Translate this page into:
Insights into the photocatalytic role of glycine-assisted sol-gel auto-combustion synthesized (Co, Mn)(Co, Mn)2O4 nanostructures for efficient visible light-driven degradation of methyl orange
⁎Corresponding author. salavati@kashanu.ac.ir (Masoud Salavati-Niasari)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Research on the use of new materials and the improvement of existing fast ways for removal of water pollutants is still challenging. This work documented rational fabrication of (Co, Mn)(Co, Mn)2O4 nanoparticles as nano-photocatalyst materials with improved charge carrier separation and strong redox potential for efficient degradation of methyl orange (MO) in wastewater. This investigation illustrated that the Co/Mn molar ratio can greatly affect the textural and structural properties. From the point of view of size and shape, the optimum product with 1:3 M ratio of Co to Mn precursors was selected as a catalyst in the photocatalysis process. The prepared highly crystallized phase of tetragonal (Co, Mn)(Co, Mn)2O4 describe an optical band gap of 1.7 eV, according to the DRS data, which make them as efficient visible driven photocatalysts. The photodegradation experiments of spinel mixed structures were assessed by investigation of photocatalyst dosages and initial MO concentrations to unravel how these operational parameters affect Co-Mn-O photocatalysis. A remarkable discoloration capability of 68.07 % could result from the catalyst dosage of 0.04 g and dye concentration of 15 mg/L after 120 min of visible light. The kinetics survey further unveiled the maximum rate constant of 0.0080 min−1 for the MO degradation. In the scavenger trials, singlet oxygen (1O2) and superoxide (●O2−) radicals were principal reactive oxygen species (ROS) responsible for pollutant photodegradation in the presence of pure (Co, Mn)(Co, Mn)2O4 structures. In addition, the cyclic performance of catalyst depicted a removal efficiency of 60.1 % over 5 runs.
Keywords
Cobalt-Manganese Oxides Spinel
Nanostructures
Methyl Orange
Photodegradation Efficiency
1 Introduction
Access to water has been the major issue for the creatures of the planet (Yaqoob et al., 2020). Humans have always looked for additional sources of water. Nowadays, the reduction of fresh and potable water resources has involved people in many troubles (Aslam et al., 2019a; Joseph et al., 2019; Rahimi-Nasrabadi et al., 2019). Dyes are known as persistent organic compounds in textiles, cosmetics, plastics, etc, so they have coloring and binding ability on the surface of substrates through several approaches like physisorption, covalent bonds and generation of metal ions/salt-based metal complexes (Behvandi et al., 2020; Bonthula et al., 2023; Hosny et al., 2023; Taifi et al., 2022). Therefore, because of their stability and their resistance to biodegradability, it is necessary to decrease potential hazards of dyes to agricultural production, aquatic organisms, and human health. As a monoazo dye, methyl orange (MO) with sulfur group and aromaticity can result in harmful diseases such as eye irritation and skin problems (Onwudiwe et al., 2023). In the meantime, researchers, along with technologists, have paid special attention to achieve various methods to prevent water wastage, and treat polluted water (Hasan and Muhammad, 2020; Shanmugam et al., 2019). Through the years, several initiatives have been taken to incorporate a variety of wastewater treatment technologies, including conventional filtration, coagulation-flocculation, and biological treatment systems (Marsooli et al., 2020; Obotey Ezugbe and Rathilal, 2020; Mahdi et al., 2021; Aljeboree et al., 2021; Ganduh et al., 2021). In order to execute the photocatalysis process, the catalyst material must possess the capacity to be stimulated by visible or ultraviolet light (Amin Marsooli et al., 2020; Naik et al., 2023). In fact, the ability to generate electron-holes is the primary stage of photocatalysis. When semiconductor materials are exposed to UV or visible light, they can initiate a range of reactions in aqueous environments, resulting in the eradication of pollutants from water (Al-Mamun et al., 2019; Badvi and Javanbakht, 2021; Jabbar and Graimed, 2022). The semiconductors used in the photocatalytic process include a wide range of materials (Bloise et al., 2009; Rostami et al., 2021). Nanotechnology has enabled researchers to use nanomaterials in a variety of water and wastewater treatment technologies (Aslam et al., 2019b; Taghipour et al., 2019). Nanomaterials can be utilized to optimize water treatment processes, including membrane and filtration, precipitation and, notably, photocatalysis (Ahmad et al., 2022; Aragaw et al., 2021; Ren et al., 2021). Transition metal oxides in different structures are one of the great families of semiconductors (Karthikeyan et al., 2020; Wang et al., 2020). Cobalt manganese based-spinel oxides namely MnCo2O4 and CoMn2O4 introduce unique electrical features and multiple oxidation states, which play a vital role in photocatalytic domain (Kihal et al., 2024; Mkhalid et al., 2023). However, owing to high recombination of photogenerated charge carrier, the photodegradation efficiency of theses compositions is still not up to expectations. As a MnxCo3-xO4 bimetallic oxide with x = 1.5, (Co, Mn)(Co, Mn)2O4, has drawn the focus of many researchers due to its low cost, availability, stability, and metal centers with redox-activity, making it a useful heterogeneous catalyst and battery anode material (Yu, L. et al., 2013; Zhao et al., 2014). Numerous ways have been documented for the production of (Co, Mn)(Co, Mn)2O4, including the ball mill (Masi et al., 2016), precipitation (Tang et al., 2017), co-precipitation, and sol–gel (Liang et al., 1998). In this project, we propose a novel and facile programmable process for the synthesis spinel-type binary transition metal oxides, namely (Co, Mn)(Co, Mn)2O4 nanostructures in the presence of glycine as both fuel and structure-directing agent. More encouragingly, the impact of the (Co, Mn) precursor ratio on the compositional and structural superiority was deeply evaluated. Based on the literature review, this project aims to investigate less studied applications of these materials, including photocatalytic activity. The photocatalysis performances of optimized Co–Mn–O nanostructures were systematically investigated through diverse operational parameters including effect of dye concentration, and catalyst dosage in the degradation of MO dye under visible light. The possible photocatalytic mechanism of the products was discussed based on experimental results.
2 Experimental
2.1 Materials
All of the chemical components used in this study including cobalt(II) nitrate hexahydrate (Co(NO3)2·6H2O), manganese(II) nitrate tetrahydrate (Mn(NO3)2·4H2O), glycine (C2H5NO2) and tetraethylenepentamine (TEPA), ethylenediaminetetraacetic acid (EDTA), benzoic acid (BA), 1,4-Benzoquinone (BQ) and sodium azide (NaN3), were obtained from Merck Company (Darmstadt, Germany) and applied without any purification. In addition, MO (C14H14N3NaO3S) employed as a contaminant, was obtained from Aldrich (Chemical Co., Milwaukee, WI, USA).
2.2 Characterization of (Co,Mn)(Co,Mn)2O4 nanostructures
2.2.1 X-ray diffraction (XRD)
The type of structure and the purity of the as-synthesized nanoparticles were confirmed by collecting XRD patterns with a diffractometer of the Philips company with X’PertPro monochromatized Cu Kα radiation (λ = 1.54 Å), in the 2θ values between 10 and 80 with the step size of 0.050. To analyze the acquired data for different samples, PANalytical HighScore Plus software was applied. By applying the Scherrer equation (Holzwarth and Gibson, 2011):
where β is the width of the observed diffraction peak at its half maximum intensity (FWHM), K is the shape factor, which takes a value of about 0.9, and λ is the X-ray wavelength (CuKα radiation, equals to 0.154 nm), the size of the crystallites for samples can be estimated.
2.2.1.1 Fourier-transform infrared (FT-IR) spectroscopy
For FT-IR analysis, a spectrophotometer (Shimadzu Varian 4300) was employed for studying the functional group composition of as-fabricated cobalt–manganese oxide spinel nanostructures in the range between 400 and 4000 cm−1 (Nicolet Magna-IR 550, Potassium bromide (KBr) pellets) at 4 cm−1 resolution. For this process, the sample were obtained by a mixture form 5 mg of (Co,Mn)(Co,Mn)2O4 and 95 mg of KBr and grounding by an agate mortar and subsequently pressing under a pressure of 2 tons with a pelletizer. Then, the resulting pellet was placed in the path of the IR beam.
2.2.1.2 Field emission scanning electron microscopy (FE-SEM)and energy dispersive X-ray (EDS) studies
An examination of the morphology and distribution of nanoparticles was conducted via FE-SEM (Sigma 300 Zeiss) using the secondary electron (SE) detector and an accelerating voltage of 10.0 kV. To support better imaging quality, samples for FE-SEM studies were covered by a 5-nm thick gold layer. EDS instrument was utilized to characterize the elemental constituents and purity of synthesized samples with 20 kV stimulating charge.
2.2.1.3 Transmission electron microscopy (TEM)
Additional morphological features for optimum samples were recorded by TEM measurement (Philips EM208) with an accelerating voltage of 200 kV.
2.2.1.4 Vibrating sample magnetometer (VSM)
The magnetic properties of the synthesized nanoparticles were studied using a magnetometer device produced by Meghnatis Daghigh Kavir Company from Iran.
2.2.1.5 Diffuse reflectance spectroscopy (DRS)
The UV–vis diffuse reflectance analysis was executed by utilizing a UV–vis spectrophotometer (Shimadzu, UV2550, Japan). The optical bandgap energy of (Co,Mn)(Co,Mn)2O4 semiconductor can be obtained from Tauc method by the following:
where light frequency, Planck constant, absorbance, material constant, and optical bandgap are referred to as υ, h, α, A, and B.G., respectively.
2.3 Synthesis of mixed spinel structure (Co,Mn)(Co,Mn)2O4 nanomaterial
For the synthesis of (Co, Mn)(Co, Mn)2O4 via simple sol–gel auto-combustion method, 1.0 g of Co(NO3)2·6H2O was dissolved in 10 mL of deionized water. Next, 2.05 g of Mn(NO3)2·4H2O was combined into the above mixture. Then, nitrate solution with 3:1 M ratio of Mn:Co was mixed using a magnetic stirrer for 5 min. After that, 0.82 g of glycine as an amino acid was slowly added to the container containing the metal ions. TEPA was used to adjust the pH of the medium to 10. The temperature of hot plate was set at 80 °C with continuous stirring (800 rpm). After 90 min, a red gel was obtained. The resulting gel was transferred to an oven to drying process. The dried gel was first placed in an air furnace at 450 °C for 3 h. The obtained powder underwent a temperature of 750 °C for duration of 5 h in the following step (Scheme 1). In Table 1, stoichiometric ratios and method information have been reported.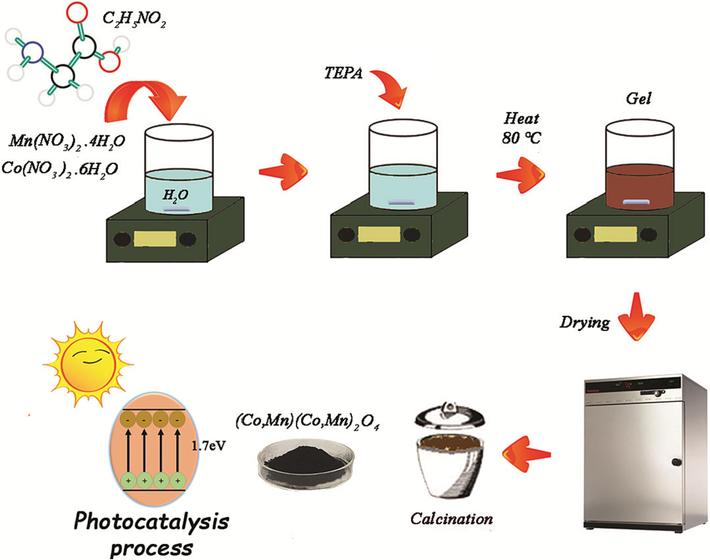
Schematic model of the formation for auto-comstion synthesized (Co, Mn)(Co, Mn)2O4 nanoparticles in the presence of glycine as fuel.
No.
Co:Mn ratio
Co: Glycine ratio
Phase purity
Average Crystallite size/XRD (nm)
Average Particle Size/SEM (nm)
1
2:3
1:2
(Co, Mn)(Co, Mn)2O4/MnO2
31.5
84.63
2
1:3
1:3
(Co, Mn)(Co, Mn)2O4
30
74.11
3
3:3
1:3
(Co, Mn)(Co, Mn)2O4/MnCo2O4
20
88.49
2.4 Photocatalysis process
0.04 g of the optimal samples was combined with 40 mL of the dye solution, having different concentrations of 5, 10, 15 and 20 mg/L (pH = 7), to initiate the photocatalysis process. The photocatalysis process began with the aeration of the solution for duration of 20 min. Following aeration, a sample was taken and then the sample container was exposed to visible light (20 cm away). During reaction, an Osram 400 W visible light (containing a wavelength in the range of 400 to 780 nm) was employed. Sampling was performed at 10 min intervals; however, initial sampling was performed after 20 min. All portions of the photocatalysis process were executed without external light and at ambient temperature. The absorbance was documented by means of a UV–Visible spectrophotometer in order to evaluate the discoloration of the dyes. The following formula was used to calculate the discoloration percentage:
Where A0 and At are the absorbance values of the dye solution at 0 and t min, respectively.
3 Result and discussion
3.1. Crystal structure
The crystal structure and formation of the intended structure of the synthesized samples were determined through X-ray diffraction analysis. Fig. 1 compares the resulting XRD patterns of mixed manganese cobalt oxides with a spinel structure synthesized at diverse conditions. As seen in Fig. 1(a-c), the (Co, Mn)(Co, Mn)2O4 structures (JCPDS PDF Card No. 00–018-0408) were formed as main phase for three different samples. Based on previous reported literatures (Lin et al., 2021; Lu et al., 2023), the presence of the (Co, Mn)(Co, Mn)2O4 phase is confirmed by the diffraction signals at 2θ = 18.2°, 29.3°, 31.3°, 32.8°, 36.4°, 38.7°, 44.8°, 50.5°, 51.5°, 54.4°, 56.6°, 59.0°, 60.5°, 65.1°, and 74.8° assigned to the (1 1 1), (2 0 2), (2 2 0), (1 1 3), (3 1 1), (0 0 4), (4 0 0), (2 2 4), (3 3 2), (2 0 5), (3 3 3), (5 1 1), (4 0 4), (4 4 0), and (5 3 3) planes, respectively. Noticeably, it could be observed that the molar ratio of Co and Mn precursors can effectively govern the phase purity and crystallographic information of the products. As shown in Fig. 1a, the XRD pattern confirms mixed crystal structures of the (Co, Mn)(Co, Mn)2O4 and MnO2 by adjusting the Co:Mn molar ratio of 2:3 in the precursor mixture. In sample No. 1, the corresponding reflection (1 1 0) of MnO2 (JCPDS PDF Card No. 00–050-0866) can be detected at 28.8°. However, when the molar ratio of Co to Mn reduced from 2:
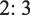 3 to 1
3 to 1
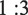 :3 within the mixture, highly crystallized phase of tetragonal (Co, Mn)(Co, Mn)2O4 was obtained without other impurities (Fig. 1b). Whereas, under the same reaction conditions of Co/Mn molar ratio (Fig. 1c), the diffraction peaks in the XRD pattern of samples can be indexed to the two phases of the (Co, Mn)(Co, Mn)2O4 and MnCo2O4 (JCPDS PDF Card No. 00–023-1237). In general, the XRD results showed that calcination at 750 °C helps increase the crystallinity of the desired compound. Furthermore, using the data collected from XRD analysis and using the Scherrer equation (Holzwarth and Gibson, 2011), the size of the crystallites for samples No. 1, 2, and 3 was calculated to be near 31.5, 30, and 20 nm, respectively.
:3 within the mixture, highly crystallized phase of tetragonal (Co, Mn)(Co, Mn)2O4 was obtained without other impurities (Fig. 1b). Whereas, under the same reaction conditions of Co/Mn molar ratio (Fig. 1c), the diffraction peaks in the XRD pattern of samples can be indexed to the two phases of the (Co, Mn)(Co, Mn)2O4 and MnCo2O4 (JCPDS PDF Card No. 00–023-1237). In general, the XRD results showed that calcination at 750 °C helps increase the crystallinity of the desired compound. Furthermore, using the data collected from XRD analysis and using the Scherrer equation (Holzwarth and Gibson, 2011), the size of the crystallites for samples No. 1, 2, and 3 was calculated to be near 31.5, 30, and 20 nm, respectively.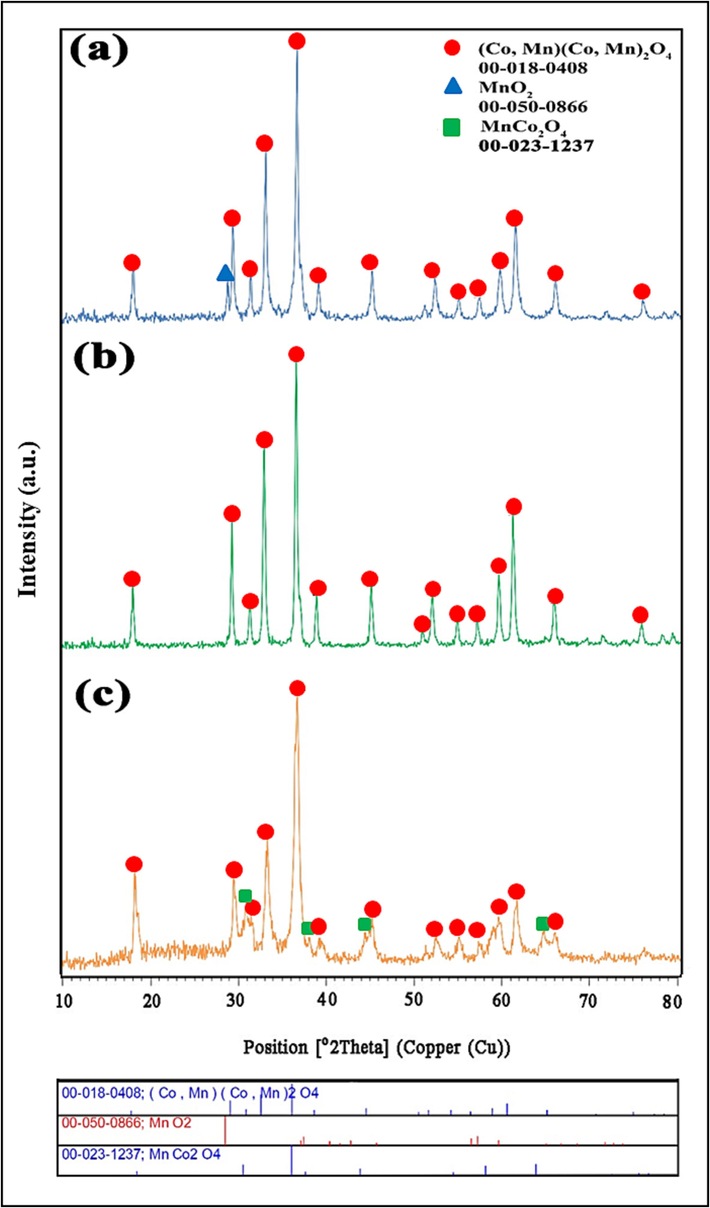
XRD patterns of Mn-Co spinel oxides synthesized in the different ratios of precursors (Co: Mn) (a) 2:3, (b) 1:3, and (c) 3:3.
3.2. Bond structure
A deeper understanding of the functional groups of organic compounds can be gained through FT-IR analysis. The FT-IR spectrum of the as-synthesized (Co, Mn)(Co, Mn)2O4 is illustrated in Figure S1. The absorption peaks at 3432 and 1640 cm−1 are related to the stretching and bending vibrational absorptions of O–H, respectively (Monsef and Salavati-Niasari, 2023). The peaks at 505 and 619 cm−1 corresponded to the stretching vibration of Co-O and Mn-O, respectively (Topka et al., 2021). Furthermore, an EDS analysis was implemented to guarantee the quality of the synthesized particles. The results of the EDS analysis are shown in Figure S2(a-c). As seen, EDS analyses confirms Co, Mn and O elements. The Au peak is observed in the EDS spectra because of the coating of the sample before SEM analysis.
3.3. Morphology studies
Considering that different conditions used in the synthesis of nanoparticles, SEM analysis was employed to check the morphology of the products. Fig. 2(a-i) shows the SEM images and particle size distribution plots obtained from samples No. 1, 2 and 3 in two magnifications. As it is clear from the images, the particles in all three samples are spherical, although this was predictable because of the use of glycine in all three samples. In sample No. 2 (Fig. 2 (d, e)), the particles are monodispersed and smaller than the other two samples. The reason for this occurrence can be attributed to the using the optimum amounts of glycine and metal precursors during the reaction. In samples No. 1 (Fig. 2 (a, b)) and 3 (Fig. 2 (g, h)), according to the images, the particles adhere together and are agglomerated. By examining the results of XRD and SEM analyses and the importance of particle size and morphology, sample No. 2 was selected as the optimal sample and other analyses were applied for this sample. The form of the particles was investigated in depth using TEM images. TEM images obtained from (Co, Mn)(Co, Mn)2O4 nanoparticles (sample No. 2) are shown in Fig. 3. The images revealed that the nanoparticles were sticking together and agglomerated.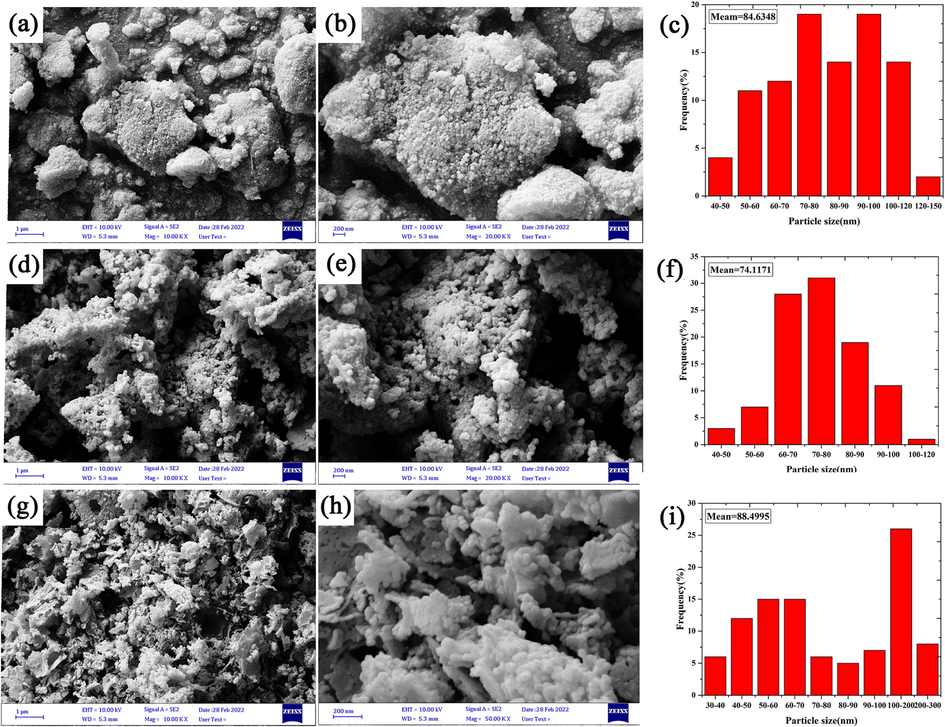
FE-SEM micrographs and particle size distribution histograms of Mn-Co spinel oxides synthesized in the different ratios of precursors (Co: Mn) (a-c) 2:3, (d-f) 1:3, and (g-i) 3:3.
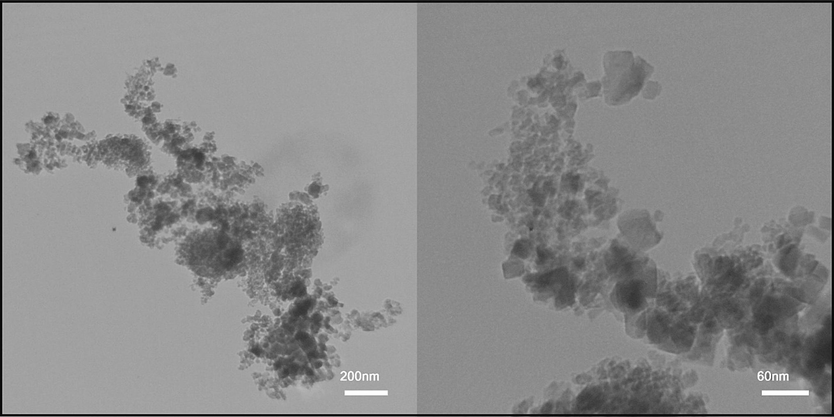
TEM images of the (Co, Mn)(Co, Mn)2O4 nanoparticles (sample No. 2).
3.4. Magnetic properties
The magnetic properties of nanomaterials, which can be understood through the use of VSM analysis, can be a valuable element in the study of photocatalytic virtues. The magnetic properties of catalysts can help in their better collection. The magnetization evaluation as a function of the applied field of the (Co, Mn)(Co, Mn)2O4 nanoparticles (sample No. 2) was discussed at 300 K and is shown in Fig. 4. The hysteresis loop with the coercive field of 100 Oe and remnant magnetization of 0.012 emu.g−1 for sample No. 2 was obtained. The results of the VSM study demonstrated dual magnetic behavior, ferromagnetic nature in low field and paramagnetic nature in up field, of the (Co, Mn)(Co, Mn)2O4 nanoparticles.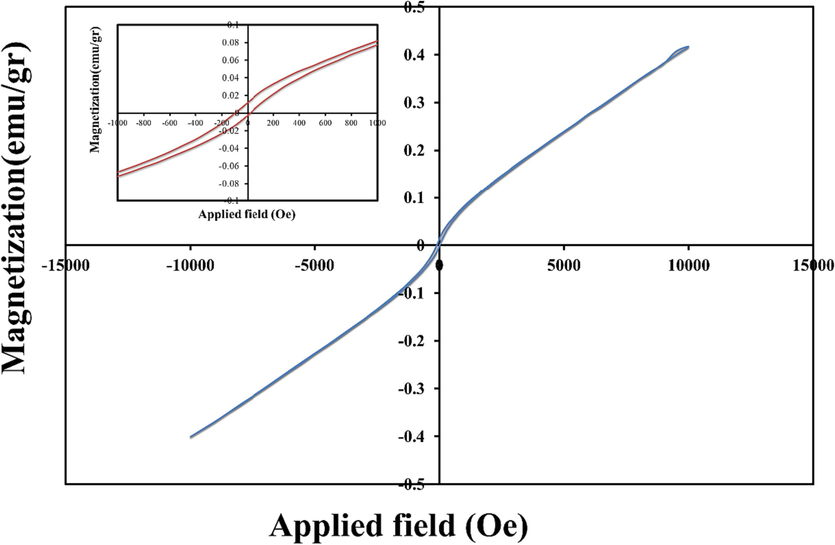
Magnetization versus applied magnetic field at room temperature (inset shows the magnified hysteresis loop) of the (Co, Mn)(Co, Mn)2O4 nanoparticles (sample No. 2).
3.5. Optical features
Understanding the optical properties of nanoparticles is an essential component when examining their photocatalytic activity. A complete survey of the optical properties of the (Co, Mn)(Co, Mn)2O4 nanoparticles (sample No. 2) was conducted through the utilization of DRS analysis and the relative results is illustrated in Figure S3. The absorption spectrum of optimized product depicts a light absorption edge in wider range from 200 to 700 nm. By using the Tauc’s equation (Tauc et al., 1966), the estimated band-gap for these nanoparticles was calculated to be about 1.7 eV which is showed in inset of Figure S3. In light of the above analysis, it recommended that the synthesized (Co, Mn)(Co, Mn)2O4 nanoparticles can be introduced as an effective and promising catalyst in the advanced photocatalysis applications.
3.6. Photoactivity of catalyst
Thoroughly researching the photocatalysis process necessitates carrying out several steps, such as assessing the effect of dye adsorption on the catalyst, the impact of pH and temperature, the impact of the photocatalyst, and so on (Boruah et al., 2019; Jamdar et al., 2024). Generally, to elucidate the photodegradation mechanism of a semiconductor, possibility of three ways such as (i) photolysis, (ii) dye photosensitization, and (iii) photocatalysis are examined (Jiang et al., 2015) (Scheme 2). It can be found that the negatively charged electron on the excited dye molecules is trapped by the oxygen molecules to create a singlet oxygen atom, which has strong oxidation capability to photolysis pure dye. An initial experiment was conducted to assess the effect of visible light on the discoloration of MO for 120 min at room temperature in order to evaluate the effect of light itself, namely photolysis. In this process, the dye was slightly degraded under visible (6.0 %) light. On the other hand, the organic dye photosensitization activity involves the structural stability of dye molecules during indirect process of degradation (Yu, C. et al., 2013), in which the adsorbed MO are stimulated to produce photoinduced electrons under energy of the irradiating light and then transfer them to the conduction band of the semiconductor, resulting in further oxidative transformations. Afterwards, the experiment was carried out in the dark to evaluate the adsorption of the dye onto the catalysts. The purpose of this process was to measure the amount of dye that was adsorbed onto (Co, Mn)(Co, Mn)2O4 nanoparticles. Fig. 5a describes the decolorization performances of MO solution with (Co, Mn)(Co, Mn)2O4 nano-catalyst under dark and visible light illumination. In the dark, the MO adsorption rate over synthesized (Co, Mn)(Co, Mn)2O4 nanoparticles showed the percentage of 15.28 % after 120 min. It is clear that the possibility of both process namely, photolysis and dye photosensitization in MO decomposition cannot be ignored. Subsequently, photocatalysis was implemented to eliminate MO from the aqueous solution under visible lights. As seen from the Fig. 5a, discoloration under light source showed a higher removal percentage (68.07 %). In the following, four different concentrations of MO (5, 10, 15, and 20 mg/L) were investigated under visible light in the photocatalysis process. The designed model for the pollutant’s concentration showed an effective role on the photodegradation yield. As it is clear in Fig. 5c, the photodegradation efficiencies of dye solution within 120 min of visible light irradiation were found to be 34.21 %, 58.52 %, 68.07 %, and 53.35 %, corresponding to 5, 10, 15, and 20 mg/L of pollutant, respectively. The highest discoloration percentage was achieved at the concentration of 15 mg/L. This findings exhibits that the saturation of the (Co, Mn)(Co, Mn)2O4 surface with excessive dye molecules can be the fact for low photocatalytic decolorization. However, when the initial contaminant concentration was less that the certain amount (5 and 10 mg/L), high relative number of reactive sites on the surface of the (Co, Mn)(Co, Mn)2O4 could provide the rapid degradation of MO (Cui et al., 2021). After employing the optimized concentration of contaminant solution, three different amounts of photocatalyst (0.02, 0.04, and 0.06 g) were used to check the effect of the catalyst loading on discoloration. As shown in Fig. 5e, while increasing the dosage of the (Co, Mn)(Co, Mn)2O4 nanophotocatalysts from 0.02 to 0.04 g, the change in photodegradation efficiency could show an increase rate form 32.77 % to 68.07 %, but precautions are needed. This enhancement is due to the combined effect of higher surface area and more active site of synthesized catalyst, which can easily absorb photons to promote the final efficiency (Cui et al., 2021). When the (Co, Mn)(Co, Mn)2O4 dosage exceeded the amount of 0.04 g, the removal rate of MO started to decline (43.57 %). In this case, reducing the penetration of light and light scattering may be reason for the limitation of the concentration of photo-generated carriers (Panahi et al., 2023a). To further elucidate the photocatalytic degradation kinetics of the resultant samples, the Langmuir-Hinshelwood first-order reaction kinetics model was adapted under a variety of photocatalytic reaction conditions, which is described below (Shanmugam et al., 2023):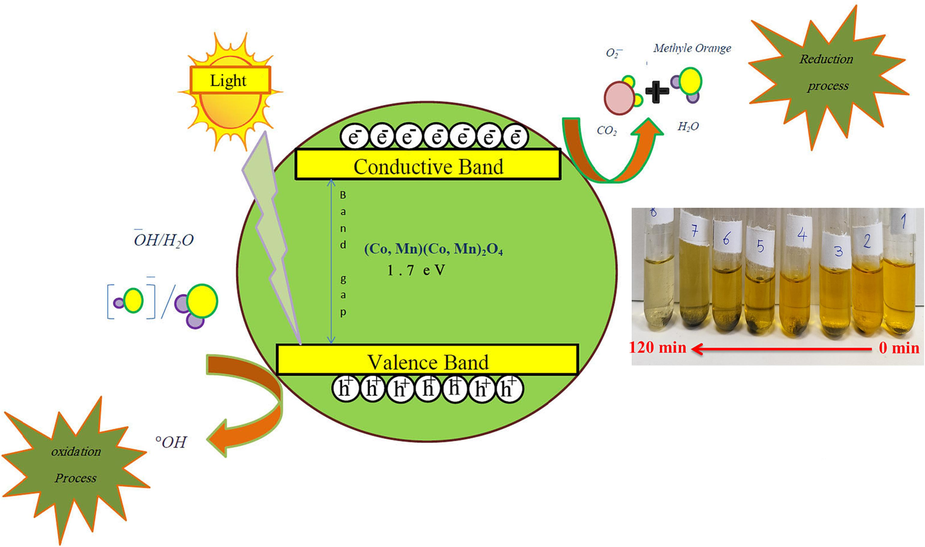
Schematic of charge transfer mechanism in (Co, Mn)(Co, Mn)2O4 nanoparticles under visible region.
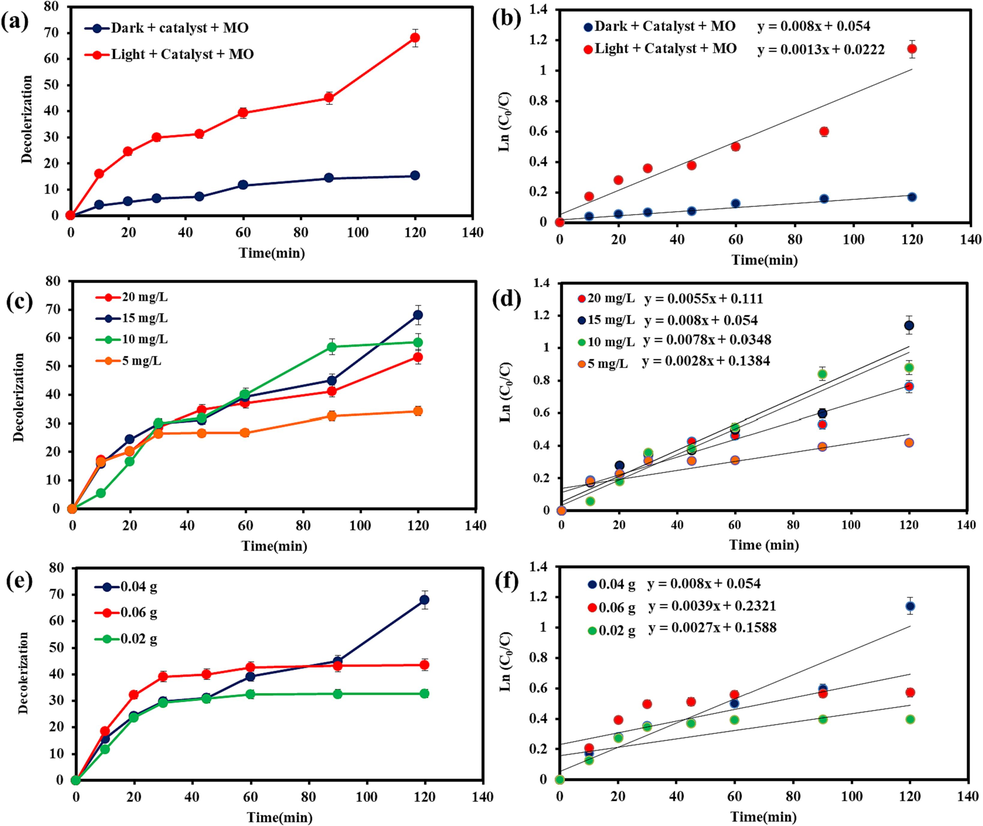
Photocatalytic activities and kinetic linear simulation plots of the (Co, Mn)(Co, Mn)2O4 nanoparticles (a, b) Investigating the MO removal in the dark and visible light after 120 min, (c, d) Investigating the effect of different concentrations of MO, and (e, f) Investigating the effect of different amounts of catalyst on the photocatalysis process under visible radiation.
Where C0 and C represent initial and final MO concentration in the solution at an initial irradiation time of t = 0 and at time of t, respectively, whereas k expresses the degradation rate constant. From the logarithmic analysis of the absorbance content vs the irradiation time (Fig. 5(b, d, f)), it can be observed that the maximum linear fitted value in the form of straight line confirm superior photocatalytic efficiency (0.008 min−1) for (Co,Mn)(Co,Mn)2O4 nanostructures at 15 mg/L of MO molecules with 0.04 g of catalyst. In Table 2, the comparison of photoactivity conditions of the as-obtained (Co,Mn)(Co,Mn)2O4 nanoparticle with previously reported papers has been summarized.
Photocatalyst
Method
Targeted Pollutants
Light source
Contact time
Percentage%
Ref.
Cu-MnO2
Chemical synthesis
Brilliant Green
visible light
180 min
73.1 %
(Mondal et al., 2019)
CoMn2O4
Co-precipitation
Congo Red
Direct sunlight
120 min
52.78 %
(Mark et al., 2021)
Co3O4
Co-precipitation
Methyl orange
visible light
120 min
64.4 %
(Zeid et al., 2023)
MnO2
Wet-chemical
Tetracycline
Visible light
60 min
36.41 %
(Du et al., 2021)
CoMoO4
Refluxing
Rhodamine B
Visible light
300 min
37.4 %
(Pirhashemi and Habibi-Yangjeh, 2018)
Mn0.2Co0.8Zr0.15Fe1.85O4
Co-precipitation
Alizarin Yellow R
Visible light
120 min
52.45 %
(Ahmed et al., 2021)
(Co, Mn)(Co, Mn)2O4
Combustion
Methyl orange
Visible light
120 min
68.07 %,
This work
To further distinguish the role of active free radicals in the photodegradation rate, the relative mechanism was analyzed via the trapping experiments of radicals. Different compounds are employed to ensnare free radicals (Munawar et al., 2020; Schneider et al., 2020). In this content, BQ, EDTA, BA, and NaN3 were used to remove superoxide (●O2−) radicals, holes (h+), hydroxyl (●OH) radicals and singlet oxygen (1O2), respectively. As shown in Fig. 6, the photocatalytic activity of the (Co, Mn)(Co, Mn)2O4 nanoparticles is decreased significantly in the presence of BQ. In addition, NaN3 also decreased the discoloration rate, but not intensively. Therefore, it can be concluded that ●O2− and 1O2 species have more important role in the photocatalytic discoloration of MO by using (Co, Mn)(Co, Mn)2O4 nanoparticles under visible radiation. The possible photocatalytic mechanism for removal of MO dye by Co–Mn–O nanostructures can be summarized as (Karami et al., 2022; Panahi et al., 2023b):
MO + hv → MO*(5)
MO*+(Co,Mn)(Co,Mn)2O4 → (Co,Mn)(Co,Mn)2O4-1 + MO+1(6)
(Co,Mn)(Co,Mn)2O4-1 + O2 → (Co,Mn)(Co,Mn)2O4 + O2•−(7)
(Co,Mn)(Co,Mn)2O4 + hv → (Co,Mn)(Co,Mn)2O4(hVB++eCB−)(8)
H2O + h+ → H++HO•(9)
OH−(ads) + h+ → HO•(10)
O2(ads) + e−+H+ → HO2•(11)
2H++O2•− → H2O2(12)
HO2•+H2O → H2O2 + HO•(13)
2HO2• → H2O2 + O2(14)
O2•−+HO2• → HO2−+O2(15)
HO2−+H+ → H2O2(16)
H2O2(ads) + e− → HO•+OH−(17)
H2O2 + O2•− → HO•+OH−+O2(18)
O2•−+h+ → O2(19)
2H2O + 2 h+ → H2O2 + 2H+(20)
H2O2(ads) + 2 h+ → O2 + 2H+(21)
H2O2(ads) + h+ → HO2•+H+(22)
HO2•+H2O2 → HO•+H2O + O2(23)
MO + OH• → Degradation products(e.g.,CO2,H2O,H2)(24)
MO + hVB+ → Oxidation products(25)
MO + eCB− → Reduction products(26)
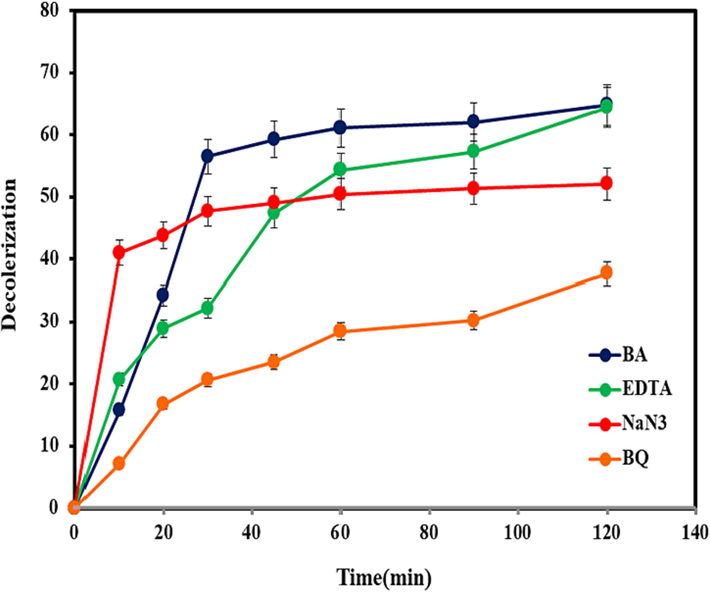
Photocatalytic activity of the (Co, Mn)(Co, Mn)2O4 nanoparticles (sample No. 2) in the presence of scavengers.
Fig. 7(a, b) illustrates the photo-stability and reusing of as-formed (Co, Mn)(Co, Mn)2O4 nanoparticles under visible light. After recycling process, Co-Mn-O powders were centrifuged, washed with distilled water, and then dried at 60 °C for 20 h. After that, the second run of photoactivity test was accomplished in the presence of previously used (Co, Mn)(Co, Mn)2O4 structures and fresh MO solution. As illustrated in Fig. 7a, the removal efficiency expresses 60.1 % after five repetitions of photodegradation performance. The XRD pattern of the reused (Co, Mn)(Co, Mn)2O4 nano-catalysts was carried out to test the impacts of light and high temperatures during drying on structural degradation. The diffraction peaks of sample in Fig. 7b confirm that the host composition is related to (Co, Mn)(Co, Mn)2O4 (JCPDS No. 00–018-0408, tetragonal structure).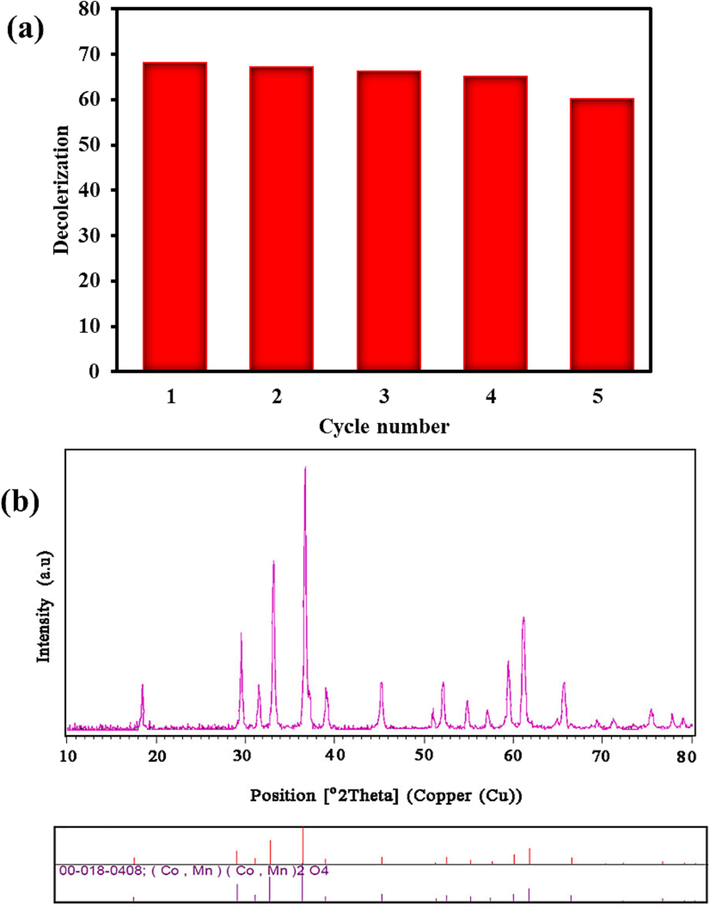
(a) Recycle efficiency and (b) XRD pattern of the (Co, Mn)(Co, Mn)2O4 after five cycles of photocatalytic reaction.
4 Conclusion
To conclude, the employing glycine as both fuel and capping agent in facile auto-combustion method enabled the successful synthesis of the (Co, Mn)(Co, Mn)2O4-based compounds. Several synthesis conditions, including modifications in the proportion of ingredients (Co:Mn) and the addition of glycine, were employed to create particles with desired size and shape. In accordance with XRD and FESEM analyses, the sample prepared with a ratio of 1 to 3 of Co:Mn precursors with no impurity was selected as the optimal sample (Sample No.2). Optical band gap of tetragonal (Co, Mn)(Co, Mn)2O4 phase was found to be 1.7 eV, which is suitable for visible-light-driven degradation of MO as pollutant model. The dye concentration and the amount of catalyst were examined in order to perform the photocatalysis test in the most optimal way. The photocatalysis test at a dye concentration of 15 mg/L in the presence of 0.04 g of the catalyst with 68.07 % discoloration was the best result after 120 min. Based on the kinetic model, the highest-fitted reaction rate constant was calculated to be 0.008 min−1. An investigation of the photocatalysis process was done with the utilization of scavengers during the photocatalysis test. The results showed that ●O2− and 1O2 species have important role in the discoloration of MO by using (Co, Mn)(Co, Mn)2O4 nanoparticles under visible radiation. The MO photodegradation yield of the host Co-Mn-O nanostructures depicted an imperceptible reduction (∼7.97 %) following the five successive cycles under visible region, suggesting broad potential possibilities of mixed spinel structure (Co,Mn)(Co,Mn)2O4 nanomaterial for environmental remediation applications. That is to say, these compounds have propitious ability to keep their catalytic activity as well as structural stability. Research on new methods and nanomaterials for the elimination of water impurities will be pursued.
CRediT authorship contribution statement
Ghazal Oroumi: Software, Investigation, Methodology, Formal analysis. Foroozan Samimi: Formal analysis, Data curation, Software. Makarim A. Mahdi: Writing – review & editing, Software, Data curation, Validation. Elmuez A. Dawi: Writing – review & editing, Software, Conceptualization. Masoud Salavati-Niasari: Software, Formal analysis, Methodology, Writing – review & editing, Writing – original draft, Conceptualization, Supervision, Project administration, Investigation, Data curation, Validation, Resources, Visualization, Funding acquisition.
Acknowledgements
Authors are grateful to the council of Iran National Science Foundation (97017837) and University of Kashan for supporting this work by Grant No. (159271/GO1).
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Nanofiltration membrane processes for water recycling, reuse and product recovery within various industries: A review. J. Water Process Eng.. 2022;45:102478
- [Google Scholar]
- Facile synthesis of Zr4+ substituted Mn0. 2Co0. 8Fe2− xO4 nanoparticles and their composites with reduced graphene oxide for enhanced photocatalytic performance under visible light irradiation. Synth. Met.. 2021;277:116766
- [Google Scholar]
- Aseel M. Aljeboree, R. A. Mohammed, M. Mahdi, Layth S. Jasim, A. Alkaim, Synthesis, Characterization of P(CH/AA-co-AM) and Adsorptive Removal of Pb (II) ions from Aqueous Solution: Thermodynamic Study. NeuroQuantology 19(7) (2021) 137-143. 10.14704/nq.2021.19.7.NQ21096.
- Photocatalytic activity improvement and application of UV-TiO2 photocatalysis in textile wastewater treatment: A review. J. Environ. Chem. Eng.. 2019;7(5):103248
- [Google Scholar]
- Synthesis of magnetic Fe3O4/ZnWO4 and Fe3O4/ZnWO4/CeVO4 nanoparticles: the photocatalytic effects on organic pollutants upon irradiation with UV-Vis light. Catalysts. 2020;10(5):494.
- [Google Scholar]
- Iron-based nanoparticles in wastewater treatment: A review on synthesis methods, applications, and removal mechanisms. J. Saudi Chem. Soc.. 2021;25(8):101280
- [Google Scholar]
- Synthesis of novel g-C3N4 microrods: A metal-free visible-light-driven photocatalyst. Mater. Sci. Energy Technol.. 2019;2(3):401-407.
- [Google Scholar]
- Synthesis of WO3· H2O spherical particles for efficient photocatalytic properties under visible light source. Mater. Sci. Energy Technol.. 2019;2(2):187-193.
- [Google Scholar]
- Enhanced photocatalytic degradation of dye contaminants with TiO2 immobilized on ZSM-5 zeolite modified with nickel nanoparticles. J. Clean. Prod.. 2021;280:124518
- [Google Scholar]
- Synthesis and characterization of Sm2 (MoO4) 3, Sm2 (MoO4) 3/GO and Sm2 (MoO4) 3/C3N4 nanostructures for improved photocatalytic performance and their anti-cancer the MCF-7 cells. Polyhedron. 2020;180:114424
- [Google Scholar]
- Flux growth and characterization of Ti-and Ni-doped forsterite single crystals. Crystal Research and Technology: J. Exp. Ind. Crystallogr.. 2009;44(5):463-468.
- [Google Scholar]
- Recent Advances in Copper-Based Materials for Sustainable Environmental Applications. Sustain. Chem.. 2023;4(3):246-271.
- [Google Scholar]
- Enhanced photocatalysis and bacterial inhibition in Nb 2 O 5 via versatile doping with metals (Sr, Y, Zr, and Ag): a critical assessment. Nanoscale Adv.. 2019;1(7):2748-2760.
- [Google Scholar]
- Synthesis of a novel Type-II In2S3/Bi2MoO6 heterojunction photocatalyst: Excellent photocatalytic performance and degradation mechanism for Rhodamine B. Sep. Purif. Technol.. 2021;255:117758
- [Google Scholar]
- Construction of Z-scheme g-C3N4/MnO2/GO ternary photocatalyst with enhanced photodegradation ability of tetracycline hydrochloride under visible light radiation. Environ. Res.. 2021;200:111427
- [Google Scholar]
- Selective spectrophotometric determination of 4-amino antipyrine antibiotics in pure forms and their pharmaceutical formulations. International Journal of Drug Delivery Technology. 2021;11(2):371-375.
- [Google Scholar]
- A review of biological drinking water treatment technologies for contaminants removal from polluted water resources. J. Water Process Eng.. 2020;33:101035
- [Google Scholar]
- Holzwarth, U., Gibson, N., 2011. The Scherrer equation versus the'Debye-Scherrer equation'. Nat. Nanotechnol. 6(9), 534-534.
- Adsorption of polluted dyes from water by transition metal oxides: A review. Appl. Surf. Sci. Adv.. 2023;15:100395
- [Google Scholar]
- Recent developments in industrial organic degradation via semiconductor heterojunctions and the parameters affecting the photocatalytic process: A review study. J. Water Process Eng.. 2022;47:102671
- [Google Scholar]
- Unraveling the potential of sonochemically achieved DyMnO3/Dy2O3 nanocomposites as highly efficient visible-light-driven photocatalysts in decolorization of organic contamination. Ecotoxicol. Environ. Saf.. 2024;269:115801
- [Google Scholar]
- Controlled hydrothermal synthesis of BiOxCly/BiOmIn composites exhibiting visible-light photocatalytic degradation of crystal violet. J. Hazard. Mater.. 2015;283:787-805.
- [Google Scholar]
- Removal of heavy metals from water sources in the developing world using low-cost materials: A review. Chemosphere. 2019;229:142-159.
- [Google Scholar]
- UV-light-induced photocatalytic response of Pechini sol–gel synthesized erbium vanadate nanostructures toward degradation of colored pollutants. Environ. Technol. Innov.. 2022;28:102947
- [Google Scholar]
- Recent advances in semiconductor metal oxides with enhanced methods for solar photocatalytic applications. J. Alloys Compd.. 2020;828:154281
- [Google Scholar]
- CoMn2O4: A new spinel photocatalyst for hydrogen photo-generation under visible light irradiation. J. Photochem. Photobiol. a: Chem.. 2024;446:115170
- [Google Scholar]
- CO hydrogenation over nanometer spinel-type Co/Mn complex oxides prepared by sol-gel method. Appl. Catal. a: Gen.. 1998;166(1):191-199.
- [Google Scholar]
- Oxygen vacancy engineering of carbon-encapsulated (Co, Mn)(Co, Mn) 2O4 from metal-organic framework towards boosted lithium storage. Chem. Eng. J.. 2021;425:130661
- [Google Scholar]
- Properties of g-C3N4 modified mixed spinel structure (Co, Mn)(Co, Mn) 2O4 cathode material for supercapacitor. J. Solid State Chem.. 2023;322:123973
- [Google Scholar]
- Makarim A. Mahdi , Aseel M. Aljeboree , Layth S. Jasim , Ayad F. Alkaim, Synthesis, Characterization and Adsorption Studies of a Graphene Oxide/Polyacrylic Acid Nanocomposite Hydrogel. NeuroQuantology, 19 (9) (2021) 46-54. 10.14704/nq.2021.19.9.NQ21136.
- Mark, J.A.M., Venkatachalam, A., Pramothkumar, A., Senthilkumar, N., Jothivenkatachalam, K., prince Jesuraj, J., 2021. Investigation on structural, optical and photocatalytic activity of CoMn2O4 nanoparticles prepared via simple co-precipitation method. Phys. B: Condens. Matter. 601, 412349.
- Marsooli, M.A., Rahimi-Nasrabadi, M., Fasihi-Ramandi, M., Adib, K., Eghbali-Arani, M., Ahmadi, F., Sohouli, E., Sobhani nasab, A., Mirhosseini, S.A., Gangali, M.R., 2020. Preparation of Fe3O4/SiO2/TiO2/CeVO4 nanocomposites: investigation of photocatalytic effects on organic pollutants, bacterial environments, and new potential therapeutic candidate against cancer cells. Front. pharmacol. 11, 192.
- Mechanochemical processing of Mn and Co oxides: an alternative way to synthesize mixed spinels for protective coating. J. Am. Ceram. Soc.. 2016;99(1):308-314.
- [Google Scholar]
- Nanoheterojunctions MnCo2O4 nanoparticles anchored on mesoporous TiO2 networks for superior photocatalytic ability. Mater. Res. Bull.. 2023;167:112439
- [Google Scholar]
- Size engineered Cu-doped α-MnO2 nanoparticles for exaggerated photocatalytic activity and energy storage application. Mater. Res. Bull.. 2019;115:159-169.
- [Google Scholar]
- Fundamental understanding on cyclability behaviors of ammonium vanadate nanoarchitectures as cathode materials for Li-ion batteries: A comparative insight of dual modification of precursor and morphology. J. Energy Storage. 2023;74:109395
- [Google Scholar]
- Novel tri-phase heterostructured ZnO–Yb2O3–Pr2O3 nanocomposite; structural, optical, photocatalytic and antibacterial studies. Ceram. Int.. 2020;46(8):11101-11114.
- [Google Scholar]
- Multifunctional La10Si6O27: Tb3+ tailored material for photoluminescence, photocatalysis and electrochemical sensing applications. Appl. Surf. Sci. Adv.. 2023;14:100392
- [Google Scholar]
- Dual S-scheme heterojunction g-C3N4/Bi2S3/CuS composite with enhanced photocatalytic activity for methyl orange degradation. Inorg. Chem. Commun.. 2023;155:111075
- [Google Scholar]
- Simple sonochemical synthesis, characterization of TmVO4 nanostructure in the presence of Schiff-base ligands and investigation of its potential in the removal of toxic dyes. Ultrason. Sonochem.. 2023;95:106362
- [Google Scholar]
- TmVO4/Fe2O3 nanocomposites: Sonochemical synthesis, characterization, and investigation of photocatalytic activity. Int. J. Hydrog. Energy. 2023;48(10):3916-3930.
- [Google Scholar]
- Facile fabrication of novel ZnO/CoMoO4 nanocomposites: Highly efficient visible-light-responsive photocatalysts in degradations of different contaminants. J. Photochem. Photobiol. a: Chem.. 2018;363:31-43.
- [Google Scholar]
- Preparation of Co 2 TiO 4/CoTiO 3/Polyaniline ternary nano-hybrids for enhanced destruction of agriculture poison and organic dyes under visible-light irradiation. J. Mater. Sci: Mater. Electron.. 2019;30:15854-15868.
- [Google Scholar]
- Recent advances of photocatalytic application in water treatment: A review. Nanomaterials. 2021;11(7):1804.
- [Google Scholar]
- A facile preparation of ZnFe 2 O 4–CuO-N/B/RGO and ZnFe 2 O 4–CuO–C 3 N 4 ternary heterojunction nanophotocatalyst: characterization, biocompatibility, photo-Fenton-like degradation of MO and magnetic properties. J. Mater. Sci: Mater. Electron.. 2021;32:5457-5472.
- [Google Scholar]
- Use of scavenger agents in heterogeneous photocatalysis: truths, half-truths, and misinterpretations. Phys. Chem. Chem. Phys.. 2020;22(27):15723-15733.
- [Google Scholar]
- Heterogeneous form of poly (4-vinyl pyridine) beads based dendrimer stabilized Ag, Au and PdNPs catalyst for reduction of trypan blue. Mater. Sci. Energy Technol.. 2019;2(3):532-542.
- [Google Scholar]
- Visible-light induced photocatalytic removal of methylene blue dye by copper oxide decorated zinc oxide nanorods. Mater. Sci. Energy Technol.. 2023;6:359-367.
- [Google Scholar]
- Engineering nanomaterials for water and wastewater treatment: review of classifications, properties and applications. New J. Chem.. 2019;43(21):7902-7927.
- [Google Scholar]
- Taifi A., Alkadir O. K. A., Oda A. A., Aljeboree A. M., Al Bayaa A. L., Alkaim A. F., Abed S. A. “Biosorption by Environmental, Natural and Acid-Activated Orange Peels as Low-Cost Aadsorbent: Optimization of Disperse Blue 183 as a Model”;(2022) IOP Conference Series: Earth and Environmental Science, 1029 (1), art. no. 012009. DOI: 10.1088/1755-1315/1029/1/012009.
- Scalable integration of highly uniform MnxCo3− xO4 nanosheet array onto ceramic monolithic substrates for low-temperature propane oxidation. ChemCatChem. 2017;9(21):4112-4119.
- [Google Scholar]
- Optical properties and electronic structure of amorphous germanium. Phys. Status Solidi B. 1966;15(2):627-637.
- [Google Scholar]
- Hydrothermal deposition as a novel method for the preparation of Co–Mn mixed oxide catalysts supported on stainless steel meshes: application to VOC oxidation. Environ. Sci. Pollut. Res. 2021:1-12.
- [Google Scholar]
- Perovskite oxide based electrodes for high-performance photoelectrochemical water splitting. Angew. Chem. Int. Ed.. 2020;59(1):136-152.
- [Google Scholar]
- Role of nanomaterials in the treatment of wastewater: a review. Water. 2020;12(2):495.
- [Google Scholar]
- Stable Au25 (SR) 18/TiO2 composite nanostructure with enhanced visible light photocatalytic activity. J. Phys. Chem. Lett.. 2013;4(17):2847-2852.
- [Google Scholar]
- Controlled synthesis of hierarchical Co x Mn 3–x O 4 array micro-/nanostructures with tunable morphology and composition as integrated electrodes for lithium-ion batteries. Energy Environ. Sci.. 2013;6(9):2664-2671.
- [Google Scholar]
- A comparative study of single and bi-doped Co3O4 nanocatalysts for the photodegradation of methyl orange dye. J. Mol. Struct.. 2023;1293:136203
- [Google Scholar]
- Spinel Mn–Co oxide in N-doped carbon nanotubes as a bifunctional electrocatalyst synthesized by oxidative cutting. J. Am. Chem. Soc.. 2014;136(21):7551-7554.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2023.105588.
Appendix A
Supplementary material
The following are the Supplementary data to this article:Supplementary data 1
Supplementary data 1







