Translate this page into:
Synthesis and In-silico Simulation of Some New Bis-thiazole Derivatives and Their Preliminary Antimicrobial Profile: Investigation of Hydrazonoyl Chloride Addition to Hydroxy-Functionalized Bis-carbazones
⁎Corresponding author. sahussain@imamu.edu.sa (Sami A. Al-Hussain), zeinab.a.muhammad@gmail.com (Zeinab A. Muhammad)
-
Received: ,
Accepted: ,
This article was originally published by Elsevier and was migrated to Scientific Scholar after the change of Publisher.
Abstract
Abstract
Two new series of bis-thiazoles 6a-f and bis-thiazolones 9a-d were prepared via reacting bis-thiosemicarbazone 3 with hydrazonoyl chloride derivatives 4a-f and 7a-d, respectively, in dioxane under basic conditions. Another group of bis-thiazole derivatives 12a-h was prepared by reacting bis-thiosemicarbazone 3 with each of phenacyl bromide derivatives 10a-h under a similar reaction protocol. A plausible mechanism was proposed. Structural elucidation of the new products was established using both elemental and spectrometric/spectroscopic analyses. Structural, electronic, and the pharmacological characteristics of the prepared molecules were investigated with DFT calculations. Antibacterial and antifungal activities of the new bis-thiazoles were screened and compared with vancomycin and amphotericin B as antimicrobial standards. Molecular docking studies on the promising candidate compounds, with the lowest minimum inhibitory concentrations (MIC), were performed with SAP2 of C. albicans and FabI of S. aureus and P. aeruginosa.
Keywords
Carbazone
Hydrazonoyl chlorides
Bis-thiazoles
Antimicrobial
DFT
In-silico studies
1 Introduction
Thiazole derivatives have attracted the attention of the scientific community in general and pharmaceutical chemists in particular (Potewar et al., 2007; Rajanarendar et al., 2012). This interest is largely fueled by the thiazole diverse pharmacological and biological activities. Many thiazoles are well-known anti-inflammatory, antimicrobial, antibacterial, analgesic, antihypertensive, herbicidal and/or anti-HIV agents (Abdel-Wahab et al., 2008; Gomha and Khalil, 2012; Verma and Saraf, 2008). The thiazole ring system represents the active handle in many biologically-active heterocyclic compounds (Abdel-Wahab et al., 2008; Abdelhamid et al., 2015; Dawood and Gomha, 2015; Gomha et al., 2015; Gomha et al., 2015b; Gomha and Khalil, 2012; Potewar et al., 2007; Rajanarendar et al., 2012; Sayed et al., 2020; Verma and Saraf, 2008). Various thiazoles are biologically active scaffolds (Verma and Saraf, 2008), making them perfect candidates for library screening in medicinal chemistry (Oka et al., 2012; Watanabe and Uesugi, 2013). Many bis-thiazoles are also as reactive as their thiazole counterparts and their biological activity as antibacterial, antifungal, and anticancer were reported (Auterhoff, 1972; Franklin et al., 2008; Kouatly et al., 2009; Limban et al., 2012, 2011, 2008; Olar et al., 2010). In this study, our main focus is to construct some novel bis-thiazoles with high bioactivity profile. Accordingly, the starting carbazone is tagged with a hydroxyl group, which could be further functionalized as needed (Ou et al., 2013). This –OH handle could potentially be used to fine-tune structure–activity relationship (SAR). Herein, we introduce the preparation strategy of novel bis-thiazoles 6a-g and 12a-c as well as bis-thiazolone derivatives 9a-e from synthetically simple bis-thiosemicarbazone derivative 3, tagged with a hydroxyl group, with various hydrazonoyl chlorides 4a-g, α-haloketones 10a-c, and hydrazonoyl chlorides 4a-g under basic reaction conditions. All new bis-heterocycles were structurally elucidated by elemental and spectral (1H NMR, 13C NMR, IR, and MS) analysis techniques. Furthermore, the biological profiles of the new bis-heterocycles were evaluated and the results were followed by in-silico docking studies and DFT calculations. Some of the new bis-thiazoles were good candidates as antimicrobial prototypes and could be further developed to target drug-resistant bacteria.
2 Results and discussion
2.1 Reaction chemistry
To kick-start our synthetic strategy, the common bis-thiosemicarbazone precursor 3 was constructed when one equivalent of hydroxyl-tagged bis-aldehyde 1 was condensed with two equivalents of thiosemicarbazide 2 in refluxing ethanol, acidified with HCl, for 2 h to give the new hydroxyl-bearing bis-thiosemicarbazone 3 as depicted in Scheme 1. The bis-aldehyde 1 itself was synthesized via reacting two equivalents of salicylaldehyde with epichlorohydrin in NaOH solution as previously reported by Bin, et al. (Zhao et al., 1996) (Scheme 1).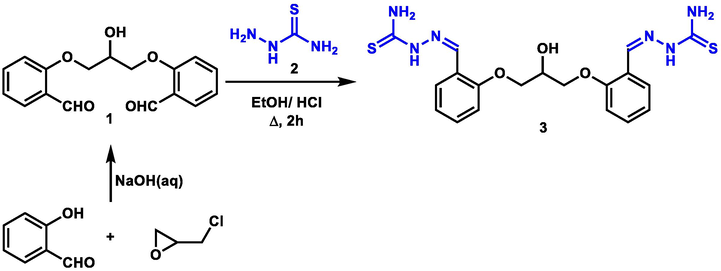
Synthesis of bis-thiosemicarbazone3.
The chemical reactivity of the new hydroxyl-bearing bis-thiosemicarbazone 3 towards a host of hydrazonoyl chlorides 4 was evaluated to construct a new group of bis-thiazoles. Therefore, reaction of bis-thiosemicarbazone 3 with hydrazonoyl chlorides 4a-g in refluxing dioxane in the presence of catalytic triethylamine delivered the target bis-thiazoles 6a-g (Scheme 2). Elucidation of all the new bis-thiazole derivatives was executed using various spectral analyses. For example, the IR spectrum of compound 6f revealed typical –NH absorption at the expected wave number v 3410 cm−1. Also, 1H NMR spectrum of 6f revealed four typical singlets for CH3, CH-OH, CH = N, and NH groups at δ 2.60, 5.36, 8.51, and 11.36 ppm, respectively. The characteristic multiplet for the two CH2 groups is visible at δ 4.19–4.25 ppm, in addition to the C-H multiplet shown at δ 5.40–5.45 ppm.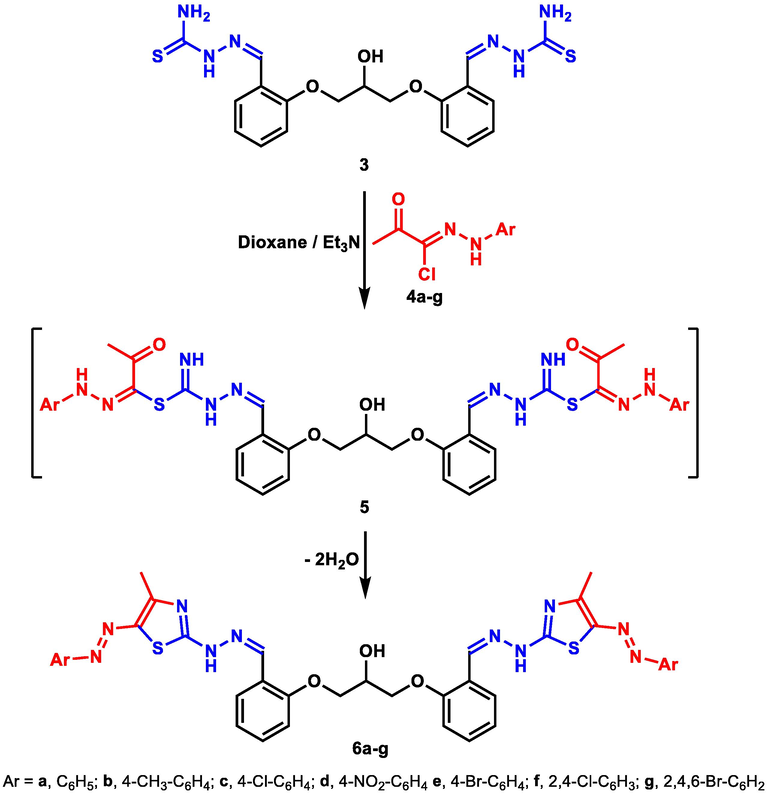
Synthesis of bis-thiazolesderivatives 6a-g.
In a similar route, hydroxyl-tagged bis-thiosemicarbazone 3 reacted with N-aryl-2-oxopropanehydrazonoyl halides 7a-e (the ester counterparts of compounds 4a-e) in refluxing dioxane and catalytic triethylamine, leading to the formation of the expected bis-thiazole derivatives 9a-e (Scheme 3). Elucidation of the new bis-heterocycles 9a-e was substantiated typically by extensive spectral analysis. For example, the IR spectrum of derivative 9c showed the presence of –NH absorption at 3396 and 3281 cm-1characteristic for the four –NH groups. Further structural confirmation of the isolated bis-thiazole derivatives 9a-e was based on their 1H NMR spectra which contained four singlets for the CH-OH, CH = N and 2NH groups near δ 5.36, 8.50, 9.42, and 11.37 ppm, respectively.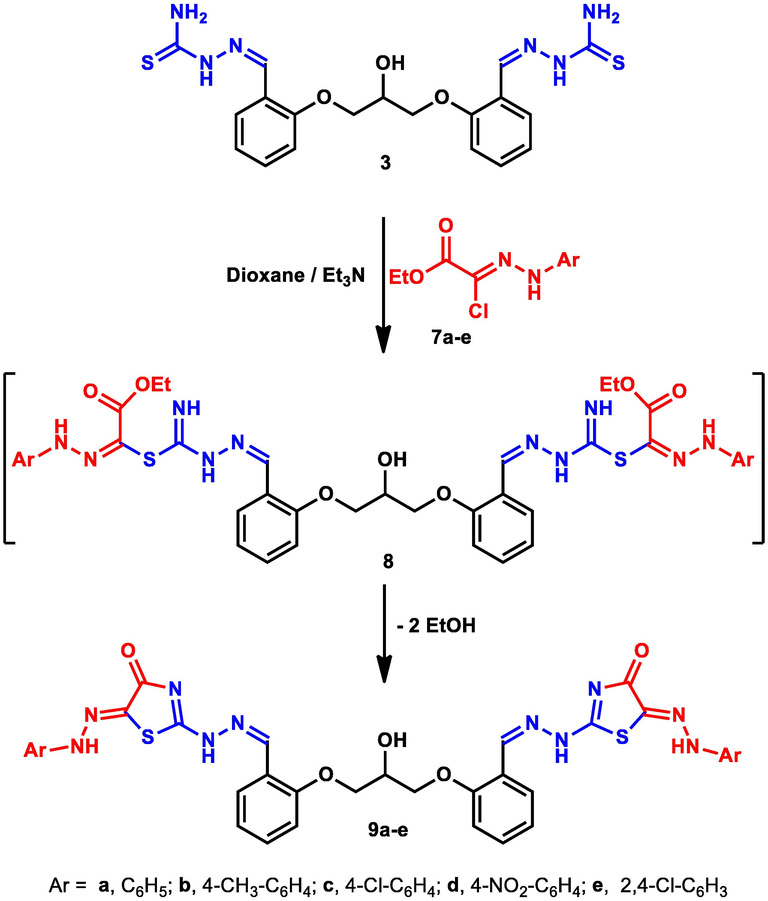
Synthesis of bis-thiazole derivatives 9a-e.
In a similar fashion, the reaction of compound 3 with phenacyl bromides 10a-c in dioxane under reflux using catalytic triethylamine, afforded bis-thiazoles 12a-c (Scheme 4). Structural analysis of the new bis-thiazoles was established using both spectral and elemental analyses. For example, the IR spectrum of product 12c revealed –NH absorption at 3412 cm−1. Moreover, the 1H NMR spectra of the isolated compounds 12a-c showed the characteristic four singlets at δ 3.78, 5.59, 8.93, and 11.35 ppm due to 2OCH3, CH-OH, thiazole-CH and 2NH, respectively. Also, two multiplets were shown at δ4.21–4.24 and 6.43–6.45 corresponding to the 2CH2 and the CH-OH groups, respectivly.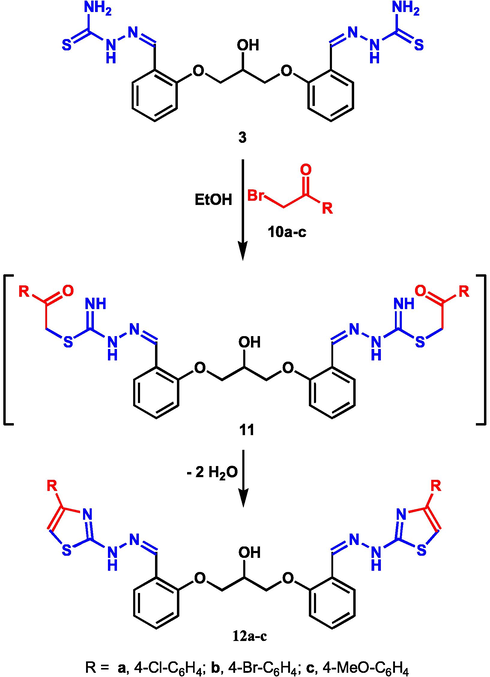
Synthesis of bis-thiazole derivatives 12a-c.
2.2 Molecular geometries
The molecular geometries of the studied compounds were subjected to energy minimization by implementing the B3LYP/6-311G level of theory. The obtained optimized structures and their corresponding frontier molecular orbitals (FMOs) are illustrated in Fig. 1. The calculated total energies are −83,764.15, −221,680.97, −83,579.12, −108,592.06 and −213,585.72 eV for 6c, 6f, 9b, 9d, and 12c, respectively. Based on their relative energy values, compounds stability follows the following order: 6f > 12c > 9d > 6c > 9b. The quantum mechanical descriptors of the investigated molecules are collected in Table 1.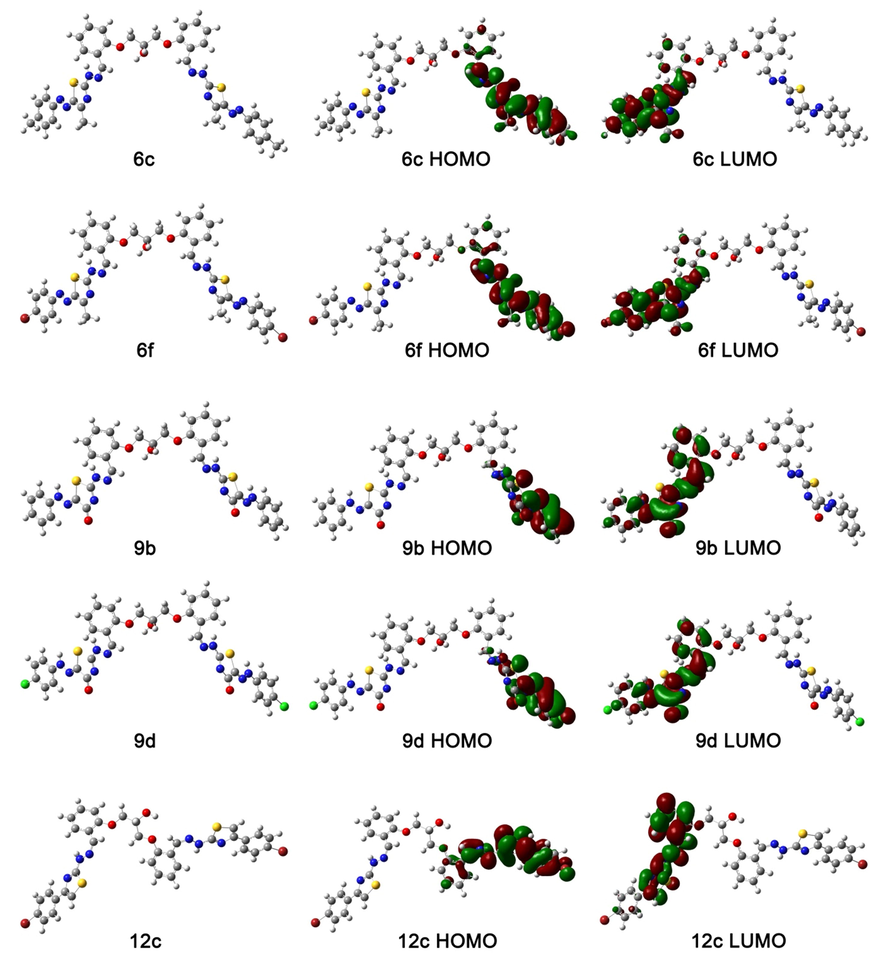
Optimized molecular geometries of compounds 6c, 6f, 9b, 9d and 12c and their corresponding MOs. The isovalue used for visualization of the MOs is 0.02. Color codes: C: grey, O: red, H: white, N: blue, Cl: green, Br: brown, S: yellow.
Quantum parameter
6c
6f
9b
9d
12c
Total energy (eV)
−83,764.15
−221,680.97
−83,579.12
−108,592.06
−213,585.72
EHOMO (eV)
−5.47
−5.70
−5.89
−6.10
−5.82
ELUMO (eV)
−2.48
−2.76
−2.43
−2.60
−1.92
ΔE (eV)
2.99
2.94
3.46
3.5
3.9
Ionization energy, I (eV)
5.47
5.70
5.89
6.10
5.82
Electron affinity, A (eV)
2.48
2.76
2.43
2.60
1.92
Mulliken electronegativity, χ
3.975
4.23
4.16
4.35
3.87
Chemical potential, μ (eV/mol)
−3.975
−4.23
−4.16
−4.35
−3.87
Softness, S
0.67
0.68
0.58
0.57
0.51
Hardness, η
1.50
1.47
1.73
1.75
1.95
Electrophilicity index, ω
5.28
6.09
5.00
5.41
3.84
From the DFT energy calculations, some quantum chemical descriptors (Table 1) can be obtained that give information about the reactivity, complex formation with transition metals, the ability to donate/accept electrons, etc. For instance, ionization energy (I) and electron affinity (A) are two properties that can indicate the reactivity of the molecules. They can be calculated as follows: I = − EHOMO and A = − ELUMO (Abo Dena et al., 2019). Moreover, the complexation with transition metals requires having information about the softness (S) and/or hardness (η) of the molecules. Hardness is calculated from η = (I − A)/2 and softness is calculated as the reciprocal of hardness. Furthermore, during charge-transfer reactions, chemical potential (μ = − (I + A)/2), Mullikan electronegativity (χ = (I + A)/2) and electrophilicity index (ω = μ2/2η) measure the lowering in energy that result from the maximal electron flow from the donor molecule to the acceptor molecule (Abo Dena and Abdel Gaber, 2017; Abo Dena and Hassan, 2016; Hassan and Abo Dena, 2014).
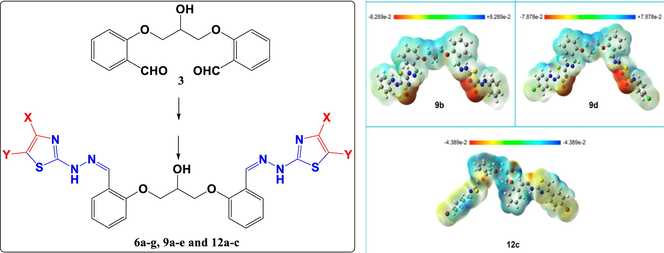
In order to make it easier to predict the chemical behavior/reactivity of the investigated organic molecules, the electrostatic potential maps (EPMs) were visualized (Fig. 2). In EPMs, areas of low electrostatic potential energy are shown in red color (nucleophilic sites), while blue areas indicate parts of low electron density (electrophilic sites). The compounds of interest contain areas susceptible for electrophilic addition, for instance the N atom of the thiazole rings and the OH groups. These are considered as electron-rich areas due to the localization of electron lone pairs on nitrogen and oxygen atoms. On the other hand, common functional groups or molecular parts that act as suitable sites for nucleophilic attack are represented by all aromatic and/or aliphatic hydrogens due to the strong electron attraction by the attached aromatic groups.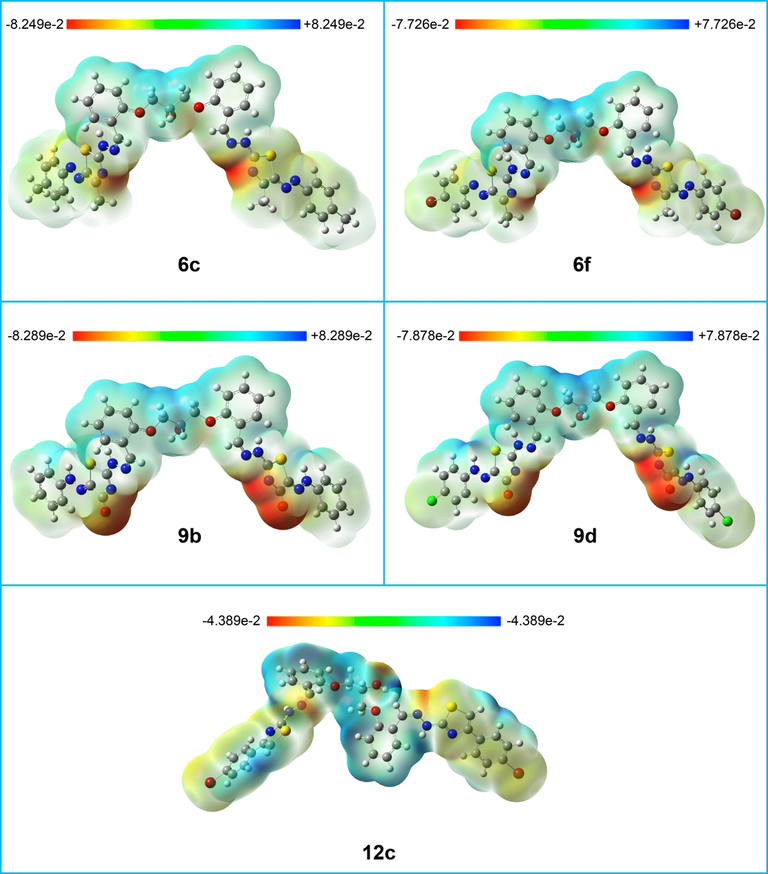
Electrostatic potential maps of compounds 6c, 6f, 9b, 9d, and 12c. The isocalue of the electrostatic potntial ssurfaces is 0.0004.
Interestingly, Frontier molecular orbitals are not only used for calculating the above-mentioned quantum mechanical descriptors, but also they can give information about light excitability and electron transfer through the molecules. HOMO refers to the highest occupied molecular orbital, while LUMO is an abbreviation for the lowest unoccupied molecular orbital. It is clear from the data illustrated in Table 1 that compound 6f (ΔE = 2.94 eV) has the highest light excitability as well as the highest heterogeneous electronic charge transfer among all of the investigated candidate compounds. Meanwhile, compound 12c (ΔE = 3.9) demonstrates the lowest light excitability and the lowest ability to catalyze heterogeneous electronic charge transfer. Due to the bilateral symmetry of the molecules, HOMOs are always delocalized in an opposite side to LUMOs of the same molecule. According to the above-mentioned ΔE values, it is expected that compound 12c will absorb higher light energy (i.e. shorter wavelength) than compound 6f.
2.3 XTT assay results
The cell proliferation kit II (XTT) assay was performed to find the minimum amounts (a.k.a. minimum inhibitory concentration, MIC) of the compounds that inhibit cell viability/metabolism of P. aeruginosa, S. aureus and C. albicans. The results obtained from the XTT assay are summarized in Table 2. Obviously, compound 9b has excellent antibacterial (P. aeruginosa, MIC: 0.12 – 1.95 μg/mL) and antifungal (C. albicans, MIC: 0.98 – 3.90 μg/mL) activities, as reflected by the MIC values, compared to the standard antibiotic molecules, vancomycin, amikacin, and amphotericin B. In addition, molecule 12c demonstrated an acceptable antibacterial activity against S. aureus (MIC: 0.98 – 1.95 μg/mL).
Sample
Minimum inhibitory concentration (µg/mL)
Gram positive bacteria
Gram negative bacteria
Fungus
Methicillin-susceptible Staphylococcus aureus ATCC 25,923
Methicillin-resistant Staphylococcus aureus (MRSA) ATCC-BAA-1720
Sensitive Pseudomonasaeruginosa ATCC10145
Penicillin and Cephalosporins-resistant Pseudomonas aeruginosa ATCC BAA-2108
Azole- sensitive Candida albicans ATCC 18,804
Azole-Resistant Candida albicans ATCC 10,231
3
31.25
125
NA
NA
250
NA
6a
1.95
15.63
62.5
NA
125
NA
6b
7.81
31.25
31.25
NA
125
NA
6c
0.98
1.95
1.95
15.63
62.5
NA
6d
1.95
7.81
7.81
62.5
31.25
NA
6f
0.49
1.95
1.95
7.81
15.63
NA
6g
1.95
3.9
31.25
125
15.63
NA
9a
1.95
15.63
31.25
NA
NA
NA
9b
0.12
0.49
0.24
1.95
0.98
3.9
9c
0.98
3.9
3.9
7.81
3.9
15.63
9d
0.98
1.95
0.98
3.9
7.81
NA
9e
1.95
3.9
3.9
15.63
15.63
NA
12a
1.95
7.81
15.63
NA
62.5
NA
12b
7.81
15.63
15.63
NA
15.63
NA
12c
0.98
1.95
15.63
62.5
NA
NA
Vancomycin
0.24
0.98
0.49
3.9
ND
Amikacin
0.98
7.81
0.24
3.9
ND
Amphotericin B
ND
0.24
1.95
Table 2 showed that:
-
Most of the studied molecules demonstrated greater antimicrobial activities against bacteria than fungi.
-
All molecules showed different degrees of biological activities towards antibiotic-resistant Staphylococcus aureus (MRSA) TCC BAA-1720. The activity of compound 9b (0.49 µg/mL) was greater than the standard antibiotic (0.98 µg/mL); however, compounds 6c, 6f, 9d and 12c showed the same effect (1.95 µg/mL).
-
The activity of compound 9b against the antibiotic-sensitive Pseudomonas aeruginosa ATCC 10145 and the antibiotic-resistant Pseudomonas aeruginosa ATCC BAA-2108 was greater than that of vancomycin that showed an MIC value for the antibiotic-resistant strain similar to that of compound 9d.
-
On the other hand, all compounds did not have antimicrobial activity against Azole- resistant Candida albicans ATCC10231 fungus except compound 9b.
-
Compounds 9b demonstrated a high antimicrobial effect against the antibiotic-resistant P. aeruginosa ATCC BAA-2108, and compound 9d shows an acceptable antimicrobial activity in case of P. aeruginosa and S. aureus (MIC: 0.12 and 0.49 μg/mL, respectively).
-
What’s more, the antimicrobial activity of molecule 3 is very powerful as revealed from its very low MIC value. The compound was able to act against all resistant bacterial (P. aeruginosa and S. aureus, MIC: 0.49 and 3.9 μg/mL, respectively) and fungal (C. albicans, MIC: ND μg/mL) strains.
-
Most of the synthesized molecules show very strong (MIC < 10 µg/mL) biological activity and few of them demonstrate either strong (MIC 10–25 µg/mL) or good (MIC 26–125 µg/mL) bioactivity according to the ranking reported elsewhere (O’Donnell et al., 2010).
2.4 Molecular docking
Molecular docking is a molecular modeling technique that helps in studying how two or more molecules fit together; for instance, a drug and an enzyme. It represents a potent tool in drug design and discovery of lead molecules. In the present study, this technique was applied to the investigated compounds to predict their biological activities. Reportedly, thiazoles and bis-thiazoles usually have powerful biological activities against C. albicans and S. aureus. Accordingly, those microorganisms were significantly influenced by treatment with the synthesized bis-thiazoles. The investigated compounds showed high toxicity to Pseudomonas aeruginosa. Consequently, we have chosen the most suitable microbial receptor proteins that can be influenced by bis-thiazole ligands based on a thorough literature review (Mahmoud et al., 2020).
Secreted Aspartic Proteinase (SAP) plays a significant role as an important virulence factor in C. albicans disseminated/mucosal infections. The attachment/invasion of different Candida species, particularly C. albicans, is thought to be attributed to this protein, and hence it plays a crucial role in their pathogenicity. That is why SAPs can be a suitable target for drug intervention for candidiasis (Cutfield et al., 1995).
S. aureus is a common Gram-positive microbe which infects wounds resulting in increased incidence of staphylococcal scalded skin syndrome (a reaction of the skin to a staphylococcal exotoxin that can be absorbed into the blood stream) (Priyadarshi et al., 2010). Enoyl-[acyl-carrier-protein] reductase (FabI) is an enzyme of the FAS II system (a group of fatty acid synthases that catalyze fatty acids synthesis many types of bacteria and plants).
This enzyme is also crucial for other types of bacteria such as Pseudomonas aeruginosa. Accordingly, SAP and FabI were selected for with the studied molecules in order to relate the in-silico results with those of the experimental antibacterial tests.
Docking results indicated that the theoretical binding energies of the ligands are consistent with the experimental findings. For SAP (C. albicans), compound 9b showed the best binding as indicated by the calculated energy of binding (-10.304 kcal/mol). However, compound 12c demonstrated the highest binding energy (B.E., −8.736 kcal/mol) with the S. aureus protein, (FabI).
Finally, FabI receptor of P. aeruginosa shows the best affinity to compound 9b with a calculated B.E. of −9.583 kcal/mol. It is worth mentioning that the arene-H dominated the types of receptor-ligand interactions in all of the above-mentioned cases, Fig. 3.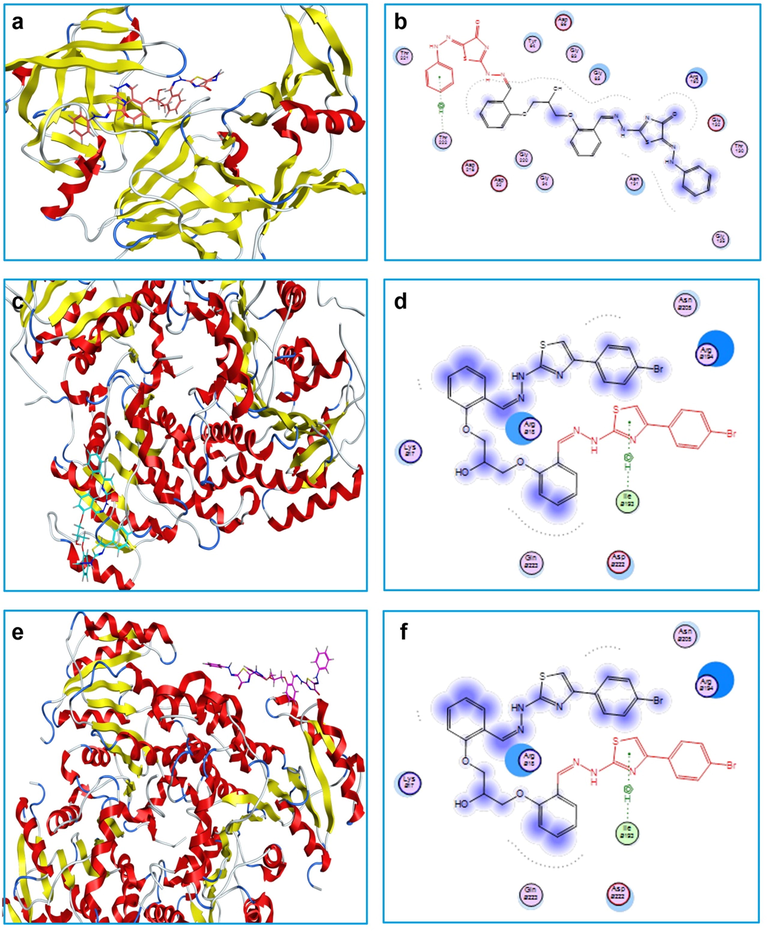
Best receptor-ligand interaction poses (a, c and e) and ligand interaction diagrams (b, d and f) of compounds 9b (with SAP of C. albicans), 12c (with FabI of S. aureus) and 9b (with FabI of P. aeruginosa), respectively.
Clearly, the empirical MIC values of the investigated compounds are in good agreement with the theoretically computed binding energies. Consequently, compound 9b may be suitable for inhibiting C. albicans and P. aeruginosa growth, while compound 12c is suitable for retarding the growth of S. aureus.
3 Materials and methods
3.1 Synthesis and characterization
Melting points were determined on a Gallenkamp apparatus and are uncorrected. IR spectra were recorded via the KBr-disc method using a Pye-Unicam SP300 spectrophotometer. 1H and 13C NMR spectra were recorded in deuterated DMSO‑d6 using a Varian Gemini 300 NMR spectrometer (300 MHz for 1H NMR and 75 MHz for 13C NMR) and the chemical shifts were related to that of the solvent DMSO‑d6. Mass spectra were recorded with the aid of a GCMS-Q1000-EX Shimadzu and GCMS 5988-A HP spectrometers by applying an ionizing voltage of 70 eV. Elemental analyses were performed at the Microanalytical Centre, Faculty of Science, Cairo University, Giza, Egypt.
3.2 Synthesis of Bis-thiosemicarbazone derivative 3
A mixture of Hydroxy-bis-aldeyde1(3.0031 g, 0.01 mol), thiosemicarbazide(0.01 mol) and a few drops of conc. HCl in ethanol (30 mL) was refluxed for 3 h. The solid product which formed after cooling was collected and recrystallized from ethanol/dioxane as yellow crystals,72% yield; mp 229–230 °C; IR (KBr): v 3426 (OH), 3356,3252, 3147 (NH2 and NH), 3062, 2986 (C-H) cm−1; 1H NMR (DMSO‑d6):δ4.19–4.25 (m, 5H, 2CH2, CH-OH), 5.39 (s, 1H, OH),6.96–6.98 (t, 2H, Ar-H), 7.10–7.12 (d, 2H, Ar-H), 7.35–7.39 (t, 2H, Ar-H), 7.92 (s, 2H, 2NH), 8.05–8.07 (d, 2H, Ar-H), 8.12 (s, 2H, 2NH), 8.50 (s, 2H, 2CH = N-), 11.37 (s, 2H, 2NH) ppm; 13C NMR (DMSO‑d6): δ68.2 (CH-OH), 69.7(CH2O), 113.1, 121.2, 122.8, 126.5, 131.7, 139.0, 157.5 (Ar-C and N = C), 178.2 (C = S) ppm; MS m/z (%): 446 (M+, 16), 435 (33), 410 (1 0 0), 373 (51), 320 (78), 299 (35), 210 (46), 126 (58), 60 (66)ppm; Anal. Calcd for C19H22N6O3S2(446.54): C, 51.11; H, 4.97; N, 18.82. Found: C, 51.45; H, 4.65; N, 18.57%.
3.2.1 Synthesis of bis-thiazole derivatives 6a-g, 9a-e and 12a-c
General procedure:To a stirred solution of thiosemicarbazone derivative 3(1.0 g, 2.5 mmol) and hydrazonoyl chloride derivative 4a-g, 7a-e,orphenacyl bromide 10a-c (2.5 mmol) in dioxane (30 mL), was added triethylamine (0.7 mL) and the mixture was refluxed for 5 h. The precipitated triethylamine hydrochloride was filtered off, and the solvent of the filtrate was subjected to evaporattion under reduced pressure. The residue was triturated with methanol. The formed solid was collected by filtration, washed with water, dried, and recrystallized from dioxane to afford the corresponding bis-thiazole derivatives 6a-g,9a-e or 12a-c respectively.
3.2.2 1,3-Bis(2-((2-(4-methyl-5-(phenyldiazenyl)thiazol-2-yl)hydrazono)methyl)phenoxy)propan-2-ol (6a)
Red solid, 74% yield; mp. 190–191 °C; IR (KBr): v 3407 (2NH), 3037, 2933 (C-H) cm−1; 1H- NMR (DMSO‑d6):δ 2.45 (s, 6H, 2CH3), 4.19–4.28 (m, 4H, 2CH2), 5.37 (s, 1H, OH), 5.70–5.71 (m, 1H, CH-OH), 6.96–7.92 (m, 18H, Ar-H), 8.50 (s, 2H, 2CH = N), 11.37 (s, 2H, 2NH) ppm; MS m/z (%): 731 (M+, 30), 621 (47), 594 (44), 515 (36), 489 (1 0 0), 383 (23), 353 (31), 245 (54), 164 (16), 41 (65).Anal. Calcd for C37H34N10O3S2(730.87): C, 60.81; H, 4.69; N, 19.16. Found: C, 60.53; H, 4.40; N, 18.85%.
3.2.3 1,3-Bis(2-((2-(4-methyl-5-(p-tolyldiazenyl)thiazol-2-yl)hydrazono)methyl)phenoxy)propan-2-ol (6b)
Red solid, 71% yield; mp. 186–187 °C; IR (KBr): v 3410 (2NH), 3024, 2921 (C-H) cm−1; 1H- NMR (DMSO‑d6):δ1.90 (s, 6H, 2CH3), 2.50 (s, 6H, 2CH3), 4.73–4.76 (m, 4H, 2CH2), 5.36 (m, 1H, CH-OH), 5.64 (s, 1H, OH), 7.03–8.05 (m, 16H, Ar-H), 8.12 (s, 2H, 2CH = N-), 10.41 (s, 2H, 2NH) ppm; 13C-NMR (DMSO-d6): δ 16.7, 18.9, 19.8, 22.5, 58.6, 65.4, 112.9, 114.1, 116.6, 120.7, 128.6, 129.9, 131.7, 132.5, 139.5, 143.9, 157.5 (Ar-H and C=N) ppm; MS m/z (%): 759 (M+, 26), 716 (31), 670 (34), 617 (53), 596 (65), 510 (28), 480 (61), 407 (60), 380 (55), 313 (72), 289 (68), 226 (34), 179 (55), 102 (33), 43 (1 0 0).Anal. Calcd for C39H38N10O3S2 (758.92): C, 61.72; H, 5.05; N, 18.46. Found: C, 61.50; H, 4.78; N, 18.28%.
3.2.4 1,3-Bis(2-((2-(5-((4-chlorophenyl)diazenyl)-4-methylthiazol-2-yl)hydrazono)methyl) phenoxy)propan-2-ol (6c)
Red solid, 74% yield; mp. 151–152 °C; IR (KBr):v 3406 (2NH), 3098, 2927 (C-H) cm−1; 1H NMR (DMSO‑d6):δ 2.57 (s, 6H, 2CH3), 4.21–4.26 (m, 4H, 2CH2), 5.40 (s, 1H, OH),5.69–5.70 (m, 1H, CH-OH), 6.94–7.53 (m, 16H, Ar-H), 8.50 (s, 2H, 2CH = N-), 10.66 (s, 2H, 2NH) ppm;MS m/z (%): 799 (M+, 22), 720 (45), 687 (63), 608 (39), 545 (1 0 0), 480 (69), 325 (34), 207 (21), 167 (44), 149 (84), 135 (70), 44 (51). Anal. Calcd for C37H32Cl2N10O3S2 (799.75): C, 55.57; H, 4.03; N, 17.51. Found: C, 55.35; H, 3.75; N, 17.25%.
3.2.5 1,3-Bis(2-((2-(4-methyl-5-((4-nitrophenyl)diazenyl)thiazol-2-yl)hydrazono)methyl)phenoxy) propan-2-ol (6d)
Red solid, 76% yield; mp. 169–170 °C; IR (KBr): v 3412, 333 (2NH), 3073, 2925 (CH) cm−1; 1H- NMR (DMSO‑d6):δ 2.35 (s, 6H, 2CH3), 4.19–4.28 (m, 4H, 2CH2), 5.35 (s, 1H, OH), 5.68–5.70 (m, 1H, CH-OH), 7.01–8.12 (m, 16H, Ar-H), 8.95 (s, 2H, 2CH = N-), 11.36 (s, 2H, 2NH); MS m/z (%): 820 (M+, 10), 792 (22), 671 (18), 595 (20), 460 (16), 358 (19), 291 (20), 181 (41), 91 (51), 66 (78), 45 (1 0 0).Anal. Calcd for C37H32N12O7S2(820.86): C, 54.14; H, 3.93; N, 20.48. Found: C, 54.45; H, 3.65; N, 20.20 %.
3.2.6 1,3-Bis(2-((2-(5-((4-bromophenyl)diazenyl)-4-methylthiazol-2-yl)hydrazono)methyl) phenoxy)propan-2-ol (6e)
Red solid, 78% yield; mp. 148–149 °C; IR (KBr): v 3418 (2NH), 3074, 2925 (CH) cm−1; 1H NMR (DMSO‑d6):δ 2.56 (s, 6H, 2CH3), 4.03–4.33 (m, 5H, 2CH2, CH-OH), 5.62 (s, 1H, OH), 7.05–7.97 (m, 16H, Ar-H), 8.96 (s, 2H, 2CH = N-), 10.62 (s, 2H, 2NH); 13C-NMR (DMSO-d6): δ 18.9, 23.6, 63.2, 68.0, 116.5, 118.4, 120.1, 121.3, 122.4, 123.7, 125.6, 126.3, 128.1, 132.3, 139.1, 170.8 (Ar-H and C=N) ppm; MS m/z (%): 888 (M+, 25), 785 (48), 659 (27), 508 (28), 413 (1 0 0), 303 (31), 258 (50), 182 (56), 126 (65), 104 (75), 62 (92).Anal. Calcd for C37H32Br2N10O3S2(888.66): C, 50.01; H, 3.63; N, 15.76. Found: C, 49.76; H, 3.45; N, 15.55%.
3.2.7 1,3-Bis(2-((2-(5-((2,4-dichlorophenyl)diazenyl)-4-methylthiazol-2-yl)hydrazono)methyl) phenoxy)propan-2-ol (6f)
Red solid, 69% yield; mp. 180–181 °C; IR (KBr): v 3410 (2NH), 3300, 2929 (CH) cm−1; 1H NMR (DMSO‑d6):δ 2.60 (s, 6H, 2CH3), 4.19–4.25 (m, 4H, 2CH2), 5.36 (s, 1H, OH), 5.40–5.42 (m, 1H, CH-OH), 6.94–8.12 (m, 14H, Ar-H), 8.51 (s, 2H, 2CH = N-), 11.36 (s, 2H, 2NH); MS m/z (%): 868 (M+, 11), 852 (24), 816 (39), 763 (20), 728 (17), 658 (19), 567 (13), 422 (1 0 0), 386 (28), 243 (73), 148 (20), 85 (40).Anal. Calcd for C37H30Cl4N10O3S2 (868.63): C, 51.16; H, 3.48; N, 16.13. Found: C, 51.53; H, 3.15; N, 15.89%.
3.2.8 1,3-Bis(2-((2-(4-methyl-5-((2,4,6-tribromophenyl)diazenyl)thiazol-2-yl)hydrazono)methyl) phenoxy)propan-2-ol (6 g)
Red solid, 70% yield; mp. 163–164 °C; IR (KBr):v 3402 (2NH), 3175, 2923 (CH) cm−1; 1H NMR (DMSO‑d6):δ 2.50 (s, 6H, 2CH3), 4.19–4.25 (m, 4H, 2CH2), 4.53 (s, 1H, OH), 5.65 (m, 1H, CH-OH), 6.94–8.13 (m, 12H, Ar-H), 8.50 (s, 2H, 2CH = N-), 11.36 (s, 2H, 2NH)ppm; 13C-NMR (DMSO-d6): δ 28.9, 32.1, 65.6, 89.5, 113.5, 116.6, 118.3, 120.9, 123.7, 125.5, 128.2, 129.9, 130.8, 34.3, 134.9, 147.9 (Ar-H and C=N) ppm; MS m/z (%): 1204 (M+, 18), 994 (13), 891 (18), 730 (30), 693 (26), 660 (33), 545 (38), 478 (76), 406 (35), 368 (55), 341 (23), 204 (19), 176 (23), 116 (1 0 0), 96 (42).Anal. Calcd for C37H28Br6N10O3S2 (1204.24): C, 36.90; H, 2.34; N, 11.63. Found: C, 37.17; H, 2.11; N, 11.51 %.
3.2.9 2,2′-(((((2-Hydroxypropane-1,3-diyl)bis(oxy))bis(2,1-phenylene))bis(methanylylidene)) bis(hydrazin-1-yl-2-ylidene))bis(5-(2-phenylhydrazono)thiazol-4(5H)-one) (9a)
Yellow solid, 76% yield; mp. 170–172 °C; IR (KBr): v 3396, 3281 (4NH), 3173, 3033, 2975 (CH), 1678 (2C = O) cm−1; 1H NMR (DMSO‑d6):δ4.02–4.21 (m, 5H, 2CH2, CH-OH), 5.42 (s, 1H, OH), 7.01–7.92 (m, 18H, Ar-H), 8.82 (s, 2H, 2CH = N-), 10.81 (s, 2H, 2NH), 11.36 (s, 2H, 2NH) ppm; MS m/z (%): 734 (M+, 35), 582 (48), 651 (16), 563 (34), 519 (14), 448 (1 0 0), 422 (68), 412 (31), 349 (39), 317 (22), 292 (20), 218 (26), 166 (57), 143 (39), 80 (38). Anal. Calcd for C35H30N10O5S2 (734.81): C, 57.21; H, 4.12; N, 19.06. Found: C, 57.00; H, 3.94; N, 18.88 %.
3.2.10 2,2′-(((((2-Hydroxypropane-1,3-diyl)bis(oxy))bis(2,1-phenylene))bis(methanylylidene))bis (hydrazin-1-yl-2-ylidene))bis(5-(2-(p-tolyl)hydrazono)thiazol-4(5H)-one) (9b)
Yellow solid, 73% yield; mp. 175–176 °C; IR (KBr): v 3403, 3286 (4NH), 3073, 2975 (CH), 1689 (2C = O) cm−1; 1H NMR (DMSO‑d6):δ 2.42 (s, 6H, 2CH3), 4.05–4.35 (m, 4H, 2CH2, CH-OH), 5.40 (s, 1H, OH), 6.94–7.91 (m, 16H, Ar-H), 8.50 (s, 2H, 2CH = N-), 10.26 (s, 2H, 2NH), 11.36 (s, 2H, 2NH) ppm; 13C-NMR (DMSO-d6): δ 14.4, 28.8, 32.0, 61.9, 115.7, 116.5, 120.7, 122.4, 127.5, 129.6, 133.2, 137.9, 142.1, 143.1, 152.1 (Ar-H and C=N) , 162 (2C=O) ppm; MS m/z (%): 763 (M+, 7), 737 (8), 639 (8), 562 (15), 509 (13), 412 (1 0 0), 385 (15), 216 (35), 164 (22), 120 (24), 91 (27), 61 (20). Anal. Calcd for C37H34N10O5S2 (762.86): C, 58.26; H, 4.49; N, 18.36. Found: C, 58.55; H, 4.24; N, 18.14%.
3.2.11 2,2′-(((((2-Hydroxypropane-1,3-diyl)bis(oxy))bis(2,1-phenylene))bis(methanylylidene))bis (hydrazin-1-yl-2-ylidene))bis(5-(2-(4-chlorophenyl)hydrazono)thiazol-4(5H)-one) (9c)
Yellow solid, 74% yield; mp. 200–201 °C; IR (KBr): v 3430, 3263 (4NH), 3157, 2978 (CH), 1707 (C = O) cm−1; 1H NMR (DMSO‑d6):δ4.03–4.24 (m, 5H, 2CH2, CH-OH), 5.36 (s, 1H, OH), 6.94–8.14 (m, 16H, Ar-H), 8.50 (s, 2H, 2CH = N-), 10.64 (s, 2H, 2NH), 11.36 (s, 2H, 2NH) ppm; 13C-NMR (DMSO-d6): δ 63.2, 67.9, 69.6, 113.0, 121.2, 122.8, 125.3, 126.5, 129.5, 130.2, 131.8, 139.0, 157.5, 163.5 (Ar-H and C=N), 178.1 (2C=O) ppm; MS m/z (%): 803 (M+, 7), 764 (8), 667 (6), 602 (13), 544 (3), 421 (10), 333 (95), 318 (64), 251 (53), 214 (28), 173 (36), 147 (69), 118 (58), 91 (48), 65 (1 0 0).Anal. Calcd for C35H28Cl2N10O5S2 (803.69): C, 52.31; H, 3.51; N, 17.43. Found: C, 52.55; H, 3.30; N, 17.16%.
3.2.12 2,2′-(((((2-Hydroxypropane-1,3-diyl)bis(oxy))bis(2,1-phenylene))bis(methanylylidene))bis (hydrazin-1-yl-2-ylidene))bis(5-(2-(4-nitrophenyl)hydrazono)thiazol-4(5H)-one) (9d)
Yellow solid, 70% yield; mp. 190–192 °C; IR (KBr):v 3446, 3204 (4NH), 3058, 2947 (C-H), 1708 (C = O) cm−1; 1H NMR (DMSO‑d6):δ 2.22 (s, 6H, 2CH3), 4.17–4.28 (m, 5H, 2CH2, CH-OH), 5.64 (s, 1H, OH), 6.94–8.06 (m, 16H, Ar-H), 8.80 (s, 2H, 2CH = N-), 10.54 (s, 2H, 2NH), 11.35 (s, 2H, 2NH) ppm; MS m/z (%): 825 (M+, 60), 737 (35), 707 (25), 650 (29), 551 (48), 513 (30), 474 (61), 388 (31), 341 (35), 268 (30), 214 (32), 199 (23), 75 (88), 57 (1 0 0). Anal. Calcd for C35H28N12O9S2(824.80): C, 50.97; H, 3.42; N, 20.38;. Found: C, 51.19; H, 3.19; N, 20.17%.
3.2.13 2,2′-(((((2-Hydroxypropane-1,3-diyl)bis(oxy))bis(2,1-phenylene))bis(methanylylidene))bis (hydrazin-1-yl-2-ylidene))bis(5-(2-(2,4-dichlorophenyl)hydrazono)thiazol-4(5H)-one) (9e)
Yellow solid, 73% yield; mp. 188–189 °C; IR (KBr): v 3425, 3264 (4NH), 3154, 2974 (CH), 1716 (2C = O) cm−1; 1H NMR (DMSO‑d6):δ4.22–4.25 (m, 5H, 2CH2, CH-OH), 5.37 (s, 1H, OH), 6.94–8.12 (m, 14H, Ar-H), 8.50 (s, 2H, 2CH = N-), 9.42 (s, 2H, 2NH), 11.37 (s, 2H, 2NH) ppm: MS m/z (%): 872 (M+, 26), 828 (92), 763 (22), 752 (35), 678 (26), 608 (37), 576 (16), 431 (23), 310 (1 0 0), 280 (70), 138 (62), 111 (53), 77 (50) . Anal. Calcd for C35H26Cl4N10O5S2(872.58): C, 48.18; H, 3.00; N, 16.05. Found: C, 48.36; H, 2.84; N, 15.85%.
3.2.14 1,3-Bis(2-((2-(4-(4-chlorophenyl)thiazol-2-yl)hydrazono)methyl)phenoxy)propan-2-ol (12a)
Brown solid, 70% yield; mp. 255–256 °C; IR (KBr): v 3412 (2NH), 3062, 2928 (CH) cm−1; 1H- NMR (DMSO‑d6):δ4.25–4.29 (m, 5H, 2CH2, CH-OH), 5.70 (s, 1H, OH), 7.28 (s, 2H, 2CH-thiazole-H), 7.55–8.36 (m, 16H, Ar-H), 8.79 (s, 2H, 2CH = N-), 10.44 (s, 2H, 2NH) ppm; 13C-NMR (DMSO-d6): δ 55.7, 56.2, 114.2, 115.1, 117.9, 119.4, 121.3, 122.3, 125.0, 130.6, 132.4, 136.8, 160.4, 165.1, 166.9 (Ar-H and C=N) ppm; MS m/z (%): 715 (M+, 31), 696 (18), 615 (22), 573 (43), 500 (39), 480 (44), 422 (49), 396 (32), 360 (31), 210 (1 0 0), 171 (37), 143 (20), 117 (42). Anal. Calcd for C35H28Cl2N6O3S2(715.67): C, 58.74; H, 3.94; N, 11.74. Found: C, 59.00; H, 3.72; N, 11.52%.
3.2.15 1,3-Bis(2-((2-(4-(4-bromophenyl)thiazol-2-yl)hydrazono)methyl)phenoxy)propan-2-ol (12b)
Brown solid, 76% yield; mp. 250–251 °C; IR (KBr): v 3387 (2NH), 3063, 2926 (CH) cm−1; 1H- NMR (DMSO‑d6):δ 2.58 (s, 6H, 2CH3), 4.12–4.22 (m, 5H, 2CH2, CH-OH), 5.65 (s, 1H, OH), 7.27 (s, 2H, 2CH-thiazole-H), 7.47–8.21 (m, 16H, Ar-H), 8.40 (s, 2H, 2CH = N-), 11.96 (s, 2H, 2NH) ppm; 13C-NMR (DMSO-d6): δ 32.8, 34.8, 66.8, 114.7, 118.2, 121.4, 128.7, 130.9, 131.1, 132.6, 133.8, 144.8, 153.3, 161.0, 162.6 (Ar-H and C=N) ppm; MS m/z (%): 804 (M+, 16), 774 (27), 741 (37), 663 (22), 607 (28), 575 (23), 512 (41), 483 (40), 465 (63), 348 (74), 283 (30), 186 (1 0 0), 166 (58), 102 (46), 75 (35). Anal. Calcd for C35H28Br2N6O3S2(804.58): C, 52.25; H, 3.51; N, 10.45. Found: C, 52.51; H, 3.33; N, 10.19%.
3.2.16 1,3-Bis(2-((2-(4-(4-methoxyphenyl)thiazol-2-yl)hydrazono)methyl)phenoxy)propan-2-ol (12c)
Brown solid, 65% yield; mp. 269–270 °C; IR (KBr):v 3403 (2NH), 3068, 2936 (CH) cm−1; 1H NMR (DMSO‑d6):δ 3.78 (s, 6H, 2OCH3), 4.21–4.24 (m, 5H, 2CH2, CH-OH), 5.59 (s, 1H, OH), 6.43–8.06 (m, 16H, Ar-H), 8.93 (s, 2H, 2CH-thiazole-H), 8.48 (s, 2H, 2CH = N-), 11.35 (s, 2H, 2NH) ppm; MS m/z (%): 706 (M+, 26), 688 (30), 642 (25), 576 (19), 512 (22), 480 (33), 422 (35), 355 (1 0 0), 313 (27), 239 (22), 151 (39), 101 (42). Anal. Calcd for C37H34N6O5S2(706.84): C, 62.87; H, 4.85; N, 11.89. Found: C, 63.20; H, 4.59; N, 11.61%.
3.3 In-vitro XTT assay
XTT assay (Al-Bakri and Afifi, 2007; Kuhn et al., 2003; Liang et al., 2012; Monteiro et al., 2012) is a non-radioactive spectrophotometric assay usually used for measuring cell viability and proliferation via the assessment of the cellular metabolic activity. The assay depends on the chemical reduction of the XTT dye (a yellow tetrazolium salt) to an orange formazan dye by living cells. The minimal inhibitory concentration values represent the lowest concentrations of test or standard drugs (vancomycin and amikacin for bacteria, and amphotericin B for fungi) that completely inhibit microbial growth. For determining the MIC values, the microdilution method was applied. Briefly, the microbial inoculum was prepared and the concentrations of the suspensions were adjusted to 106 CFU/mL. Solutions of the standard and test molecules were prepared in dimethyl sulfoxide (DMSO) and subsequent two-fold dilutions were carried out in a 96-well microplate. Each well of the microplate included 40 μL of the Brain Heart Infusion (BHI) growth medium, 10 μL of the microbial inoculum and 50 μL of the investigated molecules diluted to final concentrations of 1000–0.12 µg/mL. Pure DMSO was used as a blank. The plates were incubated for 24 h at 37 °C; thereafter, 40 μL of XTT tetrazolium salt were added. Consequently, the plates were incubated at 37 °C in a dark environment for 1 h, and then the color change was measured spectrophotometrically at 492 nm using a microtiterplate reader (Tecan Sunrise absorbance reader; Tecan UK, Reading, United Kingdom). MIC values were determined as the minimum concentrations that caused the largest color change compared to the blank.
3.4 Theoretical calculations
Geomtery optimizations were implemented, using DFT algorithms at the B3LYP/6-311G level of theory, with the aid of Gaussian 09 W package (Gomha et al., 2016a). The obtained molecular geometries and their corresponding molecular orbitals were visualized using GaussView 5.0.9 package. Becke’s three-parameter hybrid method (B3LYP) demonstrates more accurate results than the pure Hartree-Fock method (Gomha et al., 2016b). B3LYP represents the combinaton of the correlation functional of Lee, Yang and Parr with Becke’s three parameters (Lee et al., 1988).
3.5 Molecular docking
Molecular docking was performed using Molecular Operating Environment (MOE) 2014 software (Group, 2014). Compounds that showed the lowest MIC values in the XTT assay were selected and docked with the corresponding target proteins. The compounds were subjected to energy minimzation using the MMFF94x force field prior to docking. High resolution 3D molecular structures of the receptors (Table 3) were obtained from the Protein Data Bank (PDB).
PDB ID
Receptor name
Resolution
Organism
1EAG
Secreted aspartic proteinase (SAP2) from Candida albicanscomplexed with A70450
X-Ray Diffraction, 2.1 Å
Candida albicans
3GR6
Crystal structure of the Staphylococcus aureusenoyl-acyl carrier protein reductase (FabI) in complex with NADP and triclosan
X-Ray Diffraction, 2.28 Å
Staphylococcus aureus
4NR0
Crystal structure of the Pseudomonas aeruginosaenoyl-acyl carrier protein reductase (FabI) in complex with NAD + and triclosan
X-Ray Diffraction, 1.779 Å
Pseudomonas aeruginosa
4 Conclusions
Finally, we succeeded to synthesize novel carbazones as precursors for the preparation of a novel series of bis-thiazole and bis-thiazolone derivatives. The mechanistic pathways and the chemical structures of all synthesized derivatives were explained and confirmed relying on the available spectral data. The antimicrobial activity results of the tested bis-thiazole and bis-thiazolone showed that some derivatives have moderate to no activity against the tested fungal and bacterial strains. Only one derivative (9b), being the most active against all bacteria and fungi, was equipotent to the standard antibiotic, vancomycin. Molecular docking simulations of the prepared compounds revealed the activity of compouns 9b (with SAP of C. albicans), 12c (with FabI of S. aureus) and 9b (with FabI of P. aeruginosa), respectively. DFT calculations were employed to obtain information about geometries, chemical reactivity and optical/electronic properties of the synthesized compounds. The candidate molecules are promising antimicrobial agents and can be used as lead compounds for the preparation of novel antibiotics for antibiotic-resistant bacterial species.
Author contributions
S.M.G. and R. M. K. conceived the experiment(s); S.M.G., R.M.K., A.S.A.D., M.M.A., S.A.A., M.E.Z and Z.A.M. conducted the experiment(s); S.M.G., R. M. K. and Z.A.M. analyzed the results. All authors reviewed the manuscript.
Acknowledgment
The authors extend their appreciation to the Deanship of Scientific Research at Imam Mohammad Ibn Saud Islamic University for funding this work through Research Group no-RG. 21-09-76.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- Synthesis and reactions of thiosemicarbazides, triazoles, and Schiff bases as antihypertensive α-blocking agents. Monatshefte für Chemie - Chem. Mon.. 2008;139(9):1083-1090.
- [CrossRef] [Google Scholar]
- Synthesis and Antimicrobial Evaluation of Some Novel Thiazole, 1,3,4-Thiadiazole and Pyrido[2,3-d][1,2,4]triazolo[4,3-a]pyrimidine Derivatives Incorporating Pyrazole Moiety. Heterocycles. 2015;91:2126-2142.
- [CrossRef] [Google Scholar]
- In vitro drug interaction of levocetirizine and diclofenac: Theoretical and spectroscopic studies. Spectrochim. Acta - Part A Mol. Biomol. Spectrosc.. 2017;181:239-248.
- [CrossRef] [Google Scholar]
- Experimental and quantum mechanical studies on the ion–pair of levocetirizine and bromocresol green in aqueous solutions. Spectrochim. Acta Part A Mol. Biomol. Spectrosc.. 2016;163:108-114.
- [CrossRef] [Google Scholar]
- Abo Dena, A.S., Muhammad, Z.A., Hassan, W.M.I., 2019. Spectroscopic, DFT studies and electronic properties of novel functionalized bis-1,3,4-thiadiazoles. Chem. Pap. https://doi.org/10.1007/s11696-019-00833-7
- Evaluation of antimicrobial activity of selected plant extracts by rapid XTT colorimetry and bacterial enumeration. J. Microbiol. Methods. 2007;68(1):19-25.
- [CrossRef] [Google Scholar]
- Auterhoff, H., 1972. Textbook of Organic Medicinal and Pharmaceutical Chemistry. Herausgegeben von Ch.O. Wilson, O. Gisvold und R.G. Doerge. 6. Auflage, 1053 Seiten. J.B. Lippincott Co, Philadelphia and Toronto, und Blackwell Scientific Publications, Oxford and Edinburgh 1971. Arch. Pharm. (Weinheim). 305, 316. https://doi.org/10.1002/ardp.19723050418
- The crystal structure of a major secreted aspartic proteinase from Candida albicans in complexes with two inhibitors. Structure. 1995;3(11):1261-1271.
- [Google Scholar]
- Synthesis and Anti-cancer Activity of 1,3,4-Thiadiazole and 1,3-Thiazole Derivatives Having 1,3,4-Oxadiazole Moiety. J. Heterocycl. Chem.. 2015;52:1400-1405.
- [CrossRef] [Google Scholar]
- 2-Amino-5-thiazolyl motif: A novel scaffold for designing anti-inflammatory agents of diverse structures. Eur. J. Med. Chem.. 2008;43(1):129-134.
- [CrossRef] [Google Scholar]
- Heterocyclisation of 2,5-diacetyl-3,4-disubstituted-thieno[2,3-b]Thiophene Bis-Thiosemicarbazones Leading to Bis-Thiazoles and Bis-1,3,4-thiadiazoles as Anti-breast Cancer Agents. J. Chem. Res.. 2016;40(2):120-125.
- [CrossRef] [Google Scholar]
- Synthesis and Characterization of Some New Bis-Pyrazolyl-Thiazoles Incorporating the Thiophene Moiety as Potent Anti-Tumor Agents. Int. J. Mol. Sci.. 2016;17(9):1499.
- [CrossRef] [Google Scholar]
- Synthesis and Antihypertensive α-Blocking Activity Evaluation of Thiazole Derivatives Bearing Pyrazole Moiety. Heterocycles. 2015;91:1763-1773.
- [CrossRef] [Google Scholar]
- A convenient ultrasound-promoted synthesis of some new thiazole derivatives bearing a coumarin nucleus and their cytotoxic activity. Molecules. 2012;17:9335-9347.
- [CrossRef] [Google Scholar]
- Synthesis and anticancer activity of arylazothiazoles and 1,3,4-thiadiazoles using chitosan-grafted-poly(4-vinylpyridine) as a novel copolymer basic catalyst. Chem. Heterocycl. Compd.. 2015;51(11-12):1030-1038.
- [CrossRef] [Google Scholar]
- Group, C.C., 2014. Molecular Operating Environment.
- Unraveling the nature of interaction between substituted phenol and amiodarone. Anal. Chem.. 2014;86(3):1881-1886.
- [CrossRef] [Google Scholar]
- Adamantane derivatives of thiazolyl-N-substituted amide, as possible non-steroidal anti-inflammatory agents. Eur. J. Med. Chem.. 2009;44(3):1198-1204.
- [CrossRef] [Google Scholar]
- Uses and limitations of the XTT assay in studies of Candida growth and metabolism. J. Clin. Microbiol.. 2003;41(1):506-508.
- [CrossRef] [Google Scholar]
- Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B. 1988;37(2):785-789.
- [CrossRef] [Google Scholar]
- Antimicrobial activities of endophytic fungi isolated from Ophiopogon japonicus (Liliaceae) BMC Complement. Altern. Med.. 2012;12:238.
- [CrossRef] [Google Scholar]
- Antimicrobial Activity of Some New Thioureides Derived from 2-(4-Chlorophenoxymethyl)benzoic Acid. Molecules. 2008;13(3):567-580.
- [CrossRef] [Google Scholar]
- Optimized Anti-pathogenic Agents Based on Core/Shell Nanostructures and 2-((4-Ethylphenoxy)ethyl)-N-(substituted-phenylcarbamothioyl)-benzamides. Int. J. Mol. Sci.. 2012;13:12584-12597.
- [CrossRef] [Google Scholar]
- Synthesis, Spectroscopic Properties and Antipathogenic Activity of New Thiourea Derivatives. Molecules. 2011;16:7593-7607.
- [CrossRef] [Google Scholar]
- Synthesis, Antimicrobial Evaluation and Molecular Docking of New Functionalized Bis(1,3,4-Thiadiazole) and Bis(Thiazole) Derivatives. Polycycl. Aromat. Compd. 2020:1-13.
- [CrossRef] [Google Scholar]
- A new approach to drug discovery: high-throughput screening of microbial natural extracts against Aspergillus fumigatus using resazurin. J. Biomol. Screen.. 2012;17(4):542-549.
- [CrossRef] [Google Scholar]
- A study of the antimicrobial activity of selected synthetic and naturally occurring quinolines. Int. J. Antimicrob. Agents. 2010;35(1):30-38.
- [CrossRef] [Google Scholar]
- Discovery and optimization of a series of 2-aminothiazole-oxazoles as potent phosphoinositide 3-kinase gamma inhibitors. Bioorg. Med. Chem. Lett.. 2012;22:7534-7538.
- [CrossRef] [Google Scholar]
- N, N-dimethylbiguanide complexes displaying low cytotoxicity as potential large spectrum antimicrobial agents. Eur. J. Med. Chem.. 2010;45(7):3027-3034.
- [CrossRef] [Google Scholar]
- Synthesis and characterization of macrocyclic compounds with a hydroxyl functional group. Chinese Chem. Lett.. 2013;24(10):869-872.
- [CrossRef] [Google Scholar]
- Efficient synthesis of 2,4-disubstituted thiazoles using ionic liquid under ambient conditions: a practical approach towards the synthesis of Fanetizole. Tetrahedron. 2007;63:11066-11069.
- [CrossRef] [Google Scholar]
- Structural insights into Staphylococcus aureus enoyl-ACP reductase (FabI), in complex with NADP and triclosan. Proteins Struct. Funct. Bioinforma.. 2010;78(2):480-486.
- [CrossRef] [Google Scholar]
- Rajanarendar, E., Ramakrishna, S., Murthy], K. [Rama, 2012. Synthesis of novel isoxazolyl bis-thiazolo[3,2-a]pyrimidines. Chinese Chem. Lett. 23, 899–902. https://doi.org/https://doi.org/10.1016/j.cclet.2012.06.029
- One-Pot Synthesis of Novel Thiazoles as Potential Anti-Cancer Agents. Drug Des. Devel. Ther.. 2020;14:1363-1375.
- [CrossRef] [Google Scholar]
- 4-Thiazolidinone – A biologically active scaffold. Eur. J. Med. Chem.. 2008;43:897-905.
- [CrossRef] [Google Scholar]
- Small-molecule inhibitors of SREBP activation – potential for new treatment of metabolic disorders. Med. Chem. Commun.. 2013;4:1422-1433.
- [CrossRef] [Google Scholar]
- Studies on the syntheses of hydroxy-bearing benzo-azacrown ethers and their complexing behaviour. Polyhedron. 1996;15:1197-1202.
- [CrossRef] [Google Scholar]
Appendix A
Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.arabjc.2021.103396.
Appendix A
Supplementary data
The following are the Supplementary data to this article:







