Translate this page into:
Metabolomics techniques applied in the investigation of phenolic acids from the agro-industrial by-product of Carapa guianensis Aubl
⁎Corresponding author. alberdan@ufpa.br (Alberdan Silva Santos)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Processing of Carapa guianensis seeds to obtain oil on an industrial scale generates a significant amount of by-product, approximately 66% w/w, which is called cake and is a potential source of biomolecules, including simple phenolic structures. For this reason, studies were carried out on the chemical profiles of hydrolyzed extract from this agro-industrial by-product through High Performance Thin-Layer Chromatography (HPTLC) and Gas Chromatography coupled to Mass Spectrometry (GC–MS). These techniques were used to detect metabolic classes and/or groups, and to identify, for the first time, thirteen simple phenolic acids in this by-product. The sample antioxidant capacity was determined by methods of 2,2-diphenyl-1-picrylhydrazyl (DPPH•)and 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS•+) radicals direct sequestration. The hydrolyzed fraction showed a total of 63.47% in the relative abundance of the total of compounds, standing out: p-hydroxybenzoic acid (39.19%) and protocatechuic acid (3,4-dihydroxybenzoic acid) (5.62%), both from hydroxybenzoic acids and 3-(3,4-dihydroxyphenyl)lactic acid, (7.76%) hydroxycinnamic acids derivatives. In these results, the fraction rich in simple phenolic acids was obtained, attributing the prominent behavior of this matrix antioxidant activity, expressed by (IC50: of 16.42 µg/mL and 6.52 µg/mL for DPPH• and ABTS•+ radicals, respectively). The research demonstrated an alternative to applicability that involves sustainability from agro-industrial. These techniques were used to detect metabolic classes and/or groups, and to identify, for the first time, thirteen simple phenolic acids in this by-product, generating a process capable of converting biomass into a bioproduct, consisting of bioactive compounds, in addition to adding value to the industrial chain.
Keywords
Andiroba
Phenolics source
Metabolomics
Antioxidant capacity
Carapa
- HPTLC
-
High Performance Thin-Layer Chromatography
- GC–MS
-
Gas Chromatography coupled to Mass Spectrometry
- DPPH•
-
2,2-diphenyl-1-picrylhydrazyl
- ABTS•+
-
2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt
Abbreviations
1 Introduction
The chemical constitution of Carapa genus species seeds presents several groups of lipid molecules, highlighting the saponifiable compounds: oleic, palmitic, stearic, and linoleic acids; in addition to non-saponifiable compounds, such as steroids, triterpenes, and especially tetranortriterpenes, or limonoids, which are Meliaceae family chemotaxonomic markers (Ambrozin et al., 2006). Due to this metabolic diversity, chemical studies have been carried out in several species of this genus, especially in Carapa guianensis Aubl., known in the northern region of Brazil as “andirobeira” or “andiroba”, since the species is used as a source of bioactive compounds. The oil extracted from its seeds is used by local communities, as it has anti-inflammatory, analgesic, antiarthritic, antitumor, larvicidal, and antimicrobial properties (Graham et al., 2000; Miranda Jr et al., 2012; Nascimento et al., 2019; Penido et al., 2006).
C. guianensis oil is one of the main non-timber forest products in the Amazon region. It is sold mainly in open markets in the northern region of Brazil and markets in other regions of the country, as well as in the supply of export trade, being supplied as an input for pharmaceutical and cosmetic industries in Europe and the USA (Mendonça and Ferraz, 2007). Andiroba oil, on an industrial scale, is obtained by seeds (almonds and shells) cold pressing without the addition of organic solvents, which consists of a continuous and clean extraction, free of any chemical additive, generating a solid by-product (Mendonça et al., 2020). The by-product quality is a reflection of the main product characteristic. The “andiroba oil”, which is disputed by the cosmetics industry as raw material rich in emollient compounds, as the seed pressing process allows greater efficiency in the extraction of lipid metabolites (Uitterhaegen and Evon, 2017), however, many metabolites remain trapped in the seed cell wall, substances of greater molecular mass, mainly: polysaccharides, phenolic compounds, proteins, and bound lipids. In this case, cold pressing removes 34% w/w of extra virgin oil and a significant percentage of residual cake remains, approximately 66% w/w, of which only a small part is used in the production of repellent candles (Mendonça et al., 2020; Mendonça and Ferraz, 2007), the rest is discarded, burned, used in composting or rarely used as a supplement for pig feed.
The use of agro-industrial by-products is increasingly accentuated, focusing on sustainability requirements and scarcity of resources, in addition to the fact that by-products derived from husks and seeds after industrial processing may also contain fibers and bioactive compounds (Pelizer et al., 2007). In this way, the use of by-products originating from raw materials from renewable sources presents itself with great potential directed to several applications with prospects of generating wealth, in addition to showing itself as an alternative for the valorization of the production chain through by-products generation in the formulation of medicines, cosmetics, and food supplements. Thus, husks and seeds have an important use and numerous technological applications as they are rich in phenolic compounds, with polyphenols, oligophenols, and monophenols (Borges et al., 2019; Castro et al., 2018).
Although the presence of monophenols (simple phenolic acids) in plants is common, there are still no reports in the literature on the identification of these metabolites in Carapa genus species until this moment. Simple phenolic acids identified in the C. guianensis agro-industrial by-product biomass are derived from hydroxybenzoic and hydroxycinnamic acids formed in the shikimate biosynthesis route from deamination of L-phenylalanine and L-tyrosine aromatic amino acids, producing phenylpropanoids, cinnamic acid, and p-coumaric acid, respectively and their derivatives, being p-coumaric, caffeic, ferulic and sinapic acids (hydroxycinnamic acids) the most common. Except for caffeic acid, these hydroxycinnamic acids are essential for lignin formation, acting as precursors after undergoing reduction. On the other hand, in the cell wall, especially p-coumaric and ferulic acids, through ester bond, perform the function of connectors of hemicellulose with lignin. These metabolites can still be observed, both in free form and as esters, binding to other structures, as seen in rosmarinic acid – 3-(3,4-dihydroxyphenyl) lactic acid with caffeic acid (del Río et al., 2007; Dewick, 2009; Ferguson et al., 2003; Lozovaya et al., 1999; Marchiosi et al., 2020; Reinoso et al., 2018). Certainly, these metabolites also play the role of precursors of hydroxybenzoic acids due to the loss of two olefinic carbons in the side chain, that is, p-coumaric and ferulic acids derive the respective p-hydroxybenzoic and vanillic acids, in addition to the fact that cinnamic acids can be converted into benzoic acids and their derivatives (Cartea et al., 2011; Dewick, 2009; Heleno et al., 2015; Kumar and Goel, 2019; Marchiosi et al., 2020).
Phenolics are compounds of antioxidant nature, as they have the ability to inhibit oxidative cascade, responsible for deleterious health effects, which transforms them as inputs in the composition of numerous drugs with biological properties, such as: healing, anti-inflammatory, anti-tumor, anti-diabetic, neuroprotective, hepatoprotective, etc., as well as they are widely used in dietary supplements and as additives in food preservation (Kumar and Goel, 2019; Neha et al., 2019; Ou and Kwok, 2004; Prior et al., 2005) In pharmaceutical and cosmetic formulations, preservatives based on p-hydroxybenzoic acid stand out (one of the compounds identified with greater abundance in area in the C. guianensis agro-industrial by-product biomass), which serves as a precursor for the production of its esters, called parabens, characterized by having a broad spectrum of activity against filamentous fungi, yeasts and bacteria; they have hydrophilic character, without organoleptic properties (colorless, odorless and tasteless) and low toxicity, which makes their use relatively safe according to regulatory agencies (Fernandes et al., 2013; Soni et al., 2005). They are responsible for increasing product lifetime on shelves since they have the function of preventing the proliferation of microorganisms, which cause numerous pathologies, or even prevent the compromise of quality due to final product degradation (Soni et al., 2005).
Therefore, due to this range of applicability, the use of simple phenolic acids occupies a strategic place in different industrial branches as they are essential components applied in the production of cosmetics, pharmaceutical products, and food (Liu et al., 2019; Salvachúa et al., 2018).
The objective of this work was to investigate the chemical profile of C. guianensis hydrolyzed extract from its agro-industrial by-product, describing for the first time the simple phenolic acids that occur in this species. In this way, this work proposes a new contribution to the species metabolomic study, as well as the valorization of its productive chain from the metabolic description of bioactive substances in hydrolyzed extract, allowing the description of a by-product that can be directed to cosmetic, food and pharmaceutical applications.
2 Material and methods
2.1 Reagents and materials
All chemical reagents and solvents used in the extractions, chromatographic and spectroscopic analysis were analytical grade and were provided by TEDIA® COMPANY (Fairfield, USA). Ultrapure water with a resistivity of 18.3 MΩ/cm3 produced in a Scholar UV UP 900 model water purification system (BIOHUMAN, Curitiba, Brazil) was used for the preparation of the alkaline and acidic solutions and in the elution system. Thin- layer chromatography (TLC) analysis was performed on SiliaPlate TLC chromatographic plates, with aluminum support, silica, 200 µm, 20 × 20 cm, F 254 (SiliCycle Inc., Quebec, Canada). Gallic acid, Trolox, DPPH (1,1-diphenyl-2-picrylhydrazyl), and ABTS [2,2′-Azino-bis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt were purchased from Sigma Aldrich (St Louis, MO, USA).
2.2 Obtaining and processing of the agro-industrial by-product
The cake resulting from Carapa guianensis seeds mechanical pressing was acquired from local industry, in the municipality of Ananindeua, in the state of Pará, the northern region of Brazil, georeferenced coordinates 1° 22′ 26.8″ S; 48° 23′ 41.3″ W, SisGen registration, number A3B8C54. This cake was dehydrated in a kiln with a temperature (T) of 45 °C and humidity (U%) of 20% until it reached a constant mass. The dehydrated material was crushed and sieved in a 40 mesh and then the resulting sample was vacuum packed and stored at 25 °C.
2.3 Sample treatment
The dehydrated sample was subjected to successive extractions, to separate constituents other than phenolic acids from the sample, obeying the increasing gradient of polarity, with organic solvents: hexane (Hex), ethyl acetate (AcOEt), and ethanol (EtOH). It was used an aliquot of 1 g to 10 mL of solvent per step. Each extraction was carried out by a simple batch and the solvents were renewed twice more, totaling 3 batches per extractor solvent. Each extraction lasted 60 min at 40 °C under ultrasound-assisted agitation in the UNIQUE equipment, model USC 5000 A, frequency of 40 kHz, 155 Watts RMS-USA.
2.4 Extraction of simple phenolic acids (ester-linked)
In an aliquot of 100 mg sample (after treatment, item 2.3) 400 µL of NaOH (4 M), were added. Then, the mixture was stirred at maximum speed on a vortex mixer (Vision Bionex KMC-1300 V, Indonesia) for 30 s. The mixture was subjected to ultrasound-assisted agitation in the UNIQUE equipment, model USC 5000 A, 40 kHz frequency, 155 Watts RMS-USA at 70 °C for 60 min and cooled for 10 min. Subsequently, 500 µL of HCl (6 M) was added, and the aforementioned homogenization, ultrasound-assisted agitation, and cooling steps were repeated. pH was measured to ensure a value less than or equal to 3, and then, 400 µL of AcOEt were added, stirred in a vortex mixer for 30 s, centrifuged for 2 min at 10000 rpm (Microhemato® centrifuge, mod. 2410, Fanen®, São Paulo, Brazil), and the supernatant, collected. This procedure was carried out in five batches, and the supernatant solutions have been brought together. Next, the solvent was evaporated in an automated system at 5 psi and 35 °C for 20 min, and the hydrolyzed extract was stored for further analysis.
2.5 Characterization of hydrolyzed extract
The characterization of the hydrolyzed extract was carried out through High Performance Thin-Layer Chromatography (HPTLC – CAMAG®, Muttenz, Switzerland) and Gas Chromatography coupled to Mass Spectrometry (GC–MS – Thermo Fisher Scientific™, USA) analytical techniques, both associated with the identification of compounds classes respectively present.
2.5.1 HPTLC analysis
The chemical profile of the sample's metabolic classes was performed by HPTLC. 10 mg aliquots of hydrolyzed extract and 1 mg of each of the standards: β-amyrin (identification for triterpenes), 7-deacetoxy-7-oxogedunin (identification for limonoids), and gallic acid (identification for phenolic compounds), were solubilized, separately, in 1000 mL of methanol each. Next, the solutions were applied to Aluminum F-254 60 Å silica gel plates through ATS 4 (Automatic TLC Sample 4) application module, using the spay-on mode. Injections of 50 µg/spot (5 µL) of hydrolyzed extract and 5 µg/spot (5 µL) of adopted standards were performed. After applications, the chromatoplates were eluted in glass vat by using the combination of the system containing the mobile phase: Dichloromethane/Methanol/HCOOH/H2O (7.5:1.5:0.5:0.5), with 70 mm chromatographic path. Then, each plate was revealed with selective reagents to terpenes/limonoids (vanillin sulfuric acid – VSA) and phenolic compounds (Fast Blue Salt – FBS), followed by photo documentation by using TLC Visualizer module in white light. For data processing, it was used WinCats – Planar Chromatography Manager, version 1.46 software.
2.5.2 GC–MS analysis
The sample chromatographic profile was analyzed by using GC–MS. A 2 mg aliquot of sample was derivatized with 100 µL of N, O-Bis(trimethylsilyl)trifluoroacetamide (BSTFA) + 1% of trimethylchlorosilane (TMCS). Then, the sample was agitated in a vortex mixer for 1 min in maximum agitation and submitted to a UNIQUE, model USC 5000 A of 40 kHz frequency, 155 Watts RMS ultrasonic bath for 60 min at 60 °C. Subsequently, 400 µL of AcOEt was added to the system and stirred in a vortex mixer for 1 min and centrifuged for 2 min at 10000 rpm. The supernatant was transferred to a 2 mL glass flask with lid and septum and then analyzed on the Thermo Scientific Trace 1300 gas chromatograph (GC) coupled to a Thermo Scientific MS-ISQ Single Quadrupole mass spectrometer (MS) with autosampler. AI 1310, equipped with ZB-5HT INFERNO (30 m × 0.25 mm × 0.10 µm) capillary column and using Helium as carrier gas at a flow rate of 1 mL/min. The sample injection was 1.0 µL in Splitless mode. The injector operated at 250° C and the oven temperature programming started at 200 °C, remaining for 2 min and rising to 300 °C (20 °C/min) and maintaining for 10 min. The MS-ISQ operated with an interface at 275 °C, ionization's source at 230 °C, using a solvent wait for 5 min with mass range (50–1000 Da), and electronic ionization at 70 eV. The compounds identifications were carried out by comparing mass spectra with those of commercial libraries, NIST2011, WILEY2009, and FAMES2011.
2.6 In vitro evaluation of antioxidant capacity
The evaluation of the antioxidant capacity of hydrolyzed extract and its constituents was carried out based on the adaptation of the methods described by Załuski et al. (2018) and Sridhar and Charles (2019) for in situ analysis of plant samples followed by its quantification.
2.6.1 Direct bioautography assay (HPTLC)
The bioautography method allows the evaluation of the antioxidant capacity of complex matrices, such as plant extracts individually for each of their constituents, allowing to associate the chromatographic separation obtained by HPTLC with indicative reactions of biological activities. For the antioxidant assay by bioautography, the same parameters adopted in item 2.5.1 were used, with gallic acid as standard (positive control). After elution, the plate was revealed with 0.5% DPPH• (1,1-diphenyl-2-picryl-hydrazyl) solution and stored for 60 min protected from light and oxygen at 25 °C. The plate photo documentation was made through the Visualizer module under visible radiation, with bioactive compounds appearing with yellow-white coloring (Załuski et al., 2018).
2.6.2 DPPH• assay
The ability of the hydrolyzed extract to sequester DPPH• (1,1-diphenyl-2-picrylhydrazyl) radicals was determined by the method described by Sridhar and Charles (2019), with some modifications. The quantification was performed by spectrophotometry, by using ELISA EspectraMax i3 reader (Molecular Devices Inc. - Sunnyvale, CA, USA) in a 96-well microplate. The extract effect was evaluated at concentrations of 1, 5, 25, 50, and 100 µg/mL under DPPH• solution at 60 µg/mL adjusted to absorbance to 0.866. The ELISA plate was incubated for 30 min in the dark and at room temperature. After the incubation period, the absorbance of the solutions was obtained by adopting a wavelength of 517 nm. Results were expressed by the effective concentration for the sequestration of half of the free radicals (IC50). The experiments were carried out in triplicate and using trolox as the positive control (Sigma Aldrich – St Louis, MO, USA). The inhibition result was calculated in sequestered radicals percentage, according to equation (1):
In which Acontrol is the absorbance of the control and Aextract is the absorbance of the hydrolyzed extract
2.6.3 ABTS•+ assay
The antioxidant activity assay of hydrolyzed extract facing the radical (ABTS•+) cation used SpectraMax® i3, SoftMax® Pro7 spectrophotometer (Molecular Devices, Sunnyvale, CA, USA), and the adaptation of the method described by Sridhar and Charles (2019). The stock solution of radical cation was prepared by reacting the aqueous solution ABTS (7 mM) with 2.45 mM of potassium persulfate (Sigma Aldrich – St Louis, MO, USA) in equal amounts. This reaction lasted 16 h, at room temperature and in the shelter of light. It was subsequently diluted and adjusted to an absorbance of 0.510 ± 0.011, at a wavelength of 734 nm. The 60 µg/mL ABTS•+ solution volume was added in extract and positive control trolox (1, 5, 25, 50 and 100 µg/mL) concentrations in a 96-well microplate. The reaction medium was incubated at room temperature for 10 min in the shelter of light, and its absorbances were determined at a wavelength of 734 nm. As in DPPH• assay, item 2.5.1., results were expressed as a function of free radicals sequestered, calculated according to equation (1) described previously.
2.6.4 Statistical analysis
IC50 values of hydrolyzed extract and standard were estimated using a nonlinear regression model built by graphical plotting of inhibition percentage results in function of samples concentration logarithm. The determinations were performed in triplicate and data obtained were subjected to statistical analysis by using GraphPad Prism® software version 8.0.1 (GraphPad Software Inc. – San Diego, CA, USA). Data were treated by non-linear regression by adopting a logistic equation with 5 parameters and the result was plotted according to dose–response inhibition of antioxidant capacity of the pattern (Trolox) and the hydrolyzed extract. All statistical tests were performed with level significance with p < 0.05.
3 Results and discussion
3.1 Characterization by HPTLC
HPTLC chemical profile of hydrolyzed extract allowed to evaluate the metabolic complexity of phenolic and non-phenolic compounds present in the cell wall after alkaline hydrolysis. By comparing retention factors, Rf, of patterns used with those recorded in the extract, and by analyzing the bands after selective chemical developers use (Karthika et al., 2014; Salazar et al., 2018), it was possible to detect the presence of chemical markers that indicate the importance of the method applied (Fig. 1).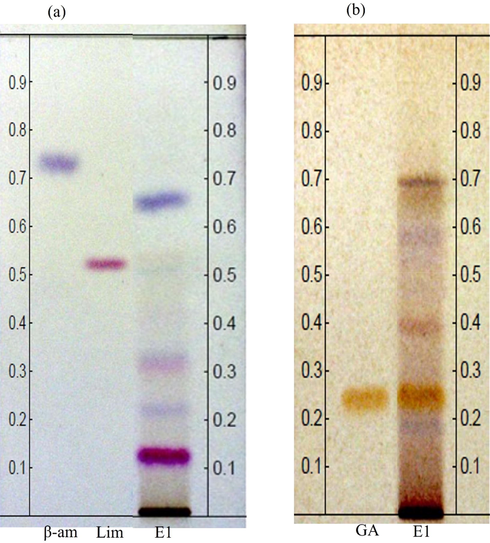
Chemical profile via HPTLC. Chromatoplate analysis, visualized in white light. (a) TLC plate, β- amirine (β-am), 7-deacetoxy-7-oxogedunine (Lim) and C. guianensis hydrolyzed extract (E1); derivatized with VSA reagent. (b) TLC plate, gallic acid (GA) and C. guianensis hydrolyzed extract (E1), derivatized with FBS reagent.
Based on the methods described by Karthika et al. (2014) and Salazar et al. (2018);, the use of VSA solution as a chemical developer, allows the detection of fatty acids (blue), in addition to terpenes, limonoids, and steroids (purple). Thus, it is possible to determine the low occurrence of substances of these classes, because, after the revelation of the plate with VSA, few bands were formed with blue and/or purple tones. The Rf comparison of β-amyrin (β-am) and 7-deacetoxy-7-oxogedunin (Lim) standards, Rf 0.72 and 0.53 respectively, allowed to observe the absence of β-amirine standard in the extract, as well as, based on the mass ratio and staining intensity, it is possible to determine the presence in a low quantity of limonoid 7-deacetoxy-7-oxogedunin in the extract (0.52) (Fig. 1a). In this same sense, it is possible to observe the presence of fatty acids (0.35), terpenes, steroids (0.68), and possibly saponin co-eluting with another compound also in Rf (0.13), even in low concentrations.
When using the selective developer for phenolic compounds (FBS), it was possible to observe the diversity of these compounds in the hydrolyzed extract (Fig. 1b), evidenced by the formation of bands with yellow or brown tones (Salazar et al., 2018), which shows the efficiency of the hydrolysis applied for the release of non-structural phenolic compounds linked by ester to the cell wall polymers of the seeds of the species. Thus, the chemical profile of this matrix is following the histochemical studies carried out by (Santos, 2020), which indicate the presence of non-structural phenolic substances in the composition of the envelope of C. guianensis seeds and these are responsible for the protection of the embryo. And in the literature, reports that prove the existence of phenolic compounds in extracts obtained from the seeds of the species of Carapa spp have not yet been found. As for phenolics present in the extract, the band in Rf 0.23 stands out, with an intense brown coloring, suggestive of marked concentration. Through standard use, gallic acid (GA), it is possible to determine, by comparing Rfs, that the chromatographic band in 0.23 corresponds to the same phenolic compound. Gallic acid is the precursor of tannins, but it is classified with the C6-C3 structure and is derived from hydroxybenzoic acid including gallic, protocatechuic, vanillic, syringic, and gentisic acids. In the same sense, phenolics with C6-C3 structures present a central structure of hydroxycinnamic acid, including caffeic, p-coumaric, ferulic, and sinapic acids (Sinosaki et al., 2020). Gallic acid is a metabolite that draws attention to its versatile applications as it can combat oxidative cascade related to the most diverse pathologies (Kumar and Goel, 2019; Neha et al., 2019; Ou and Kwok, 2004). Certainly, this metabolite is also used as raw material in the composition of skincare line products due to its ability to inhibit tyrosinase, presenting anti-melanogenic activity. This simple phenolic acid is also fundamental in the composition of biofilm packaging for food preservation since studies related to this area demonstrate that biofilms having gallic acid as a chemical constituent of these polymers have a higher moisture barrier property, resistance to traction, thermal stability, and greater protection from UV light (Kim, 2007; Pokhrel and Yadav, 2019; Zhang et al., 2019).
3.2 Characterization by GC–MS
The characterization of C. guianensis hydrolyzed extract, by GC–MS, allowed the identification of 21 organic compounds, among them, 13 simple phenolic acids which presented a total relative abundance of 63.47% stand out, confirming the efficiency of the hydrolysis method applied in the agro-industrial by-product, which allowed the breaking of the bonds of esters and the release of the simple phenolic acids from the cell wall, besides, it allowed, in a pioneering way, the validation of simple phenolic acids hypothesis in the chemical composition of Carapa genus species, because so far, no work had reported the presence of this class in the seeds. The other identified substances (36.53%) correspond to chemical classes commonly found in C. guianensis industrial oil, such as terpenes, steroids, limonoids, in addition to fatty acids (Nascimento et al., 2019), indicating that even after industrial processing, these substances are still present in the resulting biomass (Table 1). RT = Retention times. Total phenolic acids = 63.47%. Total other organic compounds: terpenes, steroids, limonoids and fatty acids = 36.53%. MI = molecular ion.
Simple Phenolic acid profile
Abundance, fragmentation and molecular ion
Phenolic Compounds
RT
%
m/z (abundance)
MI
1
4-hydroxybenzoic acid
14.51
39.19
267 (100); 223 (98); 73 (96)
282 (M+)
2
4-hydroxy-phenyl acetic acid
14.66
0.18
73 (100); 43 (81); 46 (26)
296 (M+)
3
4-hydroxy-3-methoxybenzoic acid
16.13
1.67
73 (100); 297 (33); 267 (25)
312 (M+)
4
3,4-dihydroxybenzoic acid
16.97
5.62
73 (100); 193 (67); 45 (24)
370 (M+)
5
3,4-dihydroxyphenyl acetic acid
17.05
1.09
73 (100); 179 (26); 75 (14)
384 (M+)
6
4-hydroxy-3,5-dimethoxybenzoic acid
17.84
0.36
73 (100); 43 (35); 45 (21)
342 (M+)
7
3-(4-hydroxyphenyl)lactic acid
17.92
0.43
73 (100); 179 (47); 147 (17)
398 (M+)
8
4-hydroxycinnamic acid
18.24
0.10
73 (100), 75 (34), 45 (30)
308 (M+)
9
3,4-dihydroxyhydrocinnamic acid
18.44
3.82
73 (100); 179 (63); 75 (20)
398 (M+)
10
3,4,5-trihydroxybenzoic acid
18.71
1.52
73 (100); 281 (19); 45 (12)
458 (M+)
11
Caffeic acid
19.94
1.57
73 (100), 219 (82), 396 (76)
396 (M + )
12
3-(3,4-dihydroxyphenyl)lactic acid
20.03
7.76
73 (100); 267 (89); 179 (33)
486 (M+)
13
Ferulic acid
20.11
0.16
73 (100); 249 (58); 75 (23)
338 (M+)
Total
63.47
Among the phenolic substances found, 5 compounds derived from hydroxybenzoic acids were evidenced (Fig. 2), such as 4-hydroxybenzoic acid (p-hydroxybenzoic acid), silylated m/z 282 (M + ) identified as being the main constituent of the extract, with a relative abundance of 39.19%. Besides them, it was found: 3,4-dihydroxybenzoic acid (protocatechuic acid) (5.62%), 4-hydroxy-3-methoxybenzoic acid (vanillic acid) (1.67%), 3,4,5-trihydroxybenzoic acid (gallic acid) (1.52%) and 4-hydroxy-3,5-dimethoxybenzoic acid (0.36%) (Table 1).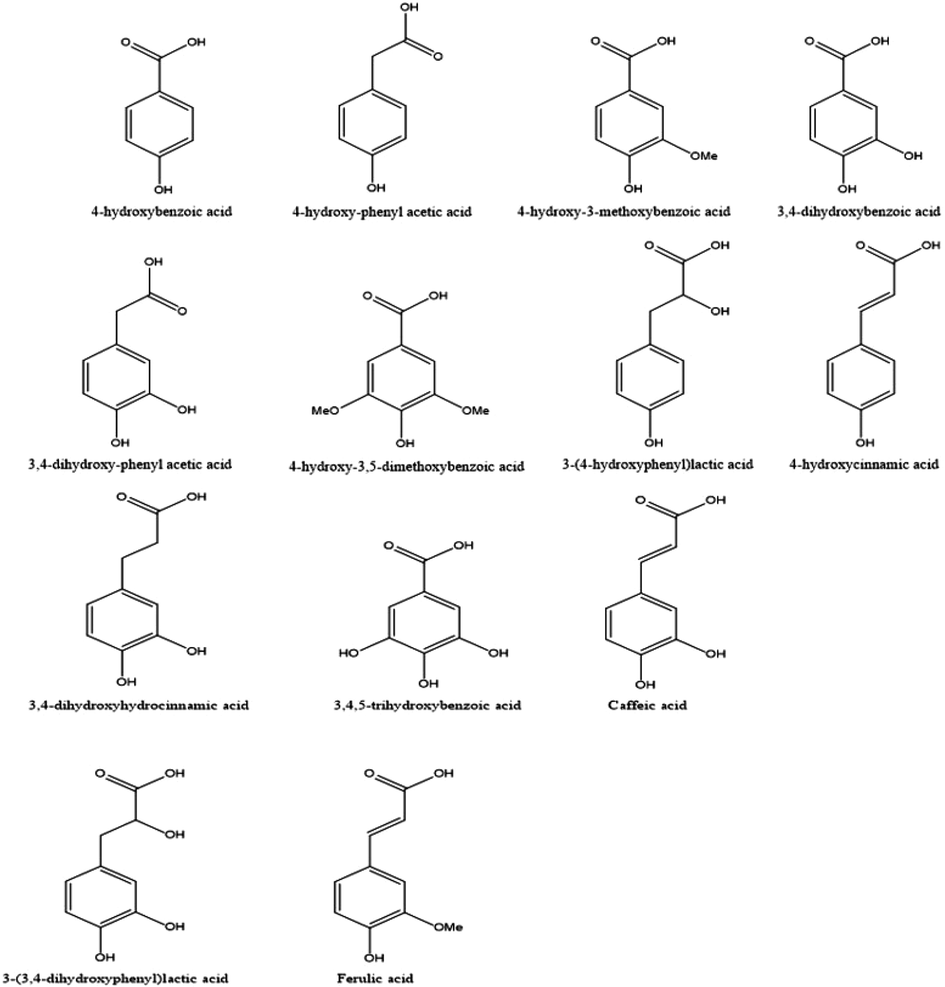
Simple phenolic acids structures characterized in the C. guianensis hydrolyzed extract via GC–MS.
The analysis also indicated the presence of phenolic compounds derived from phenyl-lactic acids and phenylacetic acids, among them, their respective: 3- (3,4-dihydroxyphenyl) lactic acid (Danshensu) which stands out with approximately 7.76% abundance and 3-(4-hydroxyphenyl) lactic acid (0.43%), both are coenzyme A esters, which are involved in the formation of rosmarinic acid (Deve et al., 2014; Dewick, 2009; Zhao et al., 2008), in addition to 3,4-dihydroxyphenyl acetic acid (1.09%) and 4-hydroxy-phenyl acetic acid (0.18%). The phenyl-lactic acids and phenylacetic acids are compounds derived from 4-hydroxyphenylpyruvic acid, an important metabolite formed from the amino acid L-tyrosine. Danshensu is an important major antioxidant agent present in the roots of Salvia miltiorrhiza, it is widely used in traditional Chinese medicine, due to its cardiovascular and protective effects against oxidative damage (Zhao et al., 2008). And the antihyperglycemic effects of Artocarpus heterophyllus species are associated with the 4-hydroxy-phenyl acetic acid in its composition (Deve et al., 2014).
It was also possible to observe the 3,4-dihydroxyhydrocinnamic acid (3.82%), in addition to the characterization of the most common phenylpropanoids derivatives: caffeic acid (1.57%), ferulic acid (0.16%), and 4-hydroxycinnamic acid (p-coumaric acid) (0.1%), from these latter ones, with caffeic acid exception, they are primordial acids for lignin formation. They are also the ones that, in the cell wall, establish, through ester bonds, the connection of hemicellulose with lignin (del Río et al., 2007; Ferguson et al., 2003; Lozovaya et al., 1999; Marchiosi et al., 2020; Reinoso et al., 2018; Sun et al., 2002) (Table 1).
Due to their antioxidant nature, simple phenolic acids are considered multipurpose biomolecules, as they are used for therapeutic purposes, in the formulation of dietary supplements, food gels, and edible films. In the cosmetic sector, they are used as raw materials, in the formulation of skincare lines, applied as antimelanogenic agents, and photoprotection (Kim, 2007; Kumar and Goel, 2019; Neha et al., 2019; Oliveira et al., 2021; Ou and Kwok, 2004).
Thus, the research highlights the unprecedented discovery of these compounds in the seeds of Carapa genus, especially in C. guianensis agro-industrial by-product, which refers to the valorization of this biomass rich in bioactive compounds, since they are metabolites that can be targeted in a wide industrial applications spectrum, admitting the valorization of this by-product, which until now has not presented an economical application of significant added value.
3.3 Antioxidant capacity of hydrolyzed extract
The antioxidant capacity of the hydrolyzed extract of C. guianensis was determined from the scavenging of DPPH• and ABTS•+ radicals, in which through the minimal inhibitory concentration for the scavenging of 50% of the radicals (IC50), it highlighted the expressive capacity of hydrolyzed extract in scavenging free radicals, DPPH• (IC50 16.42 µg/mL) and ABTS•+ (IC50 6.52 µg/mL), the results show values close to those found for the Trolox positive control, IC50: 7.31 µg/mL (DPPH•) and 3.75 µg/ml (ABTS•+) (table 2). The comparative analysis of the points of the inhibition curve of the hydrolyzed extract and the trolox against the radical solutions (Fig. 3), allowed us to identify the similar behavior between the analytes, mainly in the scavenging of ABTS•+ radicals (Fig. 3b), in which it is observed the overlapping of the curves, thus emphasizing the important antioxidant activity of the hydrolyzed extract. Values were expressed by the concentration required for the decay of 50% of free radicals. All experiments presented significance with p < 0.05.
Samples
DPPH• (IC50 μg/mL)
ABTS•+ (IC50 μg/mL)
Trolox
7.31 ± 0,88
3.75 ± 0,40
Hydrolyzed extract
16.42 ± 0,98
6.52 ± 0,77
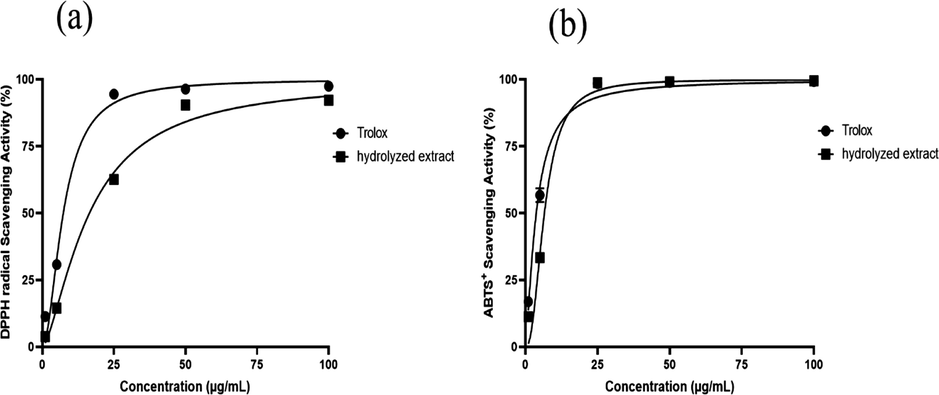
Graphs with inhibition levels for the radicals from 1, 5, 25, 50 and 100 µg/mL concentrations of C. guianensis hydrolyzed extract and positive control trolox; (a) DPPH• and (b) ABTS•+.
The antioxidant activity determined by spectroscopy may be closely related to the phenolic acids identified by GC–MS and HPTLC, because, according to, simple phenolic acids are the major contributors to total antioxidant activity present in wheat (90%), corn (87%) and oats (58%), which may enable the use of these compounds in the formulation of functional foods. In addition, being strong allies in the prevention and/or treatment of diseases and enzyme inhibitors. In this way, the study highlights the important and unpublished discovery of these compounds in Carapa genus hydrolyzed extract, proposing a new contribution to the species metabolomic study, as well as the valorization of its productive chain from the metabolic description of bioactive substances in the hydrolyzed extract, besides, it is confirmed the C. guianensis agro-industrial by-product potential as a supplier of a bioproduct constituted by bioactive compounds that can be directed in food, pharmaceutical, and cosmetic applications.
3.4 Antioxidant activity by direct bioautography assay (HPTLC-DB)
Antioxidant assay by direct bioautography confirmed the capacity of the constituents of the hydrolyzed extract to scavenge DPPH• radicals, The in situ analysis used in the same chromatographic parameters as the chemical profile (item 2.6.1), allowed to attribute the antioxidant action of the extract to the various phenolic compounds identified by HPTLC and GC–MS, result evidenced by yellow-white bands throughout the chromatographic path, especially in the Rf's equivalent to the phenolic compounds identified in the chemical profile by HPTLC (Fig. 4).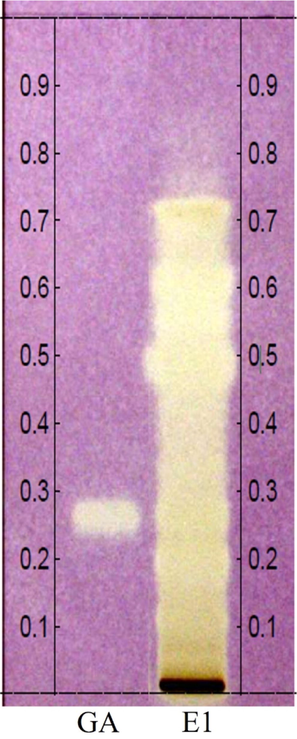
Antioxidants direct bioautography by HPTLC. Gallic acid (GA) and C. guianensis hydrolyzed extract (E1). Derivatized chromatoplate with DPPH• solution.
In addition, it was possible to observe the same behavior of the positive control employed, gallic acid, in which both the pattern band and the region referring to the gallic acid occurring in the hydrolyzed extract presented the same stabilizing action as the DPPH• radical (Fig. 4), which is in accordance with Kumar and Goel, (2019), simple phenolic acids are important antioxidant compounds because they are capable of eliminating free radicals, responsible for the effects of the oxidative cascade, characterizing them as multipurpose agents, that is, their use is associated with the composition of several medicines, in addition to other branches of application, for example, as occurs with gallic acid, which can be a raw material in the composition of cosmetics or can also be used as a constituent of biofilm packaging, and as for p-hydroxybenzoic, vanillic, caffeic acids, they can be used in the formulation of photoprotection (Kim, 2007; Oliveira et al., 2021; Pokhrel and Yadav, 2019; Zhang et al., 2019).
4 Conclusion
This was the first study of the composition of simple phenolic acids of the species Carapa guianensis, especially its agro-industrial by-product after obtaining the oil from the seeds of the species. After the extraction of the non-structural phenolic compounds linked by ester to the seed cell wall polymers, its chemical profile was found, which can be directed towards the investigation of bioactive compounds, mainly simple phenolic acids. Among the hydroxybenzoic acids identified, p-hydroxybenzoic acid (39.19%) stands out as the major compound, an important constituent in the formulation of cosmetic products. In the analysis, it was possible to characterize the hydroxycinnamic acid derivatives as 3,4-dihydroxyhydrocinnamic, caffeic, ferulic, 4-hydroxycinnamic acids. In addition to the derivatives of phenylacetic and phenyl-lactic acids, both are coenzyme A esters, which are involved in the formation of rosmarinic acid. The 3-(3,4-dihydroxyphenyl)lactic acid (Danshensu) (7.76%) is the compound responsible for the antioxidant property of Salvia species. The phenolic acids identified in the by-product of C. guianensis are the major contributors to the antioxidant nature of certain foods, they are also the same responsible for the prominent behavior of the antioxidant activity present in this matrix, quantitatively noticed through the DPPH• and ABTS•+ free radicals capture assays, results demonstrated in this research, that is, the study indicates an alternative for an application that involves sustainability and the search for new sources of substances, through a process capable of converting considerable biomass (66% of the agro-industrial by-product of C. guianensis) in a bioproduct, consisting of phenolic acids, possibly bioactive, and at the same time adding value to the industrial chain.
Acknowledgments
The authors would like to thank CNPq and PROPESP/UFPA for granting a scholarship and financial support.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Limonoids from andiroba oil and Cedrela fissilis and their insecticidal activity. J. Braz. Chem. Soc.. 2006;17:542-547.
- [CrossRef] [Google Scholar]
- Mosquiticidal and repellent potential of formulations containing wood residue extracts of a Neotropical plant, Tabebuia heptaphylla. Ind. Crop. Prod.. 2019;129:424-433.
- [CrossRef] [Google Scholar]
- Green coffee seed residue: a sustainable source of antioxidant compounds. Food Chem.. 2018;246:48-57.
- [CrossRef] [Google Scholar]
- Composition of non-woody plant lignins and cinnamic acids by Py-GC/MS, Py/TMAH and FT-IR. J. Anal. Appl. Pyrol.. 2007;79(1-2):39-46.
- [CrossRef] [Google Scholar]
- Extraction process optimization of polyphenols from Indian Citrus sinensis – as novel antiglycative agents in the management of diabetes mellitus. J. Diabetes Metab. Disord.. 2014;13:1-10.
- [Google Scholar]
- Dewick, P.M., 2009. Medicinal Natural Products: A Biosynthetic Approach, 3rd ed., England.
- Bacterial antimutagenesis by hydroxycinnamic acids from plant cell walls. Mutat. Res.. 2003;542(1-2):49-58.
- [CrossRef] [Google Scholar]
- Estudo das relações entre estrutura e atividade de parabenos: uma aula prática. Quim. Nova. 2013;36(6):890-893.
- [Google Scholar]
- Plants used against cancer – an extension of the work of Jonathan Hartwell. J. Ethnopharmacol.. 2000;73(3):347-377.
- [CrossRef] [Google Scholar]
- Bioactivity of phenolic acids: metabolites versus parent compounds: a review. Food Chem.. 2015;173:501-513.
- [CrossRef] [Google Scholar]
- TLC and HPTLC Fingerprint profiles of different bioactive components from the tuber of solena amplexicaulis. J. Pharmacogn. Phytochem.. 2014;3:198-206.
- [Google Scholar]
- Antimelanogenic and antioxidant properties of gallic acid. Biol. Pharm. Bull.. 2007;30(6):1052-1055.
- [Google Scholar]
- Phenolic acids: natural versatile molecules with promising therapeutic applications. Biotechnol. Reports. 2019;24:e00370.
- [CrossRef] [Google Scholar]
- Hydroxycinnamic acids release during bioconversion of corn stover and their effects on lignocellulolytic enzymes. Bioresour. Technol.. 2019;294:122116.
- [CrossRef] [Google Scholar]
- Cold alkali can extract phenolic acids that are ether-linked to cell wall components in dicotyledonous plants (buckwheat, soybean, and flax) Phytochemistry. 1999;50(3):395-400.
- [Google Scholar]
- Biosynthesis and metabolic actions of simple phenolic acids in plants. Phytochem. Rev.. 2020;19(4):865-906.
- [CrossRef] [Google Scholar]
- Extração de óleo de andiroba por prensa: rendimento e qualidade de óleo de sementes submetidas a diferentes teores de água e temperaturas de secagem. Sci. For.. 2020;48(125)
- [CrossRef] [Google Scholar]
- Óleo de andiroba: processo tradicional da extração, uso e aspectos sociais no estado do Amazonas. Brasil. Acta Amaz.. 2007;37(3):353-364.
- [CrossRef] [Google Scholar]
- Antiplasmodial activity of the andiroba (Carapa guianensis Aubl., Meliaceae) oil and its limonoid-rich fraction. J. Ethnopharmacol.. 2012;142:679-683.
- [CrossRef] [Google Scholar]
- Lipidomic profiles from seed oil of Carapa guianensis Aubl. and Carapa vasquezii Kenfack and implications for the control of phytopathogenic fungi. Ind. Crops Prod.. 2019;129:67-73.
- [CrossRef] [Google Scholar]
- Medicinal prospects of antioxidants: a review. Eur. J. Med. Chem.. 2019;178:687-704.
- [CrossRef] [Google Scholar]
- Photoprotective and antiglycation activities of non-toxic Cocos nucifera Linn. (Arecaceae) husk fiber ethanol extract and its phenol chemical composition. Ind. Crop. Prod.. 2021;162:113246.
- [CrossRef] [Google Scholar]
- Ferulic acid: pharmaceutical functions, preparation, and applications in foods. J. Sci. Food Agric.. 2004;84(11):1261-1269.
- [CrossRef] [Google Scholar]
- Utilização de resíduos agro-industriais em processos biotecnológicos como perspectia de redução do impacto ambiental. J. Technol. Manag. Innov.. 2007;2:118-127.
- [Google Scholar]
- Antiinflammatory effects of natural tetranortriterpenoids isolated from Carapa guianensis Aublet on zymosan-induced arthritis in mice. Inflamm. Res.. 2006;55(11):457-464.
- [CrossRef] [Google Scholar]
- Functionalization of chitosan polymer and their applications. J. Macromol. Sci. parte A Pure Appl. Chem.. 2019;56(5):450-475.
- [Google Scholar]
- Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem.. 2005;53(10):4290-4302.
- [Google Scholar]
- Fate of p - hydroxycinnamates and structural characteristics of residual hemicelluloses and lignin during alkaline – sulfite chemithermomechanical pretreatment of sugarcane bagasse. Biotechnol. Biofuels. 2018;11(1)
- [CrossRef] [Google Scholar]
- Chemical composition, antioxidant activity, neuroprotective and anti-inflammatory effects of cipó-pucá (Cissus sicyoides L.) extracts obtained from supercritical extraction. J. Supercrit. Fluids. 2018;138:36-45.
- [CrossRef] [Google Scholar]
- Bioprocess development for muconic acid production from aromatic compounds and lignin. Green Chem.. 2018;20(21):5007-5019.
- [CrossRef] [Google Scholar]
- Structural study of phenolic acids by triple quadrupole mass spectrometry with electrospray ionization in negative mode and H/D isotopic exchange. J. Braz. Chem. Soc.. 2020;31:402-408.
- [Google Scholar]
- Santos, A.S., 2020. Aspectos químico, biológico, botânico, sanzonal, microbiológico e biotecnológico das sementes de espécies de andirobeiras (Carapa spp.), 1 ed., ed. Appis, Curitiba. ISBN 978-65-5523-150-2.
- Safety assessment of esters of p-hydroxybenzoic acid (parabens) Food Chem. Toxicol.. 2005;43(7):985-1015.
- [CrossRef] [Google Scholar]
- In vitro, antioxidant activity of Kyoho grape extracts in DPPH• and ABTS•+ assays: estimation methods for EC50 using advanced statistical programs. Food Chem.. 2019;275:41-49.
- [CrossRef] [Google Scholar]
- Ester and ether linkages between hydroxycinnamic acids and lignins from wheat, rice, rye, and barley straws, maize stems, and fast-growing poplar wood. Ind. Crops Prod.. 2002;15(3):179-188.
- [Google Scholar]
- Twin-screw extrusion technology for vegetable oil extraction: a review. J. Food Eng.. 2017;212:190-200.
- [CrossRef] [Google Scholar]
- HPTLC-profiling of eleutherosides, mechanism of antioxidative action of eleutheroside E1, the PAMPA test with LC/MS detection, and the structure–activity relationship. Saudi J. Biol. Sci.. 2018;25(3):520-528.
- [CrossRef] [Google Scholar]
- Effect of grafting method on the physical property and antioxidant potential of chitosan film functionalized with gallic acid. Food Hydrocoll.. 2019;89:1-10.
- [CrossRef] [Google Scholar]
- Characterization of the radical scavenging and antioxidant activities of danshensu and salvianolic acid B. Food Chem. Toxicol.. 2008;46(1):73-81.
- [CrossRef] [Google Scholar]







