Translate this page into:
Selective separation of trace nickel(II) and gold(I) ions via hollow fiber supported liquid membrane enhanced by synergistic extractants D2EHPA/TBP
⁎Corresponding author. ura.p@chula.ac.th (Ura Pancharoen)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Selective separation of Ni(II) and Au(I) ions, in trace amounts, from the real rinse wastewater of the ENIG plating process via HFSLM is investigated. Synergistic and antagonistic effects on extraction efficiency using a combination of the two extractants D2EHPA/TBP are examined. Effect of inorganic acid types on stripping efficiency have been theoretically explained. A novel approach as regards structural chemistry and reaction mechanisms in a synergistic system is provided. Optimized separation parameters are presented.
Abstract
This work presents the selective and simultaneous separation of nickel (Ni2+) and gold ([Au(CN)2]−) ions, in trace amounts, from alkaline solution via hollow fiber supported liquid membrane (HFSLM) technique. HFSLM is challengingly carried out in real rinse wastewater generated by the ENIG plating process. The influence of various chemical parameters, including the type of extractant and their concentrations, molar ratios of mixed extractant as well as type of strippant, are also studied. The organophosphorus extractant mixtures of D2EHPA and TBP provide a synergistic effect for target Ni2+ ions but has an antagonistic effect as regards the extraction of non-target [Au(CN)2]− ions. Compared to other inorganic acids, HCl is seen to be the most suitable strippant for the selective stripping. Results demonstrate that percentages of extraction and stripping of Ni2+ ions achieved 85.7 and 83.2%, respectively. In contrast, percentages of extraction and stripping of non-target [Au(CN)2]− ions attained 15.6 and 1.94%.
Keywords
Hollow fiber supported liquid membrane
Electroless nickel immersion gold plating
Rinse wastewater
Gold(I)
Nickel(II)
1 Introduction
Electroless nickel immersion gold (ENIG) is a flat and solderable metal plating process applied in the manufacture of printed circuit boards (PCBs), especially flexible circuit types and ceramic substrates. Recently, ENIG plating has received much attention since it meets the requirements for lead-free assembly (Nichols, 2020; Roberts and Johal, 2007). The ENIG process consists of a series of unitary and separate processes, from substrate acid cleaning and activation to the electroless Ni deposition and immersion gold plating, as illustrated in Fig. 1 (Roberts and Johal, 2007). In the case of PCBs, a nickel-phosphorus (Ni-P) metal layer is necessary to protect copper (Cu) lines from quick oxidation in order to guarantee good resistance to corrosion and mechanical properties. To prevent the oxidation of Ni surfaces and to assure better conductivity, Au layers can be deposited simply by immersing the Ni-P layers in the suitable solution. The ENIG plating process involves galvanic displacement reaction whereby Ni atoms dissolve from the substrate into the solution while Au ions are reduced on the electroless nickel substrate (Accogli et al., 2020; Osaka et al., 2000).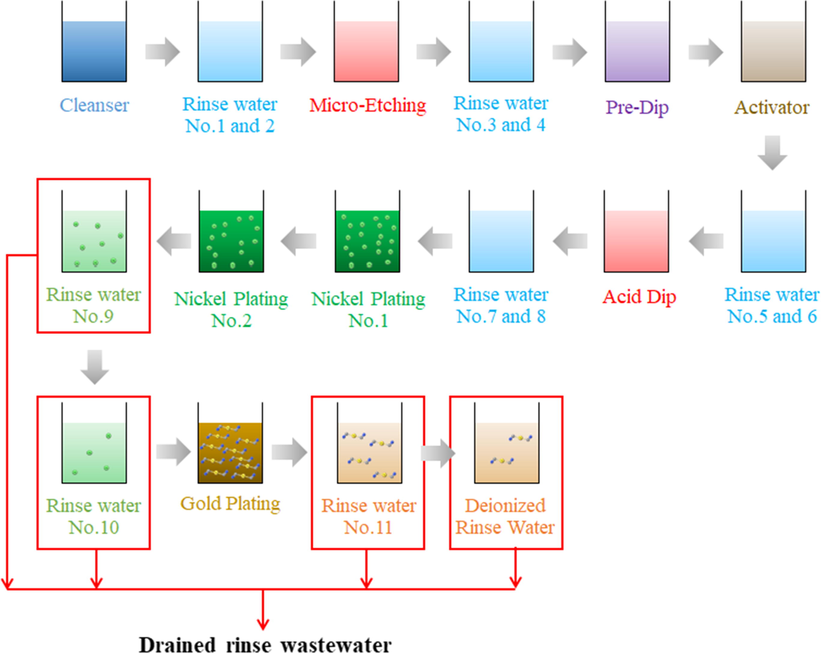
Schematic diagram of the ENIG plating process on PCBs.
In general, rinse water baths are used to remove the excess Ni and Au plating solutions, which still adhere to the electroplated parts. Excess solutions contain very small but economically significant quantities of precious metals viz. Ni2+ and [Au(CN)2]− ions. Owing to its outstanding physical and chemical properties, Ni is considered a highly valued metal for its wide range of applications (Sun et al., 2009). The attributes of Ni such as high-temperature stability, strength, ductility, toughness, recyclability, as well as catalytic and electromagnetic properties help achieve sustainability. Unfortunately, Ni is also a toxic and hazardous heavy metal. Exposure to Ni has direct and serious consequences for human beings viz. cancer in the respiratory system, headaches, nausea, skin allergies, lung fibrosis, etc (Cangul et al., 2002; Seilkop and Oller, 2003). Thus, the necessity to remove Ni is not only due to the rise in environmental awareness but also due to stringent legislation regarding the disposal of toxic substances. Likewise, Au is not only valuable as a noble metal in economic activity, but also widely used in various fields (Khosravi et al., 2017; Morcali et al., 2015). Due to continued buoyant demand and a relatively high stable price, Au has been the focus of intense activity in the areas of exploration and metallurgy. Both the diminishing availability of mineral resources and the increasing necessity for Au metal emphasize the importance of its recovery from scrap material and wastewater (Brooks, 2018; Hussain and Khan, 2011). Such demand has led companies to focus on their ability to clean up the wastewater.
Conventional methods in the treatment of wastewater include chemical precipitation, coagulation and flocculation, ion exchange, adsorption, electrochemical treatment, and photocatalysis (Barakat, 2011; Fu and Wang, 2011; Gunatilake, 2015; Gupta et al., 2012; Kurniawan et al., 2006). However, these methods have several disadvantages such as high capital and operational costs, and generate metal sludge, which requires further treatment.
At present, the use of liquid membrane techniques in wastewater treatment has become increasingly applied. The HFSLM technique is very promising having several advantages: its simplicity, and cost-effectiveness (Sunsandee et al., 2012; Wannachod et al., 2015), low energy consumption and less chemicals used compared with traditional solvent extraction and other membrane processes (Es’haghi and Azmoodeh, 2010; Es’haghi et al., 2017; Lothongkum et al., 2013). Furthermore, the HFSLM system can simultaneously perform extraction, stripping and regeneration in a single-unit operation (Chaudhury, 2018; Panja et al., 2011; Vijayalakshmi et al., 2015). Due to its high surface area and mass transfer area of the liquid membrane module (approximately 104 m2/m3), HFSLM can effectively attain a high separation rate, high selectivity, and high overall separation (Kedari et al., 2020; Ni’am et al., 2020). HFSLM can be regarded as a green technology over conventional methods on account of its reusability of liquid membrane and zero discharge of effluent. Accordingly, HFSLM is a cutting edge system that has been successfully applied in mass separation processes, including extraction and recovery of target metal ions at trace concentration level (Duan et al., 2017; Zaheri et al., 2015), removal of contaminants from wastewater (Kazemi et al., 2014; Mohdee et al., 2021), and extraction of drugs and amino acids (Manna et al., 2013; Sunsandee et al., 2021). However, no research has fully investigated the use of the HFSLM technique for the simultaneous and selective separation of Ni2+ cations and [Au(CN)2]− anions from rinse wastewater after plating of the ENIG process. To the best of our knowledge, this work is new and authentic.
Di-(2-ethylhexyl) phosphoric acid (D2EHPA) is one of the organophosphoric extractants widely used as an extractant or carrier for the reactive extraction of several metal ions such as Ni2+ (Talebi et al., 2012), Cu2+ (Chang et al., 2010), Cd2+ (Jha et al., 2012), Fe3+ (Jin et al., 2014), Mn2+ (Sousa Junior et al., 2010) and Zn2+ (Jafari et al., 2018). Besides, tributyl phosphate (TBP) is known to be one of the major neutral or solvating extractants and an effective organic phase modifier in numerous applications for extraction of several metal ions such as Ni2+ (Duan et al., 2017), Cd2+ (Nowier et al., 2000), Cu2+ (Datta et al., 2016), Co2+ (Amani et al., 2017), Fe3+ (Chaturabul et al., 2015) and Zn2+ (Vahidi et al., 2009). It is noted that the addition of TBP to the organic phase containing the D2EHPA extractant improves phase separation by modifying the extraction mechanism, thereby promoting the efficient separation of heavy metals (Azizitorghabeh et al., 2017; Gajda and Bogacki, 2007; Haghshenas Fatmehsari et al., 2009; Keshavarz Alamdari et al., 2004; Lee et al., 2019; Meng et al., 2021; Shakib et al., 2021; Sharma et al., 2016b; Zheng et al., 2018).
This present work aims to evaluate the performance of HFSLM and the synergistic binary mixture of D2EHPA/TBP extractants as an effective way to simultaneously and selectively separate valuable metal ions, in trace amounts, of Ni2+ and [Au(CN)2]− from the real rinse wastewater produced by the electroless nickel immersion gold (ENIG) plating process. The effects of several process parameters have been investigated e.g. the concentrations and molar ratios of D2EHPA and TBP extractants in the liquid membrane phase, types of strippant, and pH of stripping solutions.
2 Experimental
2.1 Chemicals and reagents
The real rinse wastewater containing Ni2+ and [Au(CN)2]− ions as an aqueous feed solution generated by the ENIG plating process was supplied by Mektec Manufacturing Corporation (Thailand) Ltd. In Table 1 below, the typical compositions analyzed by Mektec Manufacturing Corp. are given. The extractants D2EHPA and TBP were commercially obtained from Merck. The extractants were diluted in commercial grade kerosene for preparation of the organic liquid membrane phase. Kerosene was employed as a liquid membrane diluent for D2EHPA and TBP extractants due to its low viscosity, availability and non-polar characteristic. The inorganic acids viz. HCl, H2SO4 and HNO3 were employed as aqueous stripping solutions. Distilled water was used for preparing all stripping solutions. As listed in Table 2, information of all chemicals used in this study are detailed. These chemicals were used without further purification.
Component
Chemical formula
Concentration
% Relative molar concentration
mg/L
M
Aurodicyanate ions
[Au(CN)2]−
25
0.1 × 10−3
0.23
Nickel(II) ion
Ni2+
15
0.3 × 10−3
0.59
Lead(II) ion
Pb2+
1
0.01 × 10−3
0.02
Potassium ion
K+
667
17.1 × 10−3
39.35
Sodium ion
Na+
59
2.6 × 10−3
5.93
Cyanide ion
CN−
18
0.7 × 10−3
1.64
Sulfate ion
SO42−
25
0.3 × 10−3
0.59
Hydroxide ion
OH−
276
16.2 × 10−3
37.48
Hypophosphate ion
H2PO3-
45
0.6 × 10−3
1.27
Acetate ion
CH3COO−
0.4
0.01 × 10−3
0.02
Pyrophosphate
P2O74−
88
0.5 × 10−3
1.17
Hydrazine
N2H4
163
5.1 × 10−3
11.71
Chemicals
Chemical formula
Molar mass
CAS No.
Purity, %wt.
Purification method
Source
Di-(2-ethylhexyl) phosphoric acid (D2EHPA)
C16H35O4P
322.42
298–07-7
≥95.0 (Synthesis Grade)
None
Merck
Tributyl Phosphate (TBP)
C12H27O4P
266.31
126–73-8
≥99.0 (Synthesis Grade)
None
Merck
Kerosene, Exxols™ D40
C6-C16
143
64742–47-8
≥98.0 (Commercial Grade)
None
ExxonMobil
Distilled water
H2O
18.02
7732–18-5
>99.99 (Analytical Grade)
None
Merck
Concentration, %wt.
Hydrochloric acid
HCl
36.46
7647–01-0
37.0–38.0 (Analytical Grade)
None
Merck
Nitric acid
HNO3
63.01
7697–37-2
≥65.0 (Analytical Grade)
None
Merck
Sulfuric acid
H2SO4
98.08
7664–93-9
95.0–97.0 (Analytical Grade)
None
Merck
2.2 Apparatus and experimental procedure
Liqui-Cel® Extra-Flow consisting of 35,000 woven fibers of hydrophobic microporous polypropylene was selected as the solid support for the liquid membrane. The membrane had an inside diameter of 240 µm, a thickness of 60 µm, an effective length of 15 cm, a porosity of 30%, an average pore size of 0.03 µm, an effective surface area of 1.4 m2, and tortuosity factor of 2.6 (Chaturabul et al., 2015; Mohdee et al., 2020). Woven fibers provide more uniform spacing, leading to a higher mass transfer coefficient than that obtained from a single fiber, in comparison with the flat sheet supported liquid membrane technique (FSSLM) (Pirom et al., 2017; Wannachod et al., 2016).
The separation process of metal ions (Ni2+ and [Au(CN)2]−) via a single module HFSLM system was carried out using the same method as reported previously (Chaturabul et al., 2015). First, 500 mL of the organic solution as liquid membrane was prepared by dissolving a certain amount of the extractants (D2EHPA and/or TBP) in kerosene to obtain the desired concentration, ranging from 0.01 to 1.00 M. Then, the organic membrane solution was pumped counter-currently through both tube and shell sides of the hollow fiber module before use in order for the liquid membrane to embed in the micropores of the hollow fibers. Subsequently, the solution was recirculated at an equal flow rate of 100 mL/min for 40 min. After impregnation, 500 mL of distilled water was slowly fed through the system to remove all excess organic solution from the support membrane surface. Consequently, 1 L of the real rinse wastewater, as feed solution, and 1 L of stripping solution was pumped counter-currently at an equal flow rate of 200 mL/min into the tube and shell sides of the hollow fiber module, respectively. The feed solution of pH 8.6 ± 0.05 consisted of 15 mg/L of Ni2+, 25 mg/L of [Au(CN)2]− and other contaminated ions e.g. hydroxide (OH−), cyanide (CN−) and sulfate (SO42−) anions (Table 1). In Fig. 2, the HFSLM setup is illustrated. Both feed and stripping solutions were recirculated to each reservoir via recycling mode for 2 h. Then, 7 mL samples of feed and stripping solutions were collected to analyze the concentration of Ni2+ and [Au(CN)2]− ions using an inductively coupled plasma (ICP) technique (Optima 2100 DV, Perkin Elmer). All experiments were controlled at T = 303 ± 1 K for both feed and stripping solutions using a digital hotplate stirrer (DAIHAN, MSH-20D) and checked by a precision Pt-100 thermocouple having an accuracy of ±0.1 K.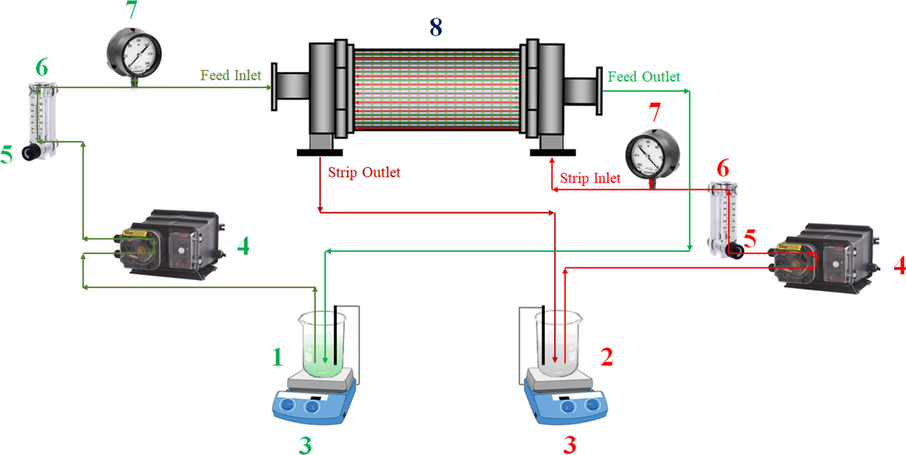
Schematic flow diagram of the separation system via HFSLM: 1 feed solution reservoir, 2 stripping solution reservoir, 3 hotplate and stirrer with Pt-100 temperature sensor, 4 peristaltic pump, 5 flow regulator valve, 6 flow meter, 7 pressure gauge, and 8 hollow fiber module.
The extractability of Ni2+ and [Au(CN)2]− ions in this work was calculated by the percentage of extraction (%E) and stripping (%St) using Eqs. (1) and (2):
The distribution ratio (D) is defined as the concentration of metal ions (Ni2+ and [Au(CN)2]−) in the organic phase of the liquid membrane to that in the aqueous phase after extraction and stripping. It can be determined, as expressed:
In the organic phase, a higher distribution ratio is proportional to higher Ni2+ and [Au(CN)2]− ions concentrations, thus enhancing the extraction of Ni2+ and [Au(CN)2]− ions from aqueous feed phase to organic liquid membrane phase.
The
in the organic phase of the liquid membrane layer can be obtained by subtracting the aqueous concentration from the initial concentration, as shown (Aggett et al., 1969):
In order to enhance extraction efficiency and selectivity, synergism of mixed extractants was used to overcome the problems from utilizing a single extractant. Synergistic extraction is defined as the cooperation of two extractants in transferring metal ions from the aqueous feed phase to the organic liquid membrane phase. The synergistic coefficient (SC) is defined as the distribution ratio (D) of metal ions (Ni2+ and [Au(CN)2]−) by the mixture of D2EHPA and TBP extractants (Dmix) to the sum of the distribution ratio by the single extractants D2EHPA (DD2EHPA) and TBP (DTBP), as shown in Eq. (5):
Extractant synergism is established when Dmix is greater when a single extractant is used (SC > 1). An antagonistic effect occurs vice versa (SC < 1) (R. Sarkar, 2014; Špadina et al., 2019).
3 Results and discussion
3.1 Effect of type of extractant
Due to their cost benefits as well as proof of performances, the two extractants viz. D2EHPA and TBP were selected as both a suitable acidic and neutral extractant for the extraction of Ni2+ ions. In Table 3, the extractability of Ni2+ and [Au(CN)2]− ions by a single extractant across HFSLM are tabulated.
Extractant
%Extraction
Distribution ratio (D)
Selectivity (SNi/Au)
Synergistic Coefficients (SC)
Ni2+
[Au(CN)2]−
Ni2+
[Au(CN)2]−
Ni2+
[Au(CN)2]−
0.5 M D2EHPA
56.18 ± 0.89
24.21 ± 0.57
1.282 ± 0.043
0.3194 ± 0.0096
2.321 ± 0.021
–
–
0.5 M TBP
52.68 ± 0.73
31.54 ± 0.85
1.113 ± 0.035
0.4607 ± 0.0172
1.670 ± 0.075
–
–
0.25 M D2EHPA/0.25 M TBP
85.70 ± 1.53
15.65 ± 0.62
5.993 ± 0.631
0.1855 ± 0.0084
5.476 ± 0.143
2.502 ± 0.248
0.2378 ± 0.0014
As results show in Table 3, both D2EHPA, an acidic type extractant, and TBP, a solvating type extractant are capable of extracting Ni2+ and [Au(CN)2]− ions from the real rinse wastewater of the ENIG plating process. However, when these two extractants are used, the distribution ratio of each ion (DNi and DAu) as well as the selectivity of Ni2+ relative to [Au(CN)2]− ions (SNi/Au) are found to be quite different, especially [Au(CN)2]− ions. At equal concentration of 0.5 M, D2EHPA attained a slightly higher extraction percentage (ENi, D2EHPA 56.18%) and distribution ratio (DNi, D2EHPA 1.282) of Ni2+ ions more than TBP (ENi, TBP 52.68% and DNi, TBP 1.113). Furthermore, selective extraction in terms of selectivity of Ni2+ relative to [Au(CN)2]− ions is improved when D2EHPA is used (SNi/Au, D2EHPA 2.321), as a result of its less extractability of [Au(CN)2]− ions than TBP. The non-target [Au(CN)2]− ions were also extracted reaching a high percentage by D2EHPA (EAu, D2EHPA 24.21%) and TBP (EAu, TBP 31.54%). Such outcomes can affect the purity of Ni2+ in the aqueous stripping phase and loss of valuable Au metal, thus reduction of [Au(CN)2]− extraction from the aqueous feed phase is needed.
D2EHPA is an acidic extractant, offering both hydrogen bond donor and acceptor sites while TBP, a neutral extractant, only offers a single acceptor site per molecule. At an organic-aqueous membrane interface of liquid membrane, the polar hydroxyl group (–OH) of D2EHPA can be deprotonated to become a negative charge on the oxygen atom (P—O−) (Bapat and Dalvi, 2019). The deprotonated form of D2EHPA has high affinity to strongly interact with the positive charges on Ni2+ ions. Furthermore, Ni2+ ions prefer to substitute the hydrogen atom in the P—O—H group of D2EHPA and combined with the P⚌O group via the cation exchange mechanism. Thus, D2EHPA extractant was found to give better efficiency for Ni2+ ions extraction over TBP in terms of distribution ratio and selectivity.
The chemical reactions of Ni2+ ions with dimeric form of D2EHPA extractant via cation exchange mechanism are as follows (Chauhan and Patel, 2014; Gajda and Bogacki, 2007; Gharabaghi et al., 2013):
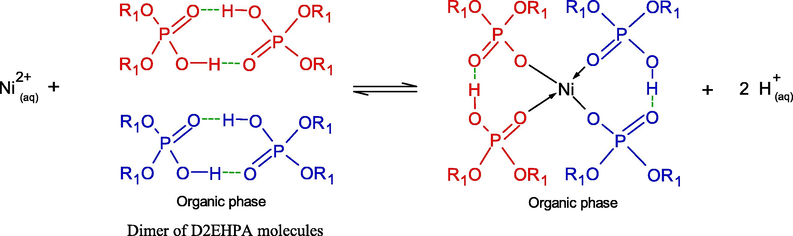 where R1 denotes 2-ethylhexyl group (C8H17) as in Eq. (6).
where R1 denotes 2-ethylhexyl group (C8H17) as in Eq. (6).
In the literature, the reaction mechanism of Ni2+ ions in aqueous basic solution with the TBP extractant has never been reported. Consequently, the reactive extraction of Ni2+ applying TBP extractant in aqueous basic solution, mainly composed of the hydroxide ions (OH−) with 16.2 mM and 37.48 %relative molar concentration (Table 1), is proposed in this study.
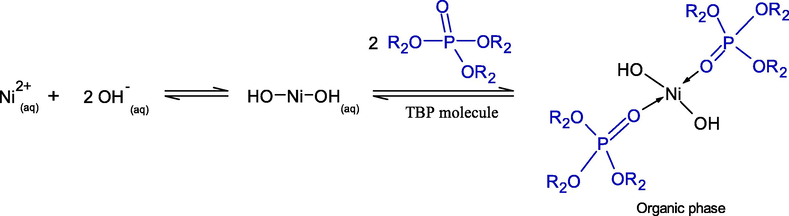
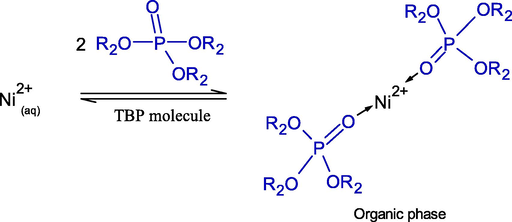 where R2 denotes butyl group (C4H9) as in Eqs. (7) and (8).
where R2 denotes butyl group (C4H9) as in Eqs. (7) and (8).
Owing to its chemical stability, low aqueous solubility and high loading characteristics, D2EHPA proved to be the most ideal extractant for Ni2+ ions extraction (H. Narita, 2006). Besides, since Ni2+ ions extraction is highly pH dependent, a higher acidic extractant like D2EHPA is seen to be preferable. Due to its high solubility and degree of protonation, D2EHPA provides the highest acidity constant (Duan et al., 2017). Thus, the H-atom in the hydroxyl group (–OH) of D2EHPA is easily substituted by Ni2+ ions.
Henceforth, extraction by solvation is carried out by a solvating extractant. In nature, a solvating extractant has a weak base, extracting either neutral metal complexes or acids by forming a solvate.
In the rinse wastewater of the ENIG plating process, Au(I) is present as the complex of [Au(CN)2]− anions whereas Ni2+ is found as cations. When aqueous feed solution is basic, [Au(CN)2]− ions can be extracted via anion exchange with several amine extractants (Kordosky et al., 1992; Mooiman and Miller, 1986; Sastre et al., 1999), quaternary ammonium compounds (Yang et al., 2008) and ionic liquids (Yang et al., 2018; Yang et al., 2015). It can be seen that D2EHPA and/or TBP is a suitable choice of extractant for selectively separating Ni2+ from [Au(CN)2]− ions via HFSLM.
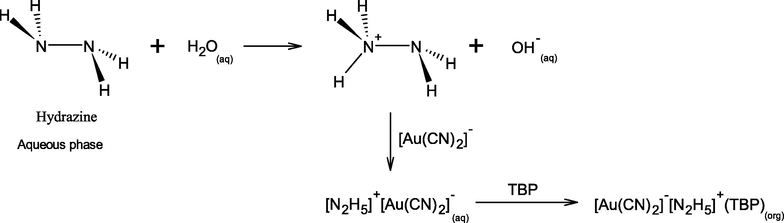
(Tromp et al., 1988)

3.2 Effect of mixed extractants
Amongst the single extractant types studied in Section 3.1, it was found that both D2EHPA and TBP extractants were best for the extraction of Ni2+ ions via HFSLM having good selectivity of Ni2+ relative to [Au(CN)2]− (SNi/Au). As depicted in Table 3 and Fig. 4, mixing of 0.25 M D2EHPA and 0.25 M TBP extractants are investigated in order to improve the extraction of Ni2+ ions and the selectivity (SNi/Au).
According to Fig. 4a, it is evident that using the mixture of D2EHPA/TBP extractants yielded the highest Ni2+ ions extraction efficiency followed by the single extractants i.e. D2EHPA and TBP, respectively. In Table 3, the synergistic coefficient of the D2EHPA/TBP extractants for Ni2+ ions extraction (SCNi > 1) indicates that synergism was found to occur. Thus, maximum extraction (%ENi) of 85.70%, distribution ratio (Dmix, Ni) of 5.993 and a selectivity (SNi/Au) of 5.476 were achieved (Fig. 4b). Furthermore, the synergistic mixture D2EHPA/TBP can transport the Ni2+ ions much faster than a single extractant as seen in Table 4 as well as Figs. 3 and 5. Accordingly, extraction of Ni2+ ions can rapidly reach 50% within 2.5 min, and then 80% within 28 min. Thus, separation time of Ni2+ ions via HSFLM can be reduced compared with using D2EHPA and TBP extractant, respectively. In contrast, extraction of [Au(CN)2]− ions by the mixture D2EHPA/TBP expressed an antagonistic effect behavior (synergistic coefficient, SCAu < 1) as in Table 3. Transportation of [Au(CN)2]− ions from the aqueous feed phase via HFSLM decreased significantly. At equal extraction percentage of 10% for [Au(CN)2]− ions (%ENi), it took a separation time of 69 min when using the mixture D2EHPA/TBP. This outcome was clearly slower than when D2EHPA and TBP extractants were used (Table 4). Thus, as regards selective separation, the combination of D2EHPA/TBP extractants proved to be the most suitable ions carrier system via HFSLM as a result of their synergism to target Ni2+ ions as well as their antagonism to non-target [Au(CN)2]− ions for improving %ENi, DNi and SNi/Au.
Extractant
Separation time at %ENi, min
Separation time at %EAu, min
40%
50%
55%
80%
1%
5%
10%
15%
20%
0.5 M D2EHPA
9.2
64
106
–
4.2
37
49
67
97
0.5 M TBP
52
107
–
–
2.9
9.4
30
54
71
0.25 M D2EHPA/0.25 M TBP
1.6
2.5
2.9
28
10
46
69
110
–
![%Extraction and concentration of Ni2+ and [Au(CN)2]− ions in aqueous feed phase with continuous separation time (a) 0.5 M D2EHPA extractant (b) 0.5 M TBP extractant: feed solution; 15 mg/L Ni2+, 25 mg/L [Au(CN)2]−, pH 8.6 ± 0.05 | organic solution; 0.5 M D2EHPA in kerosene | stripping solution; 0.5 M HCl, pH 0.38 ± 0.05 | flow ratefeed = flow ratestripping = 200 mL/min | separation time 2 h | T = 303 ± 1 K.](/content/184/2021/14/12/img/10.1016_j.arabjc.2021.103427-fig4.png)
%Extraction and concentration of Ni2+ and [Au(CN)2]− ions in aqueous feed phase with continuous separation time (a) 0.5 M D2EHPA extractant (b) 0.5 M TBP extractant: feed solution; 15 mg/L Ni2+, 25 mg/L [Au(CN)2]−, pH 8.6 ± 0.05 | organic solution; 0.5 M D2EHPA in kerosene | stripping solution; 0.5 M HCl, pH 0.38 ± 0.05 | flow ratefeed = flow ratestripping = 200 mL/min | separation time 2 h | T = 303 ± 1 K.
![Effect of mixed extractants on Ni2+ and [Au(CN)2]− ions extraction from aqueous feed phase via HFSLM: feed solution; 15 mg/L Ni2+, 25 mg/L [Au(CN)2]−, pH 8.6 ± 0.05 | organic solution; 0.5 M of total extractant concentration in kerosene | stripping solution; 0.5 M HCl, pH 0.38 ± 0.05 | flow ratefeed = flow ratestripping = 200 mL/min | separation time 2 h | T = 303 ± 1 K.](/content/184/2021/14/12/img/10.1016_j.arabjc.2021.103427-fig5.png)
Effect of mixed extractants on Ni2+ and [Au(CN)2]− ions extraction from aqueous feed phase via HFSLM: feed solution; 15 mg/L Ni2+, 25 mg/L [Au(CN)2]−, pH 8.6 ± 0.05 | organic solution; 0.5 M of total extractant concentration in kerosene | stripping solution; 0.5 M HCl, pH 0.38 ± 0.05 | flow ratefeed = flow ratestripping = 200 mL/min | separation time 2 h | T = 303 ± 1 K.
![%Extraction and concentration of Ni2+ and [Au(CN)2]− ions in aqueous feed phase over separation time: feed solution; 15 mg/L Ni2+, 25 mg/L [Au(CN)2]−, pH 8.6 ± 0.05 | organic solution; 0.25 M D2EHPA and 0.25 M TBP extractants in kerosene | stripping solution; 0.5 M HCl, pH 0.38 ± 0.05 | flow ratefeed = flow ratestripping = 200 mL/min | separation time 2 h | T = 303 ± 1 K.](/content/184/2021/14/12/img/10.1016_j.arabjc.2021.103427-fig6.png)
%Extraction and concentration of Ni2+ and [Au(CN)2]− ions in aqueous feed phase over separation time: feed solution; 15 mg/L Ni2+, 25 mg/L [Au(CN)2]−, pH 8.6 ± 0.05 | organic solution; 0.25 M D2EHPA and 0.25 M TBP extractants in kerosene | stripping solution; 0.5 M HCl, pH 0.38 ± 0.05 | flow ratefeed = flow ratestripping = 200 mL/min | separation time 2 h | T = 303 ± 1 K.
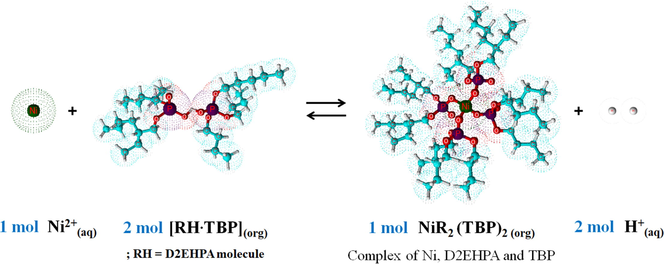
As in Eq. (6), the D2EHPA molecules were strongly attracted via H-bonding in dimer form. D2EHPA is a strong acid, the majority of the molecules are dimerized in nonpolar diluents such as kerosene, and its aqueous solubility is also extremely low (Biswas et al., 2000). The synergistic effect of the mixture D2EHPA/TBP in enhancing Ni2+ extraction is proposed via the replacement of two D2EHPA molecules in Ni∙(D2EHPA)4 complex by two TBP molecules to form adducts with Ni complexes of Ni∙(D2EHPA)2(TBP)2 (Flett, 2005; Gajda and Bogacki, 2007). The reactive extraction mechanism of Ni2+ ions by mixed D2EHPA/TBP extractants is expressed in Eq. (11):
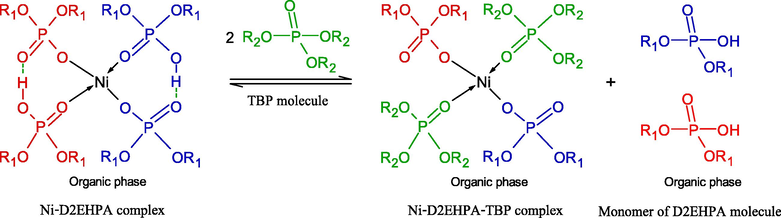
As calculated via Van der Waals radii, the molecular volume of the TBP molecule proved to be smaller than that of D2EHPA molecule by about 50 Å, as shown in Table 5 (Kolarik, 1982). Note 1 Molecular volumes were calculated via the Van der Waals radii.
Extractant
Molecular volume1, Å3
Negative charge on the oxygen atom of phosphoryl functional group
Dipole moment, Debye
D2EHPA
311.3
−0.873
2.74
TBP
263.7
−0.779
3.10
Therefore, the TBP molecule can easily replace the D2EHPA molecule in the Ni∙(D2EHPA)4 complex (Gajda and Bogacki, 2007). As shown in Table 5, the greater dipole moment of the TBP molecule indicates its greater polarity.
According to Eq. (11), the remaining two molecules of monomeric D2EHPA can be dissociated at the interface of the organic membrane-aqueous phases, and then form a complex with one ion of Ni2+ as in Eq. (12): (Biswas et al., 2000; Gajda and Bogacki, 2007; Ghosh et al., 2018):
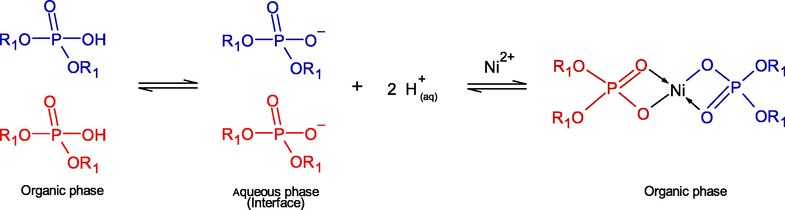
In this study, the rinse wastewater from the ENIG process is a slightly basic effluent having pH of 8.6 ± 0.05. Thus, deprotonation of the monomeric D2EHPA molecules is quite favorable. It is proved that D2EHPA extractant is suitable for Ni2+ ions extraction in the basic aqueous solution (Gharabaghi et al., 2013).
As observed in Eqs. (11) and (12), when the mixture D2EHPA/TBP is used, it can be seen that four molecules of D2EHPA are able to form a complex with two ions of Ni2+. Thus, this outcome increases the extractability for Ni2+ ions more than when a single D2EHPA extractant is used such that four D2EHPA molecules form a tetrameric complex with one Ni2+ ion as in Eq. (6). Furthermore, two molecules of TBP can also extract one Ni2+ ion via the complex of Ni(OH)2∙(TBP)2 or Ni∙(TBP)2 as in Eq. (7).
At equal total molar concentration or number of extractant molecules, it is found that the mixed extractants of four D2EHPA and four TBP molecules (i.e. 0.25 M D2EHPA/0.25 M TBP) are able to extract three Ni2+ ions while a single type extractant of eight D2EHPA molecules (i.e. 0.5 M D2EHPA) form a complex with two Ni2+ ions. Thus, the extractability of Ni2+ ions by the mixture D2EHPA/TBP proved to be 1.5 times more than when the single D2EHPA extractant system was used.
In the case of [Au(CN)2]− ions, it is noted that extraction efficiency by the mixture D2EHPA/TBP decreased. This antagonistic effect may be a result of less H+ ions generated by D2EHPA dissociation as its lower concentration (i.e. from 0.5 M to 0.25 M) and by TBP molecules in preferring to form a Ni∙(D2EHPA)2(TBP)2 complex. The supported liquid membrane system containing the combination of D2EHPA/TBP extractants is suitable for further investigation due to their synergistic effect (SCNi > 1), high efficiency of Ni2+ ions extraction (ENi > 80%), improved distribution ratio (DNi) as well as selectivity of Ni2+ relative to [Au(CN)2]− ions (SNi/Au).
In general, D2EHPA contains mono-(2-ethylhexyl) phosphoric acid (M2EHPA) as impurities due to poor purification of the reagent and/or due to hydrolysis of D2EHPA during prolonged storage. (Haghshenas Fatmehsari et al., 2009) found that the presence of M2EHPA causes the extraction curves of these metals to shift to lower pH values depending on the physico-chemistry properties of the metal. In other words, the presence of M2EHPA changes the thermodynamic conditions of extraction and produces variable changes in the shifting of the extraction curves that for some metals are in order Cd > Cu > Co > Ni > Zn. This impurity sometimes affects separation efficiency. This antagonistic effect may cause trouble with the solvent extraction.
3.3 Effect of molar ratio of mixed D2EHPA/TBP extractant
As proved in Section 3.2, when the synergistic mixture D2EHPA/TBP increased, extraction of Ni2+ ions was found to increase significantly. Fig. 6 and Table 6 show the effect of the combination of D2EHPA/TBP extractants at various molar ratios as regards the extraction percentages (%E) of Ni2+ and [Au(CN)2]− ions. At equal total concentration of 0.5 M, it is seen that extraction of Ni2+ ions increased from 56.18 to 85.70 % when TBP concentration increased from 0.05 to 0.25 M and the molar ratio of the mixture D2EHPA/TBP decreased from 9:1 to 1:1, respectively. Furthermore, the distribution ratio of Ni2+ ions (DNi) and selectivity of Ni2+ relative to [Au(CN)2]− ions (SNi/Au) also increased as TBP concentration increased. Thus, the optimum combination obtained for the mixed extractants in the liquid membrane for this HFSLM studied is an equal molar concentration (1:1 ratio) of 0.25 M D2EHPA and 0.25 M TBP.![Effect of concentration and molar ratio of the D2EHPA/ TBP synergistic extractants: feed solution; 15 mg/L Ni2+, 25 mg/L [Au(CN)2]−, pH 8.6 ± 0.05 | organic solution; mixed D2EHPA/TBP extractant in kerosene | stripping solution; 0.5 M HCl, pH 0.38 ± 0.05 | flow ratefeed = flow ratestripping = 200 mL/min | separation time 2 h | T = 303 ± 1 K.](/content/184/2021/14/12/img/10.1016_j.arabjc.2021.103427-fig7.png)
Effect of concentration and molar ratio of the D2EHPA/ TBP synergistic extractants: feed solution; 15 mg/L Ni2+, 25 mg/L [Au(CN)2]−, pH 8.6 ± 0.05 | organic solution; mixed D2EHPA/TBP extractant in kerosene | stripping solution; 0.5 M HCl, pH 0.38 ± 0.05 | flow ratefeed = flow ratestripping = 200 mL/min | separation time 2 h | T = 303 ± 1 K.
Extractant Concentration
Molar ratio of D2EHPA/TBP
%Extraction
Distribution ratio (D)
Selectivity (SNi/Au)
Synergistic Coefficients (SC)
Ni2+
[Au(CN)2]−
Ni2+
[Au(CN)2]−
Ni2+
[Au(CN)2]−
0.50 M D2EHPA
–
56.18 ± 0.89
24.21 ± 0.57
1.282 ± 0.035
0.3194 ± 0.0096
2.321 ± 0.021
–
–
0.45 M D2EHPA/0.05 M TBP
9:1
71.32 ± 0.44
28.16 ± 0.28
2.487 ± 0.048
0.3920 ± 0.0057
2.533 ± 0.038
1.038 ± 0.017
0.5024 ± 0.0274
0.40 M D2EHPA/0.10 M TBP
4:1
72.59 ± 0.20
21.39 ± 0.54
2.648 ± 0.022
0.2721 ± 0.0090
3.394 ± 0.084
1.106 ± 0.006
0.3488 ± 0.0261
0.35 M D2EHPA/0.15 M TBP
2.33:1
74.83 ± 0.39
19.57 ± 0.19
2.973 ± 0.069
0.2433 ± 0.0028
3.824 ± 0.064
1.241 ± 0.033
0.3119 ± 0.0078
0.30 M D2EHPA/0.20 M TBP
1.5:1
81.16 ± 0.28
17.42 ± 0.14
4.308 ± 0.093
0.2109 ± 0.0019
4.659 ± 0.063
1.798 ± 0.044
0.2704 ± 0.0074
0.25 M D2EHPA/0.25 M TBP
1:1
85.70 ± 0.18
15.65 ± 0.62
5.993 ± 0.631
0.1855 ± 0.0084
5.476 ± 0.143
2.502 ± 0.248
0.2378 ± 0.0014
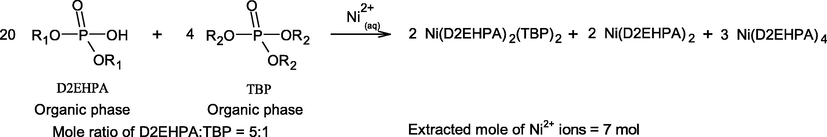
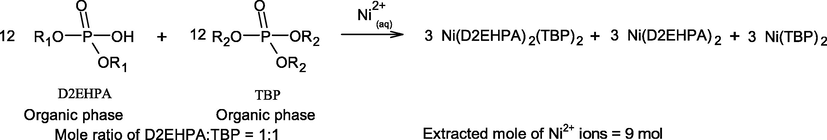
According to Eq. (11), the role of TBP extractant is to disrupt the dimers or aggregation of the D2EHPA molecules. Two D2EHPA molecules and two TBP molecules can form a complex with the Ni2+ ions as Ni∙(D2EHPA)2(TBP)2. Hence, two monomeric molecules of D2EHPA become available for complexation with other Ni2+ ions, which enhances the extraction efficiency (%ENi) as in Eq. (11). Furthermore, at equal molar concentration in comparison with D2EHPA, TBP extractant can solvate Ni2+ ions by using two TBP molecules as a form of Ni(OH)2∙(TBP)2 or Ni∙(TBP)2, Eq. (7). However, four molecules of D2EHPA are required for extraction with Ni2+ when the extractant is taken alone as in Eq. (6). For example, at equal number of extractant molecules expressed in Eqs. (13) and (14), it can be proved that the mixture of D2EHPA/TBP at 1:1 ratio provides higher extractability of Ni2+ ions in comparison with using D2EHPA/TBP ratio of 5:1.
As for the extraction of [Au(CN)2]− ions, increasing the extractant TBP as well as decreasing D2EHPA in molar concentration from 9:1 to 1:1 at equal molar concentration practically decreases the degree of extraction. Results arise due to the reduction of H+ ions generated after Ni2+ extraction with D2EHPA molecules as in Eqs. (6) and (11). Thus, the protonation mechanism of hydrazine (N2H4) to form hydrazinium (N2H5+) ions, for [Au(CN)2]− complexation as in Eq. (8), is decreased. Furthermore, although concentration of TBP increased, its molecule contributes to form Ni-complexes such as Ni∙(D2EHPA)2(TBP)2, Ni(OH)2∙(TBP)2 and/or Ni∙(TBP)2. These extractive reactions are competitive as in Eqs. (8) and (9), whereby the complex of K+[Au(CN)2]−(TBP) and [N2H5]+ [Au(CN)2]−(TBP) are formed.
3.4 Effect of extractant concentration
As presented in Table 7 and Figs. 7a to 7c, the effect of concentration of D2EHPA and TBP as well as the mixture D2EHPA/TBP was investigated by varying their concentration from 0.01 to 1.0 M.
Extractant
Extraction, %
Distribution ratio (D)
Selectivity (SNi/Au)
Synergistic Coefficients (SC)
Ni2+
[Au(CN)2]−
Ni2+
[Au(CN)2]−
Ni2+
[Au(CN)2]−
0.01 M D2EHPA
1.813 ± 0.396
0.8402 ± 0.0533
0.01846 ± 0.00422
0.008473 ± 0.000543
2.158 ± 0.206
–
–
0.025 M D2EHPA
4.620 ± 0.460
2.010 ± 0.330
0.04844 ± 0.00521
0.02051 ± 0.00354
2.299 ± 0.185
–
–
0.05 M D2EHPA
9.161 ± 0.720
7.203 ± 0.266
0.1008 ± 0.0084
0.07762 ± 0.00320
1.272 ± 0.129
–
–
0.10 M D2EHPA
18.32 ± 0.440
13.67 ± 0.56
0.2243 ± 0.0064
0.1583 ± 0.0032
1.340 ± 0.027
–
–
0.25 M D2EHPA
31.75 ± 0.750
19.94 ± 0.65
0.4652 ± 0.0169
0.2491 ± 0.0072
1.592 ± 0.100
–
–
0.50 M D2EHPA
56.18 ± 0.89
24.21 ± 0.57
1.282 ± 0.043
0.3194 ± 0.0096
2.321 ± 0.021
–
–
1.00 M D2EHPA
58.21 ± 0.40
25.17 ± 0.61
1.393 ± 0.022
0.3364 ± 0.0113
2.313 ± 0.064
–
–
0.01 M TBP
0.9503 ± 0.0832
1.040 ± 0.153
0.009594 ± 0.000851
0.01051 ± 0.00157
0.9138 ± 0.0385
–
–
0.025 M TBP
3.420 ± 0.140
2.214 ± 0.262
0.03541 ± 0.00142
0.02264 ± 0.00283
1.545 ± 0.089
–
–
0.05 M TBP
5.807 ± 0.398
3.541 ± 0.320
0.06165 ± 0.00462
0.03671 ± 0.00354
1.640 ± 0.009
–
–
0.10 M TBP
11.40 ± 0.53
9.703 ± 0.432
0.1287 ± 0.0070
0.1075 ± 0.0055
1.175 ± 0.004
–
–
0.25 M TBP
21.45 ± 0.52
15.12 ± 0.29
0.2731 ± 0.0087
0.1781 ± 0.0042
1.419 ± 0.008
–
–
0.50 M TBP
52.68 ± 0.73
31.54 ± 0.85
1.113 ± 0.035
0.4607 ± 0.0172
1.670 ± 0.075
–
–
1.00 M TBP
53.05 ± 0.32
33.17 ± 0.39
1.130 ± 0.015
0.4963 ± 0.0084
1.599 ± 0.030
–
–
0.005 M D2EHPA/0.005 M TBP
4.650 ± 0.450
1.250 ± 0.083
0.04877 ± 0.09510
0.01266 ± 0.00086
3.720 ± 0.066
1.713 ± 0.038
0.6852 ± 0.0390
0.0125 M D2EHPA/0.0125 M TBP
14.11 ± 0.37
3.230 ± 0.293
0.1643 ± 0.0048
0.03434 ± 0.00324
4.250 ± 0.169
1.858 ± 0.035
0.8476 ± 0.0251
0.025 M D2EHPA/0.025 M TBP
22.42 ± 0.03
6.710 ± 0.430
0.2890 ± 0.0006
0.07193 ± 0.00509
3.341 ± 0.141
1.797 ± 0.046
0.6115 ± 0.0270
0.05 M D2EHPA/0.05 M TBP
39.20 ± 0.60
9.710 ± 0.430
0.6447 ± 0.0170
0.1075 ± 0.0054
4.037 ± 0.082
1.820 ± 0.055
0.4008 ± 0.0270
0.125 M D2EHPA/0.125 M TBP
59.52 ± 0.99
12.56 ± 0.48
1.470 ± 0.079
0.1436 ± 0.0065
4.739 ± 0.073
2.000 ± 0.034
0.3348 ± 0.0214
0.25 M D2EHPA/0.25 M TBP
85.70 ± 1.53
15.65 ± 0.62
5.993 ± 0.631
0.1855 ± 0.0084
5.476 ± 0.143
2.502 ± 0.248
0.2378 ± 0.0014
0.50 M D2EHPA/0.50 M TBP
86.80 ± 1.13
16.27 ± 0.58
6.576 ± 0.379
0.1943 ± 0.0085
5.335 ± 0.232
2.606 ± 0.141
0.2323 ± 0.0101
![Effect of D2EHPA extractant concentrations: feed solution; 15 mg/L Ni2+, 25 mg/L [Au(CN)2]−, pH 8.6 ± 0.05 | organic solution; D2EHPA in kerosene | stripping solution; 0.5 M HCl, pH 0.38 ± 0.05 | flow ratefeed = flow ratestripping = 200 mL/min | separation time 2 h | T = 303 ± 1 K.](/content/184/2021/14/12/img/10.1016_j.arabjc.2021.103427-fig8.png)
Effect of D2EHPA extractant concentrations: feed solution; 15 mg/L Ni2+, 25 mg/L [Au(CN)2]−, pH 8.6 ± 0.05 | organic solution; D2EHPA in kerosene | stripping solution; 0.5 M HCl, pH 0.38 ± 0.05 | flow ratefeed = flow ratestripping = 200 mL/min | separation time 2 h | T = 303 ± 1 K.
![Effect of TBP extractant concentrations: feed solution; 15 mg/L Ni2+, 25 mg/L [Au(CN)2]−, pH 8.6 ± 0.05 | organic solution; TBP in kerosene | stripping solution; 0.5 M HCl, pH 0.38 ± 0.05 | flow ratefeed = flow ratestripping = 200 mL/min | separation time 2 h | T = 303 ± 1 K.](/content/184/2021/14/12/img/10.1016_j.arabjc.2021.103427-fig9.png)
Effect of TBP extractant concentrations: feed solution; 15 mg/L Ni2+, 25 mg/L [Au(CN)2]−, pH 8.6 ± 0.05 | organic solution; TBP in kerosene | stripping solution; 0.5 M HCl, pH 0.38 ± 0.05 | flow ratefeed = flow ratestripping = 200 mL/min | separation time 2 h | T = 303 ± 1 K.
![Effect of mixed D2EHPA and TBP extractant concentrations (1:1 ratio): feed solution; 15 mg/L Ni2+, 25 mg/L [Au(CN)2]−, pH 8.6 ± 0.05 | organic solution; mixed of D2EHPA and TBP extractant in kerosene | stripping solution; 0.5 M HCl, pH 0.38 ± 0.05 | flow ratefeed = flow ratestripping = 200 mL/min | separation time 2 h | T = 303 ± 1 K.](/content/184/2021/14/12/img/10.1016_j.arabjc.2021.103427-fig10.png)
Effect of mixed D2EHPA and TBP extractant concentrations (1:1 ratio): feed solution; 15 mg/L Ni2+, 25 mg/L [Au(CN)2]−, pH 8.6 ± 0.05 | organic solution; mixed of D2EHPA and TBP extractant in kerosene | stripping solution; 0.5 M HCl, pH 0.38 ± 0.05 | flow ratefeed = flow ratestripping = 200 mL/min | separation time 2 h | T = 303 ± 1 K.
Overall, results demonstrate that by increasing the concentration of the extractant from 0.01 to 1.00 M, for the single D2EHPA, TBP and synergistic D2EHPA/TBP system, the extraction of Ni2+ and [Au(CN)2]− ions increased. Beyond 0.50 M and up to 1.00 M, it is seen that the slight difference in extraction of Ni2+ and [Au(CN)2]− ions reveals that the plateau stage was reached. Thus, 0.5 M concentration of the extractant proved to be sufficient and economical for the extraction of Ni2+ and [Au(CN)2]− ions in trace amount.
3.5 Effect of the type of strippant
The type of strippant or stripping agent is one of the key factors for the back extraction or stripping of metal ions from a thin layer of the organic liquid membrane. The loaded organic phase was stripped using different acidic solutions. In Table 8, stripping efficiencies of the various strippants i.e. distilled water (H2O) and the strong inorganic acids (HCl, H2SO4 and HNO3) are presented. In the case of the synergistic D2EHPA/TBP extractants, stripping percentages of the target Ni2+ ions when using different strippants were found to be in the order: HCl > H2SO4 > H2O > HNO3. It was difficult to strip Ni2+ ions from the loaded D2EHPA/TBP using distilled water. Furthermore, stripping performances of the non-target [Au(CN)2]− ions were less than 2% for all three strong inorganic acids. Thus, HCl was selected as the most suitable strippant for use in the HFSLM system due to the results, which affirm the highest selectivity of Ni2+ relative to [Au(CN)2]− ions in the stripping solution.
Strippant
pH at 303 K
pKa
Organic extractant
%Stripping
Distribution Ratio (D)
Selectivity (SNi/Au)
Ni2+
[Au(CN)2]−
Ni2+
[Au(CN)2]−
HCl
0.44 ± 0.02
−6.11
0.25 M D2EHPA/0.25 M TBP
85.70 ± 0.18
15.65 ± 0.62
5.993 ± 0.631
0.1855 ± 0.0084
5.476 ± 0.143
0.50 M D2EHPA
56.18 ± 0.89
24.21 ± 0.57
1.282 ± 0.043
0.3194 ± 0.0096
2.321 ± 0.021
0.50 M TBP
52.68 ± 0.73
31.54 ± 0.85
1.113 ± 0.035
0.4607 ± 0.0172
1.670 ± 0.075
HNO3
0.42 ± 0.02
−1.38
0.25 M D2EHPA/0.25 M TBP
4.473 ± 0.158
1.941 ± 0.020
0.04682 ± 0.00164
0.01979 ± 0.00028
2.304 ± 0.213
0.50 M D2EHPA
7.353 ± 0.549
2.220 ± 0.260
0.07937 ± 0.00660
0.02270 ± 0.00281
3.312 ± 0.288
0.50 M TBP
2.441 ± 0.520
1.554 ± 0.149
0.02502 ± 0.00562
0.01579 ± 0.00161
1.571 ± 0.412
H2SO4
0.43 ± 0.02
−3.00
0.25 M D2EHPA/0.25 M TBP
23.85 ± 0.38
1.284 ± 0.239
0.3132 ± 0.0069
0.01301 ± 0.00254
18.57 ± 0.41
0.50 M D2EHPA
9.862 ± 0.379
2.432 ± 0.521
0.1094 ± 0.0048
0.02493 ± 0.00564
4.055 ± 0.017
0.50 M TBP
4.723 ± 0.092
1.230 ± 0.521
0.0496 ± 0.0011
0.01245 ± 0.00071
3.840 ± 0.0478
H2O
5.86 ± 0.02
14.0
0.25 M D2EHPA/0.25 M TBP
3.050 ± 0.317
5.006 ± 0.331
0.03146 ± 0.00355
0.05270 ± 0.00379
0.6093 ± 0.0084
0.50 M D2EHPA
4.731 ± 0.090
9.403 ± 0.532
0.04966 ± 0.00107
0.1038 ± 0.0067
0.5031 ± 0.0200
0.50 M TBP
0.9906 ± 0.036
12.21 ± 0.263
0.01001 ± 0.00037
0.1391 ± 0.0035
0.08113 ± 0.00108
To understand these observations in detail, awareness of the reaction mechanism between the metal-extractant complex and strippant is indispensable. The stripping mechanisms of Ni2+-extractant complexes by inorganic acids (HA), which occur at the organic-aqueous interface can be represented, as in Eqs. (15) to (18).
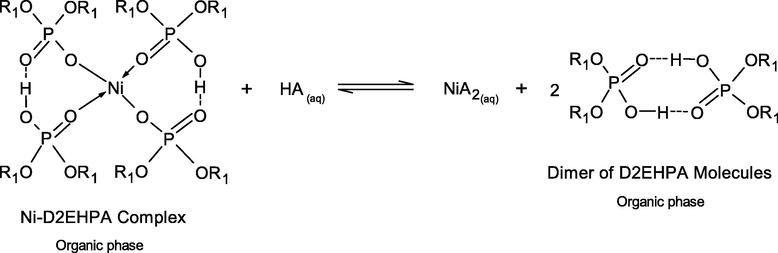
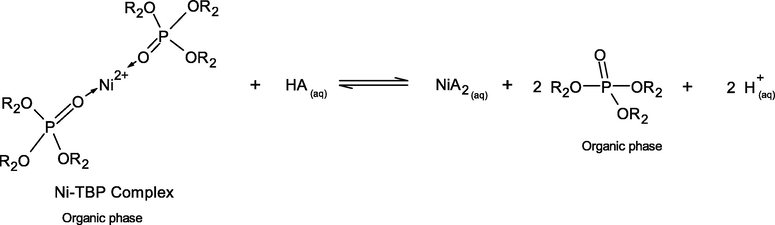
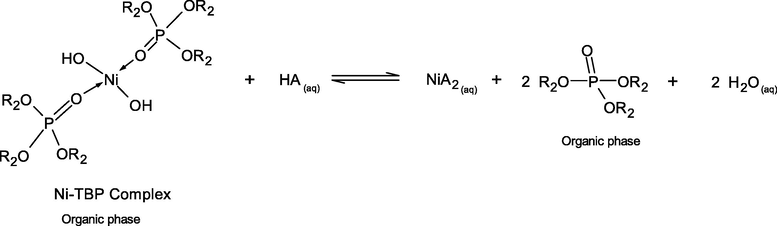
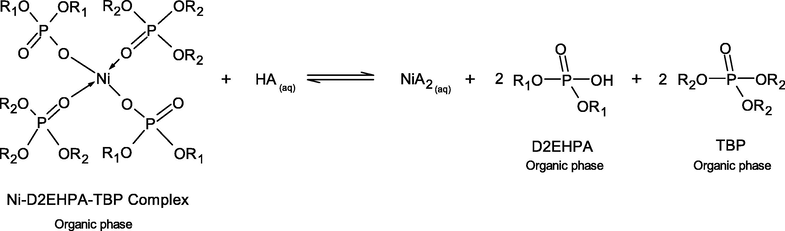
The case of different inorganic acids resulting in different stripping percentage of Ni2+-extractant complexes can be explained by the effect of pKa of these acids. In simple terms, pKa is a number that shows how weak or strong an acid is. A strong acid will have a pKa of less than zero. The lower the value of pKa, the stronger the acid and the greater its ability to donate its protons. The extraction of Ni2+ by D2EHPA and TBP extractants from the real rinse wastewater of the ENIG plating process is carried out in basic alkaline solution at pH 8.5 ± 0.05. However, higher acid conditions are more favorable. D2EHPA has highly acidic properties, due to the presence of oxygen atoms bonding to the phosphorus atom and the alkyl radicals. In Eqs. (15) and (18), the strong interaction of O-Ni-O bonding between D2EHPA and Ni proved difficult for stripping in the mild acid. From Table 8, the order of pKa of the three inorganic acids are listed as follows: HCl > H2SO4 > HNO3. Thus, HCl is the most effective strippant.
3.6 Effect of stripping agent concentration
Since stripping depends on the nature of the metal complexes in the loaded organic phase, the nature and the concentration of acidic stripping solutions affect stripping efficiency. The effect of HCl concentration towards selective and synergistic stripping of Ni2+ ions was studied in the range of 0.001 to 1.00 M with pH from 3.01 to 0.1, as exhibited in Table 9 as well as Figs. 8 and 9.
Strippant Concentration
pH at 303 K
%Stripping
Distribution Ratio (D)
Selectivity (SNi/Au)
Ni2+
[Au(CN)2]−
Ni2+
[Au(CN)2]−
0.001 M HCl
3.01 ± 0.02
9.683 ± 0.273
1.134 ± 0.255
0.1072 ± 0.0035
0.01147 ± 0.00262
8.536 ± 1.374
0.01 M HCl
2.02 ± 0.02
13.06 ± 0.18
1.336 ± 0.021
0.1502 ± 0.0023
0.01354 ± 0.00022
9.773 ± 0.281
0.05 M HCl
1.37 ± 0.02
45.12 ± 0.63
1.558 ± 0.014
0.8221 ± 0.0220
0.01583 ± 0.00014
28.95 ± 0.20
0.10 M HCl
1.09 ± 0.02
72.21 ± 0.60
1.579 ± 0.040
2.598 ± 0.086
0.01605 ± 0.00041
45.72 ± 0.62
0.25 M HCl
0.70 ± 0.02
79.18 ± 0.61
1.819 ± 0.061
3.802 ± 0.161
0.01853 ± 0.00063
43.53 ± 0.94
0.50 M HCl
0.38 ± 0.02
83.21 ± 0.26
1.940 ± 0.023
4.956 ± 0.112
0.01978 ± 0.00024
42.89 ± 0.32
1.00 M HCl
0.10 ± 0.02
84.73 ± 0.42
2.010 ± 0.137
5.549 ± 0.217
0.02051 ± 0.00142
42.15 ± 3.33
![Effect of strippant concentration: feed solution; 15 mg/L Ni2+, 25 mg/L [Au(CN)2]−, pH 8.6 ± 0.05 | organic solution; mixture of 0.25 M D2EHPA and 0.25 M TBP extractants in kerosene | stripping solution; HCl, | flow ratefeed = flow ratestripping = 200 mL/min | separation time 2 h | T = 303 ± 1 K.](/content/184/2021/14/12/img/10.1016_j.arabjc.2021.103427-fig11.png)
Effect of strippant concentration: feed solution; 15 mg/L Ni2+, 25 mg/L [Au(CN)2]−, pH 8.6 ± 0.05 | organic solution; mixture of 0.25 M D2EHPA and 0.25 M TBP extractants in kerosene | stripping solution; HCl, | flow ratefeed = flow ratestripping = 200 mL/min | separation time 2 h | T = 303 ± 1 K.
![%Stripping of Ni2+ and [Au(CN)2]− ions via HFSLM with various pH of HCl strippant: feed solution; 15 mg/L Ni2+, 25 mg/L [Au(CN)2]−, pH 8.6 ± 0.05 | organic solution; mixture of 0.25 M D2EHPA and 0.25 M TBP extractants in kerosene | stripping solution; HCl, | flow ratefeed = flow ratestripping = 200 mL/min | separation time 2 h | T = 303 ± 1 K.](/content/184/2021/14/12/img/10.1016_j.arabjc.2021.103427-fig12.png)
%Stripping of Ni2+ and [Au(CN)2]− ions via HFSLM with various pH of HCl strippant: feed solution; 15 mg/L Ni2+, 25 mg/L [Au(CN)2]−, pH 8.6 ± 0.05 | organic solution; mixture of 0.25 M D2EHPA and 0.25 M TBP extractants in kerosene | stripping solution; HCl, | flow ratefeed = flow ratestripping = 200 mL/min | separation time 2 h | T = 303 ± 1 K.
As presented in Table 9, the increase in acid concentration increased the stripping percentage of Ni2+ ions. It is observed that Ni2+ stripping increased up to 85%; maximum HCl concentration was 1.00 M. Beyond 0.50 M HCl, stripping slightly increased. In Fig. 9, when pH of the stripping solution decreased, the stripping efficiency of Ni2+ increased. 0.50 M HCl proved to be enough for selective stripping of Ni2+ from [Au(CN)2]− ions in trace amount.
3.7 Equilibrium study of Ni2+ extraction via HFSLM
The extraction of Ni2+ ions using single extractants D2EHPA and TBP as well as the synergistic extractant mixture D2EHPA/TBP can be written as shown in Appendix A.
According to Eqs. (A10) and (A12), plots between
in y-axis and
in x-axis provide the equilibrium constant (Kex) from the y-axis intersection as shown in Table 10.
Extractant
Kex
D2EHPA
3.719 mol/L
TBP
1.479 (mol/L)−1
D2EHPA/TBP (1:1 ratio)
11.35
3.8 Mass transfer coefficient for Ni2+ ions extraction from the real rinse wastewater of the ENIG plating process via HFSLM
For the recycling mode of operation both feed and strip phases are continuously recirculated from the respective reservoirs. The resistances across the feed–membrane interface, membrane pore and strip–membrane interface are related as follows (Sharma et al., 2016a). Detail calculation on mass transfer coefficients and mass balance have shown in Appendix B.
Eqs. (B28) to (B30) in Appendix B is applied to calculate the diffusion flux (JD) and the mass transfer coefficient for Ni2+ and [Au(CN)2]− ions via the liquid membrane. The results are shown in Table 11.
Ni2+ ions extraction from aqueous feed phase to organic liquid membrane phase
Extractant
JD mmol/m2∙s
Kex m/s
0.5 M D2EHPA
1.42 × 10−5
12.7 × 10−5
0.5 M TBP
1.34 × 10−5
11.0 × 10−5
0.25 M D2EHPA/0.25 M TBP
2.17 × 10−5
59.4 × 10−5
[Au(CN)2]− ions extraction from aqueous feed phase to organic liquid membrane phase
0.5 M D2EHPA
0.305 × 10−5
3.17 × 10−5
0.5 M TBP
0.397 × 10−5
4.57 × 10−5
0.25 M D2EHPA/0.25 M TBP
0.197 × 10−5
1.84 × 10−5
3.9 Determination of the reaction order and the reaction rate constant for Ni2+ and [Au(CN)2]− extraction from the real rinse wastewater of the ENIG plating process via HFSLM
The reaction order (n) and the reaction rate constant for Ni2+ and [Au(CN)2]− extraction (
) were verified by integration and graphical method as shown in Table 12.
Ni2+ ions extraction from aqueous feed phase to organic liquid membrane phase
Extractant
Rate order
Reaction rate constant
R2
Remark
0.5 M D2EHPA
0
1.6 × 10−3
mmol∙L−1∙min−1
0.5786
1
9 × 10−3
min−1
0.7224
2
2 × 10−5
L∙ mmol−1∙min−1
0.8396
Best fit
0.5 M TBP
0
1.4 × 10−3
mmol∙L−1∙min−1
0.8574
1
7.4 × 10−3
min−1
0.9187
2
1 × 10−5
L∙ mmol−1∙min−1
0.9631
Best fit
0.25 M D2EHPA/0.25 M TBP
0
2.6 × 10−3
mmol∙L−1∙min−1
0.3932
1
2.22 × 10−2
min−1
0.6043
2
7 × 10−5
L∙ mmol−1∙min−1
0.7927
Best fit
[Au(CN)2]− ions extraction from aqueous feed phase to organic liquid membrane phase
0.5 M D2EHPA
0
3 × 10−4
mmol∙L−1∙min−1
0.9821
Best fit
1
2.3 × 10−3
min−1
0.9808
2
5 × 10−7
L∙ mmol−1∙min−1
0.9772
0.5 M TBP
0
3 × 10−4
mmol∙L−1∙min−1
0.9934
1
3.2 × 10−3
min−1
0.9958
Best fit
2
7 × 10−7
L∙ mmol−1∙min−1
0.9928
0.25 M D2EHPA/0.25 M TBP
0
2 × 10−4
mmol∙L−1∙min−1
0.9821
Best fit
1
1.4 × 10−3
min−1
0.9815
2
3 × 10−7
L∙ mmol−1∙min−1
0.9801
4 Conclusions
In this paper, results demonstrate that HFSLM could successfully separate Ni2+ and [Au(CN)2]− from the real rinse wastewater of the ENIG plating process. It is evident that the binary mixtures having acidic/neutral extractants of D2EHPA/TBP were found to be capable of selectively extracting both Ni2+ and [Au(CN)2]− ions at low concentrations from the feed solution viz. up to 85.70% and 15.65%, respectively. Optimum conditions proved to be 0.25 M D2EHPA and 0.25 M TBP in kerosene in the liquid membrane phase. Subsequently, 0.5 M HCl was employed as a stripping agent for the back extraction of Ni2+ and for [Au(CN)2]− ions from the organic phase. The HFSLM technique illustrates the selective extraction of Ni2+ ions over other conventional techniques. The successful separation of Ni2+ ions from the real rinse wastewater of the ENIG plating process via HFSLM proved its worth that it can be a most useful technique in the integrated operation of the ENIG plating process.
Acknowledgements
This work was supported by the Research and Researcher for Industry (RRI) of Thailand Research Fund (TRF) [grant numbers PHD60I005]; Mektec Manufacturing corporation (Thailand) ltd.; the Mass Separation Laboratory, Department of Chemical Engineering, Chulalongkorn University as well as the Research Cess Fund (Malaysia–Thailand Joint Authority).
References
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2021.103427.
Appendix A
Supplementary material
The following are the Supplementary data to this article:







