Translate this page into:
Quality evaluation for Dipacus asperoides from Enshi areas and optimization extraction of saponins and organic acids and its application
⁎Corresponding authors at: College of Biological Science and Technology, Hubei Minzu University, China (Z. Zeng). yangwn@mail.ccnu.edu.cn (Wannian Yang), zengzhi11@outlook.com (Zhi Zeng)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Dipacus asperoides (DA), a traditional perennial herb, has distributed wildly in China, especially Wuhe Xuduan known as genuine herb. To evaluate DA’s quality, we collected ten samples from eight counties in Enshi and determined the contents of total saponins (TS) and asperosaponin VI (Asp. VI) using vanillin - glacial acetic acid - perchloric acid with spectrophotometry and ultra-high performance liquid chromatography (UPLC) separately. Subsequently, we carried out the optimization extraction of TS, Asp. VI and several organic acids such as loganic acid (LA), chlorogenic acid (ChA) and caffeic acid (CA) in the cultivated DA root grown at Hefeng and the optimized method was applied to detect the contents of TS, LA, ChA, CA and Asp. VI in flowers, leaves, stem and root of the wild and cultured DA. The results showed that DA grown at Hefeng was superior to the other samples according to the two characteristic components. In addition, grown at the same place of Hefeng, the content of Asp. VI in cultivated DA was 84% higher than that of the wild one. Moreover, leaves and flowers were rich in TS, Asp. VI and some organic acids, second only to that of root, suggesting that flowers and leaves of DA would be employed for further application.
Keywords
Dipacus asperoides
Total saponins
Asperosaponin VI
Organic acid
1 Introduction
Dipsacus asper Wall. (DA), belonging to Dispsacus, as a perennial herb, is known as Xuduan or Himalayan Teasel Root and widely distributed in China. DA has been recorded in ‘Shennong Bencao Jing’ and used as traditional Chinese herb for hundreds of years. DA is mainly rich in triterpenes and their saponins, alkaloids, iridoids, organic acids and oils, polysaccharides (Gao et al. 2010, Chen et al. 2013, Du et al. 2014, Huang et al. 2014) and so on. Researchers (Tan et al. 2006, Wei et al. 2011) have suggested that total saponins (TS) and asperosaponin VI (Asp. VI) (Fig. 1) are the representative active compounds in DA and little reported in other plants (Gao et al. 2020). On the basis of those components, DA has played important roles in pharmacology and pharmacodynamic for a long time, including neuroprotective action (Qian et al. 2002), cancer prevention against human osteosarcoma (Chen et al. 2013, Chang et al. 2015), antioxidant and free radical scavenging activities (Cong et al. 2013, Tan et al. 2015), improvement of learning memory abilities (He and Qiu 2005), no cytotoxic activity (Zhou et al. 2009, Ji et al. 2012), inhibitory effects on collagen-induced arthritis (Jung et al. 2012) et al.. Moreover, Asp. VI has exhibited excellent advantages in reducing cerebral ischemic injury (Chen et al. 2013), protecting roles of acute myocardial infarction (Li et al. 2010), promoting angiogenesis and accelerating wound healing (Wang et al., 2018), stimulating or promoting stem cells differentiation (Ding et al. 2019, Niu et al. 2021), protecting against bone destructions in collagen induced arthritis (Liu et al. 2019), being antidepressant effects (Zhang et al., 2020) and inhibiting LPS-induced inflammatory response (Zhang et al., 2020) et al.. Therefore, the TS and Asp. VI are considered to be the main elements in DA and often used for its quality control. Researches (Du et al. 2014, Ma et al. 2018) have also reported that some organic acids are found in it such as loganic acid (LA) and chlorogenic acid (ChA), caffeic acid (CA) (Fig. 1) and so on. LA has played important role in antiadipogenic effects (Park et al. 2018), antioxidant and cytoprotective properties (Abirami et al. 2019), osteoprotective effects (Park et al. 2020) et al. ChA and CA are also responsible for anti-hepatitis B virus activity (Wang et al. 2009), cardio-protective and antioxidant properties (Agunloye et al. 2019), attenuating insulin resistance and modulate glucose uptake in HepG2 cells (Chen et al. 2019), and synergistic effect on growth inhibition of a resistant Listeria monocytogenes strain (Zhang et al., 2020) and so on. However, little attention has been paid for the comprehensive extraction of saponins and organic acids in DA. To the best of our knowledge, there is also no reports on the determination of these active constituents.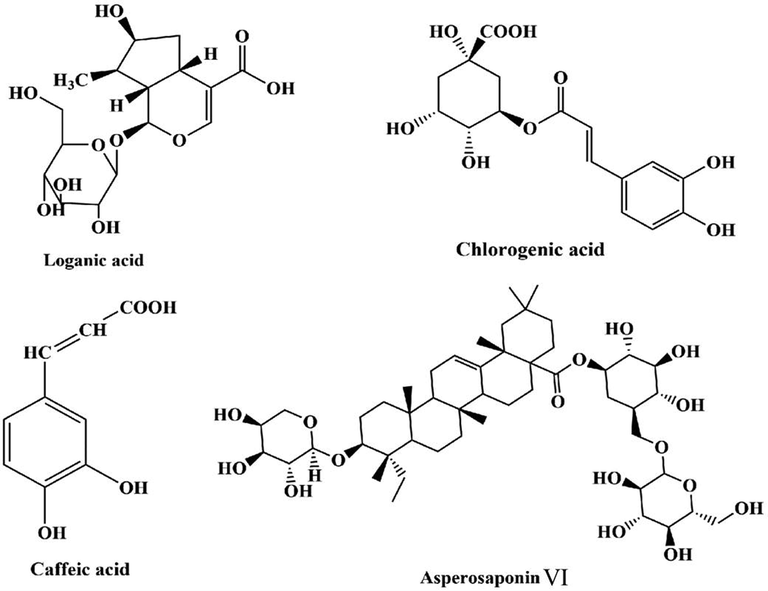
Structures of the organic acids that detected and asperosaponin VI.
Response surface methodology (RSM) is an effective and reliable way for some complicated processes. Moreover, RSM is also a labor- and time-saving approach that can obtain more information on interactions of the factors as a series of experiments containing multiple factors were conducted simultaneously (Belwal et al. 2016, Albuquerque et al. 2017, Kashyap et al. 2019). To gain a high extraction yield, RSM was employed to optimize the extraction of TS, LA, ChA, CA and Asp. VI in DA, which would lay the foundation for the further studies. The root of DA as a traditional Chinese herb, has been used for hundreds of years, however, research on the other parts such as leaves and flowers of the plant has not been reported up to now.
DA was also wildly distributed in a lot of areas in Enshi. To evaluate where was the better place for DA’s growing, it is very necessary to carry out the work of DA’ authentic evaluation, which would be beneficial for local residents to guide them planting the herb scientifically and reasonably. Additionally, planting herbs would also help people to get rid of poverty, if possible. Therefore, vanillin - glacial acetic acid - perchloric acid chromogenic system with spectrophotometry was used to detect the content of TS and ultra-high performance liquid chromatography (UPLC) was employed as an efficient tool to determine that of Asp. VI, LA, ChA and CA. As described above, TS and Asp. VI are the representative components in DA so that the two components were considered as the indicators to evaluate the ten samples collected from ten places in Enshi. Otherwise, the optimization extraction was applied to determine the flowers, leaves, stem and root of DA, which can provide more useful information for its further exploiting and utilization.
2 Materials and methods
2.1 Plant materials
All the DA samples were collected from different population in Enshi, distinguished by Professor Wanfu Zhang from Hubei Minzu University. Samples were cleaned, classified, air-dried with moisture less than 5% and milled and passed through a 0.25 mm sieve for following studies. Samples were dried without moisture at 60 °C before used. The information on each sample was summarized in Table S1 and distribution of each sample were seen in Fig. 2.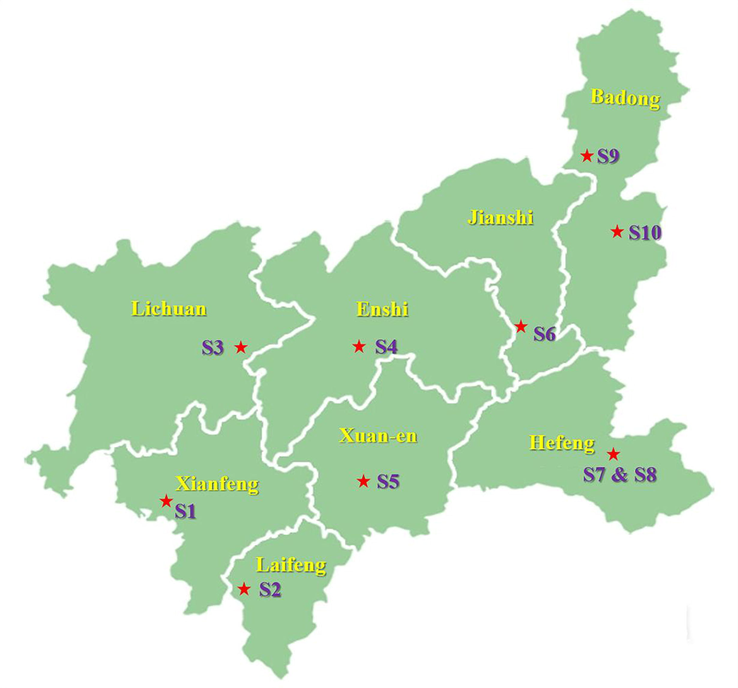
Distribution diagram of samples collected. S1–S10 represented each sample collected from the nine places where were Pingbaying of Xianfeng, Dahe of Laifeng, Shibanling of Lichuan, Shuanghe of Enshi, Chunmuying of Xuan-en, Guandian of Jianshi, Qincaoping of Hefeng (S7 & S8), Lvcongpo of Badong and Yesanguan of Badong, respectively.
2.2 Chemicals and regents
Standards of oleanolic acid (OA), Asp. VI, CA, ChA and LA were purchased from National Drug Reference Standards Database. Acetonitrile and formic acid were chromatographically pure and water was purified and distilled by ourselves. All other reagents needed were analytical grade unless otherwise stated.
2.3 Samples prepared for evaluation
Each DA sample powder of 0.50 g was extracted by 70% ethanol for three times with an ultrasonic bath for 60 min, in which of the first time the powder was soaked for 30 min firstly. The extracts were filtered and combined finally for subsequent analysis. The ten samples were extracted according to the above method. Moreover, the contents of TS and Asp. VI were separately determined for the evaluation of their qualities and genuineness. The extract solutions of samples were stored at 4 °C before used. The detailed detection methods of TS and Asp. VI were described in Sections 2.5 and 2.6, respectively.
2.4 Optimization
2.4.1 Variables selection
According to the reports (Du et al. 2014, Sun et al. 2014), we found that DA was rich in TS and Asp. VI. Moreover, organic acids such as ChA, CA and LA are also the representative components. Therefore, the contents of the five components were selected as the response values for the optimized extraction. Due to their chemical properties, solvent concentration (X1), extraction time (X2), ratio of solvent to sample (X3) and extraction temperature (X4) were chosen as subjects of the work. Moreover, researches (He et al. 2016, Oniszczuk and Olech 2016) have reported that ethanol was the most favorable solvent for the extraction of various phenolic compounds from different plants. Considering the edible safety and friendly to the operator and environment and its application in future, ethanol is the best choice and best discounts integrated into account.
2.4.2 Box-Behnken design (BBD)
Box-Behnken Design (BBD) was widely used in process optimizations such as natural component extractions (Oniszczuk and Olech 2016, Pinela et al. 2016, Chaiwut et al. 2019). A three-level four factor BBD was chosen to evaluate the comprehensive effect of the four factors and the experiment design was summarized in Table 3. Each dependent parameter was fitted to a second-order polynomial model exhibited in the following equation: where Y is the response variable, Xi and Xj are the independent variables respectively, and k is the number of tested variable (k = 4). Regression coefficient is defined as β0 for intercept, βi for linear, βii for quadratic and βij for cross product term.
Design-Expert. V8.0.6 (Stat-Ease, Inc) was employed for the analysis of variance (ANOVA) which was used to determine individual linear, quadratic and interaction regression coefficient, and for doing 3D response surface graphs. The coefficient of determination (R2) was applied for estimation the fitness of the polynomial equation to the response, and the significance of the dependent variables was analyzed by calculating the F value at p < 0.05.
The conditions of optimization extract process were performed according to the Table 3. Each response value was detected by the same method described subsequently. Each sample was pretreated by ultra-sonication at room temperature for 5 min with the power of 100 w (TH-100 BQX, Ji Ning Tian Hua ultra-sonication electronic instrument Ltd.). Then put them into water bath for some time.
2.5 Detection of total saponins
OA was chosen as the standard to determination the content of TS. OA was resolved by methanol to 0.1504 mg/mL and stored at 4 °C before used. Detection of total saponins was referred to Tang et al. (2018) with some modifies. The determination procedure was as follows: 10 µL of crude extracts was dried by nitrogen. Then 40 μL 5% vanilline-glacial acetic acid and 120 μL perchloric acid were added and mixed. The mixture was incubated at 60 °C water bath for 20 min and cooled down to room temperature. The final mixture was scanned from 500 nm to 600 nm to determine the maximum absorption wavelength of the extraction of DA. All the samples were determined at the maximum absorption wavelength.
2.6 Determination of asperosaponin VI, chlorogenic acid, caffeic acid and loganic acid
Asp. VI, ChA, CA and LA were determined by UPLC with a reversed -phase XBridge column (C18, 5 μm, 4.6 × 250 mm), carried out on a Thermo U3000. The maximum absorbent wavelength of Asp. VI is 212 nm while that of organic acids are 254 nm. However, these organic acids can be also detected under the absorbent wavelength of 212 nm. Therefore, we established a UPLC method with detection wavelength of 212 nm for simultaneous determination of each organic acid and Asp. VI. To keep the pH of mobile phase constant and wash the column expediently, gradient elution was employed using solvent A (acetonitrile), B (water containing 0.5% (v/v) formic acid) and C (water). The elution procedures were as follows: 0 min, 10% A and 80% C; 6.0 min, 12% A and 78% C and keep for 1 min; 12.0 min, 20% A and 70% C; 22.0 min, 90% A and 10% B; 25.0 min, 100% A, during which phase B was kept 10% during the analysis that could make the pH stable. The flow rate was kept at 0.8 mL/min and 10 μL of samples or standard solution were injected. The column temperature was kept at 30 °C. The formic acid in mobile phase could prevent organic acids from dissociating and improve the peak shape. The target materials were identified by comparing retention times with the standards, tested under the same condition. Each solution was in triplicate.
3 Results and discussion
3.1 Methods validation
Such parameters as linearity, repeatability, recovery, etc. were measured to validate the determination method of LA, ChA, CA and Asp. VI, respectively (Summarized in Table 1). Due to the weak ultraviolet absorption, the linear range of the four standards were narrow when we established the regression equation. The LODs and LOQs at a signal-to noise ratio (S/N) of 3 and 10 varied from 3.97–17.8 ng/mL to 13.2–59.4 ng/mL. The relative standard deviations (RSDs) of the peak areas and retention times were measured by injecting three times of standard solutions and were in the range of 0.03–0.15 and 0.16–0.25, respectively, which showed that the repeatability of the method was stable. Recoveries were carried out by spiking blank samples with a known concentration of standard solutions, calculated by (measured value – endogenous value) / added value × 100%, which were not lower than 93.5%. All the results demonstrated that the proposed method was favorably suitable for monitoring the content of these components. Note: LA, ChA, CA and Asp. VI represent loganic acid, chlorogenic acid, caffeic acid and asperosaponin VI, respectively.
Components
Regression equation
R2
Linear range (mg/mL)
Repeatability R.S.D (%) (n = 3)
LOD (ng/mL)
LOQ (ng/mL)
Recovery (%)
Retention time
Peak area
LA
Y = 473.91x + 1.9492
0.9999
0.080–0.800
0.03
0.16
9.90
33.0
93.5
ChA
Y = 894.26x + 21.55
0.9890
0.050–0.200
0.09
0.23
3.97
13.2
96.8
CA
Y = 2814.4x + 3.6212
0.9996
0.020–0.080
0.06
0.19
16.40
54.6
95.2
Asp. VI
Y = 527.31x − 10.091
0.9951
0.100–0.800
0.15
0.25
17.80
59.4
95.9
3.2 The maximum absorption wavelength of total saponins in DA samples
The solution of oleanolic acid and sample were treated as described in 2.5. The final solutions were scanned from 500 nm to 600 nm. The results showed that both the standard and sample solution own the maximum wavelength at 546 nm (Fig. 3). Therefore, 546 nm was chosen as the detection wavelength of TS.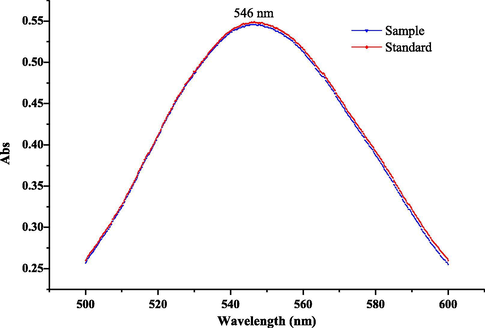
The scanning wavelength of sample and standard solutions. The final concentration of the standard was 9.4 × 10−3 mg/mL.
3.3 The contents of total saponins and asperosaponin VI in DA from different areas in Enshi
The contents of TS and Asp. VI of ten DA samples collected from nine areas of Enshi were shown in Fig. 4 A & B, respectively. From Fig. 4 A, we found that TS were rich in those DAs growing at the altitude varied from 1233 to 1626 m except the cultivated (S7). The TS contents of them (S1, S3, S4, S6 and S10) have no significant differences (p > 0.05). The DA grown at lower altitude (S2) or higher altitude (S5, S8 and S9) are all not as well as the plants at middle altitudes, which may be related to its habitat that is warm, cooler and cold-resistant without high temperature. However, DA was cultivated at altitude of 1730 and its TS content can be up to 74.86 ± 0.74 mg/g, which is the highest one and has significant differences (p < 0.05, p < 0.01) or even highly significant differences (p < 0.001) compared with the other nine wild samples.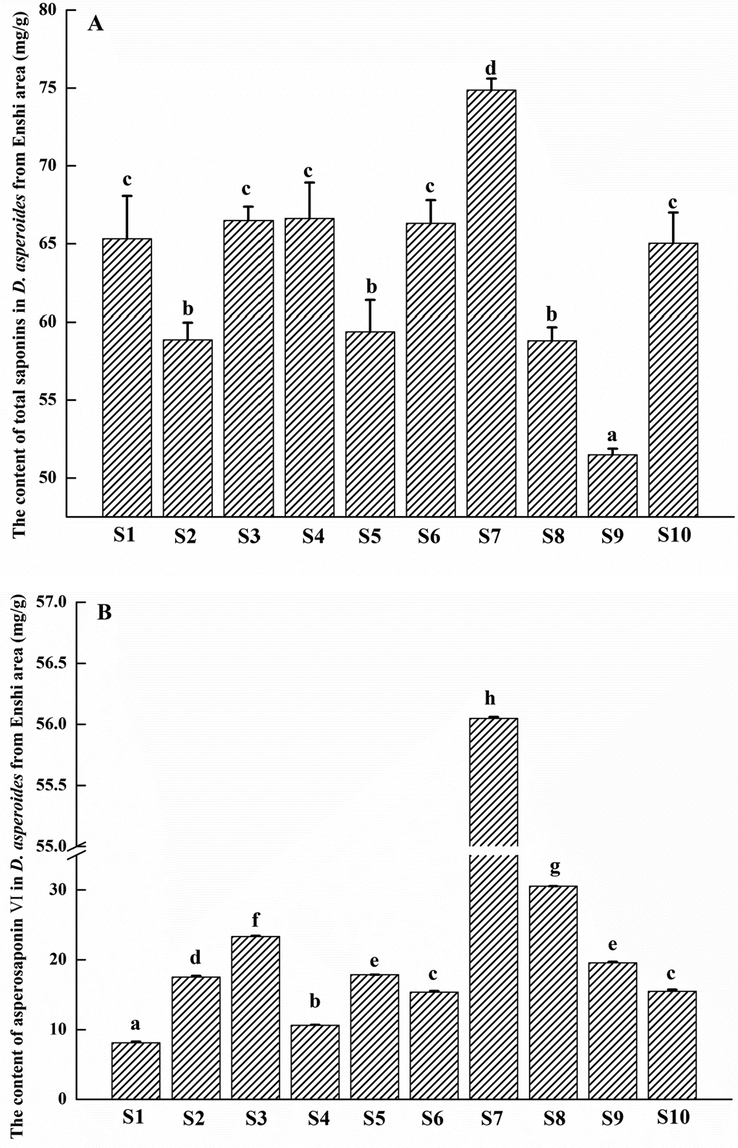
The contents of total saponins (A) and asperosaponin VI (B) in D. asperaides from Enshi areas.
Asp. VI is an important role in DA chemical components with some unique physiological activities and functions such as attenuating cardiac dysfunction and myocardial fibrosis in a rat model of chronic myocardial infarction (Li et al. 2012), providing significant cardioprotective effects against myocardial ischemia in rats (Li et al. 2010), promoting proliferation of 3T3-L1 cells and inhibiting it preadipocytes differentiation (Chen et al. 2013), protecting against bone destructions(Liu et al. 2019) and promoting angiogenesis and accelerating wound healing in rat (Wang et al., 2018) etc.. From Fig. 4 B, we found that the content of Asp. VI was highest in DA grown in Hefeng, whether it was planted or wild. There were highly significant differences (p < 0.001) between the planted and wild plant, nevertheless, which didn’t show similar phenomenon to that of TS on the altitude. Hefeng, a county of Enshi, has been verified to be a genuineness area where is good for DA’s growing with the best qualities.
Moreover, the contents of TS in DA from different areas had no direct relations with that of Asp. VI. That is to say, that the content of TS is high is not suggested that the content of Asp. VI would be higher. The contents of TS in S1, S3 and S4 were similar and have no significant difference (p > 0.05), while the contents of Asp. VI in the three samples were S3 > S4 > S1 (p < 0.05, p < 0.01, p < 0.001). On the contrary, the contents of Asp. VI in S5 and S9 were semblable (p > 0.05), nevertheless the contents of TS have significant difference (p < 0.05).
To sum up, DA which grows in Hefeng is better than the other areas evaluated only from their contents of TS and Asp. VI. DA grown there is also called “Wuhe Xuduan” and considered to be an authentic medicine.
3.4 Optimization of the extraction of total saponins, asperosaponin VI and organic acid in DA
Based on the consequence of Section 3.3, the subsequent experiments were carried out taking the Wuhe Xuduan as the research object. The root of cultivated DA in Hefeng was used for optimizing the extraction conditions.
Researches (Zhu et al. 2014, Belwal et al. 2016, Oniszczuk and Olech 2016, Šamec et al. 2016) suggest that methanol is a superior solvent for most organic acids such as caffeic acid, vanilic acid and protocatechuic acid and so on and Asp. VI. Considering the safety and friendship to the environment and operators, ethanol was chosen as the extract solution at last. Optimization of the extraction was carried out by RSM. Ethanol concentration (X1), extract temperature (X2) and time (X3) and ratio of liquid to solid (X4) were taken into consideration as the factors (Table 2). The contents of TS, LA, ChA, CA and Asp. VI were recorded as YTS, YTS, YLA, YChA, YCA and YAsp. VI and taken as the response value, seperately. We summarized the response values of each monitoring indicators and the results of ANOVA in Table 2 and Table 3, respectively. Each model was significant and the lack of fit was non-significant. The response surface plots of 3D graphs were shown in Supplementary Fig. S1, which could show the interaction between independent variables, if any. Note: X1: ethanol concentration (%); X2: Temperature (°C); X3: Extraction time (min); X4: Ratio of liquid to solid (g/mL). Level of significance: * p < 0.05, ** p < 0.01, *** p < 0.001.
Num.
Extraction conditions
Response value
Concentration (%) (X1)
Temperature (°C) (X2)
Time (min) (X3)
Ratio of liquid to solid (X4)
YTS (mg/g)
YLA (mg/g)
YChA (mg/g)
YCA (mg/g)
YAsp. VI (mg/g)
1
0(55)
−1(20)
−1(20)
0(25)
78.01
11.02
2.97
0.65
65.96
2
1(75)
0(40)
0(30)
−1(15)
35.45
3.53
0.98
0.26
32.57
3
0(55)
−1(20)
0(30)
1(35)
70.41
9.71
2.53
0.66
66.41
4
0(55)
0(40)
0(30)
0(25)
37.44
5.72
1.51
0.40
39.41
5
−1(35)
0(40)
0(30)
−1(15)
7.98
3.28
0.78
0.21
4.88
6
0(55)
1(60)
−1(20)
0(25)
15.87
3.63
1.72
0.30
24.85
7
0(55)
0(40)
0(30)
0(25)
49.25
6.95
2.97
0.51
47.73
8
0(55)
−1(20)
0(30)
−1(15)
14.67
2.56
0.58
0.21
18.47
9
−1(35)
0(40)
1(40)
0(25)
9.22
5.73
1.32
0.35
8.03
10
0(55)
0(40)
0(30)
0(25)
19.22
3.37
2.65
0.38
25.77
11
−1(35)
−1(20)
0(30)
0(25)
60.66
16.91
3.14
1.05
15.06
12
0(55)
0(40)
−1(20)
1(35)
36.82
5.75
1.36
0.34
44.09
13
0(55)
0(40)
0(30)
0(25)
44.56
5.97
1.54
0.45
41.80
14
−1(35)
0(40)
0(30)
1(35)
18.86
7.21
1.72
0.47
12.15
15
1(75)
0(40)
0(30)
1(35)
19.08
2.52
2.18
0.50
27.14
16
0(55)
−1(20)
1(40)
0(25)
78.23
10.23
2.84
0.75
64.24
17
0(55)
0(40)
1(40)
−1(15)
31.27
4.51
0.32
0.34
31.48
18
0(55)
1(60)
0(30)
−1(15)
27.28
5.04
1.45
0.40
30.41
19
0(55)
1(60)
0(30)
1(35)
37.24
5.79
1.42
0.49
43.43
20
0(55)
0(40)
−1(20)
−1(15)
12.04
2.26
0.48
0.19
17.46
21
1(75)
1(60)
0(30)
0(25)
25.00
2.94
1.55
0.53
29.83
22
−1(35)
1(60)
0(30)
0(25)
0.76
2.40
1.33
0.40
6.09
23
1(75)
0(40)
−1(20)
0(25)
30.53
3.53
0.74
0.25
35.16
24
−1(35)
0(40)
−1(20)
0(25)
15.96
5.65
1.37
0.38
10.61
25
0(55)
0(40)
0(30)
0(25)
35.57
4.76
1.11
0.18
34.19
26
0(55)
0(40)
1(40)
1(35)
7.49
2.39
1.12
0.22
17.32
27
0(55)
1(60)
1(40)
0(25)
89.94
10.66
3.12
0.75
66.93
28
1(75)
−1(20)
0(30)
0(25)
104.47
11.05
3.57
0.70
80.67
29
1(75)
0(40)
1(40)
0(25)
51.34
4.83
2.32
0.58
45.03
Terms
YTS
YLA
YChA
YCA
YAsp. VI
Intercept
X0
37.21
89.39
1.96
0.38
31.28
Linear
X1
12.70*
−17.31
0.14
−0.003
13.39***
X2
−17.53**
−41.99**
−0.42*
−0.094*
−7.55*
X3
6.52
8.82
0.20
0.073
2.41
X4
5.10
17.45
0.48*
0.089*
5.18
Quadratic
X12
−7.80
0.91
−0.059
0.060
−11.41***
X22
20.11*
46.35*
0.59*
0.21**
9.87*
X32
1.88
2.17
−0.22
−0.012
1.45
X42
−15.39
−29.59
−0.82**
−0.11
−7.41
Interactions
X1 X2
−4.89
25.95
−0.054
0.12
−8.68
X1 X3
6.89
4.97
0.41
0.091
2.59
X1 X4
−6.81
−20.08
0.066
0.002
−2.64
X2 X3
18.46
31.79
0.38
0.087
9.09
X2 X4
−11.45
−26.02
−0.49
−0.089
−7.24
X3 X4
−12.14
−22.76
−0.020
−0.070
−8.46
R2
0.7550
0.7440
−0.15
0.7337
0.8337
F Value (Model)
3.08*
2.89*
2.52*
3.47**
5.02**
F Value (Lack of fit)
3.32
4.31
0.58
1.27
2.39
Predicted value
97.93
16.70
3.17
0.95
70.84
Experimental value
93.15 ± 2.37
15.54 ± 1.49
2.81 ± 0.32
1.05 ± 0.51
71.08 ± 1.54
For TS, the final model in which the non-significant variables were removed was as follows:
The ethanol concentration (X1), a factor that would be greatly associated with the polarity of the extract, was significant (p < 0.05) and so was the quadratic term of extract temperature (X22) (p < 0.05). Both of X1 and X22 positively affected the extraction yield of TS while X2 was at p < 0.01 with negative affection. As shown in Supplementary Fig. S1 1a–1f, the higher the ethanol concentration was, the higher extraction yield of TS was. However, high temperature was an unwelcome factor for improving the TS extraction yield.
For LA, the final model, in which the non-significant variables were removed, was as follows:
LA, as shown in Fig. 1, is an iridoid with five hydroxyls and a carboxyl but not a phenolic acid. LA is widely distributed in Gentiana species such as G. apiata and G. macrophylla etc. (Cao et al. 2012, Cui et al. 2012), and also rich in DA. LA has played an important role in increasing the plasma L-arginine/ADMA ratio and decreasing levels of ADMA in rabbits fed on a high-cholesterol diet (Sozański et al. 2019), scavenging free radical activity and having temarkable cyto-protective effect against heavy metal mediated toxicity (Abirami et al., 2019), and having antiadipogenic effects in vitro and in vivo (Park et al. 2018) and so on.
During its extraction, temperature (X2) negatively affected the extraction yield of LA (p < 0.01) while its quadratic term (X22) is significant (p < 0.05) with positive influence. From supplementary Fig. S1 2a–2f, we found that lower temperature and ethanol concentration were beneficial for improving the extraction yield of LA, which may be related to its special structure described above.
For ChA and CA, mathematical models were built, obtaining the following second-order polynomial equations:
ChA and CA are important secondary metabolites widely distributed in plants with many important pharmacological activities. The results of ANOVA showed that temperature (X2) negatively affected the extraction yields of ChA and CA with p < 0.05 while their quadratic terms were significant and positive (for ChA, p < 0.05; for CA, p < 0.01). Generally, the bigger the ratio of liquid to solid is, the extraction yield is higher to some extent. In this study, we also found that the ratio of liquid to solid had improved the extraction yields of ChA and CA positively (p < 0.05). The quadratic term of X4 (X42), however, negatively affected the extraction yield of ChA with p < 0.01, which was not found in that of CA.
Asp. VI is a characteristic component in DA and plays an important role in modern pharmacology. Therefore, we chose it as a response value to investigate the comprehensive extraction for further work. For Asp. VI, the final model was as follows with removing the non-significant variables:
The consequences exhibited that ethanol concentration (X1) and its quadratic term (X12) were remarkable significant difference (p < 0.001), however, X1 positively affected the extraction yield of Asp. VI while X12 did negatively. Moreover, temperature (X2) and its quadratic term (X22) were significant (p < 0.05). Those results showed that Asp. VI is more soluble to organic agents and could not bear high temperature.
In short, when the extraction conditions were as follows: ethanol concentration (X1) 44.76%, extraction temperature (X2) 20 °C, extraction time (X3) 20 min and the ratio of liquid to solid (X4) 35 mL/g, each of the response value can reach up to its optimization. In order to carry out the test experiments conveniently, we adjusted the ethanol concentration to 45%. Samples were pretreated with ultra-sonication for 5 min, which could save a lot of time and improve the extraction yields. Therefore, 20 min can reach up to a good extraction effect. Other factors were the optimal ones. The experimental values were in good agreement with the predicted ones (The predicted and experimental values were summarized in Table 3).
3.5 Comparison analysis of the saponins and organic acids between the wild and cultivated DAs
The optimization conditions were applied to determine the contents of TS, LA, ChA, CA and Asp. VI in flowers, leaves, stem and root of Wuhe Xuduan, respectively. The flowers, leaves, stem and root of wild Wuhe Xuduan were recorded as WF, WL, WS and WR in turn. Those parts of cultured Wuhe Xuduan were severally named CF, CL, CS and CR. The determination results were summarized in Table 4 and representative chromatogram was shown in Fig. 5.
Sample
TS
LA
ChA
CA
Asp. VI
WF
16.97 ± 0.43
1.64 ± 0.03
1.06 ± 0.01
0.27 ± 0.01
2.57 ± 0.02
WL
19.67 ± 3.33
4.78 ± 0.42
2.87 ± 0.14
0.32 ± 0.01
10.01 ± 0.21
WS
6.46 ± 0.44
3.70 ± 0.23
1.71 ± 0.02
0.29 ± 0.01
2.44 ± 0.10
WR
64.46 ± 3.90
7.21 ± 1.01
4.53 ± 0.03
0.50 ± 0.04
36.94 ± 1.39
CF
19.31 ± 2.36
11.24 ± 0.07
1.65 ± 0.02
0.33 ± 0.01
5.81 ± 0.19
CL
22.50 ± 2.42
16.74 ± 1.87
3.37 ± 0.49
0.72 ± 0.27
18.74 ± 0.83
CS
9.79 ± 2.60
2.97 ± 0.27
1.26 ± 0.06
0.29 ± 0.03
4.76 ± 0.16
CR
93.15 ± 2.37
15.54 ± 1.49
2.81 ± 0.32
1.05 ± 0.51
71.08 ± 1.54
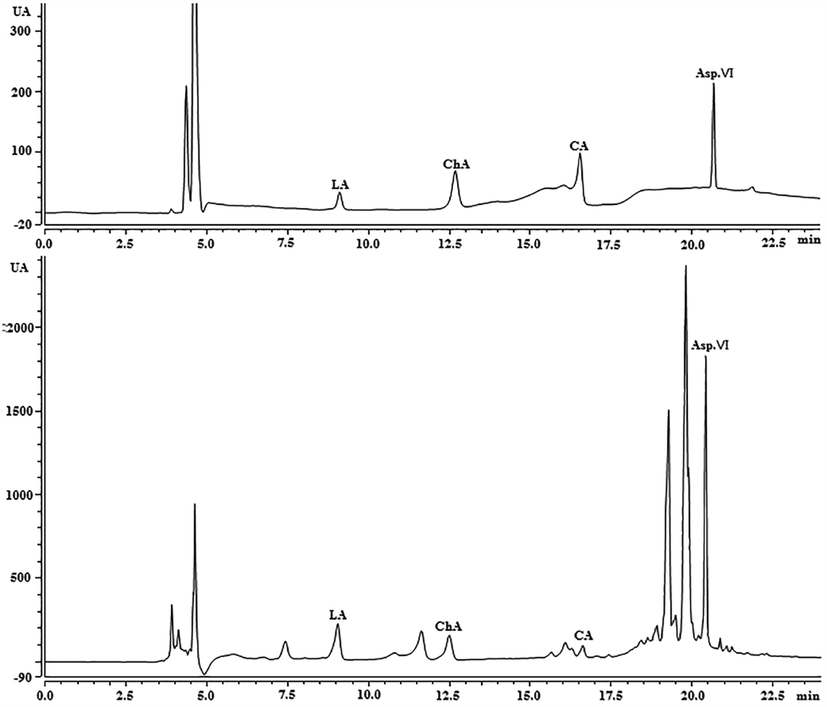
The representative UPLC chromatogram of the standards and the root of D. asperaides.
The root of DA was used to be the officinal parts in all of the plant. To enlarge the availability of the plant, we performed the work to find whether there were suitable parts to be exploited for further utilization and conserve resources avoiding unnecessary waste. The results showed that the contents of TS and Asp. VI distributed in the plant were root > leaves > flowers > stem in both the wild and cultivated, moreover, the content of TS and Asp. VI in WR were 3.28 and 3.69 times of that in WL and that in CR were 4.14 and 3.79 times of that in CL, respectively. What’s more, WF and CF were also rich in TS and Asp. VI.
In wild DA, the contents of LA, ChA and CA were root > leaves > stem > flowers, which might be due to the fact that the ChA and CA were mainly biosynthesized in root where there were amount of plant fungi and bacteria through the shikimic acid pathway (Mandal et al. 2010). Moreover, leaves were the reaction central of photosynthesis and the important sites for respiration, during which a lot of intermediated metabolites were produced that could be participate in the synthesis of those components. In the cultured DA, the contents of LA and ChA were leaves > root > flowers > stem while that of CA was root > leaves > flowers > stem. What’s more, there were only a small difference in the amount of these ingredients in leaves and flowers. These consequences in cultured DA suggested that leaves and flowers were the potential resource.
Based on the above findings, flowers and leaves of DA would be employed for further application with their high contents of these characteristic components. Our team have also investigated the selenium enrichment in DA and found that flowers were the highest selenium enrichment when they grow in the low selenium content soil while leaves were the highest one as they grow in the high selenium content soil (Wan et al. 2020). Enshi is famous for selenium in the world and appropriate selenium is also benefit for humans’ healthy. Therefore, the next step for us is to work on the further development of the flowers and leaves combined with selenium, whether to be exploited into some health products or used for pharmaceutic adjuvants.
4 Conclusion
In this study, we found that DA, grown in Hefeng, Enshi, was the best one compared with those grown in other places just considering the contents of TS and Asp. VI in all the ten samples. Subsequently, we carried out the optimization experiments taking the cultivated root from Hefeng as example and also applied the method to determine the contents of TS, LA, ChA, CA and Asp. VI to investigate whether there was significant difference in these component contents between the wild DA and the cultivated one. Moreover, to the best of knowledge, it is the first time that these ingredients were determined synchronously in each part of DA, which improved more information for researchers to put these having been ignored parts into further studies. The consequences showed that leaves and flowers were the potential resources which could be applied for further ultilization and development such as health products with some special functions.
Acknowledgements
This work was supported by Natural Science Foundation of Hubei Province (2015CFB485) and Hubei Min Zu University PhD Start-up Fund (4188023).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Antioxidant and cytoprotective properties of loganic acid isolated from seeds of Strychnos potatorum L. against heavy metal induced toxicity in PBMC model. Drug Chem. Toxicol. 2019:1-11.
- [CrossRef] [Google Scholar]
- Cardio-protective and antioxidant properties of caffeic acid and chlorogenic acid: mechanistic role of angiotensin converting enzyme, cholinesterase and arginase activities in cyclosporine induced hypertensive rats. Biomed. Pharmacother.. 2019;109:450-458.
- [CrossRef] [Google Scholar]
- Catechin-based extract optimization obtained from Arbutus unedo L. fruits using maceration/microwave/ultrasound extraction techniques. Ind. Crop Prod.. 2017;95:404-415.
- [CrossRef] [Google Scholar]
- Optimization extraction conditions for improving phenolic content and antioxidant activity in Berberis asiatica fruits using response surface methodology (RSM) Food Chem.. 2016;207:115-124.
- [CrossRef] [Google Scholar]
- Comparative analysis of contents of four iridoid glucosides in different organs of four species of Gentiana L. J. Plant Resour. Environ.. 2012;21:58-63.
- [Google Scholar]
- Optimization of polysaccharide extraction from Okra (Abelmoschus esculentus) by using response surface methodology. J. Food Sci. Agric. Technol.. 2019;5:99-105.
- [Google Scholar]
- The effects of injectable calcium silicate-based composites with the Chinese herb on an osteogenic accelerator in vitro. Biomed. Mater.. 2015;10:055004
- [CrossRef] [Google Scholar]
- Dipsacus asperoides polysaccharide induces apoptosis in osteosarcoma cells by modulating the PI3K/Akt pathway. Carbohydr. Polym.. 2013;95:780-784.
- [CrossRef] [Google Scholar]
- Chlorogenic acid and caffeic acid from Sonchus oleraceus Linn synergistically attenuate insulin resistance and modulate glucose uptake in HepG2 cells. Food Chem. Toxicol.. 2019;127:182-187.
- [CrossRef] [Google Scholar]
- Effect of asperosaponin VI on proliferation and differentiation of 3T3-L1cells. Chinese Pharmacol. Bull.. 2013;29:1150-1154.
- [Google Scholar]
- Effect of asperosaponin VI on proliferation and differentiation of 3T3-L1 cells. Chinese Pharmacol. Bull.. 2013;29:1150-1154.
- [CrossRef] [Google Scholar]
- Attenuation of renal ischemia/reperfusion injury by a polysaccharide from the roots of Dipsacus asperoides. Int. J. Biol. Macromol.. 2013;56:14-19.
- [CrossRef] [Google Scholar]
- Simultaneous determination of loganic acid and gentiopicroside in Gentianae apiatae Herba by Dual-wavelength HPLC. Chinese Trad. Patent Med.. 2012;34:1315-1318.
- [Google Scholar]
- Asperosaponin VI stimulates osteogenic differentiation of rat adipose-derived stem cells. Regen. Ther.. 2019;11:17-24.
- [CrossRef] [Google Scholar]
- Component analysis of crude and sweated Dipsaci Radix based on HPLC-ESI-MS. Chinese Trad. Herbal Drugs.. 2014;45:3251-3254.
- [CrossRef] [Google Scholar]
- Discrimination and quality evaluation of fifteen components in Stauntonia hexaphylla leaves from different harvest time by HPLC–PDA–ESI–MS/MS and ELSD coupled with multivariate statistical analysis and anti-inflammatory activity evaluation. Appl. Biol. Chem.. 2020;63
- [CrossRef] [Google Scholar]
- Advances of studies on chemical constituents and pharmacological actions of Dipsacus Asperoides. Asia-Pacific Trad. Med.. 2010;6:142-146.
- [Google Scholar]
- Optimization of Ultrasound-Assisted Extraction of phenolic compounds and anthocyanins from blueberry (Vaccinium ashei) wine pomace. Food Chem.. 2016;204:70-76.
- [CrossRef] [Google Scholar]
- Effects of extract from Dipsacus Asperoides on antioxidation and learning memory abilities in D-galaetose induced mice. China Pharm.. 2005;8:185-187.
- [Google Scholar]
- LC/MS/MS determination and pharmacokinetic studies of six compounds in rat plasma following oral administration of the single and combined extracts of Eucommia ulmoides and Dipsacus asperoides. Chinese J. Nat. Med.. 2014;12:469-476.
- [CrossRef] [Google Scholar]
- Triterpene saponins from the roots of Dipsacus asper and their protective effects against the Abeta(25–35) induced cytotoxicity in PC12 cells. Fitoterapia. 2012;83:843-848.
- [CrossRef] [Google Scholar]
- Inhibitory effects of the root extract of Dipsacus asperoides C.Y. Cheng et al T.M.Ai on collagen-induced arthritis in mice. J. Ethnopharmacol.. 2012;139:98-103.
- [CrossRef] [Google Scholar]
- Ultrasound assisted synthesis of biodiesel from karanja oil by interesterification: intensification studies and optimization using RSM. Ultrason. Sonochem.. 2019;50:36-45.
- [CrossRef] [Google Scholar]
- Long-term oral Asperosaponin VI attenuates cardiac dysfunction, myocardial fibrosis in a rat model of chronic myocardial infarction. Food Chem. Toxicol.. 2012;50:1432-1438.
- [CrossRef] [Google Scholar]
- Protective roles of Asperosaponin VI, a triterpene saponin isolated from Dipsacus asper Wall on acute myocardial infarction in rats. Eur. J. Pharmacol.. 2010;627:235-241.
- [CrossRef] [Google Scholar]
- Asperosaponin VI protects against bone destructions in collagen induced arthritis by inhibiting osteoclastogenesis. Phytomedicine. 2019;63:153006
- [CrossRef] [Google Scholar]
- Simultaneous determination of seven components in Dipsaci Radix by HPLC. Chinese J. Exp. Trad. Med. Formulae.. 2018;24:55-60.
- [CrossRef] [Google Scholar]
- Phenolic acids act as signaling molecules in plant-microbe symbioses. Plant Signal Behav.. 2010;5:359-368.
- [CrossRef] [Google Scholar]
- In the presence of TGF-beta1, Asperosaponin VI promotes human mesenchymal stem cell differentiation into nucleus pulposus like- cells. BMC Complement Med. Ther.. 2021;21:32.
- [CrossRef] [Google Scholar]
- Optimization of ultrasound-assisted extraction and LC-ESI–MS/MS analysis of phenolic acids from Brassica oleracea L. var. sabellica. Ind. Crop Prod.. 2016;83:359-363.
- [CrossRef] [Google Scholar]
- Antiadipogenic effects of loganic acid in 3T3-L1 preadipocytes and ovariectomized mice. Molecules. 2018;23
- [CrossRef] [Google Scholar]
- Osteoprotective effects of loganic acid on osteoblastic and osteoclastic cells and osteoporosis-induced mice. Int. J. Mol. Sci.. 2020;22
- [CrossRef] [Google Scholar]
- Microwave-assisted extraction of phenolic acids and flavonoids and production of antioxidant ingredients from tomato: a nutraceutical-oriented optimization study. Sep. Purif. Technol.. 2016;164:114-124.
- [CrossRef] [Google Scholar]
- The effects of the total saponin of Dipsacus asperoides on the damage of cultured neurons induced by β-amyloid protein 25–35. Anatom. Sci. Int.. 2002;77:196-200.
- [Google Scholar]
- Phenolic acids significantly contribute to antioxidant potency of Gynostemma pentaphyllum aqueous and methanol extracts. Ind. Crop Prod.. 2016;84:104-107.
- [CrossRef] [Google Scholar]
- The iridoid loganic acid and anthocyanins from the cornelian cherry (Cornus mas L.) fruit increase the plasma l-arginine/ADMA ratio and decrease levels of ADMA in rabbits fed a high-cholesterol diet. Phytomedicine. 2019;52:1-11.
- [CrossRef] [Google Scholar]
- Comparising analysis of components in volatile oils of nutmeg and prepared nutmeg by GC-MS. China J. Chinese Mater. Med.. 2006;31
- [Google Scholar]
- Antioxidant activity and optimization of extraction of polysaccharide from the roots of Dipsacus asperoides. Int. J. Biol. Macromol.. 2015;81:332-339.
- [CrossRef] [Google Scholar]
- Hemostasis and uterine contraction promoting effect of the extract from drugs in the Zi-Yin-Tiao-Jing granule, a traditional Chinese compound preparation. J. Ethnopharmacol.. 2018;211:278-284.
- [CrossRef] [Google Scholar]
- Selenium contents and characteristics of selenium enrichment of wild Dipsacus asperoides in Enshi. Soils.. 2020;52:294-299.
- [CrossRef] [Google Scholar]
- Asperosaponin VI promotes angiogenesis and accelerates wound healing in rats via up-regulating HIF-1alpha/VEGF signaling. Acta Pharmacol. Sin.. 2018;39:393-404.
- [CrossRef] [Google Scholar]
- Anti-hepatitis B virus activity of chlorogenic acid, quinic acid and caffeic acid in vivo and in vitro. Antiviral Res.. 2009;83:186-190.
- [CrossRef] [Google Scholar]
- Chromatographic fingerprint of Dipsacus asper and analysis by HPLC-MS. China J. Chinese Mater. Med.. 2011;36:169-174.
- [Google Scholar]
- Synergistic effect of chlorogenic acid and caffeic acid with fosfomycin on growth inhibition of a resistant Listeria monocytogenes strain. ACS Omega. 2020;5:7537-7544.
- [CrossRef] [Google Scholar]
- The antidepressant effects of asperosaponin VI are mediated by the suppression of microglial activation and reduction of TLR4/NF-kappaB-induced IDO expression. Psychopharmacology. 2020;237:2531-2545.
- [CrossRef] [Google Scholar]
- Asperosaponin VI inhibits LPS-induced inflammatory response by activating PPAR-gamma pathway in primary microglia. Saudi J. Biol. Sci.. 2020;27:3138-3144.
- [CrossRef] [Google Scholar]
- Akebia saponin D, a saponin component from Dipsacus asper Wall, protects PC 12 cells against amyloid-β induced cytotoxicity. Cell Biol. Int.. 2009;33:1102-1110.
- [CrossRef] [Google Scholar]
- Optimization of ultrasonic extraction of asperosaponin VI from Dipsaci Radix by response surface methodology. Modern. Tradit. Chinese Med. Mater. Med.. 2014;16:1777-1783.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2021.103107.
Appendix A
Supplementary material
The following are the Supplementary data to this article:







