Translate this page into:
Spectroscopic and TDDFT studies of N-phenyl-N′-(3-triazolyl)thiourea) compounds with transition metal ions
⁎Corresponding author. mansour@sci.cu.edu.eg (Ahmed M. Mansour),
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Reaction of N-phenyl-N′-(3-triazolyl)thiourea) (H3L) with M(ClO4)2 (M = Co(II) (1), Ni(II) (2) and Cu(II) (3)) afforded [M(H2L)2] complexes, which were characterized experimentally and theoretically using different analytical, and spectral tools. The susceptibility of Staphylococcus aureus and Escherichia coli bacteria towards H3L and its complexes was evaluated. The thiourea ligand coordinates to the 3d-metal ions via C–S−, and triazole nitrogen yielding coordination compounds between the tetrahedral, and square-planar geometries (“flattened” tetrahedron, D2d symmetry). Full geometry optimization, vibrational analyses, and natural bond orbital analyses of the proposed conformations of 1–3 were executed at B3LYP/def2-SVP to gain some knowledge about the local minima structures, natural charge of the coordinated metal ion, electronic configuration as well as the hybridization of M–L bonds. The electronic structures of the local minimum structures of 1–3 were investigated by time-dependent density functional theory calculations. Coordination of the thiourea ligand to Co(II) and Cu(II) ions did alter the toxicity against the tested microbes, while chelation of Ni(II) ion gave rise to inactive species.
Keywords
Antibacterial activity
NBO
TDDFT
Thiourea ligands
Triazole
1 Introduction
The coordination chemistry of thiourea, and their derivatives received considerable interest due to their valuable industrial, (Fuerst and Jacobsen, 2005; Khaled, 2009; Fontás et al., 2005) and biological applications. (Zhao et al., 2009; Sunduru et al., 2009; Han et al., 2006; Mansour, 2016; Manjula et al., 2009; Dixit et al., 2006) Metal complexes of some thiourea derivatives exhibited effective ani-inflammatory, (Zhao et al., 2009) antimalarial, (Sunduru et al., 2009) antimicrobial (Han et al., 2006; Mansour, 2016) and cytotoxic activities. (Manjula et al., 2009; Dixit et al., 2006) Urease, the nickel-containing metalloenzyme, catalyzes the hydrolysis of urea in some plants and animals forming ammonia and CO2. Subsequently, some thiourea based Ni(II) complexes were investigated as models for the nickel center of enzymes. (Halcrow and Christou, 1994) Some thiourea derivatives exhibited respectable inhibitory activity towards tyrosine kinases, protein tyrosine kinases, and NADH oxidase (Hubbard and Till, 2000). Alternatively, the applications of thiourea based compounds were extended to cover the areas of catalysis, (Fuerst and Jacobsen, 2005) corrosion inhibitors, (Khaled, 2009) and water treatment to remove the heavy metal ion. (Fontás et al., 2005) Some thiourea derivatives were investigated and used as ionophores for anion sensing. (Liu et al., 2005; Blažek Bregovic et al., 2015) Photoactivatable properties of tricarbonyl thiourea manganese(I) compounds, capable of releasing CO upon the irradiation at 525–468 nm, were recently reported. (Mansour and Shehab, 2018; Mansour and Friedrich, 2017) The combination of soft chelation thione group and strong σ-donating ligand, Br− weaken the Mn–CO bond and facilitated the CO release. “Piano-stool” Ru(η6-p-Cym)2+ complexes functionalized with thiourea derivatives exhibited excellent toxicity to Staphylococcus aureus, which influenced by the type of the heterocyclic arm. (Mansour and Radacki, 2020) On the other hand, triazole-based compounds showed significant biological activity as antifungal, (Papakonstantinou-Garoufalias et al., 1998) and antimicrobial agents (Colanceska-Ragenovic et al., 2001).
In the present contribution, it is motivating to include triazole moiety into the framework of thiourea via the synthesis of N-phenyl-N′-(3-triazolyl)thiourea) (H3L, Scheme 1). The crystal structure, and the antimicrobial properties of H3L were reported by Stefanska et al. (2012). H3L ligand plays an important role in inhibition of the enzymatic properties of copper-containing enzyme, Dopamine fi-hydroxylase (DBH), (Goldstein et al., 1965) which is essential to catalyze the final step of the conversion of dopamine to norepinephrine Cl, through the uptake of the copper from the DBH enzyme. Coordination of H3L to Pd(II) and Pt(II) ion gave rise to inactive species. (Mansour, 2018) The solid metal complexes of H3L with Co(II), Ni(II), Cu(II), and Cd(II) chlorides were reported elsewhere (Salman, 2001) assuming that H3L behaved as a neutral bidentate ligand towards the latter mentioned Mn+ ions via the thione, and triazole nitrogen that is inconsistent with our findings.
Synthesis of some thiourea based 3d-transition metal complexes 1–3.
Herein, the ligational behavior of H3L towards some 3d-transition metal ions was investigated both experimentally, and theoretically using different analytical and spectroscopic tools. Afterward, geometry optimization, and vibrational analyses of the proposed structures of 1–3, in the ground state, were performed to obtain some knowledge about the local minima structures. Natural bond orbital analyses (Reed et al., 1988) were executed to obtain some information about the intramolecular H-bond interactions, hybridization of M–L bonds, electronic configuration, and charge of the coordinated metal ion. To understand the observed absorption electronic transitions, time-dependent density functional theory (TDDFT) calculations were performed. Finally, the antibacterial activity of the compounds was assessed against Staphylococcus aureus and Escherichia coli.
2 Experimental section
2.1 General procedures
Phenyl isothiocyanate, 2-amino-1,3,4-triazole, pyridine, metal perchlorates (Co(ClO4)2·6H2O, Ni(ClO4)2·6H2O, and Cu(ClO4)2·6H2O) and organic solvents were purchased from the commercial sources and used as received. The thiourea ligand was prepared by following the previously reported method. (Mansour, 2018) Potassium bromide pellets of the ligand and complexes were subjected to IR analysis using Jasco FTIR 460 plus. Micro-elemental analyses were determined on an Automatic Analyzer CHNS, Vario EL III Elementar. Electronic absorption spectra were recorded on OPTIZEN POP automate spectrophotometer. The magnetic susceptibility values of the paramagnetic complexes were determined using Sherwood scientific magnetic balance using Gouy method. (Hilal and Fredericks, 1954) The molar conductivity values were obtained by digital Jenway 4310 (cell constant is 1.02). X-band EPR measurements were executed on solid samples at 298 K using a Bruker EMX spectrometer. The magnetic modulation frequency was 100 kHz, and the microwave power was set to 0.201 mW. The g-values were recorded by referencing to a diphenylpicrylhyrdazyl (DPPH) sample with g = 2.0036. The modulation amplitude was fitted at 4 Gauss while the microwave frequency was verified as 9.775 GHz. Thermogravimetric analysis (TGA) was executed under dinitrogen atmosphere (20 mL min−1) in platinum crucible with a heating rate of 10 °C min−1 using a Shimadzu DTG-60H simultaneous DTG/TG apparatus.
2.2 Synthetic procedures
To a round flask charged with H3L ligand (2 mmol, 436 mg), and acetone (20 mL), 5 mL aqueous solution of metal perchlorate (1 mmol; Co(ClO4)2·6H2O (365 mg), Ni(ClO4)2·6H2O (366 mg) and Cu(ClO4)2·6H2O (370 mg) was added and then the pH of the reaction mixtures was adjusted at around 8.0 by adding few drops of ammonia. Then the solutions were heated to reflux for 4 h, whereupon precipitation occurred. The solid products were collected by filtration, washed with diethyl ether, and dried under vacuum. Stock solutions (10−3 M) of complexes 1–3 (Scheme 1) in DMF were prepared and their molar conductance values were recorded and compared to the reported data for 1:1 (65–90 Ω−1 cm2 mol−1) and 1:2 (130–170 Ω−1 cm2mol−1) electrolytes in the same solvent at the same temperature. (Mansour, 2014) The conductance values of 1–3 were found to be in the range of 13.8–41.2 Ω−1 cm2mol−1 reflecting the non-ionic nature of the complexes and rules out the probability of presence of the perchlorate ion(s) in the secondary coordination sphere.
-
[Co(H2L)2]·0.5H2O (1): Brown, yield 75% (759 mg, 1.5 mmol). IR (FTR, cm−1): ν = 3399 (br, NH), 3211 (br, NH), 3024 (w, CH), 1563 (w, –S–C = N–thiol/CC), 1520 (w, CC), 1487, 1397, 1310 (m, /C S/C–Hβ), 1257, 1173, 1083 (br, C–N/C S), 754 (m, C S). C18H16CoN10S2·0.5H2O: C 42.86, H 3.40, N 27.77, found C 43.22, H 3.44, N 27.92. UV/Vis (DMF, 10−4 M, nm): 260, 280, 570. ESI-MS (positive mode, acetone): 496.0558 {M + 1}+ (M: molecular mass). Effective magnetic moment, μeff (μB, 298 K): 2.79. Molar conductance (DMF, 10−3 M, Ω−1cm2 mol−1): 41.2.
-
[Ni(H2L)2] (2): Buff, yield 79% (788 mg, 1.57 mmol). IR (FTR, cm−1): ν = 3386 (br, NH), 3228 (br, NH), 3030 (w, CH), 1594 (m, –S–C = N–thiol), 1530 (w, CC), 1494, 1396, 1311 (m, /C S/C–Hβ), 1209, 1086 (br, C–N/C S), 747 (m, C S). C18H16NiN10S2: C 43.66, H 3.26, N 28.28, found C 43.22, H 3.28, N 27.92. UV/Vis (DMF, 10−4 M, nm): 255, 295, 370, 445. ESI-MS (positive mode, acetone): 494.0935 {M + 1}+. μeff (μB, 298 K): 0.78. Molar conductance (DMF, 10−3 M, Ω−1cm2mol−1): 13.8.
-
[Cu(H2L)2]·2H2O (3): Dark-brown, 76% (812 mg, 1.51 mmol). IR (FTR, cm−1): ν = 3399 (br, NH), 3299 (br, NH), 3026 (w, CH), 1572 (w, –S–C = N–thiol/CC), 1493, 1361, 1313 (m, /C S/C–Hβ), 1259, 1096 (br, C–N/C S), 743 (m, C S). C18H16CuN10S2·2H2O: C 40.33, H 3.76, N 26.13, found C 40.32, H 3.44, N 25.85. UV/Vis (DMF, 10−4 M, nm): 300, 335, 390, 405, 470, 620. ESI-MS (positive mode, acetone): 501.1330 {M + 1}+. μeff (μB, 298 K): 1.44. Molar conductance (DMF, 10−3 M, Ω−1 cm2mol−1): 20.3.
2.3 DFT/NBO/TDDFT calculations
Full geometry optimization of the proposed four-coordinated conformations of 1–3, in the ground state, and their vibrational analyses were carried out by Becke 3-parameter (exchange) Lee–Yang–Parr (B3LYP) functional, (Becke, 1993) and def2-SVP basis set using Gaussian03 package (Frisch et al., 2003). For time dependent density functional theory (TDDFT) calculations, the 30 singlet excited states were considered and calculated at CAM-B3LYP (with long range correction) (Yanai et al., 2004)/def2-SVP level of theory. Dimethyl sulfoxide was incorporated in the TDDFT calculations using the polarized continuum model as implemented in Gaussian03. Natural population analyses were executed using NBO method at B3LYP/def2-SVP level of theory. Local minimum structures, electronic spectra, and frontier molecular orbitals were visualized by Gaussview03 (Frisch et al., 2000).
2.4 Antibacterial activity
The antibacterial activities of the functionalized thiourea ligand and its coordination compounds against cultures of Staphylococcus aureus and Escherichia coli microbes were assessed using a modified Kirby-Bauer disc diffusion method (Abdel-Ghani and Mansour, 2012) at the concentration of 20 mg mL−1, while keeping the final DMSO concentration to a maximum of 0.5% and serially diluted 1:2 fold for 8 times. The toxicity of the compounds was compared to the standard tetracycline. In this assay, the centrifuged pellets of bacteria from a 24 h old culture comprising of approximately 104–106 CFU mL−1 (CFU: colony forming unit) were distributed on the surface of Mueller-Hinton Agar plates (evaluated for composition and pH). Then, the wells were contaminated with 10 mL of prepared inocula to get 106 CFU mL−1. Petri plates were made by flowing 100 mL of seeded nutrient agar. Plates were covered and incubated at 37 °C for 18 h. DMSO (0.1 mL) was used as control under the same conditions for each microorganism, subtracting the diameter of the inhibition zone obtained with the solvent from that observed in each case. The antibacterial activities were determined as a mean of three replicates.
3 Results & discussion
3.1 Synthesis and spectral characterization
The transition metal complexes 1–3 (Scheme 1) were prepared by heating to reflux the slightly alkaline reaction mixtures of the metal perchlorates and H3L, in 75% (v/v) acetone–water mixture, where complexes [M(H2L)2] (M = Co(II) (1), Ni(II) (2) and Cu(II) (3)) were collected by filtration. Their proposed structures were deduced from elemental analysis, TGA, UV/Vis, IR, ESR, magnetic and conductivity measurements. The vibrational spectrum of the free ligand (Fig. S1) shows two broad bands at 3381, and 3207 cm−1 assigned to the ν(NH)Ph and ν(NH)triazole−exo modes as well as a group of bands in the range of 1570–700 cm−1 having a contribution from –NH–C = S group as follows: 1540 (β(N–H)/β(C = C)) I), 1385 (ν( )/ν(C = S)/β(C–H)) II), 1018 (ν(C–N)/ν(C–S)) III), and 837 cm−1 (δ(C = S) IV) (Mansour, 2016; Mansour, 2018). The ν(NH)triazole−endo could not be identified owing to the tautomeric structure of the triazole ring. The absence of ν(S–H) mode at around 2570 cm−1 in the IR spectrum of the free ligand as well as the presence of a signal at δ 177.0 ppm in the 13C NMR spectrum confirm the existence of H3L in the thione form in both the solution and solid states. Coordination of H3L to the metal ion induced considerable spectral changes in the vibrational ranges of 1630–1300, and 900–700 cm−1. A new band assigned to ν(S–C = N-thiol) at 1594 cm−1 in the spectrum of 2 or allocated for ν(S–C = N-thiol)/ν(CC) at 1563 and 1572 cm−1 in the spectra of 1, and 3 is observed, which may be attributed to enolization of –NH–C = S group into –N = C–SH, ionization of SH group, and coordination to metal ion (Fig. S1). The shift of ν(NH)Ph mode to higher wave numbers (3386–3399 cm−1) rules out the role of the phenyl-NH group in the metal complex formation. The participation of the triazole nitrogen in the chelation inhibits the tautomeric behaviour within the moiety and leads to grown of a new band in the range of 3299–3211 cm−1 assigned to the ν(NH)triazole−endo mode. IR studies indicated that the H3L coordinates to Mn+ via C–S−, and triazole nitrogen.
The electronic absorption spectrum of the free ligand, in DMF, (Fig. S2) displays two electronic transitions at 271, and 310 nm (Mansour, 2018). In the highest energy region of the electronic spectrum of 1, two prominent bands corresponding to the intra-ligand transitions are observed at 260 and 280 nm. In addition, a broad shoulder is seen at around 570 nm in the spectrum of 1 (Fig. S2), which may be assigned to 4A2→4T1(P) (ν3) in tetrahedral Co(II) geometry (Kowalkowska-Zedler et al., 2020; Vašák et al., 1981). The effective magnetic moment of 1 is 2.79 μB. Generally, octahedral Co(II) complexes exhibit μeff values around 4.7 μB owing to the large orbital contribution, while the square-planar complexes show values around 1.7 μB corresponding to presence of an unpaired electron. Tetrahedral Co(II) complexes have μeff values usually equivalent to the population of three unpaired electrons that may be affected by orbital contribution. The observed μeff of 1 further complements the electronic findings of the distorted tetrahedral field. The μeff value of 2 is 0.78 μB suggesting a highly probability of presence of a mixture of square-planar/tetrahedral (Al-Awadi et al., 2006; Kandil et al., 2002) or existence of Ni(II) ion in a flattened tetrahedral field. By assuming the presence of a mixture of the two four-coordinated geometries, the tetrahedral percentage (Nt) in the proposed mixture is 5.6% according to the following relation, Nt = [(μeff)2/(3.3)2] × 100, where μeff value for ideal tetrahedral Ni(II) complexes is 3.3 μB. The electronic spectrum of 2 shows four transitions at 255, 295, 370, and 445 nm (Fig. S2). The two highest energy bands at 255, and 295 nm are assigned to π–π* transitions within the ligand framework. The shoulder at 370 nm is allocated for LMCT (S(σ) → Ni(II)) and the broad band at 445 nm is assigned to a combination of LMCT (N(σ) → Ni(II))/ (1A1g→ 1B1g) transitions (Royer et al., 1972). For Cu(II) complex 3, the μeff (1.44 μB) is slightly lower than the expected value for the regular spin only value of d9 metal ion revealing the probability of presence of weak antiferromagnetic interactions between the neighbouring species (Mansour, 2013). The electronic absorption spectrum of 3 (Fig. S2), in DMF, is characterized by four transitions in the visible region at 390, 405, 470, and 620 nm. Generally, square-planar Cu(II) coordination compounds are characterized by a broad band at around 650 nm corresponding to the unresolved d-d transitions, while tetrahedral complexes have no electronic transitions in the 500–1000 nm region (Sreekanth and Prathapachandra-Kurup, 2003). The transitions at 405, 470, and 620 nm may be assigned to LMCT (S(σ) → Cu(II))) (Dhara et al., 2004), LMCT(N(π) → Cu(II)) (Sreekanth and Kurup, 2003) and (2B1g → 2E1g and 2B1g → 2A1g (Lever, 1984; Garg et al., 1993) within the square-planar field. Therefore, in the solution state, Cu(II) ion of 3 may be surrounded by four ligands, which are arranged in a planar form.
The thermal decompositions of complexes (1–3) were examined in the temperature range from the ambient up to 1000 °C, under pure dinitrogen atmosphere, using a heating rate of 10 °C/min (Fig. S3). For 1, the decomposition starts at about 50 °C and proceeds through three complicated thermal steps maximally at 79, 283, and 489 °C. The first step is accompanied by a mass loss of 1.67% (calcd. 1.79%) corresponding to desorption of 0·.5H2O. The thermal steps bring the total mass loss up to 65.45% (calcd. 66.20%) leaving carbonized CoS2 (Mansour, 2014). In the case of 2, no thermal decomposition is observed below 130 °C revealing the absence of any volatile solvents and/or water of hydration. The thermogravimetric analysis of 2 shows four stages maximally at 242, 416, 488, and 782 °C. For 3, the mass loss has been taken in four thermal steps maximally at 77, 189, 238, and 484 °C. The first thermal stage up to 110 °C, with a mass loss of 6.52% (calcd. 6.71%), may be attributed to desorption of two hydrated water molecules. It seems that compounds 2 and 3 are hardly to be decomposed in the temperature range up to 1000 °C under an inert atmosphere with a total mass loss of 54.61% and 58.22%, respectively, as no plateau is reached and no stable Ni(II) or Cu(II) residue may be proposed to be existed at 1000 °C. This behaviour has been reported with similar complexes under the same experimental conditions (Mansour, 2016; Faraglia et al., 1990).
The solid-state X-band EPR spectrum of Cu(II) complex 3 (Fig. 1), at the room temperature is axial with two well defined
2.30 (
= 129 × 10−4 cm−1) and
2.05 values and
(2.0023) indicating that the unpaired electron may be present in the
orbital. The degree of the distortion from the tetrahedral geometry can be deduced from the f value (
), where f = 105–135 cm is recognized for the idea square-planar geometry and f > 135 cm indicates the presence of a distorted tetrahedral geometry (Sundaravel et al., 2009). It is worthy mention that the f value of 3 is 178 cm suggesting presence of Cu(II) ion in the tetrahedron field in the solid state, and this has been further supported by quantum chemical calculations (vide infra).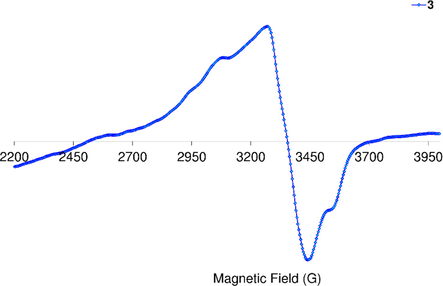
EPR spectrum of Cu(II) complex, 3.
The spectroscopic, magnetic, and analytical data of the complexes, prepared from the reaction of H3L, and metal perchlorate salts in a slightly basic media, indicated the formation [M(H2L)2] compounds. Searching in the literature showed that the reaction between H3L and metal chloride salts afforded compounds of the type [M(H3L)nCl2] (M = Co(II), Ni(II), and Cu(II); n = 1 and 2). (Salman, 2001) In comparison, changing of the experimental conditions e.g. pH of the medium, and/or using of metal salts of weakly coordinating anions e.g. ClO4− affect the coordinating ability of our tribasic ligand, and increase the probability of the departure of the anions of the metal salts from the coordination spheres of the complexes compared to the published complexes, which were prepared from the same ligand, but with different metal salts and experimental conditions.
3.2 DFT/NBO analysis
In the previous section, the analytical and spectroscopic data indicated that compounds 1–3 are present in a ''flattened'' tetrahedron field, in which the thiourea ligand coordinates to the title metal ions, as a mono-negatively bidentate ligand, via C–S−, and triazole nitrogen. However, there is still two questions that have not been experimentally answered as follows: which nitrogen of the triazole ring is involvement in the complex formation (N(1) vs. N(3))? and which geometrical isomer (cis- vs. trans-) is the local minimum structure of the complex?. To address these two questions, full geometry optimizations of the proposed structures of 1–3, in the ground state, were executed at B3LYP/def2-SVP level of theory. As shown in Table 1, conformations I, and II have been assigned to the trans- and cis-(H2L, H2L) arrangements of N(3)-coordinated ligands, while conformation III has been designated to the structure in which N(1) atom of the triazole is involved in the complex formation. The resulting geometries were checked as local minima via the harmonic frequency analysis (Table 2). The conformations I and III of complex 1 show imaginary frequencies and consequently they cannot be classified as local minima structures. * Conformations I, and II have been assigned to the trans- and cis-(H2L, H2L) arrangements of N3-coordinated ligands, while conformation III has been designated to the structure in which N1 atom of the triazole is involved in the complex formation.
1
2
3
1
2
3
I
II
III
I
II
III
I
II
III
Electronic Energy (EE)
−1391.10107
−3421.05816
−3421.05743
−3546.60879
−3546.59615
−3546.59787
−3678.74758
−3678.73578
−3678.73819
Zero-point Energy Correction
0.494759
0.344814
0.345598
0.345925
0.345432
0.346205
0.344758
0.343773
0.344452
Thermal Correction to Energy
0.502383
0.371859
0.371520
0.372355
0.372186
0.372704
0.368529
0.371391
0.371870
Thermal Correction to Enthalpy
0.503327
0.372804
0.372464
0.373299
0.373130
0.373649
0.369473
0.372335
0.372815
Thermal Correction to Free Energy
0.456834
0.281420
0.284288
0.284157
0.283764
0.284797
0.290006
0.278956
0.279265
EE + Zero-point Energy
−1390.60631
−3420.71334
−3420.71183
−3546.26286
−3546.25072
−3546.25167
−3678.40282
−3678.39200
−3678.39374
EE + Thermal Energy Correction
−1390.59868
−3420.68630
−3420.68591
−3546.23643
−3546.22396
−3546.22517
−3678.37905
−3678.36438
−3678.36632
EE + Thermal Enthalpy Correction
−1390.59774
−3420.68535
−3420.68496
−3546.23549
−3546.22302
−3546.22422
−3678.37811
−3678.36344
−3678.36538
EE + Thermal Free Energy Correction
−1390.64423
−3420.77674
−3420.77314
−3546.32463
−3546.31238
−3546.31308
−3678.45757
−3678.45682
−3678.45893
Imaginary frequencies
32
0
1
0
0
0
4
0
0
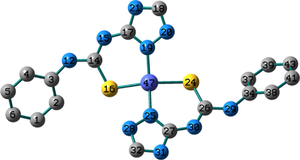
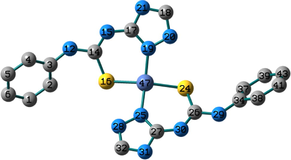
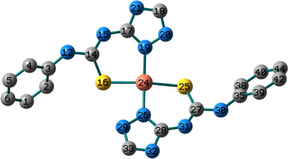
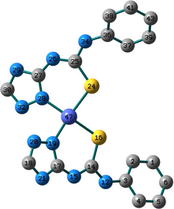
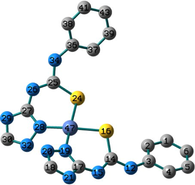
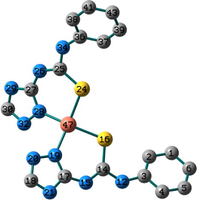
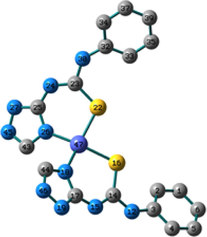
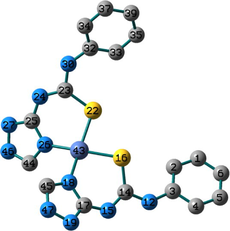
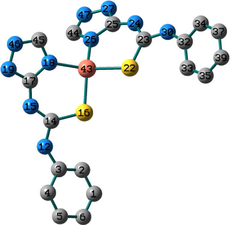
Conformation II has no imaginary vibrations as well as lower sum of electronic and zero-point energy values and consequently it may be characterized as a local minimum structure of the synthesized Co(II) complex. In the case of the Ni(II) complex 2, we did not find any imaginary modes in the three optimized structures and thus the sum of the electronic and zero-point energy values was compared. As shown in Table 2, conformation I, incorporating two N(3)-coordinated ligands in a trans-arrangement, is the local minimum structure of the nickel complex. For 3, only conformations II and III show no imaginary frequencies. Conformation III of the Cu(II) complex is characterized as a local minimum structure because it has a lower energy value among the three optimized structures. To conclude, the complexes adapt three different conformations (Fig. 2) based on the data of the quantum chemical calculations.
Structures of the local minima structures of 1–3 based on the data of geometry optimization and vibrational analyses.
Selected bond lengths and bond angles of all the optimized conformations of 1–3 are given in Table 3. Compounds 1–3 contain metal ion in a distorted tetrahedral geometry, in which the two thiourea ligands bound M2+ iso-energetically as the bonds (M–N and M–S) are equivalent. To get an insight into the distortion of the tetrahedral geometry, the four coordinated geometry index, τ4 (Yang et al., 2007), calculated as follows, (τ4 = (360–(α + β))/141) (where α and β are the two largest bond angles around the central metal ion), can be applied to evaluate the distortion between the ideal four coordinated geometries; tetrahedral (τ4 = 1) and square-planar (τ4 = 0). As shown in Table 3, the τ4 values are in the range of 0.13–0.45. The local minimum structures of complexes 1–3, based on the previously mentioned vibrational analysis and ground-state energy comparison, have τ4 values of 0.35, 0.28, and 0.45 indicating that the geometries of the complexes may be considered as an intermediate between the square planar and tetrahedral. In the case of conformation I of compound 2 (Fig. 2), the triazole NH group participates in intramolecular hydrogen bond with C–S–M via H–N…S with distance of 2.30922 Å, and angle of 121.1 °.
1
2
3
I
II
III
I
II
III
I
II
III
M–S
2.25726
2.23813
2.24028
2.23994
2.22476
2.22792
2.34521
2.30510
2.30562
M–N
1.92770
1.92460
1.95393
1.90674
1.90395
1.91464
1.97466
1.99974
2.01325
S–M–S
152.7
89.9
87.6
155.7
85.7
85.3
153.0
92.3
91.6
S–M–Nin
92.2
93.1
93.0
91.4
91.1
91.6
91.4
92.9
94.0
S–M–Nexo
90.9
154.8
157.5
91.9
170.4
169.4
94.3
150.9
147.9
N–M–N
166.9
94.6
95.1
164.2
93.1
93.6
155.2
96.1
97.6
τ4*
0.28
0.35
0.32
0.28
0.13
0.15
0.37
0.41
0.45
The calculated vibrational spectra of the stable conformations of 1–3 are shown in (Fig. S4). In general, the calculated wavenumbers are expected to be higher than the experimental finding because of the basis set truncation and the neglection of the electron correlation and mechanical anharmonicity especially in the range of the bending modes. (Rauhut and Pulay, 1995) To compensate these limitations, scaling methods should be introduced. Uniform scaling (0.96) was introduced for B3LYP/def2-SVP method. The scaled IR modes of NH, C = N, M–S and M–N bonds are found in the ranges of 3424–3427, 1560–1581, 356–361, and 331–342 cm−1.
The natural charge, nature of bonding, type of hybridization and strength of the bonds (M–N and M–S) of the local minimum structures of 1–3 were deduced from the natural bond orbital (NBO) analysis of Weinhold and co-workers (Reed et al., 1988) and second order perturbation theory analysis of Fock Matrix in NBO Basis obtained at B3LYP/def2-SVP level of theory. The electronic arrangement of the metal center in cobalt(II) complex 1 is [Ar]4s0.393d7.584p0.565 s0.01, with a natural charge of 0.46222e. The occupancies of the 3d-orbitals are as follows: 0.98828 1.16688 1.64564 1.85639 1.92636. The σ(Co–S) bond is formed from sp6.41d0.01 hybrid on S atom (a mixture of 13.48% s, 86.40% p and 0.12% d) and sp3.25d2.29 hybrid on Co (a mixture of 32.58% s, 25.13% p and 42.29% d) and is polarized towards S atom (74.79%). Triazole nitrogen bounds metal center via a lone pair interaction. Both the σ(Co–S16) → σ*(Co–S24) and σ(Co–S24) → σ*(Co–S16) interactions are equal with E2 value of 1.32 kcal mol−1 (E2: second order interaction energy). The triazole interactions are also equal and the lone pair interaction LP(1)N19 with σ*(Co–S24) has E2 value of 1.73 kcal mol−1. The natural charge of Ni in 2 is 0.44184e. The electronic configuration of Ni(II) ion in 2 is [Ar]4s0.413d8.634p0.515 s0.01 and the occupancies of 3d orbitals are 1.95706 1.97954 1.95604 0.82553 1.91507. The σ(Ni–S) bond is created from sp7.50d0.01 hybrid on S atom (comprised of 11.76% s, 88.13% p and 0.11% d) and sp4.05d3.06 hybrid on Ni (a mixture of 12.33% s, 49.90% p and 37.76% d) and is polarized towards S atom (77.51%). The σ(Ni–S) bond is more polarized than σ(Co–S) bond because of the difference in the electronegativity between the two metal ions. The E2 values of σ(Ni–S16) → σ*(Ni–S24) and LP(1)N19 → RY*(1)Ni are 3.81 and 0.36 kcalmol−1, respectively. Compared to 1, the larger the E2 values of 2, the more intensive is the interaction between Ni(II) ion and coordination sites.
For 3, the electronic arrangement of Cu is [Ar]4s0.423d9.464p0.49, the valence electrons are 10.37780 and Rydberg electrons are 0.00506. This agrees well with the natural charge on Cu ion (0.62021e), which corresponds to the difference between 28.37979e and the total number of electrons in the free copper(II) ion. The occupancies of Cu-3d subshells are 1.62771 1.97958 1.89038 1.98588 1.98142. The interactions of the nitrogen and suphur coordination sites with Cu(II) ion may be considered as charge transfer. The E2 values of LP(1)(S16) → RY*(1)Cu and LP(1)N18 → RY*(1)Cu are 0.09 and 0.23 kcalmol−1, respectively.
3.3 TDDFT
The nature of the electronic absorption transitions shown in the experimental UV/Vis spectra of the local minimum structures of 1–3 has been investigated by executing time-dependent density functional theory (TDDFT) calculations. The lowest 30 singlet–singlet spin allowed excitation states were computed using CAM-B3LYP functional with long range interaction and def2-SVP basis set. The computed wavelengths including d-d transitions and their assignments are given in Table S1. In 250–800 nm range, the calculated spectrum of 1 shows two characteristic transitions at 735, and 486 nm corresponding to HOMO–4(β)/ HOMO–5(β) → LUMO(β) and HOMO–4(α) → LUMO + 2(α), respectively. As shown in Fig. 3, the bands at 486 and 735 nm have the nature of d–d transitions.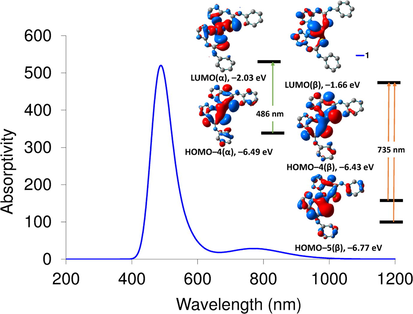
Computed electronic spectrum of 1 at CAM-B3LYP/def2-SVP and frontier molecular orbitals of some selected transitions.
The TDDFT spectrum of 2 displays two main transitions at 387 and 306 nm corresponding to HOMO → LUMO and HOMO–6 → LUMO, respectively. As shown in Fig. 4, both HOMO and HOMO–6 orbitals are contained upon d(Ni), while LUMO is composed of π* of phenyl ring. Thus, the transitions observed in the calculated spectrum of 2 are MLCT. The calculated spectrum of 3 shows three main transitions at 398, 317, and 269 nm corresponding to HOMO(α) → LUMO + 1(α)/HOMO(β) → LUMO + 2(β), HOMO–4(β) → LUMO(β) and HOMO(β)/ HOMO–2(β) → LUMO(β). HOMO and HOMO–2 orbitals with β-spin are mainly S(π)/ligand π-orbitals (Fig. 5), whereas LUMO(β) orbital is coming from d(Cu)/S(π*)/Triazole N(π*). Thus, the transition at 398 nm is LMCT/ LLCT. As shown in Fig. 5, the transition at 317 nm has a nature of d-d/MLCT.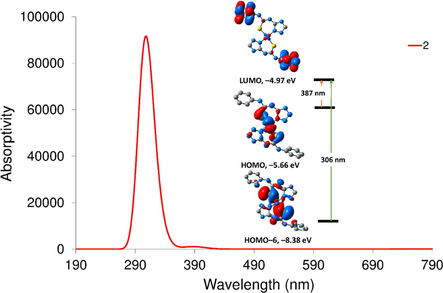
Computed electronic spectrum of 2 at CAM-B3LYP/def2-SVP and frontier molecular orbitals of some selected transitions.
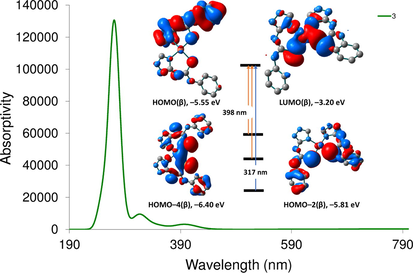
Computed electronic spectrum of 3 at CAM-B3LYP/def2-SVP and frontier molecular orbitals of some selected transitions.
3.4 Antibacterial activity
Recently, screening efforts have showed an unexpectedly high hit rate for coordination and organometallic compounds (9.9%) compared with the purely organic compounds (0.87%) submitted to the same assays. (Frei et al., 2020) Minimum inhibitory concentrations (MICs) of the tested transition metal-based compounds (Mn+ = Mn, Co, Zn, Ru, Ag, Ir, and Pt) against both Gram-positive and Gram-negative bacteria were observed, with activities down to the nanomolar range against methicillin resistant S. aureus (MRSA). The susceptibility of Staphylococcus aureus, a gram-positive bacterium, and Escherichia coli, a gram-negative microbe, towards the free ligand and its transition metal complexes was assessed at 20 mgmL−1. The potency of the compounds was compared with the standard tetracycline. The free thiourea functionalized ligand exhibits similar moderate toxicity to the tested bacteria. The possible mechanism of action of thiourea compounds functionalized with triazole moiety may be attributed to the inhibition of bacterial biofilm formation. (Stefańska et al., 2016) Coordination of the ligand to either Co(II) or Cu(II) did not alter the potency, while chelation of Ni(II) ion gives rise to inactive compound (Fig. 6). The inactivity of 2 compares well with those of Pd(II) and Pt(II) complexes of the same ligand. (Mansour, 2018) The difference between the activities of the synthesized compounds may be attributed to the variation of the lipophilicity values, where the diffusion of the compound is low and consequently, they cannot inhibit the growth of the microbe. (Anacona et al., 2019)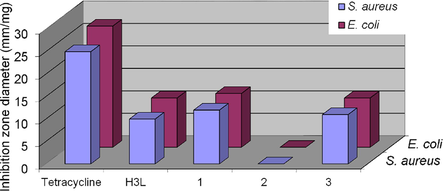
Representative and comparison of the susceptibilities of S. aureus and E. coli bacteria towards the thiourea ligand and its complexes. DMSO was used as a control and its inhibition zone was withdrawing from that of each case.
4 Conclusion
Binary metal complexes derived from reaction of N-phenyl-N′-(3-triazolyl)thiourea) (H3L) with M(ClO4)2 (M = Co(II), Ni(II) and Cu(II)) were synthesized in a pure form and good yield. The analytical and spectral tools showed that the thiourea ligand behaved as mono-negative N,S-bidentate via C–S− and triazole nitrogen affording [M(H2L)2] complexes having geometries between tetrahedral and square-planar (''flattened'' tetrahedron, D2d symmetry). The local minima structures of the proposed structures were determined with the aid of quantum chemical calculations. Analysis of the calculated vibrational spectra and comparison of the sum of the electronic and zero-point energy values of the different optimized conformations gave some knowledge about which nitrogen of the triazole ring in the complex formation (N(1) vs. N(3)) is involvment and which geometrical isomer (cis- vs. trans-) the local minimum structure is. According to the quantum chemical calculations, the complexes adapt three different conformations in which Co(II) and Ni(II) ions are coordinated to two N(3)-triazole atoms and C–S− in cis- and trans- arrangements, while Cu(II) ion is coordinated to N(1)-triazole atom and C–S− in cis-arrangement. Time-dependent density functional theory calculations were executed to understand the observed electronic transitions and to get an insight into the expected transitions. The ligand and its complexes were screened for their potential antibacterial activity against Staphylococcus aureus, and Escherichia coli. The free ligand exhibited similar moderate activity against the tested microbes in which the activity did not change by coordination to Co(II) and Cu(II) ions, but diminished by formation of Ni(II) complex. The possible mechanism of action of thiourea compounds functionalized with triazole moiety may be attributed to the inhibition of bacterial biofilm formation.
Author contributions
The authors have equal contribution to the present work.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Novel palladium(II) and platinum(II) complexes with 1H-benzimidazol-2-ylmethyl-N-(4-bromo-phenyl)-amine: Structural studies and anticancer activity. Eur. J. Med. Chem.. 2012;47:399.
- [Google Scholar]
- Synthesis and spectroscopic characterization of nickel (II) complexes of 1-benzotriazol-1-yl-[(pX-phenyl) hydrazone] propan-2-one. Spectrochim. Acta, Part A. 2006;65:36.
- [Google Scholar]
- Antibacterial activity of transition metal complexes containing a tridentate NNO phenoxymethylpenicillin-based Schiff base. An Anti-MRSA iron(II) complex. Appl. Organomet. Chem.. 2019;33:e4744
- [Google Scholar]
- Density functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993;98:5648.
- [Google Scholar]
- Anion binding with urea and thiourea derivatives. Coord. Chem. Rev.. 2015;295(1):80.
- [Google Scholar]
- K. Colanceska-Ragenovic, V. Dimova, V. Kakurinov, D. Gabor Molnar, A. Buzarovska. Synthesis, antibacterial and antifungal activity of 4-substituted-5-aryl-1, 2, 4-triazoles, Molecules 2001, 6, 815.
- Copper (II) complexes of new tetradentate NSNO pyridylthioazophenol ligands: synthesis, spectral characterization and crystal structure. Polyhedron. 2004;23:2457.
- [Google Scholar]
- Synthesis of 1-[3-(4-benzotriazol-1/2-yl-3-fluoro-phenyl)-2-oxo-oxazolidin-5-ylmethyl]-3-substituted-thiourea derivatives as antituberculosis agents. Eur. J. Med. Chem.. 2006;41:423.
- [Google Scholar]
- Palladium (II) and platinum (II) halide complexes with 2, 6-dimethyl-4H-pyran-4-thione. Trans. Met. Chem.. 1990;15:242.
- [Google Scholar]
- Selective recovery and preconcentration of mercury with benzoylthiourea-solid supported liquid membrane system. Anal. Chim. Acta. 2005;547:255.
- [Google Scholar]
- Metal complexes as a promising source for new antibiotics. Chem. Sci.. 2020;11:2627.
- [Google Scholar]
- Frisch, M.J. , Trucks, G.W. , Schlegel, H.B. , Scuseria, G.E., Robb, M.A., Cheeseman, J.R. , Zakrzewski, V.G., Montgomery, J.A., Stratmann, R.E. , Burant, J.C., Dapprich, S., Millam, J.M., Daniels, A.D., Kudin, K.N., Strain, M.C., Farkas, O. , Tomasi, J., Barone, V., Cossi, M., Cammi, R., Mennucci, B., Pomelli, C., Adamo, C., Clifford, S.,Ochterski, J., Petersson, G.A., Ayala, P.Y., Cui, Q., Morokuma, K., Malick, D.K., Rabuck, A.D., Raghavachari, K., Foresman, J.B., Cioslowski, J., Ortiz, J.V., Baboul, A.G., Stefanov, B.B., Liu, G., Liashenko, A., Piskorz, P. , Komaromi, I., Gomperts, R., Martin, R.L., Fox, D.J., Keith,T. , Al-Laham, M.A., Peng, C.Y., Nanayakkara, A., Gonzalez, C., Challacombe, M. , Gill, P.M.W., Johnson, B. G., Chen, W., Wong, M.W., Andres, J.L., Head-Gordon, M., Replogle, E.S., Pople, J.A., 2003. GAUSSIAN 03 (Revision A.9), Gaussian, Inc., Pittsburgh.
- Gaussview User Manual. Pittsburgh, PA: Gaussian Inc; 2000.
- Thiourea-catalyzed enantioselective cyanosilylation of ketones. J. Am. Chem. Soc.. 2005;124:12964.
- [Google Scholar]
- Synthesis and spectroscopic studies of Cu(II) complexes of some ligands containing the amide group. J. Coord. Chem.. 1993;29:33.
- [Google Scholar]
- Inhibition of dopamine β-hydroxylase by some new thiourea derivatives. J. Hid. Chew.. 1965;240:2066.
- [Google Scholar]
- Synthesis and biological evaluation of 1β-methylcarbapenems having cyclic thiourea moieties and their related compounds. Eur. J. Med. Chem.. 2006;41:825.
- [Google Scholar]
- Magnetic susceptibility as measured by Gouy's method with the specimen in a fixed position. J. Chem. Soc.. 1954;785
- [Google Scholar]
- Cobalt (II, III) and copper (II) complexes of 3-(2-furylidene) hydrazino-5, 6-diphenyl-1, 2, 4-triazine. Trans. Met. Chem.. 2002;27:398.
- [Google Scholar]
- Experimental and atomistic simulation studies of corrosion inhibition of copper by a new benzotriazole derivative in acid medium. Electrochim. Acta. 2009;54(18):4345-4352.
- [Google Scholar]
- D. Kowalkowska-Zedler, A. Dotega, N. Nedelko, R. Łyszczek, P. Aleshkevych, I. Demchenko, J. Łuczak, A. Ślawska-Waniewska, A. Pladzyk. Structural, magnetic and spectral properties of tetrahedral cobalt (II) silanethiolates: a variety of structures and manifestation of field-induced slow magnetic relaxation, Dalton Trans., 2020, 49, 697; b) A.M. Mansour, N.T. Abdel-Ghani and M.S. Ragab, Appl. Organometal. Chem., 2020, 34, e5995.
- Inorganic electronic spectroscopy. Amsterdam: Elsevier; 1984.
- Cholic-acid-based fluorescent sensor for dicarboxylates and acidic amino acids in aqueous solutions. Org. Lett.. 2005;7:5825.
- [Google Scholar]
- Synthesis and antitumor activity of optically active thiourea and their 2-aminobenzothiazole derivatives: A novel class of anticancer agents. Eur. J. Med. Chem.. 2009;44:2923.
- [Google Scholar]
- What happens when (1H-benzimidazol-2-ylmethyl)-N-phenyl amine is added to copper (II) acetate? Spectroscopic, magnetic, and DFT studies. Inorg. Chim. Acta. 2013;408:186.
- [Google Scholar]
- Crystal structure, DFT, spectroscopic and biological activity evaluation of analgin complexes with Co (II), Ni (II) and Cu (II) Dalton Trans.. 2014;43:15950.
- [Google Scholar]
- Thermal, spectral, DFT and biological activity evaluation of Co(II), Ni(II) and Cu(II) complexes of N,S-chelated benzotriazole ligand. J. Therm. Anal. Calorim.. 2016;123(1):571-581.
- [Google Scholar]
- Structural studies and biological activity evaluation of Pd (II), Pt (II) and Ru (II) complexes containing N-phenyl, N-(3-triazolyl) thiourea. Appl. Organometal. Chem.. 2018;32:e3928
- [Google Scholar]
- Blue-light induced CO releasing properties of thiourea based manganese (I) carbonyl complexes. Polyhedron. 2017;131:13.
- [Google Scholar]
- Protein binding affinity of biologically active thiourea based half-sandwich Ru (II) cymene complexes. Polyhedron. 2020;175:114175
- [Google Scholar]
- Reactivity of visible-light induced CO releasing thiourea-based Mn (I) tricarbonyl bromide (CORM-NS1) towards lysozyme. Inorg. Chim. Acta. 2018;480:159.
- [Google Scholar]
- Papakonstantinou-Garoufalias, S.S., Todoulou, O.G., Filippatos, E.C. , Papadaki-Valiraki, A.E., Chytiroglou-Lada. A., 1998. Synthesis, antifungal activity and antibacterial evaluation of some 3-piperazinylmethyl-5-aryl-1H-1, 2, 4-triazoles. Arzneimittelforschung 1998, 48, 1019.
- Transferable scaling factors for density functional derived vibrational force fields. J. Phys. Chem.. 1995;99:3093.
- [Google Scholar]
- Intermolecular interactions from a natural bond orbital, donor-acceptor viewpoint. Chem. Rev.. 1988;88:899.
- [Google Scholar]
- The electronic structure of square-planar nickel(II) and copper(II) complexes. Inorg. Chim. Acta. 1972;6:307.
- [Google Scholar]
- Spectral and thermal characterization of divalent transition metal complexes with some triazolylthiourea derivatives. Phosphorus Sulfur Silicon Relat Elem. 2001;173(1):193.
- [Google Scholar]
- Structural and spectral studies on four coordinate copper (II) complexes of 2-benzoylpyridine N (4), N (4)-(butane-1, 4-diyl) thiosemicarbazone. Polyhedron. 2003;22:3321.
- [Google Scholar]
- Structural and spectral studies on four coordinate copper (II) complexes of 2-benzoylpyridine N (4), N (4)-(butane-1, 4-diyl) thiosemicarbazone. Polyhedron. 2003;22:3321.
- [Google Scholar]
- Med. Chem.. 2016;12:478.
- Eur. J. Med. Chem.. 2012;55:205.
- Synthesis, structures, spectral and electrochemical properties of copper (II) complexes of sterically hindered Schiff base ligands. Inorg Chim Acta. 2009;362:199.
- [Google Scholar]
- Synthesis of novel thiourea, thiazolidinedione and thioparabanic acid derivatives of 4-aminoquinoline as potent antimalarials. Bioorg. Med. Chem. Lett.. 2009;19:2570.
- [Google Scholar]
- Spectral studies of cobalt (II)-and nickel (II)-metallothionein. Biochem.. 1981;20:6659.
- [Google Scholar]
- A new hybrid exchange–correlation functional using the Coulomb-attenuating method (CAM-B3LYP) Chem. Phys. Lett.. 2004;393:51.
- [Google Scholar]
- Structural variation in copper (I) complexes with pyridylmethylamide ligands: structural analysis with a new four-coordinate geometry index, τ 4. Dalton Trans.. 2007;955
- [Google Scholar]
- Synthesis of thiourea derivatives as CCR4 antagonists. Chin. Chem. Lett.. 2009;20:296.
- [Google Scholar]
Appendix A
Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2020.102932.
Appendix A
Supplementary material
The following are the Supplementary data to this article:







