Translate this page into:
Immobilization of bismuth oxychloride on cellulose nanocrystal for photocatalytic sulfonylation of arylacetylenic acids with sodium arylsulfinates under visible light
⁎Corresponding authors at: Key Laboratory of Magnetic Molecules & Magnetic Information Materials Ministry of Education School of Chemical and Material, Science Shanxi Normal University, Taiyuan 030006, PR China chenww@sxnu.edu.cn (Wenwen Chen), jiajf@dns.sxnu.edu.cn (Jianfeng Jia)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Peer review under responsibility of King Saud University.
Abstract
As an ideal matrix material, cellulose nanocrystal (CNC) has abundant hydroxyl groups and high specific surface area, which are beneficial to construct oxygen vacancies in BiOCl and achieve high dispersion of BiOCl on the surface of the matrix. Herein, we used a facile method to prepare a kind of BiOCl/CNC composite, which exhibited excellent visible light catalytic performance and recyclability in the preparation of arylacetylene sulfones.
Abstract
A Stable and efficient BiOCl/CNC photocatalytic composite was prepared. The CNC is conducive to the establishment of oxygen vacancies of BiOCl/CNC. BiOCl/CNC had a good photocatalytic performance in the sulfonylation reaction.
Abstract
As a promising semiconductor photocatalyst, BiOCl has been widely used in the field of environmental protection. However, due to its weak ability to absorb visible light, the application of BiOCl in other important photocatalytic fields has been significantly limited, such as organic synthesis. In this work, a facile method was used to prepare a highly efficient heterogeneous nano-photocatalyst BiOCl/cellulose nanocrystal (CNC). Subsequently, the BiOCl/CNC was verified by XPS, ESR, BET and other characterization methods. The results show that not only the strong interaction between BiOCl and CNC increases the visible light absorption intensity of the composite, but also the combination of BiOCl and CNC makes the specific area of the catalyst more than twofold. In addition, a large number of hydroxyl groups contained in CNC can be combined with the B—O bond in BiOCl through hydrogen bonds, forming abundant oxygen vacancies on BiOCl/CNC. Excitedly, these changes enable BiOCl/CNC to exhibit excellent photocatalytic performance and regeneration performance in the sulfonation reaction of arylacetylene acid and sodium arylsulfinate, with a yield of up to 96%. This work represents a step towards a low-cost, environmentally friendly composite of cellulose and BiOCl, which will provide useful enlightenment for future exploration in related fields.
Keywords
Cellulose nanocrystal
Bismuth oxychloride
Photocatalyst
Sulfonation reaction
1 Introduction
Using photocatalyst to capture and convert solar energy for chemical fuel storage and industrial applications is a paramount way to achieve sustainable development of human society (Fava et al., 2016; Xu et al., 2017). As a classical two-dimensional semiconductor material, BiOCl not only has low cost, nontoxicity, morphological variation and convenient preparation, but also has high chemical stability and catalytic activity (Han, 2021). In addition, in the unique layered structure of BiOCl, [Bi2O2]2+ is connected with halogen atom layer through intermolecular force, and this unique structure can facilitate the generation of an internal self-built electric field between Cl atom layer and [Bi2O2]2+ layer, which will greatly promote the separation of electrons and holes and improve the photocatalytic performance (Hojamberdiev et al., 2020; Liu et al., 2021). Based on these advantages, BiOCl has capture extensive attention and has been widely studied in the field of environmental protection, including sewage treatment (Gao et al., 2018; Gao et al., 2020; Gao et al., 2021; Liu et al., 2021; Monga and Basu, 2021), degradation of organic dyes (Guerrero et al., 2014; Zhang et al., 2014; Zhang et al., 2020) and purification of polluted air (Wu et al., 2020). Regrettably, the application of BiOCl in other important visible light photocatalytic fields has been greatly limited, such as organic synthesis, which may be attributed to its difficult to produce active electrons and holes to stimulate organic reactants under visible light as a wide band gap semiconductor (Han et al., 2019; Yang et al., 2021). In view of this, it is of great significance to develop a BiOCl-based photocatalyst that can be effectively used in organic synthesis and other important visible light catalysis fields.
As we all know, the BiOCl can be combined with a variety of matrices to form BiOCl/matrix composite materials, such as BiOCl/TCPP (Huang et al., 2021) and BiOCl/polyaniline (PANI) (Mansor et al., 2021), to achieve significant improvement of the spectral absorption range and photocatalytic efficiency. However, these matrix carriers are usually non degradable and unsustainable, and may even introduce new toxins in the application. Another fact that can not be ignored is that most matrices need to be produced through fossil energy and cannot fundamentally solve environmental problems. As a green, sustainable and renewable resource, cellulose not only has high hydrophilicity, permeability, transparency, physical and chemical resistance and thermal stability, but also can firmly fix the catalyst through its own large number of hydroxyl groups (Kamel and Khattab, 2021; Shi et al., 2021). In addition, the abundant hydroxyl contained in cellulose may interact with the low-energy Bi—O bonds in BiOCl through hydrogen bond to build a large number of oxygen vacancies on the surface of BiOCl, which is considered to be an important means to improve the photocatalytic activity of BiOCl (Li et al., 2018). Among a variety of cellulose, cellulose nanocrystal (CNC) deserves more attention because it not only contains a large number of hydroxyl groups, but also its specific surface area is nearly 100 times larger than that of other types of cellulose (Hamad, 2018; Ng et al., 2021), which makes it possible for CNC as matrix to significantly improve photocatalytic performance (Du et al., 2018; Elfeky et al., 2020; Tian et al., 2019; Zhan et al., 2018). Therefore, the preparation of composites with BiOCl by CNC is of great significance to improve the photocatalyst performance of BiOCl and expand its photocatalytic fields.
Sulfones have a broad spectrum of biological activities and are extensive used in the fields of chemistry, medicine, pesticide, material science (Llamas et al., 2006; Sulzer-Mosse et al., 2009; Yang et al., 2008; Zhu et al., 2008). At the same time, due to their ability to stabilize α-carbanions and strong electron-withdrawing properties, sulfones have been favored by synthetic chemists for so long. Arylacetylenic sulfones is an important derivative of sulfone, which has prominent applications in synthesis, e. g., as dienes in cycloadditions (Riddell and Tam, 2006; Zhao and Beaudry, 2014; Zhou et al., 2015), in the synthesis of disubstituted alkynesand in the alkynylation of diverse structures of biological interest (Meadows and Gervay-Hague, 2006; Todoroki et al., 2014). Due to the important application value of arylacetylenic sulfones, a variety of synthetic methods have been developed in recent years. However, the traditional synthesis methods of arylacetylenic sulfones encounter some limitations, such as harsh synthesis conditions, cumbersome synthesis process, the need of stoichiometric oxidants (Chen et al., 2017; Meesin et al., 2016; Wang et al., 2017; Zhong et al., 2020). Compared with traditional synthesis and electrocatalysis, photocatalysis utilize light energy to stimulate electrons to initiate chemical reactions, which can realize the breaking and recombination of chemical bonds under mild conditions. Predictably, photocatalysis will be a more coveted method to synthesize arylacetylenic sulfones, but it has never been achieved so far.
Herein, CNC has abundant hydroxyl groups and high specific surface area, which plays an important role in increasing the active surface area and improving the performance of photocatalyst. In addition, the high physical and chemical stability of CNC also helps to improve the recycling capacity of the composites. As expected, BiOCl/CNC has demonstrated excellent photocatalytic performance and reusability in the sulfonylation reaction of aryl acetyl acid and sodium arylsulfinate, then, a series of control experiments have proposed the possibility photocatalytic reaction mechanism.
2 Experimental
2.1 Preparation of CNC
According to previously reported literature (Hu et al., 2017), CNC was prepared by acid hydrolysis of microcrystalline cellulose (MCC) as follows: MCC (10 g) was slowly added to a beaker containing 150 mL sulfuric acid of 64 wt% and stirred continuously. MCC was completely dispersed in water and heated at 45 °C until MCC was completely hydrolyzed. The resulting yellow solution was diluted 7-fold by ultrapure water, left standing and the upper clear solution was poured away. The remaining suspension was washed via centrifugation until it was no longer layered. The obtained sample was dialyzed against ultrapure water until neutral. Finally, the obtained CNC suspension was subjected to ultrasonic treatment at room temperature for 5–10 min and stored in cold storage for next experiments.
2.2 Fabrication of BiOCl/CNC nanocomposite
BiOCl/CNC nanocomposite was obtained by in situ synthesis at room temperature (rt). In this process, 1.0 mmol KCl was added into 20 mL CNC solution under magnetic stirring. 1.0 mmol of Bi(NO3)3·5H2O was completely dissolved in 5 mL ethylene glycol (EG) by ultrasonication. Then the suspension was dropwise in CNC solution and stirred at rt for 1 h. Subsequently, the deposit was collected and washed via centrifugation and dried with vacuum drying at 60 °C for 8 h. Similarly, BiOCl was obtained by the same way except that the 1.0 mmol KCl was added into 20 mL ultrapure water instead of CNC solution.
2.3 Characterization and reactant preparation
The methods for characterization and organic reactant preparation are shown in Supplementary Data.
3 Results and discussion
3.1 Characterization and study on the interaction between BiOCl and CNC in the photocatalyst
The possible growth mechanism of BiOCl/CNC was proposed as manifested in Scheme 1. Nanorod-shaped nanocellulose and lamellar BiOCl self-assembled into three-dimensional BiOCl/CNC nanosheets. In the specific growth process, Bi3+ was preferentially strongly adsorbed onto the microporous structure of CNC due to a large number of hydroxyl groups in the molecular chain of CNC. In addition, hydrogen bonding and electron interaction can prevent the aggregation of Bi3+ and promote the formation of oxygen vacancies (Tian et al., 2019; Li et al., 2018), and then three-dimensional BiOCl/CNC nanosheets were formed through self-assembly, thereby improving its photocatalytic performance.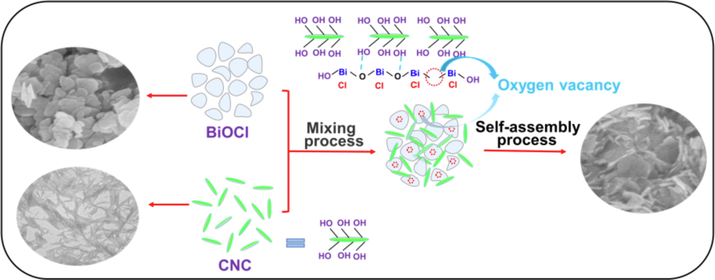
Schematic illustration of the growth mechanism of BiOCl/CNC.
X-ray diffractometer (XRD) pattern of BiOCl/CNC (Fig. S1a) is similar to BiOCl, which is indicative of the lattice structure of BiOCl will not be changed by the combination with CNC. Compared with pure BiOCl, there are significant changes that the stronger (1 1 0) diffraction peak corresponding to 2θ = 32.5°, which shows that the prepared BiOCl/CNC presents high percentage of exposed (1 1 0) facets. Previous studies have demonstrated that the increased exposure of the (1 1 0) facet in the BiOCl structure can improve the separation efficiency of photogenerated carriers, which is beneficial to enhance the photocatalytic activity of BiOCl/CNC (Wang et al., 2019). As described in Fig. S1b, the functional groups of BiOCl and BiOCl/CNC and their interactions were analyzed by FT-IR. The absorbance bands at ∼3200–3500 cm−1, 2850–3000 cm−1 and 1059–1162 cm−1 can be assigned to hydroxyl groups, C—H stretching and C—O—C stretching in CNC, respectively. The peak at 524 cm−1 may be indexed to Bi—O bond vibration (Gao et al., 2015) and the peak at 1419 cm−1 is ascribed to Bi—O bond stretching vibration (Soleimani et al., 2017). Combined with XRD results, it can be concluded that BiOCl is successfully loaded onto CNC and there is a strong interaction between the two interfaces.
Subsequently, scanning electron microscope (SEM) and transmission electron microscopy (TEM) were used to analyze the morphology and microstructure of BiOCl and BiOCl/CNC. As described in Fig. 1a, the surface of BiOCl nanosheets is smooth and stacked with each other without the addition of CNC. Interestingly, its morphology becomes three-dimensional cross-type thin nanosheets with the addition of CNC (Fig. 1b, 1c), which is attributed to the dried CNC assemble into a hierarchical structure. The HRTEM images shows two types lattice stripes with lattice spacings of 0.275 and 0.344 nm, matching (1 1 0) and (1 0 1) facets of BiOCl, respectively (Fig. 1d) (Jia et al., 2021; Wu et al., 2021), which matched with the XRD. And the element mapping of BiOCl/CNC composite further scientific evidence for the uniform distribution of Bi, O and Cl elements on the material (Fig. 1e–1h). Thermogravimetric analysis (TGA) was performed to evaluate the thermal stability of BiOCl/CNC. As shown in Fig. S2, BiOCl/CNC has high thermal stability below 240 ℃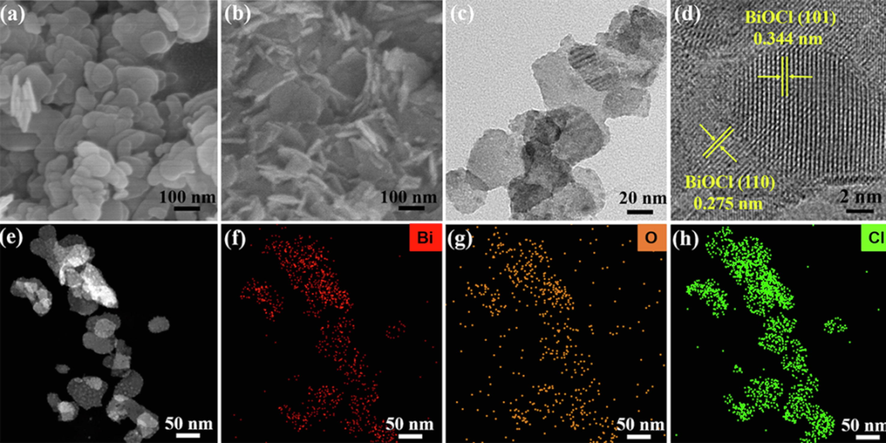
SEM images of (a) BiOCl, (b) BiOCl/CNC, TEM images of (c) BiOCl/CNC, HRTEM images of (d) BiOCl/CNC; Element mapping of Bi, O and Cl for BiOCl/CNC composite (e-h).
Then the chemical states of the elements in the samples were analyzed by X-ray photoelectron spectroscopy (XPS). The binding energy of C 1s detected at 288.7 eV, 286.5 eV and 284.8 eV were attributed to C = O, O—C—O and C—OH, respectively, indicating that the carbon comes from cellulose (Fig. S3a) (Du et al., 2019). Obviously, the carbon content of BiOCl/CNC is greater than that of BiOCl, indicating that their combination is successful. Fig. S3b shows the binding energy values of Bi 4f7/2 and Bi 4f5/2 are observed at 164.5 eV and 159.2 eV, which ascribing to the Bi3+ in BiOCl (Asadzadeh-Khaneghah et al., 2019; Liang et al., 2020; Jiang et al., 2022;). It should be noted that the interaction between BiOCl and CNC may result in the peak of BiOCl/CNC to be higher than that of BiOCl (Ma et al., 2018). As shown in Fig. S3c, the peaks at 199.5 eV and 197.9 eV in BiOCl, which are well ascribed to Cl 2p1/2 and Cl 2p3/2, respectively. Interestingly, two peaks become to 199.7 eV and 198.1 eV in BiOCl/CNC, which due to the tighter combination of Cl− and BiOCl in the material (Gao et al., 2019). As depicted in Fig. 2a, three distinct prominent peal peaks at 533.3 eV, 531.9 eV and 529.9 eV, which correspond to chemisorbed oxygen, oxygen vacancy and lattice oxygen, respectively (Song et al., 2017). Obviously, the oxygen vacancy peak shifted to high binding energy to 532.2 eV in BiOCl/CNC attributed to the interaction between BiOCl and CNC (Wang et al., 2013). In addition, it can be seen the content of vacant oxygen on the surface increases significantly, which may be due to the strong interfacial hydrogen bonds between CNC and BiOCl weakening the surface Bi—O bonds (Li et al., 2020). In order to further confirm the existence of oxygen vacancy in BiOCl/CNC, we characterized it by electron spin-resonance spectroscopy (ESR) and the signal of BiOCl and BiOCl/CNC at g = 2.00, which is confirmed as oxygen vacancy (Fig. 2b) (Wu et al., 2021). Notably, the intensity of ESR signal for BiOCl/CNC is greater than that of BiOCl, indicating that BiOCl/CNC has more oxygen vacancies, which is conducive to the improvement of photocatalytic performance (Ma et al., 2017). And UV–vis absorption spectrum shows that BiOCl/CNC has stronger visible light (400–800 nm) absorption than BiOCl, which is due to the increase of oxygen vacancies (Fig. 2c) (Li et al., 2018). As shown in the inset, the band gap energy of BiOCl and BiOCl/CNC were calculated as 3.68 and 3.54 eV, respectively (Kong et al., 2021). In addition, the specific surface areas and pore structure of the photocatalysts were analyzed through the study of N2 adsorption/desorption. Test results indicate there is a significant hysteresis loop belonging to H-III of type IV, suggesting the presence of mesopores structure, which allows small organic molecules to pass through (Fig. 2d) (Hou et al., 2018). The BET surface area of BiOCl and BiOCl/CNC were calculated to be 23.46 m2g−1 and 53.79 m2g−1, respectively, which is evident that the addition of CNC increases the surface area compared to BiOCl. Generally speaking, higher surface area can make the photogenerated charge transfer faster and can absorb more active substances and reactants to improve the reaction efficiency. The pore size distribution of the samples falls in the range of 2–8 nm.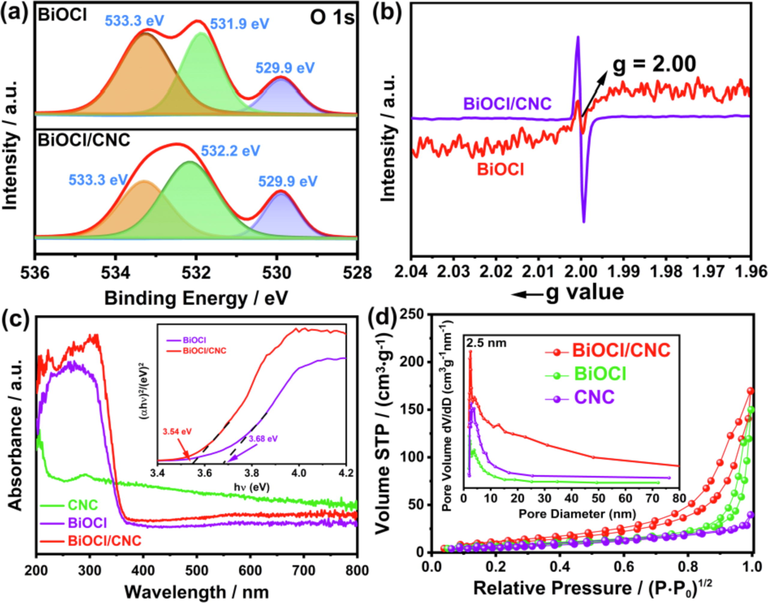
(a) XPS spectra in the O1s region of BiOCl and BiOCl/CNC. (b) ESR spectra of BiOCl and BiOCl/CNC. (c) UV–vis absorption spectra of the samples (inset: plot of bandgap). (d) Nitrogen adsorption–desorption isotherms (insert: pore size distribution).
Furthermore, the transient photocurrent response, electrochemical impedance spectroscopy (EIS) and the photoluminescence (PL) were applied to examine the photogenerated electron migration barrier and separation efficiency of photogenerated charge carriers. As shown in Fig. S4a, BiOCl/CNC showed the highest photocurrent density, which reflects the most effective charge separation. CNC showed a flat curve due to the balanced rate of charge diffusion within the CNC and rate of reaction at the interface between CNC and electrolyte. BiOCl showed a charging curvature photocurrent, which means that the rate of charge transfer with the BiOCl was higher than the rate of the surface reaction. However, BiOCl/CNC presented a blocking curvature, beacuse the rate of surface reaction is limiting the photocurrent.
Fig. S4b depicts the EIS Nyquist plots of BiOCl/CNC, BiOCl and CNC, obviously, BiOCl/CNC has the smallest arc radius in comparison with BiOCl and CNC, which suggests that the combination of CNC and BiOCl can improve the efficiency of charge separation and transfer (Wang et al., 2020). Consistence with EIS, the PL spectrum of BiOCl/CNC in Fig. S4c illustrates the weaker intensity than BiOCl, implying lower recombination rate of electrons-holes and higher photoactivity of the photocatalyst (Li et al., 2021; Wang et al., 2013). Based on the transient photocurrent response, EIS and PL results, the composite material may exhibit better photocatalytic performance than BiOCl due to the strong interaction between BiOCl and CNC.
3.2 Photocatalytic performance test of BiOCl/CNC to sulfonylation of arylacetylenic acids with sodium arylsulfinates
Table 1 lists the exploratory experimental results of arylacetylene sulfone prepared by sulfonation of arylacetylene acid and sodium sulfite under different conditions. Initially, 3-phenylpropiolic acid (1a, 0.5 mmol) was treated with sodium p-toluenesulfinate (2a, 1.0 mmol), BiOCl/CNC (20 mg) in THF (4.5 mL) and the solution was stirred for 20 h under white LED light in the presence of oxygen, however, target compound was not synthesized (Table 1, entry 1). Interestingly, the desired compound was obtained in 73% yield when iodine (0.25 eq.) was added as an initiator (Table 1, entry 2). If iodine was added to the reaction without BiOCl/CNC, the yield was significantly reduced to 44% (Table 1, entry 3), indicating the synergistic effect of BiOCl/CNC and iodine. And the yield of product will drastically decrease when exposed to air, even in the presence of iodine and BiOCl/CNC (Table 1, entry 4), which indicates that oxygen is one of the essential factors to achieve this reaction. Subsequently, the reaction was carried without visible light, only 10% of the product was obtained, indicating the light source is also the key factor (Table 1, entry 5). The yield increased from 73% to 89% when the dosage of iodine was added to 0.5 eq., and further increase of the amount of iodine did not give rise to a significantly improvement in yield (Table 1, entries 6 and 7). As a result, the appropriate amount of iodine (0.5 eq.) was conducive to the reaction. On the basis, the yield of the product decreases significantly without BiOCl/CNC in the reaction (Table 1, entry 8), which result further confirming the necessity of the BiOCl/CNC. In summary, iodine, BiOCl/CNC, oxygen and LED light are all indispensable to achieve the product efficiently. When the photocatalyst was replaced by pure BiOCl and CNC, the yields of the target product were not optimistic (Table 1, entries 9 and 10).
Entry
I2(eq.)
BiOCl/CNC
Solvent
Light
Yielda(%)
1
—
20 mg
THF
On
—
2
0.25
20 mg
THF
On
73
3
0.25
—
THF
On
44
4b
0.25
20 mg
THF
On
26
5
0.25
20 mg
THF
Dark
10
6
0.5
20 mg
THF
On
89
7
1.0
20 mg
THF
On
87
8
0.5
—
THF
On
52
9c
0.5
20 mg
THF
On
68
10d
0.5
20 mg
THF
On
65
11
0.5
15 mg
THF
On
72
12
0.5
25 mg
THF
On
88
13e
0.5
20 mg
THF
On
60
14f
0.5
20 mg
THF
On
88
15
0.5
20 mg
THF
Red
17
16
0.5
20 mg
THF
Blue
80
17
0.5
20 mg
THF
Green
25
18
0.5
20 mg
CH3CN
On
40
19
0.5
20 mg
EtOH
On
37
20
0.5
20 mg
DMAc
On
32
21
0.5
20 mg
DMF
On
23
22
0.5
20 mg
DMSO
On
trace
23
0.5
20 mg
DCE
On
—
24
0.5
20 mg
1,4-Dioxane
On
trace
25
0.5
20 mg
Toluene
On
trace
Subsequently, the reaction efficiency was not further improved after a series of adjustment of the amount of BiOCl/CNC (Table 1, entries 11 and 12). If the reaction time was shortened or prolonged to 16 h or 24 h, the yield became 60% and 88%, respectively (Table 1, entries 13 and 14). The influence of different color light sources on the reaction was explored, and the result showed that white LED light was the best light source (Table 1, entries 6, 15–17), and various solvents were screened (Table 1, entries 18–25). Eventually, THF was considered to be an optimum solvent for increasing the yield of 3a (89% yield), and the optimized reaction conditions was determined in entry 6 of Table 1. According to the above series of conditional experiments, the optimal reaction conditions are as follows: in the presence of 20 mg of BiOCl/CNC and 0.5 equivalent of iodine, 30 W white LED lamp and THF were used as the light source and solvent, the reaction was carried out at rt for 20 h.
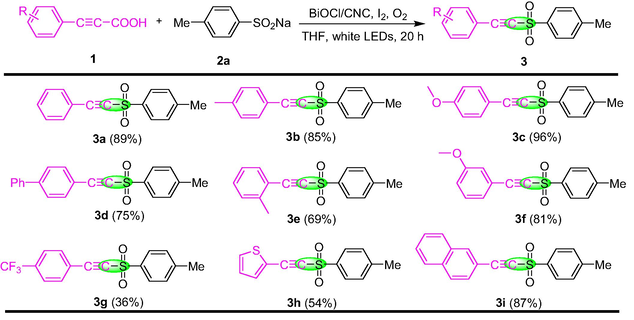
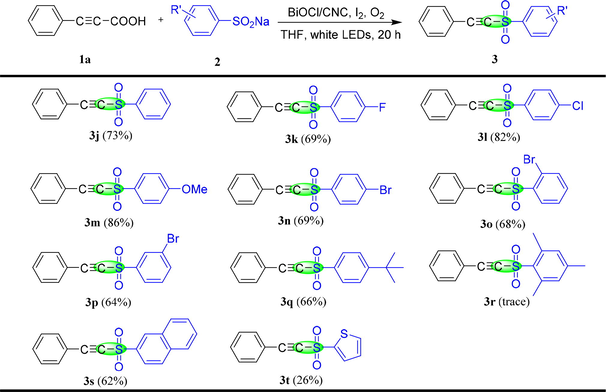
Under the optimal reaction conditions, the generality and functional group compatibility of the transformation were tested, and the results are summarized in Table 2 and Table 3. First of all, the scope of the various arylacetylenic acids with 2a was investigated (Table 2). Generally speaking, arylacetylenic acids contain a broad range of electron donating groups could successfully delivered the desired compounds 3a-3d in satisfactory yields (75%−96%). However, when the electron donating groups on arylacetylene acid were located in ortho and meta positions (3e and 3f), the yield of the reaction decreased, suggesting that the steric hindrance has a greater impact on the reaction results than the electron effect. If the arylacetylenic acid contains a strong electron withdrawing group (p-CF3), the conversion yield of 3 g was significantly reduced (36%). In addition, thiophen-2-ylpropioic acid and naphthalene-2-ylpropioic acid also possess outstanding adaptability. The yields of 3h and 3i were 54% and 87%, respectively. Subsequently, the sulfonation reaction of 1a with a series of sodium sulfonates were tried under the optimum conditions, which was summarized in Table 3. Sodium sulfonates containing electron donating group or electron withdrawing group could not significantly affect the reaction results, and all could be obtained products 3j-3q in satisfactory yields (64%−86%). Notably, it was found that the spatial effect of substituents on sodium sulfonate was weaker than that of arylacetylene acid by analyzing the reaction results of 3n, 3o and 3p. However, if 1,3,5-trimethyl-2 - (2-phenyl-ethynylsulfonyl) - benzene is used in the reaction, only trace product 3r could be detected by TLC due to the large space hindrance effect of substituents. Moreover, sodium naphthalene-2-sulfinate and sodium thiophene-2-sulfinate were also provide the desired product 3s and 3t in 62% and 26% yields, respectively.
To evaluate the recyclability of the BiOCl/CNC photocatalyst, a recycling experiment is shown in Fig. 3a. The used BiOCl/CNC was washed with water and ethanol in turn, after natural drying, it can be directly used for the next round of reaction without adding new catalyst. After four cycles of photocatalytic experiments, the yield by recycled BiOCl/CNC could still reach 53%, indicating a good recycling performance. The XRD (Fig. S5a) and IR (Fig. S5b) results of the recovered BiOCl/CNC showed that the diffraction and vibration peaks were not significantly different from those of the fresh BiOCl/CNC, indicating that the BiOCl / CNC had excellent photostability. In addition, the morphology of cycled sample maintained a complete lamellar structure by the TEM characterization (Fig. S6a). Elemental analysis also showed uniform distribution of elements on the cycled sample (Fig. S6b-e). All these results can demonstrate the photostability of the composites. Thus, the decrease of catalyst activity during recycling can be speculated to the unavoidable loss of catalyst during recovery.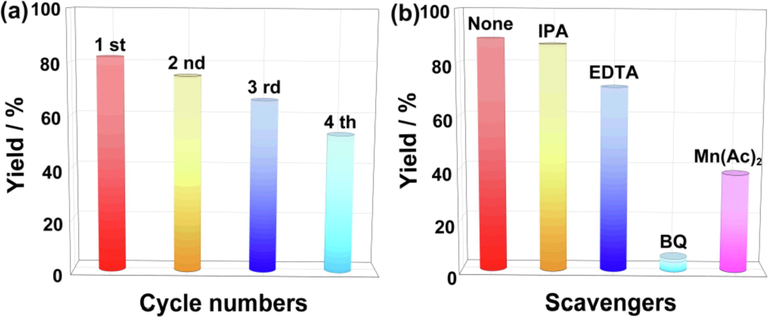
(a) The cycling experiment of BiOCl/CNC. (b) Radical trapping experiment of sulfonylation of arylacetylenic acid with sodium arylsulfinate.
3.3 Photocatalytic mechanism study of BiOCl/CNC to sulfonylation of arylacetylenic acids with sodium arylsulfinates
In order to explore the predominant active species involved in the process of photocatalytic reduction, isopropyl alcohol (IPA), EDTA-2Na, benzoquinone (BQ) and Mn(Ac)2 were applied as the sacrificial agents for hydroxyl radical (•OH), holes (h+), superoxide radical (•O2−) and photogenerated electrons (e−), respectively. In addition to adding scavengers, the capture tests were carried out under the same conditions as the photocatalytic test. Obviously, the introduction of BQ significantly decreased the yield of arylacetylenic sulfones, and the yield decreased to 38% in the presence of Mn(Ac)2 (Fig. 3b), which proved that •O2− and e− are the predominant reactive species in this reaction. Then, a systematic control experiments were carried out in Scheme 2 for profound study the reaction mechanism. The radical inhibitors including 2,2,6,6-tetramethyl-1-piperidinyloxy (TEMPO) and hydroquinone were added into the reaction to verify whether the reaction pathway was radical reaction. As shown in Scheme 2a, TEMPO almost ceased the reaction, while hydroquinone hindered the reaction, which indicating the reaction probably involves a radical process. Notably, the corresponding compounds were obtained in 60% and 45% yields (Scheme 2b, 2c) when phenylacetylene (1b) and (iodoethynyl)benzene (1c) were used instead of 3-phenylpropiolic acid (1a). Armed with this result, we deduced that 1b or 1c might be the intermediate during the reaction. In order to further verify this inference, 1a was used as the only reactant in the absence of 2a and reacted under standard conditions (Scheme 2d). However, no isolable products were detected by TLC, suggesting either 1b or 1c unlikely to be the intermediate under the standard conditions.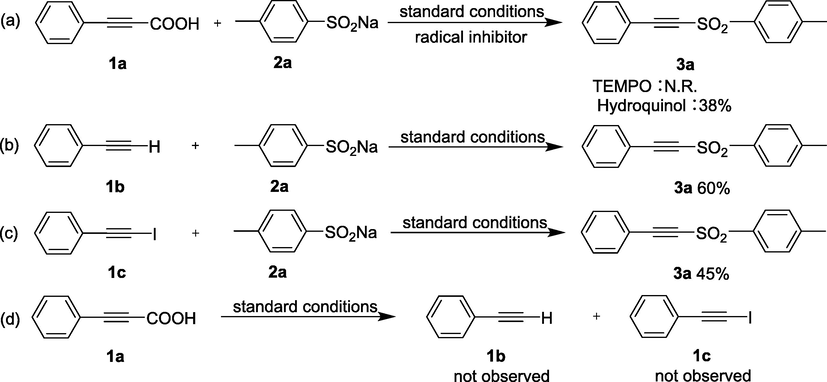
Control experiments of sulfonylation of arylacetylenic acids with sodium arylsulfinates.
According to the experimental results, we proposed the feasible mechanism is outlined in Scheme 3. The BiOCl/CNC first became motivated to produce photogenerated electrons (e−) and holes (h+) pairs under visible light. The electrons in the CB of BiOCl/CNC converted O2 into •O2−, which could further oxidize iodine molecule to form iodine free radical. Subsequently, sodium p-toluenesulfonate was preferentially oxidized by iodine radical to oxygen-center sulfonyl radical A, which could be resonant converted to sulfur-center sulfonyl radical B. Subsequently, the attack of B on 3-phenylpropiolic acid produced intermediate C, which could be quickly decarboxylated to produce intermediate D. Then, the desired product 3a is obtained by eliminating hydrogen radicals. Finally, I− reacted with IO3− to form I2 to maintain the catalytic cycle.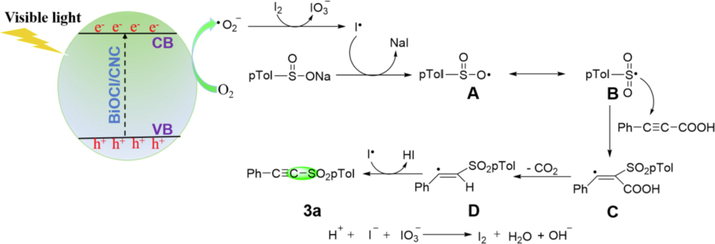
Proposed mechanism of sulfonylation of arylacetylenic acid with sodium arylsulfinate over BiOCl/CNC.
4 Conclusion
In summary, the photocatalytic synthesis of arylacetylene sulfone has been successfully achieved through the development of highly efficient and recyclable heterogeneous photocatalyst BiOCl/CNC. The substrates of the reaction had wide applicability and the yield could reach 96%. It is worth noting that the photocatalyst had good recovery capacity, which is beneficial to improve the economics of industrial synthesis. The successful development of the photocatalytic synthesis method provides an efficient and green approach for the preparation of arylacetylenic sulfones, and provides a sustainable opportunity for industrial applications.
CRediT authorship contribution statement
Xiaoxia Wang: Conceptualization, Methodology, Data curation, Visualization, Writing – original draft. Xueting Li: Investigation, Formal analysis. Baoqiang Tian: Investigation. He Xiao: Funding acquisition. Wenwen Chen: Methodology, Writing – review & editing. Haishun Wu: Investigation. Jianfeng Jia: Methodology, Supervision, Writing – review & editing.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 21571119), the Applied Basic Research Project of Shanxi Province (No. 201901D211393), the graduate Education Innovation Project of Shanxi Province (No. 2020BY081) and the graduate Education Innovation Project of Shanxi Normal University (2019XBY013 and 2019XBY016).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Novel g-C3N4 nanosheets/CDs/BiOCl photocatalysts with exceptional activity under visible light. J. Am. Ceram. 2019;102:1435-1453.
- [CrossRef] [Google Scholar]
- MnO2 -promoted oxidative radical sulfonylation of haloalkynes with sulfonyl hydrazides: C(sp)-S bond formation towards alkynyl sulfones. Chem. Asian J.. 2017;12:1875-1878.
- [CrossRef] [Google Scholar]
- Advanced photocatalytic performance of novel BiOBr/BiOI/cellulose composites for the removal of organic pollutant. Cellulose. 2019;26:5543-5557.
- [CrossRef] [Google Scholar]
- Facile preparation of BiOBr/cellulose composites by in situ synthesis and its enhanced photocatalytic activity under visible-light. Carbohydr. Polym.. 2018;195:393-400.
- [CrossRef] [Google Scholar]
- Multifunctional cellulose nanocrystal /metal oxide hybrid, photo-degradation, antibacterial and larvicidal activities. Carbohydr. Polym.. 2020;230:115711.
- [CrossRef] [Google Scholar]
- Reductive umpolung of carbonyl derivatives with visible-light photoredox catalysis: direct access to vicinal diamines and amino alcohols via α-amino radicals and ketyl radicals. Angew. Chem. Int. Ed.. 2016;55:6776-6779.
- [CrossRef] [Google Scholar]
- Surprise in the phosphate modification of BiOCl with oxygen vacancy: In situ construction of hierarchical Z-scheme BiOCl-OV-BiPO4 photocatalyst for the degradation of carbamazepine. Chem. Eng. J.. 2019;360:1320-1329.
- [CrossRef] [Google Scholar]
- Rapid synthesis of hierarchical BiOCl microspheres for efficient photocatalytic degradation of carbamazepine under simulated solar irradiation. Chem. Eng. J.. 2015;263:419-426.
- [CrossRef] [Google Scholar]
- Highly efficient and visible-light-driven BiOCl for photocatalytic degradation of carbamazepine. J. Alloys Compd.. 2018;757:455-465.
- [CrossRef] [Google Scholar]
- TBAOH assisted synthesis of ultrathin BiOCl nanosheets with enhanced charge separation efficiency for superior photocatalytic activity in carbamazepine degradation. J. Colloid Interface Sci.. 2020;570:242-250.
- [CrossRef] [Google Scholar]
- Facile synthesis of cobalt doped BiOCl ultrathin nanosheets as superior photocatalyst for degradation of carbamazepine under visible light. J. Solid State Chem.. 2021;298:122131
- [CrossRef] [Google Scholar]
- Facile in situ synthesis of BiOCl nanoplates stacked to highly porous TiO2: a synergistic combination for environmental remediation. ACS Appl. Mater. Interfaces. 2014;6:13994-14000.
- [CrossRef] [Google Scholar]
- Cellulose nanocrystals properties, production and applications, in: applications of cellulose nanocrystals. MRS Bulletin 2018:155.
- [CrossRef] [Google Scholar]
- Visible light induced selective aerobic formation of N-benzylidene benzylamine over 2-aminoterephthalic acid sensitized {110}-facetted BiOCl nanosheets. ChemCatChem. 2019;11:6425-6430.
- [CrossRef] [Google Scholar]
- Advances in preparation methods of bismuth-based photocatalysts. Chem. Eng. J.. 2021;414
- [CrossRef] [Google Scholar]
- Tuning the morphological structure, light absorption, and photocatalytic activity of Bi2WO6 and Bi2WO6-BiOCl through cerium doping. Arab. J. Chem.. 2020;13:2844-2857.
- [CrossRef] [Google Scholar]
- Hollow irregular octahedra-like NiCo2O4 cages composed of mesoporous nanosheets as a superior anode material for lithium-ion batteries. Chem. Eng. J.. 2018;350:29-36.
- [CrossRef] [Google Scholar]
- Physical study of the primary and secondary photothermal events in gold/cellulose nanocrystals (AuNP/CNC) nanocomposites embedded in PVA matrices. ACS Sustain. Chem. Eng.. 2017;5:1601-1609.
- [CrossRef] [Google Scholar]
- Organic-inorganic TCPP/BiOCl hybrids with accelerated interfacial charge separation for boosted photocatalytic performance. Colloids Surf. A Physicochem. Eng. Asp.. 2021;616:126367
- [CrossRef] [Google Scholar]
- Rapid degradation of ciprofloxacin over BiOCl: Insight into the molecular structure transformation and antibacterial activity elimination. Sep. Purif. Technol.. 2021;257:117872
- [CrossRef] [Google Scholar]
- Preparation of magnetically retrievable flower-like AgBr/BiOBr/NiFe2O4 direct Z-scheme heterojunction photocatalyst with enhanced visible-light photoactivity. Colloids Surf. A Physicochem. Eng. Asp.. 2022;633
- [CrossRef] [Google Scholar]
- Recent advances in cellulose supported metal nanoparticles as green and sustainable catalysis for organic synthesis. Cellulose. 2021;28:4545-4574.
- [CrossRef] [Google Scholar]
- AgFeO2 nanoparticle/ZnIn2S4 microsphere p–n heterojunctions with hierarchical nanostructures for efficient visible-light-driven H2 evolution. ACS Sustain. Chem. Eng.. 2021;9:2673-2683.
- [CrossRef] [Google Scholar]
- Surface hydrogen bond network spatially confined BiOCl oxygen vacancy for photocatalysis. Sci. Bull.. 2020;65:1916-1923.
- [CrossRef] [Google Scholar]
- Oxygen Vacancy-Mediated Photocatalysis of BiOCl: Reactivity, Selectivity, and Perspectives. Angew. Chem. Int. Ed.. 2018;57:122-138.
- [CrossRef] [Google Scholar]
- The simultaneous adsorption, activation and in situ reduction of carbon dioxide over Au-loading BiOCl with rich oxygen vacancies. Nanoscale. 2021;13:2585-2592.
- [CrossRef] [Google Scholar]
- Deposition-precipitation synthesis of Yb3+/Er3+ co-doped BiOBr/AgBr heterojunction photocatalysts with enhanced photocatalytic activity under Vis/NIR light irradiation. Sep. Purif. Technol.. 2020;238:116450
- [CrossRef] [Google Scholar]
- Synthesis of direct Z-Scheme Bi3NbO7/BiOCl photocatalysts with enhanced activity for CIP degradation and Cr(VI) reduction under visible light irradiation. Sep. Purif. Technol.. 2021;276:119255
- [CrossRef] [Google Scholar]
- Catalytic enantioselective 1,3-dipolar cycloaddition of azomethine ylides with vinyl sulfones. Org. Lett.. 2006;8:1795-1798.
- [CrossRef] [Google Scholar]
- Enhanced photocatalytic activity of BiOCl by C70 modification and mechanism insight. Appl. Surf. Sci.. 2018;443:497-505.
- [CrossRef] [Google Scholar]
- Oxygen vacancies induced exciton dissociation of flexible BiOCl nanosheets for effective photocatalytic CO2 conversion. J. Mater. Chem. A. 2017;5:24995-25004.
- [CrossRef] [Google Scholar]
- PANI/BiOCl nanocomposite induced efficient visible-light photocatalytic activity. J. Mater. Sci. Mater. Electron. 2021
- [CrossRef] [Google Scholar]
- Vinyl sulfones: synthetic preparations and medicinal chemistry applications. Med. Res. Rev.. 2006;26:793-814.
- [CrossRef] [Google Scholar]
- Iodine-catalyzed sulfonylation of arylacetylenic acids and arylacetylenes with sodium sulfinates: synthesis of arylacetylenic sulfones. J. Org. Chem.. 2016;81:2744-2752.
- [CrossRef] [Google Scholar]
- Single-crystalline 2D BiOCl nanorods decorated with 2D MoS2 nanosheets for visible light-driven photocatalytic detoxification of organic and inorganic pollutants. FlatChem. 2021;28:100267
- [CrossRef] [Google Scholar]
- A review on cellulose nanocrystals production and characterization methods from Elaeis guineensis empty fruit bunches. Arab. J. Chem.. 2021;14:103319
- [CrossRef] [Google Scholar]
- Ruthenium-catalyzed [2+2] cycloadditions of alkynyl sulfides and alkynyl sulfones. J. Org. Chem.. 2006;71:1934-1937.
- [CrossRef] [Google Scholar]
- Construction of Ag–ZnO/cellulose nanocomposites via tunable cellulose size for improving photocatalytic performance. J. Clean. Prod.. 2021;288:125089
- [CrossRef] [Google Scholar]
- Hydrothermal synthesis, structural and catalytic studies of CuBi2O4 nanoparticles. J. Nanoanalysis.. 2017;20:585-593.
- [CrossRef] [Google Scholar]
- Efficient photocatalytic defluorination of perfluorooctanoic acid over BiOCl nanosheets via a hole direct oxidation mechanism. Chem. Eng. J.. 2017;317:925-934.
- [CrossRef] [Google Scholar]
- Enantioselective organocatalytic conjugate addition of aldehydes to vinyl sulfones and vinyl phosphonates as challenging michael acceptors. Chem. Eur. J.. 2009;15:3204-3220.
- [CrossRef] [Google Scholar]
- Cellulose nanofibrils enable flower-like BiOCl for high-performance photocatalysis under visible-light irradiation. Appl. Surf. Sci.. 2019;464:606-615.
- [CrossRef] [Google Scholar]
- Total synthesis of (-)-4-hydroxyzinowol. J. Org. Chem.. 2014;79:8835-8849.
- [CrossRef] [Google Scholar]
- Direct cross-coupling of aryl alkynyliodines with arylsulfinic acids leading to alkynyl sulfones under catalyst-free conditions. Tetrahedron Lett.. 2017;58:4799-4802.
- [CrossRef] [Google Scholar]
- Novel BiOCl–C3N4 heterojunction photocatalysts: In situ preparation via an ionic-liquid-assisted solvent-thermal route and their visible-light photocatalytic activities. Chem. Eng. J.. 2013;234:361-371.
- [CrossRef] [Google Scholar]
- Rapid synthesis of BiOCl graded microspheres with highly exposed (110) facets and oxygen vacancies at room temperature to enhance visible light photocatalytic activity. Catal. Commun.. 2019;130:105769.
- [CrossRef] [Google Scholar]
- CTAB-assisted solvothermal construction of hierarchical Bi2MoO6/Bi5O7Br with improved photocatalytic performances. Sep. Purif. Technol.. 2020;242:116775.
- [CrossRef] [Google Scholar]
- Construction of BiOCl/CuBi2O4 S-scheme heterojunction with oxygen vacancy for enhanced photocatalytic diclofenac degradation and nitric oxide removal. Chem. Eng. J.. 2021;411:128555.
- [CrossRef] [Google Scholar]
- Graphene oxide-BiOCl nanoparticle composites as catalysts for oxidation of volatile organic compounds in nonthermal plasmas. ACS Appl. Nano Mater.. 2020;3:9363-9374.
- [CrossRef] [Google Scholar]
- Selective oxidation of 5-hydroxymethylfurfural to 2,5-furandicarboxylic acid using O2 and a photocatalyst of Co-thioporphyrazine bonded to g-C3N4. J. Am. Chem. Soc.. 2017;139:14775-14782.
- [CrossRef] [Google Scholar]
- Enantioselective total synthesis of lycopodine. J. Am. Chem. Soc.. 2008;130:9238-9239.
- [CrossRef] [Google Scholar]
- Synthesis, functional modifications, and diversified applications of hybrid BiOCl-based heterogeneous photocatalysts: a review. Cryst. Growth Des.. 2021;21:6576-6618.
- [CrossRef] [Google Scholar]
- Magnetic recoverable MnFe2O4/cellulose nanocrystal composites as an efficient catalyst for decomposition of methylene blue. Ind. Crops. Prod.. 2018;122:422-429.
- [CrossRef] [Google Scholar]
- Facile in situ chemical transformation synthesis, boosted charge separation, and increased photocatalytic activity of BiPO4/BiOCl p-n heterojunction photocatalysts under simulated sunlight irradiation. J. Phys. Chem. Solids. 2020;147:109630.
- [CrossRef] [Google Scholar]
- Synthesis of a highly efficient BiOCI single-crystal nanodisk photocatalyst with exposing 001 facets. ACS Appl. Mater. Interfaces. 2014;6:7766-7772.
- [CrossRef] [Google Scholar]
- Enantioselective and regioselective pyrone Diels-Alder reactions of vinyl sulfones: total synthesis of (+)-cavicularin. Angew. Chem. Int. Ed.. 2014;53:10500-10503.
- [CrossRef] [Google Scholar]
- Electrochemical decarboxylative sulfonylation of arylacetylenic acids with sodium arylsulfinates: Access to arylacetylenic sulfones. Synth. Commun.. 2020;50:161-167.
- [CrossRef] [Google Scholar]
- Rhodium(III)-catalyzed [3+2] annulative coupling between oximes and electron-deficient alkynes. Sci. China. Chem.. 2015;58:1297-1301.
- [CrossRef] [Google Scholar]
- Asymmetric organocatalytic Michael addition of ketones to vinyl sulfone. ChemComm 2008:6315-6317.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2022.103708.
Appendix A
Supplementary data
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1