Translate this page into:
Synergistic effect of Thiourea and HCl on Palladium (II) recovery: An investigation on Chemical structures and thermodynamic stability via DFT
⁎Corresponding author. Vudhichai.P@Chula.ac.th (Vudhichai Parasuk),
⁎⁎Corresponding author. ura.p@chula.ac.th (Ura Pancharoen)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
This work duly investigates the recovery of Pd (II) chlorocomplexes from industrial wastewater. Chemical structures and thermodynamic stabilities of the complex formed are evaluated via density functional theory (DFT). By applying synergistic solutions of thiourea mixed with hydrochloric acid (HCl), the stripping reaction of Pd (II) in the loaded Aliquat 336 occurs and Pd (II) chlorocomplexes coordinated thiourea ligands are formed, thus 80.19% of Pd (II) chlorocomplexes can be recovered. The aim of this study is to gain a better understanding of the stripping mechanisms and the structure of the complexes formed via the synergistic system. Such an understanding is still limited since little research has been conducted in this field. Owing to their molecular geometry, ligand coordination and donor groups play a vital role in the reactivity of palladium (II) complex. Quantum models have been developed to evaluate the chemical structure and thermodynamics stability of ((NH2)2CS·PdCl2) namely: (i) DFT with B3LYP/6-31g(d,p) and MP2/6-31g(d,p) basis set, (ii) MP2 with cc-pVTZ basis set and (iii) CCSD(T)/cc-pVTZ. Results demonstrate that the highest geometric stability exhibited is the structure of Pd-S bonding with 180° Cl-Pd-Cl. The distance (r) and angle (a) of the selected geometrical parameters for (NH2)2CS·PdCl2 complex are reported. Additionally, FTIR and UV–vis spectroscopies have been conducted to analyze the sampling solutions. Further, the calculated vibrational frequencies and experimental spectroscopic results show good agreement with the optimized geometry.
Keywords
Palladium recovery
Synergistic solution
Stripping mechanism
DFT
MP2
CCSD(T)
1 Introduction
Recently, Pd has received much attention owing to its increased demand and limited natural resources (Cieszynska & Wieczorek, 2018, Assunção et al., 2016). Pd belongs to the platinum group metals (PGMs), which is most valued for their wide range of electronic applications. On account of its distinguished physical and chemical properties, Pd has been employed in diverse processing areas (Cieszynska & Wieczorek, 2018, Amin, 2016, Khan et al., 2014, Huang et al., 2020). Much research has been carried out into the extraction and stripping of Pd (II) via the liquid–liquid extraction process using various types of extractants and strippants (Rane and Venugopal, 2006, Pan and Zhang, 2009, Swain et al., 2010, Wongkaew et al., 2015, Nguyen et al., 2016). Pd (II) chlorocomplexes play a significant role in various reactions of Pd ions in chloride medium systems. Kavitha and Reddy (2016) reported that Pd complexes have become the subject of intensive research. Of late, synergistic solutions have attracted considerable attention involving stripping reaction as regards metal recovery. It is acknowledged that the stripping efficiency of Pd (II) in the loaded organic phase can be enhanced by applying synergistic strippants of thiourea mixed with HCl (Ortet and Paiva, 2015, Senthil et al., 2017, Das et al., 2014, Lee et al., 2010). Nevertheless, specific details describing the mechanism of the synergistic reaction having Pd organometallic complex are still lacking. In addition, the chemical structure of the complex is rather limited. Thus, an investigation into the mechanism and Pd (II) complex form is necessary and therefore encourages interest in the design of Pd (II) complexes.
Thiourea is an interesting class of ligand and can form a stable coordinated complex with noble metals in various pathways i.e. neutral, mono/dianionic ligands, monodentate or chelating ligands (Smith et al., 2013, Eaton & Zaw, 1975, Mansour & Shehab, 2021). Thiourea is a good nucleophilicity ligand owing to its 2 donor atoms in the molecule (S and N). Thiourea has a soft nature and has shown selectively trap with noble metals in extraction and stripping processes (Telmore et al., 2018, Bogojeski et al., 2015). Cherrat et al. (2020) also evaluated heavy metals removals via urea phosphate. Results showed that urea phosphate was successfully utilized to remove heavy metals into the residual liquor. It has been acknowledge that thiourea effectively coordinated through Sulphur atom with noble metals viz Pt, Pd etc (Schiessl et al., 2005). Many researches have been conducted on metals recovery using thiourea ligand (Sunsandee et al., 2021, Fatiha et al., 2016, Mohdee et al., 2020, Truong et al., 2017, Senthil et al., 2017). Based on the concept of HSAB theory (hard and soft acid and base theory) Pd (II), as a soft acid, favors to form complexation with a soft donor base such as N and S atoms. In consideration of this, an investigation on thiourea as a ligand is carried out; owing to it was found to quantitatively trap Pd (II) in the loaded organic phase. An influential goal of this research is to understand the effect of ligands on the electronic structure and their reactivity on Pd (II) complex. Due to their molecular geometry, ligand coordination and their donor groups have an important role to play as regard the reactivity of the metal center (Geri and York, 2018, Biswas et al., 2017, Senn and Ziegler, 2004). Many types of ligand complexes with Pd have been carried out and their geometries have been calculated via DFT (Geri and York, 2018, Ruiz et al., 2018, Deligonul and Gray, 2013, Al-Noaimi et al., 2016).
Computational chemistry enables a greater understanding of molecular structural chemistry. DFT calculations, for instance, can predict structures accurately and can determine the energy of molecules (Lehtola et al., 2018, Chermette, 1999, Gross and Dreizler, 1995, Sperger et al., 2015, Omar et al., 2021, Rahnama et al. 2021). Quantum calculation relies on Schrödinger’s equation which can provide electronic energy and electron densities of molecules; thereby permitting molecular geometry and their electronic configuration to be obtained (Jäger et al., 2015). DFT focuses on molecular charge density and their electronic properties at ground state, on account of Hohenberg-Kohn theorems (Chermette, 1999, Chen et al., 2019). The accuracy of thermodynamic stability can be increased by developing various methods. The Moller-Plesset is a method based on perturbation theory which includes electronic correlation developed from the Hartree–Fock method (HF) by considering the interaction between electrons. Correlation energy is a measure of the interaction between electrons or a movement of one electron which is affected by other electrons (Cremer, 2011). The coupled cluster (CC) theory, with single and double and perturbative triple excitations, is denoted as CCSD(T). CCSD(T) is the gold-standard in quantum chemistry which represents a compromise in accuracy results (Jurečka et al., 2006, Řezáč et al., 2013). Furthermore, by assessing the CCSD(T) method, it can help to solve the problem of configuration interaction in the complex studied (Raghavachari et al., 1989).
Herein, the thermodynamic complexation of Pd (II) coordinated with thiourea ligand ((NH2)2CS·PdCl2) is proposed. Thermodynamic stabilities and the highest stable structures are examined. The chlorocomplex (NH2)2CS·PdCl2 is formed by the stripping reaction of Pd in the loaded Aliquat 336 using the synergistic solution between thiourea and HCl by way of the solvation system. Analysis of molecular interaction and the structure of Pd (II), as it reacts with synergistic molecules, is also investigated. An attempt to find the possible mechanisms used to recover Pd (II) chlorocomplexes from industrial wastewater via synergistic solutions is sought. Thus, various types of strippants have been applied in order to investigate the recovery efficiency of Pd (II) ions viz HCl, HNO3, H2SO4 and thiourea. A Fourier transform infrared spectroscopic method with attenuated total reflectance (FTIR-ATR) (Nicolet 6700, Thermo scientific) is employed to indicate chemical bonding and chemical species in the sampling solutions. FTIR imaging using ATR mode provides a highly versatile image and requires less steps or no sample preparation, due to the penetration depth of IR light is independent of sample thickness. Moreover, FTIR-ATR can be applied to avoid the problem of strong attenuation of the IR signal in highly absorbing media such as in aqueous solutions. UV–Vis spectroscopy (Carry 5000, Aligent), a quantitative determination of different analytes, is also applied. Besides, three quantum models are implemented to evaluate the structural stabilities: namely, (i) DFT with B3LYP/6-31g(d,p) and MP2/6-31g(d,p) basis set, (ii) MP2 with correlation-consistent triple-zeta basis set (cc-pVTZ) as well as (iii) CCSD(T)/cc-pVTZ.
2 Computational details
Computational chemistry has been employed to elucidate and compare the structural stabilities of Pd (II) coordinated with thiourea ligands. DFT calculation having the hybrid functional B3LYP, which is based on quantum mechanics, has been selected to investigate such structures (Lehtola et al., 2018, Chermette, 1999, Gross and Dreizler, 1995, Sperger et al., 2015, Schlangen and Schwarz, 2010). The 6-31g(d,p) basis set was used for C, N, H, S and Cl atoms. Likewise, both the Stuttgard-Dresden (SDD) basis set for valence electrons and the effective core potential (ECP) function for core electrons were utilized to study the electronic structures of Pd (II) complexes (Hansen et al., 2009). In association with B3LYP, the 6-31g(d,p) basis set was employed. All calculations were performed using the Gaussian09 program suite.
All calculations were carried out using a polarized continuum model (PCM) and water was chosen as the solvent. The total energy obtained from quantum mechanics is the internal energy at 0 K. To compare thermal stabilities of structures at 298 K and 1 atm, absolute, Gibbs free energy was evaluated for each structure through statistical mechanics (Atkins and Paula, 2006). To confirm the DFT calculations (B3LYP/6-31g(d,p)), geometry optimization using MP2 calculations, having the same basis set (MP2/6-31g(d,p)), was performed. Energies were more accurately determined using single-point MP2 and CCSD(T) methods applying the cc-pVTZ basis set for non-transition metal and SDD for Pd. Free energy at CCSD(T) level was estimated via vibrational frequency from MP2.
3 Results and discussion
3.1 Influence of types of strippant
It is acknowledged that types of strippant are a crucial factor to ensure the extraction process. A sufficient strippant can strip out the target metal ions from the organometallic complex effectively. Additionally, Stripping reaction is the reverse of extraction reaction; therefore, it is also used to recover metal and reuse of an extractant. Various types of the potential stripping solutions have been previously investigated. With adequate strippant, target metal was completely recovered in the stripping solution. On the other hand, when the strippant is not strong enough, target metal could not be recovered in the stripping phase due to it is not efficient enough to destabilize the organometallic complex to release target metal into the stripping solution (Luis, 2018, Žilnik and Likozar, 2019, Sharma et al., 2020).
Stripping procedure can be performed as follows: an equal volume of Pd (II) in the loaded Aliquat 336 solution (organic phase) and strippant solution (aqueous phase) were mechanically shaken at ambient condition. Subsequently, the solutions were left for the phase separation. Thereafter, sampling solutions were collected in order to measure the concentration of metal ions via Inductively Coupled Plasma Spectrometry (Carry 5000, Aligent, Santa Clara, CA, USA). Various types of strippants were employed to investigate the recovery of Pd (II) viz. HNO3, H2SO4, HCl, thiourea and synergistic solutions of thiourea mixed with HCl. Optimal conditions for the section on extraction followed on from previous work (Mohdee et al., 2019). The recovery percentage of Pd (II) can be defined in terms of the following equation:
In Fig. 1, the efficiency of Pd (II) recovery (in terms of concentration ratio) using various types of strippants is presented. Herein, the recovery percentage of Pd (II) from high to low was in the order: synergistic solution (0.5 M (NH2)2CS + 0.1 M HCl) (80.19%) > 0.5 M (NH2)2CS (62.35%) > 5 M HNO3 (35.29%) > 5 M HCl (22.07%) > 5 M H2SO4 (17.44%). As shown, the synergistic solution: 0.5 M (NH2)2CS + 0.1 M HCl exhibited the highest efficiency in trapping Pd (II) ions.
Influence of stripping solutions on Pd (II) recovery.
The stripping mechanisms of Pd (II) in the loaded Aliquat 336 using thiourea can be expressed as:
The equilibrium constant for the stripping reaction (Kst) of Pd (II) can be written, as shown in Eq. (3):
The corresponding amount of Pd (II) in the organic phase can be calculated from the mass balance, as shown in Eq. (4):
In Table 1, values of equilibrium constants for the stripping reaction are presented.
Kst
5 M HNO3
5 M H2SO4
5 M HCl
0.5 M (NH2)2CS
0.5 M (NH2)2CS + 0.1 M HCl
2.14 × 10−4
1.06 × 10−4
1.34 × 10−4
0.0378
0.0487
3.2 Molecular structure and thermodynamic stability of (NH2)2CS·PdCl2 complex
3.2.1 B3LYP/6-31g(d,p) and MP2/6-31g(d,p) calculations
As shown in Fig. 2, the optimized geometry of the ((NH2)2CS·PdCl2) complex was manipulated in singlet and triplet ground state via DFTB3LYP and MP2/6-31g(d,p). In Table 2, the thermodynamic stabilities of the complexes are tabulated. It is noted that Pd (II) can form a coordination bond with both N and S atoms; Pd (II) ion is a soft Lewis acid, which tends to bind together with a soft donor base like N and S atoms (Bandyopadhyay et al., 2015). In general, N is considered as being half-way between a hard base and a soft base. As obtained from calculations, eight lowest structures of ((NH2)2CS·PdCl2) complex were formed. Thus, the structure having Pd-S bonding was found to be the most stable. It is acknowledged that the coordination chemistry between noble metals and thiourea occurs via electrophilic reaction through S atom The chemistry of the Pd (II) corresponding ligand is as follows: Pd-S > N-Pd-S > Pd-N, which was in agreement with previous research (Bogojeski et al., 2015, Bandyopadhyay et al., 2015). In this study, the Complex V (Pd-S bonding) was found to have the lowest value of relative energy, indicating that this structure was the highest stable geometry of ((NH2)2CS·PdCl2) complex. The complex stability from high to low was in the order: Complex V (Pd-S bonding, singlet state) > Complex IV (S-Pd-N bonding) > Complex VIII (Pd-S bonding, triplet state) > Complex III (Pd-N bonding, Pd-Cl = 180°) > Complex I (Pd-N bonding, Pd-Cl = 90°) > Complex II (N-Pd-N bonding) > Complex VI (Pd-N bonding, triplet state) > Complex VII (Pd-S bonding, triplet state).
Optimized molecular structure of (NH2)2CS·PdCl2 complex.

Optimized molecular structure of (NH2)2CS·PdCl2 complex.
Complex
ΔE
ΔE + ZPE
ΔH
ΔG
ΔE
ΔE + ZPE
ΔH
ΔG
B3LYP/6-31g(d,p)
MP2/6-31g(d,p)
Singlet
I
17.87
19.02
18.61
19.17
15.53
16.00
15.83
15.62
II
22.72
23.60
23.12
24.06
14.73
15.44
14.91
16.00
III
16.41
17.53
17.07
17.82
15.74
16.48
16.12
16.77
IV
10.80
11.38
11.11
11.48
0.08
0.68
0.33
0.94
V
0.00
0.00
0.00
0.00
0.00
0.00
0.00
0.00
Triplet
VI
25.32
25.89
25.79
24.43
12.67
12.98
13.06
11.48
VII
71.70
71.54
71.30
70.93
71.09
71.33
70.94
71.02
VIII
11.25
11.11
11.30
9.79
4.74
4.15
4.53
2.68
Additionally, MP2 calculations were carried out to confirm DFT. MP2 is a widely used method for approximating the correlation energy of molecules by demonstrating structural parameters and interaction energies. MP2 is consistent with a single minimum that corresponds to a gauche conformation for the molecule (Badawi et al., 2004, Bouteiller et al., 2009, Beni et al., 2011). It is significant that MP2/6-31g(d,p) results agreed with those of DFT. Such results yielded similar ordering for the stability of the ((NH2)2CS·PdCl2) complexes. However, when B3LYP was used, the structure V was found to be too stable. Moreover, when MP2 was implemented, the triplet state of Pd-S bonding (Complex VIII) was observed to be extremely stable having the free energy of 2.68 kcal/mol above the singlet state. As for the geometry optimization of complex VII, its structure was found to have a similar structure to complex V and VIII. Further, Complex I is the cis-isomer of the Complex III whilst Complex VI is the triplet state of Complex III. After optimization, the whole structure becomes cis-isomer; the structure did not include the trans-isomer for the triplet state.
In Table 3, both the distance (r) in angstrom and angle (a) in degree of selected geometrical parameters for (NH2)2CS·PdCl2 complex, obtained from B3LYP/6-31G(d), were calculated.
Complex
r(Pd-Cl)
r(Pd-N)
r(Pd-S)
a(Cl-Pd-Cl)
a(Cl-Pd-N)
a(Cl-Pd-S)
I
2.312
2.119
–
94.74
176.86
–
II
2.321
2.165
–
93.09
101.68
–
III
2.329
2.057
–
177.85
90.23
–
IV
2.339
2.124
2.376
93.09
96.28
98.21
V
2.332
–
2.286
172.47
–
91.84
VI
2.408
2.255
–
109.42
94.94
–
VII
2.322
–
2.411
124.74
–
113.95
VIII
2.415
–
2443
109.74
–
98.09
3.2.2 MP2/cc-pVTZ calculations
To confirm the validity of the previous section, a second-order Moller-Plesset theory (MP2) along with the correlation-consistent triple-zeta basis set (cc-pVTZ) (Dunning, 1989, Kendall et al., 1992) was carried out. The molecular geometric stability of (NH2)2CS·PdCl2 was further investigated. For the calculation, four chemical geometries were considered i.e. Pd-S bonding at singlet (S) and triplet (T) states, S-Pd-N bonding at singlet state (S) and N-Pd-N bonding at singlet state (S). The results obtained from MP2/cc-pVTZ calculations were found to be in agreement with B3LYP method, indicating that Pd-S bonding proved to be the highest stable form of (NH2)2CS·PdCl2. The order of structural stability was as follows: Pd-S bonding (S) > S-Pd-N bonding (S) > Pd-S bonding (T) > N-Pd-N bonding (S). However, the S-Pd-N bonding (S) became less stable since it was now 4.25 (in free energy) kcal/mol higher than the lowest structure while B3LYP/6-31g(d,p) was only 0.94 kcal/mol above. Values of < S2 > from B3LYP calculations for the triplet state complexes were found to be ∼ 2.0, which indicated very low spin contamination. Likewise, values of < S2 > applying MP2 calculations were found to be similar to the B3LYP values. Moreover, it was noted that the steric effect was one of the factors that affected the structure of the organometallic chemistry. The steric ligand–metal–ligand bond angle effect involved steric interactions between the ligands. It was seen that the steric effect enhanced the rate of reductive elimination, thus allowing for more orbital overlap (Marcone and Moloy, 1998, Hartwig, 2010). In Table 4, using MP2/cc-pVTZ calculations, the thermodynamic stabilities of the complexes are shown. In Table 5, the distance (r) and angle (a) of the selected geometrical parameters for (NH2)2CS·PdCl2 complex are depicted. S = singlet spin, T = triplet spin.
Complex
Energy value (kcal/mol)
ΔE
ΔH
ΔG
Complex V: Pd-S bonding (S)
0.00
0.00
0.00
Complex IV: S-Pd-N bonding (S)
3.28
3.58
4.25
Complex VIII: Pd-S bonding (T)
6.50
6.30
4.55
Complex II: N-Pd-N bonding (S)
19.01
19.21
20.03
MP2
r(Pd-Cl)
r(Pd-N)
r(Pd-S)
a(Cl-Pd-Cl)
a(Cl-Pd-N)
a(Cl-Pd-S)
I
2.274
2.09
–
93.81
177.94
–
II
2.29
2.189
–
93.77
100.57
–
III
2.299
2.022
–
178.55
90.42
–
IV
2.303
2.095
2.342
94.69
96.51
97.77
V
2.323
–
2.252
174.67
–
90.98
VI
2.439
2.285
–
102.88
94.07
–
VII
2.393
–
2.473
160.91
–
91.101
VIII
2.443
–
2.479
103.09
–
98.92
3.2.3 CCSD(T)/cc-pVTZ calculations
Geometric stability optimization of the Pd complex was also computed by the gold standard method: CCSD(T), which confirmed the accuracy of the computational calculations. Four chemical geometries, as mentioned above in MP2 calculation, were exploited. In Table 6, estimated energy values are shown. Results show that Pd-S bonding achieved the highest stable form of (NH2)2CS·PdCl2, which was in good agreement with the results obtained from DFT and MP2/cc-pVTZ calculations. However, the order of the stability changed as follows: Pd-S bonding (S) > Pd-S bonding (T) > S-Pd-N bonding (S) > N-Pd-N bonding (S). S = single spin, T = triplet spin.
Complex
Energy value (kcal/mol)
ΔE
ΔH*
ΔG*
Complex V: Pd-S bonding (S)
0.00
0.00
0.00
Complex IV: S-Pd-N bonding (S)
4.26
4.57
5.23
Complex VIII: Pd-S bonding (T)
6.28
6.08
4.33
Complex II: N-Pd-N bonding (S)
19.04
19.24
20.07
Accordingly, the free energy at CCSD(T) level was estimated. In the case of R = methyl (CH3), the calculated reaction energy of the stripping reaction at MP2/6-31g(d,p) (not including ZPE), was found to be 15.92 kcal/mol. All three quantum models used in this work demonstrated that the highest geometric stability of (NH2)2CS·PdCl2 complex proved to be the Pd-S bonding. Hence, DFT results ensured a greater and better understanding of both molecular structural chemistry and the reaction mechanisms.
3.3 Stripping mechanism
As for the hard and soft acids and bases theory, functional groups containing S, O or N donor atoms interact strongly with Pd (II) ions, which can be considered as soft acids (Hubicki et al., 2008). Thus, thiourea as a coordination ligand is capable of coordination with Pd (II) chlorocomplexes.
As a rule, the coordination chemistry between noble metals such as Pt (II), Pd (II) and Co (III) and thiourea occurs via electrophilic reaction through S atom. In acidic condition, thiourea is easily protonated. It has been reported that the protonated thiourea at S atom is found to be more stable than at N atom. Computational chemistry also proved that the protonation at S atom over the protonation at N atom is favorably observed via diverse ab initio as well as at DFT level, using a wide range of basis sets (Schiessl et al., 2005). In Fig. 3, the structures of thiourea and protonated thiourea are depicted.
Structures of thiourea (a) and protonated thiourea (b).
Herein, the mechanism of the synergistic stripping is discussed based on the optimized products obtained via computational calculations (Complex V: Pd-S bonding). As for thiourea, it is noted that its general structure C⚌S exhibited high polarity property (Sahu et al., 2011). Ghazali et al. (2013) recorded that C⚌S bonding becomes weak due to the electron density of C⚌S from the ligand had been donated to the metal center. Thus, the interaction between Pd—C—S becomes stronger since the empty π* orbital of the metal had been occupied.
In Figs. 4 and 5, a schema of the proposed stripping mechanism using thiourea and protonated thiourea are depicted.
In the case of reaction with thiourea:
Schema of thiourea trapped Pd in the loaded Aliquat 336.
In the case of reaction with protonated thiourea: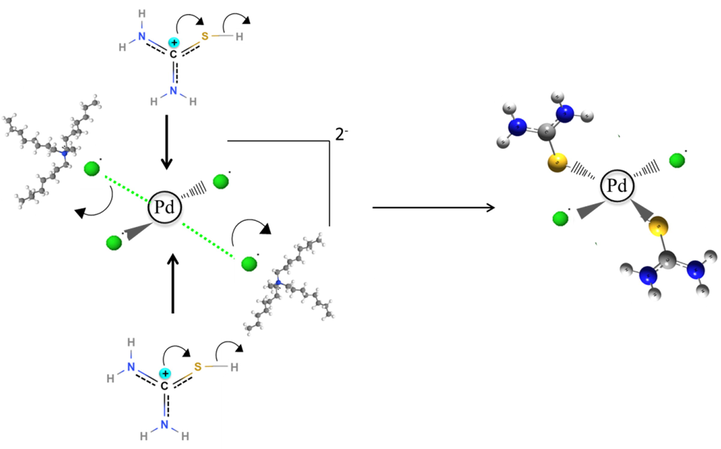
Schema of protonated thiourea trapped Pd in the loaded Aliquat 336.
3.4 Spectroscopy analysis
3.4.1 FTIR spectroscopy
As shown in Fig. 6, the experimental wavenumbers obtained from FTIR spectroscopy of Pd (II) coordinated thiourea ligands are observed. In comparison, MP2/cc-pVTZ method was conducted in order to calculate the infrared vibrational frequencies of Complex V. As regards the experimental peak observed at 3235 cm−1, it is attributed to N—H stretching. Thus, the corresponding calculated vibrational frequencies: are ʋ(N—H)(sym. Stretching) 3574, 3469 cm−1 and ʋ(N—H)(asym. Stretching) 3652, 3709 cm−1. The experimental band that appeared at 687 cm−1 is attributed to NH2 scissoring, according to the calculated value at ʋ(NH2)scissor 477 cm−1. The experimental peaks of C—S and C—N were found to be in the range of 1400–1500 cm−1 which corresponded to the calculated frequencies at ʋ(C—S)stretching 1422 cm−1 and ʋ(C—N)stretching 1546 cm−1 (Silverstein et al., 2014, Pavia et al., 2008). The calculated peaks at ʋ(Pd—Cl)(sym. Stretching) 294 cm−1 and ʋ(Pd—Cl)(asym. Stretching) 330 cm−1, do not appear in the IR spectrum. Such a frequency was reported in the range of 300–400 cm−1 (Bandyopadhyay et al., 2015, Espinal et al., 2009). However, this frequency was not observed due to the limitation of the window (400–4000 cm−1) of the spectrometer used. The band that appeared at 1622 cm−1 established the presence of C⚌N vibration (Bandyopadhyay et al., 2015, Bandyopadhyay et al., 2016, Bandyopadhyay et al., 2017). Gupta (2016) reported previously that the vibrational frequencies as calculated by the quantum mechanical methods proved to be higher than the experimental frequencies. Hence, it was found that the calculated IR spectra were in good agreement with the corresponding experimental spectra. Results obtained from FT-IR spectrum confirmed that the (NH2)2CS·PdCl2 complex was formed via the stripping reaction of Pd in the loaded Aliquat 336 along with the synergistic solutions (thiourea mixed with HCl). In addition, the peak of C—S bonding confirmed good agreement with the predicted molecular geometry results obtained. In Table 7, experimental versus calculated infrared vibrational frequencies are presented.
FTIR spectrum of solution containing Pd (II) coordinated thiourea ligands.
Functional group assigned
Wavenumber (cm−1)
Experimental
Calculated
ʋ(N—H)(stretching)
ʋ(N—H)(sym. Stretching)
ʋ(N—H)(asym. Stretching)
3235
3574, 3469
3652, 3709
ʋ(NH2)(scissoring)
687
477
ʋ(C—S)(stretching)
1400–1500
1422
ʋ(C—N)(stretching)
1400–1500
1546
ʋ(Pd—Cl)(sym. Stretching)
ʋ(Pd—Cl)(asym. Stretching)
300–400
294
330
It is evident that the metal complex comprised of N and S donor nucleophiles Schiff bases provided a miscellany of structural chemistry (Zimmerman et al., 2011, Tarafder et al., 2002). Molecular geometry and ligands coordination as well as their donor groups play a significant role in the physicochemical property of a complex. Of late, C—S bonding has been highlighted owing to its usefulness in wide chemical processes. C—S bonding especially combined with Pd (II) ions has been emphasized in the design to synthesize new ligands. Such ligands consisting of N and S donor nucleophiles can provide a better understanding of theoretical chemistry (Biswas et al., 2017, Karmakar et al., 1993).
3.4.2 UV–vis spectroscopy
In Fig. 7, the UV–vis spectrum of initial feed solution is depicted since various forms of Pd chlorocomplexes can be found in chloride medium (Hubicki et al., 2008). Therefore, UV–vis spectroscopy was conducted to determine the species of Pd (II) chlorocomplexes. As observed, the absorbance peaks at 232.83, 281.83 and 313.01 nm corresponded to PdCl42−, which was in agreement with previous research (Wojnicki et al., 2011). Hubicki et al. (2008) also acknowledged that the predominant species of Pd (II) in chloride medium was PdCl42−. Thus, the stability constants of the Pd (II) chlorocomplexes are as follows:
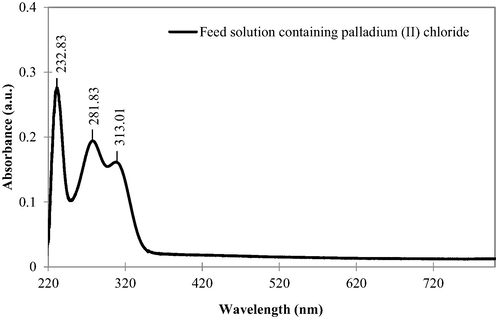
UV–vis spectrum of initial feed solution contained Pd (II).
In Figs. 8–10, absorbance peaks of HCl, thiourea and synergistic solutions, before and after stripping reaction, are provided. In the case of thiourea and synergistic solutions, results show that the absorbance peaks shifted to the left hand side and approached the peak positions of PdCl42-, indicating that the strippants had the ability to trap Pd (II) ions. As for HCl solution, the peak position was found to be invisible. Such an anomaly may result from the small amount of HCl molecules that absorb the light. Hence, thiourea played a major role in Pd (II) ions stripping.
UV–vis spectra of HCl solution before and after stripping reaction.

UV–vis spectra of thiourea solution before and after stripping reaction.

UV–vis spectra of synergistic solutions before and after stripping reaction.
3.5 Kinetic study on palladium (II) recovery via synergistic solutions
The possible stripping reactions are shown as follows:
Reaction with thiourea:
Reaction with protonated thiourea:
At low concentration of thiourea;
4 Conclusions
In this study, both optimized geometry and thermodynamics stability of the (NH2)2CS·PdCl2 complex were comprehensively analyzed via computational calculations. Eight lowest structures of ((NH2)2CS·PdCl2) complex were formed. The structure having Pd-S bonding demonstrated the highest geometric stability. Values of 〈S2〉 from B3LYP and MP2 calculations for the triplet state complex confirmed very low spin contamination. It was noted that FTIR results were found to be in good agreement with the computational chemistry. UV–vis confirmed that the strippants exhibited an ability to trap Pd (II). Schiff bases provided a variety of chemical structures which played an important role in the complex’s physicochemical property. C—S bonding having Pd (II) has been highlighted to synthesize new ligands, which consist of N and S donor nucleophiles. This work provides a novel approach for a better understanding of the structural chemistry and reaction mechanisms in the synergistic system.
Acknowledgements
The authors gratefully acknowledge the financial support by Ratchadaphiseksomphot Fund (Chulalongkorn University for Postdoctoral Fellowship). Really thanks to Mektec Manufacturing Corporation (Thailand) Ltd., Center of Excellence in Computational Chemistry (Department of Chemistry, Faculty of Science, Chulalongkorn University) and Separation Laboratory (Department of Chemical Engineering, Faculty of Engineering, Chulalongkorn University) for their kind support.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Utility of cloud-point preconcentration and spectrophotometry for determination of trace amounts of palladium (II) and their analytical applications. Arab. J. Chem.. 2016;9:S326-S333.
- [Google Scholar]
- Palladium(II) complexes incorporating phenylazoarylmethine ancillary ligands: Synthesis, spectraland antitumor activity. Arab. J. Chem.. 2016;9:S1503-S1509.
- [Google Scholar]
- A bridge between liquid–liquid extraction and the use of bacterial communities for palladium and platinum recovery as nanosized metal sulphides. Hydrometallurgy. 2016;163:40-48.
- [Google Scholar]
- Atkins, P., Paula, J.D., 2006. Chapter 17: Statistical thermodynamics 2: applications, in: Atkins, P., Paula, J.D. (Ed.), Atkins' Physical Chemistry, eighth ed., W. H. Freeman and Company, The United States of America, pp. 589–619.
- DFT-B3LYP versus MP2, MP3 and MP4 calculations of the structural stability of azidoketene OZCZCH–NNN. J. Mol. Struct.. 2004;712:131-138.
- [Google Scholar]
- Synthesis, structure, spectral characterization, electrochemistry and evaluation of antibacterial potentiality of a novel oxime-based palladium (II) compound. Eur. J. Med. Chem.. 2015;89:59-66.
- [Google Scholar]
- Synthesis, structure, DFT calculations, electrochemistry, fluorescence, DNA binding and molecular docking aspects of a novel oxime based ligand and its palladium (II) complex. J. Photochem. Photobio., B: Bio.. 2016;160:336-346.
- [Google Scholar]
- Comparative study of an osazone based ligand and its palladium (II) complex with human serum albumin: a spectroscopic, thermodynamic and molecular docking approach. J. Photochem. Photobio., B: Bio.. 2017;173:1-11.
- [Google Scholar]
- MP2, DFT and ab initio calculations on thioxanthone. Spectrochimica Acta Part A. 2011;82:49-55.
- [Google Scholar]
- Synthesis of palladium (II) complex with NNS donor Schiff base ligand via C-S bond cleavage: X-ray structure, electrochemistry and DFT computation. J. Mol. Structure.. 2017;1142:110-115.
- [Google Scholar]
- Palladium (II) complexes with highly basic imidazolin-2-imines and their reactivity toward small bio-molecules. Dalton. Trans.. 2015;44:17346-17359.
- [Google Scholar]
- Evaluation of MP2, DFT, and DFT-D methods for the prediction of infrared spectra of peptides. J. Phys. Chem.. 2009;113(22):6301-6307.
- [Google Scholar]
- Density functional theory study of small Ag cluster adsorbed on graphyne. App. Surf. Sci.. 2019;465:93-102.
- [Google Scholar]
- Chemical reactivity indexes in density functional theory. J. Comput. Chem.. 1999;20:129-154.
- [Google Scholar]
- Wet synthesis of high purity crystalline urea phosphate from untreated Moroccan industrial phosphoric acid. Mor. J. Chem.. 2020;8(4):1024-1032.
- [Google Scholar]
- Extraction and separation of palladium(II), platinum(IV), gold(III) and rhodium(III) using piperidine-based extractants. Hydrometallurgy. 2018;175:359-366.
- [Google Scholar]
- Møller-Plesset perturbation theory: from small molecule methods to methods for thousands of atoms: Møller-Plesset perturbation theory. WIREs Comput. Mol. Sci.. 2011;1(4):509-530.
- [CrossRef] [Google Scholar]
- Evaluation of novel ligand dithiodiglycolamide (DTDGA) for separation and recovery of palladium from simulated spent catalyst dissolver solution. Sep. Purif. Technol.. 2014;125:151-155.
- [Google Scholar]
- Azadipyrromethene complexes of d8 metal centers: rhodium (I), iridium (I), palladium (II), and platinum (II) Inorg. Chem.. 2013;52:13048-13057.
- [Google Scholar]
- Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen. J. Chem. Phys.. 1989;90:1007-1023.
- [Google Scholar]
- The structure and stability of Ni (II) complexes of Thiourea and related ligands. Can. J. Chem.. 1975;53:633-643.
- [Google Scholar]
- Unexpected formation of [Pd(bedp)][Pd2Cl4(l-SMe)2] (bedp = 1,4-bis[2-(3,5-dime-thyl-1-pyrazolyl)ethyl]piperazine). Structural characterisation and spectroscopic properties. J. Ros. Inorg. Chem. Commun.. 2009;12:368-370.
- [Google Scholar]
- Highly selective transport of palladium from electroplating wastewater using emulsion liquid membrane process. J. Taiwan Inst. Chem. Eng.. 2016;64:134-141.
- [Google Scholar]
- A systematic examination of ligand basicity effects on bonding in palladium (0)- and palladium (II)-ethylene complexes. Inorg. Chim. Acta.. 2018;483:191-202.
- [Google Scholar]
- Synthesis and characterisation of palladium (II) complex of (N-pentanoylthioreido)-N’-propanoic acid and its application as catalyst in heck cross coupling reaction. Aust. J. Basic Appl. Sci.. 2013;7(9):149-155.
- [Google Scholar]
- Chapter 1: Fundamental aspects and approximate functionals. In: Gross E.K.U., Dreizler R.M., eds. Density Functional Theory. The United States of America: Plenum Press; 1995. p. :3-236.
- [Google Scholar]
- Gupta, V.P., 2016. Chapter 8: Vibrational Frequencies and Intensities. In: Gupta, V.P. (Ed.), Principles and Applications of Quantum Chemistry, Academic Press, pp. 247–289.
- Computational study on the selectivity of donor/acceptor-substituted rhodium carbenoids. J. Org. Chem.. 2009;74:6555-6563.
- [Google Scholar]
- Hartwig, J.F., 2010. Organotransition Metal Chemistry, from Bonding to Catalysis. University Science Books. pp. 321. ISBN 978-1-891389-53-5.
- Adsorption behavior of Pd(II) ions from aqueous solution onto pyromellitic acid modified-UiO-66-NH2. Arab. J. Chem.. 2020;13:7007-7019.
- [Google Scholar]
- Studies of removal of palladium (II) ions from chloride solutionson weakly and strongly basic anion exchangers. J. Hazard. Mater.. 2008;159:280-286.
- [Google Scholar]
- Using computational chemistry to design Ru photosensitizers with directional charge transfer. Coor. Chem. Rev.. 2015;304–305:146-165.
- [Google Scholar]
- Benchmark database of accurate (MP2 and CCSD(T) complete basis set limit) interaction energies of small model complexes, DNA base pairs, and amino acid pairs. Phys. Chem. Chem. Phys.. 2006;8:1985-1993.
- [Google Scholar]
- Nickel complexes of tridentate ligands incorporating thioether and triazene-1-oxide functions. Synthesis, structure and metal redox. Polyhedron. 1993;12:2325-2329.
- [Google Scholar]
- Pd (II) complexes bearing chromone based Schiff bases: synthesis, characterisation and biological activity studies. Arab. J. Chem.. 2016;9:640-648.
- [Google Scholar]
- Electron affinities of the first-row atoms revisited. Systematic basis sets and wave functions. J. Chem. Phys.. 1992;96:6796-6806.
- [Google Scholar]
- Biogenic synthesis of palladium nanoparticles using Pulicaria glutinosa extract and their catalytic activity towards the Suzuki coupling reaction. Dalton Trans.. 2014;43:9026-9031.
- [Google Scholar]
- Solvent extraction separation and recovery of palladium and platinum from chloride leach liquors of spent automobile catalyst. Sep. Purif. Technol.. 2010;73:213-218.
- [Google Scholar]
- Recent developments in LIBXC-A comprehensive library of functionals for density functional theory. SoftwareX. 2018;7:1-5.
- [Google Scholar]
- Chapter 4 – Gas permeation and supported liquid membranes. In: Patricia Luis, Fundamental Modelling of Membrane Systems. Elsevier; 2018. p. :103-151.
- [Google Scholar]
- Spectroscopic and TDDFT studies of N-phenyl-N0-(3-triazolyl)thiourea) compounds with transition metal ions. Arab. J. Chem.. 2021;14:102932.
- [CrossRef] [Google Scholar]
- Kinetic study of reductive elimination from the complexes (Diphosphine)Pd(R)(CN) J. Am. Chem. Soc.. 1998;120:8527.
- [Google Scholar]
- Synergistic strippants of Pd (II) ions in the presence of chloride medium from wastewater of electroless plating process via solvating system: kinetics and thermodynamics study. Sep. Sci. Tech.. 2019;54:2971-2982.
- [Google Scholar]
- A numerical and experimental investigation on the selective separation of Pd (II) from wastewater using Aliquat 336 via hollow fiber supported liquid membrane. J. Environ. Chem. Eng.. 2020;8(5):104234.
- [CrossRef] [Google Scholar]
- Separation of Pt (IV), Pd (II), Rh (III) and Ir (IV) from concentrated hydrochloric acid solutions by solvent extraction. Hydrometallurgy. 2016;164:71-77.
- [Google Scholar]
- Part II: Impact of ionic liquids as anticorrosives and additives on Ni-Co alloy electrodeposition: experimental and DFT study. Arab. J. Chem.. 2021;14:102909.
- [CrossRef] [Google Scholar]
- Liquid-liquid extraction of palladium (II) from chloride media by N, N'-dimethyl-N, N'-dicyclohexylthiodiglycolamide. Sep. Purif. Technol.. 2015;156:363-368.
- [Google Scholar]
- Solvent extraction and separation of palladium (II) and platinum (IV) from hydrochloric acid medium with dibutyl sulfoxide. Miner. Eng.. 2009;22:1271-1276.
- [Google Scholar]
- Chapter 2: infared spectroscopy. In: Pavia D.L., Lampman G.M., Kriz G.S., Vyvyan J.R., eds. Introduction to Spectroscopy (fourth ed.). Brooks/Cole, The United States of America; 2008. p. :15-104.
- [Google Scholar]
- A fifth-order perturbation comparison of electron correlation theories. Chem. Phy. Lett.. 1989;157(6):479-483.
- [Google Scholar]
- Theoretical study of the catalytic effect of TM-C4H4and TM-C5H5(TM = Cr, Ti, V, Sc) on the activation of O2 at the cathode and CH3OH at the anode in ‘‘CH3OH-O2fuel cell via DFT computational method. Arab. J. Chem.. 2021;14:103062.
- [CrossRef] [Google Scholar]
- Study on the extraction of palladium (II) and platinum (IV) using LIX 84I. Hydrometallurgy. 2006;84:54-59.
- [Google Scholar]
- CCSD[T] describes noncovalent interactions better than the CCSD(T), CCSD(TQ), and CCSDT methods. J. Chem. Theory Comput.. 2013;8:9(1):364-369.
- [Google Scholar]
- Benzylic complexes of palladium (II): bonding modes and pentacoordination for steric relie. Organometallics. 2018;37:1074-1085.
- [Google Scholar]
- Oxidation of thiourea and substituted thioureas: a review. J. Sulfur Chem. 2011:1-27.
- [Google Scholar]
- Experimental and theoretical approaches to the protonation of Thiourea: a convenient nucleophile in coordination chemistry revisited. Z. Anorg. Allg. Chem.. 2005;631:2812-2819.
- [Google Scholar]
- Selective CH versus OH bond activation of CH3OH upon electrospraying methanolic solutions of MX2 (M=Fe Co, Ni; X=Br, I): a DFT study. ChemCatChem.. 2010;2:799-802.
- [Google Scholar]
- Oxidative addition of aryl halides to palladium (0) complexes: a density-functional study including solvation. Organometallics. 2004;23:2980-2988.
- [Google Scholar]
- High selectivity and extractability of palladium from chloride leach liquors of an automotive catalyst residue by azothiacalix[4]arene derivative. Hydrometallurgy. 2017;169:478-487.
- [Google Scholar]
- Chapter 2: infared spectroscopy. In: Silverstein R.M., Webster F.X., Kiemle D.J., Bryce D.L., eds. Spectrometric Identification of Organic Compounds (eighth ed.). Wiley, The United States of America; 2014. p. :71-125.
- [Google Scholar]
- Cycloaurated gold (III) complexes with monoanionic thiourea ligands. Inorg. Chim. Acta.. 2013;408:27-32.
- [Google Scholar]
- Computational studies of synthetically relevant homogeneous organometallic catalysis involving Ni, Pd, Ir, and Rh: an overview of commonly employed DFT methods and mechanistic insights. Chem. Rev.. 2015;115:9532-9586.
- [Google Scholar]
- Extraction and carrier mediated transport of urea using noncyclic receptors through liquid membrane systems. Arab. J. Chem.. 2020;14(3):4764-4770.
- [Google Scholar]
- Separation of homogeneous palladium catalystsfrom pharmaceutical industry wastewater by usingsynergistic recovery phase via HFSLM system. Arab. J. Chem.. 2021;14:103024.
- [CrossRef] [Google Scholar]
- Separation of platinum and palladium from chloride solution by solvent extraction using Alamine 300. Hydrometallurgy. 2010;104:1-7.
- [Google Scholar]
- Coordination chemistry and bioactivity of some metal complexes containing two isomeric bidentate NS Schiff bases derived from S-benzyldithiocarbazate and the X-ray crystal structures of S-benzyl-β-N-(5-methyl-2-furylmethylene)dithiocarbazate and bis[S-benzyl-β-N-(2-furylmethylketone)dithiocarbazato]cadmium(II) Polyhedron. 2002;21:2691-2698.
- [Google Scholar]
- Study on complexation of palladium with thiourea-based ligands and its determination in simulated high-level liquid waste using solid phase extraction-electrospray mass spectrometry. J. Radio. Nuclear Chem.. 2018;318:1249-1259.
- [Google Scholar]
- Extraction of palladium(II) from hydrochloric acid solutions by solvent extraction with mixtures containing either cyanex 301 or LIX 63. Metals. 2017;7(12):541.
- [CrossRef] [Google Scholar]
- Kinetics of palladium (II) chloride complex reduction in aqueous solutions using dimethylamineborane. Hydrometallurgy. 2011;110:56-61.
- [Google Scholar]
- Effect of diluent polarity on membrane stability in the separation of trace Pd(II) from wastewater by HFSLM using LIX84-I. J. Indust. Eng. Chem.. 2015;21:212-220.
- [Google Scholar]
- Tridentate N2S ligand from 2,2 '-dithiodibenzaldehyde and N, N-dimethylethylenediamine: synthesis, structure, and characterization of a Ni (II) complex with relevance to Ni superoxide dismutase. Inorg. Chim. Acta.. 2011;373(1):54-61.
- [Google Scholar]
- Back-extraction process operation and modeling through thermodynamic equilibrium solubility of valeric acid in aqueous and organic phase mixtures. Sep. Pur. Technol.. 2019;222:125-135.
- [Google Scholar]







