Translate this page into:
Influence of suspended natural sands on the photolysis of ciprofloxacin in water
⁎Corresponding authors. cjdxwqfscience@163.com (Qingfeng Wu), li@uwp.edu (Zhaohui Li)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
A comprehensive understanding on the phototransformation of antibiotics in aqueous environments requires consideration of particulate matters contained in natural waters. However, relevant studies on this issue are still limited. Considering the wide distribution of natural sands in natural waters, especially in rainy seasons, the photolysis of CIP in water containing natural sands was investigated, and the influence of natural sands on photolysis was evaluated. The photolytic kinetics showed that the natural sands suspended in water notably accelerated the photodegradation of CIP, and the affecting degree depended on their compositions. The scavenger experiments combined with electron paramagnetic resonance (EPR) experiments showed that 1O2, O2−•, and ·OH generated in the natural sand systems under irradiation, and responsible for the enhanced photodegradation of CIP. The H2O2 and HCl treatment experiments, together with deoxygenation experiments, confirmed that the production of reactive radicals was mainly ascribed to the photoactive oxides and natural organic matter (NOM) in natural sands. The photoproducts of CIP in the presence of natural sands were identified by LC-MS, and the possible degradation pathways were proposed. This study suggested that particulate matters contained in natural waters was a important factor influencing the phototransformation of pollutants, and should be considered in assessing their fate in aqueous environments.
Keywords
Antibiotic
Photolysis
Reactive oxygen species
Natural sand
Photoproduct
1 Introduction
China is the largest producer and consumer of antibiotics in the world. According to a survey conducted by Zhao et al.(2010), the total production of antibiotics in China in 2013 was nearly 248,000 tons, and the antibiotic usage was about 162,000 tons. Currently, problems caused by the production and use of antibiotics are receiving more and more attention. Owing to incomplete metabolization in human and animal bodies, a large amount of antibiotics used are excreted through urine and feces, and finally enter aquatic environments via different pathways such as discharge of sewage effluents containing antibiotic (Nikolaou et al., 2007), direct discharge of untreated wastewaters (Chang et al., 2010), or release from animal husbandry and aquaculture (Luo et al., 2011; Zou et al., 2011). Chronic exposure to low levels of antibiotics may lead to adverse effects to the ecosystem and human health, including development of antibiotic resistance, genotoxicity, aquatic toxicity, and endocrine disruption (Daughton et al., 1999; Fent et al. 2006). Therefore, it is very important to understand the fate and transformation of antibiotics in aqueous environments.
Natural water system is a major sink for released antibiotics. When they enter the aquatic environment, photolysis is one of the most important degradation pathways. Previously, many studies have investigated the photochemical degradation of antibiotics in aquatic environments, but mainly centered on direct or indirect photolysis in pure aqueous phase (ultrapure water, buffers, or natural waters) (Sturini et al., 2012; Wang et al., 2012; Babić et al., 2013; Lin et al., 2013). Actually, natural water bodies generally contain a certain amount of particulate materials such as clays, silts, sands, and organic substances, which can affect the photochemical behavior of pollutants (Mathew and Khan, 1996). Therefore, a comprehensive understanding on phototransformation of antibiotics in natural waters requires consideration of these indigenous materials. An early study demonstrated that the photolysis rate in the presence of suspended sediment was more rapid than in clear water, which was ascribed to the increased diffuseness of light caused by scattering (Miller and ZePP, 1979). Photolysis of organic compounds in the water containing clays (kaolinite, montmorillonite) indicated that the photodegradation was accelerated in the presence of clays, and the reactive radicals such as hydroxyl radicals (·OH) and singlet oxygen (1O2) generated by exposure of the suspended clays to sunlight irradiation were responsible for the enhanced photodegradation (Katagi, 1993; Kong and Ferry, 2003; Mathew and Khan, 1996). In addition, the generation of superoxide anion (O2−•) and hydroxyl radical (·OH) in the water containing clays under irradiation were verified by electron paramagnetic resonance (EPR) technique and laser flash photolysis (Gournis et al., 2002; Zhang et al., 2008). The common opinions for the effects of particulate matters on photolysis of pollutants in water were that particulate matters may either inhibit the photolysis by quenching the excited states of organic molecules or light shielding (Oliver et al., 1979), or promote the photolysis by photoinduced oxidation or efficient light scattering (Miller and Zepp, 1979; Katagi, 1993; Mathew and Khan, 1996).
Overall, understanding about the effects of particulate matters on photolysis was still limited. More studies need to be done to enrich the knowledge in this field. Natural sand, a complex composed of inorganic and organic matters, widely distributed in aquatic environments especially in rainy season. To our knowledge, influence of natural sands on photochemical behavior of pollutants in water are still unknown. Thus, we planned to investigate its effects on the photolysis of ciprofloxacin (CIP). CIP is a second generation fluroquinolone (FQ), and widely used in human and veterinary medicine due to its broad activity spectrum against gram-positive and gram-negative bacteria. Traditional wastewater treatment methods cannot effectively remove it from wastewater, leading to the continuous release of CIP into natural waters. It has been frequently detected at concentrations ranging from ngL−1 in secondary wastewater effluents and surface waters, to μgL−1 level in untreated hospital sewage (Kümmerer, 2009). Furthermore, CIP is chemically stable due to the quinolone ring stability, and resistant to hydrolysis and increased temperature. In many studies, CIP was chosen as a representative antibiotic. The main goals of this study were to investigate the effects of suspended natural sands on the photolysis of CIP, and to further elucidate the intrinsic mechanisms. We attempt to (1) examine the possible effects of suspended natural sands on the photolysis of CIP in water; (2) to elucidate the affecting mechanisms based on experimental results and analysis; (3) determine the photoproducts of CIP in the water containing natural sands, and speculate the possible photolysis pathways. This research will help us have a better understanding about the fate and transformation of antibiotics in natural waters.
2 Experimental
2.1 Materials
Sea sand (SS) and desert sand (DS) samples (Fig.S1) collected from ocean beach (Shenzhen) and Tenggeli desert in China were used in this study. Ciprofloxacin hydrochloride (purity > 98%) was purchased from J&K Chemical Co., Ltd (Beijing, China). High performance liquid chromatography (HPLC) grade chemical reagents including acetonitrile, acetic acid, triethylamine, isopropanol (IPA), 1, 4-diazabicyclo [2.2.2] octane (DABCO), and p-benzoquinone (PBQ) were supplied by Sinopharm Chemical Reagent Co., Ltd (Shanghai, China). The spin trapping reagents used for electron paramagnetic resonance (EPR) experiments, 5,5-dimethyl-1-pyrroline N-oxide (DMPO) and 2,2,6,6-tetramethylpiperidine (TEMP), were supplied by Sigma-Aldrich (Shanghai, China). Ultrapure water (18 MΩcm−1) was used to prepare all aqueous solutions.
2.2 Treatment and characterization of natural sands
The samples were first passed through 0.15 and 0.25 mm sieves to obtain a uniform particle size. Then, the obtained samples were cleaned by Milli-Q water for several times, dried in a oven at 50 °C for 24 h, and stored in a desiccator prior to use. No further chemical treatments were conducted on the samples to preserve their natural attributes. Powder X-ray diffraction (XRD) analysis were performed with a Bruker D8 advanced diffractometer equipped with Cu-Kα radiation, monitoring in the range from 2θ = 5 to 800, and the collected data were processed with MDI Jade software (version 6.5). The XRD analysis showed that the DS sand mainly contained quartz, as well as a certain amount of albite and gismondine, while the main component of SS sand was quartz (Fig.S2). The elemental composition analysis of sand samples were carried out on a Rigaku-ZSX Primus II X-ray fluorescence (XRF) spectrometer. The total organic carbon (TOC) content, an indicator of natural organic matter (NOM) in natural sand samples, was measured using a TOC analyzer (LECO CS230, America). The results of XRF and TOC analysis were shown in Table 1.
Composition
% content
SS
DS
SiO2
93.3
77.4
Al2O3
2.15
10.6
CaO
2.95
2.9
K2O
0.93
2.79
Fe2O3
0.502
2.16
Na2O
–
2.14
MgO
–
1.37
TiO2
–
0.266
TOC
0.019
0.011
To remove the photoactive metal oxides in the sand sample, the 10 g sample was added into 200 mL HCl (1 M) solution, then the suspension was stirred continuously for 2 h until no bubbles come out of the solution. This procedure was repeated 3 times. At the end of HCl treatment, the sample was washed several times with ultrapure water until no HCl residual was detected with AgNO3, and then dried at 50℃ for 24 h. For the removal of NOM, the 10 g sample was mixed with 200 mL H2O2 (30%) solution, and then the procedure similar to the above was applied. Finally, the H2O2 treated sample was washed several times with ultrapure water and dried at 100℃ for 24 h, ensuring the complete removal of H2O2 residual.
2.3 Photolysis experiments
Photolysis experiments were performed in a PR22-25 photochemical reactor (PerfectLight, China), which is equipped with a xenon light (PLS-SXE300UV) and a water-cooling system (DC-0506). The output wavelength of xenon light was in the range between 300 and 800 nm, and the light power entering the reactor was nearly 120 mWcm−2. During the irradiation time, the suspension was kept stirring constantly at 1400 rpm, and the temperature in the reactor was maintained at 24 °C through circulating water. For each experiment, 0.5 g sand sample and 100 mL CIP solution (30 mgL−1) were mixed in the 250 mL reactor, and then magnetically stirred for 1 h to establish the adsorption equilibrium. At the end of equilibrium, the suspension began to be irradiated. The suspension pH was maintained at around 6.5 by adding 50 mM HCl or NaOH. During irradiation, a 2-mL aliquot of the suspension was collected at 15 -min intervals, filtered through a 0.45 μm membrane filter, and then measured using HPLC or LC-MS. Dark control experiments were carried out in the same manner as that for regular experiments, except with no exposure to irradiation. All experiments were performed in duplicate for each condition.
2.4 Analytical determination and photoproduct identification
The CIP concentration measurement was achieved on a Shimadzu HPLC system (LC-20) with a C18 ODS reversed phase column (4.6 mm × 150 mm, 5 μm) and a UV–Vis detector (SPD20). An isocratic elution was performed at a flow rate 0.8 mL/min, with a mobile phase composed of 25 mM acetic acid and acetonitrile (60:40, v/v) with pH adjusted to 6.0 ± 0.1 using triethylamine. Oven temperature was kept at 40 °C and the detector wavelength was set to 276 nm. The injection volume was 20 μL.
Photoproducts were analyzed on a Thermo Scientific Q Exactive mass spectrometer paired with Ultimate 3000 UPLC system. The analytical column was Thermo Scientific Hypersil GOLD (2.1 mm × 100 mm, 3 μm). The mobile phase consisting of solvent A (acetonitrile) and solvent B (0.1% formic acid) was used to elute the column with a optimized gradient program: from 5% to 35% A (0–10 min); 35% A (10–14 min); 35%-5% A (14–14.1 min); 5% A (14.1–20 min). The flow rate and injection volume were 0.25 mL/min and 5 μL, respectively. The optimized MS parameters were set as follows: spray voltage 3200 V; capillary temperature 300 °C; sheath gas pressure 40 arb; aux gas pressure 15 arb; probe heater temperature 350 °C; S-lens RF level 50 V.
2.5 Determination of reactive oxygen species in photochemical reaction
Reactive oxygen species (ROS) including O2−•, ·OH, and 1O2 were determined on a Bruker EPR spectrometer (ESP300E) equipped with a Quanta-Ray Nd: YAG laser system as the irradiation light source (λ = 355 nm) at ambient temperature. The operating parameters were set as follows: center field 3227.67 G; microwave frequency 9054.62 MHz; power 0.998 mW. In quenching experiments, DABCO, IPA, and PBQ were employed as 1O2, ·OH, and O2−• scavengers, respectively.
3 Results and discussion
3.1 Accelerated photodegradation of CIP in the presence of natural sands
Fig. 1 displays the photolytic kinetics of CIP with and without addition of natural sands, together with the corresponding dark controls. In the dark control without natural sands, no obvious loss of CIP was observed in 75 min, indicating that the degradation caused by hydrolysis or pyrolysis was negligible. While in the dark control with natural sands, about 5–10% of CIP loss was observed, which was presumed to be due to adsorption on the surface of natural sands. In pure water phase, about 5% of CIP was degraded via direct photolysis after exposure to simulated sunlight. The regression analysis indicated that the photolysis followed the pseudo-first-order kinetics with a rate constant (KCIP) of (0.14 ± 0.01) × 10−2 min−1. However, when the DS and SS sands were added, the degradation rate of CIP increased to 80% and 70%, with the rate constants increased to 2.06 × 10−2 and 1.58 × 10−2 min−1, respectively (Table 2). Obviously, the presence of natural sands greatly promoted the photolysis of CIP in water, which was similar to that previously observed in sediment and clay suspensions (Miller and ZePP, 1979;Katagi, 1993; Kong and Ferry, 2003; Mathew and Khan, 1996). In contrast, the photolysis in the DS sand system exhibited a higher efficiency relative to the SS sand system, which might be due to their different physicochemical compositions. All the results above indicated that natural sand, an indigenous material of water body, could significantly affect the photolysis of antibiotics in water, and different natural sands had different effects. Therefore, the role of suspended natural sands should be considered as an important factor in evaluating the phototransformation of pollutants in aqueous environments.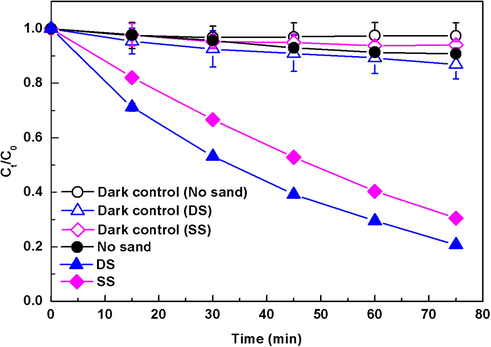
Photolysis of CIP (30 mgL−1) in the presence of natural sands (0.5 g) under simulated solar light. The pH of suspension was maintained at around 6.5 by adding 50 mM HCl or NaOH. Ct/C0 is the ratio of the concentration of DP at t time to the initial concentration of DP.
Reaction Condition
KCIP (min−1)
R2
t1/2 (min)
No sand
(0.14 ± 0.01) × 10−2
0.97
495.1
DS sand
(2.06 ± 0.04) × 10−2
0.999
33.6
SS sand
(1.58 ± 0.06) × 10−2
0.993
43.9
Pure Quartz
(0.12 ± 0.06) × 10−2
0.988
577.6
DS sand + IPA
(1.45 ± 0.02) × 10−2
0.999
47.8
DS sand + PBQ
(0.88 ± 0.06) × 10−2
0.98
78.77
DS sand + DABCO
(0.30 ± 0.04) × 10−2
0.947
231.1
SS sand + IPA
(1.17 ± 0.04) × 10−2
0.995
59.2
SS sand + PBQ
(0.76 ± 0.03) × 10−2
0.993
91.2
SS sand + DABCO
(0.36 ± 0.05) × 10−2
0.918
192.5
DS sand treated with H2O2
(0.92 ± 0.08) × 10−2
0.968
75.3
DS sand treated with HCl
(0.18 ± 0.03) × 10−2
0.897
385.1
SS sand treated with H2O2
(0.65 ± 0.03) × 10−2
0.991
106.6
SS sand treated with HCl
(0.16 ± 0.02) × 10−2
0.949
433.2
DS sand + N2
(7.5 ± 0.4) × 10−2
0.989
9.2
SS sand + N2
(3.6 ± 0.2) × 10−2
0.987
20.1
3.2 Photocatalytic mechanisms of CIP in the presence of natural sands
3.2.1 Study of reactive species
As indicated by the XRF and TOC analysis, the natural sand samples also contained a small amount of iron oxide, titanium dioxide, and natural organic matter (NOM) in addition to silica (Table 1). As we known, these components are photoactive, and can induce the generation of reactive oxygen species (ROS) such as ·OH, 1O2 and O2−• under irradiation. Thus, it is reasonable to speculate that the ROS are produced in the irradiated natural sand system, and contribute to the enhanced photodegradation of CIP. To determine the ROS generated in the natural sand system, the EPR spin-trapping technique was employed. As shown in Fig. 2, no obvious signal was detected in dark, but after 10 min irradiation, the characteristic peaks of TEMP-1O2, DMPO-O2−•, and DMPO-OH adducts appeared, and gradually increased with the prolonging of irradiation time (Kou et al., 2009; Yao et al., 2013; Qi et al., 2016). This result clearly showed the generation of 1O2, ·OH, and O2−• in the present system.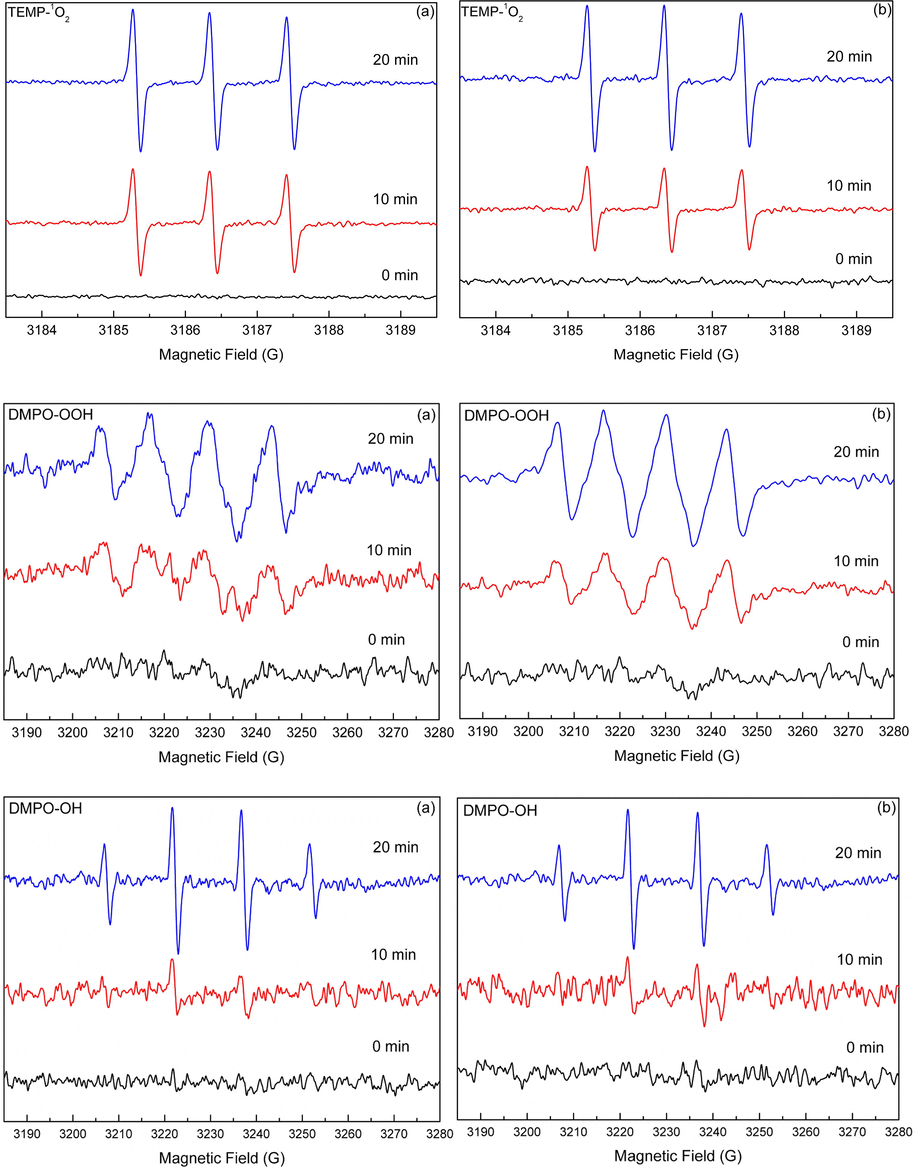
EPR spectral changes of the TEMP-1O2, DMPO-O2−•, DMPO-OH adducts generated in the DS (a) and SS (b) systems under simulated solar light irradiation.
Moreover, scavenger experiments were also conducted to quantitatively evaluate the role of each reactive radical in the photoreaction. Fig. 3 showed the photolysis of CIP in the presence of natural sands with addition of IPA (·OH scavenger), DABCO (1O2 scavenger), and PBQ (O2−• scavenger). In both DS and SS systems, the addition of DABCO drastically inhibited the photodegradation of CIP, indicating a very important role played by 1O2 in the photoreaction, which was consistent with the strong signals of TEMP-1O2 adducts. Also, the addition of PBQ and IPA showed a certain inhibitory effect on CIP photodegradation, suggesting that the contribution of O2−• and ·OH to the enhanced photodegradation of CIP. The EPR analysis combined with scavenger experiments demonstrated that the 1O2, O2−•, and ·OH were generated in the natural sand system, and responsible for the enhanced photodegradation of CIP.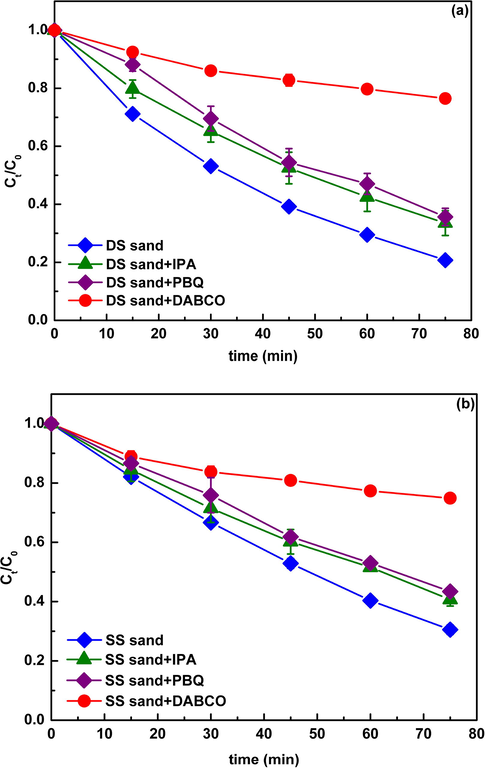
Suppressed photodegradation of CIP (30 mgL−1) in the presence of DS sand (a) and SS sand (b) by the radical scavengers. The initial concentration of IPA (·OH), DABCO (O2−•), and PBQ (1O2) were 30, 2, and 0.1 mM, respectively. Ct/C0 is the ratio of the concentration of DP at t time to the initial concentration of DP.
3.2.2 H2O2 and HCl-treated experiments and deoxygenation experiments
To further investigate the mechanisms of the generation of ROS, several designed experiments were performed. As the predominant component of natural sands, the role of silica in the generation of reactive radicals was determined by examining the photolysis of CIP in the presence of pure silica (Fig. 4). The result demonstrated that silica itself could not promote the photodegradation of CIP, but slightly inhibited it, which might be due to the light shielding of natural sand particles. In contrast to this, the addition of SiO2 particles to solutions of anthracene in cyclohexane increased the photolysis rate of anthracene, which was attributed to the polarity/polarizability of the SiO2/cyclohexane interface (Zingg and Sigman, 1993). Considering the slight suppression of SiO2 on the photolysis of DP, it could be speculated that the generation of reactive radicals was not due to silica, but other components such as the photoactive metal oxides or NOM.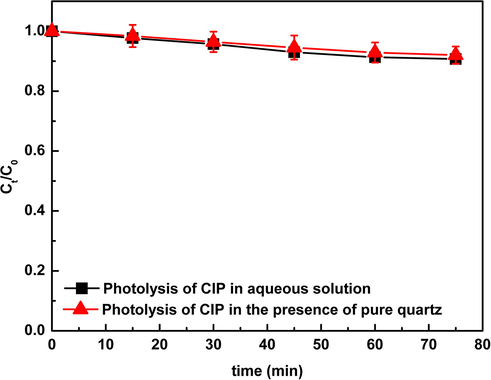
Photolysis of CIP (30 mgL−1) in the presence of pure quartz (0.5 g) under simulated solar light. Ct/C0 is the ratio of the concentration of DP at t time to the initial concentration of DP.
Many studies have indicated that,NOM,as an important photosensitizer, could induce the production of reactive radicals including O2−•, ·OH, 1O2, eaq− (hydrated electron), and the triplet-state NOM via the energy or electron transfer mechanism under irradiation (Haag et al., 1986; Zepp et al., 1987; Boule et al., 1999; Goldstone et al., 2000). To explore the role of NOM in the production of reactive radicals, the sand samples were treated with H2O2 to remove the NOM, and then the photolysis of CIP in the presence of H2O2 treated sand samples was conducted. Obviously, the removal of NOM significantly reduced the CIP degradation (Fig. 5), with the rate constants decreasing from 2.06 × 10−2 and 1.58 × 10−3 min−1 to 0.92 × 10−3 and 0.65 × 10−3 min−1, respectively (Table 2). This result verified the important role of NOM in natural sand in the production of reactive radicals. In general,the way that NOM induces the generation of reactive radicals is mainly through the triplet-state NOM excited by irradiation (Xu et al., 2011; Jacobs et., 2011; Wenk et al., 2013; Parker et al., 2013; Sharpless and Blough, 2014). In order to examine whether this process occurred in the present system, deoxygenation experiments were carried out. As shown in Fig. 6, the contrast of the photolysis of CIP in the suspension with and without deoxygenation showed that the degradation of CIP was greatly promoted in the deoxygenation condition. Considering dissolved oxygen (DO) is an efficient triple-state quencher (Halladja et al., 2007; Chen et al., 2009), this result can be interpreted that the elimination of DO reduces the quenching of the triplet-state NOM, thereby benefiting the generation of free radicals. This result further confirmed the production of reactive radicals was through the triplet- state NOM excited by irradiation.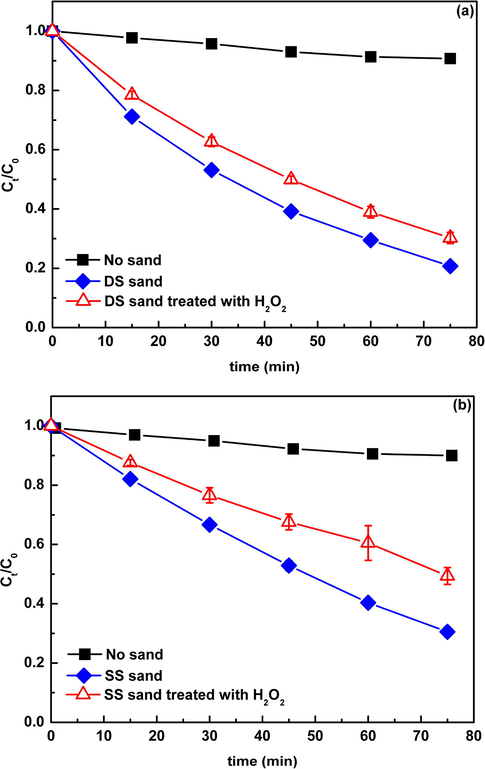
The contrast of the photodegradation of CIP (30 mgL−1) in the presence of DS (a) and SS (b) (0.5 g) before and after H2O2 treatment. Ct/C0 is the ratio of the concentration of DP at t time to the initial concentration of DP.
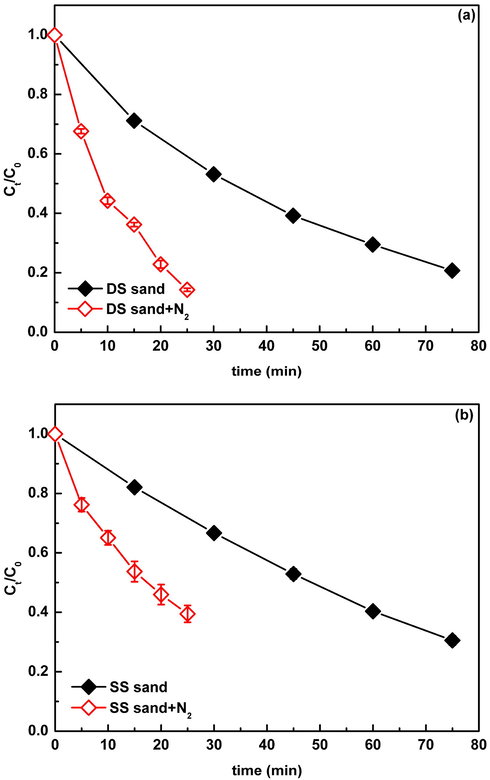
The contrast of CIP photdegradation (30 mgL−1) in the presence of DS (a) and SS (b) (0.5 g) before and after deoxygenation. Ct/C0 is the ratio of the concentration of DP at t time to the initial concentration of DP.
Besides NOM, the photoactive oxides (iron oxide, titanium dioxide) in natural sands could also induce the generation of O2−• and ·OH under irradiation. To ascertain the role of these oxides, the sand samples were further treated with 1 M HCl to remove the photoactive oxides, and then the photolysis of CIP in the presence of HCl treated sand sample was investigated. The results showed that the HCl treatment drastically decrease the degradation of CIP, suggesting a significant contribution of the photoactive oxides towards the generation of reactive radicals (Fig. 7). According to this result, it could be speculated that the higher constant rate in the DS sand system compared with the SS sand system might be attributed to the higher content of photoactive oxides as indicated by XRF analysis.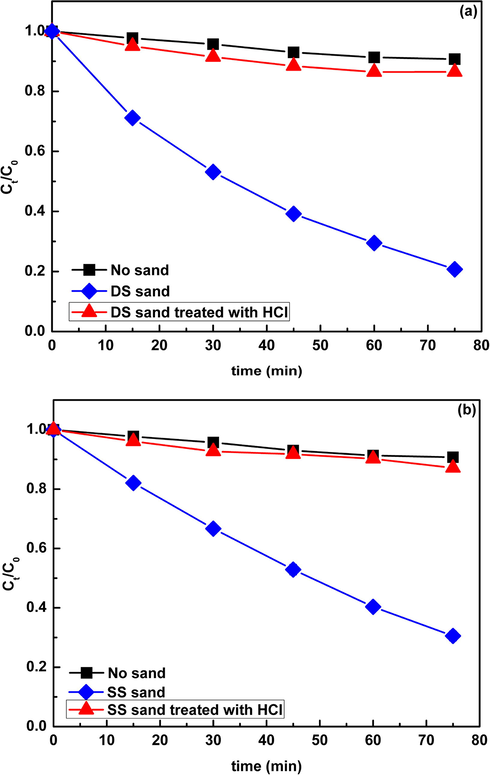
The contrast of the photodegradation of CIP (30 mgL−1) in the presence of DS (a) and SS (b) (0.5 g) before and after 1 M HCl treatment. Ct/C0 is the ratio of the concentration of DP at t time to the initial concentration of DP.
In summary, the influence of natural sand particles on the photolysis of CIP was attributed to the NOM and photoactive oxides contained in natural sands. Under irradiation, the NOM in natural sands was excited to a triplet-state NOM, and further induced the production of reactive radicals via electron or energy transfer. Meanwhile, the photoactive oxides also produced reactive radicals via photoexcited electrons or holes (Li et al., 2018; Li et al., 2019). Then, these reactive radicals further reacted with CIP, leading to the enhanced degradation of CIP.
3.3 Photoproducts and proposed degradation pathways
To further study the mechanism of the photodegradation of CIP in the presence of natural sands, the photoproducts of CIP were separated and identified by HPLC-ESI-MS (Fig.S3). The molecular masses and proposed structures of the photoproducts were determined on the basis of mass data and previous studies. As shown in Table 3, photolysis of CIP in natural sand suspension produced 5 major products, all of which have also been detected in previous studies (paul et al., 2010; An et al., 2010; Guo et al. 2013; Haddad et al. 2014; Salma et al., 2016; Wu et al., 2018). As proposed in these studies, cleavage of piperazinering, defluorination, substitution of fluorine, and hydroxylation of the quinolone core were the main photodegradation pathways for CIP. Accordingly, the formation of P1 could be attributed to the photosubstitution of the fluorine atom in CIP molecule by ·OH. The further hydroxylation of the quinolone core of P1 led to the generation of P3. For product P4, it was also detected in the study by Salma et al. (2016), and its structure was elucidated via deuterated CIP. Following their suggestions, an O-O peroxide ring was formed between the superoxide radical anion and the piperazine ring, resulting in the formation of P4. Under further irradiation, this ring can be opened through further oxidation forming P5 and P2 (Salma et al. 2016). The possible photodegradation pathways for CIP in the presence of natural sands were illustrated in Fig. 8. Based on the identified photoproducts and proposed degradation pathways, it could be confirmed that the ·OH and O2−• participated in the phototransformation of CIP in the natural sand systems.
Product
Retention time (min)
[M + H]+ (m/z)
Proposed structure
CIP
6.42
332
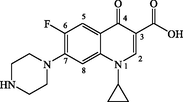
P1
5.13
330

P2
5.39
288
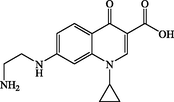
P3
6.01
346
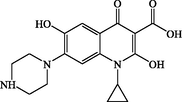
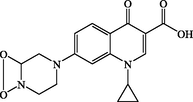
P4
7.76
344
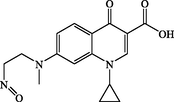
P5
8.48
316
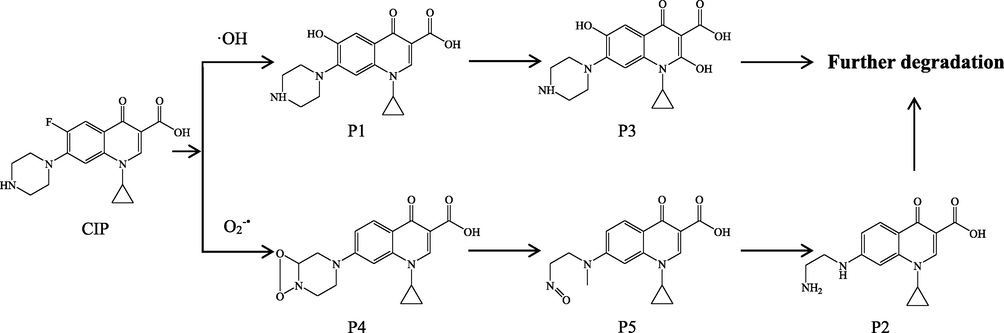
Proposed photolysis pathways for CIP in the presence of natural sands.
4 Conclusion
Understanding the influence of suspended particulate matters on the photolysis of pollutants in water was still limited. In this work, the photolysis of CIP in water containing natural sands was investigated. The results showed that the natural sands (SS and SD) suspended in water significantly accelerated the photodegradation of CIP. The information obtained from EPR analysis and designed experiments revealed that the O2−•, ·OH and 1O2 induced by the NOM and photoactive oxides in natural sands were mainly responsible for the accelerated degradation of CIP. Photoproduct analysis indicated that O2−• and ·OH participated in the transformation of CIP, and photosubstitution of the fluorine atom, addition of hydroxyl group to the quinolone core, and cleavage of piperazine moiety were proved to be the main degradation pathways. This work suggested that suspended natural sand had a significant influence on the phototransformation of antibiotics in water. Thus, its role need to be considered in accessing the transformation and fate of pollutants in aqueous environments.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant 41403083), the Key Project of Science and Technology of Hubei Provincial Department of Education (Grant D20141305), the Natural Science Foundation of Hubei Province, China (Grant 2019CFB225), and the Science Foundation of Education Commission of Hubei Province of China (Grant T2020008).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Kinetics and mechanism of advanced oxidation processes (AOPs) in degradation of ciprofloxacin in water. Appl. Catal. B: Environ.. 2010;94(3-4):288-294.
- [Google Scholar]
- Photolytic degradation of norfloxacin, enrofloxacin and ciprofloxacin in various aqueous media. Chemosphere. 2013;91(11):1635-1642.
- [Google Scholar]
- Phototransformations induced in aquatic media byNO3−/NO2−, Fe(III) and humic substances. In: Andrey G., ed. Barceló, Damià, Kostianoy. TheHandbook of Environmental Chemistry: Springer; 1999. p. :204-205.
- [Google Scholar]
- Determination of antibiotics in sewage from hospitals, nursery and slaughter house, waste water treatment plant and source water in Chongqing region of Three Gorge Reservoir in China. Environ. Pollut.. 2010;158(5):1444-1450.
- [Google Scholar]
- Indirect photodegradation of amine drugs in aqueous solution under simulated sunlight. Environ. Sci. Technol.. 2009;43(8):2760-2765.
- [Google Scholar]
- Pharmaceuticals and personal care products in the environment: agents of subtle change? Environ. Health Perspec.. 1999;107(suppl 6):907-938.
- [Google Scholar]
- Chemistry of superoxide radical in seawater: CDOM associated sink of superoxide in coastal waters. Environ. Sci. Technol.. 2000;34:1043-1048.
- [Google Scholar]
- Formation of hydroxyl radicals catalyzed by clay surfaces. Phys. Chem. Minerals. 2002;29(2):155-158.
- [Google Scholar]
- Photochemical degradation of ciprofloxacin in UV and UV/H2O2 process: kinetics, parameters, and products. Environ. Sci. Pollut. Res.. 2013;20(5):3202-3213.
- [Google Scholar]
- Singlet oxygen in surface waters. 3. Photochemical formation and steady-state concentrations in various types of waters. Environ. Sci. Technol.. 1986;20(4):341-348.
- [Google Scholar]
- Characterization of photo-transformation products of the antibiotic drug Ciprofloxacin with liquid chromatography-tandem mass spectrometry in combination with accurate mass determination using an LTQ-Orbitrap. Chemosphere. 2014;115:40-46.
- [Google Scholar]
- Inhibition of humic substances mediated photooxygenation of furfuryl alcohol by 2,4,6-trimethylphenol. Evidence for reactivity of the phenol with humic triplet excited states. Environ. Sci. Technol.. 2007;41(17):6066-6073.
- [Google Scholar]
- Fulvic acid mediated photolysis of ibuprofen in water. Water Res.. 2011;45(15):4449-4458.
- [Google Scholar]
- Photodegradation of esfenvalerate in clay suspension. J. Agric. Food Chem.. 1993;41:2178-2183.
- [Google Scholar]
- Effects of salinity on the photolysis of chrysene adsorbed to a smectite clay. Environ. Sci. Technol.. 2003;37:4894-4900.
- [Google Scholar]
- Visible-light-induced photocatalytic oxidation of polycyclic aromatic hydrocarbons over tantalum oxynitride photocatalysts. Environ. Sci. Technol.. 2009;43(8):2919-2924.
- [Google Scholar]
- Natural wolframite as a novel visible-light photocatalyst towards organics degradation and bacterial inactivation. Catal. today. 2019;358:177-183.
- [Google Scholar]
- Photocatalytic removal of NO by Z-scheme mineral based heterojunction intermediated by carbon quantum dots. Appl. Surf. Sci.. 2018;456:835-844.
- [Google Scholar]
- Lin, A.Y.-C., Wang, X.-H., Lee, W.-N., 2013. Phototransformation determines the fate of 5-fuorouracil and cyclophosphamide in natural surface waters. Environ. Sci. Technol. 47, 4140–4112.
- Occurrence and transport of tetracycline, sulfonamide, quinolone, and macrolide antibiotics in the Haihe River Basin. China. Environ. Sci. Technol.. 2011;45(5):1827-1833.
- [Google Scholar]
- Photodegradation of metolachlor in water in the presence of soil mineral and organic constituents. J. Agric. Food Chem.. 1996;44(12):3996-4000.
- [Google Scholar]
- Effects of suspended sediments on photolysis rates of dissolved pollutants. Water Res.. 1979;13:453-459.
- [Google Scholar]
- Occurrence patterns of pharmaceuticals in water and wastewater environments. Anal. Bioanal. Chem.. 2007;387(4):1225-1234.
- [Google Scholar]
- Effect of suspended sediments on the photolysis of organics in water. Environ. Sci. Technol.. 1979;13:1075-1077.
- [Google Scholar]
- Influence of ionic strength on triplet-state natural organic matter loss by energy transfer and electron transfer pathways. Environ. Sci. Technol.. 2013;47(19):10987-10994.
- [Google Scholar]
- Photolytic and photocatalytic decomposition of aqueous ciprofloxacin: Transformation products and residual antibacterial activity. Water Res.. 2010;44(10):3121-3132.
- [Google Scholar]
- Activation of peroxymonosulfate by base: Impilcations for the degradation of organic pollutants. Chemosphere. 2016;151:280-288.
- [Google Scholar]
- Dependence of transformation product formation on pH during photolytic and photocatalytic degradation of ciprofloxacin. J. Hazard. Mater.. 2016;313:49-59.
- [Google Scholar]
- The importance of charge-transfer interactions in determining chromophoric dissolved organic matter (CDOM) optical and photochemical properties. Environ. Sci-Proc Imp.. 2014;16(4):654-671.
- [Google Scholar]
- Influence of an SiO2/cyclohexane interface on the photochemisitry of anthracene. Photochem. Photobiol.. 1993;57:453-459.
- [Google Scholar]
- Photodegradation of fluoroquinolones in surface water and antimicrobial activity of the photoproducts. Water Res.. 2012;46(17):5575-5582.
- [Google Scholar]
- Phototransformation of cephalosporin antibiotics in an aqueous environment results in higher toxicity. Environ. Sci. Technol.. 2012;46(22):12417-12426.
- [Google Scholar]
- Quenching of excited triplet states by dissolved natural organic matter. Environ. Sci. Technol.. 2013;47(22):12802-12810.
- [Google Scholar]
- Photodegradation of ciprofloxacin adsorbed in the intracrystalline space of montmorillonite. J. Hazard. Mater.. 2018;359:414-420.
- [Google Scholar]
- Photosensitized degradation of amoxicillin in natural organic matter isolate solutions. Water Res.. 2011;45(2):632-638.
- [Google Scholar]
- Efficient removal of dyes using heterogeneous Fenton catalysts based on activated carbon fibers with enhanced activity. Chem. Eng. Sci.. 2013;101:424-431.
- [Google Scholar]
- Photoproduction of hydrated electrons from natural organic solutes in aquatic environments. Environ. Sci. Technol.. 1987;21(5):485-490.
- [Google Scholar]
- Evidence of the hydroxyl radical formation upon the photolysis of an iron-rich clay in aqueous solutions. React. Kinet. Catal. Lett.. 2008;94(2):207-218.
- [Google Scholar]
- Occurrence and a screening-level risk assessment of human pharmaceuticals in the pearl river system, south China. Environ. Toxicol. Chem.. 2010;29:1377-1384.
- [Google Scholar]
- Occurrence and distribution of antibiotics in coastal water of the Bohai Bay, China: impacts of river discharge and aquaculture activities. Environ. Pollut.. 2011;159(10):2913-2920.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2021.103369.
Appendix A
Supplementary material
The following are the Supplementary data to this article:







