (1 M KCl), respectively). Cyclic voltammetry (CV) and galvanostatic charge/discharge (GCD) curves were obtained in 3 M H2SO4 electrolyte (-0.6 to 0.2 V, vs. Ag/AgCl). A range of 0.01 Hz to 100 kHz and a fixed potential amplitude of 5 mV were employed for electrochemical impedance spectroscopy (EIS). Finally, a symmetric supercapacitor was built using Ti3C2Tx@C/carbon cloth as both the positive electrode and negative electrode [16]. To prepare this symmetric device, PET was used to encapsulate two Ti3C2Tx@C/carbon cloth electrodes. The two electrodes were then separated using a 3 M PVA/H2SO4 gel electrolyte.
Gravimetric specific capacitance (Cg) was calculated based on the charge-discharge curves using Eq. (1):
Where I is the gravimetric current (A), t represents the discharge time (s), m is the mass of the working electrode (g), and ΔV is the potential window (V). The value of Cg was also calculated by utilizing the CV curves with Eq. (2):
Where m is the mass of the working electrode (g), v is the potential scan rate (V s-1), V is the potential window (V), and i is the current (A).For calculations involving the symmetric supercapacitor, the total anode+cathode mass was used for the mass of the working electrode. The energy density (Eg) and power density (Pg) of the symmetric supercapacitor were determined using Eqs. (3) and (4):
Where ∆t is the discharge time (h) and m is the total working electrode mass (kg).
3. Results and Discussion
The process for preparing porous Ti3C2Tx@C aerogel has been shown in Figure 1. First, the Al layer in the Ti3AlC2 phase is removed using a LiF/HCl mixture to obtain multilayer Ti3C2Tx nanosheets. In this etching step, hydrophilic groups (-F, -O, and -OH) are used to terminate Ti3C2Tx [16]. The Ti atoms have different electron gravities than the O/F atoms, which means that the end of the Ti3C2Tx nanosheets carry more charge. Consequently, the Ti3C2Tx nanosheets are negatively charged. This provides the Ti3C2Tx nanosheets with good water dispersion properties due to electrostatic repulsion [17]. Next, gallic acid solution (GA) is added to the Ti3C2Tx MXene suspension and evenly mixed, followed by the addition of ZnCl2 solution. The introduction of Zn2+ into the Ti3C2Tx MXene@GA suspension breaks its electrostatic balance. At the same time, Zn2+ acts as a cross-linker, enabling the formation of a three-dimensional porous Ti3C2Tx MXene@GA structure. The obtained Ti3C2Tx@GA hydrogel then undergoes freeze-drying to obtain a Ti3C2Tx@GA aerogel. Finally, the Ti3C2Tx@C aerogel containing intercalated carbon dots is obtained by carbonizing the Ti3C2Tx@GA aerogel at 500°C.
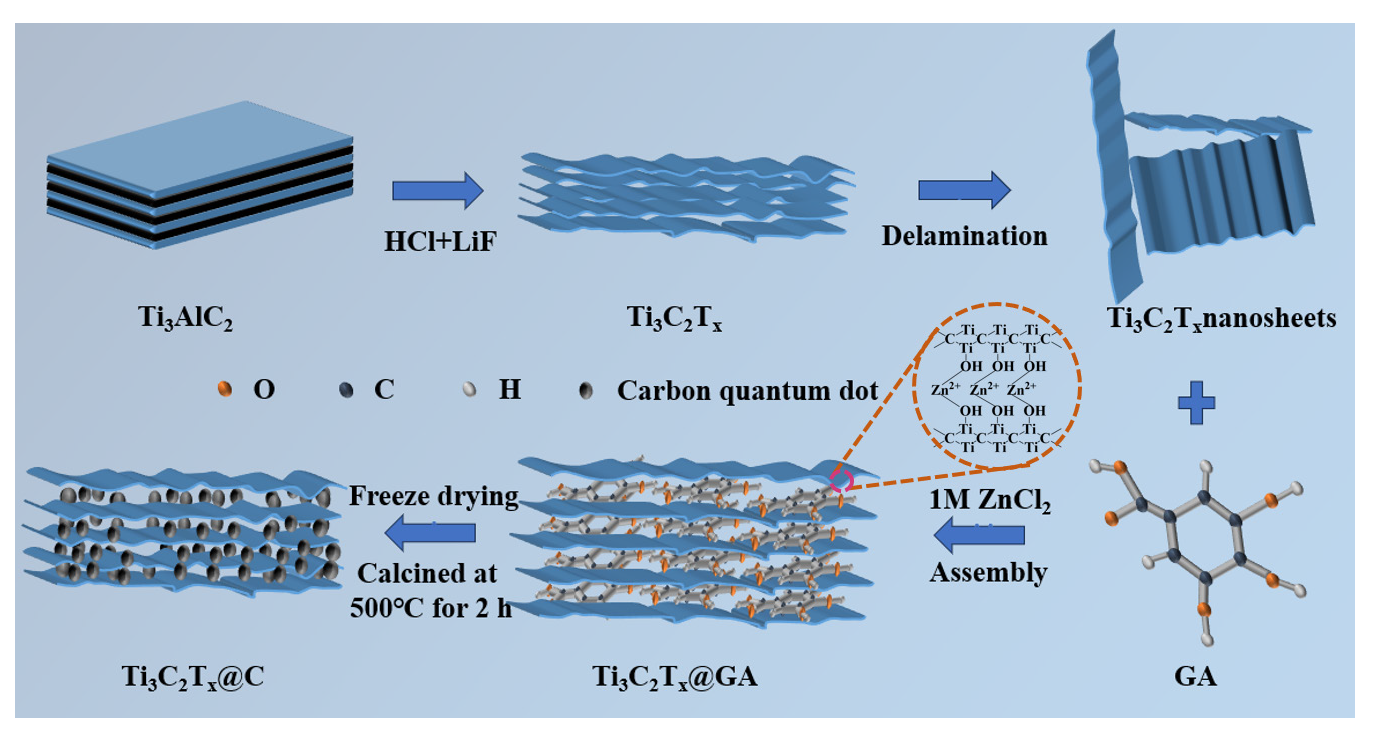
- Preparation process for obtaining the three-dimensional porousTi3C2Tx@Caerogel.
In the Figure 2, the surface topography changes caused by pore formation in the Ti3C2Tx MXene that have been shown in Figure 2(a). Large-sized MXene nanosheets with a typical layered structure can be observed. The MXene sheets are oriented in a face-to-face manner, so the Ti3C2Tx MXene has a dense structure, which may limit the availability of active sites for electrolyte ions. In contrast, a three-dimensional macroporous structure (macropore diameters of 5–10 μm) can be observed in the Ti3C2Tx@C and Ti3C2Tx@GAaerogel (Figure 2b and 2c). Both Ti3C2Tx and Ti3C2Tx@C have type-IV nitrogen–desorption isotherms with H3 hysteresis loops (Figure S1a), indicating mesoporosity. Ti3C2Tx@C has a larger specific surface area than pure Ti3C2Tx ((35.57 vs. 29.69 m2g-1). The barret-joyner-halenda (BJH) pore size distribution of Ti3C2Tx@C (Figure S1b) confirms its mesoporosity [18]. The presence of the intercalated carbon dots causes the MXene nanosheets in Ti3C2Tx@C to become more disordered. The 3D macroporous structure of Ti3C2Tx@C is attributed to the Zn2+ acting as a cross-linking agent for the -OH on the surface of Ti3C2Tx and the -OH on GA. This structure facilitates electrolyte penetration and ion accessibility on the surface of the MXene, which is expected to provide superior capacitance and rate performance. The microstructure of the Ti3C2Tx@C powder was observed by transmission electron microscope (TEM) (Figure 2e). The carbonized Ti3C2Tx@C nanosheets contain a distribution of many nanometer-sized particles. These nanoparticles are carbon, as indicated by the 0.43 nm lattice spacing (Figure 2f). Thus, Ti3C2Tx@C contains intercalated carbon particles. Element mapping demonstrates the uniform distribution of Zn in Ti3C2Tx@C (Figure 2g).
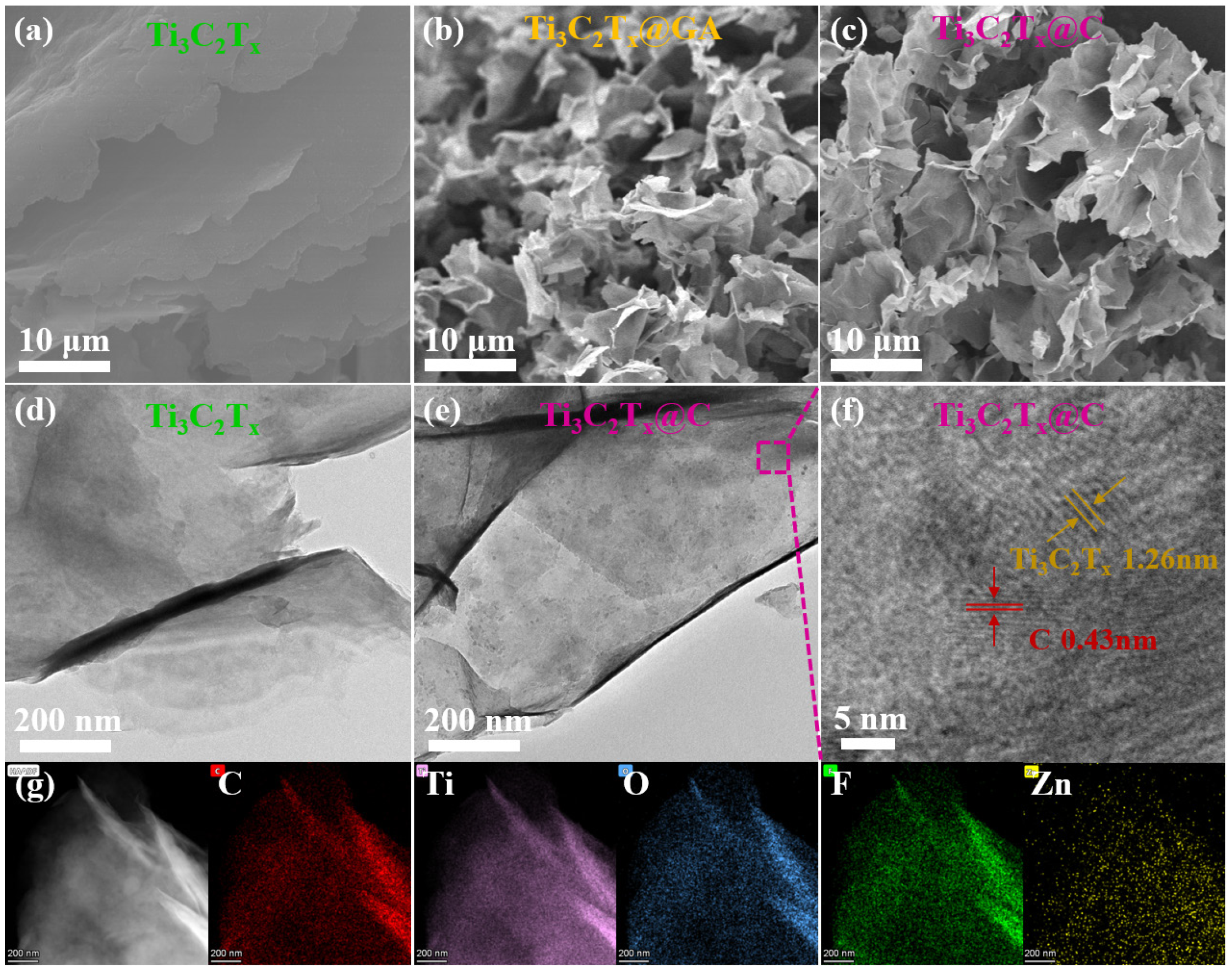
- SEM micrographs of (a) Ti3C2Tx, (b) Ti3C2Tx@GA and (c) Ti3C2Tx@C. TEM micrographs of (d) Ti3C2Tx and (e) Ti3C2Tx@C. (f) HRTEM micrographs of Ti3C2Tx@C. (g) EDX elemental mapping images of C, Ti, O, F, and Zn for Ti3C2Tx@C. EDX: Energy dispersive X-ray spectroscopy.
XRD was used to evaluate the atomic structures of Ti3C2Tx@C and Ti3C2Tx, as displayed in Figure 3(a). After etching with LiF-HCl, the (002) peak of the Ti3AlC2 MAX phase is shifted to 7.28° (indicating 12.13 Å d-spacing), and the (104) peak vanishes, proving that 2D Ti3C2Tx was successfully synthesized [19]. Compared with Ti3C2Tx, the (002) peak of the Ti3C2Tx@C aerogel shifts to a lower 2θ value by 6.36°(indicating 13.85 Å d-spacing). Thus, Ti3C2Tx@C has wider d-spacing, which can be ascribed to the carbon dots intercalated between the Ti3C2Tx MXene nanosheets. This is favorable for making the structure more accessible to the electrolyte [15]. The XPS survey spectra of Ti3C2TxandTi3C2Tx@C demonstrate the successful addition of Zn2+ to Ti3C2Tx@C (Figure 3b). The Ti 2p spectra (Figure 3c) show deconvoluted peak pairs at 454.76 and 460.56 eV (Ti-C), 455.46 and 461.24 eV (Ti2+), 456.56 eV and 462.71 eV (Ti3+), and 459.03 eV and 464.21 eV (TiO2) [16]. Due to the presence of Zn2+ as a cross-linker, Ti3C2Tx@C exhibits stronger Ti2+ peaks. The C1s spectrum of Ti3C2Tx@C (Figure 3d) has a stronger C-C bond peak than Ti3C2Tx, which confirms the large number of carbon dots in Ti3C2Tx@C. The O 1s spectrum of Ti3C2Tx@C contains a slightly smaller C-Ti-OH bond peak compared to that of Ti3C2Tx (Figure 3e), which can also be ascribed to Zn2+ cross-linking the -OH on the MXene surface with the -OH on GA. The Zn 2p spectrum of Ti3C2Tx@C confirms that Zn2+ has been successfully inserted into the Ti3C2Tx layers (Figure 3f).
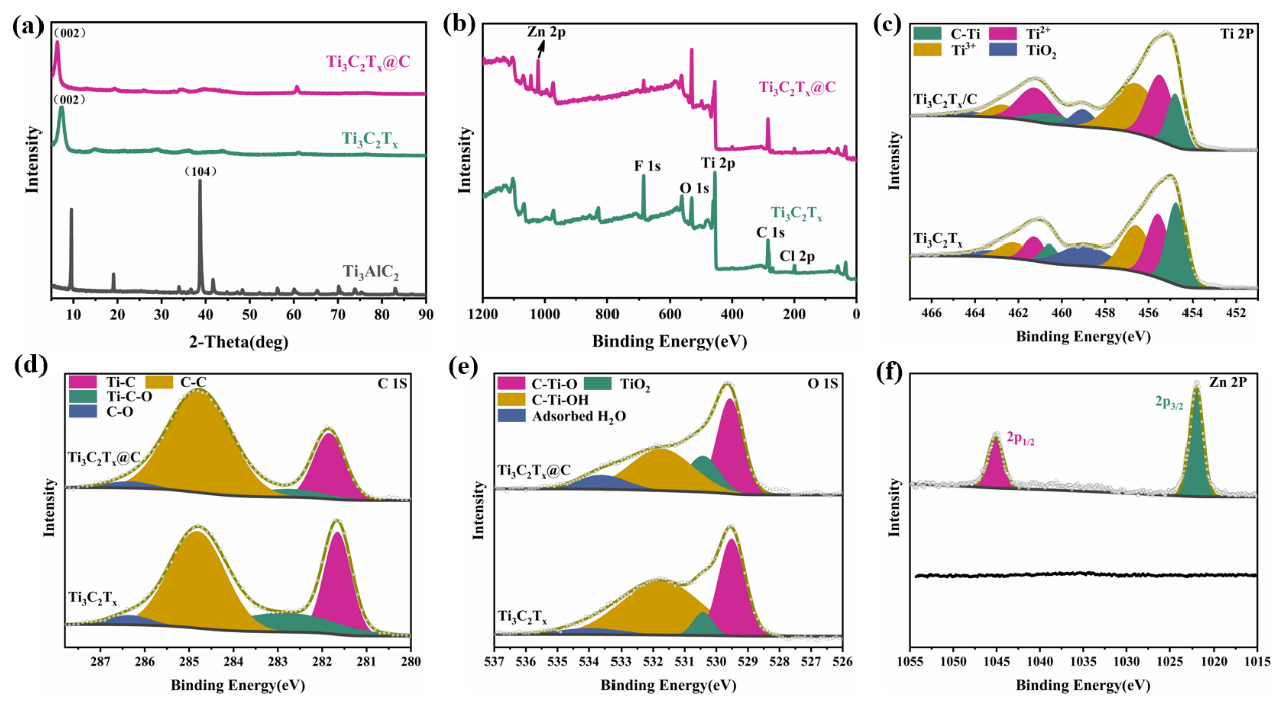
- (a) X-ray diffractograms of Ti3C2Tx and Ti3C2Tx@C. (b) XPS survey, (c) Ti 2p, (d) C 1s, (e) O 1s, and (f) Zn 2p spectra of Ti3C2Tx and Ti3C2Tx@C.
The porous, three-dimensional morphology and larger layer spacing of the Ti3C2Tx@C aerogel favors the more rapid transport of electrolyte ions in shorter ion diffusion channels [17,20]. In addition, the numerous carbon dots in Ti3C2Tx@C provide excellent electron transport pathways between the Ti3C2Tx nanosheets. The CV analysis of the prepared electrodes is exhibited in Figure 4(a). Reversible Faraday pseudocapacitive behavior mainly contributes to the capacitance of Ti3C2Tx and Ti3C2Tx@C, as demonstrated by the pair of redox peaks for both materials [21,22]. Ti3C2Tx@C has a higher Cg than Ti3C2Tx, as indicated by the larger CV curve area. The GCD analysis of the Ti3C2Tx and Ti3C2Tx@C electrodes is displayed in Figure 4(b). These curves are not linear, indicating that both materials exhibit contributions from Faradaic pseudocapacitance and electric double-layer capacitance [23]. The higher Cg of Ti3C2Tx@C is confirmed by its longer discharge time. Ti3C2Tx@C was further studied by obtaining multiple CV curves at varying scan rate values (Figure 4c). Within 2–100 mVs-1, no significant distortion in the curve shape is observed. Thus, Ti3C2Tx@C has superb rate performance and low internal resistance [16]. Ti3C2Tx (Figure S2a) shows a similar CV curve trend. Moreover, GCD curves acquired at various current density values are also consistent for both samples (Figure 4d and Figure S2b). The calculated Cg values of both electrode materials are displayed in Figure 4(e). Ti3C2Tx@C displays significantly higher Cg values than Ti3C2Tx at all scan rates (393.7 vs. 315.5 Fg-1 at 2 mVs-1). This difference is attributed to the three-dimensional porous structure of Ti3C2Tx@C, which hinders the stacking of layers, opens more electrodynamically active sites, and promotes faster electrolyte ion transport [18,24]. Additionally, the carbon dots in Ti3C2Tx@C act as fast electron transfer channels that accelerate electron transfer between the Ti3C2Tx nanosheets. At 200 mVs-1, Ti3C2Tx@C still retains a Cg of 329.3 F g-1, while that of Ti3C2Tx is only 272.3 Fg-1. The GCD curves acquired in the current density range of 1–100 Ag-1 display a similar trend (Figure 4f). At 1 A g-1, Ti3C2Tx@C exhibits a notably high Cg of 436 Fg-1, and when the current density increases to 100 A g-1, this value only decreases to 292.5 Fg-1. However, the Cg of the Ti3C2Tx electrode at 100 Ag-1 declines to 257.5 Fg-1. Therefore, Ti3C2Tx@C has better rate performance, which can be explained by three main reasons. First, the self-assembly of the Ti3C2Tx sheets with GA via Zn2+ cross-linking forms a three-dimensional porous structure, which inhibits Ti3C2Tx layer stacking. This enables the exposure of more electrochemically active sites. Second, the formed Ti3C2Tx@C structure facilitates electrolyte ion transport, which ensures excellent rate performance. Third, the carbon dots in Ti3C2Tx@C accelerate electron transport between the Ti3C2Tx nanosheets by providing electron transport paths. A Cg value comparison between this work and other MXene-based electrode materials is shown in Table S1. Compared to these other materials, Ti3C2Tx@C has a superb Cg and exceptional rate performance [11,25-27].
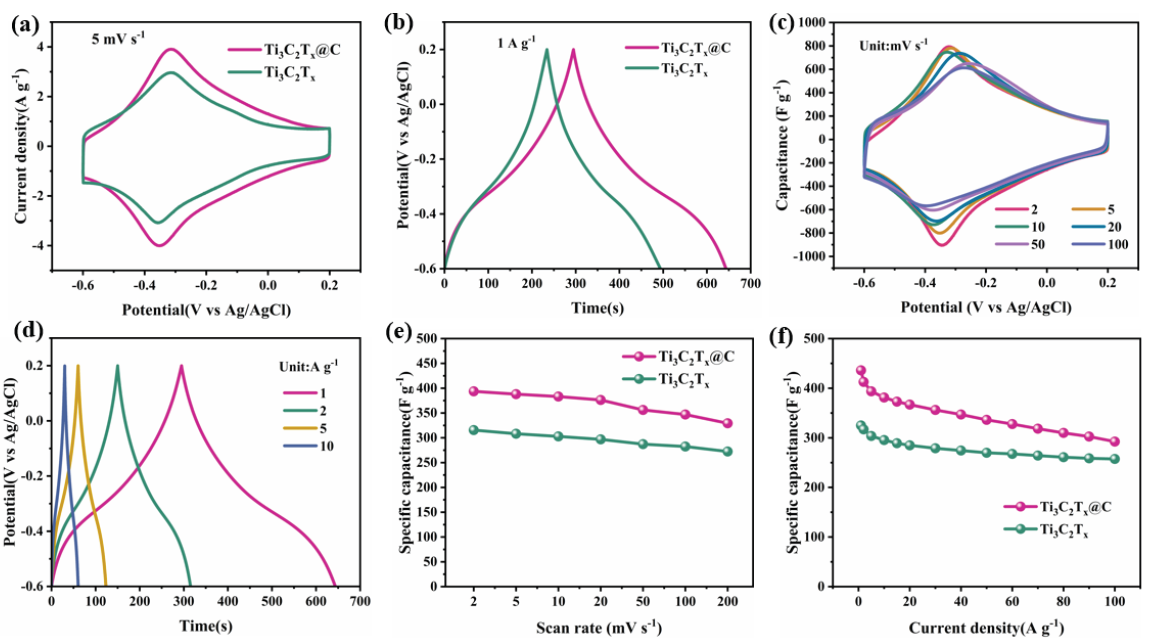
- Electrochemical analysis ofTi3C2Tx and Ti3C2Tx@Celectrodes: (a) CV (5 mV s–1) and (b) GCD (1 A g–1) curves obtained in 3 М H2SO4 electrolyte. (c) CV curves of Ti3C2Tx@Cat various scan rate values. (d) GCD curves of Ti3C2Tx@Cunder different current density values. Rate performance of Ti3C2Tx and Ti3C2Tx@Celectrodes at different (e) scanning rate and (f) current density values.
A log (i) vs. log(ν) plot was developed to study the charge storage kinetics and distinguish the charge storage mechanisms of Ti3C2Tx@C [17]. The peak current was obtained using the following Eq. (5):
Where the variables a and b are obtained by plotting log(i) and log(ν). A capacitatively controlled charge storage mechanism is revealed by b = 1, and a diffusion-controlled charge storage process is revealed by b = 0.5 [28]. As displayed in Figure 5(a), the anode peak b value of Ti3C2Tx@C is 0.94, while the cathode peak b value is 0.89. Consequently, the storage of charges in Ti3C2Tx@C is mainly related to its fast capacitance behavior. At 20 mVs-1, capacitive control accounts for 82.45% of overall charge storage in the Ti3C2Tx@C electrode (Figure 5b). From 2–50 mVs-1, the capacitive control contribution increases from 72.31% to 87.87% with increasing scan rate (Figure 5c). This indicates that the storage of charge in Ti3C2Tx@C is primarily governed by Faraday pseudocapacitance behavior rather than a diffusion-controlled process [29]. The ion diffusion kinetics of the synthesized electrodes were evaluated using EIS. Figure 5(d) exhibits the Nyquist plots of Ti3C2Tx and Ti3C2Tx@C. Ti3C2Tx@C has a negligible semicircle, indicating low charge transfer resistance. The equivalent circuit fitting parameters in Table S2 and Table S3 show that Ti3C2Tx@C has a lower charge transfer resistance than Ti3C2Tx. This is mainly because the intercalation of carbon dots in Ti3C2Tx@C facilitates electron transfer between the Ti3C2Tx nanosheets. In addition, the low-frequency linear region of the Ti3C2Tx@C Nyquist plot has a higher slope. This is because the 3D porous structure of the Ti3C2Tx@C electrode accelerates the transport of electrolyte ions [30]. Bode plots of the Ti3C2Tx and Ti3C2Tx@C electrode samples are displayed in Figure 5(e). These electrode materials both exhibit phase angles close to -90°, indicating that their capacitive properties are close to the ideal state [31]. The relaxation time constant τ0 of the Ti3C2Tx@C electrode (1.25 s) is shorter than that of the Ti3C2Tx electrode (1.84 s), indicating that electrolyte ions are more rapidly transported within Ti3C2Tx@C. The Ti3C2Tx@C electrode also has excellent cyclic stability (Figure 5f), retaining more than 90.3% of its Cg even after 5,000 cycles, while the Cg of Ti3C2Tx only retains 87.6%. To further confirm the stability of the MXene structure, the Ti3C2Tx@C electrode that underwent cyclic stability tests was characterized by SEM. After long-term cycling, the porous structure of Ti3C2Tx@C collapses to a certain extent, but the basic morphology is still retained (Figure S3).
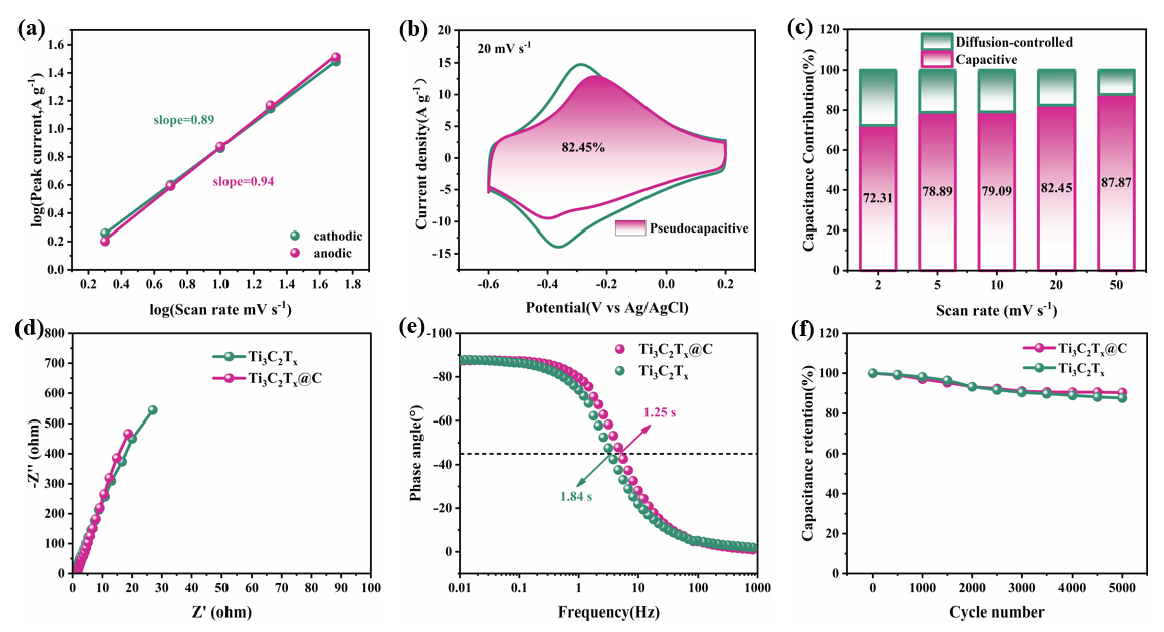
- Electrochemical evaluation of Ti3C2Tx@Celectrode: (a) Peak current vs. scan rate log-log plot. (b) Capacitance contributions at 20 mV s-1. (c) Contributions of capacitive control and diffusion control to total capacitance at different scan rate values. (d) Nyquist and (e) Bode plots of Ti3C2Tx and Ti3C2Tx@C. (f) Cyclic stability of Ti3C2Tx@C and Ti3C2Tx at 100A g-1.
To investigate the potential for using Ti3C2Tx@C in practical applications, a symmetric supercapacitor was prepared [32-39]. CV curves of this symmetric supercapacitor from 2–50 mVs-1 are shown in Figure 6(a). With the increase in scan rate, no significant distortion was observed at 20 mVs-1; however, a significant distortion occurred at 50 mV s-1, indicating that the device has good reversibility and rate performance. A similar trend can be seen in the GCD curves acquired from 1–5 Ag-1 (Figure 6b). The calculated Cg values have been shown in Figure 6(c). This symmetric supercapacitor achieves a Cg of 153.25 Fg-1 at 2 mVs-1. Moreover, a Cg of 45.29 Fg-1 is still reached at 50 mVs-1. Next, a cycling test was performed at 50 mVs-1 to study the stability of the symmetric supercapacitor (Figure 6d). After 5000 continuous cycles of charging and discharging, 90.1% of the original Cg value is still retained, confirming the good cycle stability of the prepared device. A Ragone plot (Figure 6e) is used to show the gravimetric Eg and Pg values of the symmetric supercapacitor. The highest Eg of 21.28 Whkg-1 is attained at a Pg of 153.25 Wkg-1. Moreover, an Eg of 14.63 Whkg-1 is still retained when Pg increases to 526.75 Wkg-1. The practical use of this symmetric supercapacitor design was demonstrated by connecting four symmetric supercapacitor devices in series. These devices are capable of successfully lighting an light emitting diode (LED) (Figure 6f).
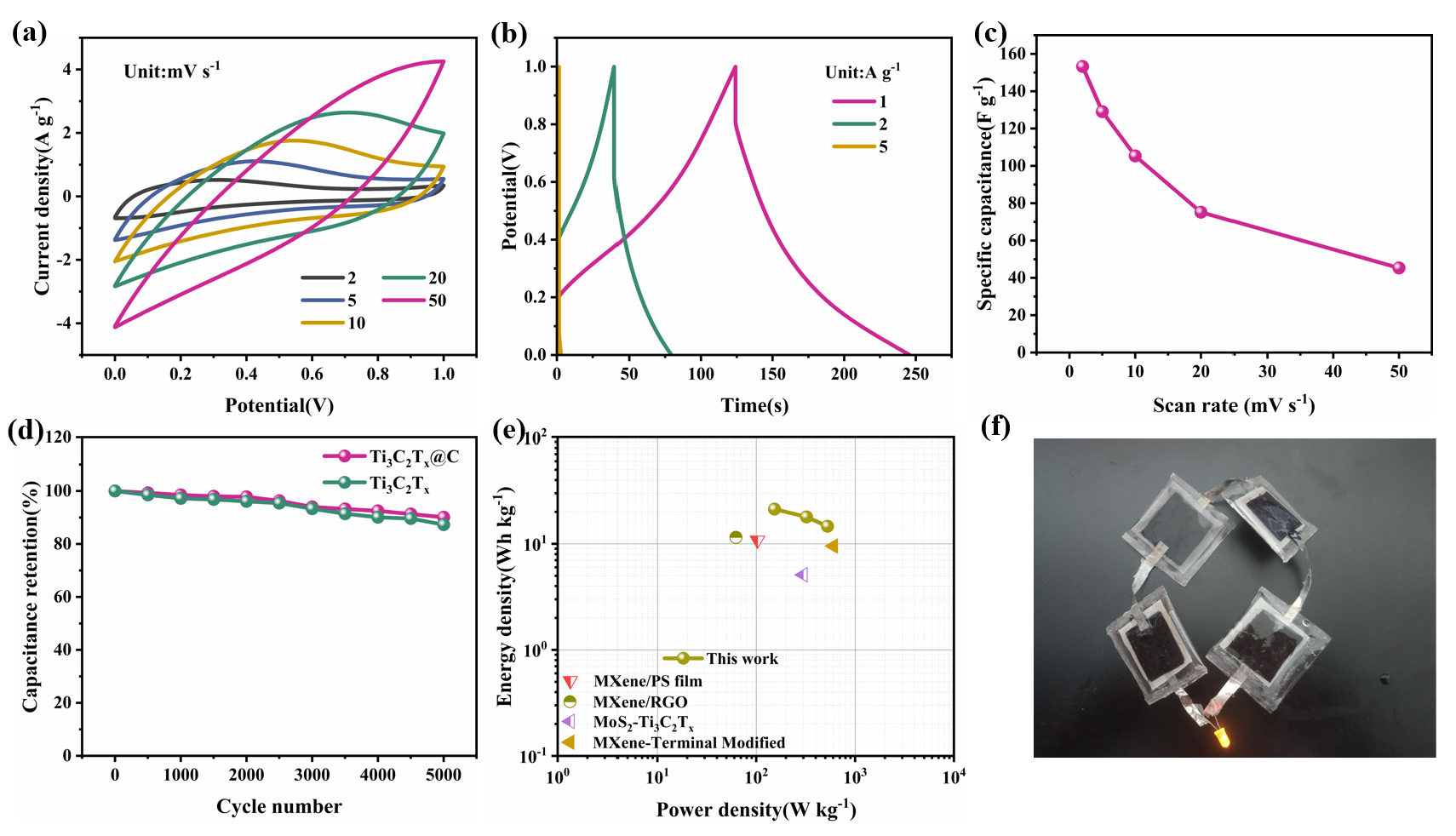
- Performance of Ti3C2Tx@C-based symmetric supercapacitor. (a) CV curves at different scanning rate values. (b) GCD curves obtained at different current density values. (c) Rate performance at various scanning rate values. (d) Cycling of Ti3C2Tx@C and Ti3C2Tx at 50 mV s-1. (e) Comparative Ragone plot. (f) Application of four Ti3C2Tx@C-based symmetric supercapacitors in series.
4. Conclusions
In summary, a three-dimensional porous carbon dot-intercalated Ti3C2Tx MXene material was prepared by a simple carbon dots intercalation strategy. The -OH on the Ti3C2Tx MXene nanosheet surface was cross-linked with the -OH on GA under the action of Zn2+, forming a three-dimensional structure. Next, Ga-derived carbon dot particles were embedded into the Ti3C2Tx MXene via carbonization, leading to larger layer spacing and an active surface accessible to ions. Moreover, these carbon dots acted as excellent electron transport channels, which accelerated electron transport between the Ti3C2Tx MXene nanosheets. Due to this favorable design, the Ti3C2Tx@C electrode had a Cg of 393.7 Fg-1 at 2 mVs-1 and maintained 83.7% of this value at 200 mVs-1. Moreover, the electrode showed 90.3% Cg retention after 5000 charging and discharging cycles, indicating superb cycle stability. A Ti3C2Tx@C/carbon cloth symmetric supercapacitor displayed a high Eg of 21.28 Whkg-1 under a Pg of 153.25 Whkg-1. The results and analysis presented in this study offer a useful approach for efficiently preparing MXene electrodes with high performance. However, due to the limitation of the MXene material preparation process, large-scale manufacturing of Ti3C2Tx@C composites has not been possible. In addition, due to the high activity of MXene, it is also necessary to continue to improve the long-term cyclic stability of Ti3C2Tx@C composites.
Acknowledgment
This work was supported financially by Key Research and Development Project of Henan Province (Science and Technology Research Project, 232102241032), Natural Science Foundation of Henan Province (242300420565), Henan Provincial Science and Technology Research Project (242102320357), Nanyang Programs for Science and Technology Development (23KJGG240)
CRediT authorship contribution statement
Qiaonan Yu and Qiang Liu contributed significantly to analysis and project funding support; Zhongliang Liu performed the experiment; Keliang Wu contributed to the conception of the study and wrote the manuscript; Bingke Li performed the methodology; Niuniu Gu design of data analyses and Dongmei Yang provided macro guidance.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Declaration of Generative AI and AI-assisted technologies in the writing process
I acknowledge using generative AI/AI-assisted tools in drafting. Responsibility for originality, accuracy, and ethics remains mine. Work reflects my intent; AI use is transparent.
Supplementary data
Supplementary data/material includes 3 Figures and 3 Table can be found online at https://dx.doi.org/10.25259/AJC_264_2024.
References
- Nitrogen-doped Ti3C2Tx MXene prepared by thermal decomposition of ammonium salts and its application in flexible quasi-solid-state supercapacitor. Chemical Engineering Journal. 2023;458:141338. https://doi.org/10.1016/j.cej.2023.141338
- [Google Scholar]
- 3D MXene architectures for efficient energy storage and conversion. Advanced Functional Materials. 2020;30:2000842. https://doi.org/10.1002/adfm.202000842
- [Google Scholar]
- Fabrication of cobaltous sulfide nanoparticle-modified 3D MXene/Carbon foam hybrid aerogels for all-solid-state supercapacitors. ACS Applied Materials & Interfaces. 2021;13:28222-28230. https://doi.org/10.1021/acsami.1c05904
- [Google Scholar]
- Roles of metal ions in MXene synthesis, processing and applications: A perspective. Advanced Science (Weinheim, Baden-Wurttemberg, Germany). 2022;9:e2200296. https://doi.org/10.1002/advs.202200296
- [Google Scholar]
- Interfacial structure design of MXene‐based nanomaterials for electrochemical energy storage and conversion. InfoMat. 2020;2:1057-1076. https://doi.org/10.1002/inf2.12118
- [Google Scholar]
- 3D printed MXene aerogels with truly 3D macrostructure and highly engineered microstructure for enhanced electrical and electrochemical performance. Advanced Materials (Deerfield Beach, Fla.). 2022;34:e2104980. https://doi.org/10.1002/adma.202104980
- [Google Scholar]
- The assembly of MXenes from 2D to 3D. Advanced Science (Weinheim, Baden-Wurttemberg, Germany). 2020;7:1903077. https://doi.org/10.1002/advs.201903077
- [Google Scholar]
- Ultralight MXene/carbon nanotube composite aerogel for high-performance flexible supercapacitor. Advanced Composites and Hybrid Materials. 2023;6:108. https://doi.org/10.1007/s42114-023-00675-8
- [Google Scholar]
- Confined hydrothermal assembly of hierarchical porous MXene/RGO composite fibers with enhanced oxidation resistance for high‐performance wearable supercapacitors. Small 2025 https://doi.org/10.1002/smll.202412378
- [Google Scholar]
- Fast gelation of Ti3 C2 tx MXene initiated by metal ions. Advanced Materials (Deerfield Beach, Fla.). 2019;31:e1902432. https://doi.org/10.1002/adma.201902432
- [Google Scholar]
- Hierarchical heterostructures of MXene and mesoporous hollow carbon sphere for improved ion accessibility and rate performance. Chemical Engineering Journal. 2024;494:153246. https://doi.org/10.1016/j.cej.2024.153246
- [Google Scholar]
- Spontaneous three-dimensional self-assembly of MXene and graphene for impressive energy and rate performance pseudocapacitors. Electrochimica Acta. 2021;391:138959. https://doi.org/10.1016/j.electacta.2021.138959
- [Google Scholar]
- Room-temperature assembled MXene-based aerogels for high mass-loading sodium-ion storage. Nano-micro Letters. 2021;14:37. https://doi.org/10.1007/s40820-021-00781-6
- [Google Scholar]
- Flexible carbon dots‐Intercalated MXene film electrode with outstanding volumetric performance for supercapacitors. Advanced Functional Materials. 2023;33:2209918. https://doi.org/10.1002/adfm.202209918
- [Google Scholar]
- 3D porous MXene induced by zinc-assisted electrodeposition for flexible all-solid-state supercapacitors. Journal of Alloys and Compounds. 2024;997:174426. https://doi.org/10.1016/j.jallcom.2024.174426
- [Google Scholar]
- Three-dimensional hierarchical porous MXene aerogel with outstanding rate performance for flexible supercapacitors. Journal of Energy Storage. 2024;93:112194. https://doi.org/10.1016/j.est.2024.112194
- [Google Scholar]
- Hierarchically porous 3D freestanding holey-MXene framework via mild oxidation of self-assembled MXene hydrogel for ultrafast pseudocapacitive energy storage. ACS Nano. 2024;18:3707-3719. https://doi.org/10.1021/acsnano.3c11551
- [Google Scholar]
- Interface‐Anchored covalent organic frameworks@Amino‐Modified ti3C2Tx MXene on nylon 6 film for high‐performance deformable supercapacitors. Angewandte Chemie International Edition. 2023;62:e202307195. https://doi.org/10.1002/anie.202307195
- [Google Scholar]
- A rationally designed hetero-assembly of 2D/2D nitrogen-doped MXene/Graphene via supercritical fluid processing for high energy durable supercapacitors. Chemical Engineering Journal. 2023;474:145505. https://doi.org/10.1016/j.cej.2023.145505
- [Google Scholar]
- Qualitative electrochemical impedance spectroscopy study of ion transport into sub-nanometer carbon pores in electrochemical double layer capacitor electrodes. Electrochimica Acta. 2010;55:7489-7494. https://doi.org/10.1016/j.electacta.2010.01.003
- [Google Scholar]
- ti3C2Tx MXene/carbon composites for advanced supercapacitors: Synthesis, progress, and perspectives. Carbon Energy. 2024;6:e501. https://doi.org/10.1002/cey2.501
- [Google Scholar]
- Unraveling the role of metal vacancy sites and doped nitrogen in enhancing pseudocapacitance performance of defective MXene. Small (Weinheim an der Bergstrasse, Germany). 2024;20:e2307408. https://doi.org/10.1002/smll.202307408
- [Google Scholar]
- Vertically aligned graphene-MXene nanosheets based electrodes for high electrochemical performance asymmetric supercapacitor. Chemical Engineering Journal. 2024;482:149063. https://doi.org/10.1016/j.cej.2024.149063
- [Google Scholar]
- Sandwich-like high-performance Ti3C2Tx MXene/NiCO2O4 nanosphere composites for asymmetric supercapacitor application. Journal of Energy Storage. 2024;86:111097. https://doi.org/10.1016/j.est.2024.111097
- [Google Scholar]
- Annealing modification of MXene films with mechanically strong structures and high electrochemical performance for supercapacitor applications. Journal of Power Sources. 2020;470:228356. https://doi.org/10.1016/j.jpowsour.2020.228356
- [Google Scholar]
- Mixed-dimensional heterostructure of few-layer MXene based vertical aligned moS2 nanosheets for enhanced supercapacitor performance. Journal of Alloys and Compounds. 2021;859:157797. https://doi.org/10.1016/j.jallcom.2020.157797
- [Google Scholar]
- Optimizing ion pathway in titanium carbide MXene for practical high‐Rate supercapacitor. Advanced Energy Materials. 2021;11:2003025. https://doi.org/10.1002/aenm.202003025
- [Google Scholar]
- Multidimensional nanostructural engineering of MXene-based composite films for high-performance supercapacitors. Energy & Fuels. 2024;38:5493-5505. https://doi.org/10.1021/acs.energyfuels.3c03870
- [Google Scholar]
- Unveiling the energy storage mechanism of MXenes under acidic conditions through transitions of surface functionalizations. The Journal of Physical Chemistry C. 2024;128:2352-2361. https://doi.org/10.1021/acs.jpcc.3c06956
- [Google Scholar]
- Cation-induced Ti3C2Tx MXene hydrogel for capacitive energy storage. Chemical Engineering Journal. 2022;433:134488. https://doi.org/10.1016/j.cej.2021.134488
- [Google Scholar]
- 3D porous oxidation‐Resistant MXene/Graphene architectures induced by in situ zinc template toward high‐Performance supercapacitors. Advanced Functional Materials. 2021;31:2101087. https://doi.org/10.1002/adfm.202101087
- [Google Scholar]
- Facile tailoring of surface terminations of MXenes by doping nb element: Toward extraordinary pseudocapacitance performance. ACS Applied Materials & Interfaces. 2023;15:15367-15376. https://doi.org/10.1021/acsami.2c21838
- [Google Scholar]
- Lithiophilic v2CTx/MoO3 hosts with electronic/Ionic dual conductive gradients for ultrahigh‐rate lithium metal anodes. Advanced Functional Materials. 2024;34:2400348. https://doi.org/10.1002/adfm.202400348
- [Google Scholar]
- V2CTx MXene artificial solid electrolyte interphases toward dendrite-free lithium metal anodes. ACS Sustainable Chemistry & Engineering. 2021;9:9961-9. https://doi.org/10.1021/acssuschemeng.1c03904
- [Google Scholar]
- In situ growth of nanorod-shaped ni,Co-MOF on Mo2CTx MXene surface to realize enhanced energy storage for supercapacitors. ACS Applied Materials & Interfaces. 2024;16:49380-49391. https://doi.org/10.1021/acsami.4c09616
- [Google Scholar]
- Defect engineered Ti3C2Tx MXene electrodes by phosphorus doping with enhanced kinetics for supercapacitors. Electrochimica Acta. 2022;435:141372. https://doi.org/10.1016/j.electacta.2022.141372
- [Google Scholar]
- High performance MXene/MnCo2O4 supercapacitor device for powering small robotics. ACS Applied Electronic Materials. 2024;6:7339-7345. https://doi.org/10.1021/acsaelm.4c01204
- [Google Scholar]
- Hydrothermal synthesis of layered niS2/Ti3C2Tx composite electrode for supercapacitors. Materials Chemistry and Physics. 2022;291:126733. https://doi.org/10.1016/j.matchemphys.2022.126733
- [Google Scholar]
- In situ grown VO2/V2C MXene and its supercapacitor applications. Journal of Energy Storage. 2024;88:111484. https://doi.org/10.1016/j.est.2024.111484
- [Google Scholar]







