Translate this page into:
Lactamomethylation of alkylphenols: Synthesis and quantum-chemical study of the reaction pathway
⁎Corresponding author. vorstepan@yandex.ru (Stepan V. Vorobyev)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Six novel lactamomethyl derivatives of 2,5-dimethylphenol and 2,3,5-trimethylphenol were prepared with moderate yields by the reaction of corresponding phenols with 1-(hydroxymethyl)lactams in the presence of an acid catalyst. In all cases, the substitution occurred at position 4 to the phenolic hydroxyl group. The structures of all synthesized compounds were confirmed by FT-IR, 1H and 13C NMR, 2D NMR and elemental analysis. The selectivity and possible pathways of the lactamomethylation reaction were studied by quantum-chemical methods. In silico calculations showed that the substitution at para-position to the hydroxyl group of the corresponding phenols was more preferable due to the higher stability of forming intermediates.
Keywords
Organic synthesis
Phenols
Lactams
Spectroscopic techniques
Quantum-chemical calculations
Mechanism study
1 Introduction
Oxidation affects the properties of fuels and lubricating oils, leading to corrosion and premature wear of equipment. Oxidation is a radical process that can be inhibited by antioxidants (Burton and Ingold, 1981), such as phenols (Shahidi et al., 1992). Naturally occurring and synthetic phenols are widely used as additives to biofuels (Varatharajan and Pushparani, 2018, Uğuz et al., 2019), pharmaceuticals (Tsai and Lee, 2008, Soto et al., 2017) and foods (Raccach, 1984, Medeiros et al., 2010) because of their ability to interrupt chain reactions by forming stable radicals that do not oxidize organic compounds (Leopoldini et al., 2004). These stable radicals can also react with hydroperoxides formed by auto-oxidation (Ingold, 1961). Electron-donating substituents in phenyl ring enhance the antioxidant properties by facilitating the transfer of the hydrogen atom from the OH group to free radicals (Torres de Pinedo et al., 2007, Osipova et al., 2020). In addition, phenols exhibit diverse biological activity, including antimicrobial (Magnani et al., 2014), antitumor (Ryu et al., 1994, Nile et al., 2017) and analgesic (Hidaka et al., 1986, Pawełczyk et al., 2020).
The use of phenols as additives in lubricating oils is limited by their thermal stability (Santos et al., 2012), which can be increased by introducing a heterocyclic moiety to the phenyl ring (Koshelev et al., 1995). We have previously reported (Vorobyev et al., 2018, Vorobyev et al., 2019) the synthesis of lactamomethyl derivatives of phenols. Lactams were chosen as heterocyclic fragments due to their nootropic (Ostrovskaya et al., 1993, Berestovitskaya et al., 2018), antihypoxic (Bakibaev et al., 1997), anticonlvusant (Malawska, 2005) and other biological effects (Saldívar-González et al., 2019). In particular, we demonstrated that the introduction of a lactamomethyl fragment to the phenyl ring generally decreases the energy of the ArO–H bond, enhancing the antioxidant properties (Vorobyev et al., 2018).
Amidoalkyl derivatives of phenols (in particular, lactamomethyl ones) can be synthesized by Tscherniak–Einhorn reaction (Mudududdla et al., 2012, Schramm et al., 2015). Tscherniak–Einhorn reaction is a type of Mannich reaction, so similar compounds containing moieties of nitrogen heterocycles can be produced by Mannich reaction (Yo. Omura et al., 2001; Biersack et al., 2018, Farooq et al., 2021). Aminomethylation of alkylphenols, such as thymol and 2,3,5-trimethylphenol, has also been reported (Strubell and Baumgärtel, 1962, Burckhalter et al., 1946). These phenols have two possible reaction sites (ortho- and para-positions to the hydroxyl group), but the composition of target compounds was confirmed by elemental analysis only, leaving the question of the reaction selectivity unanswered. Later it was shown that thymol can form either ortho- or para-substituted products, depending on the structure of the amine reactant (Inci Gul et al., 2016).
Although the mechanism of Tscherniak–Einhorn reaction was studied previously (Zaugg et al., 1969, Barry et al., 1977) for some amides, there is a lack of such information about lactamomethylation of aromatic compounds and especially phenols.
In this work we report the synthesis of lactamomethyl derivatives of alkylphenols and quantum-chemical study of the possible reaction pathways.
2 Material and methods
2.1 Measurement and reagents
In this article, 2,5-dimethylphenol (compound 1) and 2,3,5-trimethylphenol (compound 2) were the substrates for the lactamomethylation reaction. Common reagents and solvents were purchased from Acros and Sigma–Aldrich. 1-(hydroxymethyl)pyrrolidin-2-one, 1-(hydroxymethyl)azepan-2-one, 1-(hydroxymethyl)piperidin-2-one were synthesized as described previously (Vorobyev et al., 2018). The melting points were determined using a Stuart SMP30 instrument. The IR spectra were recorded using an Agilent Carry 600 spectrometer equipped with an attenuated total reflectance (ATR) device. The 1H and 13C NMR spectra were recorded at ambient temperature using a Bruker Avance II 300 spectrometer (1H, 300 MHz; 13C, 75 MHz) in CDCl3; Me4Si was used as the internal reference. 2D NMR spectra were recorded using a Bruker Avance II 600 instrument (1H, 600 MHz; 13C, 150 MHz) under the same conditions. Elemental analysis was performed using a Vario MicroCube apparatus.
2.2 General procedure for the preparation of lactamomethyl derivatives of alkylphenols
A solution of the corresponding phenol (0.01 mol), 1-(hydroxymethyl)lactam (0.01 mol) and trifluoroacetic acid (4 mL) in chloroform (20 mL) was refluxed for 48 h, then the reaction mixture was cooled and poured into toluene (75 mL). The resulting solution was washed with aqueous sodium bicarbonate to neutral pH. The organic layer was dried over calcined magnesium sulfate, and the volatiles were removed in vacuum. The residue was allowed to crystallize under hexane, and the product was recrystallized from toluene/cyclohexane (2:1).
2.2.1 1-(4-hydroxy-2,5-dimethylbenzyl)pyrrolidin-2-one (3)
White powder with m.p. 158–159 °C (yield 51%).
1H NMR (CDCl3, δ, ppm, 3JHH, Hz): 1.98 (p, 2H, 4-CH2 in lactam, J = 6.6); 2.21 (s, 6H, two Ar-CH3); 2.49 (t, 2H, 3-CH2 in lactam, J = 7.7); 3.22 (3, 2H, 5-CH2 in lactam, J = 6.6); 4.41 (s, 2H, Ar-CH2-N); 6.71 (s, 1H, Ar-H); 6.90 (s, 1H, Ar-H).
13C NMR (CDCl3, δ, ppm): 15.52 (Ar-CH3 at position 2); 17.66 (4-CH2 in lactam); 18.91 (Ar-CH3 at position 5); 31.21 (3-CH2 in lactam); 44.47 (NCH2Ar), 46.65 (5-CH2 in lactam); 117.10 (6-C in phenyl ring), 121.66 (2-C in phenyl ring), 124.83 (4-C in phenyl ring), 132.34 (3-C in phenyl ring), 135.40 (5-C in phenyl ring), 154.44 (1-C in phenyl ring); 175.05 (C = O).
FT-IR, ν, cm−1: 3186 (O–H), 1660 (C = O), 1573 (C = C), 1511 (C-C), 1294 (Car-O).
Calc., %: C 71.21, H 7.81, N 6.39. Found, %: C 71.67, H 7.69, N 6.28. C13H17NO2
2.2.2 1-(4-hydroxy-2,5-dimethylbenzyl)piperidin-2-one (4)
White powder with m.p. 171–172 °C (yield 67%).
1H NMR (CDCl3, δ, ppm, 3JHH, Hz): 1.77 (m, 4H, 4,5-CH2 in lactam); 2.17 (s, 3H, Ar-CH3); 2.19 (s, 3H, Ar-CH3); 2.49 (t, 2H, 3-CH2 in lactam, J = 6.0); 3.10 (t, 2H, 6-CH2 in lactam, J = 5.5); 4.57 (s, 2H, Ar-CH2-N); 6.67 (s, 1H, Ar-H); 6.84 (s, 1H, Ar-H).
13C NMR (CDCl3, δ, ppm): 15.59 (Ar-CH3 at position 2); 19.00 (Ar-CH3 at position 5); 21.18 (4-CH2 in lactam); 23.05 (5-CH2 in lactam); 32.26 (3-CH2 in lactam); 46.57 (NCH2Ar), 47.53 (6-CH2 in lactam); 117.13 (6-C in phenyl ring), 121.48 (2-C in phenyl ring), 125.28 (4-C in phenyl ring), 131.91 (3-C in phenyl ring), 135.39 (5-C in phenyl ring), 154.22 (1-C in phenyl ring); 170.17 (C = O).
FT-IR, ν, cm−1: 3391 (O–H), 1622 (C = O), 1602 (C = C), 1251 (C-O), 1072 (Car-O).
Calc., %: C 72.07, H 8.21, N 6.00. Found, %: C 72.59, H 7.99, N 5.98. C14H19NO2
2.2.3 1-(4-hydroxy-2,5-dimethylbenzyl)azepan-2-one (5)
White powder with m.p. 177–179 °C (yield 59%).
1H NMR (CDCl3, δ, ppm, 3JHH, Hz): 1.42 (m, 2H, 5-CH2 in lactam); 1.69 (m, 4H, 4,6-CH2 in lactam); 2.19 (s, 6H, two Ar-CH3); 2.62 (m, 2H, 3-CH2 in lactam); 3.24 (t, 2H, 7-CH2 in lactam, J = 4.5); 4.53 (s, 2H, Ar-CH2-N); 6.67 (s, 1H, Ar-H); 6.85 (s, 1H, Ar-H).
13C NMR (CDCl3, δ, ppm): 15.57 (Ar-CH3 at position 2); 19.02 (Ar-CH3 at position 5); 23.40 (4-CH2 in lactam); 27.70 (5-CH2 in lactam); 29.91 (6-CH2 in lactam); 37.11 (3-CH2 in lactam); 47.58 (NCH2Ar), 48.23 (7-CH2 in lactam); 117.19 (6-C in phenyl ring), 121.42 (2-C in phenyl ring), 125.81 (4-C in phenyl ring), 132.37 (3-C in phenyl ring), 135.50 (5-C in phenyl ring), 154.21 (1-C in phenyl ring); 176.18 (C = O).
FT-IR, ν, cm−1: 3410 (O–H), 1632 (C = O), 1612 (C = C), 1261 (C-O), 1045 (Car-O).
Calc., %: C 72.84, H 8.56, N 5.66. Found, %: C 72.59, H 8.79, N 5.41. C15H21NO2
2.2.4 1-(4-hydroxy-2,3,6-trimethylbenzyl)pyrrolidin-2-one (6)
White powder with m.p. 163–165 °C (yield 62%).
1H NMR (CDCl3, δ, ppm, 3JHH, Hz): 1.92 (p, 2H, 4-CH2 in lactam, J = 6.8); 2.17 (s, 3H, Ar-CH3); 2.22 (s, 3H, Ar-CH3); 2.27 (s, 3H, Ar-CH3); 2.47 (t, 2H, 3-CH2 in lactam, J = 7.8); 3.09 (t, 2H, 5-CH2 in lactam, J = 6.8); 4.54 (s, 2H, Ar-CH2-N); 6.59 (s, 1H, Ar-H).
13C NMR (CDCl3, δ, ppm): 12.00 (Ar-CH3 at position 2); 16.05 (Ar-CH3 at position 3); 17.85 (4-CH2 in lactam); 20.21 (Ar-CH3 at position 5); 31.22 (3-CH2 in lactam); 40.79 (NCH2Ar); 45.78 (5-CH2 in lactam); 114.76 (6-C in phenyl ring); 120.92 (2-C in phenyl ring); 123.62 (4-C in phenyl ring); 135.76 (5-C in phenyl ring); 138.08 (3-C in phenyl ring); 153.57 (1-C in phenyl ring); 174.69 (C = O).
FT-IR, ν, cm−1: 3154 (O–H), 1655 (C = O), 1592 (C = C), 1250 (C-C), 1093 (Car-O).
Calc., %: C 72.07, H 8.21, N 6.00. Found, %: 71.81, H 8.39, N 6.02. C14H19NO2.
2.2.5 1-(4-hydroxy-2,3,6-trimethylbenzyl)piperidin-2-one (7)
White powder with m.p. 222–225 °C (yield 52%).
1H NMR (CDCl3, δ, ppm, 3JHH, Hz): 1.61–1.83 (m, 4H, 4,5-CH2 in lactam); 2.17 (s, 6H, two Ar-CH3); 2.23 (s, 3H, Ar-CH3); 2.46 (t, 2H, 2H, 3-CH2 in lactam, J = 6.2); 2.89 (t, 2H, 6-CH2 in lactam, J = 5.5); 4.73 (s, 2H, Ar-CH2-N); 6.58 (s, 1H, Ar-H).
13C NMR (CDCl3, δ, ppm): 11.99 (Ar-CH3 at position 2); 16.05 (Ar-CH3 at position 3); 20.33 (Ar-CH3 at position 5); 21.17 (4-CH2 in lactam); 23.18 (5-CH2 in lactam); 32.39 (3-CH2 in lactam); 43.33 (NCH2Ar); 44.59 (6-CH2 in lactam); 114.70 (6-C in phenyl ring); 120.84 (2-C in phenyl ring); 124.08 (4-C in phenyl ring); 136.18 (5-C in phenyl ring); 138.45 (3-C in phenyl ring); 153.39 (1-C in phenyl ring); 169.88 (C = O).
FT-IR, ν, cm−1: 3435 (O–H), 1609 (C = O), 1593 (C = C), 1242 (Car-O).
Calc., %: C 72.84, H 8.56, N 5.66. Found, %: C 72.61, H 8.83, N 5.33. C15H21NO2
2.2.6 1-(4-hydroxy-2,3,6-trimethylbenzyl)azepan-2-one (8)
Yellow powder with m.p. 143–145 °C (yield 68%).
1H NMR (CDCl3, δ, ppm, 3JHH, Hz): 1.33 (m, 2H, 5-CH2 in lactam); 1.67 (m, 4H, 4,6-CH2 in lactam); 2.16 (s, 3H, Ar-CH3); 2.18 (s, 3H, Ar-CH3); 2.23 (s, 3H, Ar-CH3); 2.59 (t, 2H, 3-CH2 in lactam, J = 5.9); 3.02 (m, 2H, 7-CH2 in lactam, J = 5.9); 4.66 (s, 2H, Ar-CH2-N); 6.61 (s, 1H, Ar).
13C NMR (CDCl3, δ, ppm): 12.06 (Ar-CH3 at position 2); 16.16 (Ar-CH3 at position 3); 20.32 (Ar-CH3 at position 5); 23.42 (4-CH2 in lactam); 27.85 (5-CH2 in lactam); 29.94 (6-CH2 in lactam); 37.25 (3-CH2 in lactam); 43.55 (NCH2Ar); 45.36 (7-CH2 in lactam); 114.84 (6-C in phenyl ring); 123.00 (2-C in phenyl ring); 124.22 (4-C in phenyl ring); 136.05 (5-C in phenyl ring); 138.39 (3-C in phenyl ring); 153.71 (1-C in phenyl ring); 176.14 (C = O).
FT-IR, ν, cm−1: 3442 (O–H), 1616 (C = O), 1596 (C = C), 1280 (Car-O).
Calc., %: C 73.53, H 8.87, N 8.36. Found, %: 73.69, H 9.01, N 8.08. C16H23NO2.
2.3 Computational details
Quantum-chemical calculations were performed using Gaussian09 software (Gaussian 09, Revision D.01); the results were visualized by GaussView 6.0.16 (Roy D. Dennington II et al., 2016). Geometry optimization was carried out with the M06-2X functional using the 6-311G(d,p) basis set. The influence of solvation on σ-complexes stability was studied with IEF-PCM model with M06-2X functional and 6-311G(d,p) basis set. The M06-2X functional was chosen for studying the lactamomethylation pathway because it was used previously for the aromatic electrophilic substitution calculations and produced better results (in thermochemistry, kinetics and non-covalent interactions) than B3LYP (Oliveira and Esteves, 2011, Liljenberg et al., 2017). The optimized geometries were characterized by minima on the potential energy surface (PES) with no imaginary vibrational frequencies, whereas the transition states were characterized by the presence of a single imaginary frequency. Transition state calculations were carried out using synchronous transit-guided quasi-Newton (STQN) method (QST2) based on optimized structures of the reactant and product. All calculations were carried out for the gas phase (T = 298.15 K, P = 1 atm). Intrinsic reaction coordinate (IRC) calculations were provided to confirm that the transition state indeed connects the reactants and the products.
NBO analysis for the starting phenols was carried out with the B3LYP method using 6-311G(d,p) basis set in gas phase, as this method was previously shown (Metri et al., 2012) to give the most accurate results.
Vibration frequencies were calculated with B3LYP DFT method in 6-311+G (2d,p) basis set. These calculations were performed for a free molecule in vacuum, while the experimental frequencies are in the solid state. In order to improve the agreement with the experimental data, theoretical values were scaled with 0.9613 (Razak et al., 2015), which allows to consider the formation of hydrogen bonds. Theoretical vibrational spectra of the target compounds were interpreted by means of potential energy distribution (PED) with VEDA program (Jamroz, 2010).
NMR chemical shifts for target compounds were calculated by B3LYP/6-311+G (2d,p). The obtained data were scaled according to CHESHIRE CCAT method (Lodewyk et al., 2012), combining gas-phase optimization and SMD-solvation (chloroform) model for NMR calculations. B3LYP/6-311+G (2d,p) was chosen due to its high root mean square deviation value. Theoretical and experimental chemical shifts were compared, the agreement between them was estimated by calculation of mean absolute deviation (MAD) and root-mean-square (RMS) deviation.
3 Results and discussion
3.1 Synthesis of the target compounds
In our previous work (Vorobyev et al., 2018) we synthesized a series of lactamomethyl derivatives of 2,4-di-tert-butylphenol and thymol. In the first case there was only one expected product while thymol could produce two possible isomers. According to 1H NMR data, the substitution took place at position 4 (para-) to the hydroxyl group. Quantum-chemical study of the lactamomethylation of thymol was also considered (Vorobyev et al., 2021).
In this work, we continued the study of lactamomethylation and synthesized novel derivatives of 2,5-dimethyl- and 2,3,5-trimethylphenols (1 and 2, respectively), which also had two possible reaction sites (Scheme 1). For the both phenols, para-substitution was observed.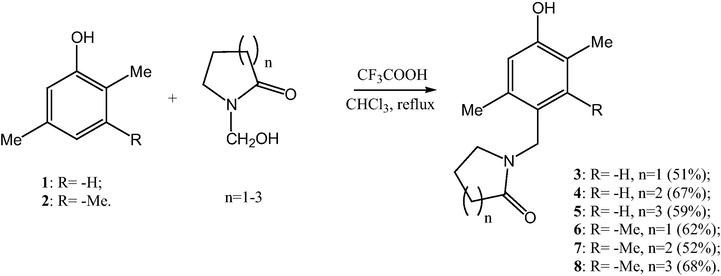
Lactamomethylation of 2,5-dimethylphenol 1 and 2,3,5-trimethylphenol 2.
Lactamomethyl derivatives of alkylphenols can also be obtained in acetic acid saturated with gaseous HCl (Idel et al., 1979). We have found that under these conditions the reaction of 1-(hydroxymethyl)pyrrolidine-2-one with 2,3,5-trimethylphenol 2 gives the same product 6 as at reflux in chloroform (Scheme 1), experimental procedure is given in Supplementary Materials. Since the yields and reaction times were almost identical for both methods, we can assume that solvation does not play an important role in this reaction.
3.2 IR spectra
The vibrational assignments of the normal modes were made using the PED. In the FT-IR spectra of the synthesized compounds, the absorptions of C=O bonds in lactam rings were observed between 1655 and 1615 cm−1, which correlates with literature and calculated data (Pretsch et al., 2000, Kuruvilla et al., 2018). Broad bands of stretching vibrations of hydroxyl groups in substituted phenols were observed at 3450–3150 cm−1. The medium intensity bands at 1573–1612 cm−1 and 1455–1510 cm−1 were assigned to stretching vibrations of aromatic rings. Strong bands of stretching vibrations of C-O in phenol rings were observed at 1251–1294 and 1093–1045 cm−1. The out-of-plane deformational vibrations of the C-H bonds in aromatic rings were observed at 900–855 cm−1 (Muthu and Renuga, 2014). The experimental IR spectra generally correlate well with the calculated ones. Observed and calculated IR spectra, IR bands with scaled wavenumbers and assignments are given in supplementary materials.
3.3 NMR spectra
The 1H NMR spectra of 2,5-dimethylphenol derivatives 3–5 had two singlets at 6.60–6.85 ppm, suggesting that the electrophilic substitution took place at position 4 to hydroxyl group.
The 13C NMR spectra showed all the expected signals. The lowest 13C chemical shifts of carbonyl groups (ca. 170 ppm) were observed for valerolactam derivatives and the highest (ca. 176 ppm) for caprolactam derivatives.
These data are in agreement with theoretical values obtained by DFT calculations. The largest deviation from experimental chemical shift for 1H calculated values is observed for the compound 8, while 13C shows the maximum deviation in case of 5. Tables of calculated and experimental chemical shifts, mean absolute deviations (MAD) and root-mean-square (RMS) deviations are listed in Supplementary materials.
Compounds 6–8 have only one hydrogen atom in the phenyl ring and it has no visible interactions to the other protons. Unfortunately, even DFT calculations of chemical shifts for ortho- and para-isomers of the compound 6 are nearly identical. However, the substitution is likely to take placeat position 4 because of the lower MAD and RMS values for para-isomer in 1H-shift calculations (0.15 and 0.18, respectively, compared to 0.2 and 0.25 for the ortho-isomer). For 13C calculations this difference is even greater: 1.53 and 1.73 vs. 2.11 and 2.51 for para- and ortho-isomers, respectively.
In order to determine the structures of 6–8 we used 2D NMR methods. In particular, the HSQC (1H, 13C) spectrum was used to establish single-bond correlations between hydrogen and carbon atoms (Fig. 1). The increment calculations for the 13C NMR spectra (Pretsch et al., 2000) also suggested that the substitution took place at position 4.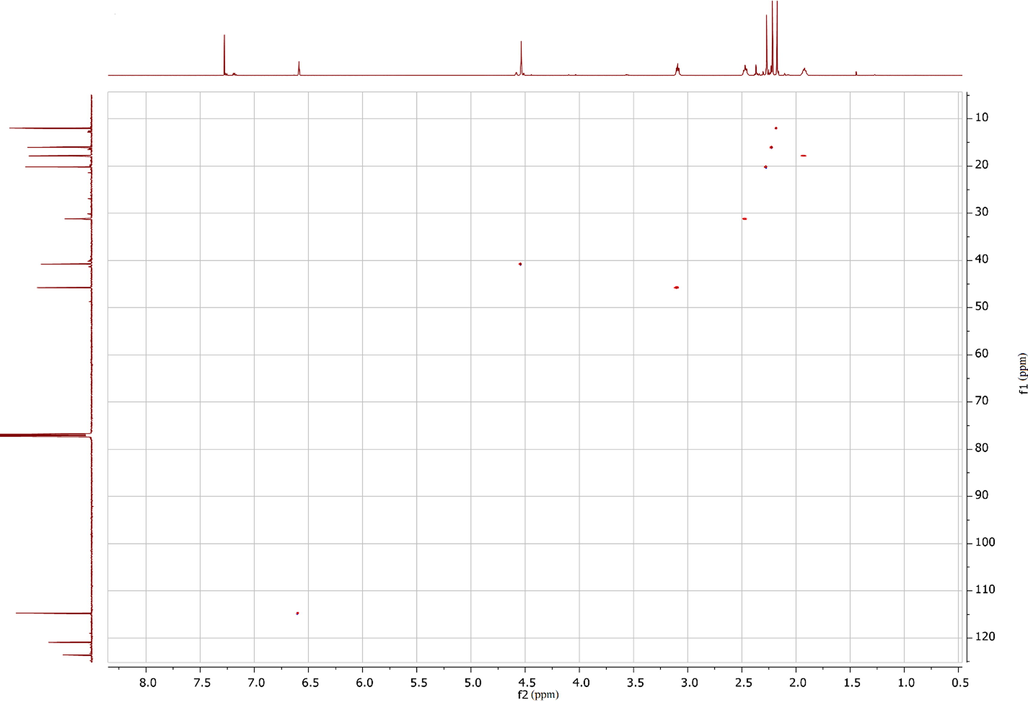
HSQC (1H, 13C) spectrum of compound 10.
The HMBC (1H; 13C) spectrum (Fig. 2) was used to assign the signals to specific methyl groups. The 1H signal at 2.17 ppm belongs to hydrogen atoms of the methyl group at position 2, which interact with carbon 1 of the aromatic ring (cross-peak 2.17, 153.57). They also interact with carbons 2 and 3 (2.17, 120.92 and 2.17, 138.08, respectively). The 1H signal at 2.22 ppm is produced by hydrogen atoms of the methyl group at position 3, as it shows similar correlations (2.22, 120.92 and 2.22, 138.08). Thus, the signal at 2.27 ppm belongs to hydrogen atoms of the methyl group at position 5.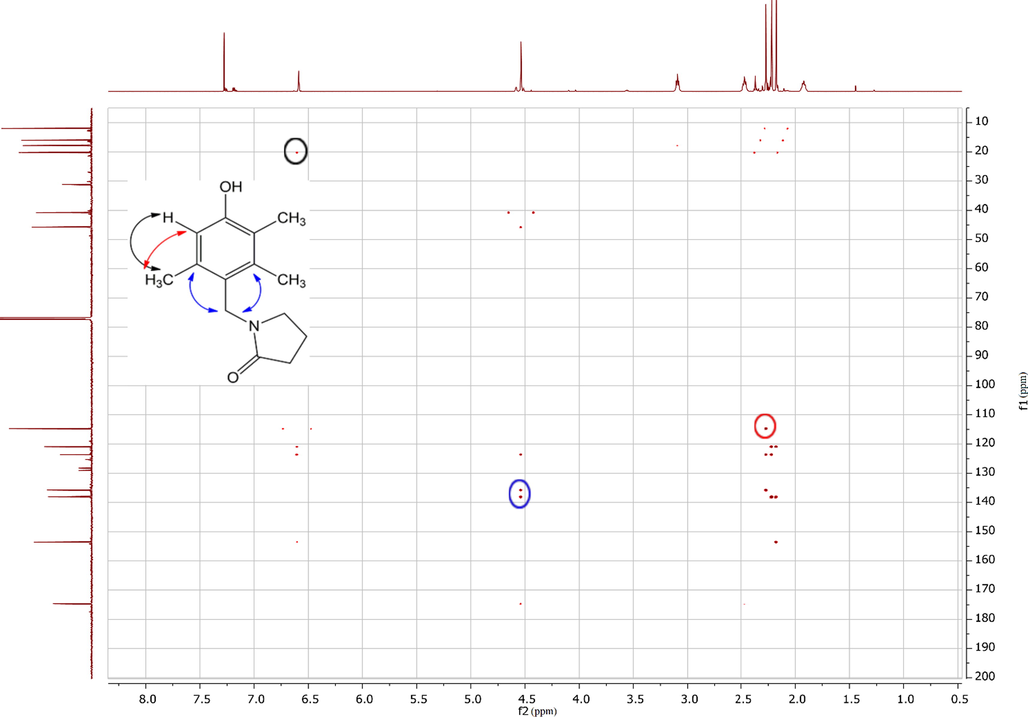
HMBC (1H, 13C) spectrum of compound 6.
In the case of ortho-substitution, the HMBC spectrum would show 2JCH correlations between the single aromatic hydrogen atom and two aromatic carbon atoms bonded to methyl groups. Furthermore, 3JCH correlations between the aromatic hydrogen atom and two carbon atoms of adjacent methyl groups would also be observed.
In contrast, HMBC (1H, 13C) spectrum (Fig. 2) shows the following 3JCH correlations: (6.59, 20.21) between the aromatic proton and the carbon atom of the methyl group at position 5; (2.27, 114.76) between hydrogen atoms of the methyl group at position 5 and the carbon atom with the aromatic hydrogen; (4.54, 135.76) and (4.54, 138.08), which correspond to the interactions of hydrogen atoms of the “methylene bridge” between phenyl and lactam rings with two carbon atoms in the phenyl ring linked to methyl groups. These correlations suggest that the substitution in the phenyl ring took place at position 4 (para-) to hydroxyl group.
3.4 Quantum-chemical calculations
Lactamomethylation of aromatic compounds is an electrophilic substitution reaction, since it is a variant of amidoalkylation (similar to Tscherniak-Einhorn reaction) (Barry et al., 1977, Chung et al., 2008). As it was mentioned above, we assumed that solvation had little effect on the reaction progress, so all quantum-chemical calculations were performed for the gas phase to reduce the computation time.
In order to study the mechanism of lactamomethylation of phenols we firstly estimated partial atomic charges and localization of frontier orbitals in starting phenols.
For the two considered phenols (2,5-dimethylphenol 1 and 2,3,5-trimethylphenol 2) the obtained results were very similar. The greatest negative partial charge (NBO scheme) was located on the carbon atom at ortho-position to the hydroxyl group in each compound, although the charge at para-position had comparable value (Fig. 3):
Partial NBO atomic charges in 2,5-dimethylphenol 1 and 2,3,5-trimethylphenol 2.
Frontier orbitals analysis showed that the Highest Occupied Molecular Orbital (HOMO) is located on the carbon atoms of the phenyl ring (Fig. 4).
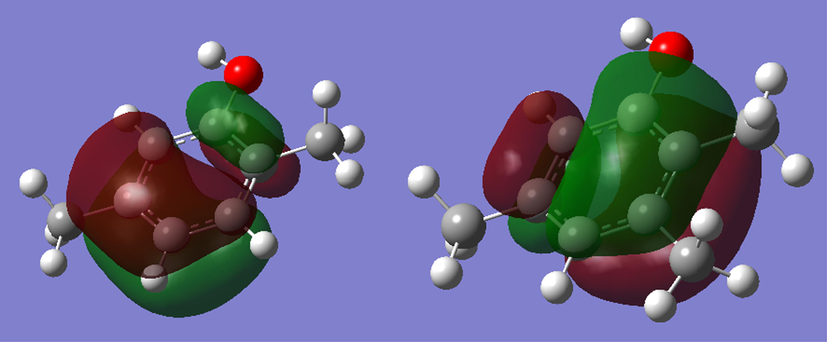
HOMO localization in 2,5-dimethylphenol 1 and 2,3,5-trimethylphenol 2.
As a result, the analysis of the partial charge distribution and MO localization shows that the pathway of lactamomethylation cannot be predicted properly by static factors, such as electron structures of the reactants. Another key parameter for electrophilic substitution is the stability of the intermediates (dynamic factor). The reaction is likely to proceed through the formation of the lowest-energy intermediate. Previously it was shown (Shi, 2017) that the zero-point corrected energies of the reaction system could be used to estimate the pathway of the reaction.
We assumed that lactamomethylation involves typical steps (Liljenberg et al., 2010, Liljenberg et al., 2017) for the electrophilic substitution: formation of the π-complex, followed by the σ-complex through the transition state (TS) with subsequent formation of the products via another transition state. Firstly, we considered the reaction of 2,5-dimethylphenol 1 with the pyrrolidone derivative.
The comparison of the two possible intermediates for 1 shows that the σ-complex at para-position (3b) is more stable, with the energy 2.2 kcal/mol (9.22 kJ/mol) lower than that for the complex at ortho-position (3a, Fig. 5). Moreover, in the case of ortho-substitution the energy minimum corresponds not to the σ-complex but to the π-complex, which does not lead to the formation of the expected bond. Both complexes have no imaginary frequencies, so they represent true energy minima.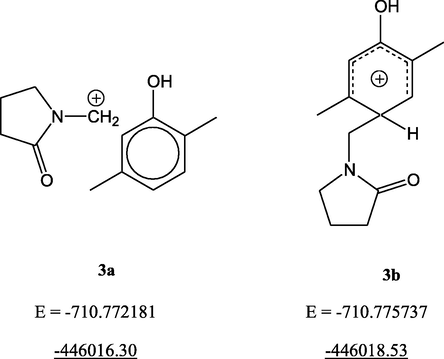
Zero-point corrected energy values (atomic units, a.u. and kcal/mol, underlined) for 2,5-dimethylphenol intermediates.
The energy profile of the reaction of 2,5-dimethylphenol 1 with the pyrrolidone derivative at para-position (Fig. 6) shows that the formation of the π-complex (I) significantly decreases the energy of the system by 14.0 kcal/mol (58.8 kJ/mol) compared to reactants. The transition state (TS1) for the conversion into σ-complex (II) is very close in energy to the π-complex (the difference is only 0.4 kcal/mol, or 1.68 kJ/mol), so the barrier for conversion into (II) is very low. TS1 is characterized by one imaginary frequency (-115 cm−1). The intermediate σ-complex has a free energy that is 9.6 kcal/mol (40.2 kJ/mol) lower than that of the TS1. The formation of the products is preceded by the second transition state (TS2) with the imaginary frequency of −137 cm−1. As the TS1 is similar to I, TS2 has similar energy to the σ-complex II.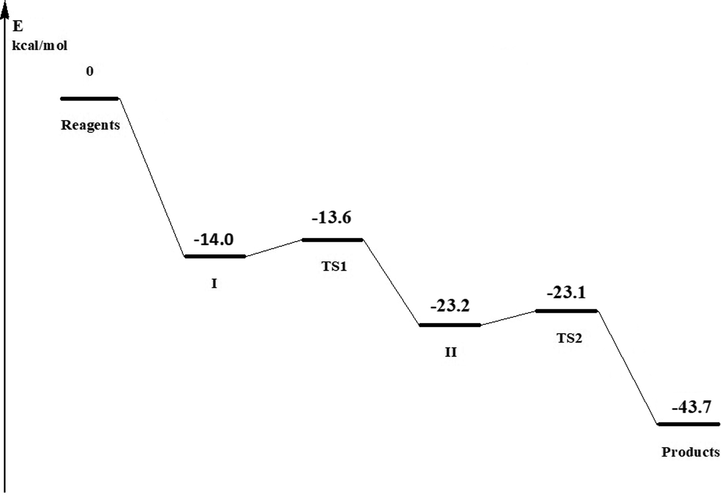
Zero-point corrected energies (kcal/mol) of the stationary points on the PES (shown relative to the reactants) for the gas phase lactamomethylation of 2,5-dimethylphenol 1 by pyrrolidone derivative.
A similar picture is observed for other target compounds (3–8). The substitution proceeds through the more stable σ-complex, which forms in the reaction at the para-position, in comparison to π-complex with higher energy for the ortho-intermediate. Therefore, the complexes of 2,3,5-trimethylphenol derivatives with valerolactam and caprolactam are also more stable when the substitution takes place at para-position. Valerolactam forms a σ-complex at para-position, which has only a 0.5 kcal/mol (2.1 kJ/mol) lower energy than the π-complex at ortho-position. In the case of caprolactam the energy difference is even greater, 13.2 kcal/mol (55.4 kJ/mol) (Table 1). Noteworthy, there is no observable correlation between the energy differences of intermediates and the structures of reactants.
Compound
Intermediate energy E, a.u.
Energy difference Epara–Eortho, a.u. (kcal/mol)
ortho-substitution
para-substitution
3
−710.772181
−710.775737
−0.003556 (−2.2)
4
−750.050906
−750.053234
−0.002328 (−1.5)
5
−789.326086
−789.327433
−0.001347 (−0.8)
6
−750.058059
−750.060571
−0.002512 (−1.6)
7
−789.337842
−789.338643
−0.000801 (−0.5)
8
−828.586048
−828.607080
−0.021032 (−13.2)
In order to prove our suggestion that solvation has no significant influence on the efficiency of the quantum-chemical calculations, we calculated σ-complex energies for compound 6 in two solvents: chloroform and acetic acid by using IEF-PCM model. In chloroform the σ-complex at para-position is more stable, with the energy 2.45 kcal/mol (10.3 kJ/mol) lower than that for the complex at ortho-position. Very similar values were obtained for the calculations in acetic acid. Intermediate σ-complex at para-position has the energy 2.2 kcal/mol (9.4 kJ/mol) lower compared to ortho-isomer (Table 2). Hence, gas-phase calculations give accurate results and may be used for the study of the reaction pathway.
Solvent
Intermediate energy E, a.u.
Energy difference Epara–Eortho, a.u. (kcal/mol)
ortho-substitution
para-substitution
Choloform
−750.112508
−750.116410
−0.004202 (−2.45)
Acetic acid
−750.116677
−750.120235
−0.003558 (−2.2)
4 Conclusion
Six novel lactamomethyl derivatives of 2,5-dimethyl- and 2,3,5-trimethylphenols were synthesized with moderate yields of 51–68%. All products were characterized by FT-IR, NMR (1H, 13C and 2D: COSY, HSQC and HMBC) and elemental analysis. Calculated and experimental IR and NMR data showed good agreement. The reaction selectivity and yields were not affected by the solvent, as similar results were obtained in chloroform and acetic acid.
M06-2X method was used for quantum-chemical study of the lactamomethylation pathway. The substitution at para-position to the hydroxyl group produces the most stable σ-complexes and thus is preferable to ortho-substitution, which cannot proceed beyond π-complexes without input of energy.
5 Authors contribution
SV: concept, synthesis and purification, structure elucidation (NMR), quantum-chemical calculations, manuscript preparation. OP: FT-IR experiments, data analysis, manuscript preparation. SB: data analysis, supervision, manuscript preparation. VK: concept, supervision, manuscript preparation. All authors have read and agreed to the final version of the manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could influence the work reported in this paper.
References
- Antihypoxic properties of organic compounds (A review) Pharm. Chem. J.. 1997;31:53-67.
- [CrossRef] [Google Scholar]
- The amidoalkylation of aromatic hydrocarbons. Tetrahedron. 1977;33(4):369-372.
- [CrossRef] [Google Scholar]
- O.S. Vasil’eva, E.S. Ostroglyadov, S.M. Aleksandrova, I.N. Tyurenkov, O.V. Merkushenkova, V.V. Bagmetova, Synthesis and Neuropsychotropic Activity of Indole-Containing Gamma-Aminobutyric Acid Derivatives. Pharm. Chem. J.. 2018;52:392-396.
- [CrossRef] [Google Scholar]
- Recent developments concerning the application of the Mannich reaction for drug design. Expert Opin. Drug Discov.. 2018;13:39-49.
- [CrossRef] [Google Scholar]
- J.H. Burckhalter, F.H. Tendick, E.M. Jones, W.F. Holcomb, A.L. Rawlins, Aminoalkylphenols as Antimalarials. I. Simply Substituted α-Aminocresols, J. Am. Chem. Soc., 68 (10) (1946), pp. 1894-1901, 10.1021/ja01214a008
- Autoxidation of biological molecules. 1. Antioxidant activity of vitamin E and related chain-breaking phenolic antioxidants in vitro. J. Am. Chem. Soc. 1981:6472-6477.
- [CrossRef] [Google Scholar]
- Synthesis development of an aminomethylcycline antibiotic via an electronically tuned acyliminium Friedel-Crafts reaction. Tetrahedron Lett.. 2008;49(42):6095-6100.
- [CrossRef] [Google Scholar]
- Synthesis, characterization and biological evaluation of N-Mannich base derivatives of 2-phenyl-2-imidazoline as potential antioxidants, enzyme inhibitors, antimicrobials, cytotoxic and anti-inflammatory agents. Arabian J. Chem.. 2021;14(4):103050
- [CrossRef] [Google Scholar]
- Gaussian 09, Revision D.01, M.J. Frisch; G.W. Trucks; H.B. Schlegel; G.E. Scuseria; M.A. Robb; J.R. Cheeseman; G. Scalmani; V. Barone; B. Mennucci; G.A. Petersson; H. Nakatsuji; M. Caricato,; X. Li; H.P. Hratchian; A.F. Izmaylov; J. Bloino; G. Zheng; J.L. Sonnenberg; M. Hada; M. Ehara; K. Toyota; R. Fukuda; J. Hasegawa; M. Ishida; T. Nakajima; Y. Honda; O. Kitao; H. Nakai; T. Vreven; J.A. Montgomery, Jr.; J.E. Peralta; F. Ogliaro; M. Bearpark,; J.J. Heyd; E. Brothers; K.N. Kudin; V.N. Staroverov; R. Kobayashi; J. Normand; K. Raghavachari; A. Rendell; J.C. Burant; S.S. Iyengar; J. Tomasi; M. Cossi; N. Rega; J.M. Millam; M. Klene; J.E. Knox; J.B. Cross; V. Bakken; C. Adamo; J. Jaramillo; R. Gomperts; R.E. Stratmann; O. Yazyev; A.J. Austin; R. Cammi; C. Pomelli; J.W. Ochterski; R.L. Martin; K. Morokuma; V.G. Zakrzewski; G.A. Voth; P. Salvador; J.J. Dannenberg; S. Dapprich; A.D. Daniels; Ö. Farkas; J.B. Foresman; J.V. Ortiz; J. Cioslowski; D.J. Fox. Gaussian, Inc., Wallingford CT. 2009.
- Analgesic and anti-inflammatory activities in rats of α-(3,5-di-t-butyl-4-hydroxybenzylidene)-γ-butyrolactone (KME-4), and its intestinal damage. J. Pharm. Pharmacol.. 1986;38(10):748-753.
- [CrossRef] [Google Scholar]
- Preparation of polycarbonates using phenols amidoalkylated in the 4-position as chain-breaking agents. Patent GB1547333 1979
- [Google Scholar]
- H. Inci Gul, C. Yamali, A.T. Yasa, E. Unluer, H. Sakagami, M. Tanc, C.T. Supuran, Carbonic anhydrase inhibition and cytotoxicity studies of Mannich base derivatives of thymol, J. Enzyme Inhib. Med. Chem., 31 (6) (2016), pp. 1375-1380, 10.3109/14756366.2016.1140755
- Inhibition of the Autoxidation of Organic Substances in the Liquid Phase. Chem. Rev.. 1961;61:563-589.
- [CrossRef] [Google Scholar]
- M.H. Jamroz, Vibrational Energy Distribution Analysis VEDA 4, Warsaw, 2004-2010
- Effect of azoles and sym-triazines with hindered phenol fragments on protective properties of turbine oils. Chem. Technol. Fuels Oils. 1995;31:26-29.
- [CrossRef] [Google Scholar]
- Vibrational spectroscopic (FT-IR, FT-Raman) and quantum mechanical study of 4-(2-chlorophenyl)-2-ethyl-9-methyl-6H-thieno[3,2-f] [1,2,4]triazolo[4,3-a][1,4] diazepine. J. Mol. Struct.. 2018;1157:519-529.
- [CrossRef] [Google Scholar]
- Antioxidant Properties of Phenolic Compounds: H-Atom versus Electron Transfer Mechanism. J. Phys. Chem. A. 2004;108(22):4916-4922.
- [CrossRef] [Google Scholar]
- Validation of a Computational Model for Predicting the Site for Electrophilic Substitution in Aromatic Systems. J. Org. Chem.. 2010;75(14):4696-4705.
- [CrossRef] [Google Scholar]
- M. Liljenberg, J. Halldin Stenlid, T. Brinck, Mechanism and regioselectivity of electrophilic aromatic nitration in solution: the validity of the transition state approach, J. Mol. Model., 24 (2017), 15, 10.1007/s00894-017-3561-z
- Computational Prediction of 1H and 13C Chemical Shifts: A Useful Tool for Natural Product, Mechanistic, and Synthetic Organic Chemistry. Chem. Rev.. 2012;112:1839-1862.
- [CrossRef] [Google Scholar]
- Caffeic acid: a review of its potential use in medications and cosmetics. Anal. Methods. 2014;6:3203-3210.
- [CrossRef] [Google Scholar]
- Simultaneous voltammetric determination of phenolic antioxidants in food using a boron-doped diamond electrode. Food Chem.. 2010;123(3):886-891.
- [CrossRef] [Google Scholar]
- Processable Star-Shaped Molecules with Triphenylamine Core as Hole-Transporting Materials: Experimental and Theoretical Approach. J. Phys. Chem. C. 2012;116(5):3765-3772.
- [CrossRef] [Google Scholar]
- ortho-Amidoalkylation of Phenols via Tandem One-Pot Approach Involving Oxazine Intermediate. J. Org. Chem.. 2012;77(19):8821-8827.
- [CrossRef] [Google Scholar]
- Vibrational spectra and normal coordinate analysis of 2-hydroxy-3-(2-methoxyphenoxy) propyl carbamate. Spectrochim. Acta A. 2014;132(11):313-325.
- [CrossRef] [Google Scholar]
- Sh.H. Nile, A.Sh. Nile, Y.-S. Keum, Total phenolics, antioxidant, antitumor, and enzyme inhibitory activity of Indian medicinal and aromatic plants extracted with different extraction methods, 3 Biotech, 7 (2017), 76, 10.1007/s13205-017-0706-9
- Interaction of allylic carbocations with benzene: a theoretical model of carbocationic intermediates in terpene biosynthesis. J. Braz. Chem. Soc.. 2011;22(10):1979-1986.
- [CrossRef] [Google Scholar]
- Yo. Omura, Yo. Taruno, Ya. Irisa, M. Morimoto, H. Saimoto, Yo. Shigemasa, Regioselective Mannich reaction of phenolic compounds and its application to the synthesis of new chitosan derivatives, Tetrahedron Lett., 42 (41) (2001), pp. 7273-7275, 10.1016/S0040-4039(01)01491-5.
- Antioxidant activity of some organosulfur compounds in vitro. Arabian J. Chem.. 2020;14(4):103068
- [CrossRef] [Google Scholar]
- Synthesis and neurotropic activity of a number of N-[di(n-propyl)acetyl]lactams. Pharm. Chem. J.. 1993;27:546-550.
- [CrossRef] [Google Scholar]
- A. Pawełczyk, D. Olender, K. Sowa-Kasprzak, L. Zaprutko, Linked drug-drug conjugates based on triterpene and phenol structures. Rational synthesis, molecular properties, toxicity and bioactivity prediction, Arabian J. Chem., 13 (12) (2020), pp. 8793-8806, 10.1016/j.arabjc.2020.10.009
- E. Pretsch, P. Bühlmann, C. Affolter, Structure determination of organic compounds, 3rd edn. (Springer-Verlag, Berlin Heideberg, 2000), pp. 71-160, 245-312, 10.1007/978-3-662-62439-5_1
- The antimicrobial activity of phenolic antioxidants in foods: a review. J. Food Saf.. 1984;6(3):141-170.
- [CrossRef] [Google Scholar]
- Synthesis, Characterization, and Structural Investigations of 1-(3-morpholinopropyl)-3-(4-chlorobenzoyl)thiourea monohydrate and 1-(3-morpholinopropyl)-3-(4-methylbenzoyl)thiourea monohydrate. Mol. Cryst. Liq. Cryst.. 2015;616:151-175.
- [CrossRef] [Google Scholar]
- Keith and John M. Semichem Inc, Shawnee Mission, KS: Millam; 2016.
- Antitumor activity of some phenolic components in plants. Arch. Pharm. Res.. 1994;17:42-44.
- [CrossRef] [Google Scholar]
- Exploring the chemical space and the bioactivity profile of lactams: a chemoinformatic study. RSC Adv.. 2019;9:27105-27116.
- [CrossRef] [Google Scholar]
- Commercial antioxidants and thermal stability evaluations. Fuel. 2012;97:638-643.
- [CrossRef] [Google Scholar]
- L.F. Monteiro Leite Ciscato, P. Oesau, R. Krieg, J.F. Richter, I. Navizet, D. Roca-Sanjuán, D. Weiss, R. Beckert, Investigations on the synthesis and chemiluminescence of novel 2-coumaranones – II, ARKIVOC. V 2015:44-59.
- [CrossRef] [Google Scholar]
- Sulfonation mechanism of benzene with SO3 in sulfuric acid or oleum or aprotic solvent: Obeying the transition state theory via a trimolecular electrophilic substitution clarified by density functional theory calculation. Comput. Theor. Chem.. 2017;1112:111-122.
- [CrossRef] [Google Scholar]
- Relevance of Natural Phenolics from Grape and Derivative Products in the Formulation of Cosmetics. Cosmetics. 2017;2(3):259-276.
- [CrossRef] [Google Scholar]
- Über p-Cymol und seine Derivate. XXXI. Mannich-Reaktionen mit Thymol und Thiocarvacrol. J. Prakt. Chem.. 1962;16:277-283.
- [CrossRef] [Google Scholar]
- Synthesis and evaluation of new phenolic-based antioxidants: Structure–activity relationship. Food Chem.. 2007;103(1):55-61.
- [CrossRef] [Google Scholar]
- Determination of Antioxidants and Preservatives in Cosmetics by SPME Combined with GC–MS. Chromatographia. 2008;67:425-431.
- [CrossRef] [Google Scholar]
- G. Uğuz, A.E. Atabani, M.N. Mohammed, S. Shobana, S. Uğuz, G. Kumar, Ala'a H. Al-Muhtaseb, Fuel stability of biodiesel from waste cooking oil: A comparative evaluation with various antioxidants using FT-IR and DSC techniques, Biocatal. Agric. Biotechnol., 21 (2019), 101283, 10.1016/j.bcab.2019.101283
- Screening of antioxidant additives for biodiesel fuels. Renew. Sustain. Energy Rev.. 2018;82:2017-2028.
- [CrossRef] [Google Scholar]
- Synthesis of alkylphenols lactamomethyl derivatives (in Russian) Butlerov Comm.. 2018;54(6):124-131.
- [Google Scholar]
- S.V. Vorobyev, O.V. Primerova, L.V. Ivanova, V.D. Ryabov, V.N. Koshelev, Facile synthesis of phenolic derivatives, containing lactamomethyl substituents, Izv. Vyssh. Uchebn. Zaved. Khim. Khim. Tekhnol. (Russ. J. Chem. Chem. Technology), 62 (10) (2019), pp. 40-48, 10.6060/ivkkt.20196210.5930
- Lactamomethylation of Phenols: Synthesis, In Silico Study of Reactivity and Possible Applications. Chem. Proc.. 2021;3(1):81.
- [CrossRef] [Google Scholar]
- H.E. Zaugg, R.W. DeNet, J.E. Fraser, A.M. Kotre, Tscherniac-Einhorn reaction. II. Kinetics and mechanism, J. Org. Chem., 34 (1) (1969), pp. 14-18, 10.1021/jo00838a005.
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2021.103424.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







