Translate this page into:
Iron oxide nanopowder based electrochemical sensor for sensitive voltammetric quantification of midodrine
⁎Corresponding author at: Department of Chemistry, College of Science, Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia. N_elmetwaly00@yahoo.com (Nashwa M. El-Metwaly)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
The present work describes the fabrication and electrochemical characterization of a novel iron oxide nanopowder-based carbon paste electrodes (CPE) for the sensitive voltammetric quantification of midodrine (MD) in pharmaceutical formulations and biological fluids. At the electrode surface, the postulated reaction mechanism involved the irreversible oxidation of the terminal amino group of midodrine through the participation of two-electron as elucidated by the sweep rate studies and molecular orbital calculations. Calibration graphs were linear in the MD concentration range from 0.16 × 10-6 to 2.12 × 10-6 mol L-1 with LOD 0.04 × 10-6 mol L-1. Prolonged stability with an operational lifetime of more than 4 months and high measurement reproducibility were the promising futures of the fabricated sensors. The proposed electroanalytical approach was free from interference and suggested for assaying of midodrine in its pharmaceutical formulations and biological samples with recovery agreed with the official methods.
Keywords
Midodrine
Iron oxide nanoparticles
Carbon paste sensor
Differential pulse voltammetry
1 Introduction
Midodrine (MD, 2-amino N-[2-(2,5-dimethoxyphenyl)-2-hydroxy-ethyl]-acetamide) is an α- adrenergic agonist which induces arterial and venous vasoconstriction process causing constriction of the blood vessels and raising blood pressure accompanied by the case of reduction in the heart rate. Midodrine is administrated for the treatment of Cirrhosis, Hepatorenal syndrome, and orthostatic hypotension (O’Neil et al. 2006; Gilden, 2012; Krishna et al., 2018; McTavish & Goa, 1989; Pittner, 1983).
Midodrine is a pro-drug of desglymidodrine (DMAE) which was synthesized through the attachment of glycine to the functional amine of DMAE. Thus, midodrine is converted primarily into its active metabolite “desglymidodrine” by H+-coupled peptide transporter 1 (hPEPT1) which activates the α-adrenergic receptors within the blood vessels, thereby, increasing the vascular tone and correspondingly blood pressure (Mcclellan et al., 1998; Rautio et al., 2008; Yoshida et al., 2003).
Few chromatographic methods were reported in the literature for midodrine analysis (Krishna et al., 2018). High performance liquid chromatographic (HPLC) with UV detection was developed for estimation of MD (Barth et al., 2013; Dhote et al., 2018). Derivatization of MD with 3, 4-dihydro-6,7-dimethoxy-4-methyl-3-oxoquinoxaline-2-carbonyl forms a fluorescent species was introduced as a base for fluorometric HPLC method for detection of midodrine in human plasma (Yoshida et al., 2003). Stability- indicating reversed phase chromatographic approaches were reported for detection of the midodrine in presence of its degradation products (Jain et al., 2016a; Salunke & Damle, 2016; Byran et al., 2021). Two spectrophotometric methods based on measuring of MD absorption at 302.0 nm or its ruhemann purple colored complex with ninhydrin at 576.5 nm were validated for the quantification of MD in bulk and pharmaceutical preparations (Jain et al., 2016b; El-Gindy et al., 2008). Consumption of the toxic organic solvents and the tedious sample preparation protocols using expensive instrumentation obstacles the application of chromatographic and spectrophotometric methods for routine analysis of MD in pharmaceutical formulations and biological samples.
Electroanalytical techniques accompanied with electrochemical sensors are capable for the detection of pharmaceutical and biologically active compounds with improved sensitivity and selectivity with simple pretreatment steps and instrumentation requirements. Electroanalytical sensors were reported as efficient analytical tools for routine evaluating pharmaceutically active compounds in bulk formulations and biological fluids (Ozkan, 2012; Ozkan et al., 2015; Ziyatdinova et al., 2015; Angnes, 2015; Siddiqui et al., 2017). Polyvinyl chloride midodrine potentiometric sensors were constructed with the incorporation of phosphotungstate as cation exchanger and β-cyclodextrin as molecular recognition element (Elzanfaly et al., 2013). The cited sensors showed fast and stable response with cationic Nernstian compliance within the midodrine concentration range from 5 × 10-5 to 1 × 10-1 mol L-1. More recently, surface modification of carbon paste electrodes with β-cyclodextrin/gold nanoparticle offered electrocatalytic activity toward MD oxidation at pH 3.0. The modified sensor was introduced for differential pulse voltammetric determination of midodrine within the concentration range from 3.0 × 10-6 to 3.2 × 10-4 mol L-1 and LOD 5.17 × 10-7 mol L-1 (Salem, 2020).
Within the last three decades carbon pate electrodes (CPEs) were motivated as a promising working electrode with the possibility of bulk/surface modification and simple regeneration of the electrode surface compared with other solid metallic or carbonaceous electrodes (Švancara et al., 2001; Švancara et al., 2009; Svancara et al., 2019). CPEs are heterogeneous electrodes fabricated through intimate blending of the synthetic carbon powder with a suitable liquid binder where the resulting paste was packed in a piston like holder (Svancara et al., 2019). Carbon paste electrodes found sounding publications for determination of many pharmaceutical and biologically active molecules (Švancara et al., 2001; Švancara et al., 2009; Svancara et al., 2019; Ghanei-Motlagh & Baghayeri 2020; Ghanei-Motlagh et al., 2019; Baghayeri, et al., 2017).
Metal oxides nanostructure, with their unique futures and electrocatalytic activity towards the oxidation of the target analytes were reported for developing of sensitive voltammetric sensors for quantification of many pharmaceutical and biologically active compounds (Agnihotri et al., 2021; Qian et al., 2021). The present research study aimed to introduce iron oxide nanoparticles based sensors for differential pulse voltammetric determination of midodrine with improved sensitivity and selectivity. The achieved enhanced performance introduced the proposed analytical protocol for assaying of MD in pharmaceutical and biological samples with acceptable recoveries compared to the spectrophotometric methods.
2 Experimental
2.1 Drug and chemicals
The standard midodrine hydrochloride sample [C12H19N2O4Cl, 290.78 g mol−1, purity 99.5 ± 1.1%] was supplied by Nile Company for Pharmaceuticals and Chemical Industries (Cairo, Egypt). Desglymidodrine was prepared through acidic hydrolysis of midodrine in HCl as described in details elsewhere (Quaglia et al., 2004; Salunke & Damle, 2016). Components of the carbon paste working electrode were paraffin oil (Merck) and graphite sheet (Aldrich). Multiwall carbon nanotubes (MWCNTs, Sigma), iron oxide nanopowder (<50 nm), copper oxide, and zinc oxide nanopowder were supplied from Sigma-Aldrich.
2.2 Sample analysis
Midodrine tablets (assigned to 2.5 mg midodrine/tablet, Nile Company for Pharmaceuticals and Chemical Industries) were purchased from local market. Two tablets were weighed, ground, dissolved in water and filtered.
The fresh serum samples from healthy volunteers were centrifuged, and spiked with different aliquots of MD standard solution. Spiked samples were treated with acetonitrile to precipitate protein, diluted with water, vortexed and centrifuged for removal of residual protein. Urine samples were spiked with MD standard solution, treated with methanol, vortexed and centrifuged for removal of the protein. The MD contents in sample solutions were assayed voltammetrically and spectrophometrically at 290 nm (Salunke & Damle, 2016) after suitable dilution with water.
2.3 Apparatus and working electrodes
Metrohm voltammetric analyzer (797 VA) accompanied with platinum wire as an auxiliary electrode and double junction silver/silver chloride reference electrode was established for voltammetric measurements. The working CPE was fabricated through mixing of paraffin oil (80 µL), 0.19 g of graphite powder and 10 mg iron oxide nanoparticles. The produced paste was packed into a piston like Teflon holder as described in details elsewhere (Svancara et al., 2019). The electrode surface was regenerated via polishing with a wet filter paper.
2.4 Procedures
To the supporting electrolyte adjusted to pH 8.0, different aliquots of the midodrine stock solution were added and the differential pulse voltammograms were recorded at the optimized electroanalytical parameters as follows: deposition potential of −0.25 V for 60 s; pulse height 50 mV; pulse width 100 ms; pulse duration 40 ms and scan rate 40 mV s−1. Eventually, calibration graphs were illustrated by plotting the recorded peak heights (µA) versus the MD concentration in micromolar range.
3 Results and discussion
3.1 Electrochemical oxidation of MD
On the bare carbon paste electrode, midodrine showed an anodic oxidation peak at about 1.011 V with no reduction peak in the opposite cathodic direction suggesting the irreversibility of the midodrine oxidation process (Fig. 1). To improve the sensitivity of the bare electrodes, different nanostructures were incorporated with different nanostructures during mixing of the carbon paste components (Svancara et al., 2019). It is well established that nanostructured metal oxides showed electrocatalytic effect towards oxidation of target analyte which in turn improve the sensor performance (Agnihotri et al., 2021; Qian et al., 2021; Kranz, 2019; Sevinc & Ozkan, 2018). The electrochemical behavior of midodrine was explored in the presence of iron oxide, zinc oxide and copper oxide nanopowder in addition to MWCNTs (Fig. 1). MWCNTs showed 5 fold increase of the peak current which may be explained on the basis of the enhancement of the electroactive surface area and facilitation of the electron transfer process. Among the tested metal oxides nanopowder, incorporation of iron oxide nanopowder amplified the peak current by about 10 folds compared to the bare electrode with a noticeable shifting of the MD oxidation potential towards the negative direction by about 25 mV due to its electrocatalytic effect towards midodrine oxidation. Moreover, the electroactive surface area of the bulk modified senors were etimated by recording the cyclic voltammogrames of ferricyanide at different sweep rates ranged from 0.010 to 0.100 Vs−1 following Randles-Sevik equation (Zhang & Wang, 2000). The estimated surface area were 0.027, 0.162, 0.075, 0.066 and 0.209 cm2 for CPE, MWCNTs/CPE, CuO/CPE, ZnO/CPE and FeO/CPE, respectively.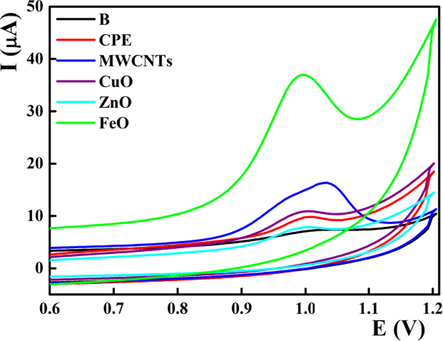
Electrochemical oxidation of 1.6 × 10-6 mol L-1 midodrine at different nanostructures based carbon paste electrodes at pH 8 with scan rate 0.040 V s−1.
3.2 Influence of pH
Midodrine showed an apparent pKa value of 7.8, therefore its electrochemical oxidation will be pH dependent. The peak heights increased gradually with the pH value to reach its maximum value at pH 8.0 (Fig. 2 a, b). Moreover, the peak potential was shifted towards more positive potential at lower pH value confirming the rule of proton in the midodrine oxidation. The recorded sub-Nernstian slope value in the pH range from 4.0 to 9.0 (EV = 1.1130 – 0.0211 [pH], r = 0.9937) suggested the unequal numbers of protons and electrons involved in the electrooxidation of midodrine.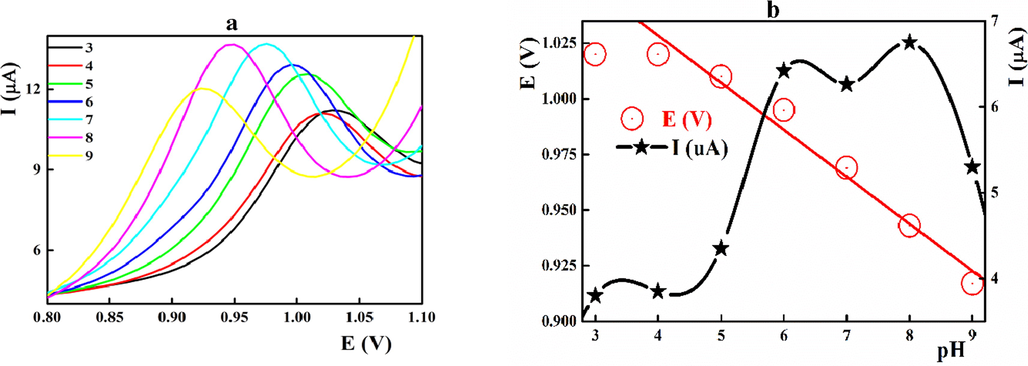
a) Effect of pH on the differential pulse voltammograms of 1.6 × 10-6 mol L-1 midodrine at different pH values; b) peak current and potential against the pH value. Scan rate 0.040 V s−1.
3.3 Effect of the sweep rate
Cyclic voltammograms of midodrine were scanned at different sweep rates to postulate its oxidation mechanism (Fig. 3a). The peak height was improved linearly (r = 0.99225) with the scan rate as illustrated in Fig. 3 b. The linear relationship between log value of the peak current (log I(µA)) and the log value of the san rate (log ν (Vs−1)) showed a slope value of 0.9527 suggesting the adsorption-controlled reaction mechanism at the electrode surface (Fig. 3 c). In addition, the peak potential was shifted to the positive direction with the sweep rate (Fig. 3d) sustaining the irreversibility of MD oxidation at the electrode surface (Zhang & Wang, 2000; Elgrishi et al., 2018). The number of electrons participated in the oxidation process was estimated from the linear relationship between the peak potential and the log υ was (EV = 1.0659 + 0.0561 [log (ʋ/Vs−1)], r = 0.9935) and found to be 2.207 (Laviron, 1972).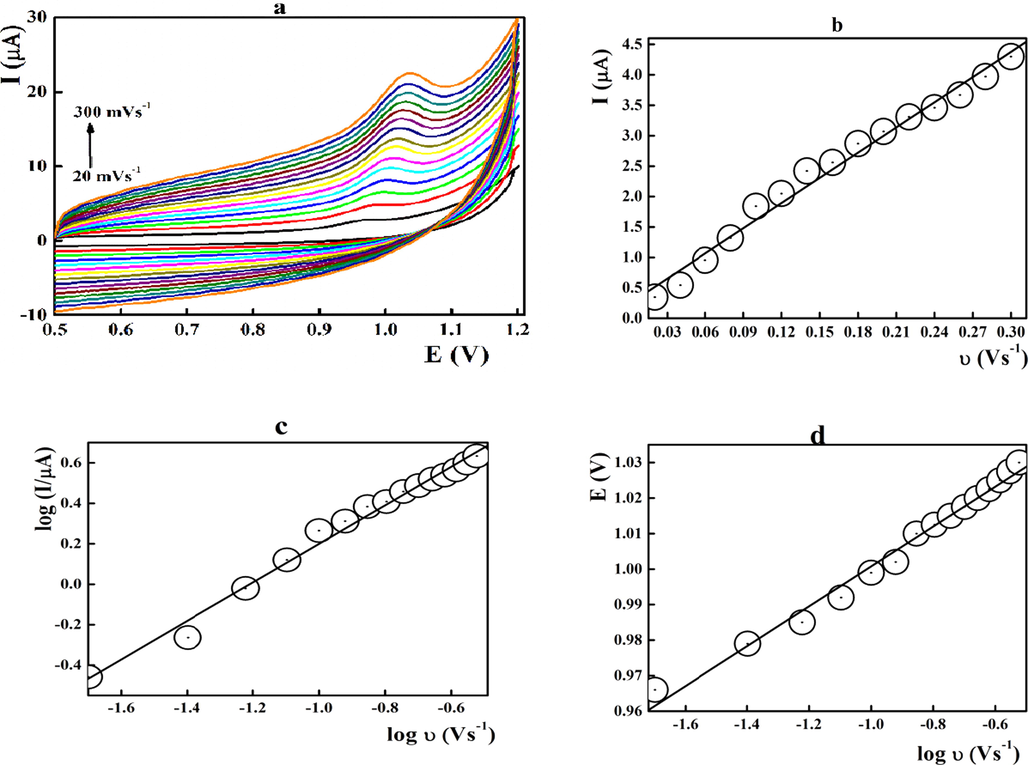
Voltammetric behaviour of 1.6 × 10-6 mol L-1 midodrine at the FeO/CPE surface performed at different sweep rates at pH 8.0.
Based on the adsorption-controlled reaction mechanism, the influence of both deposition potential and time was checked. The data achieved (S1) revealed the selection of −0.25 V as the optimum deposition potential for 60 s as deposition time.
Based on the molecular orbital calculations (Jouikov & Simonet, 2007) (S2), the irreversible electrochemical oxidation of midodrine undergoes through oxidation of the terminal amino group (nitrogen atom 18) which possess the highest electron density accompanied with the transferring of two electrons (scheme 1). The suggested mechanism disagrees with that reported based on the oxidation of the terminal OH group at pH 3.0 on the β-CD/Au/CPE (Salem, 2020).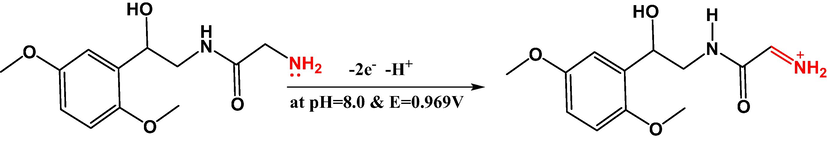
Electrochemical oxidation of midodrine at the FeO modified electrode at pH 8.
3.4 Analytical characterizations
At the optimum measuring conditions, successive aliquots of the midodrine stock solution were added to the measuring cell at pH 8.0 covering a final midodrine concentration range from 0.16 × 10-6 to 2.12 × 10-6 mol L-1. The recorded peak heights were plotted against midodrine concentrations with a linear relationship (r = 0.9997) and LOD of 0.04 × 10-6 mol L-1 (Fig. 4 & Table 1). The achieved low standard deviation and high correlation coefficient values confirms the applicability of the fabricated sensors for the determination of midodrine within the selected concentration range. Compared with the midodrine sensor based on gold nanoparticles/β-cyclodextrin composite (Salem, 2020), the presented sensor showed about 25 fold improved sensitivity indicated by the LOD value and optimal working at pH 8.0 near the biological value. Moreover, according to the modification protocol through bulk modification with iron oxide, regeneration and obtaining a new working surface can be performed through polishing with a wet filter paper.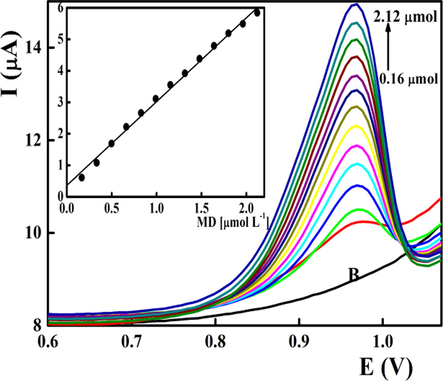
Differential pulse voltammetric determination of midodrine in BR buffer pH 8.0 applying FeO/CPE. Scan rate was 0.040 V s−1.
Parameters
pH
8.0
E (V)
0.969
Linear range (mol L-1)
0.16 × 10-6 − 2.12 × 10-6
Slope (μAcm−2)
2.52
S slope (μAcm−2)
0.02
Intercept (μA L-1 mol)
0.24
S intercept (μA L-1 mol)
0.03
R
0.9997
LOD (mol L-1)
0.04 × 10-6
LOQ (mol L-1)
0.12 × 10-6
N
20
RSD %
0.94
Repeatability of the peak current
0.59
Reproducibility of the peak current
1. 14
Repeatability of the peak potential
1.05
Reproducibility of the peak potential
0.861
Intra-day repeatability
0.66
Inter-day repeatability
1.20
Electrode stability after 4 weeks
0.712
3.5 Reproducibility of measurement and operational lifetime
The ease of modification and regeneration of the carbon paste electrode surface represents one of the promising futures of carbon paste electrodes (Svancara et al., 2019). Bulk modification mode with iron oxidation offers a prolonged operational lifetime and reproducibility of measurements. This regeneration protocol offers successful recovering of the sensor performance with removal of the memory effect rather than the surface modification mode. The operational lifetime was estimated by recording the voltammograms of 1.6 × 10-6 mol L-1 midodrine after different storage periods at 4 °C. The recorded peak current was stable within 3 months of consecutive measurements with RSD 0.712 %. Prolonged storage resulted in deterioration of the sensor performance by about 5.0 %. Successive 7 replicate measurements for 1.6 × 10-6 mol L-1 of midodrine showed limited RSD value of 0.66 %.
3.6 Specificity and interference study
To evaluate stability, midodrine hydrochloride was subjected to force degradation under the condition of acid, base and neutral hydrolysis following the ICH guidelines (Elzanfaly et al., 2013; Sattar et al., 2013; ICH Q1A, 2003). According to the suggested mechanism (Elzanfaly et al., 2013; Sattar et al., 2013), midodrine was hydrolyzed forming desglymidodrine which still containing an electroactive amino group that can be oxidized with oxidation peak at 0.999 V (S3, S4). The linear relationship for desglymidodrine (I (µA) = 0.35 + 2.04 [DMAE], r = 0.9945] within the concentration range from 1 × 10-6 to 4.5 × 10-6 mol L-1.
Moreover, the interference of contaminates and excipients that regularly exist in the pharmaceutical formulations such as citric acid, starch, glucose and some metal ions was investigated. High tolerance limits (500 fold excess of interferent) were recorded with average recovery about 95% of the peak current. Selecting iron oxide as modifier, uric acid (UA) and ascorbic acid (AA) showed two oxidation peaks at 0.12 and 0.38 V, respectively. As midodrine showed oxidation peak at 0.969 V, simultaneous determination of these compounds can be performed.
3.7 Sample analysis
The fabricated FeO/CPE sensors showed improved sensitivity and selectivity for midodrine over the tested interferents that usually present in tablets or biological samples. Therefore, the analytical applicability of the proposed sensor was tested for assaying of midodrine in pharmaceutical formulations, serum and urine samples.
Samples were spiked with known aliquots of MD standard solution and the MD contents were assayed voltammetrically and spectrophometrically at 290 nm (Salunke & Damle, 2016) after suitable dilution with water. The adequacy of this sensor for the quantification of midodrine in pharmaceutical formulations and biological samples was demonstrated by acceptable recoveries and e relative standard deviation values (Table 2).
Parameters
Taken(µ molL-1)
Found(µ molL-1)
bias
Recovery
Ref. [12]
0.50
0.49
0.01
98.00
98.05
1.00
1.01
−0.01
100.51
100.60
3.00
3.01
−0.01
100.33
99.97
Mean
99.61
99.54
Variance
1.96
1.76
Observations
3
3
t Stat
0.6
t Critical two-tail
2.78
F
1.11
F Critical
19
Serum
Pnarameters
Taken
(µ molL-1)Found
(µ molL-1)bias
Recovery
Ref. [12]
0.5
0.51
0.01
102
99.8
1.5
1.51
0.01
100.67
99.79
2
1.99
0.01
99.5
100.7
2.5
2.52
0.02
100.8
99.84
Mean
100.74
100.03
Variance
1.04
0.20
Observations
4
4
t Stat
1.27
t Critical two-tail
2.45
F
5.26
F Critical
9.28
Urine
Parameters
Taken
(µ molL-1)Found
(µ molL-1)bias
Recovery
Ref. [12]
2
2.01
0.01
99.50
99.40
3.1
3.09
0.01
100.32
100.35
Mean
99.88
99.91
Variance
0.45
0.34
Observations
2
2
t Stat
0.48
t Critical two-tail
4.30
F
0.45
F Critical
0.75
4 Conclusion
The construction and electrochemical performance of iron oxide nanoparticles based carbon paste sensors were evaluated for differential pulse voltammetric determination of midodrine. The electrocatalytic effect of iron oxide toward the electrochemical oxidation of midodrine followed an adsorption-controlled reaction mechanism with participation of two electrons. Calibration graphs were linear in the midodrine concentration range from 0.16 × 10-6 to 2.12 × 10-6 mol L-1 with LOD 0.04 × 10-6 mol L-1. Comprehensive investigation of the possible interference and the possible applicability of the fabricated sensors for assaying of midodrine in pharmaceutical formulations and biological fluids were established. Improved sensitivity [about 25 fold] was reported compared with the previously published midodrine sensor based on gold nanoparticles/β-cyclodextrin composite with working pH value near the biological value.
Acknowledgment
This research was funded by the Deanship of Scientific research at Princess Nourah bint Abdulrahman University through the Fast-track Research Funding Program.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2021.103446.
Appendix A
Supplementary material
The following are the Supplementary data to this article:







