Translate this page into:
Highly efficient and metal-free synthesis of tri- and tetrasubstituted imidazole catalyzed by 3-picolinic acid
⁎Corresponding author at: Organic Material Synthesis Division, Kayrbat Research Institute (KRI), Bogra 5810, Bangladesh. mazamsarker@gmail.com (Abu Zafar Al Munsur)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
Abstract
3-Picolinic acid is an efficient organo-catalyst for a one-pot three-component synthesis of 2,4,5-triaryl substituted imidazole. Moreover, the utility of this catalyst has been extended to the four-component synthesis of 1,2,4,5-tetra-substituted imidazole. The pivotal advantages of this process are easy purification, cost-effectiveness, and high yielding, above all environmentally benign protocol.
Keywords
Imidazole
3-Picolinic acid
Metal-free
Organo-catalyst
Green synthesis
1 Introduction
Today the synthesis, reactions, and biological properties of substituted imidazole cover a wide area of modern heterocyclic chemistry (Grimmett, 1984). Notably, compounds with imidazole core units have many pharmacological properties and play a vital role in varying biochemical processes (Liverton et al., 1999; Lombardino and Wiseman, 1974). Recent research has uncovered that highly substituted imidazole derivatives endow good photo-physical properties which result in their potential application in materials chemistry (Kulkarni et al., 2004). Moreover, appropriately substituted imidazole are extensively used as glucagon receptors (Black et al., 1974; Khanna et al., 1997), CB1 cannabinoid receptor antagonists (Lee et al., 1994), and in some cases antibacterial (Antolini et al., 1999), antitumor (Wang et al., 2002), and pesticides (Sharma et al., 2020). In the last decade, the advancement of green chemistry and organometallic catalysis have exploited the utility of imidazole as ionic liquids (Tao et al., 2016) and N-heterocyclic carbenes (Kühl, 2007; Kureja et al., 2019). Based on the above facts, lots of synthetic routes are available in the literature to synthesize imidazole analogs including ionic liquids (Akbari, 2016), [Hmim]TFA (MaGee et al., 2013), NH4OAc (Wu et al., 2010), iodine (Kidwai et al., 2006), tons of metal-based heterogeneous catalyst (Daraji et al., 2019; Kerru et al., 2019), and microwave irradiation (Zhou et al., 2010) are most notable. Recently, a metal-free method carried out by pivalic acid (Raj and Singh, 2009) has attracted a lot in condensing three or four components to imidazole instead of benzil with excellent yields.
However, most of these methods are challenged by one or more limitations such as harsh reaction conditions, tedious isolation technique, unsatisfactory yield, limited examples, expensive and detrimental metal catalysts, which limit their use due to the heavy impact on environmentally benign processes. Therefore, the development of mild, economically convenient, and complementary approaches for 2,4,5-trisubstituted and 1,2,4,5-tetrasubstituted imidazole derivatives are highly recommendable.
3-Picolinic acid is an important building block of co-enzymes (NAD and NADP) which are essential by all living cells. It has widely used in the fields of medicine, agriculture, and the food industry (Alkayeva et al., 2015).
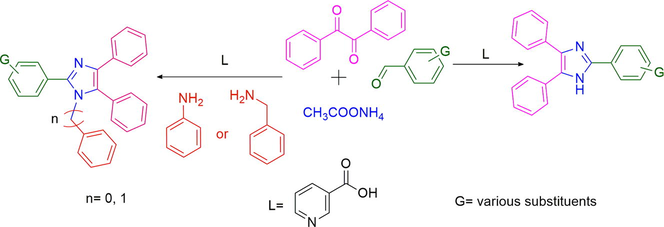
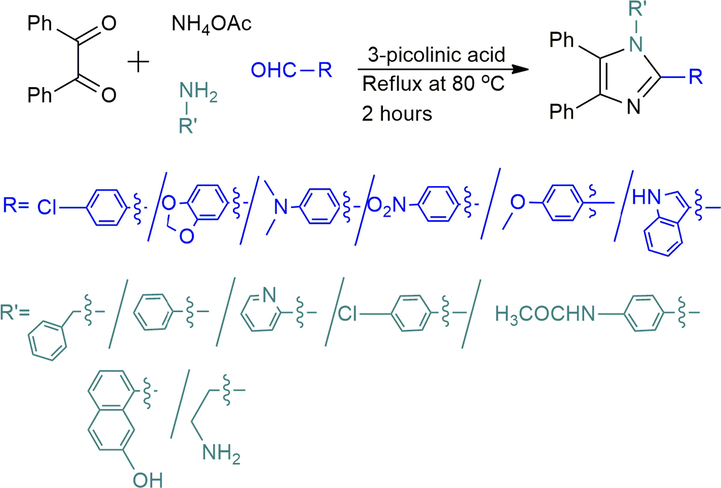
Nowadays, organocatalysts (D’Souza and Müller, 2007; Dömling, 2006) have drawn much attention in different organic transformations owing to its experimental simplicity, facile handling, cost-effectiveness, and above all excellent solubility in organic solvents or water. Despite that, there are not too many organocatalysts mediated multicomponent reactions for imidazole synthesis being reported to date. By considering the current demands of different tri- and tetrasubstituted imidazole in many aspects, to explore a new organo-catalyst and to forestall possible difficulty of the typical procedures, we herein, reported a convenient multicomponent route (Scheme 1) to condense benzil or benzoin, aldehydes, ammonium acetate, benzylamine, aniline or aniline derivatives mediated by 3-picolinic acid (niacin).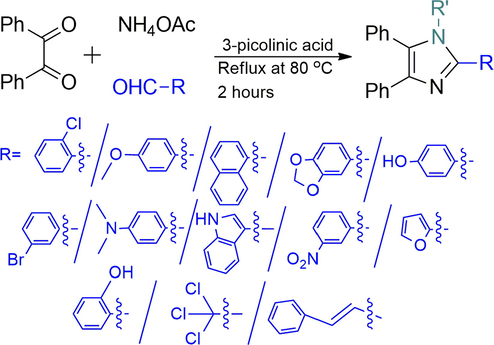
Reaction for three substituted imidazole synthesis.
2 Result and discussion
This is an extension (Roy et al., 2016) of our earlier attempts in synthesizing biologically active molecules by screening organo-catalyst via a multicomponent scaffold. During flame heating, we observed the charring of the starting materials and the products. To minimize the spoilage of the starting materials and products in this present work we wished to use a little amount of ethanol. Also, our objective was to find a new catalyst with higher efficacy than the earlier catalyst. To this end, we attempted to synthesize substituted imidazoles from benzil, benzaldehyde, and ammonium acetate in ethanol mediated by some small bifunctional molecules (o- & p-aminophenol, 2-picolinic acid, and aspartic acid; Table 1). To carry out the reaction, reacting components were mixed thoroughly with the mentioned small molecules (5–20 mol%) and heated gently over an oil bath at different temperatures with constant speed. But TLC monitoring indicated a tiny conversion of the starting materials, with the exception observed for 2-picolinic acid. The use of 2-picolinic acid afforded the product Entry I 1–50% yield despite the sincere alteration of catalyst, catalyst loading, solvents, and temperatures. Subsequently, by changing the catalyst from 2- to 3-picolinic acid to the same reaction and our delight, full conversions of the starting components were observed in TLC after 2 h reflux at 80 °C into ethanol solvent. Therefore, the feasibility of the reaction was investigated by the changes of catalyst loading and found 10 mol% catalysts are suitable for the desired conversion (Table 1).
Entry
Catalyst
Catalyst Concentration (mol%)
Solvent
Temperature/°C
Time (hour)
Yield (%)a
1
Without catalyst
–
Ethanol
RT
48
19
2
Without catalyst
–
Ethanol
40
48
21
3
Without catalyst
–
Ethanol
50
48
23
4
Without catalyst
–
Ethanol
60
48
24
5
Without catalyst
–
Ethanol
70
48
25
6
Without catalyst
–
Ethanol
Refluxes
48
25
7
Without catalyst
–
Ethanol
80
48
25
8
o-Aminophenol
5
Ethanol
40
48
18
9
o-Aminophenol
5
Ethanol
50
48
20
10
o-Aminophenol
5
Ethanol
60
48
21
11
o-Aminophenol
5
Ethanol
70
48
22
12
o-Aminophenol
5
Ethanol
Reflux
48
23
13
o-Aminophenol
5
Ethanol
80
48
23
14
o-Aminophenol
10
Ethanol
40
48
21
15
o-Aminophenol
10
Ethanol
50
48
23
16
o-Aminophenol
10
Ethanol
60
48
25
17
o-Aminophenol
10
Ethanol
70
48
27
18
o-Aminophenol
10
Ethanol
Reflux
48
26
19
o-Aminophenol
10
Ethanol
80
48
27
20
o-Aminophenol
15
Ethanol
40
48
15
21
o-Aminophenol
15
Ethanol
50
48
17
22
o-Aminophenol
15
Ethanol
60
48
22
23
o-Aminophenol
15
Ethanol
70
48
21
24
o-Aminophenol
15
Ethanol
Reflux
48
23
25
o-Aminophenol
15
Ethanol
80
48
26
26
o-Aminophenol
20
Ethanol
40
48
20
27
o-Aminophenol
20
Ethanol
50
48
21
28
o-Aminophenolo-Aminophenol
20
Ethanol
60
48
22
29
o-Aminophenol
20
Ethanol
70
48
25
30
o-Aminophenol
20
Ethanol
Reflux
48
27
31
o-Aminophenol
20
Ethanol
80
48
26
32
p-Aminophenol
5
Methanol:water (1:1)
40
48
24
33
p-Aminophenol
5
Methanol:water (1:1)
50
48
26
34
p-Aminophenol
5
Methanol:water (1:1)
60
48
28
35
p-Aminophenol
5
Methanol:water (1:1)
Reflux
48
30
36
p-Aminophenol
10
Methanol:water (1:1)
40
48
26
37
p-Aminophenol
10
Methanol:water (1:1)
50
48
28
38
p-Aminophenol
10
Methanol:water (1:1)
60
48
31
39
p-Aminophenol
10
Methanol:water (1:1)
Reflux
48
35
40
p-Aminophenol
15
Methanol:water (1:1)
40
48
23
41
p-Aminophenol
15
Methanol:water (1:1)
50
48
31
42
p-Aminophenol
15
Methanol:water (1:1)
60
48
36
43
p-Aminophenol
15
Methanol:water (1:1)
Reflux
48
43
45
p-Aminophenol
20
Methanol:water (1:1)
40
48
25
46
p-Aminophenol
20
Methanol:water (1:1)
50
48
27
47
p-Aminophenol
20
Methanol:water (1:1)
60
48
30
48
p-Aminophenol
20
Methanol:water (1:1)
Reflux
48
41
49
Aspartic acid
5
Methanol
40
48
20
50
Aspartic acid
5
Methanol
Reflux
48
25
51
Aspartic acid
10
Methanol
40
48
23
52
Aspartic acid
10
Methanol
Reflux
48
28
53
Aspartic acid
15
Methanol
40
48
21
54
Aspartic acid
15
Methanol
Reflux
48
30
55
Aspartic acid
20
Methanol
40
48
24
56
Aspartic acid
20
Methanol
Reflux
48
29
57
2-Picolinic acid
5
Ethanol:water (1:1)
35
12
20
58
2-Picolinic acid
5
Ethanol:water (1:1)
45
12
24
59
2-Picolinic acid
5
Ethanol:water (1:1)
60
12
23
60
2-Picolinic acid
5
Ethanol:water (1:1)
75
12
25
61
2-Picolinic acid
5
Ethanol:water (1:1)
Reflux
12
29
62
2-Picolinic acid
10
Ethanol:water (1:1)
35
12
35
63
2-Picolinic acid
10
Ethanol:water (1:1)
45
12
39
64
2-Picolinic acid
10
Ethanol:water (1:1)
60
12
43
65
2-Picolinic acid
10
Ethanol:water (1:1)
75
12
45
66
2-Picolinic acid
10
Ethanol:water (1:1)
Reflux
12
50
67
2-Picolinic acid
15
Ethanol:water (1:1)
35
12
29
68
2-Picolinic acid
15
Ethanol:water (1:1)
45
12
35
69
2-Picolinic acid
15
Ethanol:water (1:1)
60
12
41
70
2-Picolinic acid
15
Ethanol:water (1:1)
75
12
40
71
2-Picolinic acid
15
Ethanol:water (1:1)
Reflux
12
45
72
2-Picolinic acid
20
Ethanol:water (1:1)
35
12
31
73
2-Picolinic acid
20
Ethanol:water (1:1)
45
12
35
74
2-Picolinic acid
20
Ethanol:water (1:1)
60
12
38
75
2-Picolinic acid
20
Ethanol:water (1:1)
75
12
42
76
2-Picolinic acid
20
Ethanol:water (1:1)
Reflux
12
48
78
3-Picolinic acid
5
Ethanol
Reflux at 80
12
70
79
3-Picolinic acid
10
Ethanol
Reflux at 80
2
93
80
3-Picolinic acid
15
Ethanol
Reflux at 80
12
80
81
3-Picolinic acid
20
Ethanol
Reflux at 80
12
80
With the optimized condition, herein, we have explored the scope and limitations with other benzaldehydes and observed aryl chloride & aryl bromide (Entry I and VI in Table 2) were the compatible substrates with the yield of 90% and 88% respectively. Moreover, these two entities could provide convenient access to other products by metal-catalyzed cross-coupling reactions. Heterocyclic aldehydes (Entry IV, VIII, and X in Table 2) were endured under similar reaction conditions to obtain compound 4, 8, and 10 in 94%, 85%, and 87% yield, respectively. A few aldehydes like salicylaldehyde, chloralhydrate, and cinnamaldehyde were reacted but did not produce the expected imidazole. The presence of excess electron pushing functionalities in the benzene ring, accumulation of –ve charge by resonance in cinnamaldehyde, and the presence of three hydrolyzable —Cl atom in chloralhydrate possibly caused such failures (Roy et al., 2013). With the success of a three-component reaction, we wish to extend this methodology to other various substituted imidazole scaffolds by utilizing four-component strategies (Scheme 2).
Entry
Aldehydes
Products
Time (hour)
Yield (%)a
M.p (°C)
Reported M.p (°C)
Reference
I
2.15
90
195–197
196–198
Maleki et al. (2015)
II
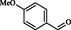
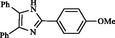
2.10
92
229–231
234
Zhou et al. (2010)
III
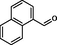
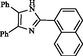
2
92
215–217
215
Roy et al. (2013)
IV
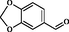
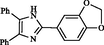
2
94
295–297
300
Zhou et al. (2010)
V
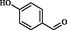
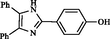
2.15
92
269–270
260–261
Kidwai et al. (2006)
VI
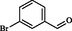
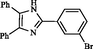
2
88
200–201
199–200
Roy et al. (2013)
VII
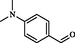
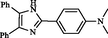
2
97
259–261
259–260
Zhou et al. (2010)
VIII

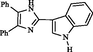
1.5
85
181–182
This Work
IX
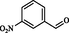
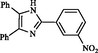
1.5
97
300
298–301
Maleki et al. (2015)
X
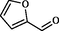
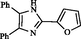
2
87
200–202
199–201
Maleki et al. (2015)
XI
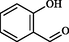
No result
48
–
–
–
XII
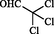
No result
47
–
–
–
XIII
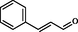
No result
48
–
–
–
Reaction for four substituted imidazole synthesis.
Under identical reaction conditions, and a stoichiometric amount of different substituted aniline derivatives as the fourth component, the reaction underwent an elegant transformation to various products in good to excellent yield. Moreover, heterocyclic amines (Entry, XXI) or other substituted amines did not create any problems during the product formation shown in Table 3. The reaction mechanism may follow a similar pattern as reported in the literature (Damavandi and Sandaroos, 2016), but in our case, 3-picolinic acid is donating its proton to aldehyde for necessary activation and on the other hand N-atom in pyridine ring abstracts protons to complete the reaction where it is necessary (shown in Fig. 1). In this manner, 3-picolinic acid is assisting the reaction in a dual way both by the rates and the yield of the products. With the same catalyst, twenty-three different tri- (Table 2) and tetrasubstituted imidazole (Table 3) were synthesized successfully without any sophisticated column chromatographic purification techniques.
Entry
Aldehydes
Amines
Products
Time (hour)
Yield (%)a
Calculated M.p (°C)
Reported M.p (°C)
Reference
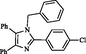
XIV
2
96
162–163
162–165
Kantevari et al. (2007)
XV
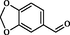
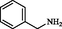
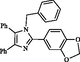
2
98
130–132
–
This Work
XVI
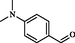
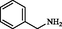
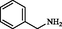
2
97
180–182
–
This Work
XVII
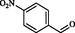
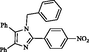
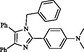
2
98
170–172
–
This Work
XVIII
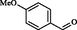
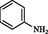
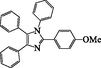
2
96
174–176
177–180
Safari and Zarnegar (2013)
XIX
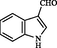
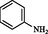
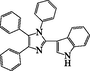
2
97
247–249
–
This Work
XX
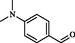
2
95
238–240
220–223
Hosseini et al. (2019)
XXI

2
90
254–256
–
This Work
XXII

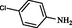
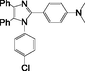
2
92
168–170
–
This Work
XXIII
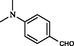
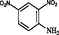
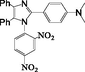
2
95
218–220
–
This Work
XXIV
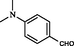
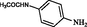
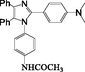
2
91
157–158
–
This Work
XXV
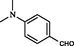
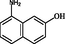
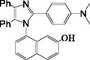
2
89
247–249
–
This Work
XXVI
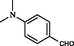

2
92
233–235
–
This Work
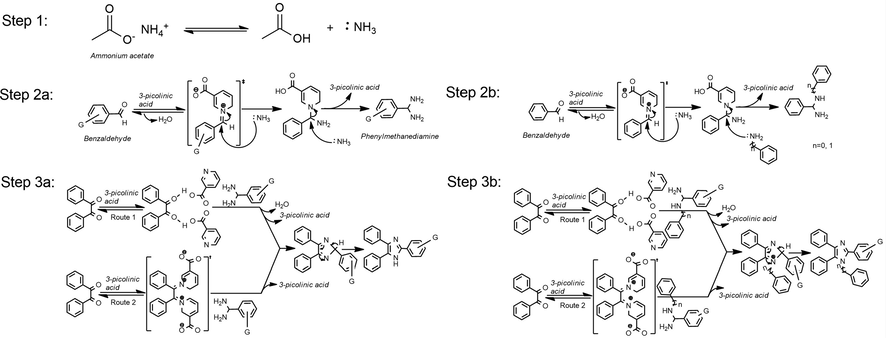
The stepwise mechanistic pathway for the multi-substituted imidazole synthesis via one-pot, three, and four components condensation reaction mediated by 3-picolinic acid.
Interestingly, benzoin also reacted in the same fashion and afforded imidazole with high yield and thereby making the reaction path shorter. Besides, the efficiency and reusability of the catalyst activity were verified for the three consecutive reactions, by drying the filtrate solutions and subsequent purifications by column chromatography, whereas two reactions were going on smoothly without any loss of catalyst activity Table 4.
Cycle
Yield (%)
Catalyst recovered (%)
Native
95
86
1st
95
83
2nd
95
85
3rd
94
82
Table 5 compares our results for the synthesis of 2-(4-Chlorophenyl)-1,4,5-triphenyl-1H-imidazole (11). with the results of different catalysts and reaction conditions obtained by other groups.
Catalyst
Conditions
Time (min)
Yield (%)
Reference
SBA-15/TFE
TFE/90 °C
180
90
Rostamnia and Zabardasti (2012)
[Poly(AMPS-co-AA)]
Solvent-free/110 °C
30
90
Mohammadi et al. (2012)
TFA
Glycerol (5 mL) MW(300)/120 °C
10
90
Khalafi-Nezhad et al. (2016)
HClO4–SiO2
Solvent-free/140 °C
8
94
Kantevari et al. (2007)
[bmim]3[GdCl6]
Solvent free/120 °C
120
94
Akbari (2016)
3-Picolinic acid
Ethanol/80 °C
120
96
This work
Many of the synthesized products are known in the literature, and for such cases, melting points were compared with the literature values.
3 Conclusion
In conclusion, the study delineates a rapid, efficient, and convenient synthesis of tri- and tetrasubstituted imidazole in a one-pot, three, and four-components condensation strategy using inexpensive, less toxic, and readily available 3-picolinic acid as an organocatalyst in ethanol. Although 3-picolinic acid is not a familiar organocatalyst in the organic transformation but this report, we have exploited it the first time in an elegant way to construct various substituted imidazole with great ease and in high yield. Some of the products are known in the literature, so far, such cases only melting points were compared with the literature values. We strongly believe that this methodology will be a great strategy annexed to the remaining methods for the synthesis of tri- and tetrasubstituted imidazole.
4 Experimental
4.1 Materials and method
The melting points of the compound were measured on an electrothermal melting point apparatus (Gallenkamp) and the melting points incorporated are uncorrected. Infrared spectra (IR) were recorded on KBr pellets for solids and neat for liquids by FT-IR 8400 PerkinElmer 883 grating spectrometer. 1H NMR spectra were recorded on AC-Bruker 500 MHz spectrometer in D6-DMSO or CDCl3, containing TMS as an internal standard. All ‘J’ values are given in Hz and chemical shifts δ- in ppm units. Reactions were monitored by thin-layer chromatographic (TLC) plates over silica gel (60 GF254. E. Merck).
4.2 General procedure for the synthesis of 2,4,5-triaryl-1H-imidazoles
A mixture of benzyl/benzoin (1 mmol), aldehyde (1 mmol), ammonium acetate (2.5 mmol), and 3-picolinic acid (12 mg, 10 mol%) in ethanol (2 mL) was stirred at reflux temperature for 2–3 hr. The reaction was monitored by thin-layer chromatography (TLC), the eluent was a mixture of solvent (Hexane & Ethyl acetate). After completion of the reaction, the mixture was cooled to room temperature, neutralized with 5% NaHCO3 solution, diluted with water, and poured on crushed ice. The obtained crude solid product was filtered, dried, and recrystallized from ethanol.
4.2.1 Spectral data for imidazole derivatives
Entry I: 2-(2-Chlorophenyl)-4,5-diphenyl-1H-imidazole (1). Mp: 195–197 °C. 1H NMR (CDCl3, 500 MHz): 10.48 (br.s, 1H, NH), 8.38 (dd, J = 2.5, 8 Hz, 2H, Ar—H), 7.68–7.29 (m, 11H, Ar—H), 2.08 (s, 1H); 13C NMR (300 MHz, DMSO‑d6): 143.3, 130.4, 128.9, 128.5, 128.3, 128.3, 127.8, 127.4, 127, 100; HRMS: calcd. for C21H15ClN2, 330.8123; found: 330.8121.
4.2.2 General procedure for the synthesis of 1,2,4,5-tetra-substituted imidazole:
A mixture of benzyl/benzoin (1 mmol), aldehyde (1 mmol), ammonium acetate (2.5 mmol), amine (1 mmol), and 3-Picolinic acid (12 mg, 10 mol%) in ethanol (2 mL) was stirred at reflux temperature for 2–3 hr. The progress of the reaction was monitored by TLC. After completion of the reaction, the mixture was cooled to room temperature, neutralized with 5% NaHCO3 solution, diluted with water, and poured on crushed ice. The obtained crude solid product was filtered, dried, and recrystallized from ethanol.
Entry XIV: 2-(4-Chlorophenyl)-1,4,5-triphenyl-1H-imidazole (11): Mp: 162–163 °C. 1H NMR (500 MHz, DMSO‑d6): 7.63–7.57 (m, 4H, Ar—H), 7.38–7.35 (m, 5H, Ar—H), 7.29–7.16 (m, 8H Ar—H), 6.84 (dd, J = 1.5, 8 Hz, 2H), 5.12 (s, 2H) ppm; 13C NMR (300 MHz, DMSO‑d6): 146.9, 138.3, 137.3, 135, 134.3, 131, 130.8, 130.4, 130.3, 129.4, 128.8, 128.8, 128.8, 128.7, 128.1, 127.5, 126.8, 126.5, 125.9, 48.3; HRMS: calcd. for C28H21ClN2, 420.9347; found: 420.93451429.
The rest of the spectral data and spectrum are included in the supporting information file.
Acknowledgment
We gratefully acknowledge the Department of Chemistry, University of Rajshahi, Bangladesh for the facility given during the present work and to the Green Chemistry Laboratory, KRICT for their necessary spectral help.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Tri(1-butyl-3-methylimidazolium) gadolinium hexachloride, ([bmim]3[GdCl6]), a magnetic ionic liquid as a green salt and reusable catalyst for the synthesis of tetrasubstituted imidazoles. Tetrahedron Lett.. 2016;57:431-434.
- [CrossRef] [Google Scholar]
- Microwave assisted heterogeneous vapor-phase oxidation of 3-picoline to nicotinic acid over vanadium-titanium oxide catalytic system. Appl. Catal. A Gen.. 2015;491:1-7.
- [CrossRef] [Google Scholar]
- Analogues of 4,5-bis(3,5-dichlorophenyl)-2-trifluoromethyl-1H-imidazole as potential antibacterial agents. Bioorg. Med. Chem. Lett.. 1999;9:1023-1028.
- [CrossRef] [Google Scholar]
- Sulphur-methylene isosterism in the developent of metiamide, a new histamine H2-receptor antagonist. Nature. 1974;248:65-67.
- [CrossRef] [Google Scholar]
- Multi-component syntheses of heterocycles by transition-metal catalysis. Chem. Soc. Rev.. 2007;36:1095-1108.
- [CrossRef] [Google Scholar]
- l-Proline-catalyzed three-component synthesis of condensed imidazoles. Arab. J. Chem.. 2016;9:S1138-S1143.
- [CrossRef] [Google Scholar]
- Synthesis and applications of 2-substituted imidazole and its derivatives: a review. J. Heterocycl. Chem.. 2019;56:2299-2317.
- [CrossRef] [Google Scholar]
- Recent developments in isocyanide based multicomponent reactions in applied chemistry. Chem. Rev.. 2006;106:17.
- [Google Scholar]
- Fe3O4@SiO2/bipyridinium nanocomposite as a magnetic and recyclable heterogeneous catalyst for the synthesis of highly substituted imidazoles via multi-component condensation strategy. Polycycl. Aromat. Compd. 2019:1-11.
- [CrossRef] [Google Scholar]
- Highly efficient, one-pot, solvent-free synthesis of tetrasubstituted imidazoles using HClO4–SiO2 as novel heterogeneous catalyst. J. Mol. Catal. A Chem.. 2007;266:109-113.
- [CrossRef] [Google Scholar]
- Recent advances in heterogeneous catalysts for the synthesis of imidazole derivatives. Synth. Commun.. 2019;49:2437-2459.
- [CrossRef] [Google Scholar]
- A new more atom-efficient multi-component approach to tetrasubstituted imidazoles: one-pot condensation of nitriles, amines and benzoin. RSC Adv.. 2016;6:67281-67289.
- [CrossRef] [Google Scholar]
- 1,2-Diarylimidazoles as potent, cyclooxygenase-2 selective, and orally active antiinflammatory agents. J. Med. Chem.. 1997;40:1634-1647.
- [CrossRef] [Google Scholar]
- Efficient elemental iodine catalyzed one-pot synthesis of 2,4,5-triarylimidazoles. Monatshefte für Chemie/Chem. Mon.. 2006;137:1189-1194.
- [CrossRef] [Google Scholar]
- The chemistry of functionalised N-heterocyclic carbenes. Chem. Soc. Rev.. 2007;36:592-607.
- [CrossRef] [Google Scholar]
- Electron transport materials for organic light-emitting diodes. Chem. Mater.. 2004;16:4556-4573.
- [CrossRef] [Google Scholar]
- Imidazolium-benzimidazolates as convenient sources of donor-functionalised normal and abnormal N-heterocyclic carbenes. Chem. Commun.. 2019;55:9705-9708.
- [CrossRef] [Google Scholar]
- A protein kinase involved in the regulation of inflammatory cytokine biosynthesis. Nature. 1994;372:739-746.
- [CrossRef] [Google Scholar]
- Design and synthesis of potent, selective, and orally bioavailable tetrasubstituted imidazole inhibitors of p38 mitogen-activated protein kinase. J. Med. Chem.. 1999;42:2180-2190.
- [CrossRef] [Google Scholar]
- Preparation and antiinflammatory activity of some nonacidic trisubstituted imidazoles. J. Med. Chem.. 1974;17:1182-1188.
- [CrossRef] [Google Scholar]
- Highly efficient and eco-friendly synthesis of 2-alkyl and 2-aryl-4,5-diphenyl-1H-imidazoles under mild conditions. Tetrahedron Lett.. 2013;54:2591-2594.
- [CrossRef] [Google Scholar]
- Design, preparation and characterization of urea-functionalized Fe3O4/SiO2 magnetic nanocatalyst and application for the one-pot multicomponent synthesis of substituted imidazole derivatives. Catal. Commun.. 2015;69:29-33.
- [CrossRef] [Google Scholar]
- A highly efficient and reusable heterogeneous catalyst for the one-pot synthesis of tetrasubstituted imidazoles. Appl. Catal. A, Gen.. 2012;429–430:73-78.
- [CrossRef] [Google Scholar]
- SBA-15/TFE (SBA-15/2,2,2-trifluoroethanol) as a suitable and effective metal-free catalyst for the preparation of the tri- and tetra-substituted imidazoles via one-pot multicomponent method. J. Fluor. Chem.. 2012;144:69-72.
- [CrossRef] [Google Scholar]
- Rapid access of some trisubstituted imidazoles from benzil condensed with aldehydes and ammonium acetate catalyzed by L-cysteine. Indian J. Chem. – Sect. B Org. Med. Chem.. 2013;52:153-159.
- [CrossRef] [Google Scholar]
- Efficient and convenient synthesis of 1H-pyrazolo[1,2-b]phthalazine-5,10-dione derivatives mediated by L-proline. Synth. Commun.. 2016;46
- [CrossRef] [Google Scholar]
- Comptes Rendus Chimie Immobilized ionic liquid on superparamagnetic nanoparticles as an effective catalyst for the synthesis of tetrasubstituted imidazoles under solvent-free conditions and microwave irradiation. Comptes Rendus – Chim.. 2013;16:920-928.
- [CrossRef] [Google Scholar]
- Glycosylated-imidazole aldoximes as reactivators of pesticides inhibited AChE: Synthesis and in-vitro reactivation study. Environ. Toxicol. Pharmacol.. 2020;80:103454.
- [CrossRef] [Google Scholar]
- Using imidazolium-based ionic liquids as dual solvent-catalysts for sustainable synthesis of vitamin esters: inspiration from bio- and organo-catalysis. Green Chem.. 2016;18:1240-1248.
- [CrossRef] [Google Scholar]
- Potent, orally active heterocycle-based combretastatin a-4 analogues: synthesis, structure–activity relationship, pharmacokinetics, and in vivo antitumor activity evaluation. J. Med. Chem.. 2002;45:1697-1711.
- [CrossRef] [Google Scholar]
- Practical multi-component synthesis of Di- or tri-aryl (heteraryl) substituted 2-(pyridin-2-yl)imidazoles from simple building blocks. J. Comb. Chem.. 2010;12:829-835.
- [CrossRef] [Google Scholar]
- Facile method for one-step synthesis of 2,4,5-triarylimidazoles under catalyst-free, solvent-free, and microwave-irradiation conditions. Synth. Commun.. 2010;40:1134-1141.
- [CrossRef] [Google Scholar]
- Grimmett, M.R., 1984. 4.07 – Imidazoles and their benzo derivatives: (ii) Reactivity. In: Katritzky, A.R., Rees, C.W.B.T.-C.H.C. (Eds.), Pergamon, Oxford, pp. 373–456. https://doi.org/10.1016/B978-008096519-2.00075-8.
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2020.10.010.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary Data 1
Supplementary Data 1







